Cyclodextrins: Enhancing Drug Delivery, Solubility and Bioavailability for Modern Therapeutics
Abstract
1. Introduction
2. The Effect of Cyclodextrins over the Encapsulated Active Substance
2.1. Changes in Physical-Chemical Properties
2.2. Change in Solubility
2.2.1. Mechanism of Action of Cyclodextrins in Improving Solubility
2.2.2. Impact on Bioavailability
- Oral formulations
- Ophthalmic formulations
- Parenteral formulations
- Solutions for chronic diseases
2.3. A Reduction in Side Effects
2.3.1. Stabilizing and Protecting the Drug
2.3.2. Controlled Release of the Drug
2.4. Reducing Local Toxicity
2.5. Detoxifying Active Compounds
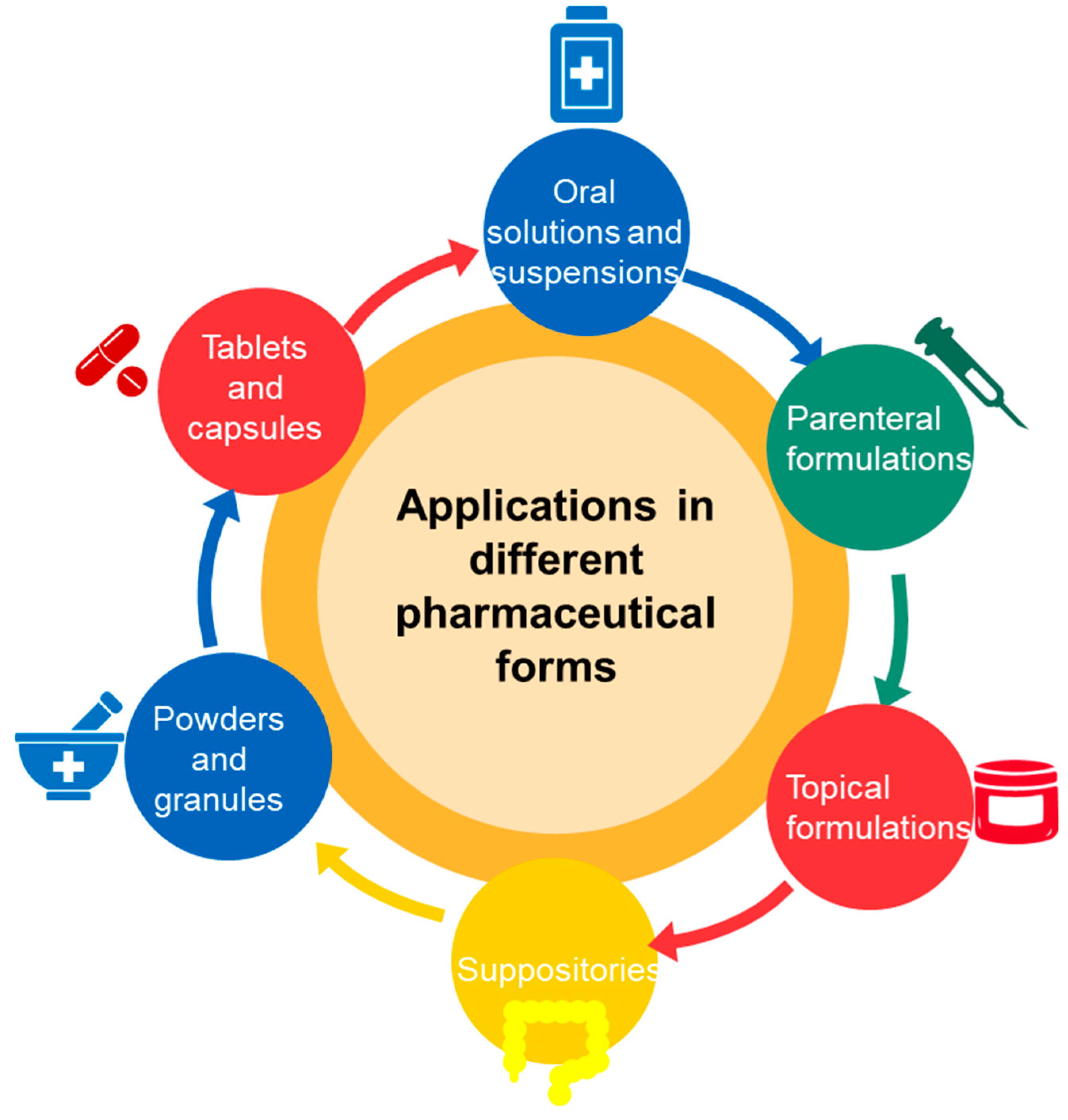
3. Pharmaceutical Forms with Cyclodextrins
3.1. Tablets
3.2. Capsules
| Active Substance | Trade Name | Cyclodextrin | Effect of Cyclodextrin | Indication | Reference |
|---|---|---|---|---|---|
| 4-Androstenediol | Androtest | HP-β-CD | Better water solubility of inclusion complex, increased bioavailability | Supplement | [88] |
| Benexate HCl | Ulgut, Lonmiel | β-CD | Increase in solubility and strong antiulcer activity | Gastritis | [89] |
| Curcumin extract | Curcumin Extract 45 | γ-CD | Improved absorption of curcuminoids and bioavailability | Supplement | [90] |
| Fingolimod | Fingolimod beta | β-CD | Up to 20x increase in solubility | Multiple sclerosis | [66,91] |
| Omeprazole | Omebeta, Losamel, enteric capsule | β-CD | Improved bioavailability | Ulcers | [87] |
3.3. Powders and Granules
3.4. Oral Solutions and Suspensions
| Active Substance | Trade Name | Cyclodextrin | Effect of Cyclodextrin | Indication | Reference |
|---|---|---|---|---|---|
| Itraconazole | Sporanox | HP-β-CD | Increase water solubility and bioavailability | Fungal infections | [103,104] |
| Larotrectinib sulfate | Vitrakvi | HP-β-CD | Solubilizing agent | Pediatric cancer | [106] |
| Midazolam | Ozalin | γ-CD | Increase midazolam solubility, mask bitter taste of solution | Premedication before anesthesia, in pediatric population | [107,108] |
| Piroxicam | Flogene | β-CD | Increased solubility, fast absorption and improved gastric tolerability | Analgesic for pediatric use | [86,109] |
3.5. Parenteral Formulations
| Active Substance | Trade Name and Formulation | Cyclodextrin | Effect of Cyclodextrin | Indication | Reference |
|---|---|---|---|---|---|
| Amiodarone | Nexterone intravenous solution | SBE-β-CD | Solubilizing agent. The inclusion complex solution is chemically stable upon storage time | Treatment and prophylaxis of frequently recurring ventricular fibrillation | [116] |
| Aripiprazole | Abilify, solution for injection (intramuscular) | SBE-β-CD | Increased water solubility | Schizophrenia | [69,113] |
| Diclofenac | Dyloject® (intramuscular and intravenous use) | HP-β-CD | Increase the solubility of the drug, resulting in less clinical thrombophlebitis | Rheumatic and non-rheumatic pain | [117,118] |
| Diclofenac | Akis subcutaneous and intramuscular use) | HP-β-CD | Good stability, solubility, and bioavailability of the preparations | Rheumatic and non-rheumatic pain | [117,118] |
| Fosphenytoin | Sesquient, Cerebyx | SBE-β-CD | Improved stability and solubility | Epilepsy | [114,115] |
| PGE1 (Alprostadil) | Alprostadil Alphadex, Caverject intracavernous injection | α-CD | Increased stability | Buerger disease, male erectile dysfunction | [66,81,119] |
| Posaconazole | Noxafil | SBE-β-CD | Improved solubility and stability | Anti-infective applications | [120] |
| Progesterone | Lubion® solution for intramuscular and subcutaneous injection | HP-β-CD | Increase in the solubility of progesterone, resulting in improved bioavailability | Luteal phase support during assisted reproductive technology | [118] |
| Remdesivir | Veklury, powder for solution for infusion | SBE-β-CD | Increased solubility, stability, bioavailability | COVID-19 | [81,121,122] |
| Voriconazole | Vfend, powder for solution for infusion | SBE-β-CD | Increased solubility, better safety profile (no deterioration of renal function) in patients with renal failure | Fungal infections | [123,124] |
| Ziprasidone mesylate | Geodon, powder for solution for injection | SBE-β-CD | Solubilizing agent | Schizophrenia | [125] |
3.6. Topical Formulations
| Active Substance | Trade Name | Cyclodextrin | Effect of Cyclodextrin | Indication | Reference |
|---|---|---|---|---|---|
| Benzoyl peroxide | Nujevi Acne | γ-CD | Decrease drug degradation | Acne | [132] |
| Dexamethasone | Glymesason, ointment | β-CD | Increased solubility up to 33 fold in a 1:1 complex | Dermatitis | [66,81] |
| Metronidazole | Metrogel, gel | β-CD | Solubility enhancer | Rosacea | [135] |
| Salicylic acid | Age Defying Blemish Treatment, Lipo™ CD-SA | HP-β-CD | Reduces skin irritation and stinging | Skin care | [136] |
| Active Substance | Trade Name | Cyclodextrin | Effect of Cyclodextrin over the Active Substance | Indication | Reference |
|---|---|---|---|---|---|
| Chloramphenicol | Clorocil | Methyl-β-cyclodextrin | Increased stability | Eye infections | [137,138] |
| Diclofenac | Voltaren Ophtha Eye Drops | HP-γ-CD | Drug solubility enhancer and penetration promoter, reduction in ocular toxicity | Pain and inflammation after ocular surgery and seasonal allergic conjunctivitis | [69,139] |
| Lanosterol | Lanomax | HP-β-CD | Increased water solubility, improved bioavailability | Cataract therapy for dogs | [140] |
| Levocabastine | Allergiflash | HP-β-CD | Solubilizing agent, increases bioavailability | Allergic conjunctivitis | [141] |
| Olopatadine | Opatanol | HP-β-CD | Increases stability by preventing precipitation or crystallization | Allergic conjunctivitis | [142] |
3.7. Suppositories
3.8. Aerosols
3.9. Transdermal Patches
3.10. Nanotechnology-Based Drug Delivery Systems
4. The Use of Cyclodextrins in Various Diseases
4.1. Neurodegenerative Disorders
4.1.1. Improved Drug Delivery to the Brain
4.1.2. Niemann–Pick Disease Type C (NPC)
4.1.3. Alzheimer’s Disease
4.1.4. Parkinson’s Disease
4.1.5. A Reduction in Oxidative Stress and Neuroinflammation
4.2. Respiratory Diseases
4.2.1. Improving the Delivery of Inhalation Drugs
4.2.2. Reducing Corticosteroid Toxicity
4.2.3. Cystic Fibrosis
4.2.4. Chronic Obstructive Pulmonary Disease (COPD)
4.2.5. Reducing the Irritating Effects of Inhaled Drugs
4.3. Cancer Therapy
4.3.1. Improving Drug Solubility and Bioavailability
4.3.2. A Reduction in Toxicity Associated with Chemotherapy
4.3.3. Targeted Therapy
4.3.4. Bioactive Molecules Transport (Genes or RNA)
4.3.5. Reducing Drug Resistance
4.4. Cardiovascular Diseases
4.4.1. Reducing Cardiac Toxicity
4.4.2. Regulating Lipid Metabolism
4.4.3. Formulation of Antihypertensive Medication
4.4.4. Therapy of Heart Failure
4.4.5. Prevention of Cardiovascular Inflammation
4.5. Infectious Diseases
4.5.1. Improving the Solubility of Antibiotics
4.5.2. Side Effects Reduction
4.5.3. Targeted Drug Delivery
4.5.4. Treatment of Fungal Infections
4.5.5. Combating Drug Resistance
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sarabia-Vallejo, Á.; Caja, M.D.M.; Olives, A.I.; Martín, M.A.; Menéndez, J.C. Cyclodextrin Inclusion Complexes for Improved Drug Bioavailability and Activity: Synthetic and Analytical Aspects. Pharmaceutics 2023, 15, 2345. [Google Scholar] [CrossRef]
- Gonzalez Pereira, A.; Carpena, M.; García Oliveira, P.; Mejuto, J.C.; Prieto, M.A.; Simal Gandara, J. Main Applications of Cyclodextrins in the Food Industry as the Compounds of Choice to Form Host-Guest Complexes. Int. J. Mol. Sci. 2021, 22, 1339. [Google Scholar] [CrossRef]
- Tsunoda, C.; Hasegawa, K.; Hiroshige, R.; Kasai, T.; Yokoyama, H.; Goto, S. Effect of cyclodextrin complex formation on solubility changes of each drug due to intermolecular interactions between acidic NSAIDs and basic H2 blockers. Mol. Pharm. 2023, 20, 5032–5042. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Roy, A.; Roy, K.; Roy, M.N. Study to explore the mechanism to form inclusion complexes of β-cyclodextrin with vitamin molecules. Sci. Rep. 2016, 6, 35764. [Google Scholar] [CrossRef]
- Ghitman, J.; Voicu, S.I. Controlled drug delivery mediated by cyclodextrin-based supramolecular self-assembled carriers: From design to clinical performances. Carbohydr. Polym. Technol. Appl. 2023, 5, 100266. [Google Scholar] [CrossRef]
- Vilanova, N.; Solans, C. Vitamin A Palmitate-β-cyclodextrin inclusion complexes: Characterization, protection and emulsification properties. Food Chem. 2015, 175, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Michalska, P.; Wojnicz, A.; Ruiz-Nuño, A.; Abril, S.; Buendia, I.; León, R. Inclusion complex of ITH12674 with 2-hydroxypropyl-β-cyclodextrin: Preparation, physical characterization and pharmacological effect. Carbohydr Polym. 2017, 157, 94–104. [Google Scholar] [CrossRef]
- Jamrógiewicz, M.; Józefowicz, M. Preparation and Characterization of Indomethacin Supramolecular Systems with β-Cyclodextrin in Order to Estimate Photostability Improvement. Molecules 2021, 26, 7436. [Google Scholar] [CrossRef] [PubMed]
- Sabari, C.L.; Rajamohan, R.; Prabu, S.; Sivakumar, K. Molecular encapsulation of lidocaine and procaine into β-cyclodexrin cavity: In vitro cytotoxic evaluation. J. Macromol. Sci. A 2019, 56, 215–224. [Google Scholar] [CrossRef]
- Luppi, F.; Cavaye, H.; Dossi, E. Nitrated Cross-linked β-Cyclodextrin Binders Exhibiting Low Glass Transition Tempratures. Propellants Explos. Pyrotech. 2018, 43, 1023–1031. [Google Scholar] [CrossRef]
- Szejtli, J.; Bolla-Pusztai, E.; Szabó, P.; Ferenczy, T. Enhancement of stability and biological effect on cholecalciferol by β-cyclodextrin complexation. Pharmazie 1980, 35, 779–787. [Google Scholar]
- Real, D.A.; Bolaños, K.; Priotti, J.; Yutronic, N.; Kogan, M.J.; Sierpe, R.; Donoso-González, O., O. Cyclodextrin-Modified Nanomaterials for Drug Delivery: Classification and Advances in Controlled Release and Bioavailability. Pharmaceutics 2021, 13, 2131. [Google Scholar] [CrossRef] [PubMed]
- Manne, A.S.N.; Hegde, A.R.; Raut, S.Y.; Rao, R.R.; Kulkarni, V.I.; Mutalik, S. Hot liquid extrusion assisted drug-cyclodextrin complexation: A novel continuous manufacturing method for solubility and bioavailability enhancement of drugs. Drug Deliv. Transl. Res. 2021, 11, 1273–1287. [Google Scholar] [CrossRef]
- Rajagopalan, N.; Chen, S.C.; Chow, W.-S. A study of the inclusion complex of amphotericin-B with γ-cyclodextrin. Int. J. Pharm. 1986, 29, 161–168. [Google Scholar] [CrossRef]
- Cirri, M.; Mennini, N.; Nerli, G.; Rubia, J.; Casalone, E.; Melani, F.; Maestrelli, F.; Mura, P. Combined Use of Cyclodextrins and Amino Acids for the Development of Cefixime Oral Solutions for Pediatric Use. Pharmaceutics 2021, 13, 1923. [Google Scholar] [CrossRef] [PubMed]
- Yurtdaş-Kırımlıoğlu, G. Spray dried nanospheres for inclusion complexes of cefpodoxime proxetil with β-cyclodextrin, 2-hydroxypopyl-β-cyclodextrin and methyl-β-cyclodextrin: Improved dissolution and enhanced antibacterial activity. Drug Dev. Ind. Pharm. 2021, 47, 1261–1278. [Google Scholar] [CrossRef]
- Liu, H.; Gao, C.; Qu, Z.; Liu, M.; Zhao, S. Preparation Method of Ceftiofur Acid Long-Acting Injection. Patent CN103230364A, 11 June 2014. [Google Scholar]
- Racaniello, G.F.; Balenzano, G.; Arduino, I.; Iacobazzi, R.M.; Lopalco, A.; Lopedota, A.A.; Sigurdsson, H.H.; Denora, N. Chitosan and Anionic Solubility Enhancer Sulfobutylether-β-Cyclodextrin-Based Nanoparticles as Dexamethasone Ophthalmic Delivery System for Anti-Inflammatory Therapy. Pharmaceutics 2024, 16, 277. [Google Scholar] [CrossRef] [PubMed]
- Oshite, Y.; Wada-Hirai, A.; Ichii, R.; Kuroda, C.; Hasegawa, K.; Hiroshige, R.; Yokoyama, H.; Tsuchida, T.; Goto, S. Comparative study on the effects of the inclusion complexes of non-steroidal anti-inflammatory drugs with 2-hydroxypropyl-β-cyclodextrins on dissociation rates and supersaturation. RSC Pharm. 2024, 1, 80–97. [Google Scholar] [CrossRef]
- Upadhyay, C.; D’Souza, A.; Patel, P.; Verma, V.; Upadhayay, K.K.; Bharkatiya, M. Inclusion Complex of Ibuprofen-β-Cyclodextrin Incorporated in Gel for Mucosal Delivery: Optimization Using an Experimental Design. AAPS PharmSciTech 2023, 24, 100. [Google Scholar] [CrossRef]
- Şuta, L.-M.; Ridichie, A.; Ledeţi, A.; Temereancă, C.; Ledeţi, I.; Muntean, D.; Rădulescu, M.; Văruţ, R.-M.; Watz, C.; Crăineanu, F.; et al. Host–Guest Complexation of Itraconazole with Cyclodextrins for Bioavailability Enhancement. Pharmaceutics 2024, 16, 560. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Li, B.; Yang, S.; Ma, P.; Wang, Z. Preparation and Cyclodextrin Solubilization of the Antibacterial Agent Benzoyl Metronidazole. Sci. World J. 2013, 2013, 306476. [Google Scholar] [CrossRef] [PubMed]
- Jagdale, S.C.; Jadhav, V.N.; Chabukswar, A.R.; Kuchekar, B.S. Solubility enhancement, physicochemical characterization and formulation of fast-dissolving tablet of nifedipine-betacyclodextrin complexes. Braz. J. Pharm. Sci. 2012, 48, 131–145. [Google Scholar] [CrossRef]
- Mognetti, B.; Barberis, A.; Marino, S.; Berta, G.N.; Francia, S.D.; Trotta, F.; Cavalli, R. In vitro enhancement of anticancer activity of paclitaxel by a cremophor free cyclodextrin-based nanosponge formulation. J. Incl. Phenom. Macrocycl. Chem. 2012, 74, 201–210. [Google Scholar] [CrossRef]
- Jagdale, S.C.; Kulkarni, A.; Chabukswar, A.; Kuchekar, B. Design and Evaluation of Microwave Induced Solid Dispersion of Tinidazole and Molecular Modelling with β-cyclodextrin. Lett. Drug Des. Discov. 2016, 13, 781–792. [Google Scholar] [CrossRef]
- Sandilya, A.A.; Natarajan, U.; Priya, M.H. Molecular View into the Cyclodextrin Cavity: Structure and Hydration. ACS Omega 2020, 5, 25655–25667. [Google Scholar] [CrossRef] [PubMed]
- Musuc, A.M. Cyclodextrins: Advances in Chemistry, Toxicology, and Multifaceted Applications. Molecules 2024, 29, 5319. [Google Scholar] [CrossRef]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Skiba, M.; Bouchal, F.; Boukhris, T.; Bounoure, F.; Fessi, H.; Chaffai, N.; Lahiani-Skiba, M. Pharmacokinetic study of an oral piroxicam formulation containing different molar ratios of β-cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2013, 75, 311–314. [Google Scholar] [CrossRef]
- Islam, M.S.; Narurkar, M.M. The Effect of 2-Hydroxypropyl-βcyclodextrin on the Solubility, Stability and Dissolution Rate of Famotidine. Drug Dev. Ind. Pharm. 1991, 17, 1229–1239. [Google Scholar] [CrossRef]
- Wang, X.; Parvathaneni, V.; Shukla, S.K.; Kanabar, D.D.; Muth, A.; Gupta, V. Cyclodextrin Complexation for Enhanced Stability and Non-Invasive Pulmonary Delivery of Resveratrol—Applications in Non-Small Cell Lung Cancer Treatment. AAPS PharmSciTech 2020, 21, 183. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lin, H.S.; Chan, S.Y.; Ho, P.C. Biopharmaceutics of β-cyclodextrin derivative-based formulations of acitretin in Sprague–Dawley rats. J. Pharm. Sci. 2004, 93, 805–815. [Google Scholar] [CrossRef]
- Brewster, M.E.; Vandecruys, R.; Peeters, J.; Neeskens, P.; Verreck, G.; Loftsson, T. Comparative interaction of 2-hydroxypropyl-β-cyclodextrin and sulfobutylether-β-cyclodextrin with itraconazole: Phase-solubility behavior and stabilization of supersaturated drug solutions. Eur. J. Pharm. Sci. 2008, 34, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Morina, D.; Sessevmez, M.; Sinani, G.; Mülazımoğlu, L.; Cevher, E. Oral tablet formulations containing cyclodextrin complexes of poorly water soluble cefdinir to enhance its bioavailability. J. Drug Deliv. Sci. Technol. 2020, 57, 101742. [Google Scholar] [CrossRef]
- Kesavan, K.; Kant, S.; Singh, P.N.; Pandit, J.K. Effect of hydroxypropyl-β-cyclodextrin on the ocular bioavailability of dexamethasone from a pH-induced mucoadhesive hydrogel. Curr. Eye Res. 2011, 36, 918–929. [Google Scholar] [CrossRef] [PubMed]
- Soliman, O.A.E.A.; Mohamed, E.A.M.; El-Dahan, M.S.; Khatera, N.A.A. Potential use of cyclodextrin complexes for enhanced stability, anti-inflammatory efficacy, and ocular bioavailability of loteprednol etabonate. AAPS PharmSciTech 2017, 18, 1228–1241. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Jain, S.; Pant, A.; Sharma, A.; Kumar, R.; Singla, N.; Suttee, A.; Kumar, S.; Barnwal, R.P.; Katare, O.P.; et al. Cyclodextrin Derivative Enhances the Ophthalmic Delivery of Poorly Soluble Azithromycin. ACS Omega 2022, 7, 23050–23060. [Google Scholar] [CrossRef]
- Ferreira, L.; Campos, J.; Veiga, F.; Cardoso, C.; Paiva-Santos, A.C. Cyclodextrin-based delivery systems in parenteral formulations: A critical update review. Eur. J. Pharm. Biopharm. 2022, 178, 35–52. [Google Scholar] [CrossRef] [PubMed]
- López-Castillo, C.; Rodríguez-Fernández, C.; Córdoba, M.; Torrado, J.J. Permeability Characteristics of a New Antifungal Topical Amphotericin B Formulation with γ-Cyclodextrins. Molecules 2018, 23, 3349. [Google Scholar] [CrossRef] [PubMed]
- Egan, T.D.; Kern, S.E.; Johnson, K.B.; Pace, N.L. The pharmacokinetics and pharmacodynamics of propofol in a modified cyclodextrin formulation (Captisol®) versus propofol in a lipid formulation (Diprivano®): An electroencephalographic and hemodynamic study in a porcine model. Anesth. Analg. 2003, 97, 72–79. [Google Scholar] [CrossRef]
- Motoyama, K.; Onodera, R.; Okamatsu, A.; Higashi, T.; Kariya, R.; Okada, S.; Arima, H. Potential use of the complex of doxorubicin with folate-conjugated methyl-β-cyclodextrin for tumor-selective cancer chemotherapy. J. Drug Target. 2014, 22, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Hyun, H.; Lee, S.; Lim, W.; Jo, D.; Jung, J.S.; Jo, G.; Kim, S.Y.; Lee, D.-W.; Um, S.; Yang, D.H.; et al. Engineered beta-cyclodextrin-based carrier for targeted doxorubicin delivery in breast cancer therapy in vivo. J. Ind. Eng. Chem. 2019, 70, 145–151. [Google Scholar] [CrossRef]
- Miranda, G.M.; Santos, V.O.R.E.; Bessa, J.R.; Teles, Y.C.F.; Yahouédéhou, S.C.M.A.; Goncalves, M.S.; Ribeiro-Filho, J. Inclusion Complexes of Non-Steroidal Anti-Inflammatory Drugs with Cyclodextrins: A Systematic Review. Biomolecules 2021, 11, 361. [Google Scholar] [CrossRef]
- Frijlink, H.W.; Franssen, E.J.; Eissens, A.C.; Oosting, R.; Lerk, C.F.; Meijer, D.K. The effects of cyclodextrins on the disposition of intravenously injected drugs in the rat. Pharm. Res. 1991, 8, 380–384. [Google Scholar] [CrossRef]
- Pomponio, R.; Gotti, R.; Fiori, J.; Cavrini, V.; Mura, P.; Cirri, M.; Maestrelli, F. Photostability studies on nicardipine-cyclodextrin complexes by capillary electrophoresis. J. Pharm. Biomed. Anal. 2004, 35, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Saokham, P.; Burapapadh, K.; Praphanwittaya, P.; Loftsson, T. Characterization and Evaluation of Ternary Complexes of Ascorbic Acid with γ-Cyclodextrin and Poly(vinyl alcohol). Int. J. Mol. Sci. 2020, 21, 4399. [Google Scholar] [CrossRef] [PubMed]
- Belhocine, Y.; Rahali, S.; Allal, H.; Assaba, I.M.; Ghoniem, M.G.; Ali, F.A.M. A Dispersion Corrected DFT Investigation of the Inclusion Complexation of Dexamethasone with β-Cyclodextrin and Molecular Docking Study of Its Potential Activity against COVID-19. Molecules 2021, 26, 7622. [Google Scholar] [CrossRef]
- Hirayama, F.; Uekama, K. Cyclodextrin-based controlled drug release system. Adv. Drug Deliv. Rev. 1999, 36, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Yoshinari, M.; Matsuzaka, K.; Hashimoto, S.; Ishihara, K.; Inoue, T.; Oda, Y.; Ide, T.; Tanaka, T. Controlled Release of Simvastatin Acid Using Cyclodextrin Inclusion System. Dent. Mater. J. 2007, 26, 451–456. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zheng, D.; Xia, L.; Ji, H.; Jin, Z.; Bai, Y. A Cyclodextrin-Based Controlled Release System in the Simulation of In Vitro Small Intestine. Molecules 2020, 25, 1212. [Google Scholar] [CrossRef]
- Fernandes, C.M.; Ramos, P.; Falcão, A.C.; Veiga, F.J. Hydrophilic and hydrophobic cyclodextrins in a new sustained release oral formulation of nicardipine: In vitro evaluation and bioavailability studies in rabbits. J. Control. Release 2003, 88, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Chowdary, K.P.R.; Kamalakara, R.G. Controlled release of nifedipine from mucoadhesive tablets of its inclusion complexes with beta-cyclodextrin. Pharmazie 2003, 58, 721–724. [Google Scholar] [PubMed]
- Moya-Ortega, M.D.; Alves, T.F.; Alvarez-Lorenzo, C.; Concheiro, A.; Stefánsson, E.; Thorsteinsdóttir, M.; Loftsson, T. Dexamethasone eye drops containing γ-cyclodextrin-based nanogels. Int. J. Pharm. 2013, 441, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, B.; Sun, J. The Solubility-Permeability Trade-Off of Progesterone With Cyclodextrins Under Physiological Conditions: Experimental Observations and Computer Simulations. J. Pharm. Sci. 2018, 107, 488–494. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, X.; Hu, W.; Bai, Y.; Zhang, L. Preparation and evaluation of naringenin-loaded sulfobutylether-β-cyclodextrin/chitosan nanoparticles for ocular drug delivery. Carbohydr. Polym. 2016, 149, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Cutrín-Gómez, E.; Conde-Penedo, A.; Anguiano-Igea, S.; Gómez-Amoza, J.L.; Otero-Espinar, F.J. Optimization of Drug Permeation from 8% Ciclopirox Cyclodextrin/Poloxamer-Soluble Polypseudorotaxane-Based Nail Lacquers. Pharmaceutics 2020, 12, 231. [Google Scholar] [CrossRef] [PubMed]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef] [PubMed]
- Kfoury, M.; Lichtfouse, E.; Fourmentin, S. The revival of cyclodextrins as active pharmaceutical ingredients. Environ. Chem. Lett. 2024, 23, 1–6. [Google Scholar] [CrossRef]
- Jakšić, D.; Šegvić Klarić, M.; Rimac, H.; Kerep, R.; Piantanida, I. Cyclodextrin-Based Displacement Strategy of Sterigmatocystin from Serum Albumin as a Novel Approach for Acute Poisoning Detoxification. Int. J. Mol. Sci. 2023, 24, 4485. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Yamada, Y.; Ishitsuka, Y.; Matsuo, M.; Shiraishi, K.; Wada, K.; Uchio, Y.; Kondo, Y.; Takeo, T.; Nakagata, N.; et al. Efficacy of 2-Hydroxypropyl-β-cyclodextrin in Niemann-Pick Disease Type C Model Mice and Its Pharmacokinetic Analysis in a Patient with the Disease. Biol. Pharm. Bull. 2015, 38, 844–851, Erratum in Biol. Pharm. Bull. 2016, 39, 455. [Google Scholar] [CrossRef]
- Kalakuntla, R.K.; Wille, T.; Le Provost, R.; Letort, S.; Reiter, G.; Müller, S.; Thiermann, H.; Worek, F.; Gouhier, G.; Lafont, O.; et al. New modified β-cyclodextrin derivatives as detoxifying agents of chemical warfare agents (I). Synthesis and preliminary screening: Evaluation of the detoxification using a half-quantitative enzymatic assay. Toxicol. Lett. 2013, 216, 200–205. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Saber, H. Recent advances in the treatment of organophosphorous poisonings. Iran. J. Med. Sci. 2012, 37, 74–91. [Google Scholar] [PubMed] [PubMed Central]
- Conceição, J.; Adeoye, O.; Cabral-Marques, H.M.; Sousa Lobo, J.M. Cyclodextrins as excipients in tablet formulations. Drug Discov. Today 2018, 23, 1274–1284. [Google Scholar] [CrossRef]
- Araj, S.K.; Szeleszczuk, Ł. A Review on Cyclodextrins/Estrogens Inclusion Complexes. Int. J. Mol. Sci. 2023, 24, 8780. [Google Scholar] [CrossRef] [PubMed]
- Adamkiewicz, L.; Szeleszczuk, Ł. Review of Applications of Cyclodextrins as Taste-Masking Excipients for Pharmaceutical Purposes. Molecules 2023, 28, 6964. [Google Scholar] [CrossRef]
- Kali, G.; Haddadzadegan, S.; Bernkop-Schnürch, A. Cyclodextrins and derivatives in drug delivery: New developments, relevant clinical trials, and advanced products. Carbohydr. Polym. 2024, 324, 121500. [Google Scholar] [CrossRef]
- Paczkowska, M.; Mizera, M.; Lewandowska, K.; Kozak, M.; Miklaszewski, A.; Cielecka-Piontek, J. Effects of inclusion of cetirizine hydrochloride in β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2018, 91, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Akito, E.; Nakajima, Y.; Horioka, M. Nitroglycerine Inclusion Compounds with Cyclodextrin and Composition Containing Same. Patent US4073931A, 14 February 1978. [Google Scholar]
- Puskás, I.; Szente, L.; Szőcs, L.; Fenyvesi, É. Recent List of Cyclodextrin-Containing Drug Products. Period. Polytech. Chem. Eng. 2023, 67, 11–17. [Google Scholar] [CrossRef]
- Inoue, R. Pharmaceutical Composition Containing Olmesartan Medoxomil. Patent JP5871984B2, 1 March 2016. [Google Scholar]
- Cuypers, S.; Berwaer, M.; Fanara, D.; Barillaro, V.; Larbanoix, M.; Dargelas, F. Pharmaceutical Compositions Comprising 2-Oxo-1-pyrrolidine Derivatives. Patent US20110275693A1, 10 November 2011. [Google Scholar]
- Carneiro, S.B.; Costa Duarte, F.Í.; Heimfarth, L.; Siqueira Quintans, J.D.S.; Quintans-Júnior, L.J.; Veiga Júnior, V.F.D.; Neves de Lima, Á.A. Cyclodextrin–Drug Inclusion Complexes: In Vivo and In Vitro Approaches. Int. J. Mol. Sci. 2019, 20, 642. [Google Scholar] [CrossRef] [PubMed]
- Stojanov, M.; Wimmer, R.; Larsen, K.L. Study of the inclusion complexes formed between cetirizine and α-, β-, and γ-cyclodextrin and evaluation on their taste-masking properties. J. Pharm. Sci. 2011, 100, 3177–3185. [Google Scholar] [CrossRef] [PubMed]
- Stojanov, M.; Larsen, K.L. Cetirizine release from cyclodextrin formulated compressed chewing gum. Drug Dev. Ind. Pharm. 2012, 38, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, M.; Joginapalli, S.; Billa, P.R. Desloratadine-Containing Formulation Stabilized with Cyclodextrin. Patent WO2007/096733, 30 August 2007. [Google Scholar]
- Backensfeld, T.; Heil, W. Beta-Cyclodextrin-Drospirenone Inclusion Complexes. Patent EP1353699B1, 22 October 2003. [Google Scholar]
- Patel, A.R.; Vavia, P.R. Preparation and evaluation of taste masked famotidine formulation using drug/β-cyclodextrin/polymer ternary complexation approach. AAPS PharmSciTech 2008, 9, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Bocanegra, M.; Seijas, A.; Yibirín, M.G. Efficacy and tolerability of conventional nimesulide versus Beta-cyclodextrin nimesulide in patients with pain after surgical dental extraction: A multicenter, prospective, randomized, double-blind, double-dummy study. Curr. Ther. Res. Clin. Exp. 2003, 64, 279–289. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vizzardi, M.; Sagarriga Visconti, C.; Pedrotti, L.; Marzano, N.; Berruto, M.; Scotti, A. Nimesulide beta cyclodextrin (nimesulide-betadex) versus nimesulide in the treatment of pain after arthroscopic surgery. Curr. Ther. Res. 1998, 59, 162–171. [Google Scholar] [CrossRef]
- Umemura, M.; Ueda, H.; Tomono, K.; Nagai, T. Effect of diethyl-beta-cyclodextrin on the release of nitroglycerin from formulations. Drug Des. Deliv. 1990, 6, 297–310. [Google Scholar] [PubMed]
- Rincón-López, J.; Almanza-Arjona, Y.C.; Riascos, A.P.; Rojas-Aguirre, Y. Technological evolution of cyclodextrins in the pharmaceutical field. J. Drug Deliv. Sci. Technol. 2021, 61, 102156. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Sekiya, N.; Katayama, K.; Narutaki, S.; Yamamoto, M.; Iohara, D.; Hirayama, F.; Uekama, K. Stabilizing effect of β-cyclodextrin on Limaprost, a PGE1 derivative, in Limaprost alfadex tablets (Opalmon®) in highly humid conditions. Chem. Pharm. Bull. 2014, 62, 786–792. [Google Scholar] [CrossRef][Green Version]
- Chavanpatil, M.; Dawre, F.D.; Shakleya, D.S.; Vavia, P.R. Enhancement of Oral Bioavailability of Rofecoxib Using β-Cyclodextrin. J. Incl. Phenom. Macrocycl. 2002, 44, 145–149. [Google Scholar] [CrossRef]
- Abou-Taleb, A.; Abdel-Rhman, A.; Samy, E.; Tawfeek, H. Interaction of rofecoxib with β-cyclodextrin and HP-β-cyclodextrin in aquoues solution and in solid state. Bull. Pharm. Sci. Assiut Univ. 2006, 29, 236–252. [Google Scholar] [CrossRef][Green Version]
- Cohen, G.; Dubois, J.L. New Complexes of Tiaprofenic Acid or Its Insoluble or Partially Soluble Esters with Cyclodextrines or Their Derivates. Patent EP0449722A1, 27 March 1990. [Google Scholar]
- Bhargav, A. Beta-Cyclodextrin As An Excipient In Drug Formulation. Asian J. Pharm. Res. Develop. 2021, 9, 122–127. [Google Scholar] [CrossRef]
- Geng, L.; Han, L.; Huang, L.; Wu, Z.; Wu, Z.; Qi, X. High Anti-Acid Omeprazole Lightweight Capsule for Gastro-Enteric System Acid-Related Disorders Treatment. J. Clin. Gastroenterol. Treat. 2019, 5, 068. [Google Scholar] [CrossRef]
- Schwarz, H.D.; Engelke, A.; Wenz, G. Solubilizing steroidal drugs by β-cyclodextrin derivatives. Int. J. Pharm. 2017, 531, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Muranushi, N.; Yoshida, M.; Kinoshita, H.; Hirose, F.; Fukuda, T.; Doteuchi, M.; Yamada, H. Studies on benexate.CD: Effect of inclusion compound formation on the antiulcer activity of benexate, the effective ingredient of benexate·CD. Nihon Yakurigaku Zasshi. 1988, 91, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Purpura, M.; Lowery, R.P.; Wilson, J.M.; Mannan, H.; Münch, G.; Razmovski-Naumovski, V. Analysis of different innovative formulations of curcumin for improved relative oral bioavailability in human subjects. Eur. J. Nutr. 2018, 57, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Garibyan, A.A.; Delyagina, E.S.; Antipova, M.L.; Odintsova, E.G.; Petrenko, V.E.; Terekhova, I.V. Experimental and Theoretical Investigation of Inclusion Complexes of β-Cyclodextrin with Fingolimod. Russ. J. Phys. Chem. 2023, 97, 469–476. [Google Scholar] [CrossRef]
- Rassu, G.; Sorrenti, M.; Catenacci, L.; Pavan, B.; Ferraro, L.; Gavini, E.; Bonferoni, M.C.; Giunchedi, P.; Dalpiaz, A. Versatile Nasal Application of Cyclodextrins: Excipients and/or Actives? Pharmaceutics 2021, 13, 1180. [Google Scholar] [CrossRef] [PubMed]
- Arias, M.J.; Moyano, J.R.; Muñoz, P.; Ginés, J.M.; Justo, A.; Giordano, F. Study of omeprazole-γ-cyclodextrin complexation in the solid state. Drug. Dev. Ind. Pharm. 2000, 26, 253–259. [Google Scholar] [CrossRef]
- Reno, F.E.; Normand, P.; McInally, K.; Silo, S.; Stotland, P.; Triest, M.; Carballo, D.; Piché, C. A novel nasal powder formulation of glucagon: Toxicology studies in animal models. BMC Pharmacol. Toxicol. 2015, 16, 29. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Varga, P.; Németh, A.; Zeiringer, S.; Roblegg, E.; Budai-Szűcs, M.; Balla-Bartos, C.; Ambrus, R. Formulation and investigation of differently charged β-cyclodextrin-based meloxicam potassium containing nasal powders. Eur. J. Pharm. Sci. 2024, 202, 106879. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, V.B.; Patel, J.K. Cyclodextrin inclusion complex to enhance solubility of poorly water soluble drugs: A review. Int. J. Pharm. Sci. Res. 2013, 4, 68–76. [Google Scholar] [CrossRef]
- Hu, Y.; Xing, K.; Li, X.; Sang, S.; McClements, D.J.; Chen, L.; Long, J.; Jiao, A.; Xu, X.; Wang, J.; et al. Cyclodextrin carboxylate improves the stability and activity of nisin in a wider range of application conditions. npj Sci. Food 2023, 7, 20. [Google Scholar] [CrossRef]
- Popielec, A.; Loftsson, T. Effects of cyclodextrins on the chemical stability of drugs. Int. J. Pharm. 2017, 531, 532–542. [Google Scholar] [CrossRef]
- Mady, F.M.; Abou-Taleb, A.E.; Khaled, K.A.; Yamasaki, K.; Iohara, D.; Ishiguro, T.; Hirayama, F.; Uekama, K.; Otagiri, M. Enhancement of the aqueous solubility and masking the bitter taste of famotidine using drug/SBE-β-CyD/povidone K30 complexation approach. J. Pharm. Sci. 2010, 99, 4285–4294. [Google Scholar] [CrossRef] [PubMed]
- Malaquias, L.F.B.; Sá-Barreto, L.C.L.; Freire, D.O.; Silva, I.C.R.; Karan, K.; Durig, T.; Lima, E.M.; Marreto, R.N.; Gelfuso, G.M.; Gratieri, T.; et al. Taste masking and rheology improvement of drug complexed with β-cyclodextrin and hydroxypropyl-β-cyclodextrin by hot-melt extrusion. Carbohydr. Polym. 2018, 185, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Cirri, M.; Mura, P.; Benedetti, S.; Buratti, S. Development of a Hydroxypropyl-β-Cyclodextrin-Based Liquid Formulation for the Oral Administration of Propranolol in Pediatric Therapy. Pharmaceutics 2023, 15, 2217. [Google Scholar] [CrossRef] [PubMed]
- Markarian, B.M.; Cohen, L.G. Solution Inbuprofen Complexes, Compositions and Processes for Preparing the Same. Patent EP0274444A2, 13 July 1988. [Google Scholar]
- Loftsson, T.; Brewster, M.E.; Másson, M. Role of cyclodextrins in improving oral drug delivery. Am. J. Drug Deliv. 2004, 2, 261–275. [Google Scholar] [CrossRef]
- Alsarra, I.A.; Alanazi, F.K.; Ahmed, S.M.; Bosela, A.A.; Alhamed, S.S.; Mowafy, H.A.; Neau, S.H. Comparative study of itraconazole-cyclodextrin inclusion complex and its commercial product. Arch. Pharm. Res. 2010, 33, 1009–1017. [Google Scholar] [CrossRef]
- Shehata, I.; Al-Marzouqi, A.H.; Jobe, B.; Dowaidar, A. Enhancement of aqueous solubility of itraconazole by complexation with cyclodextrins using supercritical carbon dioxide. Can. J. Chem. 2005, 83, 1833–1838. [Google Scholar] [CrossRef]
- Cox, M.; Nanda, N. Methods of Treating Pediatric Cancers. Patent US11191766B2, 7 December 2021. [Google Scholar]
- Marçon, F.; Mathiron, D.; Pilard, S.; Lemaire-Hurtel, A.-S.; Dubaele, J.-M.; Djedaini-Pilard, F. Development and formulation of a 0.2% oral solution of midazolam containing γ-cyclodextrin. Int. J. Pharm. 2009, 379, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Lyseng-Williamson, K.A. Midazolam oral solution (Ozalin®): A profile of its use for procedural sedation or premedication before anaesthesia in children. Drugs Ther. Perspect. 2019, 35, 255–262. [Google Scholar] [CrossRef]
- Miranda, J.C.; Martins, T.E.A.; Veiga, F.; Ferraz, H.G. Cyclodextrins and ternary complexes: Technology to improve solubility of poorly soluble drugs. Braz. J. Pharm. Sci. 2011, 47, 665–681. [Google Scholar] [CrossRef]
- Loftsson, T.; Duchene, D. Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Carli, F.; Chiesi, P. Process for Preparing Piroxicam/Cyclodextrin Complexes, the Products Obtained and Their Pharmaceutical Compositions. Patent US5164380A, 17 November 1992. [Google Scholar]
- Jayaweera, S.P.E.; Wanigasinghe Kanakanamge, S.P.; Rajalingam, D.; Silva, G.N. Carfilzomib: A Promising Proteasome Inhibitor for the Treatment of Relapsed and Refractory Multiple Myeloma. Front. Oncol. 2021, 11, 740796. [Google Scholar] [CrossRef] [PubMed]
- Nerurkar, M.; Naringrekar, V. Aripiprazole Complex Formulation and Method. Patent US7550445B2, 23 June 2009. [Google Scholar]
- Tao, M.; Xu, L.; Shujuan, M. Stable Fosphenytoin Composition of Sodium and Preparation Thereof. Patent CN106265501A, 4 January 2017. [Google Scholar]
- Narisawa, S.; Stella, V.J. Increased Shelf-Life of Fosphenytoin: Solubilization of a Degradant, Phenytoin, through Complexation with (SBE)7M-β-CD. J. Pharm. Sci. 1998, 87, 926–930. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Parasmal, S.M.; Vasoya, M.M.; Kane, P.; Bhowmick, S.B.; Thennati, R. Parenteral Dosage Form of Amiodarone. Patent WO2017149552A1, 8 September 2017. [Google Scholar]
- Colucci, R.D.; Wright, C.; Mermelstein, F.H.; Gawarecki, D.G.; Carr, D.B. Dyloject®, a novel injectable diclofenac solubilised with cyclodextrin: Reduced incidence of thrombophlebitis compared to injectable diclofenac solubilised with polyethylene glycol and benzyl alcohol. Acute Pain 2009, 11, 15–21. [Google Scholar] [CrossRef]
- Scavone, C.; Bonagura, A.C.; Fiorentino, S.; Cimmaruta, D.; Cenami, R.; Torella, M.; Fossati, T.; Rossi, F. Efficacy and Safety Profile of Diclofenac/Cyclodextrin and Progesterone/Cyclodextrin Formulations: A Review of the Literature Data. Drugs R D 2016, 16, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Lucia Appleton, S.; Navarro-Orcajada, S.; Martínez-Navarro, F.J.; Caldera, F.; López-Nicolás, J.M.; Trotta, F.; Matencio, A. Cyclodextrins as Anti-inflammatory Agents: Basis, Drugs and Perspectives. Biomolecules 2021, 11, 1384. [Google Scholar] [CrossRef]
- Heimbecher, S.K.; Monteith, D.; Pipkin, J.D. Posaconazole Intravenous Solution Formulations Stabilized by Substituted β-Cyclodextrin. Patent US9358297B2, 7 June 2016. [Google Scholar]
- Anitha, A.; Rajamohan, R.; Murugan, M.; Seo, J.H. Inclusion Complexation of Remdesivir with Cyclodextrins: A Comprehensive Review on Combating Coronavirus Resistance—Current State and Future Perspectives. Molecules 2024, 29, 4782. [Google Scholar] [CrossRef] [PubMed]
- Várnai, B.; Malanga, M.; Sohajda, T.; Béni, S. Molecular interactions in remdesivir-cyclodextrin systems. J. Pharm. Biomed. Anal. 2021, 209, 114482. [Google Scholar] [CrossRef] [PubMed]
- Soe, H.M.S.H.; Kerdpol, K.; Rungrotmongkol, T.; Pruksakorn, P.; Autthateinchai, R.; Wet-Osot, S.; Loftsson, T.; Jansook, P. Voriconazole Eye Drops: Enhanced Solubility and Stability through Ternary Voriconazole/Sulfobutyl Ether β-Cyclodextrin/Polyvinyl Alcohol Complexes. Int. J. Mol. Sci. 2023, 24, 2343. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kwon, J.C.; Park, C.; Han, S.; Yim, D.S.; Choi, J.K.; Cho, S.Y.; Lee, H.J.; Park, S.H.; Choi, S.M.; et al. Therapeutic drug monitoring and safety of intravenous voriconazole formulated with sulfobutylether β-cyclodextrin in haematological patients with renal impairment. Mycoses 2016, 59, 644–651. [Google Scholar] [CrossRef]
- Kim, Y.; Oksanen, D.A.; Massefski, W., Jr.; Blake, J.F.; Duffy, E.M.; Chrunyk, B. Inclusion complexation of ziprasidone mesylate with β-cyclodextrin sulfobutyl ether. J. Pharm. Sci. 1998, 87, 1560–1567. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Masson, M. Cyclodextrins in topical drug formulations: Theory and practice. Int. J. Pharm. 2001, 225, 15–30. [Google Scholar] [CrossRef]
- Siefert, B.; Keipert, S. Influence of Alpha-cyclodextrin and hydroxyalkylated β-cyclodextrin derivatives on the in vitro corneal uptake and permeation of aqueous pilocarpine HCl solutions. J. Pharm. Sci. 1997, 86, 716–720. [Google Scholar] [CrossRef] [PubMed]
- Masson, M.; Loftsson, T.; Masson, G.; Stefansson, E. Cyclodextrins as permeation enhancers: Some theoretical evaluations and in vitro testing. J. Control. Release 1999, 59, 107–118. [Google Scholar] [CrossRef]
- Zuo, Z.; Kwon, G.; Stevenson, B.; Diakur, J.; Wiebe, L.I. Flutamide-hydroxypropyl--cyclodextrin complex: Formulation, physical characterization, and absorption using the Caco-2 in vitro model. J. Pharm. Pharmaceut. Sci. 2000, 3, 220–227. [Google Scholar]
- Salústio, P.J.; Pontes, P.; Conduto, C.; Sanches, I.; Carvalho, C.; Arrais, J.; Marques, H.M. Advanced technologies for oral controlled release: Cyclodextrins for oral controlled release. AAPS PharmSciTech 2011, 12, 1276–1292. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Stefánsson, E. Cyclodextrins in eye drop formulations: Enhanced topical delivery of corticosteroids to the eye. Acta Ophthalmol. Scand. 2002, 80, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Aiassa, V.; Garnero, C.; Zoppi, A.; Longhi, M.R. Cyclodextrins and Their Derivatives as Drug Stability Modifiers. Pharmaceuticals 2023, 16, 1074. [Google Scholar] [CrossRef] [PubMed]
- Dahabra, L.; Broadberry, G.; Le Gresley, A.; Najlah, M.; Khoder, M. Sunscreens Containing Cyclodextrin Inclusion Complexes for Enhanced Efficiency: A Strategy for Skin Cancer Prevention. Molecules 2021, 26, 1698. [Google Scholar] [CrossRef] [PubMed]
- Perassinoto, N.L.; Raponi, M.R.B.; Shitara, J.L.Y.; Kumayama, T.M. Composition Comprising Cyclodextrin as Uv- and Ir-Radiation Screen Agent. Patent EP2981336A1, 10 February 2016. [Google Scholar]
- Chang, Y.; Dow, G.J. Aqueous Compositions Containing Metronidazole. Patent 2470492C, 23 February 2010. [Google Scholar]
- Epstein, H. Skin Care Composition Including Cyclodextrin Materials and Method for Treating Skin Therewith. Patent US5885593A, 23 March 1999. [Google Scholar]
- Zuorro, A.; Fidaleo, M.; Lavecchia, R. Solubility Enhancement and Antibacterial Activity of Chloramphenicol Included in Modified β-Cyclodextrins. Bull. Korean Chem. Soc. 2010, 31, 3460–3462. [Google Scholar] [CrossRef][Green Version]
- Boczar, D.; Michalska, K. Cyclodextrin Inclusion Complexes with Antibiotics and Antibacterial Agents as Drug-Delivery Systems-A Pharmaceutical Perspective. Pharmaceutics 2022, 14, 1389. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, H.; Fathalla, Z.; Moharram, H.; Ali, T.F.S.; Pierscionek, B. Cyclodextrin Enhances Corneal Tolerability and Reduces Ocular Toxicity Caused by Diclofenac. Oxid. Med. Cell. Longev. 2018, 2018, 5260976. [Google Scholar] [CrossRef]
- Qinyuan, Z.; Yīdàn, J.E. Lanosterol Compound Ophthalmic Preparation. Patent WO2018036522A1, 1 March 2018. [Google Scholar]
- Tutwiler, G.F.; Espino, R.L. Aqueous Solution of Levocabastine for Ophthalmic Use. Patent WO1995025518A1, 28 September 1995. [Google Scholar]
- Bhowmick, S.B.; Laddha, R.N.; Khopade, S. Inclusion Complex of Olopatadine and Cyclodextrin. Patent WO2008015695A2, 7 February 2008. [Google Scholar]
- Tiwari, G.; Tiwari, R.; Rai, A.K. Cyclodextrins in delivery systems: Applications. J. Pharm. Bioallied. Sci. 2010, 2, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Kowari, K.; Hirosawa, I.; Kurai, H.; Utoguchi, N.; Fujii, M.; Watanabe, Y. Pharmacokinetics and pharmacodynamics of human chorionic gonadotropin (hCG) after rectal administration of hollow-type suppositories containing hCG. Biol. Pharm. Bull. 2002, 25, 678. [Google Scholar] [CrossRef] [PubMed]
- Saitani, E.; Selianitis, D.; Pippa, N.; Pispas, S.; Valsami, G. Cyclodextrins-block copolymer drug delivery systems: From design and development to preclinical studies. Nanotechnol. Rev. 2024, 13, 20230204. [Google Scholar] [CrossRef]
- Buvat, J.; Costa, P.; Morlier, D.; Lecocq, B.; Stegmann, B.; Albrecht, D. Double-blind multicenter study comparing alprostadil α-cyclodextrin with moxisylyte chlorhydrate in patients with chronic erectile dysfunction. J. Urol. 1998, 159, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Gowthamarajan, K.; Kulkarni, T.G.; Venkateswaran, G.; Samanta, M.K.; Suresh, B. Formulation and dissolution properties of meloxicam solid dispersion incorporated suppositories. Indian J. Pharm. Sci. 2002, 64, 525–528. [Google Scholar]
- Dufour, G.; Bigazzi, W.; Wong, N.; Boschini, F.; De Tullio, P.; Piel, G.; Cataldo, D.; Evrard, B. Interest of cyclodextrins in spray-dried microparticles formulation for sustained pulmonary delivery of budesonide. Int. J. Pharm. 2015, 495, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Pipkin, J.D.; Zimmerer, R.O.; Thompson, D.O.; Mosher, G.L. Inhalant Formulation Containing Sulfoalkyl Ether γ-Cyclodextrin and Corticosteroid. Patent WO2008005692A2, 10 January 2008. [Google Scholar]
- Davaran, S.; Rashidi, M.R.; Khandaghi, R.; Hashemi, M. Development of a novel prolonged-release nicotine transdermal patch. Pharmacol. Res. 2005, 51, 233–237. [Google Scholar] [CrossRef]
- Chulurks, S.; Jitapunkul, K.; Katanyutanon, S.; Toochinda, P.; Lawtrakul, L. Stability Enhancement and Skin Permeation Application of Nicotine by Forming Inclusion Complex with β-Cyclodextrin and Methyl-β-Cyclodextrin. Sci. Pharm. 2021, 89, 43. [Google Scholar] [CrossRef]
- Bourdon, F.; Lecoeur, M.; Leconte, L.; Ultré, V.; Kouach, M.; Odou, P.; Vaccher, C.; Foulon, C. Evaluation of Pentravan®, Pentravan® Plus, Phytobase®, Lipovan® and Pluronic Lecithin Organogel for the transdermal administration of antiemetic drugs to treat chemotherapy-induced nausea and vomiting at the hospital. Int. J. Pharm. 2016, 515, 774–787. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Zhang, X.; Liu, C.; Xiao, M.; Li, H.; Fan, J.; Fu, P.; Wang, S.; Zan, F.; Wu, G. Polypseudorotaxane functionalized magnetic nanoparticles as a dual responsive carrier for roxithromycin delivery. Mater. Sci. Eng. C 2019, 99, 159–170. [Google Scholar] [CrossRef]
- Rezaei, A.; Khavari, S.; Sami, M. Incorporation of thyme essential oil into the β-cyclodextrin nanosponges: Preparation, characterization and antibacterial activity. J. Mol. Struct. 2021, 1241, 130610. [Google Scholar] [CrossRef]
- Haley, R.M.; Gottardi, R.; Langer, R.; Mitchell, M.J. Cyclodextrins in drug delivery: Applications in gene and combination therapy. Drug. Deliv. Transl. Res. 2020, 10, 661–677. [Google Scholar] [CrossRef]
- Farrokhi, F.; Karami, Z.; Mahani, E.S.; Heydari, A. Delivery of DNAzyme targeting c-Myc gene using β-cyclodextrin polymer nanocarrier for therapeutic application in human breast cancer cell line. J. Drug Deliv. Sci. Technol. 2018, 47, 477–484. [Google Scholar] [CrossRef]
- Almeida, B.; Domingues, C.; Mascarenhas-Melo, F.; Silva, I.; Jarak, I.; Veiga, F.; Figueiras, A. The Role of Cyclodextrins in COVID-19 Therapy—A Literature Review. Int. J. Mol. Sci. 2023, 24, 2974. [Google Scholar] [CrossRef]
- Zokaei, E.; Badoei-Dalfrad, A.; Ansari, M.; Karami, Z.; Eslaminejad, T.; Nematollahi-Mahani, S.N. Therapeutic Potential of DNAzyme Loaded on Chitosan/Cyclodextrin Nanoparticle to Recovery of Chemosensitivity in the MCF-7 Cell Line. Appl. Biochem. Biotechnol. 2019, 187, 708–723. [Google Scholar] [CrossRef]
- Boudad, H.; Legrand, P.; Lebas, G.; Cheron, M.; Duchêne, D.; Ponchel, G. Combined hydroxypropyl-β-cyclodextrin and poly(alkylcyanoacrylate) nanoparticles intended for oral administration of saquinavir. Int. J. Pharm. 2001, 218, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Braga, S.S.; Barbosa, J.S.; Santos, N.E.; El-Saleh, F.; Paz, F.A.A. Cyclodextrins in Antiviral Therapeutics and Vaccines. Pharmaceutics 2021, 13, 409, Erratum in Pharmaceutics 2022, 14, 499. [Google Scholar] [CrossRef] [PubMed]
- Castagne, D.; Fillet, M.; Delattre, L.; Evrard, B.; Nusgens, B.; Piel, B. Study of the cholesterol extraction capacity of β-cyclodextrin and its derivatives, relationships with their effects on endothelial cell viability and on membrane models. J. Incl. Phenom. Macrocycl. Chem. 2009, 63, 225–231. [Google Scholar] [CrossRef]
- Gould, S.; Scott, R.C. 2-Hydroxypropyl-β-cyclodextrin (HP-β-CD): A toxicology review. Food Chem. Toxicol. 2005, 43, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, R.; Taharabaru, T.; Nishida, T.; Ohno, Y.; Maeda, Y.; Sato, M.; Ishikura, K.; Yanagihara, K.; Takagi, H.; Nakamura, T.; et al. Lactose-appended β-cyclodextrin as an effective nanocarrier for brain delivery. J. Control. Release 2020, 328, 722–735. [Google Scholar] [CrossRef]
- Jeandet, P.; Sobarzo-Sánchez, E.; Uddin, M.S.; Bru, R.; Clément, C.; Jacquard, C.; Nabavi, S.F.; Khayatkashani, M.; Batiha, G.E.; Khan, H.; et al. Resveratrol and cyclodextrins, an easy alliance: Applications in nanomedicine, green chemistry and biotechnology. Biotechnol. Adv. 2021, 53, 107844. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Ye, X.; Su, W.; Wang, Y. Curcumin alleviates Alzheimer’s disease by inhibiting inflammatory response, oxidative stress and activating the AMPK pathway. J. Chem. Neuroanat. 2023, 134, 102363. [Google Scholar] [CrossRef] [PubMed]
- Labanca, F.; Ullah, H.; Khan, H.; Milella, L.; Xiao, J.; Dajic-Stevanovic, Z.; Jeandet, P. Therapeutic and Mechanistic Effects of Curcumin in Huntington’s Disease. Curr Neuropharmacol. 2021, 19, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Ben Mihoub, A.; Acherar, S.; Frochot, C.; Malaplate, C.; Yen, F.T.; Arab-Tehrany, E. Synthesis of New Water Soluble β-Cyclodextrin@Curcumin Conjugates and In Vitro Safety Evaluation in Primary Cultures of Rat Cortical Neurons. Int. J. Mol. Sci. 2021, 22, 3255. [Google Scholar] [CrossRef] [PubMed]
- Braga, S.S. Molecular Mind Games: The Medicinal Action of Cyclodextrins in Neurodegenerative Diseases. Biomolecules 2023, 13, 666. [Google Scholar] [CrossRef] [PubMed]
- Camargo, F.; Erickson, R.P.; Garver, W.S.; Hossain, G.S.; Carbone, P.N.; Heidenreich, R.A.; Blanchard, J. Cyclodextrins in the treatment of a mouse model of Niemann-Pick C disease. Life Sci. 2001, 70, 131–142. [Google Scholar] [CrossRef]
- Aqul, A.; Liu, B.; Ramirez, C.M.; Pieper, A.A.; Estill, S.J.; Burns, D.K.; Liu, B.; Repa, J.J.; Turley, S.D.; Dietschy, J.M. Unesterified cholesterol accumulation in late endosomes/lysosomes causes neurodegeneration and is prevented by driving cholesterol export from this compartment. J. Neurosci. 2011, 31, 9404–9413. [Google Scholar] [CrossRef] [PubMed]
- Vite, C.; Mauldin, E.; Ward, S.; Stein, V.; Prociuk, M.; Haskins, M.E.; Strattan, R.; Kao, M.; Ory, D.; Walkley, S.U.; et al. Intrathecal cyclodextrin therapy of feline Niemann-Pick Type C disease. Mol. Genet. Metab. 2011, 102, S44. [Google Scholar] [CrossRef]
- Ory, D.S.; Ottinger, E.A.; Farhat, N.Y.; King, K.A.; Jiang, X.; Weissfeld, L.; Berry-Kravis, E.; Davidson, C.D.; Bianconi, S.; Keener, L.A.; et al. Intrathecal 2-hydroxypropyl-β-cyclodextrin decreases neurological disease progression in Niemann-Pick disease, type C1: A non-randomised, open-label, phase 1–2 trial. Lancet 2017, 390, 1758–1768. [Google Scholar] [CrossRef]
- Matsuo, M.; Sakakibara, T.; Sakiyama, Y.; So, T.; Kosuga, M.; Kakiuchi, T.; Ichinose, F.; Nakamura, T.; Ishitsuka, Y.; Irie, T. Long-term efficacy of intrathecal cyclodextrin in patients with Niemann-Pick disease type C. Brain Dev. 2024, 46, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Gosselet, F.; Saint-Pol, J.; Candela, P.; Fenart, L. Amyloid-β peptides, Alzheimer’s disease and the blood-brain barrier. Curr. Alzheimer Res. 2013, 10, 1015–1033. [Google Scholar] [CrossRef]
- Selkoe, D.J. Alzheimer’s disease: Genes, proteins, and therapy. Physiol. Rev. 2001, 81, 741–766. [Google Scholar] [CrossRef]
- Shepardson, N.E.; Shankar, G.M.; Selkoe, D.J. Cholesterol level and statin use in Alzheimer disease: I. Review of epidemiological and preclinical studies. Arch. Neurol. 2011, 68, 1239–1244. [Google Scholar] [CrossRef]
- Wong, K.H.; Xie, Y.; Huang, X.; Kadota, K.; Yao, X.; Yu, Y.; Chen, X.; Lu, A.; Yang, Z. Delivering Crocetin across the Blood-Brain Barrier by Using γ-Cyclodextrin to Treat Alzheimer’s Disease. Sci. Rep. 2020, 10, 3654. [Google Scholar] [CrossRef]
- Lu, Z.; Cheng, B.; Hu, Y.; Zhang, Y.; Zou, G. Complexation of resveratrol with cyclodextrins: Solubility and antioxidant activity. Food Chem. 2009, 113, 17–20. [Google Scholar] [CrossRef]
- Agarwal, T.; Manandhar, S.; Kumar, H.; Famurewa, A.C.; Gurram, P.C.; Suggala, R.S.; Sankhe, R.; Mudgal, J.; Pai, S.R. Oxyresveratrol-β-cyclodextrin mitigates streptozotocin-induced Alzheimer’s model cognitive impairment, histone deacetylase activity in rats: In silico & in vivo studies. Sci. Rep. 2024, 14, 9897. [Google Scholar] [CrossRef]
- Barros, M.C.F.; Ribeiro, A.C.F.; Esteso, M.A. Cyclodextrins in Parkinson’s Disease. Biomolecules 2019, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Rezaeisadat, M.; Salehi, N.; Bordbar, A.-K. Inclusion of Levodopa into β-Cyclodextrin: A Comprehensive Computational Study. ACS Omega 2021, 6, 23814–23825. [Google Scholar] [CrossRef]
- Jarazo, J.; Barmpa, K.; Modamio, J.; Saraiva, C.; Sabaté-Soler, S.; Rosety, I.; Griesbeck, A.; Skwirblies, F.; Zaffaroni, G.; Smits, L.M.; et al. Parkinson’s disease phenotypes in patient neuronal cultures and brain organoids improved by 2-hydroxypropyl-β-cyclodextrin treatment. Mov. Disord. 2022, 37, 80–94. [Google Scholar] [CrossRef] [PubMed]
- Aborode, A.T.; Pustake, M.; Awuah, W.A.; Alwerdani, M.; Shah, P.; Yarlagadda, R.; Ahmad, S.; Silva Correia, I.F.; Chandra, A.; Nansubuga, E.P.; et al. Targeting Oxidative Stress Mechanisms to Treat Alzheimer’s and Parkinson’s Disease: A Critical Review. Oxid. Med. Cell. Longev. 2022, 2022, 7934442. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, N.; Sugiyama, H.; Shimosegawa, H.; Nakane, R.; Ishida, Y.; Uekaji, Y.; Nakata, D.; Pallauf, K.; Rimbach, G.; Terao, K.; et al. Analysis of the Enhanced Stability of R(+)-Alpha Lipoic Acid by the Complex Formation with Cyclodextrins. Int. J. Mol. Sci. 2013, 14, 3639–3655. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, N.; Okamoto, H.; Furune, T.; Uekaji, Y.; Terao, K.; Uchida, R.; Iwamoto, K.; Miyajima, A.; Hirota, T.; Sakamoto, N. Bioavailability of an R-α-Lipoic Acid/γ-Cyclodextrin Complex in Healthy Volunteers. Int. J. Mol. Sci. 2016, 17, 949. [Google Scholar] [CrossRef] [PubMed]
- Memudu, A.E.; Adanike, R.P. Alpha lipoic acid reverses scopolamine-induced spatial memory loss and pyramidal cell neurodegeneration in the prefrontal cortex of Wistar rats. IBRO Neurosci. Rep. 2022, 13, 1–8. [Google Scholar] [CrossRef]
- Racz, C.P.; Santa, S.; Tomoaia-Cotisel, M.; Borodi, G.; Kacso, I.; Pirnau, A.; Bratu, I. Inclusion of a-lipoic acid in b-cyclodextrin. Physical–chemical and structural characterization. J. Incl. Phenom. Macrocycl. Chem. 2013, 76, 193–199. [Google Scholar] [CrossRef]
- Cataldo, D.; Evrard, B.; Noel, A.; Foldart, J.-M. Use of Cyclodextrin for Treatment and Prevention of Bronchial Inflammatory Diseases. Patent US9034846B2, 19 May 2015. [Google Scholar]
- Evrard, B.; Bertholet, P.; Gueders, M.; Flament, M.-P.; Piel, G.; Delattre, L.; Gayot, A.; Leterme, P.; Foidart, J.-M.; Cataldo, D. Cyclodextrins as a potential carrier in drug nebulization. J. Control. Release 2004, 96, 403–410. [Google Scholar] [CrossRef]
- Michailidou, G.; Papageorgiou, G.Z.; Bikiaris, D.N. β-Cyclodextrin Inclusion Complexes of Budesonide with Enhanced Bioavailability for COPD Treatment. Appl. Sci. 2021, 11, 12085. [Google Scholar] [CrossRef]
- Bayiha, J.C.; Evrard, B.; Cataldo, D.; De Tullio, P.; Mingeot-Leclercq, M.P. The Budesonide-Hydroxypropyl-β-Cyclodextrin Complex Attenuates ROS Generation, IL-8 Release and Cell Death Induced by Oxidant and Inflammatory Stress. Study on A549 and A-THP-1 Cells. Molecules 2020, 25, 4882. [Google Scholar] [CrossRef] [PubMed]
- Dogbe, M.G.; Mafilaza, A.Y.; Eleutério, C.V.; Cabral-Marques, H.; Simões, S.; Gaspar, M.M. Pharmaceutical Benefits of Fluticasone Propionate Association to Delivery Systems: In Vitro and In Vivo Evaluation. Pharmaceutics 2019, 11, 521. [Google Scholar] [CrossRef] [PubMed]
- Challoner, P.; Rodriguez, C.; Tarara, T.E.; Lord, J.D.; Samara, E. Tobramycin Formualtions for Treatment of Endobronchial Infections. Patent EP1765288B1, 7 November 2012. [Google Scholar]
- Gunasekara, L.; Al-Saiedy, M.; Green, F.; Pratt, R.; Bjornson, C.; Yang, A.; Michael Schoel, W.; Mitchell, I.; Brindle, M.; Montgomery, M.; et al. Pulmonary surfactant dysfunction in pediatric cystic fibrosis: Mechanisms and reversal with a lipid-sequestering drug. J. Cyst. Fibros. 2017, 16, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Asztemborska, M.; Ceborska, M.; Pietrzak, M. Complexation of tropane alkaloids by cyclodextrins. Carbohydr. Polym. 2019, 209, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Srichana, T.; Suedee, R.; Reanmongkol, W. Cyclodextrin as a potential drug carrier in salbutamol dry powder aerosols: The in-vitro deposition and toxicity studies of the complexes. Respir. Med. 2001, 95, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Jarho, P.; Másson, M.; Järvinen, T. Cyclodextrins in drug delivery. Expert Opin. Drug Deliv. 2005, 2, 335–351. [Google Scholar] [CrossRef]
- Wei, X.; Yu, C.-Y.; Wei, H. Application of Cyclodextrin for Cancer Immunotherapy. Molecules 2023, 28, 5610. [Google Scholar] [CrossRef] [PubMed]
- Stella, V.J.; Rajewski, R.A. Sulfobutylether-β-cyclodextrin. Int. J. Pharm. 2020, 583, 119396. [Google Scholar] [CrossRef] [PubMed]
- Păduraru, D.N.; Niculescu, A.G.; Bolocan, A.; Andronic, O.; Grumezescu, A.M.; Bîrlă, R. An Updated Overview of Cyclodextrin-Based Drug Delivery Systems for Cancer Therapy. Pharmaceutics 2022, 14, 1748. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Shen, Y.; Jin, F.; Du, Y.Z.; Ying, X.Y. Paclitaxel/hydroxypropyl-β-cyclodextrin complex-loaded liposomes for overcoming multidrug resistance in cancer chemotherapy. J. Liposome Res. 2020, 30, 12–20. [Google Scholar] [CrossRef]
- Mi, Y.; Zhang, J.; Tan, W.; Miao, Q.; Li, Q.; Guo, Z. Preparation of Doxorubicin-Loaded Carboxymethyl-β-Cyclodextrin/Chitosan Nanoparticles with Antioxidant, Antitumor Activities and pH-Sensitive Release. Mar. Drugs 2022, 20, 278. [Google Scholar] [CrossRef]
- Zhang, J.-Q.; Li, K.; Jiang, K.-M.; Cong, Y.-W.; Pu, S.-P.; Xie, X.-G.; Jin, Y.; Lin, J. Development of an oral satraplatin pharmaceutical formulation by encapsulation with cyclodextrin. RSC Adv. 2016, 6, 17074–17082. [Google Scholar] [CrossRef]
- Shukla, J.; Sharma, U.; Kar, R.; Varma, I.K.; Juyal, S.; Jagannathan, N.R.; Bandopadhyaya, G.P. Tamoxifen-2-hydroxylpropyl-β-cyclodextrin-aggregated nanoassembly for nonbreast estrogen-receptor-positive cancer therapy. Nanomedicine 2009, 4, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, S.P.; Balakrishnan, S.B.; Ganesan, V.; Munisamy, M.; Kuppu, S.V.; Narayanan, V.; Baskaralingam, V.; Jeyachandran, S.; Thambusamy, S. In-vitro dissolution and microbial inhibition studies on anticancer drug etoposide with β-cyclodextrin. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Möller, K.; Macaulay, B.; Bein, T. Curcumin Encapsulated in Crosslinked Cyclodextrin Nanoparticles Enables Immediate Inhibition of Cell Growth and Efficient Killing of Cancer Cells. Nanomaterials 2021, 11, 489. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Tu, J.; Zhao, J.; Zhang, Y.; Li, T.; Zhang, Y.; Zhang, P.; Ling, G.; Ji, J. Dual-targeted and esterase-responsive cyclodextrin-based host-guest nanocomposites for enhanced antitumor therapy. Colloids Surf. B Biointerfaces 2025, 246, 114371. [Google Scholar] [CrossRef]
- Liang, W.; Huang, Y.; Lu, D.; Ma, X.; Gong, T.; Cui, X.; Yu, B.; Yang, C.; Dong, C.; Shuang, S. β-Cyclodextrin–Hyaluronic Acid Polymer Functionalized Magnetic Graphene Oxide Nanocomposites for Targeted Photo-Chemotherapy of Tumor Cells. Polymers 2019, 11, 133. [Google Scholar] [CrossRef]
- Lai, W.F. Cyclodextrins in non-viral gene delivery. Biomaterials 2014, 35, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Xiao, F.; Song, L.; Sun, B.; Sun, D.; Chu, D.; Wang, L.; Han, S.; Yu, Z.; O’Driscoll, C.M.; et al. A folate-targeted PEGylated cyclodextrin-based nanoformulation achieves co-delivery of docetaxel and siRNA for colorectal cancer. Int. J. Pharm. 2021, 606, 120888. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Russell, E.G.; Darcy, R.; Cotter, T.G.; McKenna, S.L.; Cahill, M.R.; O’Driscoll, C.M. Antibody-Targeted Cyclodextrin-Based Nanoparticles for siRNA Delivery in the Treatment of Acute Myeloid Leukemia: Physicochemical Characteristics, in Vitro Mechanistic Studies, and ex Vivo Patient Derived Therapeutic Efficacy. Mol. Pharm. 2017, 14, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Qin, Y.; Tu, K.; Xu, C.; Li, Z.; Zhang, Z. Star-shaped polymer of β-cyclodextrin-g-vitamin E TPGS for doxorubicin delivery and multidrug resistance inhibition. Colloids Surf. B Biointerfaces 2018, 169, 10–19. [Google Scholar] [CrossRef]
- Zimmer, S.; Grebe, A.; Bakke, S.S.; Bode, N.; Halvorsen, B.; Ulas, T.; Skjelland, M.; De Nardo, D.; Labzin, L.I.; Kerksiek, A.; et al. Cyclodextrin promotes atherosclerosis regression via macrophage reprogramming. Sci. Transl. Med. 2016, 8, 333ra50. [Google Scholar] [CrossRef]
- Viale, M.; Giglio, V.; Monticone, M.; Maric, I.; Lentini, G.; Rocco, M.; Vecchio, G. New doxorubicin nanocarriers based on cyclodextrins. Investig. New Drugs 2017, 35, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Durço, A.O.; Souza, D.S.; Rhana, P.; Costa, A.D.; Marques, L.P.; Santos, L.A.B.O.; de Souza Araujo, A.A.; de Aragão Batista, M.V.; Roman-Campos, D.; Santos, M.R.V.D. d-Limonene complexed with cyclodextrin attenuates cardiac arrhythmias in an experimental model of doxorubicin-induced cardiotoxicity: Possible involvement of calcium/calmodulin-dependent protein kinase type II. Toxicol. Appl. Pharmacol. 2023, 474, 116609. [Google Scholar] [CrossRef] [PubMed]
- Coisne, C.; Tilloy, S.; Monflier, E.; Wils, D.; Fenart, L.; Gosselet, F. Cyclodextrins as Emerging Therapeutic Tools in the Treatment of Cholesterol-Associated Vascular and Neurodegenerative Diseases. Molecules 2016, 21, 1748. [Google Scholar] [CrossRef] [PubMed]
- Hodgetts, K.; Howes, L.; Fang, Y.Y.; Song, Z.M. 2-Hydroxypropyl-β-Cyclodextrin Induces Rapid Regression of Atherosclerotic plaque and Reduces Hyperlipidaemia in Adult with Cardiovascular Disease. Cardiol. Res. Cardiovasc. Med. 2024, 9, 261. [Google Scholar] [CrossRef]
- Ozon, E.A.; Novac, M.; Gheorghe, D.; Musuc, A.M.; Mitu, M.A.; Sarbu, I.; Anuta, V.; Rusu, A.; Petrescu, S.; Atkinson, I.; et al. Formation and Physico-Chemical Evaluation of Nifedipine-hydroxypropyl-β-cyclodextrin and Nifedipine-methyl-β-cyclodextrin: The Development of Orodispersible Tablets. Pharmaceuticals 2022, 15, 993. [Google Scholar] [CrossRef] [PubMed]
- Bećirevićc-Laćan, M.; Filipovic-Grcic, J.; Skalko-Basnet, N.; Jalšenjak, J. Dissolution Characteristics of Nifedipine Complexes with β-Cyclodextrins. Drug Dev. Ind. Pharm. 1996, 22, 1231–1236. [Google Scholar] [CrossRef]
- Zoppi, A.; Garnero, C.; Linck, Y.G.; Chattah, K.A.; Monti, G.A.; Longhi, M.R. Enalapril:β-CD complex: Stability enhancement in solid state. Carbohydr. Polym. 2011, 86, 716–721. [Google Scholar] [CrossRef]
- Cirri, M.; Mennini, N.; Maestrelli, F.; Mura, P.; Ghelardini, C.; Mannelli, L. Developmentand in vivo evaluation of an innovative “Hydrochlorothiazide-in Cyclodextrins-in Solid Lipid Nanoparticles” formulation with sustained release and enhanced oral bioavailability for potential hypertension treatment in pediatrics. Int. J. Pharm. 2017, 521, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Sharma, R.; Kumar Jain, D.; Saraf, A. Enhancement of oral bioavailability of poorly water soluble carvedilol by chitosan nanoparticles: Optimization and pharmacokinetic study. Int. J. Biol. Macromol. 2019, 135, 246–260. [Google Scholar] [CrossRef]
- Anjani, Q.K.; Sabri, A.H.B.; Hamid, K.A.; Moreno-Castellanos, N.; Li, H.; Donnelly, R.F. Tip loaded cyclodextrin-carvedilol complexes microarray patches. Carbohydr. Polym. 2023, 320, 121194. [Google Scholar] [CrossRef] [PubMed]
- Tehran, M.M.; Kovanen, P.T.; Xu, S.; Jamialahmadi, T.; Sahebkar, A. Cyclodextrins: Potential therapeutics against atherosclerosis. Pharmacol. Ther. 2020, 214, 107620. [Google Scholar] [CrossRef] [PubMed]
- Becktel, D.A.; Zbesko, J.C.; Frye, J.B.; Chung, A.G.; Hayes, M.; Calderon, K.; Grover, J.W.; Li, A.; Garcia, F.G.; Tavera-Garcia, M.A.; et al. Repeated Administration of 2-Hydroxypropyl-β-Cyclodextrin (HPβCD) Attenuates the Chronic Inflammatory Response to Experimental Stroke. J. Neurosci. 2022, 42, 325–348. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-based pharmaceutics: Past, present and future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef]
- Aleem, O.; Kuchekar, B.; Pore, Y.; Late, S. Effect of β-cyclodextrin and hydroxypropyl β-cyclodextrin complexation on physicochemical properties and antimicrobial activity of cefdinir. J. Pharm. Biomed. Anal. 2008, 47, 535–540. [Google Scholar] [CrossRef]
- Zhao, M.-R.; Wang, L.-S.; Liu, H.-W.; Wang, Y.-J.; Yang, H. Preparation, physicochemical characterization and in vitro dissolution studies of azithromycin-cyclodextrin inclusion complexes. J. Incl. Phenom. Macrocycl. Chem. 2016, 85, 137–149. [Google Scholar] [CrossRef]
- Salem, I.I.; Düzgünes, N. Efficacies of cyclodextrin-complexed and liposome-encapsulated clarithromycin against Mycobacterium avium complex infection in human macrophages. Int. J. Pharm. 2003, 250, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Guyot, M.; Fawaz, F.; Bildet, J.; Bonini, F.; Lagueny, A.M. Physicochemical characterization and dissolution of norfloxacin/cyclodextrin inclusion compounds and PEG solid dispersions. Int. J. Pharm. 1995, 123, 53–63. [Google Scholar] [CrossRef]
- Aiassa, V.; Zoppi, A.; Becerra, M.C.; Albesa, I.; Longhi, M.R. Enhanced inhibition of bacterial biofilm formation and reduced leukocyte toxicity by chloramphenicol:β-cyclodextrin:N-acetylcysteine complex. Carbohydr. Polym. 2016, 152, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Skiba, M.; Bounoure, F.; Barbot, C.; Arnaud, P.; Skiba, M. Development of cyclodextrin microspheres for pulmonary drug delivery. J. Pharm. Pharm. Sci. 2005, 8, 409–418. [Google Scholar] [PubMed]
- De Gaetano, F.; Marino, A.; Marchetta, A.; Bongiorno, C.; Zagami, R.; Cristiano, M.C.; Paolino, D.; Pistarà, V.; Ventura, C.A. Development of Chitosan/Cyclodextrin Nanospheres for Levofloxacin Ocular Delivery. Pharmaceutics 2021, 13, 1293. [Google Scholar] [CrossRef] [PubMed]
- Masood, F.; Yasin, T.; Bukhari, H.; Mujahid, M. Characterization and application of roxithromycin loaded cyclodextrin based nanoparticles for treatment of multidrug resistant bacteria. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Montero, E.; Rosa dos Santos, J.-F.; Torres-Labandeira, J.J.; Concheiro, A.; Alvarez-Lorenzo, C. Sertaconazole-Loaded Cyclodextrin–Polysaccharide Hydrogels as Antifungal Devices. Open Drug Deliv. J. 2009, 3, 1–9. [Google Scholar] [CrossRef]
- Athanassiou, G.; Michaleas, S.; Lada-Chitiroglou, E.; Tsitsa, T.; Antoniadou-Vyza, E. Antimicrobial activity of β-lactam antibiotics against clinical pathogens after molecular inclusion in several cyclodextrins. A novel approach to bacterial resistance. J. Pharm. Pharmacol. 2003, 55, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.-Z. Methicillin/per-6-(4-methoxylbenzyl)-amino-6-deoxy-β-cyclodextrin 1:1 complex and its potentiation in vitro against methicillin-resistant Staphylococcus aureus. J. Antibiot. 2013, 66, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Ozon, E.A.; Mati, E.; Karampelas, O.; Anuta, V.; Sarbu, I.; Musuc, A.M.; Mitran, R.A.; Culita, D.C.; Atkinson, I.; Anastasescu, M.; et al. The development of an innovative method to improve the dissolution performance of rivaroxaban. Heliyon 2024, 10, e33162. [Google Scholar] [CrossRef] [PubMed]
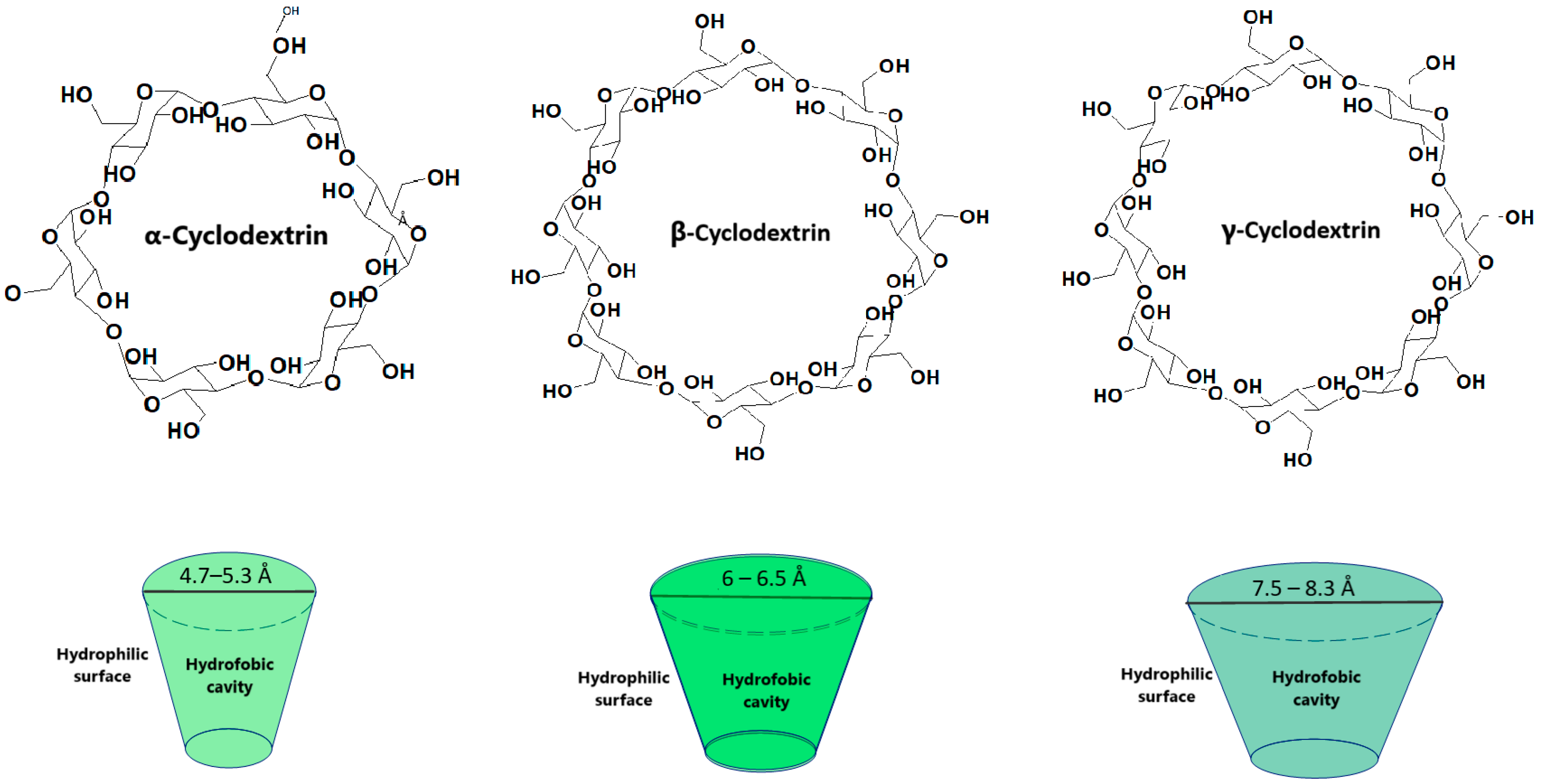

| Active Substance | Water Solubility (mg/mL) | Solubility When Associated with β-Cyclodextrin (mg/mL) | Cyclodextrin Used | Reference |
|---|---|---|---|---|
| Amphotericin B | 0.001 | 0.15 | β-cyclodextrin sulfobutyl ether (SBE-β-CD) | [14] |
| Cefixime | 0.76 | 17.5 | SBE-β-CD | [15] |
| Cefpodoxime Proxetil | 0.241 | 0.637 | β-CD | [16] |
| Ceftiofur | 0.03 | 2.18 | HP-β-CD | [17] |
| Dexamethasone | 0.1 | 2.5 | β-CD | [18] |
| Diclofenac | 4.0 | 20.0 | HP-β-CD | [19] |
| Ibuprofen | 0.1 | 10.0 | Methyl-β-cyclodextrin (M-β-CD) | [20] |
| ITH12674 | 0.31 | 10.7 | HP-β-CD | [7] |
| Itraconazole | 0.001 | 4–5 | HP-β-CD | [21] |
| Metronidazole benzoate | 0.14 | 1.39 | β-CD | [22] |
| Nifedipine | 0.02 | 1.5 | β-CD | [23] |
| Paclitaxel | 0.003 | 2.0 | HP-β-CD | [24] |
| Tinidazole | 3.76 | 36.89 | β-CD | [25] |
| Active Substance | Trade Name and Formulation | Cyclodextrin | Effect of Cyclodextrin over the Active Substance | Indication | Reference |
|---|---|---|---|---|---|
| Brivaracetam | Brivlera | β-CD | Improved drug dissolution | Epilepsy | [71,72] |
| Cetirizine | Zyrtec, WalZyr, Revicet One-day tablets, chewing tablets | β-CD | Higher stability against oxidative degradation of the drug, increased solubility, masking of the unpleasant bitter taste of the drug | Allergy | [66,67,73,74] |
| Desloratadine | Desloratadine | β-CD | Increased stability (no coloration), minimum N-, formyl desloratadine formation | Allergy | [75] |
| Ethinylestradiol/ Drospirenone | Yaz® | β-CD | Enhance water solubility | Contraception | [64,76] |
| Famotidine | Famotidine OD Tablets | β-CD | Increased water solubility, mask bitter taste | Ulcer | [77] |
| Nimesulide | Nimedex | β-CD | Increased water solubility and dissolution rate, leading to rapid absorption of the drug, more rapid onset of action compared to nimesulide | Pain after arthroscopic surgery | [78,79] |
| Nitroglycerin | Nitropen, sublingual tablet | β-CD | Low volatility of nitroglycerine | Angina pectoris | [68,80] |
| Limaprost alfadex (Prostaglandin E1 analogue) | Opalmon tablets | β-CD | Increased stability against humidity | Pain, ulcer | [66,81,82] |
| Dinoprostone (Prostaglandin E2 derivative) | Prostarmon E (sublingual tablets), Dinoproston- betadex | β-CD | Increased stability, improved drug dissolution in saliva and organoleptic properties | Induction of labor | [66,81] |
| Rofecoxib | Rofizgel | β-CD | Improved solubility and dissolution rate | Arthritis | [66,83,84] |
| Tiaprofenic acid | Surgamyl | β-CD | Increase in solubility, absorption and bioavailability, masking bitter taste | Pain management | [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicolaescu, O.E.; Belu, I.; Mocanu, A.G.; Manda, V.C.; Rău, G.; Pîrvu, A.S.; Ionescu, C.; Ciulu-Costinescu, F.; Popescu, M.; Ciocîlteu, M.V. Cyclodextrins: Enhancing Drug Delivery, Solubility and Bioavailability for Modern Therapeutics. Pharmaceutics 2025, 17, 288. https://doi.org/10.3390/pharmaceutics17030288
Nicolaescu OE, Belu I, Mocanu AG, Manda VC, Rău G, Pîrvu AS, Ionescu C, Ciulu-Costinescu F, Popescu M, Ciocîlteu MV. Cyclodextrins: Enhancing Drug Delivery, Solubility and Bioavailability for Modern Therapeutics. Pharmaceutics. 2025; 17(3):288. https://doi.org/10.3390/pharmaceutics17030288
Chicago/Turabian StyleNicolaescu, Oana Elena, Ionela Belu, Andreea Gabriela Mocanu, Valentin Costel Manda, Gabriela Rău, Andreea Silvia Pîrvu, Cătălina Ionescu, Felicia Ciulu-Costinescu, Mariana Popescu, and Maria Viorica Ciocîlteu. 2025. "Cyclodextrins: Enhancing Drug Delivery, Solubility and Bioavailability for Modern Therapeutics" Pharmaceutics 17, no. 3: 288. https://doi.org/10.3390/pharmaceutics17030288
APA StyleNicolaescu, O. E., Belu, I., Mocanu, A. G., Manda, V. C., Rău, G., Pîrvu, A. S., Ionescu, C., Ciulu-Costinescu, F., Popescu, M., & Ciocîlteu, M. V. (2025). Cyclodextrins: Enhancing Drug Delivery, Solubility and Bioavailability for Modern Therapeutics. Pharmaceutics, 17(3), 288. https://doi.org/10.3390/pharmaceutics17030288







