Evaluation of Preclinical Efficacy of Curcumin-Loaded Bicosome Systems in Amelioration of Oral Mucositis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of the Systems
2.2.1. Bicelles
2.2.2. Bicosome Systems
2.3. Determination of Particle Size and Polydispersity Index (PDI)
2.4. Determination of Encapsulation Efficiency (EE)
2.5. Cryogenic Transmission Electron Microscopy (Cryo-TEM)
2.6. In Vitro Drug Release Profile
2.7. Curcumin Release Kinetics
2.8. Ex Vivo Drug Permeation and Skin Retention
2.9. Cell Viability Assesment
qRT-PCR
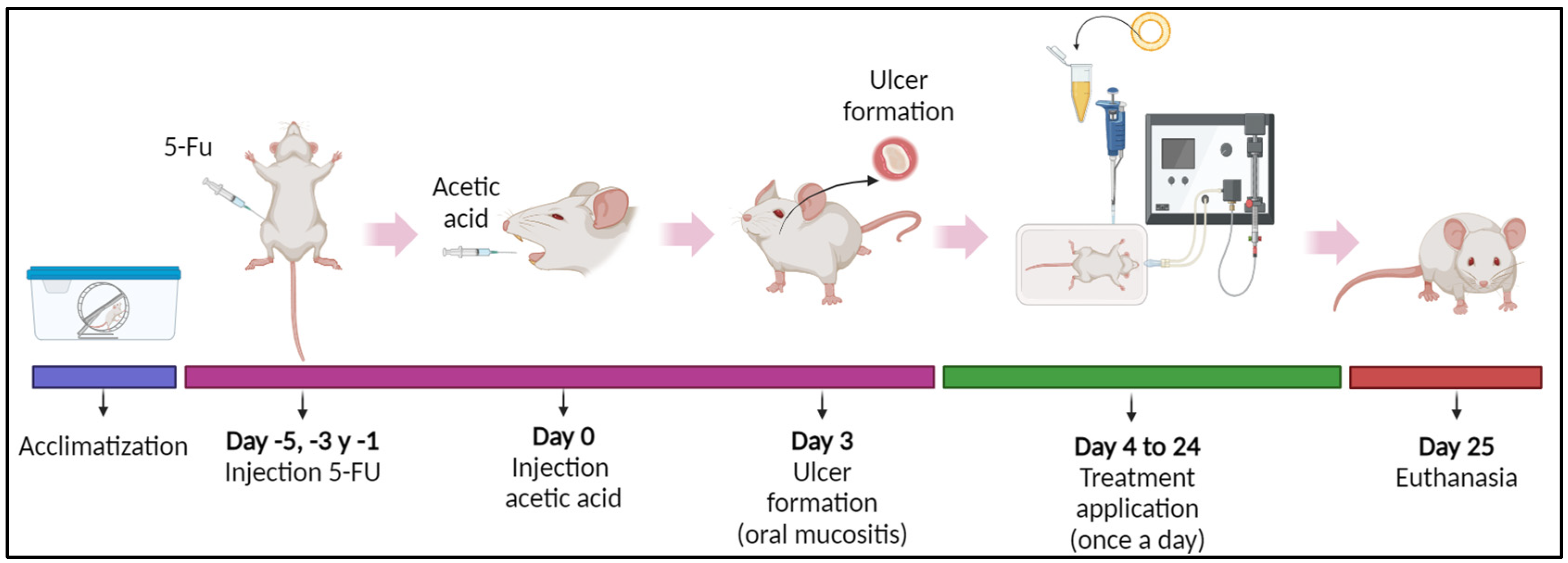
2.10. Animal Models
2.10.1. Experimental Design
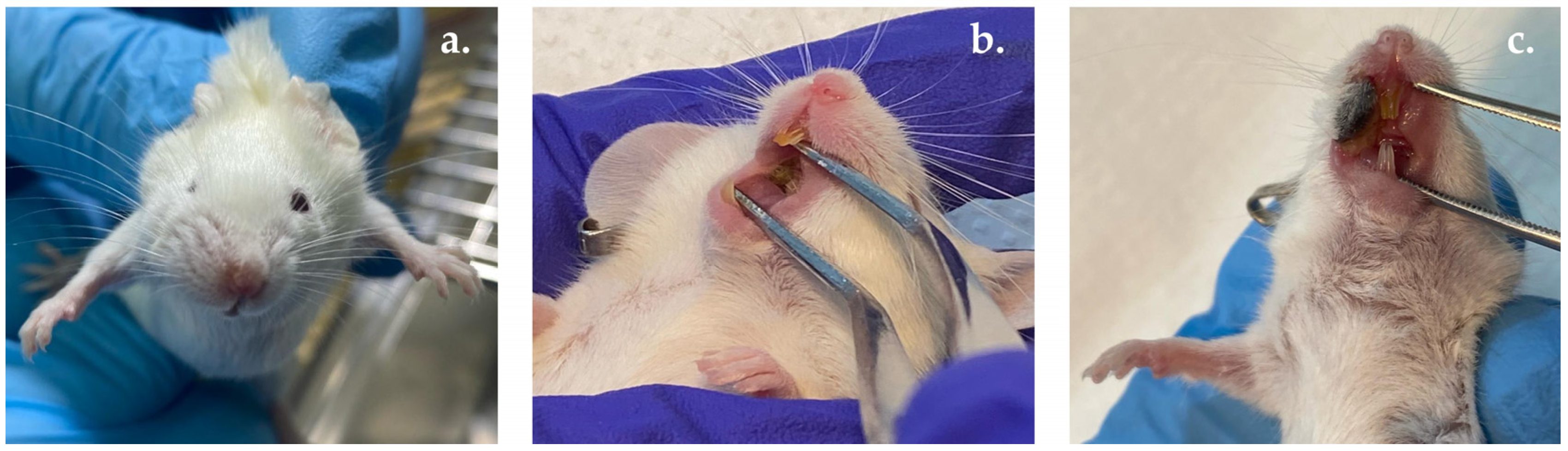
2.10.2. OM Induction
2.10.3. Estimation of OM Severity Score
2.11. Histological Analysis
2.12. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Bicelles and Bicosomes
3.1.1. Particle Size, Polydispersity Index (PDI), and Encapsulation Efficiency (EE)
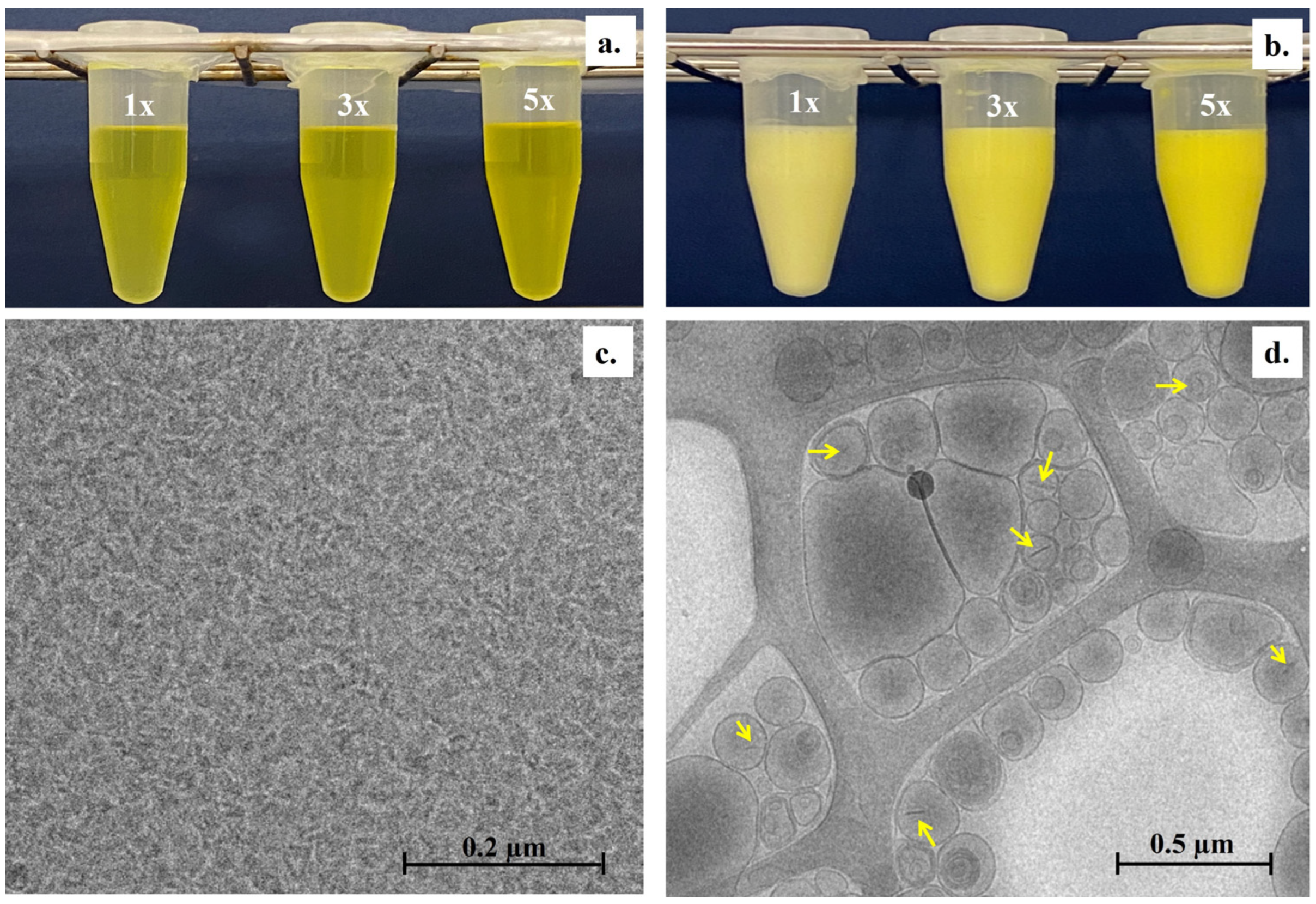
3.1.2. Visual Appearance and Microscopic Structure
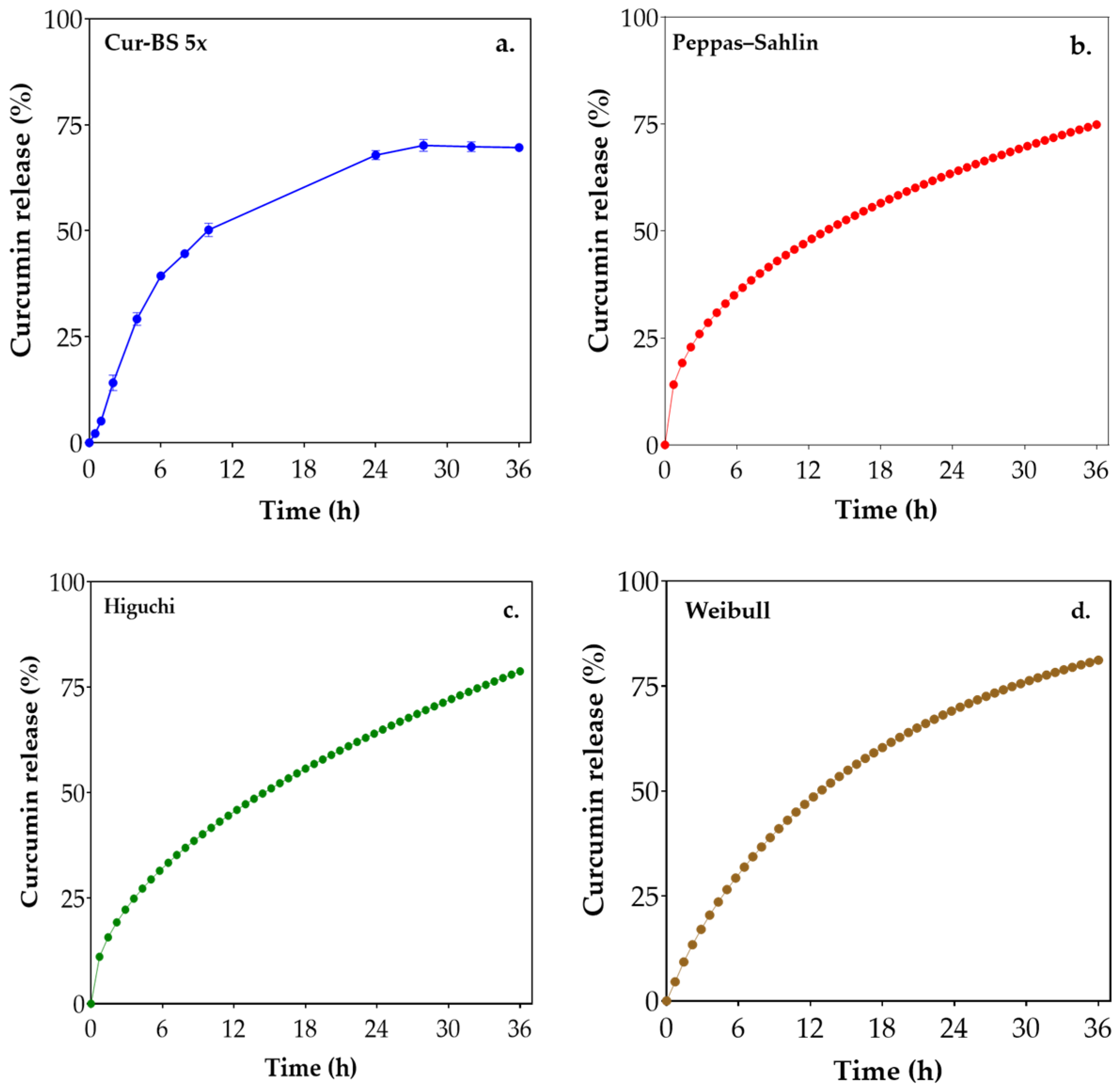
3.2. In Vitro Curcumin Release Profile
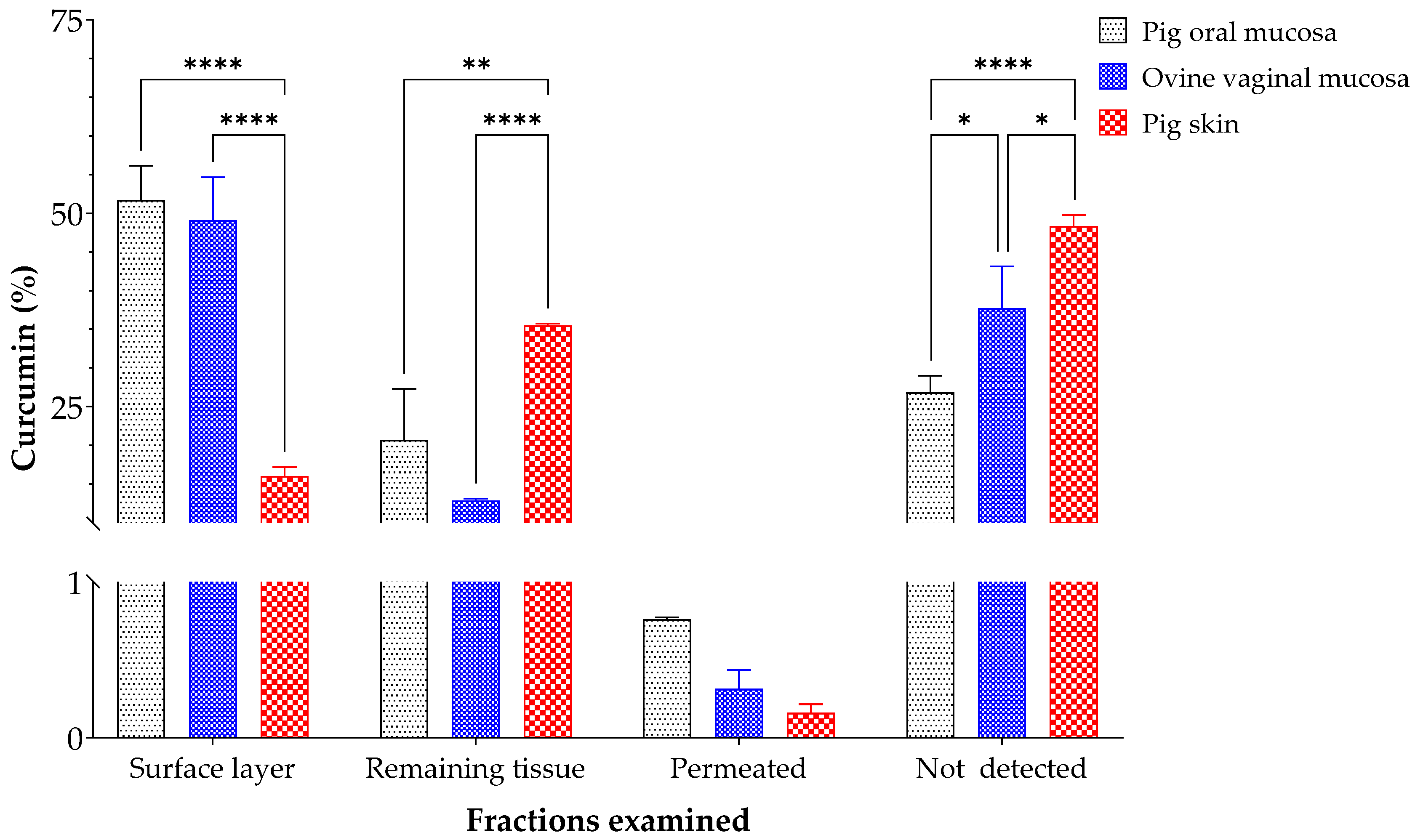
3.3. Ex Vivo Curcumin Permeation Assays
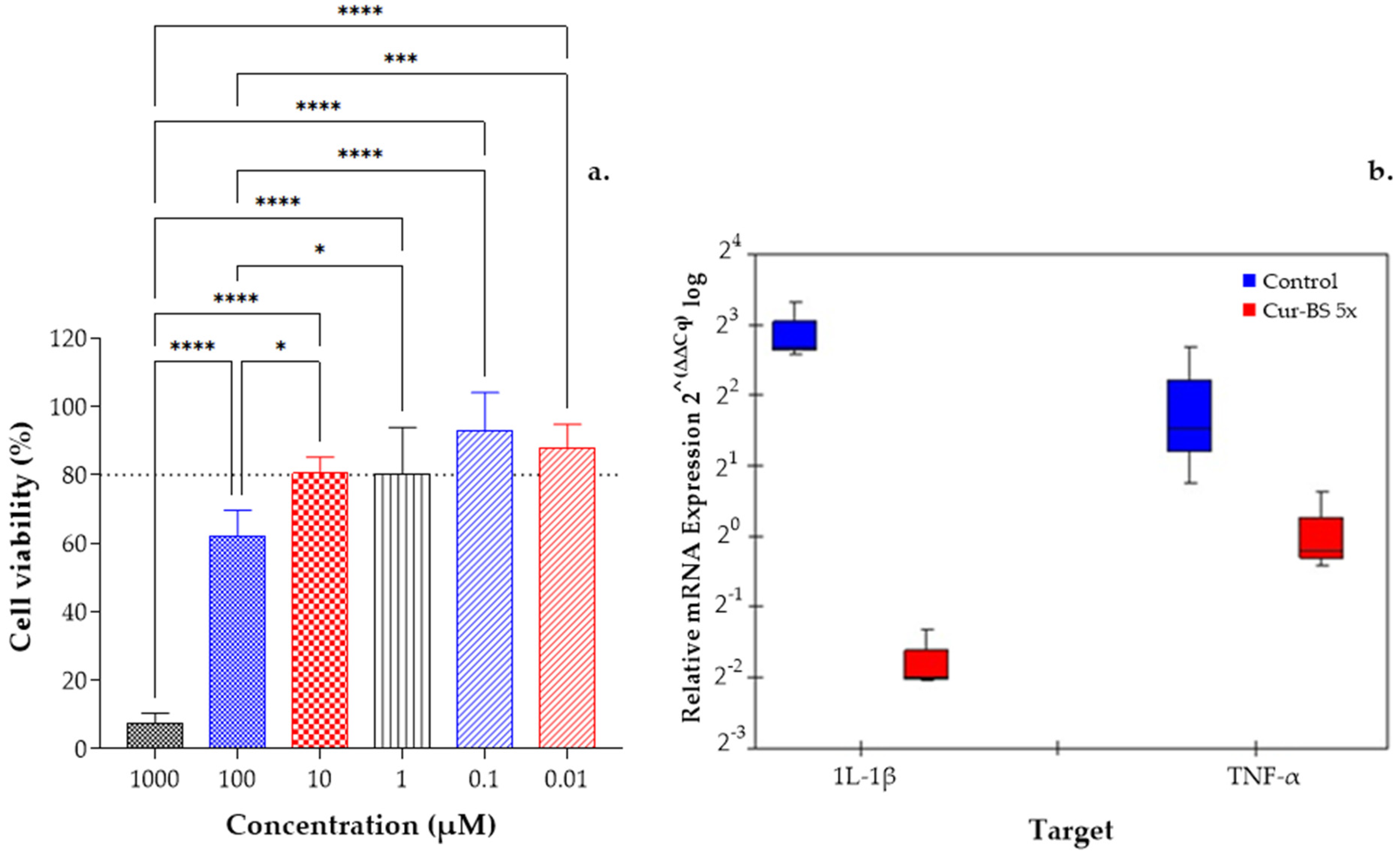
3.4. In Vitro Anti-Inflammatory Efficacy
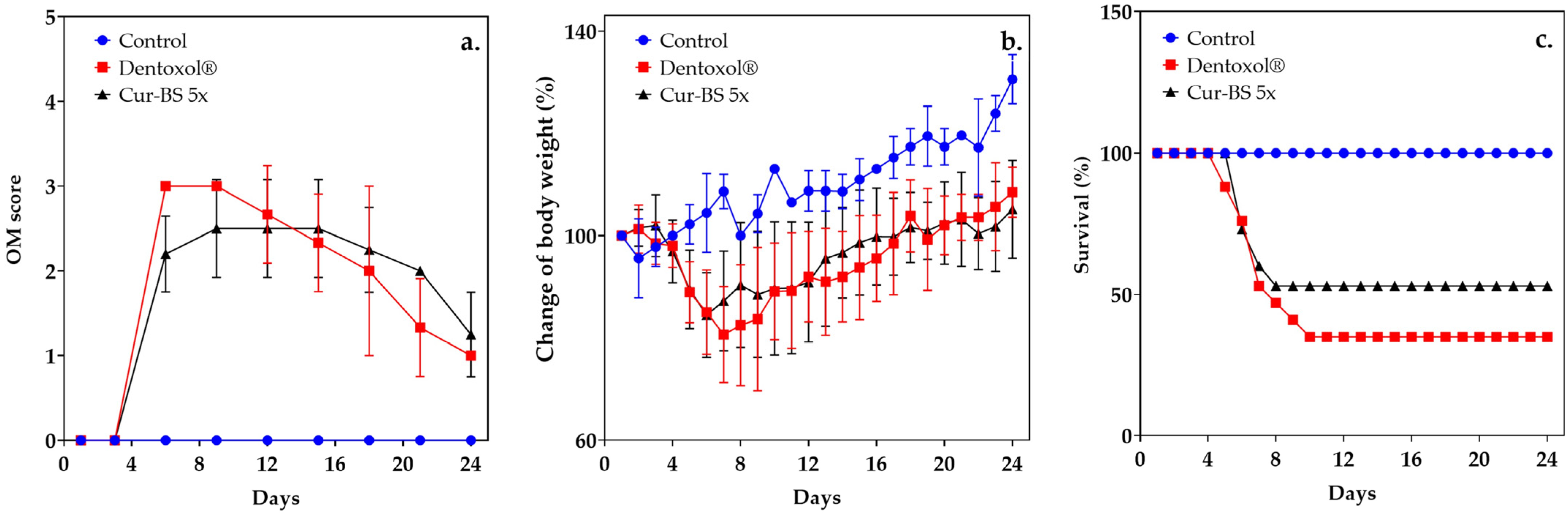
3.5. Assessment of Mucositis
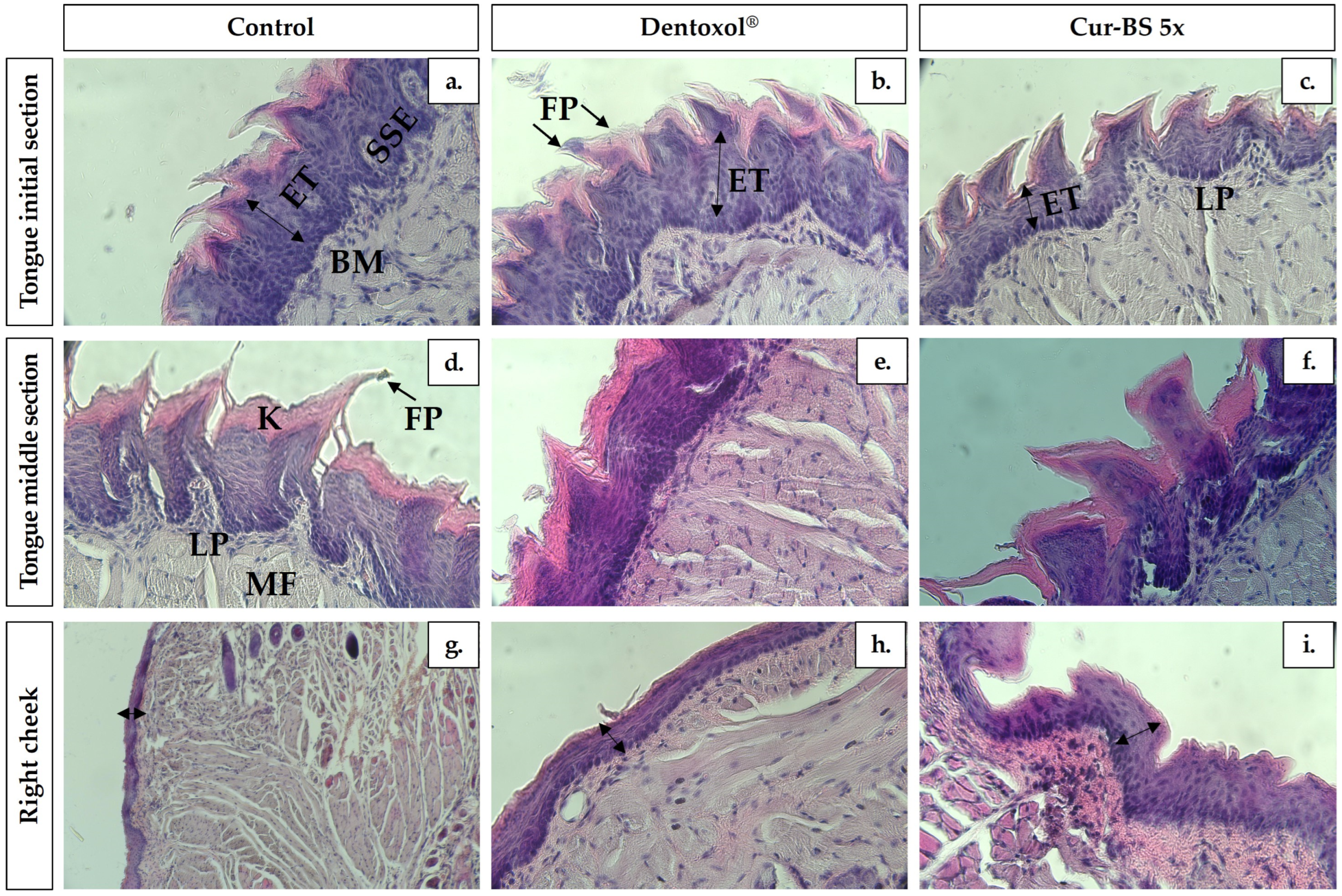
3.6. Histological Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hong, C.H.L.; Gueiros, L.A.; Fulton, J.S.; Cheng, K.K.F.; Kandwal, A.; Galiti, D.; Fall–Dickson, J.M.; Johansen, J.; Ameringer, S.; Kataoka, T.; et al. Systematic review of basic oral care for the management of oral mucositis in cancer patients and clinical practice guidelines. Support. Care Cancer 2019, 27, 3949–3967. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Goyal, S.; Kim, M.S.; Cabrales, P.; Lybeck, M.; Caroen, S.; Oronsky, N.; Burbano, E.; Carter, C.; Oronsky, A. A review of clinical radioprotection and chemoprotection for oral mucositis. Transl. Oncol. 2018, 11, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Xiong, Y.; Lu, B.; Wang, Y.; Zhang, D.; Feng, J.; Chen, L.; Zhang, Z. Application of in situ mucoadhesive hydrogel with anti-inflammatory and pro-repairing dual properties for the treatment of chemotherapy-induced oral mucositis. ACS Appl. Mater. Interfaces 2024, 16, 35949–35963. [Google Scholar] [CrossRef]
- Shah, N.M. Chapter thirteen—Oral mucositis as a target for antioxidant biomaterial therapy. In Oxidative Stress and Biomaterials; Academic Press: Cambridge, MA, USA, 2016; pp. 351–371. [Google Scholar]
- Ramezani, V.; Ghadirian, S.; Shabani, M.; Boroumand, M.A.; Daneshvar, R.; Saghafi, F. Efficacy of curcumin for amelioration of radiotherapy-induced oral mucositis: A preliminary randomized controlled clinical trial. BMC Cancer 2023, 23, 354. [Google Scholar] [CrossRef]
- Smith, M.M.; Knight, E.T.; Al–Harthi, L.; Leichter, J.W. Chronic periodontitis and implant dentistry. Periodontol. 2000 2017, 74, 63–73. [Google Scholar] [CrossRef]
- D’Aiuto, F.; Gable, D.; Syed, Z.; Allen, Y.; Wanyonyi, K.L.; White, S.; Gallagher, J.E. Evidence summary: The relationship between oral diseases and diabetes. Br. Dent. J. 2017, 222, 944–948. [Google Scholar] [CrossRef]
- De Sanctis, V.; Bossi, P.; Sanguineti, G.; Trippa, F.; Ferrari, D.; Bacigalupof, A.; Ripamonti, C.I.; Buglione, M.; Pergolizzii, S.; Langendjikj, J.A.; et al. Mucositis in head and neck cancer patients treated with radiotherapy and systemic therapies: Literature review and consensus statements. Crit. Rev. Oncol. Hematol. 2016, 100, 147–166. [Google Scholar] [CrossRef]
- Werner, H.; Hakeberg, M.; Dahlström, L.; Eriksson, M.; Sjögren, P.; Strandell, A.; Svanberg, T.; Svensson, L.; Wide Boman, U. Psychological interventions for poor oral health. J. Dent. Res. 2016, 95, 506–514. [Google Scholar] [CrossRef]
- Pulito, C.; Cristaudo, A.; Porta, C.L.; Zapperi, S.; Blandino, G.; Morrone, A.; Strano, S. Oral mucositis: The hidden side of cancer therapy. J. Exp. Clin. Cancer Res. 2020, 39, 210. [Google Scholar] [CrossRef]
- Elad, S.; Yarom, N.; Zadik, Y.; Kuten-Shorrer, M.; Sonis, S.T. The broadening scope of oral mucositis and oral ulcerative mucosal toxicities of anticancer therapies. Cancer J. Clin. 2022, 72, 57–77. [Google Scholar] [CrossRef] [PubMed]
- Konuk–Sener, D.K.; Aydin, M.; Cangur, S.; Guven, E. The effect of oral care with chlorhexidine, vitamin e and honey on mucositis in pediatric intensive care patients: A randomized controlled trial. J. Pediatr. Nurs. 2019, 45, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Chen, R.; Lin, G.; Ran, K.; Zhang, Y.; Yang, J.; Pan, H.; Shangguan, J.; Zhao, Y.; Xu, H. In situ mucoadhesive hydrogel capturing tripeptide kpv: The anti-inflammatory, antibacterial and repairing effect on chemotherapy-induced oral mucositis. Biomater. Sci. 2021, 10, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.C.; Cunha, J.D.; Ferreira, D.C.; Reis, S.; Costa, P.J. Challenges in the local delivery of peptides and proteins for oral mucositis management. Eur. J. Pharm. Biopharm. 2018, 128, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Sculley, D.; Wallace, J.; Turner, A.; Ferraris, C.; Veysey, M.; Lucock, M.; Beckett, E.L. Micronutrients and bioactive compounds in oral inflammatory diseases. J. Nutr. Intermed. Metab. 2019, 18, 100105. [Google Scholar] [CrossRef]
- Cuba, L.; Salum, F.; Cherubini, K.; Zancanaro, M.A. Antioxidant agents: A future alternative approach in the prevention and treatment of radiation–induced oral mucositis? Altern. Ther. Health Med. 2015, 21, 36–41. [Google Scholar]
- Moslemi, D.; Nokhandani, A.M.; Otaghsaraei, M.T.; Moghadamnia, Y.; Kazemi, S.; Moghadamnia, A.A. Management of chemo/radiation–induced oral mucositis in patients with head and neck cancer: A review of the current literature. Radiat. Oncol. 2016, 120, 13–20. [Google Scholar] [CrossRef]
- Kia, S.J.; Basirat, M.; Saedi, H.S.; Arab, S.A. Effects of nanomicelle curcumin capsules on prevention and treatment of oral mucosits in patients under chemotherapy with or without head and neck radiotherapy: A randomized clinical trial. BMC Complement. Med. Ther. 2021, 21, 232. [Google Scholar] [CrossRef]
- Soni, T.P.; Gupta, A.K.; Sharma, L.M.; Singhal, H.; Sharma, S.; Gothwal, R.S. A randomized, placebo-controlled study to evaluate the effect of bio-enhanced turmeric formulation on radiation-induced oral mucositis. J. Otorhinolaryngol. Relat. Spec. 2022, 84, 103–113. [Google Scholar] [CrossRef]
- Thomas, P.L.; Kang, H.K.; Rishi, K.S. Randomized control study of the effects of turmeric mouthwash on oral Health status, treatment-induced mucositis, and associated oral dysfunctions among patients with head and neck cancer. Cancer Nurs. 2023, 46, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Sivani, B.M.; Azzeh, M.; Patnaik, R.; Pantea Stoian, A.; Rizzo, M.; Banerjee, Y. Reconnoitering the therapeutic role of curcumin in disease prevention and treatment: Lessons learnt and future directions. Metabolites 2022, 12, 639. [Google Scholar] [CrossRef] [PubMed]
- Elad, S.; Meidan, I.; Sellam, G.; Simaan, S.; Zeevi, I.; Waldman, E.; Weintraub, M.; Revel-Vilk, S. Topical curcumin for the prevention of oral mucositis in pediatric patients: Case series. Altern. Ther. Health Med. 2013, 19, 21–24. [Google Scholar]
- Zhang, X.; Sun, D.; Qin, N.; Liu, M.; Zhang, J.; Li, J. Comparative prevention potential of 10 mouthwashes on intolerable oral mucositis in cancer patients: A Bayesian network analysis. Oral. Oncol. 2020, 107, 104751. [Google Scholar] [CrossRef] [PubMed]
- Panchatcharam, M.; Miriyala, S.; Gayathri, V.S.; Suguna, L. Curcumin improves wound healing by modulating collagen and decreasing reactive oxygen species. Mol. Cell. Biochem. 2006, 290, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the golden nutraceutical: Multitargeting for multiple chronic diseases. Br. J. Pharmacol. 2017, 174, 1325–1348. [Google Scholar] [CrossRef]
- Vergara, D.; López, O.; Sanhueza, C.; Chávez-Aravena, C.; Villagra, J.; Bustamante, M.; Acevedo, F. Co-Encapsulation of curcumin and α-tocopherol in bicosome systems: Physicochemical properties and biological activity. Pharmaceutics 2023, 15, 1912. [Google Scholar] [CrossRef] [PubMed]
- Fernández, E.; Hostachy, S.; Sandt, C.; Rodríguez, G.; Bertrand, H.C.; Clede, S.; Cócera, M.; de la Maza, A.; Lambert, F.; Policar, C.; et al. Monitoring bicosomes containing antioxidants innormal and irradiated skin. RSC Adv. 2016, 6, 72559–72567. [Google Scholar] [CrossRef]
- Moner, V.; Fernández, E.; Rodríguez, G.; Cócera, M.; Barbosa-Barros, L.; de la Maza, A.; López, O. Lamellar body mimetic system: An up-to-down repairing strategy of the stratum corneum lipid structure. Int. J. Pharm. 2016, 519, 135–143. [Google Scholar] [CrossRef]
- Liu, W.; Ye, A.; Liu, W.; Liu, C.; Singh, H. Stability during in vitro digestion of lactoferrin-loaded liposomes prepared from milk fat globule membrane-derived phospholipids. J. Dairy Sci. 2013, 96, 2061–2070. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Huang, T.; Li, J.; Li, A.; Li, C.; Huang, X.; Li, D.; Wang, S.; Liang, M. Optimization of differentiation and transcriptomic profile of THP-1 cells into macrophage by PMA. PLoS ONE 2023, 18, e0286056. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef]
- Adaileh, F.; Alshaer, W.; Nsairat, H.; Alqudah, D.A.; Wehaibi, S.; Daoud, F.; Al-Buqain, R.; Alsotari, S.; Al Bawab, A.; Odeh, F. Curcumin-loaded γ -cyclodextrin-grafted hyaluronic acid nanoassimblies: In vitro investigation of anti-proliferative, wound healing, and anti-inflammatory potential. J. Drug Deliv. Sci. Technol. 2023, 87, 104886. [Google Scholar] [CrossRef]
- Sonis, S.T.; Peterson, R.L.; Edwards, L.J.; Lucey, C.A.; Wang, L.; Mason, L.; Login, G.; Ymamkawa, M.; Moses, G.; Bouchard, P.; et al. Defining mechanisms of action of interleukin-11 on the progression of radiation-induced oral mucositis in hamsters. Oral. Oncol. 2000, 36, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.I.; Celentano, A.; Paolini, R.; Low, J.T.; McCullough, M.J.; O’ Reilly, L.A.; Cirillo, N. Characterization of a novel dual murine model of chemotherapy-induced oral and intestinal mucositis. Sci. Rep. 2023, 13, 1396. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, G.; Soria, G.; Coll, E.; Rubio, L.; Barbosa-Barros, L.; López-Iglesias, C.; Planas, A.; De la Maza, A.; López, O. Bicosomes: Bicelles in Dilute Systems. Biophys. J. 2020, 99, 480–488. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Putri, D.C.; Dwiastuti, R.; Marchaban, M.; Nugroho, A.K. Optimization of mixing temperature and sonication duration in liposome preparation. J. Pharm. Sci. Commun. 2017, 14, 79–85. [Google Scholar] [CrossRef]
- Bahari, L.A.; Hamishehkar, H. The impact of variables on particle size of solid lipid nanoparticles and nanostructured lipid carriers; a comparative literature review. Adv. Pharm. Bull. 2016, 6, 143. [Google Scholar] [CrossRef]
- Gravina, C.; Piccolella, S.; Alonso, C.; Martí, M.; Formato, M.; Pacifico, S.; Coderch, L.; Esposito, A. Encapsulation of Lavandula austroapennina N.G. Passal., Tundis & Upson extracts: Focus on leaf and stem enriched liposome for cosmeceutical innovation. Ind. Crops Prod. 2024, 213, 118362. [Google Scholar]
- Moner, V.; Fernández, E.; Calpena, A.C.; Garcia-Herrera, A.; Cócera, M.; López, O. A lamellar body mimetic system for the treatment of oxazolone-induced atopic dermatitis in hairless mice. J. Dermatol. Sci. 2018, 90, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, C.T.; Alemán, A.; Zapata, J.E.; Montero, M.P.; Gómez-Guillén, M.C. Characterization and storage stability of spray dried soy-rapeseed lecithin/trehalose liposomes loaded with a tilapia viscera hydrolysate. Innov. Food Sci. Emerg. Technol. 2021, 71, 102708. [Google Scholar] [CrossRef]
- Laomeephol, C.; Ferreira, H.; Kanokpanont, S.; Neves, N.M.; Kobayashi, H.; Damrongsakkul, S. Dual-functional liposomes for curcumin delivery and accelerating silk fibroin hydrogel formation. Int. J. Pharm. 2020, 589, 119844. [Google Scholar] [CrossRef]
- Chen, W.T.; Wu, H.T.; Chang, I.C.; Chen, H.W.; Fang, W.P. Preparation of curcumin-loaded liposome with high bioavailability by a novel method of high pressure processing. Chem. Phys. Lipids. 2022, 244, 105191. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Jain, S.K. In vitro release kinetics model fitting of liposomes: An insight. Chem. Phys. Lipids. 2016, 201, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Tai, K.; Rappolt, M.; He, X.; Wei, Y.; Zhu, S.; Zhang, J.; Mao, L.; Gao, X.; Yuan, F. Effect of β-sitosterol on the curcumin-loaded liposomes: Vesicle characteristics, physicochemical stability, in vitro release and bioavailability. Food Chem. 2019, 293, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.L.; Peng, S.F.; Chen, X.; Zhu, Y.Q.; Zou, L.Q.; Liu, W.; Liu, C.M. Pluronics modified liposomes for curcumin encapsulation: Sustained release, stability and bioaccessibility. Food Res. Int. 2018, 108, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, M.L. (Ed.) 5—Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodhead Publishing: Cambridge, UK, 2015. [Google Scholar]
- Hussain, A.; Altamimi, M.A.; Saleh Alneef, Y. HSPiP and QbD oriented optimized stearylamine-elastic liposomes for topical delivery of ketoconazole to treat deep seated fungal infections: In vitro and ex vivo evaluations. Int. J. Pharm-X 2024, 100279. [Google Scholar] [CrossRef] [PubMed]
- Valle, M.J.J.; Rondon, A.M.; Zarzuelo, A.; Coutinho, P.; de Abreu, A.C.; Sánchez, A. Resveratrol liposomes in buccal formulations, an approach to overcome drawbacks limiting the application of the phytoactive molecule for chemoprevention and treatment of oral cancer. J. Drug Deliv. Sci. Technol. 2024, 98, 105910. [Google Scholar] [CrossRef]
- Tran, N.P.; Tran, P.; Yoo, S.Y.; Tangchang, W.; Lee, S.; Lee, J.Y.; Son, H.Y.; Park, J.S. Sialic acid-decorated liposomes enhance the anti-cancer efficacy of docetaxel in tumor-associated macrophages. Biomater. Adv. 2023, 154, 213606. [Google Scholar] [CrossRef] [PubMed]
- Pelikh, O.; Keck, C.M. Hair follicle targeting and dermal drug delivery with curcumin drug nanocrystals—Essential influence of excipients. Nanomaterials 2020, 10, 2323. [Google Scholar] [CrossRef] [PubMed]
- Monteiro-Riviere, N.A.; Larese Filon, F. Adverse Effects of Engineered Nanomaterials, 2nd ed.; Fadeel, B., Pietroiusti, A., Shvedova, A.A., Eds.; Chapter 15—Skin; Academic Press: Cambridge, MA, USA, 2017; pp. 357–380. ISBN 9780128091999. [Google Scholar]
- Souto, E.B.; Fangueiro, J.F.; Fernandes, A.R.; Cano, A.; Sanchez-Lopez, E.; Garcia, M.L.; Severino, P.; Paganelli, M.O.; Chaud, M.V.; Silva, A.M. Physicochemical and biopharmaceutical aspects influencing skin permeation and role of SLN and NLC for skin drug delivery. Heliyon 2022, 11, e08938. [Google Scholar] [CrossRef]
- Abla, M.J.; Banga, A.K. Quantification of skin penetration of antioxidants of varying lipophilicity. Int. J. Cosmet. Sci. 2013, 35, 19–26. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Q.; Zhang, Z.; Yuan, L.; Liu, X.; Zhou, L. Preparation of curcumin-loaded liposomes and evaluation of their skin permeation and pharmacodynamics. Molecules 2012, 17, 5972–5987. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Zhang, J.; Zhang, J. Enhancing Transdermal delivery of curcumin-based ionic liquid liposomes for application in psoriasis. ACS Appl. Bio Mater. 2023, 6, 5864–5873. [Google Scholar] [CrossRef]
- Tang, Q.; Dong, M.; Xu, Z.; Xue, N.; Jiang, R.; Wei, X.; Gu, J.; Li, Y.; Xin, R.; Wang, J.; et al. Red blood cell-mimicking liposomes loading curcumin promote diabetic wound healing. J. Ophthalmol. Clin. Res. 2023, 361, 871–884. [Google Scholar] [CrossRef]
- Huang, S.; Xu, D.; Zhang, L.; Hao, L.; Jia, Y.; Zhang, X.; Cheng, T.; Chen, J. Therapeutic effects of curcumin liposomes and nanocrystals on inflammatory osteolysis: In vitro and in vivo comparative study. Pharmacol. Res. 2023, 192, 106778. [Google Scholar] [CrossRef] [PubMed]
- Vaezi, H.; Rabbani, S.; Mortazavi, S.A.; Kamalinejad, M.; Haeri, A. Fabrication, in vitro, and in vivo characterization of mucoadhesive berberine-loaded blended wafers for treatment of chemotherapy-induced oral mucositis. AAPS PharmSciTech 2023, 24, 19. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.S.; Abueva, C.; Padalhin, A.; Park, S.Y.; Yoo, S.H.; Seo, H.H.; Chung, P.S.; Woo, S.H. Oral ulcer treatment using human tonsil-derived mesenchymal stem cells encapsulated in trimethyl chitosan hydrogel: An animal model study. Stem Cell Res. Ther. 2024, 8, 15–103. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, Y.; Takeuchi, I.; Terada, H.; Makino, K. A Mouse Model for Oral Mucositis Induced by Cancer Chemotherapy. Anticancer Res. 2018, 38, 307–312. [Google Scholar]
- Dipalma, G.; Inchingolo, A.M.; Latini, G.; Ferrante, L.; Nardelli, P.; Malcangi, G.; Trilli, I.; Inchingolo, F.; Palermo, A.; Inchingolo, A.D. The effectiveness of curcumin in treating oral mucositis related to radiation and chemotherapy: A systematic review. Antioxidants 2024, 13, 1160. [Google Scholar] [CrossRef] [PubMed]
- Krilov, D.; Kosović, M.; Serec, K. Spectroscopic studies of alpha tocopherol interaction with a model liposome and its influence on oxidation dynamics. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 129, 588–593. [Google Scholar] [CrossRef]
- Berardesca, E.; Cameli, N. Vitamin E supplementation in inflammatory skin diseases. Dermatol. Ther. 2021, 34, e15160. [Google Scholar] [CrossRef] [PubMed]
- Praça, F.G.; Viegas, J.S.R.; Peh, H.Y.; Garbinc, T.N.; Medina, W.S.G.; Bentley, M.V.L.B. Microemulsion co-delivering vitamin A and vitamin E as a new platform for topical treatment of acute skin inflammation. Mater. Sci. Eng. C 2020, 110, 110639. [Google Scholar] [CrossRef] [PubMed]
- Horikoshi, Y.; Kamizaki, K.; Hanaki, T.; Morimoto, M.; Kitagawa, Y.; Nakaso, K.; Kusumoto, C.; Matsura, T. α-tocoferol promotes HaCaT keratinocytewound repair through the regulation of polarityproteins leading to the polarized cell migration. Biofactors 2018, 44, 180–191. [Google Scholar] [CrossRef] [PubMed]
| Bicosome System | DHPC/DPPC Relación Molar (q) | Cur (μM) | α-Toc (μM) | Lipoid P-100/Chol Ratio | Total Bicelle Lipid Concentration (%w/v) | Total Liposome Lipid Concentration (%w/v) | Total BS Lipid Concentration (%w/v) |
|---|---|---|---|---|---|---|---|
| Cur-BS 1× | 3.5:1 | 180 | 600 | 8:2 | 6 | 14 | 20 |
| Cur-BS 3× | 540 | ||||||
| Cur-BS 5× | 900 |
| Gene Target | Sequences | |
|---|---|---|
| Forward 5′-3′ | Reverse 5′-3′ | |
| GAPDH | TGGGGAAGGTGAAGGTCGGA | GGGATCTCGCTGCTGGAAGA |
| TNF-α | CTCTTCTGCCTGCTGCACTTTG | ATGGGCTACAGGCTTGTCACTC |
| IL-1β | CCACAGACCTTCCAGGAGAATG | GTGCAGTTCAGTGATCGTACAGG |
| Cur-Bicelle | Particle Size (nm) | Volume (%) | Polydispersity Index (PDI) |
|---|---|---|---|
| System 1× | 15.2 ± 0.2 a | 99.8 ± 0.06 a | 0.22 ± 0.02 b |
| System 3× | 15.1 ± 0.1 a | 99.6 ± 0.06 b | 0.34 ± 0.01 a |
| System 5× | 16.2 ± 0.2 b | 99.9 ± 0.06 a | 0.18 ± 0.01 c |
| Bicosome Systems | Peak 1 | Peak 2 | EE Cur (%) | ||
|---|---|---|---|---|---|
| Particle Size (nm) | Volume (%) | Particle Size (nm) | Volume (%) | ||
| Cur-BS 1× | 383 ± 7 a | 40 ± 1 b | 41 ± 1 a | 60 ± 1 a | 36 ± 2.6 c |
| Cur-BS 3× | 376 ± 22 a | 42 ± 7 a | 46 ± 10 a | 58 ± 7 a | 56 ± 0.4 b |
| Cur-BS 5× | 390 ± 3 a | 52 ± 2 a | 52 ± 1 a | 48 ± 1 a | 70 ± 4.1 a |
| Peppas–Sahlin Model | Higuchi Model | Weibull Model | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R2 | K1 | K2 | m | R2 | Kh | R2 | α | β | Ti | |
| Cur-BS x5 | 0.95 | 16.66 | −0.34 | 0.45 | 0.94 | 13.13 | 0.94 | 12.28 | 0.84 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vergara, D.; Sanhueza, C.; Méndez, S.; Bustamante, M.; Vega, B.; Acevedo, F.; López, O. Evaluation of Preclinical Efficacy of Curcumin-Loaded Bicosome Systems in Amelioration of Oral Mucositis. Pharmaceutics 2025, 17, 181. https://doi.org/10.3390/pharmaceutics17020181
Vergara D, Sanhueza C, Méndez S, Bustamante M, Vega B, Acevedo F, López O. Evaluation of Preclinical Efficacy of Curcumin-Loaded Bicosome Systems in Amelioration of Oral Mucositis. Pharmaceutics. 2025; 17(2):181. https://doi.org/10.3390/pharmaceutics17020181
Chicago/Turabian StyleVergara, Daniela, Claudia Sanhueza, Susana Méndez, Mariela Bustamante, Benjamín Vega, Francisca Acevedo, and Olga López. 2025. "Evaluation of Preclinical Efficacy of Curcumin-Loaded Bicosome Systems in Amelioration of Oral Mucositis" Pharmaceutics 17, no. 2: 181. https://doi.org/10.3390/pharmaceutics17020181
APA StyleVergara, D., Sanhueza, C., Méndez, S., Bustamante, M., Vega, B., Acevedo, F., & López, O. (2025). Evaluation of Preclinical Efficacy of Curcumin-Loaded Bicosome Systems in Amelioration of Oral Mucositis. Pharmaceutics, 17(2), 181. https://doi.org/10.3390/pharmaceutics17020181








