Validation of an LC-MS/MS Method for the Simultaneous Intracellular Quantification of the CDK4/6 Inhibitor Abemaciclib and the EZH2 Inhibitors GSK126 and Tazemetostat
Abstract
1. Introduction
2. Materials and Methods
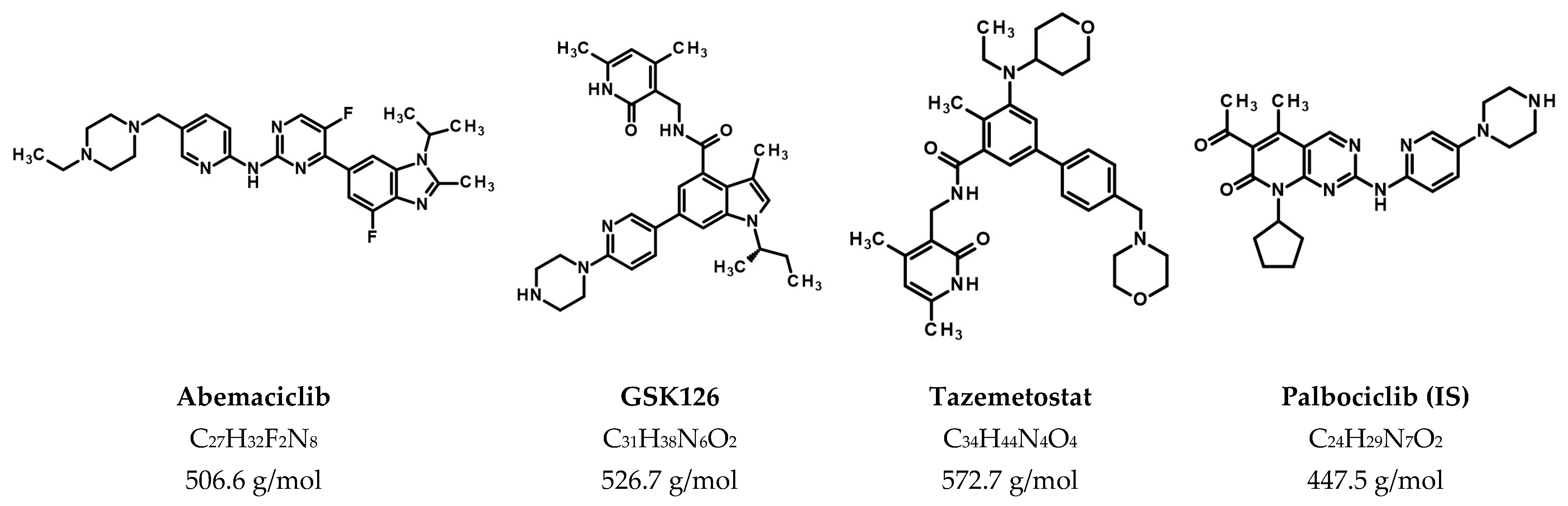
2.1. Chemicals and Reagents
2.2. Stock and Working Solutions
2.3. Sample Preparation
2.4. LC-MS/MS Instrumentation
2.5. Validation
2.5.1. Calibration Curve
2.5.2. Accuracy and Precision
2.5.3. Selectivity
2.5.4. Carryover
2.5.5. Matrix Effects
2.5.6. Dilution Integrity
2.5.7. Stability
2.5.8. Reinjection Reproducibility
2.5.9. Recovery Rate
2.6. Cell Culture
3. Results
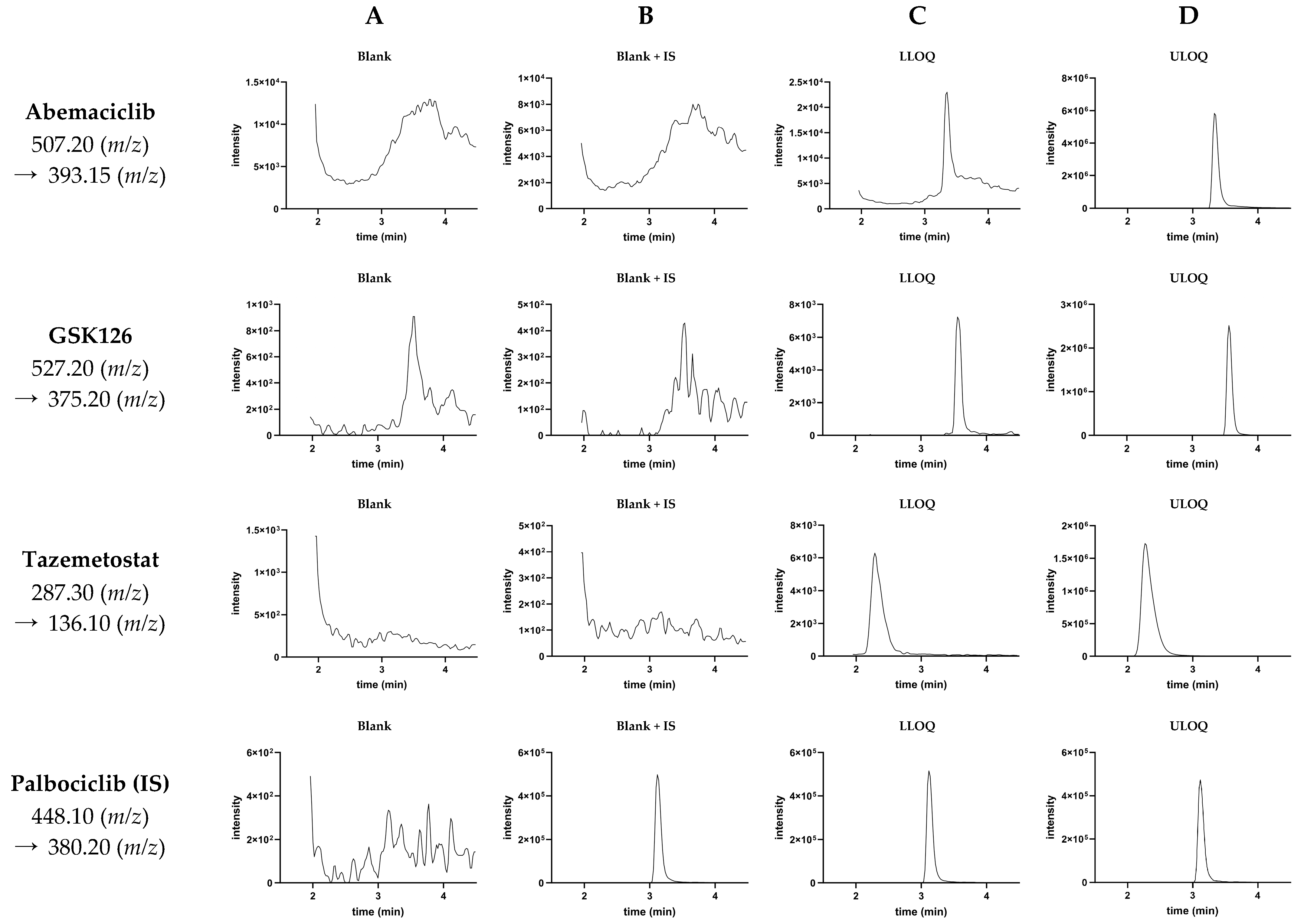
3.1. Validation of the LC-MS/MS Method
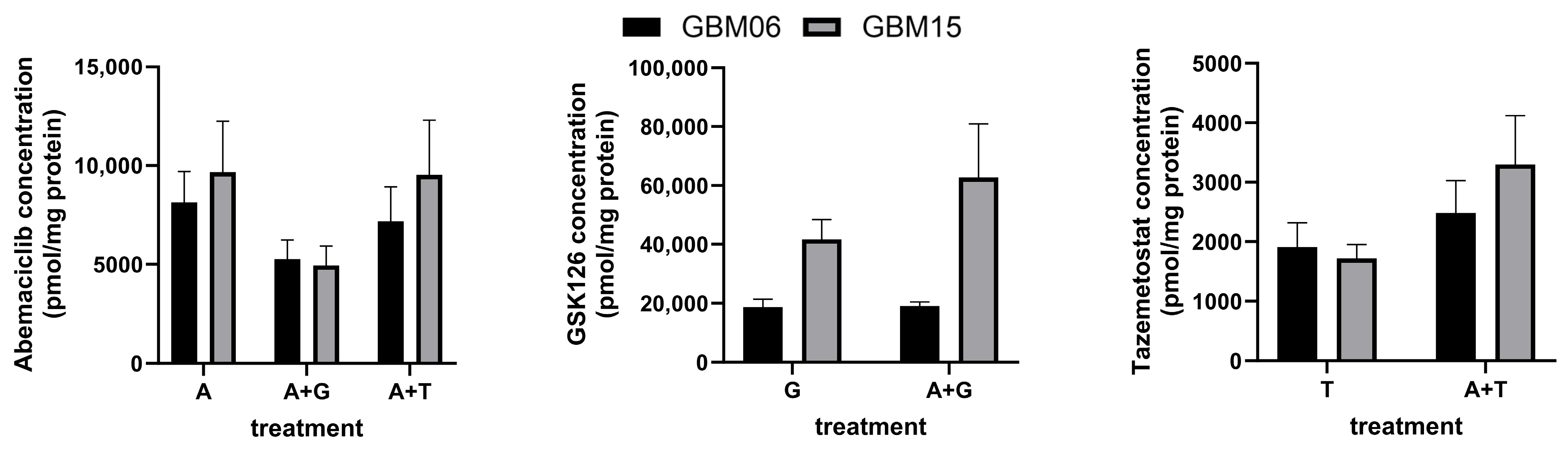
3.2. Intracellular Concentrations of Abemaciclib, GSK126 and Tazemetostat After Single and Combined Treatment of Patient-Derived GBM Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, Y.T.; Tan, Y.J.; Oon, C.E. Molecular Targeted Therapy: Treating Cancer with Specificity. Eur. J. Pharmacol. 2018, 834, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Bedard, P.L.; Hyman, D.M.; Davids, M.S.; Siu, L.L. Small Molecules, Big Impact: 20 Years of Targeted Therapy in Oncology. Lancet 2020, 395, 1078–1088. [Google Scholar] [CrossRef]
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small Molecules in Targeted Cancer Therapy: Advances, Challenges, and Future Perspectives. Signal Transduct. Target. Ther. 2021, 6, 201. [Google Scholar] [CrossRef] [PubMed]
- Van Arsdale, T.; Boshoff, C.; Arndt, K.T.; Abraham, R.T. Molecular Pathways: Targeting the Cyclin D–CDK4/6 Axis for Cancer Treatment. Clin. Cancer Res. 2015, 21, 2905–2910. [Google Scholar] [CrossRef]
- Sherr, C.J.; Beach, D.; Shapiro, G.I. Targeting CDK4 and CDK6: From Discovery to Therapy. Cancer Discov. 2016, 6, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, E.; Infante, J.R. Targeting CDK4/6 in Patients with Cancer. Cancer Treat. Rev. 2016, 45, 129–138. [Google Scholar] [CrossRef]
- Lim, J.S.J.; Turner, N.C.; Yap, T.A. CDK4/6 Inhibitors: Promising Opportunities beyond Breast Cancer. Cancer Discov. 2016, 6, 697–699. [Google Scholar] [CrossRef]
- Parylo, S.; Vennepureddy, A.; Dhar, V.; Patibandla, P.; Sokoloff, A. Role of Cyclin-Dependent Kinase 4/6 Inhibitors in the Current and Future Eras of Cancer Treatment. J. Oncol. Pharm. Pract. 2018, 25, 110–129. [Google Scholar] [CrossRef]
- Goel, S.; Bergholz, J.S.; Zhao, J.J. Targeting CDK4 and CDK6 in Cancer. Nat. Rev. Cancer 2022, 22, 356–372. [Google Scholar] [CrossRef] [PubMed]
- Corona, S.P.; Generali, D. Abemaciclib: A CDK4/6 Inhibitor for the Treatment of HR+/HER2− Advanced Breast Cancer. Drug Des. Dev. Ther. 2018, 12, 321–330. [Google Scholar] [CrossRef]
- Goetz, M.P.; Toi, M.; Campone, M.; Sohn, J.; Paluch-Shimon, S.; Huober, J.; Park, I.H.; Trédan, O.; Chen, S.-C.; Manso, L.; et al. MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J. Clin. Oncol. 2017, 35, 3638–3646. [Google Scholar] [CrossRef] [PubMed]
- Riess, C.; Koczan, D.; Schneider, B.; Linke, C.; del Moral, K.; Classen, C.F.; Maletzki, C. Cyclin-Dependent Kinase Inhibitors Exert Distinct Effects on Patient-Derived 2D and 3D Glioblastoma Cell Culture Models. Cell Death Discov. 2021, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Riess, C.; del Moral, K.; Fiebig, A.; Kaps, P.; Linke, C.; Hinz, B.; Rupprecht, A.; Frank, M.; Fiedler, T.; Koczan, D.; et al. Implementation of a Combined CDK Inhibition and Arginine-Deprivation Approach to Target Arginine-Auxotrophic Glioblastoma Multiforme Cells. Cell Death Dis. 2022, 13, 555. [Google Scholar] [CrossRef]
- Nduom, E.K.; Glod, J.; Brown, D.A.; Fagan, M.; Dalmage, M.; Heiss, J.; Steinberg, S.M.; Peer, C.; Figg, W.D.; Jackson, S. Clinical Protocol: Feasibility of Evaluating Abemaciclib Neuropharmacokinetics of Diffuse Midline Glioma Using Intratumoral Microdialysis. PLoS ONE 2023, 18, e0291068. [Google Scholar] [CrossRef]
- Rahman, R.; Trippa, L.; Lee, E.Q.; Arrillaga-Romany, I.; Fell, G.; Touat, M.; McCluskey, C.; Wiley, J.; Gaffey, S.; Drappatz, J.; et al. Inaugural Results of the Individualized Screening Trial of Innovative Glioblastoma Therapy: A Phase II Platform Trial for Newly Diagnosed Glioblastoma Using Bayesian Adaptive Randomization. J. Clin. Oncol. 2023, 41, 5524–5535. [Google Scholar] [CrossRef]
- Duan, R.; Du, W.; Guo, W. EZH2: A Novel Target for Cancer Treatment. J. Hematol. Oncol. 2020, 13, 104. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Roberts, C.W.M. Targeting EZH2 in Cancer. Nat. Med. 2016, 22, 128–134. [Google Scholar] [CrossRef]
- Orleni, M.; Beumer, J.H. Pharmacology and Pharmacokinetics of Tazemetostat. Cancer Chemother. Pharmacol. 2024, 93, 509–517. [Google Scholar] [CrossRef]
- Del Moral-Morales, A.; González-Orozco, J.C.; Hernández-Vega, A.M.; Hernández-Ortega, K.; Peña-Gutiérrez, K.M.; Camacho-Arroyo, I. EZH2 Mediates Proliferation, Migration, and Invasion Promoted by Estradiol in Human Glioblastoma Cells. Front. Endocrinol. 2022, 13, 703733. [Google Scholar] [CrossRef]
- Ratnam, N.M.; Sonnemann, H.M.; Frederico, S.C.; Chen, H.; Hutchinson, M.-K.N.D.; Dowdy, T.; Reid, C.M.; Jung, J.; Zhang, W.; Song, H.; et al. Reversing Epigenetic Gene Silencing to Overcome Immune Evasion in CNS Malignancies. Front. Oncol. 2021, 11, 719091. [Google Scholar] [CrossRef]
- Scuderi, S.A.; Filippone, A.; Basilotta, R.; Mannino, D.; Casili, G.; Capra, A.P.; Chisari, G.; Colarossi, L.; Sava, S.; Campolo, M.; et al. GSK343, an Inhibitor of Enhancer of Zeste Homolog 2, Reduces Glioblastoma Progression through Inflammatory Process Modulation: Focus on Canonical and Non-Canonical NF-ΚB/IκBα Pathways. Int. J. Mol. Sci. 2022, 23, 13915. [Google Scholar] [CrossRef]
- Sun, W.; Chen, L.; Tang, J.; Zhang, C.; Wen, Y.; Wen, W. Targeting EZH2 Depletes LMP1-Induced Activated Regulatory T Cells Enhancing Antitumor Immunity in Nasopharyngeal Carcinoma. J. Cancer Res. Ther. 2020, 16, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Lindsay, H.; Kogiso, M.; Du, Y.; Braun, F.K.; Zhang, H.; Guo, L.; Zhao, S.; Injac, S.G.; Baxter, P.A.; et al. Evaluation of an EZH2 Inhibitor in Patient-Derived Orthotopic Xenograft Models of Pediatric Brain Tumors Alone and in Combination with Chemo- and Radiation Therapies. Lab. Investig. 2022, 102, 185–193. [Google Scholar] [CrossRef]
- Freitag, T.; Kaps, P.; Ramtke, J.; Bertels, S.; Zunke, E.; Schneider, B.; Becker, A.-S.; Koczan, D.; Dubinski, D.; Freiman, T.M.; et al. Combined Inhibition of EZH2 and CDK4/6 Perturbs Endoplasmic Reticulum-Mitochondrial Homeostasis and Increases Antitumor Activity against Glioblastoma. npj Precis. Oncol. 2024, 8, 156. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Chávez, A.; Loos, N.H.C.; Lebre, M.C.; Tibben, M.M.; Rosing, H.; Beijnen, J.H.; Schinkel, A.H. ABCB1 and ABCG2 Limit Brain Penetration and, Together with CYP3A4, Total Plasma Exposure of Abemaciclib and Its Active Metabolites. Pharmacol. Res. 2022, 178, 105954. [Google Scholar] [CrossRef]
- Zhang, P.; de Gooijer, M.C.; Buil, L.C.M.; Beijnen, J.H.; Li, G.; van Tellingen, O. ABCB1 and ABCG2 Restrict the Brain Penetration of a Panel of Novel EZH2-Inhibitors. Int. J. Cancer 2015, 137, 2007–2018. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Chen, Z.; To, K.K.W.; Fang, X.; Wang, F.; Cheng, B.; Fu, L. Effect of Abemaciclib (LY2835219) on Enhancement of Chemotherapeutic Agents in ABCB1 and ABCG2 Overexpressing Cells In Vitro and In Vivo. Biochem. Pharmacol. 2017, 124, 29–42. [Google Scholar] [CrossRef]
- Budagaga, Y.; Sabet, Z.; Zhang, Y.; Novotná, E.; Hanke, I.; Rozkoš, T.; Hofman, J. Tazemetostat Synergistically Combats Multidrug Resistance by the Unique Triple Inhibition of ABCB1, ABCC1, and ABCG2 Efflux Transporters in Vitro and Ex Vivo. Biochem. Pharmacol. 2023, 216, 115769. [Google Scholar] [CrossRef] [PubMed]
- Sorf, A.; Sucha, S.; Morell, A.; Novotna, E.; Staud, F.; Zavrelova, A.; Visek, B.; Wsol, V.; Ceckova, M. Targeting Pharmacokinetic Drug Resistance in Acute Myeloid Leukemia Cells with CDK4/6 Inhibitors. Cancers 2020, 12, 1596. [Google Scholar] [CrossRef]
- Habler, K.; Vogeser, M.; Teupser, D. An UHPLC-MS/MS Method for Quantification of the CDK4/6 Inhibitor Abemaciclib in Human Serum. J. Mass Spectrom. Adv. Clin. Lab. 2022, 24, 15–21. [Google Scholar] [CrossRef]
- Wickremsinhe, E.R.; Lee, L.B. Quantification of Abemaciclib and Metabolites: Evolution of Bioanalytical Methods Supporting a Novel Oncolytic Agent. Bioanalysis 2021, 13, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Margaryan, T.; Elliott, M.; Sanai, N.; Tovmasyan, A. Simultaneous Determination of LY3214996, Abemaciclib, and M2 and M20 Metabolites in Human Plasma, Cerebrospinal Fluid, and Brain Tumor by LC-MS/MS. J. Pharm. Anal. 2022, 12, 601–609. [Google Scholar] [CrossRef]
- Soledad Poetto, A.; Posocco, B.; Zanchetta, M.; Gagno, S.; Orleni, M.; Canil, G.; Alberti, M.; Puglisi, F.; Toffoli, G. A New LC-MS/MS Method for the Simultaneous Quantification of Abemaciclib, Its Main Active Metabolites M2 and M20, and Letrozole for Therapeutic Drug Monitoring. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2022, 1207, 123403. [Google Scholar] [CrossRef]
- Habler, K.; Kalla, A.S.; Rychlik, M.; Vogeser, M.; Teupser, D. Therapeutic Drug Monitoring in Breast Cancer Therapy—LC-MS/MS Method for Quantification of the CDK4/6 Inhibitors Abemaciclib, Palbociclib, Ribociclib, and Major Metabolites Abemaciclib M20 and M2 in Human Serum. J. Pharm. Biomed. Anal. 2023, 225, 115211. [Google Scholar] [CrossRef]
- Burke, S.M.; Kamal, M.; Goey, A.K.L. Development and Validation of a Quantitative LC-MS/MS Method for CDK4/6 Inhibitors Palbociclib, Ribociclib, Abemaciclib, and Abemaciclib-M2 in Human Plasma. Ther. Drug Monit. 2024, 45, 327–336. [Google Scholar] [CrossRef]
- Posocco, B.; Zanchetta, M.; Orleni, M.; Gagno, S.; Montico, M.; Peruzzi, E.; Roncato, R.; Gerratana, L.; Corsetti, S.; Puglisi, F.; et al. Therapeutic Monitoring of Palbociclib, Ribociclib, Abemaciclib, M2, M20, and Letrozole in Human Plasma: A Novel LC-MS/MS Method. Ther. Drug Monit. 2024, 46, 485–493. [Google Scholar] [CrossRef]
- Martínez-Chávez, A.; Rosing, H.; Hillebrand, M.; Tibben, M.; Schinkel, A.H.; Beijnen, J.H. Development and Validation of a Bioanalytical Method for the Quantification of the CDK4/6 Inhibitors Abemaciclib, Palbociclib, and Ribociclib in Human and Mouse Matrices Using Liquid Chromatography-Tandem Mass Spectrometry. Anal. Bioanal. Chem. 2019, 411, 5331–5345. [Google Scholar] [CrossRef]
- Sato, Y.; Shigeta, K.; Hirasawa, T.; Sato, T.; Ogura, J.; Maekawa, M.; Ebata, A.; Hamanaka, Y.; Tada, H.; Ishida, T.; et al. Establishment of an Analytical Method for Simultaneous Quantitation of CDK4/6 Inhibitors, Aromatase Inhibitors, and an Estrogen Receptor Antagonist in Human Plasma Using LC-ESI-MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2021, 1173, 122655. [Google Scholar] [CrossRef]
- Calucica, D.M.; Manda, C.-V.; Gaman, A.M.; Raileanu, S.; Stanca, L.; Popescu, M.D.E.; Mateescu, O.G.; Bita, A.; Croitoru, O.; Neamtu, S.-D. Development of a SPE-LC-MS Method for the Quantitation of Palbociclib and Abemaciclib in Human Plasma. Molecules 2022, 27, 8604. [Google Scholar] [CrossRef]
- Turković, L.; Bočkor, L.; Ekpenyong, O.; Silovski, T.; Lovrić, M.; Crnković, S.; Nigović, B.; Sertić, M. Development and Validation of a Novel LC-MS/MS Method for the Simultaneous Determination of Abemaciclib, Palbociclib, Ribociclib, Anastrozole, Letrozole, and Fulvestrant in Plasma Samples: A Prerequisite for Personalized Breast Cancer Treatment. Pharmaceuticals 2022, 15, 614. [Google Scholar] [CrossRef]
- Turković, L.; Koraj, N.; Mlinarić, Z.; Silovski, T.; Crnković, S.; Sertić, M. Optimisation of Dispersive Liquid-Liquid Microextraction for Plasma Sample Preparation in Bioanalysis of CDK4/6 Inhibitors in Therapeutic Combinations for Breast Cancer Treatment. Heliyon 2023, 9, e18880. [Google Scholar] [CrossRef] [PubMed]
- Turković, L.; Mutavdžić Pavlović, D.; Mlinarić, Z.; Skenderović, A.; Silovski, T.; Sertić, M. Optimisation of Solid-Phase Extraction and LC-MS/MS Analysis of Six Breast Cancer Drugs in Patient Plasma Samples. Pharmaceuticals 2023, 16, 1445. [Google Scholar] [CrossRef] [PubMed]
- Mlinarić, Z.; Turković, L.; Sertić, M. Dispersive Liquid-Liquid Microextraction Followed by Sweeping Micellar Electrokinetic Chromatography-Tandem Mass Spectrometry for Determination of Six Breast Cancer Drugs in Human Plasma. J. Chromatogr. A 2024, 1718, 464698. [Google Scholar] [CrossRef] [PubMed]
- Yedlapalli, G.; Kumar, Y.G. Optimization of Bioanalytical Liquid Chromatography—Tandem Mass Spectrometric Method for Quantification of Tazemetostat: An Epithelioid Sarcoma Treatment Drug in Human Plasma. In Proceedings of the 2023 12th International Conference on System Modeling & Advancement in Research Trends (SMART), Moradabad, India, 22–23 December 2023; pp. 550–555. [Google Scholar] [CrossRef]
- Orleni, M.; Parise, R.A.; Holleran, J.L.; Amengual, J.E.; Feng, Y.; Synold, T.; Beumer, J.H. Quantitation of Tazemetostat in Human Plasma Using Liquid Chromatography–Tandem Mass Spectrometry. Biomed. Chromatogr. 2024, 38, e5903. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.J.; Geng, X.N.; Kang, Y.R.; Wang, Y.L.; Qiu, X.J. Pharmacokinetics of Herb-Drug Interactions of Plumbagin and Tazemetostat in Rats by UPLC-MS/MS. Drug Des. Dev. Ther. 2022, 16, 3385–3394. [Google Scholar] [CrossRef]
- International Council for Harmonisation. M10 Guideline for Bioanalytical Method Validation and Study Sample Analysis. 2022. 1–59. Available online: https://database.ich.org/sites/default/files/M10_Guideline_Step4_2022_0524.pdf (accessed on 7 February 2025).
- Prüser, J.L.; Ramer, R.; Wittig, F.; Ivanov, I.; Merkord, J.; Hinz, B. The Monoacylglycerol Lipase Inhibitor JZL184 Inhibits Lung Cancer Cell Invasion and Metastasis via the CB1 Cannabinoid Receptor. Mol. Cancer Ther. 2021, 20, 787–802. [Google Scholar] [CrossRef]
- Särchen, V.; Shanmugalingam, S.; Kehr, S.; Reindl, L.M.; Greze, V.; Wiedemann, S.; Boedicker, C.; Jacob, M.; Bankov, K.; Becker, N.; et al. Pediatric Multicellular Tumor Spheroid Models Illustrate a Therapeutic Potential by Combining BH3 Mimetics with Natural Killer (NK) Cell-Based Immunotherapy. Cell Death Discov. 2022, 8, 11. [Google Scholar] [CrossRef]
- Gulati, N.; Béguelin, W.; Giulino-Roth, L. Enhancer of Zeste Homolog 2 (EZH2) Inhibitors. Leuk. Lymphoma 2018, 59, 1574–1585. [Google Scholar] [CrossRef]
- Groß, E.; Hilger, R.-A.; Schümann, F.L.; Bauer, M.; Bouska, A.; Rohde, C.; Willscher, E.; Lützkendorf, J.; Müller, L.P.; Edemir, B.; et al. SAM-Competitive EZH2-Inhibitors Induce Platinum Resistance by EZH2-Independent Induction of ABC-Transporters. Cancers 2023, 15, 3043. [Google Scholar] [CrossRef]

| Time (min) | Eluent B (%) |
|---|---|
| 0.00 | 20 |
| 2.00 | 95 |
| 4.00 | 95 |
| 4.10 | 20 |
| Analyte | Precursor Ion (m/z) | Product Ion (m/z) | Collision Energy (eV) | Retention Time (min) |
|---|---|---|---|---|
| Abemaciclib | 507.20 | 393.15 | −24.0 | 3.30 |
| GSK126 | 527.20 | 375.20 | −27.0 | 3.60 |
| Tazemetostat | 287.30 | 136.10 | −15.0 | 2.30 |
| Palbociclib (IS) | 448.10 | 380.20 | −29.0 | 3.10 |
| Analyte | Calibration Range (nmol/L) | Quality Control Level Concentration (nmol/L) | ||||
|---|---|---|---|---|---|---|
| LLOQ | ULOQ | QC-LLOQ | QC-L | QC-M | QC-H | |
| Abemaciclib | 10 | 2500 | 10 | 25 | 1000 | 2000 |
| GSK126 | 50 | 12,500 | 50 | 125 | 5000 | 10,000 |
| Tazemetostat | 50 | 12,500 | 50 | 125 | 5000 | 10,000 |
| Parameter | Quality Control Level | Abemaciclib | GSK126 | Tazemetostat |
|---|---|---|---|---|
| Within-run accuracy (%, mean of n = 6) | QC-LLOQ | 106.4 | 110.9 | 100.7 |
| QC-L | 93.3 | 89.8 | 88.5 | |
| QC-M | 93.4 | 94.2 | 92.8 | |
| QC-H | 93.3 | 98.8 | 94.9 | |
| Within-run precision (% CV of n = 6) | QC-LLOQ | 3.33 | 3.21 | 4.53 |
| QC-L | 4.16 | 9.35 | 6.32 | |
| QC-M | 1.74 | 1.35 | 1.57 | |
| QC-H | 4.34 | 4.10 | 4.02 | |
| Between-run accuracy (%, mean of n = 18) | QC-LLOQ | 107.2 | 110.7 | 103.3 |
| QC-L | 92.8 | 90.0 | 92.2 | |
| QC-M | 89.1 | 90.5 | 90.2 | |
| QC-H | 101.3 | 103.3 | 97.4 | |
| Between-run precision (% CV of n = 18) | QC-LLOQ | 4.65 | 4.00 | 4.12 |
| QC-L | 4.20 | 6.23 | 5.14 | |
| QC-M | 4.94 | 4.29 | 3.26 | |
| QC-H | 6.55 | 4.63 | 3.80 |
| Storage Condition | Quality Control Level | Abemaciclib | GSK126 | Tazemetostat | |||
|---|---|---|---|---|---|---|---|
| Accuracy (%) | Precision (% CV) | Accuracy (%) | Precision (% CV) | Accuracy (%) | Precision (% CV) | ||
| Short-term stability (room temperature, 2 h) | QC-L | 108.0 | 3.38 | 101.4 | 3.73 | 102.1 | 5.22 |
| QC-H | 99.9 | 3.56 | 104.9 | 4.51 | 101.5 | 4.09 | |
| Long-term stability (−20 °C, 2 months) | QC-L | 103.9 | 1.77 | 90.28 | 7.21 | 99.5 | 2.41 |
| QC-H | 100.6 | 2.17 | 87.0 | 1.21 | 100.3 | 1.08 | |
| Freeze–thaw-stability (3 cycles, −20 °C) | QC-L | 102.4 | 4.79 | 90.8 | 5.21 | 95.9 | 3.33 |
| QC-H | 94.3 | 1.53 | 92.7 | 4.57 | 93.6 | 1.71 | |
| Autosampler stability (10 °C, 48 h) | QC-L | 90.4 | 7.57 | 87.2 | 5.53 | 86.5 | 5.39 |
| QC-H | 86.6 | 4.69 | 93.6 | 7.07 | 87.2 | 4.89 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senekowitsch, S.; Freitag, T.; Dubinski, D.; Freiman, T.M.; Maletzki, C.; Hinz, B. Validation of an LC-MS/MS Method for the Simultaneous Intracellular Quantification of the CDK4/6 Inhibitor Abemaciclib and the EZH2 Inhibitors GSK126 and Tazemetostat. Pharmaceutics 2025, 17, 433. https://doi.org/10.3390/pharmaceutics17040433
Senekowitsch S, Freitag T, Dubinski D, Freiman TM, Maletzki C, Hinz B. Validation of an LC-MS/MS Method for the Simultaneous Intracellular Quantification of the CDK4/6 Inhibitor Abemaciclib and the EZH2 Inhibitors GSK126 and Tazemetostat. Pharmaceutics. 2025; 17(4):433. https://doi.org/10.3390/pharmaceutics17040433
Chicago/Turabian StyleSenekowitsch, Stefan, Thomas Freitag, Daniel Dubinski, Thomas M. Freiman, Claudia Maletzki, and Burkhard Hinz. 2025. "Validation of an LC-MS/MS Method for the Simultaneous Intracellular Quantification of the CDK4/6 Inhibitor Abemaciclib and the EZH2 Inhibitors GSK126 and Tazemetostat" Pharmaceutics 17, no. 4: 433. https://doi.org/10.3390/pharmaceutics17040433
APA StyleSenekowitsch, S., Freitag, T., Dubinski, D., Freiman, T. M., Maletzki, C., & Hinz, B. (2025). Validation of an LC-MS/MS Method for the Simultaneous Intracellular Quantification of the CDK4/6 Inhibitor Abemaciclib and the EZH2 Inhibitors GSK126 and Tazemetostat. Pharmaceutics, 17(4), 433. https://doi.org/10.3390/pharmaceutics17040433






