Anti-Tumor Strategies of Photothermal Therapy Combined with Other Therapies Using Nanoplatforms
Abstract
1. Introduction
2. Photothermal Therapy
2.1. Operating Principles
2.2. Influence Factors
2.2.1. Light Wavelength
2.2.2. Photothermal Agent
2.2.3. Nanoplatforms
Common Nanoplatforms
Self-Assembled Nanoplatforms
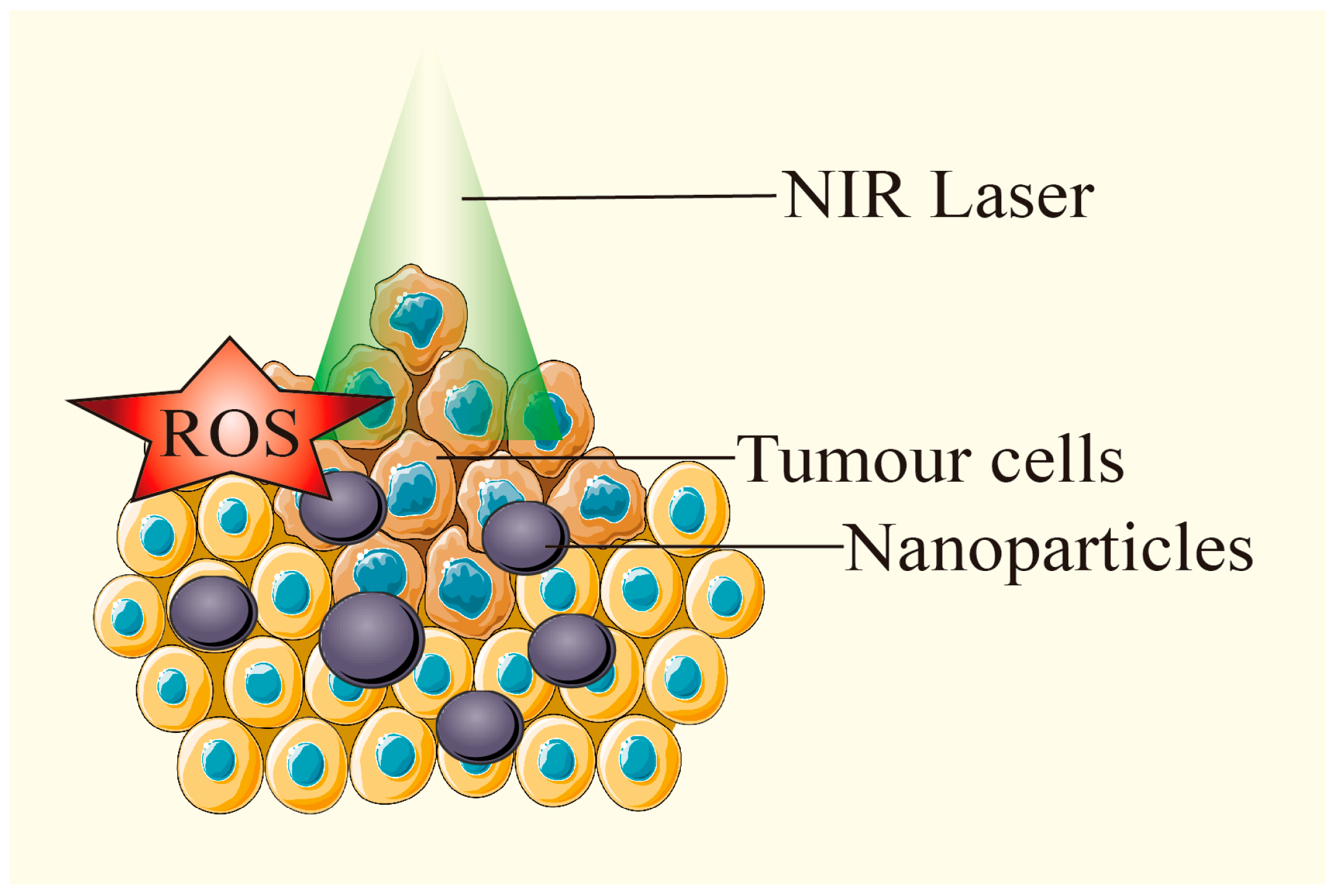
2.3. Anti-Tumor Mechanism
2.3.1. Inducing Tumor Cell Death
2.3.2. Promoting Anti-Tumor Immune Response
2.3.3. Other Anti-Tumor Mechanisms
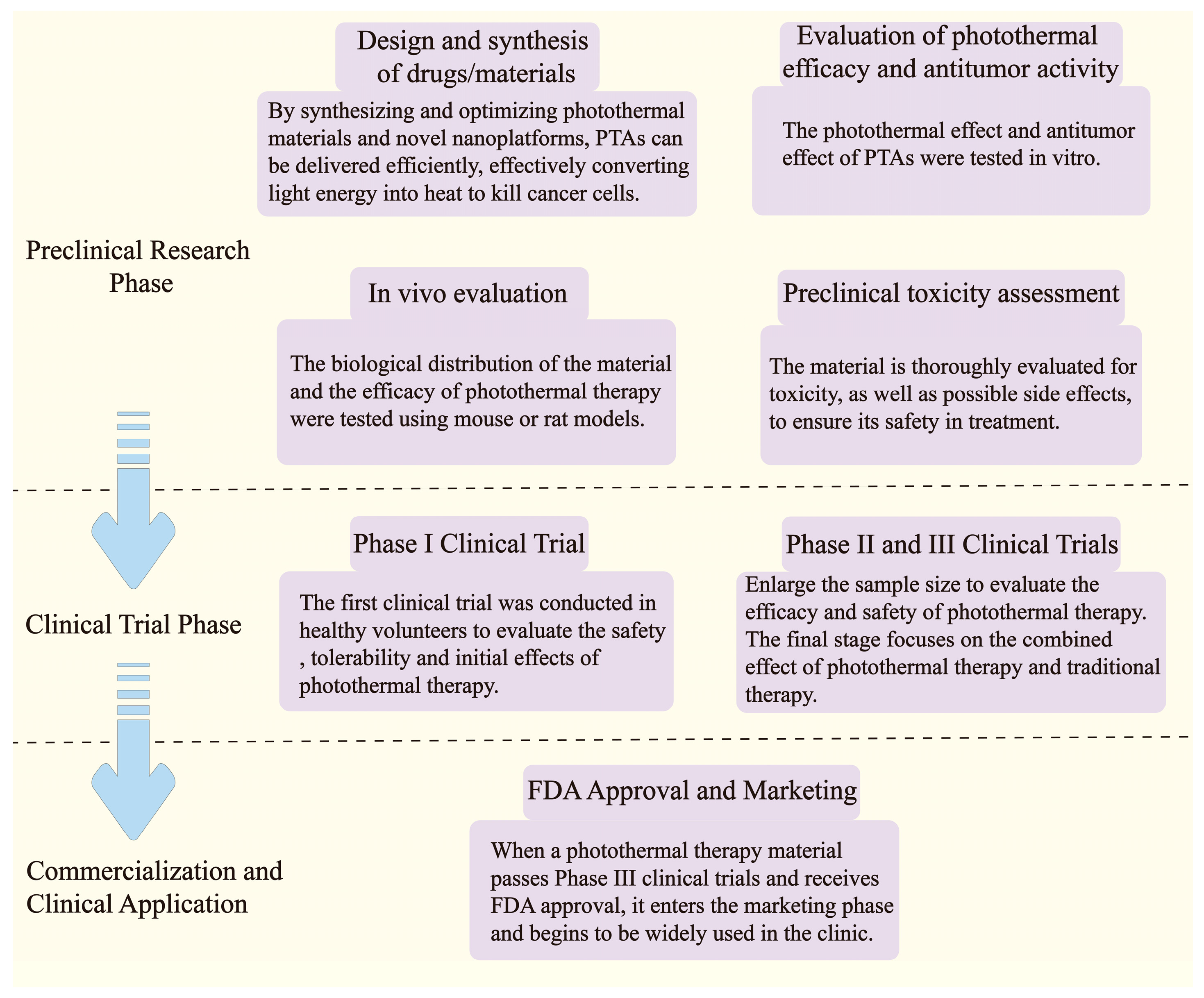
2.4. Development and Application
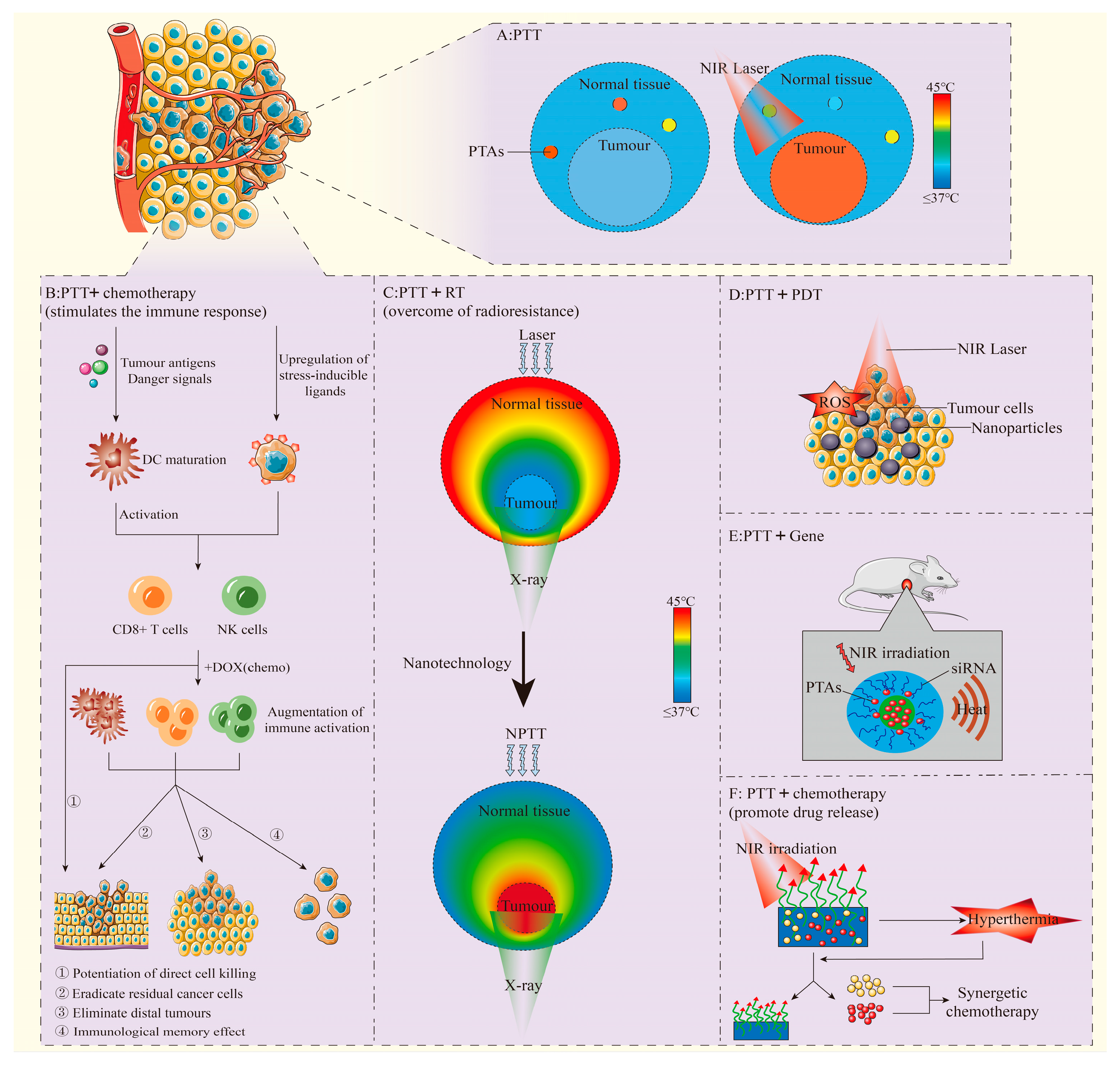
3. Photothermal Therapy in Combination with Other Therapeutic Modalities
3.1. Combination of PTT with Chemotherapy
3.1.1. Promotion of Drug Uptake and Accumulation
3.1.2. Synergy of Anti-Tumor Effects
3.1.3. Enhancement of the Responsiveness of Tumor Cells to Chemotherapy Drugs
3.1.4. Overcome of Multidrug Resistance
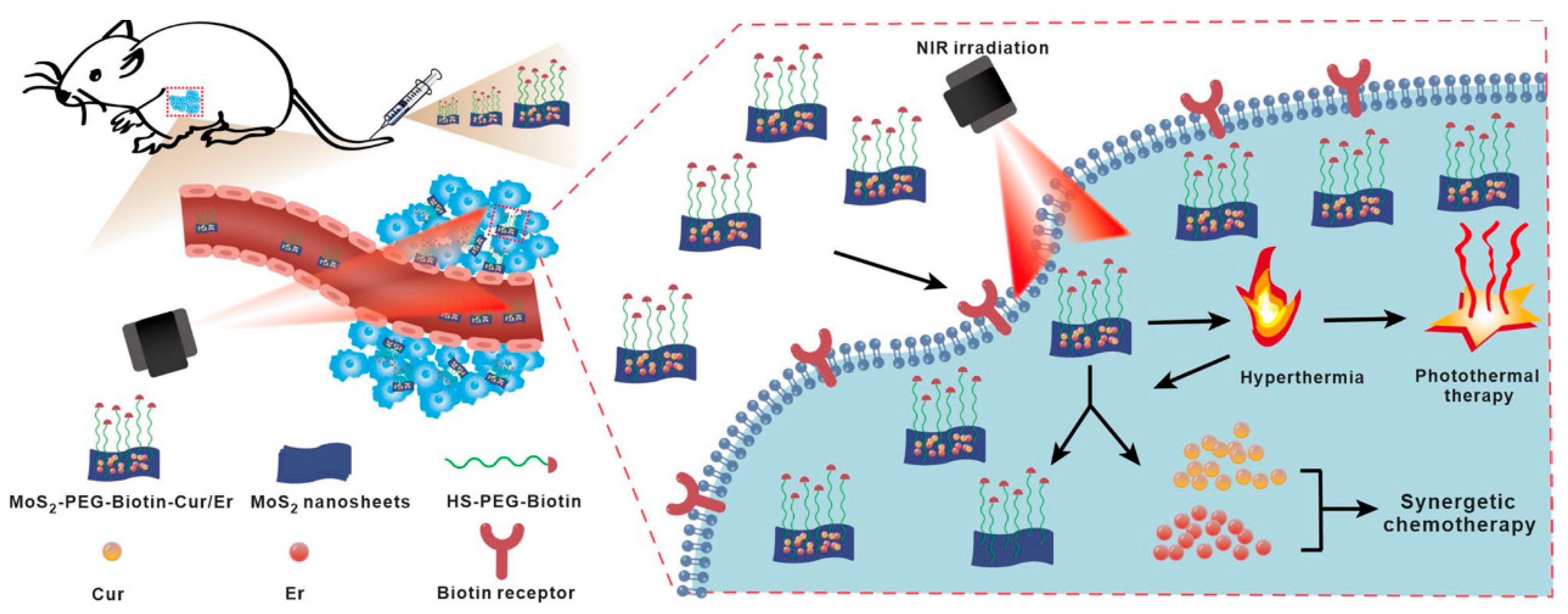
3.1.5. Pre-Clinical Studies
3.2. Combination of PTT with Immunotherapy
3.2.1. PTT in Combination with Immune Adjuvants
3.2.2. PTT in Combination with Immune Checkpoint Inhibitors
3.3. Combination of PTT with Radiotherapy
3.3.1. Increase of Radiosensitivity
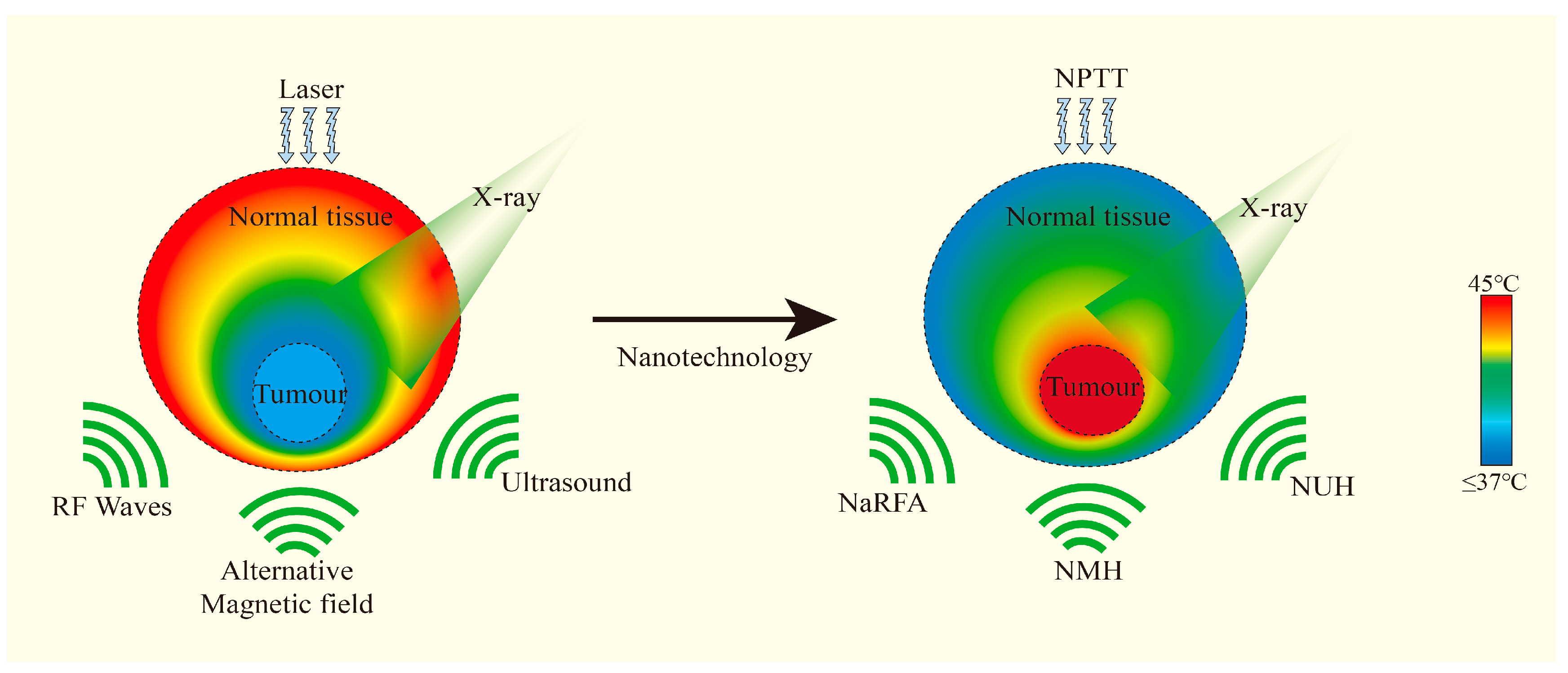
3.3.2. Overcome of Radioresistance
3.3.3. Development of Some Advanced Nanoplatforms
3.4. Conbination of PTT with Photodynamic Therapy or Sonodynamic Therapy
3.5. Combination of PTT with Gene Therapy
3.6. Combination of Chemodynamic Therapy with PTT
3.7. Combination of PTT with Other Therapies
3.7.1. PTT Combined with Gas Therapy
3.7.2. PTT Combined with Hunger Therapy
3.8. Multi-Modal Therapy Based on PTT
3.8.1. PTT Combined with PDT and Chemotherapy
3.8.2. PTT Combined with PDT and RT
3.8.3. PTT Combined with CDT and Chemotherapy
3.8.4. PTT Combined with Gene Therapy and Immunotherapy
4. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
List of Abbreviations
| AIE | aggregation induced emission |
| AP3 | ansamitocin P3 |
| ATP | adenosine triphosphate |
| BP | black phosphorus |
| BSA | bovine serum albumin |
| CDT | chemodynamic therapy |
| Ce6 | chlorin e6 |
| CeO2 | cerium dioxide |
| CuO | copper oxide |
| Cur | curcumin |
| DAMPs | damage-associated molecular patterns |
| DOX | doxorubicin |
| Er | erlotinib |
| FA | folic acid |
| GAL | galactose |
| GBP | glycopican 3 binding peptide |
| gel | incorporating hydrogel |
| GO | graphene oxide |
| GOx | glucose oxidase |
| GSH | glutathione |
| HA | hyaluronic acid |
| Hb | hemoglobin |
| HMSNs | hollow mesoporous silica nanoparticles |
| ICG | indocyanine green |
| ICPs | infinite coordination polymer |
| LM | liquid metal |
| MGO | modified graphene oxide |
| MN | metronidazole |
| mPDA | mesoporous polydopamine |
| MPE | maximum permissible exposure |
| MSNs | mesoporous silica nanoparticles |
| NIR | near-infrared |
| NPs | nanoparticles |
| PA | pheophorbide A |
| PAA | polyacrylic acid |
| PBA | phenylboronic acid |
| PBNPs | prussian blue nanoparticles |
| PDT | photodynamic therapy |
| PEG | polyethylene glycol |
| PEI | polyethylenimine |
| PHC | porphyrin-derivative hybrid complex |
| PPIX | protoporphyrin IX |
| PTAs | photothermal agents |
| PTT | photothermal therapy |
| RBL1/p107 | retinoblastoma-like protein 1 |
| RGD | aspartic acid |
| ROS | reactive oxygen species |
| RT | radiotherapy |
| SA | sodium alginate |
| SDT | sonodynamic therapy |
| SFB | sorafenib |
| SOD | superoxide dismutase |
| TCA | thyrocalcitonin |
| TF | transferrin |
| TME | tumor microenvironment |
| TPZ | tirapazamine |
| V | vanadium |
| VIO | nanoparticles |
| ZnPc | zinc phthalocyanine |
References
- Sung, H.; Ferlay, J.; Siegl, R.L.; Laversanne, M.; Soerjomataram, I.; Jemel, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Haider, T.; Pandey, V.; Banjare, N.; Gupta, P.N.; Soni, V. Drug resistance in cancer: Mechanisms and tackling strategies. Pharmacol. Rep. 2020, 72, 1125–1151. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Malviya, R. Understanding and advancement in gold nanoparticle targeted photothermal therapy of cancer. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2021, 1875, 188532. [Google Scholar] [CrossRef]
- Zou, L.; Wang, H.; He, B.; Zeng, L.; Tan, T.; Cao, H.; He, X.; Zhang, Z.; Guo, S.; Li, Y. Current Approaches of Photothermal Therapy in Treating Cancer Metastasis with Nanotherapeutics. Theranostics 2016, 6, 762–772. [Google Scholar] [CrossRef]
- Zhou, Z.; Jiang, N.; Chen, J.; Zheng, C.; Guo, Y.; Ye, R.; Qi, R.; Shen, J. Selectively down-regulated PD-L1 by albumin-phenformin nanoparticles mediated mitochondrial dysfunction to stimulate tumor-specific immunological response for enhanced mild-temperature photothermal efficacy. J. Nanobiotechnol. 2021, 19, 375. [Google Scholar] [CrossRef]
- Chang, B.; Li, D.; Ren, Y.; Qu, C.; Shi, X.; Liu, R.; Liu, H.; Tian, J.; Hu, Z.; Sun, T.; et al. A phosphorescent probe for in vivo imaging in the second near-infrared window. Nat. Biomed. Eng. 2021, 6, 629–639. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, T.; Cheng, H.; Zhang, F.; Yang, X.; Wang, S.; Zhou, J.; Ding, Y. Nanomedicine potentiates mild photothermal therapy for tumor ablation. Asian J. Pharm. Sci. 2021, 16, 738–761. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, J.; Wei, X. Editorial: Functional Nanomaterials for Cancer Diagnostics and Therapy. Front. Chem. 2021, 9, 670410. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef]
- Kok, H.P.; Crezee, J. Hyperthermia Treatment Planning: Clinical Application and Ongoing Developments. IEEE J. Electromagn. RF Microw. Med. Biol. 2021, 5, 214–222. [Google Scholar] [CrossRef]
- Nam, J.; Son, S.; Ochyl, L.J.; Kuai, R.; Schwendeman, A.; Moon, J.J. Chemo-photothermal therapy combination elicits anti-tumor immunity against advanced metastatic cancer. Nat. Commun. 2018, 9, 1074. [Google Scholar] [CrossRef] [PubMed]
- Beik, J.; Abed, Z.; Ghoreishi, F.S.; Hosseini-Nami, S.; Mehrzadi, S.; Shakeri-Zadeh, A.; Kamrava, S.K. Nanotechnology in hyperthermia cancer therapy: From fundamental principles to advanced applications. J. Control. Release 2016, 235, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lee, D.Y. Near-Infrared-Responsive Cancer Photothermal and Photodynamic Therapy Using Gold Nanoparticles. Polymers 2018, 10, 961. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.-L.; Shih, Y.-H.; Lee, P.-C.; Hsieh, T.M.-H.; Luo, T.-Y.; Shieh, M.-J. Multimodal Image-Guided Photothermal Therapy Mediated by 188Re-Labeled Micelles Containing a Cyanine-Type Photosensitizer. ACS Nano 2011, 5, 5594–5607. [Google Scholar] [CrossRef]
- Chen, Z.; Wei, X.; Zheng, Y.; Zhang, Z.; Gu, W.; Liao, W.; Zhang, H.; Wang, X.; Liu, J.; Li, H.; et al. Targeted co-delivery of curcumin and erlotinib by MoS2 nanosheets for the combination of synergetic chemotherapy and photothermal therapy of lung cancer. J. Nanobiotechnol. 2023, 21, 333. [Google Scholar] [CrossRef]
- Khan, N.U. Synthesis of gold nanorods and their performance in the field of cancer cell imaging and photothermal therapy. Cancer Nanotechnol. 2021, 12, 20. [Google Scholar] [CrossRef]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef]
- Ralph, W.; Vasilis, N. Shedding light onto live molecular targets. Nat. Med. 2003, 9, 123–128. [Google Scholar]
- Ralph, W. A clearer vision for in vivo imaging. Nat. Biotechnol. 2001, 19, 316–317. [Google Scholar]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; A El-Sayed, M. Gold Nanoparticles: Interesting Optical Properties and Recent Applications in Cancer Diagnostics and Therapy. Nanomedicine 2007, 2, 681–693. [Google Scholar] [CrossRef]
- Xia, Y.; Xiong, Y.; Lim, B.; Skrabalak, S.E. Shape-Controlled Synthesis of Metal Nanocrystals: Simple Chemistry Meets Complex Physics? Angew. Chem. Int. Ed. 2009, 48, 60–103. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tabakman, S.M.; Chen, Z.; Dai, H. Preparation of carbon nanotube bioconjugates for biomedical applications. Nat. Protoc. 2009, 4, 1372–1381. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xu, B.; Liu, Z. Carbon dot–based photonic nanomaterials for photothermal tumor therapy. Nanophototherapy 2025, 2025, 257–281. [Google Scholar]
- He, P.; Jia, M.; Yang, L.; Zhang, H.; Chen, R.; Yao, W.; Pan, Y.; Fan, Q.; Hu, W.; Huang, W. Zwitterionic Photosensitizer-Assembled Nanocluster Produces Efficient Photogenerated Radicals via Autoionization for Superior Antibacterial Photodynamic Therapy. Adv. Mater. 2025, e2418978. [Google Scholar] [CrossRef]
- Alamdari, S.G.; Amini, M.; Jalilzadeh, N.; Baradaran, B.; Mohammadzadeh, R.; Mokhtarzadeh, A.; Oroojalian, F. Recent advances in nanoparticle-based photothermal therapy for breast cancer. J. Control. Release 2022, 349, 269–303. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, H.-M.; Song, G.; Li, Z.; Zhang, X.-B. Conjugated-Polymer-Based Nanomaterials for Photothermal Therapy. ACS Appl. Polym. Mater. 2020, 2, 4258–4272. [Google Scholar] [CrossRef]
- Fan, J.; Cheng, Y.; Sun, M. Functionalized Gold Nanoparticles: Synthesis, Properties and Biomedical Applications. Chem. Rec. 2020, 20, 1474–1504. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer Cell Imaging and Photothermal Therapy in the Near-Infrared Region by Using Gold Nanorods. J. Am. Chem. Soc. 2006, 128, 2115–2120. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, S.; Zhang, G.; Sun, X.; Lee, S.-T.; Liu, Z. Graphene in Mice: Ultrahigh In Vivo Tumor Uptake and Efficient Photothermal Therapy. Nano Lett. 2010, 10, 3318–3323. [Google Scholar] [CrossRef]
- Kozics, K.; Sramkova, M.; Kopecka, K.; Begerova, P.; Manova, A.; Krivosikova, Z.; Sevcikova, Z.; Liskova, A.; Rollerova, E.; Dubaj, T.; et al. Pharmacokinetics, Biodistribution, and Biosafety of PEGylated Gold Nanoparticles In Vivo. Nanomaterials 2021, 11, 1702. [Google Scholar] [CrossRef]
- Sheng, Z.; Hu, D.; Zheng, M.; Liu, H.; Gao, D.; Gong, P.; Gao, G.; Zhang, P.; Ma, Y.; Cai, L. Smart Human Serum Albumin-Indocyanine Green Nanoparticles Generated by Programmed Assembly for Dual-Modal Imaging-Guided Cancer Synergistic Phototherapy. ACS Nano 2014, 8, 12310–12322. [Google Scholar] [CrossRef]
- Cano-Mejia, J.; Bookstaver, M.L.; Sweeney, E.E.; Jewell, C.M.; Fernandes, R. Prussian blue nanoparticle-based antigenicity and adjuvanticity trigger robust antitumor immune responses against neuroblastoma. Biomater. Sci. 2019, 7, 1875–1887. [Google Scholar] [CrossRef] [PubMed]
- Sur, S.; Steele, R.; Isbell, T.S.; Venkata, K.N.; Rateb, M.E.; Ray, R.B. Momordicine-I, a Bitter Melon Bioactive Metabolite, Displays Anti-Tumor Activity in Head and Neck Cancer Involving c-Met and Downstream Signaling. Cancers 2021, 13, 1432. [Google Scholar] [CrossRef] [PubMed]
- Xue, P.; Yang, R.; Sun, L.; Li, Q.; Zhang, L.; Xu, Z.; Kang, Y. Indocyanine Green-Conjugated Magnetic Prussian Blue Nanoparticles for Synchronous Photothermal/Photodynamic Tumor Therapy. Nano-Micro Lett. 2018, 10, 74. [Google Scholar] [CrossRef]
- Ji, B.; Cai, H.; Yang, Y.; Peng, F.; Song, M.; Sun, K.; Yan, F.; Liu, Y. Hybrid membrane camouflaged copper sulfide nanoparticles for photothermal-chemotherapy of hepatocellular carcinoma. Acta Biomater. 2020, 111, 363–372. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Liposomes as delivery agents for medical imaging. Mol. Med. Today 1996, 2, 242–249. [Google Scholar] [CrossRef]
- Gaucher, G.; Dufresne, M.-H.; Sant, V.P.; Kang, N.; Maysinger, D.; Leroux, J.-C. Block copolymer micelles: Preparation, characterization and application in drug delivery. J. Control. Release 2005, 109, 169–188. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Zhang, Q.; Franklin, J.L.; Coffey, R.J. Extracellular vesicles and nanoparticles: Emerging complexities. Trends Cell Biol. 2023, 33, 667–681. [Google Scholar] [CrossRef]
- Chang, P.; Mei, H.; Zhao, Y.; Pan, L.; Zhang, M.; Wang, X.; Cheng, L.; Zhang, L. Nature-Inspired 3D Spiral Grass Structured Graphene Quantum Dots/MXene Nanohybrids with Exceptional Photothermal-Driven Pseudo-Capacitance Improvement. Adv. Sci. 2022, 9, e2204086. [Google Scholar] [CrossRef] [PubMed]
- Macchi, S.; Jalihal, A.; Hooshmand, N.; Zubair, M.; Jenkins, S.; Alwan, N.; El-Sayed, M.; Ali, N.; Griffin, R.J.; Siraj, N. Enhanced photothermal heating and combination therapy of NIR dye via conversion to self-assembled ionic nanomaterials. J. Mater. Chem. B 2022, 10, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-J.; Zhang, W.-C.; Guo, Y.-W.; Chen, X.-Y.; Zhang, Y.-N. Metal nanoparticles as a promising technology in targeted cancer treatment. Drug Deliv. 2022, 29, 664–678. [Google Scholar] [CrossRef] [PubMed]
- Roti, J.L.R. Cellular responses to hyperthermia (40–46 °C): Cell killing and molecular events. Int. J. Hyperth. 2008, 24, 3–15. [Google Scholar] [CrossRef]
- Ahmed, A.; Tait, S.W. Targeting immunogenic cell death in cancer. Mol. Oncol. 2020, 14, 2994–3006. [Google Scholar] [CrossRef]
- Chen, J.-L.; Zhang, H.; Huang, X.-Q.; Wan, H.-Y.; Li, J.; Fan, X.-X.; Luo, K.Q.; Wang, J.; Zhu, X.-M.; Wang, J. Antiangiogenesis-Combined Photothermal Therapy in the Second Near-Infrared Window at Laser Powers Below the Skin Tolerance Threshold. Nano-Micro Lett. 2019, 11, 93. [Google Scholar] [CrossRef]
- Kim, M.; Lee Ji Choi, J.; Kim, S.Y. Reactive Oxygen Species-Responsive Nanocomposite Hydrogels for Accurate Drug Delivery and Localized PDT/PTT/chemo Synergistic Cancer Therapy. Eur. Polym. J. 2025, 224, 113683. [Google Scholar] [CrossRef]
- Cui, M.; Tang, D.; Wang, B.; Zhang, H.; Liang, G.; Xiao, H. Bioorthogonal Guided Activation of cGAS-STING by AIE Photosensitizer Nanoparticles for Targeted Tumor Therapy and Imaging. Adv. Mater. 2023, 35, e2305668. [Google Scholar] [CrossRef]
- Zhu, H.; Huang, C.; Di, J.; Chang, Z.; Li, K.; Zhang, S.; Li, X.; Wu, D. Doxorubicin-Fe(III)-Gossypol Infinite Coordination Polymer@PDA:CuO2 Composite Nanoparticles for Cost-Effective Programmed Photothermal-Chemodynamic-Coordinated Dual Drug Chemotherapy Trimodal Synergistic Tumor Therapy. ACS Nano 2023, 17, 12544–12562. [Google Scholar] [CrossRef]
- Li, Y.; Qi, H.; Geng, Y.; Li, L.; Cai, X. Research progress of organic photothermal agents delivery and synergistic therapy systems. Colloids Surf. B Biointerfaces 2024, 234, 113743. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, L.; Liang, C.; Wang, C.; Peng, R.; Liu, Z. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat. Commun. 2016, 7, 13193. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.-J.; Chen, C.; Zhao, Y.; Jia, L.; Wang, P.C. Biopharmaceutics and Therapeutic Potential of Engineered Nanomaterials. Curr. Drug Metab. 2008, 9, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, L.; Dong, H.; Liu, Y.; Wang, K.; Li, A.; Ren, T.; Shi, D.; Li, Y. Fever-Inspired Immunotherapy Based on Photothermal CpG Nanotherapeutics: The Critical Role of Mild Heat in Regulating Tumor Microenvironment. Adv. Sci. 2018, 5, 1700805. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zheng, J.; Jin, F.; Xiao, J.; Lan, N.; Xu, Z.; Yue, X.; Li, Z.; Li, C.; Cao, D.; et al. Fiber-optic drug delivery strategy for synergistic cancer photothermal-chemotherapy. Light Sci. Appl. 2024, 13, 228. [Google Scholar] [CrossRef]
- Yao, X.; Niu, X.; Ma, K.; Huang, P.; Grothe, J.; Kaskel, S.; Zhu, Y. Graphene Quantum Dots-Capped Magnetic Mesoporous Silica Nanoparticles as a Multifunctional Platform for Controlled Drug Delivery, Magnetic Hyperthermia, and Photothermal Therapy. Small 2016, 13, 1602225. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, H.; Dong, J.; Zhang, W.; Dang, G.; Yang, M.; Li, Y.; Chen, H.; Ji, H.; Dong, L. PEGylated MoS2 quantum dots for traceable and pH-responsive chemotherapeutic drug delivery. Colloids Surf. B Biointerfaces 2020, 185, 110590. [Google Scholar] [CrossRef]
- Gong, T.; Wang, X.; Ma, Q.; Li, J.; Li, M.; Huang, Y.; Liang, W.; Su, D.; Guo, R. Triformyl cholic acid and folic acid functionalized magnetic graphene oxide nanocomposites: Multiple-targeted dual-modal synergistic chemotherapy/photothermal therapy for liver cancer. J. Inorg. Biochem. 2021, 223, 111558. [Google Scholar] [CrossRef]
- Chen, J.; Xiang, Y.; Bao, R.; Zheng, Y.; Fang, Y.; Feng, J.; Wu, D.; Chen, X. Combined Photothermal Chemotherapy for Effective Treatment Against Breast Cancer in Mice Model. Int. J. Nanomed. 2024, 19, 9973–9987. [Google Scholar] [CrossRef]
- Bienia, A.; Wiecheć-Cudak, O.; Murzyn, A.A.; Krzykawska-Serda, M. Photodynamic Therapy and Hyperthermia in Combination Treatment—Neglected Forces in the Fight against Cancer. Pharmaceutics 2021, 13, 1147. [Google Scholar] [CrossRef]
- Urano, M. Invited Review: For the clinical application of thermochemotherapy given at mild temperatures. Int. J. Hyperth. 1999, 15, 79–107. [Google Scholar] [CrossRef]
- Houdaihed, L.; Evans, J.C.; Allen, C. Codelivery of Paclitaxel and Everolimus at the Optimal Synergistic Ratio: A Promising Solution for the Treatment of Breast Cancer. Mol. Pharm. 2018, 15, 3672–3681. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.-P.; Sun, H.-T.; Wang, P.; Wang, J.; Zhou, J.-H.; Cao, R.-Q.; Yue, L.; Chen, Y.-G.; Shen, F.-R. Hyperthermia enhances the efficacy of chemotherapeutic drugs in heat-sensitive cells through interfering with DNA damage repair. Ann. Transl. Med. 2022, 10, 463. [Google Scholar] [CrossRef] [PubMed]
- Issels, R.D. Hyperthermia adds to chemotherapy. Eur. J. Cancer 2008, 44, 2546–2554. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, N.; Su, Q.; Lv, Y.; Yang, C.; Zhan, H. Green Synthesis of Gold Nanoparticles and Study of Their Inhibitory Effect on Bulk Cancer Cells and Cancer Stem Cells in Breast Carcinoma. Nanomaterials 2022, 12, 3324. [Google Scholar] [CrossRef]
- Tu, Z.; Qiao, H.; Yan, Y.; Guday, G.; Chen, W.; Adeli, M.; Haag, R. Directed Graphene-Based Nanoplatforms for Hyperthermia: Overcoming Multiple Drug Resistance. Angew. Chem. Int. Ed. 2018, 57, 11198–11202. [Google Scholar] [CrossRef]
- Xing, Y.; Zhang, J.; Chen, F.; Liu, J.; Cai, K. Mesoporous polydopamine nanoparticles with co-delivery function for overcoming multidrug resistance via synergistic chemo-photothermal therapy. Nanoscale 2017, 9, 8781–8790. [Google Scholar] [CrossRef]
- Yang, K.; Feng, L.; Shi, X.; Liu, Z. Nano-graphene in biomedicine: Theranostic applications. Chem. Soc. Rev. 2013, 42, 530–547. [Google Scholar] [CrossRef]
- Rastinehad, A.R.; Anastos, H.; Wajswol, E.; Winoker, J.S.; Sfakianos, J.P.; Doppalapudi, S.K.; Carrick, M.R.; Knauer, C.J.; Taouli, B.; Lewis, S.C.; et al. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proc. Natl. Acad. Sci. USA 2019, 116, 18590–18596. [Google Scholar] [CrossRef]
- Shang, T.; Yu, X.; Han, S.; Yang, B. Nanomedicine-based tumor photothermal therapy synergized immunotherapy. Biomater. Sci. 2020, 8, 5241–5259. [Google Scholar] [CrossRef]
- Wang, S.; Huang, P.; Nie, L.; Xing, R.; Liu, D.; Wang, Z.; Lin, J.; Chen, S.; Niu, G.; Lu, G.; et al. Single Continuous Wave Laser Induced Photodynamic/Plasmonic Photothermal Therapy Using Photosensitizer-Functionalized Gold Nanostars. Adv. Mater. 2013, 25, 3055–3061. [Google Scholar] [CrossRef]
- Chen, P.-M.; Pan, W.-Y.; Wua, C.-Y.; Yeha, C.-Y.; Korupallia, C.; Luoa, P.-K.; Choua, C.-J.; Chiab, W.-T.; Sunga, H.-W. Modulation of tumor microenvironment using a TLR-7/8 agonist-loaded nanoparticle system that exerts low-temperature hyperthermia and immunotherapy for in situ cancer vaccination. Biomaterials 2020, 230, 119629. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Gao, Y.; Ouyang, Z.; Jia, B.; Shen, M.; Shi, X. Photothermal-triggered dendrimer nanovaccines boost systemic antitumor immunity. J. Control. Release 2023, 355, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.; Xu, L.; Liang, C.; Feng, L.; Xu, J.; Dong, Z.; Tian, L.; Yi, X.; Yang, K.; Liu, Z. Combined local immunostimulatory radioisotope therapy and systemic immune checkpoint blockade imparts potent antitumour responses. Nat. Biomed. Eng. 2018, 2, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.-W.; Ou, Y.-L.; Kuo, C.-C.; Tsao, H.-Y.; Lu, H.-E. Ansamitocin P3-Loaded Gold-NanoCage Conjugated with Immune Checkpoint Inhibitor to Enhance Photo-Chemo-Thermal Maturation of Dendritic Cells for Hepatocellular Carcinoma. Polymers 2021, 13, 2726. [Google Scholar] [CrossRef]
- Xie, Z.; Peng, M.; Lu, R.; Meng, X.; Liang, W.; Li, Z.; Qiu, M.; Zhang, B.; Nie, G.; Xie, N.; et al. Black phosphorus-based photothermal therapy with aCD47-mediated immune checkpoint blockade for enhanced cancer immunotherapy. Light Sci. Appl. 2020, 9, 161. [Google Scholar] [CrossRef]
- Dunne, M.; Regenold, M.; Allen, C. Hyperthermia can alter tumor physiology and improve chemo- and radio-therapy efficacy. Adv. Drug Deliv. Rev. 2020, 163, 98–124. [Google Scholar] [CrossRef]
- Krawczyk, P.M.; Eppink, B.; Essers, J.; Stap, J.; Rodermond, H.; Odijk, H.; Zelensky, A.; van Bree, C.; Stalpers, L.J.; Buist, M.R.; et al. Mild hyperthermia inhibits homologous recombination, induces BRCA2 degradation, and sensitizes cancer cells to poly (ADP-ribose) polymerase-1 inhibition. Proc. Natl. Acad. Sci. USA 2011, 108, 9851–9856. [Google Scholar] [CrossRef]
- Song, C.W.; Park, H.J.; Lee, C.K.; Griffin, R. Implications of increased tumor blood flow and oxygenation caused by mild temperature hyperthermia in tumor treatment. Int. J. Hyperth. 2005, 21, 761–767. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, J.; Fu, S.; Wu, J. Gold Nanoparticles as Radiosensitizers in Cancer Radiotherapy. Int. J. Nanomed. 2020, 15, 9407–9430. [Google Scholar] [CrossRef]
- Song, G.; Liang, C.; Yi, X.; Zhao, Q.; Cheng, L.; Yang, K.; Liu, Z. Perfluorocarbon-Loaded Hollow Bi2Se3 Nanoparticles for Timely Supply of Oxygen under Near-Infrared Light to Enhance the Radiotherapy of Cancer. Adv. Mater. 2016, 28, 2716–2723. [Google Scholar] [CrossRef]
- Gedda, G.; Yao, Y.-Y.; Chen, S.-H.; Ghule, A.V.; Ling, Y.-C.; Chang, J.-Y. Facile synthesis of gold/gadolinium-doped carbon quantum dot nanocomposites for magnetic resonance imaging and photothermal ablation therapy. J. Mater. Chem. B 2017, 5, 6282–6291. [Google Scholar] [CrossRef] [PubMed]
- Cheadle, E.J.; Lipowska-Bhalla, G.; Dovedi, S.J.; Fagnano, E.; Klein, C.; Honeychurch, J.; Illidge, T.M. A TLR7 agonist enhances the antitumor efficacy of obinutuzumab in murine lymphoma models via NK cells and CD4 T cells. Leukemia 2016, 31, 1611–1621. [Google Scholar] [CrossRef]
- Wang, S.; Riedinger, A.; Li, H.; Fu, C.; Liu, H.; Li, L.; Liu, T.; Tan, L.; Barthel, M.J.; Pugliese, G.; et al. Plasmonic Copper Sulfide Nanocrystals Exhibiting Near-Infrared Photothermal and Photodynamic Therapeutic Effects. ACS Nano 2015, 9, 1788–1800. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Chen, X. Combined Photodynamic and Photothermal Therapy and Immunotherapy for Cancer Treatment: A Review. Int. J. Nanomed. 2022, ume 17, 6427–6446. [Google Scholar] [CrossRef]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in Photodynamic Therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef] [PubMed]
- Urazaliyeva, A.; Kanabekova, P.; Beisenbayev, A.; Kulsharova, G.; Atabaev, T.; Kim, S.; Lim, C.-K. All organic nanomedicine for PDT–PTT combination therapy of cancer cells in hypoxia. Sci. Rep. 2024, 14, 17507. [Google Scholar] [CrossRef]
- Guidolin, K.; Ding, L.; Chen, J.; Wilson, B.C.; Zheng, G. Porphyrin-lipid nanovesicles (Porphysomes) are effective photosensitizers for photodynamic therapy. Nanophotonics 2021, 10, 3161–3168. [Google Scholar] [CrossRef]
- Li, S.; Yang, S.; Liu, C.; He, J.; Li, T.; Fu, C.; Meng, X.; Shao, H. Enhanced Photothermal-Photodynamic Therapy by Indocyanine Green and Curcumin-Loaded Layered MoS2 Hollow Spheres via Inhibition of P-Glycoprotein. Int. J. Nanomed. 2021, 16, 433–442. [Google Scholar] [CrossRef]
- Li, B.; Fu, Y.; Xie, M.; Feng, L.; Niu, X.; Que, L.; You, Z. Gold-based nanoparticles realize photothermal and photodynamic synergistic treatment of liver cancer and improve the anaerobic tumor microenvironment under near-infrared light. Front. Bioeng. Biotechnol. 2022, 10, 957349. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, S.; Wu, Y.; Tang, P.; Liu, J.; Liu, Z.; Shen, S.; Ren, H.; Wu, D. Highly Penetrable and On-Demand Oxygen Release with Tumor Activity Composite Nanosystem for Photothermal/Photodynamic Synergetic Therapy. ACS Nano 2020, 14, 17046–17062. [Google Scholar] [CrossRef]
- Kejík, Z.; Hajduch, J.; Abramenko, N.; Vellieux, F.; Veselá, K.; Fialová, J.L.; Petrláková, K.; Kučnirová, K.; Kaplánek, R.; Tatar, A.; et al. Cyanine dyes in the mitochondria-targeting photodynamic and photothermal therapy. Commun. Chem. 2024, 7, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, Y.; Liu, X.; Tian, Y.; Cheng, Y.; Tang, L.; Lin, H. Smart NIR-II croconaine dye-peptide for enhanced photo-sonotheranostics of hepatocellular carcinoma. Theranostics 2022, 12, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Durymanov, M.; Reineke, J. Non-viral Delivery of Nucleic Acids: Insight Into Mechanisms of Overcoming Intracellular Barriers. Front. Pharmacol. 2018, 9, 971. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Su, M.; Wang, Z.; Zhang, J. Second Near-Infrared Plasmonic Nanomaterials for Photoacoustic Imaging and Photothermal Therapy. Small 2023, 19, e2300539. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, M.; Fang, C.; Chen, X.; Liu, H.; Feng, Y.; Zhang, Y.; Min, W. A novel multifunctional gold nanorod-mediated and tumor-targeted gene silencing of GPC-3 synergizes photothermal therapy for liver cancer. Nanotechnology 2021, 32, 175101. [Google Scholar] [CrossRef]
- Yin, F.; Hu, K.; Chen, Y.; Yu, M.; Wang, D.; Wang, Q.; Yong, K.-T.; Lu, F.; Liang, Y.; Li, Z. SiRNA Delivery with PEGylated Graphene Oxide Nanosheets for Combined Photothermal and Genetherapy for Pancreatic Cancer. Theranostics 2017, 7, 1133–1148. [Google Scholar] [CrossRef]
- Zhang, L.; Li, C.; Wan, S.; Zhang, X. Nanocatalyst-Mediated Chemodynamic Tumor Therapy. Adv. Healthc. Mater. 2021, 11, 2101971. [Google Scholar] [CrossRef]
- Dang, W.; Chen, W.-C.; Ju, E.; Xu, Y.; Li, K.; Wang, H.; Wang, K.; Lv, S.; Shao, D.; Tao, Y.; et al. 3D printed hydrogel scaffolds combining glutathione depletion-induced ferroptosis and photothermia-augmented chemodynamic therapy for efficiently inhibiting postoperative tumor recurrence. J. Nanobiotechnol. 2022, 20, 266. [Google Scholar] [CrossRef]
- Yang, X.; Xiao, J.; Jiang, L.; Ran, L.; Fan, Y.; Zhang, M.; Xu, Y.; Yao, C.; An, B.; Yang, Y.; et al. A Multifunctional Vanadium-Iron-Oxide Nanoparticle Eradicates Hepatocellular Carcinoma via Targeting Tumor and Endothelial Cells. ACS Appl. Mater. Interfaces 2022, 14, 28514–28526. [Google Scholar] [CrossRef]
- Xu, Y.; Bian, J.; Liu, X.; Qian, Z.; Sun, M.; Zhang, C.; Pan, R.; Li, Q.; Sun, C.; Lin, B.; et al. Glucose-responsive enzymatic biomimetic nanodots for H2O2 self-supplied catalytic photothermal/chemodynamic anticancer therapy. Acta Biomater. 2023, 172, 441–453. [Google Scholar] [CrossRef]
- Wen, H.; Huang, X. Cuprous oxide nanoparticles-based photothermal and chemodynamic synergistic therapy inhibits proliferation and migration of gastric cancer cells in vitro, I. J. South. Med. Univ. 2022, 42, 1732–1738. [Google Scholar] [CrossRef]
- Zhang, W.-X.; Hao, Y.-N.; Gao, Y.-R.; Shu, Y.; Wang, J.-H. Mutual Benefit between Cu(II) and Polydopamine for Improving Photothermal–Chemodynamic Therapy. ACS Appl. Mater. Interfaces 2021, 13, 38127–38137. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Liu, Z.; Zhu, C.; Chen, H.; Kwok, R.T.K.; Zhang, P.; Tang, B.Z.; Cai, L.; Gong, P. H2O2-Responsive NIR-II AIE Nanobomb for Carbon Monoxide Boosting Low-Temperature Photothermal Therapy. Angew. Chem. Int. Ed. Engl. 2022, 61, e202207213. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.-H.; Qi, C.; Hu, Y.-R.; Lin, J.; Huang, P. Glucose Oxidase-Instructed Multimodal Synergistic Cancer Therapy. Adv. Mater. 2019, 31, e1808325, Correction in Adv. Mater. 2020, 32, e2003130. [Google Scholar] [CrossRef]
- Pu, Y.; Wu, W.; Zhou, B.; Xiang, H.; Yu, J.; Yin, H.; Zhang, Y.; Du, D.; Chen, Y.; Xu, H. Starvation therapy enabled “switch-on” NIR-II photothermal nanoagent for synergistic in situ photothermal immunotherapy. Nano Today 2022, 44, 101461. [Google Scholar] [CrossRef]
- Wang, Z.; Chang, Z.-M.; Shao, D.; Zhang, F.; Chen, F.; Li, L.; Ge, M.-F.; Hu, R.; Zheng, X.; Wang, Y.; et al. Janus Gold Triangle-Mesoporous Silica Nanoplatforms for Hypoxia-Activated Radio-Chemo-Photothermal Therapy of Liver Cancer. ACS Appl. Mater. Interfaces 2019, 11, 34755–34765. [Google Scholar] [CrossRef]
- Yu, X.-N.; Deng, Y.; Zhang, G.-C.; Liu, J.; Liu, T.-T.; Dong, L.; Zhu, C.-F.; Shen, X.-Z.; Li, Y.-H.; Zhu, J.-M. Sorafenib-Conjugated Zinc Phthalocyanine Based Nanocapsule for Trimodal Therapy in an Orthotopic Hepatocellular Carcinoma Xenograft Mouse Model. ACS Appl. Mater. Interfaces 2020, 12, 17193–17206. [Google Scholar] [CrossRef]
- Liu, T.; Song, Y.; Huang, Z.; Pu, X.; Wang, Y.; Yin, G.; Gou, L.; Weng, J.; Meng, X. Photothermal photodynamic therapy and enhanced radiotherapy of targeting copolymer-coated liquid metal nanoparticles on liver cancer. Colloids Surf. B Biointerfaces 2021, 207, 112023. [Google Scholar] [CrossRef]
- Lu, J.; Guo, Z.; Che, S.; Gao, F.; Gu, Z.; Xu, J.; Chi, Y.; Xu, W.; Zhang, J.; Takuya, N.; et al. Dihydroartemisinin loaded layered double hydroxide nanocomposites for tumor specific photothermal–chemodynamic therapy. J. Mater. Chem. B 2020, 8, 11082–11089. [Google Scholar] [CrossRef]



| Categories (Main Absorption Wavelength) | Common Example | Characteristics |
|---|---|---|
| Metallic nanomaterials (500–1200 nm) | Gold NPs, Silver NPs |
|
| Carbon-based nanomaterials (600–2000 nm) | Carbon Nanotubes, Carbon Quantum Dots |
|
| Organic Dyes (650–1000 nm) | IR780 dye, ICG |
|
| Conjugated Polymers (400–1100 nm) | Polystyrene, Poly (3, 4-vinyldioxylthiene -phenylethylene) |
|
| Common Nanoplatform | Classification | Characteristics | Form and Size |
|---|---|---|---|
| Polymeric Nanoparticles | Biodegradable polymeric NPs and non-biodegradable polymeric NPs |
| Generally spherical or nearly spherical, in the range of tens to hundreds of nanometers. |
| Liposomal Nanoparticles | Traditional liposomes, long-circulating liposomes, targeted liposomes, etc. |
| Usually spherical in shape, in the range of 30 to 1000 nm. |
| Micellar Nanoparticles | Block copolymer micelles, graft copolymer micelles, etc. |
| Usually spherical in shape, in the range of 10 to 100 nm. |
| Inorganic Nanoparticles | Gold NPs, graphene, quantum dots, silver NPs, carbon nanotubes, etc. |
| Usually spherical or polyhedral, ranging from 1 to 100 nm. |
| Virus-Like Nanoparticles | Nanomedicine modified by natural virus, synthetic virus-like NPs, etc. |
| Similar to the geometry of a virus, usually spherical or polyhedral, in the range of 20 to 200 nm. |
| Extracellular Vesicle Nanoparticles | Exosomes, microvesicles, etc. |
| Usually spherical or elliptical, in the range of 30 to 1000 nm. |
| Common Combination Mode | Classification | Mechanism and Characteristics |
|---|---|---|
| Combination of PTT with Chemotherapy | Promote drug uptake and accumulation | PTT can enhance tumor vascular permeability, improve drug uptake, and promote the accumulation and release of drugs at the tumor site, thus improving the efficacy of anti-tumor therapy. |
| Synergy of anti-tumor effects | PTT combined with chemotherapy can improve tumor targeting, promote drug release, and produce a synergistic therapeutic effect. | |
| Enhancement of the responsiveness of tumor cells to chemotherapy drugs | PTT changes the toxicity of chemotherapy drugs and enhances the effectiveness of chemotherapy by regulating temperature. PTT can also enhance the sensitivity of cancer cells to drugs by regulating DNA repair mechanisms. | |
| Overcome of multidrug resistance | By destroying mitochondrial function, PTT inhibits the production of ATP, reduces drug efflux, and improves the effectiveness of chemotherapy drugs against drug-resistant cancer cells. | |
| Combination of PTT with Immunotherapy | PTT in combination with immune adjuvants | The therapy has the potential to enhance anti-tumor immunity, induce apoptosis of tumor cells, alleviate immunosuppression, and prevent recurrence and metastasis. |
| PTT in combination with immune checkpoint inhibitors | The therapy enhances tumor targeting, immune response, and anti-tumor efficacy, and has shown promising results in preclinical studies and early clinical trials of advanced solid tumors. | |
| Combination of PTT with RT | Increase of radiosensitivity | PTT-induced hyperthermia can improve the sensitivity of tumor cells to radiation by inhibiting DNA repair mechanisms and increasing oxidative stress. |
| Overcoming of radioresistance | PTT leads to protein denaturation and membrane destruction, while RT induces DNA damage. The combination of the two can help overcome resistance mechanisms and improve the overall therapeutic effect. | |
| Development of some advanced nanoplatforms | This advanced nanoplatform has shown significant synergistic enhancement in PTT combined with RT sensitization, enhancing the efficacy of cancer treatment, and preclinical studies have demonstrated its potential safety and efficacy. | |
| Conbination of PTT with PDT or Sonodynamic Therapy(SDT) | Combination of PTT with PDT | PDT enhances the sensitivity of tumor cells to PTT by modulating the TME, while the heat generated by PTT can stimulate blood flow, improve oxygen delivery, and amplify the therapeutic effects of PDT. This combined effect promotes tumor cell death through both heat and ROS. |
| Conbination of PTT with SDT | Low frequency ultrasound stimulates the acoustic sensitizers gathered in the tumor to produce reactive oxygen species to kill tumor cells. | |
| Combination of PTT with Gene Therapy | PTT can break the modified gene vector, promote the regulation of gene release and expression, and exert the therapeutic effect of PTT. | |
| Combination of Chemodynamic Therapy (CDT) with PTT | PTT improves the efficiency of the Fenton reaction by increasing the temperature of the TME, and collaborates with CDT for anti-tumor. | |
| Combination of PTT with Other Therapies | PTT combined with gas therapy | Gas therapy can enhance the efficacy of PTT at low temperatures, inhibit the proliferation of tumor cells, and achieve synergistic effects. |
| PTT combined with hunger therapy | Starvation therapy limits the glucose supply to tumors, and the combination with PTT can not only “starve” tumor cells but also enhance the therapeutic effect through the thermal effect. | |
| Multi-Modal Therapy Based on PTT | PTT combined with PDT and chemotherapy | The synergies of PTT and other therapies work together through multiple mechanisms to enhance treatment effectiveness, reduce drug resistance, and minimize side effects |
| PTT combined with PDT and RT | ||
| PTT combined with CDT and chemotherapy | ||
| PTT combined with gene therapy and Immunotherapy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, R.; Wang, S.; Guo, Q.; Zhong, R.; Chen, X.; Xia, X. Anti-Tumor Strategies of Photothermal Therapy Combined with Other Therapies Using Nanoplatforms. Pharmaceutics 2025, 17, 306. https://doi.org/10.3390/pharmaceutics17030306
Xu R, Wang S, Guo Q, Zhong R, Chen X, Xia X. Anti-Tumor Strategies of Photothermal Therapy Combined with Other Therapies Using Nanoplatforms. Pharmaceutics. 2025; 17(3):306. https://doi.org/10.3390/pharmaceutics17030306
Chicago/Turabian StyleXu, Rubing, Shengmei Wang, Qiuyan Guo, Ruqian Zhong, Xi Chen, and Xinhua Xia. 2025. "Anti-Tumor Strategies of Photothermal Therapy Combined with Other Therapies Using Nanoplatforms" Pharmaceutics 17, no. 3: 306. https://doi.org/10.3390/pharmaceutics17030306
APA StyleXu, R., Wang, S., Guo, Q., Zhong, R., Chen, X., & Xia, X. (2025). Anti-Tumor Strategies of Photothermal Therapy Combined with Other Therapies Using Nanoplatforms. Pharmaceutics, 17(3), 306. https://doi.org/10.3390/pharmaceutics17030306







