Altered Pharmacokinetics of Ropivacaine in Patients Undergoing Laparoscopic Major Hepatectomy
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design and Ethics Statement
2.2. Participants
2.3. Ultrasound-Guided BD-TAP Block
2.4. Collection and Processing of Blood Samples
2.5. Liver Volumetry
2.6. Statistics
3. Results
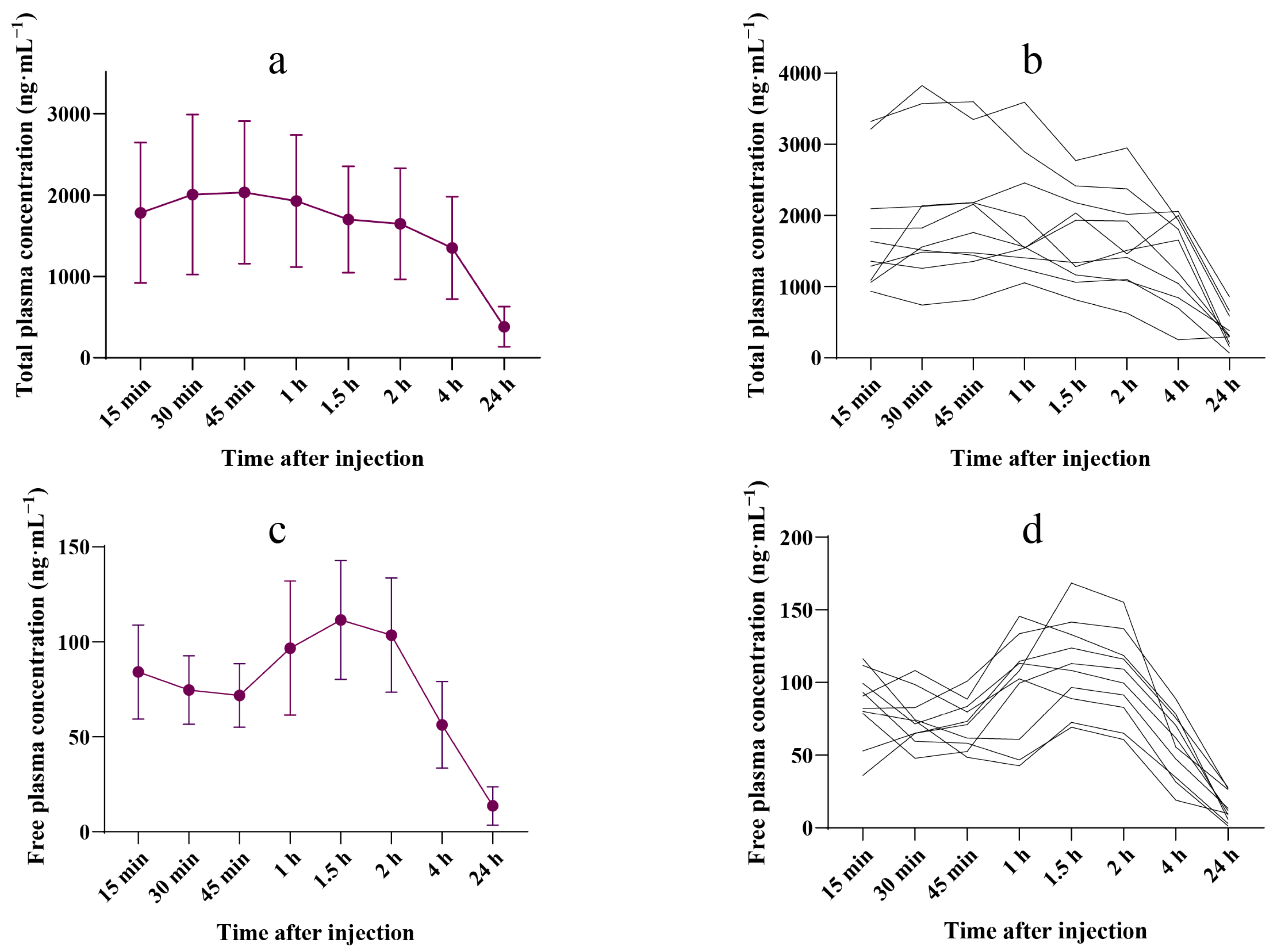
3.1. Plasma Ropivacaine Concentration
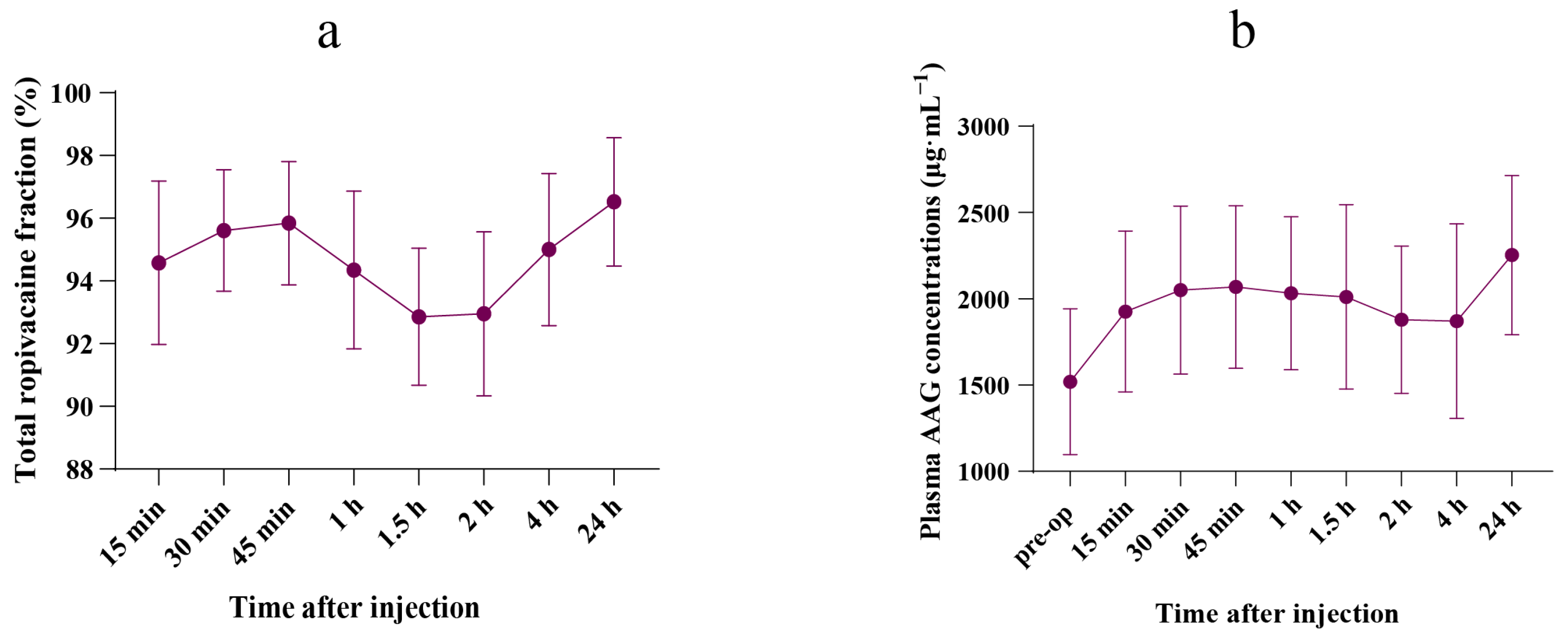
3.2. Free Ropivacaine Fraction
3.3. Plasma AAG Concentration
3.4. Liver Volumetry
3.5. Linear Regression Analysis
4. Discussion
4.1. Plasma Ropivacaine Concentrations
4.2. Is the Toxicity Threshold of Total Ropivacaine Concentration of Any Guiding Value?
4.3. Do We Need to Adjust the Dosage of Ropivacaine in an Operative Setting? Especially in Patients Undergoing Hepatectomy?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elsharydah, A.; De La Cruz, R.; Horani, S.M.; Xiao, C.Y. Utilization of Truncal Fascial Plane Nerve Blocks for Chronic Pain Management: A Narrative Review. Curr. Pain Headache Rep. 2023, 27, 149–155. [Google Scholar] [CrossRef]
- Leone, S.; Di Cianni, S.; Casati, A.; Fanelli, G. Pharmacology, toxicology, and clinical use of new long acting local anesthetics, ropivacaine and levobupivacaine. Acta Biomed. 2008, 79, 92–105. [Google Scholar]
- Jokinen, M.J.; Neuvonen, P.J.; Lindgren, L.; Höckerstedt, K.; Sjövall, J.; Breuer, O.; Askemark, Y.; Ahonen, J.; Olkkola, K.T. Pharmacokinetics of ropivacaine in patients with chronic end-stage liver disease. Anesthesiology 2007, 106, 43–55. [Google Scholar] [CrossRef]
- Hayes, P.C. Liver disease and drug disposition. Br. J. Anaesth. 1992, 68, 459–461. [Google Scholar] [CrossRef]
- Kumar, S.K.; Rao, V.; Morris, R.G.; Watts, R.W.; Westley, I.S. Ropivacaine (total and unbound) and AGP concentrations after transversus abdominis plane block for analgesia after abdominal surgery. Ther. Drug Monit. 2014, 36, 759–764. [Google Scholar] [CrossRef]
- Fournier, T.; Medjoubi-N, N.; Porquet, D. Alpha-1-acid glycoprotein. Biochim. Biophys. Acta 2000, 1482, 157–171. [Google Scholar] [CrossRef]
- Israili, Z.H.; Dayton, P.G. Human alpha-1-glycoprotein and its interactions with drugs. Drug Metab. Rev. 2001, 33, 161–235. [Google Scholar] [CrossRef]
- Børglum, J.; Maschmann, C.; Belhage, B.; Jensen, K. Ultrasound-guided bilateral dual transversus abdominis plane block: A new four-point approach. Acta Anaesthesiol. Scand. 2011, 55, 658–663. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, T.; Zhou, H.; Fei, Y.; Yu, X. The Safety and Efficacy of Ultrasound-Guided Bilateral Dual Transversus Abdominis Plane (BD-TAP) Block in ERAS Program of Laparoscopic Hepatectomy: A Prospective, Randomized, Controlled, Blinded, Clinical Study. Drug Des. Devel Ther. 2020, 14, 2889–2898. [Google Scholar] [CrossRef]
- Ollier, E.; Heritier, F.; Bonnet, C.; Hodin, S.; Beauchesne, B.; Molliex, S.; Delavenne, X. Population pharmacokinetic model of free and total ropivacaine after transversus abdominis plane nerve block in patients undergoing liver resection. Br. J. Clin. Pharmacol. 2015, 80, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Lamy, E.; Fall, F.; Boigne, L.; Gromov, K.; Fabresse, N.; Grassin-Delyle, S. Validation according to European and American regulatory agencies guidelines of an LC-MS/MS method for the quantification of free and total ropivacaine in human plasma. Clin. Chem. Lab. Med. 2020, 58, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Børglum, J.; Jensen, K.; Christensen, A.F.; Hoegberg, L.C.; Johansen, S.S.; Lönnqvist, P.-A.; Jansen, T. Distribution patterns, dermatomal anesthesia, and ropivacaine serum concentrations after bilateral dual transversus abdominis plane block. Reg. Anesth. Pain. Med. 2012, 37, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.; Baek, N.; Jensen, J.T.; Brglum, J. Bilateral dual transversus abdominis plane block providing surgical anaesthesia for abdominal wall surgery. Anaesthesia 2013, 68, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.Y.; Lu, Y.J.; Lin, Y.C.; Wei, P.L.; Kang, Y.N. Transversus abdominis plane block for laparoscopic colorectal surgery: A meta-analysis of randomised controlled trials. Int. J. Surg. 2022, 104, 106825. [Google Scholar] [CrossRef]
- Lee, A.; Fagan, D.; Lamont, M.; Tucker, G.T.; Halldin, M.; Scott, D.B. Disposition kinetics of ropivacaine in humans. Anesth. Analg. 1989, 69, 736–738. [Google Scholar] [CrossRef]
- Scott, D.B.; Lee, A.; Fagan, D.; Bowler, G.M.; Bloomfield, P.; Lundh, R. Acute toxicity of ropivacaine compared with that of bupivacaine. Anesth. Analg. 1989, 69, 563–569. [Google Scholar] [CrossRef]
- Knudsen, K.; Beckman Suurküla, M.; Blomberg, S.; Sjövall, J.; Edvardsson, N. Central nervous and cardiovascular effects of i.v. infusions of ropivacaine, bupivacaine and placebo in volunteers. Br. J. Anaesth. 1997, 78, 507–514. [Google Scholar] [CrossRef]
- Wulf, H.; Worthmann, F.; Behnke, H.; Böhle, A.S. Pharmacokinetics and pharmacodynamics of ropivacaine 2 mg/mL, 5 mg/mL, or 7.5 mg/mL after ilioinguinal blockade for inguinal hernia repair in adults. Anesth. Analg. 1999, 89, 1471–1474. [Google Scholar]
- Griffiths, J.D.; Barron, F.A.; Grant, S.; Bjorksten, A.R.; Hebbard, P.; Royse, C.F. Plasma ropivacaine concentrations after ultrasound-guided transversus abdominis plane block. Br. J. Anaesth. 2010, 105, 853–856. [Google Scholar] [CrossRef]
- Griffiths, J.D.; Le, N.V.; Grant, S.; Bjorksten, A.; Hebbard, P.; Royse, C. Symptomatic local anaesthetic toxicity and plasma ropivacaine concentrations after transversus abdominis plane block for Caesarean section. Br. J. Anaesth. 2013, 110, 996–1000. [Google Scholar] [CrossRef]
- Yokogawa, K.; Shimomura, S.; Ishizaki, J.; Shimada, T.; Fukuwal, C.; Kawada, M.; Tsubokawa, T.; Yamamoto, K.; Miyamoto, K.-I. Involvement of alpha1-acid glycoprotein in inter-individual variation of disposition kinetics of ropivacaine following epidural infusion in off-pump coronary artery bypass grafting. J. Pharm. Pharmacol. 2007, 59, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Ceciliani, F.; Pocacqua, V. The acute phase protein alpha1-acid glycoprotein: A model for altered glycosylation during diseases. Curr. Protein Pept. Sci. 2007, 8, 91–108. [Google Scholar] [CrossRef] [PubMed]
- Schönfeld, D.L.; Ravelli, R.B.; Mueller, U.; Skerra, A. The 1.8-A crystal structure of alpha1-acid glycoprotein (Orosomucoid) solved by UV RIP reveals the broad drug-binding activity of this human plasma lipocalin. J. Mol. Biol. 2008, 384, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Ung, T. Effect of alpha-1-acid glycoprotein binding on pharmacokinetics and pharmacodynamics. Curr. Drug Metab. 2013, 14, 226–238. [Google Scholar]

| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Mean (SD)/n (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| General background | |||||||||||
| Female (yes/no) | yes | yes | yes | no | yes | no | yes | no | yes | yes | 7 (70) |
| Age (year) | 52 | 56 | 71 | 61 | 62 | 35 | 58 | 73 | 31 | 67 | 56.6 (14.0) |
| Height (cm) | 162 | 152 | 150 | 171 | 160 | 182 | 161 | 181 | 163 | 161 | 164.3 (10.7) |
| Weight (kg) | 70 | 55 | 52 | 67 | 65 | 71 | 55 | 78 | 50 | 62 | 62.5 (9.2) |
| BMI (kg·m−2) | 26 | 23 | 23 | 22 | 25 | 21 | 21 | 23 | 18 | 23 | 22.5 (2.2) |
| Ropivacaine (mg) | 210 | 165 | 156 | 201 | 195 | 213 | 165 | 234 | 150 | 186 | 187.5 (27.8) |
| Ropivacaine (%) | 0.35 | 0.27 | 0.26 | 0.33 | 0.32 | 0.35 | 0.27 | 0.39 | 0.25 | 0.31 | 0.31 (0.04) |
| Blood examination | |||||||||||
| PT (s) | 12.4 | 12.2 | 13.0 | 13.5 | 12.2 | 13.0 | 12.6 | 13.5 | 13.3 | 13.1 | 12.8 (0.5) |
| ALT (U·L−1) | 13 | 49 | 7 | 15 | 23 | 43 | 10 | 22 | 10 | 45 | 23.7 (16) |
| TB (μmol·L−1) | 14.5 | 12.3 | 8.3 | 17.3 | 10.7 | 11.7 | 12.2 | 12.3 | 11 | 21.3 | 13.1 (3.7) |
| Alb (g·L−1) | 47.9 | 48.4 | 33.2 * | 40.5 | 44.3 | 46.7 | 36.6 * | 40.3 | 44.5 | 44.8 | 42.7 (4.9) |
| Liver functional reserve | |||||||||||
| liver cirrhosis (yes/no) | no | no | no | yes | yes | yes | no | no | no | no | 3 (30) |
| Child–Pugh score | A | A | A | A | A | A | A | A | A | A | |
| Surgical procedure | |||||||||||
| Surgical indications | 1 | 2 | 3 | 3 | 3 | 3 | 2 | 3 | 2 | 3 | |
| Operative time (min) | 270 | 135 | 270 | 350 | 210 | 225 | 165 | 200 | 310 | 300 | 243.5 (68.0) |
| Blood loss (mL) | 500 | 50 | 300 | 1000 | 200 | 200 | 200 | 200 | 200 | 300 | 315.0 (266.7) |
| Blood transfusion (U) | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Parameter | Unit | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Mean (SD) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total plasma ropivacaine | ||||||||||||
| AUC(0–t) | μg·L−1·h | 7960.2 | 24,639.6 | 36,138.1 | 32,950.3 | 16,734.9 | 37,106.9 | 11,903.6 | 21,654.8 | 29,790.6 | 18,525.9 | 23,740.5 (10,134.3) |
| AUC(0–∞) | μg·L−1·h | 7960.3 | 26,211.5 | 44,336.8 | 45,596.7 | 17,268.6 | 57,505.0 | 12,468.1 | 25,471.0 | 31,608.6 | 22,918.0 | 29,134.5 (15,778.5) |
| MRT(0–t) | h | 10.6 | 4.72 | 6.57 | 7.64 | 8.00 | 8.22 | 4.37 | 6.24 | 4.63 | 6.67 | 6.77 (1.95) |
| MRT(0–∞) | h | 10.6 | 6.48 | 12.4 | 17.5 | 8.72 | 22.2 | 5.62 | 10.7 | 6.26 | 12.8 | 11.3 (5.3) |
| t1/2z | h | 1.50 | 7.03 | 9.72 | 13.3 | 5.48 | 16.4 | 5.62 | 8.47 | 6.28 | 10.1 | 8.39 (4.25) |
| Tmax | h | 1.00 | 0.750 | 0.500 | 1.50 | 0.750 | 1.00 | 0.250 | 0.750 | 0.750 | 0.500 | 0.775 (0.343) |
| Vz | L·kg−1 | 0.817 | 1.16 | 0.949 | 1.26 | 1.38 | 1.24 | 1.95 | 1.44 | 0.860 | 1.92 | 1.30 (0.40) |
| CLz | L·h−1·kg−1 | 0.377 | 0.114 | 0.068 | 0.066 | 0.174 | 0.052 | 0.241 | 0.118 | 0.095 | 0.131 | 0.144 (0.099) |
| Cmax | μg·L−1 | 1055.0 | 2178.5 | 3825.0 | 2034.0 | 1761.8 | 2457.2 | 1635.0 | 2157.8 | 3597.9 | 1485.2 | 2218.7 (883.2) |
| Free plasma ropivacaine | ||||||||||||
| AUC(0–t) | μg·L−1·h | 489.9 | 597.0 | 904.9 | 1240.6 | 1084.6 | 1404.1 | 618.5 | 1142.9 | 1262.5 | 1606.4 | 1035.1 (372.7) |
| AUC(0–∞) | μg·L−1·h | 606.9 | 619.2 | 1059.7 | 1564.4 | 1206.8 | 1829.6 | 625.4 | 1223.9 | 1306.4 | 1974.8 | 1201.7 (492.2) |
| MRT(0–t) | h | 7.07 | 4.23 | 6.27 | 7.54 | 5.52 | 7.46 | 3.31 | 4.99 | 4.26 | 6.81 | 5.75 (1.51) |
| MRT(0–∞) | h | 12.6 | 5.20 | 10.5 | 13.5 | 8.43 | 14.9 | 3.59 | 6.84 | 5.16 | 12.6 | 9.33 (4.01) |
| t1/2z | h | 8.20 | 4.98 | 7.95 | 8.50 | 7.12 | 10.6 | 3.68 | 6.16 | 5.07 | 9.42 | 7.16 (2.17) |
| Tmax | h | 0.25 | 0.25 | 0.25 | 1.50 | 1.00 | 1.50 | 0.25 | 1.50 | 1.00 | 1.50 | 0.90 (0.60) |
| Vz | L·kg−1 | 58.5 | 34.8 | 32.5 | 23.5 | 25.5 | 25.0 | 25.5 | 21.8 | 16.8 | 20.7 | 28.5 (11.8) |
| CLz | L·h−1·kg−1 | 4.94 | 4.84 | 2.83 | 1.92 | 2.49 | 1.64 | 4.80 | 2.45 | 2.30 | 1.52 | 2.97 (1.36) |
| Cmax | μg·L−1 | 93.3 | 80.1 | 116.4 | 168.4 | 113.2 | 123.8 | 111.8 | 113.1 | 145.6 | 141.5 | 120.7 (25.7) |
| TLV (mL) | DLV (mL) | TFLV (mL) | RLV (mL) | CFLV (mL) | CFLV/TFLV (%) | PTRC (ng·mL−1) | PFRC (ng·mL−1) | |
|---|---|---|---|---|---|---|---|---|
| 1 | 1882.9 | 535.8 | 1347.1 | 1263.2 | 83.9 | 6.2 | 1055.0 | 93.3 |
| 2 | 871.4 | 5.1 | 866.3 | 822.7 | 43.6 | 5.0 | 2178.5 | 80.1 |
| 3 | 1074.5 | 67.6 | 1006.9 | 653.2 | 353.7 | 35.1 | 3825.0 | 116.4 |
| 4 | 1547.2 | 357.7 | 1189.5 | 886.7 | 302.8 | 25.5 | 2034.0 | 168.4 |
| 5 | 1339.4 | 171.9 | 1167.5 | 1127.9 | 39.6 | 3.4 | 1761.8 | 113.2 |
| 6 | 1136.3 | 228.4 | 907.9 | 675.0 | 232.9 | 25.7 | 2457.2 | 123.8 |
| 7 | 1010.3 | 20.5 | 989.8 | 805.1 | 184.7 | 18.7 | 1635.0 | 111.8 |
| 8 | 1443.3 | 114.3 | 1329.0 | 1273.2 | 55.8 | 4.2 | 2157.8 | 113.1 |
| 9 | 1247.6 | 14.7 | 1232.9 | 756.9 | 476.0 | 38.6 | 3597.9 | 145.6 |
| 10 | 982.4 | 21.0 | 961.4 | 694.7 | 266.7 | 27.7 | 1485.2 | 141.5 |
| Mean (SD) | 1253.5 (307.4) | 153.7 (176.1) | 1099.8 (174.9) | 895.9 (238.6) | 204.0 (149.2) | 19.0 (13.5) | 2218.7 (883.2) | 120.7 (25.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Lv, H.; Shen, J.; Ai, Z.; Liu, M.; Liu, X.; Liu, T.; Shen, B.; Yu, H.; Yu, X. Altered Pharmacokinetics of Ropivacaine in Patients Undergoing Laparoscopic Major Hepatectomy. Pharmaceutics 2025, 17, 386. https://doi.org/10.3390/pharmaceutics17030386
Zhang J, Lv H, Shen J, Ai Z, Liu M, Liu X, Liu T, Shen B, Yu H, Yu X. Altered Pharmacokinetics of Ropivacaine in Patients Undergoing Laparoscopic Major Hepatectomy. Pharmaceutics. 2025; 17(3):386. https://doi.org/10.3390/pharmaceutics17030386
Chicago/Turabian StyleZhang, Jun, Hongyuan Lv, Jiliang Shen, Zhichao Ai, Minjun Liu, Xiaorui Liu, Tieshuai Liu, Bo Shen, Hong Yu, and Xin Yu. 2025. "Altered Pharmacokinetics of Ropivacaine in Patients Undergoing Laparoscopic Major Hepatectomy" Pharmaceutics 17, no. 3: 386. https://doi.org/10.3390/pharmaceutics17030386
APA StyleZhang, J., Lv, H., Shen, J., Ai, Z., Liu, M., Liu, X., Liu, T., Shen, B., Yu, H., & Yu, X. (2025). Altered Pharmacokinetics of Ropivacaine in Patients Undergoing Laparoscopic Major Hepatectomy. Pharmaceutics, 17(3), 386. https://doi.org/10.3390/pharmaceutics17030386





