Azithromycin-Loaded Nanoparticles Incorporated in Chitosan-Based Soft Hydrogels: A Novel Approach for Dental Drug Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of AZC-Based Nanoparticles
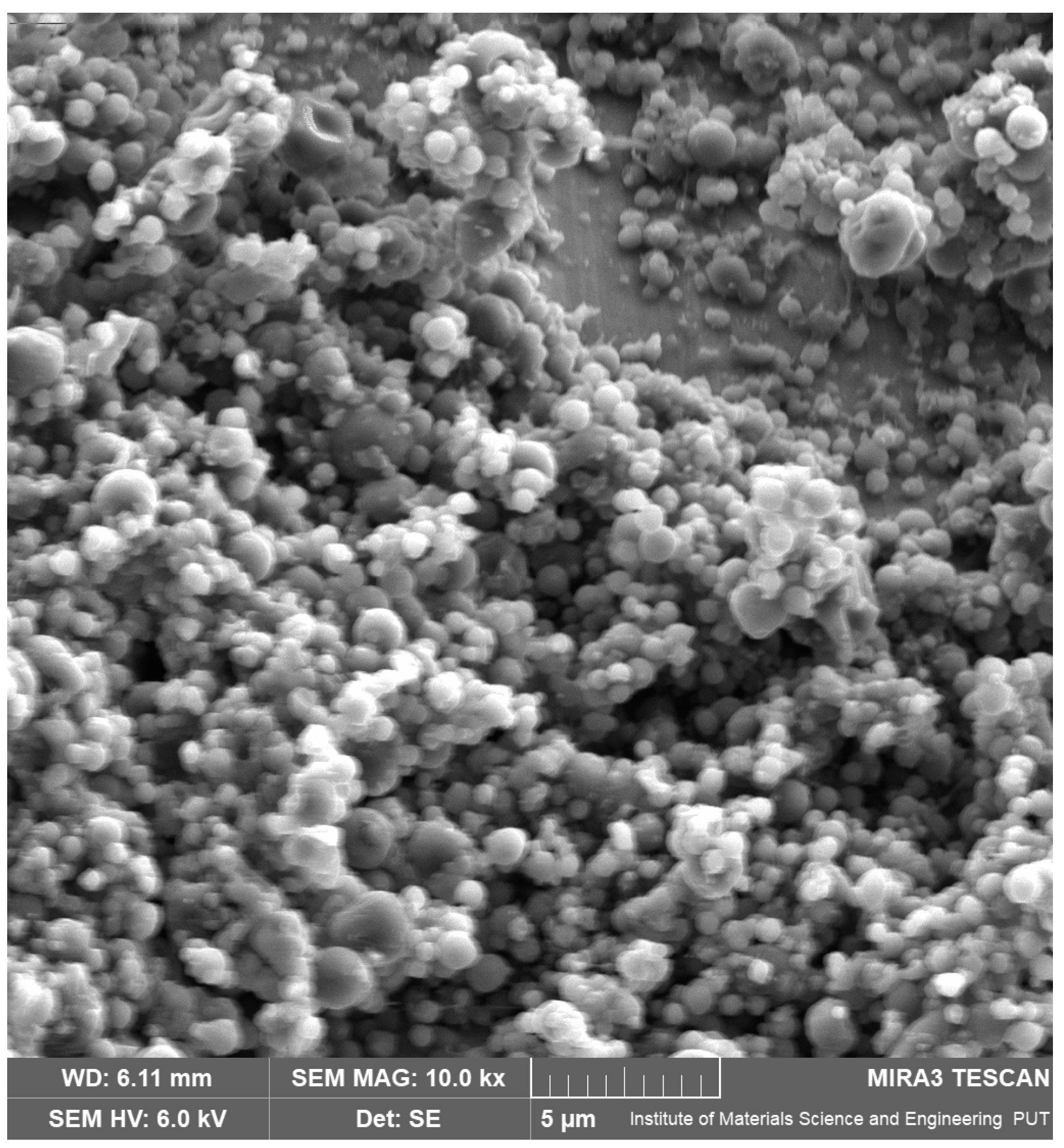
2.2.1. Scanning Electron Microscopy (SEM)
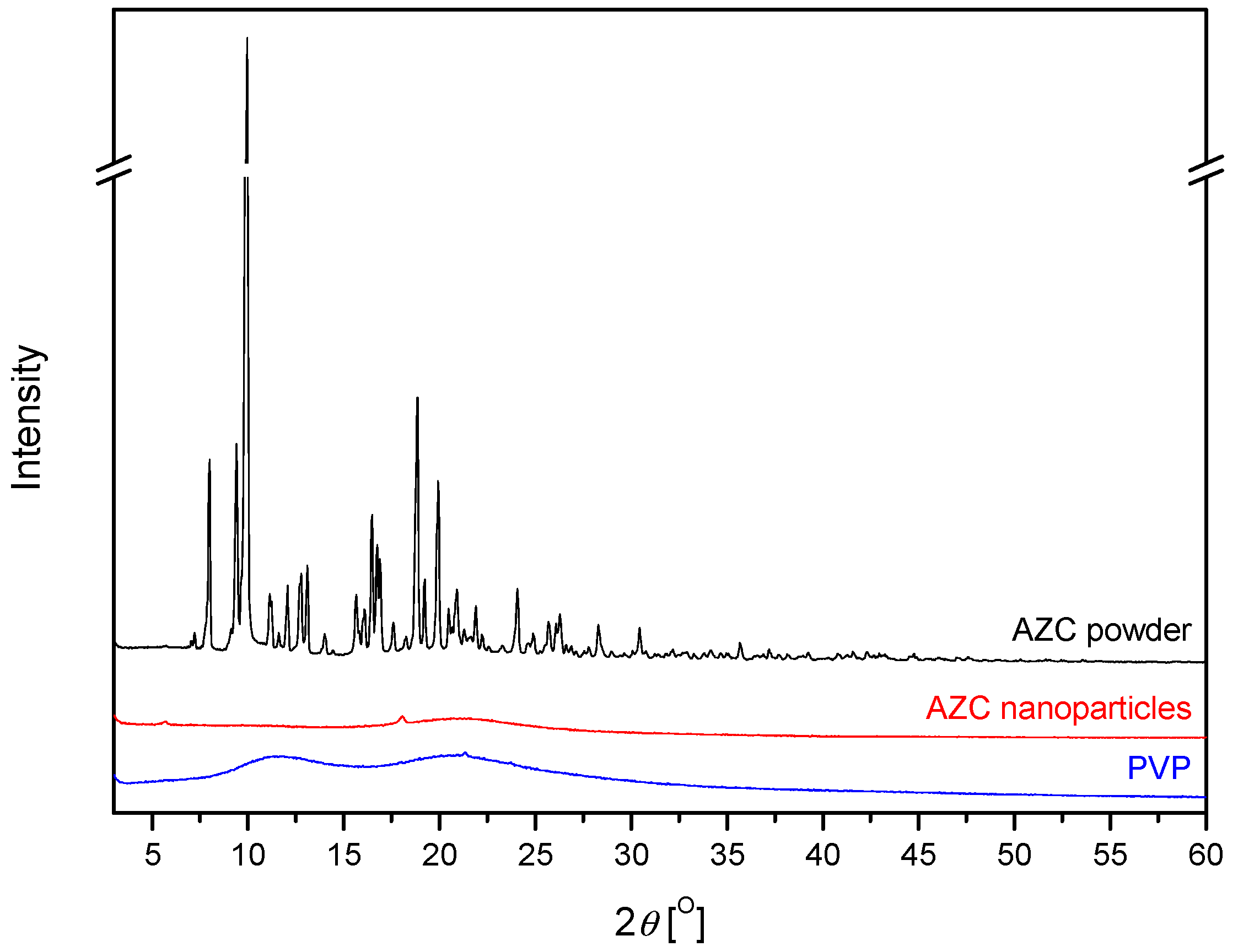
2.2.2. X-Ray Powder Diffraction (XRPD)
2.2.3. Solubility Studies
2.3. Preparation of AZC-Based Soft Hydrogels
2.4. Characteristics of Soft Hydrogels
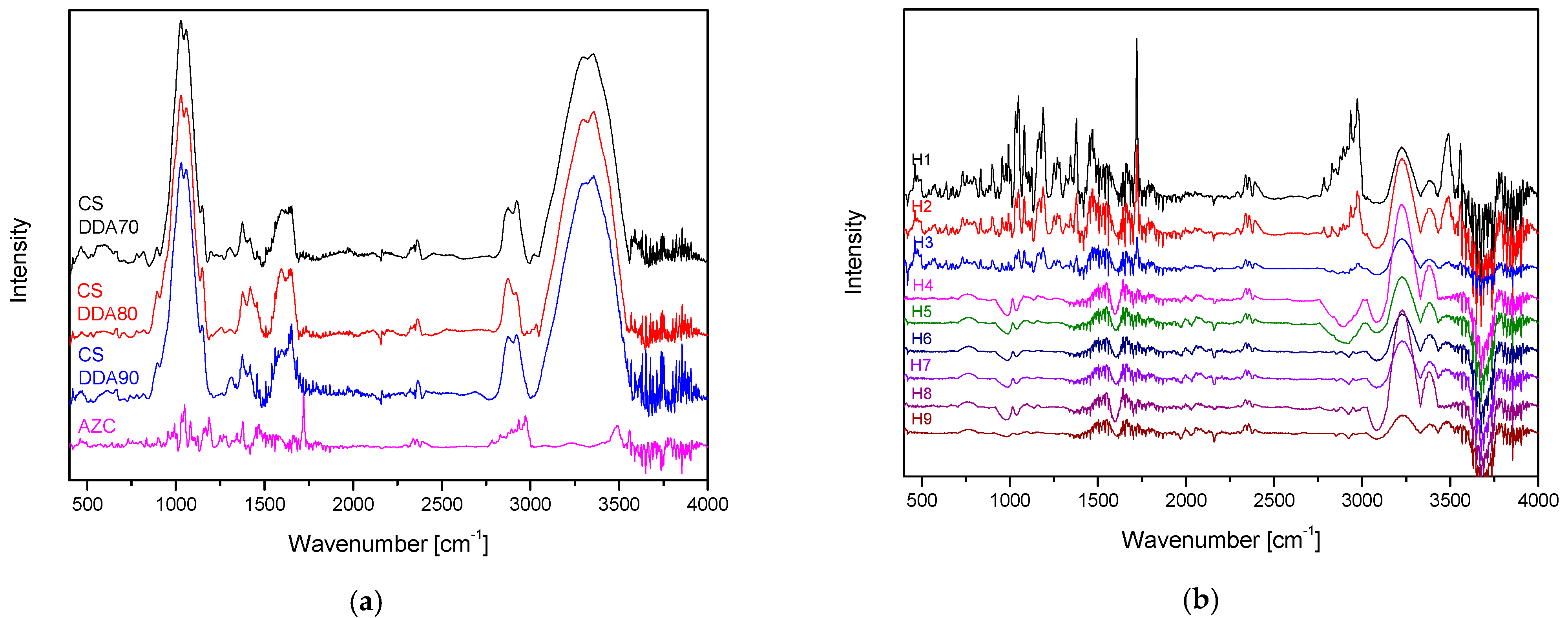
2.4.1. Fourier Transform Infrared Spectroscopy with Attenuated Total Reflectance (ATR-FTIR)
2.4.2. Rheology
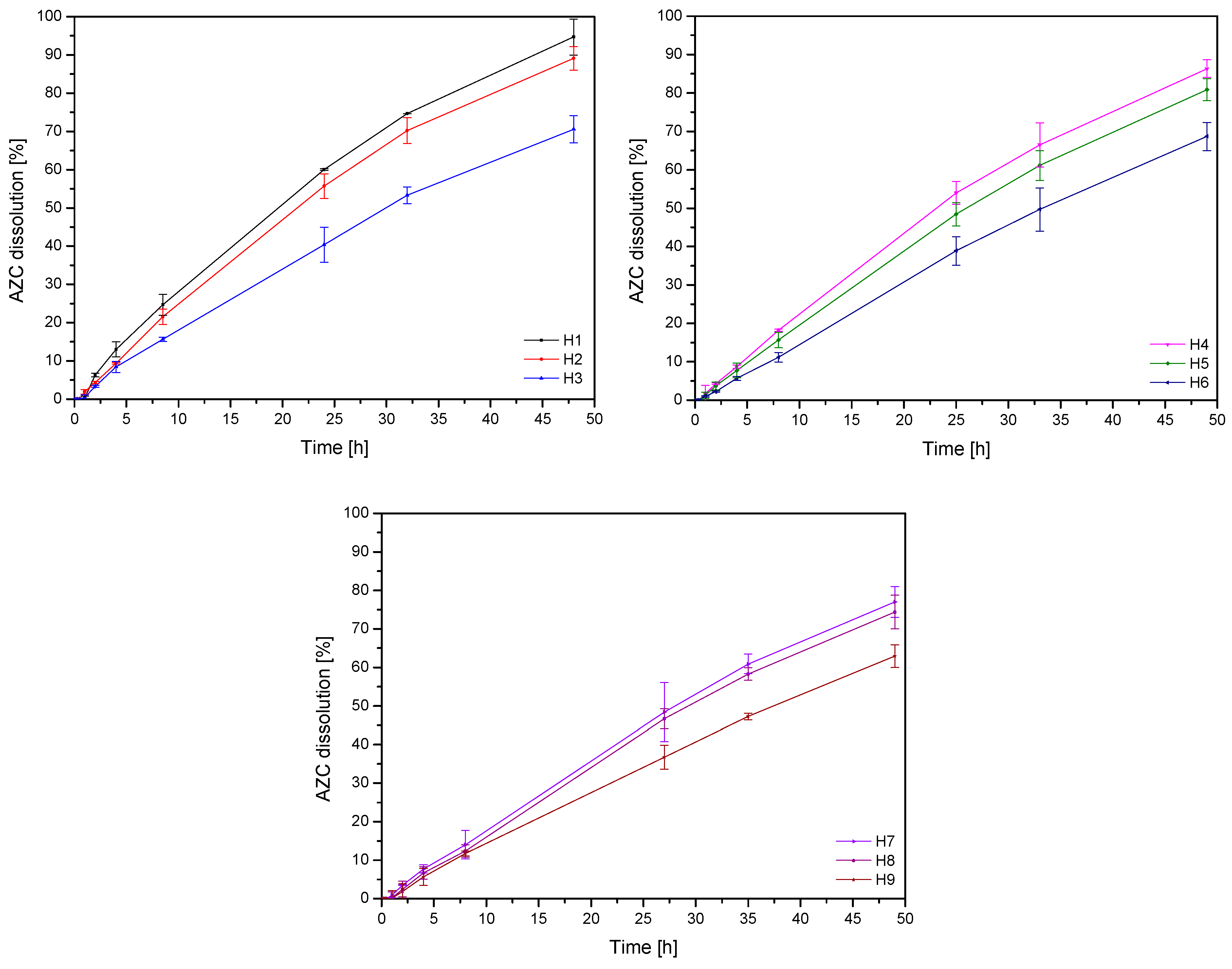
2.4.3. Diffusion Tests
2.4.4. Biological Activity
2.4.5. Microbiological Activity
2.4.6. Mucoadhesive Properties
2.5. Statistical Analysis
3. Results and Discussion
3.1. Preparation of AZC-Based Nanoparticles
3.2. Preparation of AZC-Based Soft Hydrogels
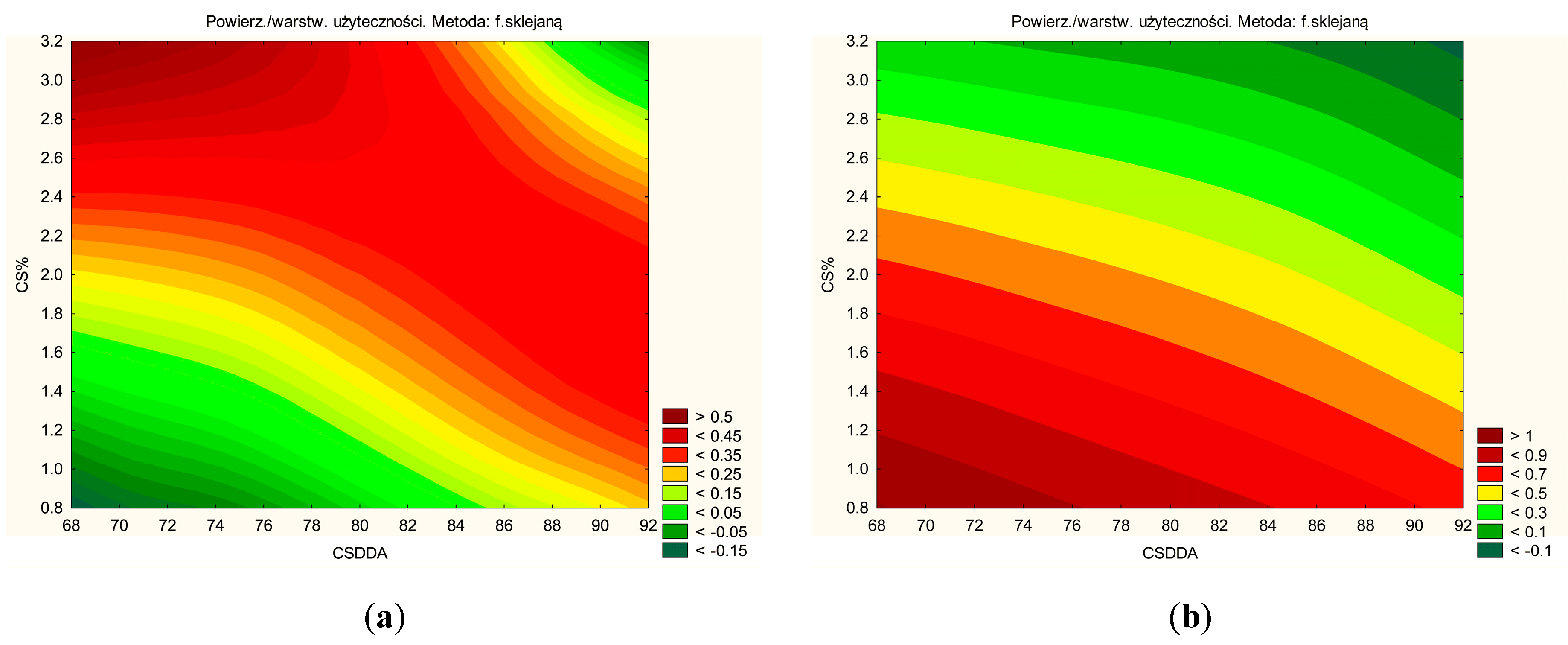
3.3. Characteristics of Soft Hydrogels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Addy, L.D.; Martin, M.V. Azithromycin and Dentistry—A Useful Agent? Br. Dent. J. 2004, 197, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, R. Periodontal Healing and Bone Regeneration in Response to Azithromycin. Aust. Dent. J. 2010, 55, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, M.; Somville, P.; Vertzoni, M.; Fotaki, N. Investigating the Critical Variables of Azithromycin Oral Absorption Using In Vitro Tests and PBPK Modeling. J. Pharm. Sci. 2021, 110, 3874–3888. [Google Scholar] [CrossRef]
- Firth, A.; Prathapan, P. Azithromycin: The First Broad-Spectrum Therapeutic. Eur. J. Med. Chem. 2020, 207, 112739. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Shah, J.; Sreeharsha, N.; Gupta, S.; Shinu, P. Emerging Role of Hydrogels in Drug Delivery Systems, Tissue Engineering and Wound Management. Pharmaceutics 2021, 13, 357. [Google Scholar] [CrossRef] [PubMed]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-Based Hydrogels Applied in Drug Delivery: An Overview. Gels 2023, 9, 523. [Google Scholar] [CrossRef]
- Bramhe, P.; Rarokar, N.; Kumbhalkar, R.; Saoji, S.; Khedekar, P. Natural and Synthetic Polymeric Hydrogel: A Bioink for 3D Bioprinting of Tissue Models. J. Drug Deliv. Sci. Technol. 2024, 101, 106204. [Google Scholar] [CrossRef]
- Chen, A.; Deng, S.; Lai, J.; Li, J.; Chen, W.; Varma, S.N.; Zhang, J.; Lei, C.; Liu, C.; Huang, L. Hydrogels for Oral Tissue Engineering: Challenges and Opportunities. Molecules 2023, 28, 3946. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef] [PubMed]
- Raghunath, I.; Koland, M.; Sarathchandran, C.; Saoji, S.; Rarokar, N. Design and Optimization of Chitosan-Coated Solid Lipid Nanoparticles Containing Insulin for Improved Intestinal Permeability Using Piperine. Int. J. Biol. Macromol. 2024, 280, 135849. [Google Scholar] [CrossRef] [PubMed]
- Paczkowska-Walendowska, M.; Cielecka-Piontek, J. Chitosan as a Functional Carrier for the Local Delivery Anti-Inflammatory Systems Containing Scutellariae Baicalensis Radix Extract. Pharmaceutics 2022, 14, 2148. [Google Scholar] [CrossRef]
- Arora, S.; Das, G.; Alqarni, M.; Grover, V.; Manzoor Baba, S.; Saluja, P.; Hassan, S.A.B.; Abdulla, A.M.; Bavabeedu, S.S.; Abullais, S.S.; et al. Role of Chitosan Hydrogels in Clinical Dentistry. Gels 2023, 9, 698. [Google Scholar] [CrossRef] [PubMed]
- Valenciana-Solís, J.A.; Gaitán-Fonseca, C.; Martínez-Castañón, G.A.; Zavala-Alonso, V.; Bermúdez-Jiménez, C.; Valenciana-Solís, J.A.; Gaitán-Fonseca, C.; Martínez-Castañón, G.A.; Zavala-Alonso, V.; Bermúdez-Jiménez, C. Uses of Chitosan-Based Hydrogels in Dentistry: A Systematic Review. Odovtos Int. J. Dent. Sci. 2024, 26, 99–112. [Google Scholar] [CrossRef]
- Reis-Prado, A.H.d.; Rahimnejad, M.; Dal-Fabbro, R.; Toledo, P.T.A.d.; Anselmi, C.; Oliveira, P.H.C.d.; Fenno, J.C.; Cintra, L.T.A.; Benetti, F.; Bottino, M.C. Injectable Thermosensitive Antibiotic-Laden Chitosan Hydrogel for Regenerative Endodontics. Bioact. Mater. 2025, 46, 406–422. [Google Scholar] [CrossRef] [PubMed]
- Kerdmanee, K.; Phaechamud, T.; Limsitthichaikoon, S. Thermoresponsive Azithromycin-Loaded Niosome Gel Based on Poloxamer 407 and Hyaluronic Interactions for Periodontitis Treatment. Pharmaceutics 2022, 14, 2032. [Google Scholar] [CrossRef]
- Ayoub, A.A.; Mahmoud, A.H.; Ribeiro, J.S.; Daghrery, A.; Xu, J.; Fenno, J.C.; Schwendeman, A.; Sasaki, H.; Dal-Fabbro, R.; Bottino, M.C. Electrospun Azithromycin-Laden Gelatin Methacryloyl Fibers for Endodontic Infection Control. Int. J. Mol. Sci. 2022, 23, 13761. [Google Scholar] [CrossRef] [PubMed]
- Abruzzo, A.; Pucci, R.; Abruzzo, P.M.; Canaider, S.; Parolin, C.; Vitali, B.; Valle, F.; Brucale, M.; Cerchiara, T.; Luppi, B.; et al. Azithromycin-Loaded Liposomes and Niosomes for the Treatment of Skin Infections: Influence of Excipients and Preparative Methods on the Functional Properties. Eur. J. Pharm. Biopharm. 2024, 197, 114233. [Google Scholar] [CrossRef]
- Hao, M.; Wang, D.; Duan, M.; Kan, S.; Li, S.; Wu, H.; Xiang, J.; Liu, W. Functional Drug-Delivery Hydrogels for Oral and Maxillofacial Wound Healing. Front. Bioeng. Biotechnol. 2023, 11, 1241660. [Google Scholar] [CrossRef]
- Amato, M.; Santonocito, S.; Polizzi, A.; Tartaglia, G.M.; Ronsivalle, V.; Viglianisi, G.; Grippaudo, C.; Isola, G. Local Delivery and Controlled Release Drugs Systems: A New Approach for the Clinical Treatment of Periodontitis Therapy. Pharmaceutics 2023, 15, 1312. [Google Scholar] [CrossRef] [PubMed]
- Franco, P.; De Marco, I. The Use of Poly(N-Vinyl Pyrrolidone) in the Delivery of Drugs: A Review. Polymers 2020, 12, 1114. [Google Scholar] [CrossRef] [PubMed]
- Paczkowska-Walendowska, M.; Gościniak, A.; Szymanowska, D.; Szwajgier, D.; Baranowska-Wójcik, E.; Szulc, P.; Dreczka, D.; Simon, M.; Cielecka-Piontek, J. Blackberry Leaves as New Functional Food? Screening Antioxidant, Anti-Inflammatory and Microbiological Activities in Correlation with Phytochemical Analysis. Antioxidants 2021, 10, 1945. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, T.M.; Ożarowski, M.; Seremak-Mrozikiewicz, A.; Wolski, H. Anti-Candida and Antibiofilm Activity of Selected Lamiaceae Essential Oils. FBL 2023, 28, 28. [Google Scholar] [CrossRef]
- Paczkowska-Walendowska, M.; Szymańska, E.; Winnicka, K.; Szwajgier, D.; Baranowska-Wójcik, E.; Ruchała, M.A.; Simon, M.; Cielecka-Piontek, J. Cyclodextrin as Functional Carrier in Development of Mucoadhesive Tablets Containing Polygoni Cuspidati Extract with Potential for Dental Applications. Pharmaceutics 2021, 13, 1916. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.A.; Zahoor, M.; Islam, N.U.; Hameed, R. Synthesis of Cefixime and Azithromycin Nanoparticles: An Attempt to Enhance Their Antimicrobial Activity and Dissolution Rate. J. Nanomater. 2016, 2016, 6909085. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Pasha, I.; Verma, A.; Kothapalli, R.; Jafar, F.; Hr, K. Formulation and Evaluation of Liquisolid Compact of Azithromycin Dihydrate. J. Res. Pharm. 2019, 23, 1022–1032. [Google Scholar] [CrossRef]
- Arora, S.C.; Sharma, P.K.; Irchhaiya, R.; Khatkar, A.; Singh, N.; Gagoria, J. Development, Characterization and Solubility Study of Solid Dispersions of Azithromycin Dihydrate by Solvent Evaporation Method. J. Adv. Pharm. Technol. Res. 2010, 1, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Sobika, M.; Paul Raj, E.; Krishnamoorthy, S.; Dash, S. Development and Evaluation of Azithromycin Dihydrate in Single and Binary Micellar Mediums. Chem. Phys. Impact 2023, 6, 100229. [Google Scholar] [CrossRef]
- Aucamp, M.; Odendaal, R.; Liebenberg, W.; Hamman, J. Amorphous Azithromycin with Improved Aqueous Solubility and Intestinal Membrane Permeability. Drug Dev. Ind. Pharm. 2015, 41, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Witkowska, K.; Paczkowska-Walendowska, M.; Plech, T.; Szymanowska, D.; Michniak-Kohn, B.; Cielecka-Piontek, J. Chitosan-Based Hydrogels for Controlled Delivery of Asiaticoside-Rich Centella Asiatica Extracts with Wound Healing Potential. Int. J. Mol. Sci. 2023, 24, 17229. [Google Scholar] [CrossRef] [PubMed]
- Robaina, N.F.; de Paula, C.E.R.; Brum, D.M.; de la Guardia, M.; Garrigues, S.; Cassella, R.J. Novel Approach for the Determination of Azithromycin in Pharmaceutical Formulations by Fourier Transform Infrared Spectroscopy in Film-through Transmission Mode. Microchem. J. 2013, 110, 301–307. [Google Scholar] [CrossRef]
- Rył, A.; Owczarz, P. Injectability of Thermosensitive, Low-Concentrated Chitosan Colloids as Flow Phenomenon through the Capillary under High Shear Rate Conditions. Polymers 2020, 12, 2260. [Google Scholar] [CrossRef] [PubMed]
- Hyun, K.; Kim, S.H.; Ahn, K.H.; Lee, S.J. Large Amplitude Oscillatory Shear as a Way to Classify the Complex Fluids. J. Non-Newton. Fluid Mech. 2002, 107, 51–65. [Google Scholar] [CrossRef]
- Kasapis, S.; Mitchell, J.; Abeysekera, R.; MacNaughtan, W. Rubber-to-Glass Transitions in High Sugar/Biopolymer Mixtures. Trends Food Sci. Technol. 2004, 15, 298–304. [Google Scholar] [CrossRef]
- Yu, Y.; Chow, D.W.Y.; Lau, C.M.L.; Zhou, G.; Back, W.; Xu, J.; Carim, S.; Chau, Y. A Bioinspired Synthetic Soft Hydrogel for the Treatment of Dry Eye. Bioeng. Transl. Med. 2021, 6, e10227. [Google Scholar] [CrossRef] [PubMed]
- Ghaffarkhah, A.; Hashemi, S.A.; Isari, A.A.; Panahi-Sarmad, M.; Jiang, F.; Russell, T.P.; Rojas, O.J.; Arjmand, M. Chemistry, Applications, and Future Prospects of Structured Liquids. Chem. Soc. Rev. 2024, 53, 9652–9717. [Google Scholar] [CrossRef] [PubMed]
- Chenite, A.; Buschmann, M.; Wang, D.; Chaput, C.; Kandani, N. Rheological Characterisation of Thermogelling Chitosan/Glycerol-Phosphate Solutions. Carbohydr. Polym. 2001, 46, 39–47. [Google Scholar] [CrossRef]
- Uddin, M.S.; Khand, S.; Dong, C. Effect of Crosslinking Agents on Chitosan Hydrogel Carriers for Drug Loading and Release for Targeted Drug Delivery. Gels 2024, 10, 421. [Google Scholar] [CrossRef]
- Hong, F.; Qiu, P.; Wang, Y.; Ren, P.; Liu, J.; Zhao, J.; Gou, D. Chitosan-Based Hydrogels: From Preparation to Applications, a Review. Food Chem. X 2024, 21, 101095. [Google Scholar] [CrossRef] [PubMed]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Muchtaridi, M. Drug Release Study of the Chitosan-Based Nanoparticles. Heliyon 2022, 8, e08674. [Google Scholar] [CrossRef]
- Desai, N.; Rana, D.; Salave, S.; Gupta, R.; Patel, P.; Karunakaran, B.; Sharma, A.; Giri, J.; Benival, D.; Kommineni, N. Chitosan: A Potential Biopolymer in Drug Delivery and Biomedical Applications. Pharmaceutics 2023, 15, 1313. [Google Scholar] [CrossRef] [PubMed]
- Safdar, R.; Omar, A.A.; Arunagiri, A.; Regupathi, I.; Thanabalan, M. Potential of Chitosan and Its Derivatives for Controlled Drug Release Applications—A Review. J. Drug Deliv. Sci. Technol. 2019, 49, 642–659. [Google Scholar] [CrossRef]
- Luan, F.; Wei, L.; Zhang, J.; Mi, Y.; Dong, F.; Li, Q.; Guo, Z. Antioxidant Activity and Antifungal Activity of Chitosan Derivatives with Propane Sulfonate Groups. Polymers 2018, 10, 395. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, H.; Yang, Z.; Duan, H.; Wang, Z.; Cheng, Z.; Song, Z.; Wu, X. Study on the Interaction of Hyaluronidase with Certain Flavonoids. J. Mol. Struct. 2021, 1241, 130686. [Google Scholar] [CrossRef]
- Pardo-Rendón, A.G.; Mejía-Méndez, J.L.; López-Mena, E.R.; Bernal-Chávez, S.A. Development and Evaluation of the Biological Activities of a Plain Mucoadhesive Hydrogel as a Potential Vehicle for Oral Mucosal Drug Delivery. Gels 2024, 10, 574. [Google Scholar] [CrossRef]
- Fraino, P.E. Using Principal Component Analysis to Explore Multi-Variable Relationships. Nat. Rev. Earth Environ. 2023, 4, 294. [Google Scholar] [CrossRef]




| No. | CS DDA | CS % |
|---|---|---|
| H1 | 70 | 1 |
| H2 | 70 | 2 |
| H3 | 70 | 3 |
| H4 | 80 | 1 |
| H5 | 80 | 2 |
| H6 | 80 | 3 |
| H7 | 90 | 1 |
| H8 | 90 | 2 |
| H9 | 90 | 3 |
| No. | n [-] | k [Pa.sn] | R2 |
|---|---|---|---|
| H1 | 0.97 | 0.37 | 0.9990 |
| H2 | 0.85 | 4.42 | 0.9933 |
| H3 | 0.72 | 21.43 | 0.9873 |
| H4 | 0.99 | 0.29 | 0.9992 |
| H5 | 0.86 | 7.04 | 0.9905 |
| H6 | 0.68 | 37.93 | 0.9854 |
| H7 | 0.98 | 0.39 | 0.9993 |
| H8 | 0.89 | 5.43 | 0.9939 |
| H9 | 0.76 | 23.97 | 0.9867 |
| No. | AZC Dissolution at 48 h [%] |
|---|---|
| H1 | 94.72 ± 4.72 a |
| H2 | 89.11 ± 3.10 a,b |
| H3 | 70.57 ± 3.56 e,f |
| H4 | 86.33 ± 2.32 b,c |
| H5 | 80.88 ± 3.87 c,d |
| H6 | 68.67 ± 3.67 f,g |
| H7 | 76.99 ± 3.98 d,e |
| H8 | 74.37 ± 4.37 d,e,f |
| H9 | 62.94 ± 2.94 g |
| Hydrogel | Mathematical Model | |||||||
|---|---|---|---|---|---|---|---|---|
| No. | Zero-Order Kinetic | First-Order Kinetic | Higuchi Kinetic | Korsmeyer-Peppas Kinetic | ||||
| K | R2 | K | R2 | K | R2 | R2 | n | |
| H1 | 2.10 | 0.98 | 0.10 | 0.67 | 9.22 | 0.77 | 0.81 | 0.57 |
| H2 | 1.98 | 0.98 | 0.09 | 0.76 | 8.58 | 0.75 | 0.93 | 0.54 |
| H3 | 1.54 | 0.99 | 0.09 | 0.74 | 6.60 | 0.75 | 0.84 | 0.52 |
| H4 | 1.91 | 0.99 | 0.09 | 0.77 | 8.21 | 0.74 | 0.92 | 0.53 |
| H5 | 1.78 | 0.99 | 0.09 | 0.79 | 7.56 | 0.73 | 0.91 | 0.52 |
| H6 | 1.49 | 0.99 | 0.09 | 0.83 | 6.23 | 0.71 | 0.88 | 0.49 |
| H7 | 1.73 | 0.99 | 0.09 | 0.79 | 7.35 | 0.73 | 0.89 | 0.52 |
| H8 | 1.68 | 0.99 | 0.10 | 0.75 | 7.06 | 0.72 | 0.78 | 0.52 |
| H9 | 1.39 | 0.99 | 0.10 | 0.72 | 5.85 | 0.72 | 0.71 | 0.51 |
| No. | Antioxidant Activity [%] | Anti-Inflammatory Activity IC50 [mg Hydrogel/mL] |
|---|---|---|
| H1 | 10.96 ± 1.25 f | 44.71 ± 3.61 a |
| H2 | 12.35 ± 1.52 e,f | 30.91 ± 2.80 b |
| H3 | 17.99 ± 2.04 b,c,d | 20.39 ± 1.92 c |
| H4 | 13.69 ± 0.80 e | 30.34 ± 3.80 b |
| H5 | 16.37 ± 1.79 d | 27.49 ± 0.97 b |
| H6 | 18.87 ± 1.14 b,c | 11.67 ± 2.20 d |
| H7 | 16.98 ± 0.64 c,d | 22.27 ± 2.82 c |
| H8 | 20.39 ± 0.50 b | 9.32 ± 0.54 d |
| H9 | 24.65 ± 1.54 a | 7.49 ± 0.67 d |
| No. | Component of Bioadhesion [cP] |
|---|---|
| H1 | 125.00 ± 7.07 e |
| H2 | 1100.00 ± 28.28 d |
| H3 | 3900.00 ± 254.56 c |
| H4 | 170.00 ± 14.14 e |
| H5 | 1250.00 ± 42.43 d |
| H6 | 4840.00 ± 311.13 b |
| H7 | 330.00 ± 14.14 e |
| H8 | 1300.00 ± 28.28 d |
| H9 | 6260.00 ± 28.28 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwiatek, J.; Paczkowska-Walendowska, M.; Rył, A.; Karpiński, T.M.; Miklaszewski, A.; Swora-Cwynar, E.; Leśna, M.; Cielecka-Piontek, J. Azithromycin-Loaded Nanoparticles Incorporated in Chitosan-Based Soft Hydrogels: A Novel Approach for Dental Drug Delivery. Pharmaceutics 2025, 17, 304. https://doi.org/10.3390/pharmaceutics17030304
Kwiatek J, Paczkowska-Walendowska M, Rył A, Karpiński TM, Miklaszewski A, Swora-Cwynar E, Leśna M, Cielecka-Piontek J. Azithromycin-Loaded Nanoparticles Incorporated in Chitosan-Based Soft Hydrogels: A Novel Approach for Dental Drug Delivery. Pharmaceutics. 2025; 17(3):304. https://doi.org/10.3390/pharmaceutics17030304
Chicago/Turabian StyleKwiatek, Jakub, Magdalena Paczkowska-Walendowska, Anna Rył, Tomasz M. Karpiński, Andrzej Miklaszewski, Ewelina Swora-Cwynar, Marta Leśna, and Judyta Cielecka-Piontek. 2025. "Azithromycin-Loaded Nanoparticles Incorporated in Chitosan-Based Soft Hydrogels: A Novel Approach for Dental Drug Delivery" Pharmaceutics 17, no. 3: 304. https://doi.org/10.3390/pharmaceutics17030304
APA StyleKwiatek, J., Paczkowska-Walendowska, M., Rył, A., Karpiński, T. M., Miklaszewski, A., Swora-Cwynar, E., Leśna, M., & Cielecka-Piontek, J. (2025). Azithromycin-Loaded Nanoparticles Incorporated in Chitosan-Based Soft Hydrogels: A Novel Approach for Dental Drug Delivery. Pharmaceutics, 17(3), 304. https://doi.org/10.3390/pharmaceutics17030304











