Oral Bioavailability Enhancement of Anti-Cancer Drugs Through Lipid Polymer Hybrid Nanoparticles
Abstract
1. Introduction
2. Anti-Cancer Drugs with Limited Bioavailability Issues
2.1. Factors Influencing Oral Bioavailability of Anti-Cancer Drugs
2.2. Planning Oral Formulation to Bypass Limited Bioavailability Issue
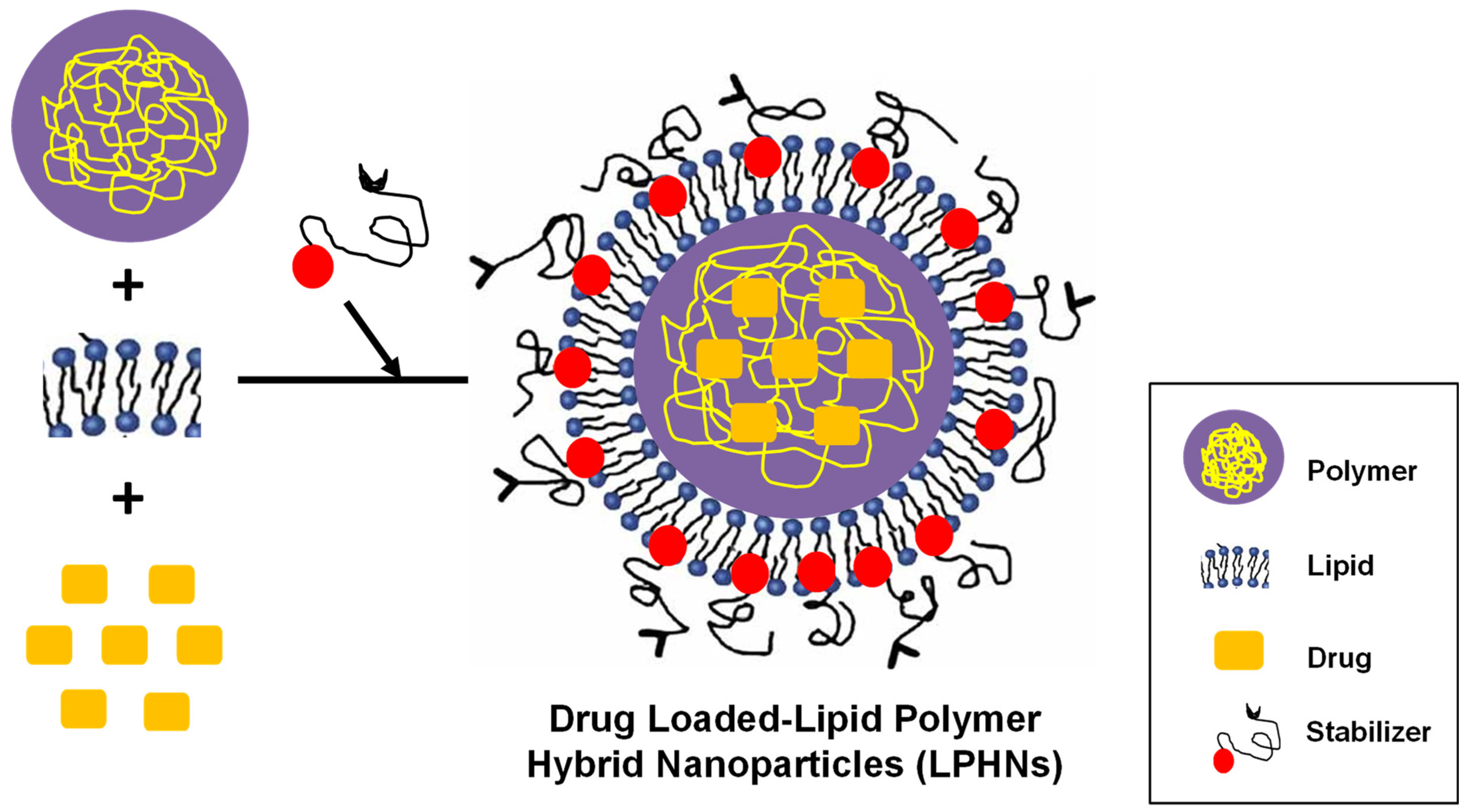
3. Lipid Polymer Hybrid Nanoparticles (LPHNs)
3.1. Basic Formulation Concept
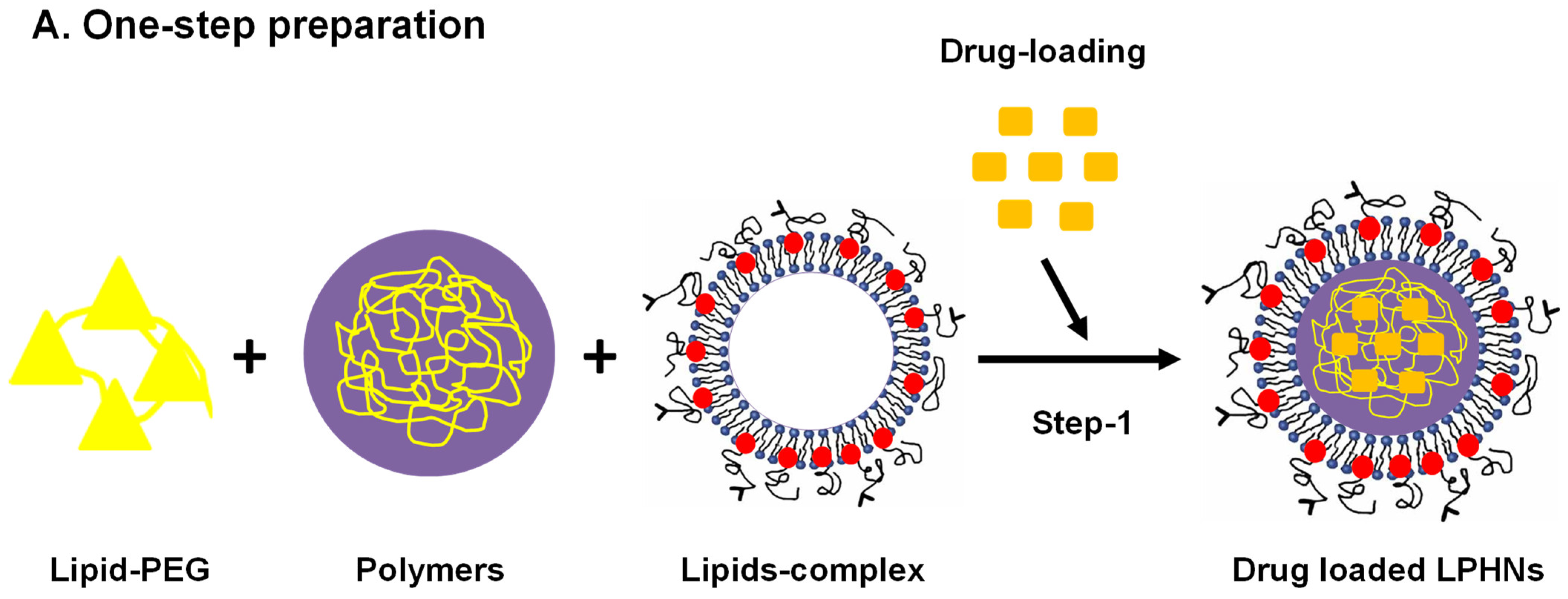
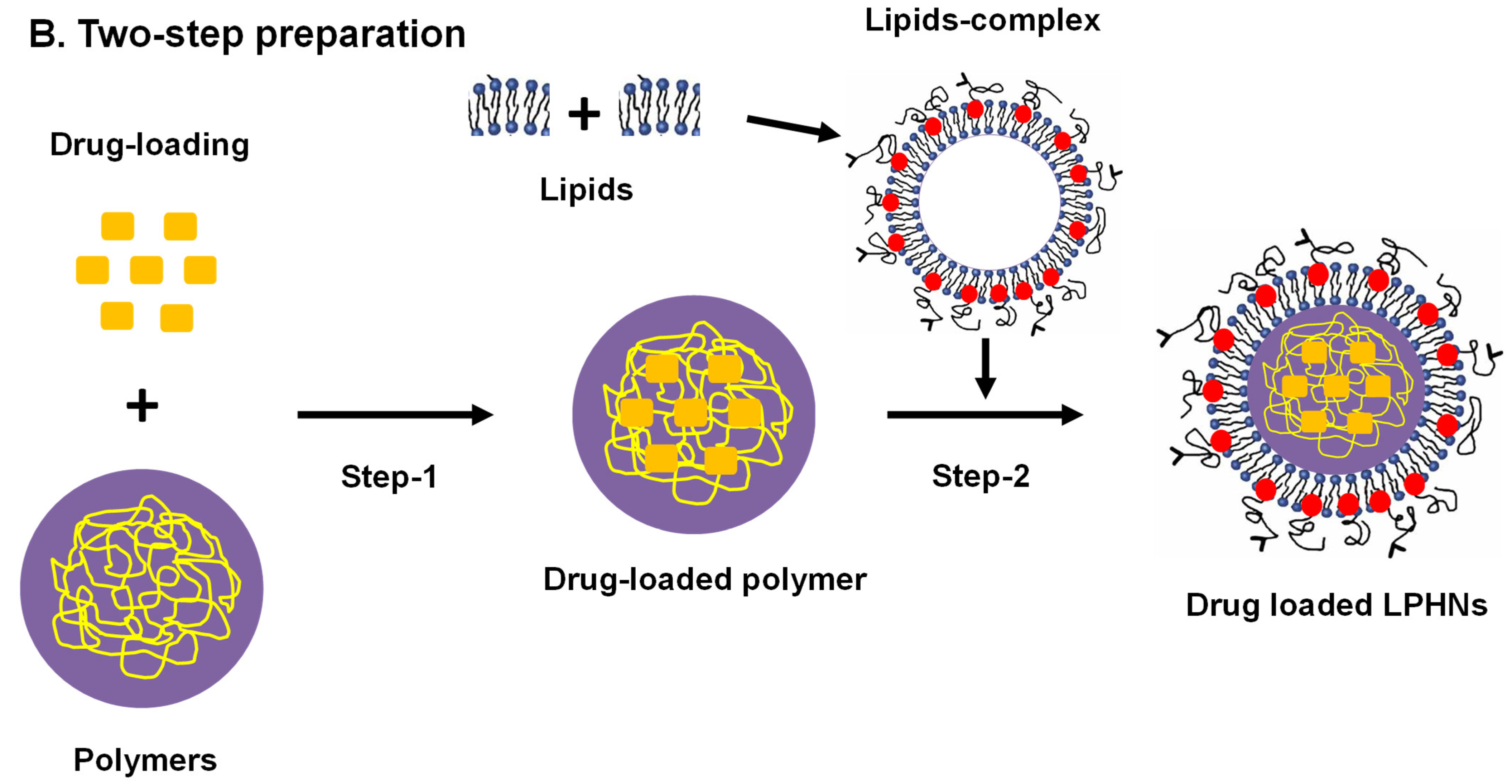
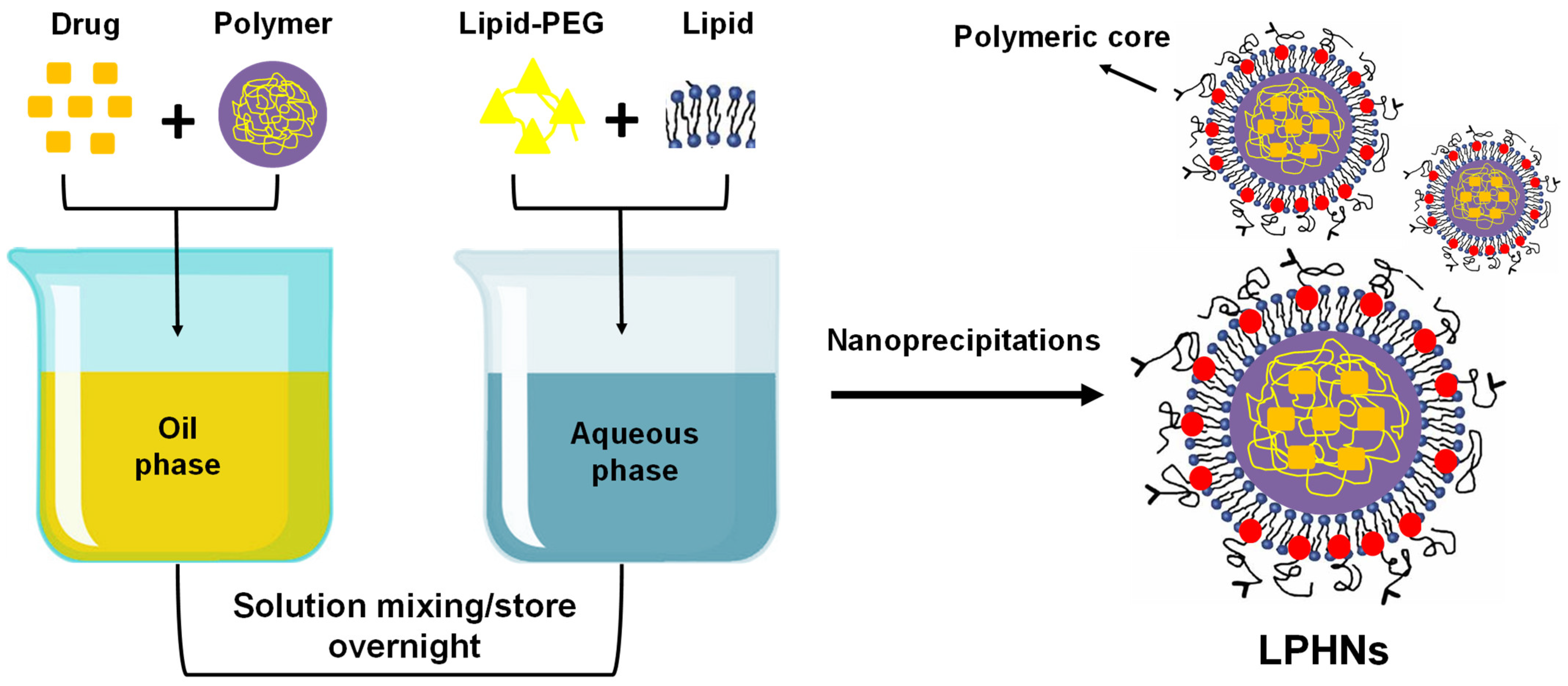
3.2. Preparation Methods
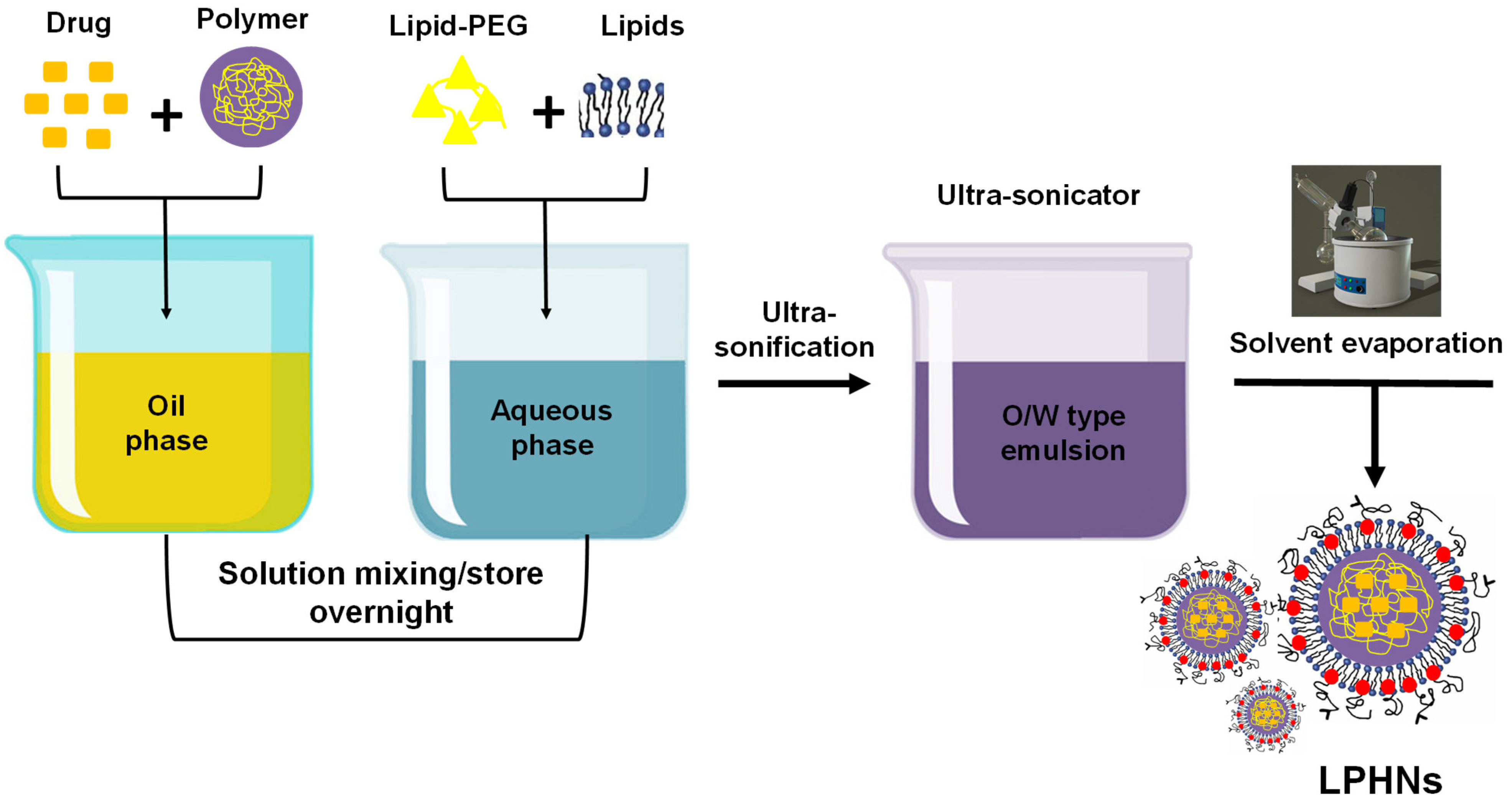
3.2.1. Single-Step Approach
3.2.2. Classical Two-Step Approach
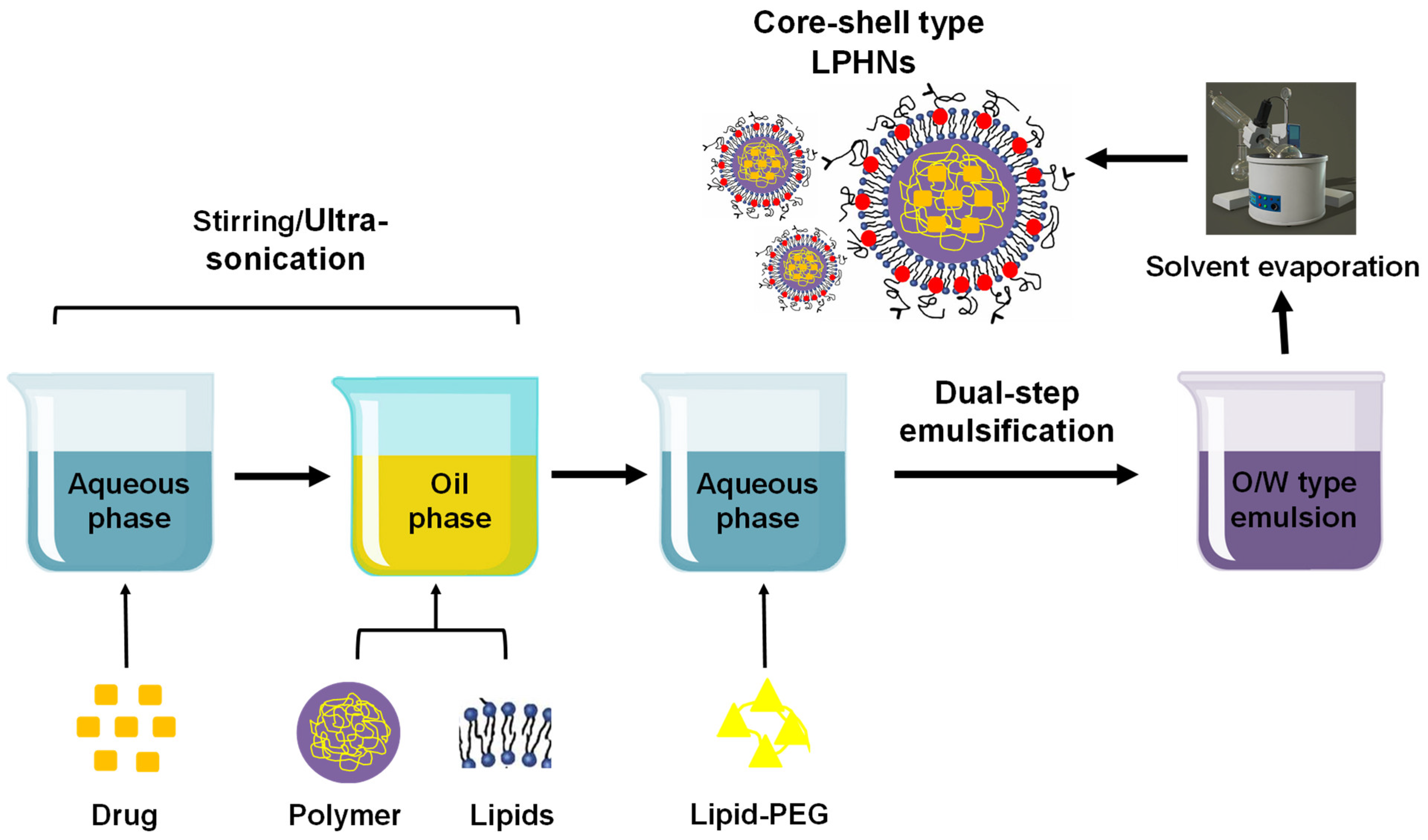
3.2.3. Modern Two-Step Approach
3.2.4. Self-Assembled Nanoprecipitation Method
3.2.5. Single Emulsification Solvent Evaporation
3.2.6. Dual Emulsification Solvent Evaporation
3.3. Characterization and Assessment of % Drug Loading (DL) and % Encapsulation Efficiency (EE)
3.4. Drug Release Mechanisms
3.5. Oral Bioavailability Enhancement (Preclinical Studies)
4. LPHN-Based Anticancer Drug Delivery
5. Limitations and Prospects
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Kawish, S.M.; Sharma, S.; Gupta, P.; Ahmad, F.J.; Iqbal, M.; Alshabrmi, F.M.; Anwer, M.K.; Fathi-karkan, S.; Rahdar, A.; Aboudzadeh, M.A. Nanoparticle-Based Drug Delivery Platform for Simultaneous Administration of Phytochemicals and Chemotherapeutics: Emerging Trends in Cancer Management. Part. Part. Syst. Charact. 2024, 41, 2400049. [Google Scholar] [CrossRef]
- Chini, C.; Bascialla, L.; Giaquinto, A.; Magni, E.; Gobba, S.; Proserpio, I.; Suter, M.; Nigro, O.; Tinelli, G.; Pinotti, G. Homcology: Home chemotherapy delivery in a simultaneous care project for frail advanced cancer patients. Support. Care Cancer 2021, 29, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Thanki, K.; Gangwal, R.P.; Sangamwar, A.T.; Jain, S. Oral delivery of anticancer drugs: Challenges and opportunities. J. Control. Release 2013, 170, 15–40. [Google Scholar] [CrossRef] [PubMed]
- Jibodh, R.A.; Lagas, J.S.; Nuijen, B.; Beijnen, J.H.; Schellens, J.H. Taxanes: Old drugs, new oral formulations. Eur. J. Pharmacol. 2013, 717, 40–46. [Google Scholar] [CrossRef]
- El Moukhtari, S.H.; Rodriguez-Nogales, C.; Blanco-Prieto, M.J. Oral lipid nanomedicines: Current status and future perspectives in cancer treatment. Adv. Drug Deliv. Rev. 2021, 173, 238–251. [Google Scholar] [CrossRef]
- Dudhipala, N.; Puchchakayala, G. Capecitabine lipid nanoparticles for anti-colon cancer activity in 1, 2-dimethylhydrazine-induced colon cancer: Preparation, cytotoxic, pharmacokinetic, and pathological evaluation. Drug Dev. Ind. Pharm. 2018, 44, 1572–1582. [Google Scholar] [CrossRef]
- Wu, L.; Li, X.; Janagam, D.R.; Lowe, T.L. Overcoming the blood-brain barrier in chemotherapy treatment of pediatric brain tumors. Pharm. Res. 2014, 31, 531–540. [Google Scholar] [CrossRef]
- Hou, J.; Sun, E.; Zhang, Z.-H.; Wang, J.; Yang, L.; Cui, L.; Ke, Z.-C.; Tan, X.-B.; Jia, X.-B.; Lv, H. Improved oral absorption and anti-lung cancer activity of paclitaxel-loaded mixed micelles. Drug Deliv. 2017, 24, 261–269. [Google Scholar] [CrossRef]
- Vaidya, F.U.; Sufiyan Chhipa, A.; Mishra, V.; Gupta, V.K.; Rawat, S.G.; Kumar, A.; Pathak, C. Molecular and cellular paradigms of multidrug resistance in cancer. Cancer Rep. 2022, 5, e1291. [Google Scholar] [CrossRef]
- Sylvester, P.W.; Akl, M.R.; Malaviya, A.; Parajuli, P.; Ananthula, S.; Tiwari, R.V.; Ayoub, N.M. Potential role of tocotrienols in the treatment and prevention of breast cancer. Biofactors 2014, 40, 49–58. [Google Scholar] [CrossRef]
- Kaur, P.; Chaurasia, C.S.; Davit, B.M.; Conner, D.P. Bioequivalence study designs for generic solid oral anticancer drug products: Scientific and regulatory considerations. J. Clin. Pharmacol. 2013, 53, 1252–1260. [Google Scholar] [CrossRef] [PubMed]
- Ananthula, S. Bioavailability and bioequivalence issues associated with oral anticancer drugs and effect on drug market. J. Bioequiv. Availab. 2014, 6, e56. [Google Scholar] [CrossRef]
- Kassaee, S.N.; Richard, D.; Ayoko, G.A.; Islam, N. Lipid polymer hybrid nanoparticles against lung cancer and their application as inhalable formulation. Nanomedicine 2024, 19, 2113–2133. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Cañadas, O.; Alonso, A.; Franzyk, H.; Thakur, A.; Pérez-Gil, J.; Foged, C. Effect of lipid-polymer hybrid nanoparticles on the biophysical function and lateral structure of pulmonary surfactant: Mechanistic in vitro studies. J. Colloid Interface Sci. 2024, 654, 1111–1123. [Google Scholar] [CrossRef]
- Chen, Y.; Deng, Y.; Zhu, C.; Xiang, C. Anti prostate cancer therapy: Aptamer-functionalized, curcumin and cabazitaxel co-delivered, tumor targeted lipid-polymer hybrid nanoparticles. Biomed. Pharmacother. 2020, 127, 110181. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, D.; Wu, Y.; Li, W.; Hu, Y.; Zhao, G.; Fu, C.; Fu, S.; Zou, L. Lipid–polymer hybrid nanoparticles for oral delivery of tartary buckwheat flavonoids. J. Agric. Food Chem. 2018, 66, 4923–4932. [Google Scholar] [CrossRef]
- Zhao, X.; Li, F.; Li, Y.; Wang, H.; Ren, H.; Chen, J.; Nie, G.; Hao, J. Co-delivery of HIF1α siRNA and gemcitabine via biocompatible lipid-polymer hybrid nanoparticles for effective treatment of pancreatic cancer. Biomaterials 2015, 46, 13–25. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Anwer, M.K.; Fatima, F.; Aldawsari, M.F.; Alalaiwe, A.; Alali, A.S.; Alharthi, A.I.; Kalam, M.A. Boosting the anticancer activity of sunitinib malate in breast cancer through lipid polymer hybrid nanoparticles approach. Polymers 2022, 14, 2459. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, D.; Dong, X.; Sun, H.; Song, C.; Wang, C.; Kong, D. Folate-modified lipid–polymer hybrid nanoparticles for targeted paclitaxel delivery. Int. J. Nanomed. 2015, 10, 2101–2114. [Google Scholar] [CrossRef]
- Seedat, N.; Kalhapure, R.S.; Mocktar, C.; Vepuri, S.; Jadhav, M.; Soliman, M.; Govender, T. Co-encapsulation of multi-lipids and polymers enhances the performance of vancomycin in lipid–polymer hybrid nanoparticles: In vitro and in silico studies. Mater. Sci. Eng. C 2016, 61, 616–630. [Google Scholar] [CrossRef]
- Liu, H.; Zhuang, Y.; Wang, P.; Zou, T.; Lan, M.; Li, L.; Liu, F.; Cai, T.; Cai, Y. Polymeric lipid hybrid nanoparticles as a delivery system enhance the antitumor effect of emodin in vitro and in vivo. J. Pharm. Sci. 2021, 110, 2986–2996. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Y. Strategies for optimizing polymer-lipid hybrid nanoparticle-mediated drug delivery. Expert Opin. Drug Deliv. 2016, 13, 609–612. [Google Scholar] [CrossRef] [PubMed]
- Bansal, T.; Akhtar, N.; Jaggi, M.; Khar, R.K.; Talegaonkar, S. Novel formulation approaches for optimising delivery of anticancer drugs based on P-glycoprotein modulation. Drug Discov. Today 2009, 14, 1067–1074. [Google Scholar] [CrossRef]
- Shuhendler, A.J.; Cheung, R.Y.; Manias, J.; Connor, A.; Rauth, A.M.; Wu, X.Y. A novel doxorubicin-mitomycin C co-encapsulated nanoparticle formulation exhibits anti-cancer synergy in multidrug resistant human breast cancer cells. Breast Cancer Res. Treat. 2010, 119, 255–269. [Google Scholar] [CrossRef]
- Yang, X.-Z.; Dou, S.; Wang, Y.-C.; Long, H.-Y.; Xiong, M.-H.; Mao, C.-Q.; Yao, Y.-D.; Wang, J. Single-step assembly of cationic lipid–polymer hybrid nanoparticles for systemic delivery of siRNA. ACS Nano 2012, 6, 4955–4965. [Google Scholar] [CrossRef]
- Ezzat, H.M.; Elnaggar, Y.S.; Abdallah, O.Y. Improved oral bioavailability of the anticancer drug catechin using chitosomes: Design, in-vitro appraisal and in-vivo studies. Int. J. Pharm. 2019, 565, 488–498. [Google Scholar] [CrossRef]
- Garg, N.K.; Tyagi, R.K.; Sharma, G.; Jain, A.; Singh, B.; Jain, S.; Katare, O. Functionalized lipid–polymer hybrid nanoparticles mediated codelivery of methotrexate and aceclofenac: A synergistic effect in breast cancer with improved pharmacokinetics attributes. Mol. Pharm. 2017, 14, 1883–1897. [Google Scholar] [CrossRef]
- Shafique, M.; Ur Rehman, M.; Kamal, Z.; Alzhrani, R.M.; Alshehri, S.; Alamri, A.H.; Bakkari, M.A.; Sabei, F.Y.; Safhi, A.Y.; Mohammed, A.M. Formulation development of lipid polymer hybrid nanoparticles of doxorubicin and its in-vitro, in-vivo and computational evaluation. Front. Pharmacol. 2023, 14, 1025013. [Google Scholar] [CrossRef]
- Khalili, L.; Dehghan, G.; Sheibani, N.; Khataee, A. Smart active-targeting of lipid-polymer hybrid nanoparticles for therapeutic applications: Recent advances and challenges. Int. J. Biol. Macromol. 2022, 213, 166–194. [Google Scholar] [CrossRef]
- Sivadasan, D.; Sultan, M.H.; Madkhali, O.; Almoshari, Y.; Thangavel, N. Polymeric lipid hybrid nanoparticles (plns) as emerging drug delivery platform—A comprehensive review of their properties, preparation methods, and therapeutic applications. Pharmaceutics 2021, 13, 1291. [Google Scholar] [CrossRef]
- Pradhan, R.; Kumari, S.; Ambati, H.; Patel, T.K.; Ghosh, B.; Puri, A.; Dubey, S.K.; Taliyan, R. Development of biotin decorated Olaparib loaded cationic lipopolymeric hybrid nanoparticle and evaluation of its anticancer effect and pharmacokinetics for triple negative breast cancer. J. Drug Deliv. Sci. Technol. 2024, 94, 105458. [Google Scholar] [CrossRef]
- Ting, Y.; Jiang, Y.; Ho, C.-T.; Huang, Q. Common delivery systems for enhancing in vivo bioavailability and biological efficacy of nutraceuticals. J. Funct. Foods 2014, 7, 112–128. [Google Scholar] [CrossRef]
- Yang, X.; Liu, K. P-gp inhibition-based strategies for modulating pharmacokinetics of anticancer drugs: An update. Curr. Drug Metab. 2016, 17, 806–826. [Google Scholar] [CrossRef] [PubMed]
- Lennernas, H. Modeling gastrointestinal drug absorption requires more in vivo biopharmaceutical data: Experience from in vivo dissolution and permeability studies in humans. Curr. Drug Metab. 2007, 8, 645–657. [Google Scholar] [CrossRef]
- Engle, K.; Kumar, G. Cancer multidrug-resistance reversal by ABCB1 inhibition: A recent update. Eur. J. Med. Chem. 2022, 239, 114542. [Google Scholar] [CrossRef]
- Krishna Vadlapatla, R.; Dutt Vadlapudi, A.; Pal, D.; Mitra, A.K. Mechanisms of drug resistance in cancer chemotherapy: Coordinated role and regulation of efflux transporters and metabolizing enzymes. Curr. Pharm. Des. 2013, 19, 7126–7140. [Google Scholar] [CrossRef]
- Eisenmann, E.D.; Talebi, Z.; Sparreboom, A.; Baker, S.D. Boosting the oral bioavailability of anticancer drugs through intentional drug–drug interactions. Basic Clin. Pharmacol. Toxicol. 2022, 130, 23–35. [Google Scholar] [CrossRef]
- Kruijtzer, C.; Beijnen, J.; Rosing, H.; ten Bokkel Huinink, W.; Schot, M.; Jewell, R.; Paul, E.; Schellens, J. Increased oral bioavailability of topotecan in combination with the breast cancer resistance protein and P-glycoprotein inhibitor GF120918. J. Clin. Oncol. 2002, 20, 2943–2950. [Google Scholar] [CrossRef]
- Rizwanullah, M.; Perwez, A.; Alam, M.; Ahmad, S.; Mir, S.R.; Rizvi, M.M.A.; Amin, S. Polymer-lipid hybrid nanoparticles of exemestane for improved oral bioavailability and anti-tumor efficacy: An extensive preclinical investigation. Int. J. Pharm. 2023, 642, 123136. [Google Scholar] [CrossRef]
- de Mattos, A.C.; Altmeyer, C.; Tominaga, T.T.; Khalil, N.M.; Mainardes, R.M. Polymeric nanoparticles for oral delivery of 5-fluorouracil: Formulation optimization, cytotoxicity assay and pre-clinical pharmacokinetics study. Eur. J. Pharm. Sci. 2016, 84, 83–91. [Google Scholar] [CrossRef]
- Budha, N.; Frymoyer, A.; Smelick, G.; Jin, J.; Yago, M.; Dresser, M.; Holden, S.; Benet, L.; Ware, J. Drug absorption interactions between oral targeted anticancer agents and PPIs: Is pH-dependent solubility the Achilles heel of targeted therapy? Clin. Pharmacol. Ther. 2012, 92, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Ashar, F.; Mohammed, A.A.S.; Selvamuthukumar, S. Enhancement of oral bioavailability of ibrutinib using a liposil nanohybrid delivery system. PLoS ONE 2024, 19, e0310492. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, S.; Bouchemal, K.; Ponchel, G. Oral delivery of anticancer drugs I: General considerations. Drug Discov. Today 2013, 18, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Sani, A.; Pourmadadi, M.; Shaghaghi, M.; Eshaghi, M.M.; Shahmollaghamsary, S.; Arshad, R.; Fathi-karkan, S.; Rahdar, A.; Jadoun, S.; Medina, D.I. Revolutionizing anticancer drug delivery: Exploring the potential of tamoxifen-loaded nanoformulations. J. Drug Deliv. Sci. Technol. 2023, 86, 104642. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Ostovar, S.; Ruiz-Pulido, G.; Hassan, D.; Souri, M.; Manicum, A.-L.E.; Behzadmehr, R.; Fathi-karkan, S.; Rahdar, A.; Medina, D.I. Novel Epirubicin-loaded Nanoformulations: Advancements in Polymeric Nanocarriers for Efficient Targeted Cellular and Subcellular Anticancer Drug Delivery. Inorg. Chem. Commun. 2023, 155, 110999. [Google Scholar] [CrossRef]
- Meylina, L.; Muchtaridi, M.; Joni, I.M.; Mohammed, A.F.A.; Wathoni, N. Nanoformulations of α-mangostin for cancer drug delivery system. Pharmaceutics 2021, 13, 1993. [Google Scholar] [CrossRef]
- Wu, B.; Yu, P.; Cui, C.; Wu, M.; Zhang, Y.; Liu, L.; Wang, C.-X.; Zhuo, R.-X.; Huang, S.-W. Folate-containing reduction-sensitive lipid–polymer hybrid nanoparticles for targeted delivery of doxorubicin. Biomater. Sci. 2015, 3, 655–664. [Google Scholar] [CrossRef]
- Song, Z.; Shi, Y.; Han, Q.; Dai, G. Endothelial growth factor receptor-targeted and reactive oxygen species-responsive lung cancer therapy by docetaxel and resveratrol encapsulated lipid-polymer hybrid nanoparticles. Biomed. Pharmacother. 2018, 105, 18–26. [Google Scholar] [CrossRef]
- Wakaskar, R.R. General overview of lipid–polymer hybrid nanoparticles, dendrimers, micelles, liposomes, spongosomes and cubosomes. J. Drug Target. 2018, 26, 311–318. [Google Scholar] [CrossRef]
- Rose, F.; Wern, J.E.; Ingvarsson, P.T.; van de Weert, M.; Andersen, P.; Follmann, F.; Foged, C. Engineering of a novel adjuvant based on lipid-polymer hybrid nanoparticles: A quality-by-design approach. J. Control. Release 2015, 210, 48–57. [Google Scholar] [CrossRef]
- Tahir, N.; Haseeb, M.T.; Madni, A.; Parveen, F.; Khan, M.M.; Khan, S.; Jan, N.; Khan, A. Lipid polymer hybrid nanoparticles: A novel approach for drug delivery. In Role of Novel Drug Delivery Vehicles in Nanobiomedicine; IntechOpen: London, UK, 2019; p. 59. [Google Scholar]
- Wang, T.; Xue, J.; Hu, Q.; Zhou, M.; Chang, C.; Luo, Y. Synthetic surfactant-and cross-linker-free preparation of highly stable lipid-polymer hybrid nanoparticles as potential oral delivery vehicles. Sci. Rep. 2017, 7, 2750. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.K.; Tandel, N.; Jadon, R.S.; Tyagi, R.K.; Katare, O.P. Lipid–polymer hybrid nanocarrier-mediated cancer therapeutics: Current status and future directions. Drug Discov. Today 2018, 23, 1610–1621. [Google Scholar] [CrossRef] [PubMed]
- Gajbhiye, K.R.; Salve, R.; Narwade, M.; Sheikh, A.; Kesharwani, P.; Gajbhiye, V. Lipid polymer hybrid nanoparticles: A custom-tailored next-generation approach for cancer therapeutics. Mol. Cancer 2023, 22, 160. [Google Scholar] [CrossRef] [PubMed]
- Sakpakdeejaroen, I.; Muanrit, P.; Panthong, S.; Ruangnoo, S. Alpha-Mangostin-Loaded Transferrin-Conjugated Lipid-Polymer Hybrid Nanoparticles: Development and Characterization for Tumor-Targeted Delivery. Sci. World J. 2022, 2022, 9217268. [Google Scholar] [CrossRef]
- Li, Q.; Xia, D.; Tao, J.; Shen, A.; He, Y.; Gan, Y.; Wang, C. Self-assembled core-shell-type lipid-polymer hybrid nanoparticles: Intracellular trafficking and relevance for oral absorption. J. Pharm. Sci. 2017, 106, 3120–3130. [Google Scholar] [CrossRef]
- Rajana, N.; Chary, P.S.; Bhavana, V.; Deshmukh, R.; Dukka, K.; Sharma, A.; Mehra, N.K. Targeted delivery and apoptosis induction of CDK-4/6 inhibitor loaded folic acid decorated lipid-polymer hybrid nanoparticles in breast cancer cells. Int. J. Pharm. 2024, 651, 123787. [Google Scholar] [CrossRef]
- Chan, J.M.; Zhang, L.; Yuet, K.P.; Liao, G.; Rhee, J.-W.; Langer, R.; Farokhzad, O.C. PLGA–lecithin–PEG core–shell nanoparticles for controlled drug delivery. Biomaterials 2009, 30, 1627–1634. [Google Scholar] [CrossRef]
- Chan, J.M.; Zhang, L.; Tong, R.; Ghosh, D.; Gao, W.; Liao, G.; Yuet, K.P.; Gray, D.; Rhee, J.-W.; Cheng, J. Spatiotemporal controlled delivery of nanoparticles to injured vasculature. Proc. Natl. Acad. Sci. USA 2010, 107, 2213–2218. [Google Scholar] [CrossRef]
- Dave, V.; Tak, K.; Sohgaura, A.; Gupta, A.; Sadhu, V.; Reddy, K.R. Lipid-polymer hybrid nanoparticles: Synthesis strategies and biomedical applications. J. Microbiol. Methods 2019, 160, 130–142. [Google Scholar] [CrossRef]
- Jain, S.; Kumar, M.; Kumar, P.; Verma, J.; Rosenholm, J.M.; Bansal, K.K.; Vaidya, A. Lipid–Polymer Hybrid Nanosystems: A Rational Fusion for Advanced Therapeutic Delivery. J. Funct. Biomater. 2023, 14, 437. [Google Scholar] [CrossRef]
- Khan, S.; Madni, A.; Aamir, M.N.; Khan, S.; Ahmad, F.-u.-D.; Basit, A.; Jan, N.; Shah, H.; Shafiq, A.; Anwar, M. Design and evaluation of sustained-release lipid-PLGA hybrid nanoparticles for enhanced anticancer efficacy of 5-fluorouracil. Part. Sci. Technol. 2023, 42, 269–287. [Google Scholar] [CrossRef]
- Sengel-Turk, C.T.; Paksoy, A.O.; Alpturk, O. The state of the art in core–shell-type lipid–polymer hybrid nanocarriers and beyond. Polym. Bull. 2023, 81, 4771–4800. [Google Scholar] [CrossRef]
- Baghel, Y.S.; Bhattacharya, S. Lipid polymeric hybrid nanoparticles: Formulation techniques and effects on glioblastoma. Pharm. Sci. 2021, 28, 174–193. [Google Scholar] [CrossRef]
- Dali, P.; Shende, P. Self-assembled lipid polymer hybrid nanoparticles using combinational drugs for migraine via intranasal route. AAPS PharmSciTech 2022, 24, 20. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, J.; Li, X.; Xu, Y.; Liu, D.; He, H.; Wang, Y.; Tang, X. Hydroxycamptothecin (HCPT)-loaded PEGlated lipid–polymer hybrid nanoparticles for effective delivery of HCPT: QbD-based development and evaluation. Drug Deliv. Transl. Res. 2022, 12, 306–324. [Google Scholar] [CrossRef]
- Surapaneni, S.G.; Ambade, A.V. Poly (N-vinylcaprolactam) containing solid lipid polymer hybrid nanoparticles for controlled delivery of a hydrophilic drug gemcitabine hydrochloride. RSC Adv. 2022, 12, 17621–17628. [Google Scholar] [CrossRef]
- Shah, S.; Famta, P.; Raghuvanshi, R.S.; Singh, S.B.; Srivastava, S. Lipid polymer hybrid nanocarriers: Insights into synthesis aspects, characterization, release mechanisms, surface functionalization and potential implications. Colloid Interface Sci. Commun. 2022, 46, 100570. [Google Scholar] [CrossRef]
- Zhao, Y.; Lin, D.; Wu, F.; Guo, L.; He, G.; Ouyang, L.; Song, X.; Huang, W.; Li, X. Discovery and in vivo evaluation of novel RGD-modified lipid-polymer hybrid nanoparticles for targeted drug delivery. Int. J. Mol. Sci. 2014, 15, 17565–17576. [Google Scholar] [CrossRef]
- Li, F.; Zhao, X.; Wang, H.; Zhao, R.; Ji, T.; Ren, H.; Anderson, G.J.; Nie, G.; Hao, J. Multiple layer-by-layer lipid-polymer hybrid nanoparticles for improved FOLFIRINOX chemotherapy in pancreatic tumor models. Adv. Funct. Mater. 2015, 25, 788–798. [Google Scholar] [CrossRef]
- Li, Y.; Wu, H.; Yang, X.; Jia, M.; Li, Y.; Huang, Y.; Lin, J.; Wu, S.; Hou, Z. Mitomycin C-soybean phosphatidylcholine complex-loaded self-assembled PEG-lipid-PLA hybrid nanoparticles for targeted drug delivery and dual-controlled drug release. Mol. Pharm. 2014, 11, 2915–2927. [Google Scholar] [CrossRef]
- Mirnezami, S.M.S.; Heydarinasab, A.; Akbarzadehkhyavi, A.; Adrjmand, M. Development and optimization of lipid-polymer hybrid nanoparticles containing melphalan using central composite design and its effect on ovarian cancer cell lines. Iran. J. Pharm. Res. IJPR 2021, 20, 213. [Google Scholar] [PubMed]
- Fu, D.; Li, C.; Huang, Y. Lipid–polymer hybrid nanoparticle-based combination treatment with cisplatin and EGFR/HER2 receptor-targeting Afatinib to enhance the treatment of nasopharyngeal carcinoma. OncoTargets Ther. 2021, 14, 2449–2461. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Madni, A.; Filipczak, N.; Pan, J.; Rehman, M.; Rai, N.; Attia, S.A.; Torchilin, V.P. Folate targeted lipid chitosan hybrid nanoparticles for enhanced anti-tumor efficacy. Nanomed. Nanotechnol. Biol. Med. 2020, 28, 102228. [Google Scholar] [CrossRef]
- Gu, L.; Shi, T.; Sun, Y.; You, C.; Wang, S.; Wen, G.; Chen, L.; Zhang, X.; Zhu, J.; Sun, B. Folate-modified, indocyanine green-loaded lipid-polymer hybrid nanoparticles for targeted delivery of cisplatin. J. Biomater. Sci. Polym. Ed. 2017, 28, 690–702. [Google Scholar] [CrossRef]
- Dong, S.; Zhou, X.; Yang, J. TAT modified and lipid–PEI hybrid nanoparticles for co-delivery of docetaxel and pDNA. Biomed. Pharmacother. 2016, 84, 954–961. [Google Scholar] [CrossRef]
- Wang, Q.; Alshaker, H.; Böhler, T.; Srivats, S.; Chao, Y.; Cooper, C.; Pchejetski, D. Core shell lipid-polymer hybrid nanoparticles with combined docetaxel and molecular targeted therapy for the treatment of metastatic prostate cancer. Sci. Rep. 2017, 7, 5901. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, P.; Zhu, T. Ovarian carcinoma biological nanotherapy: Comparison of the advantages and drawbacks of lipid, polymeric, and hybrid nanoparticles for cisplatin delivery. Biomed. Pharmacother. 2019, 109, 475–483. [Google Scholar] [CrossRef]
- Shi, K.; Zhou, J.; Zhang, Q.; Gao, H.; Liu, Y.; Zong, T.; He, Q. Arginine-glycine-aspartic acid-modified lipid-polymer hybrid nanoparticles for docetaxel delivery in glioblastoma multiforme. J. Biomed. Nanotechnol. 2015, 11, 382–391. [Google Scholar] [CrossRef]
- Zhang, L.; Chan, J.M.; Gu, F.X.; Rhee, J.-W.; Wang, A.Z.; Radovic-Moreno, A.F.; Alexis, F.; Langer, R.; Farokhzad, O.C. Self-assembled lipid− polymer hybrid nanoparticles: A robust drug delivery platform. ACS Nano 2008, 2, 1696–1702. [Google Scholar] [CrossRef]
- Tran, T.; Nguyen, H.; Yong, C.; Truong, D.; Kim, J. Synergistic therapeutic strategy of dual drug-loaded lipid polymer hybrid nanoparticles for breast cancer treatment. Indian J. Pharm. Sci. 2019, 81, 474–482. [Google Scholar] [CrossRef]
- Wong, H.L.; Rauth, A.M.; Bendayan, R.; Manias, J.L.; Ramaswamy, M.; Liu, Z.; Erhan, S.Z.; Wu, X.Y. A new polymer–lipid hybrid nanoparticle system increases cytotoxicity of doxorubicin against multidrug-resistant human breast cancer cells. Pharm. Res. 2006, 23, 1574–1585. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.; Mittal, N.K.; Balabathula, P.; Thoma, L.A.; Wood, G.C. Development and in vitro evaluation of core–shell type lipid–polymer hybrid nanoparticles for the delivery of erlotinib in non-small cell lung cancer. Eur. J. Pharm. Sci. 2016, 81, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Xing, H.; Sun, Y.; Feng, S.; Wang, S. Non-small cell lung cancer combination therapy: Hyaluronic acid modified, epidermal growth factor receptor targeted, pH sensitive lipid-polymer hybrid nanoparticles for the delivery of erlotinib plus bevacizumab. Biomed. Pharmacother. 2020, 125, 109861. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Zhou, J.; Yan, L.; Zhang, S.; Yu, D.-G. Hybrid films loaded with 5-fluorouracil and Reglan for synergistic treatment of colon cancer via asynchronous dual-drug delivery. Front. Bioeng. Biotechnol. 2024, 12, 1398730. [Google Scholar] [CrossRef]
- Yalcin, T.E.; Ilbasmis-Tamer, S.; Takka, S. Antitumor activity of gemcitabine hydrochloride loaded lipid polymer hybrid nanoparticles (LPHNs): In vitro and in vivo. Int. J. Pharm. 2020, 580, 119246. [Google Scholar] [CrossRef]
- Zhang, H.; Li, J.; Yuan, R.; Li, Y.; Zhang, Y.; Hu, X.; Qu, J.; Chen, Y.; Wang, Z.; Xia, M. Augment the efficacy of eradicating metastatic lesions and tumor proliferation in breast cancer by honokiol-loaded pH-sensitive targeted lipid nanoparticles. Colloids Surf. B Biointerfaces 2021, 207, 112008. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, J.; Fu, C.; Xie, X.; Peng, F.; You, J.; Tang, H.; Wang, Z.; Li, P.; Chen, J. iRGD-modified lipid–polymer hybrid nanoparticles loaded with isoliquiritigenin to enhance anti-breast cancer effect and tumor-targeting ability. Int. J. Nanomed. 2017, 12, 4147–4162. [Google Scholar] [CrossRef]
- Khalili, L.; Dehghan, G.; Feizi, M.A.H.; Sheibani, N.; Hamishekar, H. Development of an albumin decorated lipid-polymer hybrid nanoparticle for simultaneous delivery of methotrexate and conferone to cancer cells. Int. J. Pharm. 2021, 599, 120421. [Google Scholar] [CrossRef]
- Patel, G.; Thakur, N.S.; Kushwah, V.; Patil, M.D.; Nile, S.H.; Jain, S.; Kai, G.; Banerjee, U.C. Mycophenolate co-administration with quercetin via lipid-polymer hybrid nanoparticles for enhanced breast cancer management. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102147. [Google Scholar] [CrossRef]
- Soomherun, N.; Kreua-Ongarjnukool, N.; Niyomthai, S.T.; Chumnanvej, S. Lipid-Polymer Hybrid Nanoparticles Synthesized via Lipid-Based Surface Engineering for a robust drug delivery platform. Colloids Surf. B Biointerfaces 2024, 237, 113858. [Google Scholar] [CrossRef]
- Godara, S.; Lather, V.; Kirthanashri, S.; Awasthi, R.; Pandita, D. Lipid-PLGA hybrid nanoparticles of paclitaxel: Preparation, characterization, in vitro and in vivo evaluation. Mater. Sci. Eng. C 2020, 109, 110576. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, U.; Chashoo, G.; Sharma, P.R.; Kumar, A.; Saxena, A.K.; Vyas, S. Tailored polymer–lipid hybrid nanoparticles for the delivery of drug conjugate: Dual strategy for brain targeting. Colloids Surf. B Biointerfaces 2015, 126, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Feng, S.-S. Poly (D, L-lactide-co-glycolide)(PLGA) nanoparticles prepared by high pressure homogenization for paclitaxel chemotherapy. Int. J. Pharm. 2007, 342, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, I.P.S.; Silva, L.B.; do Amaral, R.L.F.; Chrysostomo-Massaro, T.N.; de Lima Fragelli, B.D.; de Almeida Rodolpho, J.M.; de Freitas Anibal, F.; Borra, R.C.; Paschoal, J.A.R.; Miranda, M.A. Evaluation of in vivo and in vitro efficacy of solasonine/solamargine-loaded lipid-polymer hybrid nanoparticles against bladder cancer. Int. J. Pharm. 2024, 661, 124411. [Google Scholar] [CrossRef]
- Varthya, M.; Pawar, H.; Singh, C.; Dora, C.P.; Jena, S.K.; Suresh, S. Development of novel polymer-lipid hybrid nanoparticles of tamoxifen: In vitro and in vivo evaluation. J. Nanosci. Nanotechnol. 2016, 16, 253–260. [Google Scholar] [CrossRef]
- Imam, S.S.; Gilani, S.J.; Bin Jumah, M.N.; Rizwanullah, M.; Zafar, A.; Ahmed, M.M.; Alshehri, S. Harnessing lipid polymer hybrid nanoparticles for enhanced oral bioavailability of thymoquinone: In vitro and in vivo assessments. Polymers 2022, 14, 3705. [Google Scholar] [CrossRef]
- Yang, Z.; Luo, X.; Zhang, X.; Liu, J.; Jiang, Q. Targeted delivery of 10-hydroxycamptothecin to human breast cancers by cyclic RGD-modified lipid–polymer hybrid nanoparticles. Biomed. Mater. 2013, 8, 025012. [Google Scholar] [CrossRef]
- Zhang, L.; Radovic-Moreno, A.F.; Alexis, F.; Gu, F.X.; Basto, P.A.; Bagalkot, V.; Jon, S.; Langer, R.S.; Farokhzad, O.C. Co-delivery of hydrophobic and hydrophilic drugs from nanoparticle-aptamer bioconjugates. ChemMedChem Chem. Enabling Drug Discov. 2007, 2, 1268–1271. [Google Scholar] [CrossRef]
- Farokhzad, O.C.; Cheng, J.; Teply, B.A.; Sherifi, I.; Jon, S.; Kantoff, P.W.; Richie, J.P.; Langer, R. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc. Natl. Acad. Sci. USA 2006, 103, 6315–6320. [Google Scholar] [CrossRef]
- Kong, S.D.; Sartor, M.; Hu, C.-M.J.; Zhang, W.; Zhang, L.; Jin, S. Magnetic field activated lipid–polymer hybrid nanoparticles for stimuli-responsive drug release. Acta Biomater. 2013, 9, 5447–5452. [Google Scholar] [CrossRef]
- Jadon, R.S.; Sharma, M. Docetaxel-loaded lipid-polymer hybrid nanoparticles for breast cancer therapeutics. J. Drug Deliv. Sci. Technol. 2019, 51, 475–484. [Google Scholar] [CrossRef]
- Yalcin, T.E.; Ilbasmis-Tamer, S.; Takka, S. Development and characterization of gemcitabine hydrochloride loaded lipid polymer hybrid nanoparticles (LPHNs) using central composite design. Int. J. Pharm. 2018, 548, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Soares, D.C.F.; Domingues, S.C.; Viana, D.B.; Tebaldi, M.L. Polymer-hybrid nanoparticles: Current advances in biomedical applications. Biomed. Pharmacother. 2020, 131, 110695. [Google Scholar]
- Qin, L.; Wu, H.; Xu, E.; Zhang, X.; Guan, J.; Zhao, R.; Mao, S. Exploring the potential of functional polymer-lipid hybrid nanoparticles for enhanced oral delivery of paclitaxel. Asian J. Pharm. Sci. 2021, 16, 387–395. [Google Scholar] [CrossRef]
- Subramanian, D.A.; Langer, R.; Traverso, G. Mucus interaction to improve gastrointestinal retention and pharmacokinetics of orally administered nano-drug delivery systems. J. Nanobiotechnol. 2022, 20, 362. [Google Scholar] [CrossRef]
- Kim, Y.S.; Ho, S.B. Intestinal goblet cells and mucins in health and disease: Recent insights and progress. Curr. Gastroenterol. Rep. 2010, 12, 319–330. [Google Scholar] [CrossRef]
- Ren, T.; Wang, Q.; Xu, Y.; Cong, L.; Gou, J.; Tao, X.; Zhang, Y.; He, H.; Yin, T.; Zhang, H. Enhanced oral absorption and anticancer efficacy of cabazitaxel by overcoming intestinal mucus and epithelium barriers using surface polyethylene oxide (PEO) decorated positively charged polymer-lipid hybrid nanoparticles. J. Control. Release 2018, 269, 423–438. [Google Scholar] [CrossRef]
- Tahir, N.; Madni, A.; Correia, A.; Rehman, M.; Balasubramanian, V.; Khan, M.M.; Santos, H.A. Lipid-polymer hybrid nanoparticles for controlled delivery of hydrophilic and lipophilic doxorubicin for breast cancer therapy. Int. J. Nanomed. 2019, 14, 4961–4974. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, X.; Chen, H.; Hou, X.; He, Y.; Shen, J.; Shi, J.; Feng, N. Advances in next-generation lipid-polymer hybrid nanocarriers with emphasis on polymer-modified functional liposomes and cell-based-biomimetic nanocarriers for active ingredients and fractions from Chinese medicine delivery. Nanomed. Nanotechnol. Biol. Med. 2020, 29, 102237. [Google Scholar] [CrossRef]
- Akhlaghi, S.P.; Saremi, S.; Ostad, S.N.; Dinarvand, R.; Atyabi, F. Discriminated effects of thiolated chitosan-coated pMMA paclitaxel-loaded nanoparticles on different normal and cancer cell lines. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 689–697. [Google Scholar] [CrossRef]
- Banna, G.L.; Collovà, E.; Gebbia, V.; Lipari, H.; Giuffrida, P.; Cavallaro, S.; Condorelli, R.; Buscarino, C.; Tralongo, P.; Ferraù, F. Anticancer oral therapy: Emerging related issues. Cancer Treat. Rev. 2010, 36, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Aryal, S.; Hu, C.-M.J.; Fu, V.; Zhang, L. Nanoparticle drug delivery enhances the cytotoxicity of hydrophobic–hydrophilic drug conjugates. J. Mater. Chem. 2012, 22, 994–999. [Google Scholar] [CrossRef]
- Pandita, D.; Ahuja, A.; Lather, V.; Benjamin, B.; Dutta, T.; Velpandian, T.; Khar, R.K. Development of lipid-based nanoparticles for enhancing the oral bioavailability of paclitaxel. Aaps Pharmscitech 2011, 12, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.; Saha, R.; Shanmugam, T.; Balakrishnan, B.; More, P.; Banerjee, R. Carboxymethyl-chitosan-tethered lipid vesicles: Hybrid nanoblanket for oral delivery of paclitaxel. Biomacromolecules 2013, 14, 2272–2282. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, J.; Chan, H.F.; Skibba, M.; Liang, G.; Chen, M. iRGD decorated lipid-polymer hybrid nanoparticles for targeted co-delivery of doxorubicin and sorafenib to enhance anti-hepatocellular carcinoma efficacy. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1303–1311. [Google Scholar] [CrossRef]
- Shao, Y.; Luo, W.; Guo, Q.; Li, X.; Zhang, Q.; Li, J. In vitro and in vivo effect of hyaluronic acid modified, doxorubicin and gallic acid co-delivered lipid-polymeric hybrid nano-system for leukemia therapy. Drug Des. Dev. Ther. 2019, 13, 2043–2055. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, H.; Han, L.; Qiang, Z.; Zhang, X.; Gao, W.; Zhao, K.; Song, Y. Synergistic combination therapy of lung cancer using paclitaxel-and triptolide-coloaded lipid–polymer hybrid nanoparticles. Drug Des. Dev. Ther. 2018, 12, 3199–3209. [Google Scholar] [CrossRef]
- Li, S.; Xu, Z.; Alrobaian, M.; Afzal, O.; Kazmi, I.; Almalki, W.H.; Altamimi, A.S.A.; Al-Abbasi, F.A.; Alharbi, K.S.; Altowayan, W.M. EGF-functionalized lipid–polymer hybrid nanoparticles of 5-fluorouracil and sulforaphane with enhanced bioavailability and anticancer activity against colon carcinoma. Biotechnol. Appl. Biochem. 2022, 69, 2205–2221. [Google Scholar] [CrossRef]
- Duan, R.; Li, C.; Wang, F.; Yangi, J.-C. Polymer–lipid hybrid nanoparticles-based paclitaxel and etoposide combinations for the synergistic anticancer efficacy in osteosarcoma. Colloids Surf. B Biointerfaces 2017, 159, 880–887. [Google Scholar] [CrossRef]
- Wong, H.L.; Rauth, A.M.; Bendayan, R.; Wu, X.Y. In vivo evaluation of a new polymer-lipid hybrid nanoparticle (PLN) formulation of doxorubicin in a murine solid tumor model. Eur. J. Pharm. Biopharm. 2007, 65, 300–308. [Google Scholar] [CrossRef]
- Mottaghitalab, F.; Farokhi, M.; Fatahi, Y.; Atyabi, F.; Dinarvand, R. New insights into designing hybrid nanoparticles for lung cancer: Diagnosis and treatment. J. Control. Release 2019, 295, 250–267. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.; Bhattacharjee, H.; Mittal, N.; Sah, H.; Balabathula, P.; Thoma, L.A.; Wood, G.C. Core–shell-type lipid–polymer hybrid nanoparticles as a drug delivery platform. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 474–491. [Google Scholar] [CrossRef] [PubMed]
- Cheow, W.S.; Hadinoto, K. Factors affecting drug encapsulation and stability of lipid–polymer hybrid nanoparticles. Colloids Surf. B Biointerfaces 2011, 85, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Persano, F.; Gigli, G.; Leporatti, S. Lipid-polymer hybrid nanoparticles in cancer therapy: Current overview and future directions. Nano Express 2021, 2, 012006. [Google Scholar] [CrossRef]
- Rao, S.; Prestidge, C.A. Polymer-lipid hybrid systems: Merging the benefits of polymeric and lipid-based nanocarriers to improve oral drug delivery. Expert Opin. Drug Deliv. 2016, 13, 691–707. [Google Scholar] [CrossRef]
- Mendonça, M.C.; Kont, A.; Kowalski, P.S.; O’Driscoll, C.M. Design of lipid-based nanoparticles for delivery of therapeutic nucleic acids. Drug Discov. Today 2023, 28, 103505. [Google Scholar] [CrossRef]
- Ana, R.; Mendes, M.; Sousa, J.; Pais, A.; Falcão, A.; Fortuna, A.; Vitorino, C. Rethinking carbamazepine oral delivery using polymer-lipid hybrid nanoparticles. Int. J. Pharm. 2019, 554, 352–365. [Google Scholar] [CrossRef]
- Osman, N.; Devnarain, N.; Omolo, C.A.; Fasiku, V.; Jaglal, Y.; Govender, T. Surface modification of nano-drug delivery systems for enhancing antibiotic delivery and activity. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1758. [Google Scholar] [CrossRef]
- Luk, B.T.; Fang, R.H.; Zhang, L. Lipid-and polymer-based nanostructures for cancer theranostics. Theranostics 2012, 2, 1117. [Google Scholar] [CrossRef]
- Zhang, L.; Tong, S.; Zhang, Q.; Bao, G. Lipid-encapsulated Fe3O4 nanoparticles for multimodal magnetic resonance/fluorescence imaging. ACS Appl. Nano Mater. 2020, 3, 6785–6797. [Google Scholar] [CrossRef]
- Bokare, A.; Takami, A.; Kim, J.H.; Dong, A.; Chen, A.; Valerio, R.; Gunn, S.; Erogbogbo, F. Herringbone-patterned 3D-printed devices as alternatives to microfluidics for reproducible production of lipid polymer hybrid nanoparticles. ACS Omega 2019, 4, 4650–4657. [Google Scholar] [CrossRef]
- Meyer, R.A.; Hussmann, G.P.; Peterson, N.C.; Santos, J.L.; Tuesca, A.D. A scalable and robust cationic lipid/polymer hybrid nanoparticle platform for mRNA delivery. Int. J. Pharm. 2022, 611, 121314. [Google Scholar] [CrossRef]
| Carrier System | Lipid | Polymer | Preparation Method | Size (nm) | Loaded Drug | EE (%) | Detection Method | Ref. |
|---|---|---|---|---|---|---|---|---|
| LPHNs | DLPC | PLGA and DSPE-PEG 2000 | Single emulsification solvent evaporation | 138.2 | Afatinab | 93.2 | UV-Visible spectrophotometry | [73] |
| Compritol® 888 ATO, Precirol® ATO 5, Miglyol® 812, Lecithin and Tween® 80 | Poly(d,l-lactide) | Single emulsification solvent evaporation | 141.2 | Cisplatin | 91.3 | UV-Visible spectrophotometry | [78] | |
| DLPC | PLGA and DSPE-PEG 2000 | Single emulsification solvent evaporation | 138.2 | Cisplatin | 91.4 | UV-Visible spectrophotometry | [73] | |
| Lipoid S75 | Chitosan | Ionic gelation | 258.8 | Cisplatin | 91.8 | UV-Visible spectrophotometry | [74] | |
| Lecithin | PLGA and DSPE-PEG 2000 | Single-step sonication | 94.4 | Cisplatin | 20.5 | UV-Visible spectrophotometry | [75] | |
| Lecithin, and Chol-PEG-RGD | mPEG–PLGA copolymer | W/O/W double emulsification | 216.6 | Curcumin | 96 | HPLC | [69] | |
| Soybean Lecithin | PLGA, DSPE-PEG (2000), and DSPE-PEG2000-RGD | W/O/W emulsion solvent evaporation | 110 | Docetaxel | 77.6 | HPLC | [79] | |
| Compritol, Soybean Lecithin, | PEI and PEG | Single-step solvent evaporation | 216.3 | Docetaxel | 89.1 | HPLC | [76] | |
| Phosphatidylcholine, Cholesterol, and Sphingosine FTY720 | PLGA and 18:0 PEG2000 PE | Single-step solvent evaporation | 141.5 | Docetaxel | 10 | LC-MS/MS | [77] | |
| Lecithin | PLGA, and PEG | Single-step nanoprecipitation | 19 | Docetaxel | 59 | HPLC and UV-Visible spectrophotometry | [80] | |
| Capryol 90 (Capryol), DDAB, and TPGS | PEG-b-PAsp (Poly(ethylene glycol)-block-poly(aspartic acid)) | Double-step solvent evaporation | 232.4 | Docetaxel and vorinostat (combo) | 75.8 | HPLC | [81] | |
| Stearic Acid, and Oleic Acid | Eudragit, Ethyl Cellulose, and Sodium Lauryl Sulfate | Dual-step sonication | 185.4 | Doxorubicin | 95.5 | UV-Visible spectrophotometry | [28] | |
| Epoxidized Soybean Oil, and Stearic Acid | Pluronic F68, and | Two-step solvent evaporation | 80–350 | Doxorubicin | 60-80 | UV-Visible spectrophotometry | [82] | |
| Hydrogenated soy phosphatidylcholine, and DSPE-PEG2000 | Polycaprolactone (10 kDa, 42 kDa, and 80 kDa) | Single-step sonication | 150–180 | Erlotinib | 67 | HPLC | [83] | |
| Soy phosphatidylcholine | Amine-PEG-Aldehyde (CHO-PEG-NH2, MW 2 kDa) | Single-step sonication | 100−120 | Etoposide and bevacizumab (combo) | 85.3 | UV-Visible spectrophotometry | [84] | |
| Illipe Butter, Lecithin, Phosphatidylcholine, and Cholesterol | PLGA, PEG, Eudragit, Ethyl Cellulose, and Chitosan | Coaxial electrospraying | 104.1 | 5-FU and Reglan (combo) | 91 | UV-Visible spectrophotometry | [85] | |
| Soya phosphatidylcholine, and DSPE-PEG2000 | PLGA 50:50 (MW: 38–54 kDa), PLGA 65:35 (MW: 24–38 kDa), and PVA (MW 31–50 kDa, 87–89% hydrolyzed) | Two-step solvent evaporation | ~200 | Gemicitabine | 45 | HPLC | [86] | |
| Soybean Lecithin, and Cholestrol | mPEG-PLGA (75:25), and ε-Polylysine | Two-step solvent evaporation | 60 | Gemicitabine and HIF-1 alpha SiRNA (combo) | 42 | HPLC | [17] | |
| Soybean phosphatidylcholine, and DSPE-PEG2000 | Poly(β-amino ester) | Single-step solvent evaporation | 105.3 | Honokiol | 75 | HPLC | [87] | |
| Lecithin, and DSPE-PEG-Mal | PLGA | Single-step nanoprecipitation | 137.2 | Isoliquiritigenin | 91 | HPLC | [88] | |
| Soy phosphatidylcholine, Cholesterol, and Tween 80 | Chitosan, and PLGA | Single-step sonication | 154 | Methotrexate and conferone (combo) | 85.1, and 78.4 | UV-Visible spectrophotometry | [89] | |
| Soya lecithin | PVA, and PLGA ((50:50 molar ratio) | Single-step nanoprecipitation | 162.8 | Melphalan | 94.5 | UV-Visible spectrophotometry | [72] | |
| Soybean phosphatidylcholine, and DPPE | Poly(d,l-lactide) (10 kDa) | Single-step solvent evaporation | 215.6 | Mitomycin C | 95 | UV-Visible spectrophotometry | [71] | |
| Pluronic® F-68, Soya lecithin, and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)] | PLGA | Single-step nanoprecipitation | 136 and 176 | Mycophenolic acid and quercetin | 78.2 | HPLC | [90] | |
| Lecithin, DSPE-PEG-2000, DSPE-PEG-3400-Mal and Cholesterol | mPEG-PLA (Diblock copolymer) | W/O/W double emulsion solvent evaporation | 160 | 5-FU, irinotecan, oxaliplatin | 96 | HPLC | [70] | |
| Soybean Lecithin | PLGA, PVA, and CMC | W/O/W double emulsion solvent evaporation | 176 | Paclitaxel | 92 | UV-Visible spectrophotometry | [91] | |
| Stearyl amine, and Soya lecithin | PLGA | Single step nanoprecipitation | 207 | Paclitaxel | 67.5 | HPLC | [92] | |
| DSPE, DSPE-PEG-2000,and Soybean lecithin (90–95% phosphatidylcholine) | PLGA (50:50 monomer ratio) | Nanoprecipitation technique combined with self-assembly | - | Paclitaxel | 81.3 | HPLC | [93] | |
| Polyvinyl alcohol | PLGA | Single-step nanoprecipitation | 305.4 | Paclitaxel | 62.6 | HPLC | [94] | |
| DSPE-PEG2000 | Poly(ε-caprolactone), PEG, and PCL-PEG-PCL (amphiphilic copolymer) | Single-step solvent evaporation | 279.9 | Paclitaxel | 91.1 | HPLC | [19] | |
| Illipe Butter | Chitosan | Single-step solvent evaporation | 130 | Solasonine and solamargine (combo) | 91.0 | HPLC | [95] | |
| Lecithin (70% phosphatidylcholine), and Poloxamer 407 | Low molecular weight chitosan | Single step emulsification solvent evaporation | 169.6 | Tamoxifen | 72.1 | HPLC | [96] | |
| Phospholipon 90G (PL-90G) | Chitosan (CHS; 85% deacetylated), and Poloxamer-188 (P-188) | Single-step nanoprecipitation | 179.6 | Thymoquinone | 85.4 | HPLC | [97] | |
| Capryol 90, TPGS, and DDAB | PEG-b-PAsp | Single emulsification-solvent evaporation | 232.4 | Vorinostat and docetaxel (combo) | 73.7 and 75.8 | HPLC | [81] | |
| Egg lecithin, DSPE, and DSPE-PEG | PLGA (50:50, Mw 50,000) | Single emulsification-solvent evaporation | 220.4 | 10-hydroxycamptothecin | 72.6 | UV-Visible spectrophotometry | [98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almawash, S. Oral Bioavailability Enhancement of Anti-Cancer Drugs Through Lipid Polymer Hybrid Nanoparticles. Pharmaceutics 2025, 17, 381. https://doi.org/10.3390/pharmaceutics17030381
Almawash S. Oral Bioavailability Enhancement of Anti-Cancer Drugs Through Lipid Polymer Hybrid Nanoparticles. Pharmaceutics. 2025; 17(3):381. https://doi.org/10.3390/pharmaceutics17030381
Chicago/Turabian StyleAlmawash, Saud. 2025. "Oral Bioavailability Enhancement of Anti-Cancer Drugs Through Lipid Polymer Hybrid Nanoparticles" Pharmaceutics 17, no. 3: 381. https://doi.org/10.3390/pharmaceutics17030381
APA StyleAlmawash, S. (2025). Oral Bioavailability Enhancement of Anti-Cancer Drugs Through Lipid Polymer Hybrid Nanoparticles. Pharmaceutics, 17(3), 381. https://doi.org/10.3390/pharmaceutics17030381








