Abstract
Background: Transforming growth factor-β (TGF-β) and interleukin 1β (IL-1β) are key regulators of the chondrogenic differentiation, physiology and pathology of cartilage tissue, with TGF-β promoting chondrogenesis and matrix formation, while IL-1β exerts catabolic effects, inhibiting chondrogenesis and contributing to cartilage degradation. Both cytokines alter the intracellular calcium ion (iCa2+) levels; however, the exact pathways are not known. Objectives: This study aimed to evaluate the impact of TGF-β3 and IL-1β on calcium homeostasis in human bone marrow-derived mesenchymal stem cells (hBM-MSCs) and chondrocytes during chondrogenesis. Results: TGF-β3 increased iCa2+ levels in both hBM-MSCs and chondrocytes. Furthermore, TGF-β3 increased the functional activity of L-type voltage-operated calcium channels (L-VOCCs) in hBM-MSCs but not in chondrocytes. TGF-β3 and IL-1β reduced L-VOCCs subunit CaV1.2 (CACNA1C) gene expression in chondrocytes. In hBM-MSCs, TGF-β3 and IL-1β increased SERCA pump (ATP2A2) gene expression, while in chondrocytes, this effect was observed only with TGF-β3. Conclusions: TGF-β3 increases iCa2+ both in osteoarthritic chondrocytes and hBM-MSCs during chondrogenesis. In hBM-MSCs, TGF-β3-mediated elevation in iCa2+ is related to the increased functional activity of L-VOCCs. IL-1β does not change iCa2+ in osteoarthritic chondrocytes and hBM-MSCs; however, it initiates the mechanisms leading to further downregulation of iCa2+ in both types of cells. The differential and cell-specific roles of TGF-β3 and IL-1β in the calcium homeostasis of osteoarthritic chondrocytes and hBM-MSCs during chondrogenesis may provide a new insight into future strategies for cartilage repair and osteoarthritis treatment.
1. Introduction
Molecular mechanisms that control stem cell chondrogenic differentiation, chondrocyte homeostasis and cartilage extracellular matrix (ECM) formation have been of great interest for the past few decades. Transforming growth factor-β (TGF-β) is a cytokine and among the most important growth factors in the early stage of chondrogenesis, as well as the physiology and pathology of cartilage tissue [1]. Currently, the TGF-β family of proteins is used to induce chondrogenic differentiation in various source mesenchymal stem cells (MSCs), including those that are adipose-derived (AD-MSCs) [2], menstrual blood-derived (MenMSCs) [3] and bone marrow-derived (hBM-MSCs) [3,4,5,6,7,8]. In contrast, interleukin 1β (IL-1β) is a cytokine and a key mediator of the inflammatory response that is associated with the activation of catabolic pathways in cartilage and chondrogenesis inhibition, leading the cartilage tissue to degradation and osteoarthritis (OA) development [9,10,11,12].
TGF-β and IL-1β signal through various overlapping pathways including Ca2+ signaling, Smad1/5/8 and Smad2/3 pathways. It is known that low TGF-β levels stimulate Smad2/3 signaling, maintaining the chondrogenic phenotype [13,14], while IL-1β or high levels of TGF-β activate Smad1/5/8 signaling, leading to chondrocyte hypertrophy [13,15,16].
Elevated intracellular calcium ion (iCa2+) levels have been demonstrated to improve the chondrogenic differentiation of chicken MSCs [17]. On the other hand, elevated iCa2+ can activate Ca2+/calmodulin-dependent protein kinase II (CaMKII) associated with chondrogenic hypertrophy [18,19].
Regulation of iCa2+ plays a pivotal role in both hBM-MSC chondrogenesis and chondrocyte homeostasis, thereby influencing the intracellular pathways essential for cartilage formation and maintenance and regulating key processes such as proliferation, differentiation, and ECM production [20,21,22].
TGF-β induces Ca2+ influx in murine fibroblasts, mesangial cells, insulinoma cells, and human pulmonary fibroblasts [23,24,25,26]. IL-1β also mediates the elevation of iCa2+ in bovine chondrocytes [27].
It was observed that TGF-β led to a significant elevation in iCa2+ in rat chondrocytes via a few types of voltage-operated Ca2+ channels [28], while IL-1β was shown to mediate iCa2+ elevation in rat chondrocytes via the transient receptor potential ankyrin 1 (TRPA1) cation channel [29]. However, the mechanism of TGF-β- and IL-1β-induced changes in iCa2+ in human chondrocytes and hBM-MSCs remains unclear.
Potential mediators for iCa2+ regulation in this type of cell are L-type voltage-operated calcium channels (L-VOCCs). These channels are expressed in chondrocytes [30,31,32] and are sensitive to mechanical load [33,34,35]. The α1C subunit of L-VOCCs is highly expressed in hBM-MSCs [32,36]. However, the presence of the α1C subunit does not always result in functionally active channels. Only about 15% of undifferentiated hBM-MSCs demonstrated a small dihydropyridine-sensitive calcium current, mediated by L-VOCCs, under high external calcium concentration [37], indicating a low frequency of functionally active channels [36]. This may occur because the channels can be in an inactive state.
Despite all of the studies carried out before, it is still unclear what the mechanism is behind both the anabolic (TGF-β3) and catabolic (IL-1β) protein influence on iCa2+ concentration and how it affects chondrocyte homeostasis during the development of cartilage, and/or during the onset of OA. Therefore, the aim of this study is to investigate how TGF-β3 and IL-1β affect Ca2+ homeostasis in hBM-MSCs and chondrocytes during chondrogenesis.
2. Materials and Methods
2.1. Cell Isolation and Culture
Human tissue samples were obtained in accordance with the Bioethics Committee, permission No. 158200-14-741-257, from Vilnius University Hospital Santaros Klinikos. Articular cartilage samples were obtained as tissues removed during articular surgery from 4 patients with OA (aged 64 ± 14 years) without systemic, acute or chronic comorbidities. Chondrocytes were isolated and cultured according to the established protocols as previously reported [38]. Briefly, cartilage samples were washed in PBS with 1% penicillin-streptomycin (PS) (Gibco, Life Technologies, Waltham, MA, USA), chopped into small pieces, incubated in low glucose (1 g/L) Dulbecco’s Modified Eagle media (DMEM) (Capricorn Scientific, Ebsdorfergrund, Germany) with 1% PS at 37 °C in 5% CO2. After, samples were washed and enzymatically digested: first with pronase for 1 h, then with a type II collagenase solution at 10 mL/g of tissue for 4 h, both at 37 °C in 5% CO2. Isolated chondrocytes were cultured in complete medium, consisting of low glucose DMEM, 1% PS and 10% fetal bovine serum (FBS), in a 37 °C incubator with 5% CO2, changing the medium twice a week.
hBM-MSCs were isolated according to the established protocols by the Innovative Medicine Center (IMC) from 3 donors with OA (aged 52 ± 10 years), remaining after joint replacement surgical procedures. hBM-MSCs were incubated under the same conditions as chondrocytes, with the addition of 1 ng/mL of fibroblast growth factor 2 (FGF2) (Sigma Aldrich, Burlington, MA, USA) to maintain their stem cell potential and to avoid spontaneous differentiation. Isolated hBM-MSCs were cultured with complete medium in a 37 °C incubator with 5% CO2, changing the medium twice a week.
Passage 2–3 (P2–P3) of hBM-MSCs and chondrocytes were used for all experiments.
2.2. Chondrogenic Differentiation
Proliferation media consisted of DMEM media (with 1 g/L glucose), 10% FBS and 1% PS. Chondrogenic differentiation media was applied to hBM-MSCs and chondrocytes, consisting of high glucose (4.5 g/L) DMEM media, 1% PS, 1% insulin-transferrin-selenium (Gibco, Life Technologies, Waltham, MA, USA), 350 nM L-proline (Carl Roth, Karlsruhe, Germany), 100 nM dexamethasone (Sigma Aldrich, Burlington, MA, USA) and 170 nM ascorbic acid–phosphate (Sigma Aldrich, Burlington, MA, USA).
Chondrocytes and hBM-MSCs incubated in chondrogenic media were treated for 24 h with 10 ng/mL of TGF-β3 (Gibco, Life Technologies, Waltham, MA, USA) or with 10 ng/mL of IL-1β (Prospec, Ness-Ziona, Israel).
2.3. Intracellular Calcium Levels
The evaluation of iCa2+ levels was performed by seeding hBM-MSCs and chondrocytes in 6-well plates 200,000 cells/well. After the cells reached 95% confluence, subsequent incubation with IL-1β or TGF-β3 followed for 24 h, hBM-MSCs and chondrocytes were detached with 0.25% trypsin-EDTA, counted, and transferred at a density of 100,000 cells/vial into new 1.5 mL vials. These cells were then stained with Cal-520 dye (1 µM) (Interchim, Montlucon, France) for 30 min, washed with PBS, and measured using the Luminex Guava Flow cytometer (Luminex Corporation, Austin, TX, USA). The data were analyzed using FlowJo software, version 10 (FlowJo Corp., Ashland, OR, USA).
2.4. Gene Expression Analysis
hBM-MSCs and chondrocytes were seeded in 6-well plates using 200,000 cell/well density. When cells reached 95% confluence, they were treated with IL-1β or TGF-β3 for 24 h. After, the cells were lysed using LTR lysis buffer (Qiagen, 74104, Hilden, Germany) and RNA was extracted according to the manufacturer’s instructions. The RNA concentration and purity of all samples were measured with SpectraMax i3 (Molecular Devices, San Jose, CA, USA). RNA was reverse-transcribed with a Maxima cDNA synthesis kit including dsDNase treatment (Thermo Fisher Scientific, Waltham, MA, USA). RT-qPCR reaction mixes were prepared with Maxima Probe qPCR Master Mix (Thermo Fisher Scientific, Waltham, MA, USA) and TaqMan Gene expression Assays (RPS9—Hs02339424_g1, B2M—Hs00984230_m1, CACNA1C—Hs00167681_m1, ATP2A2—Hs00544877_m1 (Thermo Fisher Scientific, Waltham, MA, USA)), and ran on the Agilent Aria MX instrument (Agilent Technologies, Santa Clara, CA, USA)) in technical triplicates starting with a denaturation step at 95 °C for 10 min followed by 40 cycles at 95 °C for 15 s of denaturation and 60 s for annealing and extension.
Relative levels of gene transcripts were calculated by subtracting the threshold cycle (Ct) of the normalizer (the geometric mean of the two housekeeping genes RPS9 and B2M) from the Ct of the gene of interest, giving the dCt values that were subsequently transformed to 2-dCt values and multiplied by 1000 to scale-up for better graphical representation.
2.5. Electrophysiological Recording
For the electrophysiological recordings, hBM-MSCs and chondrocytes were seeded at 10,000 cells per coverslip and transferred to the recording chamber of a Nikon FN-S2N microscope (Nikon, Corporation, Tokyo, Japan). Membrane currents were measured in the whole-cell configuration of the patch-clamp technique at room temperature (21 ± 1 °C) with a Multiclamp 700B amplifier (Molecular Devices, San Jose, CA, USA) under the control of Clampfit 10.5 (Molecular Devices, San Jose, CA, USA). Patch microelectrodes were pulled from borosilicate glass capillaries with a Flaming/Brown micropipette puller (Model P-1000; Sutter Instrument Co., Novato, CA, USA). For whole-cell recordings, microelectrodes were filled with internal solution consisting of (mM): KCl 130, Na aspartate 10, MgATP 3, CaCl2 0.2, EGTA 2, HEPES 5, (pH adjusted to 7.3 with KOH) [36,39]. The microelectrode tips’ resistance was ∼6–8 MΩ, when filled with internal solution. The presence of L-type calcium current (ICa,L) was investigated with Ca2+ free Krebs–Ringer external solution (mM): NaCl 150, KCl 5.4, BaCl2 10, MgCl2 2, glucose 11, HEPES 10 (pH 7.4 adjusted with NaOH), where Ca2+ was substituted with Ba2+. This substitution increases the current flow through L-VOCCs, making it easier for identification [36,40].
Nifedipine (Sigma Aldrich, Burlington, MA, USA) and Bay-K8644 (Sigma Aldrich, Burlington, MA, USA) were used as blocker and activator, respectively. Both compounds were added to the external solution at a concentration of 10 µM before the current measurements.
Membrane currents were recorded using the voltage-clamp protocol that starts from a holding potential of −40 mV and a series of depolarizing steps applied in 10 mV increments to a maximum of +10 mV [41]. For isolation of the ICa,L, we established I–V relationships for each analyzed cell using five data points of steady current of −30, −20, −10, 0, 10 mV, obtained from experimental data within 30 ms before the end of the depolarizing steps. Given that the observed peak influx of Ca2+ ions through L-VOCCs is noted at membrane potentials of 0 and +10 mV [36,42], we used three data points (−30, −20, −10 mV) to evaluate the leak conductance. The leak current at 0 and 10 mV was linearly extrapolated and then subtracted from experimental data, allowing the identification of the voltage-dependent component of the total membrane current, namely, the leak-subtracted current. The leak-subtracted and normalized to the leak current (ILSN) at +10 mV was used to evaluate the functional activity of L-VOCCs in both hBM-MSCs and chondrocytes. Negative ILSN indicates the presence of inward voltage-operated current at +10 mV, while positive ILSN—outward voltage-operated current at +10 mV.
Our approach for determining the leak conductance involved using three data points (−30, −20, −10 mV) where a minor ICa,L was already present [36,42]. This implies that obtained values of ILSN at +10 mV may be under-evaluated.
2.6. Statistical Analysis
The statistical difference between groups was evaluated using one-way analysis of variance (ANOVA) with OriginPro software, version 9.5 (OriginLab Corporation, Northampton, MA, USA), and a Student’s unpaired two-tailed t-test (Microsoft Excel) was used to calculate statistical significance. Significance was accepted when p ≤ 0.05. In the text, data are presented as mean ± standard deviation.
3. Results
3.1. Modulation of Intracellular Calcium Ion Levels in Response to TGF-β3 and IL-1β
In order to determine how TGF-β3 and IL-1β can affect iCa2+ homeostasis in hBM-MSCs and chondrocytes, we examined their effects on iCa2+. TGF-β3 significantly increased iCa2+ in both hBM-MSCs and chondrocytes, while IL-1β did not show significant changes, as compared to chondrogenic media alone (Figure 1a,b).
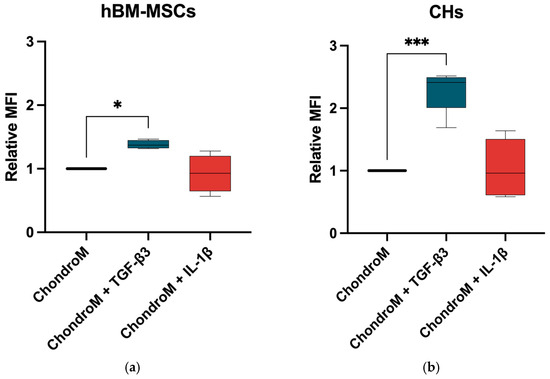
Figure 1.
iCa2+ levels in (a) hBM-MSCs and (b) chondrocytes (CHs), after staining with Cal-520. The cells were cultivated in chondrogenic media (ChondroM) with IL-1β (10 ng/mL) and TGF-β3 (10 ng/mL) for 24 h. Median fluorescence intensity (MFI) is presented as a ratio to non-stained control. Measured with Luminex Guava. Data are presented as mean ± standard deviation of three technical repeats from no fewer than three OA patient’s cells. * p < 0.05, *** p < 0.001.
3.2. Inward L-Type Calcium Current in hBM-MSCs
TGF-β3-mediated increase in iCa2+ depends on Ca2+ entry through L-VOCCs in insulinoma cells [25]. It is known that only 15% of undifferentiated hBM-MSCs demonstrated a small L-type calcium current (ICa,L) [36,37]. However, the functional role of L-VOCCs in extracellular Ca2+ entry in hBM-MSCs is currently unknown. To investigate how TGF-β3 can cause elevation of iCa2+ in hBM-MSCs, we examined its impact on the functional activity of L-VOCCs in chondrogenic media. Additionally, the influence of IL-1β on L-VOCCs functional activity in hBM-MSCs was tested.
Chondrogenic media is enriched with high glucose DMEM, 1% PS, insulin-transferrin-selenium, L-proline, dexamethasone, and other bioactive compounds. Therefore, first we tested the effect of chondrogenic media alone on the functional activity of L-VOCCs.
hBM-MSCs cultivated in proliferation media did not show significant leak-subtracted normalized current at +10 mV (ILSN) (0.006 ± 0.04) in n = 22 cells (Figure 2). Cultivation in chondrogenic media did not show significant effects on ILSN in hBM-MSCs (−0.01 ± 0.03; n = 12), compared to the proliferation media (Figure 2).
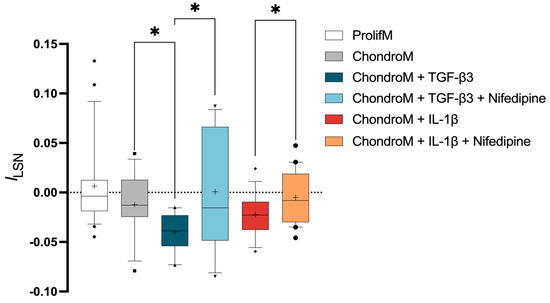
Figure 2.
ILSN in hBM-MSCs at +10 mV. The current was measured in cells cultivated in proliferation media (ProlifM) and chondrogenic media (ChondroM) with TGF-β3 (10 ng/mL) or IL-1β (10 ng/mL) for 24 h with or without nifedipine. Data, obtained from no fewer than three OA patient’s cells, are shown as a box with whiskers, indicating the median (50th), upper (90th), and lower (10th) percentiles. The number of cells for each condition are specified in the text. Outliers are represented with individual marks outside the whiskers. * p < 0.05.
3.3. Inward L-Type Calcium Current in Chondrocytes
To investigate how TGF-β3 can cause the elevation of iCa2+ in chondrocytes, we examined its impact on the functional activity of L-VOCCs. Additionally, we investigated how IL-1β influenced L-VOCCs functional activity in chondrocytes.
Chondrocytes cultivated in proliferation media did not show significant ILSN (−0.007 ± 0.02; n = 42) (Figure 3a). The application of Bay-K8644, an activator of L-VOCCs, to proliferation media did not result in a significant change in ILSN (−0.01 ± 0.01; n = 16) (Figure 3a).
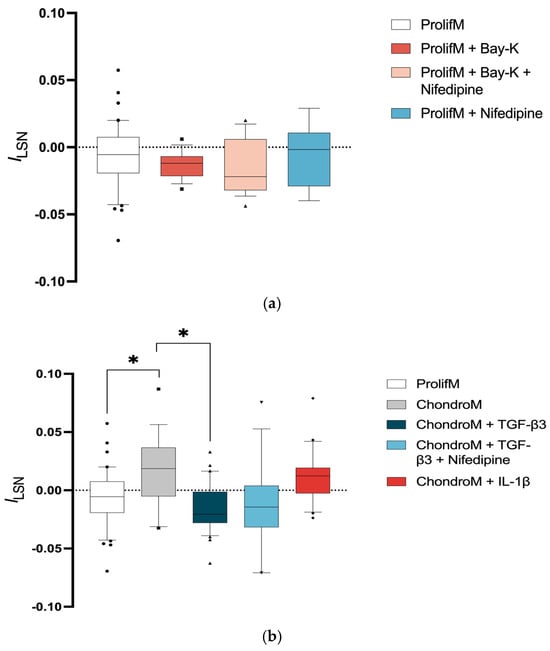
Figure 3.
ILSN in chondrocytes at +10 mV. The current was measured in cells cultivated (a) in proliferation media (ProlifM) with Bay-K8644, nifedipine, or both; (b) in proliferation and chondrogenic media (ChondroM) with TGF-β3 (10 ng/mL) or IL-1β (10 ng/mL) for 24 h with or without nifedipine. Data, obtained from no fewer than three OA patient’s cells, are shown as a box with whiskers, indicating the median (50th), upper (90th), and lower (10th) percentiles. The number of cells for each condition are specified in the text. Outliers are represented with individual marks outside the whiskers. * p < 0.05.
The inward current could be compensated by voltage-operated potassium currents [43]. Moreover, the lack of changes in ILSN after the application of Bay-K8644 may indicate that the majority of L-VOCCs were already activated. This hypothesis was tested by the application of nifedipine. Nonetheless, applying nifedipine or a combination of Bay-K8644 with nifedipine did not result in a significant change in ILSN (−0.006 ± 0.02; n = 9, −0.01 ± 0.02; n = 18, respectively) (Figure 3a).
Incubation of chondrocytes in chondrogenic media caused significantly positive ILSN (0.02 ± 0.03; n = 18), indicating the activation of voltage-operated outward current (Figure 3b).
The exposure of chondrocytes to TGF-β3 caused significantly negative ILSN (−0.02 ± 0.01; n = 31) compared with chondrocytes cultivated in chondrogenic media alone (Figure 3b). TGF-β3-mediated negative ILSN was not sensitive to nifedipine (−0.01 ± 0.04; n = 16) (Figure 3b).
The exposure of chondrocytes to IL-1β did not change ILSN (0.01 ± 0.02; n = 20) when compared to chondrogenic media alone (Figure 3b).
3.4. Effect of TGF-β3 and IL-1β on CACNA1C and ATP2A2 Gene Expression
Both TGF-β3 [44] and increased iCa2+ levels [45,46] may contribute to homeostasis of Ca2+ at the gene expression level; therefore, we investigated how these two factors influenced the gene expression of Ca2+ regulators (L-VOCCs subunit CaV1.2, encoded by the CACNA1C gene and the SERCA pump, encoded by the ATP2A2 gene) in hBM-MSCs and chondrocytes. Additionally, we analyzed IL-1β effects on the CACNA1C and ATP2A2 gene expression.
The CACNA1C gene expression was significantly downregulated in chondrocytes in response to both TGF-β3 and IL-1β; however, no effect was observed in hBM-MSCs (Figure 4a).
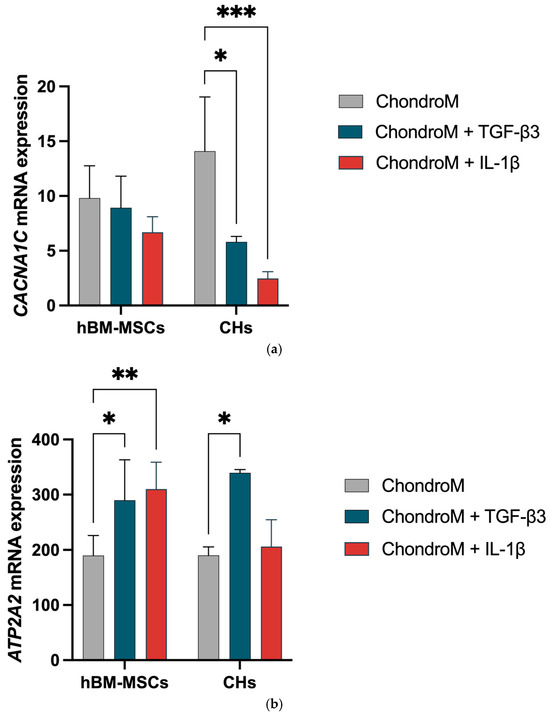
Figure 4.
The effect of IL-1β (10 ng/mL) and TGF-β3 (10 ng/mL) on hBM-MSCs and chondrocyte (CHs) Cav1.2 (CACNA1C) and SERCA2 pump (ATP2A2) gene expression. The expression ratio of (a) CACNA1C and (b) ATP2A2, were analyzed in chondrogenic media (ChondroM) with (10 ng/mL) and IL-1β (10 ng/mL). Relative transcript level after normalization to the geometric mean of housekeeping B2M and RPS9 genes are shown. Data are presented as mean ± standard deviation of three technical repeats from no fewer than three OA patient’s cells. * p < 0.05, ** p < 0.01, *** p < 0.001.
The ATP2A2 gene expression was significantly increased in hBM-hMSCs in response to both TGF-β3 and IL-1β. In chondrocytes, the ATP2A2 gene expression was significantly increased with exposure to TGF-β3 (Figure 4b).
4. Discussion
In this study, we investigated how 24 h exposure to 10 ng/mL of TGF-β3 and 10 ng/mL of IL-1β affect Ca2+ homeostasis in hBM-MSCs and chondrocytes during chondrogenesis.
Three aspects of Ca2+ homeostasis were evaluated: iCa2+ levels, ICa,L, providing insights as to whether Ca2+ is entering cells through the L-VOCCs and, finally, expression of CACNA1C and ATP2A2 genes which represent the cellular responses to the altered iCa2+ levels seeking to restore homeostasis.
We observed an increase in iCa2+ in both hBM-MSCs and chondrocytes following exposure to TGF-β3. The ICa,L in BM-hMSCs, but not in chondrocytes, was enhanced by TGF-β3. CACNA1C gene expression was significantly reduced in chondrocytes by both TGF-β3 and IL-1β, that also showed tendencies for a reduction in the CACNA1C gene in hBM-MSCs. The gene expression of ATP2A2 was increased by TGF-β3 and IL-1β in hBM-MSCs, while in chondrocytes, just by TGF-β3.
The measure of 10 ng/mL of TGF-β3 was chosen for all experiments as it is a standard concentration used to induce chondrogenic differentiation [3]. Similarly, 10 ng/mL IL-1β was chosen based on the literature as it demonstrated early degenerative changes in human chondrocytes [47]. The 24 h incubation period was selected for all experiments to study early changes in cells.
There are three known isoforms of TGF-β expressed in mammals, including TGF-β1, TGF-β2, and TGF-β3. Their sequences are 71–80% identical, and they activate signaling through the same receptors [48]. Among its three isoforms, TGF-β1 or TGF-β3 are used for the induction of chondrogenesis [38,49,50]. We chose to use TGF-β3 because it was shown to have a higher potential to induce chondrogenic differentiation than TGF-β1 [51].
TGF-β3 mediated a significant elevation in iCa2+ in both hBM-MSCs and chondrocytes. Previous studies reported an elevation in iCa2+ as early as within 1 min after incubation with TGF-β1 in chondrocytes [28]. Moreover, an increase in iCa2+ mediated by TGF-β has been observed in other cell types, including neurons [52,53], osteoblasts [54], and fibroblasts [23,55].
IL-1β mediated an increase in iCa2+ in rat chondrocytes through TRPA1 [29]. Our research demonstrated that a 24 h exposure to 10 ng/mL IL-1β does not cause a change in iCa2+ in hBM-MSCs and chondrocytes, which corresponds to a previous study in this type of cells [38].
The regulation of iCa2+ in cells depends on Ca2+ entering through channels located on the plasma membrane, with L-VOCCs being one of them. In chondrocytes, L-VOCC activation is associated with the pathogenesis of osteoarthritis (OA), making them potential therapeutic targets for alleviating OA severity [22,31]. The increase in iCa2+ mediated by TGF-β3 may be related to changes in the functional activity of L-VOCCs. Therefore, we explored L-VOCCs’ functional activity by investigating ILSN at +10 mV, which corresponds to the peak of the ICa,L [42]. The leak subtraction enabled the isolation of ICa,L from other (mainly potassium) currents present in the hBM-MSCs [36] and chondrocytes [56], while normalization minimized the effect of cell size. Incubation of hBM-MSCs with TGF-β3 resulted in an increased nifedipine-sensitive inward current, suggesting activation of L-VOCCs. This activation can result from a complex cascade reaction stimulated by TGF-β3. For example, it has been demonstrated that TGF-β increased β-adrenergic signaling [57]. The stimulation of β-adrenergic receptors is known to activate ICa,L [58].
The incubation of hBM-MSCs with IL-1β displayed a tendency to increase the inward nifedipine-sensitive current; however, this increase was not statistically significant.
The inhibition of L-VOCCs with nifedipine was reported to downregulate the proliferation of chondrocytes [38], suggesting the functional involvement of these channels. However, in our study, L-VOCCs activity was not detected in chondrocytes incubated in proliferation media. Notably, we observed a significant increase in outward currents when chondrocytes were incubated in chondrogenic media. We suppose that components of chondrogenic media, such as dexamethasone [59,60], high glucose concentration [61], or streptomycin [62], could enhance potassium outward currents, which might explain our observations. Further, incubation of chondrocytes with TGF-β3 resulted in inward currents that were not sensitive to nifedipine. One possible reason for this could be the downregulation in potassium channel expression by TGF-β [63]. The total membrane current is a sum of inward and outward currents. Therefore, the downregulation of potassium outward currents will result in the total inward membrane current.
Our data showed that neither the application of proliferation nor chondrogenic media, TGF-β3 nor IL-1β stimulation, have changed L-VOCCs activity in chondrocytes.
We explored how TGF-β3-mediated increase in iCa2+ can modulate the gene expression of the CaV1.2 (CACNA1C gene) and the SERCA pump (ATP2A2 gene), which regulate Ca2+ influx and balance it. TGF-β3 led to a significant increase in ATP2A2 gene expression in both hBM-MSCs and chondrocytes. It is important to note that ATP2A2 gene expression does not necessarily correlate with SERCA2 protein levels or the functional activity of this pump.
In chondrocytes exposed to TGF-β3 for 24 h, we found the downregulation of CACNA1C gene expression, whereas no change was observed in hBM-MSCs. Recent publications showed that treatment with TGF-β for 21 days resulted in a significant increase in CACNA1C gene expression in hBM-MSCs and OA chondrocytes [32]. Even 2 ng/mL of TGF-β for 96 h increases both gene and protein expression of L-VOCCs subunit CaV1.2 in human adipose-derived MSCs [64]. These data suggest that the effects of TGF-β3 on CACNA1C gene expression might depend on the duration of exposure to TGF-β3.
Since IL-1β regulates the gene expression of TRPA1 in rat chondrocytes, human intervertebral disc tissue and epithelial sodium channels in rat alveolar epithelial cells [29,65,66], we explored how it regulates the gene expression of CACNA1C and ATP2A2 in hBM-MSCs and chondrocytes. We observed an increase in the ATP2A2 gene expression in hBM-MSCs when exposed to IL-1β. In our study, a 24 h incubation with 10 ng/mL IL-1β resulted in a decrease in CACNA1C gene expression in chondrocytes. In contrast, previous research reported that a 48 h treatment with IL-1β at a concentration 10 times lower (1 ng/mL) than that used in our study led to an increase in CACNA1C gene expression in bovine chondrocytes [67]. The differing results regarding the effects of IL-1β on CACNA1C may be attributed to different concentrations and exposure durations. These differences highlight the need for additional studies to understand how IL-1β influences CACNA1C expression under various exposure conditions.
Both hBM-MSC and chondrocytes were obtained from tissues removed during articular surgery in OA patients. The limited availability of “healthy” cells remains a well-recognized challenge [68]. It is known that OA cartilage-derived chondrocytes exhibit altered intracellular signaling [35,69], reduced regenerative potential [35,70,71] and accelerated cellular senescence [71]. However, OA patient-derived autologous cells are commonly used in cell therapy approaches [72,73]. Moreover, the focus of the present study represents remodeling mechanisms, rather than a comparison between healthy and disease states.
In summary, our findings in hBM-MSCs indicate that TGF-β3 exposure elevates iCa2+, at least partially, due to enhanced ICa,L (Figure 5, left). While the expression of CACNA1C, a subunit of L-VOCCs, remains unchanged, we suggest that TGF-β3 exposure increases the functional activity of the L-VOCCs already present. Moreover, TGF-β3 results in the upregulation of ATP2A2 expression (Figure 5, left). Upregulation of ATP2A2 may provide the initial step in a cellular response for the reduction in elevated iCa2+. In chondrocytes, TGF-β3 exposure also elevates iCa2+ but without changes in ICa,L (Figure 5, right), indicating different pathways for iCa2+ elevation than in hBM-MSCs. Moreover, TGF-β3 downregulates CACNA1C expression while upregulating ATP2A2 (Figure 5, right). This suggests involvement of two mechanisms of elevated iCa2+ reduction in chondrocytes: by enhancing Ca2+ reuptake from the cytosol into the endoplasmic reticulum through ATP2A2 upregulation and by downregulation of CACNA1C.
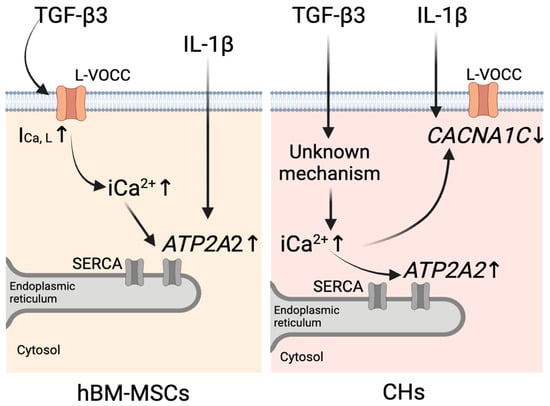
Figure 5.
Schematic representation of the proposed mechanisms of calcium homeostasis regulation by TGF-β3 and IL-1β in hBM-MSCs (left) and chondrocytes (CHs) (right). In hBM-MSCs, TGF-β3 increases intracellular calcium (iCa2+) levels by enhancing L-type calcium current (ICa,L), which leads to ATP2A2 upregulation. IL-1β upregulates ATP2A2 expression. In CHs, TGF-β3 increases iCa2+ via an unknown mechanism, which leads to upregulation of ATP2A2 and downregulation of CACNA1C. IL-1β downregulates CACNA1C expression. The image was created with BioRender.com (https://www.biorender.com/, accessed on 26 February 2025).
IL-1β did not change the levels of iCa2+ or ICa,L in either hBM-MSCs or chondrocytes. However, the upregulation of ATP2A2 gene expression observed in hBM-MSCs (Figure 5 left) and the downregulation of the CACNA1C gene in chondrocytes (Figure 5 right), as responses to stimulation with IL-1β, suggest that its mechanism of action is also associated with alterations in Ca2+ signaling, which different types of cells handle in different ways.
It is interesting to note that there is some degree of convergence of TGF-β3 and IL-1β action on calcium homeostasis: in hBM-MSCs both cytokines increase the expression of the ATP2A2 gene, while in chondrocytes, both increase the expression of the CACNA1C gene.
We conclude that both TGF-β3 and IL-1β influence iCa2+ homeostasis in hBM-MSCs and chondrocytes during chondrogenesis in a cell-type-dependent manner.
Author Contributions
A.S. investigation, formal analysis, writing original draft; A.A. supervision, validation, conceptualization, writing—review and editing; R.V. investigation, formal analysis, writing—review and editing; I.U. investigation, formal analysis, writing—review and editing; P.B. investigation, formal analysis, writing—review and editing; E.B. conceptualization, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partly funded by EU HORIZON-WIDERA-2021-ACCESS-03-01 program: “Twinning for Promoting Excellence, Ability and Knowledge to develop novel approaches for targeting inflammatory and degenerative age-related joint diseases” (nr. 101079489—TWINFLAG), and European Social Fund project according to Horizon2020 programe—ElectroMechanoActive Polymer-based Scaffolds for Heart-on-Chip (EMAPS-Cardio), No. 953138—EMAPS-Cardio.
Institutional Review Board Statement
All procedures with hBM-MSCs and chondrocytes in this study were performed in accordance with the Bioethics Committee, permission No. 158200-14-741-257, approved 2014 06 09.
Informed Consent Statement
Informed consent was obtained from all patients involved in the study.
Data Availability Statement
The data supporting intracellular calcium levels and gene expression study findings can be found at State Research Institute Centre for Innovative Medicine, Department of Regenerative Medicine. The data supporting electrophysiological study findings can be found at Vilnius University, Institute of Biosciences.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tzavlaki, K.; Moustakas, A. TGF-β Signaling. Biomolecules 2020, 10, 487. [Google Scholar] [CrossRef]
- Mehlhorn, A.T.; Niemeyer, P.; Kaschte, K.; Muller, L.; Finkenzeller, G.; Hartl, D.; Sudkamp, N.P.; Schmal, H. Differential Effects of BMP-2 and TGF-Beta1 on Chondrogenic Differentiation of Adipose Derived Stem Cells. Cell Prolif. 2007, 40, 809–823. [Google Scholar] [CrossRef]
- Uzieliene, I.; Bagdonas, E.; Hoshi, K.; Sakamoto, T.; Hikita, A.; Tachtamisevaite, Z.; Rakauskiene, G.; Kvederas, G.; Mobasheri, A.; Bernotiene, E. Different Phenotypes and Chondrogenic Responses of Human Menstrual Blood and Bone Marrow Mesenchymal Stem Cells to Activin A and TGF-Β3. Stem Cell Res. Ther. 2021, 12, 251. [Google Scholar] [CrossRef]
- Futrega, K.; Robey, P.G.; Klein, T.J.; Crawford, R.W.; Doran, M.R. A Single Day of TGF-Β1 Exposure Activates Chondrogenic and Hypertrophic Differentiation Pathways in Bone Marrow-Derived Stromal Cells. Commun. Biol. 2021, 4, 29. [Google Scholar] [CrossRef]
- Mackay, A.M.; Beck, S.C.; Murphy, J.M.; Barry, F.P.; Chichester, C.O.; Pittenger, M.F. Chondrogenic Differentiation of Cultured Human Mesenchymal Stem Cells from Marrow. Tissue Eng. 1998, 4, 415–428. [Google Scholar] [CrossRef]
- Mueller, M.B.; Fischer, M.; Zellner, J.; Berner, A.; Dienstknecht, T.; Prantl, L.; Kujat, R.; Nerlich, M.; Tuan, R.S.; Angele, P. Hypertrophy in Mesenchymal Stem Cell Chondrogenesis: Effect of TGF-β Isoforms and Chondrogenic Conditioning. CTO 2010, 192, 158–166. [Google Scholar] [CrossRef]
- Mueller, M.B.; Tuan, R.S. Functional Characterization of Hypertrophy in Chondrogenesis of Human Mesenchymal Stem Cells. Arthritis Rheum. 2008, 58, 1377–1388. [Google Scholar] [CrossRef]
- Shen, B.; Wei, A.; Tao, H.; Diwan, A.D.; Ma, D.D.F. BMP-2 Enhances TGF-Beta3-Mediated Chondrogenic Differentiation of Human Bone Marrow Multipotent Mesenchymal Stromal Cells in Alginate Bead Culture. Tissue Eng. Part A 2009, 15, 1311–1320. [Google Scholar] [CrossRef]
- Huang, X.; Zhong, L.; Hendriks, J.; Post, J.N.; Karperien, M. Different Response of Human Chondrocytes from Healthy Looking Areas and Damaged Regions to IL1β Stimulation under Different Oxygen Tension. J. Orthop. Res. 2019, 37, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Jenei-Lanzl, Z.; Meurer, A.; Zaucke, F. Interleukin-1β Signaling in Osteoarthritis—Chondrocytes in Focus. Cell. Signal. 2019, 53, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Mohamed-Ali, H. Influence of Interleukin-1 Beta, Tumour Necrosis Factor Alpha and Prostaglandin E2 on Chondrogenesis and Cartilage Matrix Breakdown in Vitro. Rheumatol. Int. 1995, 14, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Wehling, N.; Palmer, G.D.; Pilapil, C.; Liu, F.; Wells, J.W.; Müller, P.E.; Evans, C.H.; Porter, R.M. Interleukin-1 and Tumor Necrosis Factor-α Inhibit Chondrogenesis by Human Mesenchymal Stem Cells through NF-κB Dependent Pathways. Arthritis Rheum. 2009, 60, 801–812. [Google Scholar] [CrossRef]
- Remst, D.F.G.; Blaney Davidson, E.N.; Vitters, E.L.; Bank, R.A.; van den Berg, W.B.; van der Kraan, P.M. TGF-ß Induces Lysyl Hydroxylase 2b in Human Synovial Osteoarthritic Fibroblasts through ALK5 Signaling. Cell Tissue Res. 2014, 355, 163–171. [Google Scholar] [CrossRef]
- Yang, X.; Chen, L.; Xu, X.; Li, C.; Huang, C.; Deng, C.-X. TGF-β/Smad3 Signals Repress Chondrocyte Hypertrophic Differentiation and Are Required for Maintaining Articular Cartilage. J. Cell Biol. 2001, 153, 35–46. [Google Scholar] [CrossRef]
- Lu, L.; Wang, P.; Zou, Y.; Zha, Z.; Huang, H.; Guan, M.; Wu, Y.; Liu, G. IL-1β Promotes Stemness of Tumor Cells by Activating Smad/ID1 Signaling Pathway. Int. J. Med. Sci. 2020, 17, 1257–1268. [Google Scholar] [CrossRef] [PubMed]
- Blaney Davidson, E.N.; Remst, D.F.G.; Vitters, E.L.; van Beuningen, H.M.; Blom, A.B.; Goumans, M.-J.; van den Berg, W.B.; van der Kraan, P.M. Increase in ALK1/ALK5 Ratio as a Cause for Elevated MMP-13 Expression in Osteoarthritis in Humans and Mice1. J. Immunol. 2009, 182, 7937–7945. [Google Scholar] [CrossRef] [PubMed]
- Matta, C.; Fodor, J.; Szíjgyártó, Z.; Juhász, T.; Gergely, P.; Csernoch, L.; Zákány, R. Cytosolic Free Ca2+ Concentration Exhibits a Characteristic Temporal Pattern during in Vitro Cartilage Differentiation: A Possible Regulatory Role of Calcineurin in Ca-Signalling of Chondrogenic Cells. Cell Calcium 2008, 44, 310–323. [Google Scholar] [CrossRef]
- Saitta, B.; Elphingstone, J.; Limfat, S.; Shkhyan, R.; Evseenko, D. CaMKII Inhibition in Human Primary and Pluripotent Stem Cell-Derived Chondrocytes Modulates Effects of TGFβ and BMP through SMAD Signaling. Osteoarthr. Cartil. 2019, 27, 158–171. [Google Scholar] [CrossRef]
- Wuttisiriboon, K.; Tippayawat, P.; Daduang, J.; Limpaiboon, T. Ca2+/Calmodulin-Dependent Protein Kinase II Inhibitor KN-93 Enhances Chondrogenesis of Bone Marrow Mesenchymal Stem Cells and Delays Chondrogenic Hypertrophy. In Vivo 2023, 37, 667–678. [Google Scholar] [CrossRef]
- Matta, C.; Zakany, R. Calcium Signalling in Chondrogenesis: Implications for Cartilage Repair. FBS 2013, 5, 305–324. [Google Scholar] [CrossRef]
- Mobasheri, A.; Matta, C.; Uzielienè, I.; Budd, E.; Martín-Vasallo, P.; Bernotiene, E. The Chondrocyte Channelome: A Narrative Review. Jt. Bone Spine 2019, 86, 29–35. [Google Scholar] [CrossRef]
- Uzieliene, I.; Bernotas, P.; Mobasheri, A.; Bernotiene, E. The Role of Physical Stimuli on Calcium Channels in Chondrogenic Differentiation of Mesenchymal Stem Cells. Int. J. Mol. Sci. 2018, 19, 2998. [Google Scholar] [CrossRef] [PubMed]
- Alevizopoulos, A.; Dusserre, Y.; Rüegg, U.; Mermod, N. Regulation of the Transforming Growth Factor β-Responsive Transcription Factor CTF-1 by Calcineurin and Calcium/ Calmodulin-Dependent Protein Kinase IV*. J. Biol. Chem. 1997, 272, 23597–23605. [Google Scholar] [CrossRef]
- Mukherjee, S.; Kolb, M.R.J.; Duan, F.; Janssen, L.J. Transforming Growth Factor–β Evokes Ca2+ Waves and Enhances Gene Expression in Human Pulmonary Fibroblasts. Am. J. Respir. Cell Mol. Biol. 2012, 46, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, N.; Shibata, H.; Kanzaki, M.; Shiozaki, S.; Miyazaki, J.; Kobayashi, I.; Kojima, I. Calcium as a Second Messenger of the Action of Transforming Growth Factor-Beta on Insulin Secretion. Mol. Cell Endocrinol. 1996, 117, 1–6. [Google Scholar] [CrossRef] [PubMed]
- McGowan, T.A.; Madesh, M.; Zhu, Y.; Wang, L.; Russo, M.; Deelman, L.; Henning, R.; Joseph, S.; Hajnoczky, G.; Sharma, K. TGF-Beta-Induced Ca2+ Influx Involves the Type III IP3 Receptor and Regulates Actin Cytoskeleton. Am. J. Physiol. Ren. Physiol. 2002, 282, F910–F920. [Google Scholar] [CrossRef]
- Luo, L.; Cruz, T.; McCulloch, C. Interleukin 1-Induced Calcium Signalling in Chondrocytes Requires Focal Adhesions. Biochem. J. 1997, 324, 653–658. [Google Scholar] [CrossRef]
- Cailotto, F.; Reboul, P.; Sebillaud, S.; Netter, P.; Jouzeau, J.-Y.; Bianchi, A. Calcium Input Potentiates the Transforming Growth Factor (TGF)-Β1-Dependent Signaling to Promote the Export of Inorganic Pyrophosphate by Articular Chondrocyte. J. Biol. Chem. 2011, 286, 19215–19228. [Google Scholar] [CrossRef]
- Yin, S.; Zhang, L.; Ding, L.; Huang, Z.; Xu, B.; Li, X.; Wang, P.; Mao, J. Transient Receptor Potential Ankyrin 1 (Trpa1) Mediates Il-1β-Induced Apoptosis in Rat Chondrocytes via Calcium Overload and Mitochondrial Dysfunction. J. Inflamm. 2018, 15, 27. [Google Scholar] [CrossRef]
- Atsuta, Y.; Tomizawa, R.R.; Levin, M.; Tabin, C.J. L-Type Voltage-Gated Ca2+ Channel CaV1.2 Regulates Chondrogenesis during Limb Development. Proc. Natl. Acad. Sci. USA 2019, 116, 21592–21601. [Google Scholar] [CrossRef]
- Matta, C.; Zákány, R.; Mobasheri, A. Voltage-Dependent Calcium Channels in Chondrocytes: Roles in Health and Disease. Curr. Rheumatol. Rep. 2015, 17, 43. [Google Scholar] [CrossRef] [PubMed]
- Uzieliene, I.; Bironaite, D.; Miksiunas, R.; Bagdonas, E.; Vaiciuleviciute, R.; Mobasheri, A.; Bernotiene, E. The Effect of CaV1.2 Inhibitor Nifedipine on Chondrogenic Differentiation of Human Bone Marrow or Menstrual Blood-Derived Mesenchymal Stem Cells and Chondrocytes. Int. J. Mol. Sci. 2023, 24, 6730. [Google Scholar] [CrossRef]
- Li, J.; Duncan, R.L.; Burr, D.B.; Turner, C.H. L-Type Calcium Channels Mediate Mechanically Induced Bone Formation in Vivo. J. Bone Miner. Res. 2002, 17, 1795–1800. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.S.; Robling, A.G.; Farach-Carson, M.C.; Thompson, W.R. Skeletal Functions of Voltage Sensitive Calcium Channels. Curr. Osteoporos. Rep. 2021, 19, 206–221. [Google Scholar] [CrossRef] [PubMed]
- Uzieliene, I.; Bironaite, D.; Bagdonas, E.; Pachaleva, J.; Sobolev, A.; Tsai, W.-B.; Kvederas, G.; Bernotiene, E. The Effects of Mechanical Load on Chondrogenic Responses of Bone Marrow Mesenchymal Stem Cells and Chondrocytes Encapsulated in Chondroitin Sulfate-Based Hydrogel. Int. J. Mol. Sci. 2023, 24, 2915. [Google Scholar] [CrossRef]
- Heubach, J.F.; Graf, E.M.; Leutheuser, J.; Bock, M.; Balana, B.; Zahanich, I.; Christ, T.; Boxberger, S.; Wettwer, E.; Ravens, U. Electrophysiological Properties of Human Mesenchymal Stem Cells. J. Physiol. 2004, 554, 659–672. [Google Scholar] [CrossRef]
- Kawano, S.; Shoji, S.; Ichinose, S.; Yamagata, K.; Tagami, M.; Hiraoka, M. Characterization of Ca2+ Signaling Pathways in Human Mesenchymal Stem Cells. Cell Calcium 2002, 32, 165–174. [Google Scholar] [CrossRef]
- Uzieliene, I.; Bernotiene, E.; Rakauskiene, G.; Denkovskij, J.; Bagdonas, E.; Mackiewicz, Z.; Porvaneckas, N.; Kvederas, G.; Mobasheri, A. The Antihypertensive Drug Nifedipine Modulates the Metabolism of Chondrocytes and Human Bone Marrow-Derived Mesenchymal Stem Cells. Front. Endocrinol. 2019, 10, 756. [Google Scholar] [CrossRef]
- Fabiato, A.; Fabiato, F. Calculator Programs for Computing the Composition of the Solutions Containing Multiple Metals and Ligands Used for Experiments in Skinned Muscle Cells. J. Physiol. (Paris) 1979, 75, 463–505. [Google Scholar]
- Hess, P.; Tsien, R.W. Mechanism of Ion Permeation through Calcium Channels. Nature 1984, 309, 453–456. [Google Scholar] [CrossRef]
- Heubach, J.F.; Trebeß, I.; Wettwer, E.; Himmel, H.M.; Michel, M.C.; Kaumann, A.J.; Koch, W.J.; Harding, S.E.; Ravens, U. L-Type Calcium Current and Contractility in Ventricular Myocytes from Mice Overexpressing the Cardiac Β2-Adrenoceptor1. Cardiovasc. Res. 1999, 42, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xu, J.; Gong, J.; Qian, L. L-Type Calcium Channel Current up-Regulation by Chronic Stress Is Associated with Increased A1c Subunit Expression in Rat Ventricular Myocytes. Cell Stress Chaperones 2009, 14, 33–41. [Google Scholar] [CrossRef]
- Mobasheri, A.; Lewis, R.; Ferreira-Mendes, A.; Rufino, A.; Dart, C.; Barrett-Jolley, R. Potassium Channels in Articular Chondrocytes. Channels 2012, 6, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, P.; Agrawal, A.; Bhushan, R.; Chavalmane, A.K.; Kalathur, R.K.R.; Takahashi, T.; Kondaiah, P. Expression Profiling of Genes Regulated by TGF-Beta: Differential Regulation in Normal and Tumour Cells. BMC Genom. 2007, 8, 98. [Google Scholar] [CrossRef]
- Johnson, C.M.; Hill, C.S.; Chawla, S.; Treisman, R.; Bading, H. Calcium Controls Gene Expression via Three Distinct Pathways That Can Function Independently of the Ras/Mitogen-Activated Protein Kinases (ERKs) Signaling Cascade. J. Neurosci. 1997, 17, 6189–6202. [Google Scholar] [CrossRef] [PubMed]
- Puri, B.K. Calcium Signaling and Gene Expression. Adv. Exp. Med. Biol. 2020, 1131, 537–545. [Google Scholar] [CrossRef]
- Shakibaei, M.; Schulze-Tanzil, G.; John, T.; Mobasheri, A. Curcumin Protects Human Chondrocytes from IL-L1beta-Induced Inhibition of Collagen Type II and Beta1-Integrin Expression and Activation of Caspase-3: An Immunomorphological Study. Ann. Anat. 2005, 187, 487–497. [Google Scholar] [CrossRef]
- Huang, T.; Schor, S.L.; Hinck, A.P. Biological Activity Differences between TGF-Β1 and TGF-Β3 Correlate with Differences in the Rigidity and Arrangement of Their Component Monomers. Biochemistry 2014, 53, 5737–5749. [Google Scholar] [CrossRef]
- Wee, A.-S.; Lim, C.-K.; Tan, S.-L.; Ahmad, T.S.; Kamarul, T. TGF-Β1 and -Β3 for Mesenchymal Stem Cells Chondrogenic Differentiation on Poly (Vinyl Alcohol)-Chitosan-Poly (Ethylene Glycol) Scaffold. Tissue Eng. Part C Methods 2022, 28, 501–510. [Google Scholar] [CrossRef]
- Xia, P.; Wang, X.; Qu, Y.; Lin, Q.; Cheng, K.; Gao, M.; Ren, S.; Zhang, T.; Li, X. TGF-Β1-Induced Chondrogenesis of Bone Marrow Mesenchymal Stem Cells Is Promoted by Low-Intensity Pulsed Ultrasound through the Integrin-mTOR Signaling Pathway. Stem Cell Res. Ther. 2017, 8, 281. [Google Scholar] [CrossRef]
- Barry, F.; Boynton, R.E.; Liu, B.; Murphy, J.M. Chondrogenic Differentiation of Mesenchymal Stem Cells from Bone Marrow: Differentiation-Dependent Gene Expression of Matrix Components. Exp. Cell Res. 2001, 268, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, W.; Deng, H.; Li, T.; Liu, Z.; Liu, X.; Zhang, Z.; Chen, X.; Sheng, J.; Li, K. TGF-Β1 Protects Trauma-Injured Murine Cortical Neurons by Upregulating L-Type Calcium Channel Cav1.2 via the P38 Pathway. Neuroscience 2022, 492, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zheng, H.; Zhu, H.-Y.; Hu, S.; Wang, S.; Jiang, X.-H.; Xu, G.-Y. Acute Effects of TGFβ1 on Neuronal Excitability and Involvement in the Pain of Rats with Chronic Pancreatitis. J. Neurogastroenterol. Motil. 2015, 22, 333–343. [Google Scholar] [CrossRef]
- Nesti, L.J.; Caterson, E.J.; Li, W.-J.; Chang, R.; McCann, T.D.; Hoek, J.B.; Tuan, R.S. TGF-Β1 Calcium Signaling in Osteoblasts. J. Cell. Biochem. 2007, 101, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Muldoon, L.L.; Rodland, K.D.; Magun, B.E. Transforming Growth Factor Beta and Epidermal Growth Factor Alter Calcium Influx and Phosphatidylinositol Turnover in Rat-1 Fibroblasts. J. Biol. Chem. 1988, 263, 18834–18841. [Google Scholar] [CrossRef]
- Clark, R.B.; Kondo, C.; Belke, D.D.; Giles, W.R. Two-Pore Domain K+ Channels Regulate Membrane Potential of Isolated Human Articular Chondrocytes. J. Physiol. 2011, 589, 5071–5089. [Google Scholar] [CrossRef]
- Rosenkranz, S.; Flesch, M.; Amann, K.; Haeuseler, C.; Kilter, H.; Seeland, U.; Schlüter, K.-D.; Böhm, M. Alterations of β-Adrenergic Signaling and Cardiac Hypertrophy in Transgenic Mice Overexpressing TGF-Β1. Am. J. Physiol.-Heart Circ. Physiol. 2002, 283, H1253–H1262. [Google Scholar] [CrossRef]
- Ganesan, A.N.; Maack, C.; Johns, D.C.; Sidor, A.; O’Rourke, B. β-Adrenergic Stimulation of L-Type Ca2+ Channels in Cardiac Myocytes Requires the Distal Carboxyl Terminus of α1C but Not Serine 1928. Circ. Res. 2006, 98, e11–e18. [Google Scholar] [CrossRef]
- Levitan, E.S.; Hemmick, L.M.; Birnberg, N.C.; Kaczmarek, L.K. Dexamethasone Increases Potassium Channel Messenger RNA and Activity in Clonal Pituitary Cells. Mol. Endocrinol. 1991, 5, 1903–1908. [Google Scholar] [CrossRef][Green Version]
- Wang, L.; Wada, Y.; Ballan, N.; Schmeckpeper, J.; Huang, J.; Rau, C.D.; Wang, Y.; Gepstein, L.; Knollmann, B.C. Triiodothyronine and Dexamethasone Alter Potassium Channel Expression and Promote Electrophysiological Maturation of Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. J. Mol. Cell Cardiol. 2021, 161, 130–138. [Google Scholar] [CrossRef]
- Chu, K.; Cheng, Q.; Chen, C.; Au, L.; Seto, S.W.; Tuo, Y.; Motin, L.; Kwan, Y.; Leung, P.S. Angiotensin II Exerts Glucose-Dependent Effects on Kv Currents in Mouse Pancreatic β-Cells via Angiotensin II Type 2 Receptors. Am. J. Physiol. Cell Physiol. 2009, 298, C313–C323. [Google Scholar] [CrossRef] [PubMed]
- Iscla, I.; Wray, R.; Wei, S.; Posner, B.; Blount, P. Streptomycin Potency Is Dependent on MscL Channel Expression. Nat. Commun. 2014, 5, 4891. [Google Scholar] [CrossRef]
- Kaur, K.; Zarzoso, M.; Ponce-Balbuena, D.; Guerrero-Serna, G.; Hou, L.; Musa, H.; Jalife, J. TGF-Β1, Released by Myofibroblasts, Differentially Regulates Transcription and Function of Sodium and Potassium Channels in Adult Rat Ventricular Myocytes. PLoS ONE 2013, 8, e55391. [Google Scholar] [CrossRef]
- Park, W.S.; Heo, S.C.; Jeon, E.S.; Hong, D.H.; Son, Y.K.; Ko, J.-H.; Kim, H.K.; Lee, S.Y.; Kim, J.H.; Han, J. Functional Expression of Smooth Muscle-Specific Ion Channels in TGF-β1-Treated Human Adipose-Derived Mesenchymal Stem Cells. Am. J. Physiol. Cell Physiol. 2013, 305, C377–C391. [Google Scholar] [CrossRef] [PubMed]
- Kameda, T.; Zvick, J.; Vuk, M.; Sadowska, A.; Tam, W.K.; Leung, V.Y.; Bölcskei, K.; Helyes, Z.; Applegate, L.A.; Hausmann, O.N.; et al. Expression and Activity of TRPA1 and TRPV1 in the Intervertebral Disc: Association with Inflammation and Matrix Remodeling. Int. J. Mol. Sci. 2019, 20, 1767. [Google Scholar] [CrossRef]
- Roux, J.; Kawakatsu, H.; Gartland, B.; Pespeni, M.; Sheppard, D.; Matthay, M.A.; Canessa, C.M.; Pittet, J.-F. Interleukin-1β Decreases Expression of the Epithelial Sodium Channel α-Subunit in Alveolar Epithelial Cells via a P38 MAPK-Dependent Signaling Pathway*. J. Biol. Chem. 2005, 280, 18579–18589. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Zhou, Y.; Polson, S.W.; Wan, L.Q.; Wang, M.; Han, L.; Wang, L.; Lu, X.L. Identification of Chondrocyte Genes and Signaling Pathways in Response to Acute Joint Inflammation. Sci. Rep. 2019, 9, 93. [Google Scholar] [CrossRef]
- Thysen, S.; Luyten, F.P.; Lories, R.J.U. Targets, Models and Challenges in Osteoarthritis Research. Dis. Model. Mech. 2015, 8, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Grogan, S.P.; Miyaki, S.; Asahara, H.; D’Lima, D.D.; Lotz, M.K. Mesenchymal Progenitor Cell Markers in Human Articular Cartilage: Normal Distribution and Changes in Osteoarthritis. Arthritis Res. Ther. 2009, 11, R85. [Google Scholar] [CrossRef]
- Goldring, M.B. Chondrogenesis, Chondrocyte Differentiation, and Articular Cartilage Metabolism in Health and Osteoarthritis. Ther. Adv. Musculoskelet. Dis. 2012, 4, 269–285. [Google Scholar] [CrossRef]
- Martin, J.A.; Buckwalter, J.A. The Role of Chondrocyte Senescence in the Pathogenesis of Osteoarthritis and in Limiting Cartilage Repair. J. Bone Jt. Surg. Am. 2003, 85 (Suppl. S2), 106–110. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Lim, R.; Owens, B.D. Latest Advances in Chondrocyte-Based Cartilage Repair. Biomedicines 2024, 12, 1367. [Google Scholar] [CrossRef] [PubMed]
- Prodromos, C.; Finkle, S.; Rumschlag, T.; Lotus, J. Autologous Mesenchymal Stem Cell Treatment Is Consistently Effective for the Treatment of Knee Osteoarthritis: The Results of a Systematic Review of Treatment and Comparison to a Placebo Group. Medicines 2020, 7, 42. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).