Multifaceted Functional Liposomes: Theranostic Potential of Liposomal Indocyanine Green and Doxorubicin for Enhanced Anticancer Efficacy and Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of ICG/DOX Liposomes
2.2. Cell Culture
2.3. Cellular Uptake of Liposomes by A549 Cells
2.4. Photothermal Effect Validation
2.5. Photothermal Effect-Induced Drug Release
2.6. In Vitro Cell Viability Analysis
2.7. RNA Sequencing (RNA-Seq) Analysis
2.8. In Vivo Experiments
2.9. In Vivo and Ex Vivo Imaging
3. Results
3.1. Liposomal Encapsulation of ICG and DOX
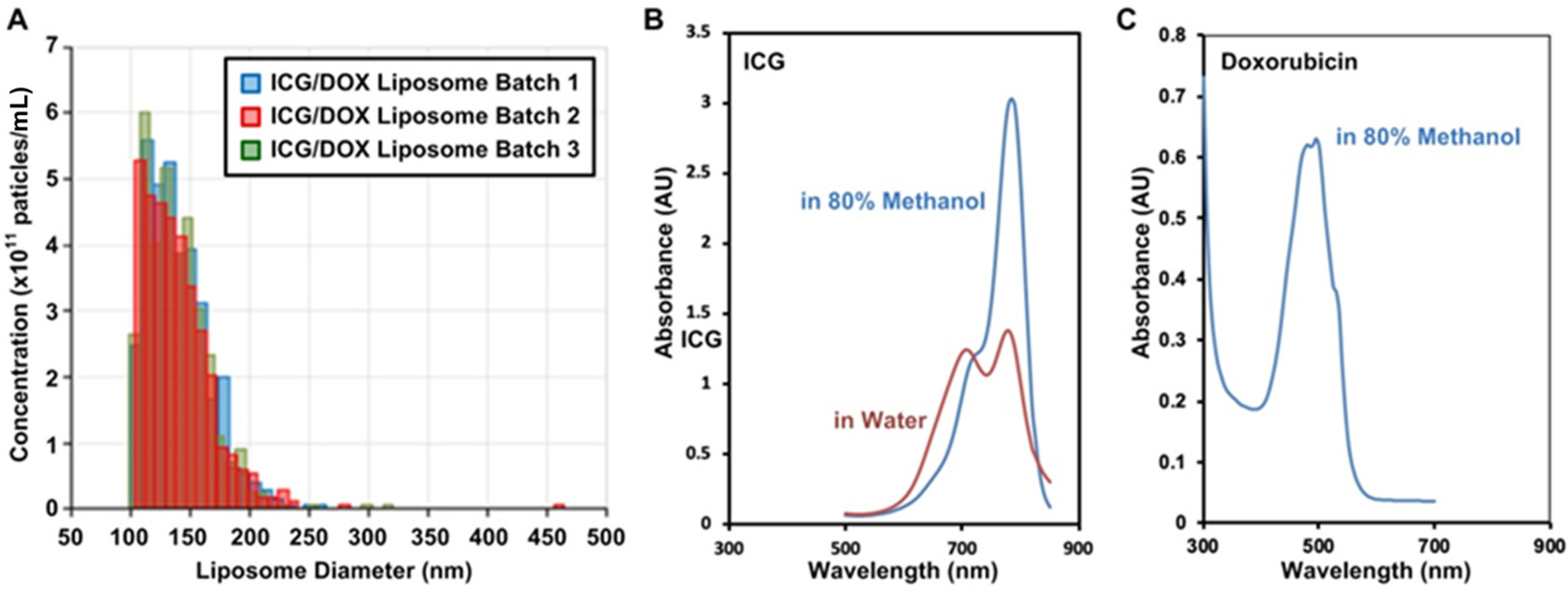
3.1.1. Liposome Properties
3.1.2. Photothermal Effect and Controlled Drug Release
3.2. In Vitro Model
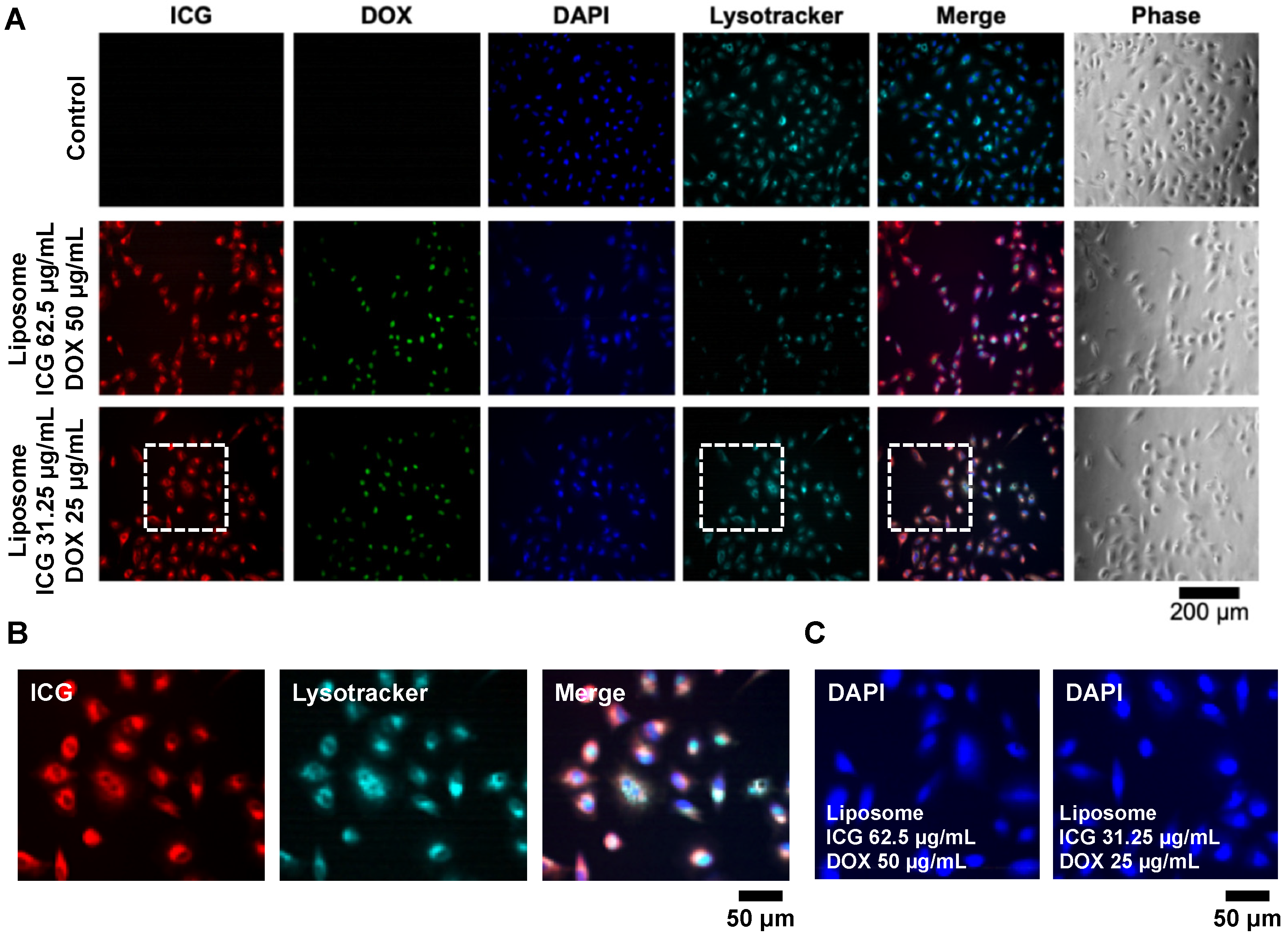
3.2.1. Lipo-ICG/DOX on A549 Cell Line
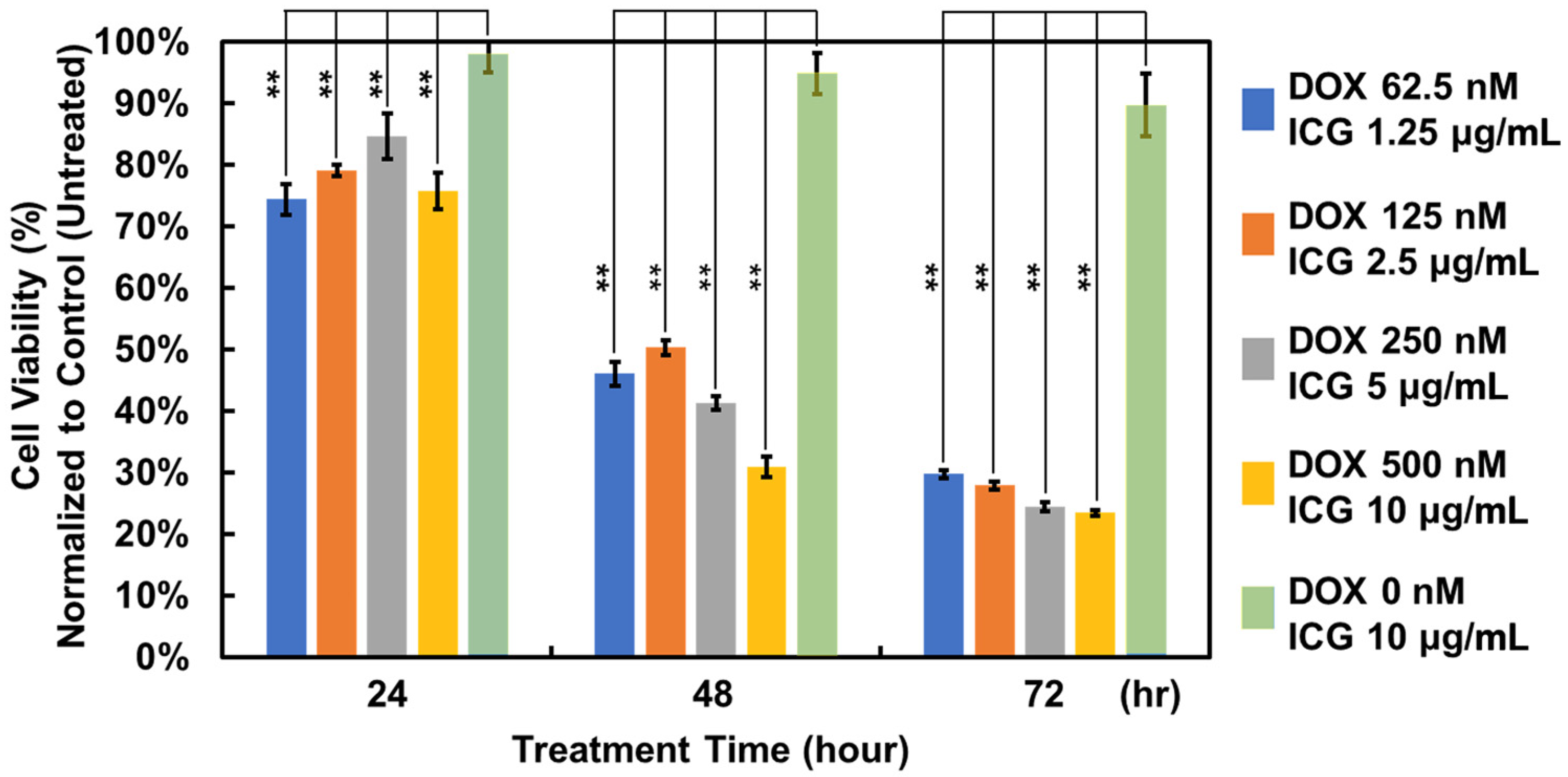
3.2.2. Treatment Efficacy
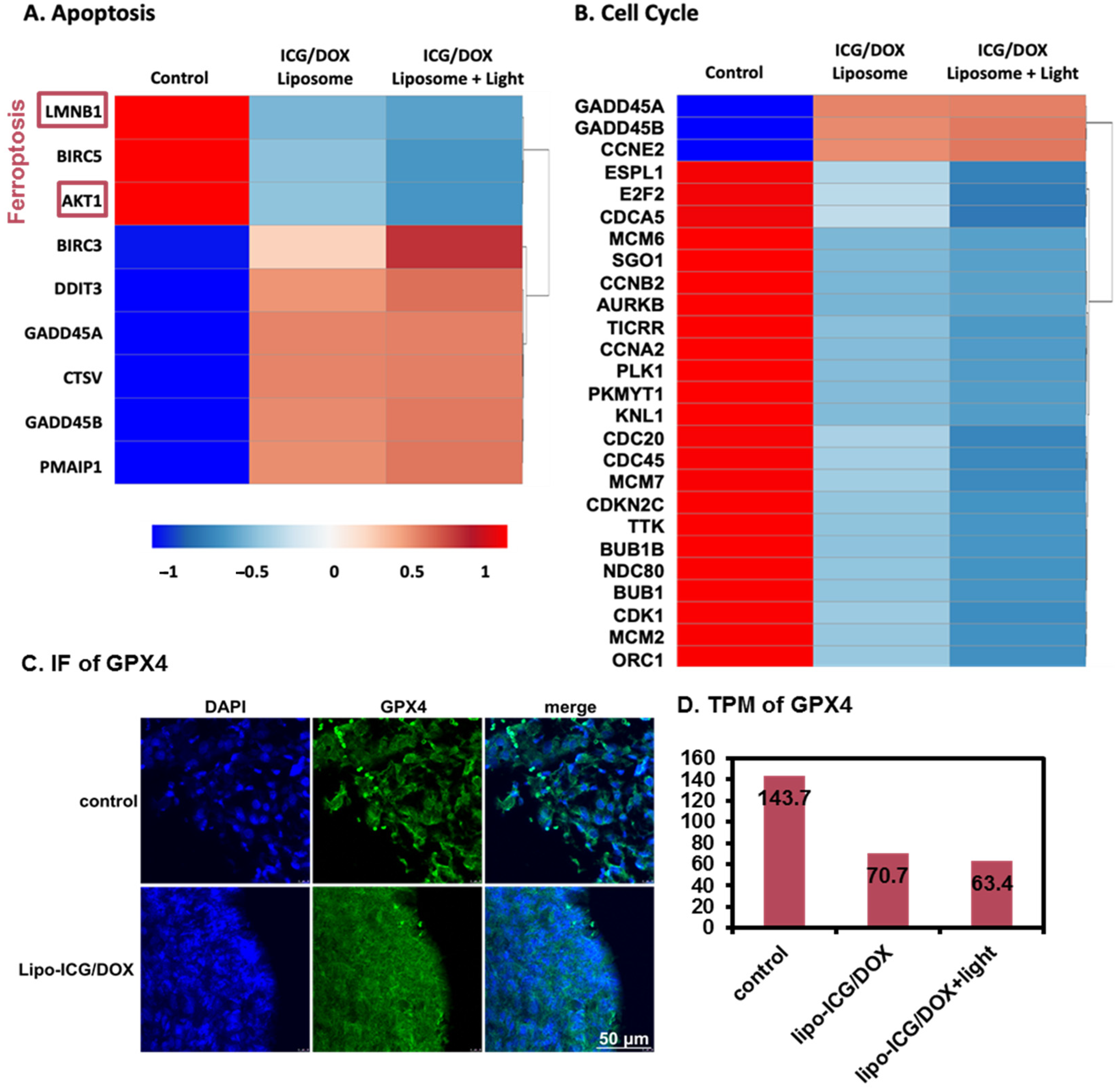
3.2.3. RNA-Seq Analysis
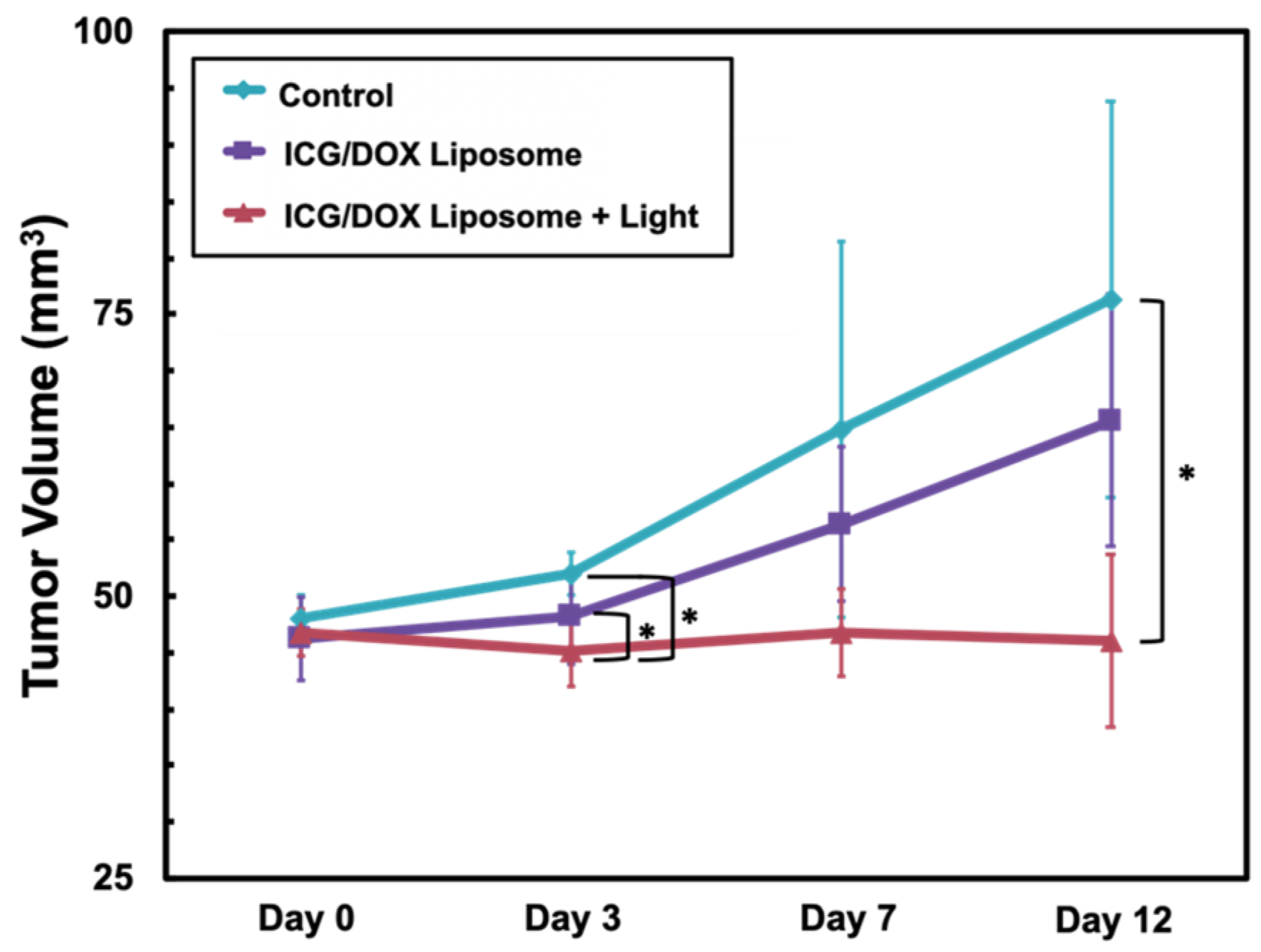
3.3. In Vivo Model
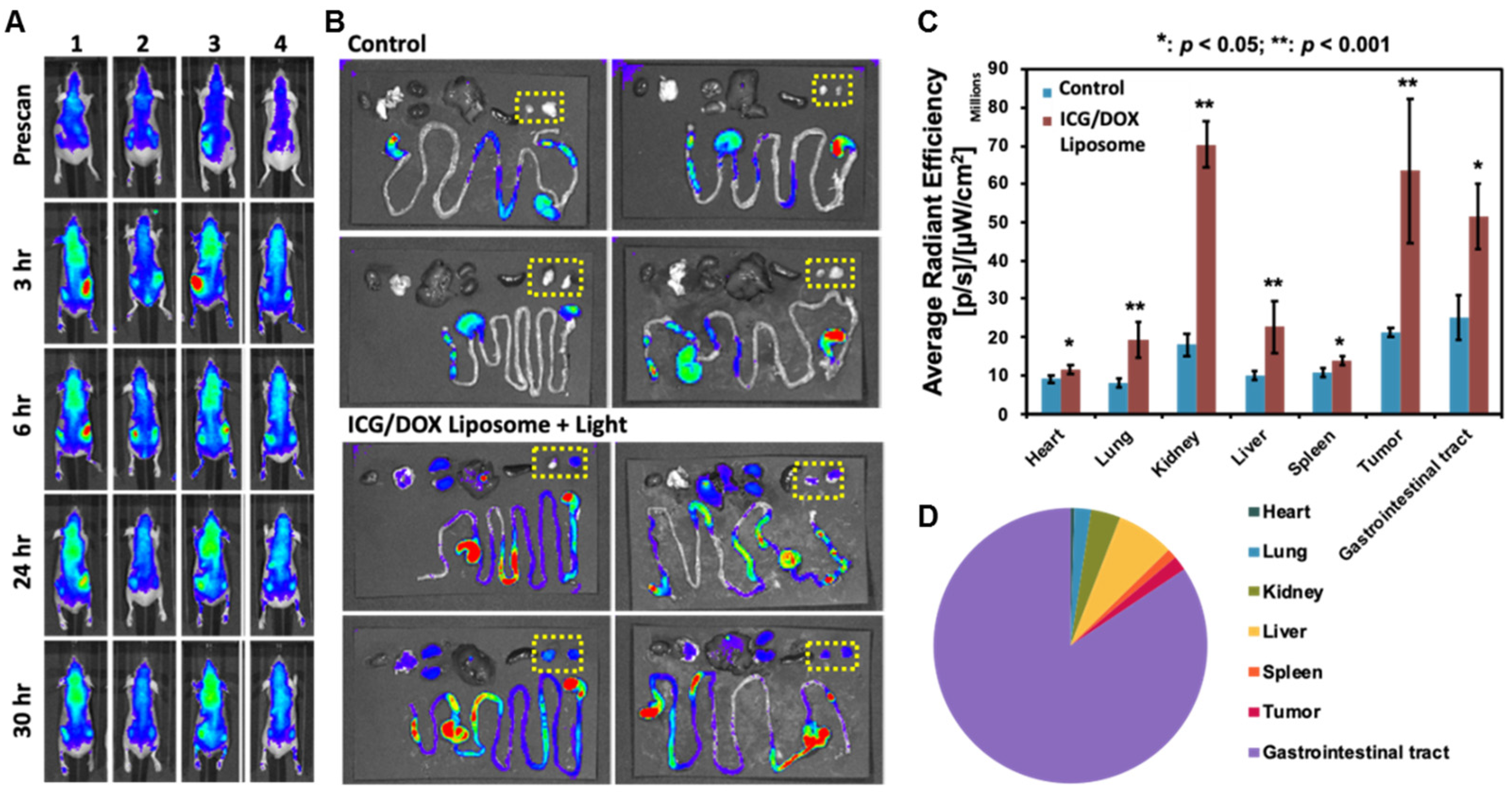
IVIS Image of Lipo-ICG/DOX in Nude Mouse
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DAPI | 4,6-diamidino-2-phenylindole |
| DLS | Dynamic Light Scattering |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DOX | Doxorubicin |
| DPPC | 1,2-dipalmitoyl-sn-glycero-3-phosphocholine |
| DSPE-PEG2000 | 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] |
| EPR | Enhanced Permeability and Retention |
| GI | Gastrointestinal |
| HSA | Human Serum Albumin |
| IACUC | Institutional Animal Care and Use Committee |
| ICG | Indocyanine Green |
| IVIS | In Vivo Imaging System |
| Lipo-ICG/DOX | Liposome Encapsulating Indocyanine Green and Doxorubicin |
| RNA-seq | RNA Sequencing |
| ROS | Reactive Oxygen Species |
References
- Alander, J.T.; Kaartinen, I.; Laakso, A.; Patila, T.; Spillmann, T.; Tuchin, V.V.; Venermo, M.; Valisuo, P. A review of indocyanine green fluorescent imaging in surgery. Int. J. Biomed. Imaging 2012, 2012, 940585. [Google Scholar] [CrossRef]
- Hsiao, J.-K.; Lu, C.-H. Indocyanine green: An old drug with novel applications. Tzu Chi. Med. J. 2021, 33, 317–322. [Google Scholar] [CrossRef]
- Tseng, H.C.; Kuo, C.Y.; Liao, W.T.; Chou, T.S.; Hsiao, J.K. Indocyanine green as a near-infrared theranostic agent for ferroptosis and apoptosis-based, photothermal, and photodynamic cancer therapy. Front. Mol. Biosci. 2022, 9, 1045885. [Google Scholar] [CrossRef] [PubMed]
- Björnsson, Ó.G.; Murphy, R.; Chadwick, V.J.E. Physicochemical studies of indocyanine green (ICG): Absorbance/concentration relationship, pH tolerance and assay precision in various solvents. Cell. Mol. Life Sci. 1982, 38, 1441–1442. [Google Scholar] [CrossRef]
- Mieog, J.S.D.; Troyan, S.L.; Hutteman, M.; Donohoe, K.J.; van der Vorst, J.R.; Stockdale, A.; Liefers, G.-J.; Choi, H.S.; Gibbs-Strauss, S.L.; Putter, H.; et al. Toward optimization of imaging system and lymphatic tracer for near-infrared fluorescent sentinel lymph node mapping in breast cancer. Ann. Surg. Oncol. 2011, 18, 2483–2491. [Google Scholar] [CrossRef] [PubMed]
- Crommelin, D.; Van Bommel, E.J.P.r. Stability of liposomes on storage: Freeze dried, frozen or as an aqueous dispersion. Pharm. Res. 1984, 1, 159–163. [Google Scholar] [CrossRef]
- Leung, S.J.; Romanowski, M.J.T. Light-activated content release from liposomes. Theranostics 2012, 2, 1020. [Google Scholar] [CrossRef]
- Lajunen, T.; Kontturi, L.S.; Viitala, L.; Manna, M.; Cramariuc, O.; Rog, T.; Bunker, A.; Laaksonen, T.; Viitala, T.; Murtomaki, L.; et al. Indocyanine green-loaded liposomes for light-triggered drug release. Mol. Pharm. 2016, 13, 2095–2107. [Google Scholar] [CrossRef]
- Stepniewski, M.; Pasenkiewicz-Gierula, M.; Rog, T.; Danne, R.; Orlowski, A.; Karttunen, M.; Urtti, A.; Yliperttula, M.; Vuorimaa, E.; Bunker, A. Study of PEGylated lipid layers as a model for PEGylated liposome surfaces: Molecular dynamics simulation and langmuir monolayer studies. Langmuir 2011, 27, 7788–7798. [Google Scholar] [CrossRef]
- Lajunen, T.; Nurmi, R.; Wilbie, D.; Ruoslahti, T.; Johansson, N.G.; Korhonen, O.; Rog, T.; Bunker, A.; Ruponen, M.; Urtti, A. The effect of light sensitizer localization on the stability of indocyanine green liposomes. J. Control. Release 2018, 284, 213–223. [Google Scholar] [CrossRef]
- Magarkar, A.; Stepniewski, M.; Karakas, E.; Rog, T.; Yliperttula, M.; Urtti, A.; Bunker, A. Molecular modeling of the PEGylated bilayer as a model for the PEGylated liposome surface in the bloodstream. In Proceedings of the NSTI Nanotechnology Conference and Expo, Santa Clara, CA, USA; 2012; pp. 293–295. [Google Scholar]
- Lin, L.; Liang, X.; Xu, Y.; Yang, Y.; Li, X.; Dai, Z. Doxorubicin and indocyanine green loaded hybrid bicelles for fluorescence imaging guided synergetic chemo/photothermal therapy. Bioconjug. Chem. 2017, 28, 2410–2419. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.B.; Sarosy, G.; Clagett-Carr, K.; Russo, M.; Leyland-Jones, B. Anthracycline analogs the past, present, and future. Cancer Chemother. Pharmacol. 1986, 18, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y. Doxil(R)—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, R.; He, J.; Yu, L.; Li, X.; Zhang, J.; Li, S.; Zhang, C.; Kagan, J.C.; Karp, J.M.; et al. Ultrasound-responsive low-dose doxorubicin liposomes trigger mitochondrial DNA release and activate cGAS-STING-mediated antitumour immunity. Nat. Commun. 2023, 14, 3877. [Google Scholar] [CrossRef]
- Xue, X.; Fang, T.; Yin, L.; Jiang, J.; He, Y.; Dai, Y.; Wang, D. Multistage delivery of CDs-DOX/ICG-loaded liposome for highly penetration and effective chemo-photothermal combination therapy. Drug Deliv. 2018, 25, 1826–1839. [Google Scholar] [CrossRef]
- Lu, W.; Liu, W.; Hu, A.; Shen, J.; Yi, H.; Cheng, Z. Combinatorial polydopamine-liposome nanoformulation as an effective anti-breast cancer therapy. Int. J.Nanomed. 2023, 18, 861–879. [Google Scholar] [CrossRef]
- Yuan, B.; Chen, N.; Zhu, Q. Emission and absorption properties of indocyanine green in Intralipid solution. J. Biomed. Opt. 2004, 9, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Dong, A.; Guo, R.; Yang, M.; Deng, L.; Zhang, J. DOX/ICG coencapsulated liposome-coated thermosensitive nanogels for NIR-triggered simultaneous drug release and photothermal effect. ACS Biomater. Sci. Eng. 2018, 4, 2424–2434. [Google Scholar] [CrossRef]
- Liao, W.T.; Chang, D.M.; Lin, M.X.; Lee, J.W.; Tung, Y.C.; Hsiao, J.K. Indocyanine-green-loaded liposomes for photodynamic and photothermal therapies: Inducing apoptosis and ferroptosis in cancer cells with implications beyond oral cancer. Pharmaceutics 2024, 16, 224. [Google Scholar] [CrossRef]
- Yan, F.; Duan, W.; Li, Y.; Wu, H.; Zhou, Y.; Pan, M.; Liu, H.; Liu, X.; Zheng, H. NIR-laser-controlled drug release from DOX/IR-780-loaded temperature-sensitive-liposomes for chemo-photothermal synergistic tumor therapy. Theranostics 2016, 6, 2337–2351. [Google Scholar] [CrossRef]
- Zylberberg, C.; Matosevic, S. Pharmaceutical liposomal drug delivery: A review of new delivery systems and a look at the regulatory landscape. Drug Deliv. 2016, 23, 3319–3329. [Google Scholar] [CrossRef] [PubMed]
- Etheridge, M.L.; Campbell, S.A.; Erdman, A.G.; Haynes, C.L.; Wolf, S.M.; McCullough, J. The big picture on nanomedicine: The state of investigational and approved nanomedicine products. Nanomedicine 2013, 9, 1–14. [Google Scholar] [CrossRef]
- Gubernator, J. Active methods of drug loading into liposomes: Recent strategies for stable drug entrapment and increased in vivo activity. Expert Opin. Drug Deliv. 2011, 8, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. Thin-film hydration followed by extrusion method for liposome preparation. Methods Mol. Biol. 2017, 1522, 17–22. [Google Scholar] [CrossRef]
- Iyaswamy, A.; Thakur, A.; Guan, X.J.; Krishnamoorthi, S.; Fung, T.Y.; Lu, K.; Gaurav, I.; Yang, Z.; Su, C.F.; Lau, K.F.; et al. Fe65-engineered neuronal exosomes encapsulating corynoxine-B ameliorate cognition and pathology of Alzheimer’s disease. Signal Transduct. Target Ther. 2023, 8, 404. [Google Scholar] [CrossRef] [PubMed]
- Frazzi, R. BIRC3 and BIRC5: Multi-faceted inhibitors in cancer. Cell Biosci. 2021, 11, 8. [Google Scholar] [CrossRef]
- Green, B.D.; Jabbour, A.M.; Sandow, J.J.; Riffkin, C.D.; Masouras, D.; Daunt, C.P.; Salmanidis, M.; Brumatti, G.; Hemmings, B.A.; Guthridge, M.A.; et al. Akt1 is the principal Akt isoform regulating apoptosis in limiting cytokine concentrations. Cell Death Differ. 2013, 20, 1341–1349. [Google Scholar] [CrossRef]
- Li, T.; Su, L.; Lei, Y.; Liu, X.; Zhang, Y.; Liu, X. DDIT3 and KAT2A proteins regulate TNFRSF10A and TNFRSF10B expression in endoplasmic reticulum stress-mediated apoptosis in human lung cancer cells. J. Biol. Chem. 2015, 290, 11108–11118. [Google Scholar] [CrossRef]
- Han, N.; Yuan, F.; Xian, P.; Liu, N.; Liu, J.; Zhang, H.; Zhang, H.; Yao, K.; Yuan, G. GADD45a mediated cell cycle inhibition is regulated by P53 in bladder cancer. Onco Targets Ther. 2019, 12, 7591–7599. [Google Scholar] [CrossRef]
- Vairapandi, M.; Balliet, A.G.; Hoffman, B.; Liebermann, D.A. GADD45b and GADD45g are cdc2/cyclinB1 kinase inhibitors with a role in S and G2/M cell cycle checkpoints induced by genotoxic stress. J. Cell Physiol. 2002, 192, 327–338. [Google Scholar] [CrossRef]
- Zhu, L.; Zeng, Q.; Wang, J.; Deng, F.; Jin, S. Cathepsin V drives lung cancer progression by shaping the immunosuppressive environment and adhesion molecules cleavage. Aging 2023, 15, 13961–13979. [Google Scholar] [CrossRef] [PubMed]
- Janus, P.; Toma-Jonik, A.; Vydra, N.; Mrowiec, K.; Korfanty, J.; Chadalski, M.; Widlak, P.; Dudek, K.; Paszek, A.; Rusin, M.; et al. Pro-death signaling of cytoprotective heat shock factor 1: Upregulation of NOXA leading to apoptosis in heat-sensitive cells. Cell Death Differ. 2020, 27, 2280–2292. [Google Scholar] [CrossRef]
- Tang, D.; Luo, H.; Xie, A.; He, Z.; Zou, B.; Xu, F.; Zhang, W.; Xu, X. Silencing LMNB1 contributes to the suppression of lung adenocarcinoma development. Cancer Manag. Res. 2021, 13, 2633–2642. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Park, S.M.; Hwang, E.M.; Baek, K.E.; Kim, I.K.; Nam, I.K.; Im, M.J.; Park, S.H.; Bae, S.; Park, J.Y.; et al. Gadd45b mediates Fas-induced apoptosis by enhancing the interaction between p38 and retinoblastoma tumor suppressor. J. Biol. Chem. 2010, 285, 25500–25505. [Google Scholar] [CrossRef] [PubMed]
- Infante, A.; Laresgoiti, U.; Fernandez-Rueda, J.; Fullaondo, A.; Galan, J.; Diaz-Uriarte, R.; Malumbres, M.; Field, S.J.; Zubiaga, A.M. E2F2 represses cell cycle regulators to maintain quiescence. Cell Cycle 2008, 7, 3915–3927. [Google Scholar] [CrossRef]
- Lee, S.Y.; Jang, C.; Lee, K.A. Polo-like kinases (plks), a key regulator of cell cycle and new potential target for cancer therapy. Dev. Reprod. 2014, 18, 65–71. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, X.; Liu, F.; Yu, H.; Zhang, Y.; Du, K.; Nan, Y.; Huang, Q. Systematic pan-cancer analysis identifies CDC45 as having an oncogenic role in human cancers. Oncol. Rep. 2022, 48, 185. [Google Scholar] [CrossRef]
- Xian, F.; Zhao, C.; Huang, C.; Bie, J.; Xu, G. The potential role of CDC20 in tumorigenesis, cancer progression and therapy: A narrative review. Medicine 2023, 102, e35038. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, W.-T.; Chang, D.-M.; Lin, M.-X.; Chou, T.-S.; Tung, Y.-C.; Hsiao, J.-K. Multifaceted Functional Liposomes: Theranostic Potential of Liposomal Indocyanine Green and Doxorubicin for Enhanced Anticancer Efficacy and Imaging. Pharmaceutics 2025, 17, 344. https://doi.org/10.3390/pharmaceutics17030344
Liao W-T, Chang D-M, Lin M-X, Chou T-S, Tung Y-C, Hsiao J-K. Multifaceted Functional Liposomes: Theranostic Potential of Liposomal Indocyanine Green and Doxorubicin for Enhanced Anticancer Efficacy and Imaging. Pharmaceutics. 2025; 17(3):344. https://doi.org/10.3390/pharmaceutics17030344
Chicago/Turabian StyleLiao, Wei-Ting, Dao-Ming Chang, Meng-Xian Lin, Te-Sen Chou, Yi-Chung Tung, and Jong-Kai Hsiao. 2025. "Multifaceted Functional Liposomes: Theranostic Potential of Liposomal Indocyanine Green and Doxorubicin for Enhanced Anticancer Efficacy and Imaging" Pharmaceutics 17, no. 3: 344. https://doi.org/10.3390/pharmaceutics17030344
APA StyleLiao, W.-T., Chang, D.-M., Lin, M.-X., Chou, T.-S., Tung, Y.-C., & Hsiao, J.-K. (2025). Multifaceted Functional Liposomes: Theranostic Potential of Liposomal Indocyanine Green and Doxorubicin for Enhanced Anticancer Efficacy and Imaging. Pharmaceutics, 17(3), 344. https://doi.org/10.3390/pharmaceutics17030344







