Ciprofloxacin-Loaded Spray-Dried Lactose Particles: Formulation Optimization and Antibacterial Efficacy
Abstract
1. Introduction
2. Materials and Methods
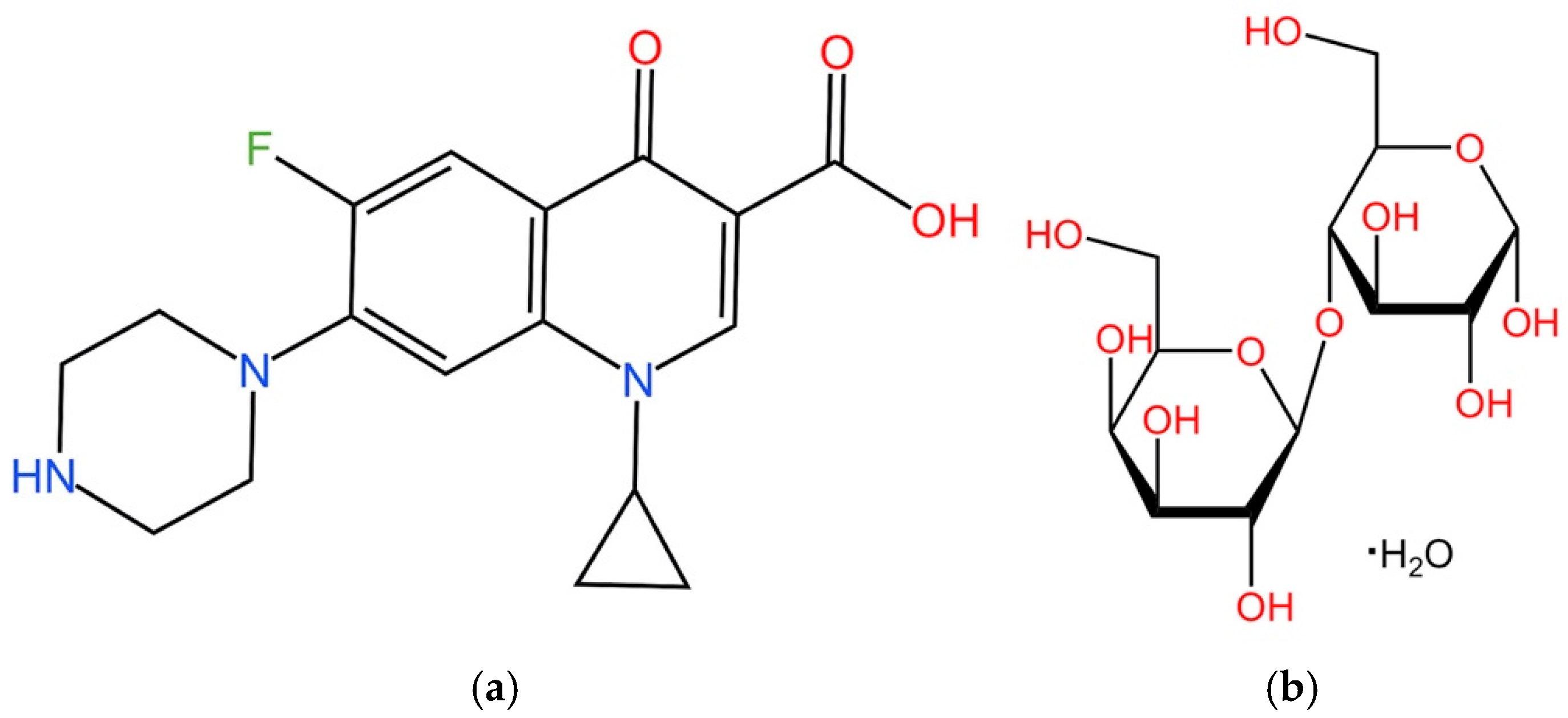
2.1. Materials
2.2. Preparation of Spray Drying Solutions
2.3. Production of SD Particles
2.4. Characterization
2.4.1. Scanning Electron Microscopy (SEM)
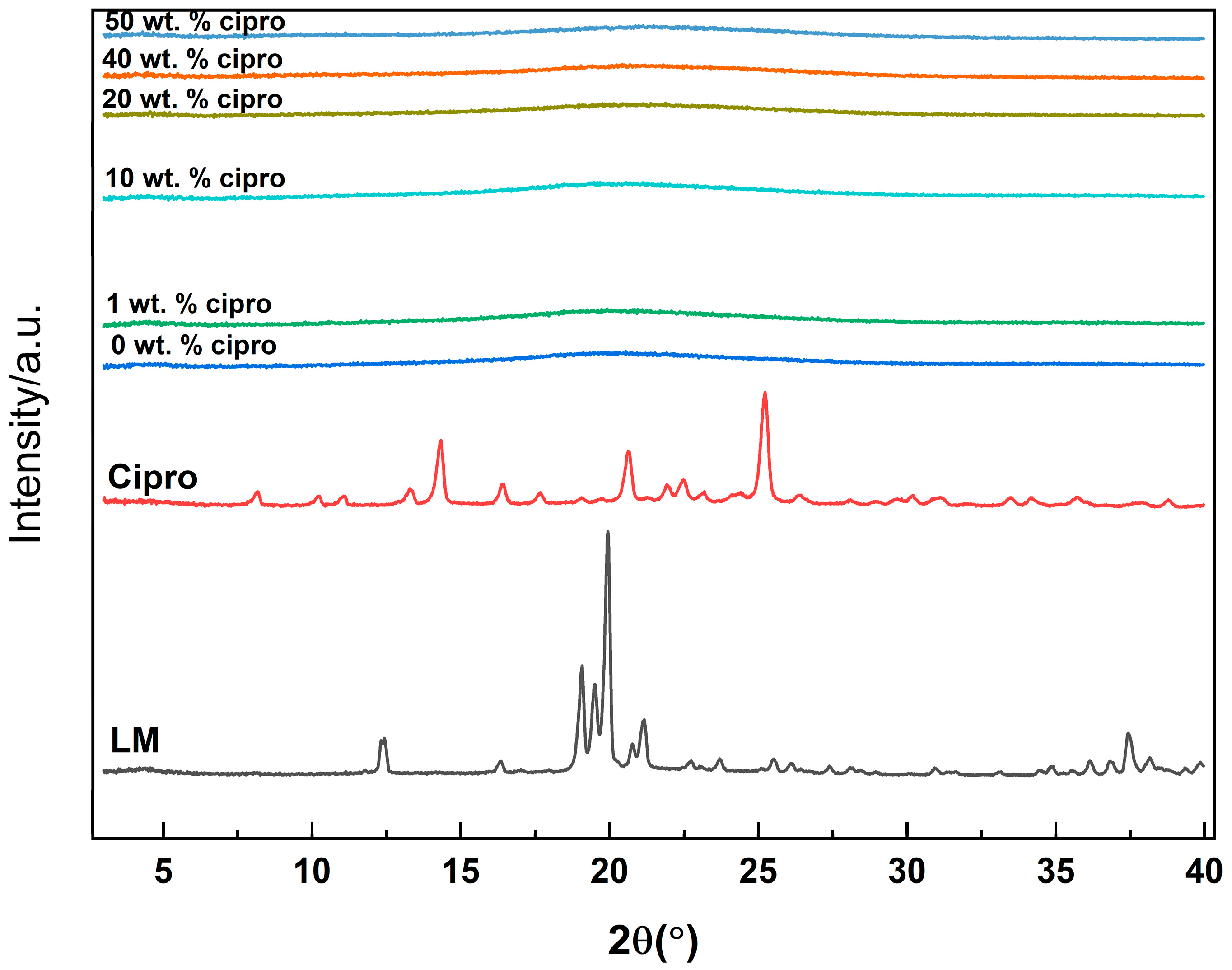
2.4.2. X-Ray Diffraction (XRD)
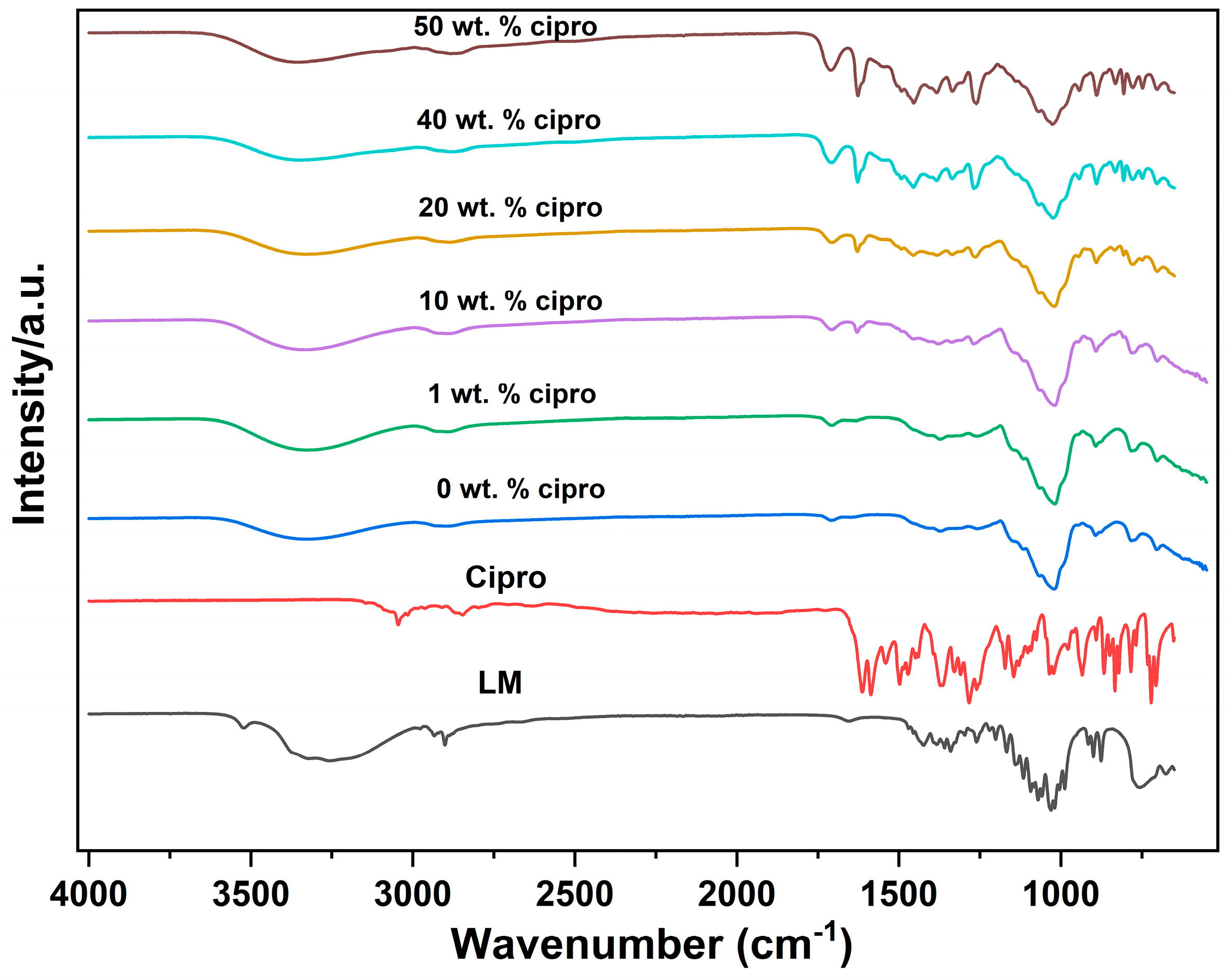
2.4.3. Fourier-Transform Infrared Spectroscopy (FTIR)
2.4.4. Thermogravimetric Analysis (TGA)
2.4.5. Differential Scanning Calorimetry (DSC)
2.4.6. Drug-Loading Determination
2.4.7. Drug Release Assays
2.4.8. Antibacterial Assays
Bacterial Culture
Antibacterial Efficacy of Cipro
Antibacterial Activity of Cipro-Loaded SD Particles
3. Results and Discussion
3.1. Formulation Optimization
3.2. Characterization
3.2.1. XRD
3.2.2. FTIR
3.2.3. Thermal Analysis
3.2.4. Drug Loading
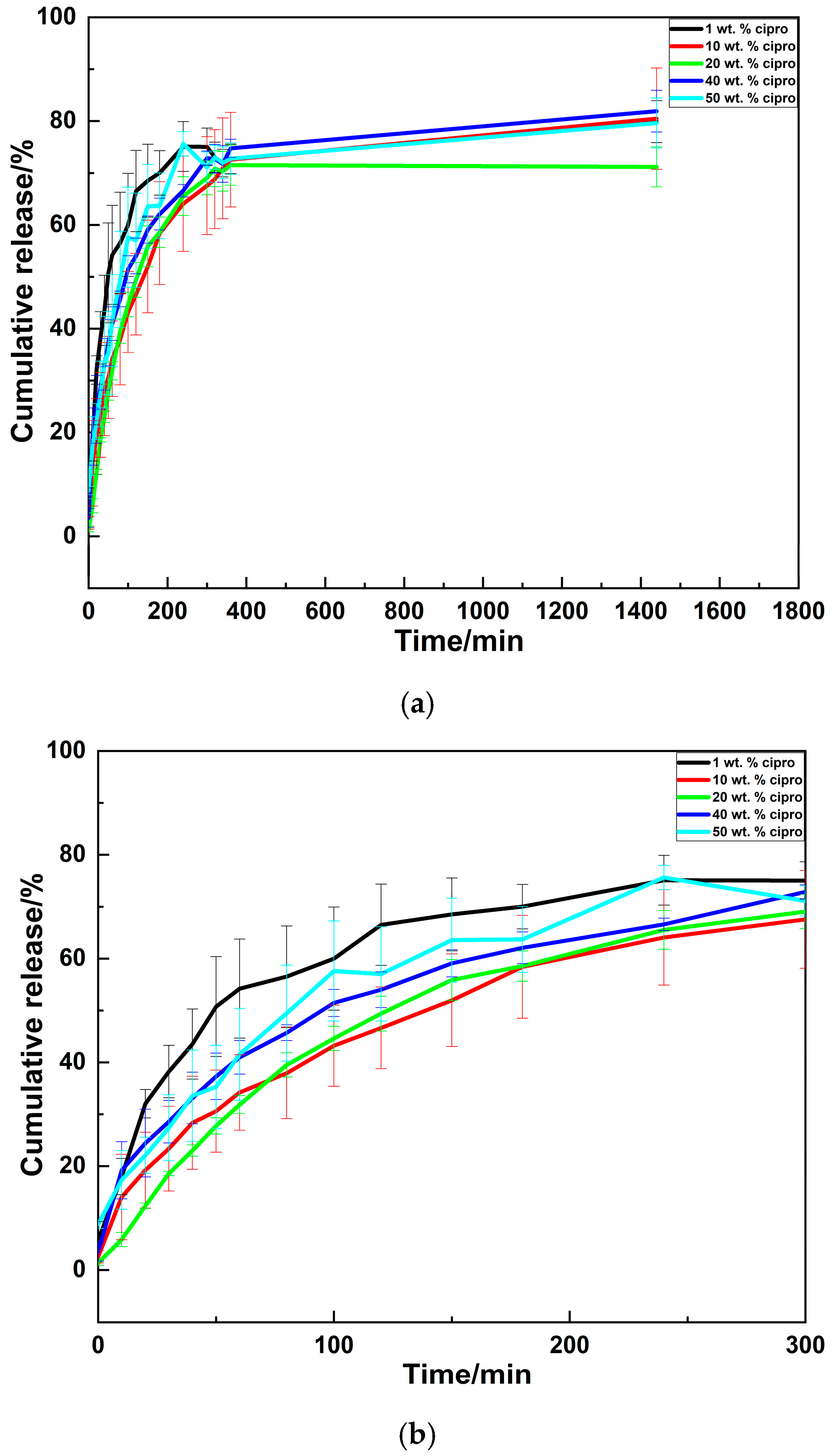
3.2.5. Drug Release Profiles
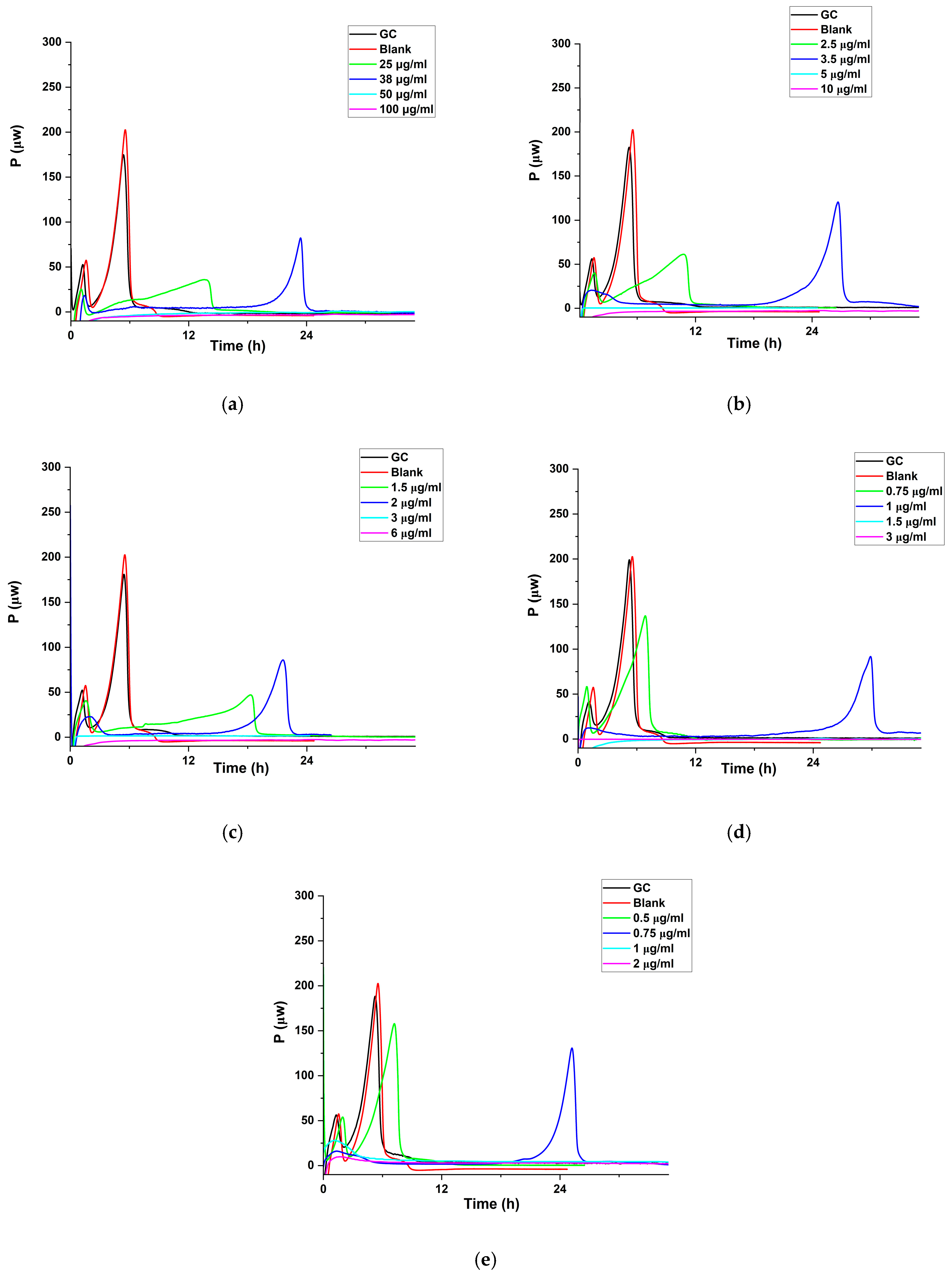
3.2.6. Antibacterial Activities
Studies with Cipro
Antibacterial Effect of Cipro-Loaded SD Particles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peres, M.A.; Macpherson, L.M.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreño, C.C.; Kearns, C. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Graydon, K.; Waterworth, C.; Miller, H.; Gunasekera, H. Global burden of hearing impairment and ear disease. J. Laryngol. Otol. 2019, 133, 18–25. [Google Scholar] [CrossRef]
- Roland, P.S.; Stroman, D.W. Microbiology of acute otitis externa. Laryngoscope 2002, 112, 1166–1177. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.V.; Barbosa, G.M.; Higashi, D.; di Micheli, G.; Rodrigues, P.H.; Simionato, M.R.L. Quantitative detection of Staphylococcus aureus, Enterococcus faecalis and Pseudomonas aeruginosa in human oral epithelial cells from subjects with periodontitis and periodontal health. J. Med. Microbiol. 2013, 62, 1592–1600. [Google Scholar] [CrossRef]
- Pollitt, E.J.; Szkuta, P.T.; Burns, N.; Foster, S.J. Staphylococcus aureus infection dynamics. PLoS Pathog. 2018, 14, e1007112. [Google Scholar] [CrossRef]
- Qin, S.; Xiao, W.; Zhou, C.; Pu, Q.; Deng, X.; Lan, L.; Liang, H.; Song, X.; Wu, M. Pseudomonas aeruginosa: Pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct. Target. Ther. 2022, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Hannley, M.T.; Denneny, J.C., III; Holzer, S.S. Use of ototopical antibiotics in treating 3 common ear diseases. Otolaryngol.—Head Neck Surg. 2000, 122, 934–940. [Google Scholar] [CrossRef]
- Ahmadi, H.; Ebrahimi, A.; Ahmadi, F. Antibiotic therapy in dentistry. Int. J. Dent. 2021, 2021, 6667624. [Google Scholar] [CrossRef]
- Tamma, P.D.; Cosgrove, S.E.; Maragakis, L.L. Combination therapy for treatment of infections with gram-negative bacteria. Clin. Microbiol. Rev. 2012, 25, 450–470. [Google Scholar] [CrossRef]
- Farhangi, M.; Kobarfard, F.; Mahboubi, A.; Vatanara, A.; Mortazavi, S.A. Preparation of an optimized ciprofloxacin-loaded chitosan nanomicelle with enhanced antibacterial activity. Drug Dev. Ind. Pharm. 2018, 44, 1273–1284. [Google Scholar] [CrossRef]
- Jannesari, M.; Varshosaz, J.; Morshed, M.; Zamani, M. Composite poly (vinyl alcohol)/poly (vinyl acetate) electrospun nanofibrous mats as a novel wound dressing matrix for controlled release of drugs. Int. J. Nanomed. 2011, 6, 993–1003. [Google Scholar]
- Charpentier, E.; Doudet, L.; Allart-Simon, I.; Colin, M.; Gangloff, S.C.; Gérard, S.; Reffuveille, F. Synergy between indoloquinolines and ciprofloxacin: An antibiofilm strategy against Pseudomonas aeruginosa. Antibiotics 2021, 10, 1205. [Google Scholar] [CrossRef] [PubMed]
- Chin, N.-X.; Neu, H.C. Ciprofloxacin, a quinolone carboxylic acid compound active against aerobic and anaerobic bacteria. Antimicrob. Agents Chemother. 1984, 25, 319–326. [Google Scholar] [CrossRef]
- Reeves, D.; Bywater, M.; Holt, H.; White, L. In-vitro studies with ciprofloxacin, a new 4-quinolone compound. J. Antimicrob. Chemother. 1984, 13, 333–346. [Google Scholar] [CrossRef]
- King, A.; Shannon, K.; Phillips, I. The in-vitro activity of ciprofloxacin compared with that of norfloxacin and nalidixic acid. J. Antimicrob. Chemother. 1984, 13, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Uhljar, L.É.; Kan, S.Y.; Radacsi, N.; Koutsos, V.; Szabó-Révész, P.; Ambrus, R. In vitro drug release, permeability, and structural test of ciprofloxacin-loaded nanofibers. Pharmaceutics 2021, 13, 556. [Google Scholar] [CrossRef]
- Breda, S.A.; Jimenez-Kairuz, A.F.; Manzo, R.H.; Olivera, M.E. Solubility behavior and biopharmaceutical classification of novel high-solubility ciprofloxacin and norfloxacin pharmaceutical derivatives. Int. J. Pharm. 2009, 371, 106–113. [Google Scholar] [CrossRef]
- Reynolds, D.; Kollef, M. The epidemiology and pathogenesis and treatment of Pseudomonas aeruginosa infections: An update. Drugs 2021, 81, 2117–2131. [Google Scholar] [CrossRef] [PubMed]
- Idrees, M.; Sawant, S.; Karodia, N.; Rahman, A. Staphylococcus aureus biofilm: Morphology, genetics, pathogenesis and treatment strategies. Int. J. Environ. Res. Public Health 2021, 18, 7602. [Google Scholar] [CrossRef]
- Ameri, M.; Maa, Y.-F. Spray drying of biopharmaceuticals: Stability and process considerations. Dry. Technol. 2006, 24, 763–768. [Google Scholar] [CrossRef]
- Santos, D.; Maurício, A.C.; Sencadas, V.; Santos, J.D.; Fernandes, M.H.; Gomes, P.S. Spray drying: An overview. Biomater.-Phys. Chem.-New Ed. 2018, 2, 9–35. [Google Scholar]
- Salama, A.H. Spray drying as an advantageous strategy for enhancing pharmaceuticals bioavailability. Drug Deliv. Transl. Res. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sosnik, A.; Seremeta, K.P. Advantages and challenges of the spray-drying technology for the production of pure drug particles and drug-loaded polymeric carriers. Adv. Colloid Interface Sci. 2015, 223, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Adi, H.; Young, P.M.; Chan, H.-K.; Stewart, P.; Agus, H.; Traini, D. Cospray dried antibiotics for dry powder lung delivery. J. Pharm. Sci. 2008, 97, 3356–3366. [Google Scholar] [CrossRef]
- Traini, D.; Young, P.M. Delivery of antibiotics to the respiratory tract: An update. Expert Opin. Drug Deliv. 2009, 6, 897–905. [Google Scholar] [CrossRef]
- Adi, H.; Young, P.M.; Chan, H.-K.; Salama, R.; Traini, D. Controlled release antibiotics for dry powder lung delivery. Drug Dev. Ind. Pharm. 2010, 36, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Healy, A.M.; Amaro, M.I.; Paluch, K.J.; Tajber, L. Dry powders for oral inhalation free of lactose carrier particles. Adv. Drug Deliv. Rev. 2014, 75, 32–52. [Google Scholar] [CrossRef] [PubMed]
- Silva-Júnior, A.A.; Scarpa, M.V.; Pestana, K.C.; Mercuri, L.P.; de Matos, J.R.; de Oliveira, A.G. Thermal analysis of biodegradable microparticles containing ciprofloxacin hydrochloride obtained by spray drying technique. Thermochim. Acta 2008, 467, 91–98. [Google Scholar] [CrossRef]
- Karimi, K.; Pallagi, E.; Szabó-Révész, P.; Csóka, I.; Ambrus, R. Development of a microparticle-based dry powder inhalation formulation of ciprofloxacin hydrochloride applying the quality by design approach. Drug Des. Dev. Ther. 2016, 10, 3331–3343. [Google Scholar] [CrossRef]
- Karimi, K.; Katona, G.; Csóka, I.; Ambrus, R. Physicochemical stability and aerosolization performance of dry powder inhalation system containing ciprofloxacin hydrochloride. J. Pharm. Biomed. Anal. 2018, 148, 73–79. [Google Scholar] [CrossRef]
- Beach, E.S.; Cui, Z.; Anastas, P.T. Green Chemistry: A design framework for sustainability. Energy Environ. Sci. 2009, 2, 1038–1049. [Google Scholar] [CrossRef]
- Razuc, M.; Piña, J.; Ramírez-Rigo, M.V. Optimization of ciprofloxacin hydrochloride spray-dried microparticles for pulmonary delivery using design of experiments. AAPS PharmSciTech 2018, 19, 3085–3096. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.M.; Paleco, R.; Traini, D.; Sencadas, V. Development of ciprofloxacin-loaded poly (vinyl alcohol) dry powder formulations for lung delivery. Int. J. Pharm. 2018, 547, 114–121. [Google Scholar] [CrossRef]
- Adi, H.; Young, P.M.; Chan, H.-K.; Agus, H.; Traini, D. Co-spray-dried mannitol–ciprofloxacin dry powder inhaler formulation for cystic fibrosis and chronic obstructive pulmonary disease. Eur. J. Pharm. Sci. 2010, 40, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Osman, R.; Kan, P.L.; Awad, G.; Mortada, N.; Abd-Elhameed, E.-S.; Alpar, O. Spray dried inhalable ciprofloxacin powder with improved aerosolisation and antimicrobial activity. Int. J. Pharm. 2013, 449, 44–58. [Google Scholar] [CrossRef]
- Silva, D.M.; Vyas, H.K.N.; Sanderson-Smith, M.L.; Sencadas, V. Development and optimization of ciprofloxacin-loaded gelatin microparticles by single-step spray-drying technique. Powder Technol. 2018, 330, 201–209. [Google Scholar] [CrossRef]
- Kaialy, W.; Alhalaweh, A.; Velaga, S.P.; Nokhodchi, A. Influence of lactose carrier particle size on the aerosol performance of budesonide from a dry powder inhaler. Powder Technol. 2012, 227, 74–85. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2012, 64, 163–174. [Google Scholar] [CrossRef]
- Elversson, J.; Millqvist-Fureby, A.; Alderborn, G.; Elofsson, U. Droplet and particle size relationship and shell thickness of inhalable lactose particles during spray drying. J. Pharm. Sci. 2003, 92, 900–910. [Google Scholar] [CrossRef]
- Mahmood, T.; Sarfraz, R.M.; Ismail, A.; Ali, M.; Khan, A.R. Pharmaceutical methods for enhancing the dissolution of poorly water-soluble drugs. ASSAY Drug Dev. Technol. 2023, 21, 65–79. [Google Scholar] [CrossRef]
- Singh, A.; Van den Mooter, G. Spray drying formulation of amorphous solid dispersions. Adv. Drug Deliv. Rev. 2016, 100, 27–50. [Google Scholar] [CrossRef]
- Edueng, K.; Bergström, C.A.; Gråsjö, J.; Mahlin, D. Long-term physical (in) stability of spray-dried amorphous drugs: Relationship with glass-forming ability and physicochemical properties. Pharmaceutics 2019, 11, 425. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Z.; Godakanda, V.U.; Chiu, Y.-J.; Angkawinitwong, U.; Patel, K.; Stapleton, P.G.; de Silva, R.M.; de Silva, K.N.; Zhu, L.-M. The effect of collection substrate on electrospun ciprofloxacin-loaded poly (vinylpyrrolidone) and ethyl cellulose nanofibers as potential wound dressing materials. Mater. Sci. Eng. C 2019, 104, 109917. [Google Scholar] [CrossRef] [PubMed]
- Topal, G.R.; Devrim, B.; Eryilmaz, M.; Bozkir, A. Design of ciprofloxacin-loaded nano-and microcomposite particles for dry powder inhaler formulations: Preparation, in vitro characterisation, and antimicrobial efficacy. J. Microencapsul. 2018, 35, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Gombás, Á.; Antal, I.; Szabó-Révész, P.; Marton, S.; Erõs, I. Quantitative determination of crystallinity of alpha-lactose monohydrate by near infrared spectroscopy (NIRS). Int. J. Pharm. 2003, 256, 25–32. [Google Scholar] [CrossRef]
- Madžarević, M.; Medarević, Đ.; Pavlović, S.; Ivković, B.; Đuriš, J.; Ibrić, S. Understanding the effect of energy density and formulation factors on the printability and characteristics of SLS Irbesartan tablets—Application of the decision tree model. Pharmaceutics 2021, 13, 1969. [Google Scholar] [CrossRef] [PubMed]
- Listiohadi, Y.; Hourigan, J.A.; Sleigh, R.W.; Steele, R.J. Thermal analysis of amorphous lactose and alpha-lactosemonohydrate. Dairy Sci. Technol. 2009, 89, 43–67. [Google Scholar] [CrossRef]
- Kumar, G.V.; Su, C.-H.; Velusamy, P. Ciprofloxacin loaded genipin cross-linked chitosan/heparin nanoparticles for drug delivery application. Mater. Lett. 2016, 180, 119–122. [Google Scholar] [CrossRef]
- Du, J.; El-Sherbiny, I.M.; Smyth, H.D. Swellable ciprofloxacin-loaded nano-in-micro hydrogel particles for local lung drug delivery. AAPS PharmSciTech 2014, 15, 1535–1544. [Google Scholar] [CrossRef]
- Schittny, A.; Philipp-Bauer, S.; Detampel, P.; Huwyler, J.; Puchkov, M. Mechanistic insights into effect of surfactants on oral bioavailability of amorphous solid dispersions. J. Control. Release 2020, 320, 214–225. [Google Scholar] [CrossRef]
- Nik Abd Rahman, N.F.N.; Zubairi, S.I.; Hashim, H.; Yaakob, H. Revolutionizing Spray Drying: An In-Depth Analysis of Surface Stickiness Trends and the Role of Physicochemical Innovations in Boosting Productivity. J. Food Qual. 2024, 2024, 8929464. [Google Scholar] [CrossRef]
- Shi, C.; Fang, Y.; Liu, Z.; Wang, Y.; Shen, L.; Zhao, L. Effect of Moisture Sorption and Lactose Type on Tablet Quality: A Hygroscopicity Study between Lactose Powder and Tablets. Mol. Pharm. 2024, 22, 544–557. [Google Scholar] [CrossRef]
- Sundar, P.S.; Chowdhury, C.; Kamarthi, S. Evaluation of human ear anatomy and functionality by axiomatic design. Biomimetics 2021, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Labiris, N.R.; Dolovich, M.B. Pulmonary drug delivery. Part II: The role of inhalant delivery devices and drug formulations in therapeutic effectiveness of aerosolized medications. Br. J. Clin. Pharmacol. 2003, 56, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Ghumman, S.A.; Bashir, S.; Noreen, S.; Khan, A.M.; Riffat, S.; Abbas, M. Polymeric microspheres of okra mucilage and alginate for the controlled release of oxcarbazepine: In vitro & in vivo evaluation. Int. J. Biol. Macromol. 2018, 111, 1156–1165. [Google Scholar]
- Costa, P.; Lobo, J.M.S. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Ahuja, N.; Katare, O.P.; Singh, B. Studies on dissolution enhancement and mathematical modeling of drug release of a poorly water-soluble drug using water-soluble carriers. Eur. J. Pharm. Biopharm. 2007, 65, 26–38. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Yuan, Z.; Han, H.; Li, T.; Li, L.; Guo, X. Chitosan cross-linked poly (acrylic acid) hydrogels: Drug release control and mechanism. Colloids Surf. B Biointerfaces 2017, 152, 252–259. [Google Scholar] [CrossRef]
- Laracuente, M.-L.; Marina, H.Y.; McHugh, K.J. Zero-order drug delivery: State of the art and future prospects. J. Control. Release 2020, 327, 834–856. [Google Scholar] [CrossRef]
- Seremeta, K.P.; Chiappetta, D.A.; Sosnik, A. Poly (ɛ-caprolactone), Eudragit® RS 100 and poly (ɛ-caprolactone)/Eudragit® RS 100 blend submicron particles for the sustained release of the antiretroviral efavirenz. Colloids Surf. B Biointerfaces 2013, 102, 441–449. [Google Scholar] [CrossRef]
- Fu, Y.; Kao, W.J. Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opin. Drug Deliv. 2010, 7, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Klose, D.; Siepmann, F.; Elkharraz, K.; Krenzlin, S.; Siepmann, J. How porosity and size affect the drug release mechanisms from PLGA-based microparticles. Int. J. Pharm. 2006, 314, 198–206. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable controlled-release polymers and polymeric nanoparticles: Mechanisms of controlling drug release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug solubility: Importance and enhancement techniques. Int. Sch. Res. Not. 2012, 2012, 195727. [Google Scholar] [CrossRef] [PubMed]
- von Ah, U.; Wirz, D.; Daniels, A. Isothermal micro calorimetry–a new method for MIC determinations: Results for 12 antibiotics and reference strains of E. coli and S. aureus. BMC Microbiol. 2009, 9, 106. [Google Scholar] [CrossRef]
- Tellapragada, C.; Hasan, B.; Antonelli, A.; Maruri, A.; de Vogel, C.; Gijón, D.; Coppi, M.; Verbon, A.; van Wamel, W.; Rossolini, G. Isothermal microcalorimetry minimal inhibitory concentration testing in extensively drug resistant Gram-negative bacilli: A multicentre study. Clin. Microbiol. Infect. 2020, 26, 1413. e1411–1413. e1417. [Google Scholar] [CrossRef] [PubMed]
- Fricke, C.; Xu, J.; Jiang, F.L.; Liu, Y.; Harms, H.; Maskow, T. Rapid culture-based detection of Legionella pneumophila using isothermal microcalorimetry with an improved evaluation method. Microb. Biotechnol. 2020, 13, 1262–1272. [Google Scholar] [CrossRef]
- Antonelli, A.; Coppi, M.; Tellapragada, C.; Hasan, B.; Maruri, A.; Gijón, D.; Morecchiato, F.; de Vogel, C.; Verbon, A.; van Wamel, W. Isothermal microcalorimetry vs. checkerboard assay to evaluate in-vitro synergism of meropenem–amikacin and meropenem–colistin combinations against multi-drug-resistant Gram-negative pathogens. Int. J. Antimicrob. Agents 2022, 60, 106668. [Google Scholar] [CrossRef]
- Butini, M.E.; Gonzalez Moreno, M.; Czuban, M.; Koliszak, A.; Tkhilaishvili, T.; Trampuz, A.; Di Luca, M. Real-time antimicrobial susceptibility assay of planktonic and biofilm bacteria by isothermal microcalorimetry. In Advances in Microbiology, Infectious Diseases and Public Health: Volume 13; Springer: Berlin/Heidelberg, Germany, 2018; pp. 61–77. [Google Scholar]
- Cirnski, K.; Coetzee, J.; Herrmann, J.; Müller, R. Metabolic profiling to determine bactericidal or bacteriostatic effects of new natural products using isothermal microcalorimetry. J. Vis. Exp. (JoVE) 2020, 164, e61703. [Google Scholar]
- Sultan, A.R.; Tavakol, M.; Lemmens-den Toom, N.A.; Croughs, P.D.; Verkaik, N.J.; Verbon, A.; van Wamel, W.J. Real time monitoring of Staphylococcus aureus biofilm sensitivity towards antibiotics with isothermal microcalorimetry. PLoS ONE 2022, 17, e0260272. [Google Scholar] [CrossRef]
- Hossain, T.J. Methods for screening and evaluation of antimicrobial activity: A review of protocols, advantages, and limitations. Eur. J. Microbiol. Immunol. 2024, 14, 97–115. [Google Scholar] [CrossRef] [PubMed]
- Gaisford, S.; Beezer, A.E.; Bishop, A.H.; Walker, M.; Parsons, D. An in vitro method for the quantitative determination of the antimicrobial efficacy of silver-containing wound dressings. Int. J. Pharm. 2009, 366, 111–116. [Google Scholar] [CrossRef]
- Kathiravan, T.; Marykala, J.; Sundaramanickam, A.; Kumaresan, S.; Balasubramanian, T. Studies on nutritional requirements of Pseudomonas aeruginosa for lipase production. Adv Appl Sci Res 2012, 3, 591–598. [Google Scholar]
- Bjarnsholt, T.; Alhede, M.; Jensen, P.Ø.; Nielsen, A.K.; Johansen, H.K.; Homøe, P.; Høiby, N.; Givskov, M.; Kirketerp-Møller, K. Antibiofilm properties of acetic acid. Adv. Wound Care 2015, 4, 363–372. [Google Scholar] [CrossRef]
- Arauzo, B.; Lobera, M.P.; Monzon, A.; Santamaria, J. Dry powder formulation for pulmonary infections: Ciprofloxacin loaded in chitosan sub-micron particles generated by electrospray. Carbohydr. Polym. 2021, 273, 118543. [Google Scholar] [CrossRef] [PubMed]
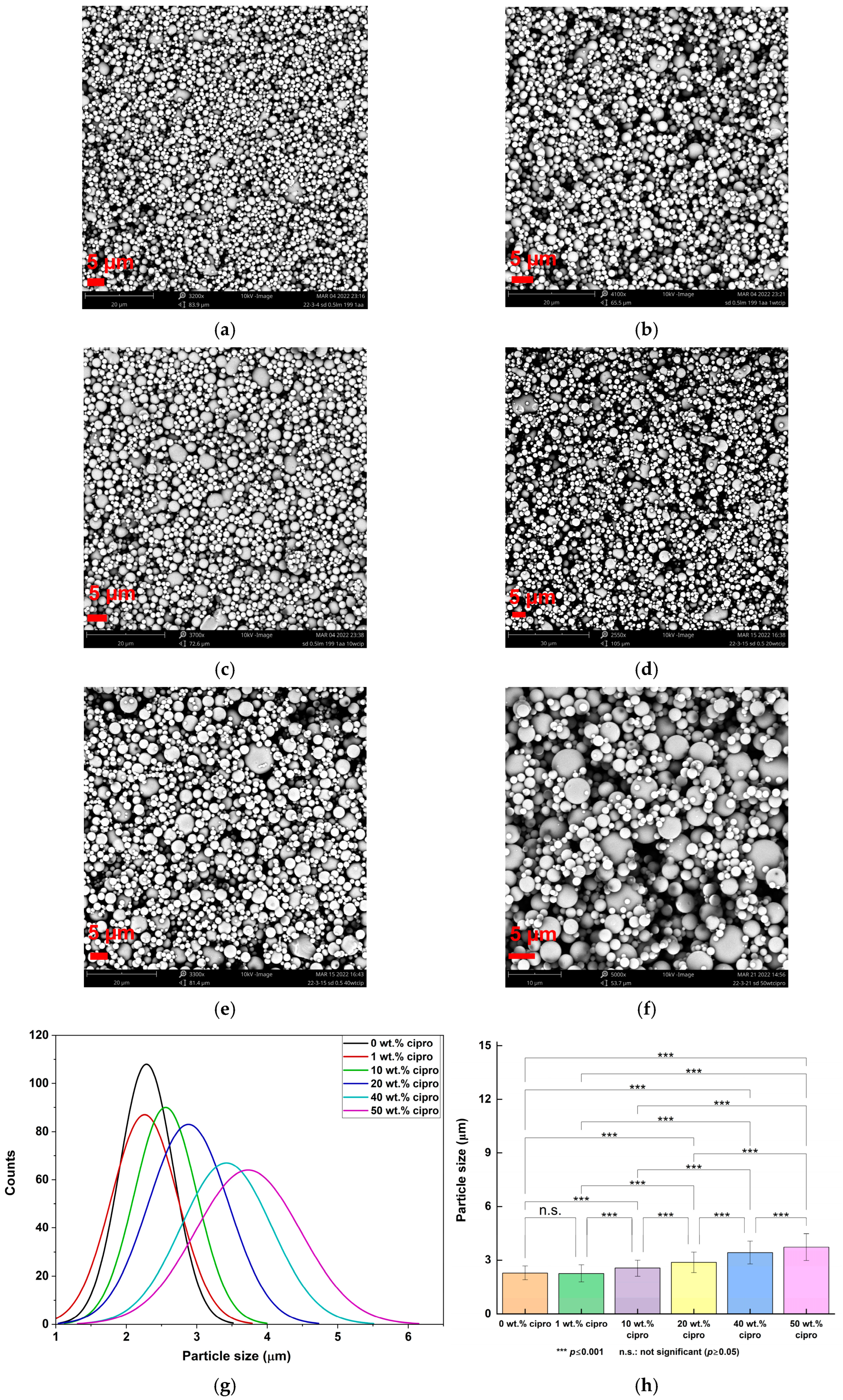

| Formulation | Mean Particle Size (μm) |
|---|---|
| 0.5% w/v LM, water/AA = 199:1 v/v | 2.29 ± 0.38 |
| 1 wt. % cipro | 2.26 ± 0.47 |
| 10 wt. % cipro | 2.56 ± 0.44 |
| 20 wt. % cipro | 2.88 ± 0.57 |
| 40 wt. % cipro | 3.42 ± 0.64 |
| 50 wt. % cipro | 3.73 ± 0.74 |
| Formulation | Drug Loading (%) | EE (%) |
|---|---|---|
| 1 wt. % cipro | 1.1 ± 0.0 | 107.0 ± 1.9 |
| 10 wt. % cipro | 9.9 ± 0.3 | 98.6 ± 2.6 |
| 20 wt. % cipro | 19.4 ± 0.2 | 96.9 ± 0.9 |
| 40 wt. % cipro | 42.1 ± 0.5 | 105.2 ± 1.2 |
| 50 wt. % cipro | 52.9 ± 1.6 | 105.7 ± 3.2 |
| Formulations | n | k (min−1) | R2 |
|---|---|---|---|
| 1 wt. % cipro | 0.603 | 0.048 | 0.981 |
| 10 wt. % cipro | 0.499 | 0.044 | 0.996 |
| 20 wt. % cipro | 0.945 | 0.007 | 0.994 |
| 40 wt. % cipro | 0.423 | 0.070 | 0.990 |
| 50 wt. % cipro | 0.485 | 0.054 | 0.982 |
| Formulation | P. aeruginosa | S. aureus | ||
|---|---|---|---|---|
| MHIC of SD (μg particles/mL) | Normalized MHIC (μg Cipro/mL) | MHIC of SD (μg particles/mL) | Normalized MHIC (μg Cipro/mL) | |
| 1 wt. % cipro | 50 | 0.54 | 80 | 0.86 |
| 10 wt. % cipro | 5 | 0.49 | 9 | 0.89 |
| 20 wt. % cipro | 3 | 0.58 | 5 | 0.97 |
| 40 wt. % cipro | 1.5 | 0.63 | 2 | 0.84 |
| 50 wt. % cipro | 1 | 0.53 | 1.6 | 0.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Gaisford, S.; Williams, G.R. Ciprofloxacin-Loaded Spray-Dried Lactose Particles: Formulation Optimization and Antibacterial Efficacy. Pharmaceutics 2025, 17, 392. https://doi.org/10.3390/pharmaceutics17030392
Liu S, Gaisford S, Williams GR. Ciprofloxacin-Loaded Spray-Dried Lactose Particles: Formulation Optimization and Antibacterial Efficacy. Pharmaceutics. 2025; 17(3):392. https://doi.org/10.3390/pharmaceutics17030392
Chicago/Turabian StyleLiu, Sai, Simon Gaisford, and Gareth R. Williams. 2025. "Ciprofloxacin-Loaded Spray-Dried Lactose Particles: Formulation Optimization and Antibacterial Efficacy" Pharmaceutics 17, no. 3: 392. https://doi.org/10.3390/pharmaceutics17030392
APA StyleLiu, S., Gaisford, S., & Williams, G. R. (2025). Ciprofloxacin-Loaded Spray-Dried Lactose Particles: Formulation Optimization and Antibacterial Efficacy. Pharmaceutics, 17(3), 392. https://doi.org/10.3390/pharmaceutics17030392









