X-Ray Irradiation Induces Oxidative Stress and Upregulates Intestinal Nrf2-Mrp2 Pathway, Leading to Decreased Intestinal Absorption of Valsartan
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Regents
2.2. LC-MS/MS Conditions
2.3. Method Validation
2.4. Animals and Treatments
2.4.1. In Vivo Pharmacokinetics Studies
2.4.2. In Vivo Excretion Studies
2.4.3. Ex Vivo Inverted Intestinal Sac Absorption Study
2.5. Cell Culture and Treatments
2.5.1. Cell Counting Kit-8 Assay
2.5.2. Cell Uptake Assay
2.6. Real-Time Quantification Polymerase Chain Reaction (RT-qPCR) Studies
2.7. Biochemical Analysis of Oxidative Stress
2.8. Statistical Analysis
3. Results
3.1. Validation of LC-MS/MS Quantitative Method
3.2. Effect of X-Ray Irradiation on the Pharmacokinetics of Valsartan in Rat Plasma
3.3. Effect of X-Ray Irradiation on the Secretion of Bile and Urinary and Fecal Excretion of Valsartan in Rats
3.4. Effect of X-Ray Irradiation on the In Vitro Absorption of Valsartan
3.5. Effect of X-Ray Irradiation on the Uptake of Valsartan in Caco-2 Cell
3.6. Effect of X-Ray Irradiation on the mRNA Expression of Transporters Related to Valsartan
3.7. Detection of Biochemical Indicators of Intestinal Oxidative Stress After Irradiation
3.8. The Impact of X-Ray Irradiation on the mRNA Expression of Nrf2 and HO-1
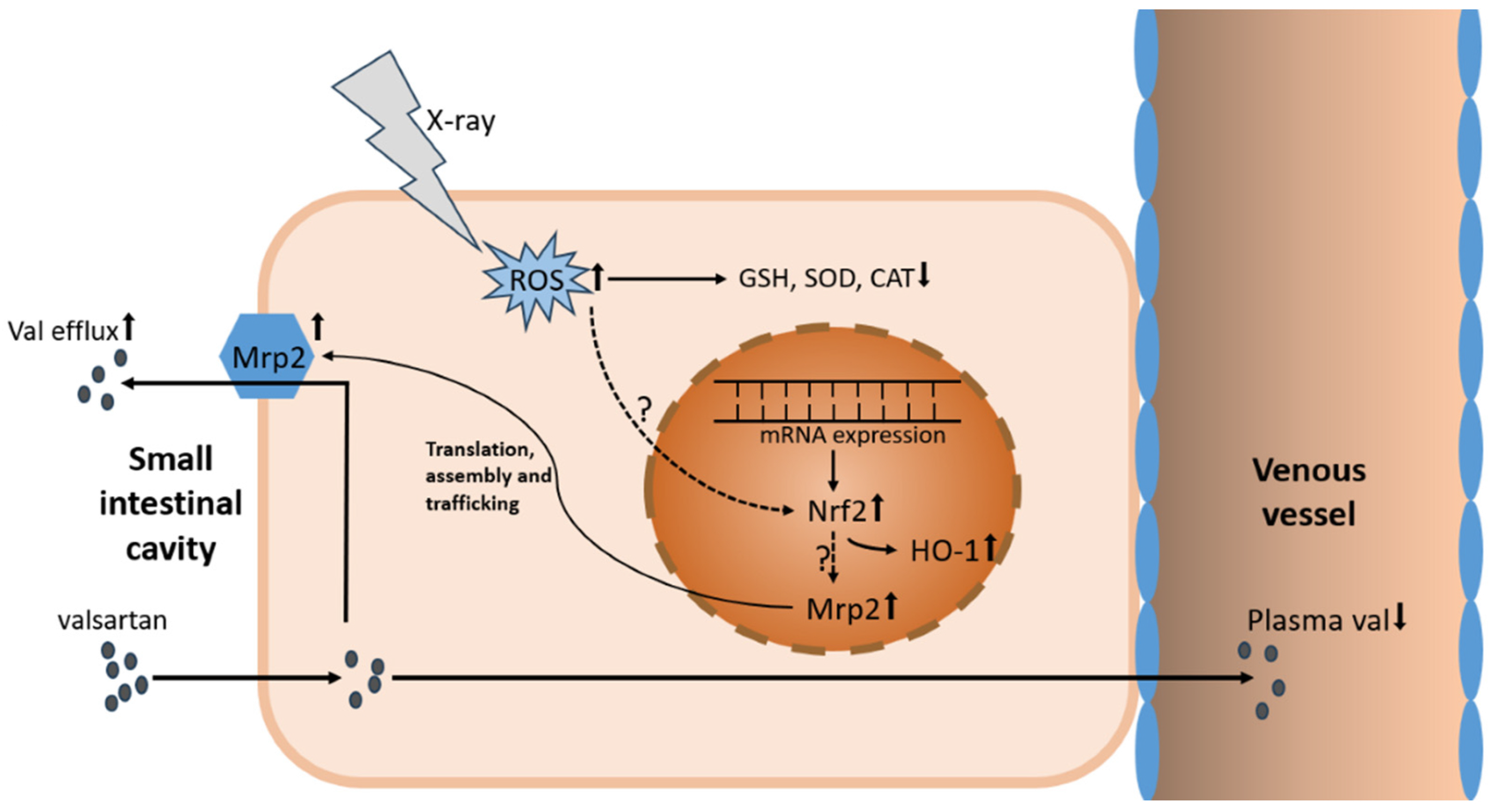
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | area under the drug–time curve |
| ARBs | angiotensin II receptor blockers |
| BCA | bicinchoninic acid assay |
| CAT | catalase |
| cck8 | cell counting kit-8 |
| CE | collision energy |
| CL | clearance rate |
| Cmax | maximum plasma concentration |
| CMC-Na | sodium carboxymethylcellulose |
| CXP | collision cell exit potential |
| DMEM | Dulbecco’s modified eagle medium |
| DP | declustering potential |
| EP | entrance potential |
| ESI | electrospray ionization |
| FBS | fetal bovine serum |
| GSH | glutathione |
| HO-1 | heme oxygenase-1 |
| HQC | high quality control |
| ICH | International Coordinating Committee for Technical Requirements for Medicinal Products for Human Use |
| IR | irradiation |
| IS | internal standard |
| KRB | Krebs–Ringer Solution |
| LC-MS/MS | liquid chromatography tandem mass spectrometry |
| LLOQ | lower limit of quantitation |
| LQC | lower quality control |
| MQC | medium quality control |
| Mrp2 | multidrug resistance-associated protein 2 |
| m/z | mass-to-charge ratio |
| Nf-κB | nuclear factor kappa-B |
| NQO1 | NADH quinone oxidoreductase 1 |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| oatp1b2 | organic anion-transporting polypeptide 1b2 |
| OATP1B1/3 | organic anion-transporting polypeptide 1B1/3 |
| PBS | phosphate-buffered saline |
| P-gp | P-glycoprotein |
| ROS | reactive oxygen species |
| RSD | relative standard deviation |
| RT-PK | radiation pharmacokinetics |
| RT-qPCR | real-time quantification polymerase chain reaction |
| SBRT | stereotactic body radiation therapy |
| SOD | superoxide dismutase |
| t1/2 | half-life |
| Tmax | peak time |
| VAL | valsartan |
| Vd | apparent volume of distribution |
| Ver | verapamil |
References
- Qiao, Y.; Wang, X.; Xin, Y.; Nian, Y.; Yang, J.; Duan, Y.; Li, X. Effect of X-ray irradiation on pharmacokinetics of irinotecan hydrochloride and expression of CES1 and CYP3A1 in rats. Fundam. Clin. Pharmacol. 2019, 33, 558–566. [Google Scholar] [CrossRef]
- Liu, Y.; Nagata, K.; Masunaga, S.; Suzuki, M.; Kashino, G.; Kinashi, Y.; Tanaka, H.; Sakurai, Y.; Maruhashi, A.; Ono, K. Gamma-ray irradiation enhanced boron-10 compound accumulation in murine tumors. J. Radiat. Res. 2009, 50, 553–557. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, J.H.; Tsai, T.H.; Chen, Y.J.; Wang, L.Y.; Liu, H.Y.; Hsieh, C.H. Local Irradiation Modulates the Pharmacokinetics of Metabolites in 5-Fluorouracil-Radiotherapy-Pharmacokinetics Phenomenon. Front. Pharmacol. 2020, 11, 141. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.Q.; Yang, F.; Zhang, D.X.; Wang, L.M.; Liu, J.F.; Zhang, A.J.; Fan, H.R. Effect of X-ray radiation on the pharmacokinetics of apatinib in vivo in rats. Front. Pharmacol. 2022, 13, 943812. [Google Scholar] [CrossRef]
- Dong, S.; Yang, F.; Zhang, Y.; Teng, Y.; Tang, W.; Liu, J.; Fan, H. Effect of X-ray irradiation on renal excretion of bestatin through down-regulating organic anion transporters via the vitamin D receptor in rats. Chem. Biol. Interact. 2024, 399, 111123. [Google Scholar] [CrossRef]
- Chen, Y.J.; Tsai, T.H.; Wang, L.Y.; Hsieh, C.H. Local Radiotherapy Affects Drug Pharmacokinetics-Exploration of a Neglected but Significant Uncertainty of Cancer Therapy. Technol. Cancer Res. Treat. 2017, 16, 705–716. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, L.; Liu, M.; Fan, J.; Hu, S. Summary of the 2022 Report on Cardiovascular Health and Diseases in China. Chin. Med. J. 2023, 136, 2899–2908. [Google Scholar] [CrossRef]
- Qiu, H.; Cao, S.; Xu, R. Cancer incidence, mortality, and burden in China: A time-trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020. Cancer Commun. 2021, 41, 1037–1048. [Google Scholar] [CrossRef]
- van Dorst, D.C.H.; Dobbin, S.J.H.; Neves, K.B.; Herrmann, J.; Herrmann, S.M.; Versmissen, J.; Mathijssen, R.H.J.; Danser, A.H.J.; Lang, N.N. Hypertension and Prohypertensive Antineoplastic Therapies in Cancer Patients. Circ. Res. 2021, 128, 1040–1061. [Google Scholar] [CrossRef]
- Souza, V.B.; Silva, E.N.; Ribeiro, M.L.; Martins Wde, A. Hypertension in patients with cancer. Arq. Bras. Cardiol. 2015, 104, 246–252. [Google Scholar] [CrossRef]
- Du, Y.X.; Li, X.; Ji, S.W.; Niu, N. Hypertension toxicity of VEGFR-TKIs in cancer treatment: Incidence, mechanisms, and management strategies. Arch. Toxicol. 2024, 99, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Koene, R.J.; Prizment, A.E.; Blaes, A.; Konety, S.H. Shared Risk Factors in Cardiovascular Disease and Cancer. Circulation 2016, 133, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Wolin, K.Y.; Carson, K.; Colditz, G.A. Obesity and cancer. Oncologist 2010, 15, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Li, N.; Yang, X.C.; Nie, X.L.; Tian, J.; Wang, D.X. Nonselective Compared with Selective α-Blockade Is Associated With Less Intraoperative Hypertension in Patients with Pheochromocytomas and Paragangliomas: A Retrospective Cohort Study with Propensity Score Matching. Anesth. Analg. 2021, 132, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Katsi, V.; Magkas, N.; Georgiopoulos, G.; Athanasiadi, E.; Virdis, A.; Masi, S.; Kliridis, P.; Hatziyanni, A.; Tsioufis, C.; Tousoulis, D. Arterial hypertension in patients under antineoplastic therapy: A systematic review. J. Hypertens. 2019, 37, 884–901. [Google Scholar] [CrossRef]
- Suter, T.M.; Ewer, M.S. Cancer drugs and the heart: Importance and management. Eur. Heart J. 2013, 34, 1102–1111. [Google Scholar] [CrossRef]
- Wu, S.; Chen, J.J.; Kudelka, A.; Lu, J.; Zhu, X. Incidence and risk of hypertension with sorafenib in patients with cancer: A systematic review and meta-analysis. Lancet Oncol. 2008, 9, 117–123. [Google Scholar] [CrossRef]
- Coleman, R.L.; Brady, M.F.; Herzog, T.J.; Sabbatini, P.; Armstrong, D.K.; Walker, J.L.; Kim, B.G.; Fujiwara, K.; Tewari, K.S.; O’Malley, D.M.; et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017, 18, 779–791. [Google Scholar] [CrossRef]
- Perren, T.J.; Swart, A.M.; Pfisterer, J.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; Kurzeder, C.; et al. A phase 3 trial of bevacizumab in ovarian cancer. N. Engl. J. Med. 2011, 365, 2484–2496. [Google Scholar] [CrossRef]
- Oza, A.M.; Selle, F.; Davidenko, I.; Korach, J.; Mendiola, C.; Pautier, P.; Chmielowska, E.; Bamias, A.; DeCensi, A.; Zvirbule, Z.; et al. Efficacy and Safety of Bevacizumab-Containing Therapy in Newly Diagnosed Ovarian Cancer: ROSiA Single-Arm Phase 3B Study. Int. J. Gynecol. Cancer 2017, 27, 50–58. [Google Scholar] [CrossRef]
- Alexandre, J.; Cautela, J.; Ederhy, S.; Damaj, G.L.; Salem, J.E.; Barlesi, F.; Farnault, L.; Charbonnier, A.; Mirabel, M.; Champiat, S.; et al. Cardiovascular Toxicity Related to Cancer Treatment: A Pragmatic Approach to the American and European Cardio-Oncology Guidelines. J. Am. Heart Assoc. 2020, 9, e018403. [Google Scholar] [CrossRef] [PubMed]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Muñoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Ghidini, A.; Cabiddu, M.; Perego, G.; Lonati, V.; Ghidini, M.; Oggionni, E.; Galli, E.; Moleri, G.; Barni, S.; et al. Effects of hypertension on cancer survival: A meta-analysis. Eur. J. Clin. Investig. 2021, 51, e13493. [Google Scholar] [CrossRef] [PubMed]
- Ettehad, D.; Emdin, C.A.; Kiran, A.; Anderson, S.G.; Callender, T.; Emberson, J.; Chalmers, J.; Rodgers, A.; Rahimi, K. Blood pressure lowering for prevention of cardiovascular disease and death: A systematic review and meta-analysis. Lancet 2016, 387, 957–967. [Google Scholar] [CrossRef]
- Li, P.C.; Huang, R.Y.; Yang, Y.C.; Hsieh, K.P.; Yang, Y.H. Prognostic impact of angiotensin-converting enzyme inhibitors and angiotensin receptors blockers in esophageal or gastric cancer patients with hypertension—A real-world study. BMC Cancer 2022, 22, 430. [Google Scholar] [CrossRef]
- Novo, S.; Lunetta, M.; Evola, S.; Novo, G. Role of ARBs in the blood hypertension therapy and prevention of cardiovascular events. Curr. Drug Targets 2009, 10, 20–25. [Google Scholar] [CrossRef]
- Gomes, T.; Song, Y.; Brede, D.A.; Xie, L.; Gutzkow, K.B.; Salbu, B.; Tollefsen, K.E. Gamma radiation induces dose-dependent oxidative stress and transcriptional alterations in the freshwater crustacean Daphnia magna. Sci. Total Environ. 2018, 628–629, 206–216. [Google Scholar] [CrossRef]
- Niu, T.; Zhi, Y.; Wei, L.; Liu, W.; Ju, X.; Pi, W.; Fu, Z.; Tong, H.; Hu, H.; Dong, J. Sirtuin 3 controls cardiac energetics and protects against oxidative stress in electromagnetic radiation-induced cardiomyopathy. Free Radic. Biol. Med. 2023, 205, 1–12. [Google Scholar] [CrossRef]
- Kita, K.; Sugita, K.; Sato, C.; Sugaya, S.; Sato, T.; Kaneda, A. Extracellular Release of Annexin A2 is Enhanced upon Oxidative Stress Response via the p38 MAPK Pathway after Low-Dose X-Ray Irradiation. Radiat. Res. 2016, 186, 79–91. [Google Scholar] [CrossRef]
- Castellví, A.; Crespo, I.; Crosas, E.; Cámara-Artigas, A.; Gavira, J.A.; Aranda, M.A.G.; Parés, X.; Farrés, J.; Juanhuix, J. Efficacy of aldose reductase inhibitors is affected by oxidative stress induced under X-ray irradiation. Sci. Rep. 2019, 9, 3177. [Google Scholar] [CrossRef]
- Xu, B.Y.; Tang, X.D.; Chen, J.; Wu, H.B.; Chen, W.S.; Chen, L. Rifampicin induces clathrin-dependent endocytosis and ubiquitin-proteasome degradation of MRP2 via oxidative stress-activated PKC-ERK/JNK/p38 and PI3K signaling pathways in HepG2 cells. Acta Pharmacol. Sin. 2020, 41, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, Y.; Uchida, Y.; Tachikawa, M.; Ohtsuki, S.; Couraud, P.O.; Suzuki, T.; Terasaki, T. Oxidative stress-induced activation of Abl and Src kinases rapidly induces P-glycoprotein internalization via phosphorylation of caveolin-1 on tyrosine-14, decreasing cortisol efflux at the blood-brain barrier. J. Cereb. Blood Flow Metab. 2020, 40, 420–436. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Hu, J.; Liu, G.; Yin, H.; Chen, M.; Miao, P.; Bai, P.; Yin, J. Altered Gene expression of ABC transporters, nuclear receptors and oxidative stress signaling in zebrafish embryos exposed to CdTe quantum dots. Environ. Pollut. 2019, 244, 588–599. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Shchulkin, A.V.; Abalenikhina, Y.V.; Kosmachevskaya, O.V.; Topunov, A.F.; Yakusheva, E.N. Regulation of P-Glycoprotein during Oxidative Stress. Antioxidants 2024, 13, 215. [Google Scholar] [CrossRef]
- Lou, Y.; Guo, Z.; Zhu, Y.; Zhang, G.; Wang, Y.; Qi, X.; Lu, L.; Liu, Z.; Wu, J. Astragali radix and its main bioactive compounds activate the Nrf2-mediated signaling pathway to induce P-glycoprotein and breast cancer resistance protein. J. Ethnopharmacol. 2019, 228, 82–91. [Google Scholar] [CrossRef]
- Wu, J.J.; Zhu, Y.F.; Guo, Z.Z.; Lou, Y.M.; He, S.G.; Guan, Y.; Zhu, L.J.; Liu, Z.Q.; Lu, L.L.; Liu, L. Aconitum alkaloids, the major components of Aconitum species, affect expression of multidrug resistance-associated protein 2 and breast cancer resistance protein by activating the Nrf2-mediated signalling pathway. Phytomedicine 2018, 44, 87–97. [Google Scholar] [CrossRef]
- McDonald, J.T.; Kim, K.; Norris, A.J.; Vlashi, E.; Phillips, T.M.; Lagadec, C.; Della Donna, L.; Ratikan, J.; Szelag, H.; Hlatky, L.; et al. Ionizing radiation activates the Nrf2 antioxidant response. Cancer Res. 2010, 70, 8886–8895. [Google Scholar] [CrossRef]
- Chioléro, A.; Burnier, M. Pharmacology of valsartan, an angiotensin II receptor antagonist. Expert Opin. Investig. Drugs 1998, 7, 1915–1925. [Google Scholar] [CrossRef]
- Yamashiro, W.; Maeda, K.; Hirouchi, M.; Adachi, Y.; Hu, Z.; Sugiyama, Y. Involvement of transporters in the hepatic uptake and biliary excretion of valsartan, a selective antagonist of the angiotensin II AT1-receptor, in humans. Drug Metab. Dispos. 2006, 34, 1247–1254. [Google Scholar] [CrossRef]
- Arana, M.R.; Tocchetti, G.N.; Rigalli, J.P.; Mottino, A.D.; Villanueva, S.S. Physiological and pathophysiological factors affecting the expression and activity of the drug transporter MRP2 in intestine. Impact on its function as membrane barrier. Pharmacol. Res. 2016, 109, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.G.; Geier, A.; Oude Elferink, R.P. ABC of oral bioavailability: Transporters as gatekeepers in the gut. Gut 2003, 52, 1788–1795. [Google Scholar] [CrossRef] [PubMed]
- Mottino, A.D.; Hoffman, T.; Jennes, L.; Vore, M. Expression and localization of multidrug resistant protein mrp2 in rat small intestine. J. Pharmacol. Exp. Ther. 2000, 293, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Mazza, T.; Roumeliotis, T.I.; Garitta, E.; Drew, D.; Rashid, S.T.; Indiveri, C.; Choudhary, J.S.; Linton, K.J.; Beis, K. Structural basis for the modulation of MRP2 activity by phosphorylation and drugs. Nat. Commun. 2024, 15, 1983. [Google Scholar] [CrossRef]
- Suzuki, H.; Sugiyama, Y. Single nucleotide polymorphisms in multidrug resistance associated protein 2 (MRP2/ABCC2): Its impact on drug disposition. Adv. Drug Deliv. Rev. 2002, 54, 1311–1331. [Google Scholar] [CrossRef]
- Lee, S.; Kwon, M.; Choi, M.K.; Song, I.S. Effects of Red Ginseng Extract on the Pharmacokinetics and Elimination of Methotrexate via Mrp2 Regulation. Molecules 2018, 23, 2948. [Google Scholar] [CrossRef]
- Kobayashi, M.; Gouda, K.; Chisaki, I.; Asada, K.; Ogura, J.; Takahashi, N.; Konishi, T.; Koshida, Y.; Sasaki, S.; Yamaguchi, H.; et al. Regulation of multidrug resistance protein 2 (MRP2, ABCC2) expression by statins: Involvement of SREBP-mediated gene regulation. Int. J. Pharm. 2013, 452, 36–41. [Google Scholar] [CrossRef]
- Du, T.; Luo, T.; Wang, J.; Sun, R.; Cai, H. Role of MRPs transporters in pharmacokinetics and intestinal toxicity of irinotecan. Food Chem. Toxicol. 2023, 182, 114171. [Google Scholar] [CrossRef]
- Zamek-Gliszczynski, M.J.; Hoffmaster, K.A.; Tian, X.; Zhao, R.; Polli, J.W.; Humphreys, J.E.; Webster, L.O.; Bridges, A.S.; Kalvass, J.C.; Brouwer, K.L. Multiple mechanisms are involved in the biliary excretion of acetaminophen sulfate in the rat: Role of Mrp2 and Bcrp1. Drug Metab. Dispos. 2005, 33, 1158–1165. [Google Scholar] [CrossRef]
- Ge, S.; Yin, T.; Xu, B.; Gao, S.; Hu, M. Curcumin Affects Phase II Disposition of Resveratrol Through Inhibiting Efflux Transporters MRP2 and BCRP. Pharm. Res. 2016, 33, 590–602. [Google Scholar] [CrossRef]
- Wang, S.; Feng, R.; Wang, S.S.; Liu, H.; Shao, C.; Li, Y.; Link, F.; Munker, S.; Liebe, R.; Meyer, C.; et al. FOXA2 prevents hyperbilirubinaemia in acute liver failure by maintaining apical MRP2 expression. Gut 2023, 72, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Keppler, D. Export pumps for glutathione S-conjugates. Free Radic. Biol. Med. 1999, 27, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Jedlitschky, G.; Hoffmann, U.; Kroemer, H.K. Structure and function of the MRP2 (ABCC2) protein and its role in drug disposition. Expert Opin. Drug Metab. Toxicol. 2006, 2, 351–366. [Google Scholar] [CrossRef]
- Goo, Y.T.; Song, S.H.; Yeom, D.W.; Chae, B.R.; Yoon, H.Y.; Kim, C.H.; Park, S.Y.; Kang, T.H.; Lee, S.; Choi, Y.W. Enhanced oral bioavailability of valsartan in rats using a supersaturable self-microemulsifying drug delivery system with P-glycoprotein inhibitors. Pharm. Dev. Technol. 2020, 25, 178–186. [Google Scholar] [CrossRef]
- Challa, V.R.; Babu, P.R.; Challa, S.R.; Johnson, B.; Maheswari, C. Pharmacokinetic interaction study between quercetin and valsartan in rats and in vitro models. Drug Dev. Ind. Pharm. 2013, 39, 865–872. [Google Scholar] [CrossRef]
- ICH Official Web Site: ICH. Available online: https://www.ich.org/page/multidisciplinary-guidelines (accessed on 27 November 2024).
- Niu, B.; Liao, K.; Zhou, Y.; Wen, T.; Quan, G.; Pan, X.; Wu, C. Application of glutathione depletion in cancer therapy: Enhanced ROS-based therapy, ferroptosis, and chemotherapy. Biomaterials 2021, 277, 121110. [Google Scholar] [CrossRef]
- Hao, M.; Liu, R. Molecular mechanism of CAT and SOD activity change under MPA-CdTe quantum dots induced oxidative stress in the mouse primary hepatocytes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 220, 117104. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Zhu, H.J.; Zhao, R.Y.; Zhou, S.Y.; Wang, M.Q.; Yang, Y.; Guo, Z.N. Remote ischemic conditioning attenuates oxidative stress and inflammation via the Nrf2/HO-1 pathway in MCAO mice. Redox Biol. 2023, 66, 102852. [Google Scholar] [CrossRef]
- Chunduri, R.H.; Dannana, G.S. Development and validation of a reliable and rapid LC-MS/MS method for simultaneous quantification of sacubitril and valsartan in rat plasma and its application to a pharmacokinetic study. Biomed. Chromatogr. 2016, 30, 1467–1475. [Google Scholar] [CrossRef]
- Lu, Z.; Zheng, X.; Ding, C.; Zou, Z.; Liang, Y.; Zhou, Y.; Li, X. Deciphering the Biological Effects of Radiotherapy in Cancer Cells. Biomolecules 2022, 12, 1167. [Google Scholar] [CrossRef]
- Kitagawa, T.; Yamamoto, J.; Tanaka, T.; Nakano, Y.; Akiba, D.; Ueta, K.; Nishizawa, S. 5-Aminolevulinic acid strongly enhances delayed intracellular production of reactive oxygen species (ROS) generated by ionizing irradiation: Quantitative analyses and visualization of intracellular ROS production in glioma cells in vitro. Oncol. Rep. 2015, 33, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wang, Y.; Li, L.; Liu, S.; Wang, C.; Yuan, Y.; Yang, G.; Chen, Y.; Cheng, J.; Lu, Y.; et al. Mitochondrial ROS promote mitochondrial dysfunction and inflammation in ischemic acute kidney injury by disrupting TFAM-mediated mtDNA maintenance. Theranostics 2021, 11, 1845–1863. [Google Scholar] [CrossRef] [PubMed]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bai, H.; Li, R.; Wang, L.; Zhang, W.; Liang, J.; Yuan, Z. High versus standard radiation dose of definitive concurrent chemoradiotherapy for esophageal cancer: A systematic review and meta-analysis of randomized clinical trials. Radiother. Oncol. 2023, 180, 109463. [Google Scholar] [CrossRef]
- Chen, J.; Bissonnette, J.P.; Craig, T.; Munoz-Schuffenegger, P.; Tadic, T.; Dawson, L.A.; Velec, M. Liver SBRT dose accumulation to assess the impact of anatomic variations on normal tissue doses and toxicity in patients treated with concurrent sorafenib. Radiother. Oncol. 2023, 182, 109588. [Google Scholar] [CrossRef]
- Colussi, D.M.; Parisot, C.; Rossolino, M.L.; Brunner, L.A.; Lefèvre, G.Y. Protein binding in plasma of valsartan, a new angiotensin II receptor antagonist. J. Clin. Pharmacol. 1997, 37, 214–221. [Google Scholar] [CrossRef]
- Waldmeier, F.; Flesch, G.; Müller, P.; Winkler, T.; Kriemler, H.P.; Bühlmayer, P.; De Gasparo, M. Pharmacokinetics, disposition and biotransformation of [14C]-radiolabelled valsartan in healthy male volunteers after a single oral dose. Xenobiotica 1997, 27, 59–71. [Google Scholar] [CrossRef]
- Yeom, D.W.; Chae, B.R.; Son, H.Y.; Kim, J.H.; Chae, J.S.; Song, S.H.; Oh, D.; Choi, Y.W. Enhanced oral bioavailability of valsartan using a polymer-based supersaturable self-microemulsifying drug delivery system. Int. J. Nanomed. 2017, 12, 3533–3545. [Google Scholar] [CrossRef]
- Arias, A.; Rigalli, J.P.; Villanueva, S.S.; Ruiz, M.L.; Luquita, M.G.; Perdomo, V.G.; Vore, M.; Catania, V.A.; Mottino, A.D. Regulation of expression and activity of multidrug resistance proteins MRP2 and MDR1 by estrogenic compounds in Caco-2 cells. Role in prevention of xenobiotic-induced cytotoxicity. Toxicology 2014, 320, 46–55. [Google Scholar] [CrossRef]
- Zhan, Y.; Zhang, Z.; Liu, Y.; Fang, Y.; Xie, Y.; Zheng, Y.; Li, G.; Liang, L.; Ding, Y. NUPR1 contributes to radiation resistance by maintaining ROS homeostasis via AhR/CYP signal axis in hepatocellular carcinoma. BMC Med. 2022, 20, 365. [Google Scholar] [CrossRef]
- Xie, Y.; Hao, H.; Wang, H.; Guo, C.; Kang, A.; Wang, G. Reversing effects of lignans on CCl4-induced hepatic CYP450 down regulation by attenuating oxidative stress. J. Ethnopharmacol. 2014, 155, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Maher, J.M.; Dieter, M.Z.; Aleksunes, L.M.; Slitt, A.L.; Guo, G.; Tanaka, Y.; Scheffer, G.L.; Chan, J.Y.; Manautou, J.E.; Chen, Y.; et al. Oxidative and electrophilic stress induces multidrug resistance-associated protein transporters via the nuclear factor-E2-related factor-2 transcriptional pathway. Hepatology 2007, 46, 1597–1610. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jian, Z.; Baskys, A.; Yang, J.; Li, J.; Guo, H.; Hei, Y.; Xian, P.; He, Z.; Li, Z.; et al. MSC-derived exosomes protect against oxidative stress-induced skin injury via adaptive regulation of the NRF2 defense system. Biomaterials 2020, 257, 120264. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, X.; Feng, Y.; Wang, Y.; Zhang, L.; Wang, Y.; Fang, Z.; Azami, N.L.B.; Sun, M.; Li, Q. Dihydromyricetin reverses MRP2-induced multidrug resistance by preventing NF-κB-Nrf2 signaling in colorectal cancer cell. Phytomedicine 2021, 82, 153414. [Google Scholar] [CrossRef]
- Schmidlin, C.J.; Shakya, A.; Dodson, M.; Chapman, E.; Zhang, D.D. The intricacies of NRF2 regulation in cancer. Semin. Cancer Biol. 2021, 76, 110–119. [Google Scholar] [CrossRef]
- Namani, A.; Li, Y.; Wang, X.J.; Tang, X. Modulation of NRF2 signaling pathway by nuclear receptors: Implications for cancer. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2014, 1843, 1875–1885. [Google Scholar] [CrossRef]
- Liu, T.; Lv, Y.F.; Zhao, J.L.; You, Q.D.; Jiang, Z.Y. Regulation of Nrf2 by phosphorylation: Consequences for biological function and therapeutic implications. Free Radic. Biol. Med. 2021, 168, 129–141. [Google Scholar] [CrossRef]
- Turpaev, K.T. Keap1-Nrf2 signaling pathway: Mechanisms of regulation and role in protection of cells against toxicity caused by xenobiotics and electrophiles. Biochemistry 2013, 78, 111–126. [Google Scholar] [CrossRef]
- Sivandzade, F.; Prasad, S.; Bhalerao, A.; Cucullo, L. NRF2 and NF-κB interplay in cerebrovascular and neurodegenerative disorders: Molecular mechanisms and possible therapeutic approaches. Redox Biol. 2019, 21, 101059. [Google Scholar] [CrossRef]
- Diao, L.; Li, N.; Brayman, T.G.; Hotz, K.J.; Lai, Y. Regulation of MRP2/ABCC2 and BSEP/ABCB11 expression in sandwich cultured human and rat hepatocytes exposed to inflammatory cytokines TNF-α, IL-6, and IL-1β. J. Biol. Chem. 2010, 285, 31185–31192. [Google Scholar] [CrossRef]
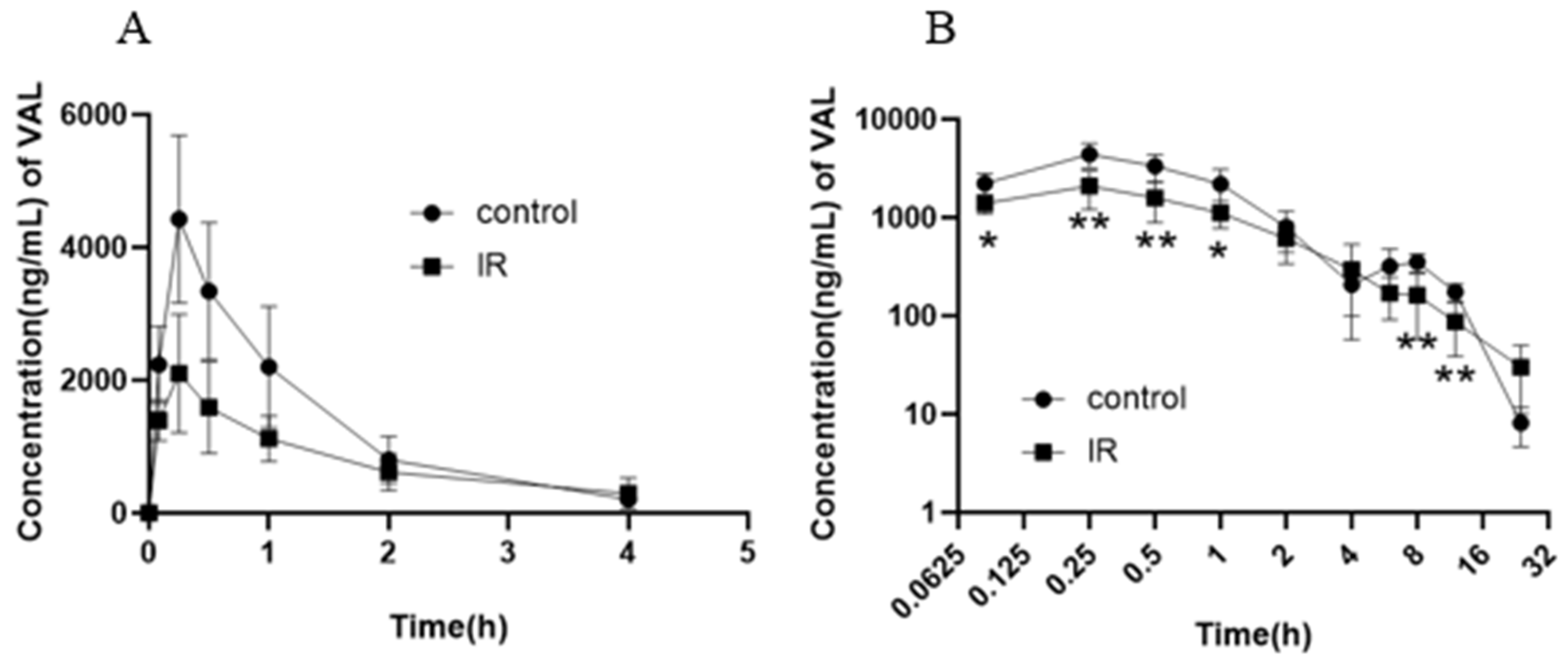
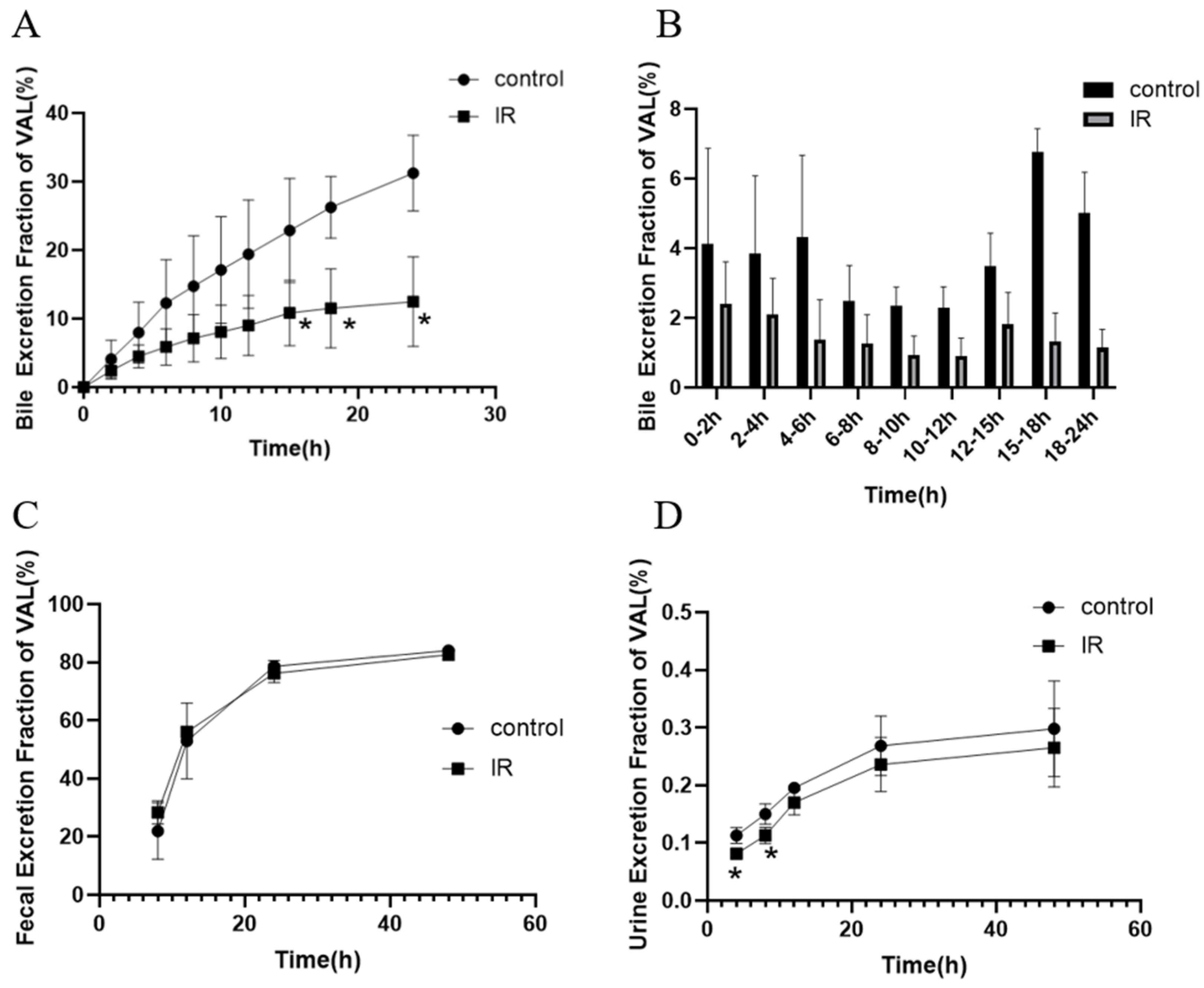
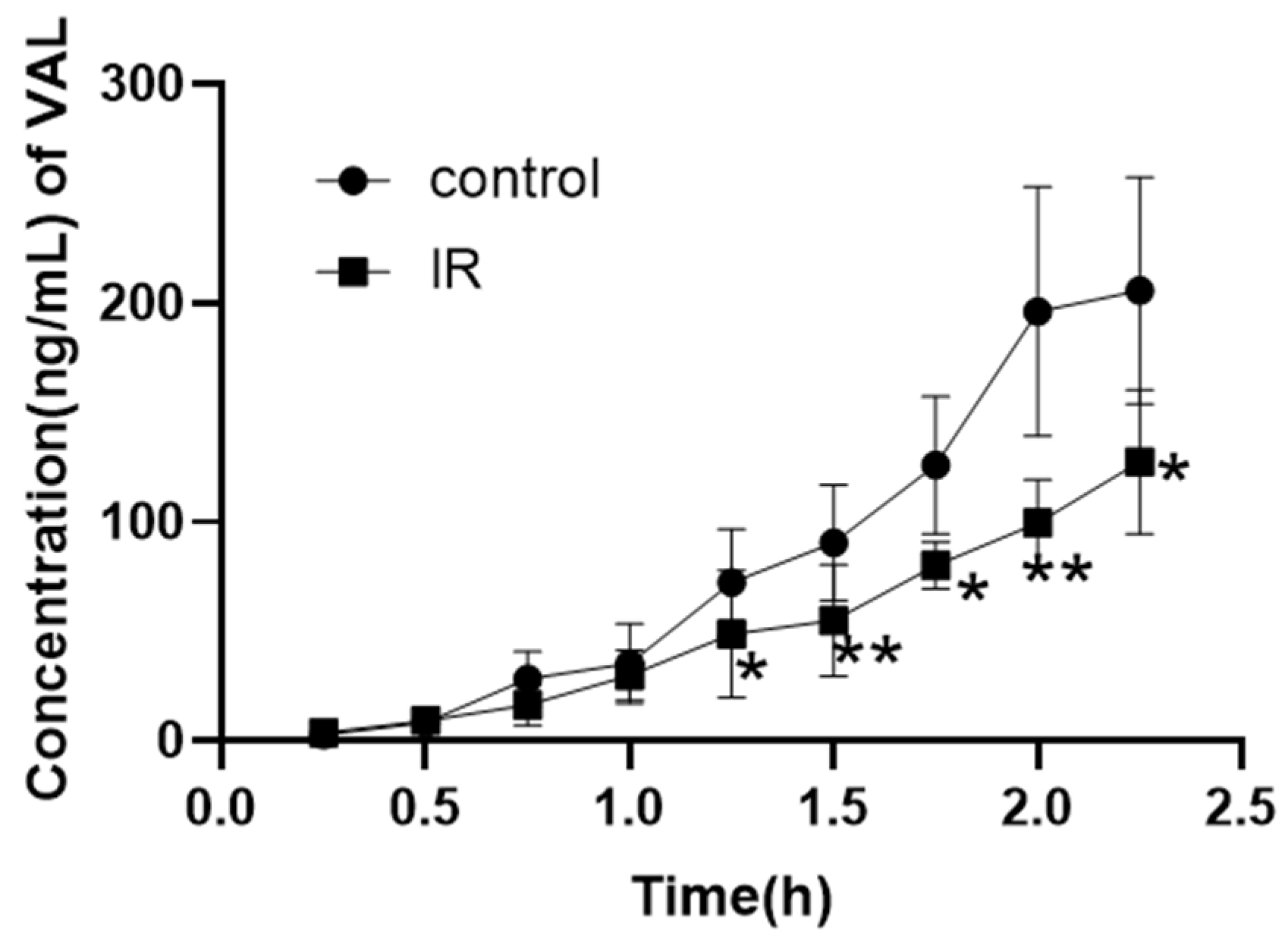
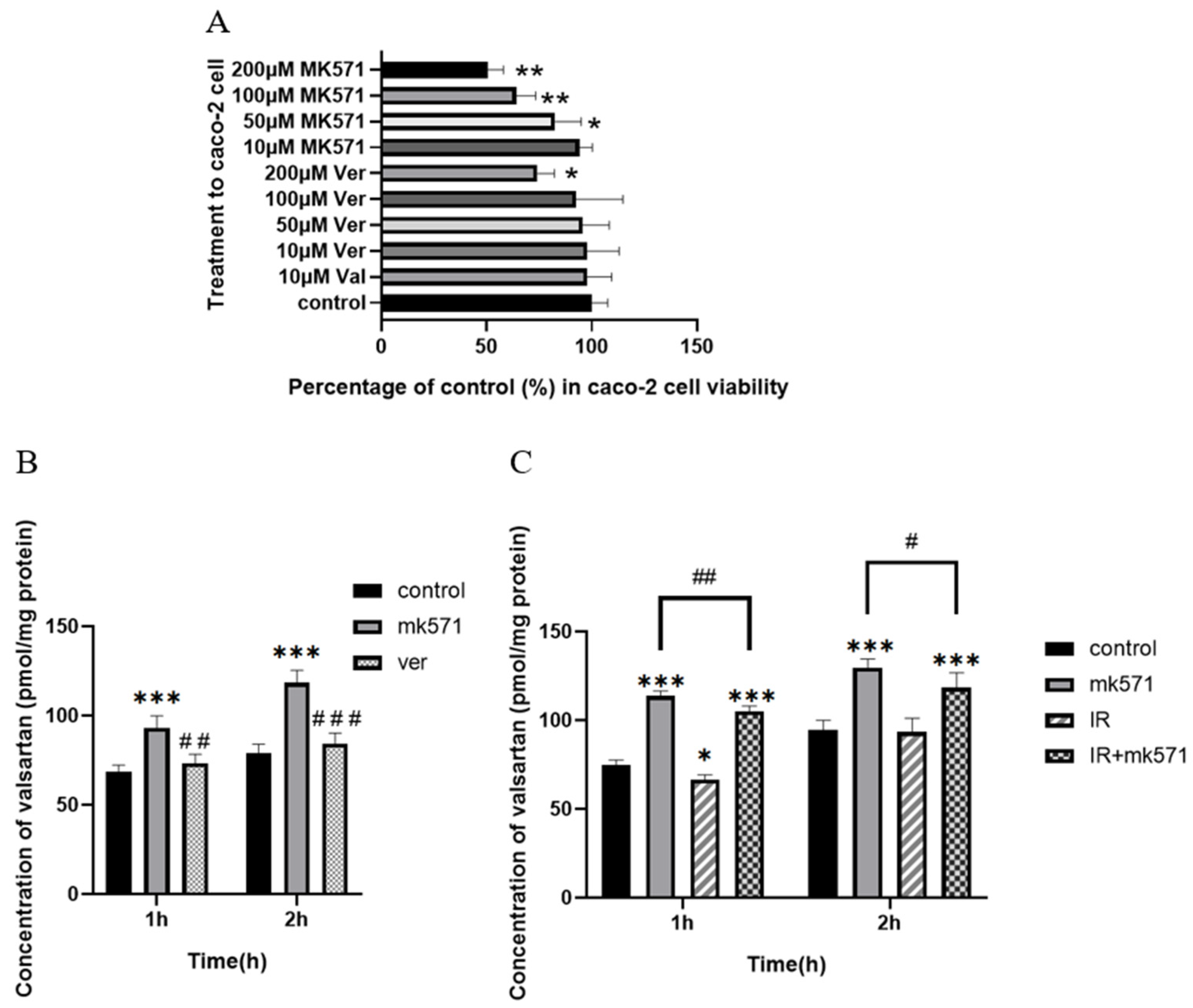

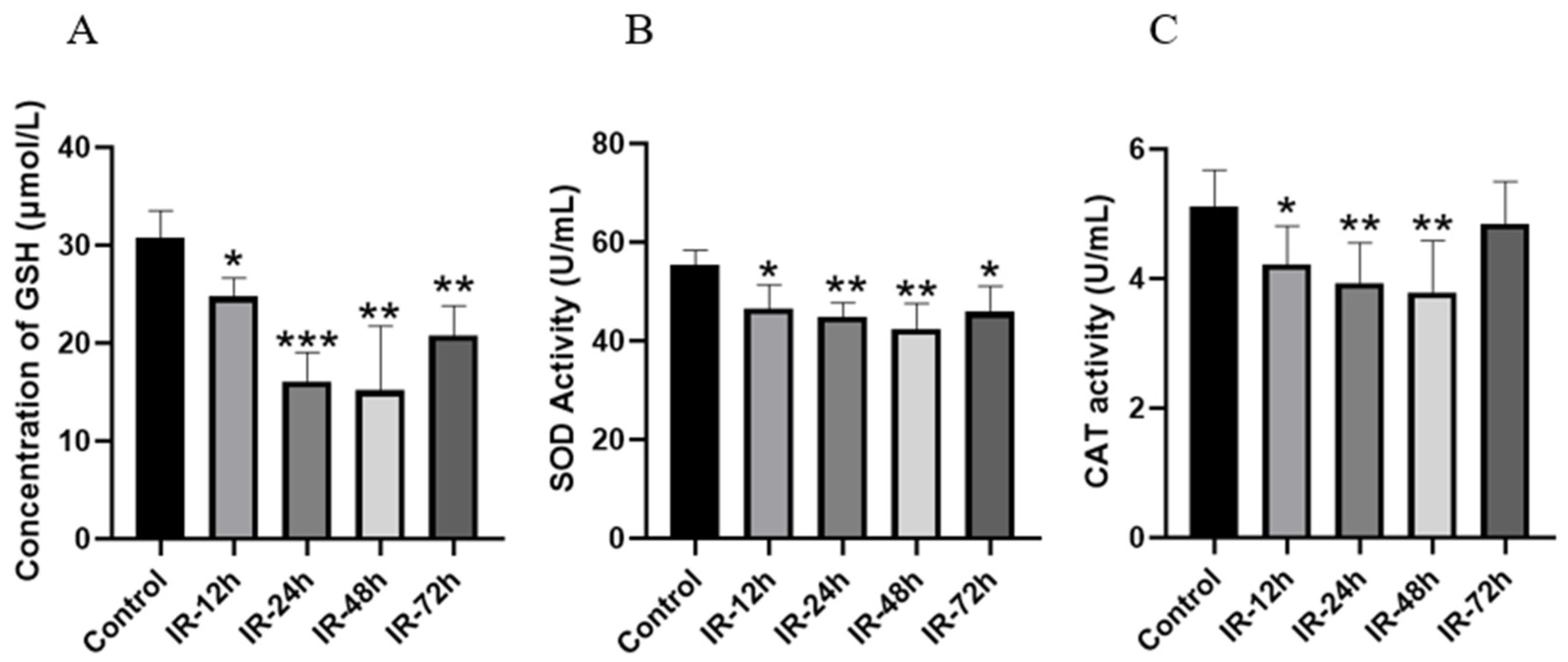

| Parameter | Control | IR-5 Gy | |
|---|---|---|---|
| t1/2 | h | 3.568 ± 0.411 | 5.032 ± 2.656 |
| Cmax | ng/mL | 4428.333 ± 1261.656 | 2131.667 ± 877.802 ** |
| Tmax | h | 0.250 ± 0.000 | 0.222 ± 0.068 |
| AUC0–24h | ng·h/mL | 8456.466 ± 1840.136 | 5269.367 ± 1826.438 * |
| AUC0–∞ | ng·h/mL | 8813.281 ± 2011.750 | 5469.390 ± 1791.823 * |
| Vd | L/kg | 6.254 ± 2.349 | 14.668 ± 9.456 |
| CL | L/h/kg | 1.200 ± 0.352 | 2.120 ± 1.110 |
| MRT0–24h | h | 3.839 ± 0.724 | 4.521 ± 1.631 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, Y.; Ma, J.; Zhang, J.; Liang, B.; Zhang, A.; Li, Y.; Dong, S.; Fan, H. X-Ray Irradiation Induces Oxidative Stress and Upregulates Intestinal Nrf2-Mrp2 Pathway, Leading to Decreased Intestinal Absorption of Valsartan. Pharmaceutics 2025, 17, 268. https://doi.org/10.3390/pharmaceutics17020268
Teng Y, Ma J, Zhang J, Liang B, Zhang A, Li Y, Dong S, Fan H. X-Ray Irradiation Induces Oxidative Stress and Upregulates Intestinal Nrf2-Mrp2 Pathway, Leading to Decreased Intestinal Absorption of Valsartan. Pharmaceutics. 2025; 17(2):268. https://doi.org/10.3390/pharmaceutics17020268
Chicago/Turabian StyleTeng, Yunhua, Jiaojiao Ma, Junxia Zhang, Bohan Liang, Aijie Zhang, Yanjie Li, Shiqi Dong, and Huirong Fan. 2025. "X-Ray Irradiation Induces Oxidative Stress and Upregulates Intestinal Nrf2-Mrp2 Pathway, Leading to Decreased Intestinal Absorption of Valsartan" Pharmaceutics 17, no. 2: 268. https://doi.org/10.3390/pharmaceutics17020268
APA StyleTeng, Y., Ma, J., Zhang, J., Liang, B., Zhang, A., Li, Y., Dong, S., & Fan, H. (2025). X-Ray Irradiation Induces Oxidative Stress and Upregulates Intestinal Nrf2-Mrp2 Pathway, Leading to Decreased Intestinal Absorption of Valsartan. Pharmaceutics, 17(2), 268. https://doi.org/10.3390/pharmaceutics17020268





