Gestational Diabetes Mellitus Does Not Change the Pharmacokinetics and Transplacental Distribution of Fluoxetine and Norfluoxetine Enantiomers
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Trial
2.2. Enantioselective Analysis of FLX and NorFLX in Plasma
2.3. Pharmacokinetic and Statistical Analysis
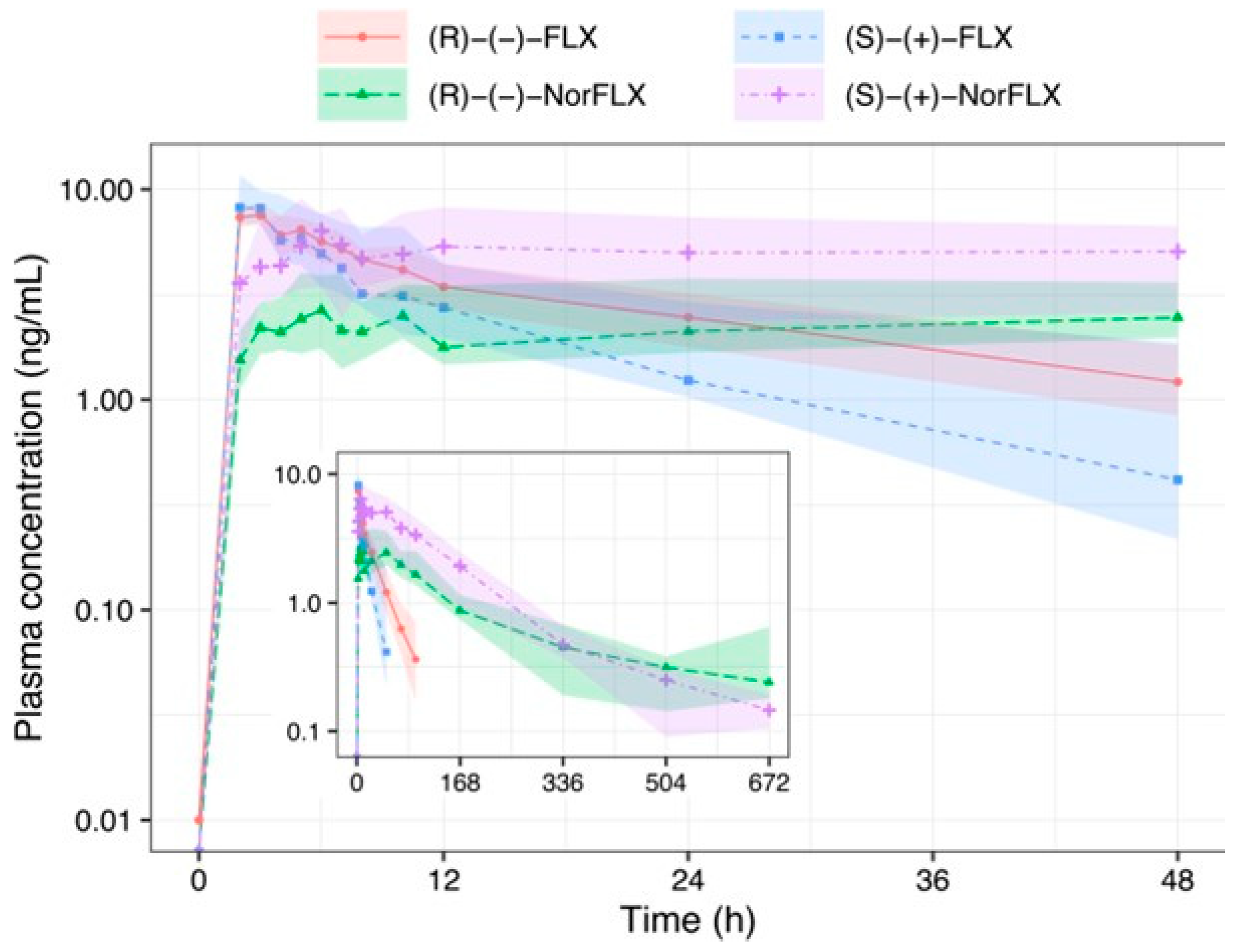
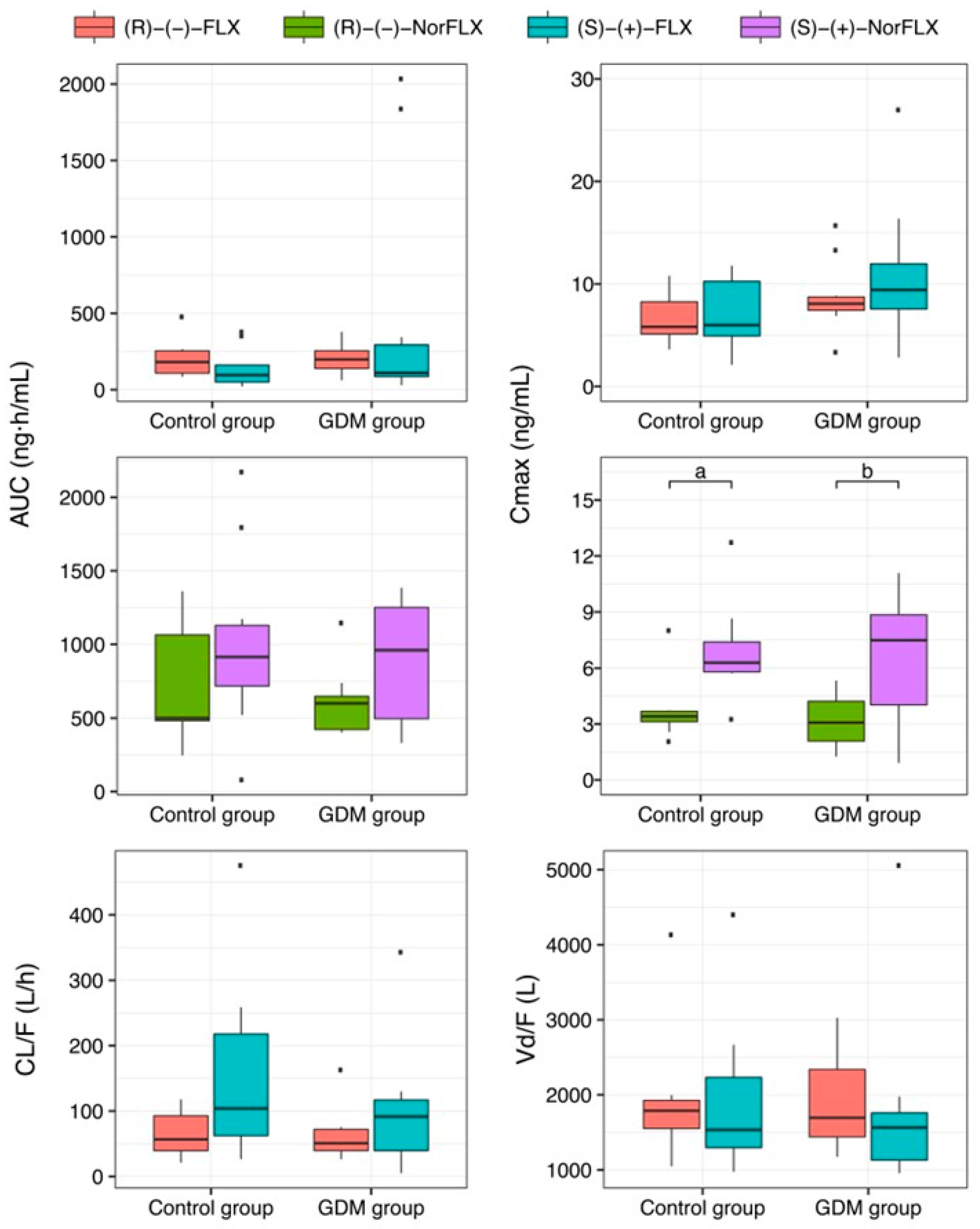
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pace, R.; Rahme, E.; Da Costa, D.; Dasgupta, K. Association between Gestational Diabetes Mellitus and Depression in Parents: A Retrospective Cohort Study. Clin. Epidemiol. 2018, 10, 1827–1838. [Google Scholar] [CrossRef]
- Woody, C.A.; Ferrari, A.J.; Siskind, D.J.; Whiteford, H.A.; Harris, M.G. A Systematic Review and Meta-Regression of the Prevalence and Incidence of Perinatal Depression. J. Affect. Disord. 2017, 219, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, L.; Gross, J.; Lanzi, S.; Quansah, D.Y.; Puder, J.; Horsch, A. How Diet, Physical Activity and Psychosocial Well-Being Interact in Women with Gestational Diabetes Mellitus: An Integrative Review. BMC Pregnancy Childbirth 2019, 19, 60. [Google Scholar] [CrossRef]
- American Diabetes Association 14. Management of Diabetes in Pregnancy: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019, 42, S165–S172. [Google Scholar] [CrossRef] [PubMed]
- Westin, A.A.; Brekke, M.; Molden, E.; Skogvoll, E.; Spigset, O. Selective Serotonin Reuptake Inhibitors and Venlafaxine in Pregnancy: Changes in Drug Disposition. PLoS ONE 2017, 12, e0181082. [Google Scholar] [CrossRef]
- Koren, G.; Ornoy, A. Clinical Implications of Selective Serotonin Reuptake Inhibitors-Selective Serotonin Norepinephrine Reuptake Inhibitors Pharmacogenetics during Pregnancy and Lactation. Pharmacogenomics 2018, 19, 1139–1145. [Google Scholar] [CrossRef]
- Fisher, S.D.; Wisner, K.L.; Clark, C.T.; Sit, D.K.; Luther, J.F.; Wisniewski, S. Factors Associated with Onset Timing, Symptoms, and Severity of Depression Identified in the Postpartum Period. J. Affect. Disord. 2016, 203, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Youn, H.; Lee, S.; Han, S.W.; Kim, L.Y.; Lee, T.-S.; Oh, M.-J.; Jeong, H.-G.; Cho, G.J. Obstetric Risk Factors for Depression during the Postpartum Period in South Korea: A Nationwide Study. J. Psychosom. Res. 2017, 102, 15–20. [Google Scholar] [CrossRef]
- Spindelegger, C.; Lanzenberger, R.; Wadsak, W.; Mien, L.K.; Stein, P.; Mitterhauser, M.; Moser, U.; Holik, A.; Pezawas, L.; Kletter, K.; et al. Influence of Escitalopram Treatment on 5-HT 1A Receptor Binding in Limbic Regions in Patients with Anxiety Disorders. Mol. Psychiatry 2009, 14, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Robakis, T.; Miller, C.; Butwick, A. Prevalence of Depression among Women of Reproductive Age in the United States. Obstet. Gynecol. 2018, 131, 671–679. [Google Scholar] [CrossRef]
- Natasha, K.; Hussain, A.; Khan, A.K.A. Prevalence of Depression among Subjects with and without Gestational Diabetes Mellitus in Bangladesh: A Hospital Based Study. J. Diabetes Metab. Disord. 2015, 14, 64. [Google Scholar] [CrossRef]
- Gao, S.; Wu, Q.; Zhang, T.; Shen, Z.; Liu, C.; Xu, X.; Ji, C.; Zhao, Y. Fluoxetine and Congenital Malformations: A Systematic Review and Meta-analysis of Cohort Studies. Br. J. Clin. Pharmacol. 2017, 83, 2134–2147. [Google Scholar] [CrossRef] [PubMed]
- Du, R.-H.; Tan, J.; Sun, X.-Y.; Lu, M.; Ding, J.-H.; Hu, G. Fluoxetine Inhibits NLRP3 Inflammasome Activation: Implication in Depression. Int. J. Neuropsychopharmacol. 2016, 19, pyw037. [Google Scholar] [CrossRef] [PubMed]
- Olivier, B.; van Oorschot, R. 5-HT1B Receptors and Aggression: A Review. Eur. J. Pharmacol. 2005, 526, 207–217. [Google Scholar] [CrossRef]
- Mårtensson, B.; Nyberg, S.; Toresson, G.; Brodin, E.; Bertilsson, L. Fluoxetine Treatment of Depression. Acta Psychiatr. Scand. 1989, 79, 586–596. [Google Scholar] [CrossRef]
- Perez-Caballero, L.; Torres-Sanchez, S.; Bravo, L.; Mico, J.A.; Berrocoso, E. Fluoxetine: A Case History of Its Discovery and Preclinical Development. Expert Opin. Drug Discov. 2014, 9, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.M.; Lanchote, V.L.; de Oliveira Filgueira, G.C.; Nardotto, G.H.B.; Duarte, G.; Cavalli, R.C.; Moisés, E.C.D. Pharmacokinetics and Transplacental Transfer of Fluoxetine Enantiomers and Their Metabolites in Pregnant Women. Clin. Pharmacol. Ther. 2019, 105, 1003–1008. [Google Scholar] [CrossRef]
- Dostalek, M.; Akhlaghi, F.; Puzanovova, M. Effect of Diabetes Mellitus on Pharmacokinetic and Pharmacodynamic Properties of Drugs. Clin. Pharmacokinet. 2012, 51, 481–499. [Google Scholar] [CrossRef]
- de Oliveira Filgueira, G.C.; Filgueira, O.A.S.; Carvalho, D.M.; Marques, M.P.; Moisés, E.C.D.; Duarte, G.; Lanchote, V.L.; Cavalli, R.C. Effect of Type 2 Diabetes Mellitus on the Pharmacokinetics and Transplacental Transfer of Nifedipine in Hypertensive Pregnant Women. Br. J. Clin. Pharmacol. 2017, 83, 1571–1579. [Google Scholar] [CrossRef]
- de Jesus Antunes, N.; Cavalli, R.C.; Marques, M.P.; Moisés, E.C.D.; Lanchote, V.L. Influence of Gestational Diabetes on the Stereoselective Pharmacokinetics and Placental Distribution of Metoprolol and Its Metabolites in Parturients. Br. J. Clin. Pharmacol. 2015, 79, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, T.M.J.P.; de Carvalho Cavalli, R.; Cunha, S.P.; de Baraldi, C.O.; Marques, M.P.; Antunes, N.J.; Godoy, A.L.P.C.; Lanchote, V.L. Influence of Gestational Diabetes Mellitus on the Stereoselective Kinetic Disposition and Metabolism of Labetalol in Hypertensive Patients. Eur. J. Clin. Pharmacol. 2011, 67, 55–61. [Google Scholar] [CrossRef] [PubMed]
- López Stewart, G. Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy: A World Health Organization Guideline. Diabetes Res. Clin. Pract. 2014, 103, 341–363. [Google Scholar] [CrossRef]
- Colagiuri, S.; Falavigna, M.; Agarwal, M.M.; Boulvain, M.; Coetzee, E.; Hod, M.; Meltzer, S.J.; Metzger, B.; Omori, Y.; Rasa, I.; et al. Strategies for Implementing the WHO Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy. Diabetes Res. Clin. Pract. 2014, 103, 364–372. [Google Scholar] [CrossRef]
- Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin No. 190: Gestational Diabetes Mellitus. Obstet. Gynecol. 2018, 131, e49–e64. [Google Scholar] [CrossRef] [PubMed]
- Hod, M.; Kapur, A.; Sacks, D.A.; Hadar, E.; Agarwal, M.; Di Renzo, G.C.; Roura, L.C.; McIntyre, H.D.; Morris, J.L.; Divakar, H. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on Gestational Diabetes Mellitus: A Pragmatic Guide for Diagnosis, Management, and Care#. Int. J. Gynecol. Obstet. 2015, 131, S173–S211. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46, S19–S40. [Google Scholar] [CrossRef] [PubMed]
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International Association of Diabetes and Pregnancy Study Groups Consensus Panel International Association of Diabetes and Pregnancy Study Groups Recommendations on the Diagnosis and Classification of Hyperglycemia in Pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.M. Estudo Farmacocinético e Análise da Distribuição Transplacentária da Fluoxetina e seu Metabólito em Gestantes Portadoras de Diabetes Mellitus Gestacional. Doctoral Thesis, Universidade De São Paulo, Faculdade de Medicina de Ribeirão Preto, Ribeirão Preto, Brazil, 2019. [Google Scholar] [CrossRef]
- Camelo, J.S., Jr.; Martinez, F.E.; Jorge, S.M.; De Sala, M.M. A New Method for Sampling Maternal Blood in the Placental Intervillous Space. Fetal Diagn. Ther. 1995, 10, 322–325. [Google Scholar] [CrossRef]
- de Barros Duarte, L.; Móises, E.C.D.; Cavalli, R.C.; Lanchote, V.L.; Duarte, G.; da Cunha, S.P. Distribution of Bupivacaine Enantiomers and Lidocaine and Its Metabolite in the Placental Intervillous Space and in the Different Maternal and Fetal Compartments in Term Pregnant Women. J. Clin. Pharmacol. 2011, 51, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.M.; Filgueira, G.C.; Marques, M.P.M.; Caris, J.A.; Duarte, G.; Cavalli, R.C.; Lanchote, V.L.; Moisés, E.C.D. Analysis of Fluoxetine and Norfluoxetine Enantiomers in Human Plasma and Amniotic Fluid by LC-MS/MS and Its Application to Clinical Pharmacokinetics in Pregnant Women. J. Res. Anal. 2017, 3, 1–9. [Google Scholar]
- Gabrielsson, J.; Weiner, D. Pharmacokinetic and Pharmacodynamic Data Analysis: Concepts and Applications, 3rd ed.; Swedish Pharmaceutical Press: Stockholm, Sweden, 2000. [Google Scholar]
- Phoenix WinNonlin® User’s Guide. Applies to: Phoenix WinNonlin 7.0, Chapter 14; Certara USA, Inc.: Princeton, NJ, USA, 2016; pp. 250–270.
- Kim, J.; Riggs, K.W.; Misri, S.; Kent, N.; Oberlander, T.F.; Grunau, R.E.; Fitzgerald, C.; Rurak, D.W. Stereoselective Disposition of Fluoxetine and Norfluoxetine during Pregnancy and Breast-Feeding. Br. J. Clin. Pharmacol. 2006, 61, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Ring, B.J.; Eckstein, J.A.; Gillespie, J.S.; Binkley, S.N.; VandenBranden, M.; Wrighton, S.A. Identification of the Human Cytochromes P450 Responsible for in Vitro Formation of R- and S-Norfluoxetine. J. Pharmacol. Exp. Ther. 2001, 297, 1044–1050. [Google Scholar]
- Nardotto, G.H.B.; Coelho, E.B.; Paiva, C.E.; Lanchote, V.L. Effects of Type 2 Diabetes Mellitus in Patients on Treatment with Glibenclamide and Metformin on Carvedilol Enantiomers Metabolism. J. Clin. Pharmacol. 2017, 57, 760–769. [Google Scholar] [CrossRef]
- Nardotto, G.H.B.; Lanchote, V.L.; Coelho, E.B.; Della Pasqua, O. Population Pharmacokinetics of Carvedilol Enantiomers and Their Metabolites in Healthy Subjects and Type-2 Diabetes Patients. Eur. J. Pharm. Sci. 2017, 109, S108–S115. [Google Scholar] [CrossRef]
- Atalah Samur, E.; Castillo, L.C.; Castro Santoro, R.; Aldea, P.A. Propuesta de un nuevo estándar de evaluación nutricional en embarazadas. Rev. Méd. Chile 1997, 125, 1429–1436. [Google Scholar]
- Calderon, I.M.P.; Damasceno, D.C.; Amorin, R.L.; Costa, R.A.A.; Brasil, M.A.M.; Rudge, M.V.C. Morphometric Study of Placental Villi and Vessels in Women with Mild Hyperglycemia or Gestational or Overt Diabetes. Diabetes Res. Clin. Pract. 2007, 78, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Rudge, M.V.; Peraçoli, J.C.; Berezowski, A.T.; Calderon, I.M.; Brasil, M.A. The Oral Glucose Tolerance Test Is a Poor Predictor of Hyperglycemia during Pregnancy. Braz. J. Med. Biol. Res. Rev. Bras. Pesqui. Medicas E Biol. 1990, 23, 1079–1089. [Google Scholar]
- Huynh, J.; Dawson, D.; Roberts, D.; Bentley-Lewis, R. A Systematic Review of Placental Pathology in Maternal Diabetes Mellitus. Placenta 2015, 36, 101–114. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Control Group [17] (n = 9) | GDM Group (n = 10) |
|---|---|---|
| Age (years) # | 30.5 (22.5–35.5) | 32.00 (29.50–37.25) |
| Gestational age (days) # | 236.5 (224.75–243.50) | 224.00 (217.75–231.25) |
| Maternal weight (kg) # | 72.65 (68.67–76.40) | 81.67 (66.25–87.72) |
| Maternal height (m) # | 1.60 (1.54–1.67) | 1.59 (1.56–1.65) |
| BMI (kg/m2) # | 29.00 (25.00–32.00) | 30.00 (28.00–35.00) |
| Newborn weight (g) * | 3220 (3180–3305) | 3255 (2975–3477) |
| Newborn height (cm) * | 49.50 (48.00–50.00) | 48.75 (47.63–49.88) |
| Placenta weight (g) * | 500 (430–600) | 507.50 (505.00–545.00) |
| Apgar index * | 9.50 (9.00–10.00) | 9.00 (8.00–9.00) |
| Total proteins (g/dL) # | 6.40 (6.20–6.60) | 6.10 (5.80–6.40) |
| Albumin (g/dL) # | 3.90 (3.82–3.92) | 3.65 (3.60–3.80) |
| α1-acid glycoprotein (mg/dL) # | 56.00 (49.05–61.20) | 50.15 (45.08–62.80) |
| Glycosylated hemoglobin (%) # | 5.55 (5.10–6.03) | 5.15 (4.57–5.25) |
| Glycemia (mg/dL) # | 87.00 (85.00–91.50) | 74.00 (72.00–84.00) |
| Control Group (n = 9) [17] | GDM Group (n = 10) | |||||||
|---|---|---|---|---|---|---|---|---|
| R-(−)-FLX | S-(+)-FLX | R-(−)-norFLX | S-(+)-norFLX | R-(−)-FLX | S-(+)-FLX | R-(−)-norFLX | S-(+)-norFLX | |
| Cmax (ng/mL) | 5.94 (5.14–9.16) | 6.05 (4.04–11.46) | 3.41 ** (3.13–3.68) | 6.29 ** (5.80–7.39) | 8.07 (7.44–8.72) | 9.42 (7.56–11.96) | 3.08 ** (2.08–4.22) | 7.48 ** (4.03–8.84) |
| tmax (h) | 4.41 (2.39–5.26) | 2.35 (1.62–3.59) | 20.60 * (14.74–23.10) | 13.66 (7.69–20.81) | 2.37 (1.38–2.81) | 1.72 (0.91–2.49) | 10.95 * (9.61–11.71) | 10.68 (8.16–25.93) |
| Ka (h−1) | 0.33 (0.24–0.55) | 0.37 (0.18–0.52) | -- | -- | 0.38 (0.24–0.58) | 0.48 (0.38–0.55) | -- | -- |
| AUC0-∞ (ng∙h/mL) | 209.20 (113.90–263.00) | 96.22 (49.61–160.94) | 500.33 (482.78–1063.96) | 942.70 (802.81–1172.15) | 197.93 (139.27–254.41) | 109.62 (85.75–293.70) | 600.39 (423.08–648.18) | 960.83 (495.88–1250.82) |
| CL/F (L/h) | 56.65 (39.45–92.71) | 103.95 (62.28–217.94) | --- | --- | 50.78 (39.58–71.96) | 91.63 (39.44–116.96) | --- | --- |
| α (h−1) | 0.18 (0.11–0.30) | 0.16 (0.11–0.36) | --- | --- | 0.375 (0.237–0.579) | 0.483 (0.382–0.546) | --- | --- |
| Vd/F (L) | 1787.81 (1552.43–1925.23) | 1533.18 (1296.74–2233.72) | --- | --- | 1695.15 (1440.18–2338.19) | 1564.90 (1130.57–1760.14) | --- | --- |
| β (h−1) | 0.03 (0.02–0.04) | 0.04 (0.03–0.07) | 0.007 0.004–0.009 | 0.008 0.005–0.011 | 0.026 (0.023–0.034) | 0.039 (0.027–0.053) | 0.005 (0.004–0.008) | 0.008 (0.006–0.009) |
| t1/2 (h) | 26.74 (18.64–32.29) | 17.21 (11.97–18.76) | 99.55 (82.87–143.85) | 83.48 (72.30–131.57) | 27.06 (20.79–29.76) | 18.41 (13.06–25.81) | 152.04 (83.15–201.08) | 90.55 (75.13–118.74) |
| R-(−) | S-(+) | R-(−) | S-(+) | |||||
| AUC0-∞ | 2.93 (2.31–5.19) | 8.78 (6.07–16.17) | 3.25 (1.98–5.01) | 8.92 (2.66–14.24) | ||||
| Control Group (n = 9) [17] | GDM Group (n = 10) | |||||||
|---|---|---|---|---|---|---|---|---|
| R-(−)-FLX | S-(+)-FLX | R-(−)-norFLX | S-(+)-norFLX | R-(−)-FLX | S-(+)-FLX | R-(−)-norFLX | S-(+)-norFLX | |
| UA/UV | 0.86 (0.70–1.04) | 0.74 (0.71–1.01) | 0.87 (0.76–1.01) | 1.00 (0.79–1.10) | 0.71 (0.46–0.98) | 0.68 (0.40–0.86) | 0.73 (0.63–1.57) | 0.82 (0.71–1.10) |
| IS/MV | 1.28 (1.14–1.38) | 1.30 (1.05–1.33) | 1.06 * (0.93–1.14) | 2.86 ** (2.52–3.69) | 0.84 (0.61–1.34) | 0.80 (0.54–1.19) | 2.61 * (1.43–4.06) | 2.12 (1.70–3.27) |
| UV/IS | 0.27 (0.19–0.30) | 0.39 ** (0.30–0.39) | 0.28 (0.21–0.38) | 0.35 ** (0.27–0.40) | 0.33 (0.26–0.35) | 0.47 (0.32–0.52) | 0.10 (0.06–0.30) | 0.48 ** (0.31–0.59) |
| UV/MV | 0.33 (0.28–0.36) | 0.44 ** (0.30–0.48) | 0.36 (0.28–0.43) | 0.38 (0.25–0.48) | 0.29 (0.19–0.36) | 0.37 (0.22–0.49) | 0.48 (0.11–0.63) | 0.35 (0.18–0.56) |
| Latency (minutes) | 198.00 (155.00–239.00) | 159.00 (138.00–212.00) | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, D.M.; Nardotto, G.H.B.; Filgueira, G.C.d.O.; Duarte, G.; Cavalli, R.C.; Lanchote, V.L.; Moisés, E.C.D. Gestational Diabetes Mellitus Does Not Change the Pharmacokinetics and Transplacental Distribution of Fluoxetine and Norfluoxetine Enantiomers. Pharmaceutics 2025, 17, 35. https://doi.org/10.3390/pharmaceutics17010035
Carvalho DM, Nardotto GHB, Filgueira GCdO, Duarte G, Cavalli RC, Lanchote VL, Moisés ECD. Gestational Diabetes Mellitus Does Not Change the Pharmacokinetics and Transplacental Distribution of Fluoxetine and Norfluoxetine Enantiomers. Pharmaceutics. 2025; 17(1):35. https://doi.org/10.3390/pharmaceutics17010035
Chicago/Turabian StyleCarvalho, Daniela Miarelli, Glauco Henrique Balthazar Nardotto, Gabriela Campos de Oliveira Filgueira, Geraldo Duarte, Ricardo Carvalho Cavalli, Vera Lucia Lanchote, and Elaine Christine Dantas Moisés. 2025. "Gestational Diabetes Mellitus Does Not Change the Pharmacokinetics and Transplacental Distribution of Fluoxetine and Norfluoxetine Enantiomers" Pharmaceutics 17, no. 1: 35. https://doi.org/10.3390/pharmaceutics17010035
APA StyleCarvalho, D. M., Nardotto, G. H. B., Filgueira, G. C. d. O., Duarte, G., Cavalli, R. C., Lanchote, V. L., & Moisés, E. C. D. (2025). Gestational Diabetes Mellitus Does Not Change the Pharmacokinetics and Transplacental Distribution of Fluoxetine and Norfluoxetine Enantiomers. Pharmaceutics, 17(1), 35. https://doi.org/10.3390/pharmaceutics17010035







