1. Introduction
Nanotechnology offers significant advantages in pulmonary drug delivery, particularly for poorly soluble drugs, by overcoming biological and physical barriers, improving solubility, and enhancing bioavailability [
1,
2]. As absorption is more efficient, lower doses can be used, thereby reducing the risk of adverse effects. Nanoparticles used in pulmonary delivery can be categorized into carrier-based and carrier-free types according to their composition and properties. Lipid-based nanoparticles, such as liposomes; solid lipid nanoparticles; and nanoemulsions are widely used for their ability to encapsulate both hydrophilic and hydrophobic drugs [
3,
4]. Polymeric nanoparticles, including PLGA and chitosan, are valued for their biodegradability, biocompatibility, and mucoadhesive properties, which enhance drug retention in the lungs [
5,
6,
7,
8]. Inorganic nanoparticles, like mesoporous silica, offer high surface area and high loading capability, suitable biocompatibility, and their surface can be functionalized [
9]. Hybrid nanoparticles, which combine materials such as lipids and polymers, are designed to optimize drug delivery by leveraging the advantages of multiple components [
10]. Each type of nanoparticle offers unique benefits, making them suitable for various therapeutic and diagnostic applications in pulmonary delivery.
Nanocrystals, a carrier-free nanotechnology, have gained increasing attention for pulmonary administration due to their ability to enhance the dissolution rate and saturation solubility of poorly soluble drugs while maintaining favorable biological properties and low toxicity [
11]. Drug nanocrystals are typically pure drug particles ranging from 1 to 1000 nm in size and are stabilized by surfactants or stabilizers without requiring additional carrier materials [
12]. This technology enhances drug adhesion to biological membranes, increases saturation solubility, and improves bioavailability due to its high surface area. Nanocrystal formulations have been explored for various administration routes, including oral, intravenous, pulmonary, percutaneous, and ocular delivery. In pulmonary drug delivery, nanocrystals offer unique advantages, such as high drug loading, improved mucus penetration, reduced macrophage clearance, and enhanced drug release, making them a promising option for inhalation administration [
13].
The top–down approach is commonly used for nanocrystal preparation, where larger solid particles are mechanically reduced to nanoscale sizes. This method includes techniques such as media milling, high-pressure homogenization, and microfluidization. Among these, wet media milling is widely employed due to its simplicity, reproducibility, and eco-friendliness, as it does not require organic solvents [
14]. Wet media milling is an established pharmaceutical technique for producing stable nanosuspensions of poorly water-soluble active pharmaceutical ingredients (APIs), with scalability further enhanced by modeling techniques to optimize industrial applications and improve efficiency [
15]. However, to obtain an inhalable dry powder, an additional solidification step is required.
Spray drying is a widely used technique for converting liquid nanosuspensions into dry powders with improved stability and ease of administration [
16]. Compared to conventional spray drying, nano spray drying offers superior control over droplet formation, resulting in more uniform particles with narrow size distribution. Unlike traditional spray dryers, which rely on pressure or centrifugal force, nano spray dryers use a vibrational nebulizer to generate uniform microdroplets, leading to higher yields even from small sample quantities [
17,
18]. Despite these advantages, nano spray drying has been underutilized in developing DPIs compared to conventional spray dryers, presenting an opportunity for improvement. The combination of wet media milling and spray drying has recently gained interest for developing nanocrystal-based dry powder inhaler (DPI) formulations.
The selection of excipients during spray drying significantly influences the physicochemical properties of the final powder. Common excipients, such as mannitol, trehalose, and leucine, play a critical role in stabilizing formulations during drying and ensuring product stability. Mannitol is commonly used as a cryoprotectant, preserving structural integrity and enhancing formulation stability [
19]. However, its tendency to crystallize can impact the aerosol performance and fine particle fraction (FPF) upon storage [
20]. In contrast, leucine improves powder dispersibility and stability in dry powder formulations by reducing particle cohesion and enhancing aerosolization [
21]. Since excipients in spray drying serve multiple roles, including thermal protection, aggregation prevention, and maintaining API bioactivity, the ideal spray-dried preparations may necessitate a combination of various excipients [
22]. Moreover, their influence on spray-dried powder should be carefully evaluated to ensure optimal characteristics and to achieve the desired powder properties.
Aerodynamic analysis plays a crucial role in DPI formulation, as the effectiveness of inhaled drugs is directly influenced by the aerodynamic properties of aerosolized particles [
23]. The aerodynamic diameter of particles determines their deposition in the respiratory tract, with optimal pulmonary delivery occurring within the 1–5 µm range. Particles within this range efficiently reach the alveolar region, ensuring effective treatment of respiratory diseases [
24]. Various factors, including particle size distribution, shape, density, and flow properties, influence aerodynamic behavior [
25]. A comprehensive evaluation of these characteristics is essential to confirm the suitability of a formulation for inhalation and therapeutic efficacy.
Stability is another key consideration in DPI formulation, as storage circumstances can significantly affect particle shape and aerosol performance [
26]. While solid formulations largely offer better stability than liquid systems, nanosized APIs are prone to aggregation, which can compromise functionality [
14]. Crystalline APIs are often preferred over amorphous forms due to their superior physical stability, though amorphous APIs can provide improved dissolution and bioavailability [
27,
28]. However, the influence of storage conditions—particularly relative humidity—on stability is highly drug specific [
29]. Despite extensive research on the stability of dry powder formulations [
20,
28,
30,
31,
32,
33], stability assessments remain essential for every newly developed DPI. Ensuring a balance between stability and performance is crucial to mitigating challenges such as crystal growth, sedimentation, and agglomeration, ultimately safeguarding the efficacy and safety of the final product.
Thus, the present study aims to evaluate the aerodynamic performance and stability of nanocrystal-based DPI formulations (NC-DPs). The nanocrystalline suspension produced via wet media milling was subjected to stability studies before undergoing nano spray drying. Box–Behnken factorial design (BBD) was employed to optimize the process parameters. The effects of excipients, including mannitol and leucine (used individually or in combination), were assessed on particle size, aerodynamic characteristics, and physicochemical stability under normal storage conditions (desiccator) and accelerated stress conditions (40 ± 2 °C and 75 ± 5% relative humadity (RH)), following International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines. Aerodynamic properties were analyzed using an Aerodynamic Particle Sizer (APS) and in silico modeling. Furthermore, the physicochemical stability of the formulations was examined using laser diffraction, scanning electron microscopy (SEM), differential scanning calorimetry (DSC), X-ray powder diffraction (XRPD), dissolution testing, and Andersen cascade impaction. By addressing these critical factors, this study aims to validate the formulation’s suitability for pulmonary drug delivery and assess its feasibility for future clinical applications.
2. Materials and Methods
2.1. Materials
Ketoprofen (KTP) (TCI Chemicals, Shanghai, China) was used as the active pharmaceutical ingredient (API) in this study. Polyvinyl alcohol (PVA) (ISP Customer Service GmbH, Cologne, Germany), a biocompatible and non-toxic polymer, and sodium dodecyl sulfate (SDS) (VWR Chemicals, Leuven, Belgium), an anionic surfactant, were employed as stabilizing agents. D-mannitol (Molar Chemicals Ltd., Budapest, Hungary) and L-leucine (AppliChem GmbH, Darmstadt, Germany) were incorporated during the nano spray drying process. Distilled water used in the experiments was obtained from a Milli-Q system (Millipore, Merck KGaA, Darmstadt, Germany).
2.2. Nanocrystal Feed Preparation
A KTP-containing nanosuspension (KTP-NS) was formulated based on a previously reported method, with a slight modification [
34]. In brief, drug particles were dispersed in an aqueous solution containing PVA (1%
w/
v) and SDS (0.1%
w/
v). The combination of steric (PVA) and electrostatic (SDS) stabilizers helps maintain nanocrystal stability [
35]. Then, wet media milling was performed in a planetary ball mill (Retsch Planetary Ball Mill PM 100 MA, Retsch GmbH, Haan, Germany) using 20.00 g of 0.3 mm zirconium dioxide (ZrO
2) beads. The milling process was carried out at 400 rpm for 90 min in a 50 mL milling chamber, with a pause every 15 min for 5 min to allow cooling.
2.3. Inhalable Nanocrystal-Based Dry Powder (NC-DP) Preparation
2.3.1. Optimization of Nano Spray Dryer Parameters by Box–Behnken Factorial Design (BBD)
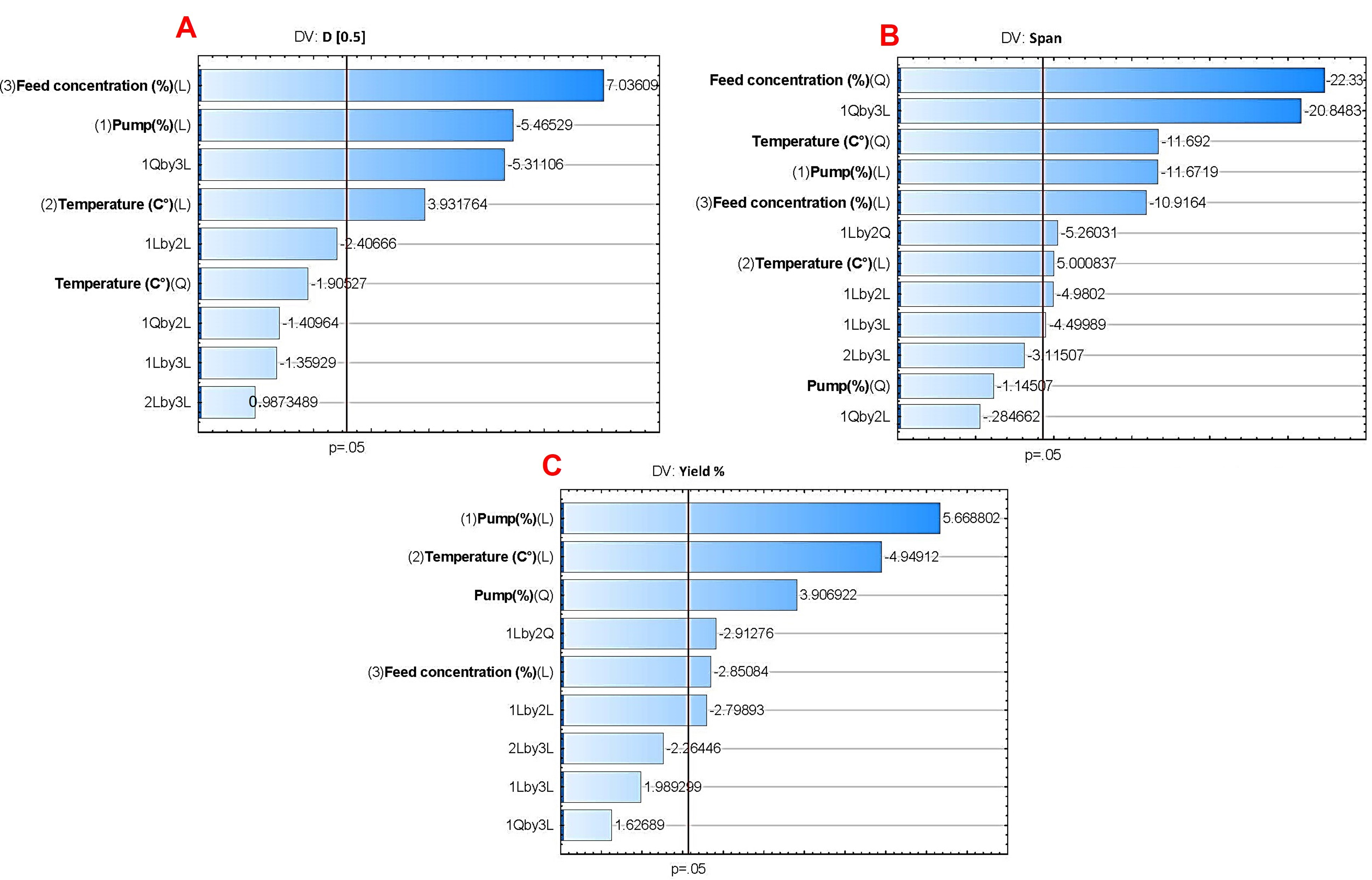
A Büchi Nano Spray Dryer equipped with a medium nebulizer (Büchi Nano Spray Dryer B-90 HP, Büchi, Flawil, Switzerland) was used to produce the NC-DPs. The Box–Behnken factorial design (BBD) was employed to optimize the parameters of the nano spray dryer, assisted by TIBCO Statistica
® 13.4 (Statsoft Hungary, Budapest, Hungary) for experimental design generation. Three independent factors were chosen: pump (%), temperature (°C), and feed concentration (%
w/v). These factors play crucial roles in the nano spray drying process. Pump dictates the droplet size during atomization [
36], thus affecting the size and uniformity of the resulting NC-DP. Temperature influences drying rate and solid-state properties, while feed concentration impacts both evaporation dynamics and yield percentage [
37]. The effect of these factors on the dependent variables (D[0.5], span, and yield percentage) was investigated at three levels, as outlined in
Table 1.
Temperature was studied at 90, 100, and 110 °C, considering the melting point of KTP is 94 °C [
38], and taking into account the boiling point of water, which is appropriate for the water-based formulation. Feed concentration was selected at the highest concentration achievable from the media milling process (10%
w/
v) [
39] and then further diluted to 5% and 2.5% (
w/
v). The pump percentages were chosen to explore a wide range of feed rates: 10% to examine slow feed rates and finer particles, 30% for an intermediate balance between droplet formation and drying efficiency, and 50% to investigate the effects of high feed rates on particle formation and yield [
36]. Moreover, mathematical models were developed for each dependent variable, and the relationship of the variables in the response was analyzed by the following second-order polynomial equation:
where y is the response variable; β
0 is a constant; β
1, β
2, and β
3 are linear coefficients; β
11, β
22, and β
33 are quadratic coefficients; β
12, β
13, and β
23 are the interaction coefficients; X
1–3 are the main effect factors; X
12, X
22, and X
32 are the quadratic effect factors; and X
1X
2, X
1X
3, and X
2X
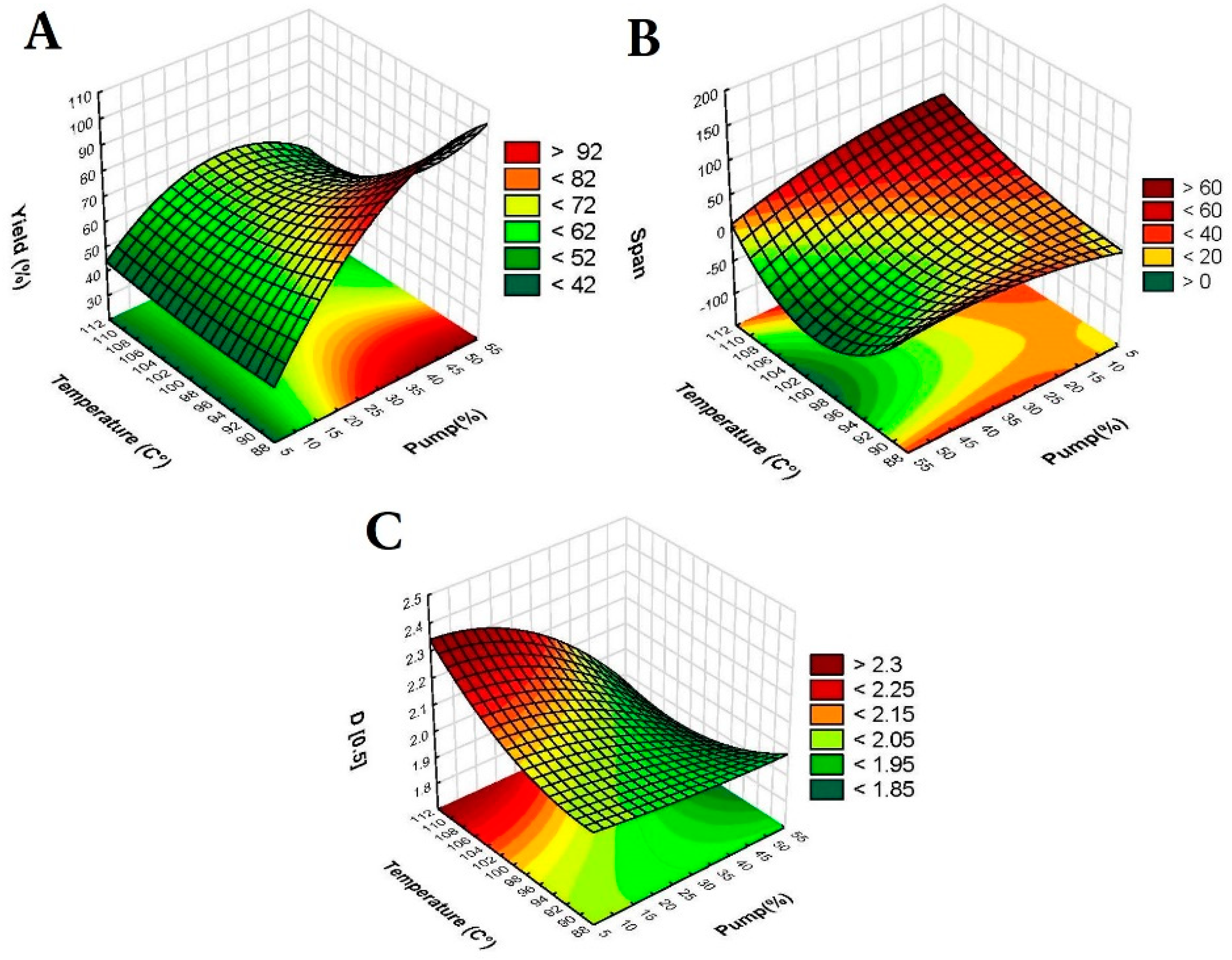
3 are the interaction effect factors. An analysis of variance (ANOVA) was conducted, with significance determined at a p-value less than 0.05, indicating statistical significance at the 95% confidence level. Response surface plots, in the form of 3D surface plots, were generated for D[0.5] (µm), span, and yield (%) based on the regression model, with one variable held constant at its center level.
2.3.2. Mannitol/Leucine Incorporation
The optimized parameters obtained from the BBD were utilized to extensively investigate the effects of leucine and mannitol, two widely employed excipients in inhaled preparations, on the properties of the NC-DP. Both mannitol and leucine are known to enhance re-dispersibility upon hydration, improve the drug dissolution, and boost aerosol performance [
40]. Using optimized nano spray dryer parameters, we evaluated the impact of excipients—mannitol (K-NC-M), leucine (K-NC-L), their combination (K-NC-ML), and the absence of excipients (K-NC)—on the particle size, aerosolization properties, and stability of NC-DPs for pulmonary delivery. In this context, a magnetic stirrer (AREC. X heating magnetic stirrer, Velp Scientifica Srl, Usmate Velate, Italy) was used for dissolving mannitol and/or leucine in the nanosuspension. Based on the factorial design study, the formulations were prepared as detailed in
Table 2; mannitol and/or leucine were added accordingly while ensuring a constant total concentration.
2.4. Stability of the Nanosuspension
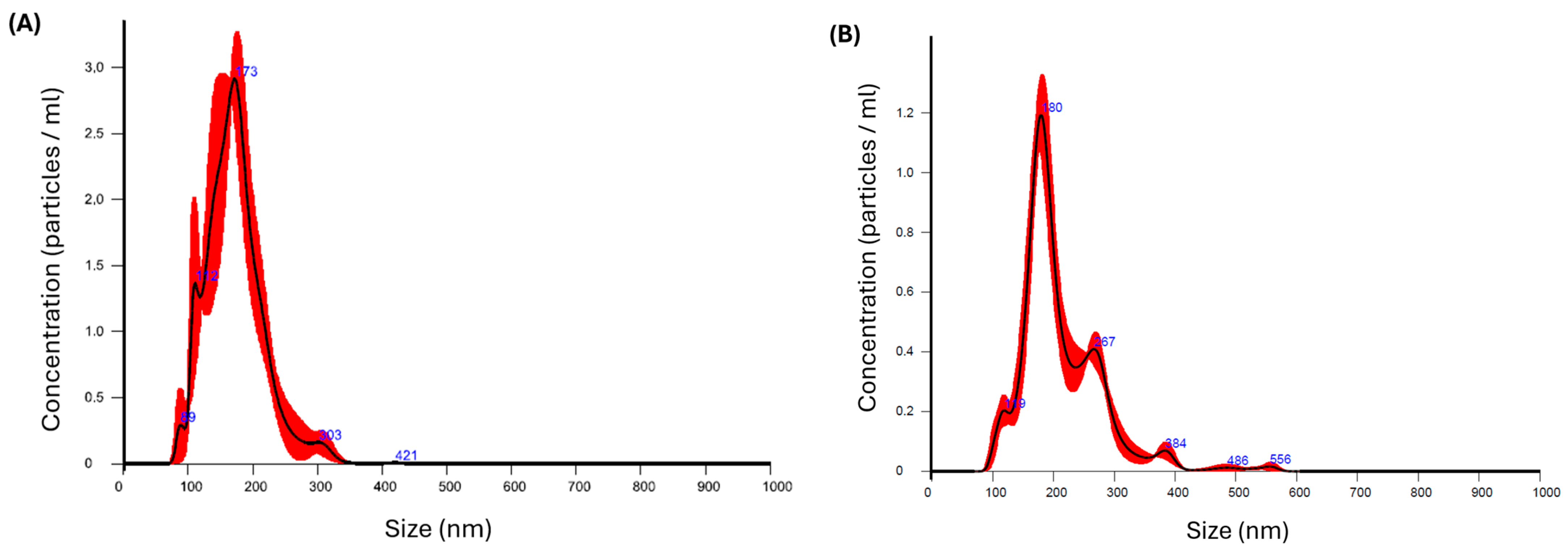
To assess the stability of the KTP-NS prior to the nano spray drying process, a short-term stability study was conducted. The nanosuspension was stored at refrigeration temperature (+4 °C) and evaluated for particle size and distribution at predefined time intervals using the NanoSight NS 3000 system (Malvern Instruments, Worcestershire, UK). Nanoparticle Tracking Analysis (NTA) was employed for precise particle size measurements. The instrument featured a 565 nm laser, a high-sensitivity sCMOS camera, and a syringe pump. The refractive index for KTP was set at 1.592, and the nanosuspension was diluted before being analyzed. The diluted sample was introduced into the instrument at a syringe pump speed of 50. Measurements were taken by recording three 30-s videos in script control mode, producing a total of 1500 frames for analysis.
The particle size distribution was characterized using the parameters D[0.1] (indicating the size below which 10% of the volume distribution falls), D[0.5] (the median particle size), and D[0.9] (the size below which 90% of the volume distribution falls). Additionally, the size distribution span was calculated using Equation (2). After confirming the stability of the NS, the nano spray dryer was employed (as detailed in
Section 2.3), and powder characterizations were conducted.
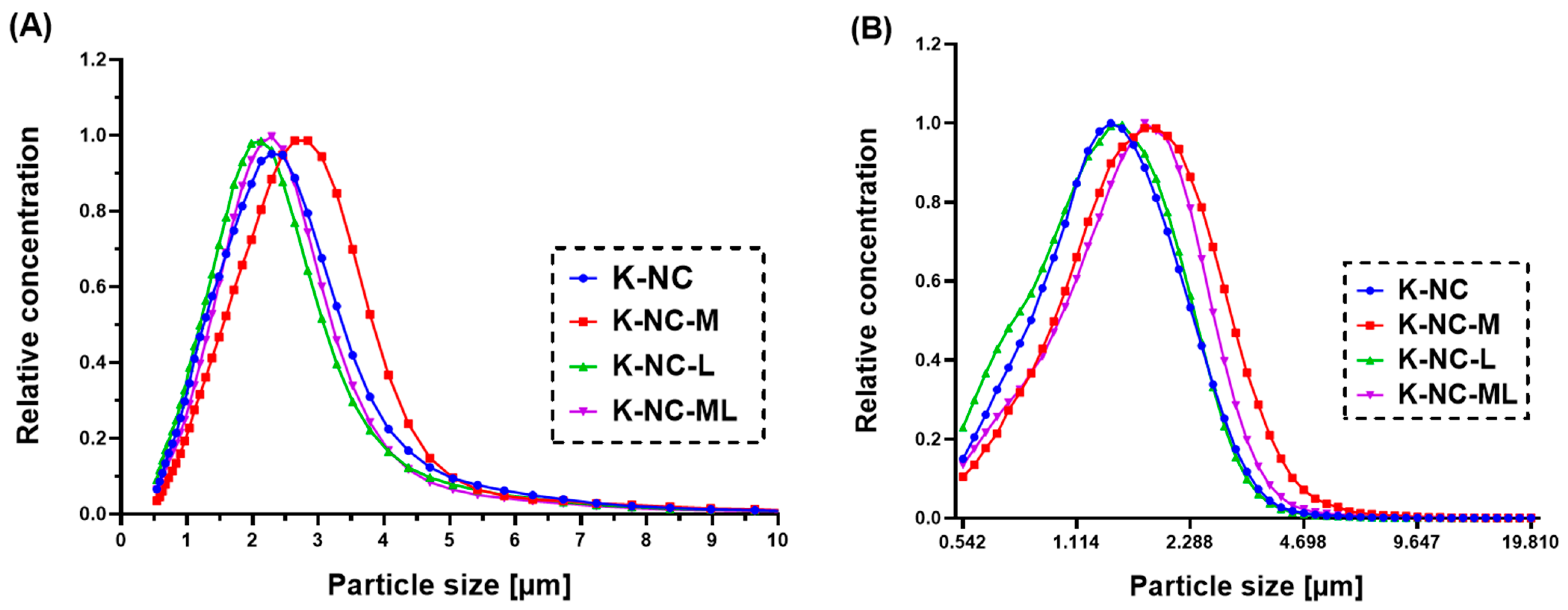
2.5. Particle Size and Yield
Laser diffraction was used to assess the particle size and distribution of the nano spray-dried NC-DPs with a Malvern Mastersizer Scirocco 2000 device (Malvern Instruments Ltd., Malvern, UK). A dry dispersion unit was employed with 0.5–1.0 g of powder placed in the feeding tray. The refractive index for KTP was set at 1.592, and the dispersion air pressure was adjusted to 3.0 bar with a vibration feed of 75%. Triplicate measurements were performed for each sample. The particle size distribution was reported using D[0.1], D[0.5], and D[0.9] values, and the span was calculated using the previously defined equation (Equation (2)). The mass ratio of dry powder collected after spray drying to the initial solid compositions before drying was calculated to determine the percentage yield of each sample (Equation (3)).
2.6. Aerosol Performance via Aerodynamic Particle Sizer
The powders were filled into size 3 Ezeeflo™ hydroxypropyl methylcellulose (HPMC) capsules (ACG-Associated Capsules Pvt. Ltd., Mumbai, India) and tested using the Breezhaler® dry powder inhaler DPI (Novartis International AG, Basel, Switzerland). The experimental setup included an induction port simulating the upper respiratory tract, a high-capacity vacuum pump (HCP5; Copley Scientific Ltd., Nottingham, UK) with a critical flow controller (TPK 2000; Copley Scientific Ltd., Nottingham, UK), and an Aerodynamic Particle Sizer (APS) (TSI Incorporated, Shoreview, MN, USA).
To simulate inhalation, the vacuum pump activated the DPI with a rectangular 4-s breathing waveform at a flow rate of 60 L/min. The APS sampled particles from the airflow via an isokinetic nozzle, using a TSI 3321 model for size distribution analysis. This instrument measures aerosol particle size distributions with aerodynamic diameters ranging from 0.5 to 20 μm across 52 channels. It determines particle sizes by measuring their time-of-flight within an accelerated flow.
The APS sampling flow rate was set at 1 L/min, with a 5-s sampling duration and no pauses. This duration was chosen based on the length of the inhalation profile and the particles’ residence time in the measurement system. A TSI 4000 thermal mass flow meter (TSI Incorporated, Shoreview, MN, USA), with a measuring range of 0.5–200 NL/min, was used to regularly verify the 60 L/min flow rate during measurements.
2.7. In Silico Aerodynamic Analysis
The latest version of the Stochastic Lung Model (SLM) was utilized to estimate drug deposition across different anatomical regions of the airways, expressed as regional deposition fractions [
41]. Numerical modeling offers several advantages over traditional scintigraphic studies, which often require large populations and face significant technical and ethical challenges [
42]. This non-invasive, reproducible, and efficient method has proven to be a valuable tool for quantifying the distribution of medications in various parts of the respiratory tract, including total, local, and regional depositions.
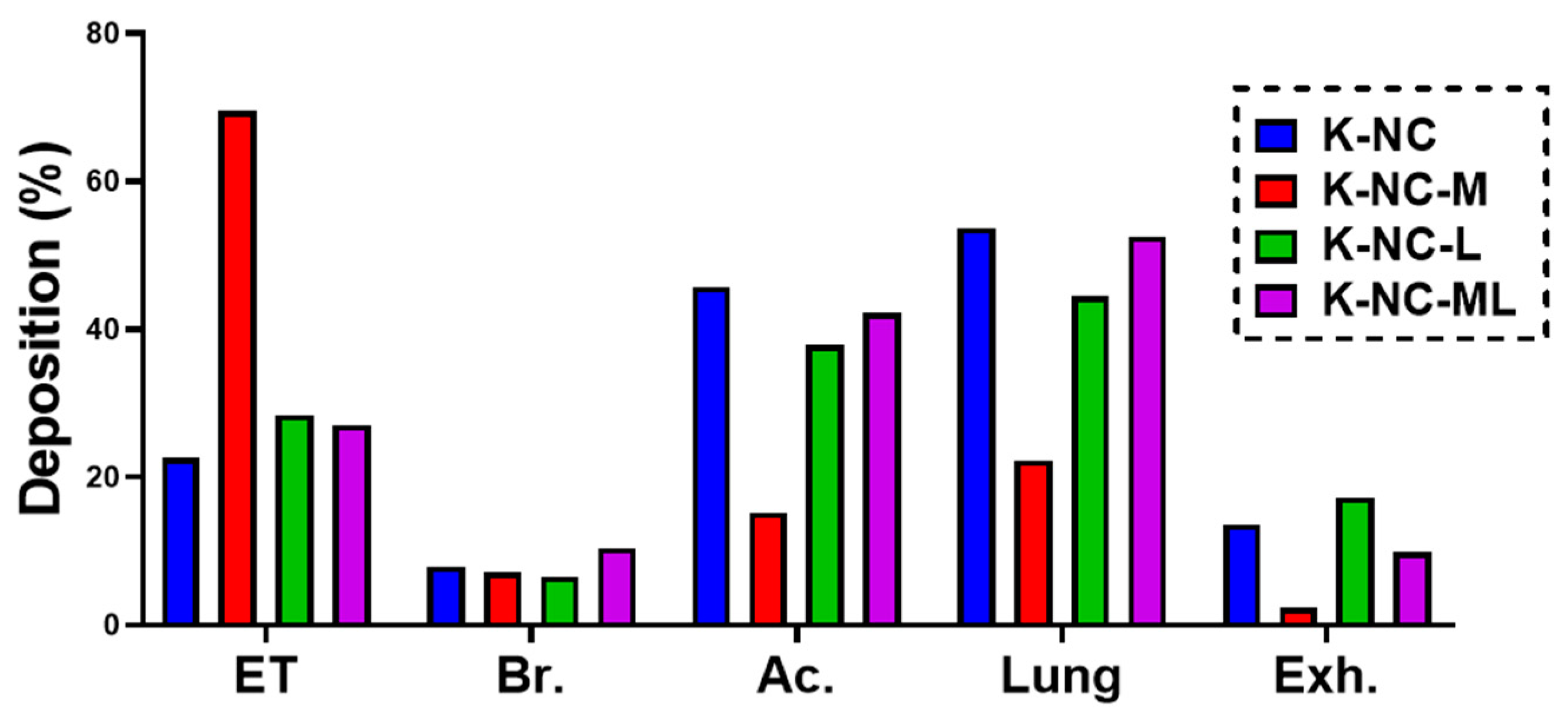
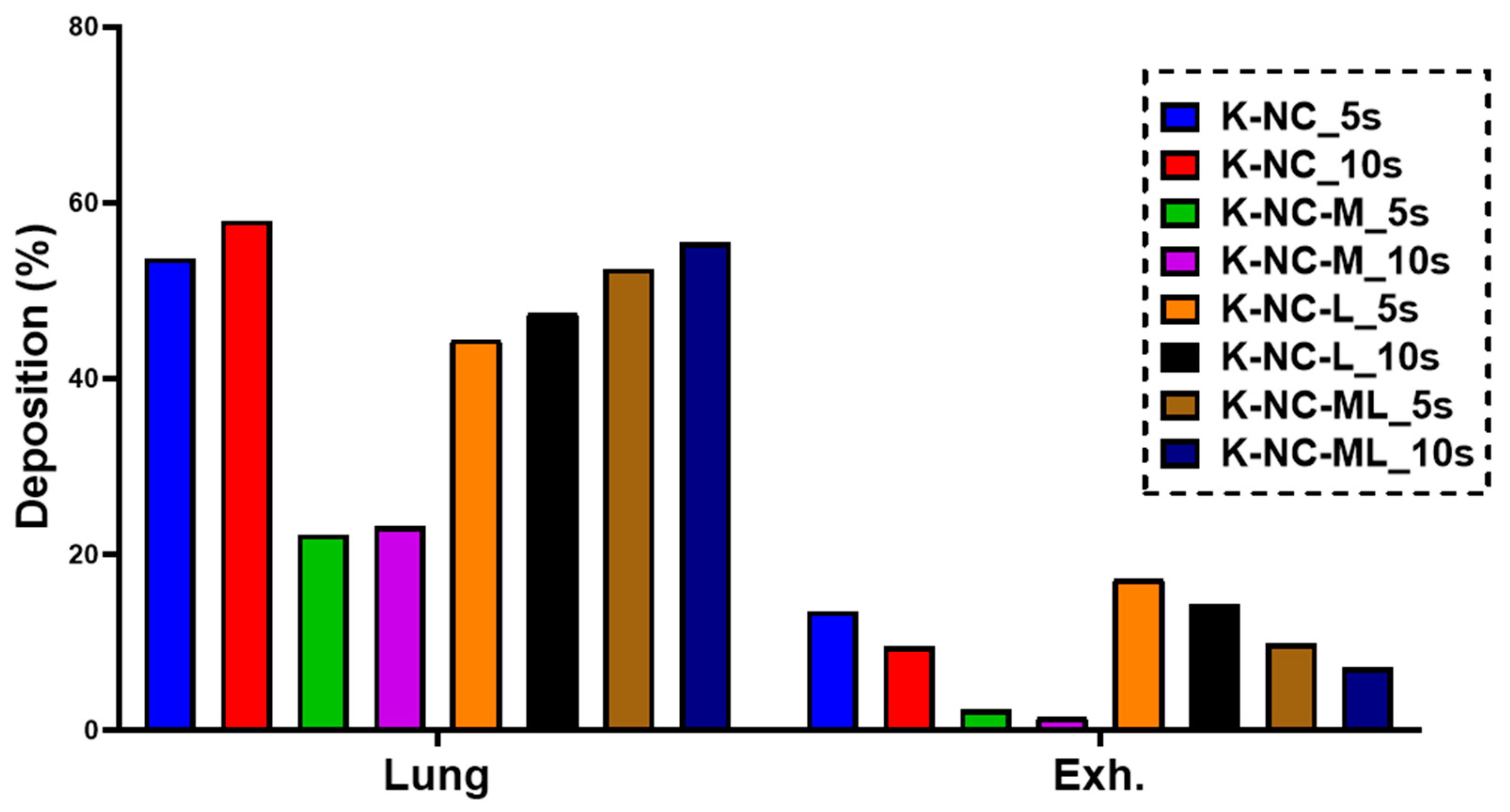
In this study, we employed a validated airway deposition model to numerically calculate the deposition fractions in the lungs and upper airways (extra-thoracic region). Deposition fraction refers to the proportion of the drug mass deposited in a specific airway region relative to the total drug mass loaded into the capsule. Lung deposition fraction is determined as the sum of the bronchial and acinar deposition fractions. Meanwhile, the exhaled fraction is calculated by subtracting the total deposited fractions (lungs, upper airways, and remaining in the device) from the metered fraction (100%).
The model incorporates a Monte Carlo approach, which uses stochastic simulations to account for the natural variability in airway morphology. By tracking approximately 10⁵ particles from inhalation to exhalation, the model can simulate their deposition in various lung regions or their exhalation. Key inputs for the model include particle characteristics and inhalation parameters. For this study, the inhalation parameters were tailored to match those of patients with chronic obstructive pulmonary disease (COPD), with a peak inhalation flow of 69.5 L/min, an inhaled volume of 1.7 L, and an inhalation time of 2.04 s [
25].
Additionally, the study investigated how breath-hold (BH) duration impacts deposition patterns by applying BH times of 5 and 10 s. The numerical deposition model used here was previously validated specifically for aerosolized medications [
43], ensuring its reliability for this application.
2.8. Long-Term Stability Under Normal Conditions
To assess the long-term stability of the NC-DPs, the four samples were stored in opaque, well-sealed glass containers at room temperature in a desiccator for six months. The particle size was evaluated using laser diffraction analysis (as described in
Section 2.5). The particle size was characterized using D[0.1], D[0.5], D[0.9], and span. The percentage change in particle size after six months of storage was calculated relative to the initial values (0 months, before storage) to quantify any changes over time (Equation (4)).
2.9. Stability Under Accelerated Conditions
Stability testing was performed in a Binder KBF 240 environmental chamber (Binder GmbH, Tuttlingen, Germany), featuring an APT.line™ preheating and cooling system for precise temperature control. The chamber maintained consistent conditions across a temperature range of 10–70 °C and RH levels of 10–80%, ensuring reproducibility throughout the study. In accordance with ICH guidelines, the stability test was carried out at 40 ± 2 °C and 75 ± 5% RH. The formulations were placed in size 3 Ezeeflo™ hydroxypropyl methylcellulose capsules (ACG-Associated Capsules Pvt. Ltd., Mumbai, India) and subjected to a 90-day stability assessment. Analytical samples were collected at three time points: initial (time zero), intermediate (30 days), and final (90 days) storage intervals.
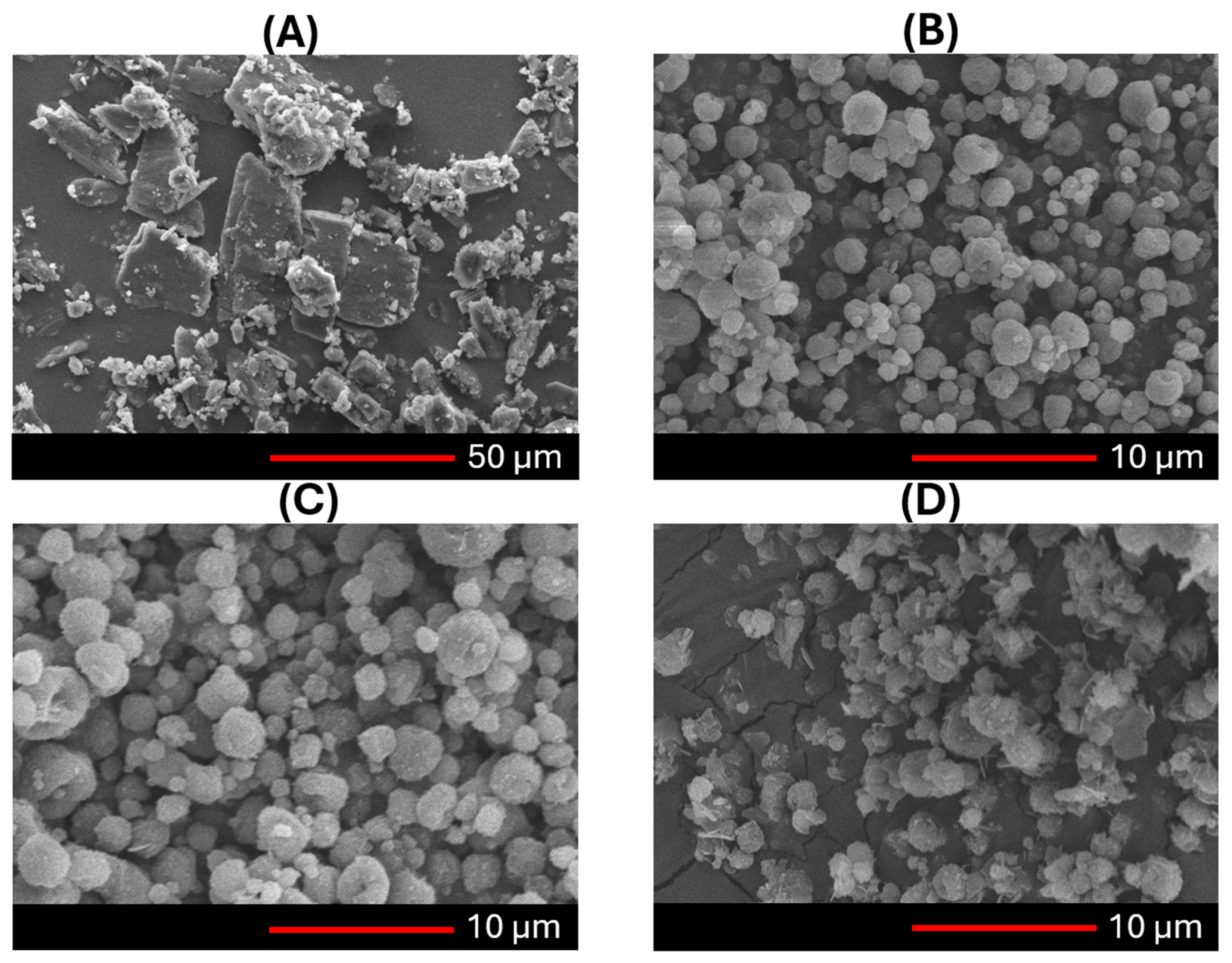
2.9.1. Morphology
The shape of both freshly prepared and stored NC-DPs samples was examined using a scanning electron microscope (SEM) (Hitachi Scientific Ltd., Tokyo, Japan). The SEM was operated at an accelerating voltage of 10 kV, and the samples were coated with a thin gold–palladium layer using a Bio-Rad sputter coater (2.0 kV, 10 mA, for 10 min). The coating process was conducted under an air pressure range of 1.3–13.0 mPa.
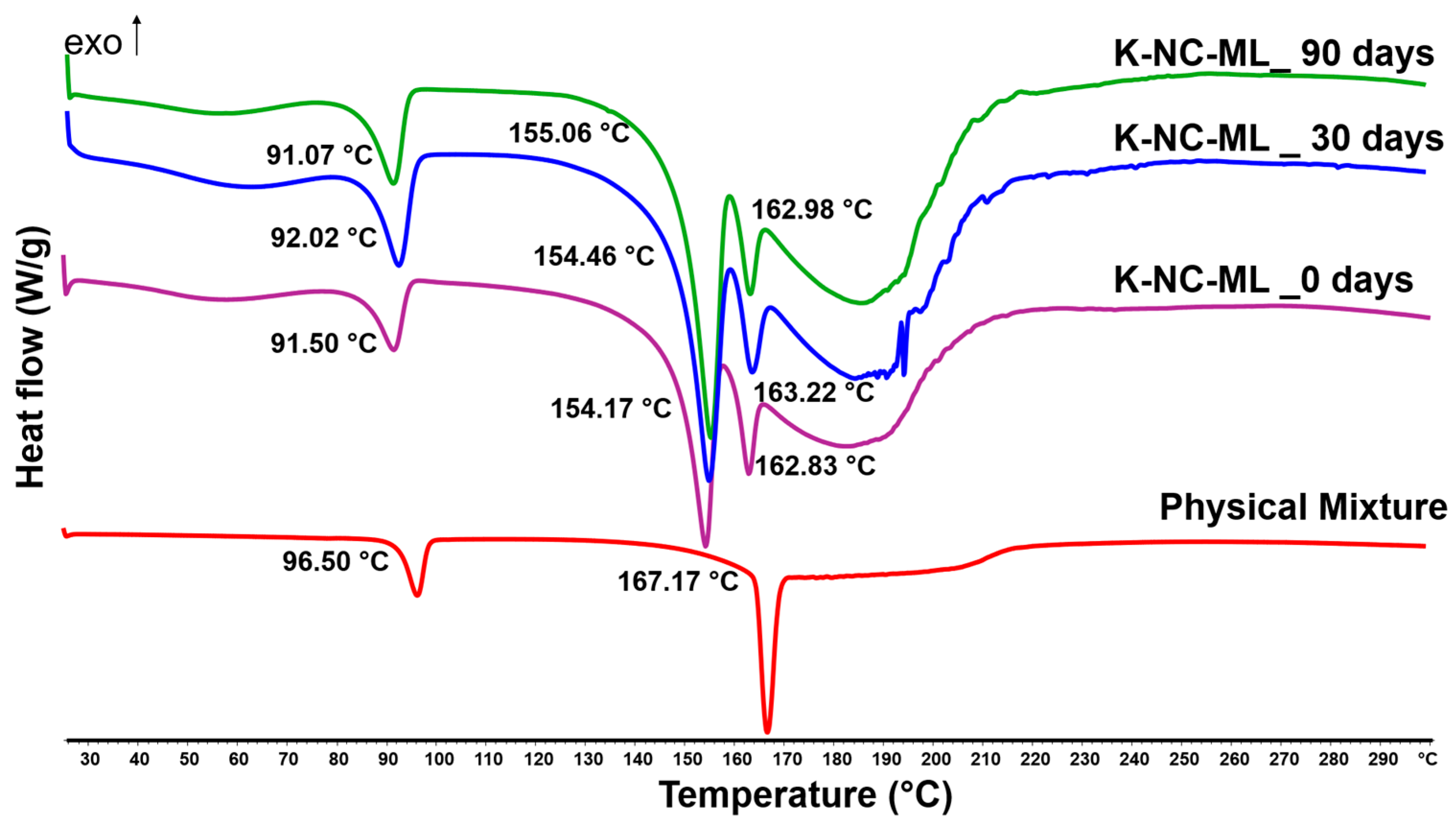
2.9.2. Thermal and Solid-State Analysis
Differential scanning calorimetry (DSC) was carried out by operating a Mettler Toledo DSC 821e system, controlled by STARe thermal analysis software version 9.3 (Mettler Inc., Schwerzenbach, Switzerland). For the analysis, approximately 2–3 mg of each sample and physical mixture (corresponding to sample compositions) was sealed in hermetically closed aluminum pans with pierced lids, while an empty pan served as the reference. The temperature was scanned from 25 °C to 300 °C at a heating rate of 10 °C/min, using argon as the carrier gas with a flow rate of 10 L/h.
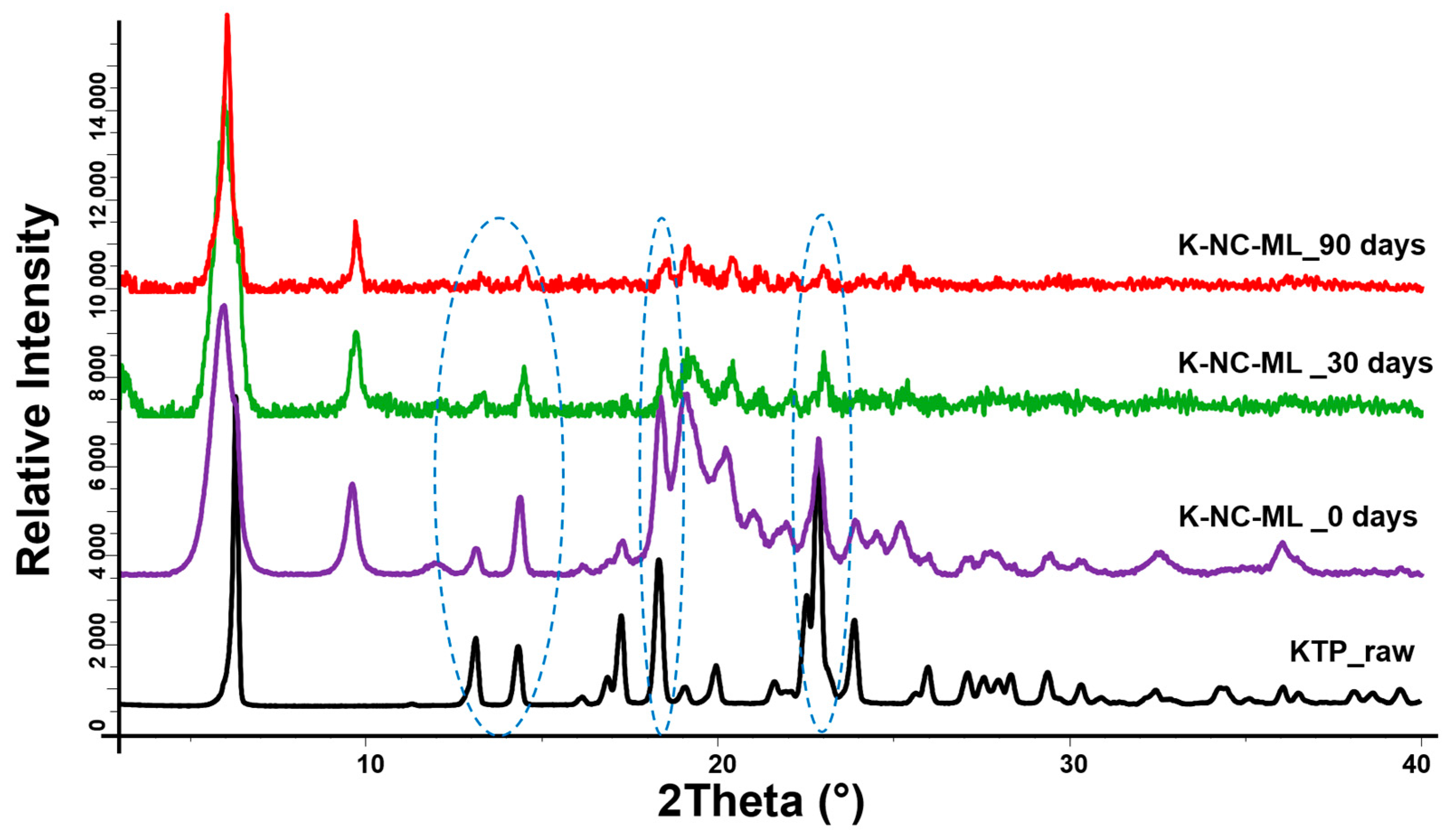
The samples and KTP raw were investigated using a BRUKER D8 Advance X-ray powder diffractometer (Bruker AXS GmbH, Karlsruhe, Germany) for structural analysis. The system utilized a Cu Kα1 radiation source (λ = 1.5406 Å) and a VÅNTEC-1 detector. Scans were performed with a copper target and nickel filter, operating at 40 kV and 40 mA. The measurements covered a 2θ range of 3° to 40°, with a step size of 0.01° and a step time of 0.1 s.
2.9.3. Aerodynamic Characterization by Andersen Cascade Impactor
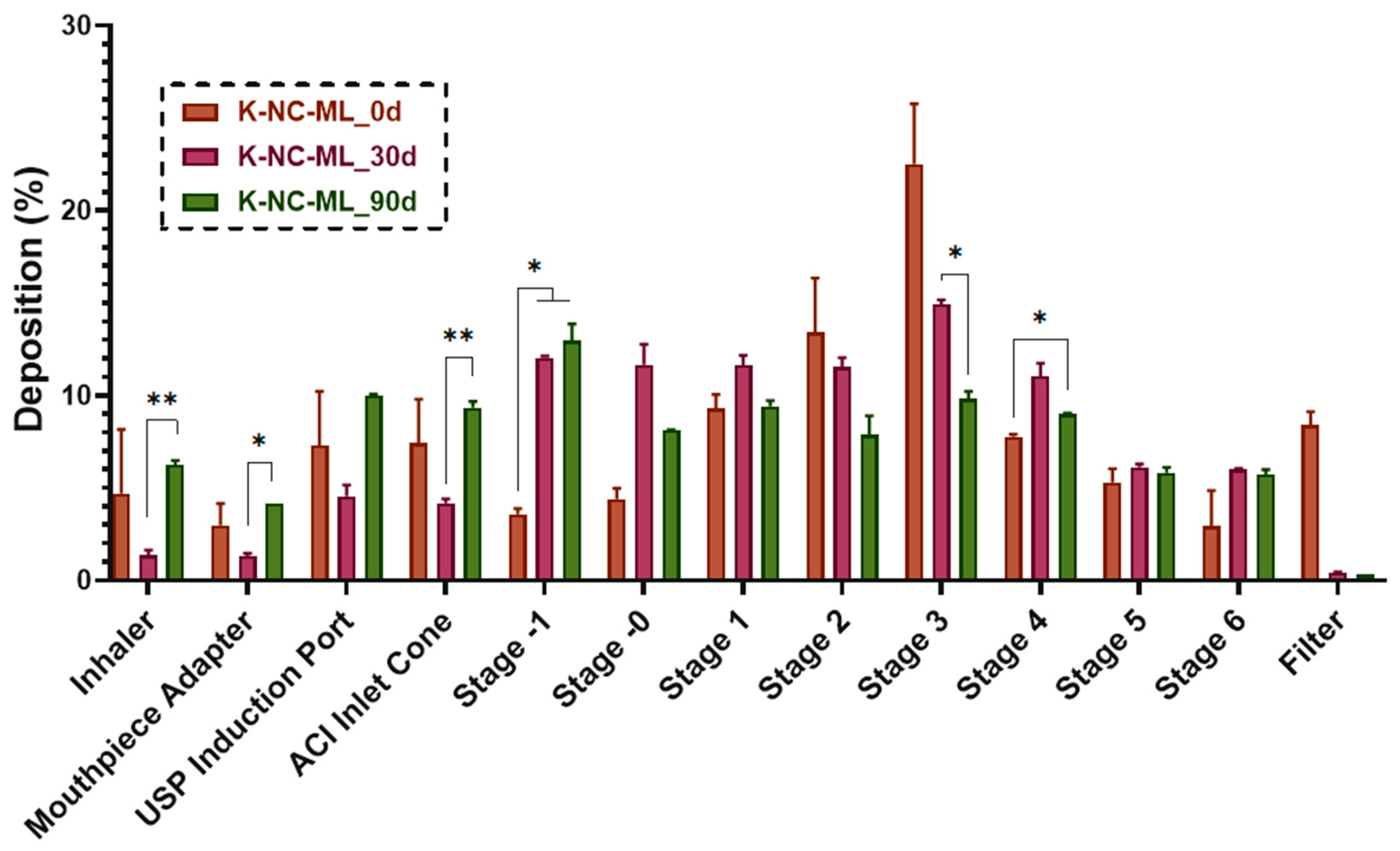
The aerosolization behavior of fresh prepared and stored NC-DP samples was evaluated using an Andersen Cascade Impactor (ACI) (Copley Scientific Ltd., Nottingham, UK). An inhalation flow rate of 60 L/min was maintained using a High-Capacity Pump (HCP5), along with a Critical Flow Controller (TPK, Copley Scientific Ltd.), with the flow rate confirmed by a mass flow meter (DFM 2000, Copley Scientific Ltd.). The samples were placed in size 3 Ezeeflo™ HPMC capsules (ACG-Associated Capsules Pvt. Ltd., Mumbai, India) and administered via a Breezhaler
® single-dose inhaler (Novartis International AG, Basel, Switzerland). Upon activation, aerosolization was carried out over a 4-s inhalation phase. To mimic lung adhesion, the ACI plates were pre-treated with a 1:99 (
v/
v%) mixture of Span 85 and cyclohexane, which was allowed to dry prior to testing. After aerosol deposition, each ACI component was washed with 50% methanol (
v/
v) to recover the deposited KTP. UV/VIS spectrophotometry (ATI-UNICAM UV/VIS Spectrophotometer, Cambridge, UK) was employed to measure KTP concentrations at 258 nm. The in vitro aerodynamic properties were analyzed using Inhalytix™ software (Copley Scientific Ltd., Nottingham, UK; Available online:
https://www.copleyscientific.com/inhaler-testing/apsd-data-analysis-software/inhalytix/, accessed on 10 October 2024) with key performance metrics calculated as follows:
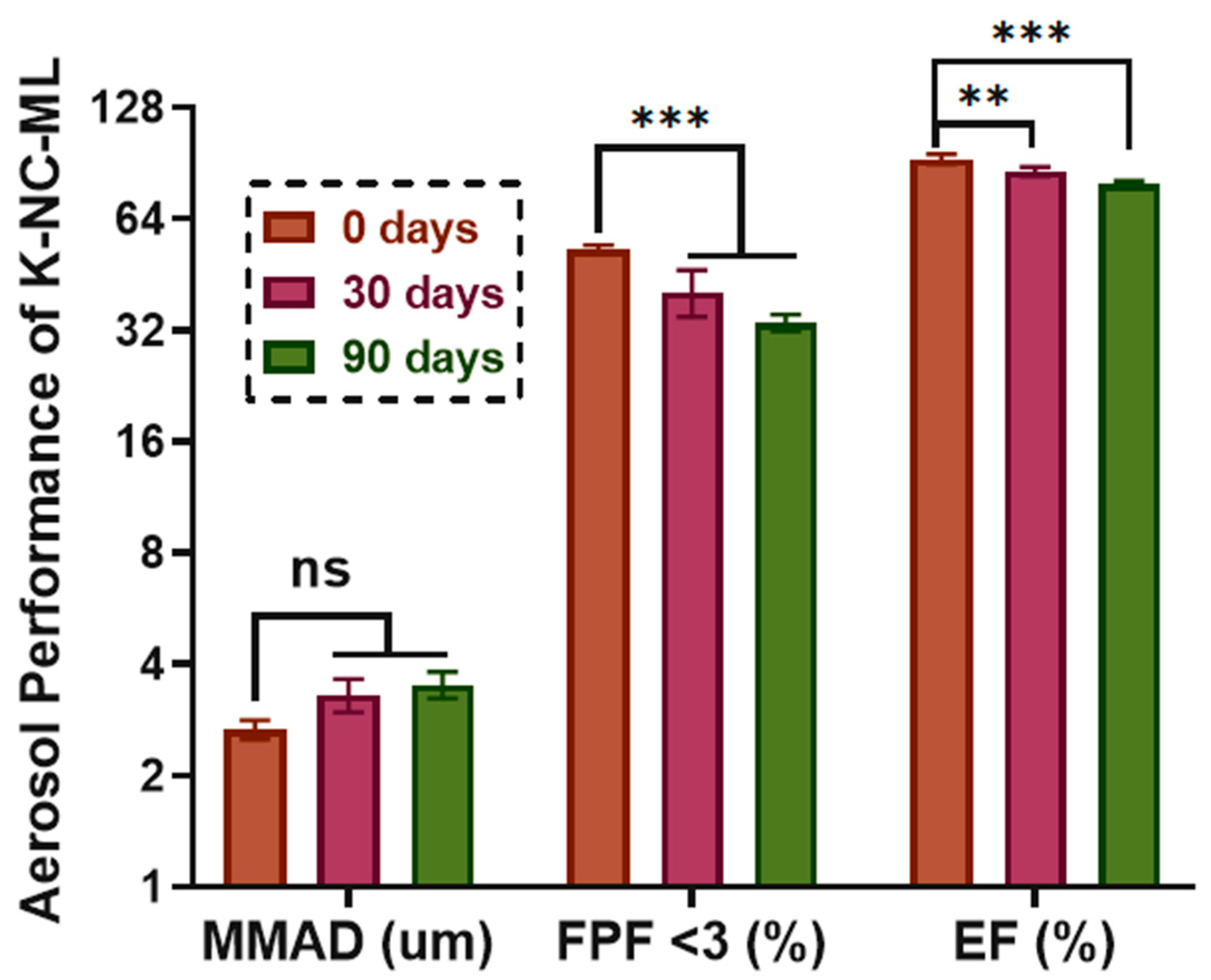
MMAD (Mass Median Aerodynamic Diameter): represents the effective particle size during inhalation.
FPF (Fine Particle Fraction): indicates the percentage of drug particles smaller than 5 µm.
EF (Emitted Fraction): denotes the percentage of drug mass reaching the impactor relative to the initial amount of KTP loaded.
2.9.4. Dissolution Test in Simulated Lung Media
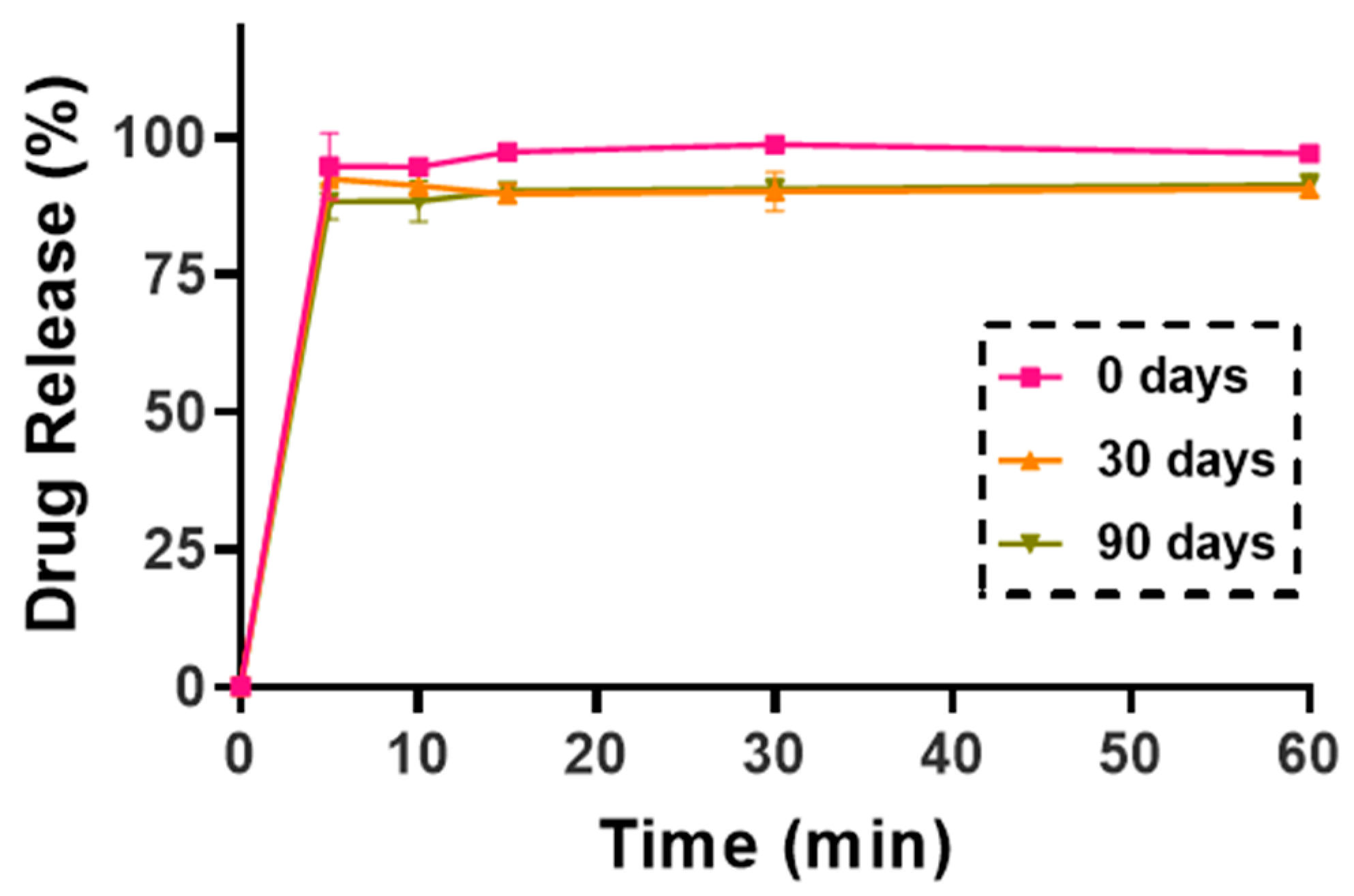
A modified paddle method (Hanson SR8 Plus, Teledyne Hanson Research, Chatsworth, CA, USA), adhering to European Pharmacopeia guidelines, was used to evaluate KTP release. To accommodate the study, 100 mL vessels and smaller paddles were substituted for the standard 1000 mL vessels. Simulated lung fluid (SLF) was prepared to mimic human airway conditions, consisting of sodium chloride (NaCl), sodium bicarbonate (NaHCO3), calcium chloride (CaCl2), sodium dihydrogen phosphate (NaH2PO4), sulfuric acid (H2SO4), and glycine, adjusted to a pH of 7.4. Each vessel was filled with 50 mL of SLF and maintained at 37 °C. The NC-DP samples, each containing 5 mg of KTP, were dispersed in the SLF, and the paddle was set to rotate at 50 rpm for 60 min. Samples of 5 mL were collected at intervals of 5, 10, 15, 30, and 60 min and immediately replaced with fresh SLF to maintain sink conditions. Collected samples were filtered using a Millex-HV syringe-driven filter unit with a 0.45 µm pore size (Millipore Corporation, Bedford, MA, USA) and analyzed via UV/VIS spectrophotometry at 258 nm (ATI-UNICAM UV/VIS Spectrophotometer, Cambridge, UK). All measurements were conducted in triplicate for accuracy and reproducibility.
2.10. Statistical Analysis
Statistical analysis was conducted using GraphPad Prism version 8.0.1 (GraphPad Software, CA, USA). A two-way ANOVA test was employed to observe statistical significance, with significant values at p ≤0.05 (* p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001).
4. Conclusions
This study demonstrated the potential of nanocrystal-based dry powders (NC-DPs) for drug delivery via inhalation by assessing their aerodynamic performance and stability. The incorporation of mannitol and leucine, either individually or in combination, significantly influenced the particle size, stability, dispersibility, and deposition behavior. While leucine in K-NC-L improved dispersibility, it also led to a higher exhaled fraction in the in silico model. In contrast, mannitol (K-NC-M) reduced exhalation but was predominantly deposited in the extra-thoracic region. Notably, the combination of mannitol and leucine (K-NC-ML) achieved an optimal balance, exhibiting low exhaled fractions, high lung deposition, and superior long-term stability. Additionally, stability assessments confirmed that K-NC-ML maintained its crystallinity, thermal behavior, release profile, and aerodynamic particle size under accelerated conditions. Hence, the combination of mannitol and leucine in K-NC-ML provided a balance between stability and aerosol performance.
Overall, these findings highlight the importance of excipient selection in optimizing DPI formulations and reinforce the feasibility of NC_DP for enhancing pulmonary drug delivery. Future research should further explore alternative excipient types in spray drying and assess stability under various storage conditions, particularly at lower relative humidity levels.