Evaluating the Impact of Minimized GnRH and PGF2α Analogues-Loaded Chitosan Nanoparticles on Ovarian Activity and Fertility of Heat-Stressed Dairy Cows
Abstract
1. Introduction
2. Materials and Methods
2.1. Hormones-Conjugated Chitosan Nanoparticles Fabrication and Evaluation
2.2. Ethical Approval
2.3. Animals and Management
2.4. Collection of Meteorological Data
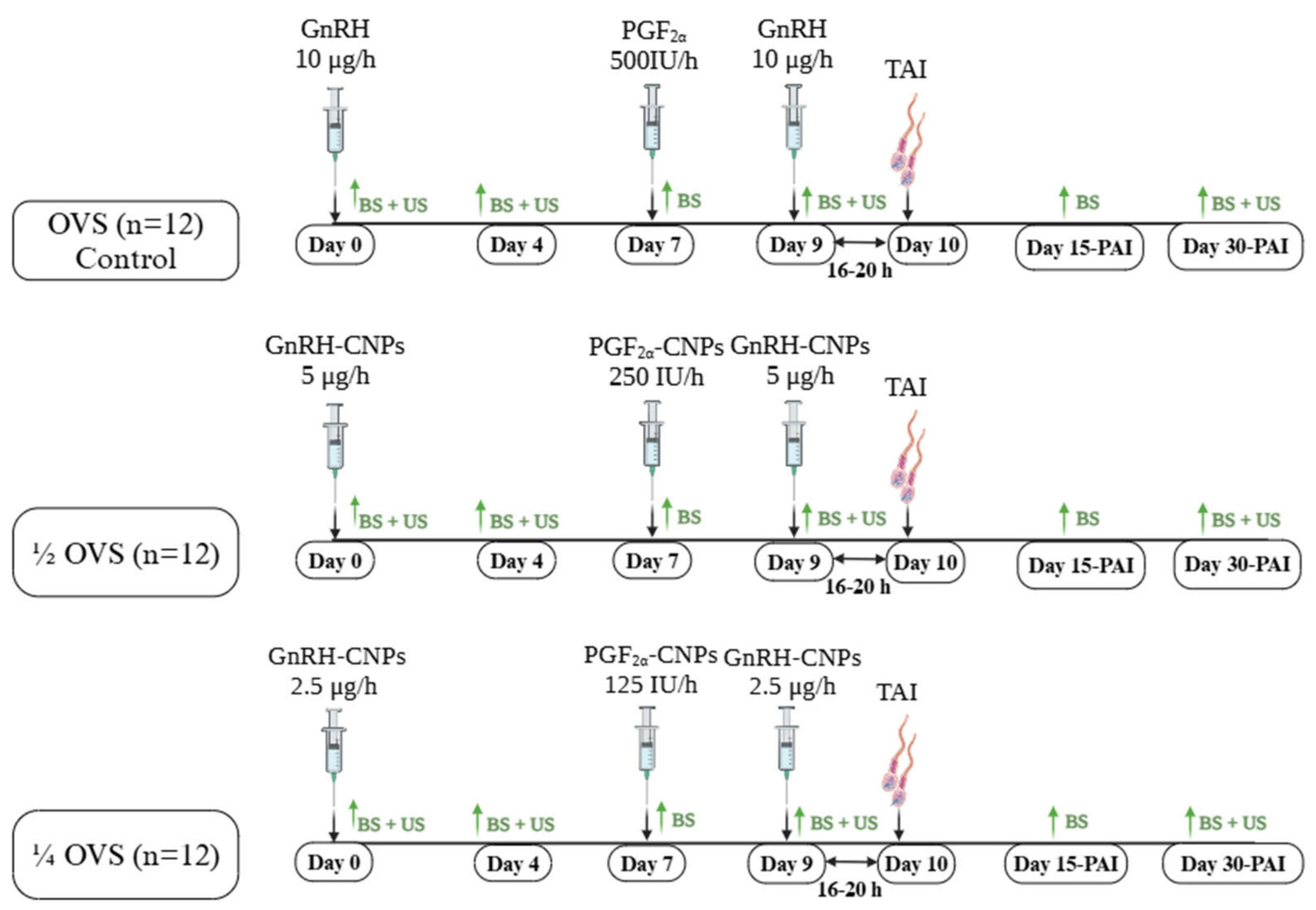
2.5. Experimental Design
2.6. Hormonal Analysis
2.7. Ultrasound Examination
2.8. Statistical Analysis
3. Results
3.1. Characterization of Chitosan and GnRH-Loaded Chitosan Nanoparticles
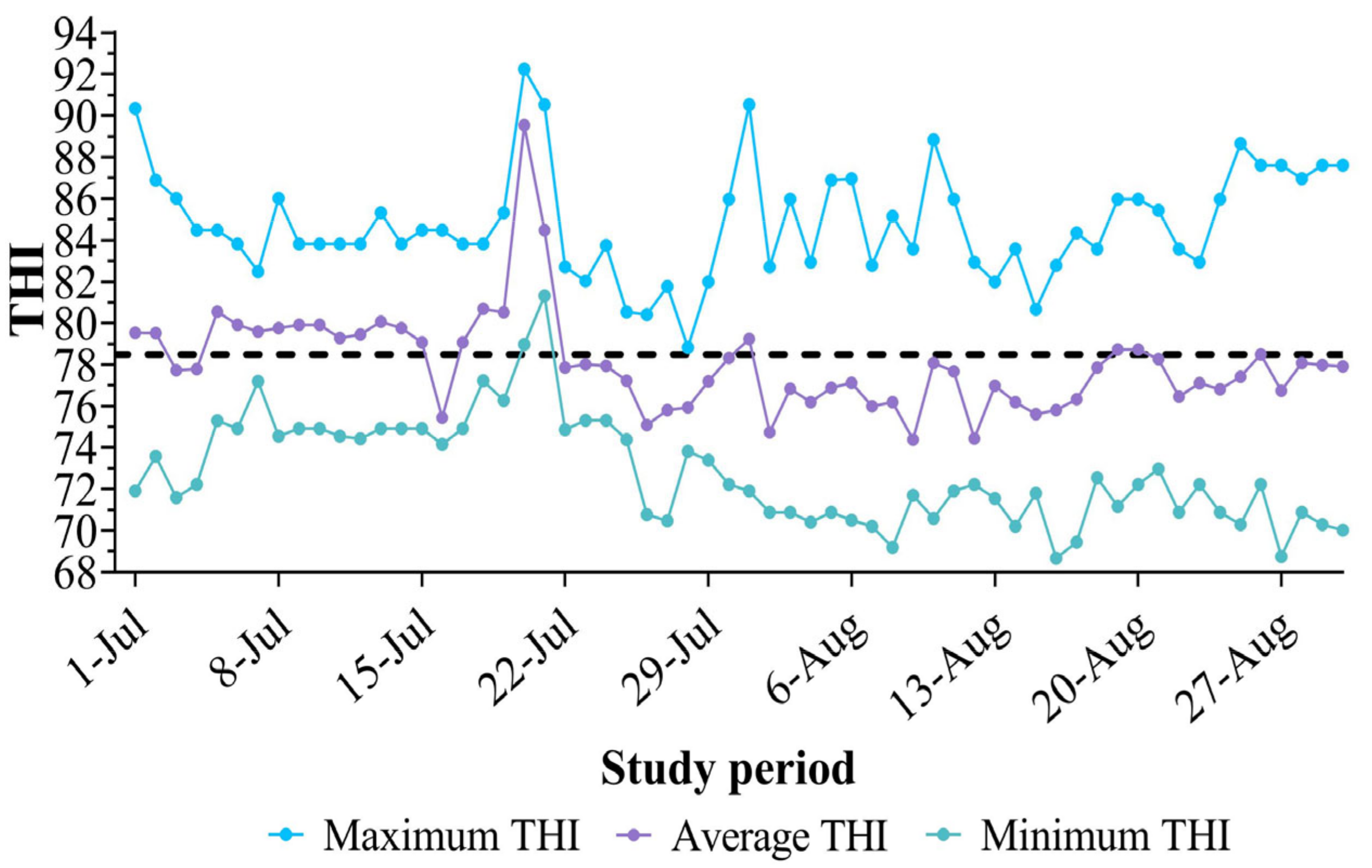
3.2. Environmental Conditions During the Study
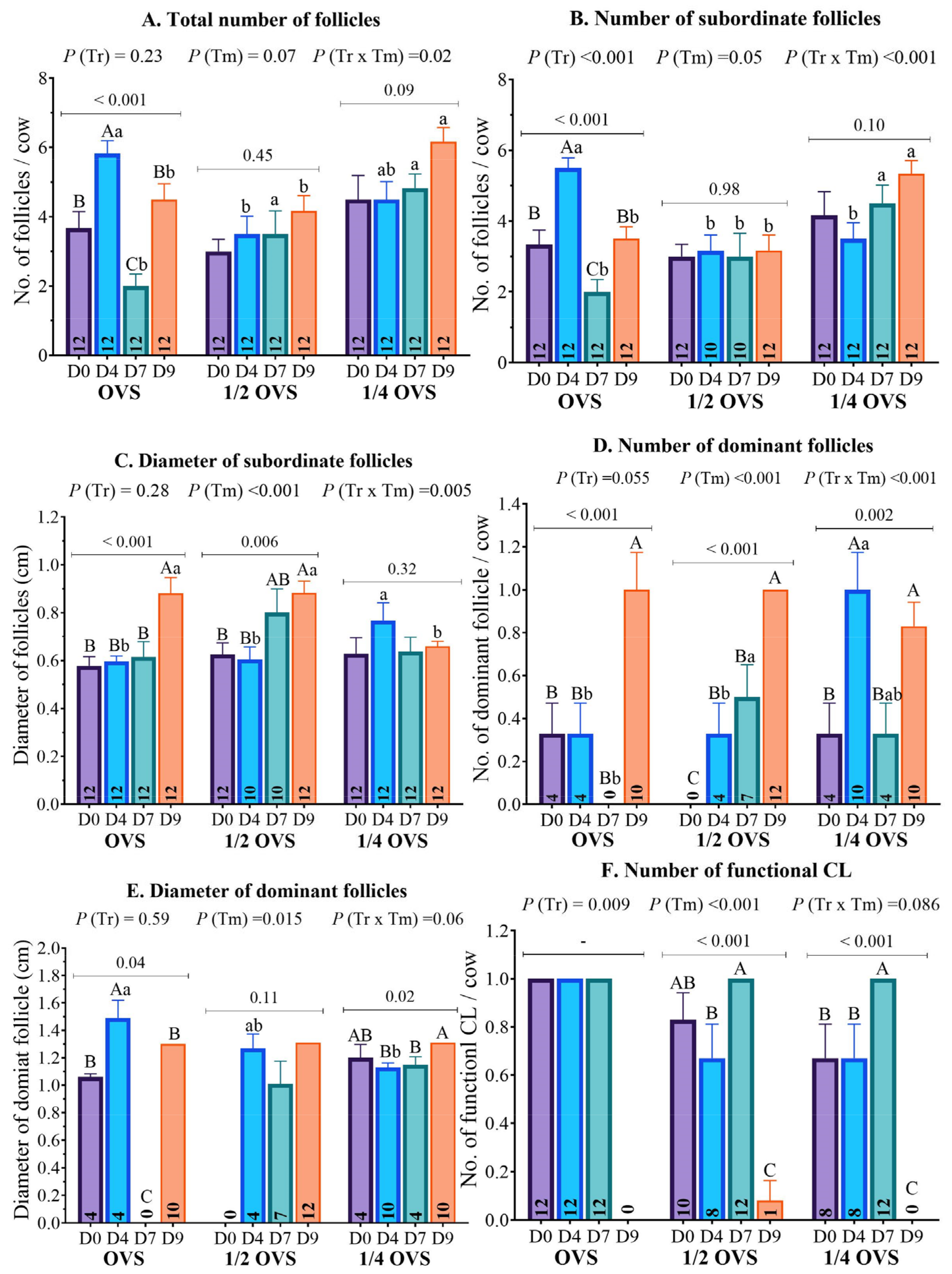
3.3. The Ovarian Structure
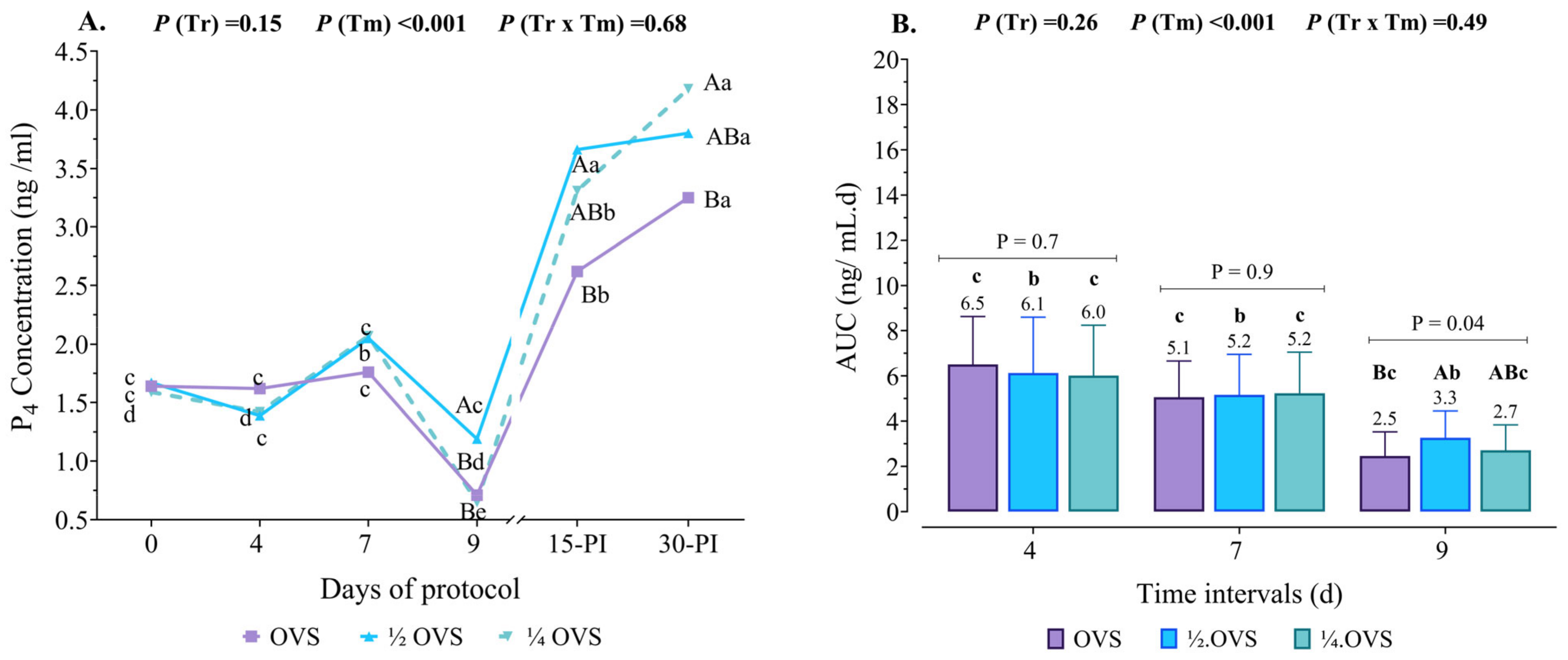
3.4. Serum P4 Profile
3.5. Pregnancy Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Stevenson, J.S.; Call, E.P.; Scoby, R.K.; Phatak, A.P. Double Insemination and Gonadotropin-Releasing Hormone Treatment of Repeat-Breeding Dairy Cattle. J. Dairy Sci. 1990, 73, 1766–1772. [Google Scholar] [CrossRef] [PubMed]
- Thatcher, W.W.; Bilby, T.R.; Bartolome, J.A.; Silvestre, F.; Staples, C.R.; Santos, J.E.P. Strategies for improving fertility in the modern dairy cow. Theriogenology 2006, 65, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Wiltbank, M.; Lopez, H.; Sartori, R.; Sangsritavong, S.; Gümen, A. Changes in reproductive physiology of lactating dairy cows due to elevated steroid metabolism. Theriogenology 2006, 65, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Sangsritavong, S.; Combs, D.K.; Sartori, R.; Armentano, L.E.; Wiltbank, M.C. High feed intake increases liver blood flow and metabolism of progesterone and estradiol-17β in dairy cattle. J. Dairy Sci. 2002, 85, 2831–2842. [Google Scholar] [CrossRef]
- Wiltbank, M.C.; Fricke, P.M.; Sangsritavong, S.; Sartori, R.; Ginther, O.J. Mechanisms that prevent and produce double ovulations in dairy cattle. J. Dairy Sci. 2000, 83, 2998–3007. [Google Scholar] [CrossRef]
- Dikmen, S.; Hansen, P.J. Is the temperature-humidity index the best indicator of heat stress in lactating dairy cows in a subtropical environment? J. Dairy Sci. 2009, 92, 109–116. [Google Scholar] [CrossRef]
- Habeeb, A.A.; Gad, A.E.; Atta, M.A. Temperature-humidity indices as indicators to heat stress of climatic conditions with relation to production and reproduction of farm animals. Int. J. Biotechnol. Recent Adv. 2018, 1, 35–50. [Google Scholar] [CrossRef]
- De Rensis, F.; Lopez-Gatius, F.; García-Ispierto, I.; Morini, G.; Scaramuzzi, R.J. Causes of declining fertility in dairy cows during the warm season. Theriogenology 2017, 91, 145–153. [Google Scholar] [CrossRef]
- Rispoli, L.A.; Edwards, J.L.; Pohler, K.G.; Russell, S.; Somiari, R.I.; Payton, R.R.; Schrick, F.N. Heat-induced hyperthermia impacts the follicular fluid proteome of the periovulatory follicle in lactating dairy cows. PLoS ONE 2019, 14, e0227095. [Google Scholar] [CrossRef]
- Sammad, A.; Umer, S.; Shi, R.; Zhu, H.; Zhao, X.; Wang, Y. Dairy cow reproduction under the influence of heat stress. J. Anim. Physiol. Anim. Nutr. 2020, 104, 978–986. [Google Scholar] [CrossRef]
- Shehab El Deen, M.A.M.M. Effects of Metabolic Stressors and High Temperature on Oocyte and Embryo Quality in High Yielding Dairy Cows. Ph.D. Thesis, Ghent University, Merelbeke, Belgium, 2011. [Google Scholar]
- Wolfenson, D.; Thatcher, W.W.; Badinga, L.; Savi0, J.D.; Meidan, R.; Lew, B.J.; Braw-Tal, R.; Berman, A. Effect of heat stress on follicular development during the estrous cycle in lactating dairy cattle. Biol. Reprod. 1995, 52, 1106–1113. [Google Scholar] [CrossRef]
- Garcia-Ispierto, I.; De Rensis, F.; Pérez-Salas, J.A.; Nunes, J.M.; Pradés, B.; Serrano-Pérez, B.; López-Gatius, F. The GnRH analogue dephereline given in a fixed-time AI protocol improves ovulation and embryo survival in dairy cows. Res. Vet. Sci. 2019, 122, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Chakravarty, A.K.; Singh, A.; Upadhyay, A.; Singh, M.; Yousuf, S. Effect of heat stress on reproductive performances of dairy cattle and buffaloes: A review. Vet. World 2016, 9, 235. [Google Scholar] [CrossRef]
- Badinga, L.; Collier, R.J.; Thatcher, W.W.; Wilcox, C.J. Effects of climatic and management factors on conception rate of dairy cattle in subtropical environment. J. Dairy Sci. 1985, 68, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Putney, D.J.; Mullins, S.; Thatcher, W.W.; Drost, M.; Gross, T.S. Embryonic development in superovulated dairy cattle exposed to elevated ambient temperatures between the onset of estrus and insemination. Anim. Reprod. Sci. 1989, 19, 37–51. [Google Scholar] [CrossRef]
- Breen, K.M.; Karsch, F.J. Does cortisol inhibit pulsatile luteinizing hormone secretion at the hypothalamic or pituitary level? Endocrinology 2004, 145, 692–698. [Google Scholar] [CrossRef]
- Wolfenson, D.; Lew, B.J.; Thatcher, W.W.; Graber, Y.; Meidan, R. Seasonal and acute heat stress effects on steroid production by dominant follicles in cows. Anim. Reprod. Sci. 1997, 47, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Hein, K.G.; Allrich, R.D. Influence of exogenous adrenocorticotropic hormone on estrous behavior in cattle. J. Anim. Sci. 1992, 70, 243–247. [Google Scholar] [CrossRef]
- Nebel, R.L.; Jobst, S.M.; Dransfield, M.B.G.; Pandolfi, S.M.; Bailey, T.L. Use of radio frequency data communication system, HeatWatch®, to describe behavioral estrus in dairy cattle. J. Dairy Sci. 1997, 80, 179. [Google Scholar]
- Armstrong, D. V Heat stress interaction with shade and cooling. J. Dairy Sci. 1994, 77, 2044–2050. [Google Scholar] [CrossRef]
- De Rensis, F.; Garcia-Ispierto, I.; López-Gatius, F. Seasonal heat stress: Clinical implications and hormone treatments for the fertility of dairy cows. Theriogenology 2015, 84, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.A.; Magee, D.D.; Tomaszewski, M.A.; Wilks, D.L.; Fourdraine, R.H. Management of summer infertility in Texas Holstein dairy cattle. Theriogenology 1996, 46, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, E.M.; Szelényi, Z.; Szenci, O. Gonadotropin-Releasing Hormone (GnRH) and Its Agonists in Bovine Reproduction I: Structure, Biosynthesis, Physiological Effects, and Its Role in Estrous Synchronization. Animals 2024, 14, 1473. [Google Scholar] [CrossRef]
- Cartmill, J.A.; El-Zarkouny, S.Z.; Hensley, B.A.; Rozell, T.G.; Smith, J.F.; Stevenson, J.S. An alternative AI breeding protocol for dairy cows exposed to elevated ambient temperatures before or after calving or both. J. Dairy Sci. 2001, 84, 799–806. [Google Scholar] [CrossRef]
- Guzeloglu, A.; Ambrose, J.D.; Kassa, T.; Diaz, T.; Thatcher, M.J.; Thatcher, W.W. Long-term follicular dynamics and biochemical characteristics of dominant follicles in dairy cows subjected to acute heat stress. Anim. Reprod. Sci. 2001, 66, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Aréchiga, C.F.; Staples, C.R.; McDowell, L.R.; Hansen, P.J. Effects of timed insemination and supplemental β-carotene on reproduction and milk yield of dairy cows under heat stress. J. Dairy Sci. 1998, 81, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Dirandeh, E. Starting Ovsynch protocol on day 6 of first postpartum estrous cycle increased fertility in dairy cows by affecting ovarian response during heat stress. Anim. Reprod. Sci. 2014, 149, 135–140. [Google Scholar] [CrossRef]
- De la Sota, R.L.; Burke, J.M.; Risco, C.A.; Moreira, F.; DeLorenzo, M.A.; Thatcher, W.W. Evaluation of timed insemination during summer heat stress in lactating dairy cattle. Theriogenology 1998, 49, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Uhm, H.B.; Jeong, J.K.; Kang, H.G.; Kim, I.H. Effect of timed artificial insemination protocols on the pregnancy rate per insemination and pregnancy loss in dairy cows and korean native cattle under heat stress. J. Vet. Clin. 2020, 37, 235–241. [Google Scholar] [CrossRef]
- Dirandeh, E.; Roodbari, A.R.; Colazo, M.G. Double-Ovsynch, compared with presynch with or without GnRH, improves fertility in heat-stressed lactating dairy cows. Theriogenology 2015, 83, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.S.; Pulley, S.L.; Mellieon, H.I., Jr. Prostaglandin F2α and gonadotropin-releasing hormone administration improve progesterone status, luteal number, and proportion of ovular and anovular dairy cows with corpora lutea before a timed artificial insemination program. J. Dairy Sci. 2012, 95, 1831–1844. [Google Scholar] [CrossRef] [PubMed]
- Amin, Y.A.; Said, A. The addition of chitosan to GnRH analog induces ovarian resumption and improves conception rates in buffaloes. Trop. Anim. Sci. J. 2021, 44, 1–9. [Google Scholar] [CrossRef]
- De Rensis, F.; Scaramuzzi, R.J. Heat stress and seasonal effects on reproduction in the dairy cow—A review. Theriogenology 2003, 60, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Rather, M.A.; Sharma, R.; Gupta, S.; Ferosekhan, S.; Ramya, V.L.; Jadhao, S.B. Chitosan-nanoconjugated hormone nanoparticles for sustained surge of gonadotropins and enhanced reproductive output in female fish. PLoS ONE 2013, 8, e57094. [Google Scholar] [CrossRef]
- Farokhzad, O.C.; Langer, R. Impact of nanotechnology on drug delivery. ACS Nano 2009, 3, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Casares-Crespo, L.; Fernández-Serrano, P.; Viudes-de-Castro, M.P. Protection of GnRH analogue by chitosan-dextran sulfate nanoparticles for intravaginal application in rabbit artificial insemination. Theriogenology 2018, 116, 49–52. [Google Scholar] [CrossRef]
- Hashem, N.M.; Sallam, S.M. Reproductive performance of goats treated with free gonadorelin or nanoconjugated gonadorelin at estrus. Domest. Anim. Endocrinol. 2020, 71, 106390. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, E.M.; Hashem, N.M.; El-Azrak, K.E.D.M.; Gonzalez-Bulnes, A.; Hassan, G.A.; Salem, M.H. Efficiency of gnrh–loaded chitosan nanoparticles for inducing lh secretion and fertile ovulations in protocols for artificial insemination in rabbit does. Animals 2021, 11, 440. [Google Scholar] [CrossRef] [PubMed]
- Gallab, R.S.; Hassanein, E.M.; Rashad, A.M.A.; El-, A.A. Maximizing the reproductive performances of anestrus dairy buffalo cows using GnRH analogue-loaded chitosan nanoparticles during the low breeding season. Anim. Reprod. Sci. 2022, 244, 107044. [Google Scholar] [CrossRef]
- Hashem, N.M.; El-Sherbiny, H.R.; Fathi, M.; Abdelnaby, E. Nanodelivery System for Ovsynch Protocol Improves Ovarian Response, Ovarian Blood Flow Doppler Velocities, and Hormonal Profile of Goats. Animals 2022, 12, 1442. [Google Scholar] [CrossRef]
- Hassanein, E.M.; Szelényi, Z.; Szenci, O. Gonadotropin-Releasing Hormone (GnRH) and Its Agonists in Bovine Reproduction II: Diverse Applications during Insemination, Post-Insemination, Pregnancy, and Postpartum Periods. Animals 2024, 14, 1575. [Google Scholar] [CrossRef]
- de Carvalho Cardoso, R.; Barbosa, L.P.; Souza, R.S.; da França, C.S.; Ribeiro, M.D.M.; Santana, A.L.A.; de Jesus, R.D.L.; dos Santos, R.S. Application of hormonal subdoses at acupoint Hou Hai in estrus synchronization protocols of goats. Semin. Ciênc. Agrár. 2018, 39, 1135–1142. [Google Scholar] [CrossRef]
- Mohammadpour Dounighi, N.; Eskandari, R.; Avadi, M.R.; Zolfagharian, H.; Mir Mohammad Sadeghi, A.; Rezayat, M. Preparation and in vitro characterization of chitosan nanoparticles containing Mesobuthus eupeus scorpion venom as an antigen delivery system. J. Venom. Anim. Toxins Incl. Trop. Dis. 2012, 18, 44–52. [Google Scholar] [CrossRef]
- McGlone, J. Guide for the Care and Use of Agricultural Animals in Research and Teaching; Federation of Animal Science Societies: Champaign, IL, USA, 2010; ISBN 1884706118. [Google Scholar]
- Wildman, E.E.; Jones, G.M.; Wagner, P.E.; Boman, R.L.; Troutt Jr, H.F.; Lesch, T.N. A dairy cow body condition scoring system and its relationship to selected production characteristics. J. Dairy Sci. 1982, 65, 495–501. [Google Scholar] [CrossRef]
- NRC Nutrient Requirements of Dairy Cattle: 2001; National Academies Press: Washington, DC, USA, 2001; ISBN 0309069971.
- Jawień, W. Searching for an optimal AUC estimation method: A never-ending task? J. Pharmacokinet. Pharmacodyn. 2014, 41, 655–673. [Google Scholar] [CrossRef] [PubMed]
- Fricke, P.M. Scanning the future—Ultrasonography as a reproductive management tool for dairy cattle. J. Dairy Sci. 2002, 85, 1918–1926. [Google Scholar] [CrossRef]
- SAS Institute Inc SAS 9. 2 Intelligence Platform: Overview; SAS Institute Inc.: Cary, NC, USA, 2009. [Google Scholar]
- Duncan, D.B. Multiple range and multiple F tests. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]
- Munari, C.; Ponzio, P.; Alkhawagah, A.R.; Schiavone, A.; Mugnai, C. Effects of an intravaginal GnRH analogue administration on rabbit reproductive parameters and welfare. Theriogenology 2019, 125, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Gan, Q.; Wang, T.; Cochrane, C.; McCarron, P. Modulation of surface charge, particle size and morphological properties of chitosan-TPP nanoparticles intended for gene delivery. Colloids Surf. B Biointerfaces 2005, 44, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.A.; Gupta, S.; Varghese, T.; Jahageerdar, S.; Nayak, S.K.; Reang, D.; Bhat, I.A.; Mahadevaswamy, C.G.; Chuphal, N.; Dar, S.A.; et al. Chitosan-hypothalamic hormonal analogue nanoconjugates enhanced the reproductive performance in Indian major carp, Labeo rohita. Front. Mar. Sci. 2023, 10, 1311158. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems—A review (Part 1). Trop. J. Pharm. Res. 2013, 12, 255–264. [Google Scholar]
- Barbari, G.R.; Dorkoosh, F.A.; Amini, M.; Sharifzadeh, M.; Atyabi, F.; Balalaie, S.; Tehrani, N.R.; Tehrani, M.R. A novel nanoemulsion-based method to produce ultrasmall, water-dispersible nanoparticles from chitosan, surface modified with cell-penetrating peptide for oral delivery of proteins and peptides. Int. J. Nanomed. 2017, 12, 3471. [Google Scholar] [CrossRef] [PubMed]
- Najafi, S.; Pazhouhnia, Z.; Ahmadi, O.; Berenjian, A.; Jafarizadeh-Malmiri, H. Chitosan nanoparticles and their applications in drug delivery: A review. Curr. Res. Drug Discov. 2014, 1, 17–25. [Google Scholar] [CrossRef]
- Schüller, L.K.; Burfeind, O.; Heuwieser, W. Impact of heat stress on conception rate of dairy cows in the moderate climate considering different temperature–humidity index thresholds, periods relative to breeding, and heat load indices. Theriogenology 2014, 81, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Johnson, H.D. Physiological Responses and Productivity of Cattle; CRC Press: Boca Raton, FL, USA, 1985. [Google Scholar]
- El-Tarabany, M.S.; El-Tarabany, A.A. Impact of thermal stress on the efficiency of ovulation synchronization protocols in Holstein cows. Anim. Reprod. Sci. 2015, 160, 138–145. [Google Scholar] [CrossRef]
- El-Tarabany, M.S.; El-Tarabany, A.A. Impact of maternal heat stress at insemination on the subsequent reproductive performance of Holstein, Brown Swiss, and their crosses. Theriogenology 2015, 84, 1523–1529. [Google Scholar] [CrossRef]
- Chebel, R.C.; Santos, J.E.P.; Reynolds, J.P.; Cerri, R.L.A.; Juchem, S.O.; Overton, M. Factors affecting conception rate after artificial insemination and pregnancy loss in lactating dairy cows. Anim. Reprod. Sci. 2004, 84, 239–255. [Google Scholar] [CrossRef]
- Wiltbank, M.C.; Sartori, R.; Herlihy, M.M.; Vasconcelos, J.L.M.; Nascimento, A.B.; Souza, A.H.; Ayres, H.; Cunha, A.P.; Keskin, A.; Guenther, J.N.; et al. Managing the dominant follicle in lactating dairy cows. Theriogenology 2011, 76, 1568–1582. [Google Scholar] [CrossRef]
- Nowicki, A.; Barański, W.; Baryczka, A.; Janowski, T. OvSynch protocol and its modifications in the reproduction management of dairy cattle herds—An update. J. Vet. Res. 2017, 61, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Bisinotto, R.S.; Chebel, R.C.; Santos, J.E.P. Follicular wave of the ovulatory follicle and not cyclic status influences fertility of dairy cows. J. Dairy Sci. 2010, 93, 3578–3587. [Google Scholar] [CrossRef] [PubMed]
- Gümen, A.; Guenther, J.N.; Wiltbank, M.C. Follicular size and response to Ovsynch versus detection of estrus in anovular and ovular lactating dairy cows. J. Dairy Sci. 2003, 86, 3184–3194. [Google Scholar] [CrossRef] [PubMed]
- Saha, R.; Bhat, I.A.; Charan, R.; Purayil, S.B.P.; Krishna, G.; Kumar, A.P.; Sharma, R. Ameliorative effect of chitosan-conjugated 17α-methyltestosterone on testicular development in Clarias batrachus. Anim. Reprod. Sci. 2018, 193, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Rastegarnia, A.; Niasari-Naslaji, A.; Hovareshti, P.; Sarhaddi, F.; Safaei, M. The effect of different doses of Gonadorelin on ovarian follicle dynamics in river buffalo (Bubalus bubalis). Theriogenology 2004, 62, 1283–1291. [Google Scholar] [CrossRef]
- Raval, R.J.; Vala, K.B.; Padodara, R.J.; Dhami, A.J.; Kavani, F.S. Evaluation of double ovsynch protocol in acyclic jaffarabadi heifers and buffaloes with respect to ovarian dynamics, hormonal profile and fertility. Indian J. Anim. Res. 2021, 55, 879–888. [Google Scholar] [CrossRef]
- Ghuman, S.; Honparkhe, M.; Singh, J. Comparison of ovsynch and progesterone-based protocol for induction of synchronized ovulation and conception rate in subestrous buffalo during low-breeding season. Iran. J. Vet. Res. 2014, 15, 375. [Google Scholar]
- Hashem, N.M.; El-Zarkouny, S.Z.; Taha, T.A.; Abo-Elezz, Z.R. Oestrous response and characterization of the ovulatory wave following oestrous synchronization using PGF2α alone or combined with GnRH in ewes. Small Rumin. Res. 2015, 129, 84–87. [Google Scholar] [CrossRef]
- Kanaya, T.; Miyagawa, S.; Kawamura, T.; Sakai, Y.; Masada, K.; Nawa, N.; Ishida, H.; Narita, J.; Toda, K.; Kuratani, T. Innovative therapeutic strategy using prostaglandin I2 agonist (ONO1301) combined with nano drug delivery system for pulmonary arterial hypertension. Sci. Rep. 2021, 11, 7292. [Google Scholar] [CrossRef] [PubMed]
- Esquivel, R.; Juárez, J.; Almada, M.; Ibarra, J.; Valdez, M.A. Synthesis and characterization of new thiolated chitosan nanoparticles obtained by ionic gelation method. Int. J. Polym. Sci. 2015, 2015, 502058. [Google Scholar] [CrossRef]
- Perry, G.A.; Smith, M.F.; Lucy, M.C.; Green, J.A.; Parks, T.E.; MacNeil, M.D.; Roberts, A.J.; Geary, T.W. Relationship between follicle size at insemination and pregnancy success. Proc. Natl. Acad. Sci. USA 2005, 102, 5268–5273. [Google Scholar] [CrossRef] [PubMed]
- Assey, R.J.; Purwantara, B.; Greve, T.; Hyttel, P.; Schmidt, M.H. Corpus luteum size and plasma progesterone levels in cattle after cloprostenol-induced luteolysis. Theriogenology 1993, 39, 1321–1330. [Google Scholar] [CrossRef]
- de Carvalho, N.A.T.; Soares, J.G.; Baruselli, P.S. Strategies to overcome seasonal anestrus in water buffalo. Theriogenology 2016, 86, 200–206. [Google Scholar] [CrossRef]
- Peters, M.W.; Pursley, J.R. Fertility of lactating dairy cows treated with ovsynch after presynchronization injections of PGF2α and GnRH. J. Dairy Sci. 2002, 85, 2403–2406. [Google Scholar] [CrossRef] [PubMed]
- Jazayeri, S.P.; Kohram, H.; Salehi, R. Hormonal responses to GnRH injection given at different stages of the estrous cycle in water buffaloes. Afr. J. Biotechnol. 2010, 9, 2169–2172. [Google Scholar]
- Roman-Ponce, H.; Thatcher, W.W.; Caton, D.; Barron, D.H.; Wilcox, C.J. Thermal stress effects on uterine blood flow in dairy cows. J. Anim. Sci. 1978, 46, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Rivera, R.M.; Hansen, P.J. Development of cultured bovine embryos after exposure to high temperatures in the physiological range. Reproduction 2001, 121, 107–115. [Google Scholar] [CrossRef] [PubMed]

| Variables | Experimental Groups | p-Value | ||
|---|---|---|---|---|
| OVS | ½ OVS | ¼ OVS | ||
| Before the 1st GnRH administration (D0) | ||||
| Number of cows | 12 | 12 | 12 | |
| Total number of follicles | 3.67 ± 0.48 (12) | 3.00 ± 0.35 (12) | 4.50 ± 0.69 (12) | 0.15 |
| Subordinate (≥0.3 to <1.0 cm) follicle number | 3.33 ± 0.41 (12) | 3.00 ± 0.35 (12) | 4.17 ± 0.66 (12) | 0.24 |
| Subordinate (≥0.3 to <1.0 cm) follicle diameter | 0.58 ± 0.04 (12) | 0.63 ± 0.05 (12) | 0.63 ± 0.07 (12) | 0.76 |
| Dominant (≥1.0 cm) follicle number | 0.33 ± 0.14 (4) | 0.00 ± 0.00 (0) | 0.33 ± 0.14 (4) | 0.07 |
| Dominant (≥1.0 cm) follicle diameter | 1.06 ± 0.02 (4) | - (0) | 1.20 ± 0.10 (4) | 0.20 |
| Functional CL number | 1.00 ± 0.00 (12) | 0.83 ± 0.11 (10) | 0.67 ± 0.14 (8) | 0.09 |
| Response to the 1st GnRH administration (D4) | ||||
| Total number of follicles | 5.83 ± 0.37 a (12) | 3.50 ± 0.52 b (12) | 4.50 ± 0.52 ab (12) | 0.005 |
| Subordinate (≥0.3 to <1.0 cm) follicle number | 5.50± 0.29 a (12) | 3.17 ± 0.44 b (10) | 3.50 ± 0.45 b (12) | <0.001 |
| Subordinate (≥0.3 to <1.0 cm) follicle diameter | 0.60 ± 0.02 b (12) | 0.61 ± 0.05 b (10) | 0.77 ± 0.07 a (12) | 0.05 |
| Dominant (≥1.0 cm) follicle number | 0.33 ± 0.14 b (4) | 0.33 ± 0.14 b (4) | 1.00 ± 0.17 a (10) | 0.005 |
| Dominant (≥1.0 cm) follicle diameter | 1.49 ± 0.13 a (4) | 1.27 ± 0.10 ab (4) | 1.09 ± 0.05 b (10) | 0.008 |
| Functional CL number | 1.00 ± 0.00 (12) | 0.67 ± 0.14 (8) | 0.67 ± 0.14 (8) | 0.08 |
| Before PGF2α administration (D7) | ||||
| Total number of follicles | 2.00 ± 0.35 b (12) | 3.50 ± 0.67 a (12) | 4.83 ± 0.41 a (12) | 0.001 |
| Subordinate (≥0.3 to <1.0 cm) follicle number | 2.00 ± 0.35 b (12) | 3.00 ± 0.65 b (10) | 4.50 ± 0.51 a (12) | 0.007 |
| Subordinate (≥0.3 to <1.0 cm) follicle diameter | 0.62 ± 0.06 (12) | 0.80 ± 0.10 (10) | 0.64 ± 0.06 (12) | 0.18 |
| Dominant (≥1.0 cm) follicle number | 0.00 ± 0.00 b (0) | 0.50 ± 0.15 a (7) | 0.33 ± 0.14 ab (4) | 0.02 |
| Dominant (≥1.0 cm) follicle diameter | - (0) | 1.01 ± 0.17 (7) | 1.13 ± 0.03 (4) | 0.56 |
| Functional CL number | 1.00 ± 0.00 (12) | 1.00 ± 0.00 (12) | 1.00 ± 0.00 (12) | - |
| Day of the 2nd GnRH and response to PGF2α administrations (D9) | ||||
| Total number of follicles | 4.50 ± 0.45 b (12) | 4.17 ± 0.44 b (12) | 6.17 ± 0.41 a (12) | 0.006 |
| Subordinate (≥0.3 to <1.0 cm) follicle number | 3.50 ± 0.34 b (12) | 3.17 ± 0.44 b (12) | 5.33 ± 0.38 a (12) | 0.001 |
| Subordinate (≥0.3 to <1.0 cm) follicle diameter | 0.88 ± 0.07 a (12) | 0.88 ± 0.05 a (12) | 0.66 ± 0.02 b (12) | 0.003 |
| Dominant (≥1.0 cm) follicle number | 1.01 ± 0.17 (10) | 1.0 ± 0.00 (12) | 0.83 ± 0.11 (10) | 0.53 |
| Dominant (≥1.0 cm) follicle diameter | 1.30 ± 0.07 (10) | 1.31 ± 0.05 (12) | 1.15 ± 0.06 (10) | 0.99 |
| Functional CL number | 0.00 ± 0.00 (0) | 0.08 ± 0.29 (1) | 0.00 ± 0.00 (0) | 0.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omar, M.E.A.; Hassanein, E.M.; Shehabeldin, A.M.; Szenci, O.; El-Shereif, A.A. Evaluating the Impact of Minimized GnRH and PGF2α Analogues-Loaded Chitosan Nanoparticles on Ovarian Activity and Fertility of Heat-Stressed Dairy Cows. Pharmaceutics 2025, 17, 274. https://doi.org/10.3390/pharmaceutics17020274
Omar MEA, Hassanein EM, Shehabeldin AM, Szenci O, El-Shereif AA. Evaluating the Impact of Minimized GnRH and PGF2α Analogues-Loaded Chitosan Nanoparticles on Ovarian Activity and Fertility of Heat-Stressed Dairy Cows. Pharmaceutics. 2025; 17(2):274. https://doi.org/10.3390/pharmaceutics17020274
Chicago/Turabian StyleOmar, Mohammed E. A., Eman M. Hassanein, Ahmed M. Shehabeldin, Ottó Szenci, and Abdelghany A. El-Shereif. 2025. "Evaluating the Impact of Minimized GnRH and PGF2α Analogues-Loaded Chitosan Nanoparticles on Ovarian Activity and Fertility of Heat-Stressed Dairy Cows" Pharmaceutics 17, no. 2: 274. https://doi.org/10.3390/pharmaceutics17020274
APA StyleOmar, M. E. A., Hassanein, E. M., Shehabeldin, A. M., Szenci, O., & El-Shereif, A. A. (2025). Evaluating the Impact of Minimized GnRH and PGF2α Analogues-Loaded Chitosan Nanoparticles on Ovarian Activity and Fertility of Heat-Stressed Dairy Cows. Pharmaceutics, 17(2), 274. https://doi.org/10.3390/pharmaceutics17020274









