A Quantitative Approach to Potency Testing for Chimeric Antigen Receptor-Encoding Lentiviral Vectors and Autologous CAR-T Cell Products, Using Flow Cytometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines, Patient Samples and Culture Conditions
2.2. Antibodies and Flow Cytometry
2.3. Jurkat Cell Transduction
2.4. Co-Culture (E:T Cells) Experiments Using Jurkat Cells
2.5. Co-Culture (E:T Cells) Experiments Using Primary T Cells
2.6. IL-2 and IFNγ ELISA
2.7. Statistics
3. Results
3.1. Selection of a Potency Assay for ARI-0001 LV Vector
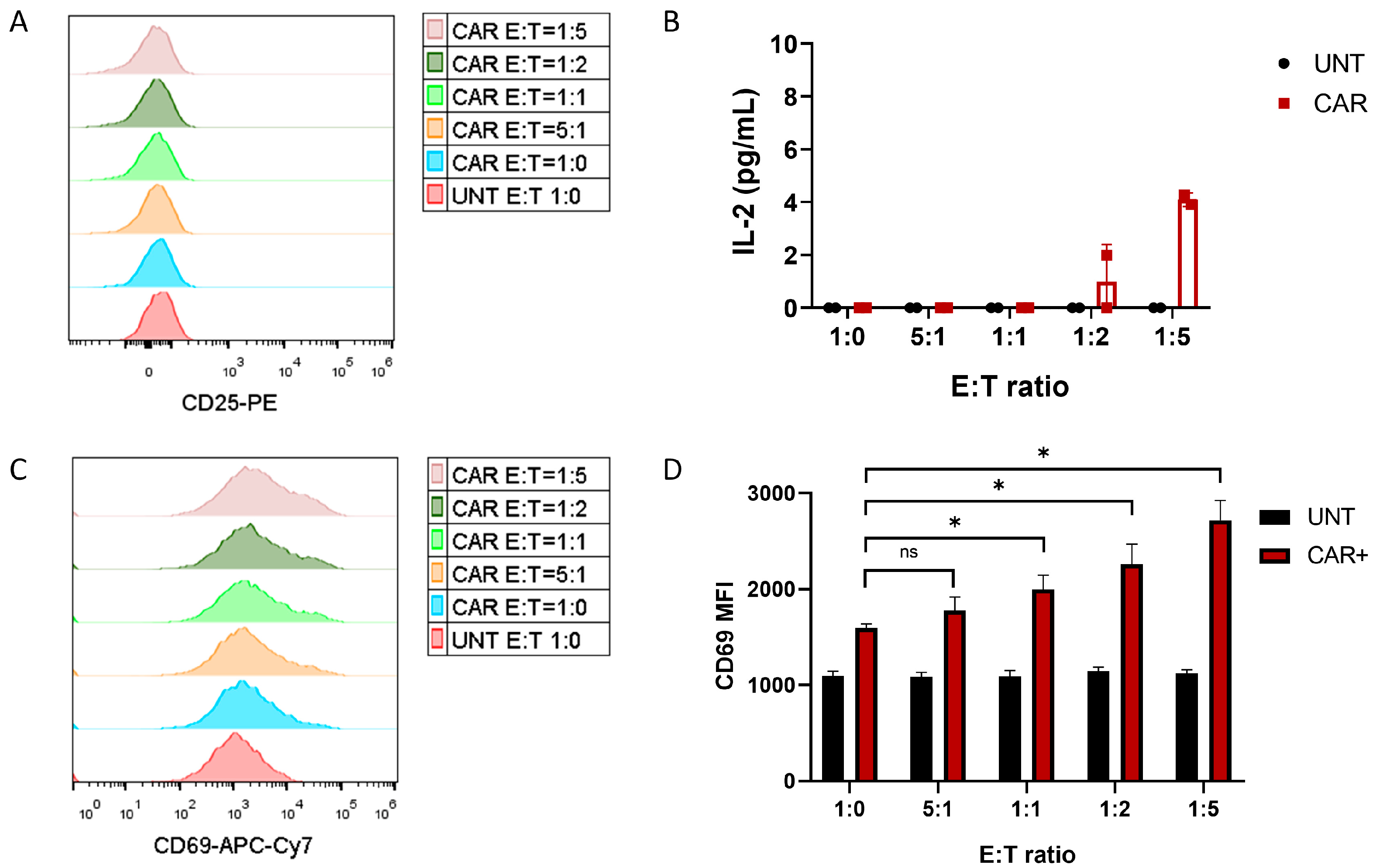
3.2. Development of CD69-Based Potency as a Quality Control-Suitable Assay
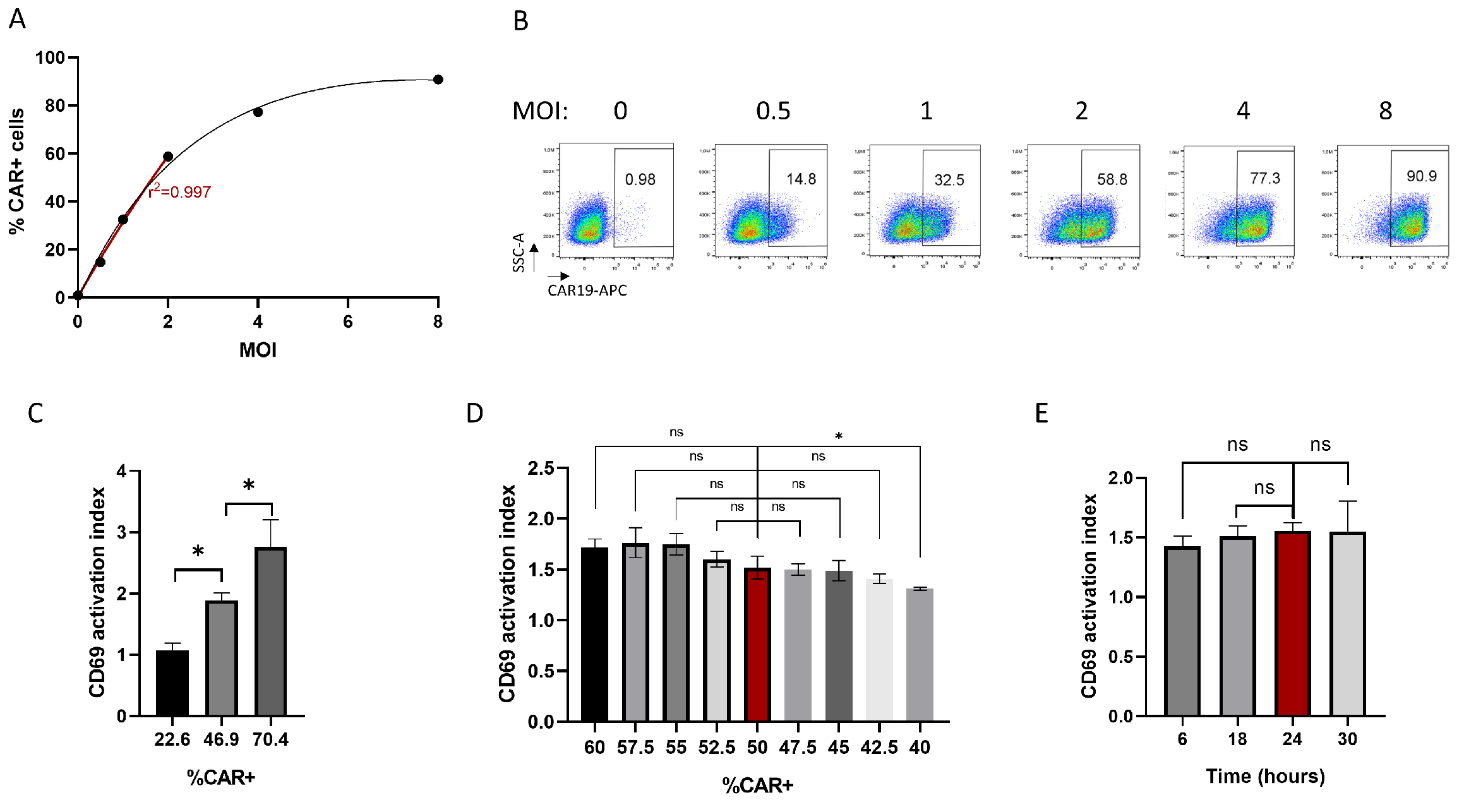
3.3. CD69-Based Potency Assay Optimization
3.4. Assay Validation
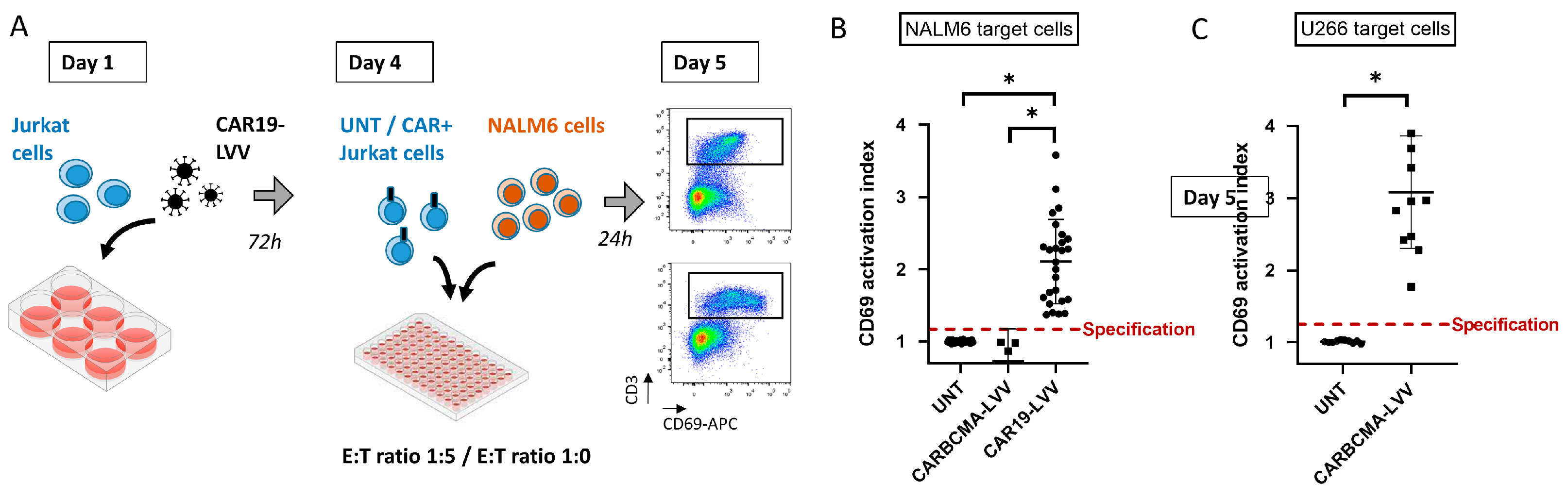
3.5. CD69-Based Potency Assay Adaptation to Other Antigen-Targeting CAR-LVV
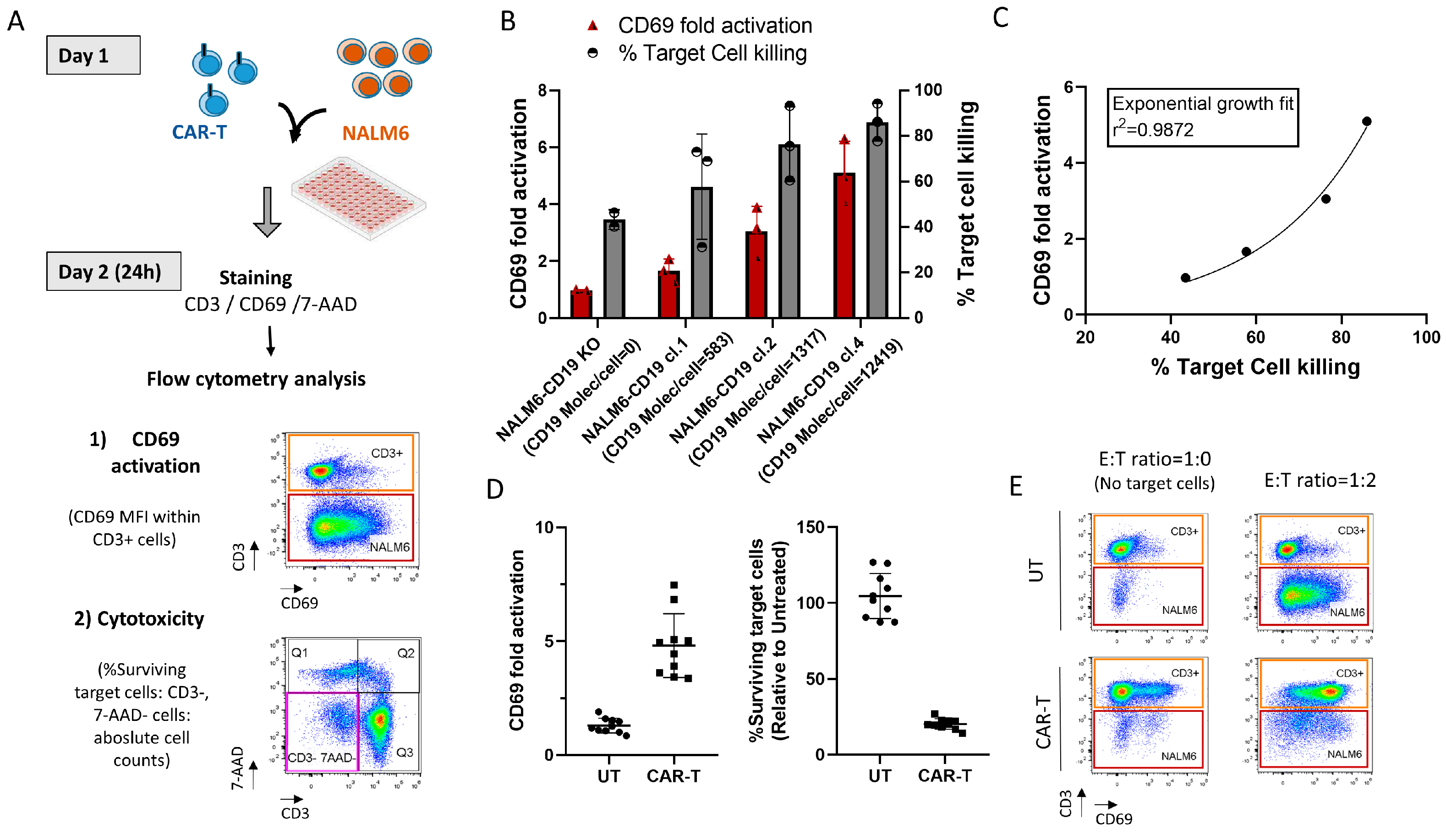
3.6. Correlation of CD69-Based Potency Assay and CAR-T Cell Cytotoxicity
3.7. CD69 Activation Assay in the Context of Autologous CAR-T Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, Z.; Anselmo, A.C.; Mitragotri, S. Viral Vector-Based Gene Therapies in the Clinic. Bioeng. Transl. Med. 2022, 7, e10258. [Google Scholar] [CrossRef] [PubMed]
- Lundstrom, K. Viral Vectors in Gene Therapy: Where Do We Stand in 2023? Viruses 2023, 15, 698. [Google Scholar] [CrossRef] [PubMed]
- Bulcha, J.T.; Wang, Y.; Ma, H.; Tai, P.W.L.; Gao, G. Viral Vector Platforms within the Gene Therapy Landscape. Signal Transduct. Target. Ther. 2021, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Torggler, R.; Margreiter, E.; Marksteiner, R.; Thurner, M. Potency Assay Development: A Keystone for Clinical Use. In Potency Assays for Advanced Stem Cell Therapy Medicinal Products; Burns, J.S., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 13–28. ISBN 978-3-031-30040-0. [Google Scholar]
- European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP) Guideline on Potency Testing of Cell Based Immunotherapy Medicinal Products for the Treatment of Cancer 3Rs Technical Update*; 2006. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-potency-testing-cell-based-immunotherapy-medicinal-products-treatment-cancer-revision-1_en.pdf (accessed on 21 February 2025).
- Gill, S.; June, C.H. Going Viral: Chimeric Antigen Receptor T-Cell Therapy for Hematological Malignancies. Immunol. Rev. 2015, 263, 68–89. [Google Scholar] [CrossRef] [PubMed]
- Barrett, D.M.; Singh, N.; Porter, D.L.; Grupp, S.A.; June, C.H. Chimeric Antigen Receptor Therapy for Cancer. Annu. Rev. Med. 2014, 65, 333–347. [Google Scholar] [CrossRef]
- Dai, H.; Wang, Y.; Lu, X.; Han, W. Chimeric Antigen Receptors Modified T-Cells for Cancer Therapy. JNCI J. Natl. Cancer Inst. 2016, 108, 439. [Google Scholar] [CrossRef]
- Sadelain, M. CAR Therapy: The CD19 Paradigm. J. Clin. Investig. 2015, 125, 3392–3400. [Google Scholar] [CrossRef]
- Brentjens, R.J.; Riviere, I.; Park, J.H.; Davila, M.L.; Wang, X.; Stefanski, J.; Taylor, C.; Yeh, R.; Bartido, S.; Borquez-Ojeda, O.; et al. Safety and Persistence of Adoptively Transferred Autologous CD19-Targeted T Cells in Patients with Relapsed or Chemotherapy Refractory B-Cell Leukemias. Blood 2011, 118, 4817–4828. [Google Scholar] [CrossRef]
- Wang, X.; Popplewell, L.L.; Wagner, J.R.; Naranjo, A.; Blanchard, M.S.; Mott, M.R.; Norris, A.P.; Wong, C.W.; Urak, R.Z.; Chang, W.-C.; et al. Phase 1 Studies of Central Memory-Derived CD19 CAR T-Cell Therapy Following Autologous HSCT in Patients with B-Cell NHL. Blood 2016, 127, 2980–2990. [Google Scholar] [CrossRef]
- Lee, D.W.; Kochenderfer, J.N.; Stetler-Stevenson, M.; Cui, Y.K.; Delbrook, C.; Feldman, S.A.; Fry, T.J.; Orentas, R.; Sabatino, M.; Shah, N.N.; et al. T Cells Expressing CD19 Chimeric Antigen Receptors for Acute Lymphoblastic Leukaemia in Children and Young Adults: A Phase 1 Dose-Escalation Trial. Lancet 2015, 385, 517–528. [Google Scholar] [CrossRef]
- Maude, S.L.; Frey, N.; Shaw, P.A.; Aplenc, R.; Barrett, D.M.; Bunin, N.J.; Chew, A.; Gonzalez, V.E.; Zheng, Z.; Lacey, S.F.; et al. Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia. N. Engl. J. Med. 2014, 371, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Kochenderfer, J.N.; Dudley, M.E.; Kassim, S.H.; Somerville, R.P.T.; Carpenter, R.O.; Maryalice, S.S.; Yang, J.C.; Phan, G.Q.; Hughes, M.S.; Sherry, R.M.; et al. Chemotherapy-Refractory Diffuse Large B-Cell Lymphoma and Indolent B-Cell Malignancies Can Be Effectively Treated with Autologous T Cells Expressing an Anti-CD19 Chimeric Antigen Receptor. J. Clin. Oncol. 2015, 33, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Kochenderfer, J.N.; Dudley, M.E.; Carpenter, R.O.; Kassim, S.H.; Rose, J.J.; Telford, W.G.; Hakim, F.T.; Halverson, D.C.; Fowler, D.H.; Hardy, N.M.; et al. Donor-Derived CD19-Targeted T Cells Cause Regression of Malignancy Persisting after Allogeneic Hematopoietic Stem Cell Transplantation. Blood 2013, 122, 4129–4139. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.H.; Liu, J.; Wang, B.Y.; Chen, Y.X.; Cao, X.M.; Yang, Y.; Zhang, Y.L.; Wang, F.X.; Zhang, P.Y.; Lei, B.; et al. A Phase 1, Open-Label Study of LCAR-B38M, a Chimeric Antigen Receptor T Cell Therapy Directed against B Cell Maturation Antigen, in Patients with Relapsed or Refractory Multiple Myeloma. J. Hematol. Oncol. 2018, 11, 141. [Google Scholar] [CrossRef] [PubMed]
- Newick, K.; O’Brien, S.; Moon, E.; Albelda, S.M. CAR T Cell Therapy for Solid Tumors. Annu. Rev. Med. 2017, 68, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.B.; Brudno, J.N.; Borie, D.; Kochenderfer, J.N. Chimeric Antigen Receptor T Cell Therapy for Autoimmune Disease. Nat. Rev. Immunol. 2024, 24, 830–845. [Google Scholar] [CrossRef]
- Morte-Romea, E.; Pesini, C.; Pellejero-Sagastizábal, G.; Letona-Giménez, S.; Martínez-Lostao, L.; Aranda, S.L.; Toyas, C.; Redrado, S.; Dolader-Ballesteros, E.; Arias, M.; et al. CAR Immunotherapy for the Treatment of Infectious Diseases: A Systematic Review. Front. Immunol. 2024, 15, 1289303. [Google Scholar] [CrossRef]
- Castella, M.; Caballero-Baños, M.; Ortiz-Maldonado, V.; González-Navarro, E.A.; Suñé, G.; Antoñana-Vidósola, A.; Boronat, A.; Marzal, B.; Millán, L.; Martín-Antonio, B.; et al. Point-of-Care CAR T-Cell Production (ARI-0001) Using a Closed Semi-Automatic Bioreactor: Experience from an Academic Phase i Clinical Trial. Front. Immunol. 2020, 11, 482. [Google Scholar] [CrossRef]
- Castella, M.; Boronat, A.; Martín-Ibáñez, R.; Rodríguez, V.; Suñé, G.; Caballero, M.; Marzal, B.; Pérez-Amill, L.; Martín-Antonio, B.; Castaño, J.; et al. Development of a Novel Anti-CD19 Chimeric Antigen Receptor: A Paradigm for an Affordable CAR T Cell Production at Academic Institutions. Mol. Ther. Methods Clin. Dev. 2019, 12, 134–144. [Google Scholar] [CrossRef]
- Salmikangas, P.; Carlsson, B.; Klumb, C.; Reimer, T.; Thirstrup, S. Potency Testing of Cell and Gene Therapy Products. Front. Med. 2023, 10, 1190016. [Google Scholar] [CrossRef]
- Capelli, C.; Cuofano, C.; Pavoni, C.; Frigerio, S.; Lisini, D.; Nava, S.; Quaroni, M.; Colombo, V.; Galli, F.; Bezukladova, S.; et al. Potency Assays and Biomarkers for Cell-Based Advanced Therapy Medicinal Products. Front. Immunol. 2023, 14, 1186224. [Google Scholar] [CrossRef] [PubMed]
- Kiesgen, S.; Messinger, J.C.; Chintala, N.K.; Tano, Z.; Adusumilli, P.S. Comparative Analysis of Assays to Measure CAR T-Cell-Mediated Cytotoxicity. Nat. Protoc. 2021, 16, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.G.; Bozenhardt, E.H.; Celluzzi, C.M.; Dobnik, D.; Grant, M.L.; Lakshmipathy, U.; Nebel, T.; Peltier, L.; Ratcliffe, A.; Sherley, J.L.; et al. Mechanism of Action, Potency and Efficacy: Considerations for Cell Therapies. J. Transl. Med. 2024, 22, 416. [Google Scholar] [CrossRef] [PubMed]
- Si, X.; Xiao, L.; Brown, C.E.; Wang, D. Preclinical Evaluation of CAR T Cell Function: In Vitro and In Vivo Models. Int. J. Mol. Sci. 2022, 23, 3154. [Google Scholar] [CrossRef] [PubMed]
- Taheri, F.H.; Hassani, M.; Sharifzadeh, Z.; Behdani, M.; Abdoli, S.; Sayadi, M.; Bagherzadeh, K.; Arashkia, A.; Abolhassani, M. Tuning Spacer Length Improves the Functionality of the Nanobody-Based VEGFR2 CAR T Cell. BMC Biotechnol. 2024, 24, 1. [Google Scholar] [CrossRef]
- Majzner, R.G.; Rietberg, S.P.; Sotillo, E.; Dong, R.; Vachharajani, V.T.; Labanieh, L.; Myklebust, J.H.; Kadapakkam, M.; Weber, E.W.; Tousley, A.M.; et al. Tuning the Antigen Density Requirement for Car T-Cell Activity. Cancer Discov. 2020, 10, 702–723. [Google Scholar] [CrossRef]
- Hassani, M.; Taheri, F.H.; Sharifzadeh, Z.; Arashkia, A.; Hadjati, J.; van Weerden, W.M.; Abdoli, S.; Modarressi, M.H.; Abolhassani, M. Engineered Jurkat Cells for Targeting Prostate-Specific Membrane Antigen on Prostate Cancer Cells by Nanobody-Based Chimeric Antigen Receptor. Iran. Biomed. J. 2020, 24, 81–88. [Google Scholar] [CrossRef]
- Bloemberg, D.; Nguyen, T.; MacLean, S.; Zafer, A.; Gadoury, C.; Gurnani, K.; Chattopadhyay, A.; Ash, J.; Lippens, J.; Harcus, D.; et al. A High-Throughput Method for Characterizing Novel Chimeric Antigen Receptors in Jurkat Cells. Mol. Ther. Methods Clin. Dev. 2020, 16, 238–254. [Google Scholar] [CrossRef]
- Committee for Medicinal Products for Human Use ICH Q2(R2) Guideline on Validation of Analytical Procedures. 2023. Available online: https://www.ema.europa.eu/en/ich-q2r2-validation-analytical-procedures-scientific-guideline (accessed on 21 February 2025).
- Selliah, N.; Nash, V.; Eck, S.; Green, C.; Oldaker, T.; Stewart, J.; Vitaliti, A.; Litwin, V. Flow Cytometry Method Validation Protocols. Curr. Protoc. 2023, 3, e868. [Google Scholar] [CrossRef]
- Collins, L.M.; Dziak, J.J.; Li, R. Design of Experiments With Multiple Independent Variables: A Resource Management Perspective on Complete and Reduced Factorial Designs. Psychol. Methods 2009, 14, 202–224. [Google Scholar] [CrossRef]
- Definition of Minimum Performance Requirements for Analytical Methods of GMO Testing European Network of GMO Laboratories (ENGL). 2008. Available online: https://gmo-crl.jrc.ec.europa.eu/doc/MPR%20Report%20Application%2020_10_2015.pdf (accessed on 21 February 2025).
- Little, T.A. Analytical Best Practices Establishing Acceptance Criteria for Analytical Methods. BioPharm Int. 2016, 29, 44–48. Available online: https://www.biopharminternational.com/view/establishing-acceptance-criteria-analytical-methods (accessed on 21 February 2025).
- Aronson, S.J.; Bakker, R.S.; Moenis, S.; van Dijk, R.; Bortolussi, G.; Collaud, F.; Shi, X.; Duijst, S.; ten Bloemendaal, L.; Ronzitti, G.; et al. A Quantitative In Vitro Potency Assay for Adeno-Associated Virus Vectors Encoding for the UGT1A1 Transgene. Mol. Ther. Methods Clin. Dev. 2020, 18, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Gonneau, C.; Wang, L.; Mitra-Kaushik, S.; Trampont, P.C.; Litwin, V. Progress towards Global Standardization for Quantitative Flow Cytometry. Bioanalysis 2021, 13, 1591–1595. [Google Scholar] [CrossRef] [PubMed]
- Devitt, K.A.; Oldaker, T.; Shah, K.; Illingworth, A. Summary of Validation Considerations with Real-Life Examples Using Both Qualitative and Semiquantitative Flow Cytometry Assays. Cytom. B Clin. Cytom. 2023, 104, 374–391. [Google Scholar] [CrossRef]
- Mizrahi, O.; Ish Shalom, E.; Baniyash, M.; Klieger, Y. Quantitative Flow Cytometry: Concerns and Recommendations in Clinic and Research. Cytom. B Clin. Cytom. 2018, 94, 211–218. [Google Scholar] [CrossRef]
- Lindsey, W.B.; Lowdell, M.W.; Marti, G.E.; Abbasi, F.; Zenger, V.; King, K.M.; Lamb, J.S. CD69 Expression as an Index of T-Cell Function: Assay Standardization, Validation and Use in Monitoring Immune Recovery. Cytotherapy 2007, 9, 123–132. [Google Scholar] [CrossRef]
- Harari-Steinfeld, R.; Abhinav Ayyadevara, V.S.S.; Cuevas, L.; Marincola, F.; Roh, K.H. Standardized In-Vitro Evaluation of CAR-T Cells Using Acellular Artificial Target Particles. Front. Immunol. 2022, 13, 994532. [Google Scholar] [CrossRef]
| CD69 Activation Index | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample 1 | Sample 2 | Sample 3 | ||||||||||
| Run | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 |
| Replicate 1 | 2.48 | 2.10 | 2.06 | 1.66 | 2.51 | 2.26 | 3.04 | 1.93 | 2.40 | 2.50 | 2.94 | 2.00 |
| Replicate 2 | 2.48 | 2.00 | 1.81 | 1.55 | 3.41 | 2.14 | 2.81 | 1.86 | 3.00 | 2.34 | 2.60 | 1.85 |
| Replicate 3 | 2.23 | 2.04 | 1.62 | 1.62 | 2.86 | 2.05 | 2.21 | 1.75 | 2.23 | 2.21 | 2.59 | 1.67 |
| Mean | 2.40 | 2.05 | 1.83 | 1.61 | 2.93 | 2.15 | 2.69 | 1.85 | 2.54 | 2.35 | 2.71 | 1.84 |
| SD | 0.15 | 0.05 | 0.22 | 0.06 | 0.45 | 0.11 | 0.43 | 0.09 | 0.40 | 0.15 | 0.20 | 0.16 |
| %CV | 6.09% | 2.50% | 12.25% | 3.67% | 15.48% | 4.94% | 15.82% | 4.88% | 15.84% | 6.22% | 7.24% | 8.87% |
| Mean %CV | 8.65% | |||||||||||
| CD69 Activation Index | Mean | SD | %CV | ||||
|---|---|---|---|---|---|---|---|
| Run | 1 | 2 | 3 | 4 | |||
| Conditions | Op 1-Inst 1 | Op 2-Inst 2 | Op 1-Inst 1 | Op 2-Inst 2 | |||
| Sample 1 | 2.40 | 2.05 | 1.83 | 1.61 | 1.97 | 0.34 | 17.08 |
| Sample 2 | 2.93 | 2.15 | 2.69 | 1.85 | 2.41 | 0.49 | 20.51 |
| Sample 3 | 2.54 | 2.35 | 2.71 | 1.84 | 2.36 | 0.38 | 15.96 |
| MESF Value | MFI | Mean | SD | %CV | ||||
|---|---|---|---|---|---|---|---|---|
| Run 1 | Run 2 | Run 3 | Run 4 | |||||
| Bead 1 | 994 | 147 | 128 | 156 | 130 | 140.25 | 13.52 | 9.64% |
| Bead 2 | 4456 | 654 | 647 | 681 | 661 | 660.75 | 14.66 | 2.22% |
| Bead 3 | 24,312 | 3511 | 3640 | 3616 | 3777 | 3636 | 109.42 | 3.01% |
| Bead 4 | 73,490 | 11,090 | 11,161 | 11,528 | 11,826 | 11,401.25 | 342.09 | 3.00% |
| r2 | 0.9998 | 1 | 0.9997 | 0.9999 | ||||
| E:T | Expected CD69 MFI | Observed CD69 MFI | % Recovery (80–120%) |
|---|---|---|---|
| 0 | 1722 | 1595.67 | 92.66 |
| 0.2 | 1675.2 | 1777.67 | 106.12 |
| 1 | 1938.1 | 1998.33 | 103.11 |
| 2 | 2154.2 | 2262.33 | 105.02 |
| 5 | 2802.5 | 2718 | 96.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mata-Molanes, J.J.; Alserawan, L.; España, C.; Guijarro, C.; López-Pecino, A.; Calderón, H.; Altuna, A.; Pérez-Amill, L.; Klein-González, N.; Fernández de Larrea, C.; et al. A Quantitative Approach to Potency Testing for Chimeric Antigen Receptor-Encoding Lentiviral Vectors and Autologous CAR-T Cell Products, Using Flow Cytometry. Pharmaceutics 2025, 17, 303. https://doi.org/10.3390/pharmaceutics17030303
Mata-Molanes JJ, Alserawan L, España C, Guijarro C, López-Pecino A, Calderón H, Altuna A, Pérez-Amill L, Klein-González N, Fernández de Larrea C, et al. A Quantitative Approach to Potency Testing for Chimeric Antigen Receptor-Encoding Lentiviral Vectors and Autologous CAR-T Cell Products, Using Flow Cytometry. Pharmaceutics. 2025; 17(3):303. https://doi.org/10.3390/pharmaceutics17030303
Chicago/Turabian StyleMata-Molanes, Juan José, Leticia Alserawan, Carolina España, Carla Guijarro, Ana López-Pecino, Hugo Calderón, Ane Altuna, Lorena Pérez-Amill, Nela Klein-González, Carlos Fernández de Larrea, and et al. 2025. "A Quantitative Approach to Potency Testing for Chimeric Antigen Receptor-Encoding Lentiviral Vectors and Autologous CAR-T Cell Products, Using Flow Cytometry" Pharmaceutics 17, no. 3: 303. https://doi.org/10.3390/pharmaceutics17030303
APA StyleMata-Molanes, J. J., Alserawan, L., España, C., Guijarro, C., López-Pecino, A., Calderón, H., Altuna, A., Pérez-Amill, L., Klein-González, N., Fernández de Larrea, C., González-Navarro, E. A., Delgado, J., Juan, M., & Castella, M. (2025). A Quantitative Approach to Potency Testing for Chimeric Antigen Receptor-Encoding Lentiviral Vectors and Autologous CAR-T Cell Products, Using Flow Cytometry. Pharmaceutics, 17(3), 303. https://doi.org/10.3390/pharmaceutics17030303








