Impact of V9302, a Competitive Antagonist of Transmembrane Glutamine Flux on Reversal of Resistance in Breast Cancer Cell Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Their Maintenance
2.2. Antiproliferative and Cytotoxicity Assays
2.3. Combination Assay
2.4. Cell Cycle Analysis
2.5. Rhodamine 123 Accumulation Assay
2.6. Pgp ATPase Assay
3. Results
3.1. Cytotoxic and Antiproliferative Activities
3.2. Combination Assay
3.3. Cell Cycle Analysis
3.4. Rhodamine 123 Accumulation Assay
3.5. Pgp ATPase Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Cancer Burden Growing, Amidst Mounting Need for Services. Available online: https://www.who.int/news/item/01-02-2024-global-cancer-burden-growing--amidst-mounting-need-for-services (accessed on 1 February 2024).
- Norsworthy, K.J.; Lee-Alonzo, R.J.; Pazdur, R. FDA Approvals in 2023: Biomarker-Positive Subsets, Equipoise and Verification of Benefit. Nat. Rev. Clin. Oncol. 2024, 21, 333–334. [Google Scholar] [CrossRef]
- de la Torre, B.G.; Albericio, F. The Pharmaceutical Industry in 2023: An Analysis of FDA Drug Approvals from the Perspective of Molecules. Molecules 2024, 29, 585. [Google Scholar] [CrossRef] [PubMed]
- Langedijk, J.; Mantel-Teeuwisse, A.K.; Slijkerman, D.S.; Schutjens, M.-H.D.B. Drug Repositioning and Repurposing: Terminology and Definitions in Literature. Drug Discov. Today 2015, 20, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Sleire, L.; Førde, H.E.; Netland, I.A.; Leiss, L.; Skeie, B.S.; Enger, P.Ø. Drug Repurposing in Cancer. Pharmacol. Res. 2017, 124, 74–91. [Google Scholar] [CrossRef]
- Chan, K.K.W.; Oza, A.M.; Siu, L.L. The Statins as Anticancer Agents. Clin. Cancer Res. 2003, 9, 10–19. [Google Scholar]
- Schelz, Z.; Muddather, H.F.; Zupkó, I. Repositioning of HMG-CoA Reductase Inhibitors as Adjuvants in the Modulation of Efflux Pump-Mediated Bacterial and Tumor Resistance. Antibiotics 2023, 12, 1468. [Google Scholar] [CrossRef]
- Barbalata, C.I.; Tefas, L.R.; Achim, M.; Tomuta, I.; Porfire, A.S. Statins in Risk-Reduction and Treatment of Cancer. World J. Clin. Oncol. 2020, 11, 573–588. [Google Scholar] [CrossRef]
- McCreight, L.J.; Bailey, C.J.; Pearson, E.R. Metformin and the Gastrointestinal Tract. Diabetologia 2016, 59, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Noto, H.; Goto, A.; Tsujimoto, T.; Noda, M. Cancer Risk in Diabetic Patients Treated with Metformin: A Systematic Review and Meta-Analysis. PLoS ONE 2012, 7, e33411. [Google Scholar] [CrossRef] [PubMed]
- Zakikhani, M.; Dowling, R.; Fantus, I.G.; Sonenberg, N.; Pollak, M. Metformin Is an AMP Kinase–Dependent Growth Inhibitor for Breast Cancer Cells. Cancer Res. 2006, 66, 10269–10273. [Google Scholar] [CrossRef]
- Han, X.; Wang, D.; Yang, L.; Wang, N.; Shen, J.; Wang, J.; Zhang, L.; Chen, L.; Gao, S.; Zong, W.-X.; et al. Activation of Polyamine Catabolism Promotes Glutamine Metabolism and Creates a Targetable Vulnerability in Lung Cancer. Proc. Natl. Acad. Sci. USA 2024, 121, e2319429121. [Google Scholar] [CrossRef] [PubMed]
- Schulte, M.L.; Fu, A.; Zhao, P.; Li, J.; Geng, L.; Smith, S.T.; Kondo, J.; Coffey, R.J.; Johnson, M.O.; Rathmell, J.C.; et al. Pharmacological Blockade of ASCT2-Dependent Glutamine Transport Leads to Antitumor Efficacy in Preclinical Models. Nat. Med. 2018, 24, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Van Geldermalsen, M.; Wang, Q.; Nagarajah, R.; Marshall, A.D.; Thoeng, A.; Gao, D.; Ritchie, W.; Feng, Y.; Bailey, C.G.; Deng, N.; et al. ASCT2/SLC1A5 Controls Glutamine Uptake and Tumour Growth in Triple-Negative Basal-like Breast Cancer. Oncogene 2016, 35, 3201–3208. [Google Scholar] [CrossRef]
- Li, Q.; Zhong, X.; Yao, W.; Yu, J.; Wang, C.; Li, Z.; Lai, S.; Qu, F.; Fu, X.; Huang, X.; et al. Inhibitor of Glutamine Metabolism V9302 Promotes ROS-Induced Autophagic Degradation of B7H3 to Enhance Antitumor Immunity. J. Biol. Chem. 2022, 298, 101753. [Google Scholar] [CrossRef]
- Zhao, L.; Rao, X.; Zheng, R.; Huang, C.; Kong, R.; Yu, X.; Cheng, H.; Li, S. Targeting Glutamine Metabolism with Photodynamic Immunotherapy for Metastatic Tumor Eradication. J. Control. Release 2023, 357, 460–471. [Google Scholar] [CrossRef]
- Peng, R.; Dong, Y.; Zheng, M.; Kang, H.; Wang, P.; Zhu, M.; Song, K.; Wu, W.; Li, F. IL-17 Promotes Osteoclast-Induced Bone Loss by Regulating Glutamine-Dependent Energy Metabolism. Cell Death Dis. 2024, 15, 111. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Ghoncheh, M.; Mohammadian-Hafshejani, A.; Salehiniya, H. Incidence and Mortality of Breast Cancer and Their Relationship to Development in Asia. Asian Pac. J. Cancer Prev. 2015, 16, 6081–6087. [Google Scholar] [CrossRef]
- Howell, A.; Anderson, A.S.; Clarke, R.B.; Duffy, S.W.; Evans, D.G.; Garcia-Closas, M.; Gescher, A.J.; Key, T.J.; Saxton, J.M.; Harvie, M.N. Risk Determination and Prevention of Breast Cancer. Breast Cancer Res. 2014, 16, 446. [Google Scholar] [CrossRef]
- Chang-Claude, J.; Andrieu, N.; Rookus, M.; Brohet, R.; Antoniou, A.C.; Peock, S.; Davidson, R.; Izatt, L.; Cole, T.; Noguès, C.; et al. Age at Menarche and Menopause and Breast Cancer Risk in the International BRCA1/2 Carrier Cohort Study. Cancer Epidemiol. Biomark. Prev. 2007, 16, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Sugiura, H.; Ando, Y.; Shiraki, N.; Yanagi, T.; Yamashita, H.; Toyama, T. Reproductive History and Breast Cancer Risk. Breast Cancer 2012, 19, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Colditz, G.A.; Bohlke, K. Priorities for the Primary Prevention of Breast Cancer. CA A Cancer J. Clin. 2014, 64, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.G.; Shenton, A.; Woodward, E.; Lalloo, F.; Howell, A.; Maher, E.R. Penetrance Estimates for BRCA1 and BRCA2based on Genetic Testing in a Clinical Cancer Genetics Service Setting: Risks of Breast/Ovarian Cancer Quoted Should Reflect the Cancer Burden in the Family. BMC Cancer 2008, 8, 155. [Google Scholar] [CrossRef]
- Sørlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; Van De Rijn, M.; Jeffrey, S.S.; et al. Gene Expression Patterns of Breast Carcinomas Distinguish Tumor Subclasses with Clinical Implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar] [CrossRef] [PubMed]
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast Cancer—Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies—An Updated Review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Stevens, D.; Pierga, J.-Y.; Lerebours, F.; Reyal, F.; Robain, M.; Asselain, B.; Rouzier, R. Impact of Adjuvant Chemotherapy on Breast Cancer Survival: A Real-World Population. PLoS ONE 2015, 10, e0132853. [Google Scholar] [CrossRef] [PubMed]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Scumaci, D.; Giuzio, F.; Saturnino, C.; Aquaro, S.; Rosano, C.; Sinicropi, M.S. Multidrug Resistance (MDR): A Widespread Phenomenon in Pharmacological Therapies. Molecules 2022, 27, 616. [Google Scholar] [CrossRef] [PubMed]
- Gennari, A.; André, F.; Barrios, C.H.; Cortés, J.; De Azambuja, E.; DeMichele, A.; Dent, R.; Fenlon, D.; Gligorov, J.; Hurvitz, S.A.; et al. ESMO Clinical Practice Guideline for the Diagnosis, Staging and Treatment of Patients with Metastatic Breast Cancer. Ann. Oncol. 2021, 32, 1475–1495. [Google Scholar] [CrossRef]
- Szakács, G.; Paterson, J.K.; Ludwig, J.A.; Booth-Genthe, C.; Gottesman, M.M. Targeting Multidrug Resistance in Cancer. Nat. Rev. Drug Discov. 2006, 5, 219–234. [Google Scholar] [CrossRef]
- Tien Kuo, M. Roles of Multidrug Resistance Genes in Breast Cancer Chemoresistance; Madame Curie Bioscience Database; Landes Bioscience: Austin, TX, USA, 2000–2013. Available online: https://www.ncbi.nlm.gov/books/NBK5989/ (accessed on 14 March 2024).
- Cripe, L.D.; Uno, H.; Paietta, E.M.; Litzow, M.R.; Ketterling, R.P.; Bennett, J.M.; Rowe, J.M.; Lazarus, H.M.; Luger, S.; Tallman, M.S. Zosuquidar, a Novel Modulator of P-Glycoprotein, Does Not Improve the Outcome of Older Patients with Newly Diagnosed Acute Myeloid Leukemia: A Randomized, Placebo-Controlled Trial of the Eastern Cooperative Oncology Group 3999. Blood 2010, 116, 4077–4085. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Cao, B.; Feng, D.; Zhou, F.; Zhang, J.; Yang, N.; Feng, S.; Wang, G.; Aa, J. The Down-Regulation of SLC7A11 Enhances ROS Induced P-Gp over-Expression and Drug Resistance in MCF-7 Breast Cancer Cells. Sci. Rep. 2017, 7, 3791. [Google Scholar] [CrossRef] [PubMed]
- Pastan, I.; Gottesman, M.M.; Ueda, K.; Lovelace, E.; Rutherford, A.V.; Willingham, M.C. A Retrovirus Carrying an MDR1 cDNA Confers Multidrug Resistance and Polarized Expression of P-Glycoprotein in MDCK Cells. Proc. Natl. Acad. Sci. USA 1988, 85, 4486–4490. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.-C. Theoretical Basis, Experimental Design, and Computerized Simulation of Synergism and Antagonism in Drug Combination Studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, D.; Szemerédi, N.; Spengler, G.; Mulhovo, S.; Dos Santos, D.; Ferreira, M.-J. Exploring the Monoterpene Indole Alkaloid Scaffold for Reversing P-Glycoprotein-Mediated Multidrug Resistance in Cancer. Pharmaceuticals 2021, 14, 862. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.-C. Drug Combination Studies and Their Synergy Quantification Using the Chou-Talalay Method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef]
- Szemerédi, N.; Dobiasová, S.; Salardón-Jiménez, N.; Kincses, A.; Nové, M.; Habibullah, G.; Sevilla-Hernández, C.; Benito-Lama, M.; Alonso-Martínez, F.-J.; Viktorová, J.; et al. Cyano- and Ketone-Containing Selenoesters as Multi-Target Compounds against Resistant Cancers. Cancers 2021, 13, 4563. [Google Scholar] [CrossRef] [PubMed]
- Weth, F.R.; Hoggarth, G.B.; Weth, A.F.; Paterson, E.; White, M.P.J.; Tan, S.T.; Peng, L.; Gray, C. Unlocking Hidden Potential: Advancements, Approaches, and Obstacles in Repurposing Drugs for Cancer Therapy. Br. J. Cancer 2024, 130, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S. Is Cancer a Genetic Disease or a Metabolic Disease? EBioMedicine 2015, 2, 478–479. [Google Scholar] [CrossRef]
- Zhu, H.-J.; Liu, G.-Q. Glutamate Up-Regulates P-Glycoprotein Expression in Rat Brain Microvessel Endothelial Cells by an NMDA Receptor-Mediated Mechanism. Life Sci. 2004, 75, 1313–1322. [Google Scholar] [CrossRef]
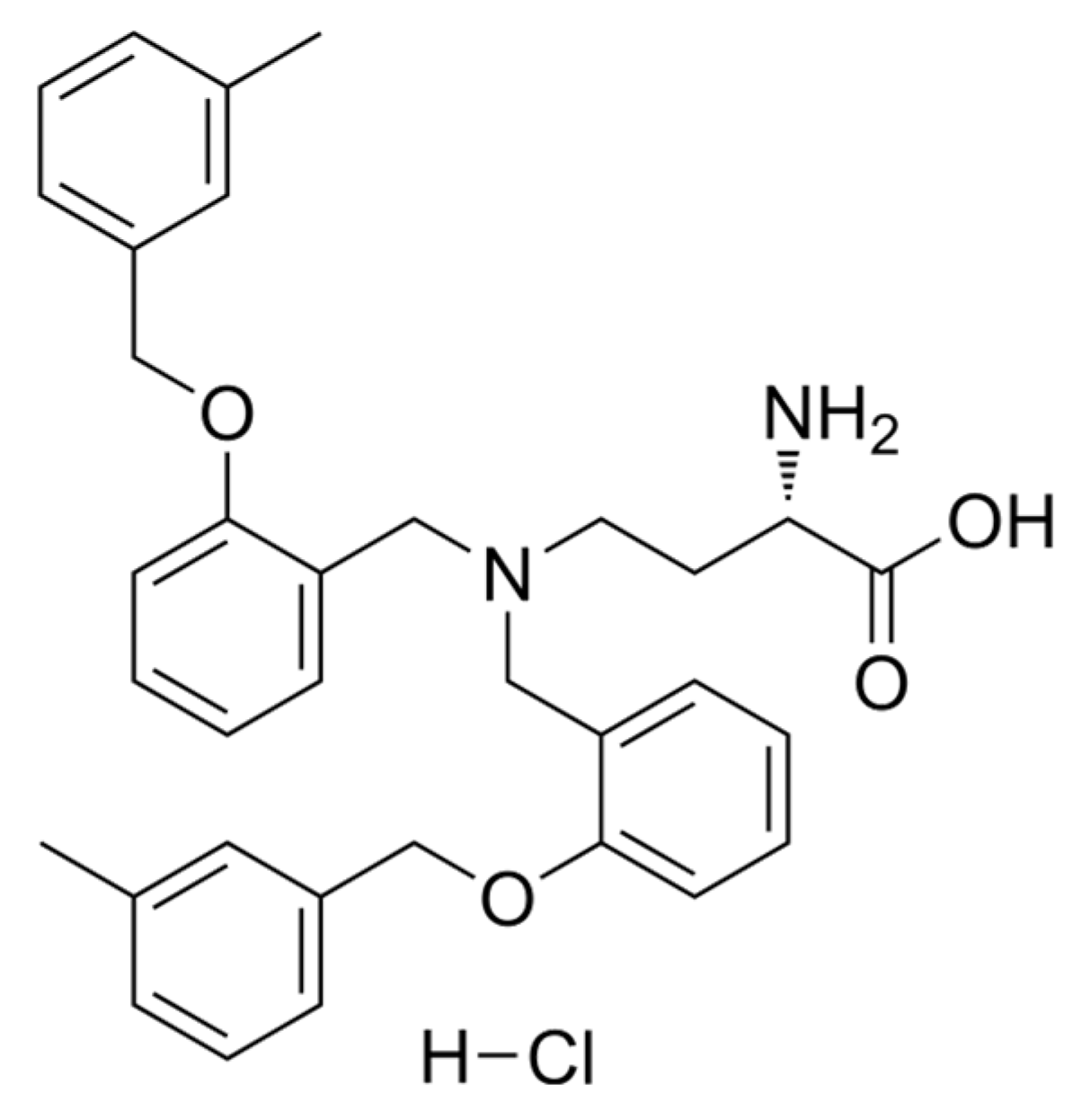



| CI | Interaction | CI | Interaction |
|---|---|---|---|
| 0–0.1 | very strong synergism | 0.9–1.1 | additive effect |
| 0.1–0.3 | strong synergism | 1.1–1.2 | slight antagonism |
| 0.3–0.7 | synergism | 1.2–1.45 | moderate antagonism |
| 1.45–3.3 | antagonism | ||
| 0.7–0.85 | moderate synergism | 3.3–10 | strong antagonism |
| 0.85–0.9 | slight synergism | >10 | very strong antagonism |
| MCF 7 | MDA-MB-231 | T-47D | KCR | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CT | AP | CT | AP | CT | AP | CT | AP | |||||||||
| IC50 | SD | IC50 | SD | IC50 | SD | IC50 | SD | IC50 | SD | IC50 | SD | IC50 | SD | IC50 | SD | |
| V9302 | 4.68 | 0.21 | 2.73 | 0.07 | 19.19 | 1.11 | 21.88 | 1.18 | 41.11 | 1.26 | 18.24 | 1.13 | >100 | - | 24.45 | 0.27 |
| DOX | 2.14 | 0.57 | 0.03 | 0 | N/A | 2.35 | 0.07 | N/A | 1.42 | 0.24 | >8.62 | - | >8.62 | - | ||
| CIS | 20.63 | 1.89 | 4.93 | 0.49 | >100 | - | 39.19 | 0.72 | >100 | - | 89.95 | 1.09 | >100 | - | 2.21 | 0.36 |
| DMSO | >2% | - | >2% | - | >2% | - | >2% | - | >2% | - | >2% | - | >2% | - | >2% | - |
| Cell Line | Compound | Chemotherapeutic Drug | Starting Concentration (µM) | Ratio * | Combination Index (CI) | SD ± | Type of Interaction at ED50 |
|---|---|---|---|---|---|---|---|
| MDA-MB-231 | V9302 | CIS | 90 µM | 0.9:1 | 0.87 | 0.063 | slight synergism |
| 1.8:1 | 0.72 | 0.166 | moderate synergism | ||||
| DOX | 20.88:1 | 0.82 | 0.014 | moderate synergism | |||
| 41.76:1 | 0.74 | 0.136 | moderate synergism | ||||
| 83.52:1 | 0.83 | 0.525 | moderate synergism | ||||
| 167.05:1 | 0.13 | 0.022 | strong synergism | ||||
| T47D | V9302 | CIS | 80 µM | 0.8:1 | 0.5 | 0.06 | synergism |
| 1.6:1 | 0.87 | 0.17 | slight synergism | ||||
| 3.2:1 | 0.66 | 0.12 | synergism | ||||
| 6.4:1 | 0.78 | 0.07 | moderate synergism | ||||
| DOX | 37.12:1 | 0.73 | 0.11 | moderate synergism | |||
| KCR | V9302 | CIS | 100 µM | 4:1 | 0.89 | 0.02 | slight synergism |
| 8:1 | 0.79 | 0.03 | moderate synergism | ||||
| 32:1 | 0.88 | 2.21 | slight synergism | ||||
| MCF-7 | V9302 | DOX | 10.92 µM | 1.27:1 | 0.31 | 0.37 | synergism |
| 2.53:1 | 0.88 | 0.19 | slight synergism | ||||
| 5.07:1 | 0.84 | 0.12 | moderate synergism | ||||
| 10.13:1 | 0.67 | 0.092 | synergism | ||||
| 20.27:1 | 0.86 | 0.1 | moderate synergism |
| IC50 (μM) of Doxorubicin | SD ± | |
|---|---|---|
| V9302 IC50 + DOX | <0.033 | - |
| V9302 IC50/2 + DOX | 0.685 | 0.050 |
| DOX | >17.24 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szemerédi, N.; Schelz, Z.; Horvath, D.A.; Rácz, B.; Szatmári, A.G.; Muddather, H.F.; Bózsity, N.; Zupkó, I.; Spengler, G. Impact of V9302, a Competitive Antagonist of Transmembrane Glutamine Flux on Reversal of Resistance in Breast Cancer Cell Lines. Pharmaceutics 2024, 16, 877. https://doi.org/10.3390/pharmaceutics16070877
Szemerédi N, Schelz Z, Horvath DA, Rácz B, Szatmári AG, Muddather HF, Bózsity N, Zupkó I, Spengler G. Impact of V9302, a Competitive Antagonist of Transmembrane Glutamine Flux on Reversal of Resistance in Breast Cancer Cell Lines. Pharmaceutics. 2024; 16(7):877. https://doi.org/10.3390/pharmaceutics16070877
Chicago/Turabian StyleSzemerédi, Nikoletta, Zsuzsanna Schelz, Dária Antónia Horvath, Bálint Rácz, András G. Szatmári, Hiba F. Muddather, Noémi Bózsity, István Zupkó, and Gabriella Spengler. 2024. "Impact of V9302, a Competitive Antagonist of Transmembrane Glutamine Flux on Reversal of Resistance in Breast Cancer Cell Lines" Pharmaceutics 16, no. 7: 877. https://doi.org/10.3390/pharmaceutics16070877
APA StyleSzemerédi, N., Schelz, Z., Horvath, D. A., Rácz, B., Szatmári, A. G., Muddather, H. F., Bózsity, N., Zupkó, I., & Spengler, G. (2024). Impact of V9302, a Competitive Antagonist of Transmembrane Glutamine Flux on Reversal of Resistance in Breast Cancer Cell Lines. Pharmaceutics, 16(7), 877. https://doi.org/10.3390/pharmaceutics16070877








