The Impact of Doxycycline as an Adjunctive Therapy on Prostate-Specific Antigen, Quality of Life, and Cognitive Function in Metastatic Prostate Cancer Patients: A Phase II Randomized Controlled Trial
Abstract
1. Introduction
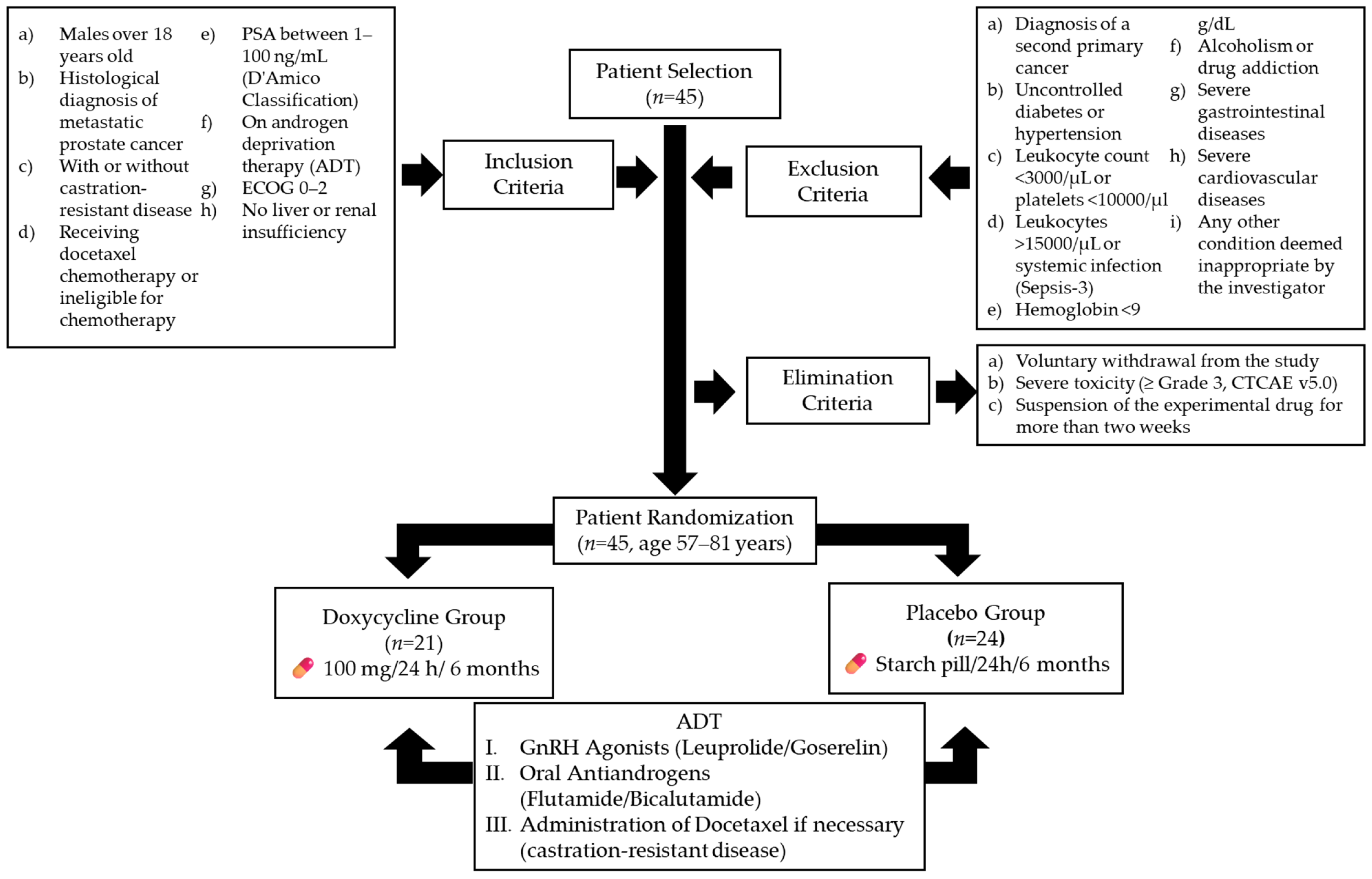
2. Materials and Methods
2.1. Study Design
2.2. Study Subjects
2.3. Outcome Measures and Follow-Up
2.4. Blinding
2.5. Sample Size
2.6. Structure–Activity Relationship Analysis
2.7. Statistical Analysis
3. Results
3.1. Participants
3.2. Treatment Effects on Prostate-Specific Antigen (PSA) Levels
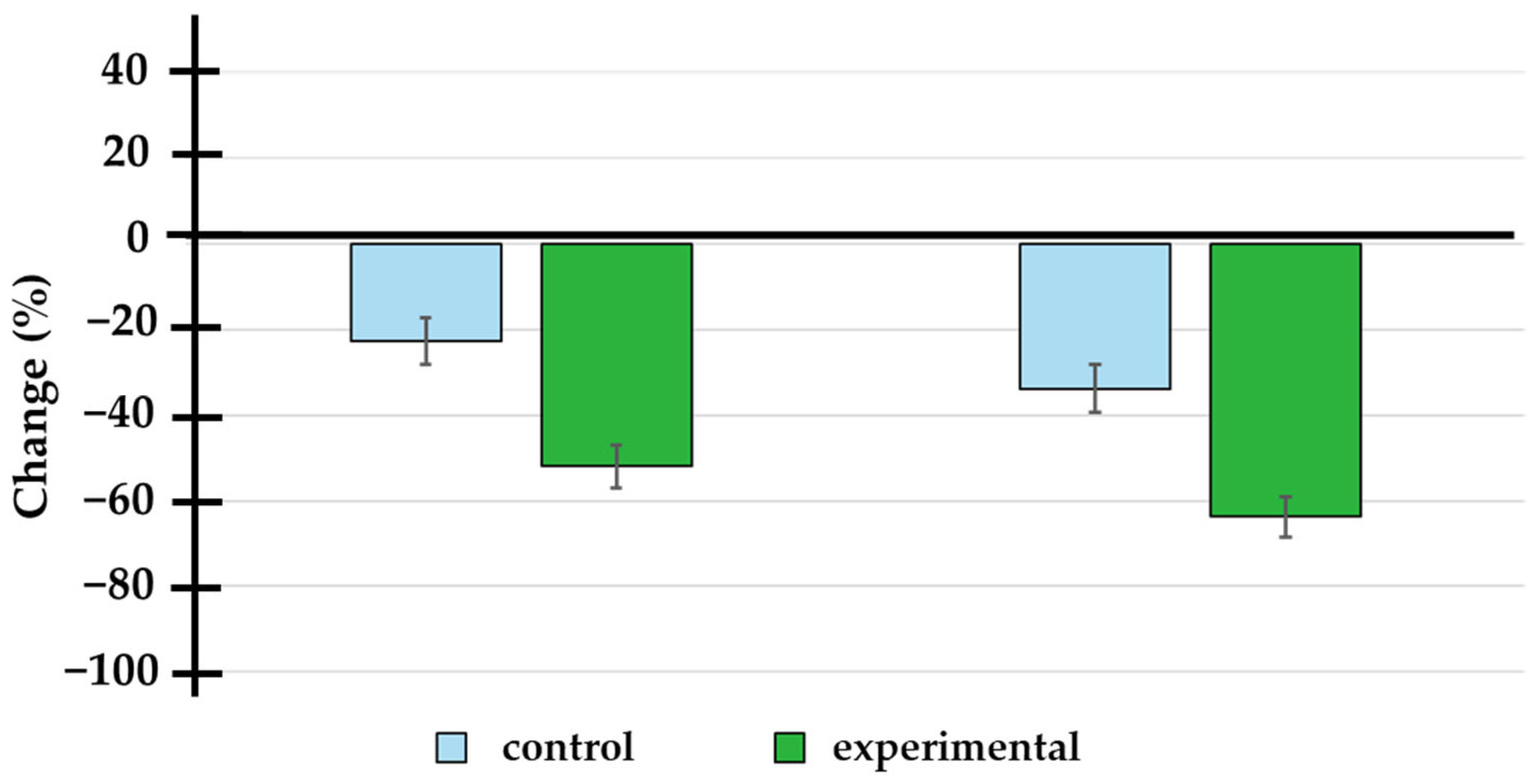
3.3. Change in PSA Levels
3.4. Therapeutic Response (≥50% Reduction in PSA Levels)
3.5. Multivariate Logistic Regression Analysis
3.6. Quality of Life and Cognitive Decline
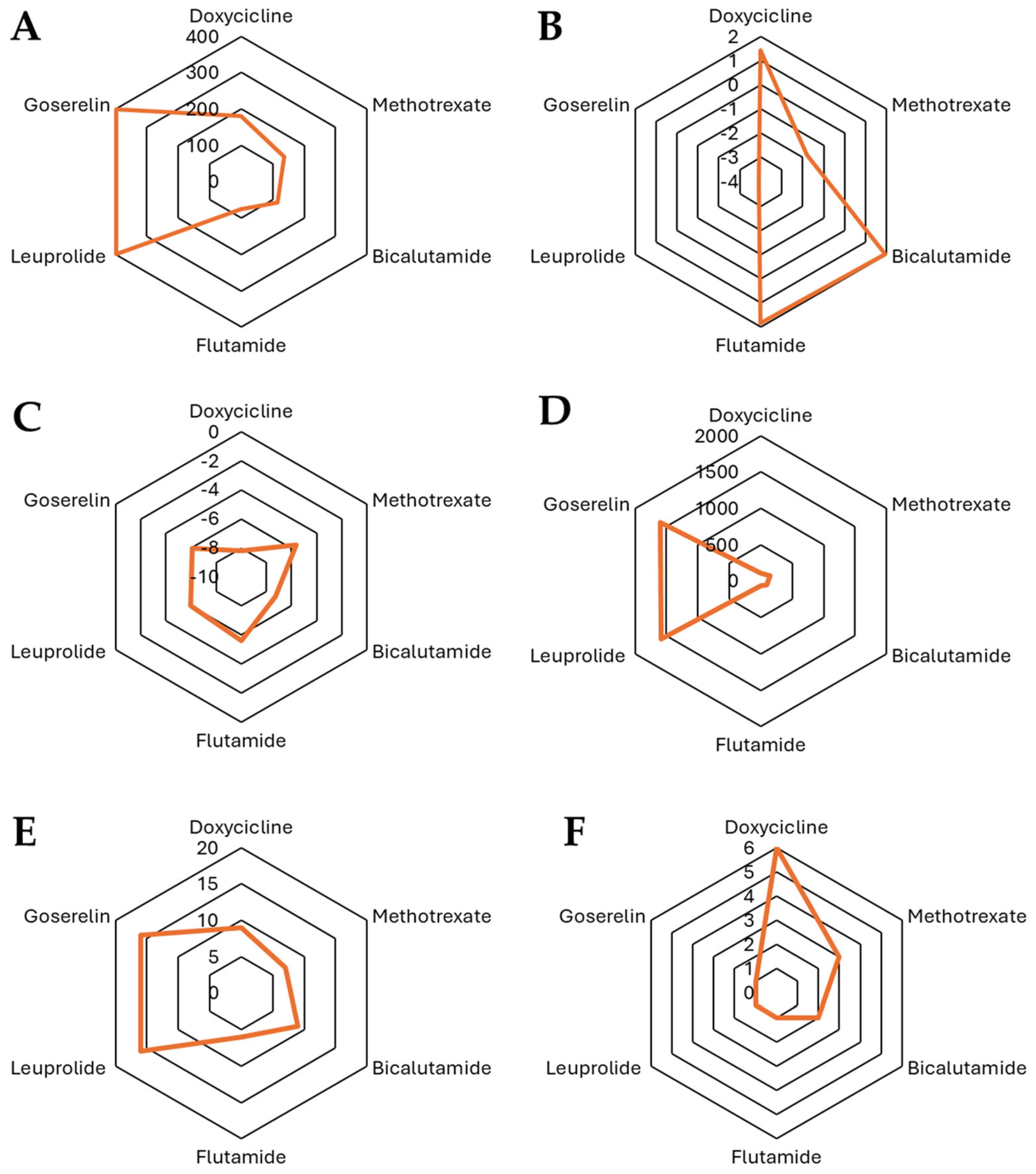
3.7. In Silico Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guzman-Esquivel, J.; Murillo-Zamora, E.; Ortiz-Mesina, M.; Galvan-Salazar, H.R.; De-Leon-Zaragoza, L.; Casarez-Price, J.C.; Delgado-Enciso, J.; Delgado-Enciso, I. Regional and National Burden of Prostate Cancer: Incidence, Mortality, Years of Life Lost, and Disability-Adjusted Life Years, in Mexico and Latin America from 1990 to 2019. Int. Urol. Nephrol. 2023, 55, 2155–2160. [Google Scholar] [CrossRef] [PubMed]
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, Regional, and National Burden of Chronic Kidney Disease, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Zhang, A.C.; Rasul, R.; Golden, A.; Feuerstein, M.A. Incidence and Mortality Trends of Metastatic Prostate Cancer: Surveillance, Epidemiology, and End Results Database Analysis. Can. Urol. Assoc. J. 2021, 15, E637–E643. [Google Scholar] [CrossRef] [PubMed]
- Devasia, T.P.; Mariotto, A.B.; Nyame, Y.A.; Etzioni, R. Estimating the Number of Men Living with Metastatic Prostate Cancer in the United States. Cancer Epidemiol. Biomark. Prev. 2023, 32, 659–665. [Google Scholar] [CrossRef]
- Jimenez-Rios, M.A.; Scavuzzo, A.; Noverón, N.R.; García-Arango, C.; Calvo-Vazquez, I.; Hurtado-Vázquez, A.; Arrieta-Rodriguez, O.G.; Davila-Jimenez, M.A.; Sighinolfi, M.C.; Rocco, B. Lethal Prostate Cancer in Mexico: Data from the Can.Prost Mexican Registry and a Project for Early Detection. Cancers 2024, 16, 3675. [Google Scholar] [CrossRef]
- Sumanasuriya, S.; De Bono, J. Treatment of Advanced Prostate Cancer—A Review of Current Therapies and Future Promise. Cold Spring Harb. Perspect. Med. 2018, 8, a030635. [Google Scholar] [CrossRef]
- Amaral, T.M.S.; Macedo, D.; Fernandes, I.; Costa, L. Castration-Resistant Prostate Cancer: Mechanisms, Targets, and Treatment. Prostate Cancer 2012, 2012, 327253. [Google Scholar] [CrossRef]
- Ritch, C.R.; Cookson, M.S. Advances in the Management of Castration Resistant Prostate Cancer. BMJ 2016, 354, i4405. [Google Scholar] [CrossRef]
- Galván-Salazar, H.R.; Soriano-Hernández, A.D.; Montes-Galindo, D.A.; Espíritu, G.C.; Guzman-Esquivel, J.; Rodríguez-Sánchez, I.P.; Newton-Sánchez, O.A.; Martinez-Fierro, M.L.; Gómez, X.G.B.; Rojas-Martínez, A.; et al. Preclinical Trial on the Use of Doxycycline for the Treatment of Adenocarcinoma of the Duodenum. Mol. Clin. Oncol. 2016, 5, 657–659. [Google Scholar] [CrossRef]
- Massa, A.; Vita, F.; Peraldo-Neia, C.; Varamo, C.; Basiricò, M.; Raggi, C.; Bernabei, P.; Erriquez, J.; Leone, F.; Aglietta, M.; et al. Doxycycline Restores Gemcitabine Sensitivity in Preclinical Models of Multidrug-Resistant Intrahepatic Cholangiocarcinoma. Cancers 2025, 17, 132. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef] [PubMed]
- Saikali, Z.; Singh, G. Doxycycline and Other Tetracyclines in the Treatment of Bone Metastasis. Anti-Cancer Drugs 2003, 14, 773–778. [Google Scholar] [CrossRef]
- Karamanolis, N.N.; Kounatidis, D.; Vallianou, N.G.; Dimitriou, K.; Tsaroucha, E.; Tsioulos, G.; Anastasiou, I.A.; Mavrothalassitis, E.; Karampela, I.; Dalamaga, M. Unraveling the Anti-Cancer Mechanisms of Antibiotics: Current Insights, Controversies, and Future Perspectives. Antibiotics 2024, 14, 9. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Uchiumi, T.; Monji, K.; Yagi, M.; Setoyama, D.; Amamoto, R.; Matsushima, Y.; Shiota, M.; Eto, M.; Kang, D. Doxycycline Induces Apoptosis via ER Stress Selectively to Cells with a Cancer Stem Cell-like Properties: Importance of Stem Cell Plasticity. Oncogenesis 2017, 6, 397. [Google Scholar] [CrossRef]
- Foroodi, F.; Duivenvoorden, W.C.; Singh, G. Interactions of Doxycycline with Chemotherapeutic Agents in Human Breast Adenocarcinoma MDA-MB-231 Cells. Anti-Cancer Drugs 2009, 20, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Tilakaratne, A.; Soory, M. Anti-Inflammatory Actions of Adjunctive Tetracyclines and Other Agents in Periodontitis and Associated Comorbidities. Open Dent. J. 2014, 8, 109–124. [Google Scholar] [CrossRef]
- Gaudreau, P.-O.; Stagg, J.; Soulières, D.; Saad, F. The Present and Future of Biomarkers in Prostate Cancer: Proteomics, Genomics, and Immunology Advancements. Biomark. Cancer 2016, 8s2, BIC.S31802. [Google Scholar] [CrossRef]
- Leslie, S.W.; Soon-Sutton, T.L.; Skelton, W.P. Prostate Cancer; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Nardo, G.; Pantziarka, P.; Conti, M. Synergistic Potential of Antibiotics with Cancer Treatments. Cancers 2024, 17, 59. [Google Scholar] [CrossRef]
- Fife, R.S.; Sledge, G.W.; Roth, B.J.; Proctor, C. Effects of Doxycycline on Human Prostate Cancer Cells in Vitro. Cancer Lett. 1998, 127, 37–41. [Google Scholar] [CrossRef]
- Gerry, C.J.; Schreiber, S.L. Chemical Probes and Drug Leads from Advances in Synthetic Planning and Methodology. Nat. Rev. Drug Discov. 2018, 17, 333–352. [Google Scholar] [CrossRef]
- Komura, H.; Watanabe, R.; Mizuguchi, K. The Trends and Future Prospective of In Silico Models from the Viewpoint of ADME Evaluation in Drug Discovery. Pharmaceutics 2023, 15, 2619. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Kwon, S.H.; Zhou, X.; Fuller, C.; Wang, X.; Vadgama, J.; Wu, Y. Overcoming Challenges in Small-Molecule Drug Bioavailability: A Review of Key Factors and Approaches. Int. J. Mol. Sci. 2024, 25, 13121. [Google Scholar] [CrossRef]
- Pandis, N.; Chung, B.; Scherer, R.W.; Elbourne, D.; Altman, D.G. CONSORT 2010 Statement: Extension Checklist for Reporting within Person Randomised Trials. BMJ 2017, 357, j2835. [Google Scholar] [CrossRef]
- Scher, H.I.; Morris, M.J.; Stadler, W.M.; Higano, C.; Basch, E.; Fizazi, K.; Antonarakis, E.S.; Beer, T.M.; Carducci, M.A.; Chi, K.N.; et al. Trial Design and Objectives for Castration-Resistant Prostate Cancer: Updated Recommendations From the Prostate Cancer Clinical Trials Working Group 3. J. Clin. Oncol. 2016, 34, 1402–1418. [Google Scholar] [CrossRef]
- Hernandez, D.J.; Nielsen, M.E.; Han, M.; Partin, A.W. Contemporary Evaluation of the D’Amico Risk Classification of Prostate Cancer. Urology 2007, 70, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and Response Criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Kok, B.; Abraldes, J. Child–Pugh Classification: Time to Abandon? Semin. Liver Dis. 2019, 39, 096–103. [Google Scholar] [CrossRef]
- El Brihi, J.; Pathak, S. Normal and Abnormal Complete Blood Count with Differential, 1st ed.; StatPearls Publishing: Treasure Island, FL, USA, 2025; Volume 1. [Google Scholar]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801. [Google Scholar] [CrossRef]
- Freites-Martinez, A.; Santana, N.; Arias-Santiago, S.; Viera, A. CTCAE Versión 5.0. Evaluación de La Gravedad de Los Eventos Adversos Dermatológicos de Las Terapias Antineoplásicas. Actas Dermosifiliogr. 2021, 112, 90–92. [Google Scholar] [CrossRef]
- Henry, N.L.; Kim, S.; Hays, R.D.; Diniz, M.A.; Luu, M.; Cecchini, R.S.; Yothers, G.; Rogatko, A.; Ganz, P.A. Toxicity Index, Patient-Reported Outcomes, and Early Discontinuation of Endocrine Therapy for Breast Cancer Risk Reduction in NRG Oncology/NSABP B-35. J. Clin. Oncol. 2021, 39, 3800–3812. [Google Scholar] [CrossRef]
- Gschwend, M.; Martin, W.; Erenmemişoğlu, A.; Scherm, M.; Dilger, C.; Tamur, U.; Kanzik, I.; Hincal, A. Pharmacokinetics and Bioequivalence Study of Doxycycline Capsules in Healthy Male Subjects. Arzneimittelforschung 2011, 57, 347–351. [Google Scholar] [CrossRef]
- Brattsand, M. Doxycycline at Low Concentrations Could Influence Your Experimental Results When Working With Prostate Cancer Cell Lines. Biomed. J. Sci. Tech. Res. 2020, 28, 21515–21519. [Google Scholar] [CrossRef]
- Ferreri, A.J.M.; Sassone, M.C.; Cangi, M.G.; Magliacane, G.; Zanussi, S.; Flospergher, E.; Marino, F.; Bongiovanni, L.; Ladetto, M.; Cavallo, F.; et al. Six-Month Doxycycline Is Safe and Effective As Upfront Monotherapy for Stage-I Malt Lymphoma of the Ocular Adnexae: Primary Endpoint Results of the IELSG39 Trial. Blood 2022, 140, 6482–6484. [Google Scholar] [CrossRef]
- Ramírez-Daffós, P.; Jiménez-Orozco, E.; Bolaños, M.; González Astorga, B.; Rubiales, S.; Ceballos-Barbancho, E.; Rodríguez García, J.M.; Reina, J.-J. A Phase 2 Study for Evaluating Doxycycline 50 Mg Once Daily and 100 Mg Once Daily as Preemptive Treatment for Skin Toxicity in Patients with Metastatic Colorectal Cancer Treated with an Anti-EGFR and Chemotherapy. Support. Care Cancer 2022, 30, 8081–8088. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.; O’Brien, J.; Medhurst, E.; Lawrentschuk, N.; Murphy, D.; Azad, A. Current Treatment Options for Newly Diagnosed Metastatic Hormone-Sensitive Prostate Cancer—A Narrative Review. Transl. Androl. Urol. 2021, 10, 3918–3930. [Google Scholar] [CrossRef]
- Estados Unidos Mexicanos. Consejo de Salubridad General Edición 2018 Del Cuadro Básico y Catálogo de Medicamentos. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5544613&fecha=23/11/2018#gsc.tab=0 (accessed on 31 July 2020).
- Guzman-Esquivel, J.; Mendoza-Hernandez, M.; Tiburcio-Jimenez, D.; Avila-Zamora, O.; Delgado-Enciso, J.; De-Leon-Zaragoza, L.; Casarez-Price, J.; Rodriguez-Sanchez, I.; Martinez-Fierro, M.; Meza-Robles, C.; et al. Decreased Biochemical Progression in Patients with Castration-resistant Prostate Cancer Using a Novel Mefenamic Acid Anti-inflammatory Therapy: A Randomized Controlled Trial. Oncol. Lett. 2020, 19, 4151–4160. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, G.; Garin, O.; Pardo, Y.; Vilagut, G.; Pont, À.; Suárez, M.; Neira, M.; Rajmil, L.; Gorostiza, I.; Ramallo-Fariña, Y.; et al. Validity of the EQ–5D–5L and Reference Norms for the Spanish Population. Qual. Life Res. 2018, 27, 2337–2348. [Google Scholar] [CrossRef] [PubMed]
- ClinCalc.com » Statistics » Post-Hoc Power Calculator Post-Hoc Power Calculator. Evaluate Statistical Power of an Existing Study. Available online: https://clincalc.com/stats/Power.aspx (accessed on 28 April 2023).
- Wang, X.; Ji, X. Sample Size Estimation in Clinical Research. Chest 2020, 158, S12–S20. [Google Scholar] [CrossRef]
- PerkinElmer Informatics PerkinElmer Informatics. ChemDraw 3D, Version 20.0; PerkinElmer Informatics: Waltham, MA, USA, 2021.
- Saxena, A.K.; Gupta, A.K.; Bhatia, K.S. Physicochemical Significance of ChemDraw and Dragon Computed Parameters: Correlation Studies in the Sets with Aliphatic and Aromatic Substituents. J. Math. Chem. 2024, 62, 2430–2455. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Schoonjans, F.; Zalata, A.; Depuydt, C.E.; Comhaire, F.H. MedCalc: A New Computer Program for Medical Statistics. Comput. Methods Programs Biomed. 1995, 48, 257–262. [Google Scholar] [CrossRef]
- Rosner, B. Fundamentals of Biostatistics, 7th ed.; Cengage Learning, Inc: Boston, MA, USA, 2010; Volume 1. [Google Scholar]
- Basaria, S.; Braga, M.; Moore, W.T. Doxycycline-Onduced Hypoglycemia in a Nondiabetic Young Man. S. Med. J. 2002, 95, 1353–1354. [Google Scholar] [CrossRef]
- Prota, A.E.; Lucena-Agell, D.; Ma, Y.; Estévez-Gallego, J.; Li, S.; Bargsten, K.; Josa-Prado, F.; Altmann, K.-H.; Gaillard, N.; Kamimura, S.; et al. Structural Insight into the Stabilization of Microtubules by Taxanes. eLife 2021, 12, e84791. [Google Scholar] [CrossRef]
- Light, A.; Carbajal, L.; Hammes, S.R. Doxycycline Mediated MMP2/9 Inhibition Blocks LH-Induced Steroidogenesis. Biol. Reprod. 2012, 87, 566. [Google Scholar] [CrossRef]
- Vinggaard, A.M.; Hnida, C.; Larsen, J.C. Environmental Polycyclic Aromatic Hydrocarbons Affect Androgen Receptor Activation in Vitro. Toxicology 2000, 145, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, S.M.; Watowich, S.J. Anthracene-Based Inhibitors of Dengue Virus NS2B–NS3 Protease. Antivir. Res. 2011, 89, 127–135. [Google Scholar] [CrossRef]
- Chen, R.-F.; Shen, Y.-C.; Huang, H.-S.; Liao, J.-F.; Ho, L.-K.; Chou, Y.-C.; Wang, W.-Y.; Chen, C.-F. Evaluation of the Anti-Inflammatory and Cytotoxic Effects of Anthraquinones and Anthracenes Derivatives in Human Leucocytes. J. Pharm. Pharmacol. 2004, 56, 915–919. [Google Scholar] [CrossRef]
- Krakauer, T.; Buckley, M. Doxycycline Is Anti-Inflammatory and Inhibits Staphylococcal Exotoxin-Induced Cytokines and Chemokines. Antimicrob. Agents Chemother. 2003, 47, 3630–3633. [Google Scholar] [CrossRef]
- Castro, M.M.; Kandasamy, A.D.; Youssef, N.; Schulz, R. Matrix Metalloproteinase Inhibitor Properties of Tetracyclines: Therapeutic Potential in Cardiovascular Diseases. Pharmacol. Res. 2011, 64, 551–560. [Google Scholar] [CrossRef]
- Park, J.G.; Kim, S.C.; Kim, Y.H.; Yang, W.S.; Kim, Y.; Hong, S.; Kim, K.-H.; Yoo, B.C.; Kim, S.H.; Kim, J.-H.; et al. Anti-Inflammatory and Antinociceptive Activities of Anthraquinone-2-Carboxylic Acid. Mediat. Inflamm. 2016, 2016, 1903849. [Google Scholar] [CrossRef]
- D’Souza, A.; Szabo, A.; Flynn, K.E.; Dhakal, B.; Chhabra, S.; Pasquini, M.C.; Weihrauch, D.; Hari, P.N. Adjuvant Doxycycline to Enhance Anti-Amyloid Effects: Results from the Dual Phase 2 Trial. EClinicalMedicine 2020, 23, 100361. [Google Scholar] [CrossRef] [PubMed]
- Nanda, N.; Dhawan, D.K.; Bhatia, A.; Mahmood, A.; Mahmood, S. Doxycycline Promotes Carcinogenesis & Metastasis via Chronic Inflammatory Pathway: An In Vivo Approach. PLoS ONE 2016, 11, e0151539. [Google Scholar] [CrossRef]
- Siregar, O.; Lelo, A.; Rahyussalim, A.J.; Ilyas, S.; Benny; Kurniawati, T.; Augustinus, Y.; Hendra; Mandagi, T.; Zufar, M.L.L.; et al. Doxycycline as a Potential MMP-1 Inhibitor for the Treatment of Spondylitis Tuberculosis: A Study in Rabbit Model. Biomed. Res. Int. 2023, 2023, 7421325. [Google Scholar] [CrossRef]
- Rahmani, M.; Negro Álvarez, S.E.; Hernández, E.B. The Potential Use of Tetracyclines in Neurodegenerative Diseases and the Role of Nano-Based Drug Delivery Systems. Eur. J. Pharm. Sci. 2022, 175, 106237. [Google Scholar] [CrossRef] [PubMed]
- Melnikov, V.; Tiburcio-Jimenez, D.; Mendoza-Hernandez, M.A.; Delgado-Enciso, J.; De-Leon-Zaragoza, L.; Guzman-Esquivel, J.; Rodriguez-Sanchez, I.P.; Martinez-Fierro, M.L.; Lara-Esqueda, A.; Delgado-Enciso, O.G.; et al. Improve Cognitive Impairment Using Mefenamic Acid Non-Steroidal Anti-Inflammatory Therapy: Additional Beneficial Effect Found in a Controlled Clinical Trial for Prostate Cancer Therapy. Am. J. Transl. Res. 2021, 13, 4535–4543. [Google Scholar]
- Nakai, Y.; Tanaka, N.; Anai, S.; Miyake, M.; Tatsumi, Y.; Fujimoto, K. A Randomized Control Trial Comparing the Efficacy of Antiandrogen Monotherapy: Flutamide vs. Bicalutamide. Horm. Cancer 2015, 6, 161–167. [Google Scholar] [CrossRef]
- Maughan, B.L.; Antonarakis, E.S. Androgen Pathway Resistance in Prostate Cancer and Therapeutic Implications. Expert Opin. Pharmacother. 2015, 16, 1521–1537. [Google Scholar] [CrossRef]
- Ali, J.; Camilleri, P.; Brown, M.B.; Hutt, A.J.; Kirton, S.B. In Silico Prediction of Aqueous Solubility Using Simple QSPR Models: The Importance of Phenol and Phenol-like Moieties. J. Chem. Inf. Model. 2012, 52, 2950–2957. [Google Scholar] [CrossRef]
- McCaffrey, G.; Staatz, W.D.; Sanchez-Covarrubias, L.; Finch, J.D.; DeMarco, K.; Laracuente, M.; Ronaldson, P.T.; Davis, T.P. P-glycoprotein Trafficking at the Blood–Brain Barrier Altered by Peripheral Inflammatory Hyperalgesia. J. Neurochem. 2012, 122, 962–975. [Google Scholar] [CrossRef]
- Fromm, M.F. P-Glycoprotein: A Defense Mechanism Limiting Oral Bioavailability and CNS Accumulation of Drugs. Int. J. Clin. Pharmacol. Ther. 2000, 38, 69–74. [Google Scholar] [CrossRef]
- Palleria, C.; Di Paolo, A.; Giofrè, C.; Caglioti, C.; Leuzzi, G.; Siniscalchi, A.; De Sarro, G.; Gallelli, L. Pharmacokinetic Drug-Drug Interaction and Their Implication in Clinical Management. J. Res. Med. Sci. 2013, 18, 601–610. [Google Scholar]
- Choi, Y.; Yu, A.-M. ABC Transporters in Multidrug Resistance and Pharmacokinetics, and Strategies for Drug Development. Curr. Pharm. Des. 2014, 20, 793–807. [Google Scholar] [CrossRef]
- Ogu, C.C.; Maxa, J.L. Drug Interactions Due to Cytochrome P450. Bayl. Univ. Med. Cent. Proc. 2000, 13, 421–423. [Google Scholar] [CrossRef]
- Stage, T.B.; Graff, M.; Wong, S.; Rasmussen, L.L.; Nielsen, F.; Pottegård, A.; Brøsen, K.; Kroetz, D.L.; Khojasteh, S.C.; Damkier, P. Dicloxacillin Induces CYP2C19, CYP2C9 and CYP3A4 in vivo and in vitro. Br. J. Clin. Pharmacol. 2018, 84, 510–519. [Google Scholar] [CrossRef]
- Schellhammer, P.F.; Sharifi, R.; Block, N.L.; Soloway, M.S.; Venner, P.M.; Patterson, A.L.; Sarosdy, M.F.; Vogelzang, N.J.; Chen, Y.; Kolvenbag, G.J.C.M.; et al. A Controlled Trial of Bicalutamide versus Flutamide, Each in Combination with Luteinizing Hormone–Releasing Hormone Analogue Therapy, in Patients with Advanced Prostate Carcinoma: Analysis of Time to Progression. Cancer 1996, 78, 2164–2169. [Google Scholar] [CrossRef]
- Upadhyay, R.K. Drug Delivery Systems, CNS Protection, and the Blood Brain Barrier. Biomed. Res. Int. 2014, 2014, 1–37. [Google Scholar] [CrossRef]
- McEwan, I.J.; Brinkmann, A.O. Androgen Physiology: Receptor and Metabolic Disorders; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Coulibaly, Y.I.; Dembele, B.; Diallo, A.A.; Lipner, E.M.; Doumbia, S.S.; Coulibaly, S.Y.; Konate, S.; Diallo, D.A.; Yalcouye, D.; Kubofcik, J.; et al. A Randomized Trial of Doxycycline for Mansonella perstans Infection. N. Engl. J. Med. 2009, 361, 1448–1458. [Google Scholar] [CrossRef]
- Bluemn, E.G.; Coleman, I.M.; Lucas, J.M.; Coleman, R.T.; Hernandez-Lopez, S.; Tharakan, R.; Bianchi-Frias, D.; Dumpit, R.F.; Kaipainen, A.; Corella, A.N.; et al. Androgen Receptor Pathway-Independent Prostate Cancer Is Sustained through FGF Signaling. Cancer Cell 2017, 32, 474–489.e6. [Google Scholar] [CrossRef]
- Skowron, K.J.; Booker, K.; Cheng, C.; Creed, S.; David, B.P.; Lazzara, P.R.; Lian, A.; Siddiqui, Z.; Speltz, T.E.; Moore, T.W. Steroid Receptor/Coactivator Binding Inhibitors: An Update. Mol. Cell. Endocrinol. 2019, 493, 110471. [Google Scholar] [CrossRef]
- Thomas Broome, S.; Musumeci, G.; Castorina, A. Doxycycline and Minocycline Act as Positive Allosteric Modulators of the PAC1 Receptor and Induce Plasminogen Activators in RT4 Schwann Cells. Appl. Sci. 2021, 11, 7673. [Google Scholar] [CrossRef]
- Bernardino, A.L.F.; Kaushal, D.; Philipp, M.T. The Antibiotics Doxycycline and Minocycline Inhibit the Inflammatory Responses to the Lyme Disease Spirochete Borrelia burgdorferi. J. Infect. Dis. 2009, 199, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Mesa, N.; Zarzuelo, A.; Gálvez, J. Minocycline: Far beyond an Antibiotic. Br. J. Pharmacol. 2013, 169, 337–352. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, F.; Chen, D.; Wang, L. Inhibition of Mitochondrial Respiration by Tigecycline Selectively Targets Thyroid Carcinoma and Increases Chemosensitivity. Clin. Exp. Pharmacol. Physiol. 2019, 46, 890–897. [Google Scholar] [CrossRef]
- Chan, P.A.; Le Brazidec, D.L.; Becasen, J.S.; Martin, H.; Kapadia, J.; Reno, H.; Bachmann, L.; Barbee, L.A. Safety of Longer-Term Doxycycline Use: A Systematic Review and Meta-Analysis with Implications for Bacterial STI Chemoprophylaxis. Sex. Transm. Dis. 2023, 50, 701–712. [Google Scholar] [CrossRef]
- Bryant, S.G.; Fisher, S.; Kluge, R.M. Increased Frequency of Doxycycline Side Effects. Pharmacother. J. Human. Pharmacol. Drug Ther. 1987, 7, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.M.; Marto, J.M.; Johnson, J.L.; Graber, E.M. A Review of Systemic Minocycline Side Effects and Topical Minocycline as a Safer Alternative for Treating Acne and Rosacea. Antibiotics 2021, 10, 757. [Google Scholar] [CrossRef] [PubMed]
- Dominic, M.R. Adverse Reactions Induced by Minocycline: A Review of Literature. Curr. Drug Saf. 2021, 16, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.Y.C.; Clements, D.; Johnson, S.R. Effect of Doxycycline on Proliferation, MMP Production, and Adhesion in LAM-Related Cells. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2010, 299, L393–L400. [Google Scholar] [CrossRef]
- Ogut, D.; Reel, B.; Gonen Korkmaz, C.; Arun, M.Z.; Cilaker Micili, S.; Ergur, B.U. Doxycycline Down-Regulates Matrix Metalloproteinase Expression and Inhibits NF-ΚB Signaling in LPS-Induced PC3 Cells. Folia Histochem. Cytobiol. 2017, 54, 171–180. [Google Scholar] [CrossRef]
- Stechmiller, J.; Cowan, L.; Schultz, G. The Role of Doxycycline as a Matrix Metalloproteinase Inhibitor for the Treatment of Chronic Wounds. Biol. Res. Nurs. 2010, 11, 336–344. [Google Scholar] [CrossRef]
- Meli, D.N.; Coimbra, R.S.; Erhart, D.G.; Loquet, G.; Bellac, C.L.; Täuber, M.G.; Neumann, U.; Leib, S.L. Doxycycline Reduces Mortality and Injury to the Brain and Cochlea in Experimental Pneumococcal Meningitis. Infect. Immun. 2006, 74, 3890–3896. [Google Scholar] [CrossRef]
- Nigam, M.; Mishra, A.P.; Deb, V.K.; Dimri, D.B.; Tiwari, V.; Bungau, S.G.; Bungau, A.F.; Radu, A.-F. Evaluation of the Association of Chronic Inflammation and Cancer: Insights and Implications. Biomed. Pharmacother. 2023, 164, 115015. [Google Scholar] [CrossRef]
- Multhoff, G.; Molls, M.; Radons, J. Chronic Inflammation in Cancer Development. Front. Immunol. 2012, 2, 98. [Google Scholar] [CrossRef]
- Balducci, C.; Santamaria, G.; La Vitola, P.; Brandi, E.; Grandi, F.; Viscomi, A.R.; Beeg, M.; Gobbi, M.; Salmona, M.; Ottonello, S.; et al. Doxycycline Counteracts Neuroinflammation Restoring Memory in Alzheimer’s Disease Mouse Models. Neurobiol. Aging 2018, 70, 128–139. [Google Scholar] [CrossRef]

| Usual Medical Care Plus | ||||
|---|---|---|---|---|
| Characteristic | All (n = 45) | Placebo (n = 24) | Doxycycline (n = 21) | p-Value |
| Age (years) | 73.0 (70.0–78.5) | 73.0 (70.0–78.0) | 73.0 (69.5–80.0) | 0.909 * |
| BMI | 28.3 (26.5–30.7) | 28.0 (26.7–30.0) | 28.8 (26.7–31.2) | 0.542 * |
| Gleason score | 8.1 (7.2–8.5) | 8.2 (7.5–8.5) | 8.0 (7.0–8.2) | 0.852 * |
| Glucose (mg/dL) | 106.0 (91.0–122.0) | 100.5 (89.5–120.7) | 110.0 (91.7–124.2) | 0.391 * |
| Docetaxel | 75.6% | 83.3% | 66.7% | 0.299 ** |
| Poor Quality of Life | 46.2% | 47.6% | 44.4% | 0.999 ** |
| MMSE score | 22.0 (21.0–25.0) | 23.0 (20.2–25.7) | 22.0 (21.0–23.5) | 0.549 * |
| Control | Doxycycline | ||||||
|---|---|---|---|---|---|---|---|
| PSA | Median | Q1 | Q3 | Median | Q1 | Q3 | p-Value * |
| Baseline | 18.50 | 11.7500 | 32.00 | 17.00 | 7.50 | 30.00 | 0.473 |
| 3 months | 17.00 | 9.2250 | 28.00 | 9.00 | 3.50 | 18.00 | 0.059 |
| 6 months | 16.0 | 7.2500 | 22.00 | 7.00 | 1.35 | 14.20 | 0.043 |
| P Baseline vs. 3 months ** | <0.001 | <0.001 | |||||
| P Baseline vs. 6 months ** | <0.001 | <0.001 | |||||
| PSA Change | Placebo | Doxycycline | p-Value * | ||||
|---|---|---|---|---|---|---|---|
| Median | Q1 | Q3 | Median | Q1 | Q3 | Control vs. Experimental | |
| 3 months | −2.80 | −7.25 | −1.00 | −8.10 | −10.00 | −2.75 | <0.001 |
| 6 months | −5.65 | −11.75 | −1.34 | −12.00 | −14.00 | −4.85 | <0.001 |
| Therapeutic Response | 95% CI | |||||||
|---|---|---|---|---|---|---|---|---|
| n | No | Yes | RR | Lower | Upper | p-Value | ||
| 3 months | Placebo | 24 | 87.5% | 12.5% | Reference | |||
| Doxycycline | 21 | 66.7% | 33.3% | 3.50 | 0.77 | 15.87 | 0.151 | |
| 6 months | Placebo | 24 | 79.2% | 20.8% | Reference | |||
| Doxycycline | 21 | 28.6% | 71.4% | 9.50 | 2.42 | 37.24 | 0.001 | |
| CI95% | ||||
|---|---|---|---|---|
| AdRR | Lower | Upper | p-Value | |
| Basal PSA | 0.977 | 0.940 | 1.014 | 0.221 |
| Age | 0.976 | 0.870 | 1.096 | 0.686 |
| Docetaxel | 0.797 | 0.127 | 5.002 | 0.808 |
| Doxycycline | 10.309 | 2.359 | 45.055 | 0.002 |
| Poor Quality of Life | All | Placebo | Doxycycline | p-Value * |
|---|---|---|---|---|
| Baseline | 46.20% | 47.6% | 44.4% | 0.999 |
| 3 months | 30.8% | 47.6% | 11.1% | 0.018 |
| 6 months | 28.6% | 42.9% | 7.1% | 0.028 |
| P Baseline vs. 3 m ** | 0.109 | 0.999 | 0.014 | |
| P Baseline vs. 6 m ** | 0.090 | 0.763 | 0.014 |
| Molecule | MW | Fraction Csp3 | # of Rotatable Bonds | # of H-Bond Acceptors | # of H-Bond Donors | MR | TPSA | iLOGP | XLOGP3 | WLOGP | MLOGP |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Doxycycline | 444.43 | 0.41 | 1 | 9 | 6 | 110.91 | 181.62 | 1.43 | 1.19 | −0.5 | −2.08 |
| Chlortetracycline | 478.88 | 0.41 | 2 | 9 | 6 | 115.23 | 181.62 | 1.92 | −0.62 | 0.33 | −1.6 |
| Demeclocycline | 464.85 | 0.38 | 2 | 9 | 6 | 110.54 | 181.62 | 1.55 | −0.56 | −0.06 | −1.82 |
| Minocycline | 457.48 | 0.43 | 3 | 8 | 5 | 118.57 | 164.63 | 1.66 | 0.05 | 0.19 | −1.6 |
| Oxytetracycline | 460.43 | 0.41 | 2 | 10 | 7 | 111.95 | 201.85 | 0.55 | −0.7 | −1.51 | −2.85 |
| Tigecycline | 585.65 | 0.52 | 8 | 10 | 7 | 154.95 | 205.76 | 2.23 | −0.19 | 0.32 | −2.05 |
| Lymecycline | 602.63 | 0.52 | 11 | 13 | 9 | 151.24 | 242.98 | 0 | −4.3 | −0.56 | −4.73 |
| Metacycline | 442.42 | 0.32 | 2 | 9 | 6 | 110.65 | 181.62 | 1.55 | −0.24 | −0.44 | −2.16 |
| Rolitetracycline | 527.57 | 0.52 | 5 | 10 | 6 | 139.04 | 170.87 | 2.69 | −0.28 | −0.02 | −1.4 |
| Bicalutamide | 430.37 | 0.22 | 7 | 9 | 2 | 93.86 | 115.64 | 1.95 | 2.31 | 5.34 | 2.47 |
| Flutamide | 276.21 | 0.36 | 5 | 6 | 1 | 64.19 | 74.92 | 1.85 | 3.35 | 4.17 | 2.03 |
| Finasteride | 372.54 | 0.83 | 3 | 2 | 2 | 113.18 | 58.2 | 3.42 | 3.03 | 3.43 | 3.46 |
| Dutasteride | 528.53 | 0.63 | 5 | 8 | 2 | 129.94 | 58.2 | 3.85 | 5.37 | 8.31 | 5.42 |
| Cyproterone acetate | 416.94 | 0.71 | 3 | 4 | 0 | 111.96 | 60.44 | 3.41 | 3.64 | 4.61 | 3.71 |
| Docetaxel | 807.88 | 0.56 | 14 | 14 | 5 | 205.25 | 224.45 | 3.33 | 2.81 | 2.94 | 1.06 |
| Leuprolide | 1237.47 | 0.24 | 20 | 16 | 1 | 1590.8 | 397.28 | −3.92 | −3.92 | −3.83 | −3.89 |
| Goserelin | 1249.47 | 0.25 | 20 | 16 | 1 | 1603.7 | 397.47 | −3.91 | −3.91 | −3.84 | −3.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzmán-Esquivel, J.; Garcia-Garcia, H.S.; Hernández-Fuentes, G.A.; Venegas-Ramírez, J.; Barajas-Mejía, C.D.; Garza-Veloz, I.; Martinez-Fierro, M.L.; Magaña-Vergara, N.E.; Guzmán-Solórzano, J.A.; Calvo-Soto, P.; et al. The Impact of Doxycycline as an Adjunctive Therapy on Prostate-Specific Antigen, Quality of Life, and Cognitive Function in Metastatic Prostate Cancer Patients: A Phase II Randomized Controlled Trial. Pharmaceutics 2025, 17, 404. https://doi.org/10.3390/pharmaceutics17040404
Guzmán-Esquivel J, Garcia-Garcia HS, Hernández-Fuentes GA, Venegas-Ramírez J, Barajas-Mejía CD, Garza-Veloz I, Martinez-Fierro ML, Magaña-Vergara NE, Guzmán-Solórzano JA, Calvo-Soto P, et al. The Impact of Doxycycline as an Adjunctive Therapy on Prostate-Specific Antigen, Quality of Life, and Cognitive Function in Metastatic Prostate Cancer Patients: A Phase II Randomized Controlled Trial. Pharmaceutics. 2025; 17(4):404. https://doi.org/10.3390/pharmaceutics17040404
Chicago/Turabian StyleGuzmán-Esquivel, José, Hossana S. Garcia-Garcia, Gustavo A. Hernández-Fuentes, Jesús Venegas-Ramírez, Carlos D. Barajas-Mejía, Idalia Garza-Veloz, Margarita L. Martinez-Fierro, Nancy E. Magaña-Vergara, José A. Guzmán-Solórzano, Patricia Calvo-Soto, and et al. 2025. "The Impact of Doxycycline as an Adjunctive Therapy on Prostate-Specific Antigen, Quality of Life, and Cognitive Function in Metastatic Prostate Cancer Patients: A Phase II Randomized Controlled Trial" Pharmaceutics 17, no. 4: 404. https://doi.org/10.3390/pharmaceutics17040404
APA StyleGuzmán-Esquivel, J., Garcia-Garcia, H. S., Hernández-Fuentes, G. A., Venegas-Ramírez, J., Barajas-Mejía, C. D., Garza-Veloz, I., Martinez-Fierro, M. L., Magaña-Vergara, N. E., Guzmán-Solórzano, J. A., Calvo-Soto, P., Avila-Zamora, O. N., Fuentes-Murguia, M., Ceja-Espíritu, G., & Delgado-Enciso, I. (2025). The Impact of Doxycycline as an Adjunctive Therapy on Prostate-Specific Antigen, Quality of Life, and Cognitive Function in Metastatic Prostate Cancer Patients: A Phase II Randomized Controlled Trial. Pharmaceutics, 17(4), 404. https://doi.org/10.3390/pharmaceutics17040404