The Role of Inorganic Nanomaterials in Overcoming Challenges in Colorectal Cancer Diagnosis and Therapy
Abstract
1. Introduction
2. Classification and Characterization of Inorganic Nanomaterials
2.1. Metal Nanomaterials
2.2. Metal Oxide Nanomaterials
2.3. Quantum Dots
2.4. Carbon-Based Nanomaterials
2.5. Magnetic Nanomaterials
2.6. Rare-Earth Nanomaterials
3. Inorganic Nanomaterials in Diagnosis and Therapy of Colorectal Cancer
3.1. Diagnostics
3.1.1. CT Imaging
3.1.2. MRI
3.1.3. Raman Endoscopic Imaging
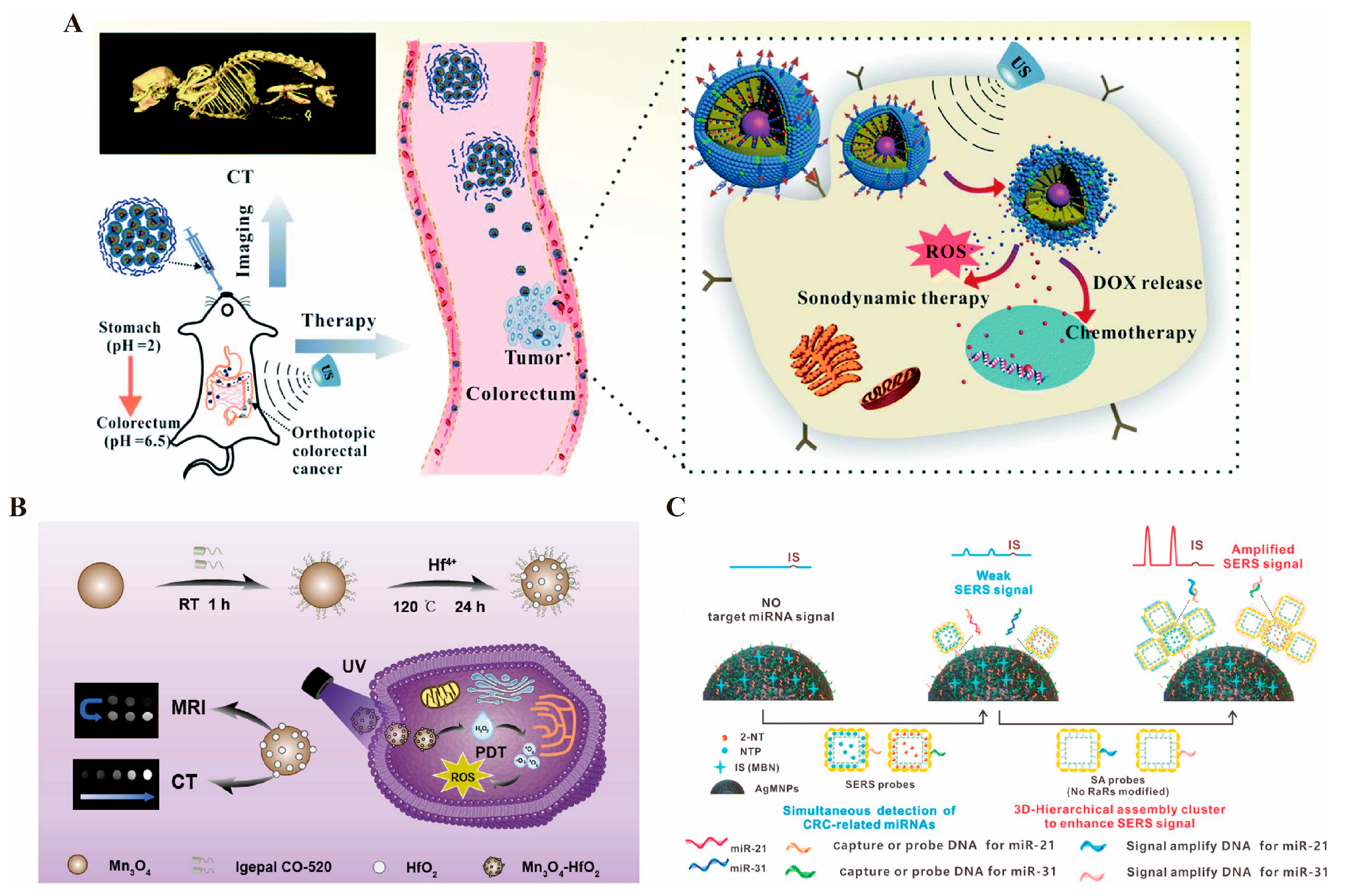
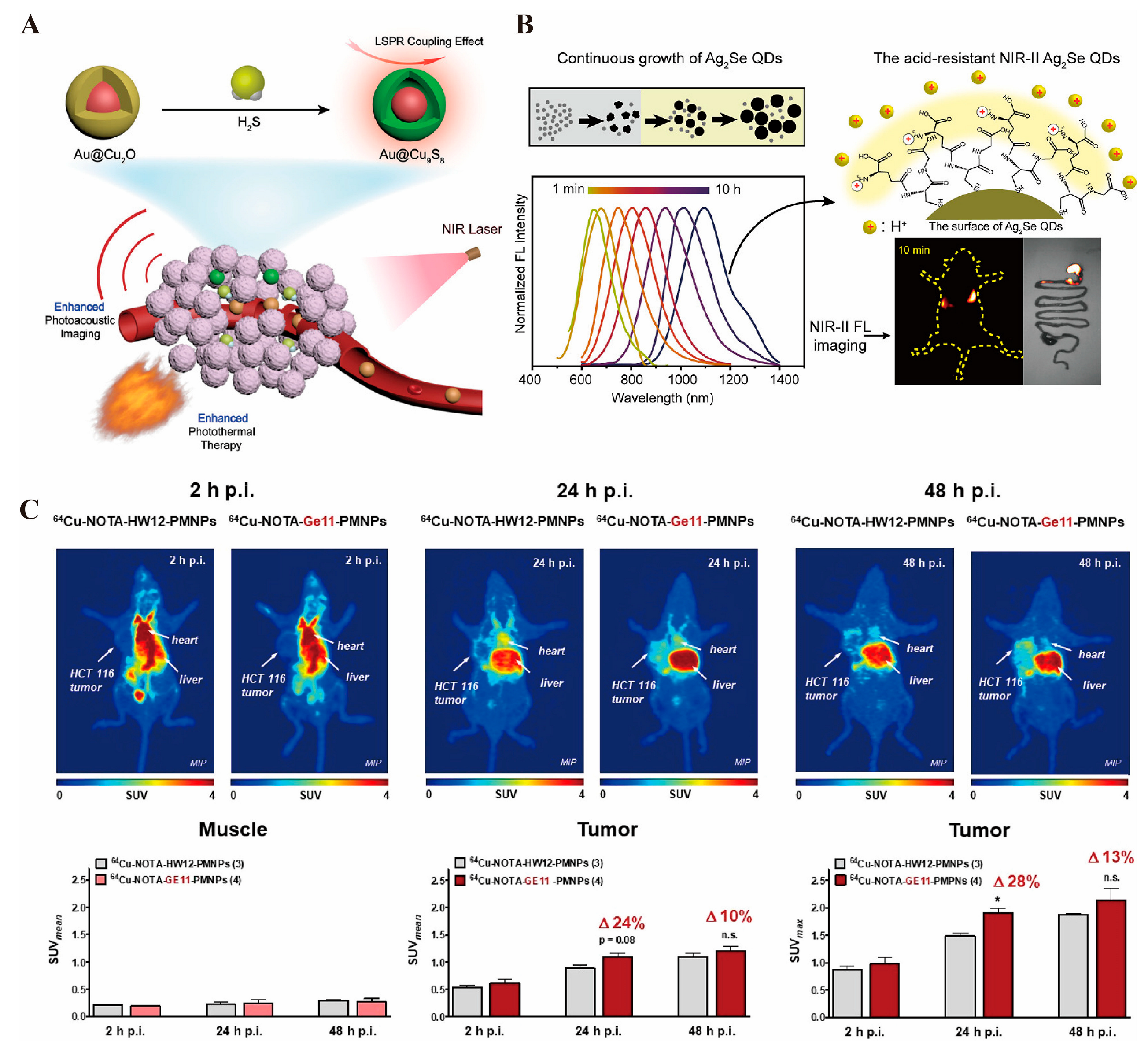
3.1.4. Photoacoustic Imaging
3.1.5. Fluorescence Imaging
3.1.6. PET Imaging
3.2. Drug Delivery
3.3. Therapy
3.3.1. Photothermal Therapy
3.3.2. Photodynamic Therapy
3.3.3. Magnetic Hyperthermia Therapy
3.3.4. Immunotherapy
4. Biocompatibility and Toxicity of Inorganic Nanomaterials
5. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G.; van de Velde, C.J.; Watanabe, T. Colorectal cancer. Nat. Rev. Dis. Primers 2015, 1, 15065. [Google Scholar] [CrossRef] [PubMed]
- Weitz, J.; Koch, M.; Debus, J.; Höhler, T.; Galle, P.R.; Büchler, M.W. Colorectal cancer. Lancet 2005, 365, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Biller, L.H.; Schrag, D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA 2021, 325, 669–685. [Google Scholar] [CrossRef]
- Ganesh, K.; Stadler, Z.K.; Cercek, A.; Mendelsohn, R.B.; Shia, J.; Segal, N.H.; Diaz, L.A., Jr. Immunotherapy in colorectal cancer: Rationale, challenges and potential. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 361–375. [Google Scholar] [CrossRef]
- Markman, J.L.; Rekechenetskiy, A.; Holler, E.; Ljubimova, J.Y. Nanomedicine therapeutic approaches to overcome cancer drug resistance. Adv. Drug Deliv. Rev. 2013, 65, 1866–1879. [Google Scholar] [CrossRef]
- Wei, G.; Wang, Y.; Yang, G.; Wang, Y.; Ju, R. Recent progress in nanomedicine for enhanced cancer chemotherapy. Theranostics 2021, 11, 6370–6392. [Google Scholar] [CrossRef]
- Pei, Z.; Chen, S.; Ding, L.; Liu, J.; Cui, X.; Li, F.; Qiu, F. Current perspectives and trend of nanomedicine in cancer: A review and bibliometric analysis. J. Control. Release Off. J. Control. Release Soc. 2022, 352, 211–241. [Google Scholar] [CrossRef]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef]
- Shi, Y.; van der Meel, R.; Chen, X.; Lammers, T. The EPR effect and beyond: Strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics 2020, 10, 7921–7924. [Google Scholar] [CrossRef]
- Ikeda-Imafuku, M.; Wang, L.L.; Rodrigues, D.; Shaha, S.; Zhao, Z.; Mitragotri, S. Strategies to improve the EPR effect: A mechanistic perspective and clinical translation. J. Control. Release Off. J. Control. Release Soc. 2022, 345, 512–536. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Chen, J.; Wang, Y.; Ji, H.; Peng, R.; Jin, L.; Wu, W. Advances in refunctionalization of erythrocyte-based nanomedicine for enhancing cancer-targeted drug delivery. Theranostics 2019, 9, 6885–6900. [Google Scholar] [CrossRef] [PubMed]
- Quan, X.; Han, Y.; Lu, P.; Ding, Y.; Wang, Q.; Li, Y.; Wei, J.; Huang, Q.; Wang, R.; Zhao, Y. Annexin V-Modified Platelet-Biomimetic Nanomedicine for Targeted Therapy of Acute Ischemic Stroke. Adv. Healthc. Mater. 2022, 11, e2200416. [Google Scholar] [CrossRef] [PubMed]
- Rizwanullah, M.; Ahmad, M.Z.; Ghoneim, M.M.; Alshehri, S.; Imam, S.S.; Md, S.; Alhakamy, N.A.; Jain, K.; Ahmad, J. Receptor-Mediated Targeted Delivery of Surface-ModifiedNanomedicine in Breast Cancer: Recent Update and Challenges. Pharmaceutics 2021, 13, 2039. [Google Scholar] [CrossRef]
- Zhu, X.; Li, S. Nanomaterials in tumor immunotherapy: New strategies and challenges. Mol. Cancer 2023, 22, 94. [Google Scholar] [CrossRef]
- Amaldoss, M.J.N.; Yang, J.L.; Koshy, P.; Unnikrishnan, A.; Sorrell, C.C. Inorganic nanoparticle-based advanced cancer therapies: Promising combination strategies. Drug Discov. Today 2022, 27, 103386. [Google Scholar] [CrossRef]
- Sowmiya, P.; Dhas, T.S.; Inbakandan, D.; Anandakumar, N.; Nalini, S.; Suganya, K.S.U.; Remya, R.R.; Karthick, V.; Kumar, C.M.V. Optically active organic and inorganic nanomaterials for biological imaging applications: A review. Micron 2023, 172, 103486. [Google Scholar] [CrossRef]
- Khalid, K.; Tan, X.; Mohd Zaid, H.F.; Tao, Y.; Lye Chew, C.; Chu, D.T.; Lam, M.K.; Ho, Y.C.; Lim, J.W.; Chin Wei, L. Advanced in developmental organic and inorganic nanomaterial: A review. Bioengineered 2020, 11, 328–355. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, A.; Zhang, Z.; Zhao, Q.; Li, J.; Mei, Y.; Yin, Y.; Wang, W. Multifunctional inorganic nanomaterials for cancer photoimmunotherapy. Cancer Commun. 2022, 42, 141–163. [Google Scholar] [CrossRef]
- Pei, Z.; Lei, H.; Cheng, L. Bioactive inorganic nanomaterials for cancer theranostics. Chem. Soc. Rev. 2023, 52, 2031–2081. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Jin, C.; Subedi, S.; Lee, C.L.; Wang, Q.; Jiang, Y.; Li, J.; Di, Y.; Fu, D. Emerging inorganic nanomaterials for pancreatic cancer diagnosis and treatment. Cancer Treat. Rev. 2012, 38, 566–579. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Wang, J.; Xiong, B.; Hu, J.; Zeng, P.; Liu, X.; Liang, H. Biologically Safe, Versatile, and Smart Bismuthene Functionalized with a Drug Delivery System Based on Red Phosphorus Quantum Dots for Cancer Theranostics. Angew. Chem. 2022, 61, e202117679. [Google Scholar] [CrossRef] [PubMed]
- Avasthi, A.; Caro, C.; Pozo-Torres, E.; Leal, M.P.; García-Martín, M.L. Magnetic Nanoparticles as MRI Contrast Agents. Top. Curr. Chem. 2020, 378, 40. [Google Scholar] [CrossRef]
- Cheng, Y.; Lu, T.; Wang, Y.; Song, Y.; Wang, S.; Lu, Q.; Yang, L.; Tan, F.; Li, J.; Li, N. Glutathione-Mediated Clearable Nanoparticles Based on Ultrasmall Gd2O3 for MSOT/CT/MR Imaging Guided Photothermal/Radio Combination Cancer Therapy. Mol. Pharm. 2019, 16, 3489–3501. [Google Scholar] [CrossRef]
- Fu, Q.; Zhu, R.; Song, J.; Yang, H.; Chen, X. Photoacoustic Imaging: Contrast Agents and Their Biomedical Applications. Adv. Mater. 2019, 31, e1805875. [Google Scholar] [CrossRef]
- Fu, Y.; Jang, M.S.; Liu, C.; Li, Y.; Lee, J.H.; Yang, H.Y. Oxygen-Generating Organic/Inorganic Self-Assembled Nanocolloids for Tumor-Activated Dual-Model Imaging-Guided Photodynamic Therapy. ACS Appl. Mater. Interfaces 2023, 15, 36013–36024. [Google Scholar] [CrossRef]
- Mi, P.; Kokuryo, D.; Cabral, H.; Kumagai, M.; Nomoto, T.; Aoki, I.; Terada, Y.; Kishimura, A.; Nishiyama, N.; Kataoka, K. Hydrothermally synthesized PEGylated calcium phosphate nanoparticles incorporating Gd-DTPA for contrast enhanced MRI diagnosis of solid tumors. J. Control. Release Off. J. Control. Release Soc. 2014, 174, 63–71. [Google Scholar] [CrossRef]
- Li, X.; Jian, M.; Sun, Y.; Zhu, Q.; Wang, Z. The Peptide Functionalized Inorganic Nanoparticles for Cancer-Related Bioanalytical and Biomedical Applications. Molecules 2021, 26, 3228. [Google Scholar] [CrossRef]
- Huang, P.; Wang, C.; Deng, H.; Zhou, Y.; Chen, X. Surface Engineering of Nanoparticles toward Cancer Theranostics. Acc. Chem. Res. 2023, 56, 1766–1779. [Google Scholar] [CrossRef]
- Peng, J.; Yang, Q.; Lei, R.; Wang, Y.; Liu, G.; Qian, Z. Preferential activation of type I interferon-mediated antitumor inflammatory signaling by CuS/MnO2/diAMP nanoparticles enhances anti-PD-1 therapy for sporadic colorectal cancer. J. Nanobiotechnol. 2024, 22, 699. [Google Scholar] [CrossRef] [PubMed]
- Nayl, A.A.; El-Fakharany, E.M.; Abd-Elhamid, A.I.; Arafa, W.A.A.; Alanazi, A.H.; Ahmed, I.M.; Abdelgawad, M.A.; Aly, A.A.; Bräse, S. Alginate-modified graphene oxide anchored with lactoperoxidase as a novel bioactive nanocombination for colorectal cancer therapy. Sci. Rep. 2024, 14, 24804. [Google Scholar] [CrossRef]
- Rivera Gil, P.; Hühn, D.; del Mercato, L.L.; Sasse, D.; Parak, W.J. Nanopharmacy: Inorganic nanoscale devices as vectors and active compounds. Pharmacol. Res. 2010, 62, 115–125. [Google Scholar] [CrossRef]
- Bonvalot, S.; Rutkowski, P.L.; Thariat, J.; Carrère, S.; Ducassou, A.; Sunyach, M.P.; Agoston, P.; Hong, A.; Mervoyer, A.; Rastrelli, M.; et al. NBTXR3, a first-in-class radioenhancer hafnium oxide nanoparticle, plus radiotherapy versus radiotherapy alone in patients with locally advanced soft-tissue sarcoma (Act.In.Sarc): A multicentre, phase 2-3, randomised, controlled trial. Lancet. Oncol. 2019, 20, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Yang, S.T.; Huang, Y.; Zeng, C.; Xin, Q.; Zeng, G.; Yang, S.; Xia, P.; Tang, X.; Tang, K. Carbon Nanoparticles-Fe(II) Complex for Efficient Tumor Inhibition with Low Toxicity by Amplifying Oxidative Stress. ACS Appl. Mater. Interfaces 2020, 12, 29094–29102. [Google Scholar] [CrossRef]
- Xie, P.; Qu, T.; Tang, K.; Huang, Y.; Zeng, G.; Yuan, H.; Xin, Q.; Zhao, Y.; Yang, J.; Zeng, C.; et al. Carbon nanoparticles-Fe(II) complex combined with sorafenib for ferroptosis-induced antitumor effects in triple-negative breast cancer. Colloids Surf. B Biointerfaces 2025, 250, 114562. [Google Scholar] [CrossRef] [PubMed]
- Gou, Z.; Tang, K.; Zeng, C.; Yuan, H.; Zhang, C.; Huang, Y.; Qu, T.; Xin, Q.; Zhao, Y.; Zeng, G.; et al. Photothermal therapy of xenografted tumor by carbon nanoparticles-Fe(II) complex. Colloids Surf. B Biointerfaces 2024, 240, 113968. [Google Scholar] [CrossRef]
- Stijns, R.C.H.; Philips, B.W.J.; Nagtegaal, I.D.; Polat, F.; de Wilt, J.H.W.; Wauters, C.A.P.; Zamecnik, P.; Fütterer, J.J.; Scheenen, T.W.J. USPIO-enhanced MRI of lymph nodes in rectal cancer: A node-to-node comparison with histopathology. Eur. J. Radiol. 2021, 138, 109636. [Google Scholar] [CrossRef]
- Koh, D.M.; Brown, G.; Temple, L.; Raja, A.; Toomey, P.; Bett, N.; Norman, A.R.; Husband, J.E. Rectal cancer: Mesorectal lymph nodes at MR imaging with USPIO versus histopathologic findings--initial observations. Radiology 2004, 231, 91–99. [Google Scholar] [CrossRef]
- Litjens, G.; Nakamoto, A.; Brosens, L.A.A.; Maas, M.C.; Scheenen, T.W.J.; Zámecnik, P.; van Geenen, E.J.M.; Prokop, M.; van Laarhoven, K.; Hermans, J.J. Ferumoxtran-10-enhanced MRI for pre-operative metastatic lymph node detection in pancreatic, duodenal, or periampullary adenocarcinoma. Eur. Radiol. 2024, 34, 7973–7984. [Google Scholar] [CrossRef]
- Zhang, M.; Shao, S.; Yue, H.; Wang, X.; Zhang, W.; Chen, F.; Zheng, L.; Xing, J.; Qin, Y. High Stability Au NPs: From Design to Application in Nanomedicine. Int. J. Nanomed. 2021, 16, 6067–6094. [Google Scholar] [CrossRef]
- Nie, P.; Zhao, Y.; Xu, H. Synthesis, applications, toxicity and toxicity mechanisms of silver nanoparticles: A review. Ecotoxicol. Environ. Saf. 2023, 253, 114636. [Google Scholar] [CrossRef] [PubMed]
- Sikder, M.; Wang, J.; Chandler, G.T.; Berti, D.; Baalousha, M. Synthesis, characterization, and environmental behaviors of monodispersed platinum nanoparticles. J. Colloid Interface Sci. 2019, 540, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Medici, S.; Peana, M.; Coradduzza, D.; Zoroddu, M.A. Gold nanoparticles and cancer: Detection, diagnosis and therapy. Semin. Cancer Biol. 2021, 76, 27–37. [Google Scholar] [CrossRef]
- Chugh, H.; Sood, D.; Chandra, I.; Tomar, V.; Dhawan, G.; Chandra, R. Role of gold and silver nanoparticles in cancer nano-medicine. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1210–1220. [Google Scholar] [CrossRef]
- Farrag, N.S.; El-Sabagh, H.A.; Al-Mahallawi, A.M.; Mamdouh, W.; Amin, A.M.; El-Bary, A.A. Improvement of doxorubicin radioiodination and in-vivo cancer suppression via loading in nanosilver system. Appl. Radiat. Isot. Incl. Data Instrum. Methods Use Agric. Ind. Med. 2022, 187, 110288. [Google Scholar] [CrossRef]
- Puja, P.; Kumar, P. A perspective on biogenic synthesis of platinum nanoparticles and their biomedical applications. Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 2019, 211, 94–99. [Google Scholar] [CrossRef]
- Paris, J.L.; Baeza, A.; Vallet-Regí, M. Overcoming the stability, toxicity, and biodegradation challenges of tumor stimuli-responsive inorganic nanoparticles for delivery of cancer therapeutics. Expert Opin. Drug Deliv. 2019, 16, 1095–1112. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, X.; Chen, L.; Gong, X.; Yang, H.; Duan, X.; Zhu, Y. Multifunctional Gold Nanoparticles in Cancer Diagnosis and Treatment. Int. J. Nanomed. 2022, 17, 2041–2067. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, C.; Wang, X.; Zhang, Y.; Wu, Y.; Dong, C.; Shuang, S. Construction of CPs@MnO2-AgNPs as a multifunctional nanosensor for glutathione sensing and cancer theranostics. Nanoscale 2019, 11, 18845–18853. [Google Scholar] [CrossRef]
- Wiesmann, N.; Tremel, W.; Brieger, J. Zinc oxide nanoparticles for therapeutic purposes in cancer medicine. J. Mater. Chem. B 2020, 8, 4973–4989. [Google Scholar] [CrossRef] [PubMed]
- Zuo, F.; Zhu, Y.; Wu, T.; Li, C.; Liu, Y.; Wu, X.; Ma, J.; Zhang, K.; Ouyang, H.; Qiu, X.; et al. Titanium Dioxide Nanomaterials: Progress in Synthesis and Application in Drug Delivery. Pharmaceutics 2024, 16, 1214. [Google Scholar] [CrossRef] [PubMed]
- Zanganeh, S.; Hutter, G.; Spitler, R.; Lenkov, O.; Mahmoudi, M.; Shaw, A.; Pajarinen, J.S.; Nejadnik, H.; Goodman, S.; Moseley, M.; et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat. Nanotechnol. 2016, 11, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, S.; Shi, X.; Shen, M. Aqueous-phase synthesis of iron oxide nanoparticles and composites for cancer diagnosis and therapy. Adv. Colloid Interface Sci. 2017, 249, 374–385. [Google Scholar] [CrossRef]
- Ibrahim, I.A.A.; Alzahrani, A.R.; Alanazi, I.M.; Shahzad, N.; Shahid, I.; Falemban, A.H.; Azlina, M.F.N.; Arulselvan, P. Chitosan biopolymer functionalized with graphene oxide and titanium dioxide with Escin metallic nanocomposites for anticancer potential against colon cancer. Int. J. Biol. Macromol. 2023, 253, 127334. [Google Scholar] [CrossRef]
- Anjum, S.; Hashim, M.; Malik, S.A.; Khan, M.; Lorenzo, J.M.; Abbasi, B.H.; Hano, C. Recent Advances in Zinc Oxide Nanoparticles (ZnO NPs) for Cancer Diagnosis, Target Drug Delivery, and Treatment. Cancers 2021, 13, 4570. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Lu, Y.; Ma, Y. ZnO nanomaterials target mitochondrial apoptosis and mitochondrial autophagy pathways in cancer cells. Cell Biochem. Funct. 2024, 42, e3909. [Google Scholar] [CrossRef]
- Fatima, H.; Jin, Z.Y.; Shao, Z.; Chen, X.J. Recent advances in ZnO-based photosensitizers: Synthesis, modification, and applications in photodynamic cancer therapy. J. Colloid Interface Sci. 2022, 621, 440–463. [Google Scholar] [CrossRef]
- Raja, G.; Cao, S.; Kim, D.H.; Kim, T.J. Mechanoregulation of titanium dioxide nanoparticles in cancer therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110303. [Google Scholar] [CrossRef]
- Ghasempour, A.; Dehghan, H.; Ataee, M.; Chen, B.; Zhao, Z.; Sedighi, M.; Guo, X.; Shahbazi, M.A. Cadmium Sulfide Nanoparticles: Preparation, Characterization, and Biomedical Applications. Molecules 2023, 28, 3857. [Google Scholar] [CrossRef]
- Nazari, O.; Gouran Orimi, P.; Chaichi, M.J.; Mohseni, M. Synthesis and characterization of cadmium selenide quantum dots doped by europium and investigation of their chemiluminescence properties and antibacterial activities. Lumin. J. Biol. Chem. Lumin. 2019, 34, 394–401. [Google Scholar] [CrossRef]
- Li, G.; Zhang, R.; Chen, K.; Dong, J.; Yang, Z.; Chen, H.; Wang, H.; Wang, H.; Lei, H.; Bao, W.; et al. Zinc sulfide nanoparticles serve as gas slow-release bioreactors for H2S therapy of ischemic stroke. Biomaterials 2025, 315, 122912. [Google Scholar] [CrossRef]
- Chen, L.L.; Zhao, L.; Wang, Z.G.; Liu, S.L.; Pang, D.W. Near-Infrared-II Quantum Dots for In Vivo Imaging and Cancer Therapy. Small 2022, 18, e2104567. [Google Scholar] [CrossRef] [PubMed]
- Yukawa, H.; Sato, K.; Baba, Y. Theranostics applications of quantum dots in regenerative medicine, cancer medicine, and infectious diseases. Adv. Drug Deliv. Rev. 2023, 200, 114863. [Google Scholar] [CrossRef]
- Pericleous, P.; Gazouli, M.; Lyberopoulou, A.; Rizos, S.; Nikiteas, N.; Efstathopoulos, E.P. Quantum dots hold promise for early cancer imaging and detection. Int. J. Cancer 2012, 131, 519–528. [Google Scholar] [CrossRef]
- Pãun, A.G.; Popescu, S.; Ungureanu, A.I.; Trusca, R.; Popp, A.; Dumitriu, C.; Buica, G.O. Anti-Tissue-Transglutaminase IgA Antibodies Presence Determination Using Electrochemical Square Wave Voltammetry and Modified Electrodes Based on Polypyrrole and Quantum Dots. Biosensors 2025, 15, 42. [Google Scholar] [CrossRef]
- Li, J.M.; Wang, Y.Y.; Zhao, M.X.; Tan, C.P.; Li, Y.Q.; Le, X.Y.; Ji, L.N.; Mao, Z.W. Multifunctional QD-based co-delivery of siRNA and doxorubicin to HeLa cells for reversal of multidrug resistance and real-time tracking. Biomaterials 2012, 33, 2780–2790. [Google Scholar] [CrossRef]
- Osorio, H.M.; Castillo-Solís, F.; Barragán, S.Y.; Rodríguez-Pólit, C.; Gonzalez-Pastor, R. Graphene Quantum Dots from Natural Carbon Sources for Drug and Gene Delivery in Cancer Treatment. Int. J. Mol. Sci. 2024, 25, 10539. [Google Scholar] [CrossRef]
- Irmania, N.; Dehvari, K.; Chang, J.Y. Multifunctional MnCuInSe/ZnS quantum dots for bioimaging and photodynamic therapy. J. Biomater. Appl. 2022, 36, 1617–1628. [Google Scholar] [CrossRef]
- Yue, L.; Li, H.; Liu, Q.; Guo, D.; Chen, J.; Sun, Q.; Xu, Y.; Wu, F. Manganese-doped carbon quantum dots for fluorometric and magnetic resonance (dual mode) bioimaging and biosensing. Mikrochim. Acta 2019, 186, 315. [Google Scholar] [CrossRef]
- Getachew, G.; Korupalli, C.; Rasal, A.S.; Dirersa, W.B.; Fahmi, M.Z.; Chang, J.Y. Highly Luminescent, Stable, and Red-Emitting CsMgxPb(1−x)I3 Quantum Dots for Dual-Modal Imaging-Guided Photodynamic Therapy and Photocatalytic Activity. ACS Appl. Mater. Interfaces 2022, 14, 278–296. [Google Scholar] [CrossRef] [PubMed]
- Zarepour, A.; Khosravi, A.; Yücel Ayten, N.; Çakır Hatır, P.; Iravani, S.; Zarrabi, A. Innovative approaches for cancer treatment: Graphene quantum dots for photodynamic and photothermal therapies. J. Mater. Chem. B 2024, 12, 4307–4334. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Zhao, M.M.; Luo, Q.W.; Zhang, Y.C.; Liu, T.T.; Yang, Z.; Liao, M.; Tu, P.; Zeng, K.W. Carbon Quantum Dots-Based Nanozyme from Coffee Induces Cancer Cell Ferroptosis to Activate Antitumor Immunity. ACS Nano 2022, 16, 9228–9239. [Google Scholar] [CrossRef] [PubMed]
- Borzooee Moghadam, N.; Avatefi, M.; Karimi, M.; Mahmoudifard, M. Graphene family in cancer therapy: Recent progress in cancer gene/drug delivery applications. J. Mater. Chem. B 2023, 11, 2568–2613. [Google Scholar] [CrossRef]
- Negri, V.; Pacheco-Torres, J.; Calle, D.; López-Larrubia, P. Carbon Nanotubes in Biomedicine. Top. Curr. Chem. 2020, 378, 15. [Google Scholar] [CrossRef]
- Bakry, R.; Vallant, R.M.; Najam-ul-Haq, M.; Rainer, M.; Szabo, Z.; Huck, C.W.; Bonn, G.K. Medicinal applications of fullerenes. Int. J. Nanomed. 2007, 2, 639–649. [Google Scholar]
- Raslan, A.; Saenz Del Burgo, L.; Ciriza, J.; Pedraz, J.L. Graphene oxide and reduced graphene oxide-based scaffolds in regenerative medicine. Int. J. Pharm. 2020, 580, 119226. [Google Scholar] [CrossRef]
- Kandhola, G.; Park, S.; Lim, J.W.; Chivers, C.; Song, Y.H.; Chung, J.H.; Kim, J.; Kim, J.W. Nanomaterial-Based Scaffolds for Tissue Engineering Applications: A Review on Graphene, Carbon Nanotubes and Nanocellulose. Tissue Eng. Regen. Med. 2023, 20, 411–433. [Google Scholar] [CrossRef]
- Zhao, H.; Ding, R.; Zhao, X.; Li, Y.; Qu, L.; Pei, H.; Yildirimer, L.; Wu, Z.; Zhang, W. Graphene-based nanomaterials for drug and/or gene delivery, bioimaging, and tissue engineering. Drug Discov. Today 2017, 22, 1302–1317. [Google Scholar] [CrossRef]
- Saranya, M.; da Silva, A.M.; Karjalainen, H.; Klinkenberg, G.; Schmid, R.; McDonagh, B.; Molesworth, P.P.; Sigfúsdóttir, M.S.; Wågbø, A.M.; Santos, S.G.; et al. Magnetic-Responsive Carbon Nanotubes Composite Scaffolds for Chondrogenic Tissue Engineering. Adv. Healthc. Mater. 2023, 12, e2301787. [Google Scholar] [CrossRef]
- Sengupta, J.; Hussain, C.M. CNT and Graphene-Based Transistor Biosensors for Cancer Detection: A Review. Biomolecules 2023, 13, 1024. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Mondal, S. Functionalized Graphene Oxide for Chemotherapeutic Drug Delivery and Cancer Treatment: A Promising Material in Nanomedicine. Int. J. Mol. Sci. 2020, 21, 6280. [Google Scholar] [CrossRef] [PubMed]
- Dilenko, H.; Bartoň Tománková, K.; Válková, L.; Hošíková, B.; Kolaříková, M.; Malina, L.; Bajgar, R.; Kolářová, H. Graphene-Based Photodynamic Therapy and Overcoming Cancer Resistance Mechanisms: A Comprehensive Review. Int. J. Nanomed. 2024, 19, 5637–5680. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, B.; Surwase, S.S.; Lee, S.S.; Park, H.; Faraji Rad, Z.; Trevaskis, N.L.; Kim, Y.C. Carbon-based nanostructures for cancer therapy and drug delivery applications. J. Mater. Chem. B 2022, 10, 9944–9967. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Zhang, Q.; Wu, X.; Wu, L.; Lin, J.H. Exploring the Interfacial Phase and π-π Stacking in Aligned Carbon Nanotube/Polyimide Nanocomposites. Nanomaterials 2020, 10, 1158. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, Q.; Yang, N. Recent Advances of Porous Graphene: Synthesis, Functionalization, and Electrochemical Applications. Small 2019, 15, e1903780. [Google Scholar] [CrossRef]
- Taheriazam, A.; Abad, G.G.Y.; Hajimazdarany, S.; Imani, M.H.; Ziaolhagh, S.; Zandieh, M.A.; Bayanzadeh, S.D.; Mirzaei, S.; Hamblin, M.R.; Entezari, M.; et al. Graphene oxide nanoarchitectures in cancer biology: Nano-modulators of autophagy and apoptosis. J. Control. Release Off. J. Control. Release Soc. 2023, 354, 503–522. [Google Scholar] [CrossRef]
- Shlapa, Y.; Siposova, K.; Veltruska, K.; Maraloiu, V.A.; Garcarova, I.; Rajnak, M.; Musatov, A.; Belous, A. Design of Magnetic Fe3O4/CeO2 “Core/Shell”-Like Nanocomposites with Pronounced Antiamyloidogenic and Antioxidant Bioactivity. ACS Appl. Mater. Interfaces 2023, 15, 49346–49361. [Google Scholar] [CrossRef]
- Liao, J.; Jia, Y.; Chen, L.; Zhou, L.; Li, Q.; Qian, Z.; Niu, D.; Li, Y.; Li, P. Magnetic/Gold Core-Shell Hybrid Particles for Targeting and Imaging-Guided Photothermal Cancer Therapy. J. Biomed. Nanotechnol. 2019, 15, 2072–2089. [Google Scholar] [CrossRef]
- Tsai, C.I.; Wang, C.Y.; Tang, J.; Hung, M.H.; Wang, K.L.; Chen, L.J. Electrical properties and magnetic response of cobalt germanosilicide nanowires. ACS Nano 2011, 5, 9552–9558. [Google Scholar] [CrossRef]
- Sonachalam, A.; Sokkalingam, R.; Giri, D.R.; Panghal, A.; Roy, S.S.; Britto Dhas, S.A.M.; Ramadoss, J.; Ganapathy, S.; Baskaran, R.B.; Ramasamy, J. Influence of shock waves on bifunctional nickel particles: Enhancing magnetic properties and supercapacitor applications. Environ. Res. 2024, 244, 117834. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Jary, J.; Machnicka, B. In vivo Biodistribution and Clearance of Magnetic Iron Oxide Nanoparticles for Medical Applications. Int. J. Nanomed. 2023, 18, 4067–4100. [Google Scholar] [CrossRef] [PubMed]
- Vangijzegem, T.; Stanicki, D.; Laurent, S. Magnetic iron oxide nanoparticles for drug delivery: Applications and characteristics. Expert Opin. Drug Deliv. 2019, 16, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, X.; Bao, L.; Liu, T.; Yuan, P.; Yang, X.; Qiu, X.; Gooding, J.J.; Bai, Y.; Xiao, J.; et al. Treatment of infarcted heart tissue via the capture and local delivery of circulating exosomes through antibody-conjugated magnetic nanoparticles. Nat. Biomed. Eng. 2020, 4, 1063–1075. [Google Scholar] [CrossRef]
- Hanoglu, S.B.; Man, E.; Harmanci, D.; Tozan Ruzgar, S.; Sanli, S.; Keles, N.A.; Ayden, A.; Tuna, B.G.; Duzgun, O.; Ozkan, O.F.; et al. Magnetic Nanoparticle-Based Electrochemical Sensing Platform Using Ferrocene-Labelled Peptide Nucleic Acid for the Early Diagnosis of Colorectal Cancer. Biosensors 2022, 12, 736. [Google Scholar] [CrossRef]
- Quarta, A.; Di Corato, R.; Manna, L.; Ragusa, A.; Pellegrino, T. Fluorescent-magnetic hybrid nanostructures: Preparation, properties, and applications in biology. IEEE Trans. Nanobiosci. 2007, 6, 298–308. [Google Scholar] [CrossRef]
- Basina, G.; Diamantopoulos, G.; Devlin, E.; Psycharis, V.; Alhassan, S.M.; Pissas, M.; Hadjipanayis, G.; Tomou, A.; Bouras, A.; Hadjipanayis, C.; et al. LAPONITE® nanodisk-“decorated” Fe3O4 nanoparticles: A biocompatible nano-hybrid with ultrafast magnetic hyperthermia and MRI contrast agent ability. J. Mater. Chem. B 2022, 10, 4935–4943. [Google Scholar] [CrossRef]
- Thong, P.Q.; Thu Huong, L.T.; Tu, N.D.; My Nhung, H.T.; Khanh, L.; Manh, D.H.; Nam, P.H.; Phuc, N.X.; Alonso, J.; Qiao, J.; et al. Multifunctional nanocarriers of Fe3O4@PLA-PEG/curcumin for MRI, magnetic hyperthermia and drug delivery. Nanomedicine 2022, 17, 1677–1693. [Google Scholar] [CrossRef]
- Minaei, S.E.; Khoei, S.; Khoee, S.; Mahdavi, S.R. Sensitization of glioblastoma cancer cells to radiotherapy and magnetic hyperthermia by targeted temozolomide-loaded magnetite tri-block copolymer nanoparticles as a nanotheranostic agent. Life Sci. 2022, 306, 120729. [Google Scholar] [CrossRef]
- Lu, H.; Wan, L.; Li, X.; Zhang, M.; Shakoor, A.; Li, W.; Zhang, X. Combined Synthesis of Cerium Oxide Particles for Effective Anti-Bacterial and Anti-Cancer Nanotherapeutics. Int. J. Nanomed. 2022, 17, 5733–5746. [Google Scholar] [CrossRef]
- Ahmad, J.; Wahab, R.; Siddiqui, M.A.; Farshori, N.N.; Saquib, Q.; Ahmad, N.; Al-Khedhairy, A.A. Neodymium oxide nanostructures and their cytotoxic evaluation in human cancer cells. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. 2022, 73, 127029. [Google Scholar] [CrossRef]
- Yang, D.; Yang, G.; Wang, X.; Lv, R.; Gai, S.; He, F.; Gulzar, A.; Yang, P. Y2O3:Yb,Er@mSiO2-CuxS double-shelled hollow spheres for enhanced chemo-/photothermal anti-cancer therapy and dual-modal imaging. Nanoscale 2015, 7, 12180–12191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, X.; Hou, C.; Shang, K.; Yang, K.; Tian, Z.; Pei, Z.; Qu, Y.; Pei, Y. Dual-responsive dithio-polydopamine coated porous CeO2 nanorods for targeted and synergistic drug delivery. Int. J. Nanomed. 2018, 13, 2161–2173. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; You, M.; Wang, F.; Wang, Z.; Gao, X.; Jing, C.; Liu, J.; Guo, M.; Li, J.; Luo, A.; et al. Multifunctional Graphdiyne-Cerium Oxide Nanozymes Facilitate MicroRNA Delivery and Attenuate Tumor Hypoxia for Highly Efficient Radiotherapy of Esophageal Cancer. Adv. Mater. 2021, 33, e2100556. [Google Scholar] [CrossRef]
- Shao, C.; Shen, A.; Zhang, M.; Meng, X.; Song, C.; Liu, Y.; Gao, X.; Wang, P.; Bu, W. Oxygen Vacancies Enhanced CeO2:Gd Nanoparticles for Sensing a Tumor Vascular Microenvironment by Magnetic Resonance Imaging. ACS Nano 2018, 12, 12629–12637. [Google Scholar] [CrossRef]
- Pi, F.; Deng, X.; Xue, Q.; Zheng, L.; Liu, H.; Yang, F.; Chen, T. Alleviating the hypoxic tumor microenvironment with MnO2-coated CeO2 nanoplatform for magnetic resonance imaging guided radiotherapy. J. Nanobiotechnol. 2023, 21, 90. [Google Scholar] [CrossRef]
- Setua, S.; Menon, D.; Asok, A.; Nair, S.; Koyakutty, M. Folate receptor targeted, rare-earth oxide nanocrystals for bi-modal fluorescence and magnetic imaging of cancer cells. Biomaterials 2010, 31, 714–729. [Google Scholar] [CrossRef]
- Chen, K.; Sun, X.; Liu, Y.; Yang, Y.; Shi, M.; Yu, J.; Zhang, S.; Shi, P. CeO2-Decorated Metal-Organic Framework for Enhanced Photodynamic Therapy. Inorg. Chem. 2022, 61, 16307–16316. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, R.; Yang, G.; Wang, Y.; Gai, S.; Zhao, X.; Huang, M.; Yang, P. CeO2 and Glucose Oxidase Co-Enriched Ti3C2Tx MXene for Hyperthermia-Augmented Nanocatalytic Cancer Therapy. ACS Appl. Mater. Interfaces 2024, 16, 9968–9979. [Google Scholar] [CrossRef]
- Dong, S.; Dong, Y.; Liu, B.; Liu, J.; Liu, S.; Zhao, Z.; Li, W.; Tian, B.; Zhao, R.; He, F.; et al. Guiding Transition Metal-Doped Hollow Cerium Tandem Nanozymes with Elaborately Regulated Multi-Enzymatic Activities for Intensive Chemodynamic Therapy. Adv. Mater. 2022, 34, e2107054. [Google Scholar] [CrossRef]
- Yang, B.; Zeng, J.; Zhao, G.; Ding, C.; Chen, L.; Huang, Y. Cascade enzyme-mimicking with spatially separated gold-ceria for dual-mode detection of superoxide anions. Biosens. Bioelectron. 2025, 267, 116847. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Lei, D.; Liu, Y.; Qin, T.; Gao, H.; Lv, W.; Liu, Q.; Qin, L.; Jin, W.; Chen, Y.; et al. NIR triggered polydopamine coated cerium dioxide nanozyme for ameliorating acute lung injury via enhanced ROS scavenging. J. Nanobiotechnol. 2024, 22, 321. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, M.; Moloudi, K.; Paydar, R.; Abed, Z.; Beik, J.; Ghaznavi, H.; Shakeri-Zadeh, A. Alginate hydrogel co-loaded with cisplatin and gold nanoparticles for computed tomography image-guided chemotherapy. J. Biomater. Appl. 2018, 33, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Bayford, R.H.; Damaso, R.; Neshatvar, N.; Ivanenko, Y.; Rademacher, T.W.; Wu, Y.; Seifnaraghi, N.; Ghali, L.; Patel, N.; Roitt, I.; et al. Locating Functionalized Gold Nanoparticles Using Electrical Impedance Tomography. IEEE Trans. Bio Med. Eng. 2022, 69, 494–502. [Google Scholar] [CrossRef]
- He, X.; Liu, F.; Liu, L.; Duan, T.; Zhang, H.; Wang, Z. Lectin-conjugated Fe2O3@Au core@Shell nanoparticles as dual mode contrast agents for in vivo detection of tumor. Mol. Pharm. 2014, 11, 738–745. [Google Scholar] [CrossRef]
- Meir, R.; Shamalov, K.; Sadan, T.; Motiei, M.; Yaari, G.; Cohen, C.J.; Popovtzer, R. Fast Image-Guided Stratification Using Anti-Programmed Death Ligand 1 Gold Nanoparticles for Cancer Immunotherapy. ACS Nano 2017, 11, 11127–11134. [Google Scholar] [CrossRef]
- Li, C.H.; Kuo, T.R.; Su, H.J.; Lai, W.Y.; Yang, P.C.; Chen, J.S.; Wang, D.Y.; Wu, Y.C.; Chen, C.C. Fluorescence-Guided Probes of Aptamer-Targeted Gold Nanoparticles with Computed Tomography Imaging Accesses for in Vivo Tumor Resection. Sci. Rep. 2015, 5, 15675. [Google Scholar] [CrossRef]
- Liu, H.; Xu, C.; Meng, M.; Li, S.; Sheng, S.; Zhang, S.; Ni, W.; Tian, H.; Wang, Q. Metal-organic framework-mediated multifunctional nanoparticles for combined chemo-photothermal therapy and enhanced immunotherapy against colorectal cancer. Acta Biomater. 2022, 144, 132–141. [Google Scholar] [CrossRef]
- Wen, D.; Dong, L.; Li, K.; Du, Y.; Deng, R.; Feng, J.; Zhang, H.; Wang, D. Selenium Vacancy Engineering Using Bi2Se3 Nanodots for Boosting Highly Efficient Photonic Hyperthermia. ACS Appl. Mater. Interfaces 2021, 13, 48378–48385. [Google Scholar] [CrossRef]
- Liang, S.; Deng, X.; Chang, Y.; Sun, C.; Shao, S.; Xie, Z.; Xiao, X.; Ma, P.; Zhang, H.; Cheng, Z.; et al. Intelligent Hollow Pt-CuS Janus Architecture for Synergistic Catalysis-Enhanced Sonodynamic and Photothermal Cancer Therapy. Nano Lett. 2019, 19, 4134–4145. [Google Scholar] [CrossRef]
- Lee, D.Y.; Kim, J.Y.; Lee, Y.; Lee, S.; Miao, W.; Kim, H.S.; Min, J.J.; Jon, S. Black Pigment Gallstone Inspired Platinum-Chelated Bilirubin Nanoparticles for Combined Photoacoustic Imaging and Photothermal Therapy of Cancers. Angew. Chem. 2017, 56, 13684–13688. [Google Scholar] [CrossRef] [PubMed]
- Alagaratnam, S.; Yang, S.Y.; Loizidou, M.; Fuller, B.; Ramesh, B. Mechano-growth Factor Expression in Colorectal Cancer Investigated With Fluorescent Gold Nanoparticles. Anticancer Res. 2019, 39, 1705–1710. [Google Scholar] [CrossRef] [PubMed]
- Khuyen, H.T.; Huong, T.T.; Van, N.D.; Huong, N.T.; Vu, N.; Lien, P.T.; Nam, P.H.; Nghia, V.X. Synthesis of Multifunctional Eu(III) Complex Doped Fe3O4/Au Nanocomposite for Dual Photo-Magnetic Hyperthermia and Fluorescence Bioimaging. Molecules 2023, 28, 749. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Wu, Q.; Wu, Y.; Xi, X.; Cao, J.; Chu, H.; Liu, Q.; Li, Y.; Wu, W.; Fang, X.; et al. Multifunctional Au Modified Ti3C2-MXene for Photothermal/Enzyme Dynamic/Immune Synergistic Therapy. Nano Lett. 2022, 22, 8321–8330. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, P.; Sun, L.; Li, C.; Petrenko, V.A.; Liu, A. Bio-mimetic nanostructure self-assembled from Au@Ag heterogeneous nanorods and phage fusion proteins for targeted tumor optical detection and photothermal therapy. Sci. Rep. 2014, 4, 6808. [Google Scholar] [CrossRef]
- Mirrahimi, M.; Abed, Z.; Beik, J.; Shiri, I.; Shiralizadeh Dezfuli, A.; Mahabadi, V.P.; Kamran Kamrava, S.; Ghaznavi, H.; Shakeri-Zadeh, A. A thermo-responsive alginate nanogel platform co-loaded with gold nanoparticles and cisplatin for combined cancer chemo-photothermal therapy. Pharmacol. Res. 2019, 143, 178–185. [Google Scholar] [CrossRef]
- He, L.; Xu, F.; Li, Y.; Jin, H.; Lo, P.C. Cupric-ion-promoted fabrication of oxygen-replenishing nanotherapeutics for synergistic chemo and photodynamic therapy against tumor hypoxia. Acta Biomater. 2023, 162, 57–71. [Google Scholar] [CrossRef]
- Lu, K.; He, C.; Lin, W. A Chlorin-Based Nanoscale Metal-Organic Framework for Photodynamic Therapy of Colon Cancers. J. Am. Chem. Soc. 2015, 137, 7600–7603. [Google Scholar] [CrossRef]
- Simelane, N.W.N.; Matlou, G.G.; Abrahamse, H. Photodynamic Therapy of Aluminum Phthalocyanine Tetra Sodium 2-Mercaptoacetate Linked to PEGylated Copper-Gold Bimetallic Nanoparticles on Colon Cancer Cells. Int. J. Mol. Sci. 2023, 24, 1902. [Google Scholar] [CrossRef]
- Yurt, F.; Ince, M.; Colak, S.G.; Ocakoglu, K.; Er, O.; Soylu, H.M.; Gunduz, C.; Avci, C.B.; Kurt, C.C. Investigation of in vitro PDT activities of zinc phthalocyanine immobilised TiO2 nanoparticles. Int. J. Pharm. 2017, 524, 467–474. [Google Scholar] [CrossRef]
- Shi, X.; Gao, K.; Xiong, S.; Gao, R. Multifunctional Transferrin Encapsulated GdF3 Nanoparticles for Sentinel Lymph Node and Tumor Imaging. Bioconjugate Chem. 2020, 31, 2576–2584. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.Y.; Cheng, K.; Xuan, Y.; Yang, X.Q.; An, J.; Hu, Y.G.; Liu, B.; Zhao, Y.D. A pH/ultrasonic dual-response step-targeting enterosoluble granule for combined sonodynamic-chemotherapy guided via gastrointestinal tract imaging in orthotopic colorectal cancer. Nanoscale 2021, 13, 4278–4294. [Google Scholar] [CrossRef]
- Jahedi, M.; Meshkini, A. Tumor tropic delivery of FU.FA@NSs using mesenchymal stem cells for synergistic chemo-photodynamic therapy of colorectal cancer. Colloids Surf. B Biointerfaces 2023, 226, 113333. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Shieh, M.J. Platinum(II) Drug-Loaded Gold Nanoshells for Chemo-Photothermal Therapy in Colorectal Cancer. ACS Appl. Mater. Interfaces 2020, 12, 4254–4264. [Google Scholar] [CrossRef] [PubMed]
- Emami, F.; Banstola, A.; Vatanara, A.; Lee, S.; Kim, J.O.; Jeong, J.H.; Yook, S. Doxorubicin and Anti-PD-L1 Antibody Conjugated Gold Nanoparticles for Colorectal Cancer Photochemotherapy. Mol. Pharm. 2019, 16, 1184–1199. [Google Scholar] [CrossRef]
- Cui, M.; Tang, Z.; Ahmad, Z.; Pan, C.; Lu, Y.; Ali, K.; Huang, S.; Lin, X.; Wahab, A.; Iqbal, M.Z.; et al. Facile synthesis of manganese-hafnium nanocomposites for multimodal MRI/CT imaging and in vitro photodynamic therapy of colon cancer. Colloids Surf. B Biointerfaces 2024, 237, 113834. [Google Scholar] [CrossRef]
- Xin, Y.; Yu, Y.; Su, M.; Li, X.; Elsabahy, M.; Gao, H. In situ-activated photothermal nanoplatform for on-demand NO gas delivery and enhanced colorectal cancer treatment. J. Control. Release Off. J. Control. Release Soc. 2023, 359, 69–84. [Google Scholar] [CrossRef]
- Yan, C.; Liu, D.; An, L.; Wang, Y.; Tian, Q.; Lin, J.; Yang, S. Magnetic-Photoacoustic Dual-Mode Probe for the Visualization of H2S in Colorectal Cancer. Anal. Chem. 2020, 92, 8254–8261. [Google Scholar] [CrossRef]
- An, L.; Wang, X.; Rui, X.; Lin, J.; Yang, H.; Tian, Q.; Tao, C.; Yang, S. The In Situ Sulfidation of Cu2 O by Endogenous H2 S for Colon Cancer Theranostics. Angew. Chem. 2018, 57, 15782–15786. [Google Scholar] [CrossRef]
- Garanina, A.S.; Naumenko, V.A.; Nikitin, A.A.; Myrovali, E.; Petukhova, A.Y.; Klimyuk, S.V.; Nalench, Y.A.; Ilyasov, A.R.; Vodopyanov, S.S.; Erofeev, A.S.; et al. Temperature-controlled magnetic nanoparticles hyperthermia inhibits primary tumor growth and metastases dissemination. Nanomed. Nanotechnol. Biol. Med. 2020, 25, 102171. [Google Scholar] [CrossRef]
- Palzer, J.; Eckstein, L.; Slabu, I.; Reisen, O.; Neumann, U.P.; Roeth, A.A. Iron Oxide Nanoparticle-Based Hyperthermia as a Treatment Option in Various Gastrointestinal Malignancies. Nanomaterials 2021, 11, 3013. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.Y.; Liu, T.Y.; Chan, T.Y.; Tsai, S.C.; Hardiansyah, A.; Huang, L.Y.; Yang, M.C.; Lu, R.H.; Jiang, J.K.; Yang, C.Y.; et al. Magnetically triggered nanovehicles for controlled drug release as a colorectal cancer therapy. Colloids Surf. B Biointerfaces 2016, 140, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Luo, Y.; Huang, L.; Bin, Y.; Liang, J.; Zhao, S. A hydrogen sulfide-activated Pd@Cu2O nanoprobe for NIR-II photoacoustic imaging of colon cancer and photothermal-enhanced ferroptosis therapy. Biosens. Bioelectron. 2024, 268, 116906. [Google Scholar] [CrossRef]
- Liu, J.; Li, L.; Zhang, B.; Xu, Z.P. MnO2-shelled Doxorubicin/Curcumin nanoformulation for enhanced colorectal cancer chemo-immunotherapy. J. Colloid Interface Sci. 2022, 617, 315–325. [Google Scholar] [CrossRef]
- Park, S.M.; Kim, D.Y.; Lee, K.H.; Shin, Y.I.; Han, S.C.; Kwon, S.M. Anti-Tumor Efficacy of Oleuropein-Loaded ZnO/Au Mesoporous Silica Nanoparticle in 5-FU-Resistant Colorectal Cancer Cells. Int. J. Nanomed. 2024, 19, 2675–2690. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.Y.; Cho, H.J. Doxorubicin-Wrapped Zinc Oxide Nanoclusters for the Therapy of Colorectal Adenocarcinoma. Nanomaterials 2017, 7, 354. [Google Scholar] [CrossRef]
- Zhang, M.; Yue, J.; Cui, R.; Ma, Z.; Wan, H.; Wang, F.; Zhu, S.; Zhou, Y.; Kuang, Y.; Zhong, Y.; et al. Bright quantum dots emitting at ∼1600 nm in the NIR-IIb window for deep tissue fluorescence imaging. Proc. Natl. Acad. Sci. USA 2018, 115, 6590–6595. [Google Scholar] [CrossRef]
- Park, Y.; Ryu, Y.M.; Jung, Y.; Wang, T.; Baek, Y.; Yoon, Y.; Bae, S.M.; Park, J.; Hwang, S.; Kim, J.; et al. Spraying quantum dot conjugates in the colon of live animals enabled rapid and multiplex cancer diagnosis using endoscopy. ACS Nano 2014, 8, 8896–8910. [Google Scholar] [CrossRef]
- Hashemkhani, M.; Demirci, G.; Bayir, A.; Muti, A.; Sennaroglu, A.; Mohammad Hadi, L.; Yaghini, E.; Loizidou, M.; MacRobert, A.J.; Yagci Acar, H. Cetuximab-Ag2S quantum dots for fluorescence imaging and highly effective combination of ALA-based photodynamic/chemo-therapy of colorectal cancer cells. Nanoscale 2021, 13, 14879–14899. [Google Scholar] [CrossRef]
- Bakalova, R.; Zhelev, Z.; Nikolova, B.; Murayama, S.; Lazarova, D.; Tsoneva, I.; Aoki, I. Lymph node mapping using quantum dot-labeled polymersomes. Gen. Physiol. Biophys. 2015, 34, 393–398. [Google Scholar] [CrossRef]
- Gazouli, M.; Bouziotis, P.; Lyberopoulou, A.; Ikonomopoulos, J.; Papalois, A.; Anagnou, N.P.; Efstathopoulos, E.P. Quantum dots-bevacizumab complexes for in vivo imaging of tumors. In Vivo 2014, 28, 1091–1095. [Google Scholar] [PubMed]
- Li, Q.; Peng, H.; Wang, J.; Wang, Y.; Guo, F. Coexpression of CdSe and CdSe/CdS quantum dots in live cells using molecular hyperspectral imaging technology. J. Biomed. Opt. 2015, 20, 110504. [Google Scholar] [CrossRef]
- He, M.; Cheng, Z.; Wang, Z.; Li, M.; Liang, H.; Liu, H.; Yu, L.; Zhao, L.; Yu, F. Controllable Regulation of Ag2 S Quantum-Dot-Mediated Protein Nanoassemblies for Imaging-Guided Synergistic PDT/PTT/Chemotherapy against Hypoxic Tumor. Adv. Healthc. Mater. 2023, 12, e2300752. [Google Scholar] [CrossRef] [PubMed]
- Abrishami, A.; Bahrami, A.R.; Nekooei, S.; Sh Saljooghi, A.; Matin, M.M. Hybridized quantum dot, silica, and gold nanoparticles for targeted chemo-radiotherapy in colorectal cancer theranostics. Commun. Biol. 2024, 7, 393. [Google Scholar] [CrossRef]
- Haider, M.; Cagliani, R.; Jagal, J.; Jayakumar, M.N.; Fayed, B.; Shakartalla, S.B.; Pasricha, R.; Greish, K.; El-Awady, R. Peptide-functionalized graphene oxide quantum dots as colorectal cancer theranostics. J. Colloid Interface Sci. 2023, 630, 698–713. [Google Scholar] [CrossRef]
- Hassanpouraghdam, Y.; Pooresmaeil, M.; Namazi, H. In-vitro evaluation of the 5-fluorouracil loaded GQDs@Bio-MOF capped with starch biopolymer for improved colon-specific delivery. Int. J. Biol. Macromol. 2022, 221, 256–267. [Google Scholar] [CrossRef]
- Khan, F.A.; Lammari, N.; Muhammad Siar, A.S.; Alkhater, K.M.; Asiri, S.; Akhtar, S.; Almansour, I.; Alamoudi, W.; Haroun, W.; Louaer, W.; et al. Quantum dots encapsulated with curcumin inhibit the growth of colon cancer, breast cancer and bacterial cells. Nanomedicine 2020, 15, 969–980. [Google Scholar] [CrossRef]
- Rakhshaei, R.; Namazi, H.; Hamishehkar, H.; Rahimi, M. Graphene quantum dot cross-linked carboxymethyl cellulose nanocomposite hydrogel for pH-sensitive oral anticancer drug delivery with potential bioimaging properties. Int. J. Biol. Macromol. 2020, 150, 1121–1129. [Google Scholar] [CrossRef]
- Lu, Y.J.; Lin, P.Y.; Huang, P.H.; Kuo, C.Y.; Shalumon, K.T.; Chen, M.Y.; Chen, J.P. Magnetic Graphene Oxide for Dual Targeted Delivery of Doxorubicin and Photothermal Therapy. Nanomaterials 2018, 8, 193. [Google Scholar] [CrossRef]
- Fu, C.; Gong, S.; Lin, L.; Bao, Y.; Li, L.; Chen, Q. Characterization and efficacy of C(60) nano-photosensitive drugs in colorectal cancer treatment. Biomed. Pharmacother. Biomed. Pharmacother. 2024, 176, 116828. [Google Scholar] [CrossRef]
- Mroz, P.; Xia, Y.; Asanuma, D.; Konopko, A.; Zhiyentayev, T.; Huang, Y.Y.; Sharma, S.K.; Dai, T.; Khan, U.J.; Wharton, T.; et al. Intraperitoneal photodynamic therapy mediated by a fullerene in a mouse model of abdominal dissemination of colon adenocarcinoma. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 965–974. [Google Scholar] [CrossRef]
- Karimi, M.; Sadeghi, E.; Zahedifar, M.; Nejati, M.; Mirzaei, H.; Hamblin, M.R. In vitro study: Green synthesis and evaluation of MgO/C-dots/DOX phosphorescent nanocomposites for photodynamic/photocatalytic therapy of tumors. Front. Bioeng. Biotechnol. 2023, 11, 1286955. [Google Scholar] [CrossRef]
- Hadi, A.S.; Haghi, M.; Barzegar, A.; Ali, M.; Feizi, H. Comparative evaluation of hesperetin-loaded graphene oxide nanosheets (Hsp-GO) as a drug delivery system for colon cancer: Synthesis and anticancer efficiency assessment. Mol. Biol. Rep. 2024, 51, 591. [Google Scholar] [CrossRef]
- Aghdam, K.J.; Sabeti, B.; Chekin, F.; Mashreghi, M. Conjugation of Doxorubicin and Carbon-based-nanostructures for Drug Delivery against HT-29 Colon Cancer Cells. Comb. Chem. High Throughput Screen. 2024, 27, 2726–2733. [Google Scholar] [CrossRef]
- Bardania, H.; Jafari, F.; Baneshi, M.; Mahmoudi, R.; Ardakani, M.T.; Safari, F.; Barmak, M.J. Folic Acid-Functionalized Albumin/Graphene Oxide Nanocomposite to Simultaneously Deliver Curcumin and 5-Fluorouracil into Human Colorectal Cancer Cells: An In Vitro Study. BioMed Res. Int. 2023, 2023, 8334102. [Google Scholar] [CrossRef]
- Krasteva, N.; Keremidarska-Markova, M.; Hristova-Panusheva, K.; Andreeva, T.; Speranza, G.; Wang, D.; Draganova-Filipova, M.; Miloshev, G.; Georgieva, M. Aminated Graphene Oxide as a Potential New Therapy for Colorectal Cancer. Oxidative Med. Cell. Longev. 2019, 2019, 3738980. [Google Scholar] [CrossRef]
- Zhang, B.; Yan, Y.; Shen, Q.; Ma, D.; Huang, L.; Cai, X.; Tan, S. A colon targeted drug delivery system based on alginate modificated graphene oxide for colorectal liver metastasis. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 185–190. [Google Scholar] [CrossRef]
- Shang, J.; Xia, Q.; Sun, Y.; Wang, H.; Chen, J.; Li, Y.; Gao, F.; Yin, P.; Yuan, Z. Bufalin-Loaded Multifunctional Photothermal Nanoparticles Inhibit the Anaerobic Glycolysis by Targeting SRC-3/HIF-1α Pathway for Improved Mild Photothermal Therapy in CRC. Int. J. Nanomed. 2024, 19, 7831–7850. [Google Scholar] [CrossRef]
- Ji, Y.; Wang, C. Magnetic iron oxide nanoparticle-loaded hydrogels for photothermal therapy of cancer cells. Front. Bioeng. Biotechnol. 2023, 11, 1130523. [Google Scholar] [CrossRef]
- Panikkanvalappil, S.R.; Bhagavatula, S.K.; Deans, K.; Jonas, O.; Rashidian, M.; Mishra, S. Enhanced Tumor Accumulation of Multimodal Magneto-Plasmonic Nanoparticles via an Implanted Micromagnet-Assisted Delivery Strategy. Adv. Healthc. Mater. 2023, 12, e2201585. [Google Scholar] [CrossRef]
- Abed, Z.; Shakeri-Zadeh, A.; Eyvazzadeh, N. Magnetic Targeting of Magneto-Plasmonic Nanoparticles and Their Effects on Temperature Profile of NIR Laser Irradiated to CT26 Tumor in BALB/C Mice. J. Biomed. Phys. Eng. 2021, 11, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Nandi, R.; Mishra, S.; Maji, T.K.; Manna, K.; Kar, P.; Banerjee, S.; Dutta, S.; Sharma, S.K.; Lemmens, P.; Saha, K.D.; et al. A novel nanohybrid for cancer theranostics: Folate sensitized Fe2O3 nanoparticles for colorectal cancer diagnosis and photodynamic therapy. J. Mater. Chem. B 2017, 5, 3927–3939. [Google Scholar] [CrossRef] [PubMed]
- Alkahtane, A.A.; Alghamdi, H.A.; Aljasham, A.T.; Alkahtani, S. A possible theranostic approach of chitosan-coated iron oxide nanoparticles against human colorectal carcinoma (HCT-116) cell line. Saudi J. Biol. Sci. 2022, 29, 154–160. [Google Scholar] [CrossRef]
- Ostroverkhov, P.V.; Semkina, A.S.; Naumenko, V.A.; Plotnikova, E.A.; Melnikov, P.A.; Abakumova, T.O.; Yakubovskaya, R.I.; Mironov, A.F.; Vodopyanov, S.S.; Abakumov, A.M.; et al. Synthesis and characterization of bacteriochlorin loaded magnetic nanoparticles (MNP) for personalized MRI guided photosensitizers delivery to tumor. J. Colloid Interface Sci. 2019, 537, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, T.; Niu, K.; Xiao, Z.; Huang, J.; Pan, X.; Sun, Y.; Wang, Y.; Ma, D.; Xie, P.; et al. Mild phototherapy mediated by manganese dioxide-loaded mesoporous polydopamine enhances immunotherapy against colorectal cancer. Biomater. Sci. 2022, 10, 3647–3656. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, H.; Liu, G.; Ma, L.; Wang, Z. The controllable growth of ultrathin MnO2 on polydopamine nanospheres as a single nanoplatform for the MRI-guided synergistic therapy of tumors. J. Mater. Chem. B 2019, 7, 7152–7161. [Google Scholar] [CrossRef]
- Wang, F.; Xu, L.; Zhang, Y.; Petrenko, V.A.; Liu, A. An efficient strategy to synthesize a multifunctional ferroferric oxide core@dye/SiO2@Au shell nanocomposite and its targeted tumor theranostics. J. Mater. Chem. B 2017, 5, 8209–8218. [Google Scholar] [CrossRef]
- Tseng, H.Y.; Lee, G.B.; Lee, C.Y.; Shih, Y.H.; Lin, X.Z. Localised heating of tumours utilising injectable magnetic nanoparticles for hyperthermia cancer therapy. IET Nanobiotechnol. 2009, 3, 46–54. [Google Scholar] [CrossRef]
- Le Renard, P.E.; Buchegger, F.; Petri-Fink, A.; Bosman, F.; Rüfenacht, D.; Hofmann, H.; Doelker, E.; Jordan, O. Local moderate magnetically induced hyperthermia using an implant formed in situ in a mouse tumor model. Int. J. Hyperth. Off. J. Eur. Soc. Hyperthermic Oncol. N. Am. Hyperth. Group 2009, 25, 229–239. [Google Scholar] [CrossRef]
- Attar, M.M.; Amanpour, S.; Haghpanahi, M.; Haddadi, M.; Rezaei, G.; Muhammadnejad, S.; HajiAkhoundzadeh, M.; Barati, T.; Sadeghi, F.; Javadi, S. Thermal analysis of magnetic nanoparticle in alternating magnetic field on human HCT-116 colon cancer cell line. Int. J. Hyperth. Off. J. Eur. Soc. Hyperthermic Oncol. N. Am. Hyperth. Group 2016, 32, 858–867. [Google Scholar] [CrossRef]
- Mannucci, S.; Ghin, L.; Conti, G.; Tambalo, S.; Lascialfari, A.; Orlando, T.; Benati, D.; Bernardi, P.; Betterle, N.; Bassi, R.; et al. Magnetic nanoparticles from Magnetospirillum gryphiswaldense increase the efficacy of thermotherapy in a model of colon carcinoma. PLoS ONE 2014, 9, e108959. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Luccioni, H.L.; Latorre-Esteves, M.; Méndez-Vega, J.; Soto, O.; Rodríguez, A.R.; Rinaldi, C.; Torres-Lugo, M. Enhanced reduction in cell viability by hyperthermia induced by magnetic nanoparticles. Int. J. Nanomed. 2011, 6, 373–380. [Google Scholar] [CrossRef]
- Kim, S.; Sundaram, A.; Mathew, A.P.; Hareshkumar, V.S.; Mohapatra, A.; Thomas, R.G.; Bui, T.T.M.; Moon, K.; Kweon, S.; Park, I.K.; et al. In situ hypoxia modulating nano-catalase for amplifying DNA damage in radiation resistive colon tumors. Biomater. Sci. 2023, 11, 6177–6192. [Google Scholar] [CrossRef] [PubMed]
- Iranpour, S.; Bahrami, A.R.; Nekooei, S.; Sh Saljooghi, A.; Matin, M.M. Improving anti-cancer drug delivery performance of magnetic mesoporous silica nanocarriers for more efficient colorectal cancer therapy. J. Nanobiotechnol. 2021, 19, 314. [Google Scholar] [CrossRef]
- Jiang, H.; Bao, Q.; Yang, T.; Yang, M.; Mao, C. Precision Treatment of Colon Cancer Using Doxorubicin-Loaded Metal-Organic-Framework-Coated Magnetic Nanoparticles. ACS Appl. Mater. Interfaces 2024, 16, 49003–49012. [Google Scholar] [CrossRef]
- Wan, X.; Zhang, Y.; Wan, Y.; Xiong, M.; Xie, A.; Liang, Y.; Wan, H. A Multifunctional Biomimetic Nanoplatform for Dual Tumor Targeting-Assisted Multimodal Therapy of Colon Cancer. ACS Nano 2024, 18, 26666–26689. [Google Scholar] [CrossRef]
- Ge, J.; Chen, L.; Huang, B.; Gao, Y.; Zhou, D.; Zhou, Y.; Chen, C.; Wen, L.; Li, Q.; Zeng, J.; et al. Anchoring Group-Mediated Radiolabeling of Inorganic Nanoparticles—A Universal Method for Constructing Nuclear Medicine Imaging Nanoprobes. ACS Appl. Mater. Interfaces 2022, 14, 8838–8846. [Google Scholar] [CrossRef]
- Cuda, T.J.; Riddell, A.D.; Liu, C.; Whitehall, V.L.; Borowsky, J.; Wyld, D.K.; Burge, M.E.; Ahern, E.; Griffin, A.; Lyons, N.J.R.; et al. PET Imaging Quantifying (68)Ga-PSMA-11 Uptake in Metastatic Colorectal Cancer. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2020, 61, 1576–1579. [Google Scholar] [CrossRef]
- Tian, R.; Zhao, S.; Liu, G.; Chen, H.; Ma, L.; You, H.; Liu, C.; Wang, Z. Construction of lanthanide-doped upconversion nanoparticle-Uelx Europaeus Agglutinin-I bioconjugates with brightness red emission for ultrasensitive in vivo imaging of colorectal tumor. Biomaterials 2019, 212, 64–72. [Google Scholar] [CrossRef]
- Zhong, Y.; Ma, Z.; Wang, F.; Wang, X.; Yang, Y.; Liu, Y.; Zhao, X.; Li, J.; Du, H.; Zhang, M.; et al. In vivo molecular imaging for immunotherapy using ultra-bright near-infrared-IIb rare-earth nanoparticles. Nat. Biotechnol. 2019, 37, 1322–1331. [Google Scholar] [CrossRef]
- Zhu, X.; Gong, Y.; Liu, Y.; Yang, C.; Wu, S.; Yuan, G.; Guo, X.; Liu, J.; Qin, X. Ru@CeO2 yolk shell nanozymes: Oxygen supply in situ enhanced dual chemotherapy combined with photothermal therapy for orthotopic/subcutaneous colorectal cancer. Biomaterials 2020, 242, 119923. [Google Scholar] [CrossRef]
- Chen, H.; Li, X.; Liu, F.; Zhang, H.; Wang, Z. Renal Clearable Peptide Functionalized NaGdF4 Nanodots for High-Efficiency Tracking Orthotopic Colorectal Tumor in Mouse. Mol. Pharm. 2017, 14, 3134–3141. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Q.Q.; Liu, Z.R.; Hu, F.; Xu, Z.Q.; Kuang, Y.; Li, C. A Novel Ce―Mn Heterojunction-Based Multi-Enzymatic Nanozyme with Cancer-Specific Enzymatic Activity and Photothermal Capacity for Efficient Tumor Combination Therapy. Adv. Funct. Mater. 2024, 35, 2414837. [Google Scholar] [CrossRef]
- Yan, J.; He, W.; Yan, S.; Niu, F.; Liu, T.; Ma, B.; Shao, Y.; Yan, Y.; Yang, G.; Lu, W.; et al. Self-Assembled Peptide-Lanthanide Nanoclusters for Safe Tumor Therapy: Overcoming and Utilizing Biological Barriers to Peptide Drug Delivery. ACS Nano 2018, 12, 2017–2026. [Google Scholar] [CrossRef]
- Mohsin, M.H.; Khashan, K.S.; Sulaiman, G.M.; Mohammed, H.A.; Qureshi, K.A.; Aspatwar, A. A novel facile synthesis of metal nitride@metal oxide (BN/Gd2O3) nanocomposite and their antibacterial and anticancer activities. Sci. Rep. 2023, 13, 22749. [Google Scholar] [CrossRef]
- Liu, A.; Li, L.; Wang, Z.; Li, X.; Liang, H.; Yang, J.; Nešić, M.D.; Yang, X.; Lin, Q. Ultrasmall Au-GRHa Nanosystem for FL/CT Dual-Mode Imaging-Guided Targeting Photothermal Therapy of Ovarian Cancer. Anal. Chem. 2025, 97, 2232–2243. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, M.; Li, P.; Wang, Y.; Fu, Q. Nanomaterial-based CT contrast agents and their applications in image-guided therapy. Theranostics 2023, 13, 483–509. [Google Scholar] [CrossRef]
- Shakeri, M.; Delavari, H.H.; Montazerabadi, A.; Yourdkhani, A. Hyaluronic acid-coated ultrasmall BiOI nanoparticles as a potentially targeted contrast agent for X-ray computed tomography. Int. J. Biol. Macromol. 2022, 217, 668–676. [Google Scholar] [CrossRef]
- Jia, T.; Xu, J.T.; Dong, S.M.; He, F.; Zhong, C.N.; Yang, G.X.; Bi, H.T.; Xu, M.S.; Hu, Y.K.; Yang, D.; et al. Mesoporous cerium oxide-coated upconversion nanoparticles for tumor-responsive chemo-photodynamic therapy and bioimaging. Chem. Sci. 2019, 10, 8618–8633. [Google Scholar] [CrossRef]
- Shoemaker, T.; Vuong, T.; Glickman, H.; Kaifi, S.; Famulari, G.; Enger, S.A. Dosimetric Considerations for Ytterbium-169, Selenium-75, and Iridium-192 Radioisotopes in High-Dose-Rate Endorectal Brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 875–883. [Google Scholar] [CrossRef]
- Xiao, Y.D.; Paudel, R.; Liu, J.; Ma, C.; Zhang, Z.S.; Zhou, S.K. MRI contrast agents: Classification and application (Review). Int. J. Mol. Med. 2016, 38, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Yon, M.; Billotey, C.; Marty, J.D. Gadolinium-based contrast agents: From gadolinium complexes to colloidal systems. Int. J. Pharm. 2019, 569, 118577. [Google Scholar] [CrossRef] [PubMed]
- Aires, A.; Fernández-Afonso, Y.; Guedes, G.; Guisasola, E.; Gutiérrez, L.; Cortajarena, A.L. Engineered Protein-Driven Synthesis of Tunable Iron Oxide Nanoparticles as T1 and T2 Magnetic Resonance Imaging Contrast Agents. Chem. Mater. A Publ. Am. Chem. Soc. 2022, 34, 10832–10841. [Google Scholar] [CrossRef]
- Knobloch, G.; Colgan, T.; Wiens, C.N.; Wang, X.; Schubert, T.; Hernando, D.; Sharma, S.D.; Reeder, S.B. Relaxivity of Ferumoxytol at 1.5 T and 3.0 T. Investig. Radiol. 2018, 53, 257–263. [Google Scholar] [CrossRef]
- Si, G.; Du, Y.; Tang, P.; Ma, G.; Jia, Z.; Zhou, X.; Mu, D.; Shen, Y.; Lu, Y.; Mao, Y.; et al. Unveiling the next generation of MRI contrast agents: Current insights and perspectives on ferumoxytol-enhanced MRI. Natl. Sci. Rev. 2024, 11, nwae057. [Google Scholar] [CrossRef]
- Wen, L.; Shi, X.; He, L.; Lu, Y.; Han, D. Manganese-enhanced MRI for the detection of metastatic potential in colorectal cancer. Eur. Radiol. Exp. 2017, 1, 21. [Google Scholar] [CrossRef]
- Wen, L.; Shi, X.; He, L.; Han, D. Manganese-Enhanced Magnetic Resonance Imaging for Detection and Characterization of Colorectal Cancers. Tomography 2018, 4, 78–83. [Google Scholar] [CrossRef]
- Wu, B.; Lu, S.T.; Yu, H.; Liao, R.F.; Li, H.; Lucie Zafitatsimo, B.V.; Li, Y.S.; Zhang, Y.; Zhu, X.L.; Liu, H.G.; et al. Gadolinium-chelate functionalized bismuth nanotheranostic agent for in vivo MRI/CT/PAI imaging-guided photothermal cancer therapy. Biomaterials 2018, 159, 37–47. [Google Scholar] [CrossRef]
- Dodo, K.; Fujita, K.; Sodeoka, M. Raman Spectroscopy for Chemical Biology Research. J. Am. Chem. Soc. 2022, 144, 19651–19667. [Google Scholar] [CrossRef]
- Harmsen, S.; Rogalla, S.; Huang, R.; Spaliviero, M.; Neuschmelting, V.; Hayakawa, Y.; Lee, Y.; Tailor, Y.; Toledo-Crow, R.; Kang, J.W.; et al. Detection of Premalignant Gastrointestinal Lesions Using Surface-Enhanced Resonance Raman Scattering-Nanoparticle Endoscopy. ACS Nano 2019, 13, 1354–1364. [Google Scholar] [CrossRef]
- Mahata, T.; Das, G.M.; Dantham, V.R. Study of surface enhanced Raman scattering of IR-780 Iodide molecules using Au-Ag bimetallic nanostructures with blunt and sharp sprouts. Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 2021, 249, 119262. [Google Scholar] [CrossRef] [PubMed]
- da Paz, M.C.; Santos Mde, F.; Santos, C.M.; da Silva, S.W.; de Souza, L.B.; Lima, E.C.; Silva, R.C.; Lucci, C.M.; Morais, P.C.; Azevedo, R.B.; et al. Anti-CEA loaded maghemite nanoparticles as a theragnostic device for colorectal cancer. Int. J. Nanomed. 2012, 7, 5271–5282. [Google Scholar] [CrossRef]
- Wu, J.; Li, S.; Ma, Y.; Zhi, W.; Chen, T.; Huang, X.; Huang, C.; Zhou, X.; Zhang, P.; Zhang, Y.; et al. 3D hierarchic interfacial assembly of Au nanocage@Au along with IS-AgMNPs for simultaneous, ultrasensitive, reliable, and quantitative SERS detection of colorectal cancer related miRNAs. Biosens. Bioelectron. 2024, 248, 115993. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, Y.H.; Xia, F.; Sawan, M. Photoacoustic imaging for monitoring of stroke diseases: A review. Photoacoustics 2021, 23, 100287. [Google Scholar] [CrossRef]
- Park, B.; Oh, D.; Kim, J.; Kim, C. Functional photoacoustic imaging: From nano- and micro- to macro-scale. Nano Converg. 2023, 10, 29. [Google Scholar] [CrossRef]
- Han, H.H.; Kim, S.J.; Kim, J.; Park, W.; Kim, C.; Kim, H.; Hahn, S.K. Bimetallic Hyaluronate-Modified Au@Pt Nanoparticles for Noninvasive Photoacoustic Imaging and Photothermal Therapy of Skin Cancer. ACS Appl. Mater. Interfaces 2023, 15, 11609–11620. [Google Scholar] [CrossRef]
- Tao, C.; An, L.; Lin, J.; Tian, Q.; Yang, S. Surface Plasmon Resonance-Enhanced Photoacoustic Imaging and Photothermal Therapy of Endogenous H2 S-Triggered Au@Cu2 O. Small 2019, 15, e1903473. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; She, W.; Zhang, X.; Liu, Y.; Liu, Y.; Jiang, P. 3-Bromopyruvate-Loaded Ti3C2 MXene/Cu2O Nanosheets for Photoacoustic Imaging-Guided and Hypoxia-Relieving Enhanced Photothermal/Chemodynamic Therapy. Anal. Chem. 2023, 95, 1710–1720. [Google Scholar] [CrossRef]
- Jin, K.T.; Yao, J.Y.; Ying, X.J.; Lin, Y.; Chen, Y.F. Nanomedicine and Early Cancer Diagnosis: Molecular Imaging using Fluorescence Nanoparticles. Curr. Top. Med. Chem. 2020, 20, 2737–2761. [Google Scholar] [CrossRef]
- Fort, M.J.; Click, S.M.; Robinson, E.H.; He, F.M.C.; Bernhardt, P.V.; Rosenthal, S.J.; Macdonald, J.E. Minimizing the Reorganization Energy of Cobalt Redox Mediators Maximizes Charge Transfer Rates from Quantum Dots. Angew. Chem 2022, 61, e202202322. [Google Scholar] [CrossRef]
- Stingel, A.M.; Leemans, J.; Hens, Z.; Geiregat, P.; Petersen, P.B. Narrow homogeneous linewidths and slow cooling dynamics across infrared intra-band transitions in n-doped HgSe colloidal quantum dots. J. Chem. Phys. 2023, 158, 114202. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Li, X.; Zhang, H.; Ji, X.; Sun, W.; Yu, Y.; Liu, Y.; Huang, J.; Sarshar, Z.; Sain, M. High quantum yield photoluminescent N-doped carbon dots for switch sensing and imaging. Talanta 2021, 222, 121663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Xia, L.; Xie, H.Y.; Zhang, Z.L.; Pang, D.W. Quantum Dot Based Biotracking and Biodetection. Anal. Chem. 2019, 91, 532–547. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.L.; Zhao, W.; Liu, Z.Y.; Ren, M.; Kong, J.; Zong, X.; Luo, M.Y.; Tang, B.; Xie, J.; Pang, D.W.; et al. Acid-Resistant Near-Infrared II Ag2Se Quantum Dots for Gastrointestinal Imaging. Anal. Chem. 2023, 95, 15540–15548. [Google Scholar] [CrossRef]
- Krasley, A.T.; Li, E.; Galeana, J.M.; Bulumulla, C.; Beyene, A.G.; Demirer, G.S. Carbon Nanomaterial Fluorescent Probes and Their Biological Applications. Chem. Rev. 2024, 124, 3085–3185. [Google Scholar] [CrossRef]
- Feng, Q.; Wilhelm, J.; Gao, J. Transistor-like Ultra-pH-Sensitive Polymeric Nanoparticles. Acc. Chem. Res. 2019, 52, 1485–1495. [Google Scholar] [CrossRef]
- Corley, D.A.; Jensen, C.D.; Marks, A.R.; Zhao, W.K.; Lee, J.K.; Doubeni, C.A.; Zauber, A.G.; de Boer, J.; Fireman, B.H.; Schottinger, J.E.; et al. Adenoma detection rate and risk of colorectal cancer and death. N. Engl. J. Med. 2014, 370, 1298–1306. [Google Scholar] [CrossRef]
- Lu, J.; Liong, M.; Li, Z.; Zink, J.I.; Tamanoi, F. Biocompatibility, biodistribution, and drug-delivery efficiency of mesoporous silica nanoparticles for cancer therapy in animals. Small 2010, 6, 1794–1805. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, X.; Ma, K.; Madajewski, B.; Benezra, M.; Zhang, L.; Phillips, E.; Turker, M.Z.; Gallazzi, F.; Penate-Medina, O.; et al. Melanocortin-1 Receptor-Targeting Ultrasmall Silica Nanoparticles for Dual-Modality Human Melanoma Imaging. ACS Appl. Mater. Interfaces 2018, 10, 4379–4393. [Google Scholar] [CrossRef]
- Prieto-Montero, R.; Prieto-Castañeda, A.; Katsumiti, A.; Cajaraville, M.P.; Agarrabeitia, A.R.; Ortiz, M.J.; Martínez-Martínez, V. Functionalization of Photosensitized Silica Nanoparticles for Advanced Photodynamic Therapy of Cancer. Int. J. Mol. Sci. 2021, 22, 6618. [Google Scholar] [CrossRef]
- Rogalla, S.; Flisikowski, K.; Gorpas, D.; Mayer, A.T.; Flisikowska, T.; Mandella, M.J.; Ma, X.; Casey, K.M.; Felt, S.A.; Saur, D.; et al. Biodegradable fluorescent nanoparticles for endoscopic detection of colorectal carcinogenesis. Adv. Funct Mater. 2019, 29, 1904992. [Google Scholar] [CrossRef] [PubMed]
- López-Mora, D.A.; Carrió, I.; Flotats, A. Digital PET vs Analog PET: Clinical Implications? Semin. Nucl. Med. 2022, 52, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Aydogan, B.; Li, J.; Rajh, T.; Chaudhary, A.; Chmura, S.J.; Pelizzari, C.; Wietholt, C.; Kurtoglu, M.; Redmond, P. AuNP-DG: Deoxyglucose-labeled gold nanoparticles as X-ray computed tomography contrast agents for cancer imaging. Mol. Imaging Biol. 2010, 12, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.; Cabral Campello, M.P.; Paulo, A. Radiolabeled Gold Nanoparticles for Imaging and Therapy of Cancer. Materials 2020, 14, 4. [Google Scholar] [CrossRef]
- Chakravarty, R.; Valdovinos, H.F.; Chen, F.; Lewis, C.M.; Ellison, P.A.; Luo, H.; Meyerand, M.E.; Nickles, R.J.; Cai, W. Intrinsically germanium-69-labeled iron oxide nanoparticles: Synthesis and in-vivo dual-modality PET/MR imaging. Adv. Mater. 2014, 26, 5119–5123. [Google Scholar] [CrossRef]
- Guo, W.; Sun, X.; Jacobson, O.; Yan, X.; Min, K.; Srivatsan, A.; Niu, G.; Kiesewetter, D.O.; Chang, J.; Chen, X. Intrinsically radioactive [64Cu]CuInS/ZnS quantum dots for PET and optical imaging: Improved radiochemical stability and controllable Cerenkov luminescence. ACS Nano 2015, 9, 488–495. [Google Scholar] [CrossRef]
- Ehman, E.C.; Johnson, G.B.; Villanueva-Meyer, J.E.; Cha, S.; Leynes, A.P.; Larson, P.E.Z.; Hope, T.A. PET/MRI: Where might it replace PET/CT? J. Magn. Reson. Imaging JMRI 2017, 46, 1247–1262. [Google Scholar] [CrossRef]
- Li, C.; Zhao, L.; Jia, L.; Ouyang, Z.; Gao, Y.; Guo, R.; Song, S.; Shi, X.; Cao, X. (68)Ga-labeled dendrimer-entrapped gold nanoparticles for PET/CT dual-modality imaging and immunotherapy of tumors. J. Mater. Chem. B 2022, 10, 3648–3656. [Google Scholar] [CrossRef]
- Paiva, I.; Mattingly, S.; Wuest, M.; Leier, S.; Vakili, M.R.; Weinfeld, M.; Lavasanifar, A.; Wuest, F. Synthesis and Analysis of (64)Cu-Labeled GE11-Modified Polymeric Micellar Nanoparticles for EGFR-Targeted Molecular Imaging in a Colorectal Cancer Model. Mol. Pharm. 2020, 17, 1470–1481. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Hari, S.K.; Gauba, A.; Shrivastava, N.; Tripathi, R.M.; Jain, S.K.; Pandey, A.K. Polymeric micelles and cancer therapy: An ingenious multimodal tumor-targeted drug delivery system. Drug Deliv. Transl. Res. 2023, 13, 135–163. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Karikó, K.; Türeci, Ö. mRNA-based therapeutics--developing a new class of drugs. Nat. Rev. Drug Discov. 2014, 13, 759–780. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Lin, Z.I.; Chen, J.A.; Xu, Z.; Gu, J.; Law, W.C.; Yang, J.H.C.; Chen, C.K. Organic/Inorganic Self-Assembled Hybrid Nano-Architectures for Cancer Therapy Applications. Macromol. Biosci. 2022, 22, e2100349. [Google Scholar] [CrossRef]
- Iranpour, S.; Bahrami, A.R.; Dayyani, M.; Saljooghi, A.S.; Matin, M.M. A potent multifunctional ZIF-8 nanoplatform developed for colorectal cancer therapy by triple-delivery of chemo/radio/targeted therapy agents. J. Mater. Chem. B 2024, 12, 1096–1114. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, J.; Zhi, Z.; Jiang, T.; Wang, S. Facile synthesis of 3D cubic mesoporous silica microspheres with a controllable pore size and their application for improved delivery of a water-insoluble drug. J. Colloid Interface Sci. 2011, 363, 410–417. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, X.; Nie, W.; Zhang, Q.; Wang, W.; Zhang, Y.; He, C. Multifunctional Redox-Responsive Mesoporous Silica Nanoparticles for Efficient Targeting Drug Delivery and Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces 2016, 8, 33829–33841. [Google Scholar] [CrossRef]
- Cao, W.; Zhang, X.; Li, R.; Li, Z.; Lu, A.; Yu, F.; Sun, L.; Wang, J.; Wang, Z.; He, H. Lipid core-shell nanoparticles co-deliver FOLFOX regimen and siPD-L1 for synergistic targeted cancer treatment. J. Control. Release Off. J. Control. Release Soc. 2024, 368, 52–65. [Google Scholar] [CrossRef]
- Li, J.; Chang, X.; Chen, X.; Gu, Z.; Zhao, F.; Chai, Z.; Zhao, Y. Toxicity of inorganic nanomaterials in biomedical imaging. Biotechnol. Adv. 2014, 32, 727–743. [Google Scholar] [CrossRef]
- Lee, C.S.; Kim, H.; Yu, J.; Yu, S.H.; Ban, S.; Oh, S.; Jeong, D.; Im, J.; Baek, M.J.; Kim, T.H. Doxorubicin-loaded oligonucleotide conjugated gold nanoparticles: A promising in vivo drug delivery system for colorectal cancer therapy. Eur. J. Med. Chem. 2017, 142, 416–423. [Google Scholar] [CrossRef]
- Go, G.; Lee, C.S.; Yoon, Y.M.; Lim, J.H.; Kim, T.H.; Lee, S.H. PrP(C) Aptamer Conjugated-Gold Nanoparticles for Targeted Delivery of Doxorubicin to Colorectal Cancer Cells. Int. J. Mol. Sci. 2021, 22, 1976. [Google Scholar] [CrossRef]
- Ouyang, Q.; Zhao, Y.; Xu, K.; He, Y.; Qin, M. Hyaluronic Acid Receptor-Mediated Nanomedicines and Targeted Therapy. Small Methods 2024, 8, e2400513. [Google Scholar] [CrossRef] [PubMed]
- Narmani, A.; Rezvani, M.; Farhood, B.; Darkhor, P.; Mohammadnejad, J.; Amini, B.; Refahi, S.; Abdi Goushbolagh, N. Folic acid functionalized nanoparticles as pharmaceutical carriers in drug delivery systems. Drug Dev. Res. 2019, 80, 404–424. [Google Scholar] [CrossRef]
- Ghasemii, K.; Darroudi, M.; Rahimmanesh, I.; Ghomi, M.; Hassanpour, M.; Sharifi, E.; Yousefiasl, S.; Ahmadi, S.; Zarrabi, A.; Borzacchiello, A.; et al. Advances in aptamer-based drug delivery vehicles for cancer therapy. Biomater. Adv. 2022, 140, 213077. [Google Scholar] [CrossRef] [PubMed]
- Li, C.M.; Haratipour, P.; Lingeman, R.G.; Perry, J.J.P.; Gu, L.; Hickey, R.J.; Malkas, L.H. Novel Peptide Therapeutic Approaches for Cancer Treatment. Cells 2021, 10, 2908. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, N.; Halder, S.; Mahata, N.; Chanda, N. Bi-Functional Gold Nanorod-Protein Conjugates with Biomimetic BSA@Folic Acid Corona for Improved Tumor Targeting and Intracellular Delivery of Therapeutic Proteins in Colon Cancer 3D Spheroids. ACS Appl. Bio Mater. 2022, 5, 1476–1488. [Google Scholar] [CrossRef]
- Pan, G.; Jia, T.T.; Huang, Q.X.; Qiu, Y.Y.; Xu, J.; Yin, P.H.; Liu, T. Mesoporous silica nanoparticles (MSNs)-based organic/inorganic hybrid nanocarriers loading 5-Fluorouracil for the treatment of colon cancer with improved anticancer efficacy. Colloids Surf. B Biointerfaces 2017, 159, 375–385. [Google Scholar] [CrossRef]
- Hu, S.; Xia, K.; Huang, X.; Zhao, Y.; Zhang, Q.; Huang, D.; Xu, W.; Chen, Z.; Wang, C.; Zhang, Z. Multifunctional CaCO3@Cur@QTX125@HA nanoparticles for effectively inhibiting growth of colorectal cancer cells. J. Nanobiotechnol. 2023, 21, 353. [Google Scholar] [CrossRef]
- Hassibian, S.; Taghdisi, S.M.; Jamshidi, Z.; Samie, A.; Alinezhad Nameghi, M.; Shayan, M.; Farrokhi, N.; Alibolandi, M.; Ramezani, M.; Dehnavi, S.M.; et al. Surface modification of hollow gold nanoparticles conducted by incorporating cancer cell membrane and AS1411 aptamer, aiming to achieve a dual-targeted therapy for colorectal cancer. Int. J. Pharm. 2024, 655, 124036. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, T.; Li, S.; Wu, Q.; Wang, K.; Xu, X.; Lu, M.; Shao, R.; Zhao, W.; Liu, H. Biomimetic Nanobomb for Synergistic Therapy with Inhibition of Cancer Stem Cells. Small 2023, 19, e2206503. [Google Scholar] [CrossRef]
- Zhao, H.; He, X.; Tan, C.; Jakhar, A.M.; He, F.; Ma, J. Chitosan-melanin complex microsphere: A potential colonic delivery system for protein drugs. Carbohydr. Polym. 2025, 348, 122886. [Google Scholar] [CrossRef]
- Wang, Z.H.; Liu, J.M.; Li, C.Y.; Wang, D.; Lv, H.; Lv, S.W.; Zhao, N.; Ma, H.; Wang, S. Bacterial Biofilm Bioinspired Persistent Luminescence Nanoparticles with Gut-Oriented Drug Delivery for Colorectal Cancer Imaging and Chemotherapy. ACS Appl. Mater. Interfaces 2019, 11, 36409–36419. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yang, H.; Fu, R.; Su, Y.; Lin, X.; Jin, X.; Du, W.; Shan, X.; Huang, G. Biomimetic Upconversion Nanoparticles and Gold Nanoparticles for Novel Simultaneous Dual-Modal Imaging-Guided Photothermal Therapy of Cancer. Cancers 2020, 12, 3136. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.; Lu, W.; Zhao, X.; Wang, H.; Zheng, Y.; Zheng, A.; Shen, Z. Chondroitin sulfate/silk fibroin hydrogel incorporating graphene oxide quantum dots with photothermal-effect promotes type H vessel-related wound healing. Carbohydr. Polym. 2024, 334, 121972. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zou, M.; Wang, H.; Zhang, L.; Xing, M.; He, M.; Jiang, H.; Zhang, Q.; Kauppinen, E.I.; Xin, F.; et al. Upconversion nanoparticles@single-walled carbon nanotubes composites as efficient self-monitored photo-thermal agents. Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 2023, 303, 123173. [Google Scholar] [CrossRef]
- Overchuk, M.; Weersink, R.A.; Wilson, B.C.; Zheng, G. Photodynamic and Photothermal Therapies: Synergy Opportunities for Nanomedicine. ACS Nano 2023, 17, 7979–8003. [Google Scholar] [CrossRef]
- Li, J.; Zhang, W.; Ji, W.; Wang, J.; Wang, N.; Wu, W.; Wu, Q.; Hou, X.; Hu, W.; Li, L. Near infrared photothermal conversion materials: Mechanism, preparation, and photothermal cancer therapy applications. J. Mater. Chem. B 2021, 9, 7909–7926. [Google Scholar] [CrossRef]
- Yang, X.Z.; Wen, L.F.; Xu, G.; Lin, H.H.; Wang, S.; Liu, J.Y. Multifunctional organic nanomaterials with ultra-high photothermal conversion efficiency for photothermal therapy and inhibition of cancer metastasis. Bioorganic Chem. 2023, 130, 106220. [Google Scholar] [CrossRef]
- Liu, R.; Miao, Y.; Wen, K.; Yang, Y.; Xu, D.; Lu, S.; Liu, Z.; Qin, H.; Zhang, X.; Zhang, Y. Salmonella Biomimetic Nanoparticles for Photothermal-Chemotherapy of Colorectal Cancer. Nano Lett. 2024, 24, 13851–13860. [Google Scholar] [CrossRef]
- Ochoa-Hugo, S.E.; Gutiérrez-Mercado, Y.K.; Canales-Aguirre, A.A.; Hernández-Gutiérrez, R. Hyperthermia on colorectal cancer: Gold nanoshells-mediated photothermal therapy. Rev. Medica Del Inst. Mex. Del Seguro Soc. 2024, 62, 1–8. [Google Scholar] [CrossRef]
- Wang, Z.; Su, Q.; Deng, W.; Wang, X.; Zhou, H.; Zhang, M.; Lin, W.; Xiao, J.; Duan, X. Morphology-Mediated Tumor Deep Penetration for Enhanced Near Infrared II Photothermal and Chemotherapy of Colorectal Cancer. ACS Nano 2024, 18, 28038–28051. [Google Scholar] [CrossRef]
- Chen, Z.; Zhuang, J.; Pang, J.; Liu, Z.; Zhang, P.; Deng, H.; Zhang, L.; Zhuang, B. Application of a cationic amylose derivative loaded with single-walled carbon nanotubes for gene delivery therapy and photothermal therapy of colorectal cancer. J. Biomed. Mater. Res. Part A 2022, 110, 1052–1061. [Google Scholar] [CrossRef]
- Cheng, Y.; Bo, H.; Qin, R.; Chen, F.; Xue, F.; An, L.; Huang, G.; Tian, Q. Hyaluronic acid-coated Bi:Cu2O: An H2S-responsive agent for colon cancer with targeted delivery and enhanced photothermal performance. J. Nanobiotechnol. 2022, 20, 346. [Google Scholar] [CrossRef]
- Huang, Z.; Song, J.; Huang, S.; Wang, S.; Shen, C.; Song, S.; Lian, J.; Ding, Y.; Gong, Y.; Zhang, Y.; et al. Phase and Defect Engineering of MoSe2 Nanosheets for Enhanced NIR-II Photothermal Immunotherapy. Nano Lett. 2024, 24, 7764–7773. [Google Scholar] [CrossRef] [PubMed]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef] [PubMed]
- Larue, L.; Myrzakhmetov, B.; Ben-Mihoub, A.; Moussaron, A.; Thomas, N.; Arnoux, P.; Baros, F.; Vanderesse, R.; Acherar, S.; Frochot, C. Fighting Hypoxia to Improve PDT. Pharmaceuticals 2019, 12, 163. [Google Scholar] [CrossRef]
- Ji, B.; Wei, M.; Yang, B. Recent advances in nanomedicines for photodynamic therapy (PDT)-driven cancer immunotherapy. Theranostics 2022, 12, 434–458. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Alzeibak, R.; Mishchenko, T.A.; Shilyagina, N.Y.; Balalaeva, I.V.; Vedunova, M.V.; Krysko, D.V. Targeting immunogenic cancer cell death by photodynamic therapy: Past, present and future. J. Immunother. Cancer 2021, 9, e001926. [Google Scholar] [CrossRef]
- Chang, M.; Hou, Z.; Jin, D.; Zhou, J.; Wang, M.; Wang, M.; Shu, M.; Ding, B.; Li, C.; Lin, J. Colorectal Tumor Microenvironment-Activated Bio-Decomposable and Metabolizable Cu2 O@CaCO3 Nanocomposites for Synergistic Oncotherapy. Adv. Mater. 2020, 32, e2004647. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, Y.; Hu, C.; Cai, L.; Pang, M. Covalent Organic Framework-Based Nanocomposite for Synergetic Photo-, Chemodynamic-, and Immunotherapies. ACS Appl. Mater. Interfaces 2020, 12, 43456–43465. [Google Scholar] [CrossRef]
- de Freitas, C.F.; Kimura, E.; Rubira, A.F.; Muniz, E.C. Curcumin and silver nanoparticles carried out from polysaccharide-based hydrogels improved the photodynamic properties of curcumin through metal-enhanced singlet oxygen effect. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 112, 110853. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Raj, B.S.; Chen, Y.; Lou, X. Novel folic acid conjugated Fe3O4-ZnO hybrid nanoparticles for targeted photodynamic therapy. Colloids Surf. B Biointerfaces 2017, 150, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xia, P.; Kurboniyon, M.S.; Fang, S.; Huang, K.; Ning, S.; Jin, G.; Zhang, L.; Wang, C. V-doped MoS2 nanozymes providing reactive oxygen species and depleting glutathione for photothermally-enhanced nanocatalytic therapy. Front. Pharmacol. 2024, 15, 1448867. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Xia, Q.; Shang, J.; He, Y.; Li, Z.; Chen, Y.; Gao, F.; Yu, X.; Yuan, Z.; et al. Photothermal Fe3O4 nanoparticles induced immunogenic ferroptosis for synergistic colorectal cancer therapy. J. Nanobiotechnol. 2024, 22, 630. [Google Scholar] [CrossRef]
- Das, P.; Colombo, M.; Prosperi, D. Recent advances in magnetic fluid hyperthermia for cancer therapy. Colloids Surf. B Biointerfaces 2019, 174, 42–55. [Google Scholar] [CrossRef]
- Paez-Muñoz, J.M.; Gámez, F.; Fernández-Afonso, Y.; Gallardo, R.; Pernia Leal, M.; Gutiérrez, L.; de la Fuente, J.M.; Caro, C.; García-Martín, M.L. Optimization of iron oxide nanoparticles for MRI-guided magnetic hyperthermia tumor therapy: Reassessing the role of shape in their magnetocaloric effect. J. Mater. Chem. B 2023, 11, 11110–11120. [Google Scholar] [CrossRef]
- Fernandes, S.; Fernandez, T.; Metze, S.; Balakrishnan, P.B.; Mai, B.T.; Conteh, J.; De Mei, C.; Turdo, A.; Di Franco, S.; Stassi, G.; et al. Magnetic Nanoparticle-Based Hyperthermia Mediates Drug Delivery and Impairs the Tumorigenic Capacity of Quiescent Colorectal Cancer Stem Cells. ACS Appl. Mater. Interfaces 2021, 13, 15959–15972. [Google Scholar] [CrossRef]
- Fang, Y.; He, Y.; Wu, C.; Zhang, M.; Gu, Z.; Zhang, J.; Liu, E.; Xu, Q.; Asrorov, A.M.; Huang, Y. Magnetism-mediated targeting hyperthermia-immunotherapy in “cold” tumor with CSF1R inhibitor. Theranostics 2021, 11, 6860–6872. [Google Scholar] [CrossRef]
- Tehrani, M.H.H.; Soltani, M.; Moradi Kashkooli, F.; Mahmoudi, M.; Raahemifar, K. Computational Modeling of Combination of Magnetic Hyperthermia and Temperature-Sensitive Liposome for Controlled Drug Release in Solid Tumor. Pharmaceutics 2021, 14, 35. [Google Scholar] [CrossRef]
- Blondy, S.; David, V.; Verdier, M.; Mathonnet, M.; Perraud, A.; Christou, N. 5-Fluorouracil resistance mechanisms in colorectal cancer: From classical pathways to promising processes. Cancer Sci. 2020, 111, 3142–3154. [Google Scholar] [CrossRef]
- Morton, D.; Seymour, M.; Magill, L.; Handley, K.; Glasbey, J.; Glimelius, B.; Palmer, A.; Seligmann, J.; Laurberg, S.; Murakami, K.; et al. Preoperative Chemotherapy for Operable Colon Cancer: Mature Results of an International Randomized Controlled Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2023, 41, 1541–1552. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Chen, H.; Deng, T.; Cai, B.; Xia, Y.; Xie, L.; Wang, H.; Huang, C. Improved Immune Response for Colorectal Cancer Therapy Triggered by Multifunctional Nanocomposites with Self-Amplifying Antitumor Ferroptosis. ACS Appl. Mater. Interfaces 2024, 16, 13481–13495. [Google Scholar] [CrossRef] [PubMed]
- Lewinski, N.; Colvin, V.; Drezek, R. Cytotoxicity of nanoparticles. Small 2008, 4, 26–49. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Li, P.; Chen, X.; Ma, Y.; Wang, R.; Ji, W.; Gu, J.; Sheng, B.; Wang, Y.; Zhang, Z. Ceria nanoparticles: Biomedical applications and toxicity. J. Zhejiang Univ. Sci. B 2024, 25, 361–388. [Google Scholar] [CrossRef]
- Cong, Y.; Baimanov, D.; Zhou, Y.; Chen, C.; Wang, L. Penetration and translocation of functional inorganic nanomaterials into biological barriers. Adv. Drug Deliv. Rev. 2022, 191, 114615. [Google Scholar] [CrossRef]
- Beurton, J.; Lavalle, P.; Pallotta, A.; Chaigneau, T.; Clarot, I.; Boudier, A. Design of surface ligands for blood compatible gold nanoparticles: Effect of charge and binding energy. Int. J. Pharm. 2020, 580, 119244. [Google Scholar] [CrossRef]
- Ai, X.; Hu, M.; Wang, Z.; Zhang, W.; Li, J.; Yang, H.; Lin, J.; Xing, B. Recent Advances of Membrane-Cloaked Nanoplatforms for Biomedical Applications. Bioconjugate Chem. 2018, 29, 838–851. [Google Scholar] [CrossRef]
- Ding, H.; Tan, P.; Fu, S.; Tian, X.; Zhang, H.; Ma, X.; Gu, Z.; Luo, K. Preparation and application of pH-responsive drug delivery systems. J. Control. Release Off. J. Control. Release Soc. 2022, 348, 206–238. [Google Scholar] [CrossRef]
- Liu, P.; Hao, L.; Liu, M.; Hu, S. Glutathione-responsive and -exhausting metal nanomedicines for robust synergistic cancer therapy. Front. Bioeng. Biotechnol. 2023, 11, 1161472. [Google Scholar] [CrossRef]
- Karakoti, A.S.; Das, S.; Thevuthasan, S.; Seal, S. PEGylated inorganic nanoparticles. Angew. Chem. 2011, 50, 1980–1994. [Google Scholar] [CrossRef]
- Ramasundaram, S.; Saravanakumar, G.; Sobha, S.; Oh, T.H. Dextran Sulfate Nanocarriers: Design, Strategies and Biomedical Applications. Int. J. Mol. Sci. 2022, 24, 355. [Google Scholar] [CrossRef] [PubMed]
- Roach, L.; Booth, M.E.; Ingram, N.; Paterson, D.A.; Batchelor, D.V.B.; Moorcroft, S.C.T.; Bushby, R.J.; Critchley, K.; Coletta, P.L.; Evans, S.D. Evaluating Phospholipid-Functionalized Gold Nanorods for In Vivo Applications. Small 2021, 17, e2006797. [Google Scholar] [CrossRef] [PubMed]
- Panday, R.; Poudel, A.J.; Li, X.; Adhikari, M.; Ullah, M.W.; Yang, G. Amphiphilic core-shell nanoparticles: Synthesis, biophysical properties, and applications. Colloids Surf. B Biointerfaces 2018, 172, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Pham, X.H.; Park, S.M.; Ham, K.M.; Kyeong, S.; Son, B.S.; Kim, J.; Hahm, E.; Kim, Y.H.; Bock, S.; Kim, W.; et al. Synthesis and Application of Silica-Coated Quantum Dots in Biomedicine. Int. J. Mol. Sci. 2021, 22, 10116. [Google Scholar] [CrossRef]
- Hauck, T.S.; Anderson, R.E.; Fischer, H.C.; Newbigging, S.; Chan, W.C. In vivo quantum-dot toxicity assessment. Small 2010, 6, 138–144. [Google Scholar] [CrossRef]
- Szurkowska, K.; Drobniewska, A.; Kolmas, J. Dual Doping of Silicon and Manganese in Hydroxyapatites: Physicochemical Properties and Preliminary Biological Studies. Materials 2019, 12, 2566. [Google Scholar] [CrossRef]
- Sharifi, S.; Behzadi, S.; Laurent, S.; Forrest, M.L.; Stroeve, P.; Mahmoudi, M. Toxicity of nanomaterials. Chem. Soc. Rev. 2012, 41, 2323–2343. [Google Scholar] [CrossRef]
- Maggini, L.; Travaglini, L.; Cabrera, I.; Castro-Hartmann, P.; De Cola, L. Biodegradable Peptide-Silica Nanodonuts. Chemistry 2016, 22, 3697–3703. [Google Scholar] [CrossRef]
- Khalifehzadeh, R.; Arami, H. Biodegradable calcium phosphate nanoparticles for cancer therapy. Adv. Colloid Interface Sci. 2020, 279, 102157. [Google Scholar] [CrossRef]
- Gomez, C.; Hallot, G.; Laurent, S.; Port, M. Medical Applications of Metallic Bismuth Nanoparticles. Pharmaceutics 2021, 13, 1793. [Google Scholar] [CrossRef]
- Whitehead, M.W.; Phillips, R.H.; Sieniawska, C.E.; Delves, H.T.; Seed, P.T.; Thompson, R.P.; Powell, J.J. Double-blind comparison of absorbable colloidal bismuth subcitrate and nonabsorbable bismuth subnitrate in the eradication of Helicobacter pylori and the relief of nonulcer dyspepsia. Helicobacter 2000, 5, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Narayana, S.; Gowda, B.H.J.; Hani, U.; Shimu, S.S.; Paul, K.; Das, A.; Ashique, S.; Ahmed, M.G.; Tarighat, M.A.; Abdi, G. Inorganic nanoparticle-based treatment approaches for colorectal cancer: Recent advancements and challenges. J. Nanobiotechnol. 2024, 22, 427. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Hu, H.; Yan, J.; Zhang, C.; Li, Y.; Wang, M.; Tan, W.; Liu, J.; Pan, Y. Multifunctional Porous Iron Oxide Nanoagents for MRI and Photothermal/Chemo Synergistic Therapy. Bioconjugate Chem. 2018, 29, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Krajewska, K.; Gołkowska, A.M.; Nowak, M.; Kozakiewicz-Latała, M.; Pudło, W.; Żak, A.; Karolewicz, B.; Khimyak, Y.Z.; Nartowski, K.P. Molecular Level Characterisation of the Surface of Carbohydrate-Functionalised Mesoporous silica Nanoparticles (MSN) as a Potential Targeted Drug Delivery System via High Resolution Magic Angle Spinning (HR-MAS) NMR Spectroscopy. Int. J. Mol. Sci. 2022, 23, 5906. [Google Scholar] [CrossRef]
- Yusefi, M.; Lee-Kiun, M.S.; Shameli, K.; Teow, S.Y.; Ali, R.R.; Siew, K.K.; Chan, H.Y.; Wong, M.M.; Lim, W.L.; Kuča, K. 5-Fluorouracil loaded magnetic cellulose bionanocomposites for potential colorectal cancer treatment. Carbohydr. Polym. 2021, 273, 118523. [Google Scholar] [CrossRef]
- Khalili-Hezarjaribi, H.; Bahrami, A.R.; Sh Saljooghi, A.; Matin, M.M. Modified mesoporous silica nanocarriers containing superparamagnetic iron oxide nanoparticle, 5-fluorouracil or oxaliplatin, and metformin as a radiosensitizer, significantly impact colorectal cancer radiation therapy. Int. J. Pharm. 2024, 666, 124838. [Google Scholar] [CrossRef]
- Safwat, M.A.; Soliman, G.M.; Sayed, D.; Attia, M.A. Gold nanoparticles enhance 5-fluorouracil anticancer efficacy against colorectal cancer cells. Int. J. Pharm. 2016, 513, 648–658. [Google Scholar] [CrossRef]
- Li, M.; Yang, J.; Yao, X.; Li, X.; Xu, Z.; Tang, S.; Sun, B.; Lin, S.; Yang, C.; Liu, J. Multifunctional Mesoporous Silica-Coated Gold Nanorods Mediate Mild Photothermal Heating-Enhanced Gene/Immunotherapy for Colorectal Cancer. Pharmaceutics 2023, 15, 854. [Google Scholar] [CrossRef]
- Darroudi, M.; Gholami, M.; Rezayi, M.; Khazaei, M. An overview and bibliometric analysis on the colorectal cancer therapy by magnetic functionalized nanoparticles for the responsive and targeted drug delivery. J. Nanobiotechnol. 2021, 19, 399. [Google Scholar] [CrossRef]
- Chen, L.; Xie, J.; Wu, H.; Zang, F.; Ma, M.; Hua, Z.; Gu, N.; Zhang, Y. Improving sensitivity of magnetic resonance imaging by using a dual-targeted magnetic iron oxide nanoprobe. Colloids Surf. B Biointerfaces 2018, 161, 339–346. [Google Scholar] [CrossRef]
- Franceschi, E.; Seidel, C.; Sahm, F.; Pajtler, K.W.; Hau, P. How we treat medulloblastoma in adults. ESMO Open 2021, 6, 100173. [Google Scholar] [CrossRef] [PubMed]
- John, C.; Jain, K.; Masanam, H.B.; Narasimhan, A.K.; Natarajan, A. Recent Trends and Opportunities for the Targeted Immuno-Nanomaterials for Cancer Theranostics Applications. Micromachines 2022, 13, 2217. [Google Scholar] [CrossRef] [PubMed]
- Bhinder, B.; Gilvary, C.; Madhukar, N.S.; Elemento, O. Artificial Intelligence in Cancer Research and Precision Medicine. Cancer Discov. 2021, 11, 900–915. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.; Wu, E.; Zhang, A.; Alizadeh, A.A.; Zou, J. From patterns to patients: Advances in clinical machine learning for cancer diagnosis, prognosis, and treatment. Cell 2023, 186, 1772–1791. [Google Scholar] [CrossRef]
- Nehru, S.; Misra, R.; Bhaswant, M. Multifaceted Engineered Biomimetic Nanorobots Toward Cancer Management. ACS Biomater. Sci. Eng. 2022, 8, 444–459. [Google Scholar] [CrossRef]

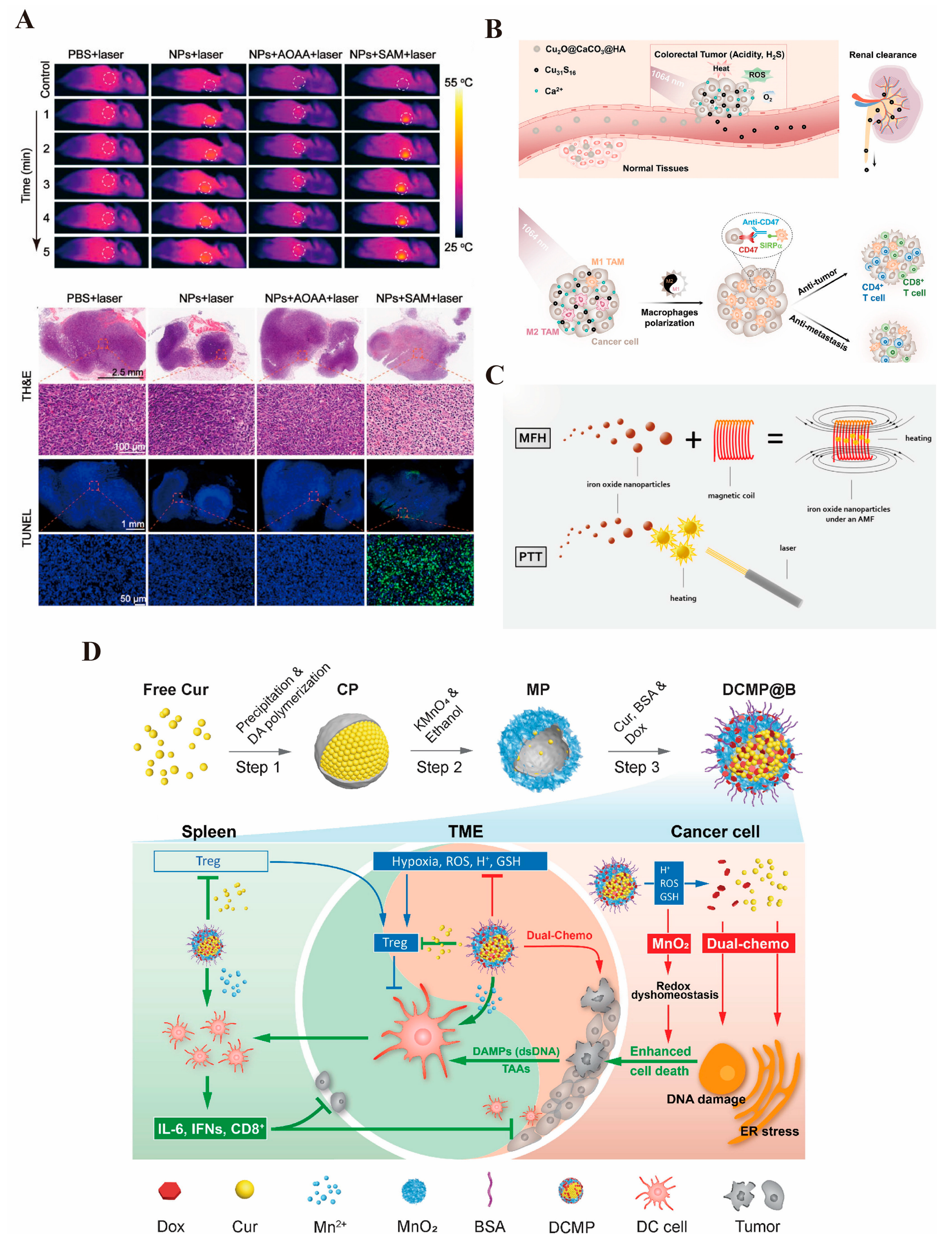
| Status | Initial Time | Categories | Aim | Clinical Trial/Reference |
|---|---|---|---|---|
| Completed | 2010 | Fe3O4 nanoparticles | Thermal ablation | [33] |
| Completed | 2015 | USPIO contrast agent (ferumoxtran-10) | Lymph node metastases | NCT02751606 [40] |
| Completed | 2017 | Carbon nanoparticles | Tumor localization and lymph node mapping | NCT03350945 |
| Completed | 2019 | Hafnium oxide nanoparticles | Radio therapy | NCT02379845 [34] |
| Completed | 2021 | Carbon nanoparticle | Lymph node tracer | NCT04759820 |
| Completed | 2022 | Carbon nanoparticles | Lymph node tracing and surgery guiding | NCT06783985 |
| Ongoing | 2023 | Carbon nanoparticle-loaded iron [CNSI-Fe (II)] | Ferroptosis | NCT06048367 [35,36,37] |
| Ongoing | 2024 | Ultrasmall superparamagnetic iron oxide (USPIO) | Evaluation of lymph node metastasis and staging | NCT06693375 [38,39] |
| Categories | Physical Property | Diagnosis in Colorectal Cancer | Treatment of Colorectal Cancer |
|---|---|---|---|
| Metal Nanomaterials | Optical properties | CT [113,114,115,116,117], photoacoustic imaging [118,119,120], fluorescence imaging [117,121,122,123] | Photothermal [118,119,121,124,125,126], photodynamic [127,128,129,130] |
| Magnetic properties | MRI [131] | Magnetothermal [123] | |
| Acoustical property | Acoustic force [120,132] | ||
| High specific surface area and surface effect, biocompatible | Targeted drug delivery [133,134,135] | ||
| Metal Oxide Nanomaterials | Optical properties | CT [136], photoacoustic imaging [137,138,139], fluorescence imaging [123] | Photothermal [123,137,139], photodynamic [136] |
| Magnetic properties | MRI [114,136,138] | Magnetothermal [140,141,142] | |
| Catalytic properties | Nano-enzymatic activity [143] | ||
| High specific surface area and surface effect, biocompatible | Targeted drug delivery [144,145,146] | ||
| Quantum Dots | Optical properties | Fluorescence imaging [147,148,149,150,151], molecular probes [152] | Photothermal [119,153] |
| High specific surface area and surface effect, biocompatible | Targeted drug delivery [151,153,154,155,156,157,158] | ||
| Carbon-based Nanomaterials | Optical properties | Photoacoustic imaging [124] | Photothermal [124,159], photodynamic [160,161,162] |
| High specific surface area and surface effect, biocompatible | Targeted drug delivery [158,160,162,163,164,165,166,167] | ||
| Magnetic Nanomaterials | Optical properties | Photothermal [168,169,170,171] Photodynamic [172,173] | |
| Magnetic properties | MRI [174,175,176,177] | Magnetothermal [178,179,180,181,182] | |
| Catalytic properties | Nano-enzymatic activity [183] | ||
| High specific surface area and surface effect, biocompatible | Targeted drug delivery [174,184,185,186] | ||
| Rare-earth Nanomaterials | Optical properties | PET-CT [187,188], fluorescence imaging [189,190] | Photothermal [191] |
| Magnetic properties | MRI [192] | ||
| Catalytic properties | Nano-enzymatic activity [191,193] | ||
| High specific surface area and surface effect, biocompatible | Targeted drug delivery [192,194,195] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Wang, H.; Zou, F.; Gu, J.; Deng, S.; Cao, Y.; Cai, K. The Role of Inorganic Nanomaterials in Overcoming Challenges in Colorectal Cancer Diagnosis and Therapy. Pharmaceutics 2025, 17, 409. https://doi.org/10.3390/pharmaceutics17040409
Wang J, Wang H, Zou F, Gu J, Deng S, Cao Y, Cai K. The Role of Inorganic Nanomaterials in Overcoming Challenges in Colorectal Cancer Diagnosis and Therapy. Pharmaceutics. 2025; 17(4):409. https://doi.org/10.3390/pharmaceutics17040409
Chicago/Turabian StyleWang, Jun, Hanwenchen Wang, Falong Zou, Junnan Gu, Shenghe Deng, Yinghao Cao, and Kailin Cai. 2025. "The Role of Inorganic Nanomaterials in Overcoming Challenges in Colorectal Cancer Diagnosis and Therapy" Pharmaceutics 17, no. 4: 409. https://doi.org/10.3390/pharmaceutics17040409
APA StyleWang, J., Wang, H., Zou, F., Gu, J., Deng, S., Cao, Y., & Cai, K. (2025). The Role of Inorganic Nanomaterials in Overcoming Challenges in Colorectal Cancer Diagnosis and Therapy. Pharmaceutics, 17(4), 409. https://doi.org/10.3390/pharmaceutics17040409








