Abstract
Cancer is considered as the second leading cause of death worldwide. Chemotherapy, radiotherapy, immunotherapy, and targeted drug delivery are the main treatment options for treating cancers. Chemotherapy drugs are either available for oral or parenteral use. Oral chemotherapy, also known as chemotherapy at home, is more likely to improve patient compliance and convenience. Oral anti-cancer drugs have bioavailability issues associated with lower aqueous solubility, first-pass metabolism, poor intestinal permeability and drug absorption, and degradation of the drug throughout its journey in the gastrointestinal tract. A highly developed carrier system known as lipid polymer hybrid nanoparticles (LPHNs) has been introduced. These nanocarriers enhance drug stability, solubility, and absorption, and reduce first-pass metabolism. Consequently, this will have a positive impact on oral bioavailability enhancement. This article provides an in-depth analysis of LPHNs as a novel drug delivery system for anti-cancer agents. It discusses an overview of the limited bioavailability of anti-cancer drugs, their reasons and consequences, LPHNs based anti-cancer drug delivery, conventional and modern preparation methods as well as their drug loading and entrapment efficiencies. In addition, this article also gives an insight into the mechanistic approach to oral bioavailability enhancement, potential applications in anti-cancer drug delivery, limitations, and future prospects of LPHNs in anti-cancer drug delivery.
1. Introduction
Cancer remains a leading cause of death worldwide, demanding continuous innovation in therapeutic strategies. Significant efforts have been made to advance cancer treatment modalities, particularly chemotherapy; however, challenges persist in achieving optimal therapeutic outcomes. Chemotherapy, a cornerstone of cancer treatment, can be administered through either parenteral or oral routes [1]. While parenteral administration ensures rapid systemic drug delivery, it often requires hospital visits, invasive procedures, and careful monitoring, which can impact patient compliance. On the other hand, oral chemotherapy, often referred to as “chemotherapy at home”, offers a more convenient alternative and potentially improves the quality of life for cancer patients [2]. In contrast to the present intravenous methods, oral delivery of anti-cancer drugs may improve patient compliance, safety, and convenience [3].
Numerous chemotherapy drugs, such as taxanes (e.g., paclitaxel and docetaxel), have poor oral bioavailability due to limited solubility, first-pass metabolism, and poor absorption in the gastrointestinal (GI) tract. For example, oral paclitaxel faces significant challenges with solubility and bioavailability, limiting its therapeutic potential [4,5]. Oral anti-cancer drugs often encounter physiological and pharmacokinetic barriers, including chemical instability, efflux transport, and first-pass metabolism, leading to reduced absorption and variability in pharmacokinetics and pharmacodynamics [6,7]. The desired therapeutic effect of anti-cancer drugs may be compromised if a significant amount is not absorbed from the gastrointestinal tract or if the drug is eliminated before reaching the tumor site, leading to reduced treatment effectiveness and suboptimal tumor control [8]. Additionally, drug resistance may arise as a result of the low availability of drug molecules for the target sites of the cancerous cells [9]. Variations in drug absorption among patients, influenced by factors such as genetic makeup, metabolic processes, and concurrent medications, can complicate the determination of optimal dosages, resulting in subpar therapeutic outcomes. Moreover, the high cost of anti-cancer medications, combined with low bioavailability, may require higher doses or more frequent administration, escalating treatment expenses and placing a significant burden on both patients and healthcare systems [10,11,12]. Therefore, choosing a suitable nanocarrier delivery system is of the utmost importance to overcome issues associated with conventional drug formulations and attain high-efficiency oral delivery.
Lipid-polymer hybrid nanoparticles (LPHNs) have emerged as a promising nanocarrier system to overcome these challenges, offering a versatile platform for improving oral bioavailability and optimizing cancer treatment outcomes [13]. LPHNs are core-shell nanoparticles composed of a lipid shell surrounding a polymer core [14]. The properties of polymeric nanoparticles and liposomes have been integrated to develop LPHNs. The LPHNs consist of a lipid coating encompassing a polymer core that is loaded with a drug [15]. This unique structure enables the extended circulation of the drug in the bloodstream and provides protection during its passage through the complex gastrointestinal environment. Additionally, the design of LPHNs effectively prevents water from accessing the drug-containing core [16,17,18]. Typically, they improve the stability of drugs in biological fluids, as well as versatile drug loading, controlled release capacity, high cellular absorption efficiency, desirable pharmacokinetics, and extended circulation half-life [19,20]. LPHNs have become a highly developed nanocarrier system that can overcome various challenges associated with oral anti-cancer drug delivery [21].
In order to solve the problems of loading water-soluble, ionic drugs in the hydrophobic solid lipid phase and achieving proper loading and sustained release of such drugs, LPHNs were first developed in the late 1990s and early 2000 [22]. Since then, a number of formulations have been designed to encapsulate a single anti-cancer drug in combination with a chemosensitizer, an anti-cancer drug with a P-glycoprotein inhibitor, or a combination of two anti-cancer drugs that work synergistically [23,24,25]. Additionally, the formulation of siRNA or siRNA paired with anti-cancer drugs has been developed for administering biological products such as gene therapy and immunotherapy. Promising findings from recent studies have demonstrated that LPHN-based anti-cancer drug delivery avoids efflux transporter-mediated multidrug resistance (MDR) in cancer cells and increases anti-tumor efficacy, enhances the bioavailability, while also decreasing the systemic toxicity of the anti-cancer drugs [26,27,28].
To date, multiple studies have attempted to report the outcomes of preclinical studies and lab experiments in the area of LPHN synthesis methods, composition, drug loading strategies, and therapeutic potentials [29,30,31]. However, the mechanistic explanations for the oral bioavailability enhancement of chemotherapy drugs through the utilization of LPHNs are not compiled in a single study. The present review aims to cover the explanation and state-of-the-art process through which LPHNs enhance the oral bioavailability of chemotherapy drugs.
2. Anti-Cancer Drugs with Limited Bioavailability Issues
2.1. Factors Influencing Oral Bioavailability of Anti-Cancer Drugs
The term “bioavailability” refers to a drug’s in vivo performance and defines how quickly and thoroughly it reaches systemic circulation, thus, making it available for therapeutic action [32]. Drug stability in the gastrointestinal tract, its solubility in water, rate of dissolution, intestinal epithelium permeability, stability against metabolic enzymes (intestinal and liver), and P-glycoprotein efflux pump are the main factors influencing the oral bioavailability of the drug [33,34]. Most anti-cancer drugs have bioavailability issues due to the P-glycoprotein efflux effect. Numerous chemotherapy drugs, such as camptothecin, topotecan, topoisomerase II inhibitors (etoposide and teniposide), doxorubicin, anthracyclines and vinca alkaloids, face issues including reduced bioavailability and interpatient variability [35].
Drug-metabolizing enzymes, drug efflux pumps and transporters are the main physiological variables affecting anti-cancer drugs’ oral bioavailability [36]. For example, a cytotoxic drug called etoposide is used for treating several kinds of cancers including small-cell lung cancer, different types of lymphomas, and germ-cell tumors. The oral bioavailability of etoposide is around 47–76% [37]. The oral bioavailability of etoposide is constrained by a number of variables, including enzyme metabolism (CYP3A4) and instability in the stomach and intestinal fluids. The oral bioavailability of topotecan, a Topoisomerase-I inhibitor, is only 40% [38]. Exemestane, an irreversible aromatase inhibitor, is primarily used as a first-line therapy for estrogen receptor-positive breast cancer patients. However, complex physicochemical characteristics limit its oral bioavailability (<10%) and anti-breast cancer efficacy [39]. Additionally, an antimetabolite called fluorouracil (5-FU) is used to treat a number of solid tumors. Even though oral and intravenous preparations of 5-FU exist, its oral absorption is lower and oral bioavailability is very unpredictable and variable [40]. Poor water solubility is a characteristic of small-molecule tyrosine kinase inhibitors, including pazopanib, vemurafenib, and lapatinib, which is a crucial factor for their limited absorption and bioavailability. The bioavailability of erlotinib, gefitinib, and pazopanib is negatively affected by antacid co-administration. In the case of taking tyrosine kinase inhibitors with antacids, the area under the curve decreases by 50% [41]. Ibrutinib has a remarkably poor bioavailability (2.7%), which contributes to the large interpatient variability in drug exposure with oral treatment [42]. In light of the aforementioned chemotherapeutic drugs with limited bioavailability, it becomes evident that an advanced drug delivery approach is imperative. The poor bioavailability exhibited by these drugs highlights the necessity for a more sophisticated method of administering them.
2.2. Planning Oral Formulation to Bypass Limited Bioavailability Issue
Complex formulation issues, such as decreased aqueous solubility, degradation by enzymes in the GI tract, first-pass metabolism of the drug by the liver cytochrome P450 (CYP3A4) and P-glycoprotein (intestine), and poor drug permeability through intestinal walls, should be addressed when planning oral delivery of such drug agents [43]. Pharmaceutical nanotechnology also known as chemotherapeutic engineering, cancer nanotechnology, or nanomedicine suggests that oral chemotherapy can be carried out by formulating the drugs in a variety of nanocarriers, such as LPHNs, solid lipid nanoparticles (SLNs), nanoemulsion and micelles, which are able to avoid P-gp recognition and non-specific cellular uptake of the loaded drug and facilitate prolonged, regulated, and targeted chemotherapy [44,45,46].
3. Lipid Polymer Hybrid Nanoparticles (LPHNs)
3.1. Basic Formulation Concept
The LPHNs are composed of three parts; a monolayer of phospholipids around the core to enhance overall biological compatibility, a hydrophilic polymeric layer outside the lipid to ensure formulation stability, and a biodegradable hydrophobic polymeric core to encapsulate hydrophobic drugs (as illustrated in Figure 1) [47,48]. The lipid shell helps to provide stability and appropriate biocompatibility, while the polymeric core is employed for carrying a variety of small molecules (drugs). Because of their outstanding biodegradability and biocompatibility, poly (lactic-co-glycolic acid) (PLGA) along with polycaprolactone (PCL) are the most utilized polymers. Lipids come in a variety of forms and can be utilized to create nanoparticles. Zwitterionic, cationic, anionic, and neutral phospholipids, such as lecithin, 1,2-dipalmitoyl-sn-glycero-3-phosphocholine or 1,2-dio-leoyl-sn-glycero-3-phosphoethanolamine, cholesterol, and myristic acid, as well as polyethylene glycol conjugate, are some of the frequently used lipids [49,50].
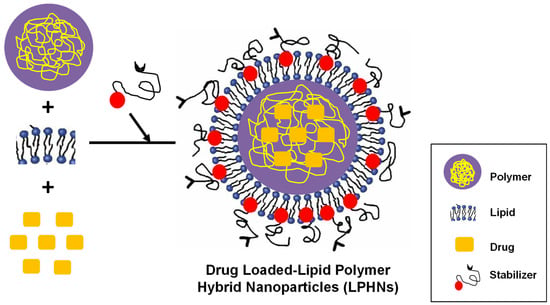
Figure 1.
Composition of LPHNs. They are composed of three parts; (i) A monolayer of phospholipids around the core, (ii) A hydrophilic polymeric layer outside the lipid core, and (iii) A biodegradable hydrophobic polymeric core encapsulating drug agent.
Several formulation processes have been reported and are used to produce LPHNs. These methods are based on the chemical and physical characteristics of the underlying components as well as the desired therapeutic outcome [51,52]. The ionic strength of the continuous phase, which is the aqueous medium containing the lipid vesicles and polymer NPs, the charge of the lipid formulation, and additional factors including size homogeneity of preformed lipid vesicles, the ratio of lipid vesicles to polymeric nanoparticles, all affect the physical properties such as size homogeneity and colloidal stability of LPHNs [53]. LPHNs may generally be synthesized using two different methods. In the two-step method, a polymer core and lipid shell are made separately first and subsequently combined at the end, while the alternative single-step method uses a one-step approach that involves single-step nanoprecipitation and self-assembly to create hybrid nanoparticles. The surface of the resultant LPHNs is often further functionalized with targeting ligands enabling payload distribution to specific cells or tissues for biomedical purposes (active targeting) [29,53].
3.2. Preparation Methods
3.2.1. Single-Step Approach
LPHNs with a lipid monolayer shell are frequently produced using the one-step method. In this technique, lipids and lipid PEG conjugates are dissolved in an aqueous solution whereas free polymers and hydrophobic drugs are dissolved in an organic solvent, and the used organic solvent must be a water-miscible solvent, such as acetonitrile. A small quantity of water-miscible organic solvent can be added to the aqueous solution to help with the solubilization of phospholipids in the solution. Following that, the gradual addition of the polymer solution is made to the lipid aqueous solution. Accelerated diffusion of the organic solvent into the aqueous solution allows the polymer to precipitate as nanoparticles. Through hydrophobic interactions, the lipids and lipid polyethylene glycol (PEG) will self-assemble on the surface of polymer nanoparticles (Figure 2A) [54,55].
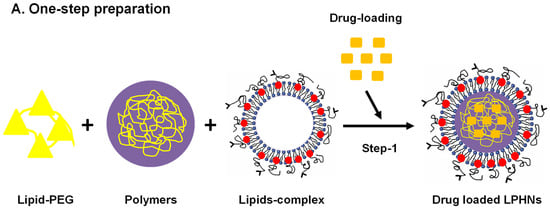
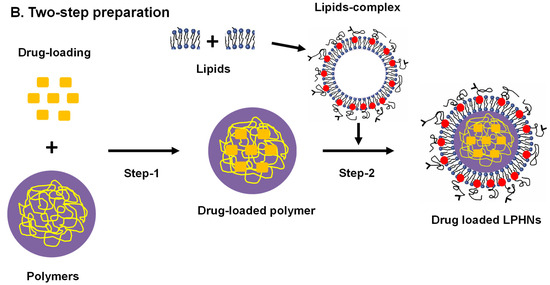
Figure 2.
Schematic illustration of drug-loaded LPHN preparations, (A) Single-step (B) Two-step preparation. PEG: polyethylene glycol.
The hydrophilic group of lipids extends into the surrounding aqueous environment, whereas the hydrophobic groups of lipids adhere to the hydrophobic polymer core. The lipid PEG conjugate is involved in the self-assembly process, as its PEG molecules are positioned on the outside while its lipid moiety is inserted inside the lipid monolayer for a stabilizing core. Lipids and lipid PEG conjugates may self-assemble at a temperature above the lipid phase transition. A hydrophobic polymer like PLGA should be utilized since the self-assembled lipid monolayer occurs because of hydrophobic interactions. This one-step self-assembly method is an inexpensive, feasible, and accurate method for producing LPHNs [56]. As an example, a recent study reported the preparation of folic acid decorated palbociclib loaded LPHNs using a single-step approach involving PLGA, with a lactic acid to glycolic ratio of (65:35)], and L-α phosphatidylcholine, respectively. The resulting LPHNs exhibited particle size of 143.36 ± 5.24 nm, 0.172 ± 0.004, zeta potential of −16.84 ± 0.27 mV, and % encapsulation efficiency of 93.12 ± 0.43, and an approximately 9, 11-fold reduction in IC50 values compared to free palbociclib in MCF-7 and MDA-MB-231 cells at 48 h [57].
3.2.2. Classical Two-Step Approach
Most small-scale LPHN preparations are carried out utilizing conventional approaches. These procedures are usually employed in small-scale preparations for hybrid nanoparticles (Figure 2B). By using high-pressure homogenization, nanoprecipitation, or solvent evaporation during emulsification, polymeric nanoparticles can be developed. There are two types of conventional two-step methodologies. The thin lipid film can be dried by dissolving it inside an organic solvent like chloroform and evaporating it in a rotary evaporator or it can be transformed into preformed lipid vesicles by hydrating it. Differential centrifugation is employed in the purification process to separate free lipids and LPHNs [58,59].
3.2.3. Modern Two-Step Approach
This novel approach is mostly used for developing a significant amount of LPHNs. The procedure includes mixing lipid vesicles with polymeric nanoparticles. Innovative techniques such as spray drying and soft lithography particle molding are used to create LPHNs. The 400–500 nm nanoparticles are produced by spray drying and then distributed in dichloromethane, an organic solvent that includes a wide range of lipid molecules. To make dry powdered LPHNs, lipid polymeric solutions are spray-dried using the processes of spray drying (SD) and spray freeze drying (SFD) [60,61].
Next, the organic solvent polymer, a sheet of Polyethylene terephthalate, is coated with PLGA that has been dissolved. Polyethylene terephthalate is heated in close contact with a soft lithography molding method, also known as particle replication in non-wetting templates (PRINT mold), which causes the polymer to flow into the mold and solidify when the temperature is lowered to room temperature. After that, LPHNs are created by removing nanoparticles from the mold and separating them from Polyethylene terephthalate sheets that had been coated with poly (vinyl alcohol) (PVA) using an aqueous lipid solution. After freeze-drying, these particles must exhibit a (+) 5 mV zeta potential and be needle-shaped and in 200 nm length [62,63].
3.2.4. Self-Assembled Nanoprecipitation Method
By employing this self-assembled nanoprecipitation technique, significant yields of LPHNs smaller than 100 nm may be achieved. The lipid shell and polymer core are made separately in two steps, and they are then combined to form a bilayer. A drug, polymer, and lipid are combined with each other in one phase during the self-assembly nanoprecipitation process to produce a monolayer of nanoparticles (Figure 3). LPHNs of dextran sulphate are prepared using a self-assembled nanoprecipitation approach to increase the efficacy of vincristine sulphate encapsulation and oral bioavailability [64,65].
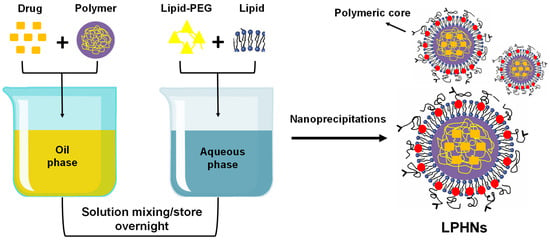
Figure 3.
Preparation of LPHNs using the nanoprecipitation (self-assembled) method.
3.2.5. Single Emulsification Solvent Evaporation
In this method, the polymer and drug to be encapsulated are dissolved in the organic (oil) phase. Using ultrasonic stirring, the solution is then gradually added to a lipid-water dispersion medium, forming an oil-in-water (o/w) emulsion. A rotary evaporator is employed to evaporate the organic solvent under reduced pressure, resulting in the formation of a polymer core, while the lipid-PEG conjugates self-assemble around the core (Figure 4). This technique is preferred over nanoprecipitation for the formulation of various LPHNs due to its ability to form stable emulsions. It is commonly used in the preparation of LPHNs loaded with nucleic acids for cancer treatment [18,66].
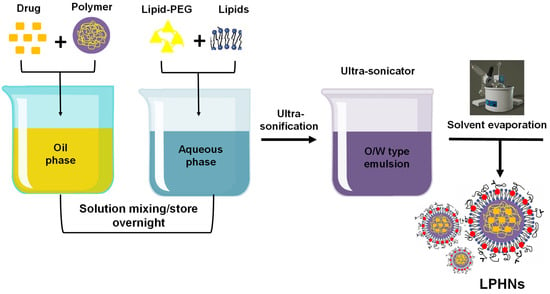
Figure 4.
Preparation of LPHNs using the single-step emulsification solvent evaporation technique.
3.2.6. Dual Emulsification Solvent Evaporation
The two-fold emulsification solvent evaporation procedure is the most efficient method for the production of polymeric nanoparticles. This method is essential to develop core-shell type LPHNs as well as polymeric nanoparticles. It provides a high encapsulation capability for both hydrophobic and hydrophilic drugs. This method is mostly used for determining drugs that are easily soluble in water but insoluble in the organic phase. The creation of (w/o/w) emulsion often employs this approach. A drug is first combined with an aqueous polymeric solution and an organic solvent that contains a lipid to form a (w/o) emulsion. A new (w/o/w) emulsion originates by shifting the emulsion to another aqueous medium. LPHNs are produced when the oil phase of an emulsion is evaporated using a rotary evaporator (Figure 5). This method is used to encapsulate nucleic acids and chemotherapy drugs [61,67].
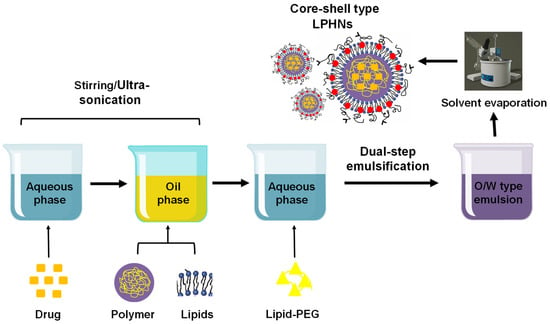
Figure 5.
Preparation of LPHNs using the dual-step emulsification solvent evaporation technique.
3.3. Characterization and Assessment of % Drug Loading (DL) and % Encapsulation Efficiency (EE)
Characterization techniques are employed to evaluate various aspects of LPHNs, including their size, surface charge, morphology, drug loading, release kinetics, and biocompatibility. Methods like dynamic light scattering, electron microscopy, and spectroscopy are utilized to analyze particle size, shape, and surface properties. Drug loading and encapsulation efficiency can be assessed using chromatography or spectrophotometry. In vitro release studies examine controlled release patterns, while cell viability assays and in vivo studies investigate biocompatibility and toxicity. These characterization methods collectively provide valuable insights to optimize and comprehend the properties of LPHNs as effective drug delivery systems for chemotherapeutic drug agents [68].
In the context of drug delivery systems like LPHNs, the purpose of drug loading (DL) and encapsulation efficiency (EE) is to assess the effectiveness of these nanoparticles (NPs) in loading and delivering chemotherapeutic drugs. DL refers to the amount of drug that can be loaded into the NPs, while EE measures the efficiency of drug encapsulation within the NPs. The DL and EE of the NPs are important indexes for drug delivery systems. The DL and EE of LPHNs determined for paclitaxel were 27.71% and 92.24%. The high DL and EE values prove the effectiveness of the NPs of the lipid monolayer shell and polymeric core to load anti-cancer drugs [19].
Based on the data from Table 1, the LPHNs demonstrate a wide range of EE% for various anti-cancer drugs, making them versatile carriers for chemotherapeutic agents. Various researchers have attempted to formulate LPHNs using different lipid and polymer compositions. The EE% of these drugs varies depending on the type of lipid, polymer, and drug that was loaded, as well as the method of preparation. The preparation method with the highest encapsulation efficiency (EE%) is W/O/W double emulsification used in the preparation of curcumin loaded in a mPEG-PLGA copolymer [69], and 5-FU, irinotecan, oxaliplatin loaded in mPEG-PLA opolymer [70], which showed an EE% of 96% for both studies. Similarly, using dual-step sonication [28], single-step solvent evaporation [71], and single-step nanoprecipitation [72] also showed the highest EE% of 95.5 [28], 95 [71], and 94.5% [72], respectively. For loaded drugs, the EE of afatinib-loaded LPHNs was found to be 93.2%, while cisplatin-loaded LPHNs had EE values ranging from 20.5% to 91.8%, depending on the carrier composition and method of preparation [73,74,75]. Other drugs like curcumin [69], docetaxel [76,77], and paclitaxel [19], also showed significant EE values, with curcumin-loaded LPHNs reaching an impressive 96% EE using HPLC detection [69], while docetaxel-loaded LPHNs had EE values between 10% and 89.1%, depending on the formulation [76,77]. Additionally, other agents like 5-FU, afatinib, bevacizumab, doxorubicin, etoposide, gemcitabine, honokiol, isoliquiritigenin, methotrexate, melphalan, mitomycin C, mycophenolic acid, solasonine and solamargine, tamoxifen, thymoquinone, vorinostat, and 10-hydroxycamptothecin are loaded into LPHNs using various approaches which exhibited a varying EE%, making them promising candidates for combination therapy (Table 1).

Table 1.
LPHN-loaded anti-cancer drugs, composition (type of lipid, and polymer), preparation method, size, EE (%), and detection method.
3.4. Drug Release Mechanisms
The hydrophobic drugs can be carried by the LPHNs with high loading yields, and their dissolution kinetics can be controlled. A significant number of hydrophobic drugs may be directly enclosed within the hydrophobic polymer core or chemically bonded to the polymer chains in the hydrophobic polymer core. The lipid shell is predicted to improve drug loading yield by preventing tiny drug molecules from readily diffusing out of the polymer core and reducing the rate of water entering the polymer core, which delays the breakdown of the polymer and the release of the drug from the LPHN [99].
Drug diffusion and polymer erosion are generally employed to release drugs from the LPHNs. The hydrolysis of the linkers between the drug and polymer chains and subsequent drug diffusion regulates the release of drug molecules. The degradation rate and particle size affect the drug release profile from the hybrid nanoparticles [100]. In addition, a remote radio frequency magnetic field is also used for drug release from the LPHN system. For instance, the magnetic field-activated LPHN nanosystem loaded with camptothecin showed long-term stability in terms of particle size and polydispersity index in phosphate-buffered saline, and stimuli-responsive drug release [101]. Typically, drug release studies are carried out using the dialysis technique. To put it briefly, a large volume release medium at 37 °C with moderate agitation is placed in a dialysis cassette carrying drug-loaded nanoparticles. The drug molecules will frequently leak into the release media from the nanoparticles. For quantification, the drug molecules which have been released or which remain inside the nanoparticles are collected at various time points using analytical methods including high-performance liquid chromatography (HPLC) and mass spectrometry [102,103].
3.5. Oral Bioavailability Enhancement (Preclinical Studies)
LPHNs can enhance the oral bioavailability of anti-cancer drugs via several mechanisms. LPHNs protect the encapsulated drug from degradation in harsh gastrointestinal environments, such as low pH and enzymatic degradation [60,104]. Paclitaxel-loaded LPHNs showed an absolute bioavailability of 21.95% compared with that of paclitaxel conventional formulation, which had an oral bioavailability of 4.75%. Cytochrome 450 and P-glycoprotein (P-gp) inhibitors incorporation further improved the oral bioavailability of paclitaxel to 42.60%, and bioavailability almost increased almost eight-fold [105]. LPHNs can also increase the solubility of many anti-cancer hydrophobic drugs. Subsequently, it leads to their improved absorption and oral bioavailability [104]. Another special characteristic of LPHN formulation is to target specific cancer cells by modifying the surface of the nanoparticles with ligands such as folic acid, and folate, which bind to specific receptors on the cancer cell surface [19,57]. Subsequently, bioadhesive systems can extend the retention time of the insoluble drugs, causing it to remain for a longer period of time in the GI tract, so they are frequently used to improve oral drug absorption. The interaction of nanoparticles with mucin improves mucus penetration and enhances bioavailability [106]. Mucin is the main component of mucus, which is secreted by the mucus layer overlying the intestinal epithelium. Intestinal epithelium is considered the first line of oral absorptive barriers [107]. LPHNs of cabazitaxel showed higher mucus penetration in comparison to free cabazitaxel. The oral bioavailability of cabazitaxel was elevated from 7.7% to 56.6% (7.3 times higher) [108].
LPHNs can be designed to release the encapsulated drug in a controlled manner, providing sustained drug delivery and making it available in systemic blood circulation for a longer period of time, which results in improved bioavailability and reduces the frequency of dosing [86,109]. Tamoxifen-loaded-LPHNs exhibited enhanced oral bioavailability with a considerable increase in area under the curve (AUC) (1277.46 vs. 585.01 ng/mL·h) in comparison to tamoxifen. LPHNs showed a markedly extended half-life (27.87 25.72 vs. 17.42 12.04 h) and an enhanced mean residence duration (40.1 h) with a heightened Tmax in comparison to pure tamoxifen. As a result, tamoxifen’s bioavailability improved by more than two-fold when incorporated into LPHNs [96].
If a drug is capable of passing through the intestinal mucus barrier, it can be absorbed in the epithelium more effectively and delivered more quickly, resulting in enhanced bioavailability [110]. Because of the potent electrostatic interactions between positively charged NPs and the negatively charged sialic groups of mucins, it has been predicted that positively charged NPs have a high affinity for mucus [111]. It was found that muco-adhesion of the positively charged paclitaxel-loaded LPHNs showed an absolute bioavailability of 21.95% compared with that of paclitaxel conventional formulation, which had an oral bioavailability of 4.75% [105].
4. LPHN-Based Anticancer Drug Delivery
Oral chemotherapeutic drug use has been steadily increasing. The ease of home-based therapy is the main reason for choosing anti-cancer oral therapy over intravenous therapy. The primary benefits of using oral anti-cancer drugs among oncologists include enhanced compliance among patients, drug tolerability, and ease of use. Oral chemotherapeutic drugs bioavailability variability among patients may be of greater significance than intravenous agents [112].
Various cytotoxic drug combinations, such as gemcitabine and paclitaxel tested on pancreatic cancer cell lines (XPA3), cisplatin and paclitaxel tested on ovarian cancer cell lines (A2780), and doxorubicin and camptothecin tested on breast cancer cell lines (MDA-MB-435), have been co-delivered using LPHNs as a drug delivery platform. Considering LPHN-based drug delivery, enhanced intracellular entry via endocytosis consequently overcomes their poor cellular absorption. Conjugated paclitaxel and cisplatin LPHNs demonstrated increased cytotoxicity on human ovarian cancer cells compared to free drug combinations [113].
The poor water solubility and oral bioavailability of phytochemicals like thymoquinone (THQ) restrict their use in clinical applications. The optimized LPHNs showed remarkable qualities for the best oral THQ delivery. THQ-LPHNs demonstrated exceptional gastrointestinal stability as well as storage stability under various environmental conditions. In both gastric and intestinal media, THQ-LPHNs showed nearly identical release characteristics, with an initial rapid release lasting 4 h and a subsequent sustained release lasting up to 48 h. After oral administration, THQ-LPHNs demonstrated a 4.74-fold higher bioavailability than the standard THQ solution [97].
Male Wistar rats were administered paclitaxel (10 mg/kg) orally, which resulted in a maximum plasma concentration (Cmax) of 2581 ng/mL. In contrast, paclitaxel-loaded LPHNs demonstrated a three-fold increase in drug concentration, reaching a Cmax of 7609 ng/mL, indicating a three-fold higher bioavailability [92]. When compared to the free paclitaxel solution, Pandita et al. concluded that the drug exposure in tissues and plasma was 2-fold and 10-fold greater following oral administration of the paclitaxel-loaded LPHNs [114]. For oral delivery, paclitaxel-loaded LPHNs with a size range of 200–300 nm was developed to withstand challenging GIT conditions and increase paclitaxel’s bioavailability. Comparatively, an increase in bioavailability and elimination half-life of 1.5 and 5.5 times, respectively, was reported [115]. When compared to free paclitaxel solution, the drug exposure in plasma and tissues after the oral administration of paclitaxel LPHNs was 10 and 2 times greater, respectively [114].
The oral bioavailability of cabazitaxel was elevated from 7.7% to 56.6% (7.3 times higher) when administered through LPHNs [108]. When administered using an LPHN formulation, cisplatin exhibited a half-life (T1/2) of 5.4 h and a Cmax of 17.1 L/kg/h, whereas the free drug had a far lower half-life of 1.7 h and a Cmax of 2.3 L/kg/h. Once cisplatin was administered through LPHNs, there was an approximately three-fold increase in the half-life and a seven-fold rise in Cmax. According to an in-vivo pharmacokinetics analysis, the AUCs for cisplatin-LPHNs and free cisplatin were 247 and 16 mg/L/h, respectively [78]. Only small quantities of doxorubicin and sorafinib were able to be found after 12 h of administration, which indicates that doxorubicin and sorafinib were quickly eliminated in their free form. On the other hand, drugs loaded into LPHNs showed extended retention for up to 36 h [116]. The targeted LPHNs carrying a doxorubicin and gallic acid combo coated with hyaluronic acid showed enhanced cytotoxicity and exhibited higher distribution in tumor compared to free drug in acute myeloid leukemia bearing mice. No significant differences in drug distribution were found in the other organs [117].
Folate-modified LPHNs for targeted paclitaxel delivery showed greater tumor growth inhibition (65.78%) compared to paclitaxel-loaded LPHNs (48.38%) [19]. Paclitaxel and etoposide combinations were far more cytotoxic than either drug alone. Similar to this fact, LPHN-based administration showed much boosted cancer cell death (~45%). The therapeutic efficacy of etoposide and paclitaxel for treating osteosarcoma was improved when the drugs were combined in LPHNs [118]. Furthermore, 5-FU and sulforaphane-loaded LPHNs significantly enhanced the cytotoxicity against HCT 15 colorectal cancer cell lines. The dual-drug-loaded LPHNs demonstrated an increased apoptotic effect compared to free drugs, suggesting that the combination of 5-FU and sulforaphane within the LPHNs may synergistically improve the therapeutic efficacy, leading to more effective cancer cell killing [119]. The LPHN-based drug delivery of paclitaxel and etoposide was found to have synergistic anti-cancer effects against osteosarcoma [120]. When mice were intravenously given doses of 0.1 and 0.2 mg of doxorubicin-loaded LPHNs, there was a 70 and 100% delay in the development of the cancer, respectively. The cytotoxic activity of the LPHNs against solid tumors appeared helpful, and they increased the efficacy of treatment [121]. In addition, the cell cytotoxicity assay revealed significantly higher cell cytotoxicity of LPHNs against human breast cancer cell lines (MCF-7) compared to the free drugs mycophenolic acid and quercetin, and their combination in both concentrations was in a time-dependent manner [90]. Gemcitabine is also delivered via LPHNs, which prolongs blood circulation duration, which is beneficial for gemcitabine accumulation in breast cancer mice models. Furthermore, gemcitabine loaded with LPHNs showed improved anti-tumor activity in rats when compared to commercial product Gemko® [86]. For the treatment of hormone-resistant prostate cancer, LPHNs that encapsulated tumor-targeted docetaxel increased its efficacy and safety [77].
The short half-life and mean retention time, which were responsible for the poor pharmacokinetic behavior, were compared with the high elimination rate and drug clearance of free doxorubicin and sorafinib. The pharmacokinetic characteristics of both drugs were greatly improved when they were co-loaded in LPHNs, respectively. LPHN-based drug delivery showed extended retention for 36 h [116]. Erlotinib-loaded LPHNs demonstrated a significant absorption into the cytoplasm of cells, and the in-vitro cell viability experiment demonstrated a significant decrease in the proliferation of A549 cells after 72 h [83]. Squamous cell carcinoma (SCC-7), human breast adenocarcinoma (MCF-7), and human Caucasian breast adenocarcinoma (MDA-MB-231) cells had all been more efficiently inhibited by the drug-loaded carrier when docetaxel and vorinostat were administered together through LPHNs [81].
5. Limitations and Prospects
LPHNs have emerged as a potential strategy for cancer therapy and drug delivery. However, there are certain limitations that need to be addressed for their successful implementation. Firstly, there are technological requirements that must be fulfilled to achieve uniformity and repeatability on a large scale. Additionally, creating LPHNs with a consistent size distribution and diverse functionalities for enhanced in-vivo performance poses challenges, particularly in traversing multiple epithelial barriers in lung tissue [122]. The limitations of LPHNs include a limited drug loading capability, which restricts the amount of drug that can be delivered per nanoparticle, potentially impacting the treatment of larger tumors or cancer tissues [77]. Stability issues, such as aggregation or degradation, can affect the ability of LPHNs to efficiently transport drugs to the desired site [123,124]. Moreover, the targeting capability of LPHNs is often limited by factors such as surface charge, size, and availability of targeting ligands [125]. Scale-up hurdles pose challenges in producing LPHNs at a sufficiently large scale for clinical usage, which may affect their accessibility and cost [126].
Despite the aforementioned limitations, there are several promising opportunities for the future of anti-cancer drug delivery using LPHNs. These opportunities include enhancing the drug-loading capacity of LPHNs through techniques like covalent bonding, prodrug conjugation, and multistage delivery systems [127]. Moreover, researchers are also developing novel polymers and protective coatings, such as polymer-coated LPHNs, to improve their stability and prolong their circulation in the bloodstream [115,128]. Advancements in targeting capabilities are being explored, including the use of tumor-specific ligands, responsive surface coatings, and biomimetic materials which allow site-specific drug release through LPHNs [129]. Modern imaging techniques, such as fluorescence labeling and magnetic resonance imaging (MRI), enable better visualization and monitoring of LPHNs within the body which facilitates precise delivery to the target site [130,131]. Additionally, LPHNs can be utilized in combination with other therapeutic approaches like gene or immune therapies, thereby augmenting the overall efficacy of cancer treatment [131]. Lastly, in order to allow widespread clinical usage of LPHNs, researchers are also developing scalable production procedures [132,133]. In overall terms, LPHNs hold an optimistic outlook for the delivery of anti-cancer drugs, and more research and development in this area might lead to the advancement of more efficient and reliable therapies for cancer.
6. Conclusions
LPHNs have a lot of potential as an innovative and successful drug delivery technology for anti-cancer drugs having limited oral bioavailability issues. Studies have shown that LPHNs significantly improve drug bioavailability, such as paclitaxel (21.95% compared to 4.75%), cabazitaxel (7.3-fold increase from 7.7% to 56.6%), and thymoquinone (4.74-fold increase). Tamoxifen-loaded LPHNs demonstrated more than a two-fold increase in AUC (1277.46 ng/mL·h vs. 585.01 ng/mL·h) and extended half-life. Furthermore, LPHNs have enhanced drug cytotoxicity, as seen with paclitaxel and cisplatin combinations, and improved anti-tumor activity with drugs like gemcitabine, showing a significant increase in drug exposure and therapeutic effects compared to free formulations. EE% values for LPHNs also vary across different drugs and types of lipids and polymers, highlighting their versatility. For instance, LPHNs loaded with paclitaxel achieved an impressive EE% of 85%, while gemcitabine-loaded LPHNs showed a lower EE% of 56.7%. The EE% for drugs like doxorubicin and sorafenib in LPHNs has been reported to be around 75%, while LPHNs containing docetaxel achieved EE% values as high as 87%. In contrast, lower EE% values have been observed with certain formulations, such as those loaded with camptothecin, which had an EE% of approximately 55%. The lipid DLPC and the polymer PLGA and DSPE-PEG 2000 combination provide the highest EE% of 93.2% for afatinab. Similarly, Lecithin combined with PLGA and DSPE-PEG 2000 also shows a high EE% of 96% for Curcumin.
Furthermore, LPHNs have demonstrated significant improvement in cytotoxicity, with paclitaxel and cisplatin-loaded LPHNs showing enhanced cytotoxic effects in ovarian cancer cell lines (A2780), and doxorubicin-loaded LPHNs exhibiting extended retention in the body for up to 36 h. These LPHN formulations have also been shown to increase drug exposure in tissues and plasma, which is crucial for improving cancer treatment efficacy. In conclusion, LPHNs hold a bright future in the delivery of chemotherapy drugs, and more research and development in this area may result in the creation of more effective and specialized therapies for cancer. We are sure that with additional technological and manufacturing advancements, LPHNs will eventually have a significant role in the battle against cancer, moving us one step closer to uncovering a cure against cancer.
Funding
This research received no external funding.
Acknowledgments
The author would like to thank the Deanship of Scientific Research at Shaqra University for supporting this work.
Conflicts of Interest
The author declares no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Kawish, S.M.; Sharma, S.; Gupta, P.; Ahmad, F.J.; Iqbal, M.; Alshabrmi, F.M.; Anwer, M.K.; Fathi-karkan, S.; Rahdar, A.; Aboudzadeh, M.A. Nanoparticle-Based Drug Delivery Platform for Simultaneous Administration of Phytochemicals and Chemotherapeutics: Emerging Trends in Cancer Management. Part. Part. Syst. Charact. 2024, 41, 2400049. [Google Scholar] [CrossRef]
- Chini, C.; Bascialla, L.; Giaquinto, A.; Magni, E.; Gobba, S.; Proserpio, I.; Suter, M.; Nigro, O.; Tinelli, G.; Pinotti, G. Homcology: Home chemotherapy delivery in a simultaneous care project for frail advanced cancer patients. Support. Care Cancer 2021, 29, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Thanki, K.; Gangwal, R.P.; Sangamwar, A.T.; Jain, S. Oral delivery of anticancer drugs: Challenges and opportunities. J. Control. Release 2013, 170, 15–40. [Google Scholar] [CrossRef] [PubMed]
- Jibodh, R.A.; Lagas, J.S.; Nuijen, B.; Beijnen, J.H.; Schellens, J.H. Taxanes: Old drugs, new oral formulations. Eur. J. Pharmacol. 2013, 717, 40–46. [Google Scholar] [CrossRef]
- El Moukhtari, S.H.; Rodriguez-Nogales, C.; Blanco-Prieto, M.J. Oral lipid nanomedicines: Current status and future perspectives in cancer treatment. Adv. Drug Deliv. Rev. 2021, 173, 238–251. [Google Scholar] [CrossRef]
- Dudhipala, N.; Puchchakayala, G. Capecitabine lipid nanoparticles for anti-colon cancer activity in 1, 2-dimethylhydrazine-induced colon cancer: Preparation, cytotoxic, pharmacokinetic, and pathological evaluation. Drug Dev. Ind. Pharm. 2018, 44, 1572–1582. [Google Scholar] [CrossRef]
- Wu, L.; Li, X.; Janagam, D.R.; Lowe, T.L. Overcoming the blood-brain barrier in chemotherapy treatment of pediatric brain tumors. Pharm. Res. 2014, 31, 531–540. [Google Scholar] [CrossRef]
- Hou, J.; Sun, E.; Zhang, Z.-H.; Wang, J.; Yang, L.; Cui, L.; Ke, Z.-C.; Tan, X.-B.; Jia, X.-B.; Lv, H. Improved oral absorption and anti-lung cancer activity of paclitaxel-loaded mixed micelles. Drug Deliv. 2017, 24, 261–269. [Google Scholar] [CrossRef]
- Vaidya, F.U.; Sufiyan Chhipa, A.; Mishra, V.; Gupta, V.K.; Rawat, S.G.; Kumar, A.; Pathak, C. Molecular and cellular paradigms of multidrug resistance in cancer. Cancer Rep. 2022, 5, e1291. [Google Scholar] [CrossRef]
- Sylvester, P.W.; Akl, M.R.; Malaviya, A.; Parajuli, P.; Ananthula, S.; Tiwari, R.V.; Ayoub, N.M. Potential role of tocotrienols in the treatment and prevention of breast cancer. Biofactors 2014, 40, 49–58. [Google Scholar] [CrossRef]
- Kaur, P.; Chaurasia, C.S.; Davit, B.M.; Conner, D.P. Bioequivalence study designs for generic solid oral anticancer drug products: Scientific and regulatory considerations. J. Clin. Pharmacol. 2013, 53, 1252–1260. [Google Scholar] [CrossRef] [PubMed]
- Ananthula, S. Bioavailability and bioequivalence issues associated with oral anticancer drugs and effect on drug market. J. Bioequiv. Availab. 2014, 6, e56. [Google Scholar] [CrossRef]
- Kassaee, S.N.; Richard, D.; Ayoko, G.A.; Islam, N. Lipid polymer hybrid nanoparticles against lung cancer and their application as inhalable formulation. Nanomedicine 2024, 19, 2113–2133. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Cañadas, O.; Alonso, A.; Franzyk, H.; Thakur, A.; Pérez-Gil, J.; Foged, C. Effect of lipid-polymer hybrid nanoparticles on the biophysical function and lateral structure of pulmonary surfactant: Mechanistic in vitro studies. J. Colloid Interface Sci. 2024, 654, 1111–1123. [Google Scholar] [CrossRef]
- Chen, Y.; Deng, Y.; Zhu, C.; Xiang, C. Anti prostate cancer therapy: Aptamer-functionalized, curcumin and cabazitaxel co-delivered, tumor targeted lipid-polymer hybrid nanoparticles. Biomed. Pharmacother. 2020, 127, 110181. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, D.; Wu, Y.; Li, W.; Hu, Y.; Zhao, G.; Fu, C.; Fu, S.; Zou, L. Lipid–polymer hybrid nanoparticles for oral delivery of tartary buckwheat flavonoids. J. Agric. Food Chem. 2018, 66, 4923–4932. [Google Scholar] [CrossRef]
- Zhao, X.; Li, F.; Li, Y.; Wang, H.; Ren, H.; Chen, J.; Nie, G.; Hao, J. Co-delivery of HIF1α siRNA and gemcitabine via biocompatible lipid-polymer hybrid nanoparticles for effective treatment of pancreatic cancer. Biomaterials 2015, 46, 13–25. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Anwer, M.K.; Fatima, F.; Aldawsari, M.F.; Alalaiwe, A.; Alali, A.S.; Alharthi, A.I.; Kalam, M.A. Boosting the anticancer activity of sunitinib malate in breast cancer through lipid polymer hybrid nanoparticles approach. Polymers 2022, 14, 2459. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, D.; Dong, X.; Sun, H.; Song, C.; Wang, C.; Kong, D. Folate-modified lipid–polymer hybrid nanoparticles for targeted paclitaxel delivery. Int. J. Nanomed. 2015, 10, 2101–2114. [Google Scholar] [CrossRef]
- Seedat, N.; Kalhapure, R.S.; Mocktar, C.; Vepuri, S.; Jadhav, M.; Soliman, M.; Govender, T. Co-encapsulation of multi-lipids and polymers enhances the performance of vancomycin in lipid–polymer hybrid nanoparticles: In vitro and in silico studies. Mater. Sci. Eng. C 2016, 61, 616–630. [Google Scholar] [CrossRef]
- Liu, H.; Zhuang, Y.; Wang, P.; Zou, T.; Lan, M.; Li, L.; Liu, F.; Cai, T.; Cai, Y. Polymeric lipid hybrid nanoparticles as a delivery system enhance the antitumor effect of emodin in vitro and in vivo. J. Pharm. Sci. 2021, 110, 2986–2996. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Y. Strategies for optimizing polymer-lipid hybrid nanoparticle-mediated drug delivery. Expert Opin. Drug Deliv. 2016, 13, 609–612. [Google Scholar] [CrossRef] [PubMed]
- Bansal, T.; Akhtar, N.; Jaggi, M.; Khar, R.K.; Talegaonkar, S. Novel formulation approaches for optimising delivery of anticancer drugs based on P-glycoprotein modulation. Drug Discov. Today 2009, 14, 1067–1074. [Google Scholar] [CrossRef]
- Shuhendler, A.J.; Cheung, R.Y.; Manias, J.; Connor, A.; Rauth, A.M.; Wu, X.Y. A novel doxorubicin-mitomycin C co-encapsulated nanoparticle formulation exhibits anti-cancer synergy in multidrug resistant human breast cancer cells. Breast Cancer Res. Treat. 2010, 119, 255–269. [Google Scholar] [CrossRef]
- Yang, X.-Z.; Dou, S.; Wang, Y.-C.; Long, H.-Y.; Xiong, M.-H.; Mao, C.-Q.; Yao, Y.-D.; Wang, J. Single-step assembly of cationic lipid–polymer hybrid nanoparticles for systemic delivery of siRNA. ACS Nano 2012, 6, 4955–4965. [Google Scholar] [CrossRef]
- Ezzat, H.M.; Elnaggar, Y.S.; Abdallah, O.Y. Improved oral bioavailability of the anticancer drug catechin using chitosomes: Design, in-vitro appraisal and in-vivo studies. Int. J. Pharm. 2019, 565, 488–498. [Google Scholar] [CrossRef]
- Garg, N.K.; Tyagi, R.K.; Sharma, G.; Jain, A.; Singh, B.; Jain, S.; Katare, O. Functionalized lipid–polymer hybrid nanoparticles mediated codelivery of methotrexate and aceclofenac: A synergistic effect in breast cancer with improved pharmacokinetics attributes. Mol. Pharm. 2017, 14, 1883–1897. [Google Scholar] [CrossRef]
- Shafique, M.; Ur Rehman, M.; Kamal, Z.; Alzhrani, R.M.; Alshehri, S.; Alamri, A.H.; Bakkari, M.A.; Sabei, F.Y.; Safhi, A.Y.; Mohammed, A.M. Formulation development of lipid polymer hybrid nanoparticles of doxorubicin and its in-vitro, in-vivo and computational evaluation. Front. Pharmacol. 2023, 14, 1025013. [Google Scholar] [CrossRef]
- Khalili, L.; Dehghan, G.; Sheibani, N.; Khataee, A. Smart active-targeting of lipid-polymer hybrid nanoparticles for therapeutic applications: Recent advances and challenges. Int. J. Biol. Macromol. 2022, 213, 166–194. [Google Scholar] [CrossRef]
- Sivadasan, D.; Sultan, M.H.; Madkhali, O.; Almoshari, Y.; Thangavel, N. Polymeric lipid hybrid nanoparticles (plns) as emerging drug delivery platform—A comprehensive review of their properties, preparation methods, and therapeutic applications. Pharmaceutics 2021, 13, 1291. [Google Scholar] [CrossRef]
- Pradhan, R.; Kumari, S.; Ambati, H.; Patel, T.K.; Ghosh, B.; Puri, A.; Dubey, S.K.; Taliyan, R. Development of biotin decorated Olaparib loaded cationic lipopolymeric hybrid nanoparticle and evaluation of its anticancer effect and pharmacokinetics for triple negative breast cancer. J. Drug Deliv. Sci. Technol. 2024, 94, 105458. [Google Scholar] [CrossRef]
- Ting, Y.; Jiang, Y.; Ho, C.-T.; Huang, Q. Common delivery systems for enhancing in vivo bioavailability and biological efficacy of nutraceuticals. J. Funct. Foods 2014, 7, 112–128. [Google Scholar] [CrossRef]
- Yang, X.; Liu, K. P-gp inhibition-based strategies for modulating pharmacokinetics of anticancer drugs: An update. Curr. Drug Metab. 2016, 17, 806–826. [Google Scholar] [CrossRef] [PubMed]
- Lennernas, H. Modeling gastrointestinal drug absorption requires more in vivo biopharmaceutical data: Experience from in vivo dissolution and permeability studies in humans. Curr. Drug Metab. 2007, 8, 645–657. [Google Scholar] [CrossRef]
- Engle, K.; Kumar, G. Cancer multidrug-resistance reversal by ABCB1 inhibition: A recent update. Eur. J. Med. Chem. 2022, 239, 114542. [Google Scholar] [CrossRef]
- Krishna Vadlapatla, R.; Dutt Vadlapudi, A.; Pal, D.; Mitra, A.K. Mechanisms of drug resistance in cancer chemotherapy: Coordinated role and regulation of efflux transporters and metabolizing enzymes. Curr. Pharm. Des. 2013, 19, 7126–7140. [Google Scholar] [CrossRef]
- Eisenmann, E.D.; Talebi, Z.; Sparreboom, A.; Baker, S.D. Boosting the oral bioavailability of anticancer drugs through intentional drug–drug interactions. Basic Clin. Pharmacol. Toxicol. 2022, 130, 23–35. [Google Scholar] [CrossRef]
- Kruijtzer, C.; Beijnen, J.; Rosing, H.; ten Bokkel Huinink, W.; Schot, M.; Jewell, R.; Paul, E.; Schellens, J. Increased oral bioavailability of topotecan in combination with the breast cancer resistance protein and P-glycoprotein inhibitor GF120918. J. Clin. Oncol. 2002, 20, 2943–2950. [Google Scholar] [CrossRef]
- Rizwanullah, M.; Perwez, A.; Alam, M.; Ahmad, S.; Mir, S.R.; Rizvi, M.M.A.; Amin, S. Polymer-lipid hybrid nanoparticles of exemestane for improved oral bioavailability and anti-tumor efficacy: An extensive preclinical investigation. Int. J. Pharm. 2023, 642, 123136. [Google Scholar] [CrossRef]
- de Mattos, A.C.; Altmeyer, C.; Tominaga, T.T.; Khalil, N.M.; Mainardes, R.M. Polymeric nanoparticles for oral delivery of 5-fluorouracil: Formulation optimization, cytotoxicity assay and pre-clinical pharmacokinetics study. Eur. J. Pharm. Sci. 2016, 84, 83–91. [Google Scholar] [CrossRef]
- Budha, N.; Frymoyer, A.; Smelick, G.; Jin, J.; Yago, M.; Dresser, M.; Holden, S.; Benet, L.; Ware, J. Drug absorption interactions between oral targeted anticancer agents and PPIs: Is pH-dependent solubility the Achilles heel of targeted therapy? Clin. Pharmacol. Ther. 2012, 92, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Ashar, F.; Mohammed, A.A.S.; Selvamuthukumar, S. Enhancement of oral bioavailability of ibrutinib using a liposil nanohybrid delivery system. PLoS ONE 2024, 19, e0310492. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, S.; Bouchemal, K.; Ponchel, G. Oral delivery of anticancer drugs I: General considerations. Drug Discov. Today 2013, 18, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Sani, A.; Pourmadadi, M.; Shaghaghi, M.; Eshaghi, M.M.; Shahmollaghamsary, S.; Arshad, R.; Fathi-karkan, S.; Rahdar, A.; Jadoun, S.; Medina, D.I. Revolutionizing anticancer drug delivery: Exploring the potential of tamoxifen-loaded nanoformulations. J. Drug Deliv. Sci. Technol. 2023, 86, 104642. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Ostovar, S.; Ruiz-Pulido, G.; Hassan, D.; Souri, M.; Manicum, A.-L.E.; Behzadmehr, R.; Fathi-karkan, S.; Rahdar, A.; Medina, D.I. Novel Epirubicin-loaded Nanoformulations: Advancements in Polymeric Nanocarriers for Efficient Targeted Cellular and Subcellular Anticancer Drug Delivery. Inorg. Chem. Commun. 2023, 155, 110999. [Google Scholar] [CrossRef]
- Meylina, L.; Muchtaridi, M.; Joni, I.M.; Mohammed, A.F.A.; Wathoni, N. Nanoformulations of α-mangostin for cancer drug delivery system. Pharmaceutics 2021, 13, 1993. [Google Scholar] [CrossRef]
- Wu, B.; Yu, P.; Cui, C.; Wu, M.; Zhang, Y.; Liu, L.; Wang, C.-X.; Zhuo, R.-X.; Huang, S.-W. Folate-containing reduction-sensitive lipid–polymer hybrid nanoparticles for targeted delivery of doxorubicin. Biomater. Sci. 2015, 3, 655–664. [Google Scholar] [CrossRef]
- Song, Z.; Shi, Y.; Han, Q.; Dai, G. Endothelial growth factor receptor-targeted and reactive oxygen species-responsive lung cancer therapy by docetaxel and resveratrol encapsulated lipid-polymer hybrid nanoparticles. Biomed. Pharmacother. 2018, 105, 18–26. [Google Scholar] [CrossRef]
- Wakaskar, R.R. General overview of lipid–polymer hybrid nanoparticles, dendrimers, micelles, liposomes, spongosomes and cubosomes. J. Drug Target. 2018, 26, 311–318. [Google Scholar] [CrossRef]
- Rose, F.; Wern, J.E.; Ingvarsson, P.T.; van de Weert, M.; Andersen, P.; Follmann, F.; Foged, C. Engineering of a novel adjuvant based on lipid-polymer hybrid nanoparticles: A quality-by-design approach. J. Control. Release 2015, 210, 48–57. [Google Scholar] [CrossRef]
- Tahir, N.; Haseeb, M.T.; Madni, A.; Parveen, F.; Khan, M.M.; Khan, S.; Jan, N.; Khan, A. Lipid polymer hybrid nanoparticles: A novel approach for drug delivery. In Role of Novel Drug Delivery Vehicles in Nanobiomedicine; IntechOpen: London, UK, 2019; p. 59. [Google Scholar]
- Wang, T.; Xue, J.; Hu, Q.; Zhou, M.; Chang, C.; Luo, Y. Synthetic surfactant-and cross-linker-free preparation of highly stable lipid-polymer hybrid nanoparticles as potential oral delivery vehicles. Sci. Rep. 2017, 7, 2750. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.K.; Tandel, N.; Jadon, R.S.; Tyagi, R.K.; Katare, O.P. Lipid–polymer hybrid nanocarrier-mediated cancer therapeutics: Current status and future directions. Drug Discov. Today 2018, 23, 1610–1621. [Google Scholar] [CrossRef] [PubMed]
- Gajbhiye, K.R.; Salve, R.; Narwade, M.; Sheikh, A.; Kesharwani, P.; Gajbhiye, V. Lipid polymer hybrid nanoparticles: A custom-tailored next-generation approach for cancer therapeutics. Mol. Cancer 2023, 22, 160. [Google Scholar] [CrossRef] [PubMed]
- Sakpakdeejaroen, I.; Muanrit, P.; Panthong, S.; Ruangnoo, S. Alpha-Mangostin-Loaded Transferrin-Conjugated Lipid-Polymer Hybrid Nanoparticles: Development and Characterization for Tumor-Targeted Delivery. Sci. World J. 2022, 2022, 9217268. [Google Scholar] [CrossRef]
- Li, Q.; Xia, D.; Tao, J.; Shen, A.; He, Y.; Gan, Y.; Wang, C. Self-assembled core-shell-type lipid-polymer hybrid nanoparticles: Intracellular trafficking and relevance for oral absorption. J. Pharm. Sci. 2017, 106, 3120–3130. [Google Scholar] [CrossRef]
- Rajana, N.; Chary, P.S.; Bhavana, V.; Deshmukh, R.; Dukka, K.; Sharma, A.; Mehra, N.K. Targeted delivery and apoptosis induction of CDK-4/6 inhibitor loaded folic acid decorated lipid-polymer hybrid nanoparticles in breast cancer cells. Int. J. Pharm. 2024, 651, 123787. [Google Scholar] [CrossRef]
- Chan, J.M.; Zhang, L.; Yuet, K.P.; Liao, G.; Rhee, J.-W.; Langer, R.; Farokhzad, O.C. PLGA–lecithin–PEG core–shell nanoparticles for controlled drug delivery. Biomaterials 2009, 30, 1627–1634. [Google Scholar] [CrossRef]
- Chan, J.M.; Zhang, L.; Tong, R.; Ghosh, D.; Gao, W.; Liao, G.; Yuet, K.P.; Gray, D.; Rhee, J.-W.; Cheng, J. Spatiotemporal controlled delivery of nanoparticles to injured vasculature. Proc. Natl. Acad. Sci. USA 2010, 107, 2213–2218. [Google Scholar] [CrossRef]
- Dave, V.; Tak, K.; Sohgaura, A.; Gupta, A.; Sadhu, V.; Reddy, K.R. Lipid-polymer hybrid nanoparticles: Synthesis strategies and biomedical applications. J. Microbiol. Methods 2019, 160, 130–142. [Google Scholar] [CrossRef]
- Jain, S.; Kumar, M.; Kumar, P.; Verma, J.; Rosenholm, J.M.; Bansal, K.K.; Vaidya, A. Lipid–Polymer Hybrid Nanosystems: A Rational Fusion for Advanced Therapeutic Delivery. J. Funct. Biomater. 2023, 14, 437. [Google Scholar] [CrossRef]
- Khan, S.; Madni, A.; Aamir, M.N.; Khan, S.; Ahmad, F.-u.-D.; Basit, A.; Jan, N.; Shah, H.; Shafiq, A.; Anwar, M. Design and evaluation of sustained-release lipid-PLGA hybrid nanoparticles for enhanced anticancer efficacy of 5-fluorouracil. Part. Sci. Technol. 2023, 42, 269–287. [Google Scholar] [CrossRef]
- Sengel-Turk, C.T.; Paksoy, A.O.; Alpturk, O. The state of the art in core–shell-type lipid–polymer hybrid nanocarriers and beyond. Polym. Bull. 2023, 81, 4771–4800. [Google Scholar] [CrossRef]
- Baghel, Y.S.; Bhattacharya, S. Lipid polymeric hybrid nanoparticles: Formulation techniques and effects on glioblastoma. Pharm. Sci. 2021, 28, 174–193. [Google Scholar] [CrossRef]
- Dali, P.; Shende, P. Self-assembled lipid polymer hybrid nanoparticles using combinational drugs for migraine via intranasal route. AAPS PharmSciTech 2022, 24, 20. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, J.; Li, X.; Xu, Y.; Liu, D.; He, H.; Wang, Y.; Tang, X. Hydroxycamptothecin (HCPT)-loaded PEGlated lipid–polymer hybrid nanoparticles for effective delivery of HCPT: QbD-based development and evaluation. Drug Deliv. Transl. Res. 2022, 12, 306–324. [Google Scholar] [CrossRef]
- Surapaneni, S.G.; Ambade, A.V. Poly (N-vinylcaprolactam) containing solid lipid polymer hybrid nanoparticles for controlled delivery of a hydrophilic drug gemcitabine hydrochloride. RSC Adv. 2022, 12, 17621–17628. [Google Scholar] [CrossRef]
- Shah, S.; Famta, P.; Raghuvanshi, R.S.; Singh, S.B.; Srivastava, S. Lipid polymer hybrid nanocarriers: Insights into synthesis aspects, characterization, release mechanisms, surface functionalization and potential implications. Colloid Interface Sci. Commun. 2022, 46, 100570. [Google Scholar] [CrossRef]
- Zhao, Y.; Lin, D.; Wu, F.; Guo, L.; He, G.; Ouyang, L.; Song, X.; Huang, W.; Li, X. Discovery and in vivo evaluation of novel RGD-modified lipid-polymer hybrid nanoparticles for targeted drug delivery. Int. J. Mol. Sci. 2014, 15, 17565–17576. [Google Scholar] [CrossRef]
- Li, F.; Zhao, X.; Wang, H.; Zhao, R.; Ji, T.; Ren, H.; Anderson, G.J.; Nie, G.; Hao, J. Multiple layer-by-layer lipid-polymer hybrid nanoparticles for improved FOLFIRINOX chemotherapy in pancreatic tumor models. Adv. Funct. Mater. 2015, 25, 788–798. [Google Scholar] [CrossRef]
- Li, Y.; Wu, H.; Yang, X.; Jia, M.; Li, Y.; Huang, Y.; Lin, J.; Wu, S.; Hou, Z. Mitomycin C-soybean phosphatidylcholine complex-loaded self-assembled PEG-lipid-PLA hybrid nanoparticles for targeted drug delivery and dual-controlled drug release. Mol. Pharm. 2014, 11, 2915–2927. [Google Scholar] [CrossRef]
- Mirnezami, S.M.S.; Heydarinasab, A.; Akbarzadehkhyavi, A.; Adrjmand, M. Development and optimization of lipid-polymer hybrid nanoparticles containing melphalan using central composite design and its effect on ovarian cancer cell lines. Iran. J. Pharm. Res. IJPR 2021, 20, 213. [Google Scholar] [PubMed]
- Fu, D.; Li, C.; Huang, Y. Lipid–polymer hybrid nanoparticle-based combination treatment with cisplatin and EGFR/HER2 receptor-targeting Afatinib to enhance the treatment of nasopharyngeal carcinoma. OncoTargets Ther. 2021, 14, 2449–2461. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Madni, A.; Filipczak, N.; Pan, J.; Rehman, M.; Rai, N.; Attia, S.A.; Torchilin, V.P. Folate targeted lipid chitosan hybrid nanoparticles for enhanced anti-tumor efficacy. Nanomed. Nanotechnol. Biol. Med. 2020, 28, 102228. [Google Scholar] [CrossRef]
- Gu, L.; Shi, T.; Sun, Y.; You, C.; Wang, S.; Wen, G.; Chen, L.; Zhang, X.; Zhu, J.; Sun, B. Folate-modified, indocyanine green-loaded lipid-polymer hybrid nanoparticles for targeted delivery of cisplatin. J. Biomater. Sci. Polym. Ed. 2017, 28, 690–702. [Google Scholar] [CrossRef]
- Dong, S.; Zhou, X.; Yang, J. TAT modified and lipid–PEI hybrid nanoparticles for co-delivery of docetaxel and pDNA. Biomed. Pharmacother. 2016, 84, 954–961. [Google Scholar] [CrossRef]
- Wang, Q.; Alshaker, H.; Böhler, T.; Srivats, S.; Chao, Y.; Cooper, C.; Pchejetski, D. Core shell lipid-polymer hybrid nanoparticles with combined docetaxel and molecular targeted therapy for the treatment of metastatic prostate cancer. Sci. Rep. 2017, 7, 5901. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, P.; Zhu, T. Ovarian carcinoma biological nanotherapy: Comparison of the advantages and drawbacks of lipid, polymeric, and hybrid nanoparticles for cisplatin delivery. Biomed. Pharmacother. 2019, 109, 475–483. [Google Scholar] [CrossRef]
- Shi, K.; Zhou, J.; Zhang, Q.; Gao, H.; Liu, Y.; Zong, T.; He, Q. Arginine-glycine-aspartic acid-modified lipid-polymer hybrid nanoparticles for docetaxel delivery in glioblastoma multiforme. J. Biomed. Nanotechnol. 2015, 11, 382–391. [Google Scholar] [CrossRef]
- Zhang, L.; Chan, J.M.; Gu, F.X.; Rhee, J.-W.; Wang, A.Z.; Radovic-Moreno, A.F.; Alexis, F.; Langer, R.; Farokhzad, O.C. Self-assembled lipid− polymer hybrid nanoparticles: A robust drug delivery platform. ACS Nano 2008, 2, 1696–1702. [Google Scholar] [CrossRef]
- Tran, T.; Nguyen, H.; Yong, C.; Truong, D.; Kim, J. Synergistic therapeutic strategy of dual drug-loaded lipid polymer hybrid nanoparticles for breast cancer treatment. Indian J. Pharm. Sci. 2019, 81, 474–482. [Google Scholar] [CrossRef]
- Wong, H.L.; Rauth, A.M.; Bendayan, R.; Manias, J.L.; Ramaswamy, M.; Liu, Z.; Erhan, S.Z.; Wu, X.Y. A new polymer–lipid hybrid nanoparticle system increases cytotoxicity of doxorubicin against multidrug-resistant human breast cancer cells. Pharm. Res. 2006, 23, 1574–1585. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.; Mittal, N.K.; Balabathula, P.; Thoma, L.A.; Wood, G.C. Development and in vitro evaluation of core–shell type lipid–polymer hybrid nanoparticles for the delivery of erlotinib in non-small cell lung cancer. Eur. J. Pharm. Sci. 2016, 81, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Xing, H.; Sun, Y.; Feng, S.; Wang, S. Non-small cell lung cancer combination therapy: Hyaluronic acid modified, epidermal growth factor receptor targeted, pH sensitive lipid-polymer hybrid nanoparticles for the delivery of erlotinib plus bevacizumab. Biomed. Pharmacother. 2020, 125, 109861. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Zhou, J.; Yan, L.; Zhang, S.; Yu, D.-G. Hybrid films loaded with 5-fluorouracil and Reglan for synergistic treatment of colon cancer via asynchronous dual-drug delivery. Front. Bioeng. Biotechnol. 2024, 12, 1398730. [Google Scholar] [CrossRef]
- Yalcin, T.E.; Ilbasmis-Tamer, S.; Takka, S. Antitumor activity of gemcitabine hydrochloride loaded lipid polymer hybrid nanoparticles (LPHNs): In vitro and in vivo. Int. J. Pharm. 2020, 580, 119246. [Google Scholar] [CrossRef]
- Zhang, H.; Li, J.; Yuan, R.; Li, Y.; Zhang, Y.; Hu, X.; Qu, J.; Chen, Y.; Wang, Z.; Xia, M. Augment the efficacy of eradicating metastatic lesions and tumor proliferation in breast cancer by honokiol-loaded pH-sensitive targeted lipid nanoparticles. Colloids Surf. B Biointerfaces 2021, 207, 112008. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, J.; Fu, C.; Xie, X.; Peng, F.; You, J.; Tang, H.; Wang, Z.; Li, P.; Chen, J. iRGD-modified lipid–polymer hybrid nanoparticles loaded with isoliquiritigenin to enhance anti-breast cancer effect and tumor-targeting ability. Int. J. Nanomed. 2017, 12, 4147–4162. [Google Scholar] [CrossRef]
- Khalili, L.; Dehghan, G.; Feizi, M.A.H.; Sheibani, N.; Hamishekar, H. Development of an albumin decorated lipid-polymer hybrid nanoparticle for simultaneous delivery of methotrexate and conferone to cancer cells. Int. J. Pharm. 2021, 599, 120421. [Google Scholar] [CrossRef]
- Patel, G.; Thakur, N.S.; Kushwah, V.; Patil, M.D.; Nile, S.H.; Jain, S.; Kai, G.; Banerjee, U.C. Mycophenolate co-administration with quercetin via lipid-polymer hybrid nanoparticles for enhanced breast cancer management. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102147. [Google Scholar] [CrossRef]
- Soomherun, N.; Kreua-Ongarjnukool, N.; Niyomthai, S.T.; Chumnanvej, S. Lipid-Polymer Hybrid Nanoparticles Synthesized via Lipid-Based Surface Engineering for a robust drug delivery platform. Colloids Surf. B Biointerfaces 2024, 237, 113858. [Google Scholar] [CrossRef]
- Godara, S.; Lather, V.; Kirthanashri, S.; Awasthi, R.; Pandita, D. Lipid-PLGA hybrid nanoparticles of paclitaxel: Preparation, characterization, in vitro and in vivo evaluation. Mater. Sci. Eng. C 2020, 109, 110576. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, U.; Chashoo, G.; Sharma, P.R.; Kumar, A.; Saxena, A.K.; Vyas, S. Tailored polymer–lipid hybrid nanoparticles for the delivery of drug conjugate: Dual strategy for brain targeting. Colloids Surf. B Biointerfaces 2015, 126, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Feng, S.-S. Poly (D, L-lactide-co-glycolide)(PLGA) nanoparticles prepared by high pressure homogenization for paclitaxel chemotherapy. Int. J. Pharm. 2007, 342, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, I.P.S.; Silva, L.B.; do Amaral, R.L.F.; Chrysostomo-Massaro, T.N.; de Lima Fragelli, B.D.; de Almeida Rodolpho, J.M.; de Freitas Anibal, F.; Borra, R.C.; Paschoal, J.A.R.; Miranda, M.A. Evaluation of in vivo and in vitro efficacy of solasonine/solamargine-loaded lipid-polymer hybrid nanoparticles against bladder cancer. Int. J. Pharm. 2024, 661, 124411. [Google Scholar] [CrossRef]
- Varthya, M.; Pawar, H.; Singh, C.; Dora, C.P.; Jena, S.K.; Suresh, S. Development of novel polymer-lipid hybrid nanoparticles of tamoxifen: In vitro and in vivo evaluation. J. Nanosci. Nanotechnol. 2016, 16, 253–260. [Google Scholar] [CrossRef]
- Imam, S.S.; Gilani, S.J.; Bin Jumah, M.N.; Rizwanullah, M.; Zafar, A.; Ahmed, M.M.; Alshehri, S. Harnessing lipid polymer hybrid nanoparticles for enhanced oral bioavailability of thymoquinone: In vitro and in vivo assessments. Polymers 2022, 14, 3705. [Google Scholar] [CrossRef]
- Yang, Z.; Luo, X.; Zhang, X.; Liu, J.; Jiang, Q. Targeted delivery of 10-hydroxycamptothecin to human breast cancers by cyclic RGD-modified lipid–polymer hybrid nanoparticles. Biomed. Mater. 2013, 8, 025012. [Google Scholar] [CrossRef]
- Zhang, L.; Radovic-Moreno, A.F.; Alexis, F.; Gu, F.X.; Basto, P.A.; Bagalkot, V.; Jon, S.; Langer, R.S.; Farokhzad, O.C. Co-delivery of hydrophobic and hydrophilic drugs from nanoparticle-aptamer bioconjugates. ChemMedChem Chem. Enabling Drug Discov. 2007, 2, 1268–1271. [Google Scholar] [CrossRef]
- Farokhzad, O.C.; Cheng, J.; Teply, B.A.; Sherifi, I.; Jon, S.; Kantoff, P.W.; Richie, J.P.; Langer, R. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc. Natl. Acad. Sci. USA 2006, 103, 6315–6320. [Google Scholar] [CrossRef]
- Kong, S.D.; Sartor, M.; Hu, C.-M.J.; Zhang, W.; Zhang, L.; Jin, S. Magnetic field activated lipid–polymer hybrid nanoparticles for stimuli-responsive drug release. Acta Biomater. 2013, 9, 5447–5452. [Google Scholar] [CrossRef]
- Jadon, R.S.; Sharma, M. Docetaxel-loaded lipid-polymer hybrid nanoparticles for breast cancer therapeutics. J. Drug Deliv. Sci. Technol. 2019, 51, 475–484. [Google Scholar] [CrossRef]
- Yalcin, T.E.; Ilbasmis-Tamer, S.; Takka, S. Development and characterization of gemcitabine hydrochloride loaded lipid polymer hybrid nanoparticles (LPHNs) using central composite design. Int. J. Pharm. 2018, 548, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Soares, D.C.F.; Domingues, S.C.; Viana, D.B.; Tebaldi, M.L. Polymer-hybrid nanoparticles: Current advances in biomedical applications. Biomed. Pharmacother. 2020, 131, 110695. [Google Scholar]
- Qin, L.; Wu, H.; Xu, E.; Zhang, X.; Guan, J.; Zhao, R.; Mao, S. Exploring the potential of functional polymer-lipid hybrid nanoparticles for enhanced oral delivery of paclitaxel. Asian J. Pharm. Sci. 2021, 16, 387–395. [Google Scholar] [CrossRef]
- Subramanian, D.A.; Langer, R.; Traverso, G. Mucus interaction to improve gastrointestinal retention and pharmacokinetics of orally administered nano-drug delivery systems. J. Nanobiotechnol. 2022, 20, 362. [Google Scholar] [CrossRef]
- Kim, Y.S.; Ho, S.B. Intestinal goblet cells and mucins in health and disease: Recent insights and progress. Curr. Gastroenterol. Rep. 2010, 12, 319–330. [Google Scholar] [CrossRef]
- Ren, T.; Wang, Q.; Xu, Y.; Cong, L.; Gou, J.; Tao, X.; Zhang, Y.; He, H.; Yin, T.; Zhang, H. Enhanced oral absorption and anticancer efficacy of cabazitaxel by overcoming intestinal mucus and epithelium barriers using surface polyethylene oxide (PEO) decorated positively charged polymer-lipid hybrid nanoparticles. J. Control. Release 2018, 269, 423–438. [Google Scholar] [CrossRef]
- Tahir, N.; Madni, A.; Correia, A.; Rehman, M.; Balasubramanian, V.; Khan, M.M.; Santos, H.A. Lipid-polymer hybrid nanoparticles for controlled delivery of hydrophilic and lipophilic doxorubicin for breast cancer therapy. Int. J. Nanomed. 2019, 14, 4961–4974. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, X.; Chen, H.; Hou, X.; He, Y.; Shen, J.; Shi, J.; Feng, N. Advances in next-generation lipid-polymer hybrid nanocarriers with emphasis on polymer-modified functional liposomes and cell-based-biomimetic nanocarriers for active ingredients and fractions from Chinese medicine delivery. Nanomed. Nanotechnol. Biol. Med. 2020, 29, 102237. [Google Scholar] [CrossRef]
- Akhlaghi, S.P.; Saremi, S.; Ostad, S.N.; Dinarvand, R.; Atyabi, F. Discriminated effects of thiolated chitosan-coated pMMA paclitaxel-loaded nanoparticles on different normal and cancer cell lines. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 689–697. [Google Scholar] [CrossRef]
- Banna, G.L.; Collovà, E.; Gebbia, V.; Lipari, H.; Giuffrida, P.; Cavallaro, S.; Condorelli, R.; Buscarino, C.; Tralongo, P.; Ferraù, F. Anticancer oral therapy: Emerging related issues. Cancer Treat. Rev. 2010, 36, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Aryal, S.; Hu, C.-M.J.; Fu, V.; Zhang, L. Nanoparticle drug delivery enhances the cytotoxicity of hydrophobic–hydrophilic drug conjugates. J. Mater. Chem. 2012, 22, 994–999. [Google Scholar] [CrossRef]
- Pandita, D.; Ahuja, A.; Lather, V.; Benjamin, B.; Dutta, T.; Velpandian, T.; Khar, R.K. Development of lipid-based nanoparticles for enhancing the oral bioavailability of paclitaxel. Aaps Pharmscitech 2011, 12, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.; Saha, R.; Shanmugam, T.; Balakrishnan, B.; More, P.; Banerjee, R. Carboxymethyl-chitosan-tethered lipid vesicles: Hybrid nanoblanket for oral delivery of paclitaxel. Biomacromolecules 2013, 14, 2272–2282. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, J.; Chan, H.F.; Skibba, M.; Liang, G.; Chen, M. iRGD decorated lipid-polymer hybrid nanoparticles for targeted co-delivery of doxorubicin and sorafenib to enhance anti-hepatocellular carcinoma efficacy. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1303–1311. [Google Scholar] [CrossRef]
- Shao, Y.; Luo, W.; Guo, Q.; Li, X.; Zhang, Q.; Li, J. In vitro and in vivo effect of hyaluronic acid modified, doxorubicin and gallic acid co-delivered lipid-polymeric hybrid nano-system for leukemia therapy. Drug Des. Dev. Ther. 2019, 13, 2043–2055. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, H.; Han, L.; Qiang, Z.; Zhang, X.; Gao, W.; Zhao, K.; Song, Y. Synergistic combination therapy of lung cancer using paclitaxel-and triptolide-coloaded lipid–polymer hybrid nanoparticles. Drug Des. Dev. Ther. 2018, 12, 3199–3209. [Google Scholar] [CrossRef]
- Li, S.; Xu, Z.; Alrobaian, M.; Afzal, O.; Kazmi, I.; Almalki, W.H.; Altamimi, A.S.A.; Al-Abbasi, F.A.; Alharbi, K.S.; Altowayan, W.M. EGF-functionalized lipid–polymer hybrid nanoparticles of 5-fluorouracil and sulforaphane with enhanced bioavailability and anticancer activity against colon carcinoma. Biotechnol. Appl. Biochem. 2022, 69, 2205–2221. [Google Scholar] [CrossRef]
- Duan, R.; Li, C.; Wang, F.; Yangi, J.-C. Polymer–lipid hybrid nanoparticles-based paclitaxel and etoposide combinations for the synergistic anticancer efficacy in osteosarcoma. Colloids Surf. B Biointerfaces 2017, 159, 880–887. [Google Scholar] [CrossRef]
- Wong, H.L.; Rauth, A.M.; Bendayan, R.; Wu, X.Y. In vivo evaluation of a new polymer-lipid hybrid nanoparticle (PLN) formulation of doxorubicin in a murine solid tumor model. Eur. J. Pharm. Biopharm. 2007, 65, 300–308. [Google Scholar] [CrossRef]
- Mottaghitalab, F.; Farokhi, M.; Fatahi, Y.; Atyabi, F.; Dinarvand, R. New insights into designing hybrid nanoparticles for lung cancer: Diagnosis and treatment. J. Control. Release 2019, 295, 250–267. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.; Bhattacharjee, H.; Mittal, N.; Sah, H.; Balabathula, P.; Thoma, L.A.; Wood, G.C. Core–shell-type lipid–polymer hybrid nanoparticles as a drug delivery platform. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 474–491. [Google Scholar] [CrossRef] [PubMed]
- Cheow, W.S.; Hadinoto, K. Factors affecting drug encapsulation and stability of lipid–polymer hybrid nanoparticles. Colloids Surf. B Biointerfaces 2011, 85, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Persano, F.; Gigli, G.; Leporatti, S. Lipid-polymer hybrid nanoparticles in cancer therapy: Current overview and future directions. Nano Express 2021, 2, 012006. [Google Scholar] [CrossRef]
- Rao, S.; Prestidge, C.A. Polymer-lipid hybrid systems: Merging the benefits of polymeric and lipid-based nanocarriers to improve oral drug delivery. Expert Opin. Drug Deliv. 2016, 13, 691–707. [Google Scholar] [CrossRef]
- Mendonça, M.C.; Kont, A.; Kowalski, P.S.; O’Driscoll, C.M. Design of lipid-based nanoparticles for delivery of therapeutic nucleic acids. Drug Discov. Today 2023, 28, 103505. [Google Scholar] [CrossRef]
- Ana, R.; Mendes, M.; Sousa, J.; Pais, A.; Falcão, A.; Fortuna, A.; Vitorino, C. Rethinking carbamazepine oral delivery using polymer-lipid hybrid nanoparticles. Int. J. Pharm. 2019, 554, 352–365. [Google Scholar] [CrossRef]
- Osman, N.; Devnarain, N.; Omolo, C.A.; Fasiku, V.; Jaglal, Y.; Govender, T. Surface modification of nano-drug delivery systems for enhancing antibiotic delivery and activity. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1758. [Google Scholar] [CrossRef]
- Luk, B.T.; Fang, R.H.; Zhang, L. Lipid-and polymer-based nanostructures for cancer theranostics. Theranostics 2012, 2, 1117. [Google Scholar] [CrossRef]
- Zhang, L.; Tong, S.; Zhang, Q.; Bao, G. Lipid-encapsulated Fe3O4 nanoparticles for multimodal magnetic resonance/fluorescence imaging. ACS Appl. Nano Mater. 2020, 3, 6785–6797. [Google Scholar] [CrossRef]
- Bokare, A.; Takami, A.; Kim, J.H.; Dong, A.; Chen, A.; Valerio, R.; Gunn, S.; Erogbogbo, F. Herringbone-patterned 3D-printed devices as alternatives to microfluidics for reproducible production of lipid polymer hybrid nanoparticles. ACS Omega 2019, 4, 4650–4657. [Google Scholar] [CrossRef]
- Meyer, R.A.; Hussmann, G.P.; Peterson, N.C.; Santos, J.L.; Tuesca, A.D. A scalable and robust cationic lipid/polymer hybrid nanoparticle platform for mRNA delivery. Int. J. Pharm. 2022, 611, 121314. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).