In Vitro Enzymatic and Computational Assessments of Pyrazole–Isatin and Pyrazole–Indole Conjugates as Anti-Diabetic, Anti-Arthritic, and Anti-Inflammatory Agents
Abstract
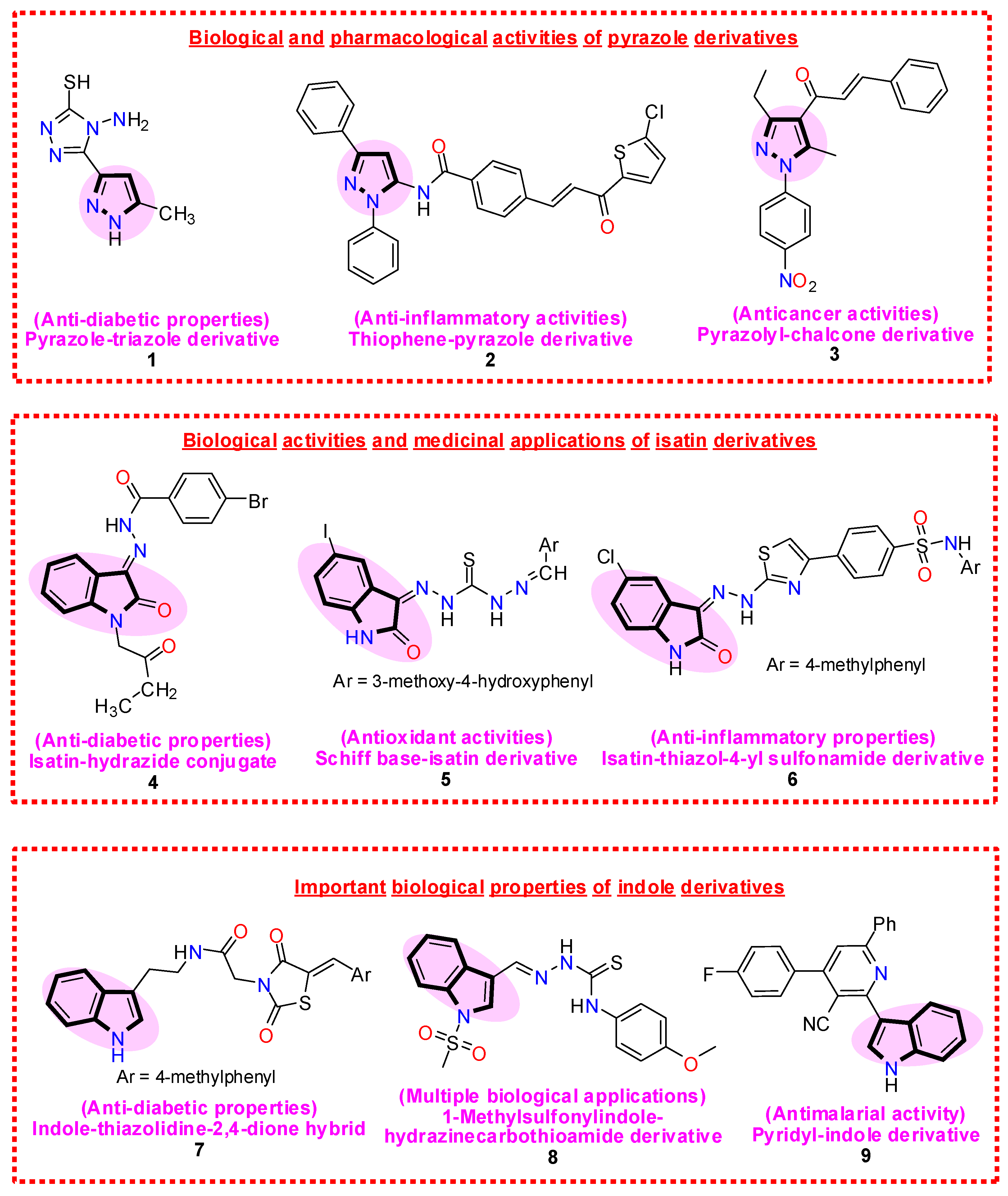
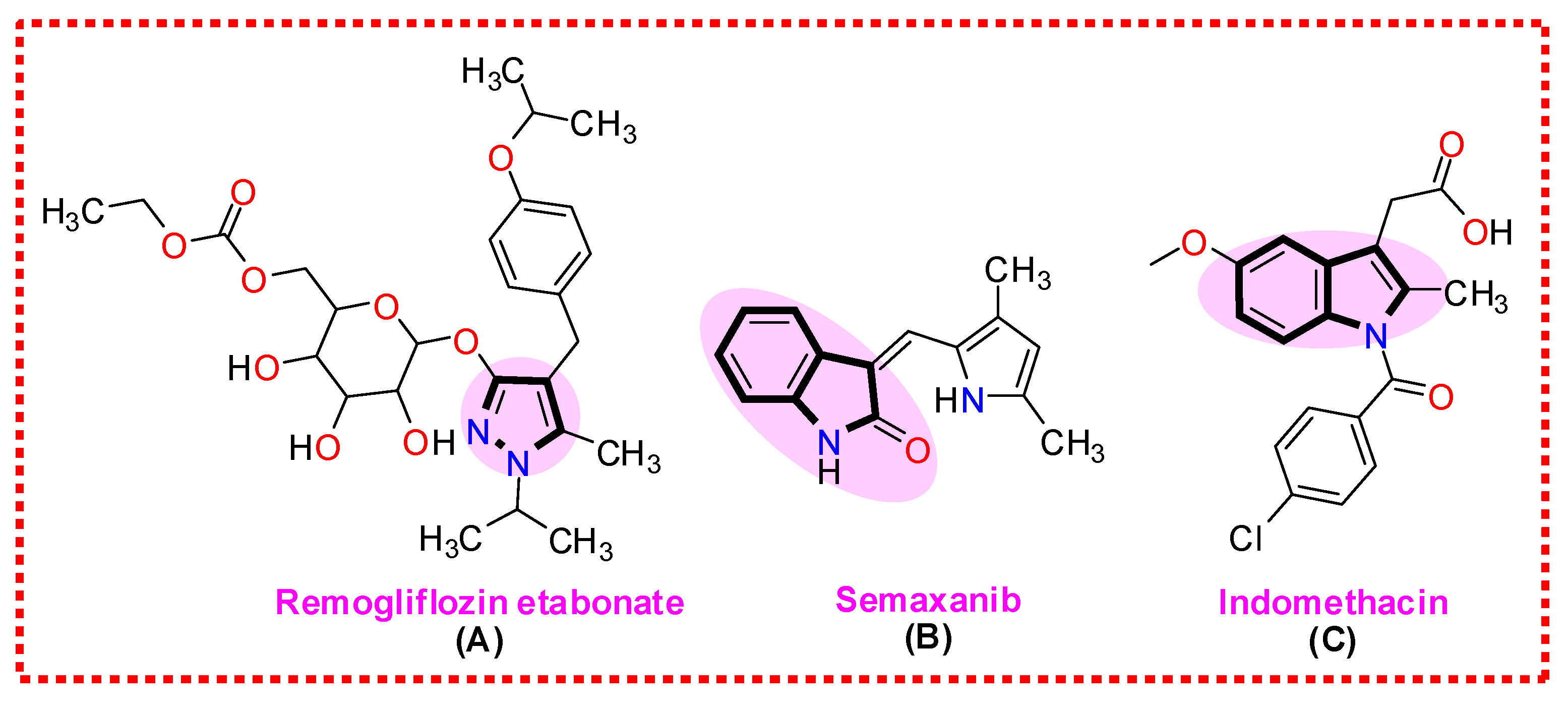
1. Introduction
2. Materials and Methods
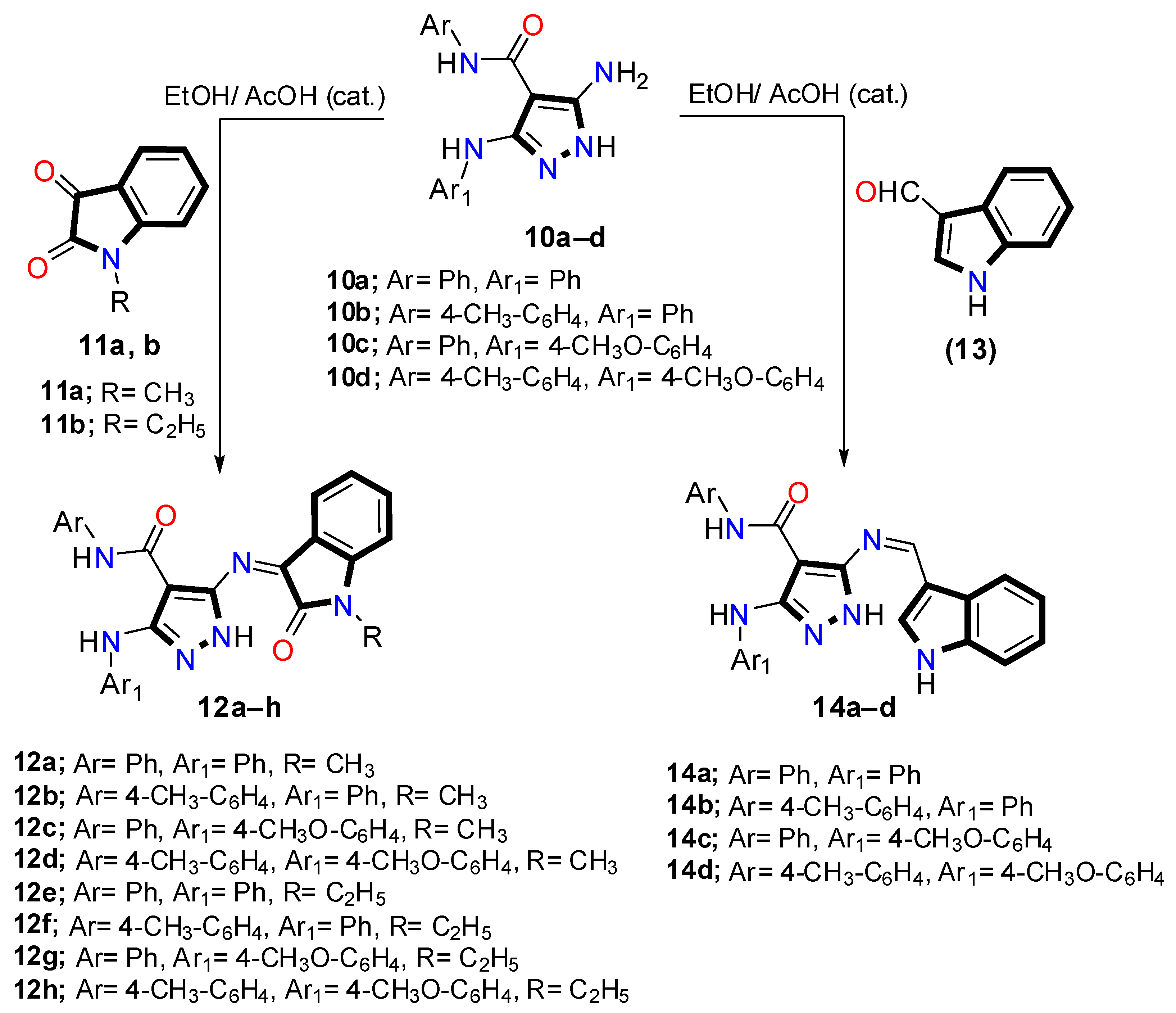
2.1. Chemistry
2.2. In Vitro Enzymatic Assessments
2.3. Computational Assessments
3. Results and Discussion
3.1. Chemistry
3.2. In Vitro Enzymatic Assessments
3.2.1. In Vitro Anti-Diabetic Assessment
3.2.2. In Vitro Anti-Arthritic Assessment
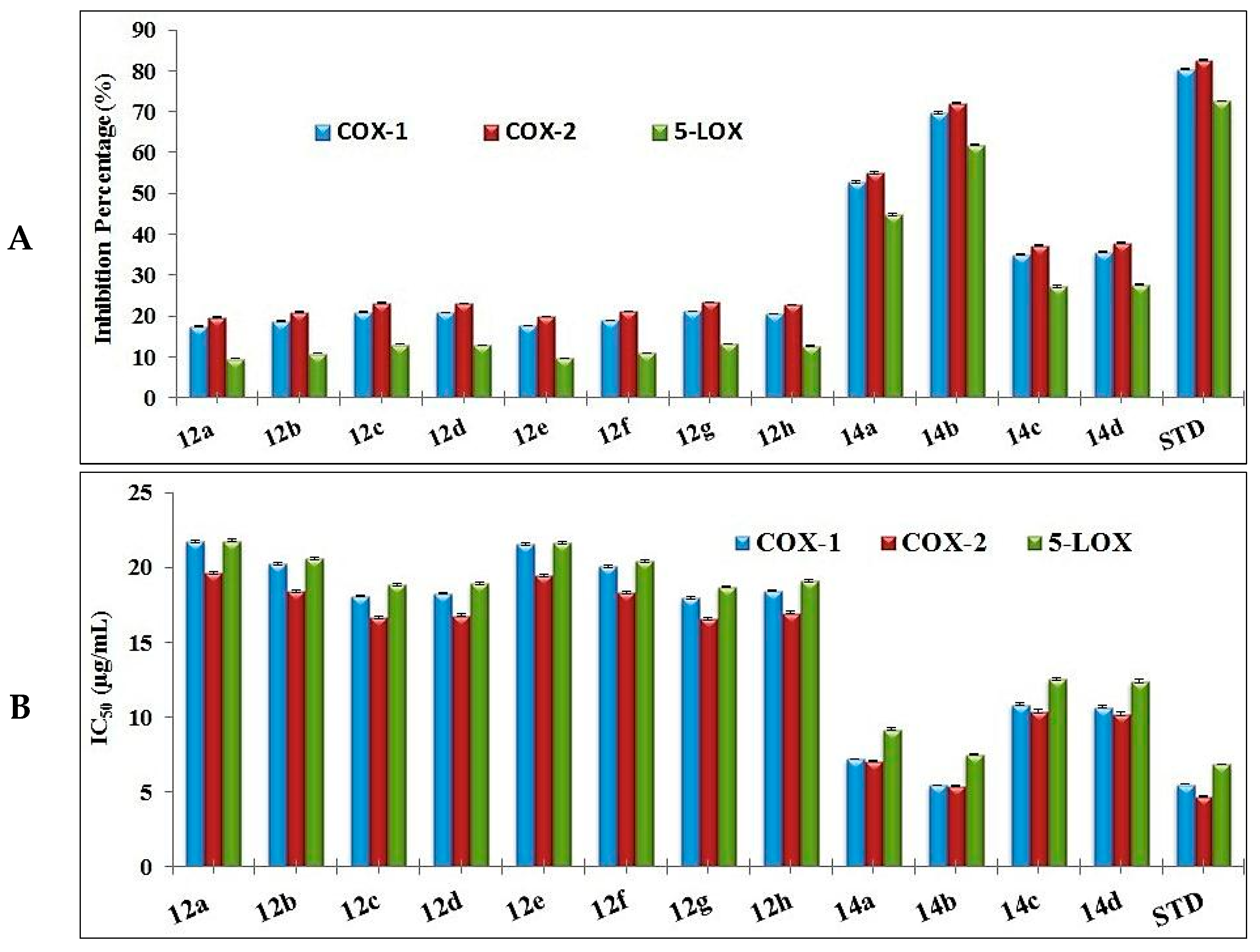
3.2.3. In Vitro Anti-Inflammatory Assessment
3.3. Computational Assessments
3.3.1. Toxicity Prediction
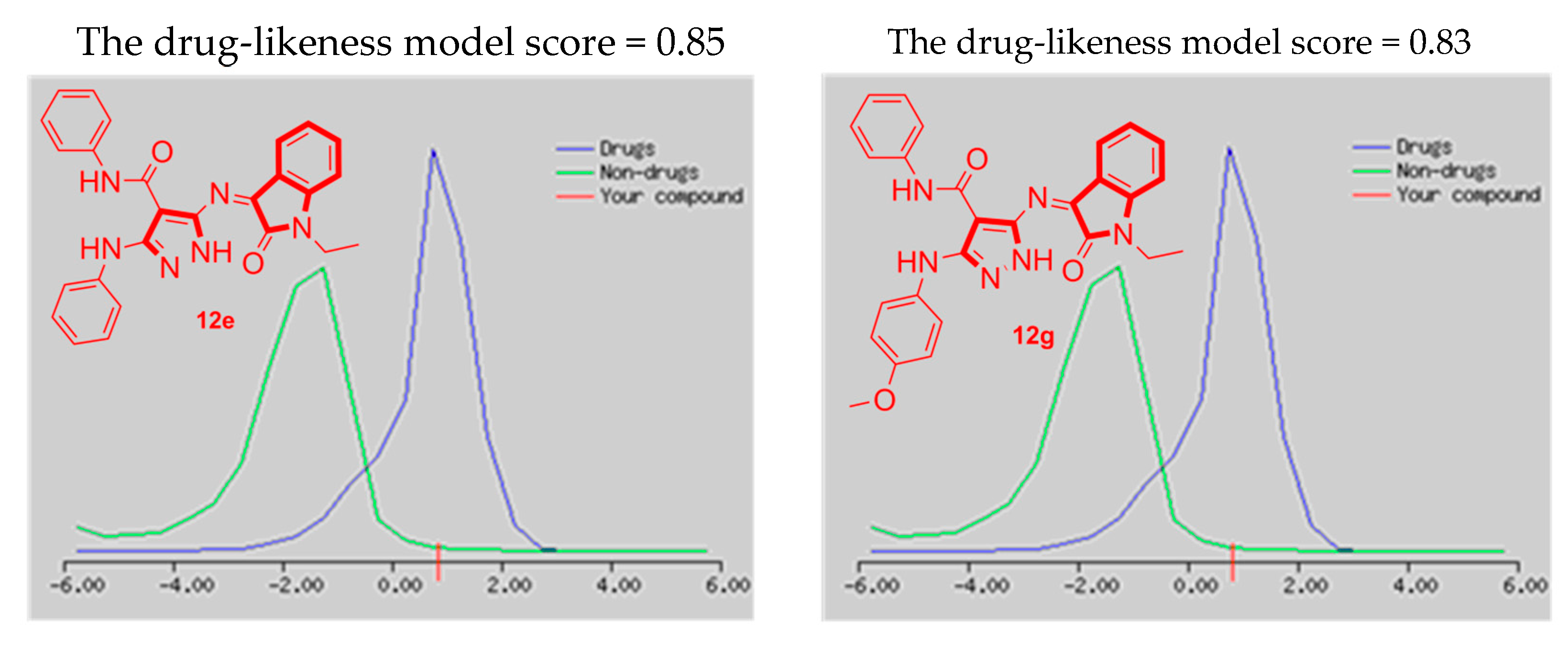
3.3.2. The Drug-Likeness Model Scores (DLMS) Prediction
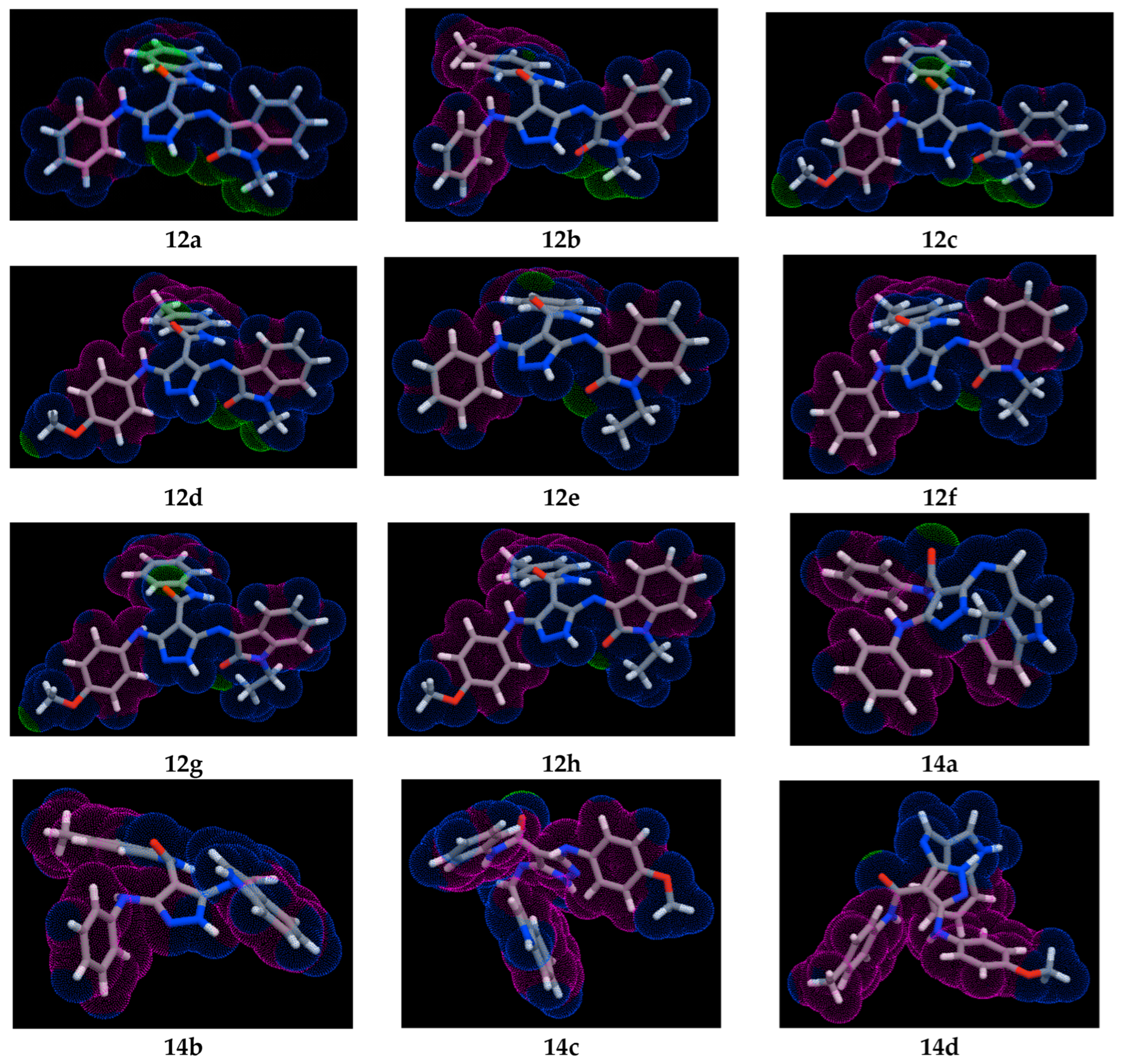
3.3.3. Molecular Lipophilicity Potential (MLP) Maps
- The three conjugates 12c, 12d, and 12g exhibit similar lipophilic and hydrophilic properties, with the lipophilic regions covering most of the surface area except for three specific areas: the carbonyl of amide (-NH-CO-), CH3O-p-C6H4-NH-, and carbonyl of isatin (C=O).
- The three conjugates 12a, 12b, and 12e possess similar surface properties (lipophilic and hydrophilic). The intermediate hydrophilic area appears at the two positions: the carbonyl of amide (-NH-CO-) and the carbonyl of isatin (C=O).
- The two conjugates 12f and 12h exhibit almost completely lipophilic properties, except for the carbonyl position of isatin (C=O), which shows intermediate hydrophilic properties.
- The three conjugates 14a, 14c, and 14d possess almost completely lipophilic surface properties, except for the carbonyl position of amide (-NH-CO-), which shows intermediate hydrophilic properties.
- The pyrazole–indole conjugate 14b possesses lipophilic surface properties.
3.3.4. Polar Surface Area (PSA) Maps and Topological Polar Surface Area (TPSA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suneja, S.; Christian, Y.; Chandra, N.C. Milieu of Diabetes in the 2nd Decade of 21st Century. J. Diabetes Metab. 2018, 9, 804. [Google Scholar] [CrossRef]
- Dowarah, J.; Singh, V.P. Anti-diabetic drugs recent approaches and advancements. Bioorg. Med. Chem. 2020, 28, 115263. [Google Scholar] [CrossRef]
- Kerru, N.; Singh-Pillay, A.; Awolade, P.; Singh, P. Current anti-diabetic agents and their molecular targets: A review. Eur. J. Med. Chem. 2018, 152, 436–488. [Google Scholar] [CrossRef] [PubMed]
- Alhawday, F.; Alminderej, F.; Ghannay, S.; Hammami, B.; Albadri, A.E.; Kadri, A.; Aouadi, K. In Silico Design, Synthesis, and Evaluation of Novel Enantiopure Isoxazolidines as Promising Dual Inhibitors of α-Amylase and α-Glucosidase. Molecules 2024, 29, 305. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Luo, J.; Tian, X.; Zhao, Y.; Li, Y.; Wu, X. Progress in Understanding Oxidative Stress, Aging, and Aging-Related Diseases. Antioxidants 2024, 13, 394. [Google Scholar] [CrossRef]
- Hassan, A.S.; Morsy, N.M.; Aboulthana, W.M.; Ragab, A. Exploring novel derivatives of isatin-based Schiff bases as multi-target agents: Design, synthesis, in vitro biological evaluation, and in silico ADMET analysis with molecular modeling simulations. RSC Adv. 2023, 13, 9281–9303. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, T.; Moe, R.H.; Kvien, T.K. The burden of disease in rheumatoid arthritis. PharmacoEconomics 2014, 32, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Alkahtani, H.M.; Almehizia, A.A.; Al-Omar, M.A.; Obaidullah, A.J.; Zen, A.A.; Hassan, A.S.; Aboulthana, W.M. In Vitro Evaluation and Bioinformatics Analysis of Schiff Bases Bearing Pyrazole Scaffold as Bioactive Agents: Antioxidant, Anti-Diabetic, Anti-Alzheimer, and Anti-Arthritic. Molecules 2023, 28, 7125. [Google Scholar] [CrossRef] [PubMed]
- Qandeel, N.A.; El-Damasy, A.K.; Sharawy, M.H.; Bayomi, S.M.; El-Gohary, N.S. Synthesis, in vivo anti-inflammatory, COX-1/COX-2 and 5-LOX inhibitory activities of new 2, 3, 4-trisubstituted thiophene derivatives. Bioorg. Chem. 2020, 102, 103890. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, P.d.-S.M.; Franco, L.S.; Montagnoli, T.L.; Fraga, C.A.M. Molecular hybridization: A powerful tool for multitarget drug discovery. Expert. Opin. Drug Discov. 2024, 19, 451–470. [Google Scholar] [CrossRef]
- Liu, C.; Cao, Y.; Zuo, Y.; Zhang, C.; Ren, S.; Zhang, X.; Wang, C.; Zeng, Y.; Ling, J.; Liu, Y.; et al. Hybridization-based discovery of novel quinazoline-2-indolinone derivatives as potent and selective PI3Kα inhibitors. J. Adv. Res. 2025, 68, 459–475. [Google Scholar] [CrossRef] [PubMed]
- Abdelazeem, N.M.; Aboulthana, W.M.; Hassan, A.S.; Almehizia, A.A.; Naglah, A.M.; Alkahtani, H.M. Synthesis, in silico ADMET prediction analysis, and pharmacological evaluation of sulfonamide derivatives tethered with pyrazole or pyridine as anti-diabetic and anti-Alzheimer’s agents. Saudi Pharm. J. 2024, 32, 102025. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.S.; Hafez, T.S.; Ali, M.M.; Khatab, T.K. Design, synthesis and cytotoxic activity of some new pyrazolines bearing benzofuran and pyrazole moieties. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 417–429. [Google Scholar]
- Shaldam, M.A.; Almahli, H.; Angeli, A.; Badi, R.M.; Khaleel, E.F.; Zain-Alabdeen, A.I.; Elsayed, Z.M.; Elkaeed, E.B.; Salem, R.; Supuran, C.T.; et al. Discovery of sulfonamide-tethered isatin derivatives as novel anticancer agents and VEGFR-2 inhibitors. J. Enzym. Inhib. Med. Chem. 2023, 38, 2203389. [Google Scholar] [CrossRef] [PubMed]
- Ebenezer, O.; Shapi, M.; Tuszynski, J.A. A review of the recent development in the synthesis and biological evaluations of pyrazole derivatives. Biomedicines 2022, 10, 1124. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.; Almehmadi, M.; Alsaiari, A.A.; Allahyani, M. Diverse Pharmacological Potential of different Substituted Pyrazole Derivatives. Curr. Org. Synth. 2024, 21, 858–888. [Google Scholar] [CrossRef]
- Vahora, M.S.; Boruah, J.J.; Das, S.P. Synthesis and Pharmacological Activities of Pyrazole and Oxadiazole Derivatives: A Review. Russ. J. Org. Chem. 2023, 59, 846–869. [Google Scholar] [CrossRef]
- Mortada, S.; Karrouchi, K.; Hamza, E.H.; Oulmidi, A.; Bhat, M.A.; Mamad, H.; Aalilou, Y.; Radi, S.; Ansar, M.H.; Masrar, A.; et al. Synthesis, structural characterizations, in vitro biological evaluation and computational investigations of pyrazole derivatives as potential antidiabetic and antioxidant agents. Sci. Rep. 2024, 14, 1312. [Google Scholar] [CrossRef]
- Khadri, M.N.; Ramu, R.; Simha, N.A.; Khanum, S.A. Synthesis, molecular docking, analgesic, anti-inflammatory, and ulcerogenic evaluation of thiophene-pyrazole candidates as COX, 5-LOX, and TNF-α inhibitors. Inflammopharmacology 2024, 32, 693–713. [Google Scholar] [CrossRef] [PubMed]
- Kamel, M.G.; Sroor, F.M.; Hanafy, M.K.; Mahrous, K.F.; Hassaneen, H.M. Design, synthesis and potent anti-pancreatic cancer activity of new pyrazole derivatives bearing chalcone, thiazole and thiadiazole moieties: Gene expression, DNA fragmentation, cell cycle arrest and SAR. RSC Adv. 2024, 14, 26954–26970. [Google Scholar] [CrossRef]
- Shu, V.A.; Eni, D.B.; Ntie-Kang, F. A survey of isatin hybrids and their biological properties. Mol. Divers. 2024, accepted. 1–24. [Google Scholar] [CrossRef]
- Rohila, Y.; Sebastian, S.; Ansari, A.; Kumar, D.; Mishra, D.K.; Gupta, M.K. A Comprehensive Review of the Diverse Spectrum Activity of 1,2,3-Triazole-linked Isatin Hybrids. Chem. Biodivers. 2024, 21, e202301612. [Google Scholar] [CrossRef]
- Abbasi, I.; Nadeem, H.; Saeed, A.; Kharl, H.A.A.; Tahir, M.N.; Naseer, M.M. Isatin-hydrazide conjugates as potent α-amylase and α-glucosidase inhibitors: Synthesis, structure and in vitro evaluations. Bioorg. Chem. 2021, 116, 105385. [Google Scholar] [CrossRef]
- Bakır, T.K.; Çavuş, M.S.; Muğlu, H.; Yakan, H. Synthesis, structure elucidation, antioxidant properties, and theoretical calculations of new Schiff bases–isatin derivatives. Res. Chem. Intermed. 2024, 50, 3937–3962. [Google Scholar] [CrossRef]
- Alkorbi, F.; Alshareef, S.A.; Abdelaziz, M.A.; Omer, N.; Jame, R.; Alatawi, I.S.; Ali, A.M.; Omran, O.A.; Bakr, R.B. Multicomponent reaction for synthesis, molecular docking, and anti-inflammatory evaluation of novel indole-thiazole hybrid derivatives. Mol. Divers. 2024, accepted. 1–12. [Google Scholar] [CrossRef]
- Raza, A.R.; Rubab, S.L.; Ashfaq, M.; Altaf, Y.; Tahir, M.N.; Rehman, M.F.u.; Aziz, T.; Alharbi, M.; Alasmari, A.F. Evaluation of Antimicrobial, Anticholinesterase Potential of Indole Derivatives and Unexpectedly Synthesized Novel Benzodiazine: Characterization, DFT and Hirshfeld Charge Analysis. Molecules 2023, 28, 5024. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Singh, R.K. Medicinal chemistry of indole derivatives: Current to future therapeutic prospectives. Bioorg. Chem. 2019, 89, 103021. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Zhang, F.; Zhu, S.; Yang, Z.; Feng, X.; Liu, Y. Photoredox-catalyzed diastereoselective dearomative prenylation and reverse-prenylation of electron-deficient indole derivatives. Nat. Commun. 2023, 14, 3876. [Google Scholar] [CrossRef] [PubMed]
- Siddique, S.; Ahmad, K.R.; Nawaz, S.K.; Raza, A.R.; Ahmad, S.N.; Ali, R.; Inayat, I.; Suleman, S.; Kanwal, M.A.; Usman, M. Evaluation of the anti-inflammatory, analgesic, anti-pyretic and anti-ulcerogenic potentials of synthetic indole derivatives. Sci. Rep. 2023, 13, 8639. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Celik, I.; Chinnam, S.; Çevik, U.A.; Tallei, T.E.; Nizam, A.; Joy, F.; Abdellattif, M.H.; Walode, S.G. Analgesic and anti-inflammatory potential of indole derivatives. Polycycl. Aromat. Compd. 2023, 43, 7732–7753. [Google Scholar] [CrossRef]
- Hu, C.; Liang, B.; Sun, J.; Li, J.; Xiong, Z.; Wang, S.H.; Xuetao, X. Synthesis and biological evaluation of indole derivatives containing thiazolidine-2, 4-dione as α-glucosidase inhibitors with antidiabetic activity. Eur. J. Med. Chem. 2024, 264, 115957. [Google Scholar] [CrossRef]
- Philoppes, J.N.; Abdelgawad, M.A.; Abourehab, M.A.S.; Sebak, M.; Darwish, M.A.; Lamie, P.F. Novel N-methylsulfonyl-indole derivatives: Biological activity and COX-2/5-LOX inhibitory effect with improved gastro protective profile and reduced cardio vascular risks. J. Enzym. Inhib. Med. Chem. 2023, 38, 246–266. [Google Scholar] [CrossRef] [PubMed]
- Elshemy, H.A.; Zaki, M.A.; Mohamed, E.I.; Khan, S.I.; Lamie, P.F. A multicomponent reaction to design antimalarial pyridyl-indole derivatives: Synthesis, biological activities and molecular docking. Bioorg. Chem. 2020, 97, 103673. [Google Scholar] [CrossRef]
- Vinod, A.; Mouli, H.M.C.; Jana, A.; Peraman, R. Unlocking therapeutic potential: Exploring indole scaffolds and their structural insights as pharmacophores in designing anti-breast cancer agents. Med. Chem. Res. 2024, 33, 1100–1132. [Google Scholar] [CrossRef]
- Nehra, B.; Kumar, M.; Singh, S.; Chawla, V.; Chawla, P.A. Sojourn of Nitrogenous Heterocycles as Promising Antileishmanial Agents: Medicinal Perspectives and Structure–Activity Relationship Studies. Chem. Afr. 2024, 7, 3485–3529. [Google Scholar] [CrossRef]
- Ahmad, G.; Sohail, M.; Bilal, M.; Rasool, N.; Qamar, M.U.; Ciurea, C.; Marceanu, L.G.; Misarca, C. N-Heterocycles as Promising Antiviral Agents: A Comprehensive Overview. Molecules 2024, 29, 2232. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Rohila, Y.; Kumar, V.; Lal, K. A mini review on pharmacological significance of isatin-1, 2, 3-triazole hybrids. Curr. Top. Med. Chem. 2023, 23, 833–847. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Al-Majid, A.M.; Sholkamy, E.N.; Yousuf, S.; Ayaz, M.; Nawaz, A.; Wadood, A.; Rehman, A.U.; Verma, V.P.; Bari, A.; et al. Synthesis, molecular docking and enzyme inhibitory approaches of some new chalcones engrafted pyrazole as potential antialzheimer, antidiabetic and antioxidant agents. J. Mol. Struct. 2022, 1269, 133843. [Google Scholar] [CrossRef]
- Mohan, V.; Mithal, A.; Joshi, S.R.; Aravind, S.R.; Chowdhury, S. Remogliflozin etabonate in the treatment of type 2 diabetes: Design, development, and place in therapy. Drug Des. Dev. Ther. 2020, 14, 2487–2501. [Google Scholar] [CrossRef]
- Paiva, R.E.F.; Vieira, E.G.; Silva, D.R.; Wegermann, C.A.; Ferreira, A.M.C. Anticancer compounds based on isatin-derivatives: Strategies to ameliorate selectivity and efficiency. Front. Mol. Biosci. 2021, 7, 627272. [Google Scholar] [CrossRef]
- Dandić, A.; Rajkovača, K.; Jozanović, M.; Pukleš, I.; Széchenyi, A.; Budetić, M.; Samardžić, M. Review of characteristics and analytical methods for determination of indomethacin. Rev. Anal. Chem. 2020, 41, 34–62. [Google Scholar] [CrossRef]
- Mukhtar, S.S.; Hassan, A.S.; Morsy, N.M.; Hafez, T.S.; Saleh, F.M.; Hassaneen, H.M. Design, synthesis, molecular prediction and biological evaluation of pyrazole-azomethine conjugates as antimicrobial agents. Synth. Commun. 2021, 51, 1564–1580. [Google Scholar] [CrossRef]
- Almehizia, A.A.; Naglah, A.M.; Zen, A.A.; Khatab, T.K.; Hassan, A.S. TCS/ZnCl2 as a controlled reagent for the Michael addition and heterocyclic cyclization based on the phenyl pyrazolone scaffold with docking validation as a Covid-19 protease inhibitor. Bull. Chem. Soc. Ethiop. 2024, 38, 1119–1127. [Google Scholar] [CrossRef]
- Hassan, A.S.; Hafez, T.S. Antimicrobial Activities of Ferrocenyl Complexes: A Review. J. Appl. Pharm. Sci. 2018, 8, 156–165. [Google Scholar]
- Hassan, A.S. Mixed isatin with 3-(2-(aryl)hydrazono)acetylacetone Mn(II), Co(II) and Ni(II) complexes: Antibacterial evaluation and molecular properties prediction. Bull. Chem. Soc. Ethiop. 2020, 34, 533–541. [Google Scholar] [CrossRef]
- Hassan, A.S.; Aboulthana, W.M. Synthesis, In Vitro Biological Investigation, and In Silico Analysis of Pyrazole-Based Derivatives as Multi-target Agents. Egypt. J. Chem. 2023, 66, 441–455. [Google Scholar] [CrossRef]
- Khatab, T.K.; Hassan, A.S. Computational molecular docking and in silico ADMET prediction studies of pyrazole derivatives as COVID-19 main protease (Mpro) and papain-like protease (PLpro) inhibitors. Bull. Chem. Soc. Ethiop. 2023, 37, 449–461. [Google Scholar] [CrossRef]
- Almehizia, A.A.; Naglah, A.M.; Aljafen, S.S.; Hassan, A.S.; Aboulthana, W.M. Assessment of the In Vitro Biological Activities of Schiff Base-Synthesized Copper Oxide Nanoparticles as an Anti-Diabetic, Anti-Alzheimer, and Anti-Cancer Agent. Pharmaceutics 2025, 17, 180. [Google Scholar] [CrossRef]
- Mukhtar, S.S.; Hassan, A.S.; Morsy, N.M.; Hafez, T.S.; Hassaneen, H.M.; Saleh, F.M. Overview on synthesis, reactions, applications, and biological activities of Schiff bases. Egypt. J. Chem. 2021, 64, 6541–6554. [Google Scholar] [CrossRef]
- Hassan, A.S.; Askar, A.A.; Nossier, E.S.; Naglah, A.M.; Moustafa, G.O.; Al-Omar, M.A. Antibacterial evaluation, in silico characters and molecular docking of Schiff bases derived from 5-aminopyrazoles. Molecules 2019, 24, 3130. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.S. Antimicrobial evaluation, in silico ADMET prediction, molecular docking, and molecular electrostatic potential of pyrazole-isatin and pyrazole-indole hybrid molecules. J. Iran. Chem. Soc. 2022, 19, 3577–3589. [Google Scholar] [CrossRef]
- Hassan, A.S.; Moustafa, G.O.; Awad, H.M.; Nossier, E.S.; Mady, M.F. Design, synthesis, anticancer evaluation, enzymatic assays, and a molecular modeling study of novel pyrazole–indole hybrids. ACS Omega 2021, 6, 12361–12374. [Google Scholar] [CrossRef] [PubMed]
- Pistia-Brueggeman, G.; Hollingsworth, R.I. A preparation and screening strategy for glycosidase inhibitors. Tetrahedron 2001, 57, 8773–8778. [Google Scholar] [CrossRef]
- Wickramaratne, M.N.; Punchihewa, J.; Wickramaratne, D. In-vitro alpha amylase inhibitory activity of the leaf extracts of Adenanthera pavonina. BMC Complement. Altern. Med. 2016, 16, 466. [Google Scholar] [CrossRef] [PubMed]
- Oyedapo, O.O.; Famurewa, A.J. Antiprotease and Membrane Stabilizing Activities of Extracts of Fagara Zanthoxyloides, Olax Subscorpioides and Tetrapleura Tetraptera. Int. J. Pharmacogn. 1995, 33, 65–69. [Google Scholar] [CrossRef]
- Das, S.; Sureshkumar, P. Effect of methanolic root extract of Blepharispermum subsesssile DC in controlling arthritic activity. Res. J. Biotechnol. 2016, 11, 65–74. [Google Scholar]
- Alaa, A.M.; El-Azab, A.S.; Abou-Zeid, L.A.; ElTahir, K.E.; Abdel-Aziz, N.I.; Ayyad, R.R.; Al-Obaid, A.M. Synthesis, anti-inflammatory, analgesic and COX-1/2 inhibition activities of anilides based on 5, 5-diphenylimidazolidine-2, 4-dione scaffold: Molecular docking studies. Eur. J. Med. Chem. 2016, 115, 121–131. [Google Scholar]
- Huang, Y.; Zhang, B.; Li, J.; Liu, H.; Zhang, Y.; Yang, Z.; Liu, W. Design, synthesis, biological evaluation and docking study of novel indole-2-amide as anti-inflammatory agents with dual inhibition of COX and 5-LOX. Eur. J. Med. Chem. 2019, 180, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Rajaselvi, N.D.; Jida, M.D.; Nair, D.B.; Sujith, S.; Beegum, N.; Nisha, A.R. Toxicity prediction and analysis of flavonoid apigenin as a histone deacetylase inhibitor: An in-silico approach. Silico Pharmacol. 2023, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Elsherif, M.A.; Hassan, A.S.; Moustafa, G.O.; Awad, H.M.; Morsy, N.M. Antimicrobial evaluation and molecular properties prediction of pyrazolines incorporating benzofuran and pyrazole moieties. J. Appl. Pharm. Sci. 2020, 10, 37–43. [Google Scholar] [CrossRef]
- Serin, S. Quantum Chemical-Based Investigations and Lipophilicity Evaluations on Some Structurally Related Quinazoline Derivatives. Orbital Electron. J. Chem. 2024, 16, 30–40. [Google Scholar] [CrossRef]
- Naveed, M.; Ain, N.U.; Aziz, T.; Saleem, A.; Shabbir, M.A.; Khan, A.A.; Albekairi, T.H. Integrated track of nano-informatics coupling with the enrichment concept in developing a novel nanoparticle targeting ERK protein in Naegleria fowleri. Open Chem. 2024, 22, 20230198. [Google Scholar] [CrossRef]
- Iduoku, K.; Ngongang, M.; Kulathunga, J.; Daghighi, A.; Casanola-Martin, G.; Simsek, S.; Rasulev, B. Phenolic Acid–β-Cyclodextrin Complexation Study to Mask Bitterness in Wheat Bran: A Machine Learning-Based QSAR Study. Foods 2024, 13, 2147. [Google Scholar] [CrossRef] [PubMed]
- Nkoana, J.K.; Mphahlele, M.J.; More, G.K.; Choong, Y.S. Exploring the 3,5-Dibromo-4,6-dimethoxychalcones and Their Flavone Derivatives as Dual α-Glucosidase and α-Amylase Inhibitors with Antioxidant and Anticancer Potential. Antioxidants 2024, 13, 1255. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Dubey, R.; Bhupal, R.; Patel, P.; Verma, S.K.; Kaya, S.; Asati, V. An insight on medicinal attributes of 1,2,3-and 1,2,4-triazole derivatives as alpha-amylase and alpha-glucosidase inhibitors. Mol. Divers. 2023, 28, 3605–3634. [Google Scholar] [CrossRef]
- Khadri, M.N.; Khamees, H.A.; Kouser, S.; Khanum, S.A. Synthesis, analgesic, anti-inflammatory, ulcerogenic evaluation, and docking study of (benzoylphenoxy)-N-{5-[2-methylphenyl-6-chlorobenzoxazole]} acetamides as COX/5-LOX inhibitor. J. Mol. Struct. 2023, 1272, 134240. [Google Scholar] [CrossRef]
- Hassan, A.S.; Morsy, N.M.; Awad, H.M.; Ragab, A. Synthesis, molecular docking, and in silico ADME prediction of some fused pyrazolo [1, 5-a] pyrimidine and pyrazole derivatives as potential antimicrobial agents. J. Iran. Chem. Soc. 2022, 19, 521–545. [Google Scholar] [CrossRef]
- Alam, M.S.; Lee, D.U.; Bari, M.L. Antibacterial and cytotoxic activities of Schiff base analogues of 4-aminoantipyrine. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 613–619. [Google Scholar] [CrossRef]
- Matyszewska, D.; Zaborowska, M.; Fontaine, P. Is lipophilicity the main factor determining the effective drug penetration through lipid membranes? Combined GIXD and PM-IRRAS studies on the example of anthracyclines. J. Mol. Liq. 2023, 385, 122324. [Google Scholar] [CrossRef]
- Schaftenaar, G.; de Vlieg, J. Quantum mechanical polar surface area. J. Comput. Aided Mol. Des. 2012, 26, 311–318. [Google Scholar] [CrossRef]
- Sakamoto, K.; Hirokawa, T. Lipid bilayer membrane permeability mechanism of the K-Ras (G12D)-inhibitory bicyclic peptide KS-58 elucidated by molecular dynamics simulations. Bioorg. Med. Chem. Lett. 2024, 100, 129649. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, Z.Y.; Uzairu, A.; Shallangwa, G.A.; Abechi, S.E. Pharmacokinetic predictions and docking studies of substituted aryl amine-based triazolopyrimidine designed inhibitors of Plasmodium falciparum dihydroorotate dehydrogenase (PfDHODH). Futur. J. Pharm. Sci. 2021, 7, 133. [Google Scholar] [CrossRef]
- Roney, M.; Singh, G.; Huq, A.K.M.M.; Forid, M.S.; Ishak, W.M.B.W.; Rullah, K.; Aluwi, M.F.F.M.; Tajuddin, S.N. Identification of pyrazole derivatives of usnic acid as novel inhibitor of SARS-CoV-2 main protease through virtual screening approaches. Mol. Biotechnol. 2024, 66, 696–706. [Google Scholar] [CrossRef]

| Pyrazole Conjugates | α-Amylase | α-Glucosidase | ||
|---|---|---|---|---|
| Inhibition (%) | IC50 (µg/mL) | Inhibition (%) | IC50 (µg/mL) | |
| 12a | 19.19 ± 0.03 | 14.42 ± 0.16 | 8.94 ± 0.03 | 17.10 ± 0.14 |
| 12b | 20.33 ± 0.03 | 13.61 ± 0.15 | 10.08 ± 0.03 | 15.16 ± 0.12 |
| 12c | 22.31 ± 0.03 | 12.40 ± 0.13 | 12.06 ± 0.03 | 12.68 ± 0.10 |
| 12d | 22.16 ± 0.03 | 12.49 ± 0.14 | 11.91 ± 0.03 | 12.84 ± 0.10 |
| 12e | 19.33 ± 0.03 | 14.32 ± 0.16 | 9.08 ± 0.03 | 16.85 ± 0.14 |
| 12f | 20.48 ± 0.03 | 13.52 ± 0.15 | 10.23 ± 0.03 | 14.95 ± 0.12 |
| 12g | 22.47 ± 0.03 | 12.32 ± 0.13 | 12.22 ± 0.03 | 12.52 ± 0.10 |
| 12h | 21.94 ± 0.03 | 12.62 ± 0.14 | 11.69 ± 0.03 | 13.08 ± 0.10 |
| 14a | 50.59 ± 0.23 | 5.47 ± 0.03 | 40.34 ± 0.23 | 3.79 ± 0.01 |
| 14b | 65.74 ± 0.23 | 4.21 ± 0.03 | 55.49 ± 0.23 | 2.76 ± 0.01 |
| 14c | 34.84 ± 0.23 | 7.94 ± 0.03 | 24.59 ± 0.23 | 6.22 ± 0.01 |
| 14d | 35.34 ± 0.23 | 7.83 ± 0.04 | 25.09 ± 0.23 | 6.09 ± 0.01 |
| Acarbose | 72.58 ± 0.16 | 3.81 ± 0.04 | 62.33 ± 0.16 | 2.45 ± 0.02 |
| Pyrazole Conjugates | Protein Denaturation | Proteinase | |
|---|---|---|---|
| Inhibition (%) | Inhibition (%) | IC50 (µg/mL) | |
| 12a | 14.40 ± 0.03 | 11.65 ± 0.03 | 23.20 ± 0.04 |
| 12b | 15.25 ± 0.03 | 12.50 ± 0.03 | 21.90 ± 0.04 |
| 12c | 16.73 ± 0.03 | 13.98 ± 0.03 | 19.96 ± 0.04 |
| 12d | 16.62 ± 0.03 | 13.87± 0.03 | 20.10 ± 0.04 |
| 12e | 14.50 ± 0.03 | 11.75 ± 0.03 | 23.04 ± 0.04 |
| 12f | 15.36 ± 0.03 | 12.61 ± 0.03 | 21.75 ± 0.04 |
| 12g | 16.85 ± 0.03 | 14.10 ± 0.03 | 19.82 ± 0.04 |
| 12h | 16.45 ± 0.03 | 13.70 ± 0.03 | 20.30 ± 0.04 |
| 14a | 37.94 ± 0.17 | 35.19 ± 0.17 | 8.80 ± 0.02 |
| 14b | 49.30 ± 0.17 | 46.55 ± 0.17 | 6.77 ± 0.01 |
| 14c | 26.13 ± 0.17 | 23.38 ± 0.17 | 12.78 ± 0.06 |
| 14d | 26.50 ± 0.17 | 23.75 ± 0.17 | 12.60 ± 0.06 |
| Diclofenac Sodium | 48.75 ± 0.04 | 46.00 ± 0.04 | 6.85 ± 0.02 |
| Pyrazole Conjugates | COX-1 | COX-2 | 5-LOX | |||
|---|---|---|---|---|---|---|
| Inhibition (%) | IC50 (µg/mL) | Inhibition (%) | IC50 (µg/mL) | Inhibition (%) | IC50 (µg/mL) | |
| 12a | 17.47 ± 0.04 | 21.74 ± 0.08 | 19.72 ± 0.04 | 19.64 ± 0.10 | 9.57 ± 0.04 | 21.79 ± 0.10 |
| 12b | 18.75 ± 0.04 | 20.25 ± 0.07 | 21.00 ± 0.04 | 18.44 ± 0.09 | 10.85 ± 0.04 | 20.59 ± 0.09 |
| 12c | 20.98 ± 0.04 | 18.10 ± 0.06 | 23.23 ± 0.04 | 16.68 ± 0.08 | 13.08 ± 0.04 | 18.83 ± 0.08 |
| 12d | 20.80 ± 0.04 | 18.26 ± 0.06 | 23.05 ± 0.04 | 16.80 ± 0.08 | 12.90 ± 0.04 | 18.95 ± 0.08 |
| 12e | 17.62 ± 0.04 | 21.55 ± 0.08 | 19.87 ± 0.04 | 19.49 ± 0.09 | 9.72 ± 0.04 | 21.64 ± 0.09 |
| 12f | 18.91 ± 0.04 | 20.08 ± 0.07 | 21.16 ± 0.04 | 18.30 ± 0.09 | 11.01 ± 0.04 | 20.45 ± 0.09 |
| 12g | 21.15 ± 0.04 | 17.95 ± 0.06 | 23.40 ± 0.04 | 16.55 ± 0.08 | 13.25 ± 0.04 | 18.70 ± 0.08 |
| 12h | 20.56 ± 0.04 | 18.47 ± 0.06 | 22.81 ± 0.04 | 16.98 ± 0.08 | 12.66 ± 0.04 | 19.13 ± 0.08 |
| 14a | 52.79 ± 0.26 | 7.19 ± 0.04 | 55.04 ± 0.26 | 7.04 ± 0.06 | 44.89 ± 0.26 | 9.19 ± 0.06 |
| 14b | 69.83 ± 0.26 | 5.44 ± 0.03 | 72.08 ± 0.26 | 5.37 ± 0.04 | 61.93 ± 0.26 | 7.52 ± 0.04 |
| 14c | 35.07 ± 0.26 | 10.83 ± 0.09 | 37.32 ± 0.26 | 10.38 ± 0.11 | 27.17 ± 0.26 | 12.53 ± 0.11 |
| 14d | 35.63 ± 0.26 | 10.66 ± 0.09 | 37.88 ± 0.26 | 10.23 ± 0.11 | 27.73 ± 0.26 | 12.38 ± 0.11 |
| STD | Indomethacin | Zileuton | ||||
| 80.51 ± 0.07 | 5.50 ± 0.01 | 82.76 ± 0.07 | 4.68 ± 0.02 | 72.61 ± 0.07 | 6.83 ± 0.02 | |
| Inhibition Percentage (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Anti-Diabetic | Anti-Arthritic | Anti-Inflammatory | |||||||
| α-Amylase | α-Glucosidase | Protein Denaturation | Proteinase | COX-1 | COX-2 | 5-LOX | |||
| Anti-diabetic assessment | Inhibition Percentage (%) | α-amylase | - | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| α-Glucosidase | 0.000 | - | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| Anti-arthritic assessment | Protein Denaturation | 0.000 | 0.000 | - | 0.000 | 0.000 | 0.000 | 0.000 | |
| Proteinase | 0.000 | 0.000 | 0.000 | - | 0.000 | 0.000 | 0.000 | ||
| Anti-inflammatory assessment | COX-1 | 0.000 | 0.000 | 0.000 | 0.000 | - | 0.000 | 0.000 | |
| COX-2 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | - | 0.000 | ||
| 5-LOX | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | - | ||
| Pyrazole Conjugates | Toxic Dose (LD50, mg kg−1) | Toxicity Class | Drug-Likeness Model Scores (DLMS) |
|---|---|---|---|
| 12a | 1000 | 4 | 0.70 |
| 12b | 1000 | 4 | 0.70 |
| 12c | 1000 | 4 | 0.71 |
| 12d | 1000 | 4 | 0.65 |
| 12e | 1000 | 4 | 0.85 |
| 12f | 1000 | 4 | 0.82 |
| 12g | 1000 | 4 | 0.83 |
| 12h | 1000 | 4 | 0.76 |
| 14a | 1000 | 4 | −0.50 |
| 14b | 1000 | 4 | −0.43 |
| 14c | 1000 | 4 | −0.37 |
| 14d | 1000 | 4 | −0.44 |
| Pyrazole Conjugates | Topological Polar Surface Area (TPSA) |
|---|---|
| 12a | 104.18 |
| 12b | 104.18 |
| 12c | 113.41 |
| 12d | 113.41 |
| 12e | 104.18 |
| 12f | 104.18 |
| 12g | 113.41 |
| 12h | 113.41 |
| 14a | 97.96 |
| 14b | 97.96 |
| 14c | 107.20 |
| 14d | 107.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naglah, A.M.; Almehizia, A.A.; Ghazwani, M.; Al-Wasidi, A.S.; Naglah, A.A.; Aboulthana, W.M.; Hassan, A.S. In Vitro Enzymatic and Computational Assessments of Pyrazole–Isatin and Pyrazole–Indole Conjugates as Anti-Diabetic, Anti-Arthritic, and Anti-Inflammatory Agents. Pharmaceutics 2025, 17, 293. https://doi.org/10.3390/pharmaceutics17030293
Naglah AM, Almehizia AA, Ghazwani M, Al-Wasidi AS, Naglah AA, Aboulthana WM, Hassan AS. In Vitro Enzymatic and Computational Assessments of Pyrazole–Isatin and Pyrazole–Indole Conjugates as Anti-Diabetic, Anti-Arthritic, and Anti-Inflammatory Agents. Pharmaceutics. 2025; 17(3):293. https://doi.org/10.3390/pharmaceutics17030293
Chicago/Turabian StyleNaglah, Ahmed M., Abdulrahman A. Almehizia, Mohammed Ghazwani, Asma S. Al-Wasidi, Abdelrahman A. Naglah, Wael M. Aboulthana, and Ashraf S. Hassan. 2025. "In Vitro Enzymatic and Computational Assessments of Pyrazole–Isatin and Pyrazole–Indole Conjugates as Anti-Diabetic, Anti-Arthritic, and Anti-Inflammatory Agents" Pharmaceutics 17, no. 3: 293. https://doi.org/10.3390/pharmaceutics17030293
APA StyleNaglah, A. M., Almehizia, A. A., Ghazwani, M., Al-Wasidi, A. S., Naglah, A. A., Aboulthana, W. M., & Hassan, A. S. (2025). In Vitro Enzymatic and Computational Assessments of Pyrazole–Isatin and Pyrazole–Indole Conjugates as Anti-Diabetic, Anti-Arthritic, and Anti-Inflammatory Agents. Pharmaceutics, 17(3), 293. https://doi.org/10.3390/pharmaceutics17030293







