Trojan Horse Delivery Strategies of Natural Medicine Monomers: Challenges and Limitations in Improving Brain Targeting
Abstract
1. Introduction
2. Major Factors Limiting Brain Targeting
2.1. Blood–Brain Barrier
2.2. Blood–Cerebrospinal Fluid Barrier
2.3. Blood–Brain Tumor Barrier
3. The Application of Natural Products in the Treatment of the CNS
3.1. Alzheimer’s Disease
3.2. Parkinson’s Disease
3.3. Stroke
3.4. Glioma
4. Application of the Trojan Horse Strategy in Drug Delivery to the Central Nervous System
4.1. Receptor-Mediated Transcytosis
4.1.1. Transferrin Receptor
4.1.2. Interleukin-13 Receptor
4.1.3. Lactoferrin Receptor
4.1.4. Low-Density Lipoprotein Receptor
4.1.5. Low-Density Lipoprotein Receptor-Related Protein
4.1.6. Integrin
4.1.7. Nicotinic Acetylcholine Receptor
4.1.8. Nucleolin
4.2. Carrier-Mediated Transport
4.2.1. Glucose Transporters
4.2.2. L-Type Amino Transporters 1
4.3. Adsorptive-Mediated Transcytosis
4.4. Other Emerging Delivery Pathways
4.4.1. Cell-Penetrating Peptides
4.4.2. Cell-Mediated Drug Delivery
Mesenchymal Stem Cells
Macrophage
Red Blood Cells
Neutrophils
5. Limitations of Trojan Horse Delivery Strategies
- The Biocompatibility of Nanocarriers: The safety of nanomaterials in the body is crucial. Some nanomaterials may trigger toxic or immune responses and could even cause long-term side effects to the human body. Therefore, it is necessary to develop safer and more efficient nanocarriers to ensure their widespread application does not adversely affect patient health.
- The Precise Control of Drug Release: The rate and timing of the drug release are crucial in disease treatment. Adjusting the drug release process according to the needs of different disease stages and targeted sites has become a current research hotspot. This requires an in-depth understanding of the molecular changes in the disease process and precise control of the carrier’s release behavior.
- Insufficient Targeting Specificity: Although nanocarriers theoretically have some targeting ability, in practical application, drug delivery may still exhibit “off-target” phenomena. This means drugs may not only concentrate in the lesion area but could also affect healthy tissues, leading to side effects. Enhancing the targeting capability of carriers and ensuring drugs act only at target sites remains a significant research topic.
- Immune Response and Long-term Toxicity: Some nanocarriers, upon entering the body, may trigger immune responses, especially during long-term treatment, where cumulative effects could lead to potential long-term toxicity. Thus, reducing immune rejection and long-term toxicity remains one of the bottlenecks in current technological development.
6. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Guo, Z.H.; Khattak, S.; Rauf, M.A.; Ansari, M.A.; Alomary, M.N.; Razak, S.; Yang, C.Y.; Wu, D.D.; Ji, X.Y. Role of Nanomedicine-Based Therapeutics in the Treatment of CNS Disorders. Molecules 2023, 28, 1283. [Google Scholar] [CrossRef] [PubMed]
- Deuschl, G.; Beghi, E.; Fazekas, F.; Varga, T.; Christoforidi, K.A.; Sipido, E.; Bassetti, C.L.; Vos, T.; Feigin, V.L. The burden of neurological diseases in Europe: An analysis for the Global Burden of Disease Study 2017. Lancet Public Health 2020, 5, e551–e567.s. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Vos, T.; Nichols, E.; Owolabi, M.O.; Carroll, W.M.; Dichgans, M.; Deuschl, G.; Parmar, P.; Brainin, M.; Murray, C. The global burden of neurological disorders: Translating evidence into policy. Lancet Neurol. 2020, 19, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Choudhari, M.; Hejmady, S.; Narayan Saha, R.; Damle, S.; Singhvi, G.; Alexander, A.; Kesharwani, P.; Kumar Dubey, S. Evolving new-age strategies to transport therapeutics across the blood-brain-barrier. Int. J. Pharm. 2021, 599, 120351. [Google Scholar] [CrossRef]
- Rahman, M.H.; Bajgai, J.; Fadriquela, A.; Sharma, S.; Trinh, T.T.; Akter, R.; Jeong, Y.J.; Goh, S.H.; Kim, C.S.; Lee, K.J. Therapeutic Potential of Natural Products in Treating Neurodegenerative Disorders and Their Future Prospects and Challenges. Molecules 2021, 26, 5327. [Google Scholar] [CrossRef]
- Rehman, M.U.; Wali, A.F.; Ahmad, A.; Shakeel, S.; Rasool, S.; Ali, R.; Rashid, S.M.; Madkhali, H.; Ganaie, M.A.; Khan, R. Neuroprotective Strategies for Neurological Disorders by Natural Products: An update. Curr. Neuropharmacol. 2019, 17, 247–267. [Google Scholar] [CrossRef]
- Deng, M.; Yan, W.; Gu, Z.; Li, Y.; Chen, L.; He, B. Anti-Neuroinflammatory Potential of Natural Products in the Treatment of Alzheimer’s Disease. Molecules 2023, 28, 1486. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Drew, J.; Berney, W.; Lei, W. Neuroprotective Natural Products for Alzheimer’s Disease. Cells 2021, 10, 1309. [Google Scholar] [CrossRef]
- Liu, J.; Li, T.; Zhong, G.; Pan, Y.; Gao, M.; Su, S.; Liang, Y.; Ma, C.; Liu, Y.; Wang, Q.; et al. Exploring the therapeutic potential of natural compounds for Alzheimer’s disease: Mechanisms of action and pharmacological properties. Biomed. Pharmacother. 2023, 166, 115406. [Google Scholar] [CrossRef] [PubMed]
- Rawal, S.U.; Patel, B.M.; Patel, M.M. New Drug Delivery Systems Developed for Brain Targeting. Drugs 2022, 82, 749–792. [Google Scholar] [CrossRef]
- Jiao, Y.; Yang, L.; Wang, R.; Song, G.; Fu, J.; Wang, J.; Gao, N.; Wang, H. Drug Delivery Across the Blood-Brain Barrier: A New Strategy for the Treatment of Neurological Diseases. Pharmaceutics 2024, 16, 1611. [Google Scholar] [CrossRef]
- Zhou, T.; Liu, Y.; Lei, K.; Liu, J.; Hu, M.; Guo, L.; Guo, Y.; Ye, Q. A “Trojan Horse” Strategy: The Preparation of Bile Acid-Modifying Irinotecan Hydrochloride Nanoliposomes for Liver-Targeted Anticancer Drug Delivery System Study. Molecules 2023, 28, 1577. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Drug and gene targeting to the brain with molecular Trojan horses. Nat. Rev. Drug Discov. 2002, 1, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.A.; Amtul, Z. Developing Trojan horses to induce, diagnose and suppress Alzheimer’s pathology. Pharmacol. Res. 2019, 149, 104471. [Google Scholar] [CrossRef]
- Lei, K.; Yuan, M.; Zhou, T.; Ye, Q.; Zeng, B.; Zhou, Q.; Wei, A.; Guo, L. Research progress in the application of bile acid-drug conjugates: A “trojan horse” strategy. Steroids 2021, 173, 108879. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Jiang, Y.; Wang, H.; Wang, J.; Shin, M.C.; Byun, Y.; He, H.; Liang, Y.; Yang, V.C. Curb challenges of the “Trojan Horse” approach: Smart strategies in achieving effective yet safe cell-penetrating peptide-based drug delivery. Adv. Drug Deliv. Rev. 2013, 65, 1299–1315. [Google Scholar] [CrossRef] [PubMed]
- Leal, A.F.; Inci, O.K.; Seyrantepe, V.; Rintz, E.; Celik, B.; Ago, Y.; León, D.; Suarez, D.A.; Alméciga-Díaz, C.J.; Tomatsu, S. Molecular Trojan Horses for treating lysosomal storage diseases. Mol. Genet. Metab. 2023, 140, 107648. [Google Scholar] [CrossRef] [PubMed]
- Azarmi, M.; Maleki, H.; Nikkam, N.; Malekinejad, H. Transcellular brain drug delivery: A review on recent advancements. Int. J. Pharm. 2020, 586, 119582. [Google Scholar] [CrossRef] [PubMed]
- Ayub, A.; Wettig, S. An Overview of Nanotechnologies for Drug Delivery to the Brain. Pharmaceutics 2022, 14, 224. [Google Scholar] [CrossRef] [PubMed]
- Lôbo, G.; Paiva, K.L.R.; Silva, A.L.G.; Simões, M.M.; Radicchi, M.A.; Báo, S.N. Nanocarriers Used in Drug Delivery to Enhance Immune System in Cancer Therapy. Pharmaceutics 2021, 13, 1167. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Liu, S.; Jia, S.; Xu, F. Emerging frontiers in drug delivery with special focus on novel techniques for targeted therapies. Biomed. Pharmacother. 2023, 165, 115049. [Google Scholar] [CrossRef] [PubMed]
- Agrahari, V.; Burnouf, P.A.; Burnouf, T.; Agrahari, V. Nanoformulation properties, characterization, and behavior in complex biological matrices: Challenges and opportunities for brain-targeted drug delivery applications and enhanced translational potential. Adv. Drug Deliv. Rev. 2019, 148, 146–180. [Google Scholar] [CrossRef]
- Jiang, Y.; Yan, C.; Li, M.; Chen, S.; Chen, Z.; Yang, L.; Luo, K. Delivery of natural products via polysaccharide-based nanocarriers for cancer therapy: A review on recent advances and future challenges. Int. J. Biol. Macromol. 2024, 278 Pt 4, 135072. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Zhao, Y.; Yue, P.; Peng, Y.; Sun, Y.; Chen, X.; Zhao, Z.; Han, B. Recent advances in drug delivery systems for targeting brain tumors. Drug Deliv. 2023, 30, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.M.; Patel, B.M. Crossing the Blood-Brain Barrier: Recent Advances in Drug Delivery to the Brain. CNS Drugs 2017, 31, 109–133. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The blood-brain barrier: Structure, regulation, and drug delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef] [PubMed]
- Pandit, R.; Chen, L.; Götz, J. The blood-brain barrier: Physiology and strategies for drug delivery. Adv. Drug Deliv. Rev. 2020, 165–166, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Persidsky, Y.; Ramirez, S.H.; Haorah, J.; Kanmogne, G.D. Blood-brain barrier: Structural components and function under physiologic and pathologic conditions. J. Neuroimmune Pharmacol. 2006, 1, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Furtado, D.; Björnmalm, M.; Ayton, S.; Bush, A.I.; Kempe, K.; Caruso, F. Overcoming the Blood-Brain Barrier: The Role of Nanomaterials in Treating Neurological Diseases. Adv. Mater. 2018, 30, e1801362. [Google Scholar] [CrossRef]
- Harilal, S.; Jose, J.; Parambi, D.G.T.; Kumar, R.; Unnikrishnan, M.K.; Uddin, M.S.; Mathew, G.E.; Pratap, R.; Marathakam, A.; Mathew, B. Revisiting the blood-brain barrier: A hard nut to crack in the transportation of drug molecules. Brain Res. Bull. 2020, 160, 121–140. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Drug transport across the blood-brain barrier. J. Cereb. Blood Flow. Metab. 2012, 32, 1959–1972. [Google Scholar] [CrossRef]
- Bellettato, C.M.; Scarpa, M. Possible strategies to cross the blood-brain barrier. Ital. J. Pediatr. 2018, 44 (Suppl. S2), 131. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, R.I.; Preda, M.D.; Niculescu, A.G.; Vladâcenco, O.; Radu, C.I.; Grumezescu, A.M.; Teleanu, D.M. Current Strategies to Enhance Delivery of Drugs across the Blood-Brain Barrier. Pharmaceutics 2022, 14, 987. [Google Scholar] [CrossRef] [PubMed]
- Schinkel, A.H. P-Glycoprotein, a gatekeeper in the blood-brain barrier. Adv. Drug Deliv. Rev. 1999, 36, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Potschka, H. Role of drug efflux transporters in the brain for drug disposition and treatment of brain diseases. Prog. Neurobiol. 2005, 76, 22–76. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Potschka, H. Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx 2005, 2, 86–98. [Google Scholar] [CrossRef]
- Löscher, W.; Potschka, H. Drug resistance in brain diseases and the role of drug efflux transporters. Nat. Rev. Neurosci. 2005, 6, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Campos-Bedolla, P.; Walter, F.R.; Veszelka, S.; Deli, M.A. Role of the blood-brain barrier in the nutrition of the central nervous system. Arch. Med. Res. 2014, 45, 610–638. [Google Scholar] [CrossRef]
- Rankovic, Z. CNS drug design: Balancing physicochemical properties for optimal brain exposure. J. Med. Chem. 2015, 58, 2584–2608. [Google Scholar] [CrossRef] [PubMed]
- Gabathuler, R. Approaches to transport therapeutic drugs across the blood-brain barrier to treat brain diseases. Neurobiol. Dis. 2010, 37, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Johanson, C.E.; Stopa, E.G.; McMillan, P.N. The blood-cerebrospinal fluid barrier: Structure and functional significance. Methods Mol. Biol. 2011, 686, 101–131. [Google Scholar] [PubMed]
- Ghersi-Egea, J.F.; Strazielle, N.; Catala, M.; Silva-Vargas, V.; Doetsch, F.; Engelhardt, B. Molecular anatomy and functions of the choroidal blood-cerebrospinal fluid barrier in health and disease. Acta Neuropathol. 2018, 135, 337–361. [Google Scholar] [CrossRef]
- Dabbagh, F.; Schroten, H.; Schwerk, C. In Vitro Models of the Blood-Cerebrospinal Fluid Barrier and Their Applications in the Development and Research of (Neuro)Pharmaceuticals. Pharmaceutics 2022, 14, 1729. [Google Scholar] [CrossRef] [PubMed]
- Madadi, A.K.; Sohn, M.J. Advances in Intrathecal Nanoparticle Delivery: Targeting the Blood-Cerebrospinal Fluid Barrier for Enhanced CNS Drug Delivery. Pharmaceuticals 2024, 17, 1070. [Google Scholar] [CrossRef]
- Modi, D.M.; Modi, A.D. Nanogel-mediated therapeutic delivery across blood-cerebrospinal fluid and blood-spinal cord barriers. Brain Disord. 2024, 15, 100151. [Google Scholar] [CrossRef]
- Zhang, S.; Gan, L.; Cao, F.; Wang, H.; Gong, P.; Ma, C.; Ren, L.; Lin, Y.; Lin, X. The barrier and interface mechanisms of the brain barrier, and brain drug delivery. Brain Res. Bull. 2022, 190, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Dai, H.; Shaik, N.; Elmquist, W.F. Drug efflux transporters in the CNS. Adv. Drug Deliv. Rev. 2003, 55, 83–105. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Zepeda, D.; Taghi, M.; Scherrmann, J.M.; Decleves, X.; Menet, M.C. ABC Transporters at the Blood-Brain Interfaces, Their Study Models, and Drug Delivery Implications in Gliomas. Pharmaceutics 2019, 12, 20. [Google Scholar] [CrossRef]
- de Boer, A.G.; Gaillard, P.J. Drug Targeting to the Brain. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 323–355. [Google Scholar] [CrossRef]
- Loryan, I.; Hammarlund-Udenaes, M.; Syvänen, S. Brain Distribution of Drugs: Pharmacokinetic Considerations. Handb. Exp. Pharmacol. 2022, 273, 121–150. [Google Scholar] [PubMed]
- Erickson, M.A.; Banks, W.A. Age-Associated Changes in the Immune System and Blood-Brain Barrier Functions. Int. J. Mol. Sci. 2019, 20, 1632. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. CSF, blood-brain barrier, and brain drug delivery. Expert Opin. Drug Deliv. 2016, 13, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Johanson, C.; Stopa, E.; McMillan, P.; Roth, D.; Funk, J.; Krinke, G. The distributional nexus of choroid plexus to cerebrospinal fluid, ependyma and brain: Toxicologic/pathologic phenomena, periventricular destabilization, and lesion spread. Toxicol. Pathol. 2011, 39, 186–212. [Google Scholar] [CrossRef] [PubMed]
- Proulx, S.T. Cerebrospinal fluid outflow: A review of the historical and contemporary evidence for arachnoid villi, perineural routes, and dural lymphatics. Cell Mol. Life Sci. 2021, 78, 2429–2457. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.K.; Mestre, H.; Nedergaard, M. Fluid transport in the brain. Physiol. Rev. 2022, 102, 1025–1151. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Drug transport in brain via the cerebrospinal fluid. Fluids Barriers CNS 2011, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.H.; Thomas, J.H. Cerebrospinal Fluid Flow. Annu. Rev. Fluid. Mech. 2023, 55, 237–264. [Google Scholar] [CrossRef]
- Marcucci, F.; Corti, A.; Ferreri, A.J.M. Breaching the Blood-Brain Tumor Barrier for Tumor Therapy. Cancers 2021, 13, 2391. [Google Scholar] [CrossRef]
- Gonzales-Aloy, E.; Ahmed-Cox, A.; Tsoli, M.; Ziegler, D.S.; Kavallaris, M. From cells to organoids: The evolution of blood-brain barrier technology for modelling drug delivery in brain cancer. Adv. Drug Deliv. Rev. 2023, 196, 114777. [Google Scholar] [CrossRef]
- Parodi, A.; Rudzińska, M.; Deviatkin, A.A.; Soond, S.M.; Baldin, A.V.; Zamyatnin, A.A., Jr. Established and Emerging Strategies for Drug Delivery Across the Blood-Brain Barrier in Brain Cancer. Pharmaceutics 2019, 11, 245. [Google Scholar] [CrossRef]
- Griffith, J.I.; Rathi, S.; Zhang, W.; Zhang, W.; Drewes, L.R.; Sarkaria, J.N.; Elmquist, W.F. Addressing BBB Heterogeneity: A New Paradigm for Drug Delivery to Brain Tumors. Pharmaceutics 2020, 12, 1205. [Google Scholar] [CrossRef] [PubMed]
- Hersh, A.M.; Alomari, S.; Tyler, B.M. Crossing the Blood-Brain Barrier: Advances in Nanoparticle Technology for Drug Delivery in Neuro-Oncology. Int. J. Mol. Sci. 2022, 23, 4153. [Google Scholar] [CrossRef] [PubMed]
- Rabah, N.; Ait Mohand, F.E.; Kravchenko-Balasha, N. Understanding Glioblastoma Signaling, Heterogeneity, Invasiveness, and Drug Delivery Barriers. Int. J. Mol. Sci. 2023, 24, 14256. [Google Scholar] [CrossRef] [PubMed]
- Roma-Rodrigues, C.; Mendes, R.; Baptista, P.V.; Fernandes, A.R. Targeting Tumor Microenvironment for Cancer Therapy. Int. J. Mol. Sci. 2019, 20, 840. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.W.; Sakurai, Y.; Harashima, H.; Bae, Y.H. Nano-sized drug carriers: Extravasation, intratumoral distribution, and their modeling. J. Control. Release 2017, 267, 31–46. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Gao, Y.; Jiang, Y.; Guan, Z.; Xie, Y.; Hu, J.; Chen, J. Barrier permeation and improved nanomedicine delivery in tumor microenvironments. Cancer Lett. 2023, 562, 216166. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.J.; Xu, P. Strategies to overcome/penetrate the BBB for systemic nanoparticle delivery to the brain/brain tumor. Adv. Drug Deliv. Rev. 2022, 191, 114619. [Google Scholar] [CrossRef] [PubMed]
- Rathi, S.; Griffith, J.I.; Zhang, W.; Zhang, W.; Oh, J.H.; Talele, S.; Sarkaria, J.N.; Elmquist, W.F. The influence of the blood-brain barrier in the treatment of brain tumours. J. Intern. Med. 2022, 292, 3–30. [Google Scholar] [CrossRef]
- Tan, X.; Zhang, K.; Shi, W.; Tang, Z. Research progress on the regulation and mechanism of borneol on the blood-brain barrier in pathological states: A narrative review focused on ischemic stroke and cerebral glioma. Transl. Cancer Res. 2023, 12, 3198–3209. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, W. Recent advances in brain tumor-targeted nano-drug delivery systems. Expert Opin. Drug Deliv. 2012, 9, 671–686. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shi, Y.; Jiang, J.; Li, C.; Zhang, H.; Zhang, X.; Jiang, T.; Wang, L.; Wang, Y.; Feng, L. Micro-Nanocarriers Based Drug Delivery Technology for Blood-Brain Barrier Crossing and Brain Tumor Targeting Therapy. Small 2022, 18, e2203678. [Google Scholar] [CrossRef] [PubMed]
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chételat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer disease. Nat. Rev. Dis. Primers 2021, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Atri, A. The Alzheimer’s Disease Clinical Spectrum: Diagnosis and Management. Med. Clin. N. Am. 2019, 103, 263–293. [Google Scholar] [CrossRef]
- Sadigh-Eteghad, S.; Sabermarouf, B.; Majdi, A.; Talebi, M.; Farhoudi, M.; Mahmoudi, J. Amyloid-beta: A crucial factor in Alzheimer’s disease. Med. Princ. Pract. 2015, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.V.F.; Loures, C.M.G.; Alves, L.C.V.; de Souza, L.C.; Borges, K.B.G.; Carvalho, M.D.G. Alzheimer’s disease: Risk factors and potentially protective measures. J. Biomed. Sci. 2019, 26, 33. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Vergallo, A.; Aguilar, L.F.; Benda, N.; Broich, K.; Cuello, A.C.; Cummings, J.; Dubois, B.; Federoff, H.J.; Fiandaca, M.; et al. Precision pharmacology for Alzheimer’s disease. Pharmacol. Res. 2018, 130, 331–365. [Google Scholar] [CrossRef]
- Yiannopoulou, K.G.; Papageorgiou, S.G. Current and future treatments for Alzheimer’s disease. Ther. Adv. Neurol. Disord. 2013, 6, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Khandker, S.S.; Alam, F.; Khalil, M.I.; Kamal, M.A.; Gan, S.H. Alzheimer’s Disease and Natural Products: Future Regimens Emerging from Nature. Curr. Top. Med. Chem. 2017, 17, 1408–1428. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, A.; Ekavali. A review on Alzheimer’s disease pathophysiology and its management: An update. Pharmacol. Rep. 2015, 67, 195–203. [Google Scholar] [CrossRef]
- Xing, M.; Cao, Q.; Wang, Y.; Xiao, H.; Zhao, J.; Zhang, Q.; Ji, A.; Song, S. Advances in Research on the Bioactivity of Alginate Oligosaccharides. Mar. Drugs 2020, 18, 144. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, G.; Feng, T.; Zhang, J.; Huang, X.; Wang, T.; Xie, Z.; Chu, X.; Yang, J.; Wang, H.; et al. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res. 2019, 29, 787–803. [Google Scholar] [CrossRef]
- Ma, L.; Jiang, X.; Huang, Q.; Chen, W.; Zhang, H.; Pei, H.; Cao, Y.; Wang, H.; Li, H. Traditional Chinese medicine for the treatment of Alzheimer’s disease: A focus on the microbiota-gut-brain axis. Biomed. Pharmacother. 2023, 165, 115244. [Google Scholar] [CrossRef]
- Sadowska-Krępa, E.; Kłapcińska, B.; Pokora, I.; Domaszewski, P.; Kempa, K.; Podgórski, T. Effects of Six-Week Ginkgo biloba Supplementation on Aerobic Performance, Blood Pro/Antioxidant Balance, and Serum Brain-Derived Neurotrophic Factor in Physically Active Men. Nutrients 2017, 9, 803. [Google Scholar] [CrossRef] [PubMed]
- Barbalho, S.M.; Direito, R.; Laurindo, L.F.; Marton, L.T.; Guiguer, E.L.; Goulart, R.A.; Tofano, R.J.; Carvalho, A.C.A.; Flato, U.A.P.; Capelluppi Tofano, V.A.; et al. Ginkgo biloba in the Aging Process: A Narrative Review. Antioxidants 2022, 11, 525. [Google Scholar] [CrossRef]
- Kandiah, N.; Ong, P.A.; Yuda, T.; Ng, L.L.; Mamun, K.; Merchant, R.A.; Chen, C.; Dominguez, J.; Marasigan, S.; Ampil, E.; et al. Treatment of dementia and mild cognitive impairment with or without cerebrovascular disease: Expert consensus on the use of Ginkgo biloba extract, EGb 761®. CNS Neurosci. Ther. 2019, 25, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Kojder, K.; Zielonka-Brzezicka, J.; Wróbel, J.; Bosiacki, M.; Fabiańska, M.; Wróbel, M.; Sołek-Pastuszka, J.; Klimowicz, A. The Use of Ginkgo biloba L. as a Neuroprotective Agent in the Alzheimer’s Disease. Front. Pharmacol. 2021, 12, 775034. [Google Scholar] [CrossRef]
- Luo, Y. Ginkgo biloba neuroprotection: Therapeutic implications in Alzheimer’s disease. J. Alzheimers Dis. 2001, 3, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Ahlemeyer, B.; Krieglstein, J. Neuroprotective effects of Ginkgo biloba extract. Cell. Mol. Life Sci. CMLS 2003, 60, 1779–1792. [Google Scholar] [CrossRef]
- Singh, S.K.; Srivastav, S.; Castellani, R.J.; Plascencia-Villa, G.; Perry, G. Neuroprotective and Antioxidant Effect of Ginkgo biloba Extract Against AD and Other Neurological Disorders. Neurotherapeutics 2019, 16, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.R.; Jong, Y.X.; Balakrishnan, M.; Bok, Z.K.; Weng, J.K.K.; Tay, K.C.; Goh, B.H.; Ong, Y.S.; Chan, K.G.; Lee, L.H.; et al. Beneficial Role of Carica papaya Extracts and Phytochemicals on Oxidative Stress and Related Diseases: A Mini Review. Biology 2021, 10, 287. [Google Scholar] [CrossRef]
- Barbagallo, M.; Marotta, F.; Dominguez, L.J. Oxidative stress in patients with Alzheimer’s disease: Effect of extracts of fermented papaya powder. Mediat. Inflamm. 2015, 2015, 624801. [Google Scholar] [CrossRef] [PubMed]
- Ververis, A.; Savvidou, G.; Ioannou, K.; Nicolaou, P.; Christodoulou, K.; Plioukas, M. Greek Sage Exhibits Neuroprotective Activity against Amyloid Beta-Induced Toxicity. Evid. Based Complement. Altern. Med. 2020, 2020, 2975284. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, I.E.; Osman, E.E.; Saeed, A.; Ming, L.C.; Goh, K.W.; Razi, P.; Abdullah, A.D.I.; Dahab, M. Plant extracts as emerging modulators of neuroinflammation and immune receptors in Alzheimer’s pathogenesis. Heliyon 2024, 10, e35943. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.V.; Krishnan, V.; Praveen, S.; Hebbar, K.B. Dietary prospects of coconut oil for the prevention and treatment of Alzheimer’s disease (AD): A review of recent evidences. Trends Food Sci. Technol. 2021, 112, 201–211. [Google Scholar] [CrossRef]
- Sandupama, P.; Munasinghe, D.; Jayasinghe, M. Coconut oil as a therapeutic treatment for alzheimer’s disease: A review. J. Future Foods 2022, 2, 41–52. [Google Scholar] [CrossRef]
- Rausch, W.D.; Liu, S.; Gille, G.; Radad, K. Neuroprotective effects of ginsenosides. Acta Neurobiol. Exp. 2006, 66, 369–375. [Google Scholar] [CrossRef]
- Zheng, M.; Xin, Y.; Li, Y.; Xu, F.; Xi, X.; Guo, H.; Cui, X.; Cao, H.; Zhang, X.; Han, C. Ginsenosides: A Potential Neuroprotective Agent. BioMed Res. Int. 2018, 2018, 8174345. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, P.; Shin, C.Y. A comprehensive review of the therapeutic and pharmacological effects of ginseng and ginsenosides in central nervous system. J. Ginseng Res. 2013, 37, 8–29. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Lee, D.; Lee, H.L.; Kim, C.E.; Jung, K.; Kang, K.S. Beneficial effects of Panax ginseng for the treatment and prevention of neurodegenerative diseases: Past findings and future directions. J. Ginseng Res. 2018, 42, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Yang, Y.; Wan, Y.; Xia, J.; Xu, J.F.; Zhang, L.; Liu, D.; Chen, L.; Tang, F.; Ao, H.; et al. New insights into the role and mechanisms of ginsenoside Rg1 in the management of Alzheimer’s disease. Biomed. Pharmacother. 2022, 152, 113207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Z.; Qian, S.S.; Zhang, Y.J.; Wang, R.Q. Salvia miltiorrhiza: A source for anti-Alzheimer’s disease drugs. Pharm. Biol. 2016, 54, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Bonaccini, L.; Karioti, A.; Bergonzi, M.C.; Bilia, A.R. Effects of Salvia miltiorrhiza on CNS Neuronal Injury and Degeneration: A Plausible Complementary Role of Tanshinones and Depsides. Planta Med. 2015, 81, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, W.; Xu, L.; Chen, L. In Salvia miltiorrhiza, phenolic acids possess protective properties against amyloid β-induced cytotoxicity, and tanshinones act as acetylcholinesterase inhibitors. Environ. Toxicol. Pharmacol. 2011, 31, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, X.; Yan, L.; Zhao, R.; An, J.; Liu, C.; Yang, H. Danshen extract (Salvia miltiorrhiza Bunge) attenuate spinal cord injury in a rat model: A metabolomic approach for the mechanism study. Phytomedicine 2019, 62, 152966. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Liu, Y.; An, Z.; Ni, J. Active Components and Pharmacological Effects of Cornus officinalis: Literature Review. Front. Pharmacol. 2021, 12, 633447. [Google Scholar] [CrossRef]
- Zhou, L.; Hou, Y.; Yang, Q.; Du, X.; Li, M.; Yuan, M.; Zhou, Z. Tetrahydroxystilbene glucoside improves the learning and memory of amyloid-β1–42-injected rats and may be connected to synaptic changes in the hippocampus. Can. J. Physiol. Pharmacol. 2012, 90, 1446–1455. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Ni, B.; Lin, H.; Zhang, M.; Li, X.; Yin, X.; Qu, C.; Ni, J. Traditional usages, botany, phytochemistry, pharmacology and toxicology of Polygonum multiflorum Thunb.: A review. J. Ethnopharmacol. 2015, 159, 158–183. [Google Scholar] [CrossRef]
- Qian, H.Q.; Wu, D.C.; Li, C.Y.; Liu, X.R.; Han, X.K.; Peng, Y.; Zhang, H.; Zhao, B.Y.; Zhao, Y. A systematic review of traditional uses, phytochemistry, pharmacology and toxicity of Epimedium koreanum Nakai. J. Ethnopharmacol. 2024, 318 Pt B, 116957. [Google Scholar] [CrossRef]
- Sabogal-Guáqueta, A.M.; Muñoz-Manco, J.I.; Ramírez-Pineda, J.R.; Lamprea-Rodriguez, M.; Osorio, E.; Cardona-Gómez, G.P. The flavonoid quercetin ameliorates Alzheimer’s disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer’s disease model mice. Neuropharmacology 2015, 93, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Ohta, K. Quercetin Regulates the Integrated Stress Response to Improve Memory. Int. J. Mol. Sci. 2019, 20, 2761. [Google Scholar] [CrossRef] [PubMed]
- Gomes, B.A.Q.; Silva, J.P.B.; Romeiro, C.F.R.; Dos Santos, S.M.; Rodrigues, C.A.; Gonçalves, P.R.; Sakai, J.T.; Mendes, P.F.S.; Varela, E.L.P.; Monteiro, M.C. Neuroprotective Mechanisms of Resveratrol in Alzheimer’s Disease: Role of SIRT1. Oxidative Med. Cell Longev. 2018, 2018, 8152373. [Google Scholar] [CrossRef]
- Chiang, M.C.; Nicol, C.J.; Cheng, Y.C. Resveratrol activation of AMPK-dependent pathways is neuroprotective in human neural stem cells against amyloid-beta-induced inflammation and oxidative stress. Neurochem. Int. 2018, 115, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Benameur, T.; Giacomucci, G.; Panaro, M.A.; Ruggiero, M.; Trotta, T.; Monda, V.; Pizzolorusso, I.; Lofrumento, D.D.; Porro, C.; Messina, G. New Promising Therapeutic Avenues of Curcumin in Brain Diseases. Molecules 2021, 27, 236. [Google Scholar] [CrossRef]
- Tang, L.; Xiang, Q.; Xiang, J.; Zhang, Y.; Li, J. Tripterygium glycoside ameliorates neuroinflammation in a mouse model of Aβ25-35-induced Alzheimer’s disease by inhibiting the phosphorylation of IκBα and p38. Bioengineered 2021, 12, 8540–8554. [Google Scholar] [CrossRef]
- Nakhate, K.T.; Bharne, A.P.; Verma, V.S.; Aru, D.N.; Kokare, D.M. Plumbagin ameliorates memory dysfunction in streptozotocin induced Alzheimer’s disease via activation of Nrf2/ARE pathway and inhibition of β-secretase. Biomed. Pharmacother. 2018, 101, 379–390. [Google Scholar] [CrossRef]
- Bhuvanendran, S.; Kumari, Y.; Othman, I.; Shaikh, M.F. Amelioration of Cognitive Deficit by Embelin in a Scopolamine-Induced Alzheimer’s Disease-Like Condition in a Rat Model. Front. Pharmacol. 2018, 9, 665. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef]
- Balestrino, R.; Schapira, A.H.V. Parkinson disease. Eur. J. Neurol. 2020, 27, 27–42. [Google Scholar] [CrossRef]
- Charvin, D.; Medori, R.; Hauser, R.A.; Rascol, O. Therapeutic strategies for Parkinson disease: Beyond dopaminergic drugs. Nat. Rev. Drug Discov. 2018, 17, 804–822. [Google Scholar] [CrossRef] [PubMed]
- Solayman, M.; Islam, M.A.; Alam, F.; Khalil, M.I.; Kamal, M.A.; Gan, S.H. Natural Products Combating Neurodegeneration: Parkinson’s Disease. Curr. Drug Metab. 2017, 18, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Si, L.; An, Y.; Zhou, J.; Lai, Y. Neuroprotective effects of baicalin and baicalein on the central nervous system and the underlying mechanisms. Heliyon 2025, 11, e41002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Du, L.; Zhang, W.; Yang, Y.; Zhou, Q.; Du, G. Therapeutic effects of baicalein on rotenone-induced Parkinson’s disease through protecting mitochondrial function and biogenesis. Sci. Rep. 2017, 7, 9968. [Google Scholar] [CrossRef] [PubMed]
- Mir, I.A.; Tiku, A.B. Chemopreventive and therapeutic potential of “naringenin,” a flavanone present in citrus fruits. Nutr. Cancer 2015, 67, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Verma, A.; Dubey, N.; Raghav, J.; Agrawal, A. Naringenin: A prospective therapeutic agent for Alzheimer’s and Parkinson’s disease. J. Food Biochem. 2022, 46, e14415. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Zheng, C.C.; Hao, J.P.; Yang, C.C.; Hu, C.Y. Icariin ameliorates behavioral deficits and neuropathology in a mouse model of multiple sclerosis. Brain Res. 2023, 1804, 148267. [Google Scholar] [CrossRef]
- Alivirdiloo, V.; Hajiabbasi, M.; Gargari, M.K.; Gargari, H.K.; Ghazi, F.; Mohammadi, M.; Rahimi, F.; Mobed, A.; Mehra, A. Neuroprotective role of nobiletin against amyloid-β (Aβ) aggregation in Parkinson and Alzheimer disease as neurodegenerative diseases of brain. Med. Chem. Res. 2024, 33, 1055–1063. [Google Scholar] [CrossRef]
- Rajasekhar, K.; Samanta, S.; Bagoband, V.; Murugan, N.A.; Govindaraju, T. Antioxidant Berberine-Derivative Inhibits Multifaceted Amyloid Toxicity. iScience 2020, 23, 101005. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Cho, K.H.; Shin, M.S.; Lee, J.M.; Cho, H.S.; Kim, C.J.; Shin, D.H.; Yang, H.J. Berberine prevents nigrostriatal dopaminergic neuronal loss and suppresses hippocampal apoptosis in mice with Parkinson’s disease. Int. J. Mol. Med. 2014, 33, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Rajeswari, V.D.; Venkatraman, G.; Ramanathan, G. Phytochemicals in Parkinson’s Disease: A Pathway to Neuroprotection and Personalized Medicine. Cell Biochem. Biophys. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Houghton, P.J.; Howes, M.J. Natural products and derivatives affecting neurotransmission relevant to Alzheimer’s and Parkinson’s disease. Neurosignals 2005, 14, 6–22. [Google Scholar] [CrossRef] [PubMed]
- Bhusal, C.K.; Uti, D.E.; Mukherjee, D.; Alqahtani, T.; Alqahtani, S.; Bhattacharya, A.; Akash, S. Unveiling Nature’s potential: Promising natural compounds in Parkinson’s disease management. Park. Relat. Disord. 2023, 115, 105799. [Google Scholar] [CrossRef]
- Campbell, B.C.V.; De Silva, D.A.; Macleod, M.R.; Coutts, S.B.; Schwamm, L.H.; Davis, S.M.; Donnan, G.A. Ischaemic stroke. Nat. Rev. Dis. Primers 2019, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- Tao, T.; Liu, M.; Chen, M.; Luo, Y.; Wang, C.; Xu, T.; Jiang, Y.; Guo, Y.; Zhang, J.H. Natural medicine in neuroprotection for ischemic stroke: Challenges and prospective. Pharmacol. Ther. 2020, 216, 107695. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Bu, T.; Li, Y.; He, Y.; Yang, F.; Zou, L. Pharmacological Activity, Pharmacokinetics, and Clinical Research Progress of Puerarin. Antioxidants 2022, 11, 2121. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.; Wadhwa, K.; Mishra, R.; Gupta, S.; Ahmad, F.; Kamal, M.; Iqbal, D.; Alsaweed, M.; Nuli, M.V.; Abomughaid, M.M.; et al. Investigating the Potential Therapeutic Mechanisms of Puerarin in Neurological Diseases. Mol. Neurobiol. 2024, 61, 10747–10769. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, J.; Yang, F.; Li, S.; Ma, W.; Chang, X.; Yang, L. Neuroprotective Effects of Quercetin on Ischemic Stroke: A Literature Review. Front. Pharmacol. 2022, 13, 854249. [Google Scholar] [CrossRef] [PubMed]
- Mokra, D.; Joskova, M.; Mokry, J. Therapeutic Effects of Green Tea Polyphenol (−)-Epigallocatechin-3-Gallate (EGCG) in Relation to Molecular Pathways Controlling Inflammation, Oxidative Stress, and Apoptosis. Int. J. Mol. Sci. 2022, 24, 340. [Google Scholar] [CrossRef]
- Mancuso, C. Panax notoginseng: Pharmacological Aspects and Toxicological Issues. Nutrients 2024, 16, 2120. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Song, H.; Zhang, C.; Wang, A.; Zhang, B.; Xiong, C.; Zhuang, X.; Zang, Y.; Li, C.; Fang, Q.; et al. Efficacy and Safety of Panax notoginseng Saponins in the Treatment of Adults With Ischemic Stroke in China: A Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e2317574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, Q.; Jin, M.; Ren, J.; Sun, X.; Zhang, Z.; Luo, Y.; Sun, X. Notoginsenoside R1 alleviates cerebral ischemia/reperfusion injury by inhibiting the TLR4/MyD88/NF-κB signaling pathway through microbiota-gut-brain axis. Phytomedicine 2024, 128, 155530. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Zhou, P.; Sun, Y.; Meng, X.; Dai, Z.; Sun, G.; Sun, X. Protective Effects and Target Network Analysis of Ginsenoside Rg1 in Cerebral Ischemia and Reperfusion Injury: A Comprehensive Overview of Experimental Studies. Cells 2018, 7, 270. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, X.; Liu, X.; Zhang, C.; Shang, W.; Xue, J.; Chen, R.; Xing, Y.; Song, D.; Xu, R. Ginsenoside Rg1 promotes cerebral angiogenesis via the PI3K/Akt/mTOR signaling pathway in ischemic mice. Eur. J. Pharmacol. 2019, 856, 172418. [Google Scholar] [CrossRef] [PubMed]
- Sarkaki, A.; Rashidi, M.; Ranjbaran, M.; Asareh Zadegan Dezfuli, A.; Shabaninejad, Z.; Behzad, E.; Adelipour, M. Therapeutic Effects of Resveratrol on Ischemia-Reperfusion Injury in the Nervous System. Neurochem. Res. 2021, 46, 3085–3102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Peng, B.; Chen, Z.; Yu, J.; Deng, G.; Bao, Y.; Ma, C.; Du, F.; Sheu, W.C.; Kimberly, W.T.; et al. Brain-targeting, acid-responsive antioxidant nanoparticles for stroke treatment and drug delivery. Bioact. Mater. 2022, 16, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Freije, W.A.; Castro-Vargas, F.E.; Fang, Z.; Horvath, S.; Cloughesy, T.; Liau, L.M.; Mischel, P.S.; Nelson, S.F. Gene expression profiling of gliomas strongly predicts survival. Cancer Res. 2004, 64, 6503–6510. [Google Scholar] [CrossRef] [PubMed]
- Vengoji, R.; Macha, M.A.; Batra, S.K.; Shonka, N.A. Natural products: A hope for glioblastoma patients. Oncotarget 2018, 9, 22194–22219. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Yang, T.; Korma, S.A.; Sitohy, M.; Abd El-Mageed, T.A.; Selim, S.; Al Jaouni, S.K.; Salem, H.M.; Mahmmod, Y.; Soliman, S.M.; et al. Impacts of turmeric and its principal bioactive curcumin on human health: Pharmaceutical, medicinal, and food applications: A comprehensive review. Front. Nutr. 2022, 9, 1040259. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.C.; Mittal, S. Antitumor Activity of Curcumin in Glioblastoma. Int. J. Mol. Sci. 2020, 21, 9435. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Butt, M.S.; Nadeem, M.; Peters, D.G.; Mubarak, M.S. Resveratrol as an anti-cancer agent: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1428–1447. [Google Scholar] [CrossRef]
- Zdioruk, M.; Jimenez-Macias, J.L.; Nowicki, M.O.; Manz, K.E.; Pennell, K.D.; Koch, M.S.; Finkelberg, T.; Wu, B.; Boucher, P.; Takeda, Y.; et al. PPRX-1701, a nanoparticle formulation of 6′-bromoindirubin acetoxime, improves delivery and shows efficacy in preclinical GBM models. Cell Rep. Med. 2023, 4, 101019. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Qiu, B.; Zhang, Y.; Hu, Y.; Wang, Z.; Guan, Z.; Qin, Y.; Sui, T.; Wu, F.; Li, B.; et al. The tumor-enriched small molecule gambogic amide suppresses glioma by targeting WDR1-dependent cytoskeleton remodeling. Signal Transduct. Target. Ther. 2023, 8, 424. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Du, T.; Zhang, Z.; Zhang, Q.; Zhang, J.; Li, W.; Jiang, J.D.; Chen, X.; Hu, H.Y. Target fishing and mechanistic insights of the natural anticancer drug candidate chlorogenic acid. Acta Pharm. Sin. B 2024, 14, 4431–4442. [Google Scholar] [CrossRef]
- Dardevet, L.; Rani, D.; Aziz, T.A.; Bazin, I.; Sabatier, J.M.; Fadl, M.; Brambilla, E.; De Waard, M. Chlorotoxin: A helpful natural scorpion peptide to diagnose glioma and fight tumor invasion. Toxins 2015, 7, 1079–1101. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.; Burks, S.R.; Frank, J.A. Chlorotoxin-A Multimodal Imaging Platform for Targeting Glioma Tumors. Toxins 2018, 10, 496. [Google Scholar] [CrossRef] [PubMed]
- Baghirov, H. Receptor-mediated transcytosis of macromolecules across the blood-brain barrier. Expert Opin. Drug Deliv. 2023, 20, 1699–1711. [Google Scholar] [CrossRef]
- Haqqani, A.S.; Bélanger, K.; Stanimirovic, D.B. Receptor-mediated transcytosis for brain delivery of therapeutics: Receptor classes and criteria. Front. Drug Deliv. 2024, 4, 1360302. [Google Scholar] [CrossRef]
- Mojarad-Jabali, S.; Farshbaf, M.; Walker, P.R.; Hemmati, S.; Fatahi, Y.; Zakeri-Milani, P.; Sarfraz, M.; Valizadeh, H. An update on actively targeted liposomes in advanced drug delivery to glioma. Int. J. Pharm. 2021, 602, 120645. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yuan, M.; Zhang, Q.; Ting Yang, Y.; Gao, H.; He, Q. Synergistic Combination of Doxorubicin and Paclitaxel Delivered by Blood Brain Barrier and Glioma Cells Dual Targeting Liposomes for Chemotherapy of Brain Glioma. Curr. Pharm. Biotechnol. 2016, 17, 636–650. [Google Scholar] [CrossRef] [PubMed]
- Song, X.L.; Liu, S.; Jiang, Y.; Gu, L.Y.; Xiao, Y.; Wang, X.; Cheng, L.; Li, X.T. Targeting vincristine plus tetrandrine liposomes modified with DSPE-PEG(2000)-transferrin in treatment of brain glioma. Eur. J. Pharm. Sci. 2017, 96, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, A.; Deshpande, P.; Pattni, B.; Torchilin, V. Transferrin-targeted, resveratrol-loaded liposomes for the treatment of glioblastoma. J. Control. Release 2018, 277, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Duan, W.; Zhang, S.; Chen, D.; Feng, J.; Qi, N. Muscone/RI7217 co-modified upward messenger DTX liposomes enhanced permeability of blood-brain barrier and targeting glioma. Theranostics 2020, 10, 4308–4322. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zeng, H.; You, Y.; Wang, R.; Tan, T.; Wang, W.; Yin, L.; Zeng, Z.; Zeng, Y.; Xie, T. Active targeting of orthotopic glioma using biomimetic liposomes co-loaded elemene and cabazitaxel modified by transferritin. J. Nanobiotechnol. 2021, 19, 289. [Google Scholar] [CrossRef] [PubMed]
- Mojarad-Jabali, S.; Farshbaf, M.; Hemmati, S.; Sarfraz, M.; Motasadizadeh, H.; Shahbazi Mojarrad, J.; Atyabi, F.; Zakeri-Milani, P.; Valizadeh, H. Comparison of three synthetic transferrin mimetic small peptides to promote the blood-brain barrier penetration of vincristine liposomes for improved glioma targeted therapy. Int. J. Pharm. 2022, 613, 121395. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wu, G.; Wang, H.; Chen, L. Pep-1&borneol-Bifunctionalized Carmustine-Loaded Micelles Enhance Anti-Glioma Efficacy Through Tumor-Targeting and BBB-Penetrating. J. Pharm. Sci. 2019, 108, 1726–1735. [Google Scholar] [PubMed]
- Wang, B.; Lv, L.; Wang, Z.; Jiang, Y.; Lv, W.; Liu, X.; Wang, Z.; Zhao, Y.; Xin, H.; Xu, Q. Improved anti-glioblastoma efficacy by IL-13Rα2 mediated copolymer nanoparticles loaded with paclitaxel. Sci. Rep. 2015, 5, 16589. [Google Scholar] [CrossRef]
- Lv, L.; Jiang, Y.; Liu, X.; Wang, B.; Lv, W.; Zhao, Y.; Shi, H.; Hu, Q.; Xin, H.; Xu, Q.; et al. Enhanced Antiglioblastoma Efficacy of Neovasculature and Glioma Cells Dual Targeted Nanoparticles. Mol. Pharm. 2016, 13, 3506–3517. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.C.; Tsao, C.W. Neuroprotection against apoptosis of SK-N-MC cells using RMP-7- and lactoferrin-grafted liposomes carrying quercetin. Int. J. Nanomed. 2017, 12, 2857–2869. [Google Scholar] [CrossRef]
- Tang, S.; Wang, A.; Yan, X.; Chu, L.; Yang, X.; Song, Y.; Sun, K.; Yu, X.; Liu, R.; Wu, Z.; et al. Brain-targeted intranasal delivery of dopamine with borneol and lactoferrin co-modified nanoparticles for treating Parkinson’s disease. Drug Deliv. 2019, 26, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Wang, A.; Hua, H.; Jiang, Y.; Wang, Y.; Mu, H.; Wu, Z.; Sun, K. Intranasal delivery of Huperzine A to the brain using lactoferrin-conjugated N-trimethylated chitosan surface-modified PLGA nanoparticles for treatment of Alzheimer’s disease. Int. J. Nanomed. 2018, 13, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Qi, N.; Duan, W.; Gao, D.; Ma, N.; Zhang, J.; Feng, J.; Li, A. “Guide” of muscone modification enhanced brain-targeting efficacy and anti-glioma effect of lactoferrin modified DTX liposomes. Bioeng. Transl. Med. 2023, 8, e10393. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-C.; Rajesh, R. Targeted delivery of rosmarinic acid across the blood–brain barrier for neuronal rescue using polyacrylamide-chitosan-poly(lactide-co-glycolide) nanoparticles with surface cross-reacting material 197 and apolipoprotein E. Int. J. Pharm. 2017, 528, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Yang, Z.; Wang, S.; Chen, J.; Liu, Q.; Tianle, H.; Hai, L.; Lu, R.; Wu, Y. Berberine and folic acid co-modified pH-sensitive cascade-targeted PTX-liposomes coated with Tween 80 for treating glioma. Bioorg. Med. Chem. 2022, 69, 116893. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Sun, X.; Mei, H.; Wang, Y.; Liao, Z.; Chen, J.; Zhang, Q.; Hu, Y.; Pang, Z.; Jiang, X. LDLR-mediated peptide-22-conjugated nanoparticles for dual-targeting therapy of brain glioma. Biomaterials 2013, 34, 9171–9182. [Google Scholar] [CrossRef]
- Sun, X.; Chen, Y.; Zhao, H.; Qiao, G.; Liu, M.; Zhang, C.; Cui, D.; Ma, L. Dual-modified cationic liposomes loaded with paclitaxel and survivin siRNA for targeted imaging and therapy of cancer stem cells in brain glioma. Drug Deliv. 2018, 25, 1718–1727. [Google Scholar] [CrossRef]
- Song, W.; Bai, L.; Yang, Y.; Wang, Y.; Xu, P.; Zhao, Y.; Zhou, X.; Li, X.; Xue, M. Long-Circulation and Brain Targeted Isoliquiritigenin Micelle Nanoparticles: Formation, Characterization, Tissue Distribution, Pharmacokinetics and Effects for Ischemic Stroke. Int. J. Nanomed. 2022, 17, 3655–3670. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shi, N.; Chen, M.; Liu, M.; Ju, R.; Liu, Y.; Kong, L.; Yu, Y.; Li, X. Angiopep-2 modified dual drug-loaded liposomes with brain targeting functionality mitigate Alzheimer’s disease-related symptoms in APP/PS-1 mice. J. Drug Target. 2023, 31, 634–645. [Google Scholar] [CrossRef]
- Liu, Y.; Ran, R.; Chen, J.; Kuang, Q.; Tang, J.; Mei, L.; Zhang, Q.; Gao, H.; Zhang, Z.; He, Q. Paclitaxel loaded liposomes decorated with a multifunctional tandem peptide for glioma targeting. Biomaterials 2014, 35, 4835–4847. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mei, L.; Yu, Q.; Xu, C.; Qiu, Y.; Yang, Y.; Shi, K.; Zhang, Q.; Gao, H.; Zhang, Z.; et al. Multifunctional Tandem Peptide Modified Paclitaxel-Loaded Liposomes for the Treatment of Vasculogenic Mimicry and Cancer Stem Cells in Malignant Glioma. ACS Appl. Mater. Interfaces 2015, 7, 16792–16801. [Google Scholar] [CrossRef] [PubMed]
- Li, X.T.; Tang, W.; Xie, H.J.; Liu, S.; Song, X.L.; Xiao, Y.; Wang, X.; Cheng, L.; Chen, G.R. The efficacy of RGD modified liposomes loaded with vinorelbine plus tetrandrine in treating resistant brain glioma. J. Liposome Res. 2019, 29, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, X.; Shen, L.; Alrobaian, M.; Panda, S.K.; Almasmoum, H.A.; Ghaith, M.M.; Almaimani, R.A.; Ibrahim, I.A.A.; Singh, T.; et al. Paclitaxel and naringenin-loaded solid lipid nanoparticles surface modified with cyclic peptides with improved tumor targeting ability in glioblastoma multiforme. Biomed. Pharmacother. 2021, 138, 111461. [Google Scholar] [CrossRef]
- Gu, G.; Gao, X.; Hu, Q.; Kang, T.; Liu, Z.; Jiang, M.; Miao, D.; Song, Q.; Yao, L.; Tu, Y.; et al. The influence of the penetrating peptide iRGD on the effect of paclitaxel-loaded MT1-AF7p-conjugated nanoparticles on glioma cells. Biomaterials 2013, 34, 5138–5148. [Google Scholar] [CrossRef]
- Shi, K.; Long, Y.; Xu, C.; Wang, Y.; Qiu, Y.; Yu, Q.; Liu, Y.; Zhang, Q.; Gao, H.; Zhang, Z.; et al. Liposomes Combined an Integrin αvβ3-Specific Vector with pH-Responsible Cell-Penetrating Property for Highly Effective Antiglioma Therapy through the Blood-Brain Barrier. ACS Appl. Mater. Interfaces 2015, 7, 21442–21454. [Google Scholar] [CrossRef]
- Xin, X.; Liu, W.; Zhang, Z.A.; Han, Y.; Qi, L.L.; Zhang, Y.Y.; Zhang, X.T.; Duan, H.X.; Chen, L.Q.; Jin, M.J.; et al. Efficient Anti-Glioma Therapy Through the Brain-Targeted RVG15-Modified Liposomes Loading Paclitaxel-Cholesterol Complex. Int. J. Nanomed. 2021, 16, 5755–5776. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, S.; Ma, C.; Li, T.; Yang, L. Preparation of baicalin-loaded ligand-modified nanoparticles for nose-to-brain delivery for neuroprotection in cerebral ischemia. Drug Deliv. 2022, 29, 1282–1298. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Tao, Y.; Payne, G.; Do, L.; Thomas, T.; Rodriguez, J.; Dou, H. Targeted delivery of nano-PTX to the brain tumor-associated macrophages. Oncotarget 2017, 8, 6564–6578. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, Z.; Zhang, G.; Lin, F.; Liu, Y.; Zhang, Y.; Feng, J.; Chen, W.; Meng, Q.; Chen, L. AS1411 Aptamer/Hyaluronic Acid-Bifunctionalized Microemulsion Co-Loading Shikonin and Docetaxel for Enhanced Antiglioma Therapy. J. Pharm. Sci. 2019, 108, 3684–3694. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, W.; Wu, G.; Kong, J.; Yuan, S.; Chen, L. A Magnetic T7 Peptide&AS1411 Aptamer-Modified Microemulsion for Triple Glioma-Targeted Delivery of Shikonin and Docetaxel. J. Pharm. Sci. 2021, 110, 2946–2954. [Google Scholar] [PubMed]
- Johnsen, K.B.; Burkhart, A.; Thomsen, L.B.; Andresen, T.L.; Moos, T. Targeting the transferrin receptor for brain drug delivery. Prog. Neurobiol. 2019, 181, 101665. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, H.; Pandey, M.; Chin, P.X.; Phang, Y.L.; Cheah, J.Y.; Ooi, S.C.; Mak, K.K.; Pichika, M.R.; Kesharwani, P.; Hussain, Z.; et al. Transferrin receptors-targeting nanocarriers for efficient targeted delivery and transcytosis of drugs into the brain tumors: A review of recent advancements and emerging trends. Drug Deliv. Transl. Res. 2018, 8, 1545–1563. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.S.; Johnsen, K.B.; Kucharz, K.; Lauritzen, M.; Moos, T. Blood-Brain Barrier Transport of Transferrin Receptor-Targeted Nanoparticles. Pharmaceutics 2022, 14, 2237. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, L.; Yin, X. Pathophysiological aspects of transferrin-A potential nano-based drug delivery signaling molecule in therapeutic target for varied diseases. Front. Pharmacol. 2024, 15, 1342181. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Zhang, B.; Yan, X.; Fan, K. Transferrin receptor 1 targeted nanomedicine for brain tumor therapy. Biomater. Sci. 2023, 11, 3394–3413. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.P.; Wang, G.Y.; Arima, K.; Guan, S.P.; Waters, M.R.; Cavenee, W.K.; Pan, E.; Aliwarga, E.; Chong, S.T.; Kok, C.Y.L.; et al. Interleukin-13 receptor alpha 2 cooperates with EGFRvIII signaling to promote glioblastoma multiforme. Nat. Commun. 2017, 8, 1913. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Jiang, Z.K.; Yang, B.; Manzuk, L.; Rosfjord, E.; Yao, J.; Lemon, L.; Noorbehesht, K.; David, J.; Puthenveetil, S.; et al. Targeting and pharmacology of an anti-IL13Rα2 antibody and antibody-drug conjugate in a melanoma xenograft model. MAbs 2021, 13, 1958662. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Ericson, K.; Chao, W.; Low, W.C. NFAT and AP1 are essential for the expression of a glioblastoma multiforme related IL-13Ra2 transcript. Cell Oncol. 2010, 32, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Puri, R.K. Analysis of the cancer genome atlas (TCGA) database identifies an inverse relationship between interleukin-13 receptor α1 and α2 gene expression and poor prognosis and drug resistance in subjects with glioblastoma multiforme. J. Neurooncol. 2018, 136, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Raza, G.; Yunus, F.U.; Mangukiya, H.B.; Merugu, S.B.; Mashausi, D.S.; Zeling, W.; Negi, H.; Zhou, B.; Roy, D.; Wu, Z.; et al. A novel target anti-interleukin-13 receptor subunit alpha-2 monoclonal antibody inhibits tumor growth and metastasis in lung cancer. Int. Immunopharmacol. 2021, 90, 107155. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Q.; Lv, L.; Fu, J.; Jiang, Y.; Xin, H.; Yao, Q. Glioma and microenvironment dual targeted nanocarrier for improved antiglioblastoma efficacy. Drug Deliv. 2017, 24, 1401–1409. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, A.; Lu, H.; Cheng, Q. The Role and Mechanism of Borneol to Open the Blood-Brain Barrier. Integr. Cancer Ther. 2018, 17, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Li, L.; Fan, L.; Fan, W.; Liu, L.; Zhang, F.; Hu, Z.; Wang, K.; Yang, L.; Wang, Z. The history, stereochemistry, ethnopharmacology and quality assessment of borneol. J. Ethnopharmacol. 2023, 300, 115697. [Google Scholar] [CrossRef]
- Song, H.; Wei, M.; Zhang, N.; Li, H.; Tan, X.; Zhang, Y.; Zheng, W. Enhanced permeability of blood-brain barrier and targeting function of brain via borneol-modified chemically solid lipid nanoparticle. Int. J. Nanomed. 2018, 13, 1869–1879. [Google Scholar] [CrossRef] [PubMed]
- Elzoghby, A.O.; Abdelmoneem, M.A.; Hassanin, I.A.; Abd Elwakil, M.M.; Elnaggar, M.A.; Mokhtar, S.; Fang, J.Y.; Elkhodairy, K.A. Lactoferrin, a multi-functional glycoprotein: Active therapeutic, drug nanocarrier & targeting ligand. Biomaterials 2020, 263, 120355. [Google Scholar]
- Teixeira, M.I.; Lopes, C.M.; Amaral, M.H.; Costa, P.C. Surface-modified lipid nanocarriers for crossing the blood-brain barrier (BBB): A current overview of active targeting in brain diseases. Colloids Surf. B Biointerfaces 2023, 221, 112999. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Ren, Y.; Lu, Q.; Wang, K.; Wu, Y.; Wang, Y.; Zhang, Y.; Cui, X.S.; Yang, Z.; Chen, Z. Lactoferrin: A glycoprotein that plays an active role in human health. Front. Nutr. 2022, 9, 1018336. [Google Scholar] [CrossRef] [PubMed]
- Friedli, M.J.; Inestrosa, N.C. Huperzine A and Its Neuroprotective Molecular Signaling in Alzheimer’s Disease. Molecules 2021, 26, 6531. [Google Scholar] [CrossRef] [PubMed]
- Actis Dato, V.; Chiabrando, G.A. The Role of Low-Density Lipoprotein Receptor-Related Protein 1 in Lipid Metabolism, Glucose Homeostasis and Inflammation. Int. J. Mol. Sci. 2018, 19, 1780. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.; Engel, D.F.; de Paula, G.C.; Dos Santos, D.B.; Lopes, J.B.; Farina, M.; Moreira, E.L.G.; de Bem, A.F. High Cholesterol Diet Exacerbates Blood-Brain Barrier Disruption in LDLr-/- Mice: Impact on Cognitive Function. J. Alzheimers Dis. 2020, 78, 97–115. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Choi, J.; Wong, G.W.; Wolfgang, M.J. Neurometabolic roles of ApoE and Ldl-R in mouse brain. J. Bioenerg. Biomembr. 2016, 48, 13–21. [Google Scholar] [CrossRef]
- Stockinger, W.; Hengstschläger-Ottnad, E.; Novak, S.; Matus, A.; Hüttinger, M.; Bauer, J.; Lassmann, H.; Schneider, W.J.; Nimpf, J. The low density lipoprotein receptor gene family. Differential expression of two alpha2-macroglobulin receptors in the brain. J. Biol. Chem. 1998, 273, 32213–32221. [Google Scholar] [CrossRef] [PubMed]
- Kanekiyo, T.; Cirrito, J.R.; Liu, C.C.; Shinohara, M.; Li, J.; Schuler, D.R.; Shinohara, M.; Holtzman, D.M.; Bu, G. Neuronal clearance of amyloid-β by endocytic receptor LRP1. J. Neurosci. 2013, 33, 19276–19283. [Google Scholar] [CrossRef]
- Srichai, M.B.; Zent, R. Integrin Structure and Function. In Cell-Extracellular Matrix Interactions in Cancer; Zent, R., Pozzi, A., Eds.; Springer: New York, NY, USA, 2010; pp. 19–41. [Google Scholar]
- Guell, K.; Bix, G.J. Brain endothelial cell specific integrins and ischemic stroke. Expert Rev. Neurother. 2014, 14, 1287–1292. [Google Scholar] [CrossRef] [PubMed]
- Liu, S. Radiolabeled multimeric cyclic RGD peptides as integrin alphavbeta3 targeted radiotracers for tumor imaging. Mol. Pharm. 2006, 3, 472–487. [Google Scholar] [CrossRef] [PubMed]
- Nieberler, M.; Reuning, U.; Reichart, F.; Notni, J.; Wester, H.J.; Schwaiger, M.; Weinmüller, M.; Räder, A.; Steiger, K.; Kessler, H. Exploring the Role of RGD-Recognizing Integrins in Cancer. Cancers 2017, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Zhang, X.; Mu, H.; Meng, Q.; Jiang, Y.; Wang, Y.; Lu, X.; Wang, A.; Liu, S.; Zhang, Y.; et al. RVG29-modified docetaxel-loaded nanoparticles for brain-targeted glioma therapy. Int. J. Pharm. 2018, 543, 179–189. [Google Scholar] [CrossRef]
- Ruan, S.; Zhou, Y.; Jiang, X.; Gao, H. Rethinking CRITID Procedure of Brain Targeting Drug Delivery: Circulation, Blood Brain Barrier Recognition, Intracellular Transport, Diseased Cell Targeting, Internalization, and Drug Release. Adv. Sci. 2021, 8, 2004025. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, D.; Wallace, T.L. A Review of the Cholinergic System and Therapeutic Approaches to Treat Brain Disorders. Curr. Top. Behav. Neurosci. 2020, 45, 1–28. [Google Scholar]
- Shen, J.X.; Yakel, J.L. Nicotinic acetylcholine receptor-mediated calcium signaling in the nervous system. Acta Pharmacol. Sin. 2009, 30, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Yakel, J.L. Cholinergic receptors: Functional role of nicotinic ACh receptors in brain circuits and disease. Pflug. Arch. 2013, 465, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Incontro, S.; Musella, M.L.; Sammari, M.; Di Scala, C.; Fantini, J.; Debanne, D. Lipids shape brain function through ion channel and receptor modulations: Physiological mechanisms and clinical perspectives. Physiol. Rev. 2025, 105, 137–207. [Google Scholar] [CrossRef]
- Dineley, K.T.; Pandya, A.A.; Yakel, J.L. Nicotinic ACh receptors as therapeutic targets in CNS disorders. Trends Pharmacol. Sci. 2015, 36, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Borroni, V.; Barrantes, F.J. Homomeric and Heteromeric α7 Nicotinic Acetylcholine Receptors in Health and Some Central Nervous System Diseases. Membranes 2021, 11, 664. [Google Scholar] [CrossRef]
- Skok, M.; Lykhmus, O. The Role of α7 Nicotinic Acetylcholine Receptors and α7-Specific Antibodies in Neuroinflammation Related to Alzheimer Disease. Curr. Pharm. Des. 2016, 22, 2035–2049. [Google Scholar] [CrossRef] [PubMed]
- Vallés, A.S.; Barrantes, F.J. Nicotinic Acetylcholine Receptor Dysfunction in Addiction and in Some Neurodegenerative and Neuropsychiatric Diseases. Cells 2023, 12, 2051. [Google Scholar] [CrossRef] [PubMed]
- Hoskin, J.L.; Al-Hasan, Y.; Sabbagh, M.N. Nicotinic Acetylcholine Receptor Agonists for the Treatment of Alzheimer’s Dementia: An Update. Nicotine Tob. Res. 2019, 21, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Crestini, A.; Carbone, E.; Rivabene, R.; Ancidoni, A.; Rosa, P.; Tata, A.M.; Fabrizi, E.; Locuratolo, N.; Vanacore, N.; Lacorte, E.; et al. A Systematic Review on Drugs Acting as Nicotinic Acetylcholine Receptor Agonists in the Treatment of Dementia. Cells 2024, 13, 237. [Google Scholar] [CrossRef] [PubMed]
- Hurst, R.; Rollema, H.; Bertrand, D. Nicotinic acetylcholine receptors: From basic science to therapeutics. Pharmacol. Ther. 2013, 137, 22–54. [Google Scholar] [CrossRef] [PubMed]
- Park, T.E.; Singh, B.; Li, H.; Lee, J.Y.; Kang, S.K.; Choi, Y.J.; Cho, C.S. Enhanced BBB permeability of osmotically active poly(mannitol-co-PEI) modified with rabies virus glycoprotein via selective stimulation of caveolar endocytosis for RNAi therapeutics in Alzheimer’s disease. Biomaterials 2015, 38, 61–71. [Google Scholar] [CrossRef]
- Son, S.; Hwang, D.W.; Singha, K.; Jeong, J.H.; Park, T.G.; Lee, D.S.; Kim, W.J. RVG peptide tethered bioreducible polyethylenimine for gene delivery to brain. J. Control. Release 2011, 155, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, R.; Han, L.; Ke, W.; Shao, K.; Ye, L.; Lou, J.; Jiang, C. Brain-targeting gene delivery and cellular internalization mechanisms for modified rabies virus glycoprotein RVG29 nanoparticles. Biomaterials 2009, 30, 4195–4202. [Google Scholar] [CrossRef] [PubMed]
- Romano, S.; Fonseca, N.; Simões, S.; Gonçalves, J.; Moreira, J.N. Nucleolin-based targeting strategies for cancer therapy: From targeted drug delivery to cytotoxic ligands. Drug Discov. Today 2019, 24, 1985–2001. [Google Scholar] [CrossRef] [PubMed]
- Brignole, C.; Bensa, V.; Fonseca, N.A.; Del Zotto, G.; Bruno, S.; Cruz, A.F.; Malaguti, F.; Carlini, B.; Morandi, F.; Calarco, E.; et al. Cell surface Nucleolin represents a novel cellular target for neuroblastoma therapy. J. Exp. Clin. Cancer Res. 2021, 40, 180. [Google Scholar] [CrossRef]
- Van den Avont, A.; Sharma-Walia, N. Anti-nucleolin aptamer AS1411: An advancing therapeutic. Front. Mol. Biosci. 2023, 10, 1217769. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.; Hwang, K.; Nam, K.M.; Kim, M.J.; Song, Y.K.; Kim, C.Y. Nucleolin-Targeting AS1411 Aptamer-Conjugated Nanospheres for Targeted Treatment of Glioblastoma. Pharmaceutics 2024, 16, 566. [Google Scholar] [CrossRef]
- Boulos, J.C.; Rahama, M.; Hegazy, M.F.; Efferth, T. Shikonin derivatives for cancer prevention and therapy. Cancer Lett. 2019, 459, 248–267. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; He, J.; Song, X.; Tan, L.; Wang, M.; Jiang, P.; Li, Y.; Cao, Z.; Peng, C. Pharmacological properties and derivatives of shikonin-A review in recent years. Pharmacol. Res. 2019, 149, 104463. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, W.; Gou, C.; Wang, X.; Wang, X.; Shao, X.; Chen, X.; Chen, Z. AS1411 binds to nucleolin via its parallel structure and disrupts the exos-miRNA-27a-mediated reciprocal activation loop between glioma and astrocytes. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167211. [Google Scholar] [CrossRef]
- Mikitsh, J.L.; Chacko, A.M. Pathways for small molecule delivery to the central nervous system across the blood-brain barrier. Perspect. Med. Chem. 2014, 6, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Pavan, B.; Dalpiaz, A. Prodrugs and endogenous transporters: Are they suitable tools for drug targeting into the central nervous system? Curr. Pharm. Des. 2011, 17, 3560–3576. [Google Scholar] [CrossRef] [PubMed]
- Markowicz-Piasecka, M.; Markiewicz, A.; Darłak, P.; Sikora, J.; Adla, S.K.; Bagina, S.; Huttunen, K.M. Current Chemical, Biological, and Physiological Views in the Development of Successful Brain-Targeted Pharmaceutics. Neurotherapeutics 2022, 19, 942–976. [Google Scholar] [CrossRef]
- Zhu, Y.; Liang, J.; Gao, C.; Wang, A.; Xia, J.; Hong, C.; Zhong, Z.; Zuo, Z.; Kim, J.; Ren, H.; et al. Multifunctional ginsenoside Rg3-based liposomes for glioma targeting therapy. J. Control. Release 2021, 330, 641–657. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Zhao, Y.; Yang, Z.; Yue, Q.; Xiao, W.; Chen, Y.; Yang, Y.; Guo, L.; Wu, Y. Liposomes actively recognizing the glucose transporter GLUT(1) and integrin α(v) β(3) for dual-targeting of glioma. Arch. Pharm. 2019, 352, e1800219. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhao, Y.; Chen, Y.; Yang, Z.; Zhang, L.; Xiao, W.; Yang, J.; Guo, L.; Wu, Y. Dual-targeting for brain-specific liposomes drug delivery system: Synthesis and preliminary evaluation. Bioorg. Med. Chem. 2018, 26, 4677–4686. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Y.; Zhao, Y.; Sun, M.-G.; Shi, J.-F.; Ju, R.-J.; Zhang, C.-X.; Li, X.-T.; Zhao, W.-Y.; Mu, L.-M.; Zeng, F.; et al. Multifunctional liposomes loaded with paclitaxel and artemether for treatment of invasive brain glioma. Biomaterials 2014, 35, 5591–5604. [Google Scholar] [CrossRef]
- Barbara, R.; Belletti, D.; Pederzoli, F.; Masoni, M.; Keller, J.; Ballestrazzi, A.; Vandelli, M.A.; Tosi, G.; Grabrucker, A.M. Novel Curcumin loaded nanoparticles engineered for Blood-Brain Barrier crossing and able to disrupt Abeta aggregates. Int. J. Pharm. 2017, 526, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, X.; Xie, C.; Zhang, M.; Ruan, H.; Wang, S.; Jiang, K.; Wang, F.; Zhan, C.; Lu, W.; et al. Nanodisk-based glioma-targeted drug delivery enabled by a stable glycopeptide. J. Control. Release 2018, 284, 26–38. [Google Scholar] [CrossRef]
- Wang, Y.; Ying, X.; Xu, H.; Yan, H.; Li, X.; Tang, H. The functional curcumin liposomes induce apoptosis in C6 glioblastoma cells and C6 glioblastoma stem cells in vitro and in animals. Int. J. Nanomed. 2017, 12, 1369–1384. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Hong, W.; Yu, M.; Li, Y.; Zheng, Y.; Ying, X. Multifunctional Targeting Liposomes of Epirubicin Plus Resveratrol Improved Therapeutic Effect on Brain Gliomas. Int. J. Nanomed. 2022, 17, 1087–1110. [Google Scholar] [CrossRef] [PubMed]
- Ying, X.; Wang, Y.; Xu, H.; Li, X.; Yan, H.; Tang, H.; Wen, C.; Li, Y. The construction of the multifunctional targeting ursolic acids liposomes and its apoptosis effects to C6 glioma stem cells. Oncotarget 2017, 8, 64129–64142. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, J.; Yu, P.; Yang, C.; Xia, C.; Deng, J.; Yu, M.; Xiang, Z.; Gan, L.; Zhu, B.; et al. A Novel Quercetin Encapsulated Glucose Modified Liposome and Its Brain-Target Antioxidative Neuroprotection Effects. Molecules 2024, 29, 607. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zheng, Z.; Wang, X.; Liu, S.; Gu, L.; Mu, J.; Zheng, X.; Li, Y.; Shen, S. Establishment and evaluation of glucose-modified nanocomposite liposomes for the treatment of cerebral malaria. J. Nanobiotechnol. 2022, 20, 318. [Google Scholar] [CrossRef] [PubMed]
- Puris, E.; Gynther, M.; Huttunen, J.; Auriola, S.; Huttunen, K.M. L-type amino acid transporter 1 utilizing prodrugs of ferulic acid revealed structural features supporting the design of prodrugs for brain delivery. Eur. J. Pharm. Sci. 2019, 129, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Montaser, A.; Markowicz-Piasecka, M.; Sikora, J.; Jalkanen, A.; Huttunen, K.M. L-type amino acid transporter 1 (LAT1)-utilizing efflux transporter inhibitors can improve the brain uptake and apoptosis-inducing effects of vinblastine in cancer cells. Int. J. Pharm. 2020, 586, 119585. [Google Scholar] [CrossRef] [PubMed]
- Vannucci, S.J.; Maher, F.; Simpson, I.A. Glucose transporter proteins in brain: Delivery of glucose to neurons and glia. Glia 1997, 21, 2–21. [Google Scholar] [CrossRef]
- Dienel, G.A. Brain Glucose Metabolism: Integration of Energetics with Function. Physiol. Rev. 2019, 99, 949–1045. [Google Scholar] [CrossRef] [PubMed]
- Temre, M.K.; Kumar, A.; Singh, S.M. An appraisal of the current status of inhibition of glucose transporters as an emerging antineoplastic approach: Promising potential of new pan-GLUT inhibitors. Front. Pharmacol. 2022, 13, 1035510. [Google Scholar] [CrossRef] [PubMed]
- Halmos, T.; Santarromana, M.; Antonakis, K.; Scherman, D. Synthesis of glucose-chlorambucil derivatives and their recognition by the human GLUT1 glucose transporter. Eur. J. Pharmacol. 1996, 318, 477–484. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, L.; Peng, Y.; Yue, Q.; Hai, L.; Guo, L.; Wang, Q.; Wu, Y. GLUT(1) -mediated venlafaxine-thiamine disulfide system-glucose conjugates with “lock-in” function for central nervous system delivery. Chem. Biol. Drug Des. 2018, 91, 707–716. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, A.; Zhang, S.; Kim, J.; Xia, J.; Zhang, F.; Wang, D.; Wang, Q.; Wang, J. Paclitaxel-loaded ginsenoside Rg3 liposomes for drug-resistant cancer therapy by dual targeting of the tumor microenvironment and cancer cells. J. Adv. Res. 2023, 49, 159–173. [Google Scholar] [CrossRef]
- Parrasia, S.; Szabò, I.; Zoratti, M.; Biasutto, L. Peptides as Pharmacological Carriers to the Brain: Promises, Shortcomings and Challenges. Mol. Pharm. 2022, 19, 3700–3729. [Google Scholar] [CrossRef] [PubMed]
- Malakoutikhah, M.; Teixidó, M.; Giralt, E. Shuttle-mediated drug delivery to the brain. Angew. Chem. Int. Ed. Engl. 2011, 50, 7998–8014. [Google Scholar] [CrossRef]
- Gosselet, F.; Loiola, R.A.; Roig, A.; Rosell, A.; Culot, M. Central nervous system delivery of molecules across the blood-brain barrier. Neurochem. Int. 2021, 144, 104952. [Google Scholar] [CrossRef] [PubMed]
- Grabrucker, A.M.; Ruozi, B.; Belletti, D.; Pederzoli, F.; Forni, F.; Vandelli, M.A.; Tosi, G. Nanoparticle transport across the blood brain barrier. Tissue Barriers 2016, 4, e1153568. [Google Scholar] [CrossRef] [PubMed]
- Tosi, G.; Fano, R.A.; Bondioli, L.; Badiali, L.; Benassi, R.; Rivasi, F.; Ruozi, B.; Forni, F.; Vandelli, M.A. Investigation on mechanisms of glycopeptide nanoparticles for drug delivery across the blood-brain barrier. Nanomedicine 2011, 6, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Fu, Q.; Zhao, Y.; Zhang, L.; Yue, Q.; Hai, L.; Guo, L.; Wu, Y. Ascorbic acid-modified brain-specific liposomes drug delivery system with “lock-in” function. Chem. Phys. Lipids 2019, 224, 104727. [Google Scholar] [CrossRef]
- Harrison, F.E.; May, J.M. Vitamin C function in the brain: Vital role of the ascorbate transporter SVCT2. Free Radic. Biol. Med. 2009, 46, 719–730. [Google Scholar] [CrossRef] [PubMed]
- May, J.M. Vitamin C transport and its role in the central nervous system. Subcell. Biochem. 2012, 56, 85–103. [Google Scholar] [PubMed]
- Portugal, C.C. Ascorbate and its transporter SVCT2: The dynamic duo’s integrated roles in CNS neurobiology and pathophysiology. Free Radic. Biol. Med. 2024, 212, 448–462. [Google Scholar] [CrossRef]
- Nualart, F.; Mack, L.; García, A.; Cisternas, P.; Bongarzone, E.R.; Heitzer, M.; Jara, N.; Martínez, F.; Ferrada, L.; Espinoza, F.; et al. Vitamin C Transporters, Recycling and the Bystander Effect in the Nervous System: SVCT2 versus Gluts. J. Stem Cell Res. Ther. 2014, 4, 209. [Google Scholar] [CrossRef]
- Gilgun-Sherki, Y.; Melamed, E.; Offen, D. Oxidative stress induced-neurodegenerative diseases: The need for antioxidants that penetrate the blood brain barrier. Neuropharmacology 2001, 40, 959–975. [Google Scholar] [CrossRef] [PubMed]
- Bay, C.; Bajraktari-Sylejmani, G.; Haefeli, W.E.; Burhenne, J.; Weiss, J.; Sauter, M. Functional Characterization of the Solute Carrier LAT-1 (SLC7A5/SLC2A3) in Human Brain Capillary Endothelial Cells with Rapid UPLC-MS/MS Quantification of Intracellular Isotopically Labelled L-Leucine. Int. J. Mol. Sci. 2022, 23, 3673. [Google Scholar] [CrossRef] [PubMed]
- Puris, E.; Gynther, M.; Auriola, S.; Huttunen, K.M. L-Type amino acid transporter 1 as a target for drug delivery. Pharm. Res. 2020, 37, 88. [Google Scholar] [CrossRef] [PubMed]
- Sgarbossa, A.; Giacomazza, D.; di Carlo, M. Ferulic Acid: A Hope for Alzheimer’s Disease Therapy from Plants. Nutrients 2015, 7, 5764–5782. [Google Scholar] [CrossRef] [PubMed]
- Li, X.T.; Tang, W.; Jiang, Y.; Wang, X.M.; Wang, Y.H.; Cheng, L.; Meng, X.S. Multifunctional targeting vinorelbine plus tetrandrine liposomes for treating brain glioma along with eliminating glioma stem cells. Oncotarget 2016, 7, 24604–24622. [Google Scholar] [CrossRef]
- Xiao, Y.; Cheng, L.; Xie, H.J.; Ju, R.J.; Wang, X.; Fu, M.; Liu, J.J.; Li, X.T. Vinorelbine cationic liposomes modified with wheat germ agglutinin for inhibiting tumor metastasis in treatment of brain glioma. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. S3), S524–S537. [Google Scholar] [CrossRef]
- Wang, X.; Gao, J.; Ouyang, X.; Wang, J.; Sun, X.; Lv, Y. Mesenchymal stem cells loaded with paclitaxel-poly(lactic-co-glycolic acid) nanoparticles for glioma-targeting therapy. Int. J. Nanomed. 2018, 13, 5231–5248. [Google Scholar] [CrossRef]
- Long, Y.; Xiang, Y.; Liu, S.; Zhang, Y.; Wan, J.; Ci, Z.; Cui, M.; Shen, L.; Li, N.; Guan, Y. Macrophage membrane modified baicalin liposomes improve brain targeting for alleviating cerebral ischemia reperfusion injury. Nanomedicine 2022, 43, 102547. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Zhao, Z.; Zhang, L.; Xue, L.; Shen, S.; Wen, Y.; Wei, Z.; Wang, L.; Kong, L.; Sun, H.; et al. Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat. Nanotechnol. 2017, 12, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Chu, X.; Cui, L.; Fu, S.; Gao, C.; Li, Y.; Sun, B. Neuronal mitochondria-targeted therapy for Alzheimer’s disease by systemic delivery of resveratrol using dual-modified novel biomimetic nanosystems. Drug Deliv. 2020, 27, 502–518. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Zhang, H.X.; He, C.P.; Fan, S.; Zhu, Y.L.; Qi, C.; Huang, N.P.; Xiao, Z.D.; Lu, Z.H.; Tannous, B.A.; et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials 2018, 150, 137–149. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Jiang, Y.; Shi, Y.; Liang, J.; Zhao, L. Curcumin-laden exosomes target ischemic brain tissue and alleviate cerebral ischemia-reperfusion injury by inhibiting ROS-mediated mitochondrial apoptosis. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 117, 111314. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Meng, S.; Song, Z.; Yang, X.; Li, X.; Guo, H.; Du, M.; Chen, J.; Zhu, Y.Z.; Wang, X. Neutrophil membrane fusogenic nanoliposomal leonurine for targeted ischemic stroke therapy via remodeling cerebral niche and restoring blood-brain barrier integrity. Mater. Today Bio 2023, 20, 100674. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.Q.; Qi, X.R.; Xiang, B.; Zhang, Y. A survey on “Trojan Horse” peptides: Opportunities, issues and controlled entry to “Troy”. J. Control. Release 2014, 194, 53–70. [Google Scholar] [CrossRef] [PubMed]
- Vives, E. Present and future of cell-penetrating peptide mediated delivery systems: “is the Trojan horse too wild to go only to Troy?”. J. Control. Release 2005, 109, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Timin, A.S.; Litvak, M.M.; Gorin, D.A.; Atochina-Vasserman, E.N.; Atochin, D.N.; Sukhorukov, G.B. Cell-Based Drug Delivery and Use of Nano-and Microcarriers for Cell Functionalization. Adv. Healthc. Mater. 2018, 7, 1700818. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Gao, C.; Gu, D.; Tang, H. Cell-based carrier for targeted hitchhiking delivery. Drug Deliv. Transl. Res. 2022, 12, 2634–2648. [Google Scholar] [CrossRef]
- Batrakova, E.V.; Gendelman, H.E.; Kabanov, A.V. Cell-mediated drug delivery. Expert Opin. Drug Deliv. 2011, 8, 415–433. [Google Scholar] [CrossRef] [PubMed]
- Ou, A.; Wang, Y.; Zhang, J.; Huang, Y. Living Cells and Cell-Derived Vesicles: A Trojan Horse Technique for Brain Delivery. Pharmaceutics 2023, 15, 1257. [Google Scholar] [CrossRef]
- Rosu, A.; Ghaemi, B.; Bulte, J.W.M.; Shakeri-Zadeh, A. Tumor-tropic Trojan horses: Using mesenchymal stem cells as cellular nanotheranostics. Theranostics 2024, 14, 571–591. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Iqbal, Z.; Lu, J.; Wang, J.; Zhang, H.; Chen, X.; Duan, L.; Xia, J. Cell-derived nanovesicle-mediated drug delivery to the brain: Principles and strategies for vesicle engineering. Mol. Ther. 2023, 31, 1207–1224. [Google Scholar] [CrossRef] [PubMed]
- Bajetto, A.; Thellung, S.; Dellacasagrande, I.; Pagano, A.; Barbieri, F.; Florio, T. Cross talk between mesenchymal and glioblastoma stem cells: Communication beyond controversies. Stem Cells Transl. Med. 2020, 9, 1310–1330. [Google Scholar] [CrossRef] [PubMed]
- Ho, I.A.; Toh, H.C.; Ng, W.H.; Teo, Y.L.; Guo, C.M.; Hui, K.M.; Lam, P.Y. Human bone marrow-derived mesenchymal stem cells suppress human glioma growth through inhibition of angiogenesis. Stem Cells 2013, 31, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Scholtemeijer, M.; Shah, K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol. Sci. 2020, 41, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Allabun, S.; Ojo, S.; Alqahtani, M.S.; Shukla, P.K.; Abbas, M.; Wechtaisong, C.; Almohiy, H.M. Enhanced Drug Delivery System Using Mesenchymal Stem Cells and Membrane-Coated Nanoparticles. Molecules 2023, 28, 2130. [Google Scholar] [CrossRef] [PubMed]
- Chulpanova, D.S.; Kitaeva, K.V.; Tazetdinova, L.G.; James, V.; Rizvanov, A.A.; Solovyeva, V.V. Application of Mesenchymal Stem Cells for Therapeutic Agent Delivery in Anti-tumor Treatment. Front. Pharmacol. 2018, 9, 259. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Li, J.; Yan, S.; Zhang, H.; Zhang, W.; Zhang, F.; Jiang, J. siRNA Delivery with Stem Cell Membrane-Coated Magnetic Nanoparticles for Imaging-Guided Photothermal Therapy and Gene Therapy. ACS Biomater. Sci. Eng. 2018, 4, 3895–3905. [Google Scholar] [CrossRef]
- Kang, S.; Bhang, S.H.; Hwang, S.; Yoon, J.K.; Song, J.; Jang, H.K.; Kim, S.; Kim, B.S. Mesenchymal Stem Cells Aggregate and Deliver Gold Nanoparticles to Tumors for Photothermal Therapy. ACS Nano 2015, 9, 9678–9690. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Y.; Tang, Y.; Wang, S.; Wang, C.; Li, Y.; Su, X.; Tian, J.; Tian, Y.; Pan, J.; et al. Synergistic Chemo-Photothermal Therapy of Breast Cancer by Mesenchymal Stem Cell-Encapsulated Yolk-Shell GNR@HPMO-PTX Nanospheres. ACS Appl. Mater. Interfaces 2016, 8, 17927–17935. [Google Scholar] [CrossRef]
- Levy, O.; Kuai, R.; Siren, E.M.J.; Bhere, D.; Milton, Y.; Nissar, N.; De Biasio, M.; Heinelt, M.; Reeve, B.; Abdi, R.; et al. Shattering barriers toward clinically meaningful MSC therapies. Sci. Adv. 2020, 6, eaba6884. [Google Scholar] [CrossRef]
- Liang, T.; Zhang, R.; Liu, X.; Ding, Q.; Wu, S.; Li, C.; Lin, Y.; Ye, Y.; Zhong, Z.; Zhou, M. Recent Advances in Macrophage-Mediated Drug Delivery Systems. Int. J. Nanomed. 2021, 16, 2703–2714. [Google Scholar] [CrossRef] [PubMed]
- Lapenna, A.; De Palma, M.; Lewis, C.E. Perivascular macrophages in health and disease. Nat. Rev. Immunol. 2018, 18, 689–702. [Google Scholar] [CrossRef]
- Wu, Y.; Wan, S.; Yang, S.; Hu, H.; Zhang, C.; Lai, J.; Zhou, J.; Chen, W.; Tang, X.; Luo, J.; et al. Macrophage cell membrane-based nanoparticles: A new promising biomimetic platform for targeted delivery and treatment. J. Nanobiotechnol. 2022, 20, 542. [Google Scholar] [CrossRef] [PubMed]
- Klyachko, N.L.; Polak, R.; Haney, M.J.; Zhao, Y.; Gomes Neto, R.J.; Hill, M.C.; Kabanov, A.V.; Cohen, R.E.; Rubner, M.F.; Batrakova, E.V. Macrophages with cellular backpacks for targeted drug delivery to the brain. Biomaterials 2017, 140, 79–87. [Google Scholar] [CrossRef]
- Fornari Laurindo, L.; Aparecido Dias, J.; Cressoni Araújo, A.; Torres Pomini, K.; Machado Galhardi, C.; Rucco Penteado Detregiachi, C.; Santos de Argollo Haber, L.; Donizeti Roque, D.; Dib Bechara, M.; Vialogo Marques de Castro, M.; et al. Immunological dimensions of neuroinflammation and microglial activation: Exploring innovative immunomodulatory approaches to mitigate neuroinflammatory progression. Front. Immunol. 2023, 14, 1305933. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Liu, R.; Jia, M.; Wang, Q.; Wu, J. Ischemia Reperfusion Injury Induced Blood Brain Barrier Dysfunction and the Involved Molecular Mechanism. Neurochem. Res. 2023, 48, 2320–2334. [Google Scholar] [CrossRef]
- Pang, B.; Dong, G.; Pang, T.; Sun, X.; Liu, X.; Nie, Y.; Chang, X. Emerging insights into the pathogenesis and therapeutic strategies for vascular endothelial injury-associated diseases: Focus on mitochondrial dysfunction. Angiogenesis 2024, 27, 623–639. [Google Scholar] [CrossRef] [PubMed]
- Muzykantov, V.R. Drug delivery by red blood cells: Vascular carriers designed by mother nature. Expert Opin. Drug Deliv. 2010, 7, 403–427. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.H.D.; Jayasinghe, M.K.; Le, A.H.; Peng, B.; Le, M.T.N. Advances in Drug Delivery Systems Based on Red Blood Cells and Their Membrane-Derived Nanoparticles. ACS Nano 2023, 17, 5187–5210. [Google Scholar] [CrossRef]
- Villa, C.H.; Anselmo, A.C.; Mitragotri, S.; Muzykantov, V. Red blood cells: Supercarriers for drugs, biologicals, and nanoparticles and inspiration for advanced delivery systems. Adv. Drug Deliv. Rev. 2016, 106 Pt A, 88–103. [Google Scholar] [CrossRef]
- Wang, H.; Zang, J.; Zhao, Z.; Zhang, Q.; Chen, S. The Advances of Neutrophil-Derived Effective Drug Delivery Systems: A Key Review of Managing Tumors and Inflammation. Int. J. Nanomed. 2021, 16, 7663–7681. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Yang, X.; Li, S.; Cheng, Z.; Wang, Y.; Zhao, J.; Zhang, C.; Li, Y.; Luo, M.; Ren, H.; et al. Accessing neuroinflammation sites: Monocyte/neutrophil-mediated drug delivery for cerebral ischemia. Sci. Adv. 2019, 5, eaau8301. [Google Scholar] [CrossRef] [PubMed]
- Enzmann, G.; Kargaran, S.; Engelhardt, B. Ischemia-reperfusion injury in stroke: Impact of the brain barriers and brain immune privilege on neutrophil function. Ther. Adv. Neurol. Disord. 2018, 11, 1756286418794184. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.F.; Phan, T.N.; Fan, C.H.; Vo Le, T.T.; Yeh, C.K. Advancements in ultrasound-mediated drug delivery for central nervous system disorders. Expert Opin. Drug Deliv. 2025, 22, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.V.; Chandrasekar, V.; Janapareddy, P.; Mathews, D.E.; Laux, P.; Luch, A.; Yang, Y.; Garcia-Canibano, B.; Balakrishnan, S.; Abinahed, J.; et al. Emerging Application of Nanorobotics and Artificial Intelligence To Cross the BBB: Advances in Design, Controlled Maneuvering, and Targeting of the Barriers. ACS Chem. Neurosci. 2021, 12, 1835–1853. [Google Scholar] [CrossRef]
- Maksymov, I.S. Gas Bubble Photonics: Manipulating Sonoluminescence Light with Fluorescent and Plasmonic Nanoparticles. Appl. Sci. 2022, 12, 8790. [Google Scholar] [CrossRef]
- Tomitaka, A.; Vashist, A.; Kolishetti, N.; Nair, M. Machine learning assisted-nanomedicine using magnetic nanoparticles for central nervous system diseases. Nanoscale Adv. 2023, 5, 4354–4367. [Google Scholar] [CrossRef] [PubMed]
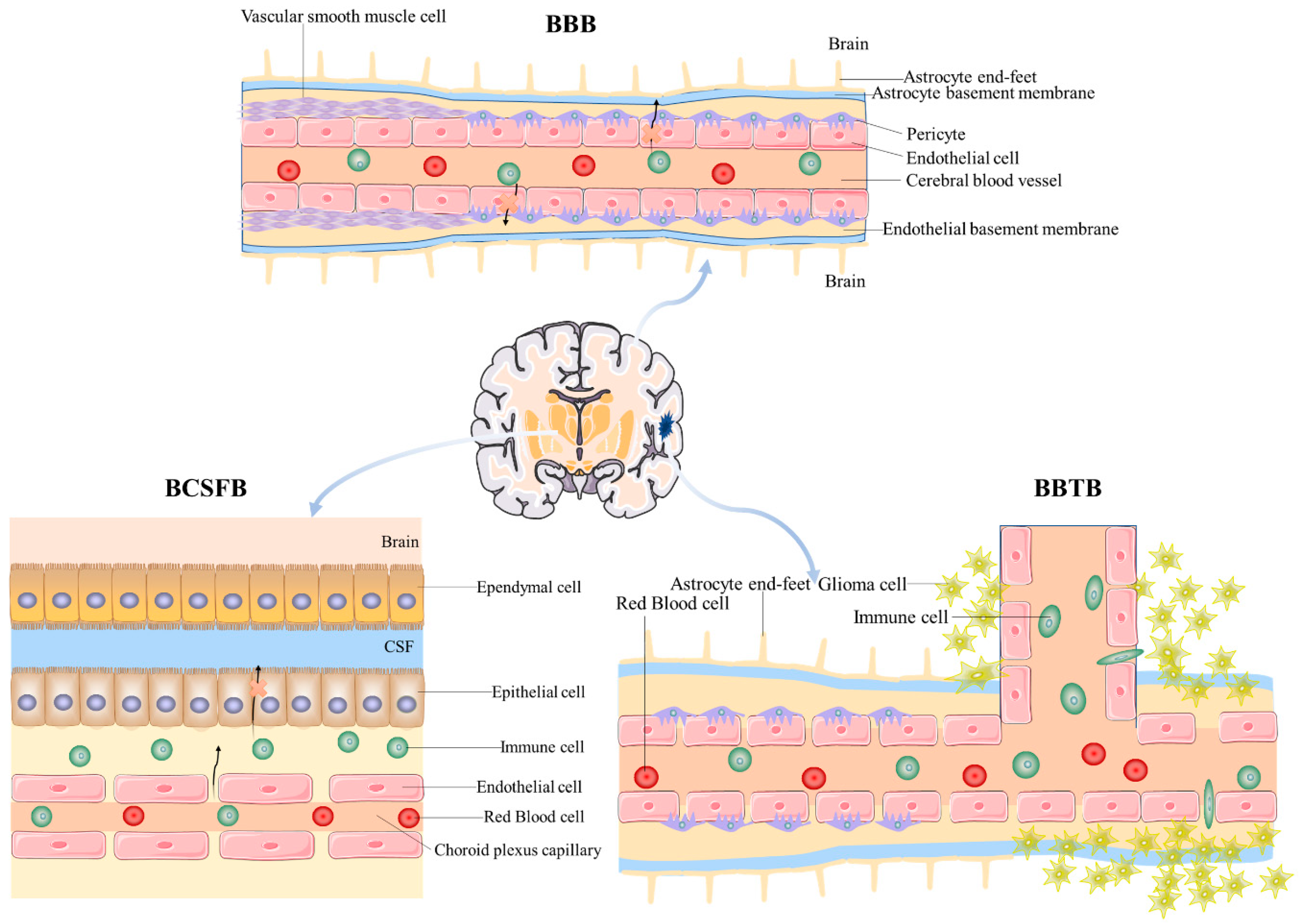
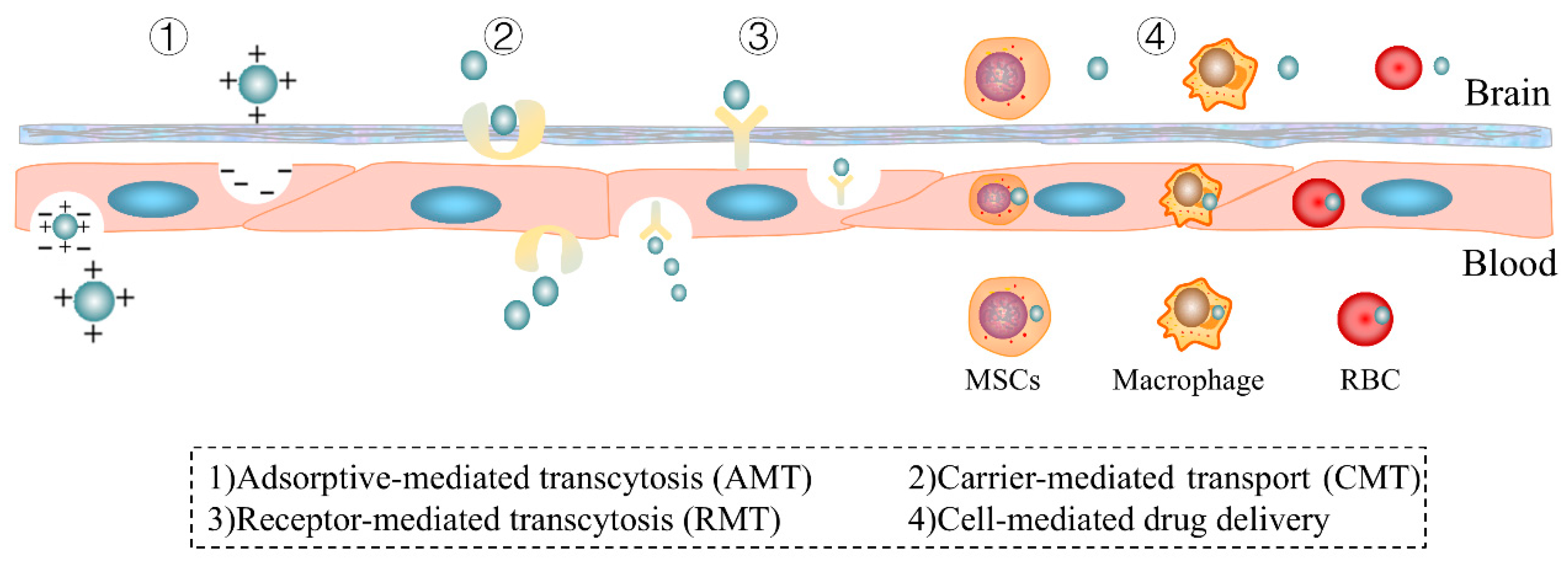
| Natural Medicine Monomers | Ligands | Receptors | Nano-Drug Delivery System | Application | Ref. |
|---|---|---|---|---|---|
| PTX | Tf | TfR | TF/TAT-PTX/DOX-LP | Glioma | [160] |
| Vincristine/Tetrandrine | Tf | TfR | Tf modified vincristine plus tetrandrine liposomes | Glioma | [161] |
| Resveratrol | Tf | TfR | f-PEG-PLA-RES nanoparticles | GBM | [162] |
| Muscone | RI7217 | TfR | Muscone and RI7217 co-modified DTX liposomes | Glioma | [163] |
| Elemene (ELE) | Tf | TfR | Tf-ELE/CTX@BLIP | Glioma | [164] |
| Vincristine (VCR) | T12, B6, and T7 | TfR | T12/B6/T7-LS/VCR | Glioma | [165] |
| Borneol | Pep-1 | IL-13R | DSPE-PEG-Bor | Glioma | [166] |
| PTX | Pep-1 | Interleukin-13 receptor α2 (IL-13Rα2) | Pep-NP-PTX | Glioma | [167] |
| PTX | Pep-1 | Interleukin-13 receptor α2 (IL-13Rα2) | PTX loaded Pep-1 and CGKRK peptide-modified PEG-PLGA nanoparticle (PC-NP-PTX) | Glioma | [168] |
| Quercetin (QU) | Lf | LfR | RMP-7-Lf-QU-LS | AD | [169] |
| Borneol | Lf | LfR | Borneol and lactoferrin co-modified nanoparticles (Lf-BNPs) | PD | [170] |
| Huperzine A | Lf | LfR | HupA Lf-TMC NPs | AD | [171] |
| Muscone | Lf | LfR | Lf-LP-Mu-DTX | Glioma | [172] |
| Rosmarinic acid | Cross-reacting material 197 (CRM197)/ApoE | Diphtheria toxin receptor/LDL-R | CRM197-ApoE-RA-PAAM-CH-PLGA | AD | [173] |
| Berberine | The attachment of plasma apolipoprotein (ApoE and ApoB) to Tween 80 | LDL-R | PTX-Tween 80-BBR + FA-Lip | Glioma | [174] |
| PTX | Peptide-22 | LDL-R | PNP–PTX | Glioma | [175] |
| PTX | Angiopep-2/A15 | LRP/CD133 | DP-CLPs-PTX-siRNA nanocomplex | Glioma | [176] |
| Isoliquiritigenin (ISL) | Angiopep-2 | LRP-1 | ISL loaded micelle prepared with DSPE-PEG2000 as the drug carrier modified with angiopep-2 | Ischemic stroke | [177] |
| Salidroside/Icariin | Angiopep-2 | LRP-1 | Ang-Sal/Ica-Lip | AD | [178] |
| PTX | R8-RGD | Integrin receptors expressed on C6 cells | PTX-R8-RGD-lipo | Glioma | [179] |
| PTX | R8-c(RGD) (R8c(RGD)-Lip) | Integrin αvβ3 | PTX-R8-c(RGD)-Lip | Vasculogenic mimicry and cancer ctem cells in malignant glioma | [180] |
| Vincristine/Tetrandrine | RGD | Integrin αvβ3 | RGD-modified vinorel- bine plus tetrandrine liposomes | Glioma | [181] |
| PTX/Naringenin | Cyclic RGD peptide sequence (Arg-Gly-Asp) | Integrin αvβ3 | RGD-modified PTX-NAR loaded SLNs | GBM | [182] |
| PTX | iRGD | Integrin αvβ3 | Co-administration of MT1-AF7p-conjugated PEG-PLA with iRGD | GBM | [183] |
| PTX | TR peptide | Integrin αvβ3 | PTX-TR-Lip | Glioma | [184] |
| PTX | RVG | nAChR | PTX-cholesterol complex (PTX-CHO) | Glioma | [185] |
| Baicalin | RVG29 peptide | nAChR | RVG29 peptide-modified BA-PEG-PLGA RNPs | Neuroprotection in cerebral ischemia | [186] |
| PTX | RVG | nAChR | RVG-PTX-NPs | Glioma | [187] |
| Shikonin | HA/AS1411 | CD44/Nucleolin | AS1411 aptamer/hyaluronic acid-bifunctionalized microemulsion co-loading shikonin and docetaxel | Glioma | [188] |
| Shikonin | AS1411/T7 (HAIYPRH) | Nucleolin/Tfr | Fe3O4@T7/AS1411/DTX&SKN-M | Glioma | [189] |
| Natural Medicine Monomers | Substrate | Transporters | Nano-Drug Delivery System | Application | Ref. |
|---|---|---|---|---|---|
| PTX | Multifunctional ginsenoside Rg3-based liposomal system (Rg3-LPs) | GLUT1 | Rg3-PTX-LPs | Glioma | [243] |
| PTX | Glucose-RGD (Glu-RGD) | GLUT1 and integrin αv β3 | PTX-Glu-RGD-Lip | Glioma | [244] |
| PTX | Glucose-vitamin C (Glu-Vc) derivative | GLUT1/SVCT2 | Glu-Vc-PTX-Lip | Glioma? | [245] |
| PTX/Artemether | MAN | GLUT1 | PTX-Artemether-MAN-TPGS1000-Lip | Glioma | [246] |
| Curcumin | Glycopeptide (g7) | GLUT1 | g7-PLGA-NPs-Cur | AD | [247] |
| PTX | Glycosylated A7R derivative | GLUT1 | 9G-A7R-PTX | Glioma | [248] |
| Curcumin | MAN | GLUT1 | Curcumin and quinacrine liposomes modified with MAN | Glioma | [249] |
| Resveratrol | MAN | GLUT1 | Epirubicin plus resveratrol liposomes modified with MAN (MAN-EPI-RES-L | Glioma | [250] |
| Rrsolic acids (UA)/epigallocatechin 3-gallate (EGCG) | MAN | GLUT1 | MAN-Ursolic acids plus EGCG liposomes | Glioma | [251] |
| Quercetin | Glucose | GLUT1 | Glucose-modified QU liposome (QU-Glu-Lip) | Neurodegenerative diseases (NDDs) | [252] |
| Artemisinin | Cholesterol-undecanoic acid-glucose conjugate | GLUT1 | na-ATS/TMP@lipoBX | Cerebral malaria (CM) | [253] |
| Ferulic acid (FA) | L-phenylalanine | LAT1 | L-phenylalanine-amino/ester-Ferulic acid | AD | [254] |
| Vinblastine | Derivatives of probenecid (PRB) | LAT1 | PRB-vinblastine | Improves efflux transporter-related MDR of brain-targeted anti-cancer agents | [255] |
| Natural Medicine Monomers | Electropositive Components | Nano-Drug Delivery System | Application | Ref. |
|---|---|---|---|---|
| Vincristine/ tetrandrine | Polyethylenimine (PEI)/ Vapreotide (VAP) | Vinorelbine plus tetrandrine liposomes modified with PEI and VAP | Glioma stem cells (GSCs) | [276] |
| Vinorelbine | DC-Chol | WGA (wheat germ agglutinin)-modified vinorelbine cationic liposomes | Glioma | [277] |
| Natural Medicine Monomers | Ligands | Nano-Drug Delivery System | Application | Ref. |
|---|---|---|---|---|
| PTX | R8-RGD | PTX-R8-RGD-lipo | Glioma | [179] |
| PTX | R8-c(RGD) (R8c(RGD)-Lip) | PTX-R8-c(RGD)-Lip | Vasculogenic mimicry and cancer ctem cells in malignant glioma | [180] |
| PTX | MSCs | Ptx-encapsulated PLGA nanoparticles (NPs) | Glioma | [278] |
| Baicalin | Macrophage membrane | Macrophage membrane-modified BA-LP (MM-BA-LP) | Cerebral ischemia reperfusion injury | [279] |
| PTX | Neutrophils (NEs) | PTX-NEs | Penetrates the brain and suppress the recurrence of glioma | [280] |
| Resveratrol | Red blood cell | RVG/TPP NPs@RBCm | AD | [281] |
| Curcumin | MSCs | cRGD-Exo-cur | IS | [282] |
| Curcumin | Macrophage RAW264.7 cells | Ex-cur | Alleviates cerebral ischemia-reperfusion injury | [283] |
| Leonurine | Neutrophil membrane | Leo@NM-Lipo | IS | [284] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, K.; Zhou, L.; Dan, M.; Yang, F.; Jian, T.; Xin, J.; Yu, Z.; Wang, Y. Trojan Horse Delivery Strategies of Natural Medicine Monomers: Challenges and Limitations in Improving Brain Targeting. Pharmaceutics 2025, 17, 280. https://doi.org/10.3390/pharmaceutics17030280
Lei K, Zhou L, Dan M, Yang F, Jian T, Xin J, Yu Z, Wang Y. Trojan Horse Delivery Strategies of Natural Medicine Monomers: Challenges and Limitations in Improving Brain Targeting. Pharmaceutics. 2025; 17(3):280. https://doi.org/10.3390/pharmaceutics17030280
Chicago/Turabian StyleLei, Kelu, Lanyu Zhou, Min Dan, Fei Yang, Tiantian Jian, Juan Xin, Zhigang Yu, and Yue Wang. 2025. "Trojan Horse Delivery Strategies of Natural Medicine Monomers: Challenges and Limitations in Improving Brain Targeting" Pharmaceutics 17, no. 3: 280. https://doi.org/10.3390/pharmaceutics17030280
APA StyleLei, K., Zhou, L., Dan, M., Yang, F., Jian, T., Xin, J., Yu, Z., & Wang, Y. (2025). Trojan Horse Delivery Strategies of Natural Medicine Monomers: Challenges and Limitations in Improving Brain Targeting. Pharmaceutics, 17(3), 280. https://doi.org/10.3390/pharmaceutics17030280









