Subcellular Organelle Targeting as a Novel Approach to Combat Tumor Metastasis
Abstract
1. Introduction
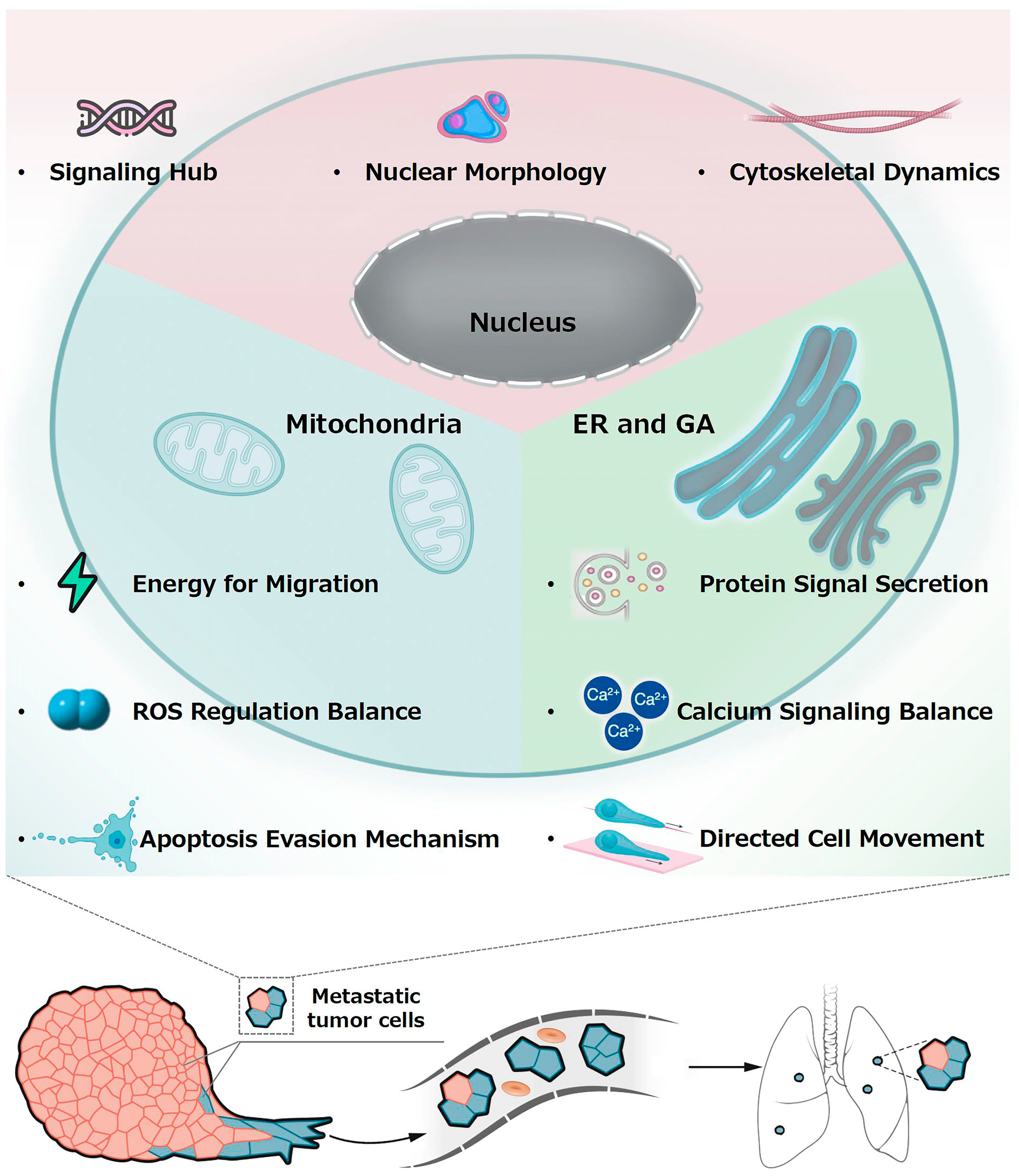
2. Potential Connection Between Subcellular Organelles and Metastasis
2.1. Nucleus and Metastasis
2.2. Mitochondria and Metastasis
2.3. Endoplasmic Reticulum, Golgi Apparatus, and Metastasis
3. Subcellular Drug Delivery Systems for Tumor Metastasis Therapy
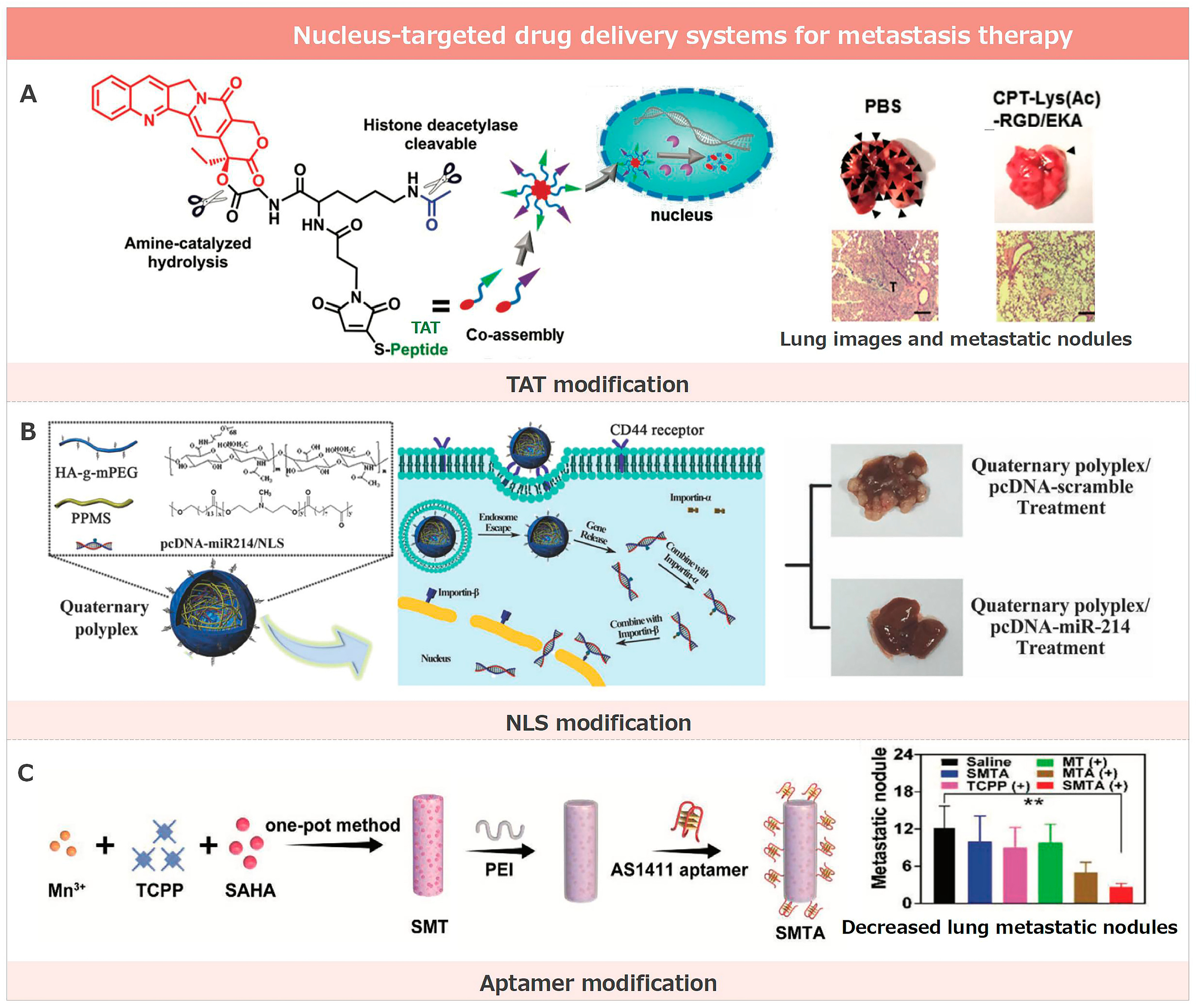
3.1. Nucleus-Targeted Drug Delivery Systems for Metastasis Therapy
3.1.1. TAT Modification
3.1.2. NLS Modification
3.1.3. Aptamer Modification
3.1.4. Other Strategies
3.2. Mitochondrial-Targeted Drug Delivery Systems for Metastasis Therapy
3.2.1. TPP Modification
3.2.2. MTS Modification
3.2.3. Other Strategies
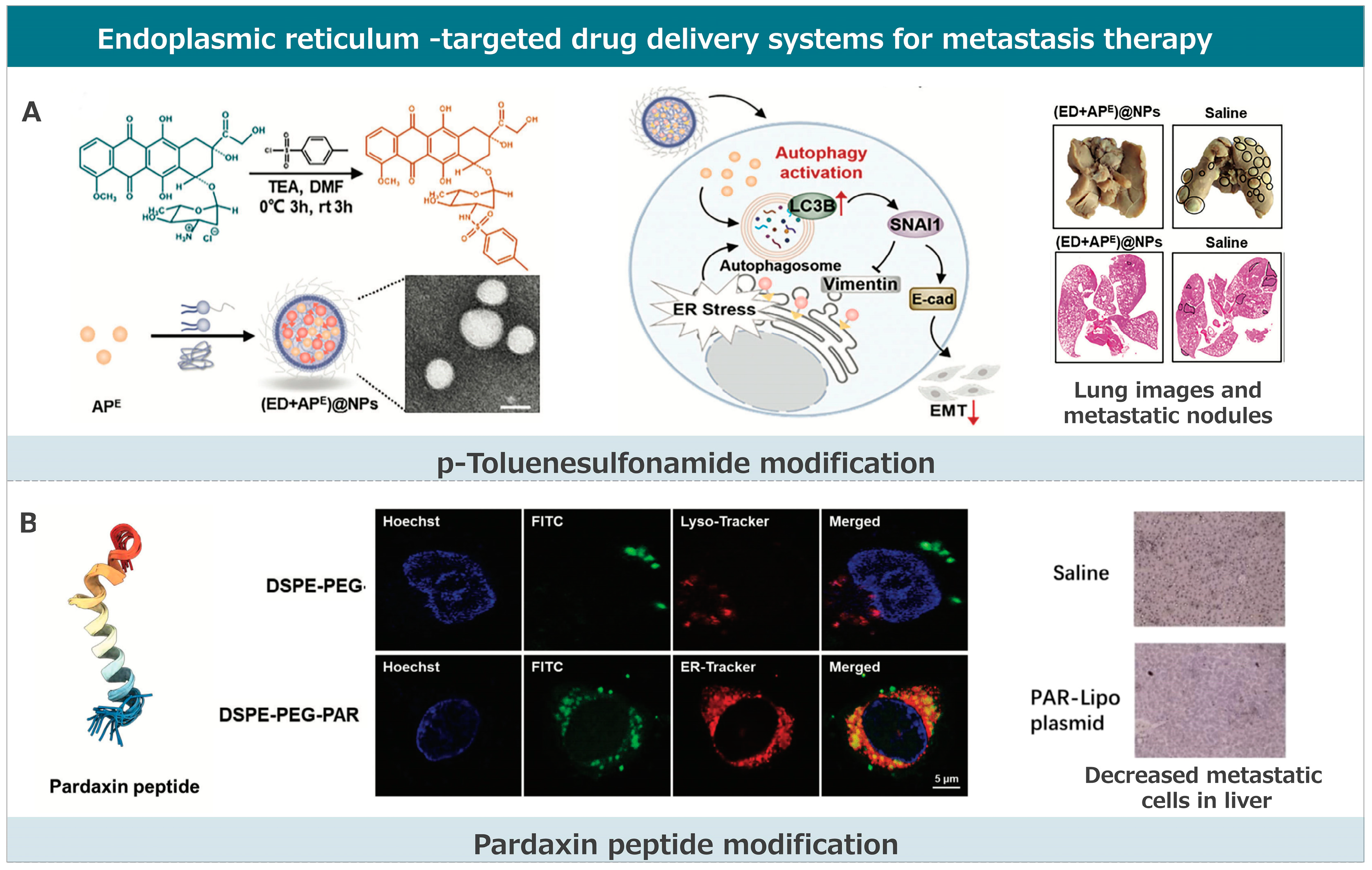
3.3. Endoplasmic Reticulum-Targeted Drug Delivery Systems for Metastasis Therapy
3.3.1. p-Toluenesulfonamide Modification
3.3.2. Pardaxin Peptide Modification
3.3.3. Other Strategies
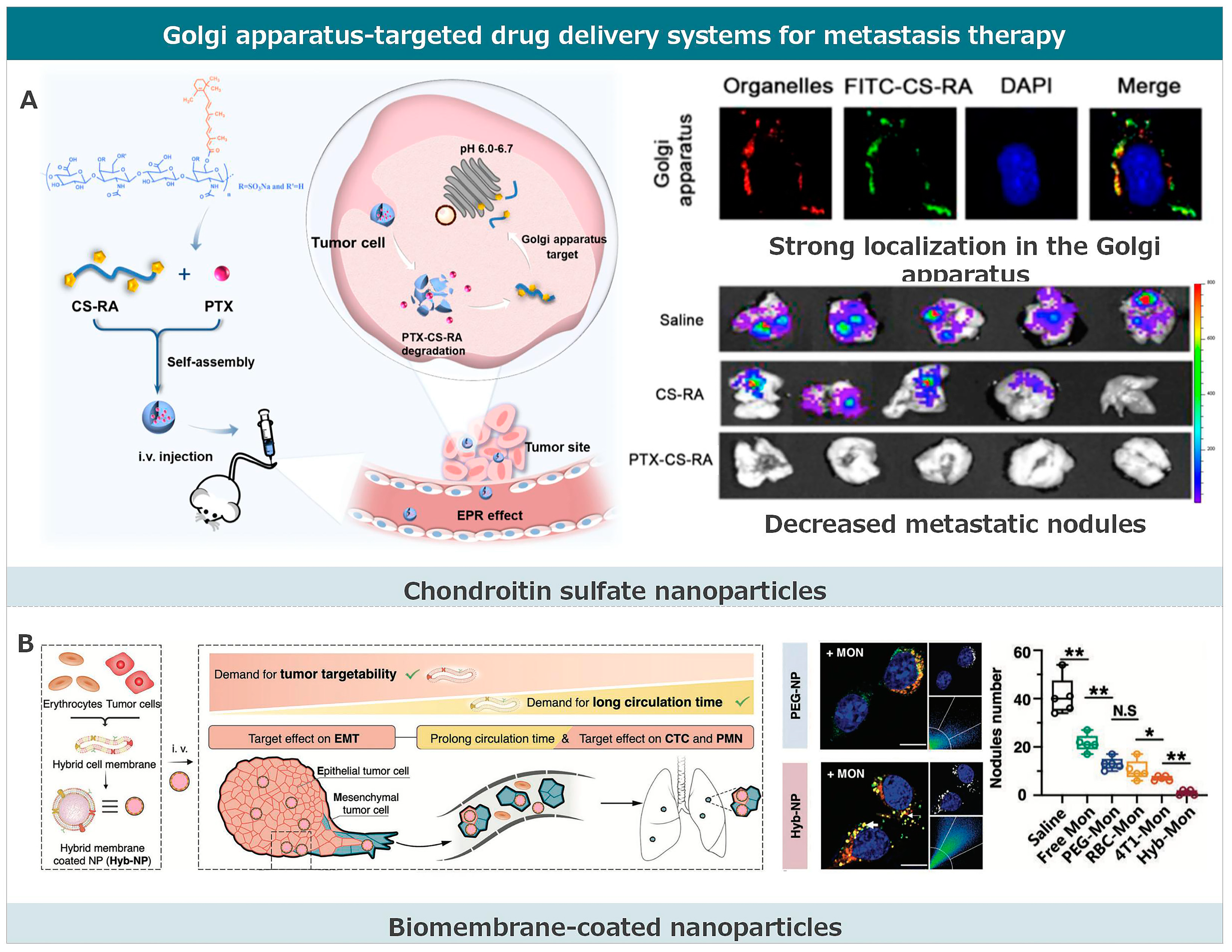
3.4. Golgi Apparatus-Targeted Drug Delivery Systems for Metastasis Therapy
3.4.1. Chondroitin Sulfate Nanoparticles
3.4.2. Biomembrane-Coated Nanoparticles
3.4.3. Platinum Complex
4. Future Prospects
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Majidpoor, J.; Mortezaee, K. Steps in metastasis: An updated review. Med. Oncol. 2021, 38, 3. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, F.; Hu, C.; Zhou, Y.; Gao, H.; Hu, J. The progress and perspective of nanoparticle-enabled tumor metastasis treatment. Acta Pharm. Sin. B 2020, 10, 2037–2053. [Google Scholar] [CrossRef]
- Gerstberger, S.; Jiang, Q.; Ganesh, K. Metastasis. Cell 2023, 186, 1564–1579. [Google Scholar] [CrossRef]
- Bakir, B.; Chiarella, A.M.; Pitarresi, J.R.; Rustgi, A.K. EMT, MET, Plasticity, and Tumor Metastasis. Trends Cell Biol. 2020, 30, 764–776. [Google Scholar] [CrossRef]
- Ganesh, K.; Massagué, J. Targeting metastatic cancer. Nat. Med. 2021, 27, 34–44. [Google Scholar] [CrossRef]
- Hu, Z.; Li, Z.; Ma, Z.; Curtis, C. Multi-cancer analysis of clonality and the timing of systemic spread in paired primary tumors and metastases. Nat. Genet. 2020, 52, 701–708. [Google Scholar] [CrossRef]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef]
- Gensbittel, V.; Kräter, M.; Harlepp, S.; Busnelli, I.; Guck, J.; Goetz, J.G. Mechanical Adaptability of Tumor Cells in Metastasis. Dev. Cell 2020, 56, 164–179. [Google Scholar] [CrossRef]
- Gong, N.; Alameh, M.-G.; El-Mayta, R.; Xue, L.; Weissman, D.; Mitchell, M.J. Enhancing in situ cancer vaccines using delivery technologies. Nat. Rev. Drug Discov. 2024, 23, 607–625. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, B.; Wang, Z.; Li, J.; Wang, F.; Kong, J.; Zhou, Z.; Huang, Y.; Li, L. Durable Attenuation of Tumor pH–Platelet Linkage Reinstates Bioorthogonal Targeting of Residual Tumors Post-Debulking. ACS Nano 2024, 18, 4520–4538. [Google Scholar] [CrossRef]
- Li, B.; Zhang, P.; Li, J.; Zhou, R.; Zhou, M.; Liu, C.; Liu, X.; Chen, L.; Li, L. Allogeneic “Zombie Cell” as Off-The-Shelf Vaccine for Postsurgical Cancer Immunotherapy. Adv. Sci. 2024, 11, e2307030. [Google Scholar] [CrossRef]
- Zhou, M.; Zuo, Q.; Huang, Y.; Li, L. Immunogenic hydrogel toolkit disturbing residual tumor “seeds” and pre-metastatic “soil” for inhibition of postoperative tumor recurrence and metastasis. Acta Pharm. Sin. B 2022, 12, 3383–3397. [Google Scholar] [CrossRef]
- Deng, M.; Wu, S.; Huang, P.; Liu, Y.; Li, C.; Zheng, J. Engineered exosomes-based theranostic strategy for tumor metastasis and recurrence. Asian J. Pharm. Sci. 2023, 18, 100870. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Fan, H.; Ti, H. Tumor microenvironment reprogramming by nanomedicine to enhance the effect of tumor immunotherapy. Asian J. Pharm. Sci. 2024, 19, 100902. [Google Scholar] [CrossRef]
- Qin, L.; Cao, J.; Shao, K.; Tong, F.; Yang, Z.; Lei, T.; Wang, Y.; Hu, C.; Umeshappa, C.S.; Gao, H.; et al. A tumor-to-lymph procedure navigated versatile gel system for combinatorial therapy against tumor recurrence and metastasis. Sci. Adv. 2020, 6, eabb3116. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, Y.; Yang, C.H.; Radford, D.C.; Wang, J.; Janát-Amsbury, M.; Kopeček, J.; Yang, J. Inhibition of Immunosuppressive Tumors by Polymer-Assisted Inductions of Immunogenic Cell Death and Multivalent PD-L1 Crosslinking. Adv. Funct. Mater. 2020, 30, 1908961. [Google Scholar] [CrossRef]
- Li, C.; Qiao, S.; Kang, M.; Gao, X.; Li, Z. Combinational use of trabectedin and pegylated liposomal doxorubicin for recurrent ovarian cancer: A meta-analysis of phase III randomized controlled trials. Am. J. Transl. Res. 2023, 15, 6675–6689. [Google Scholar]
- Yuan, Z.; Zhang, Y.; Cao, D.; Shen, K.; Li, Q.; Zhang, G.; Wu, X.; Cui, M.; Yue, Y.; Cheng, W.; et al. Pegylated liposomal doxorubicin in patients with epithelial ovarian cancer. J. Ovarian Res. 2021, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Jiang, Z.; Wang, M.; Ma, L. Current understandings and clinical translation of nanomedicines for breast cancer therapy. Adv. Drug Deliv. Rev. 2021, 180, 114034. [Google Scholar] [CrossRef]
- Yuan, H.; Guo, H.; Luan, X.; He, M.; Li, F.; Burnett, J.; Truchan, N.; Sun, D. Albumin Nanoparticle of Paclitaxel (Abraxane) Decreases while Taxol Increases Breast Cancer Stem Cells in Treatment of Triple Negative Breast Cancer. Mol. Pharm. 2020, 17, 2275–2286. [Google Scholar] [CrossRef]
- Hu, X.; Wang, B.; Zhang, J.; Wang, Z.; Sun, T.; Wang, S.; Teng, Y.; Yan, M.; Wang, X.; Jiang, Z.; et al. 282MO Abraxane plus cisplatin compared with gemcitabine plus cisplatin as first-line treatment in patients with metastatic triple-negative breast cancer (GAP): A multicenter, randomized, open-label, phase III trial. Ann. Oncol. 2020, 31, S354. [Google Scholar] [CrossRef]
- Cagel, M.; Moretton, M.A.; Bernabeu, E.; Zubillaga, M.; Lagomarsino, E.; Vanzulli, S.; Nicoud, M.B.; Medina, V.A.; Salgueiro, M.J.; Chiappetta, D.A. Antitumor efficacy and cardiotoxic effect of doxorubicin-loaded mixed micelles in 4T1 murine breast cancer model. Comparative studies using Doxil® and free doxorubicin. J. Drug Deliv. Sci. Technol. 2020, 56, 101506. [Google Scholar] [CrossRef]
- Franco, Y.L.; Vaidya, T.R.; Ait-Oudhia, S. Anticancer and cardio-protective effects of liposomal doxorubicin in the treatment of breast cancer. Breast Cancer Targets Ther. 2018, 10, 131–141. [Google Scholar] [CrossRef]
- Miele, E.; Spinelli, G.P.; Miele, E.; Tomao, F.; Tomao, S. Albumin-bound formulation of paclitaxel (Abraxane® ABI-007) in the treatment of breast cancer. Int. J. Nanomed. 2009, 4, 99–105. [Google Scholar] [CrossRef]
- Huang, S.-T.; Wang, Y.; Chen, Y.-H.; Lin, C.-T.; Li, W.-S.; Wu, H.-C. Liposomal paclitaxel induces fewer hematopoietic and cardiovascular complications than bioequivalent doses of Taxol. Int. J. Oncol. 2018, 53, 1105–1117. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Liu, C.; Li, B.; Li, J.; Zhang, P.; Huang, Y.; Li, L. Cell surface patching via CXCR4-targeted nanothreads for cancer metastasis inhibition. Nat. Commun. 2024, 15, 2763. [Google Scholar] [CrossRef]
- Venditto, V.J.; Szoka, F.C., Jr. Cancer nanomedicines: So many papers and so few drugs! Adv. Drug Deliv. Rev. 2013, 65, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.; Saura, C. Nanoparticle albumin-bound (nab™)-paclitaxel: Improving efficacy and tolerability by targeted drug delivery in metastatic breast cancer. Eur. J. Cancer Suppl. 2010, 8, 1–10. [Google Scholar] [CrossRef]
- Bernabeu, E.; Cagel, M.; Lagomarsino, E.; Moretton, M.; Chiappetta, D.A. Paclitaxel: What has been done and the challenges remain ahead. Int. J. Pharm. 2017, 526, 474–495. [Google Scholar] [CrossRef]
- Büll, C.; Boltje, T.J.; van Dinther, E.A.W.; Peters, T.; de Graaf, A.M.A.; Leusen, J.H.W.; Kreutz, M.; Figdor, C.G.; Brok, M.H.D.; Adema, G.J. Targeted Delivery of a Sialic Acid-Blocking Glycomimetic to Cancer Cells Inhibits Metastatic Spread. ACS Nano 2015, 9, 733–745. [Google Scholar] [CrossRef]
- Hetian, L.; Ping, A.; Shumei, S.; Xiaoying, L.; Luowen, H.; Jian, W.; Lin, M.; Meisheng, L.; Junshan, Y.; Chengchao, S. A Novel Peptide Isolated from a Phage Display Library Inhibits Tumor Growth and Metastasis by Blocking the Binding of Vascular Endothelial Growth Factor to Its Kinase Domain Receptor. J. Biol. Chem. 2002, 277, 43137–43142. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, Y.; Guo, J.; Li, J. A novel molecular probe 131I-K237 targeting tumor angiogenesis in human prostate cancer xenografts. Mol. Med. Rep. 2012, 12, 1363–1367. [Google Scholar] [CrossRef]
- Bell, D.R.; Weber, J.K.; Yin, W.; Huynh, T.; Duan, W.; Zhou, R. In silico design and validation of high-affinity RNA aptamers targeting epithelial cellular adhesion molecule dimers. Proc. Natl. Acad. Sci. USA 2020, 117, 8486–8493. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Xie, L.; Qiu, S.; Li, H.; Zhou, Y.; Zhang, H.; Zhang, Y.; Zhang, L.; Xie, T.; Chen, Y.; et al. Elucidation of CKAP4-remodeled cell mechanics in driving metastasis of bladder cancer through aptamer-based target discovery. Proc. Natl. Acad. Sci. USA 2022, 119, e2110500119. [Google Scholar] [CrossRef]
- Shao, X.; Meng, C.; Song, W.; Zhang, T.; Chen, Q. Subcellular visualization: Organelle-specific targeted drug delivery and discovery. Adv. Drug Deliv. Rev. 2023, 199, 114977. [Google Scholar] [CrossRef]
- Ma, X.; Gong, N.; Zhong, L.; Sun, J.; Liang, X.-J. Future of nanotherapeutics: Targeting the cellular sub-organelles. Biomaterials 2016, 97, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, H.; He, X.; Zhao, P.; Wu, T.; Xiahou, J.; Wu, Y.; Liu, Y.; Chen, Y.; Jiang, X.; et al. Blocking Spatiotemporal Crosstalk between Subcellular Organelles for Enhancing Anticancer Therapy with Nanointercepters. Adv. Mater. 2023, 35, 2211597. [Google Scholar] [CrossRef]
- McKenna, M.T.; Weis, J.A.; Barnes, S.L.; Tyson, D.R.; Miga, M.I.; Quaranta, V.; Yankeelov, T.E. A Predictive Mathematical Modeling Approach for the Study of Doxorubicin Treatment in Triple Negative Breast Cancer. Sci. Rep. 2017, 7, 5725. [Google Scholar] [CrossRef]
- Rivankar, S. An overview of doxorubicin formulations in cancer therapy. J. Cancer Res. Ther. 2014, 10, 853–858. [Google Scholar] [CrossRef]
- Xie, S.; Ogden, A.; Aneja, R.; Zhou, J. Microtubule-Binding Proteins as Promising Biomarkers of Paclitaxel Sensitivity in Cancer Chemotherapy. Med. Res. Rev. 2015, 36, 300–312. [Google Scholar] [CrossRef]
- Altieri, D.C. Mitochondria on the move: Emerging paradigms of organelle trafficking in tumour plasticity and metastasis. Br. J. Cancer 2017, 117, 301–305. [Google Scholar] [CrossRef]
- McGregor, A.L.; Hsia, C.-R.; Lammerding, J. Squish and squeeze—The nucleus as a physical barrier during migration in confined environments. Curr. Opin. Cell Biol. 2016, 40, 32–40. [Google Scholar] [CrossRef]
- Yadav, T.; Gau, D.; Roy, P. Mitochondria–actin cytoskeleton crosstalk in cell migration. J. Cell. Physiol. 2022, 237, 2387–2403. [Google Scholar] [CrossRef] [PubMed]
- Danese, A.; Marchi, S.; Vitto, V.A.M.; Modesti, L.; Leo, S.; Wieckowski, M.R.; Giorgi, C.; Pinton, P. Cancer-Related Increases and Decreases in Calcium Signaling at the Endoplasmic Reticulum-Mitochondria Interface (MAMs). In Organelles in Disease; Pedersen, S.H.F., Barber, D.L., Eds.; Springer: Cham, Switzerland, 2023; pp. 153–193. [Google Scholar]
- Short, B.; Barr, F.A. The Golgi apparatus. Curr. Biol. 2000, 10, R583–R585. [Google Scholar] [CrossRef]
- Hao, H.; Niu, J.; Xue, B.; Su, Q.P.; Liu, M.; Yang, J.; Qin, J.; Zhao, S.; Wu, C.; Sun, Y. Golgi-associated microtubules are fast cargo tracks and required for persistent cell migration. EMBO Rep. 2020, 21, e48385. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhen, W.; An, S.; Wang, S.; Hu, W.; Li, Y.; Jiang, X.; Li, J. Precise Subcellular Organelle Targeting for Boosting Endogenous-Stimuli-Mediated Tumor Therapy. Adv. Mater. 2021, 33, 2101572. [Google Scholar] [CrossRef] [PubMed]
- Escriou, V.; Carrière, M.; Scherman, D.; Wils, P. NLS bioconjugates for targeting therapeutic genes to the nucleus. Adv. Drug Deliv. Rev. 2002, 55, 295–306. [Google Scholar] [CrossRef]
- Ren, C.; Li, D.; Zhou, Q.; Hu, X. Mitochondria-targeted TPP-MoS2 with dual enzyme activity provides efficient neuroprotection through M1/M2 microglial polarization in an Alzheimer’s disease model. Biomaterials 2020, 232, 119752. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, N.; Xu, W.; Ling, G.; Zhang, P. Potential Therapies and Diagnosis Based on Golgi-targeted Nano Drug Delivery Systems. Pharmacol. Res. 2021, 175, 105861. [Google Scholar] [CrossRef]
- Li, Z.; Zou, J.; Chen, X. In Response to Precision Medicine: Current Subcellular Targeting Strategies for Cancer Therapy. Adv. Mater. 2023, 35, e2209529. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Luo, G.; Zhang, X. Recent Advances in Subcellular Targeted Cancer Therapy Based on Functional Materials. Adv. Mater. 2018, 31, e1802725. [Google Scholar] [CrossRef] [PubMed]
- Altieri, D.C. Mitochondrial dynamics and metastasis. Cell. Mol. Life Sci. 2018, 76, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Audano, M.; Pedretti, S.; Ligorio, S.; Crestani, M.; Caruso, D.; De Fabiani, E.; Mitro, N. “The Loss of Golden Touch”: Mitochondria-Organelle Interactions, Metabolism, and Cancer. Cells 2020, 9, 2519. [Google Scholar] [CrossRef] [PubMed]
- Lamond, A.I.; Earnshaw, W.C. Structure and Function in the Nucleus. Science 1998, 280, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Macara, I.G. Transport into and out of the Nucleus. Microbiol. Mol. Biol. Rev. 2001, 65, 570–594. [Google Scholar] [CrossRef] [PubMed]
- Funasaka, T.; Wong, R.W. The role of nuclear pore complex in tumor microenvironment and metastasis. Cancer Metastasis Rev. 2011, 30, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Tey, S.K.; Tse, E.Y.T.; Mao, X.; Ko, F.C.F.; Wong, A.S.T.; Lo, R.C.-L.; Ng, I.O.-L.; Yam, J.W.P. Nuclear Met promotes hepatocellular carcinoma tumorigenesis and metastasis by upregulation of TAK1 and activation of NF-κB pathway. Cancer Lett. 2017, 411, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Amin, R.; Shukla, A.; Zhu, J.J.; Kim, S.; Wang, P.; Tian, S.Z.; Tran, A.D.; Paul, D.; Cappell, S.D.; Burkett, S.; et al. Nuclear pore protein NUP210 depletion suppresses metastasis through heterochromatin-mediated disruption of tumor cell mechanical response. Nat. Commun. 2021, 12, 7216. [Google Scholar] [CrossRef] [PubMed]
- Iwaya, K.; Ogawa, H.; Kuroda, M.; Izumi, M.; Ishida, T.; Mukai, K. Cytoplasmic and/or nuclear staining of beta-catenin is associated with lung metastasis. Clin. Exp. Metastasis 2003, 20, 525–529. [Google Scholar] [CrossRef]
- Dey, N.; Barwick, B.G.; Moreno, C.S.; Ordanic-Kodani, M.; Chen, Z.; Oprea-Ilies, G.; Tang, W.; Catzavelos, C.; Kerstann, K.F.; Sledge, G.W.; et al. Wnt signaling in triple negative breast cancer is associated with metastasis. BMC Cancer 2013, 13, 537. [Google Scholar] [CrossRef]
- Miricescu, D.; Totan, A.; Stanescu-Spinu, I.-I.; Badoiu, S.C.; Stefani, C.; Greabu, M. PI3K/AKT/mTOR Signaling Pathway in Breast Cancer: From Molecular Landscape to Clinical Aspects. Int. J. Mol. Sci. 2020, 22, 173. [Google Scholar] [CrossRef]
- Aoki, M.; Fujishita, T. Oncogenic Roles of the PI3K/AKT/mTOR Axis, in Viruses, Genes, and Cancer; Hunter, E., Bister, K., Eds.; Springer: Cham, Switzerland, 2017; pp. 153–189. [Google Scholar]
- Hilger, R.; Scheulen, M.; Strumberg, D. The Ras-Raf-MEK-ERK Pathway in the Treatment of Cancer. Oncol. Res. Treat. 2002, 25, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Hoelz, A.; Debler, E.W.; Blobel, G. The Structure of the Nuclear Pore Complex. Annu. Rev. Biochem. 2011, 80, 613–643. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.S.; Vadlamudi, R.K. Role of Estrogen Receptor Signaling in Breast Cancer Metastasis. Int. J. Breast Cancer 2011, 2012, 65469. [Google Scholar] [CrossRef]
- Jevtić, P.; Edens, L.J.; Vuković, L.D.; Levy, D.L. Sizing and shaping the nucleus: Mechanisms and significance. Curr. Opin. Cell Biol. 2014, 28, 16–27. [Google Scholar] [CrossRef]
- Friedl, P.; Wolf, K.; Lammerding, J. Nuclear mechanics during cell migration. Curr. Opin. Cell Biol. 2010, 23, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Vlodavsky, I.; Lui, G.; Gospodarowicz, D. Morphological appearance, growth behavior and migratory activity of human tumor cells maintained on extracellular matrix versus plastic. Cell 1980, 19, 607–616. [Google Scholar] [CrossRef]
- Dey, P. Cancer nucleus: Morphology and beyond. Diagn. Cytopathol. 2010, 38, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Fischer, E.G. Nuclear Morphology and the Biology of Cancer Cells. Acta Cytol. 2020, 64, 511–519. [Google Scholar] [CrossRef]
- Wu, P.-H.; Phillip, J.M.; Khatau, S.B.; Chen, W.-C.; Stirman, J.; Rosseel, S.; Tschudi, K.; Van Patten, J.; Wong, M.; Gupta, S.; et al. Evolution of cellular morpho-phenotypes in cancer metastasis. Sci. Rep. 2015, 5, 18437. [Google Scholar] [CrossRef]
- Wolberg, W.H.; Street, W.N.; Mangasarian, O.L. Importance of nuclear morphology in breast cancer prognosis. Clin. Cancer Res. 1999, 5, 3542–3548. [Google Scholar]
- Travis, W.D. Update on small cell carcinoma and its differentiation from squamous cell carcinoma and other non-small cell carcinomas. Mod. Pathol. 2012, 25, S18–S30. [Google Scholar] [CrossRef] [PubMed]
- Okudela, K. An association between nuclear morphology and immunohistochemical expression of p53 and p16INK4A in lung cancer cells. Med. Mol. Morphol. 2013, 47, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Junker, K.; Wiethege, T.; Müller, K.-M. Pathology of small-cell lung cancer. J. Cancer Res. Clin. Oncol. 2000, 126, 361–368. [Google Scholar] [CrossRef]
- Muff, R.; Nigg, N.; Gruber, P.; Walters, D.; Born, W.; Fuchs, B. Altered morphology, nuclear stability and adhesion of highly metastatic derivatives of osteoblast-like SAOS-2 osteosarcoma cells. Anticancer. Res. 2008, 27, 3973–3979. [Google Scholar]
- Lyons, S.M.; Alizadeh, E.; Mannheimer, J.; Schuamberg, K.; Castle, J.; Schroder, B.; Turk, P.; Thamm, D.; Prasad, A. Changes in cell shape are correlated with metastatic potential in murine and human osteosarcomas. Biol. Open 2016, 5, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Debes, J.D.; Sebo, T.J.; Heemers, H.V.; Kipp, B.R.; Haugen, D.A.L.; Lohse, C.M.; Tindall, D.J. p300 Modulates Nuclear Morphology in Prostate Cancer. Cancer Res. 2005, 65, 708–712. [Google Scholar] [CrossRef] [PubMed]
- Veltri, R.W.; Christudass, C.S.; Isharwal, S. Nuclear morphometry, nucleomics and prostate cancer progression. Asian J. Androl. 2012, 14, 375–384. [Google Scholar] [CrossRef]
- Ikeguchi, M.; Sakatani, T.; Endo, K.; Makino, M.; Kaibara, N. Computerized nuclear morphometry is a useful technique for evaluating the high metastatic potential of colorectal adenocarcinoma. Cancer 1999, 86, 1944–1951. [Google Scholar] [CrossRef]
- Chow, K.-H.; Factor, R.E.; Ullman, K.S. The nuclear envelope environment and its cancer connections. Nat. Rev. Cancer 2012, 12, 196–209. [Google Scholar] [CrossRef]
- Calero-Cuenca, F.J.; Janota, C.S.; Gomes, E.R. Dealing with the nucleus during cell migration. Curr. Opin. Cell Biol. 2018, 50, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.C.; Yang, J. Forcing through Tumor Metastasis: The Interplay between Tissue Rigidity and Epithelial–Mesenchymal Transition. Trends Cell Biol. 2015, 26, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Wright, L.E.; Pagnotti, G.M.; Uzer, G.; Powell, K.M.; Wallace, J.M.; Sankar, U.; Rubin, C.T.; Mohammad, K.; Guise, T.A.; et al. Mechanical suppression of breast cancer cell invasion and paracrine signaling to osteoclasts requires nucleo-cytoskeletal connectivity. Bone Res. 2020, 8, 40. [Google Scholar] [CrossRef]
- Burke, B.; Stewart, C.L. The nuclear lamins: Flexibility in function. Nat. Rev. Mol. Cell Biol. 2012, 14, 13–24. [Google Scholar] [CrossRef]
- Stefanello, S.T.; Luchtefeld, I.; Liashkovich, I.; Pethö, Z.; Azzam, I.; Bulk, E.; Rosso, G.; Döhlinger, L.; Hesse, B.; Oeckinghaus, A.; et al. Impact of the Nuclear Envelope on Malignant Transformation, Motility, and Survival of Lung Cancer Cells. Adv. Sci. 2021, 8, 2102757. [Google Scholar] [CrossRef] [PubMed]
- Johns, D.R. Mitochondrial DNA and Disease. N. Engl. J. Med. 1995, 333, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Porporato, P.E.; Filigheddu, N.; Pedro, J.M.B.-S.; Kroemer, G.; Galluzzi, L. Mitochondrial metabolism and cancer. Cell Res. 2018, 28, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Nagdas, S.; Kashatus, J.A.; Nascimento, A.; Hussain, S.S.; Trainor, R.E.; Pollock, S.R.; Adair, S.J.; Michaels, A.D.; Sesaki, H.; Stelow, E.B.; et al. Drp1 Promotes KRas-Driven Metabolic Changes to Drive Pancreatic Tumor Growth. Cell Rep. 2019, 28, 1845–1859.e5. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L.; Cao, H.; Wang, G.; Lyu, Y.; Sun, X.; An, J.; Wu, Z.; Huang, Q.; Liu, B.; Xing, J. Drp1-mediated mitochondrial fission promotes cell proliferation through crosstalk of p53 and NF-κB pathways in hepatocellular carcinoma. Oncotarget 2016, 7, 65001–65011. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhu, G.; Zhao, R.; Li, Y.; Li, H.; Liu, Y.; Jin, N.; Li, X.; Li, Y.; Liu, T. Deapioplatycodin D inhibits glioblastoma cell proliferation by inducing BNIP3L-mediated incomplete mitophagy. Cancer Cell Int. 2025, 25, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.J.; Loebel, F.; Juratli, T.A.; Tummala, S.S.; Williams, E.A.; Batchelor, T.T.; Arrillaga-Romany, I.; Cahill, D.P. Accelerated progression of IDH mutant glioma after first recurrence. Neuro-Oncology 2019, 21, 669–677. [Google Scholar] [CrossRef] [PubMed]
- DiMauro, S.; Davidzon, G. Mitochondrial DNA and disease. Ann. Med. 2005, 37, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.V.; Dong, L.; Neuzil, J. Mitochondrial DNA in Tumor Initiation, Progression, and Metastasis: Role of Horizontal mtDNA Transfer. Cancer Res. 2015, 75, 3203–3208. [Google Scholar] [CrossRef] [PubMed]
- Stagg, J.; Smyth, M.J. Extracellular adenosine triphosphate and adenosine in cancer. Oncogene 2010, 29, 5346–5358. [Google Scholar] [CrossRef]
- Forsythe, J.A.; Jiang, B.-H.; Iyer, N.V.; Agani, F.; Leung, S.W.; Koos, R.D.; Semenza, G.L. Activation of Vascular Endothelial Growth Factor Gene Transcription by Hypoxia-Inducible Factor. Mol. Cell. Biol. 1996, 16, 4604–4613. [Google Scholar] [CrossRef]
- Mizukami, Y.; Li, J.; Zhang, X.; Zimmer, M.A.; Iliopoulos, O.; Chung, D.C. Hypoxia-Inducible Factor-1-Independent Regulation of Vascular Endothelial Growth Factor by Hypoxia in Colon Cancer. Cancer Res. 2004, 64, 1765–1772. [Google Scholar] [CrossRef]
- Wilson, W.R.; Hay, M.P. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer 2011, 11, 393–410. [Google Scholar] [CrossRef] [PubMed]
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia 2015, 3, 83–92. [Google Scholar] [CrossRef]
- Paul, S.; Ghosh, S.; Kumar, S. Tumor glycolysis, an essential sweet tooth of tumor cells. Semin. Cancer Biol. 2022, 86, 1216–1230. [Google Scholar] [CrossRef]
- Liang, L.; Li, W.; Li, X.; Jin, X.; Liao, Q.; Li, Y.; Zhou, Y. ‘Reverse Warburg effect’ of cancer-associated fibroblasts (Review). Int. J. Oncol. 2022, 60, 67. [Google Scholar] [CrossRef] [PubMed]
- Hirschhaeuser, F.; Sattler, U.G.A.; Mueller-Klieser, W. Lactate: A Metabolic Key Player in Cancer. Cancer Res. 2011, 71, 6921–6925. [Google Scholar] [CrossRef]
- Wang, Y.; Kohane, D.S. External triggering and triggered targeting strategies for drug delivery. Nat. Rev. Mater. 2017, 2, 17020. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Meng, Y.; Xu, X.; Zuo, D. The role of glycolysis and lactate in the induction of tumor-associated macrophages immunosuppressive phenotype. Int. Immunopharmacol. 2022, 110, 108994. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.J.; Vignali, P.D.A.; Mullett, S.J.; Overacre-Delgoffe, A.E.; Peralta, R.M.; Grebinoski, S.; Menk, A.V.; Rittenhouse, N.L.; DePeaux, K.; Whetstone, R.D.; et al. Metabolic support of tumour-infiltrating regulatory T cells by lactic acid. Nature 2021, 591, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Papa, S.; Skulachev, V.P. Reactive oxygen species, mitochondria, apoptosis and aging. In Detection of Mitochondrial Diseases; Gellerich, F.N., Zierz, S., Eds.; Springer: Boston, MA, USA, 1997; pp. 305–319. [Google Scholar]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Svineng, G.; Ravuri, C.; Rikardsen, O.; Huseby, N.-E.; Winberg, J.-O. The Role of Reactive Oxygen Species in Integrin and Matrix Metalloproteinase Expression and Function. Connect. Tissue Res. 2008, 49, 197–202. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef]
- Nishikawa, M. Reactive oxygen species in tumor metastasis. Cancer Lett. 2008, 266, 53–59. [Google Scholar] [CrossRef]
- Moloney, J.N.; Cotter, T.G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef]
- Pelicano, H.; Carney, D.; Huang, P. ROS stress in cancer cells and therapeutic implications. Drug Resist. Updates 2004, 7, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Raza, M.H.; Siraj, S.; Arshad, A.; Waheed, U.; Aldakheel, F.; Alduraywish, S.; Arshad, M. ROS-modulated therapeutic approaches in cancer treatment. J. Cancer Res. Clin. Oncol. 2017, 143, 1789–1809. [Google Scholar] [CrossRef]
- Kale, J.; Osterlund, E.J.; Andrews, D.W. BCL-2 family proteins: Changing partners in the dance towards death. Cell Death Differ. 2018, 25, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, K.; Chen, G.; Lindsten, T.; White, E. BAX and BAK mediate p53-independent suppression of tumorigenesis. Cancer Cell 2002, 2, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Gurel, P.S.; Hatch, A.L.; Higgs, H.N. Connecting the Cytoskeleton to the Endoplasmic Reticulum and Golgi. Curr. Biol. 2014, 24, R660–R672. [Google Scholar] [CrossRef] [PubMed]
- Howley, B.V.; Link, L.A.; Grelet, S.; El-Sabban, M.; Howe, P.H. A CREB3-regulated ER–Golgi trafficking signature promotes metastatic progression in breast cancer. Oncogene 2017, 37, 1308–1325. [Google Scholar] [CrossRef]
- Hsieh, H.-L.; Wang, H.-H.; Wu, W.-B.; Chu, P.-J.; Yang, C.-M. Transforming growth factor-β1 induces matrix metalloproteinase-9 and cell migration in astrocytes: Roles of ROS-dependent ERK- and JNK-NF-κB pathways. J. Neuroinflamm. 2010, 7, 88. [Google Scholar] [CrossRef]
- Dijke, P.T.; Arthur, H.M. Extracellular control of TGFβ signalling in vascular development and disease. Nat. Rev. Mol. Cell Biol. 2007, 8, 857–869. [Google Scholar] [CrossRef]
- Navid, F.; Colbert, R.A. Causes and consequences of endoplasmic reticulum stress in rheumatic disease. Nat. Rev. Rheumatol. 2016, 13, 25–40. [Google Scholar] [CrossRef]
- Urra, H.; Dufey, E.; Avril, T.; Chevet, E.; Hetz, C. Endoplasmic Reticulum Stress and the Hallmarks of Cancer. Trends Cancer 2016, 2, 252–262. [Google Scholar] [CrossRef]
- Chen, X.; Cubillos-Ruiz, J.R. Endoplasmic reticulum stress signals in the tumour and its microenvironment. Nat. Rev. Cancer 2020, 21, 71–88. [Google Scholar] [CrossRef]
- Feng, Y.-X.; Sokol, E.S.; Del Vecchio, C.A.; Sanduja, S.; Claessen, J.H.; Proia, T.A.; Jin, D.X.; Reinhardt, F.; Ploegh, H.L.; Wang, Q.; et al. Epithelial-to-Mesenchymal Transition Activates PERK–eIF2α and Sensitizes Cells to Endoplasmic Reticulum Stress. Cancer Discov. 2014, 4, 702–715. [Google Scholar] [CrossRef] [PubMed]
- Krebs, J.; Agellon, L.B.; Michalak, M. Ca2+ homeostasis and endoplasmic reticulum (ER) stress: An integrated view of calcium signaling. Biochem. Biophys. Res. Commun. 2015, 460, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Urra, H.; Henriquez, D.R.; Cánovas, J.; Villarroel-Campos, D.; Carreras-Sureda, A.; Pulgar, E.; Molina, E.; Hazari, Y.M.; Limia, C.M.; Alvarez-Rojas, S.; et al. IRE1α governs cytoskeleton remodelling and cell migration through a direct interaction with filamin A. Nat. Cell Biol. 2018, 20, 942–953. [Google Scholar] [CrossRef]
- Prudent, J.; Popgeorgiev, N.; Gadet, R.; Deygas, M.; Rimokh, R.; Gillet, G. Mitochondrial Ca2+ uptake controls actin cytoskeleton dynamics during cell migration. Sci. Rep. 2016, 6, 36570. [Google Scholar] [CrossRef]
- Orrenius, S.; Zhivotovsky, B.; Nicotera, P. Regulation of cell death: The calcium–apoptosis link. Nat. Rev. Mol. Cell Biol. 2003, 4, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Missiroli, S.; Patergnani, S.; Caroccia, N.; Pedriali, G.; Perrone, M.; Previati, M.; Wieckowski, M.R.; Giorgi, C. Mitochondria-associated membranes (MAMs) and inflammation. Cell Death Dis. 2018, 9, 329. [Google Scholar] [CrossRef]
- Lunz, V.; Romanin, C.; Frischauf, I. STIM1 activation of Orai. Cell Calcium. 2019, 77, 29–38. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Chen, Y.-F.; Chen, Y.-T.; Chiu, W.-T.; Shen, M.-R. The STIM1-Orai1 pathway of store-operated Ca2+ entry controls the checkpoint in cell cycle G1/S transition. Sci. Rep. 2016, 6, 22142. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Kumar, G.; Co, C.C.; Ho, C.-C. Geometric Control of Cell Migration. Sci. Rep. 2013, 3, 2827. [Google Scholar] [CrossRef]
- Millarte, V.; Farhan, H. The Golgi in Cell Migration: Regulation by Signal Transduction and Its Implications for Cancer Cell Metastasis. Sci. World J. 2012, 2012, 498278. [Google Scholar] [CrossRef]
- Anitei, M.; Hoflack, B. Bridging membrane and cytoskeleton dynamics in the secretory and endocytic pathways. Nat. Cell Biol. 2011, 14, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, R.; Warner, A.N.; Fradette, J.F.; Gibbons, D.L. Dance of The Golgi: Understanding Golgi Dynamics in Cancer Metastasis. Cells 2022, 11, 1484. [Google Scholar] [CrossRef]
- Whitehead, C.A.; Kaye, A.H.; Drummond, K.J.; Widodo, S.S.; Mantamadiotis, T.; Vella, L.J.; Stylli, S.S. Extracellular vesicles and their role in glioblastoma. Crit. Rev. Clin. Lab. Sci. 2019, 57, 227–252. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, M.; Pavlopoulou, A.; Georgakilas, A.G.; Kyrodimos, E. The challenge of drug resistance in cancer treatment: A current overview. Clin. Exp. Metastasis 2018, 35, 309–318. [Google Scholar] [CrossRef]
- Wan, L.; Pantel, K.; Kang, Y. Tumor metastasis: Moving new biological insights into the clinic. Nat. Med. 2013, 19, 1450–1464. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Shestov, A.A.; Worth, A.J.; Nath, K.; Nelson, D.S.; Leeper, D.B.; Glickson, J.D.; Blair, I.A. Inhibition of Mitochondrial Complex II by the Anticancer Agent Lonidamine. J. Biol. Chem. 2016, 291, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Lytton, J.; Westlin, M.; Hanley, M.R. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J. Biol. Chem. 1991, 266, 17067–17071. [Google Scholar] [CrossRef]
- Zhang, G.F.; Driouich, A.; Staehelin, L.A. Effect of monensin on plant Golgi: Re-examination of the monensin-induced changes in cisternal architecture and functional activities of the Golgi apparatus of sycamore suspension-cultured cells. J. Cell Sci. 1993, 104, 819–831. [Google Scholar] [CrossRef]
- Xiang, Y.; Chen, L.; Liu, C.; Yi, X.; Li, L.; Huang, Y. Redirecting Chemotherapeutics to the Endoplasmic Reticulum Increases Tumor Immunogenicity and Potentiates Anti-PD-L1 Therapy. Small 2021, 18, 2104591. [Google Scholar] [CrossRef]
- Li, Q.; Yang, J.; Chen, C.; Lin, X.; Zhou, M.; Zhou, Z.; Huang, Y. A novel mitochondrial targeted hybrid peptide modified HPMA copolymers for breast cancer metastasis suppression. J. Control. Release 2020, 325, 38–51. [Google Scholar] [CrossRef]
- Huo, S.; Jin, S.; Ma, X.; Xue, X.; Yang, K.; Kumar, A.; Wang, P.C.; Zhang, J.; Hu, Z.; Liang, X.-J. Ultrasmall Gold Nanoparticles as Carriers for Nucleus-Based Gene Therapy Due to Size-Dependent Nuclear Entry. ACS Nano 2014, 8, 5852–5862. [Google Scholar] [CrossRef] [PubMed]
- Hoye, A.T.; Davoren, J.E.; Wipf, P.; Fink, M.P.; Kagan, V.E. Targeting Mitochondria. Accounts Chem. Res. 2008, 41, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, S.; Wu, J.; Jin, X.; You, J. Pharmaceutical strategies for endoplasmic reticulum-targeting and their prospects of application. J. Control. Release 2020, 329, 337–352. [Google Scholar] [CrossRef]
- Li, H.; Zhang, P.; Luo, J.; Hu, D.; Huang, Y.; Zhang, Z.-R.; Fu, Y.; Gong, T. Chondroitin Sulfate-Linked Prodrug Nanoparticles Target the Golgi Apparatus for Cancer Metastasis Treatment. ACS Nano 2019, 13, 9386–9396. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Xiong, X.; Ming, Y.; Zhao, J.; Guo, X.; Yang, G.; Zhou, S. A Multifunctional Micellar Nanoplatform with pH-Triggered Cell Penetration and Nuclear Targeting for Effective Cancer Therapy and Inhibition to Lung Metastasis. Adv. Heal. Mater. 2018, 7, e1700974. [Google Scholar] [CrossRef]
- Bai, H.; Wang, H.; Zhou, Z.; Piao, Y.; Liu, X.; Tang, J.; Shen, X.; Shen, Y.; Zhou, Z. Histone Deacetylase-Triggered Self-Immolative Peptide-Cytotoxins for Cancer-Selective Drug Delivery. Adv. Funct. Mater. 2023, 33, 2214025. [Google Scholar] [CrossRef]
- Luo, H.-Y.; Yang, Z.; Wei, W.; Li, Y.-Q.; Pu, H.; Chen, Y.; Sheng, H.; Liu, J.; Xu, R.-H. Enzymatically synthesized poly(amino-co-ester) polyplexes for systemic delivery of pcDNA-miRNA-214 to suppress colorectal cancer liver metastasis. J. Mater. Chem. B 2018, 6, 6365–6376. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.-Y.; He, X.-Y.; Xu, C.; Ren, X.-H.; Zhuo, R.-X.; Cheng, S.-X. Peptide and Aptamer Decorated Delivery System for Targeting Delivery of Cas9/sgRNA Plasmid To Mediate Antitumor Genome Editing. ACS Appl. Mater. Interfaces 2019, 11, 23870–23879. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, K.; Wang, Y.; Jiang, W.; Cheng, H.; Wang, Q.; Xiang, T.; Zhang, Z.; Liu, J.; Shi, J. Intracellular Self-Assembly Driven Nucleus-Targeted Photo-Immune Stimulator with Chromatin Decompaction Function for Robust Innate and Adaptive Antitumor Immunity. Adv. Funct. Mater. 2022, 32, 2108883. [Google Scholar] [CrossRef]
- Su, W.; Guo, R.; Yuan, F.; Li, Y.; Li, X.; Zhang, Y.; Zhou, S.; Fan, L. Red-Emissive Carbon Quantum Dots for Nuclear Drug Delivery in Cancer Stem Cells. J. Phys. Chem. Lett. 2020, 11, 1357–1363. [Google Scholar] [CrossRef]
- Zhan, J.; Wang, Y.; Ma, S.; Qin, Q.; Wang, L.; Cai, Y.; Yang, Z. Organelle-inspired supramolecular nanomedicine to precisely abolish liver tumor growth and metastasis. Bioact. Mater. 2021, 9, 120–133. [Google Scholar] [CrossRef]
- Yang, G.; Xu, L.; Xu, J.; Zhang, R.; Song, G.; Chao, Y.; Feng, L.; Han, F.; Dong, Z.; Li, B.; et al. Smart Nanoreactors for pH-Responsive Tumor Homing, Mitochondria-Targeting, and Enhanced Photodynamic-Immunotherapy of Cancer. Nano Lett. 2018, 18, 2475–2484. [Google Scholar] [CrossRef]
- Huo, D.; Zhu, J.; Chen, G.; Chen, Q.; Zhang, C.; Luo, X.; Jiang, W.; Jiang, X.; Gu, Z.; Hu, Y. Eradication of unresectable liver metastasis through induction of tumour specific energy depletion. Nat. Commun. 2019, 10, 305. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, F.; Wen, H.; Shi, W.; Huang, Q.; Huang, Y.; Xie, J.; Li, P.; Chen, J.; Qin, L.; et al. Tumor- and mitochondria-targeted nanoparticles eradicate drug resistant lung cancer through mitochondrial pathway of apoptosis. J. Nanobiotechnology 2020, 18, 8. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Jin, Z.; Zhao, P.; Zhao, B.; Fan, M.; He, Q. A multistage assembly/disassembly strategy for tumor-targeted CO delivery. Sci. Adv. 2020, 6, eaba1362. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Lei, L.; Cao, J.; Yang, X.; Cai, S.; Tong, F.; Huang, D.; Mei, H.; Luo, K.; Gao, H.; et al. A combinational chemo-immune therapy using an enzyme-sensitive nanoplatform for dual-drug delivery to specific sites by cascade targeting. Sci. Adv. 2021, 7, eaba0776. [Google Scholar] [CrossRef]
- Peng, J.; Hu, X.; Fan, S.; Zhou, J.; Ren, S.; Sun, R.; Chen, Y.; Shen, X.; Chen, Y. Inhibition of Mitochondrial Biosynthesis Using a “Right-Side-Out” Membrane-Camouflaged Micelle to Facilitate the Therapeutic Effects of Shikonin on Triple-Negative Breast Cancer. Adv. Health Mater. 2022, 11, e2200742. [Google Scholar] [CrossRef]
- Yi, X.; Yan, Y.; Shen, X.; Li, L.; Huang, Y. Mitochondria-Targeted Delivery of Camptothecin Based on HPMA Copolymer for Metastasis Suppression. Pharmaceutics 2022, 14, 1534. [Google Scholar] [CrossRef]
- Deng, Y.; Jia, F.; Chen, X.; Jin, Q.; Ji, J. ATP Suppression by pH-Activated Mitochondria-Targeted Delivery of Nitric Oxide Nanoplatform for Drug Resistance Reversal and Metastasis Inhibition. Small 2020, 16, e2001747. [Google Scholar] [CrossRef]
- Shen, X.; Deng, Y.; Chen, L.; Liu, C.; Li, L.; Huang, Y. Modulation of Autophagy Direction to Enhance Antitumor Effect of Endoplasmic-Reticulum-Targeted Therapy: Left or Right? Adv. Sci. 2023, 10, e2301434. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Cao, Y.; Song, P.; Li, W.; Lv, Z.; Song, S.; Wang, Y.; Zhang, H. A Porous Organic Framework-Based Nanoplatform for Directional Destruction on Endoplasmic Reticulum to Enhance Tumor Immunotherapy. Adv. Funct. Mater. 2023, 33, 2307175. [Google Scholar] [CrossRef]
- Yin, H.; Yuan, X.; Luo, L.; Lu, Y.; Qin, B.; Zhang, J.; Shi, Y.; Zhu, C.; Yang, J.; Li, X.; et al. Appropriate Delivery of the CRISPR/Cas9 System through the Nonlysosomal Route: Application for Therapeutic Gene Editing. Adv. Sci. 2020, 7, 1903381. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Q.; Ji, M.-Y.; Wang, C. Endoplasmic reticulum-targeted glutathione and pH dual responsive vitamin lipid nanovesicles for tocopheryl DM1 delivery and cancer therapy. Int. J. Pharm. 2020, 582, 119331. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Jiang, P.; Shen, X.; Lyu, J.; Liu, C.; Li, L.; Huang, Y. Cascade Delivery to Golgi Apparatus and On-Site Formation of Subcellular Drug Reservoir for Cancer Metastasis Suppression. Small 2022, 19, e2204747. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.-B.; Liu, Q.; Liu, B.; Yao, H.-G.; He, J.; Tang, C.-F.; Peng, K.; Su, X.-X.; Zheng, Y.; Ding, J.-Y.; et al. A Golgi-Targeted Platinum Complex Plays a Dual Role in Autophagy Regulation for Highly Efficient Cancer Therapy. Angew. Chem. 2023, 135, e202312170. [Google Scholar] [CrossRef]
- Tammam, S.N.; Azzazy, H.M.; Lamprecht, A. How successful is nuclear targeting by nanocarriers? J. Control. Release 2016, 229, 140–153. [Google Scholar] [CrossRef]
- Rizzuti, M.; Nizzardo, M.; Zanetta, C.; Ramirez, A.; Corti, S. Therapeutic applications of the cell-penetrating HIV-1 Tat peptide. Drug Discov. Today 2015, 20, 76–85. [Google Scholar] [CrossRef]
- Pan, L.; He, Q.; Liu, J.; Chen, Y.; Ma, M.; Zhang, L.; Shi, J. Nuclear-Targeted Drug Delivery of TAT Peptide-Conjugated Monodisperse Mesoporous Silica Nanoparticles. J. Am. Chem. Soc. 2012, 134, 5722–5725. [Google Scholar] [CrossRef]
- Cartier, R.; Reszka, R. Utilization of synthetic peptides containing nuclear localization signals for nonviral gene transfer systems. Gene Ther. 2002, 9, 157–167. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.; Zhang, X.; Zhang, W.; Guo, S.; Jin, F. Recent progress of cell-penetrating peptides as new carriers for intracellular cargo delivery. J. Control. Release 2014, 174, 126–136. [Google Scholar] [CrossRef]
- Zhou, J.; Rossi, J. Aptamers as targeted therapeutics: Current potential and challenges. Nat. Rev. Drug Discov. 2017, 16, 181–202, Erratum: Nat. Rev. Drug Discov. 2017, 16, 440. [Google Scholar] [CrossRef] [PubMed]
- Dunn, M.R.; Jimenez, R.M.; Chaput, J.C. Analysis of aptamer discovery and technology. Nat. Rev. Chem. 2017, 1, 0076. [Google Scholar] [CrossRef]
- Yoon, S.; Rossi, J.J. Aptamers: Uptake mechanisms and intracellular applications. Adv. Drug Deliv. Rev. 2018, 134, 22–35. [Google Scholar] [CrossRef]
- Li, L.; Hou, J.; Liu, X.; Guo, Y.; Wu, Y.; Zhang, L.; Yang, Z. Nucleolin-targeting liposomes guided by aptamer AS1411 for the delivery of siRNA for the treatment of malignant melanomas. Biomaterials 2014, 35, 3840–3850. [Google Scholar] [CrossRef]
- Friedman, J.R.; Nunnari, J. Mitochondrial form and function. Nature 2014, 505, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.C. Mitochondria and cancer. Nat. Rev. Cancer 2012, 12, 685–698. [Google Scholar] [CrossRef]
- Fogg, V.C.; Lanning, N.J.; MacKeigan, J.P. Mitochondria in cancer: At the crossroads of life and death. Chin. J. Cancer 2011, 30, 526–539. [Google Scholar] [CrossRef]
- Avril, T.; Vauléon, E.; Chevet, E. Endoplasmic reticulum stress signaling and chemotherapy resistance in solid cancers. Oncogenesis 2017, 6, e373. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Kaufman, R.J. The impact of the endoplasmic reticulum protein-folding environment on cancer development. Nat. Rev. Cancer 2014, 14, 581–597. [Google Scholar] [CrossRef]
- Wlodkowic, D.; Skommer, J.; McGuinness, D.; Hillier, C.; Darzynkiewicz, Z. ER–Golgi network—A future target for anti-cancer therapy. Leuk. Res. 2009, 33, 1440–1447. [Google Scholar] [CrossRef]
- Zhou, H.; Sauvat, A.; Gomes-Da-Silva, L.C.; Durand, S.; Forveille, S.; Iribarren, K.; Yamazaki, T.; Souquere, S.; Bezu, L.; Müller, K.; et al. The oncolytic compound LTX-401 targets the Golgi apparatus. Cell Death Differ. 2016, 23, 2031–2041. [Google Scholar] [CrossRef] [PubMed]

| Subcellular Compartment | Targeting Mechanism | Carrier or Material | Drug | Anti-Metastasis Effect | Ref. |
|---|---|---|---|---|---|
| Nuclear | TAT modification | PEG-PCL NPs | HCPT | Effective antitumor efficacy and inhibition to lung metastasis | [149] |
| TAT modification | Self-immolative peptide-camptothecin (CPT) nanoassemblies | CPT | Potent antitumor activity by inhibiting tumor progression and metastasis in breast tumors | [150] | |
| NLS modification | HA-g-mPEG quaternary polyplexes | pcDNA-miR-214 | Highly specific therapeutic approach in the treatment of CRC liver metastasis | [151] | |
| NLS and AS1411 aptamer modification | Protamine NPs | CRISPR/Cas9 plasmid | Prevents cancer invasion and metastasis in genome-edited cells | [152] | |
| AS1411 aptamer modification | Metal–organic framework | Vorinostat and photosensitizer TCPP | Significantly enhanced efficacy for inhibiting distant metastasis in several xenograft tumor models | [153] | |
| Nitrogen groups | Carbon quantum dots | DOX | Therapeutic effect by eliminating CSCs, and shows potential in mediating metastasis | [154] | |
| Size decreasement (4–9 nm) | Peptide–drug self-assembly NPs | HCPT | Potently abolishing liver tumor growth and inhibiting lung metastasis | [155] | |
| mitochondrial | TPP modification | Silica nanoparticles | Catalase | Strong abscopal effect and promising in metastasis inhibition | [156] |
| TPP and NLS modification | MSNs | Ce6 | Effectively eliminates liver metastasis while sparing hepatocytes | [157] | |
| TPP modification | Pluronic F127-hyaluronic acid micelles | PTX | Significant antitumor efficacy in a breast cancer-bearing mouse model with lung metastasis | [158] | |
| TPP modification | MSNs | CO prodrugs | Effective inhibition of tumor growth and metastasis | [159] | |
| TPP modification | positively charged triphenylphosphonium derivatives particles (LTPT) | lonidamine (LND) dimers (LTPT) | Efficient tumor inhibition and antitumor immune response against tumor metastasis | [160] | |
| TPP modification | RBC membrane camouflaged cationic micelle | Shikonin | Profound inhibition of lung metastasis in a TNBC mouse model | [161] | |
| R8-MTS modification | HPMA | DOX | Enhanced reactive oxygen species generation and apoptosis initiation; suppressed migration and invasion of breast cancer 4T1 and MDA-MB-231 cells | [144] | |
| DEA modification | HPMA | CPT | Anti-metastasis capacity via down-regulation of various pro-metastatic proteins | [162] | |
| PEG-(KLAKLAK) 2 CGKRK modification | α-cyclodextrin-based NPs | DOX and NO prodrugs | Overcoming drug resistance and cancer metastasis | [163] | |
| ER | p-toluene sulfonyl modification | PEG–PLGA NPs | DOX | ER-targeting therapy benefits from the autophagy-enhancing strategy more than the autophagy-inhibiting strategy for antitumor and antimetastasis treatment | [164] |
| N-tosylethylenediamine modification | Porous organic framework | GOX and luminol | Effectively activates ICD-induced anti-tumor immunity to hinder the growth of distant and metastatic tumors | [165] | |
| Pardaxin modification | liposomes | CRISPR/Cas9 system | Down-regulation of cancer cell proliferation and results in fewer metastatic cancer cells in the liver | [166] | |
| Vitamin E modification | Vitamin lipid nanovesicles | Tocopheryl DM1 | Inhibits migration and suppresses tumor growth of metastatic MCF-7 mice | [167] | |
| GA | Chondroitin sulfate | Chondroitin sulfate NPs | Retinoic acid | Inhibits migration, invasion, and angiogenesis in vitro and suppresses tumor growth and metastasis in 4T1-Luc-bearing mice | [148] |
| Cell membrane camouflaged | Cell membrane camouflaged PLGA NPs | Monensin | Potential therapeutic strategy for cancer metastasis suppression | [168] | |
| Platinum complex | liposomes | Platinum complex | Migration ability of A549 cells was significantly suppressed in a dose-dependent manner | [169] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Liu, Y.; Kang, X.; Li, L.; Xiang, Y. Subcellular Organelle Targeting as a Novel Approach to Combat Tumor Metastasis. Pharmaceutics 2025, 17, 198. https://doi.org/10.3390/pharmaceutics17020198
Liu Z, Liu Y, Kang X, Li L, Xiang Y. Subcellular Organelle Targeting as a Novel Approach to Combat Tumor Metastasis. Pharmaceutics. 2025; 17(2):198. https://doi.org/10.3390/pharmaceutics17020198
Chicago/Turabian StyleLiu, Zefan, Yang Liu, Xin Kang, Lian Li, and Yucheng Xiang. 2025. "Subcellular Organelle Targeting as a Novel Approach to Combat Tumor Metastasis" Pharmaceutics 17, no. 2: 198. https://doi.org/10.3390/pharmaceutics17020198
APA StyleLiu, Z., Liu, Y., Kang, X., Li, L., & Xiang, Y. (2025). Subcellular Organelle Targeting as a Novel Approach to Combat Tumor Metastasis. Pharmaceutics, 17(2), 198. https://doi.org/10.3390/pharmaceutics17020198






