Nasal Administration of a Nanoemulsion Based on Methyl Ferulate and Eugenol Encapsulated in Chitosan Oleate: Uptake Studies in the Central Nervous System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Formulation and Characterization of CS-OA NE
2.2.1. Preparation of Nanoemulsions (Encapsulation of Fer-Me and Eugenol in CS-OA Micelles)
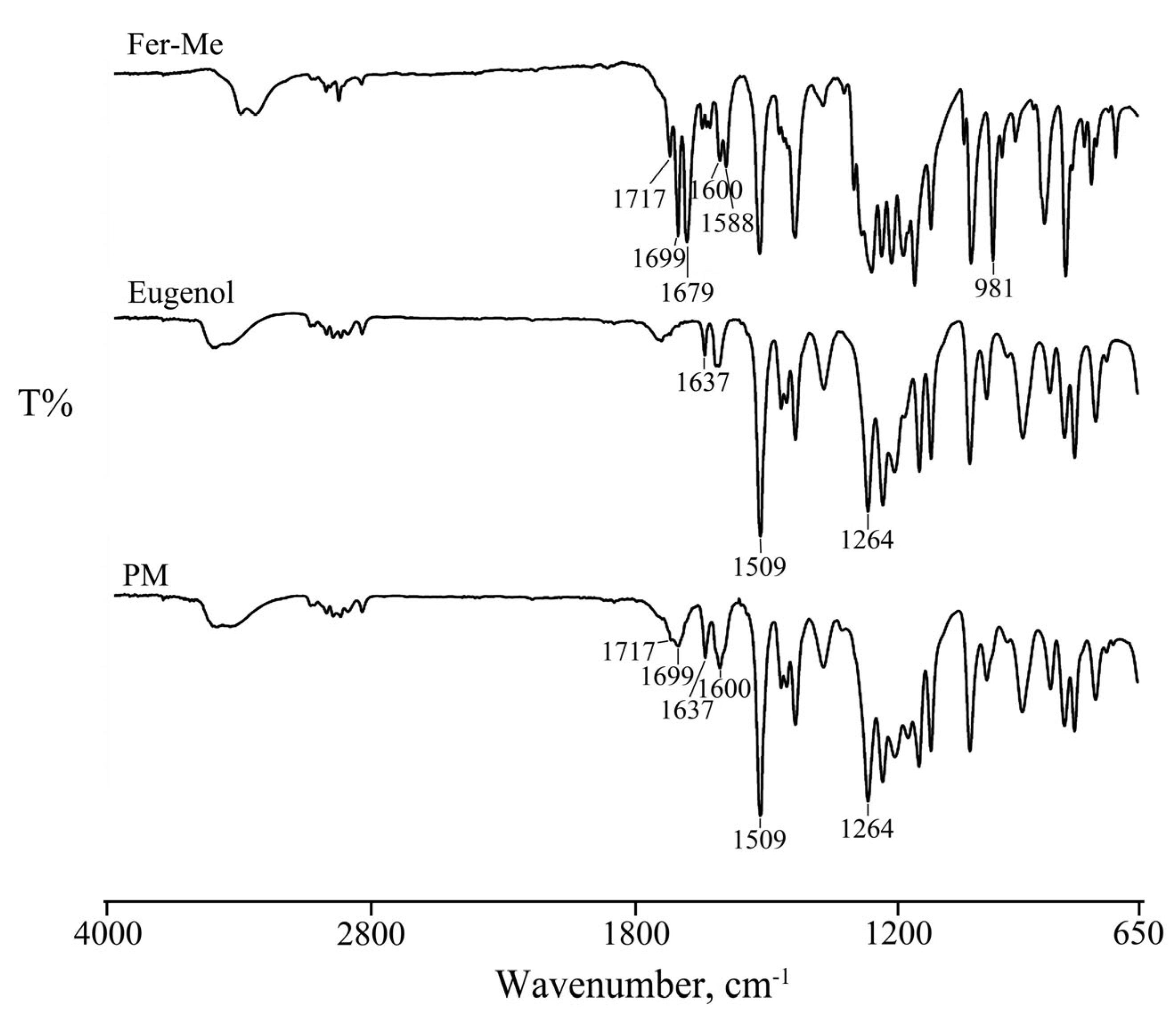
2.2.2. FT-IR Analysis
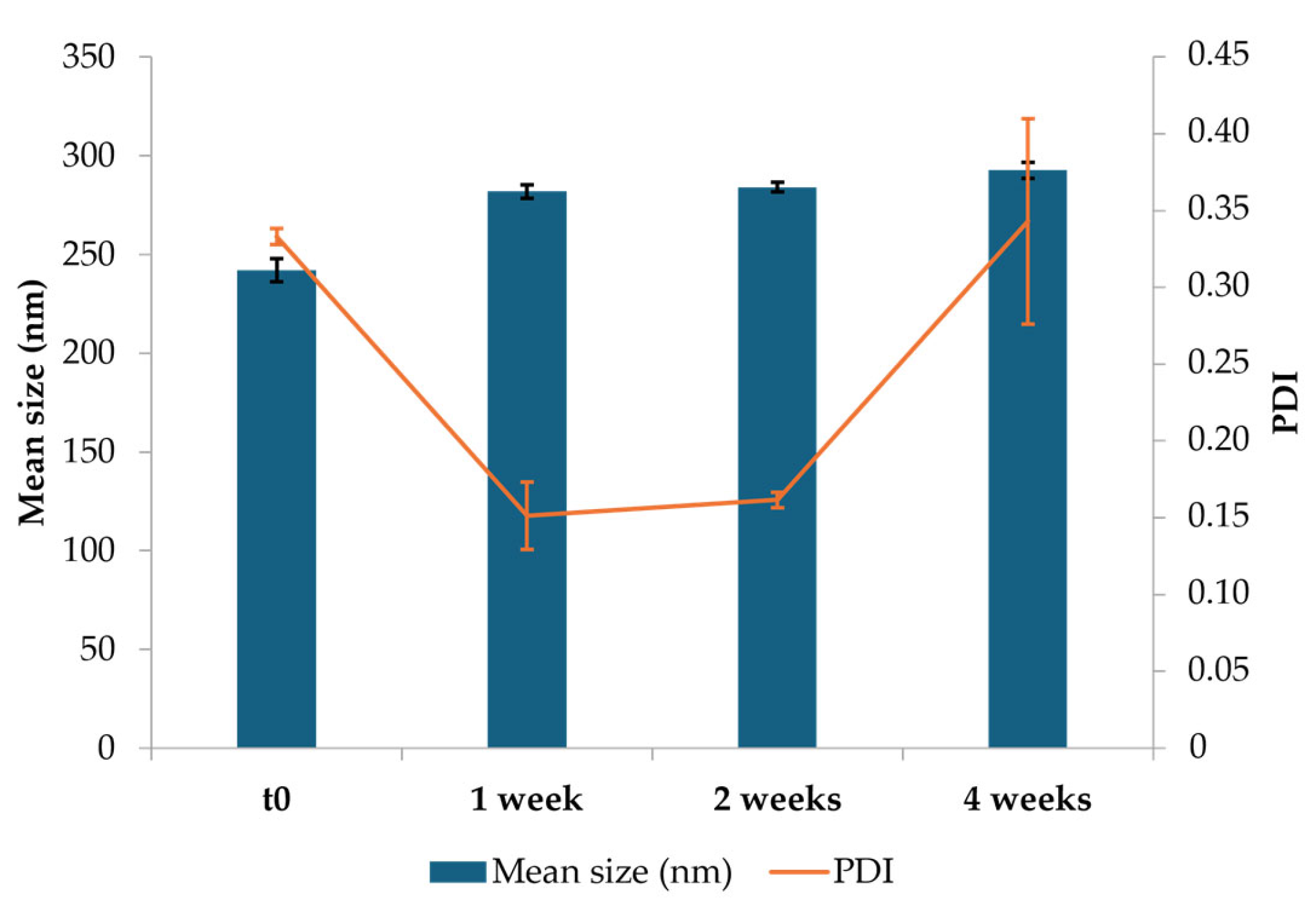
2.2.3. Particle Size Characterization
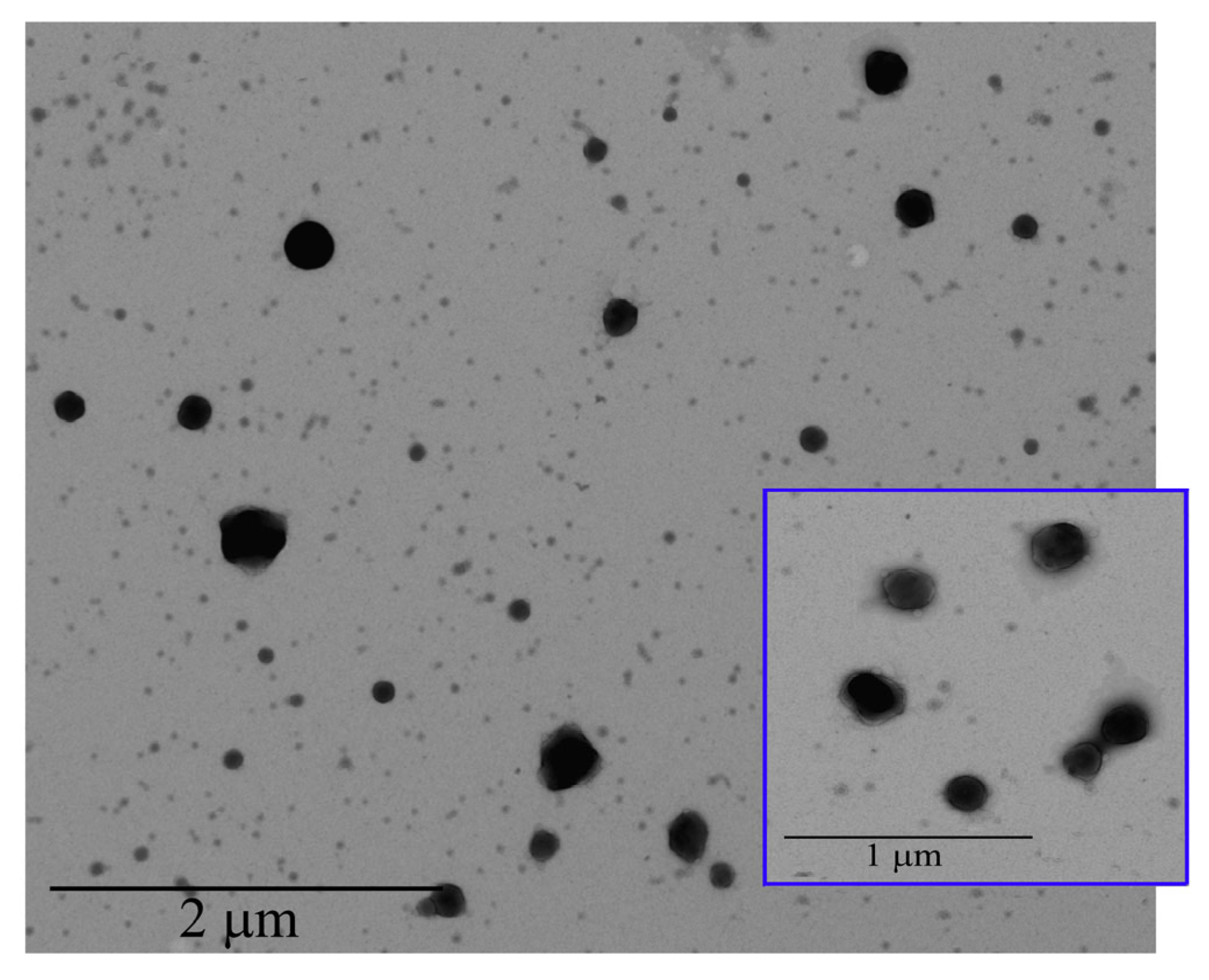
2.2.4. Transmission Electron Microscopy (TEM)
2.2.5. HPLC Analysis
2.2.6. Process Yield and Encapsulation Efficiency (EE)
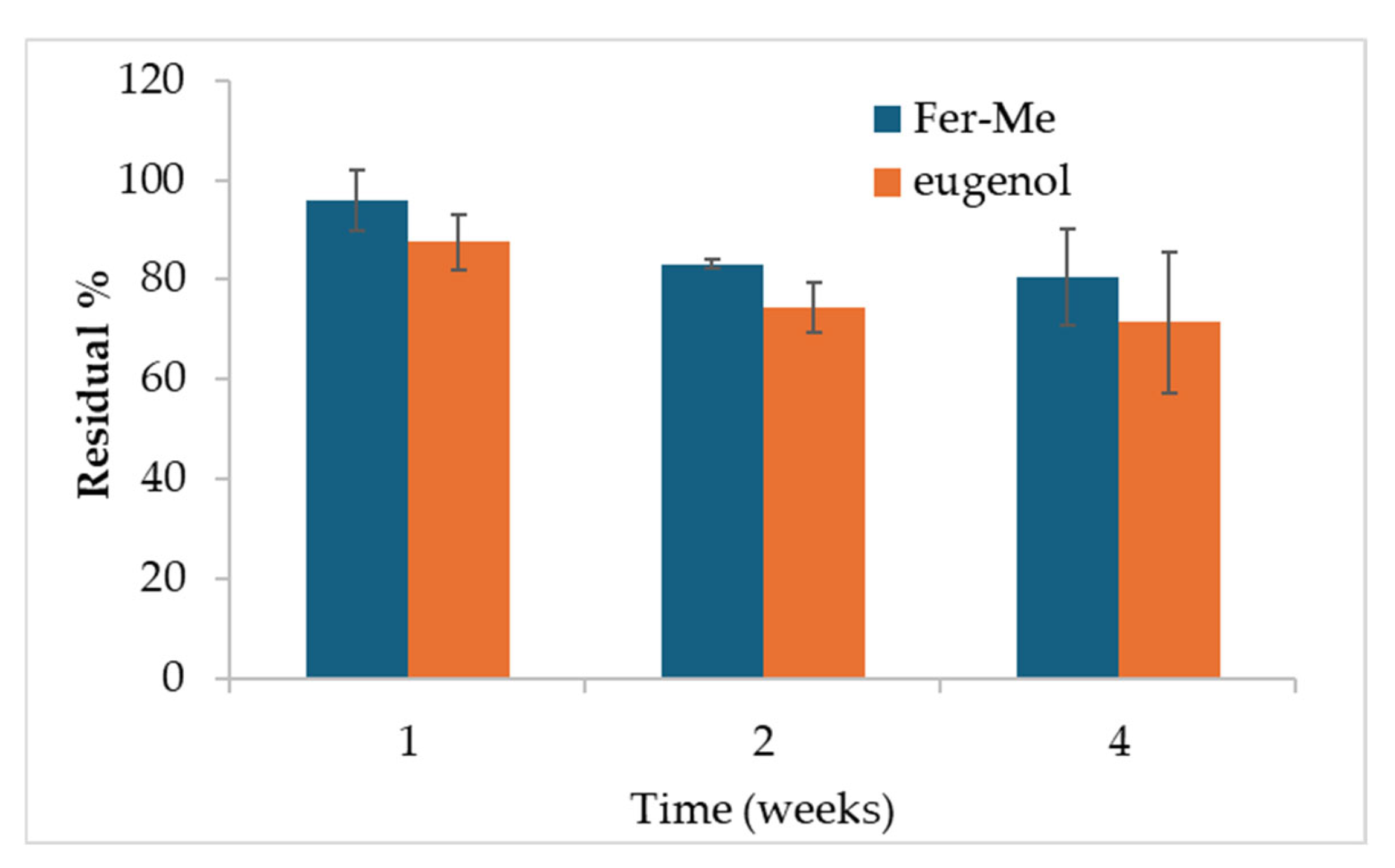
2.2.7. Drug Release
2.2.8. Mucoadhesion Test
2.3. In Vitro Cytotoxicity and Uptake Studies in RPMI 2650 Cells of CS-OA NE
2.3.1. RPMI 2650 Cell Culture
2.3.2. MTT Assay for Evaluation of Fer-Me and Eugenol Toxicity
2.3.3. Time Course of Fer-Me and Eugenol Uptake in RPMI 2650 Cells
2.4. In Vivo Administration
2.4.1. Intravenous Administration of Fer-Me
2.4.2. Nasal Administration of CS-OA NE
2.4.3. In Vivo Pharmacokinetic Calculations
2.5. Statistical Analysis
3. Results
3.1. Characterization of CS-OA NE
3.1.1. FTIR Analysis
3.1.2. Particle Size Characterization
3.1.3. Transmission Electron Microscopy (TEM) Analysis
3.1.4. Process Yield and Encapsulation Efficiency
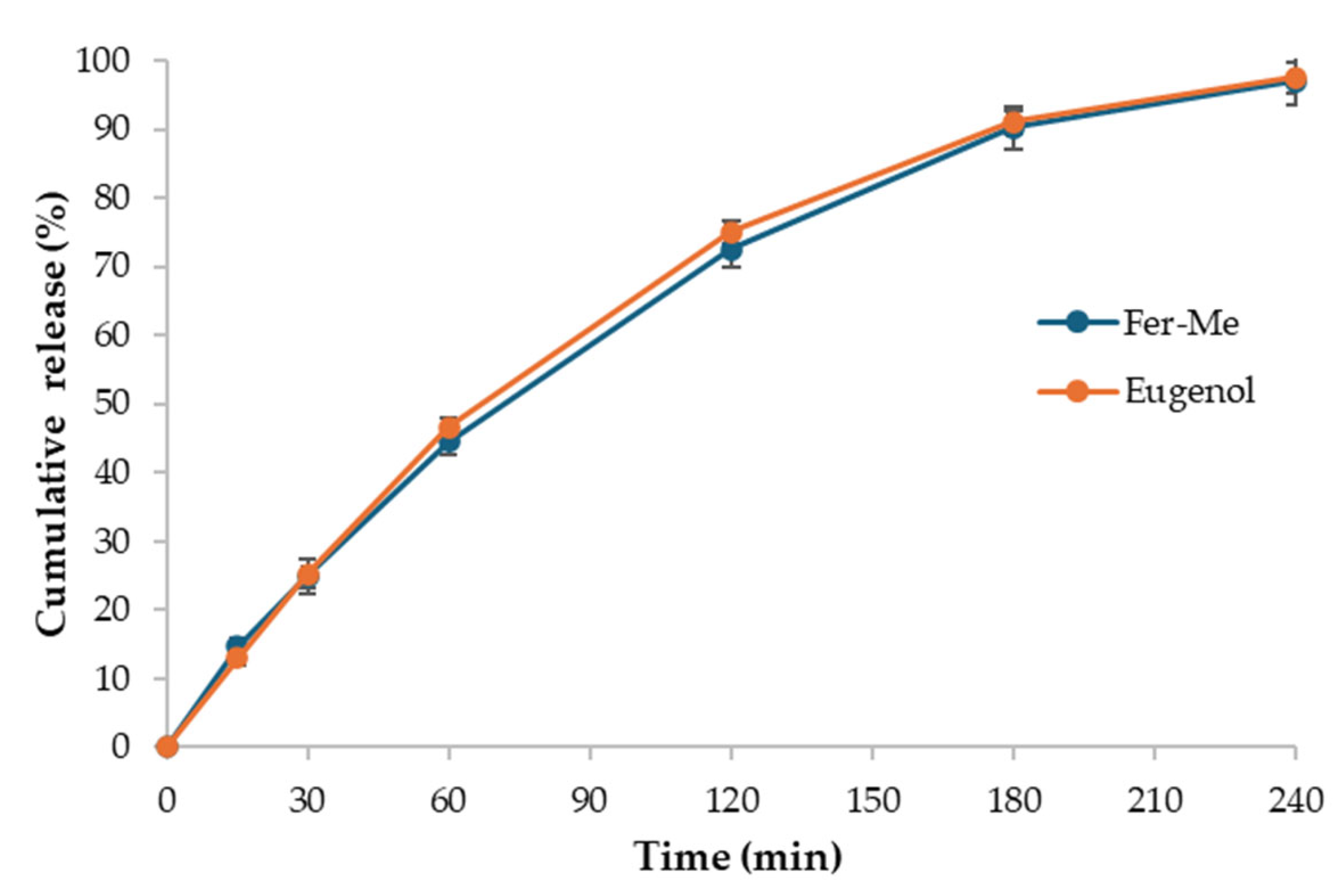
3.1.5. Drug Release
3.1.6. Mucoadhesion Test
3.2. In Vitro Cell Studies
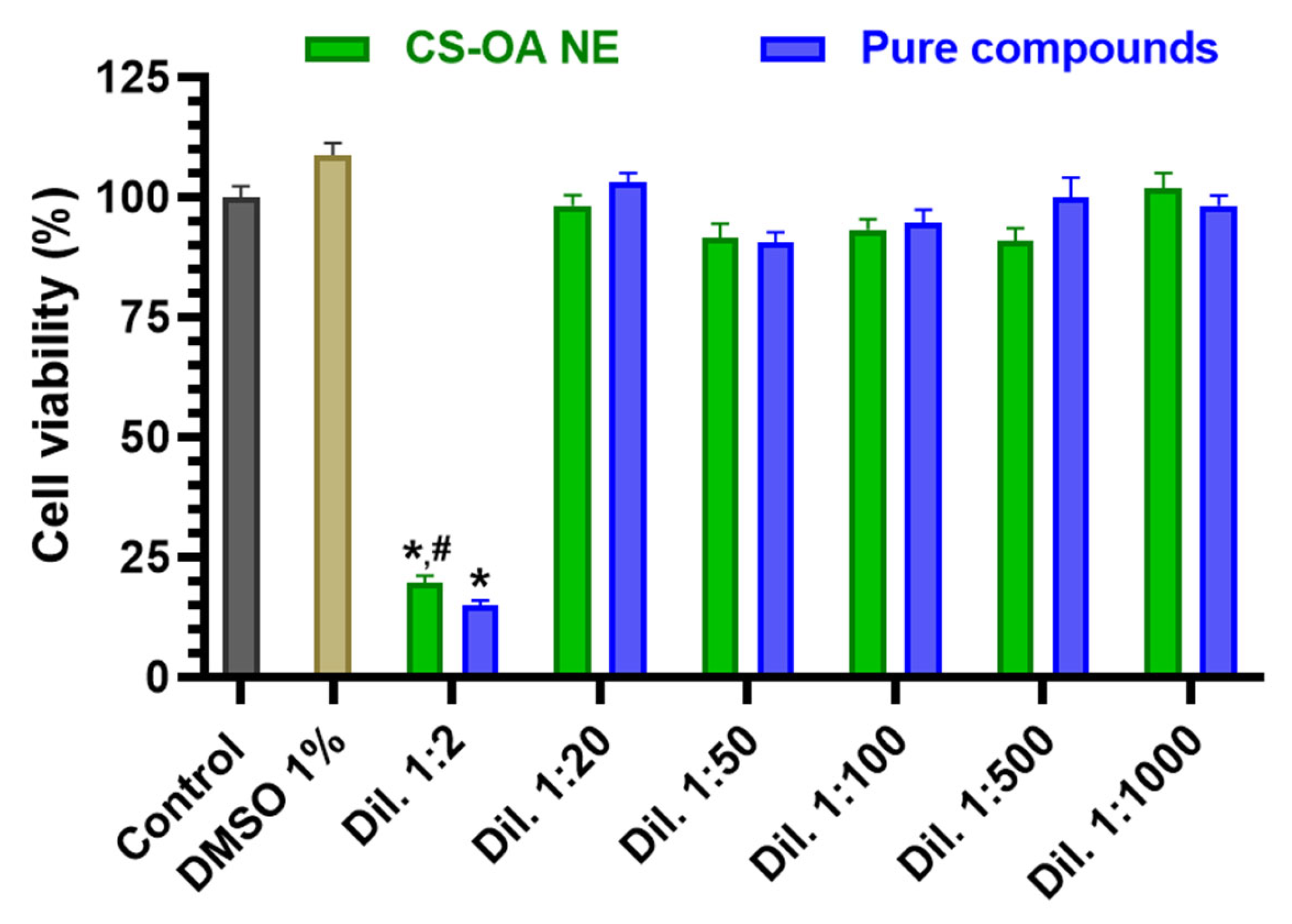
3.2.1. Evaluation of CS-OA NE or Fer-Me/Eugenol Solution Toxicity on RPMI 2650 Cells
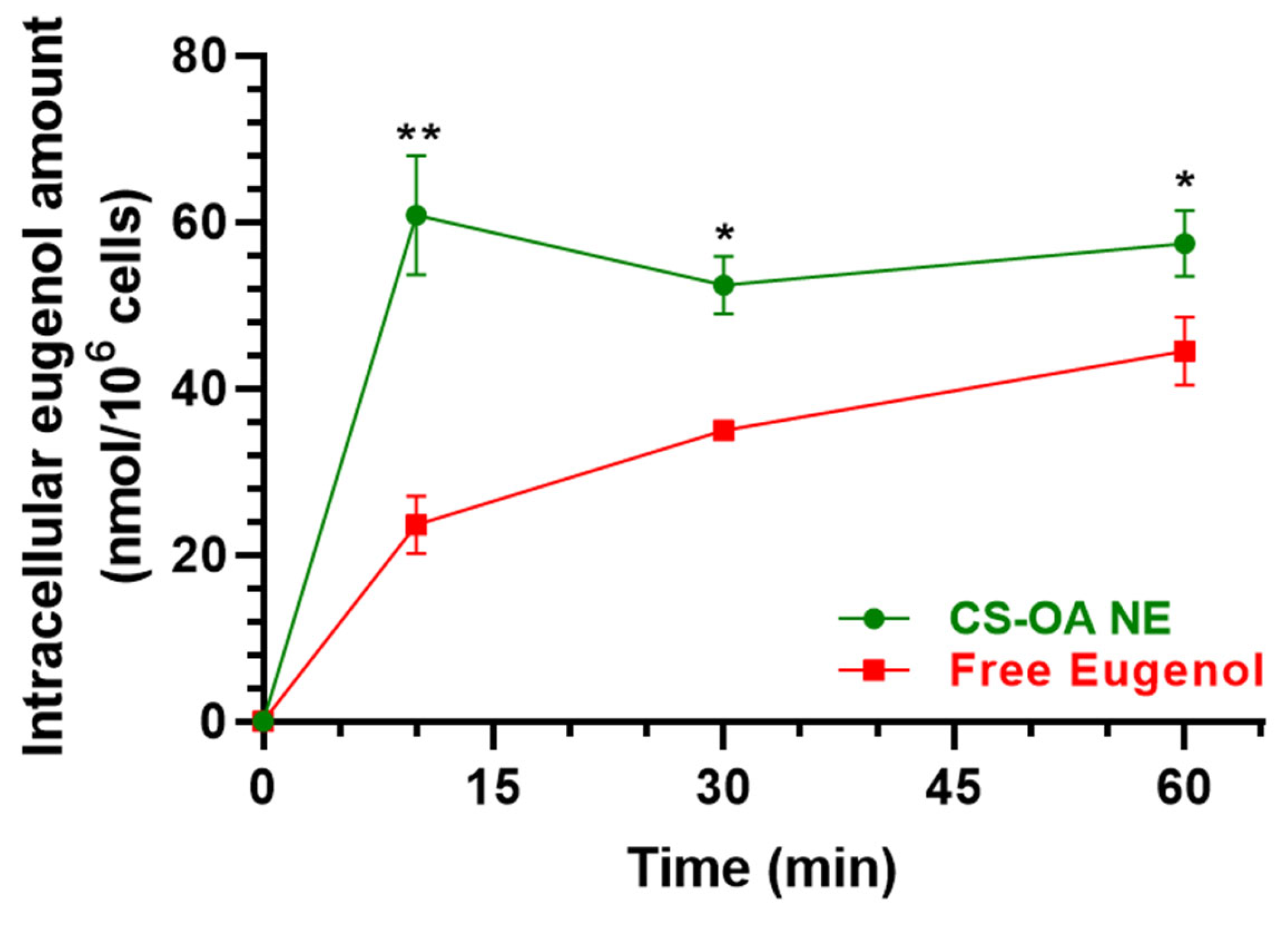
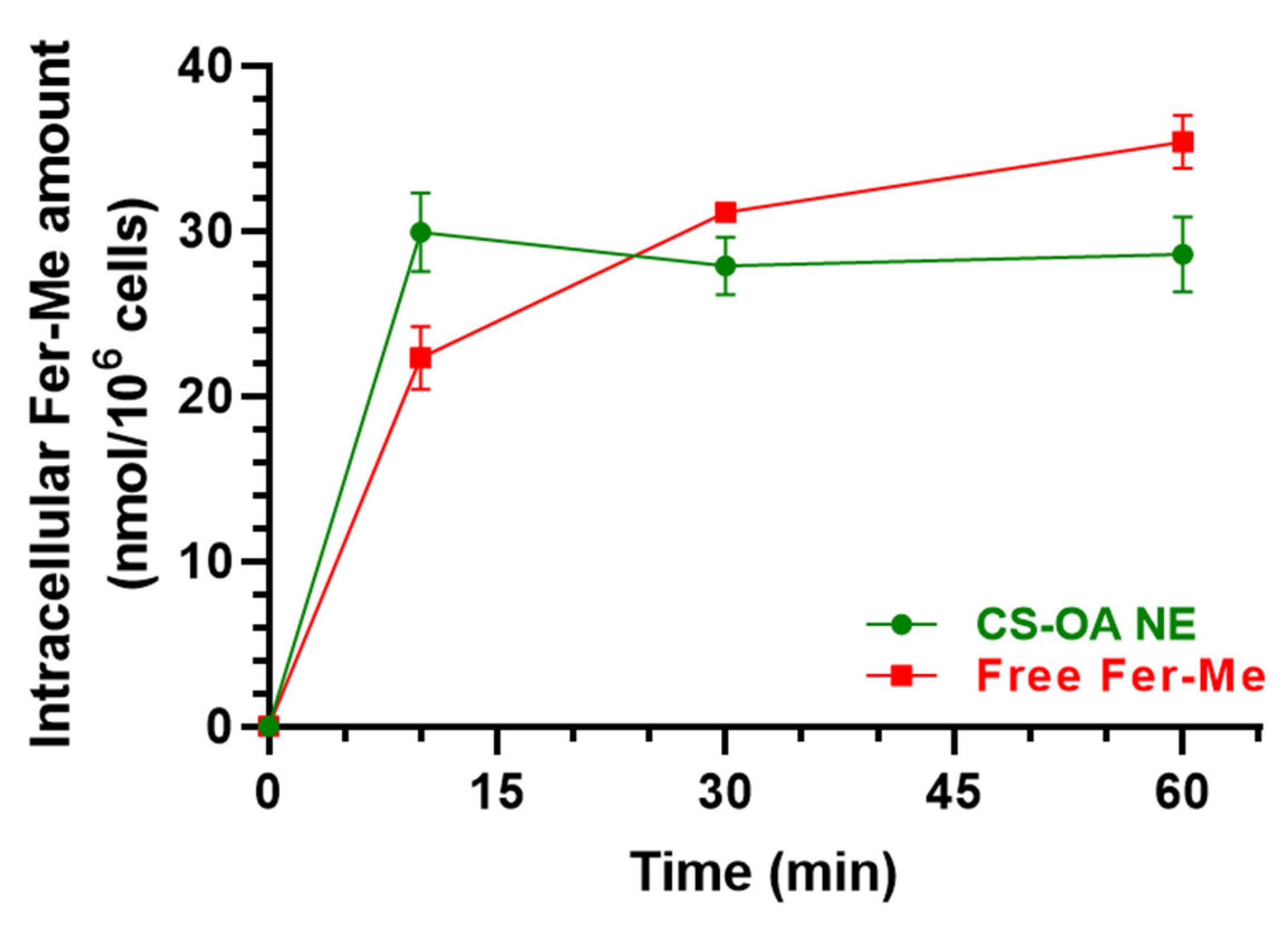
3.2.2. Evaluation of CS-OA NE or Fer-Me/Eugenol Uptake by RPMI 2650 Cells
3.3. In Vivo Studies
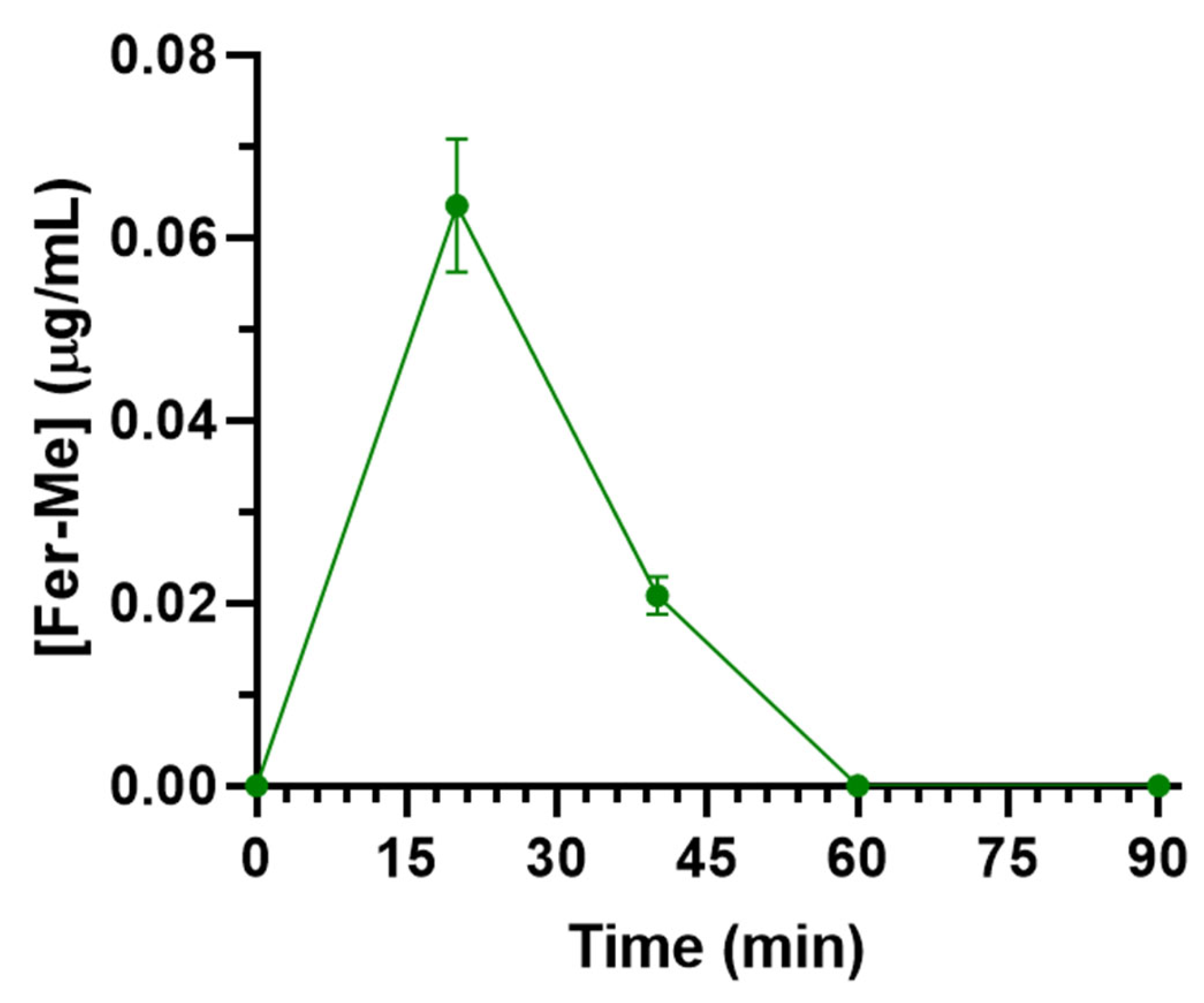
3.3.1. Intravenous Administration of Fer-Me
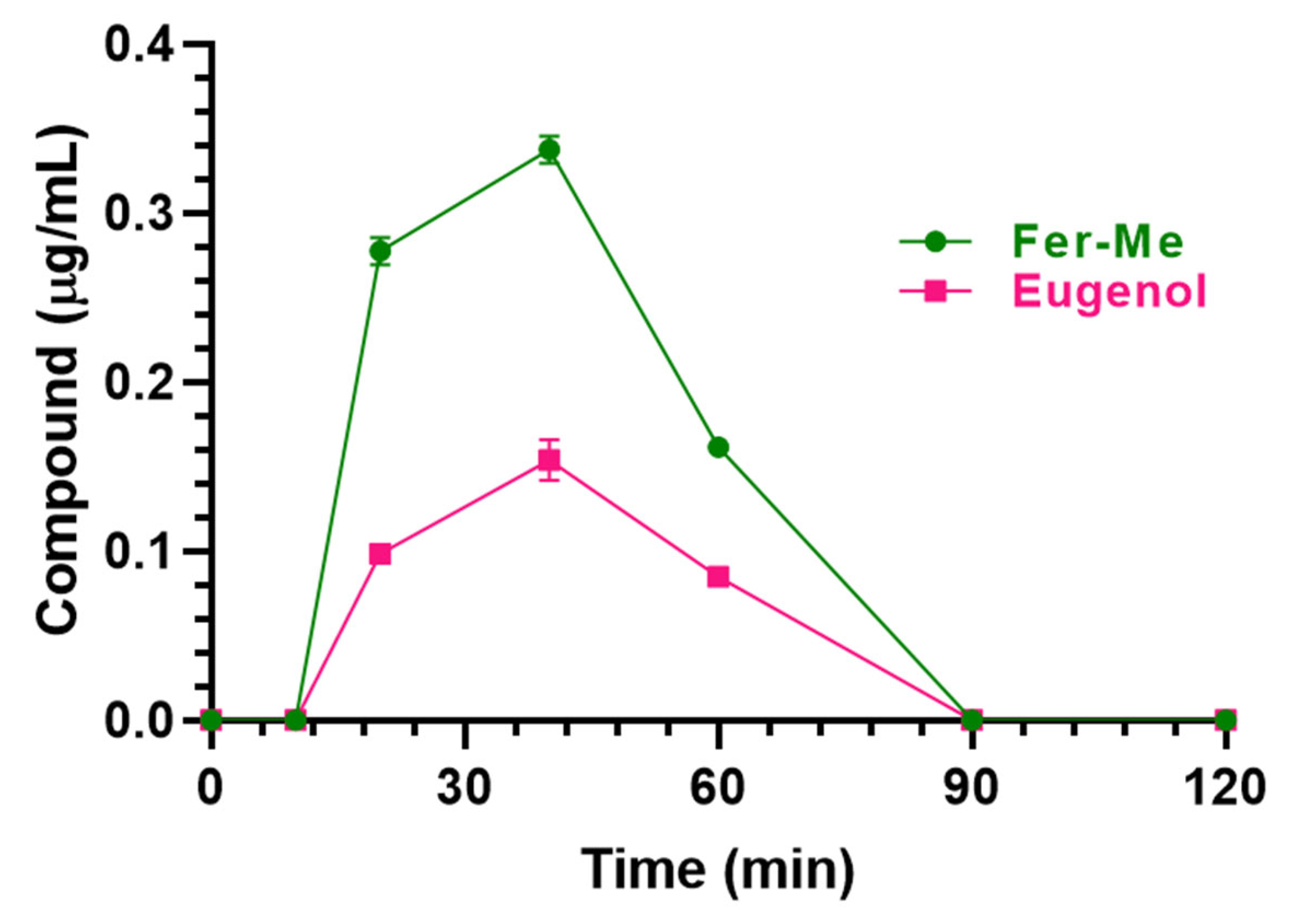
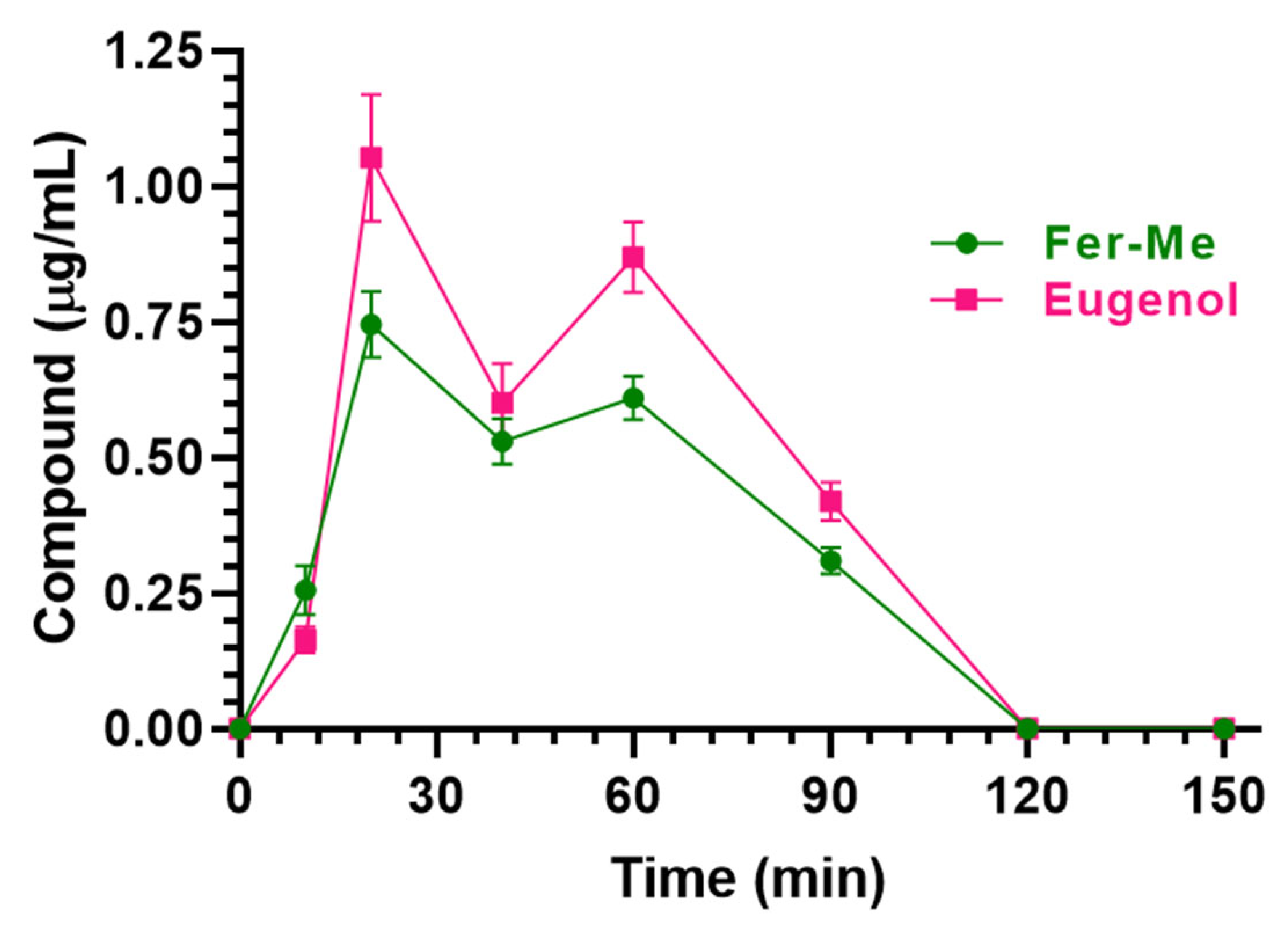
3.3.2. Nasal Administration of CS-OA NE
4. Discussion
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spisni, E.; Petrocelli, G.; Imbesi, V.; Spigarelli, R.; Azzinnari, D.; Donati Sarti, M.; Campieri, M.; Valerii, M.C. Antioxidant, anti-inflammatory, and microbial-modulating activities of essential oils: Implications in colonic pathophysiology. Int. J. Mol. Sci. 2020, 21, 4152. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as potential anti-inflammatory molecules: A review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef]
- Rekha, K.R.; Selvakumar, G.P.; Sethupathy, S.; Santha, K.; Sivakamasundari, R.I. Geraniol ameliorates the motor behavior and neurotrophic factors inadequacy in MPTP-induced mice model of Parkinson’s disease. J. Mol. Neurosci. 2013, 51, 851–862. [Google Scholar] [CrossRef]
- Li, J.; Long, X.; Hu, J.; Bi, J.; Zhou, T.; Guo, X.; Han, C.; Huang, J.; Wang, T.; Xiong, N.; et al. Multiple pathways for natural product treatment of Parkinson’s disease: A mini review. Phytomedicine 2019, 60, 152954. [Google Scholar] [CrossRef] [PubMed]
- Thapliyal, S.; Singh, T.; Handu, S.; Bisht, M.; Kumari, P.; Arya, P.; Srivastava, P.; Gandham, R. A review on potential footprints of ferulic acid for treatment of neurological disorders. Neurochem. Res. 2021, 46, 1043–1057. [Google Scholar] [CrossRef]
- Bhusal, C.K.; Uti, D.E.; Mukherjee, D.; Alqahtani, T.; Alqahtani, S.; Bhattacharya, A.; Akash, S. Unveiling nature’s potential: Promising natural compounds in Parkinson’s disease management. Parkinsonism Relat. Disord. 2023, 115, 105799. [Google Scholar] [CrossRef] [PubMed]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant properties of ferulic acid and its possible application. Skin Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef]
- Li, D.; Rui, Y.X.; Guo, S.D.; Luan, F.; Liu, R.; Zeng, N. Ferulic acid: A review of its pharmacology, pharmacokinetics and derivatives. Life Sci. 2021, 284, 119921. [Google Scholar] [CrossRef]
- Botti, G.; Bianchi, A.; Pavan, B.; Tedeschi, P.; Albanese, V.; Ferraro, L.; Spizzo, F.; Del Bianco, L.; Dalpiaz, A. Effects of microencapsulated ferulic acid or its prodrug methyl ferulate on neuroinflammation induced by muramyl dipeptide. Int. J. Environ. Res. Public Health 2022, 19, 10609. [Google Scholar] [CrossRef]
- Barboza, J.N.; da Silva Maia Bezerra Filho, C.; Silva, R.O.; Medeiros, J.V.R.; de Sousa, D.P. An overview on the anti-inflammatory potential and antioxidant profile of eugenol. Oxid. Med. Cell. Longev. 2018, 2018, 3957262. [Google Scholar] [CrossRef]
- Pavan, B.; Bianchi, A.; Botti, G.; Ferraro, L.; Valerii, M.C.; Spisni, E.; Dalpiaz, A. Pharmacokinetic and permeation studies in rat brain of natural compounds led to investigate eugenol as direct activator of dopamine release in PC12 cells. Int. J. Mol. Sci. 2023, 24, 1800. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Pacelli, C.; Giguère, N.; Bourque, M.J.; Lévesque, M.; Slack, R.S.; Trudeau, L.É. Elevated mitochondrial bioenergetics and axonal arborization size are key contributors to the vulnerability of dopamine neurons. Curr. Biol. 2015, 25, 2349–2360. [Google Scholar] [CrossRef] [PubMed]
- Flores-Ponce, X.; Velasco, I. Dopaminergic neuron metabolism: Relevance for understanding Parkinson’s disease. Metabolomics 2024, 20, 116. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Riccardi, G.; Giosuè, A.; Calabrese, I.; Vaccaro, O. Dietary recommendations for prevention of atherosclerosis. Cardiovasc. Res. 2022, 118, 1188–1204. [Google Scholar] [CrossRef]
- Bonferoni, M.C.; Sandri, G.; Rossi, S.; Usai, D.; Liakos, I.; Garzoni, A.; Fiamma, M.; Zanetti, S.; Athanassiou, A.; Caramella, C.; et al. A novel ionic amphiphilic chitosan derivative as a stabilizer of nanoemulsions: Improvement of antimicrobial activity of Cymbopogon citratus essential oil. Colloids Surf. B Biointerfaces 2017, 152, 385–392. [Google Scholar] [CrossRef]
- Bonferoni, M.C.; Ferraro, L.; Pavan, B.; Beggiato, S.; Cavalieri, E.; Giunchedi, P.; Dalpiaz, A. Uptake in the central nervous system of geraniol oil encapsulated in chitosan oleate following nasal and oral administration. Pharmaceutics 2019, 11, 106. [Google Scholar] [CrossRef]
- Perteghella, S.; Garzoni, A.; Invernizzi, A.; Sorrenti, M.; Boselli, C.; Icaro Cornaglia, A.; Dondi, D.; Lazzaroni, S.; Marrubini, G.; Caramella, C.; et al. Nanoemulsions of clove oil stabilized with chitosan oleate-antioxidant and wound-healing activity. Antioxidants 2023, 12, 273. [Google Scholar] [CrossRef]
- Botti, G.; Bianchi, A.; Dalpiaz, A.; Tedeschi, P.; Albanese, V.; Sorrenti, M.; Catenacci, L.; Bonferoni, M.C.; Beggiato, S.; Pavan, B. Dimeric ferulic acid conjugate as a prodrug for brain targeting after nasal administration of loaded solid lipid microparticles. Expert Opin. Drug Deliv. 2023, 20, 1657–1679. [Google Scholar] [CrossRef]
- Moore, G.E.; Sandberg, A.A. Studies of a human tumor cell line with a diploid karyotype. Cancer 1964, 17, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Moorhead, P.S. Human tumor cell line with a quasi-diploid karyotype (RPMI 2650). Exp. Cell Res. 1965, 39, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Dellera, E.; Bonferoni, M.C.; Sandri, G.; Rossi, S.; Ferrari, F.; Del Fante, C.; Perotti, C.; Grisoli, P.; Caramella, C. Development of chitosan oleate ionic micelles loaded with silver sulfadiazine to be associated with platelet lysate for application in wound healing. Eur. J. Pharm. Biopharm. 2014, 88, 643–650. [Google Scholar] [CrossRef]
- Sari, T.P.; Mann, B.; Kumar, R.; Singh, R.R.B.; Sharma, R.; Bhardwaj, M.; Athira, S. Preparation and characterization of nanoemulsion encapsulating curcumin. Food Hydrocoll. 2015, 43, 540–546. [Google Scholar] [CrossRef]
- He, P.; Davis, S.S.; Illum, L. In vitro evaluation of the mucoadhesive properties of chitosan microspheres. Int. J. Pharm. 1998, 166, 75–88. [Google Scholar] [CrossRef]
- Wang, N.; Brickute, D.; Braga, M.; Barnes, C.; Lu, H.; Allott, L.; Aboagye, E.O. Novel non-congeneric derivatives of the choline kinase alpha inhibitor ICL-CCIC-0019. Pharmaceutics 2021, 13, 1078. [Google Scholar] [CrossRef]
- Dalpiaz, A.; Fogagnolo, M.; Ferraro, L.; Capuzzo, A.; Pavan, B.; Rassu, G.; Salis, A.; Giunchedi, P.; Gavini, E. Nasal chitosan microparticles target a zidovudine prodrug to brain HIV sanctuaries. Antivir. Res. 2015, 123, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Rassu, G.; Soddu, E.; Cossu, M.; Brundu, A.; Cerri, G.; Marchetti, N.; Ferraro, L.; Regan, R.F.; Giunchedi, P.; Gavini, E.; et al. Solid microparticles based on chitosan or methyl-β-cyclodextrin: A first formulative approach to increase the nose-to-brain transport of deferoxamine mesylate. J. Control. Release 2015, 201, 68–77. [Google Scholar] [CrossRef]
- Truzzi, E.; Rustichelli, C.; de Oliveira Junior, E.R.; Ferraro, L.; Maretti, E.; Graziani, D.; Botti, G.; Beggiato, S.; Iannuccelli, V.; Lima, E.M.; et al. Nasal biocompatible powder of geraniol oil complexed with cyclodextrins for neurodegenerative diseases: Physicochemical characterization and in vivo evidences of nose to brain delivery. J. Control. Release 2021, 335, 191–202. [Google Scholar] [CrossRef]
- van den Berg, M.P.; Romeijn, S.G.; Verhoef, J.C.; Merkus, F.W. Serial cerebrospinal fluid sampling in a rat model to study drug uptake from the nasal cavity. J. Neurosci. Methods 2002, 116, 99–107. [Google Scholar] [CrossRef]
- Dalpiaz, A.; Ferraro, L.; Perrone, D.; Leo, E.; Iannuccelli, V.; Pavan, B.; Paganetto, G.; Beggiato, S.; Scalia, S. Brain uptake of a Zidovudine prodrug after nasal administration of solid lipid microparticles. Mol. Pharm. 2014, 11, 1550–1561. [Google Scholar] [CrossRef] [PubMed]
- Felgenhauer, K. Protein size and cerebrospinal fluid composition. Klin. Wochenschr. 1974, 52, 1158–1164. [Google Scholar] [CrossRef]
- Madu, A.; Cioffe, C.; Mian, U.; Burroughs, M.; Tuomanen, E.; Mayers, M.; Schwartz, E.; Miller, M. Pharmacokinetics of fluconazole in cerebrospinal fluid and serum of rabbits: Validation of an animal model used to measure drug concentrations in cerebrospinal fluid. Antimicrob. Agents Chemother. 1994, 38, 2111–2115. [Google Scholar] [CrossRef]
- Simovic, S.; Heard, P.; Hui, H.; Song, Y.; Peddie, F.; Davey, A.K.; Lewis, A.; Rades, T.; Prestidge, C.A. Dry hybrid lipid-silica microcapsules engineered from submicron lipid droplets and nanoparticles as a novel delivery system for poorly soluble drugs. Mol. Pharm. 2009, 6, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Tarhan, İ. A robust method for simultaneous quantification of eugenol, eugenyl acetate, and β-caryophyllene in clove essential oil by vibrational spectroscopy. Phytochemistry 2021, 191, 112928. [Google Scholar] [CrossRef]
- Aguilar, J.S.; Roy, D.; Ghazal, P.; Wagner, E.K. Dimethyl sulfoxide blocks herpes simplex virus-1 productive infection in vitro acting at different stages with positive cooperativity. Application of micro-array analysis. BMC Infect. Dis. 2002, 2, 9. [Google Scholar] [CrossRef]
- Tamagawa, S.; Sakai, D.; Schol, J.; Sako, K.; Nakamura, Y.; Matsushita, E.; Warita, T.; Hazuki, S.; Nojiri, H.; Sato, M.; et al. N-acetylcysteine attenuates oxidative stress-mediated cell viability loss induced by dimethyl sulfoxide in cryopreservation of human nucleus pulposus cells: A potential solution for mass production. JOR Spine 2022, 5, e1223. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Ahmad, R.; Alam, M.A.; Ahmad, F.J. Quantification and brain targeting of eugenol-loaded surface modified nanoparticles through intranasal route in the treatment of cerebral ischemia. Drug Res. 2018, 68, 584–595. [Google Scholar] [CrossRef]
- De Fazio, L.; Spisni, E.; Cavazza, E.; Strillacci, A.; Candela, M.; Centanni, M.; Ricci, C.; Rizzello, F.; Campieri, M.; Valerii, M.C. Dietary geraniol by oral or enema administration strongly reduces dysbiosis and systemic inflammation in dextran sulfate sodium treated mice. Front. Pharmacol. 2016, 7, 38. [Google Scholar] [CrossRef]
- Casettari, L.; Illum, L. Chitosan in nasal delivery systems for therapeutic drugs. J. Control. Release 2014, 190, 189–200. [Google Scholar] [CrossRef]
- Mak, K.K.; Kamal, M.B.; Ayuba, S.B.; Sakirolla, R.; Kang, Y.B.; Mohandas, K.; Balijepalli, M.K.; Ahmad, S.H.; Pichika, M.R. A comprehensive review on eugenol’s antimicrobial properties and industry applications: A transformation from ethnomedicine to industry. Pharmacogn. Rev. 2019, 13, 1–9. [Google Scholar] [CrossRef]
- Demisli, S.; Mitsou, E.; Pletsa, V.; Xenakis, A.; Papadimitriou, V. Development and study of nanoemulsions and nanoemulsion-based hydrogels for the encapsulation of lipophilic compounds. Nanomaterials 2020, 10, 2464. [Google Scholar] [CrossRef]
- Al Wafai, R.; El-Rabih, W.; Katerji, M.; Safi, R.; El Sabban, M.; El-Rifai, O.; Usta, J. Chemosensitivity of MCF-7 cells to eugenol: Release of cytochrome-c and lactate dehydrogenase. Sci. Rep. 2017, 7, 43730. [Google Scholar] [CrossRef]
- Sibinovska, N.; Žakelj, S.; Kristan, K. Suitability of RPMI 2650 cell models for nasal drug permeability prediction. Eur. J. Pharm. Biopharm. 2019, 145, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Gadhave, D.G.; Quadros, M.; Ugale, A.R.; Goyal, M.; Ayehunie, S.; Gupta, V. Mucoadhesive chitosan-poly (lactic-co-glycolic acid) nanoparticles for intranasal delivery of quetiapine—Development & characterization in physiologically relevant 3D tissue models. Int. J. Biol. Macromol. 2024, 267 Pt 2, 131491. [Google Scholar] [CrossRef]
- Shen, Q.; Wang, Y.; Zhang, Y. Improvement of colchicine oral bioavailability by incorporating eugenol in the nanoemulsion as an oil excipient and enhancer. Int. J. Nanomed. 2011, 6, 1237–1243. [Google Scholar] [CrossRef]
- Guenette, S.A.; Beaudry, F.; Marier, J.F.; Vachon, P. Pharmacokinetics and anesthetic activity of eugenol in male Sprague-Dawley rats. J. Vet. Pharmacol. Ther. 2006, 29, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Pavan, B.; Dalpiaz, A. Prodrugs and endogenous transporters: Are they suitable tools for drug targeting into the central nervous system? Curr. Pharm. Des. 2011, 17, 3560–3576. [Google Scholar] [CrossRef]
- Segura-Aguilar, J.; Paris, I.; Muñoz, P.; Ferrari, E.; Zecca, L.; Zucca, F.A. Protective and toxic roles of dopamine in Parkinson’s disease. J. Neurochem. 2014, 129, 898–915. [Google Scholar] [CrossRef]
- Tan, Y.Y.; Jenner, P.; Chen, S.D. Monoamine oxidase-B inhibitors for the treatment of Parkinson’s disease: Past, present, and future. J. Parkinsons Dis. 2022, 12, 477–493. [Google Scholar] [CrossRef]
- Gahtani, R.M.; Shoaib, S.; Hani, U.; Jayachithra, R.; Alomary, M.N.; Chauhan, W.; Jahan, R.; Tufail, S.; Ansari, M.A. Combating Parkinson’s disease with plant-derived polyphenols: Targeting oxidative stress and neuroinflammation. Neurochem. Int. 2024, 178, 105798. [Google Scholar] [CrossRef] [PubMed]
- Zafra-Gómez, A.; Luzón-Toro, B.; Jiménez-Diaz, I.; Ballesteros, O.; Navalón, A. Quantification of phenolic antioxidants in rat cerebrospinal fluid by GC-MS after oral administration of compounds. J. Pharm. Biomed. Anal. 2010, 53, 103–108. [Google Scholar] [CrossRef]
- Liu, C.S.; Chen, L.; Hu, Y.N.; Dai, J.L.; Ma, B.; Tang, Q.F.; Tan, X.M. Self-microemulsifying drug delivery system for improved oral delivery and hypnotic efficacy of ferulic acid. Int. J. Nanomed. 2020, 15, 2059–2070. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Egashira, Y.; Sanada, H. Digestion and absorption of ferulic acid sugar esters in rat gastrointestinal tract. J. Agric. Food Chem. 2003, 51, 5534–5539. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, C.; Zhang, Y.; Mi, S.; Wang, N. Pharmacokinetics of ferulic acid and potential interactions with Honghua and clopidogrel in rats. J. Ethnopharmacol. 2011, 137, 562–567. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, W.; Zhang, Y.; Wang, Q.; Qin, C.; Du, S.; Huang, L.; Ye, F.; Chen, L.; Zheng, T. Pharmacokinetics, bioavailability, and tissue distribution study of angoroside c and its metabolite ferulic acid in rat using UPLC-MS/MS. Front. Pharmacol. 2018, 9, 1186. [Google Scholar] [CrossRef]

| Bloodstream | |||||
|---|---|---|---|---|---|
| Administration (Dose) | Compound | Co (μg·mL−1) | t1/2 (Min) | AUC (μg·mL−1·min) | Reference |
| Fer (1 mg/kg) | Fer | 10.5 ± 1.0 | 20.3 ± 1.3 | 244 ± 13 | [20] |
| Fer-Me (10 mg/kg) | Fer-Me | 44.3 ± 1.3 | 10.08 ± 0.37 | 671 ± 34 | Current data |
| Fer | 0.209 ± 0.001 | - | 8.4 ± 0.8 | Current data | |
| Eugenol (20 mg/kg) | Eugenol | 16.5 ± 0.2 | 19.4 ± 2.1 | 174.8 ± 3.1 | [11] |
| CSF | |||||
| Administration (Dose) | Compound | Cmax (μg·mL−1) | Tmax (Min) | AUC (μg·mL−1·min) | Reference |
| Fer (1 mg/kg) | Fer | 0.08 ± 0.01 | 60 | 3.3 ± 0.3 | [20] |
| Fer-Me (10 mg/kg) | Fer-Me | 0.064 ± 0.007 | 20 | 1.69 ± 0.15 | Current data |
| Eugenol (20 mg/kg) | Eugenol | 2.79 ± 0.18 | 10 | 56.1 ± 4.2 | [11] |
| Bloodstream | ||||
|---|---|---|---|---|
| Compound (Dose) | Cmax (μg·mL−1) | Tmax (Min) | AUC (μg·mL−1·min) | F (%) |
| Fer-Me (0.4 mg/kg) | 0.34 ± 0.01 | 40 | 14.95 ± 0.23 | 56 |
| Eugenol (0.8 mg/kg) | 0.15 ± 0.01 | 40 | 6.68 ± 0.27 | 95 |
| CSF | ||||
| Compound (Dose) | Cmax (μg·mL−1) | Tmax (Min) | AUC (μg·mL−1·min) | - |
| Fer-Me (0.4 mg/kg) | 0.75 ± 0.04 | 20 | 48.89 ± 1.30 | - |
| Eugenol (0.8 mg/kg) | 1.05 ± 0.08 | 20 | 63.75 ± 2.18 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botti, G.; Catenacci, L.; Dalpiaz, A.; Randi, L.; Bonferoni, M.C.; Perteghella, S.; Beggiato, S.; Ferraro, L.; Pavan, B.; Sorrenti, M. Nasal Administration of a Nanoemulsion Based on Methyl Ferulate and Eugenol Encapsulated in Chitosan Oleate: Uptake Studies in the Central Nervous System. Pharmaceutics 2025, 17, 367. https://doi.org/10.3390/pharmaceutics17030367
Botti G, Catenacci L, Dalpiaz A, Randi L, Bonferoni MC, Perteghella S, Beggiato S, Ferraro L, Pavan B, Sorrenti M. Nasal Administration of a Nanoemulsion Based on Methyl Ferulate and Eugenol Encapsulated in Chitosan Oleate: Uptake Studies in the Central Nervous System. Pharmaceutics. 2025; 17(3):367. https://doi.org/10.3390/pharmaceutics17030367
Chicago/Turabian StyleBotti, Giada, Laura Catenacci, Alessandro Dalpiaz, Luca Randi, Maria Cristina Bonferoni, Sara Perteghella, Sarah Beggiato, Luca Ferraro, Barbara Pavan, and Milena Sorrenti. 2025. "Nasal Administration of a Nanoemulsion Based on Methyl Ferulate and Eugenol Encapsulated in Chitosan Oleate: Uptake Studies in the Central Nervous System" Pharmaceutics 17, no. 3: 367. https://doi.org/10.3390/pharmaceutics17030367
APA StyleBotti, G., Catenacci, L., Dalpiaz, A., Randi, L., Bonferoni, M. C., Perteghella, S., Beggiato, S., Ferraro, L., Pavan, B., & Sorrenti, M. (2025). Nasal Administration of a Nanoemulsion Based on Methyl Ferulate and Eugenol Encapsulated in Chitosan Oleate: Uptake Studies in the Central Nervous System. Pharmaceutics, 17(3), 367. https://doi.org/10.3390/pharmaceutics17030367











