Physiologically Based Pharmacokinetic Model of CYP2D6 Associated Interaction Between Venlafaxine and Strong Inhibitor Bupropion—The Influence of Age-Relevant Changes and Inhibitory Dose to Classify Therapeutical Success and Harm
Abstract
1. Introduction
1.1. Venlafaxine
1.2. Bupropion
2. Materials and Methods
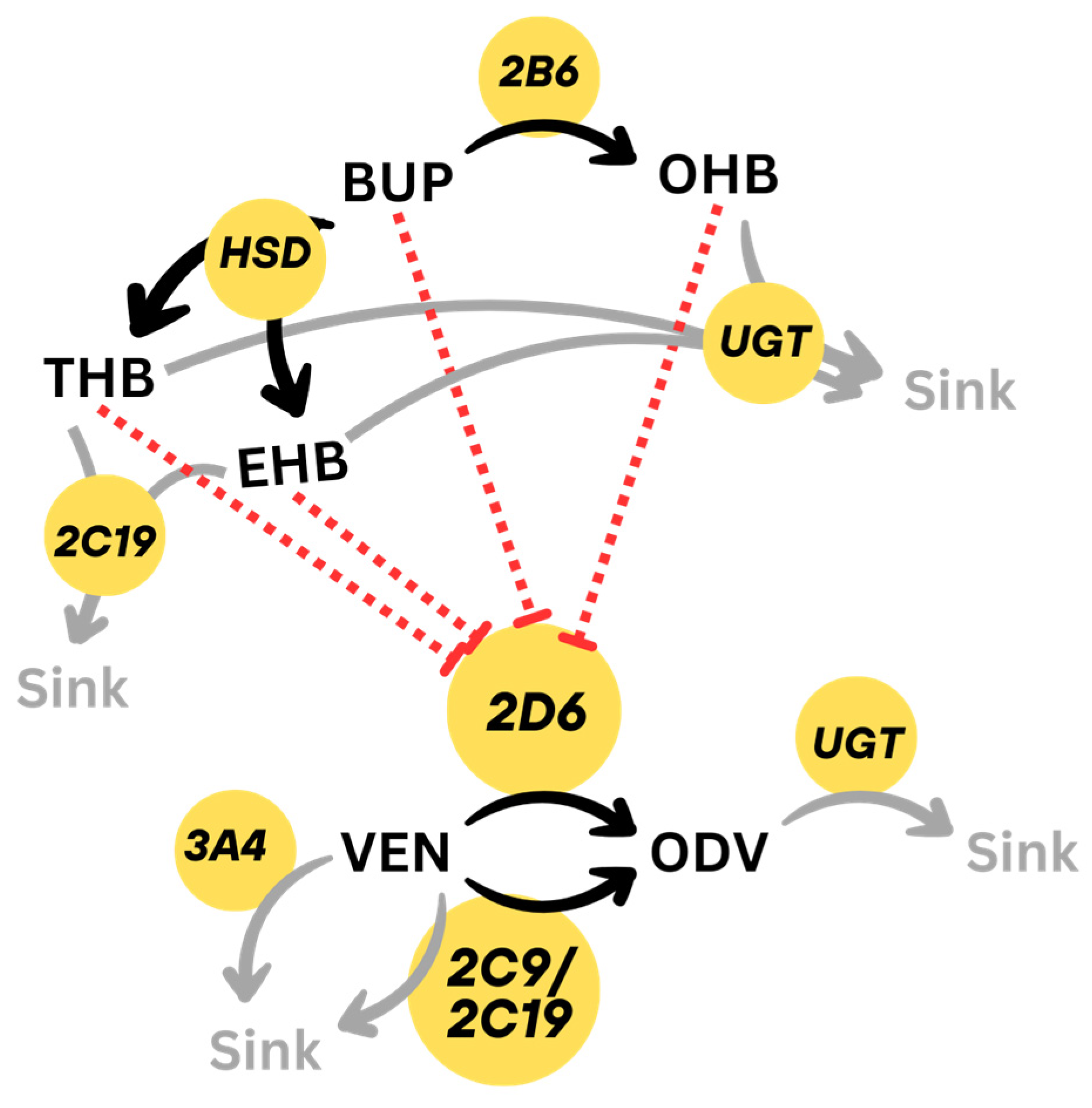
2.1. Physiologically Based Pharmacokinetics (PBPK) Modeling
2.1.1. General Workflow
2.1.2. DDI Implementation
2.2. Development of Literature-Based PBPK Model
| Parameter | VEN | ODV | Metabolism Parameter VEN → ODV | |||
|---|---|---|---|---|---|---|
| Mr [g/mol] | 277.4 [34] | 263.38 [35] | CYP | Vmax | KM | kcat |
| logP | 2.62 [2.8 [34], 2.69, 2.74 [6]] | 2.45 [2.62 [35], 2.6, 2.29 [36]] | 2D6 n.d. 2D6 EM 2D6 PM | 6.48 [7] | 23.2 [7] | 29.16 * 64.8 * 0 * |
| pKa | 9.4 [34] | 8.86 [35], 10.25 [35] | 2C9 | 0.58 [7] | 3119 [7] | 2.61 * |
| Solubility | 572 [6] | - | 2C19 | 3.78 [7] | 293 [7] | 17.01 * |
| fu [%] | 73 [6] | 70 [6] | VEN → Sink | |||
| Part. Coef. | Berezhkovskiy [37] | Berezhkovskiy [37] | CYP | Vmax | KM | kcat |
| Cell. Perm. | PK-Sim Standard | PK-Sim Standard | 3A4 | 1.23 [7] | 556 [7] | 5.54 * |
| Tdiss | 338 * | 2C9 | 10.33 [7] | 2250 [7] | 46.49 * | |
| Tlag | 81.2 * | 2C19 | 7.56 [7] | 398 [7] | 34.02 * | |
| Diss. Sh. | 0.92 * | ODV → Sink | ||||
| Clrenal | 0.05 ± 0.02 [38] | 0.12 ± 0.03 [39] | Clint [min−1] | |||
| CYP2D6 Ki | 41 ± 9.5 [40] | 40 [41] | UGT1A1 | 0.1 * | ||
| parameter | BUP | OHB | EHB | THB | ||
| Mr [g/mol] | 239.74 [36] | 255.74 [36] | 241.76 [42] | |||
| logP | 3.5 [3.6 [36], 3.2 [43], 3.5 [44]] | 1.98 [2.6 [44], 1.98 [36], 2.03 [42]] | 2.69 [2.88 [42], 2.69 [45]] | |||
| pKa | 7.9 [7.9 [46], 8.0 [43], 7.2 [44]] | 7.7 [44] | 9.6 [42] | |||
| Solubility | 312 [47] | - | - | |||
| fu [%] | 16 [14] | 23 [14] | 58 [14] | |||
| Part. Coef. | Schmitt [48] | Rodgers + Rowland [49,50] | Berezhkovskiy [37] | |||
| Cell. Perm. | PK-Sim Standard | PK-Sim Standard | Charge-dependent Schmitt | |||
| Tdiss | 170 * | |||||
| Diss. Sh. | 1.52 * | |||||
| Clrenal | 0.17 (0.12–0.21) [27] | 0.02 (0.02–0.03) [27] | 0.50 (0.39–0.61) [27] | 0.36 (0.28–0.45) [27] | ||
| CYP2D6 Ki | 0.46 * [21 [14]] | 0.41 [13.3 [14]] | 0.15 [5.4 [14]] | 0.04 [1.7 [14]] | ||
| CYP2B6 BUP → OHB | UGT2B7 OHB → Sink | CYP2C19 THB → Sink | CYP2C19 EHB → Sk. | |||
| Vmax | 3623 ± 1520 [51] † | 5550 ± 507 [12] † | 0.55 [13] | 0.40 [13] | ||
| KM | 89 ± 14 [51] | 488 ± 98.3 [12] | 13.0 [13] | 39.0 [13] | ||
| kcat | 92.9 * | 33.81 * | 1.10 * | 0.80 * | ||
| HSD11ß1 BUP → THB | UGT2B7 OHB → Sk. | UGT1A9 THB → Sink | UGT2B7 EHB → Sk. | |||
| Vmax | 11,800 ± 265 [52] † | 739 ± 59.6 [12] † | 3290 ± 269 [12] † | 2280 ± 241 [12] † | ||
| KM | 42.2 ± 3.05 [52] | 172 ± 38.9 [12] | 343 ± 37.5 [12] | 360 ± 55.2 [12] | ||
| kcat | 102.7 * | 5.27 * | 38.1 * | 52.8 * | ||
| HSD11ß1 BUP → EHB | UGT2B7 THB → Sink | UGT2B7 EHB → Sk. | ||||
| Vmax | 661 ± 54 [52] † | 358 ± 25.5 [12] † | 1740 ± 212 [12] † | |||
| KM | 66.5 ± 19.9 [52] | 248 ± 27.1 [12] | 373 ± 63.0 [12] | |||
| kcat | 38.46 * | 4.14 * | 40.3 * | |||
2.3. Development of DDI Model
2.3.1. DDI Model Evaluation
2.3.2. Excursus: Multiple Interaction of VEN, BUP and Itraconazole (ITRA)
2.4. SCHOLZ Databank’s MDDI Calculator
3. Results
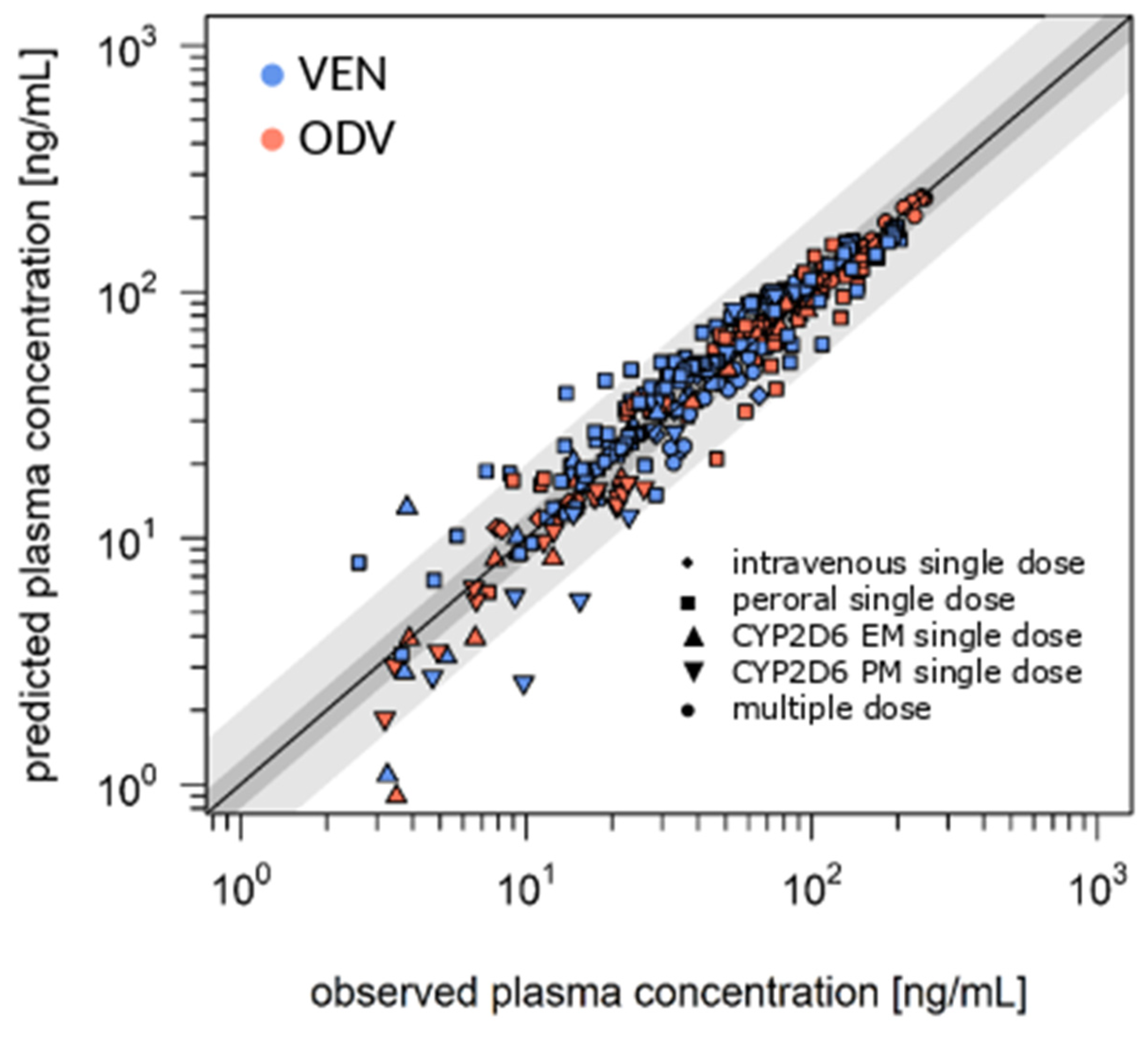
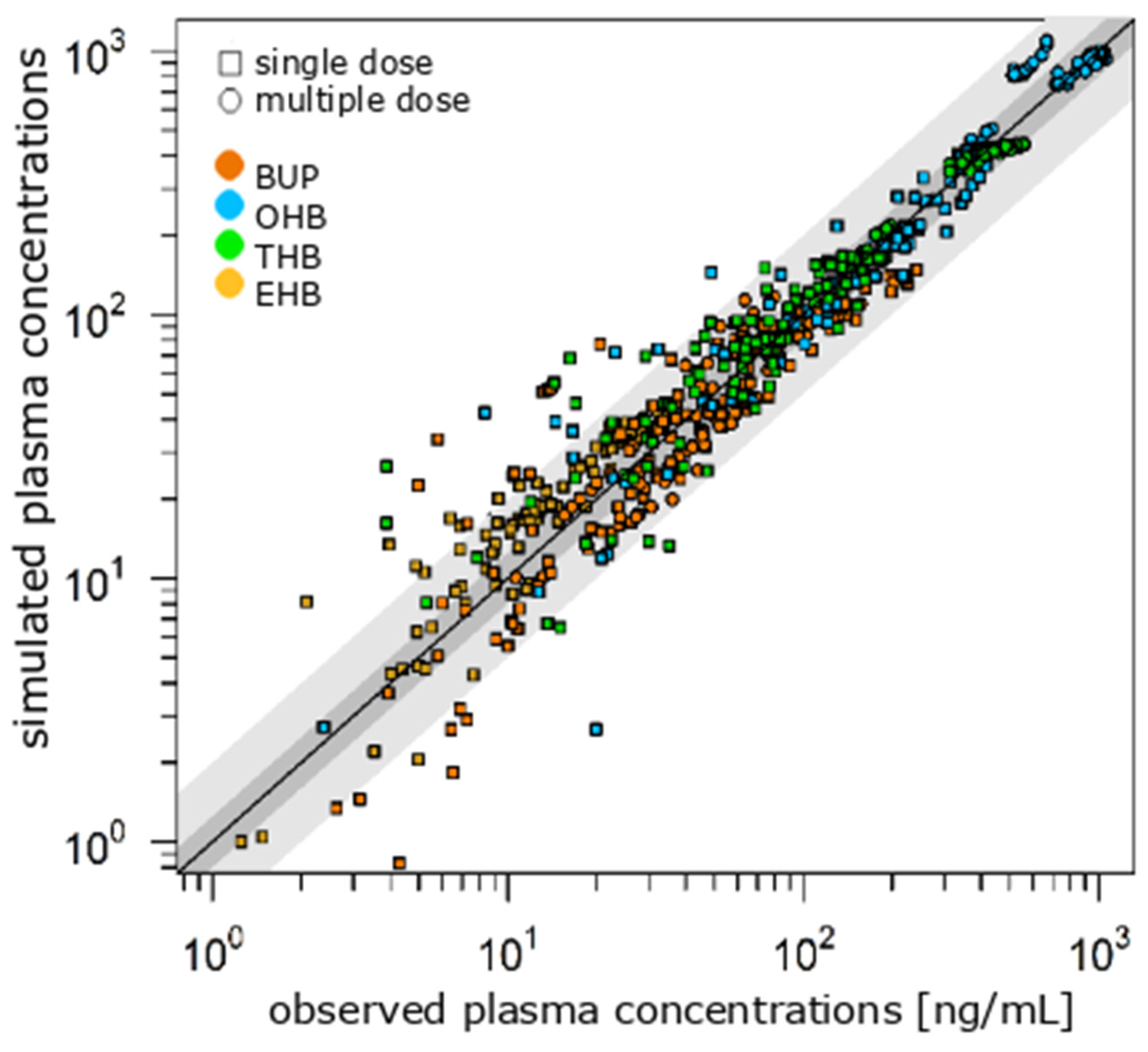
3.1. Literature-Based Model of VEN and BUP
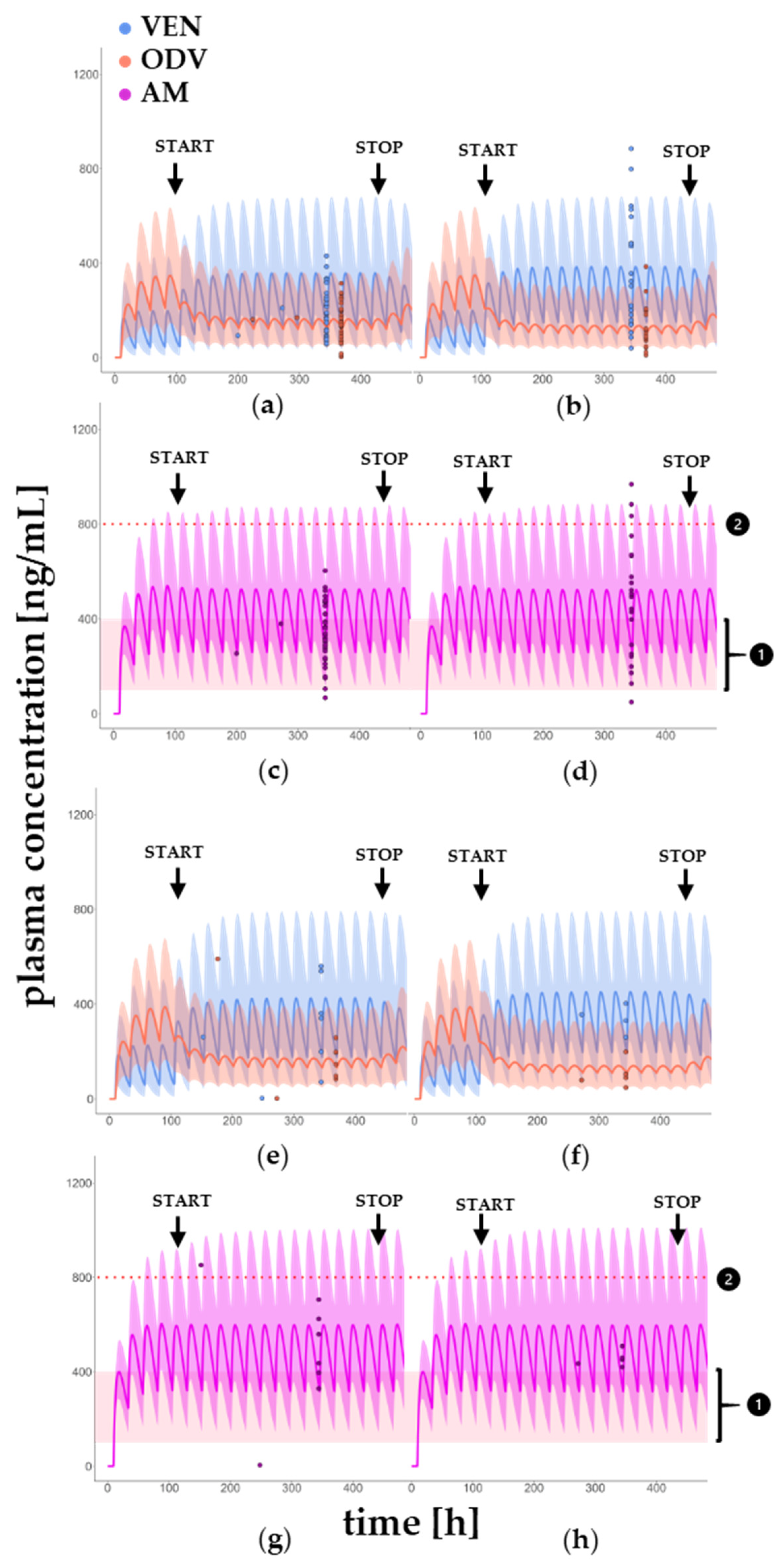
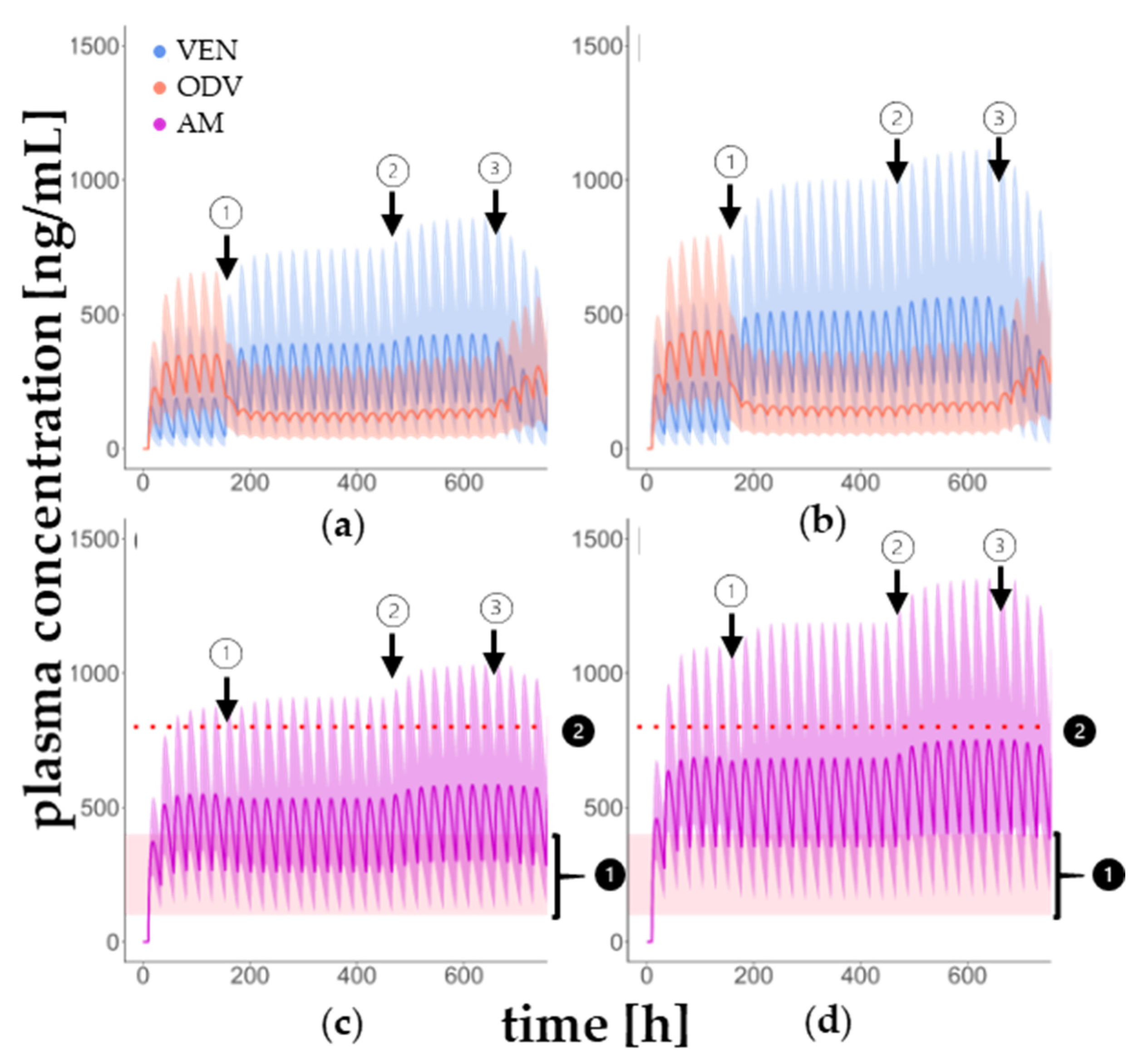
3.2. DDI Model in Younger and Older Patients
3.3. Multiple Drug–Drug Interaction of VEN, BUP and ITRA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Read, J.R.; Sharpe, L.; Modini, M.; Dear, B.F. Multimorbidity and depression: A systematic review and meta-analysis. J. Affect. Disord. 2017, 221, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J.; Hefner, G.; Normann, C.; Kaier, K.; Binder, H.; Hiemke, C.; Toto, S.; Domschke, K.; Marschollek, M.; Klimke, A. Polypharmacy and the risk of drug-drug interactions and potentially inappropriate medications in hospital psychiatry. Pharmacoepidemiol. Drug Saf. 2021, 30, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Reimche, L.; Forster, A.J.; van Walraven, C. Incidence and contributors to potential drug-drug interactions in hospitalized patients. J. Clin. Pharmacol. 2011, 51, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Guideline on the Investigation of Drug Interactions [Online]. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-investigation-drug-interactions-revision-1_en.pdf (accessed on 22 April 2024).
- Entsuah, R.; Chitra, R. A benefit-risk analysis of once-daily venlafaxine extended release (XR) and venlafaxine immediate release (IR) in outpatients with major depression. Psychopharmacol. Bull. 1997, 33, 671–676. [Google Scholar]
- Wyeth Pharmaceuticals Inc. Effexor(R) XR (Venlafaxine Hydrochloride) Extended-Release Capsules, U.S. Prescribing Information [Online]. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/020699s059lbl.pdf (accessed on 31 December 2024).
- Fogelman, S.M.; Schmider, J.; Venkatakrishnan, K.; von Moltke, L.L.; Harmatz, J.S.; Shader, R.I.; Greenblatt, D.J. O- and N-demethylation of venlafaxine in vitro by human liver microsomes and by microsomes from cDNA-transfected cells: Effect of metabolic inhibitors and SSRI antidepressants. Neuropsychopharmacology 1999, 20, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, P.; Alves, G.; Llerena, A.; Falcão, A. Venlafaxine pharmacokinetics focused on drug metabolism and potential biomarkers. Drug Metab. Drug Interact. 2014, 29, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Hiemke, C.; Bergemann, N.; Clement, H.W.; Conca, A.; Deckert, J.; Domschke, K.; Eckermann, G.; Egberts, K.; Gerlach, M.; Greiner, C.; et al. Consensus Guidelines for Therapeutic Drug Monitoring in Neuropsychopharmacology: Update 2017. Pharmacopsychiatry 2018, 51, 9–62. [Google Scholar]
- GlaxoSmithKline. Wellbutrin XL (Bupropion Hydrochloride Extended-Release Tablets): Prescribing Information [Online]. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/021515s023s024lbl.pdf (accessed on 3 June 2024).
- Coles, R.; Kharasch, E.D. Stereoselective metabolism of bupropion by cytochrome P4502B6 (CYP2B6) and human liver microsomes. Pharm. Res. 2008, 25, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Gufford, B.T.; Lu, J.B.L.; Metzger, I.F.; Jones, D.R.; Desta, Z. Stereoselective Glucuronidation of Bupropion Metabolites In Vitro and In Vivo. Drug Metab. Dispos. 2016, 44, 544–553. [Google Scholar] [CrossRef]
- Sager, J.E.; Price, L.S.L.; Isoherranen, N. Stereoselective Metabolism of Bupropion to OH-bupropion, Threohydrobupropion, Erythrohydrobupropion, and 4′-OH-bupropion in vitro. Drug Metab. Dispos. 2016, 44, 1709–1719. [Google Scholar] [CrossRef] [PubMed]
- Reese, M.J.; Wurm, R.M.; Muir, K.T.; Generaux, G.T.; St John-Williams, L.; Mcconn, D.J. An in vitro mechanistic study to elucidate the desipramine/bupropion clinical drug-drug interaction. Drug Metab. Dispos. 2008, 36, 1198–1201. [Google Scholar] [CrossRef]
- Open System Pharmacology Suite. Open Systems Pharmacology Manual [Online]. Available online: https://docs.open-systems-pharmacology.org/ (accessed on 22 April 2024).
- Food Drug Administration, U.S. Department of Health and Human Services. Clinical Drug Interaction Studies–Cytochrome P450 Enzyme- and Transporter-Mediated Drug Interactions: Guidance for Industry [Online]. Available online: https://downloads.regulations.gov/FDA-2017-D-5961-0002/attachment_1.pdf (accessed on 19 December 2024).
- Patat, A.; Troy, S.; Burke, J.; Trocherie, S.; Danjou, P.; Le Coz, F.; Allain, H.; Gandon, J.M. Absolute bioavailability and electroencephalographic effects of conventional and extended-release formulations of venlafaxine in healthy subjects. J. Clin. Pharmacol. 1998, 38, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Nichols, A.I.; Behrle, J.A.; Richards, L.S.; Parker, V.D.; Posener, J.A.; Fruncillo, R.; Paul, J. The Absolute Bioavailability of Desvenlafaxine in Healthy Subjects. J. Bioequiv. Availab. 2012, 4, 2. [Google Scholar] [CrossRef]
- Troy, S.M.; Dilea, C.; Martin, P.T.; Leister, C.A.; Fruncillo, R.J.; Chiang, S.T. Pharmacokinetics of once-daily venlafaxine extended release in healthy volunteers. Curr. Ther. Res. 1997, 58, 504–514. [Google Scholar] [CrossRef]
- Troy, S.M.; Dilea, C.; Martin, P.T.; Rosen, A.S.; Fruncillo, R.J.; Chiang, S.T. Bioavailability of once-daily venlafaxine extended releasecompared with the immediate-release formulation in healthy adult volunteers. Curr. Ther. Res. 1997, 58, 492–503. [Google Scholar] [CrossRef]
- Wright, C.W.; Aikman, M.S.; Werts, E.; Seabolt, J.; Haeusler, J.-M.C. Bioequivalence of single and multiple doses of venlafaxine extended-release tablets and capsules in the fasted and fed states: Four open-label, randomized crossover trials in healthy volunteers. Clin. Ther. 2009, 31, 2722–2734. [Google Scholar] [CrossRef]
- Preskorn, S.; Patroneva, A.; Silman, H.; Jiang, Q.; Isler, J.A.; Burczynski, M.E.; Ahmed, S.; Paul, J.; Nichols, A.I. Comparison of the pharmacokinetics of venlafaxine extended release and desvenlafaxine in extensive and poor cytochrome P450 2D6 metabolizers. J. Clin. Psychopharmacol. 2009, 29, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Nichols, A.I.; Focht, K.; Jiang, Q.; Preskorn, S.H.; Kane, C.P. Pharmacokinetics of venlafaxine extended release 75 mg and desvenlafaxine 50 mg in healthy CYP2D6 extensive and poor metabolizers: A randomized, open-label, two-period, parallel group, crossover study. Clin. Drug Investig. 2011, 31, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Oberegger, W.; Eradiri, O.; Zhou, F.; Maes, P. Modified-Release Tablet of Bupropion Hydrochloride. U.S. Patent US007537784B2, 13 June 2006. [Google Scholar]
- Woodcock, J.; Khan, M.; Yu, L.X. Withdrawal of generic budeprion for nonbioequivalence. N. Engl. J. Med. 2012, 367, 2463–2465. [Google Scholar] [CrossRef] [PubMed]
- Paiement, N.; Noonan, P.K.; Gonzalez, M.A.; Zerbe, H. Steady State Plasma Levels of Bupropion After Administration of 3 × 150 Mg Extended Release Reference Tablets and Switching to 1 × 450 Mg Extended Release 450ER Tablets. Int. J. Clin. Pharmacol. Toxicol. 2012, 1, 26–31. [Google Scholar] [CrossRef]
- Benowitz, N.L.; Zhu, A.Z.X.; Tyndale, R.F.; Dempsey, D.; Jacob, P. Influence of CYP2B6 genetic variants on plasma and urine concentrations of bupropion and metabolites at steady state. Pharmacogenet. Genom. 2013, 23, 135–141. [Google Scholar] [CrossRef]
- Schmid, Y.; Rickli, A.; Schaffner, A.; Duthaler, U.; Grouzmann, E.; Hysek, C.M.; Liechti, M.E. Interactions between bupropion and 3,4-methylenedioxymethamphetamine in healthy subjects. J. Pharmacol. Exp. Ther. 2015, 353, 102–111. [Google Scholar] [CrossRef]
- Connarn, J.N.; Flowers, S.; Kelly, M.; Luo, R.; Ward, K.M.; Harrington, G.; Moncion, I.; Kamali, M.; McInnis, M.; Feng, M.R.; et al. Pharmacokinetics and Pharmacogenomics of Bupropion in Three Different Formulations with Different Release Kinetics in Healthy Human Volunteers. AAPS J. 2017, 19, 1513–1522. [Google Scholar] [CrossRef]
- Koestlbacher, A.; Haen, E. Konbest—A Web-Based Laboratory Information Management System (LIMS) for TDM-Laboratories. Pharmacopsychiatry 2008, 41, A23. [Google Scholar] [CrossRef]
- Kneller, L.A. Precision Dosing of Risperidone and Aripiprazole in the Treatment of Schizophrenia Using a Physiologically Based Pharmakokinetic Approach: A Focus on the Genetic Polymorphism of CYP2D6; Westfälische Wilhelms Universität: Münster, Germany, 2021. [Google Scholar]
- Zhou, S.-F. Polymorphism of human cytochrome P450 2D6 and its clinical significance: Part II. Clin. Pharmacokinet. 2009, 48, 761–804. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Guideline on the Reporting of Physiologically Based Pharmacokinetic (PBPK) Modelling and Simulation [Online]. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-reporting-physiologically-based-pharmacokinetic-pbpk-modelling-simulation_en.pdf (accessed on 22 April 2024).
- Siccardi, M.; Marzolini, C.; Seden, K.; Seden, K.; Almond, L.; Kirov, A.; Khoo, S.; Owen, A.; Back, D. Prediction of drug-drug interactions between various antidepressants and efavirenz or boosted protease inhibitors using a physiologically based pharmacokinetic modelling approach. Clin. Pharmacokinet. 2013, 52, 583–592. [Google Scholar] [CrossRef]
- Lin, H.-P.; Sun, D.; Zhang, X.; Wen, H. Physiologically Based Pharmacokinetic Modeling for Substitutability Analysis of Venlafaxine Hydrochloride Extended-Release Formulations Using Different Release Mechanisms: Osmotic Pump Versus Openable Matrix. J. Pharm. Sci. 2016, 105, 3088–3096. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0, a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef] [PubMed]
- Berezhkovskiy, L.M. Volume of distribution at steady state for a linear pharmacokinetic system with peripheral elimination. J. Pharm. Sci. 2004, 93, 1628–1640. [Google Scholar] [CrossRef] [PubMed]
- Troy, S.M.; Parker, V.D.; Hicks, D.R.; Boudino, F.D.; Chiang, S.T. Pharmacokinetic interaction between multiple-dose venlafaxine and single-dose lithium. J. Clin. Pharmacol. 1996, 36, 175–181. [Google Scholar] [CrossRef]
- Baird-Bellaire, S.; Behrle, J.A.; Parker, V.D.; Patat, A.; Paul, J.; Nichols, A.I. An open-label, single-dose, parallel-group study of the effects of chronic hepatic impairment on the safety and pharmacokinetics of desvenlafaxine. Clin. Ther. 2013, 35, 782–794. [Google Scholar] [CrossRef] [PubMed]
- Ball, S.E.; Ahern, D.; Scatina, J.; Kao, J. Venlafaxine: In vitro inhibition of CYP2D6 dependent imipramine and desipramine metabolism; comparative studies with selected SSRIs, and effects on human hepatic CYP3A4, CYP2C9 and CYP1A2. Br. J. Clin. Pharmacol. 1997, 43, 619–626. [Google Scholar] [CrossRef]
- DeMaio, W.; Kane, C.P.; Nichols, A.I.; Jordan, R. Metabolism Studies of Desvenlafaxine. J. Bioequiv. Availab. 2011, 3, 151–160. Available online: https://www.researchgate.net/profile/william-demaio/publication/279241674_metabolism_studies_of_desvenlafaxine (accessed on 31 December 2024). [CrossRef]
- Xue, C.; Zhang, X.; Cai, W. Prediction of Drug-Drug Interactions with Bupropion and Its Metabolites as CYP2D6 Inhibitors Using a Physiologically-Based Pharmacokinetic Model. Pharmaceutics 2017, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- French, D.; Smollin, C.; Ruan, W.; Wong, A.; Drasner, K.; Wu, A.H.B. Partition constant and volume of distribution as predictors of clinical efficacy of lipid rescue for toxicological emergencies. Clin. Toxicol. 2011, 49, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Spaggiari, D.; Mehl, F.; Desfontaine, V.; Perrenoud, A.G.-G.; Fekete, S.; Rudaz, S.; Guillarme, D. Comparison of liquid chromatography and supercritical fluid chromatography coupled to compact single quadrupole mass spectrometer for targeted in vitro metabolism assay. J. Chromatogr. A 2014, 1371, 244–256. [Google Scholar] [CrossRef]
- Royal Society of Chemistry. Chemspider [Online]. Available online: https://www.chemspider.com/Chemical-Structure.432.html?rid=7451052c-a865-475c-85de-7fc98a96fc40 (accessed on 29 May 2024).
- Gondaliya, D.; Pundarikakshudu, K. Studies in formulation and pharmacotechnical evaluation of controlled release transdermal delivery system of bupropion. AAPS PharmSciTech 2003, 4, E3. [Google Scholar] [CrossRef][Green Version]
- Khan, S.R.; Berendt, R.T.; Ellison, C.D.; Ciavarella, A.B.; Asafu-Adjaye, E.; Khan, M.A.; Faustino, P.J. Bupropion Hydrochloride. Profiles Drug Subst. Excip. Relat. Methodol. 2016, 41, 1–30. [Google Scholar] [PubMed]
- Schmitt, W. General approach for the calculation of tissue to plasma partition coefficients. Toxicol. In Vitro 2008, 22, 457–467. [Google Scholar] [CrossRef]
- Rodgers, T.; Rowland, M. Physiologically based pharmacokinetic modelling 2, predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J. Pharm. Sci. 2006, 95, 1238–1257. [Google Scholar] [CrossRef]
- Rodgers, T.; Leahy, D.; Rowland, M. Physiologically based pharmacokinetic modeling 1, predicting the tissue distribution of moderate-to-strong bases. J. Pharm. Sci. 2005, 94, 1259–1276. [Google Scholar] [CrossRef] [PubMed]
- Hesse, L.M.; Venkatakrishnan, K.; Court, M.H.; Von Moltke, L.L.; Duan, S.X.; Shader, R.I.; Greenblatt, D.J. CYP2B6 mediates the in vitro hydroxylation of bupropion: Potential drug interactions with other antidepressants. Drug Metab. Dispos. 2000, 28, 1176–1183. [Google Scholar] [CrossRef]
- Molnari, J.C.; Myers, A.L. Carbonyl reduction of bupropion in human liver. Xenobiotica 2012, 42, 550–561. [Google Scholar] [CrossRef]
- Scholz, W.U. Zur Pharmakokinetik von Arzneimitteln bei multiplen Interaktionen. Krankenhauspharmazie 2016, 37, 497–505. [Google Scholar]
- ePrax GmbH. SCHOLZ Online Nutzerhandbuch [Online]. Available online: https://deutscher-apotheker-verlag.atlassian.net/wiki/spaces/SONO/pages/2470510686/MDDI+Calculator (accessed on 31 December 2024).
- Eap, C.B.; Lessard, E.; Baumann, P.; Brawand-Amey, M.; Yessine, M.A.; O’Hara, G.; Turgeon, J. Role of CYP2D6 in the stereoselective disposition of venlafaxine in humans. Pharmacogenet. Genom. 2003, 13, 39. Available online: https://journals.lww.com/jpharmacogenetics/fulltext/2003/01000/role_of_cyp2d6_in_the_stereoselective_disposition.6.aspx (accessed on 31 December 2024). [CrossRef]
- Hancu, G.; Lupu, D.; Milan, A.; Budău, M.; Barabás-Hajdu, E. Enantioselective analysis of venlafaxine and its active metabolites: A review on the separation methodologies. Biomed. Chromatogr. 2021, 35, e4874. [Google Scholar] [CrossRef] [PubMed]
- Klomp, S.D.; Manson, M.L.; Guchelaar, H.-J.; Swen, J.J. Phenoconversion of Cytochrome P450 Metabolism: A Systematic Review. J. Clin. Med. 2020, 9, 2890. [Google Scholar] [CrossRef]
- Kringen, M.K.; Bråten, L.S.; Haslemo, T.; Molden, E. The Influence of Combined CYP2D6 and CYP2C19 Genotypes on Venlafaxine and O-Desmethylvenlafaxine Concentrations in a Large Patient Cohort. J. Clin. Psychopharmacol. 2020, 40, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Templeton, I.E.; Thummel, K.E.; Kharasch, E.D.; Kunze, K.L.; Hoffer, C.; Nelson, W.L.; Isoherranen, N. Contribution of itraconazole metabolites to inhibition of CYP3A4 in vivo. Clin. Pharmacol. Ther. 2008, 83, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-Y.; Shon, J.-H.; Kim, K.-A.; Jung, H.-J.; Shim, J.-C.; Yoon, Y.-R.; Cha, I.-J.; Shin, J.-G. Combined effects of itraconazole and CYP2D6*10 genetic polymorphism on the pharmacokinetics and pharmacodynamics of haloperidol in healthy subjects. J. Clin. Psychopharmacol. 2006, 26, 135–142. [Google Scholar] [CrossRef]
- Lindh, J.D.; Annas, A.; Meurling, L.; Dahl, M.-L.; AL-Shurbaji, A. Effect of ketoconazole on venlafaxine plasma concentrations in extensive and poor metabolisers of debrisoquine. Eur. J. Clin. Pharmacol. 2003, 59, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Wen, X.; Backman, J.T.; Taavitsainen, P.; Neuvonen, P.J.; Kivistö, K.T. Midazolam α-Hydroxylation by Human Liver Microsomes in vitro: Inhibition by Calcium Channel Blockers, Itraconazole and Ketoconazole. Basic Clin. Pharmacol. Toxicol. 1999, 85, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Akiyoshi, T.; Kawamura, G.; Imaoka, A.; Miyazaki, M.; Guengerich, F.P.; Nakamura, K.; Yamamoto, K.; Ohtani, H. Comparison of the inhibitory effects of azole antifungals on cytochrome P450 3A4 genetic variants. Drug Metab. Pharmacokinet. 2021, 38, 100384. [Google Scholar] [CrossRef]
- Mutschler, E.; Geisslinger, G.; Kroemer, H.K.; Menzel, S.; Ruth, P. Arzneimittelwirkungen, 10th ed.; Wissenschaftliche Verlagsgesellschaft Stuttgart: Stuttgart, Germany, 2012; pp. 28,43,49. [Google Scholar]
| Substance | Ki (Model) | Ki (Literature) | Reference |
|---|---|---|---|
| bupropion | 0.46 µM | 21.0 µM | [14] |
| hydroxybupropion | 0.41 µM | 13.3 µM | [14] |
| threohydrobupropion | 0.15 µM | 5.40 µM | [14] |
| erythrohydrobupropion | 0.04 µM | 1.70 µM | [14] |
| Dose VEN [mg] | Dose BUP [mg] | N | % Female (♂/♀) | Age [Years] | Height [cm] | Weight [kg] | BMI [kg/m2] |
|---|---|---|---|---|---|---|---|
| Young patients | |||||||
| 225 † | 150 300 | 35 22 | 58.0 (24/33) | 50 (20–63) | 171 (152–193) | 90 (52–140) | 29 (19–48) |
| Elderly patients | |||||||
| 255 † | 150 300 | 8 5 | 53.8 (6/7) | 69 (65–78) | 166 (160–181) | 78 (65–112) | 28 (24–39) |
| DoseBUP [mg] | Parameter | N | %5–95 Percentile | Data Median (min–max) | Model Median (min–max) | PEmedian [%] |
|---|---|---|---|---|---|---|
| young patients | ||||||
| 150 | CVEN | 35 | 94.3 | 169 (56.3–430) | 144 (15.8–731) | −14.8 |
| CODV | 35 | 88.6 | 149 (2.25–315) | 126 (20.3–527) | −15.4 | |
| CAM | 35 | 91.4 | 335 (67.5–603) | 277 (54.0–1051) | −17.3 | |
| MRODV/VEN | 35 | - | 0.81 (0.02–2.87) | 0.85 (0.72–1.29) | 4.94 | |
| 300 | CVEN | 21 | 52.4 | 322 (38.3–882) | 162 (18.0–1013) | −49.7 |
| CODV | 21 | 76.2 | 87.8 (11.3–405) | 108 (20.3–441) | 23.0 | |
| CAM | 21 | 66.7 | 500 (49.5–968) | 281 (81.0–1177) | −43.8 | |
| MRODV/VEN | 21 | - | 0.26 (0.02–2.78) | 0.63 (0.44–1.13) | 142 | |
| elderly patients | ||||||
| 150 | CVEN | 8 | 62.5 | 302 (2.25–560) | 189 (18.0–878) | −37.4 |
| CODV | 8 | 75.0 | 171 (2.25–592) | 140 (18.0–599) | −18.1 | |
| CAM | 8 | 75.0 | 497 (4.50–853) | 340 (40.5–1031) | −31.6 | |
| MRODV/VEN | 8 | - | 0.61 (0.16–3.63) | 0.74 (0.68–1.00) | 21.3 | |
| 300 | CVEN | 5 | 100 | 356 (261–403) | 214 (22.5–891) | −39.9 |
| CODV | 5 | 100 | 90.0 (47.3–198) | 117 (13.5–518) | 30.0 | |
| CAM | 5 | 100 | 452 (421–509) | 340 (36.0–1053) | −24.8 | |
| MRODV/VEN | 5 | - | 0.26 (0.12–3.77) | 0.55 (0.58–0.60) | 112 | |
| AUCVEN [ng·h/mL] | AUC+150 mg BUP [ng·h/mL] | AUC+300 mg BUP [ng·h/mL] | AUC300/AUC150 | |||||
|---|---|---|---|---|---|---|---|---|
| young | VEN ODV AM | 2949 7086 10,240 | 6197 3543 10,031 | +110% −50.0% −2.04% | 6809 2935 9994 | +131% −58.6% −2.40% | 1.10 0.83 1.00 | +9.88% −17.2% −0.37% |
| MRAUC | 2.40 | 0.57 | −76.3% | 0.43 | −82.1% | 0.75 | −24.6% | |
| old | VEN ODV AM | 3578 8047 11,809 | 7681 3775 11,689 | +115% −53.1% −1.02% | 8297 3097 11,679 | +132% −61.5% −1.10% | 1.08 0.82 1.00 | +8.02% −18.0% −0.09% |
| MRAUC | 2.25 | 0.49 | −78.2% | 0.37 | −83.6% | 0.76 | −24.5% | |
| AUCold/AUCyoung | ||||||||
| VEN | 1.21 | +21.3% | 1.24 | +23.9% | 1.22 | +21.9% | 0.98 | |
| ODV | 1.14 | +13.6% | 1.07 | +6.55% | 1.06 | +5.52% | 0.99 | |
| AM | 1.15 | +15.3% | 1.17 | +16.5% | 1.17 | +16.9% | 1.00 | |
| MRAUC | 0.94 | −6.25% | 0.86 | −14.0% | 0.86 | −14.0% | 1.01 | |
| AUCVEN [ng·h/mL] | AUCVEN+BUP [ng·h/mL] | AUCVEN+ITRA [ng·h/mL] | AUCVEN+BUP+ITRA [ng·h/mL] | AUCMDDI/ AUCVEN+BUP | AUCMDDI/ AUCVEN+ITRA | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| young | VEN ODV AM | 2822 7131 10,441 | 6838 2913 10,128 | +142% −59.1% −3.00% | 2969 7507 11,044 | +5.23% +5.27% +5.78% | 7681 3258 11,371 | +172% −54.3% +8.91% | 1.12 1.12 1.12 | +12.3% +11.8% +12.3% | 2.59 0.43 1.03 | +159% −56.6% +3.00% |
| old | VEN ODV AM | 3845 9104 13,269 | 9199 3441 13,205 | +139% −62.2% −0.48% | 4048 9485 14,017 | +5.26% +5.23% +5.64% | 10,339 3854 14,820 | +169% −57.7% +11.7% | 1.12 1.12 1.12 | +12.4% +12.0% +12.2% | 2.55 0.41 1.06 | +155% −59.4% +5.73% |
| CmaxVEN [ng/mL] | CmaxVEN+BUP [ng/mL] | CmaxVEN+ITRA [ng/mL] | CmaxVEN+BUP+ITRA [ng/mL] | CmaxMDDI/ CmaxVEN+BUP | CmaxMDDI/ CmaxVEN+ITRA | |||||||
| young | VEN ODV AM | 189 352 549 | 390 132 533 | +106% −62.5% −2.91% | 197 367 578 | +4.32% +4.26% +5.28% | 426 147 584 | +125% −58.2% +6.38% | 1.09 1.11 1.10 | +9.23% +11.4% +9.57% | 2.16 0.40 1.01 | +116% −59.9% +1.04% |
| old | VEN ODV AM | 248 440 687 | 512 155 682 | +106% −64.8% −0.73% | 260 458 719 | +4.84% +4.09% +4.66% | 565 173 751 | +128% −60.7% +9.32% | 1.10 1.12 1.10 | +10.4% +11.6% +10.1% | 2.17 0.38 1.04 | +117% −48.1% +4.45% |
| CminVEN [ng/mL] | CminVEN+BUP [ng/mL] | CminVEN+ITRA [ng/mL] | CminVEN+BUP+ITRA [ng/mL] | CminMDDI/ CminVEN+BUP | CminMDDI/ CminVEN+ITRA | |||||||
| young | VEN ODV AM | 48.3 227 288 | 161 107 281 | +233% −52.9% −2.43% | 51.7 237 307 | +7.04% +4.41% +6.60% | 190 123 327 | +293% −45.8% +13.5% | 1.18 1.15 1.16 | +18.0% +15.0% +16.4% | 3.68 0.52 1.07 | +268% −48.1% +6.51% |
| old | VEN ODV AM | 70 293 382 | 231 127 380 | +230% −56.7% −0.52% | 75 307 406 | +7.14% +4.78% +6.28% | 268 146 435 | +283% −50.2% +13.9% | 1.16 1.15 1.14 | +16.0% +15.0% +14.5% | 3.57 0.48 1.07 | +257% −52.4% +7.14% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luecht, U.R.; Scholz, W.; Geiben, A.-K.; Haen, E.; Hempel, G. Physiologically Based Pharmacokinetic Model of CYP2D6 Associated Interaction Between Venlafaxine and Strong Inhibitor Bupropion—The Influence of Age-Relevant Changes and Inhibitory Dose to Classify Therapeutical Success and Harm. Pharmaceutics 2025, 17, 179. https://doi.org/10.3390/pharmaceutics17020179
Luecht UR, Scholz W, Geiben A-K, Haen E, Hempel G. Physiologically Based Pharmacokinetic Model of CYP2D6 Associated Interaction Between Venlafaxine and Strong Inhibitor Bupropion—The Influence of Age-Relevant Changes and Inhibitory Dose to Classify Therapeutical Success and Harm. Pharmaceutics. 2025; 17(2):179. https://doi.org/10.3390/pharmaceutics17020179
Chicago/Turabian StyleLuecht, Ulrich Ruben, Wolfgang Scholz, Ann-Kathrin Geiben, Ekkehard Haen, and Georg Hempel. 2025. "Physiologically Based Pharmacokinetic Model of CYP2D6 Associated Interaction Between Venlafaxine and Strong Inhibitor Bupropion—The Influence of Age-Relevant Changes and Inhibitory Dose to Classify Therapeutical Success and Harm" Pharmaceutics 17, no. 2: 179. https://doi.org/10.3390/pharmaceutics17020179
APA StyleLuecht, U. R., Scholz, W., Geiben, A.-K., Haen, E., & Hempel, G. (2025). Physiologically Based Pharmacokinetic Model of CYP2D6 Associated Interaction Between Venlafaxine and Strong Inhibitor Bupropion—The Influence of Age-Relevant Changes and Inhibitory Dose to Classify Therapeutical Success and Harm. Pharmaceutics, 17(2), 179. https://doi.org/10.3390/pharmaceutics17020179







