Microfluidics-Assisted Formulation of Polymeric Oxytocin Nanoparticles for Targeted Brain Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of BSA Nanoparticles Using a 5-Input Microfluidic Chip
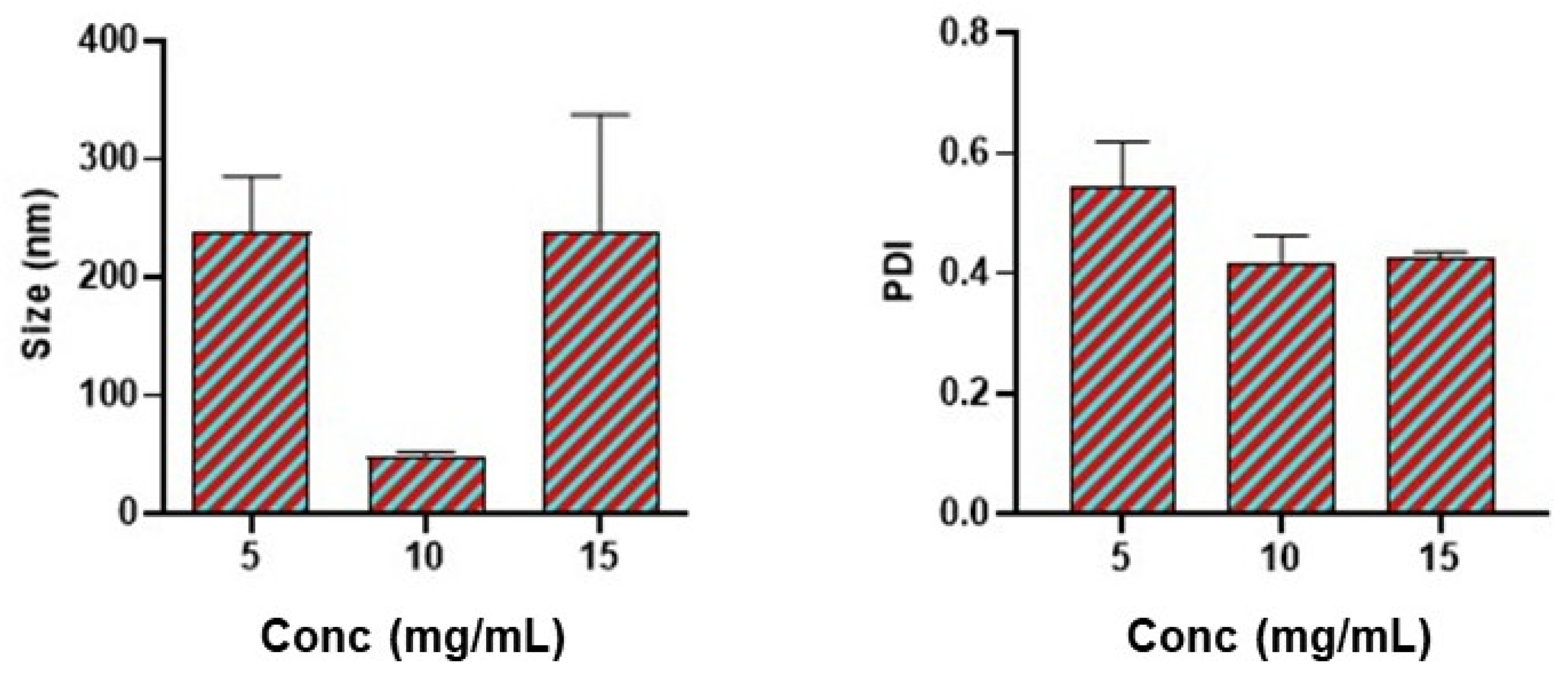
2.3. Effect of Polymer Concentration
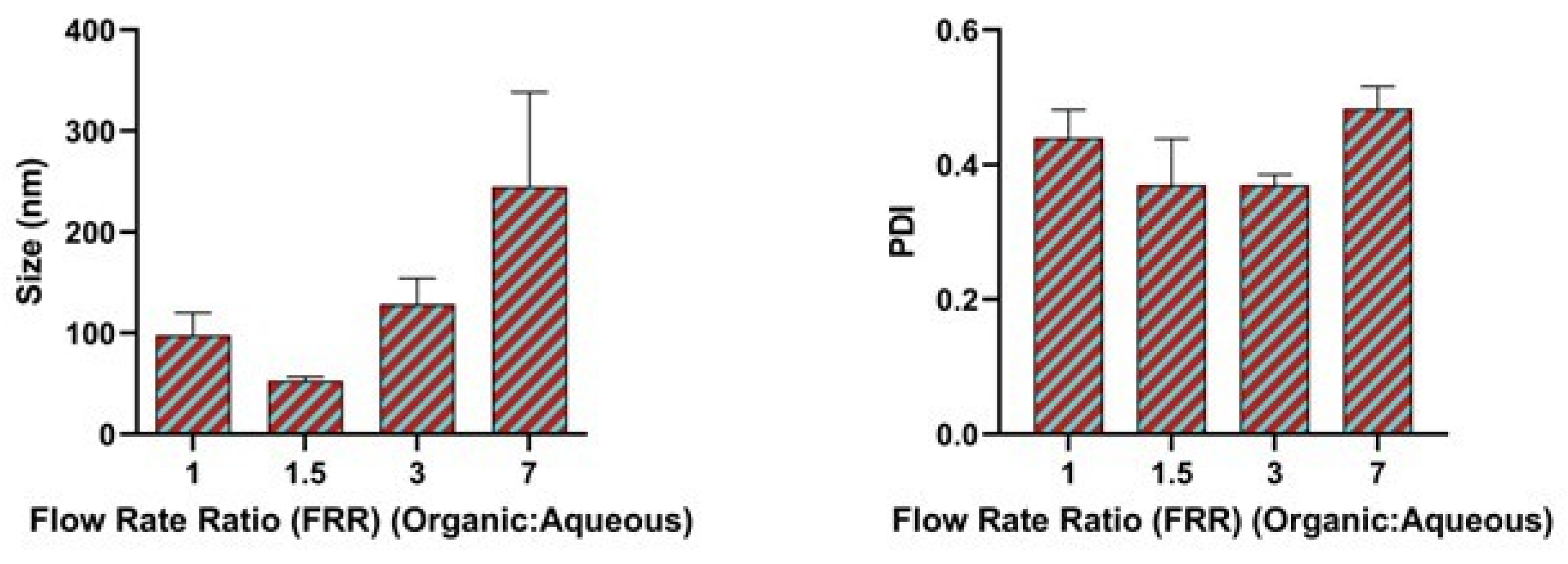
2.4. Effect of Flow Rate Ratio
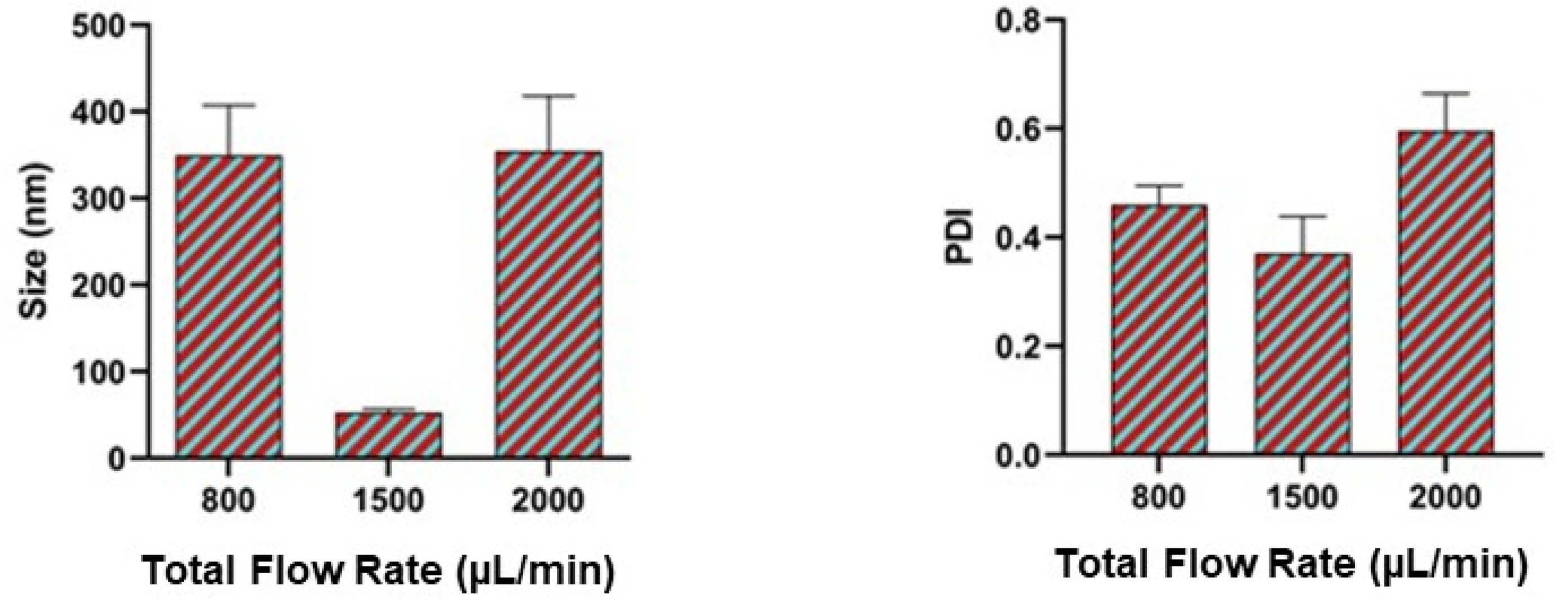
2.5. Effect of Total Flow Rate
2.6. Effect of Binary Organic Solvent Mixture
2.7. Formulation of OT-Loaded BSA NP Using a 5-Input Chip
2.8. Conjugation of BSA Polymeric Nanoparticles to Rabies Virus Glycoprotein (RVG)
2.9. Characterization of RVG Conjugated Oxytocin BSA Nanoparticles
2.10. Encapsulation Efficiency of Oxytocin in BSA Nanoparticles
2.11. In-Vitro Drug Release
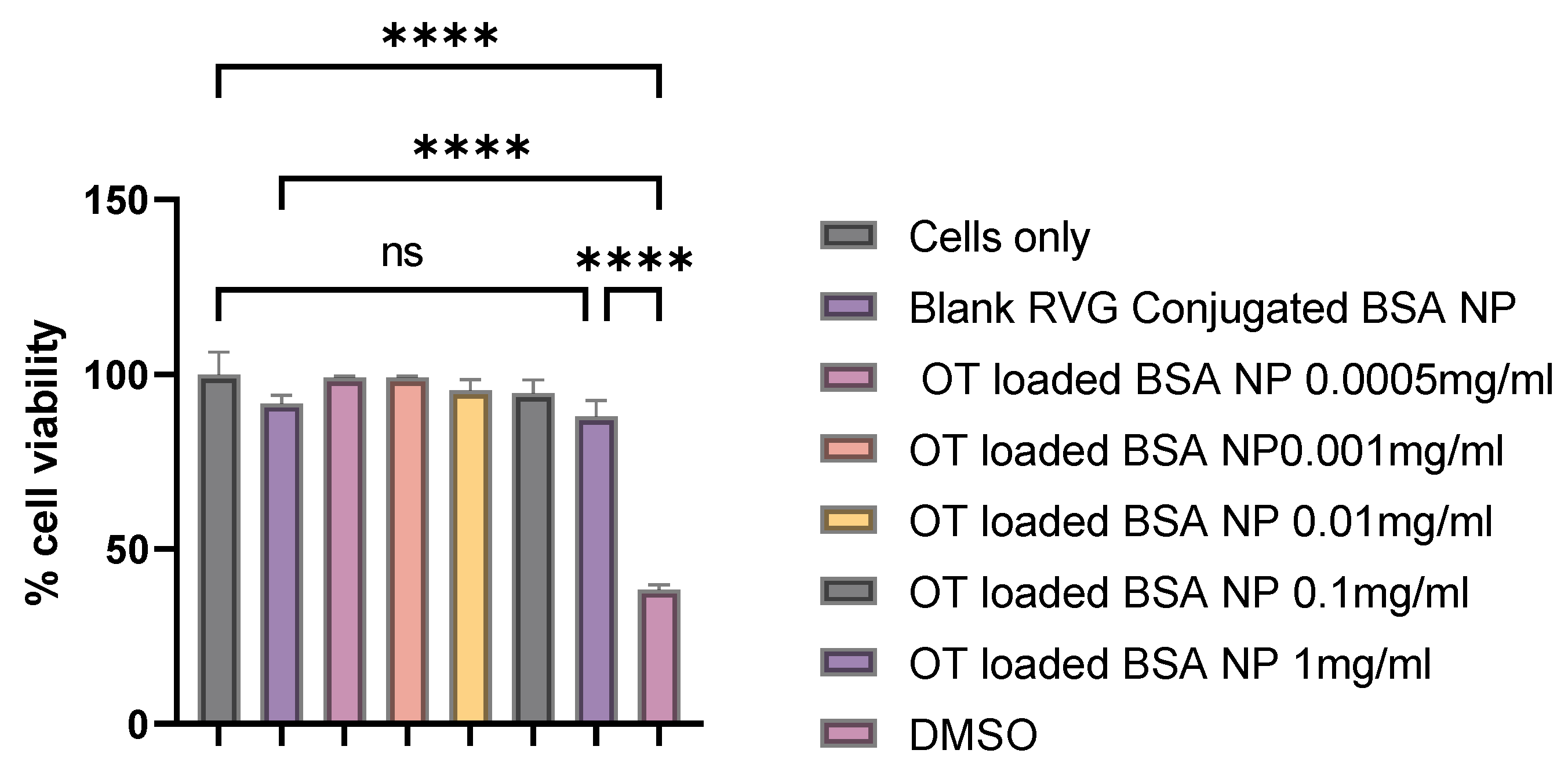
2.12. Cytotoxicity Assay
2.13. Statistical Analysis
3. Results and Discussion
3.1. Effect of Bovine Serum Albumin Concentration
3.2. Effect of Flow Rate Ratio
3.3. Effect of Total Flow Rate
3.4. Effect of Binary Organic Solvent Mixture
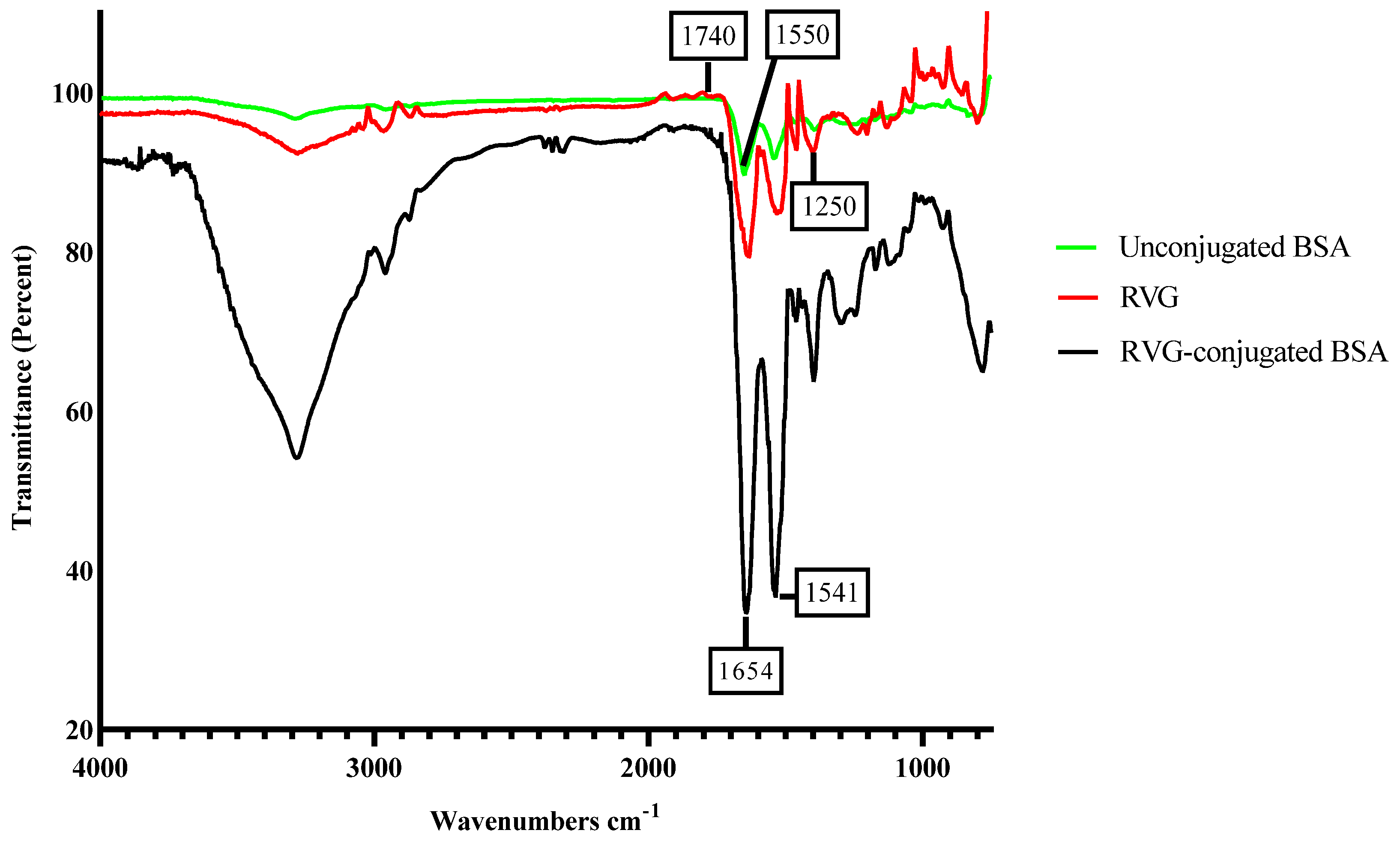
3.5. Characterization of RVG Conjugated Oxytocin BSA Nanoparticles
3.6. DLS Characterization of RVG Conjugated Oxytocin BSA Nanoparticles
3.7. Encapsulation Efficiency of Oxytocin into BSA Nanoparticles
3.8. In-Vitro Oxytocin Release Profile
3.9. Cytotoxicity Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hernández-Giottonini, K.Y.; Rodríguez-Córdova, R.J.; Gutiérrez-Valenzuela, C.A.; Peñuñuri-Miranda, O.; Zavala-Rivera, P.; Guerrero-Germán, P.; Lucero-Acuña, A. PLGA nanoparticle preparations by emulsification and nanoprecipitation techniques: Effects of formulation parameters. RSC Adv. 2020, 10, 4218–4231. [Google Scholar] [CrossRef] [PubMed]
- Rezvantalab, S.; Keshavarz Moraveji, M. Microfluidic assisted synthesis of PLGA drug delivery systems. RSC Adv. 2019, 9, 2055–2072. [Google Scholar] [CrossRef] [PubMed]
- Zoqlam, R.; Morris, C.J.; Akbar, M.; Alkilany, A.M.; Hamdallah, S.I.; Belton, P.; Qi, S. Evaluation of the Benefits of Microfluidic-Assisted Preparation of Polymeric Nanoparticles for DNA Delivery. Mater. Sci. Eng. C 2021, 127, 112243. [Google Scholar] [CrossRef]
- Luppi, B.; Bigucci, F.; Corace, G.; Delucca, A.; Cerchiara, T.; Sorrenti, M.; Catenacci, L.; Di Pietra, A.M.; Zecchi, V. Albumin nanoparticles carrying cyclodextrins for nasal delivery of the anti-Alzheimer drug tacrine. Eur. J. Pharm. Sci. 2011, 44, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Vrignaud, S.; Benoit, J.-P.; Saulnier, P. Strategies for the nanoencapsulation of hydrophilic molecules in polymer-based nanoparticles. Biomaterials 2011, 32, 8593–8604. [Google Scholar] [CrossRef]
- Han, F.Y.; Xu, W.; Kumar, V.; Cui, C.S.; Li, X.; Jiang, X.; Woodruff, T.M.; Whittaker, A.K.; Smith, M.T. Optimisation of a Microfluidic Method for the Delivery of a Small Peptide. Pharmaceutics 2021, 13, 1505. [Google Scholar] [CrossRef]
- Sharma, M.; Pachori, R.B.; Rajendra Acharya, U. A new approach to characterize epileptic seizures using analytic time-frequency flexible wavelet transform and fractal dimension. Pattern Recognit. Lett. 2017, 94, 172–179. [Google Scholar] [CrossRef]
- Hollander, E.; Novotny, S.; Hanratty, M.; Yaffe, R.; DeCaria, C.M.; Aronowitz, B.R.; Mosovich, S. Oxytocin Infusion Reduces Repetitive Behaviors in Adults with Autistic and Asperger’s Disorders. Neuropsychopharmacology 2003, 28, 193–198. [Google Scholar] [CrossRef]
- Insel, T.R. The Challenge of Translation in Social Neuroscience: A Review of Oxytocin, Vasopressin, and Affiliative Behavior. Neuron 2010, 65, 768–779. [Google Scholar] [CrossRef]
- Carson, D.S.; Guastella, A.J.; Taylor, E.R.; McGregor, I.S. A brief history of oxytocin and its role in modulating psychostimulant effects. J. Psychopharmacol. 2013, 27, 231–247. [Google Scholar] [CrossRef]
- Martins, D.A.; Mazibuko, N.; Zelaya, F.; Vasilakopoulou, S.; Loveridge, J.; Oates, A.; Maltezos, S.; Mehta, M.; Wastling, S.; Howard, M.; et al. Effects of route of administration on oxytocin-induced changes in regional cerebral blood flow in humans. Nat. Commun. 2020, 11, 1160. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Sinko, P.J. Drug delivery across the blood–brain barrier: Why is it difficult? how to measure and improve it? Expert Opin. Drug Deliv. 2006, 3, 419–435. [Google Scholar] [CrossRef]
- Bramosanti, M.; Chronopoulou, L.; Grillo, F.; Valletta, A.; Palocci, C. Microfluidic-assisted nanoprecipitation of antiviral-loaded polymeric nanoparticles. Colloids Surf. Physicochem. Eng. Asp. 2017, 532, 369–376. [Google Scholar] [CrossRef]
- Lababidi, N.; Sigal, V.; Koenneke, A.; Schwarzkopf, K.; Manz, A.; Schneider, M. Microfluidics as tool to prepare size-tunable PLGA nanoparticles with high curcumin encapsulation for efficient mucus penetration. Beilstein J. Nanotechnol. 2019, 10, 2280–2293. [Google Scholar] [CrossRef] [PubMed]
- Mares, A.G.; Pacassoni, G.; Marti, J.S.; Pujals, S.; Albertazzi, L. Formulation of tunable size PLGA-PEG nanoparticles for drug delivery using microfluidic technology. PLoS ONE 2021, 16, e0251821. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Albumin-based nanoparticles as potential controlled release drug delivery systems. J. Control. Release 2012, 157, 168–182. [Google Scholar] [CrossRef]
- da Silva, N.I.O.; Salvador, E.A.; Rodrigues Franco, I.; de Souza, G.A.P.; de Souza Morais, S.M.; Prado Rocha, R.; Dias Novaes, R.; Paiva Corsetti, P.; Malaquias, L.C.C.; Leomil Coelho, L.F. Bovine serum albumin nanoparticles induce histopathological changes and inflammatory cell recruitment in the skin of treated mice. Biomed. Pharmacother. 2018, 107, 1311–1317. [Google Scholar] [CrossRef]
- Yu, Z.; Yu, M.; Zhang, Z.; Hong, G.; Xiong, Q. Bovine serum albumin nanoparticles as controlled release carrier for local drug delivery to the inner ear. Nanoscale Res. Lett. 2014, 9, 343. [Google Scholar] [CrossRef]
- Cho, H.; Jeon, S.I.; Ahn, C.-H.; Shim, M.K.; Kim, K. Emerging Albumin-Binding Anticancer Drugs for Tumor-Targeted Drug Delivery: Current Understandings and Clinical Translation. Pharmaceutics 2022, 14, 728. [Google Scholar] [CrossRef]
- Almoustafa, H.A.; Alshawsh, M.A.; Chik, Z. Technical aspects of preparing PEG-PLGA nanoparticles as carrier for chemotherapeutic agents by nanoprecipitation method. Int. J. Pharm. 2017, 533, 275–284. [Google Scholar] [CrossRef]
- Operti, M.C.; Fecher, D.; van Dinther, E.A.W.; Grimm, S.; Jaber, R.; Figdor, C.G.; Tagit, O. A comparative assessment of continuous production techniques to generate sub-micron size PLGA particles. Int. J. Pharm. 2018, 550, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, R.; Babu, R.J.; Palakurthi, S. Nanomedicine Scale-up Technologies: Feasibilities and Challenges. AAPS PharmSciTech 2014, 15, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Son, S.; Hwang, D.W.; Singha, K.; Jeong, J.H.; Park, T.G.; Lee, D.S.; Kim, W.J. RVG peptide tethered bioreducible polyethylenimine for gene delivery to brain. J. Control. Release 2011, 155, 18–25. [Google Scholar] [CrossRef]
- Shrimal, P.; Jadeja, G.; Patel, S. A review on novel methodologies for drug nanoparticle preparation: Microfluidic approach. Chem. Eng. Res. Des. 2020, 153, 728–756. [Google Scholar] [CrossRef]
- Valencia, P.M.; Pridgen, E.M.; Rhee, M.; Langer, R.; Farokhzad, O.C.; Karnik, R. Microfluidic Platform for Combinatorial Synthesis and Optimization of Targeted Nanoparticles for Cancer Therapy. ACS Nano 2013, 7, 10671–10680. [Google Scholar] [CrossRef]
- Sanjay, S.T.; Zhou, W.; Dou, M.; Tavakoli, H.; Ma, L.; Xu, F.; Li, X. Recent advances of controlled drug delivery using microfluidic platforms. Adv. Drug Deliv. Rev. 2018, 128, 3–28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, Q.; Ma, Y.; Sun, J. Microfluidic Methods for Fabrication and Engineering of Nanoparticle Drug Delivery Systems. ACS Appl. Bio Mater. 2020, 3, 107–120. [Google Scholar] [CrossRef]
- Bally, F.; Garg, D.K.; Serra, C.A.; Hoarau, Y.; Anton, N.; Brochon, C.; Parida, D.; Vandamme, T.; Hadziioannou, G. Improved size-tunable preparation of polymeric nanoparticles by microfluidic nanoprecipitation. Polymer 2012, 53, 5045–5051. [Google Scholar] [CrossRef]
- Gourdon, B.; Declèves, X.; Péan, J.-M.; Chemin, C. Double or Simple Emulsion Process to Encapsulate Hydrophilic Oxytocin Peptide in PLA-PEG Nanoparticles. Pharm. Res. 2018, 35, 82. [Google Scholar] [CrossRef]
- Zaman, R.U.; Mulla, N.S.; Braz Gomes, K.; D’Souza, C.; Murnane, K.S.; D’Souza, M.J. Nanoparticle formulations that allow for sustained delivery and brain targeting of the neuropeptide oxytocin. Int. J. Pharm. 2018, 548, 698–706. [Google Scholar] [CrossRef]
- Oppong-Damoah, A.; Zaman, R.U.; D’Souza, M.J.; Murnane, K.S. Nanoparticle encapsulation increases the brain penetrance and duration of action of intranasal oxytocin. Horm. Behav. 2019, 108, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.C.; Shapiro, L.; Thelin, J.T.; Heaton, E.C.; Zaman, R.U.; D’Souza, M.J.; Murnane, K.S.; Escayg, A. Nanoparticle encapsulated oxytocin increases resistance to induced seizures and restores social behavior in Scn1a-derived epilepsy. Neurobiol. Dis. 2021, 147, 105147. [Google Scholar] [CrossRef]
- Panaitescu, A. Oxytocin Reduces Seizure Burden and Hippocampal Injury in a Rat Model of Perinatal Asphyxia. Acta Endocrinol. Buchar. 2018, 14, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Sahin, H.; Yucel, O.; Emik, S.; Senturk, G.E. Protective Effects of Intranasally Administrated Oxytocin-Loaded Nanoparticles on Pentylenetetrazole-Kindling Epilepsy in Terms of Seizure Severity, Memory, Neurogenesis, and Neuronal Damage. ACS Chem. Neurosci. 2022, 13, 1923–1937. [Google Scholar] [CrossRef]
- Smith, A.S.; Korgan, A.C.; Young, W.S. Oxytocin delivered nasally or intraperitoneally reaches the brain and plasma of normal and oxytocin knockout mice. Pharmacol. Res. 2019, 146, 104324. [Google Scholar] [CrossRef] [PubMed]
- Omidi, M.; Hashemi, M.; Tayebi, L. Microfluidic synthesis of PLGA/carbon quantum dot microspheres for vascular endothelial growth factor delivery. RSC Adv. 2019, 9, 33246–33256. [Google Scholar] [CrossRef]
- Cytotoxicity Assay. Available online: https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/cytotoxicity-assay (accessed on 25 March 2025).
- Sun, J.; Xianyu, Y.; Li, M.; Liu, W.; Zhang, L.; Liu, D.; Liu, C.; Hu, G.; Jiang, X. A microfluidic origami chip for synthesis of functionalized polymeric nanoparticles. Nanoscale 2013, 5, 5262. [Google Scholar] [CrossRef]
- Nair, S.C.; Vinayan, K.P.; Mangalathillam, S. Nose to Brain Delivery of Phenytoin Sodium Loaded Nano Lipid Carriers: Formulation, Drug Release, Permeation and In Vivo Pharmacokinetic Studies. Pharmaceutics 2021, 13, 1640. [Google Scholar] [CrossRef]
- Bohrey, S.; Chourasiya, V.; Pandey, A. Polymeric nanoparticles containing diazepam: Preparation, optimization, characterization, in-vitro drug release and release kinetic study. Nano Converg. 2016, 3, 3. [Google Scholar] [CrossRef]
- Tarhini, M.; Benlyamani, I.; Hamdani, S.; Agusti, G.; Fessi, H.; Greige-Gerges, H.; Bentaher, A.; Elaissari, A. Protein-Based Nanoparticle Preparation via Nanoprecipitation Method. Materials 2018, 11, 394. [Google Scholar] [CrossRef]
- Wang, G.; Siggers, K.; Zhang, S.; Jiang, H.; Xu, Z.; Zernicke, R.F.; Matyas, J.; Uludağ, H. Preparation of BMP-2 Containing Bovine Serum Albumin (BSA) Nanoparticles Stabilized by Polymer Coating. Pharm. Res. 2008, 25, 2896–2909. [Google Scholar] [CrossRef] [PubMed]
- Rahimnejad, M.; Najafpour, G.; Bakeri, G. Investigation and modeling effective parameters influencing the size of BSA protein nanoparticles as colloidal carrier. Colloids Surf. Physicochem. Eng. Asp. 2012, 412, 96–100. [Google Scholar] [CrossRef]
- Wang, R.K.; Chen, W.-C.; Campos, D.K.; Ziegler, K.J. Swelling the Micelle Core Surrounding Single-Walled Carbon Nanotubes with Water-Immiscible Organic Solvents. J. Am. Chem. Soc. 2008, 130, 16330–16337. [Google Scholar] [CrossRef]
- Joye, I.J.; McClements, D.J. Production of nanoparticles by anti-solvent precipitation for use in food systems. Trends Food Sci. Technol. 2013, 34, 109–123. [Google Scholar] [CrossRef]
- Hornig, S.; Heinze, T.; Becer, C.R.; Schubert, U.S. Synthetic polymeric nanoparticles by nanoprecipitation. J. Mater. Chem. 2009, 19, 3838. [Google Scholar] [CrossRef]
- Shen, H.; Hong, S.; Prud’homme, R.K.; Liu, Y. Self-assembling process of flash nanoprecipitation in a multi-inlet vortex mixer to produce drug-loaded polymeric nanoparticles. J. Nanopart. Res. 2011, 13, 4109–4120. [Google Scholar] [CrossRef]
- Baby, T.; Liu, Y.; Middelberg, A.P.J.; Zhao, C.-X. Fundamental studies on throughput capacities of hydrodynamic flow-focusing microfluidics for producing monodisperse polymer nanoparticles. Chem. Eng. Sci. 2017, 169, 128–139. [Google Scholar] [CrossRef]
- Gimondi, S.; Guimarães, C.F.; Vieira, S.F.; Gonçalves, V.M.F.; Tiritan, M.E.; Reis, R.L.; Ferreira, H.; Neves, N.M. Microfluidic mixing system for precise PLGA-PEG nanoparticles size control. Nanomed. Nanotechnol. Biol. Med. 2022, 40, 102482. [Google Scholar] [CrossRef]
- Zhang, L.; Chan, J.M.; Gu, F.X.; Rhee, J.-W.; Wang, A.Z.; Radovic-Moreno, A.F.; Alexis, F.; Langer, R.; Farokhzad, O.C. Self-assembled lipid--polymer hybrid nanoparticles: A robust drug delivery platform. ACS Nano 2008, 2, 1696–1702. [Google Scholar] [CrossRef]
- Hakala, T.A.; Davies, S.; Toprakcioglu, Z.; Bernardim, B.; Bernardes, G.J.L.; Knowles, T.P.J. A Microfluidic Co-Flow Route for Human Serum Albumin-Drug–Nanoparticle Assembly. Chem. Eur. J. 2020, 26, 5965–5969. [Google Scholar] [CrossRef]
- Hussain, M.H.; Abu Bakar, N.F.; Mustapa, A.N.; Low, K.-F.; Othman, N.H.; Adam, F. Synthesis of Various Size Gold Nanoparticles by Chemical Reduction Method with Different Solvent Polarity. Nanoscale Res. Lett. 2020, 15, 140. [Google Scholar] [CrossRef] [PubMed]
- Langer, K.; Balthasar, S.; Vogel, V.; Dinauer, N.; von Briesen, H.; Schubert, D. Optimization of the preparation process for human serum albumin (HSA) nanoparticles. Int. J. Pharm. 2003, 257, 169–180. [Google Scholar] [CrossRef]
- Mohammad-Beigi, H.; Shojaosadati, S.A.; Morshedi, D.; Mirzazadeh, N.; Arpanaei, A. The Effects of Organic Solvents on the Physicochemical Properties of Human Serum Albumin Nanoparticles. Iran. J. Biotechnol. 2016, 14, 45–50. [Google Scholar] [CrossRef]
- Haim Zada, M.; Rottenberg, Y.; Domb, A.J. Peptide loaded polymeric nanoparticles by non-aqueous nanoprecipitation. J. Colloid Interface Sci. 2022, 622, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Okubo, T.; Nangrejo, M.; Edirisinghe, M. Preparation of polymeric nanoparticles by novel electrospray nanoprecipitation: Novel electrospray nanoprecipitation. Polym. Int. 2015, 64, 183–187. [Google Scholar] [CrossRef]
- Lazarevic, A.; Pokrajac, D.; Marcano, A.; Melikechi, N. Support vector machine based classification of fast Fourier transform spectroscopy of proteins. In Advanced Biomedical and Clinical Diagnostic Systems VII; Mahadevan-Jansen, A., Vo-Dinh, T., Grundfest, W.S., Eds.; SPIE: San Jose, CA, USA, 2009; p. 71690C. [Google Scholar] [CrossRef]
- Chung, E.P.; Cotter, J.D.; Prakapenka, A.V.; Cook, R.L.; DiPerna, D.M.; Sirianni, R.W. Targeting Small Molecule Delivery to the Brain and Spinal Cord via Intranasal Administration of Rabies Virus Glycoprotein (RVG29)-Modified PLGA Nanoparticles. Pharmaceutics 2020, 12, 93. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Cheng, S.; Qin, F.; Fu, A.; Fu, C. Application progress of RVG peptides to facilitate the delivery of therapeutic agents into the central nervous system. RSC Adv. 2021, 11, 8505–8515. [Google Scholar] [CrossRef] [PubMed]
- Vu, H.T.H.; Streck, S.; Hook, S.M.; McDowell, A. Utilization of Microfluidics for the Preparation of Polymeric Nanoparticles for the Antioxidant Rutin: A Comparison with Bulk Production. Pharm. Nanotechnol. 2019, 7, 469–483. [Google Scholar] [CrossRef]
- Silva, A.L.; Rosalia, R.A.; Sazak, A.; Carstens, M.G.; Ossendorp, F.; Oostendorp, J.; Jiskoot, W. Optimization of encapsulation of a synthetic long peptide in PLGA nanoparticles: Low-burst release is crucial for efficient CD8+ T cell activation. Eur. J. Pharm. Biopharm. 2013, 83, 338–345. [Google Scholar] [CrossRef]
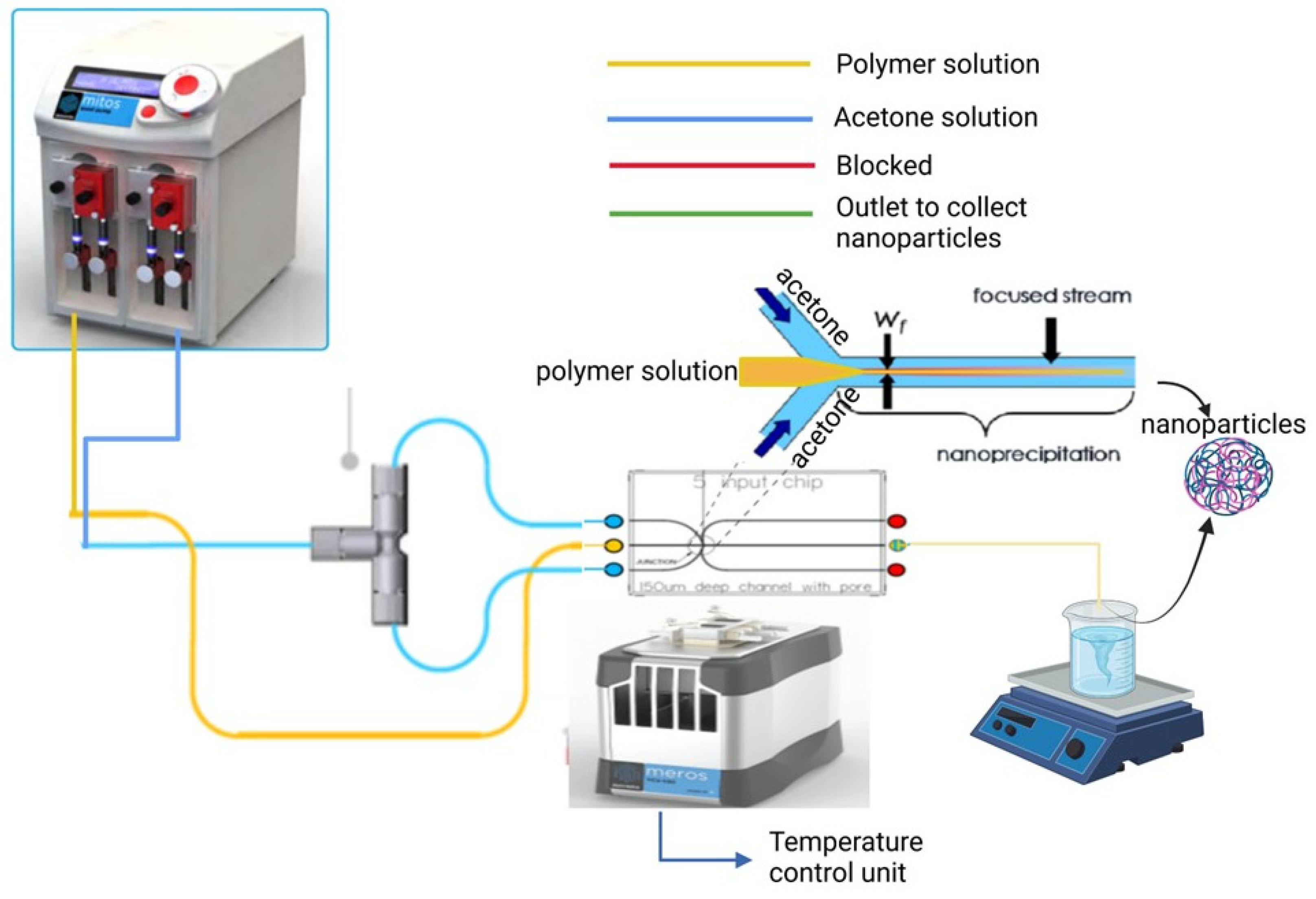
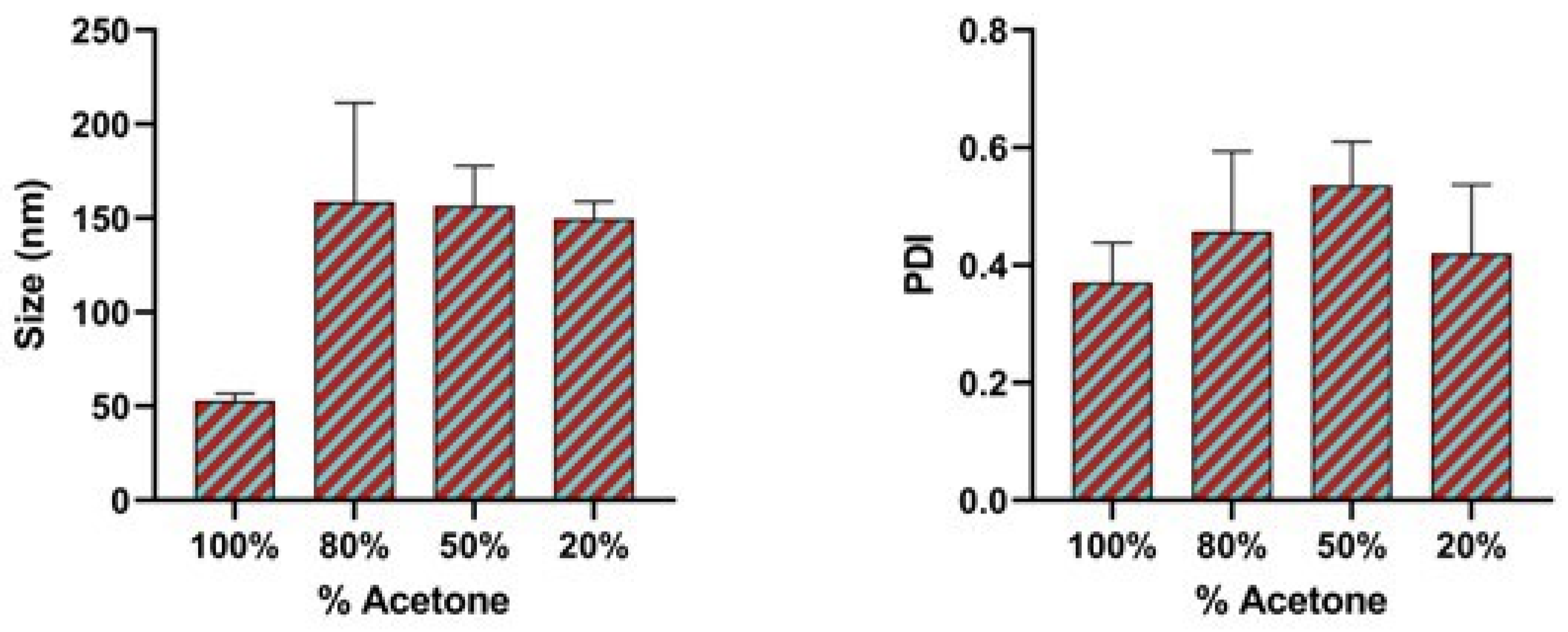


| Binary Organic Solvent Mixture | Binary Organic Solvent Mixture Polarity Index | Size (nm) | Polydispersity Index (PDI) |
|---|---|---|---|
| 100% Acetone solvent | 5.2 | 52.89 ± 3.8 | 0.37 |
| 80% acetone + 20% ethanol | 5.12 | 158.76 ± 52.5 | 0.4 |
| 50% acetone + 50% ethanol | 5.15 | 156.64 ± 21.2 | 0.5 |
| 20% acetone + 80% ethanol | 5.18 | 149.70 ± 9.05 | 0.4 |
| Model | R2 | K | n |
|---|---|---|---|
| Zero order | 0.9884 | 8.67005 | - |
| First order | 0.8584 | 0.2647 | - |
| Higuchi | 0.9634 | 17.3401 | - |
| Korsmeyer–Peppas | 0.9898 | 0.0862 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adediran, E.; Vijayanand, S.; Kale, A.; Gulani, M.; Wong, J.C.; Escayg, A.; Murnane, K.S.; D’Souza, M.J. Microfluidics-Assisted Formulation of Polymeric Oxytocin Nanoparticles for Targeted Brain Delivery. Pharmaceutics 2025, 17, 452. https://doi.org/10.3390/pharmaceutics17040452
Adediran E, Vijayanand S, Kale A, Gulani M, Wong JC, Escayg A, Murnane KS, D’Souza MJ. Microfluidics-Assisted Formulation of Polymeric Oxytocin Nanoparticles for Targeted Brain Delivery. Pharmaceutics. 2025; 17(4):452. https://doi.org/10.3390/pharmaceutics17040452
Chicago/Turabian StyleAdediran, Emmanuel, Sharon Vijayanand, Akanksha Kale, Mahek Gulani, Jennifer C. Wong, Andrew Escayg, Kevin S. Murnane, and Martin J. D’Souza. 2025. "Microfluidics-Assisted Formulation of Polymeric Oxytocin Nanoparticles for Targeted Brain Delivery" Pharmaceutics 17, no. 4: 452. https://doi.org/10.3390/pharmaceutics17040452
APA StyleAdediran, E., Vijayanand, S., Kale, A., Gulani, M., Wong, J. C., Escayg, A., Murnane, K. S., & D’Souza, M. J. (2025). Microfluidics-Assisted Formulation of Polymeric Oxytocin Nanoparticles for Targeted Brain Delivery. Pharmaceutics, 17(4), 452. https://doi.org/10.3390/pharmaceutics17040452









