Production of Prophylactic Nanoformulation for Dental Caries and Investigation of Its Effectiveness by In Vitro and In Silico Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Standardization and Characterization of CEOs and OEOs
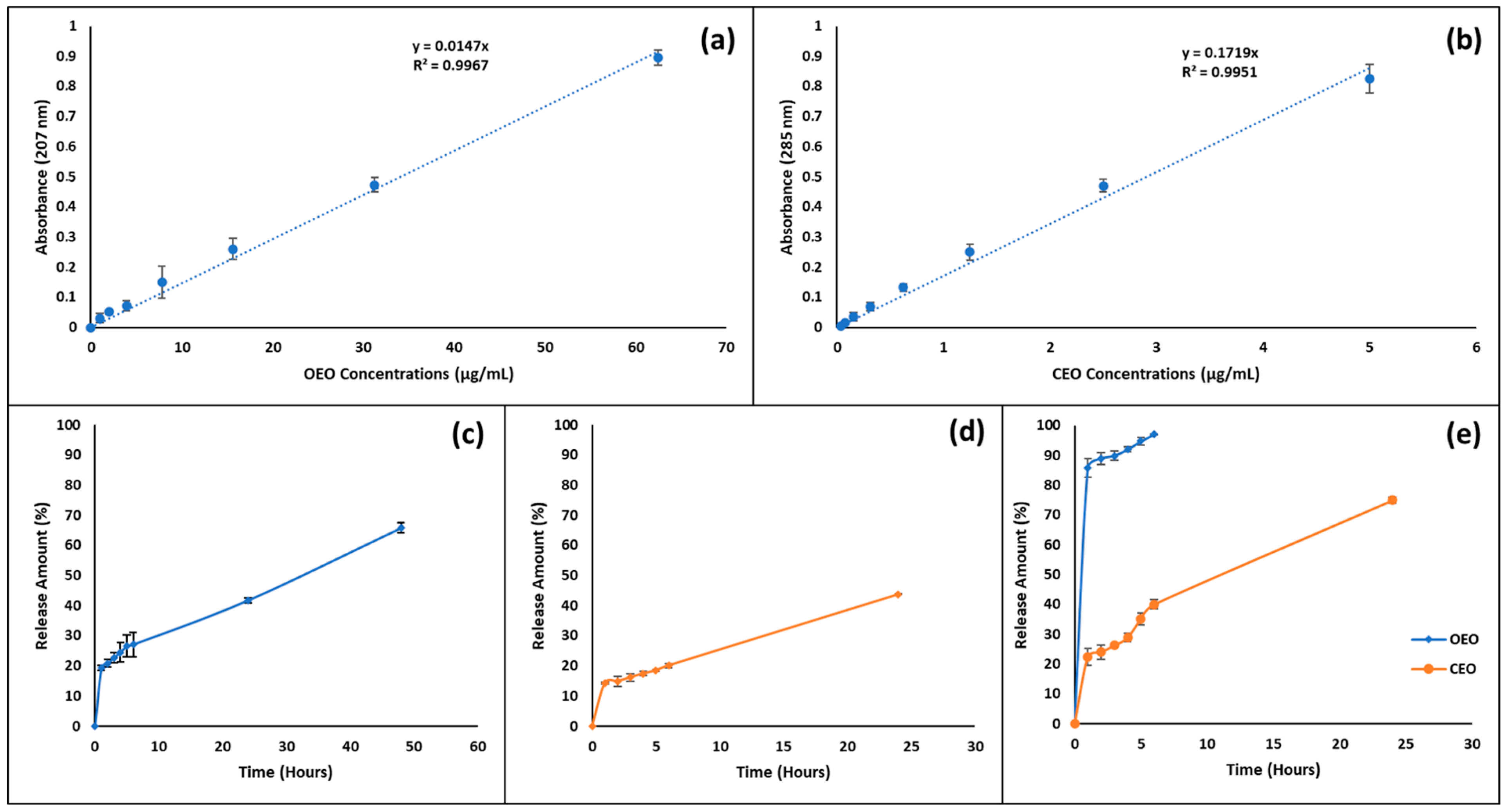
2.2.2. Determining the Standard Curve of EOs
2.2.3. Fabrication of EO-Loaded PLGA Nanoparticles
2.2.4. DLS Analysis of EO-Loaded PLGA Nanoparticles
2.2.5. TEM Analysis
2.2.6. Stability Test
2.2.7. Determination of Encapsulation Efficiency and Loading Capacity of EO-Loaded PLGA Nanoparticles
2.2.8. In Vitro Release Profile of EO-Loaded PLGA Nanoparticles
2.2.9. Ames Test
2.2.10. Determination of Minimum Inhibitory Concentration (MIC)
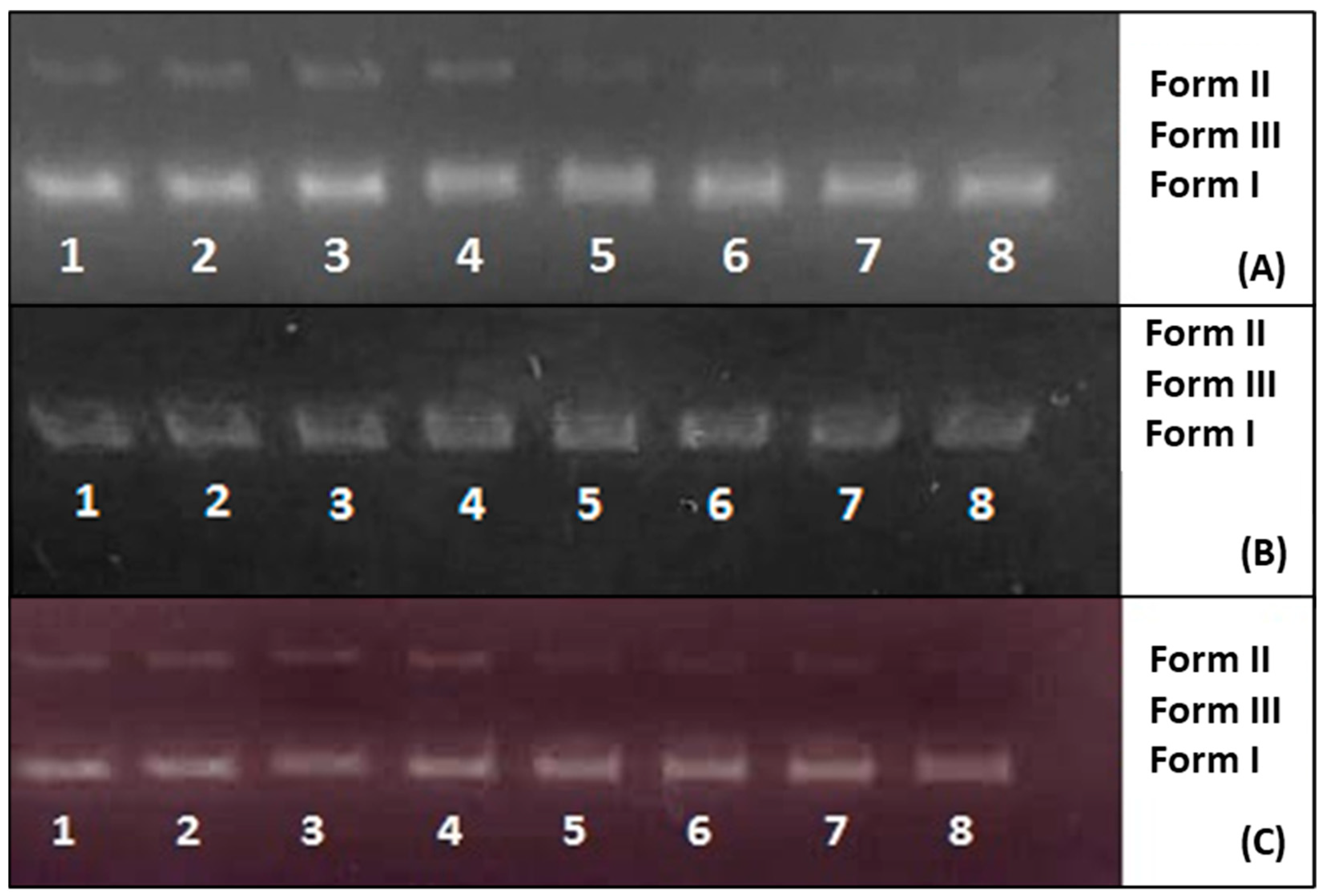
2.2.11. DNA Binding
2.2.12. DNA Cleavage
2.2.13. In Silico Molecular Docking Studies
2.2.14. Statistical Analysis
3. Results
3.1. OEO and CEO Compositions
3.2. DLS Analysis Results
3.3. TEM Analysis Results
3.4. Stability Results of EO-Loaded PLGA Nanoparticles
3.5. Determination of Encapsulation Efficiency and Loading Capacity
3.6. In Vitro Release Profile
3.7. Ames Test Results
3.8. MIC Test Results
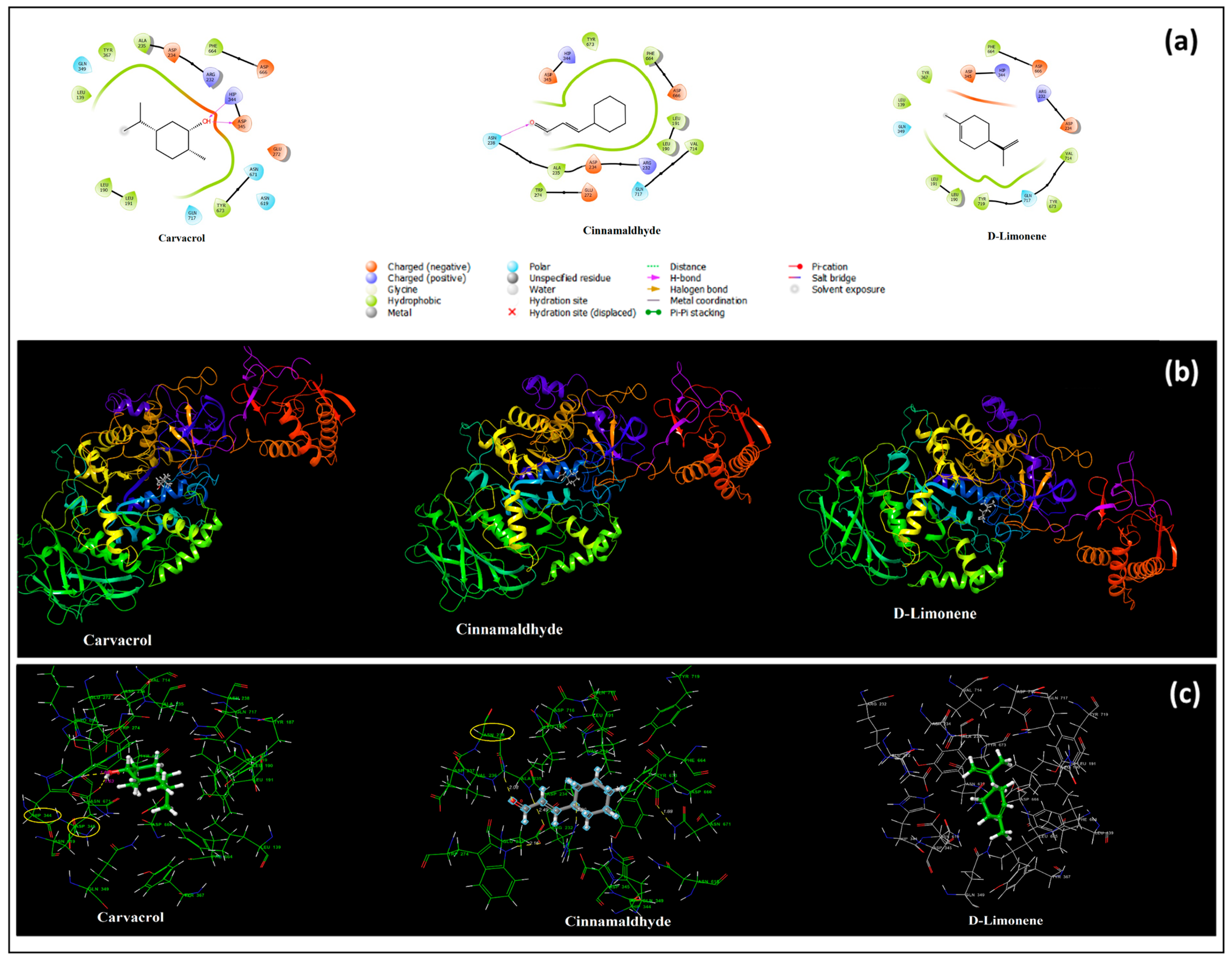
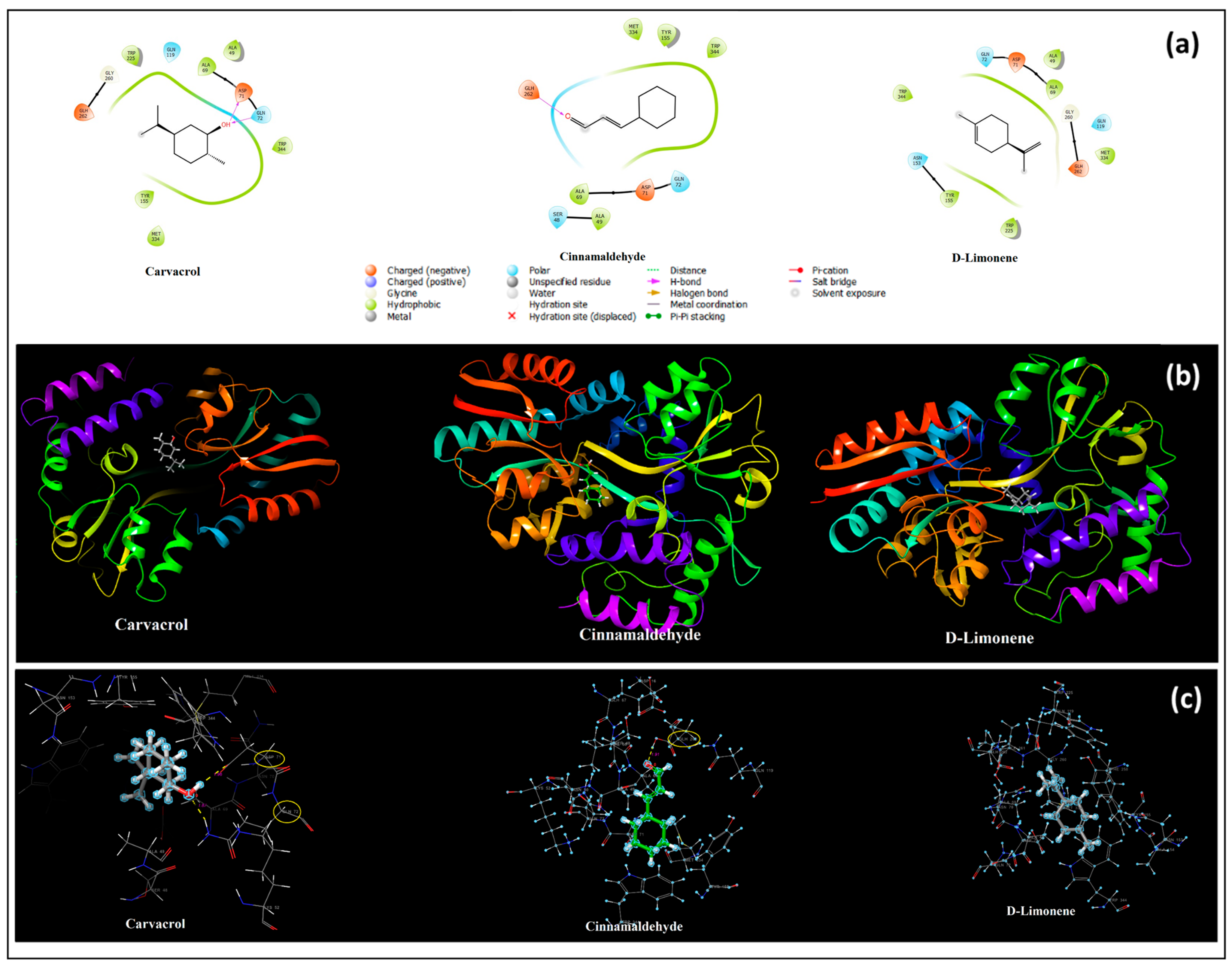
3.9. In Silico Molecular Docking Results
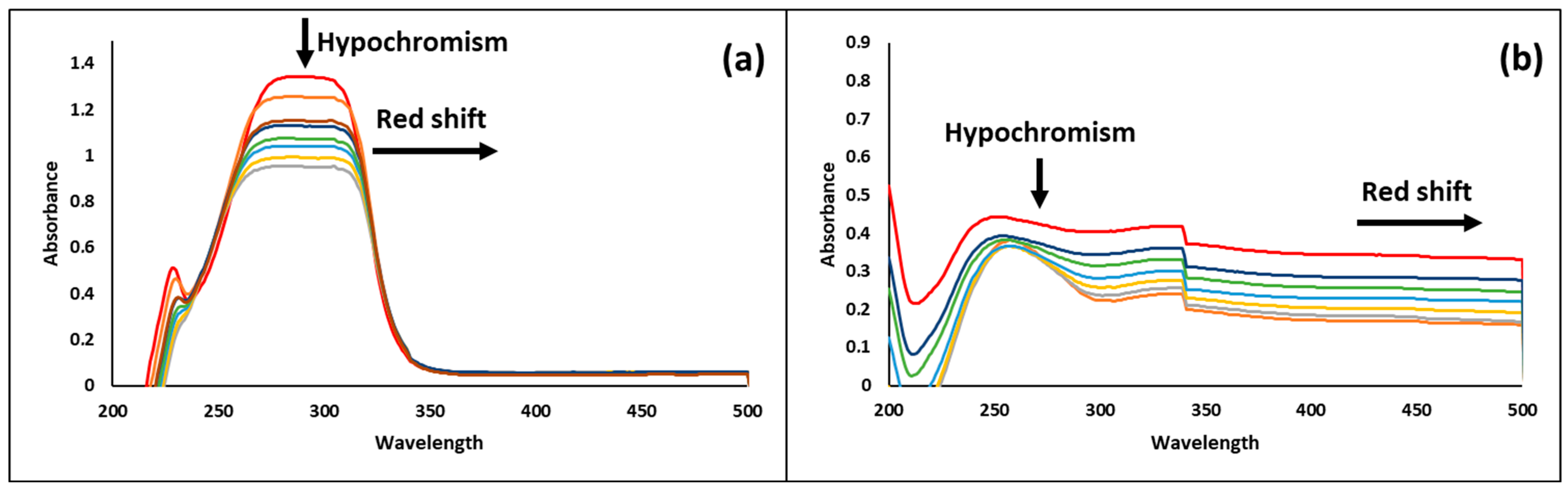
3.10. DNA Binding Results
3.11. DNA Cleavage Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loesche, W.J. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 1986, 50, 353. [Google Scholar] [CrossRef] [PubMed]
- Bader, J.D.; Shugars, D.A.; Bonito, A.J. Systematic reviews of selected dental caries diagnostic and management methods. J. Dent. Educ. 2001, 65, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Banavar Ravi, S.; Nirupad, S.; Chippagiri, P.; Pandurangappa, R. Antibacterial effects of natural herbal extracts on streptococcus mutans: Can they be potential additives in dentifrices? Int. J. Dent. 2017, 2017, 4921614. [Google Scholar] [CrossRef]
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Primers 2017, 3, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Badria, F.A.; Zidan, O.A. Natural products for dental caries prevention. J. Med. Food 2004, 7, 381–384. [Google Scholar] [CrossRef]
- Mahalakshmi, P.; Rameshkumar, A.; Sudha, G.; Dineshkumar, T.; Vinoth, H.; Malar, A. Evaluation of antimicrobial properties of Solanum xanthocarpum and Pistacia lentiscus extracts on Streptococcus mutans, Lactobacillus species and Actinomyces viscosus: An in vitro study. J. Oral Maxillofac. Pathol. JOMFP 2019, 23, 383. [Google Scholar]
- Marsh, P.; Nyvad, B. The Oral Microflora and Biofilms on Teeth; Fejerskov, O., Kidd, E.A.M., Eds.; Blackwell Publishing: Hoboken, NJ, USA, 2008. [Google Scholar]
- Eugenio Brambilla, D.; Franklin Garcia-Godoy, D.; Laura Strohmenger, O. Principles of Diagnosis and Treatment of High-Carles-Risk Subjects. Dent. Clin. N. Am. 2000, 44, 507. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.T.; Huang, X.; Ellepola, K.; Liao, S.; Li, Y. Lactobacilli and human dental caries: More than mechanical retention. Microbiology 2022, 168, 001196. [Google Scholar] [CrossRef]
- Tichy, J.; Novak, J. Extraction, assay, and analysis of antimicrobials from plants with activity against dental pathogens (Streptococcus sp.). J. Altern. Complement. Med. 1998, 4, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Choo, J.; Lee, M.; Hwang, J. Anticariogenic activity of macelignan isolated from Myristica fragrans (nutmeg) against Streptococcus mutans. Phytomedicine 2006, 13, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Bidault, P.; Chandad, F.; Grenier, D. Risk of bacterial resistance associated with systemic antibiotic therapy in periodontology. J. Can. Dent. Assoc. 2007, 73, 721–725. [Google Scholar] [PubMed]
- Lamooki, S.A.P.; Heris, F.S.; Fathi, A.; Aminianpour, N.; Zahra, J.; Ram, M.A. Prevalence and antimicrobial resistance of bacterial agents isolated from the cases of dental caries. Int. Tinnitus J. 2023, 27, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Jassam, R.A.K.M.; Abed, A.S.; Abood, E.S. Antimicrobial Susceptibility Pattern of Some Pathogenic Bacteria Isolated from Dental Caries. Egypt. J. Chem. 2022, 65, 701–714. [Google Scholar] [CrossRef]
- Rautemaa, R.; Lauhio, A.; Cullinan, M.; Seymour, G. Oral infections and systemic disease—An emerging problem in medicine. Clin. Microbiol. Infect. 2007, 13, 1041–1047. [Google Scholar] [CrossRef]
- Sirajuddin, M.; Ali, S.; Badshah, A. Drug–DNA interactions and their study by UV–Visible, fluorescence spectroscopies and cyclic voltametry. J. Photochem. Photobiol. B Biol. 2013, 124, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.A.; Jayaram, B. DNA Drug Interaction; Supercomputing Facility for Bioinformatics and Computational Biology: New Delhi, India, 2004. [Google Scholar]
- Zhou, X.-Q.; Li, Y.; Zhang, D.-Y.; Nie, Y.; Li, Z.-J.; Gu, W.; Liu, X.; Tian, J.-L.; Yan, S.-P. Copper complexes based on chiral Schiff-base ligands: DNA/BSA binding ability, DNA cleavage activity, cytotoxicity and mechanism of apoptosis. Eur. J. Med. Chem. 2016, 114, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Chitme, H.R.; Chandra, R.; Kaushik, S. Studies on anti-diarrhoeal activity of Calotropis gigantea R. Br. in experimental animals. J. Pharm. Pharm. Sci. 2004, 7, 70–75. [Google Scholar] [PubMed]
- Shetty, S.B.; Mahin-Syed-Ismail, P.; Varghese, S.; Thomas-George, B.; Kandathil-Thajuraj, P.; Baby, D.; Haleem, S.; Sreedhar, S.; Devang-Divakar, D. Antimicrobial effects of Citrus sinensis peel extracts against dental caries bacteria: An in vitro study. J. Clin. Exp. Dent. 2016, 8, e71. [Google Scholar] [CrossRef]
- Hotwani, K.; Baliga, S.; Sharma, K. Phytodentistry: Use of medicinal plants. J. Complement. Integr. Med. 2014, 11, 233–251. [Google Scholar] [CrossRef] [PubMed]
- Freires, I.A.; Denny, C.; Benso, B.; De Alencar, S.M.; Rosalen, P.L. Antibacterial activity of essential oils and their isolated constituents against cariogenic bacteria: A systematic review. Molecules 2015, 20, 7329–7358. [Google Scholar] [CrossRef] [PubMed]
- Alexa, V.T.; Galuscan, A.; Popescu, I.; Tirziu, E.; Obistioiu, D.; Floare, A.D.; Perdiou, A.; Jumanca, D. Synergistic/antagonistic potential of natural preparations based on essential oils against Streptococcus mutans from the oral cavity. Molecules 2019, 24, 4043. [Google Scholar] [CrossRef] [PubMed]
- Yudaev, P.A.; Chistyakov, E.M. Progress in dental materials: Application of natural ingredients. Russ. Chem. Rev. 2024, 93, RCR5108. [Google Scholar] [CrossRef]
- Yan, Y.; Li, X.; Zhang, C.; Lv, L.; Gao, B.; Li, M. Research progress on antibacterial activities and mechanisms of natural alkaloids: A review. Antibiotics 2021, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lan, W.; Xie, J. Natural phenolic compounds: Antimicrobial properties, antimicrobial mechanisms, and potential utilization in the preservation of aquatic products. Food Chem. 2024, 440, 138198. [Google Scholar] [CrossRef]
- Dagli, N.; Dagli, R.; Mahmoud, R.S.; Baroudi, K. Essential oils, their therapeutic properties, and implication in dentistry: A review. J. Int. Soc. Prev. Community Dent. 2015, 5, 335. [Google Scholar] [CrossRef]
- Guleria, S.; Tiku, A.; Koul, A.; Gupta, S.; Singh, G.; Razdan, V. Antioxidant and antimicrobial properties of the essential oil and extracts of Zanthoxylum alatum grown in north-western Himalaya. Sci. World J. 2013, 2013, 790580. [Google Scholar] [CrossRef] [PubMed]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 1999, 86, 985–990. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Azzimonti, B.; Cochis, A.; Beyrouthy, M.E.; Iriti, M.; Uberti, F.; Sorrentino, R.; Landini, M.M.; Rimondini, L.; Varoni, E.M. Essential oil from berries of Lebanese Juniperus excelsa M. Bieb displays similar antibacterial activity to chlorhexidine but higher cytocompatibility with human oral primary cells. Molecules 2015, 20, 9344–9357. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, A.; Yitayew, B.; Tesema, A.; Taddese, S. In vitro antimicrobial activity of essential oil of Thymus schimperi, Matricaria chamomilla, Eucalyptus globulus, and Rosmarinus officinalis. Int. J. Microbiol. 2016, 2016, 9545693. [Google Scholar] [CrossRef] [PubMed]
- Yanakiev, S. Effects of cinnamon (Cinnamomum spp.) in dentistry: A review. Molecules 2020, 25, 4184. [Google Scholar] [CrossRef]
- Esin, B. Bakteriyel Balık Patojenlerine Karşı Portakal (Citrus sinensis) Kabuğu Uçucu Yağının In vitro Antibakteriyel Etkisi. Süleyman Demirel Üniversitesi Eğirdir Su Ürünleri Fakültesi Dergisi 2018, 14, 208–214. [Google Scholar]
- Tao, N.g.; Liu, Y.j.; Zhang, M.l. Chemical composition and antimicrobial activities of essential oil from the peel of bingtang sweet orange (Citrus sinensis Osbeck). Int. J. Food Sci. Technol. 2009, 44, 1281–1285. [Google Scholar] [CrossRef]
- Ruiz-Pérez, N.J.; González-Ávila, M.; Sánchez-Navarrete, J.; Toscano-Garibay, J.D.; Moreno-Eutimio, M.A.; Sandoval-Hernández, T.; Arriaga-Alba, M. Antimycotic activity and genotoxic evaluation of Citrus sinensis and Citrus latifolia essential oils. Sci. Rep. 2016, 6, 25371. [Google Scholar] [CrossRef] [PubMed]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Ercin, E.; Kecel-Gunduz, S.; Gok, B.; Aydin, T.; Budama-Kilinc, Y.; Kartal, M. Laurus nobilis L. Essential Oil-Loaded PLGA as a Nanoformulation Candidate for Cancer Treatment. Molecules 2022, 27, 1899. [Google Scholar] [CrossRef] [PubMed]
- Froiio, F.; Ginot, L.; Paolino, D.; Lebaz, N.; Bentaher, A.; Fessi, H.; Elaissari, A. Essential oils-loaded polymer particles: Preparation, characterization and antimicrobial property. Polymers 2019, 11, 1017. [Google Scholar] [CrossRef]
- Karimi, S.M.; Sankian, M.; Khademi, F.; Tafaghodi, M. Chitosan (CHT) and trimethylchitosan (TMC) nanoparticles as adjuvant/delivery system for parenteral and nasal immunization against Mycobacterium tuberculosis (MTb) ESAT-6 antigen. Nanomed. J. 2016, 3, 223–229. [Google Scholar]
- Abdollahi, S.; Lotfipour, F. PLGA-and PLA-based polymeric nanoparticles for antimicrobial drug delivery. Biomed. Int. 2012, 3, 1–11. [Google Scholar]
- Demirbolat, I.; Karik, Ü.; Erçin, E.; Kartal, M. Gender Dependent Differences in Composition, Antioxidant and Antimicrobial Activities of Wild and Cultivated Laurus nobilis L. Leaf and Flower Essential Oils from Aegean Region of Turkey. J. Essent. Oil Bear. Plants 2020, 23, 1084–1094. [Google Scholar] [CrossRef]
- Alirezaei, M.; Ghobeh, M.; Es-haghi, A. Poly (lactic-co-glycolic acid)(PLGA)-based nanoparticles modified with chitosan-folic acid to delivery of Artemisia vulgaris L. essential oil to HT-29 cancer cells. Process Biochem. 2022, 121, 207–215. [Google Scholar] [CrossRef]
- Fraj, A.; Jaâfar, F.; Marti, M.; Coderch, L.; Ladhari, N. A comparative study of oregano (Origanum vulgare L.) essential oil-based polycaprolactone nanocapsules/microspheres: Preparation, physicochemical characterization, and storage stability. Ind. Crops Prod. 2019, 140, 111669. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Guo, X.; Wan, Q.; Teng, Y.; Liu, J. Development of rapamycin-encapsulated exosome-mimetic nanoparticles-in-PLGA microspheres for treatment of hemangiomas. Biomed. Pharmacother. 2022, 148, 112737. [Google Scholar] [CrossRef] [PubMed]
- Badawi, N.M.; Attia, Y.M.; El-Kersh, D.M.; Hammam, O.A.; Khalifa, M.K. Investigating the Impact of Optimized Trans-Cinnamic Acid-Loaded PLGA Nanoparticles on Epithelial to Mesenchymal Transition in Breast Cancer. Int. J. Nanomed. 2022, 17, 733–750. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Zandi, M.; Rezaei, M.; Farahmandghavi, F. Two-step method for encapsulation of oregano essential oil in chitosan nanoparticles: Preparation, characterization and in vitro release study. Carbohydr. Polym. 2013, 95, 50–56. [Google Scholar] [CrossRef]
- Mortelmans, K.; Zeiger, E. The Ames Salmonella/microsome mutagenicity assay. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2000, 455, 29–60. [Google Scholar] [CrossRef]
- Bostan, H.; Karaoglu, S.A. In vitro Investigation of Antibacterial Activity of Drugs Used in Sedation in Intensive Care Unit. Acta Medica Mediterr. 2019, 35, 3505–3508. [Google Scholar]
- Phillips, I.; Acar, J.; Bergan, T.; Degener, J.; Baquero, F.; Forsgren, A.; Schito, G.; Wiedemann, B.J.C.M.I. Methods for the determination of susceptibility of bacteria to antimicrobial agents. Terminology, EUCAST Definitive Document. Clin. Microbiol. Infect. 1998, 4, 291–296. [Google Scholar]
- Kowalska-Krochmal, B.; Dudek-Wicher, R. The Minimum Inhibitory Concentration of Antibiotics: Methods, Interpretation, Clinical Relevance. Pathogens 2021, 10, 165. [Google Scholar] [CrossRef] [PubMed]
- Green, D.A.; Macesic, N.; Uhlemann, A.-C.; Lopez, M.; Stump, S.; Whittier, S.; Schuetz, A.N.; Simner, P.J.; Humphries, R.M. Evaluation of calcium-enhanced media for colistin susceptibility testing by gradient agar diffusion and broth microdilution. J. Clin. Microbiol. 2020, 58, e01522-19. [Google Scholar] [CrossRef] [PubMed]
- Dufour, M.; Simmonds, R.; Bremer, P. Development of a method to quantify in vitro the synergistic activity of “natural” antimicrobials. Int. J. Food Microbiol. 2003, 85, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Egil, A.C.; Ozdemir, B.; Gok, B.; Kecel-Gunduz, S.; Budama-Kilinc, Y. Synthesis, characterization, biological activities and molecular docking of Epilobium parviflorum aqueous extract loaded chitosan nanoparticles. Int. J. Biol. Macromol. 2020, 161, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Budama-Kilinc, Y.; Kecel-Gunduz, S.; Ozdemir, B.; Bicak, B.; Akman, G.; Arvas, B.; Aydogan, F.; Yolacan, C. New nanodrug design for cancer therapy: Its synthesis, formulation, in vitro and in silico evaluations. Arch. Der Pharm. 2020, 353, e2000137. [Google Scholar] [CrossRef]
- Xavier, L.O.; Sganzerla, W.G.; Rosa, G.B.; da Rosa, C.G.; Agostinetto, L.; de Lima Veeck, A.P.; Bretanha, L.C.; Micke, G.A.; Dalla Costa, M.; Bertoldi, F.C. Chitosan packaging functionalized with Cinnamodendron dinisii essential oil loaded zein: A proposal for meat conservation. Int. J. Biol. Macromol. 2021, 169, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Rajivgandhi, G.; Saravanan, K.; Ramachandran, G.; Li, J.-L.; Yin, L.; Quero, F.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Manoharan, N. Enhanced anti-cancer activity of chitosan loaded Morinda citrifolia essential oil against A549 human lung cancer cells. Int. J. Biol. Macromol. 2020, 164, 4010–4021. [Google Scholar] [CrossRef] [PubMed]
- Jummes, B.; Sganzerla, W.G.; da Rosa, C.G.; Noronha, C.M.; Nunes, M.R.; Bertoldi, F.C.; Barreto, P.L.M. Antioxidant and antimicrobial poly-ε-caprolactone nanoparticles loaded with Cymbopogon martinii essential oil. Biocatal. Agric. Biotechnol. 2020, 23, 101499. [Google Scholar] [CrossRef]
- Kildaci, I.; Budama-Kilinc, Y.; Kecel-Gunduz, S.; Altuntas, E. Linseed oil nanoemulsions for treatment of atopic dermatitis disease: Formulation, characterization, in vitro and in silico evaluations. J. Drug Deliv. Sci. Technol. 2021, 64, 102652. [Google Scholar] [CrossRef]
- Cai, J.-X.; Liu, J.-H.; Wu, J.-Y.; Li, Y.-J.; Qiu, X.-H.; Xu, W.-J.; Xu, P.; Xiang, D.-X. Hybrid Cell Membrane-Functionalized Biomimetic Nanoparticles for Targeted Therapy of Osteosarcoma. Int. J. Nanomed. 2022, 17, 837–854. [Google Scholar] [CrossRef]
- Arunthirumeni, M.; Veerammal, V.; Shivakumar, M.S. Biocontrol efficacy of mycosynthesized selenium nanoparticle using Trichoderma sp. on insect pest Spodoptera litura. J. Clust. Sci. 2022, 33, 1645–1653. [Google Scholar] [CrossRef]
- Stewart, J.J. Optimization of parameters for semiempirical methods IV: Extension of MNDO, AM1, and PM3 to more main group elements. J. Mol. Model. 2004, 10, 155–164. [Google Scholar] [CrossRef]
- Frisch, A. Gaussian 09W Reference; Gaussian Inc.: Wallingford, CT, USA, 2009; Volume 470, 25p. [Google Scholar]
- El-Afify, M.E.; Elsayed, S.A.; Shalaby, T.I.; Toson, E.A.; El-Hendawy, A.M. Synthesis, characterization, DNA binding/cleavage, cytotoxic, apoptotic, and antibacterial activities of V (IV), Mo (VI), and Ru (II) complexes containing a bioactive ONS-donor chelating agent. Appl. Organomet. Chem. 2021, 35, e6082. [Google Scholar] [CrossRef]
- Priyadarshini, B.M.; Antipina, M.N.; Fawzy, A.S. Formulation and characterisation of poly (lactic-co-glycolic acid) encapsulated clove oil nanoparticles for dental applications. IET Nanobiotechnol. 2018, 12, 311–317. [Google Scholar] [CrossRef]
- Phuangkaew, T.; Booranabunyat, N.; Kiatkamjornwong, S.; Thanyasrisung, P.; Hoven, V.P. Amphiphilic quaternized chitosan: Synthesis, characterization, and anti-cariogenic biofilm property. Carbohydr. Polym. 2022, 277, 118882. [Google Scholar] [CrossRef] [PubMed]
- Minhaco, V.M.T.R.; Huacho, P.M.M.; Imbriani, M.J.M.; Tonon, C.C.; Chorilli, M.; de Souza Rastelli, A.N.; Spolidorio, D.M.P. Improving antimicrobial activity against endodontic biofilm after exposure to blue light-activated novel curcumin nanoparticle. Photodiagnosis Photodyn. Ther. 2023, 42, 103322. [Google Scholar] [CrossRef]
- Kaplan, M.; Öztürk, K.; Öztürk, S.C.; Tavukçuoğlu, E.; Esendağlı, G.; Calis, S. Effects of particle geometry for PLGA-based nanoparticles: Preparation and in vitro/in vivo evaluation. Pharmaceutics 2023, 15, 175. [Google Scholar] [CrossRef] [PubMed]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems-a review (Part 1). Trop. J. Pharm. Res. 2013, 12, 255–264. [Google Scholar]
- Öztürk, K.; Kaplan, M.; Çalış, S. Effects of nanoparticle size, shape, and zeta potential on drug delivery. Int. J. Pharm. 2024, 124799. [Google Scholar] [CrossRef] [PubMed]
- Pochapski, D.J.; Carvalho dos Santos, C.; Leite, G.W.; Pulcinelli, S.H.; Santilli, C.V. Zeta potential and colloidal stability predictions for inorganic nanoparticle dispersions: Effects of experimental conditions and electrokinetic models on the interpretation of results. Langmuir 2021, 37, 13379–13389. [Google Scholar] [CrossRef] [PubMed]
- Gursu, B.Y.; Dag, İ.; Dikmen, G. Antifungal and antibiofilm efficacy of cinnamaldehyde-loaded poly (DL-lactide-co-glycolide)(PLGA) nanoparticles against Candida albicans. Int. Microbiol. 2022, 25, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Gossmann, R.; Langer, K.; Mulac, D. New perspective in the formulation and characterization of didodecyldimethylammonium bromide (DMAB) stabilized poly (lactic-co-glycolic acid)(PLGA) nanoparticles. PLoS ONE 2015, 10, e0127532. [Google Scholar] [CrossRef]
- Tadros, T. Steric Stabilization; Springer: Berlin, Germany, 2013; pp. 1048–1049. [Google Scholar]
- Liu, Z.; Fu, R.; Yuying, Y. Preparation and evaluation of stable nanofluids for heat transfer application. Adv. Nanofluid Heat. Transf. 2022, 25–57. [Google Scholar] [CrossRef]
- Stromberg, Z.R.; Phipps, M.L.; Magurudeniya, H.D.; Pedersen, C.A.; Rajale, T.; Sheehan, C.J.; Courtney, S.J.; Bradfute, S.B.; Hraber, P.; Rush, M.N. Formulation of stabilizer-free, nontoxic PLGA and elastin-PLGA nanoparticle delivery systems. Int. J. Pharm. 2021, 597, 120340. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Wu, Y.; Liu, Y.; Wu, D. High drug-loading nanomedicines: Progress, current status, and prospects. Int. J. Nanomed. 2017, 12, 4085. [Google Scholar] [CrossRef]
- Iannitelli, A.; Grande, R.; Di Stefano, A.; Di Giulio, M.; Sozio, P.; Bessa, L.J.; Laserra, S.; Paolini, C.; Protasi, F.; Cellini, L. Potential antibacterial activity of carvacrol-loaded poly (DL-lactide-co-glycolide)(PLGA) nanoparticles against microbial biofilm. Int. J. Mol. Sci. 2011, 12, 5039–5051. [Google Scholar] [CrossRef] [PubMed]
- Esfandyari-Manesh, M.; Ghaedi, Z.; Asemi, M.; Khanavi, M.; Manayi, A.; Jamalifar, H.; Atyabi, F.; Dinarvand, R. Study of antimicrobial activity of anethole and carvone loaded PLGA nanoparticles. J. Pharm. Res. 2013, 7, 290–295. [Google Scholar] [CrossRef]
- Almeida, K.B.; Ramos, A.S.; Nunes, J.B.; Silva, B.O.; Ferraz, E.R.; Fernandes, A.S.; Felzenszwalb, I.; Amaral, A.C.F.; Roullin, V.G.; Falcao, D.Q. PLGA nanoparticles optimized by Box-Behnken for efficient encapsulation of therapeutic Cymbopogon citratus essential oil. Colloids Surf. B Biointerfaces 2019, 181, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Azzazy, H.M.E.-S.; Abdelnaser, A.; Al Mulla, H.; Sawy, A.M.; Shamma, S.N.; Elhusseiny, M.; Alwahibi, S.; Mahdy, N.K.; Fahmy, S.A. Essential Oils Extracted from Boswellia sacra Oleo Gum Resin Loaded into PLGA–PCL Nanoparticles: Enhanced Cytotoxic and Apoptotic Effects against Breast Cancer Cells. ACS Omega 2022, 8, 1017–1025. [Google Scholar] [CrossRef]
- Su, Z.; Sun, F.; Shi, Y.; Jiang, C.; Meng, Q.; Teng, L.; Li, Y. Effects of formulation parameters on encapsulation efficiency and release behavior of risperidone poly (D, L-lactide-co-glycolide) microsphere. Chem. Pharm. Bull. 2009, 57, 1251–1256. [Google Scholar] [CrossRef] [PubMed]
- Barichello, J.M.; Morishita, M.; Takayama, K.; Nagai, T. Encapsulation of hydrophilic and lipophilic drugs in PLGA nanoparticles by the nanoprecipitation method. Drug Dev. Ind. Pharm. 1999, 25, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, C.; Badgujar, P.C.; Gundev, P.; Upadhyay, A. Review of toxicological assessment of d-limonene, a food and cosmetics additive. Food Chem. Toxicol. 2018, 120, 668–680. [Google Scholar] [CrossRef]
- Bai, G.; Wen, X.; Niu, L. Recent Developments in Amorphous Alloy Catalysts for Hydrogenation; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Bode, K.A.; Applequist, J. A new optimization of atom polarizabilities in halomethanes, aldehydes, ketones, and amides by way of the atom dipole interaction model. J. Phys. Chem. 1996, 100, 17820–17824. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 440917, Limonene, (+)-. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/440917 (accessed on 15 January 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 637511, Cinnamaldehyde. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Cinnamaldehyde (accessed on 15 January 2025).
- Hill, L.E.; Taylor, T.M.; Gomes, C. Antimicrobial efficacy of poly (DL-lactide-co-glycolide)(PLGA) nanoparticles with entrapped cinnamon bark extract against listeria monocytogenes and salmonella typhimurium. J. Food Sci. 2013, 78, N626–N632. [Google Scholar] [CrossRef]
- Zhu, Z.; Min, T.; Zhang, X.; Wen, Y. Microencapsulation of Thymol in Poly (lactide-co-glycolide)(PLGA): Physical and Antibacterial Properties. Materials 2019, 12, 1133. [Google Scholar] [CrossRef] [PubMed]
- Gou, Q.; Favero, L.B.; Bahamyirou, S.S.; Xia, Z.; Caminati, W. Interactions between carboxylic acids and aldehydes: A rotational study of HCOOH–CH2O. J. Phys. Chem. A 2014, 118, 10738–10741. [Google Scholar] [CrossRef]
- Ogueri, K.S.; Ogueri, K.S.; Allcock, H.R.; Laurencin, C.T. A regenerative polymer blend composed of glycylglycine ethyl ester-substituted polyphosphazene and poly (lactic-co-glycolic acid). ACS Appl. Polym. Mater. 2020, 2, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Sponchiado, G.; Adam, M.L.; Silva, C.D.; Soley, B.S.; de Mello-Sampayo, C.; Cabrini, D.A.; Correr, C.J.; Otuki, M.F. Quantitative genotoxicity assays for analysis of medicinal plants: A systematic review. J. Ethnopharmacol. 2016, 178, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Ipek, E.; Zeytinoglu, H.; Okay, S.; Tuylu, B.A.; Kurkcuoglu, M.; Baser, K.H.C. Genotoxicity and antigenotoxicity of Origanum oil and carvacrol evaluated by Ames Salmonella/microsomal test. Food Chem. 2005, 93, 551–556. [Google Scholar] [CrossRef]
- Oliveira, N.d.M.S.; Resende, M.R.; Morales, D.A.; de Ragão Umbuzeiro, G.; Boriollo, M.F.G. In vitro mutagenicity assay (Ames test) and phytochemical characterization of seeds oil of Helianthus annuus Linné (sunflower). Toxicol. Rep. 2016, 3, 733–739. [Google Scholar] [CrossRef] [PubMed]
- da Silva Dantas, F.G.; de Castilho, P.F.; de Almeida-Apolonio, A.A.; de Araújo, R.P.; de Oliveira, K.M.P. Mutagenic potential of medicinal plants evaluated by the Ames Salmonella/microsome assay: A systematic review. Mutat. Res./Rev. Mutat. Res. 2020, 786, 108338. [Google Scholar] [CrossRef]
- Orhan, M.; Koç, S.; Özakin, C.; Hocekenberger, A.; Sinirtaş, M. Hastanelerde Kullanılan Tekstillerin Antibakteriyel ve Antimantar Etkinliklerinin Değerlendirilmesi. Kahramanmaraş Sütçü İmam Üniversitesi Mühendislik Bilim. Derg. 2019, 22, 19–31. [Google Scholar] [CrossRef]
- Trinh, N.-T.-T.; Dumas, E.; Thanh, M.L.; Degraeve, P.; Amara, C.B.; Gharsallaoui, A.; Oulahal, N. Effect of a Vietnamese Cinnamomum cassia essential oil and its major component trans-cinnamaldehyde on the cell viability, membrane integrity, membrane fluidity, and proton motive force of Listeria innocua. Can. J. Microbiol. 2015, 61, 263–271. [Google Scholar] [CrossRef]
- Utchariyakiat, I.; Surassmo, S.; Jaturanpinyo, M.; Khuntayaporn, P.; Chomnawang, M.T. Efficacy of cinnamon bark oil and cinnamaldehyde on anti-multidrug resistant Pseudomonas aeruginosa and the synergistic effects in combination with other antimicrobial agents. BMC Complement. Altern. Med. 2016, 16, 158. [Google Scholar] [CrossRef] [PubMed]
- Yap, P.S.X.; Krishnan, T.; Chan, K.-G.; Lim, S.H.E.J.J.o.M. Antibacterial mode of action of Cinnamomum verum bark essential oil, alone and in combination with piperacillin, against a multi-drug-resistant Escherichia coli strain. Biotechnology 2015, 25, 1299–1306. [Google Scholar] [CrossRef]
- Bae, K.-H.; Ji, J.-M.; Park, K.-L. The antibacterial component from Cinnamomi Cortex against a cariogenic bacteriumStreptococcus mutans OMZ 176. Arch. Pharmacal Res. 1992, 15, 239–241. [Google Scholar] [CrossRef]
- Pattnaik, D.; Padhan, D.; Jana, G. Evaluation of Cinnamon oil, Peppermint oil, Cardamom oil and Orange oil as antimicrobial agent. J. Pharm. Res. 2010, 3, 414–416. [Google Scholar]
- Sreepian, A.; Popruk, S.; Nutalai, D.; Phutthanu, C.; Sreepian, P.M. Antibacterial Activities and Synergistic Interaction of Citrus Essential Oils and Limonene with Gentamicin against Clinically Isolated Methicillin-Resistant Staphylococcus aureus. Sci. World J. 2022, 2022, 8418287. [Google Scholar] [CrossRef]
- Costa, M.D.S.; Rocha, J.E.; Campina, F.F.; Silva, A.R.; Da Cruz, R.P.; Pereira, R.L.; Quintans-Júnior, L.J.; De Menezes, I.R.; Adriano, A.D.S.; De Freitas, T.S. Comparative analysis of the antibacterial and drug-modulatory effect of d-limonene alone and complexed with β-cyclodextrin. Eur. J. Pharm. Sci. 2019, 128, 158–161. [Google Scholar] [CrossRef]
- Echeverry-Chica, J.; Naranjo-Díaz, A.; Araque-Marín, P. Silver nanoparticles functionalized in situ with D-Limonene: Effect on antibacterial activity. Rev. ION 2020, 33, 79–92. [Google Scholar] [CrossRef]
- Motelica, L.; Vasile, B.-S.; Ficai, A.; Surdu, A.-V.; Ficai, D.; Oprea, O.-C.; Andronescu, E.; Mustățea, G.; Ungureanu, E.L.; Dobre, A.A. Antibacterial activity of zinc oxide nanoparticles loaded with essential oils. Pharmaceutics 2023, 15, 2470. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Petrisor, G.; Oprea, O.-C.; Trușcǎ, R.-D.; Ficai, A.; Andronescu, E.; Hudita, A.; Holban, A.M. Antimicrobial hydroxyethyl-cellulose-based composite films with zinc oxide and mesoporous silica loaded with cinnamon essential oil. Pharmaceutics 2024, 16, 1225. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Li, Z.; Sun, Y.; Zhang, Y.; Wang, S.; Zhang, Q.; Cai, T.; Xiang, W.; Zeng, C.; Tang, J. D-Limonene: Promising and Sustainable Natural Bioactive Compound. Appl. Sci. 2024, 14, 4605. [Google Scholar] [CrossRef]
- Kargar, H.; Behjatmanesh-Ardakani, R.; Torabi, V.; Kashani, M.; Chavoshpour-Natanzi, Z.; Kazemi, Z.; Mirkhani, V.; Sahraei, A.; Tahir, M.N.; Ashfaq, M. Synthesis, characterization, crystal structures, DFT, TD-DFT, molecular docking and DNA binding studies of novel copper (II) and zinc (II) complexes bearing halogenated bidentate N, O-donor Schiff base ligands. Polyhedron 2021, 195, 114988. [Google Scholar] [CrossRef]
- Daravath, S.; Rambabu, A.; Ganji, N.; Ramesh, G.; Lakshmi, P.A. Spectroscopic, quantum chemical calculations, antioxidant, anticancer, antimicrobial, DNA binding and photo physical properties of bioactive Cu (II) complexes obtained from trifluoromethoxy aniline Schiff bases. J. Mol. Struct. 2022, 1249, 131601. [Google Scholar] [CrossRef]
- Ishaniya, W.; Ganeshpandian, M. Mixed ligand copper (II) complexes of diimine co-ligands: Synthesis, characterization, DNA binding and DNA cleavage activity. Mater. Today Proc. 2022, 50, 358–364. [Google Scholar] [CrossRef]
- Kecel-Gunduz, S.; Budama-Kilinc, Y.; Gok, B.; Bicak, B.; Akman, G.; Arvas, B.; Aydogan, F.; Yolacan, C. Computer-aided anticancer drug design: In vitro and in silico studies of new iminocoumarin derivative. J. Mol. Struct. 2021, 1239, 130539. [Google Scholar] [CrossRef]
- Chaveerach, U.; Meenogwa, A.; Trongpanich, Y.; Soikum, C.; Chaveerach, P. DNA binding and cleavage behaviors of copper(II) complexes with amidino-O-methylurea and N-methylphenyl-amidino-O-methylurea, and their antibacterial activities. Polyhedron 2010, 29, 731–738. [Google Scholar] [CrossRef]
- Raza, A.; Xu, X.; Xia, L.; Xia, C.; Tang, J.; Ouyang, Z. Quercetin-iron complex: Synthesis, characterization, antioxidant, DNA binding, DNA cleavage, and antibacterial activity studies. J. Fluoresc. 2016, 26, 2023–2031. [Google Scholar] [CrossRef]
- Nural, Y.; Ozdemir, S.; Yalcin, M.S.; Demir, B.; Atabey, H.; Seferoglu, Z.; Ece, A. New bis-and tetrakis-1, 2, 3-triazole derivatives: Synthesis, DNA cleavage, molecular docking, antimicrobial, antioxidant activity and acid dissociation constants. Bioorganic Med. Chem. Lett. 2022, 55, 128453. [Google Scholar] [CrossRef]
- Bicak, B.; Kecel Gunduz, S.; Budama Kilinc, Y.; Imhof, P.; Gok, B.; Akman, G.; Ozel, A.E. Structural, spectroscopic, in silico, in vitro and DNA binding evaluations of tyrosyl-lysyl-threonine. J. Biomol. Struct. Dyn. 2022, 40, 12148–12164. [Google Scholar] [CrossRef]
- Alpaslan, G.; Boyacioglu, B.; Demir, N.; Tümer, Y.; Yapar, G.; Yıldırım, N.; Yıldız, M.; Ünver, H. Synthesis, characterization, biological activity and theoretical studies of a 2-amino-6-methoxybenzothiazole-based fluorescent Schiff base. J. Mol. Struct. 2019, 1180, 170–178. [Google Scholar] [CrossRef]

| Components | Amount (%) |
|---|---|
| Alpha Pinene | 0.473 |
| Beta Pinene | 0.346 |
| Myrcene | 1.694 |
| Limonene | 97.309 |
| Aromadendren | 0.179 |
| Components | Amount (%) |
|---|---|
| Alpha Pinene | 0.790 |
| Camphene | 0.217 |
| Benzaldehyde | 0.263 |
| Beta Pinene | 0.213 |
| Phellandrene | 0.318 |
| Cymene | 1.916 |
| Limonene | 0.925 |
| 1.8-Cineole | 1.941 |
| Linalool | 2.120 |
| Terpinene-4-ol | 0.538 |
| Alpha Terpineol | 1.331 |
| Cinnamaldehyde | 70.267 |
| Eugenol | 5.220 |
| Caryophyllene | 0.316 |
| Cinnamyl Acetate | 10.581 |
| Eugenyl Acetate | 0.821 |
| Caryophyllene Oxide | 0.132 |
| Beznyl Benzaoate | 1.705 |
| NPs | Storage Conditions | DLS Analysis Parameters | Day 1 | 1st Month | 2nd Month | 3rd Month |
|---|---|---|---|---|---|---|
| OEO-loaded PLGA nanoparticles | 5 ± 3 °C | Size (nm) | 232.6 ± 2.95 | 203.6 ± 4.04 | 190.2 ± 3.46 | 189.3 ± 3.27 |
| PdI | 0.196 ± 0,06 | 0.142 ± 0.04 | 0.152 ± 0.02 | 0.134 ± 0.01 | ||
| Zeta (mV) | −7.44 ± 0.36 | −9.23 ± 0.07 | −8.59 ± 0.57 | −9.84 ± 0.39 | ||
| 25 ± 2 °C 60% RH | Size (nm) | 232.6 ± 2.95 | 202 ± 3.29 | 186.1 ±0.32 | 185.6 ± 1.97 | |
| PdI | 0.196 ± 0,06 | 0.143 ± 0.02 | 0.122 ± 0.01 | 0.125 ± 0.05 | ||
| Zeta (mV) | −7.44 ± 0.36 | −7.81 ± 0.56 | −9.84 ± 0.24 | −10.4 ± 0.17 | ||
| 40 ± 2 °C 75% RH | Size (nm) | 232.6 ± 2.95 | 215.4 ± 4.69 | 194.3 ± 2.26 | 192.1 ± 2.58 | |
| PdI | 0.196 ± 0,06 | 0.126 ± 0.04 | 0.090 ± 0.01 | 0.183 ± 0.01 | ||
| Zeta (mV) | −7.44 ± 0.36 | −9.85 ± 0.16 | −11.66 ± 0.25 | −8.42 ± 0.73 | ||
| CEO-loaded PLGA nanoparticles | 5 ± 3 °C | Size (nm) | 191.1 ± 1.06 | 198.9 ± 2.64 | 188.5 ± 2.02 | 192.4 ± 2.82 |
| PdI | 0.132 ± 0.02 | 0.183 ± 0.02 | 0.112 ± 0.03 | 0.106 ± 0.02 | ||
| Zeta (mV) | −5.20 ± 0.04 | −12.1 ± 0.80 | −9.97 ± 0.39 | −10.56 ± 0.46 | ||
| 25 ± 2 °C 60% RH | Size (nm) | 191.1 ± 1.06 | 189.2 ± 1.28 | 191.7 ± 3.67 | 190.2 ± 1.91 | |
| PdI | 0.132 ± 0.02 | 0.144 ± 0.03 | 0.096 ± 0.01 | 0.146 ± 0.02 | ||
| Zeta (mV) | −5.20 ± 0.04 | −10.66 ± 0.30 | −10.8 ± 0.55 | −11 ± 0.26 | ||
| 40 ± 2 °C 75% RH | Size (nm) | 191.1 ± 1.06 | 228.3 ± 1.92 | 188.7 ± 4.41 | 190.4 ± 1.70 | |
| PdI | 0.132 ± 0.02 | 0.214 ± 0.02 | 0.110 ± 0.02 | 0.206 ± 0.02 | ||
| Zeta (mV) | −5.20 ± 0.04 | −7.61 ± 0.34 | −12.8 ± 0.36 | −9.97 ± 0.38 | ||
| OEO-CEO-loaded PLGA nanoparticles | 5 ± 3 °C | Size (nm) | 211.8 ± 1.65 | 187.63 ± 2.73 | 243.1 ± 2.65 | 213 ± 1.76 |
| PdI | 0.152 ± 0.01 | 0.171 ± 0.01 | 0.172 ± 0.09 | 0.122 ± 0.03 | ||
| Zeta (mV) | −4.79 ± 0.03 | −9 ± 0.85 | −6.37 ± 0.45 | −8.25 ± 0.36 | ||
| 25 ± 2 °C 60% RH | Size (nm) | 211.8 ± 1.65 | 170.9 ± 0.65 | 230.3 ± 1.64 | 177.6 ± 0.32 | |
| PdI | 0.152 ± 0.01 | 0.118 ± 0.02 | 0.122 ± 0.04 | 0.105 ± 0.02 | ||
| Zeta (mV) | −4.79 ± 0.03 | −8.42 ± 0.99 | −7.23 ± 0.28 | −7.50 ± 0.39 | ||
| 40 ± 2 °C 75% RH | Size (nm) | 211.8 ± 1.65 | 176.6 ± 2.30 | 225.9 ± 2.09 | 185.2 ± 1.92 | |
| PdI | 0.152 ± 0.01 | 0.075 ± 0.03 | 0.138 ± 0.05 | 0.144 ± 0.06 | ||
| Zeta (mV) | −4.79 ± 0.03 | −7.88 ± 0.28 | −8.42 ± 0.73 | −6.82 ± 0.26 |
| Treatment | Concentrations (mg/mL) | Revertant Colonies/Plate | |
|---|---|---|---|
| TA98 Mean ± SD | TA98 Mean ± SD | ||
| OEO | 0.10 | 5.6 ± 2.08 * | 45 ± 5.0 * |
| 0.20 | 8.3 ± 1.52 * | 37 ± 2.0 * | |
| 0.40 | 274 ± 7.93 * | 39.66 ± 2.51 * | |
| 0.80 | 606 ± 4.58 * | 25.33 ± 4.93 * | |
| OEO-Loaded PLGA Nanoparticles | 0.125 | 26 ± 2.64 | 210 ± 4.58 |
| 0.25 | 28.6 ± 1.52 | 226 ± 23.2 | |
| 0.50 | 22.6 ± 1.54 | 229.6 ± 6.65 | |
| 1.00 | 24.3 ± 3.05 | 235 ± 15.0 | |
| Positive Control (NPD) | 0.005 | 847.3 ± 2.52 * | |
| Positive Control (SA) | 0.0005 | 1242 ± 21.2 * | |
| Negative Control (Water) | 25 ± 3.60 | 191 ± 8.51 | |
| Negative Control (Ethanol) | 27.6 ± 2.51 | 188 ± 3.60 | |
| Spontaneous Control | 24 ± 1.00 | 188.66 ± 3.51 | |
| Treatment | Concentrations (mg/mL) | Revertant Colonies/Plate | |
|---|---|---|---|
| TA98 Mean ± SD | TA98 Mean ± SD | ||
| CEO | 0.10 | 6 ± 1.00 * | 322 ± 7.54 * |
| 0.20 | 9.6 ± 2.08 * | 83 ± 7.0 * | |
| 0.40 | 7 ± 1.00 * | 65 ± 13.74 * | |
| 0.80 | 5 ± 2.00 * | 6 ± 1.0 * | |
| CEO-Loaded PLGA Nanoparticles | 0.125 | 31.6 ± 3.51 | 200.6 ± 2.08 |
| 0.25 | 26 ± 6.00 | 195.6 ± 5.13 | |
| 0.50 | 32 ± 2.64 | 209.3 ± 9.01 | |
| 1.00 | 31.3 ± 3.51 | 194.6 ± 25.3 | |
| Positive Control (NPD) | 0.005 | 847.3 ± 2.52 * | |
| Positive Control (SA) | 0.0005 | 1242 ± 21.2 * | |
| Negative Control (Water) | 25 ± 3.60 | 191 ± 8.51 | |
| Negative Control (Ethanol) | 27.6 ± 2.51 | 188 ± 3.60 | |
| Spontaneous Control | 24 ± 1.00 | 188.6 ± 3.51 | |
| Treatment | Concentrations (mg/mL) | Revertant Colonies/Plate | |
|---|---|---|---|
| TA98 Mean ± SD | TA98 Mean ± SD | ||
| OEO-CEO | 0.10 | 7.00 ± 2.00 * | 293 ± 3.21 * |
| 0.20 | 2.00 ± 1.00 * | 508 ± 3.78 * | |
| 0.40 | 5.33 ± 2.51 * | 82 ± 2.51 * | |
| 0.80 | 4.33 ± 2.08 * | 9.66 ± 2.51 * | |
| OEO-CEO-loaded PLGA nanoparticles | 0.125 | 21.33 ± 3.51 | 246.6 ± 9.07 |
| 0.25 | 24.33 ± 3.05 | 225 ± 5.0 | |
| 0.50 | 24.66 ± 2.30 | 228 ± 7.02 | |
| 1.00 | 23.66 ± 2.51 | 207.3 ± 2.08 | |
| Positive Control (NPD) | 0.005 | 847.33 ± 2.52 * | |
| Positive Control (SA) | 0.0005 | 1242 ± 21.2 * | |
| Negative Control (Water) | 25 ± 3.60 | 191 ± 8.51 | |
| Negative Control (Ethanol) | 27.66 ± 2.51 | 188 ± 3.60 | |
| Spontaneous Control | 24 ± 1.00 | 188.66 ± 3.51 | |
| Samples | MIC (mg/mL) | |
|---|---|---|
| L. casei | S. mutans | |
| CEO-loaded PLGA NPs | - | 0.25 |
| OEO-loaded PLGA NPs | - | - |
| CEO-OEO-loaded PLGA NPs | 0.5 | 0.5 |
| CEO | 0.40 | 0.20 |
| OEO | 0.80 | - |
| CEO-OEO | 0.20 | 0.10 |
| Blank PLGA NPs | - | - |
| Selection of Key Ingredients in EOs | The Main Active Ingredient of CEO Is Cinnamaldehyde, a Phenylpropanoid | The Main Active Ingredient of OEO Is D-Limonene | ||||
|---|---|---|---|---|---|---|
| Receptors | Streptococcus mutans PDB:3AIC | Lactobacillus casei PDB:5MTU | ||||
| Ligands | Carvacrol (Supplement) | Cinnamaldehyde | D-Limonene | Carvacrol (Supplement) | Cinnamaldehyde | D-Limonene |
| Docking score (Kcal/mol) | −5.349 | −3.693 | −3.235 | −5.818 | −4.098 | −4.221 |
| H bond interactions (Angstrom) | ASP345(1.63) HIS344(1.89) | ASN238(2.08) | - | ASP71(1.96) GLN72(1.87) | GLU262(1.91) | - |
| Salt bridge interaction | - | - | - | - | - | - |
| Pi-cation interaction | - | - | - | - | - | - |
| Hydrophobic residues | LEU139, TYR367 ALA235, PHE664, TYR673, LEU191, LEU190 | TYR673, PHE664, LEU191, LEU190, VAL714, ALA235, TRP274 | TYR367, LEU139, LEU191, LEU190, TYR719, TYR673, VAL714, PHE664 | ALA49, ALA69, TRP225, TRP344, TYR155, MET334 | MET334, TYR155, TRP344, ALA69, ALA49 | ALA49, ALA69, MET334, TRP225, TYR155, TRP344 |
| Polar residues | GLN349, ASN671, ASN619, GLN717 | ASN238, GLN717 | GLN717, GLN349 | GLN119, GLN72 | GLN72, SER48 | GLN119, GLN72, ASN153 |
| Positively charged residues | ARG232, HIP344 | ARG232, HIP344 | ARG232, HIP344 | - | - | - |
| Negatively charged residues | ASP234, ASP666, ASP345, GLU272 | ASP234, ASP666, ASP345, GLU272 | ASP234, ASP666, ASP345, | ASP71, GLH262 | ASP71, GLH262 | ASP71, GLH262 |
| Glycine | - | - | - | GLY260 | - | GLY260 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budama-Kilinc, Y.; Kurtur, O.B.; Gok, B.; Kecel-Gunduz, S.; Alpay-Karaoglu, S.; Yılmaz Atalı, P.; Kartal, M. Production of Prophylactic Nanoformulation for Dental Caries and Investigation of Its Effectiveness by In Vitro and In Silico Methods. Pharmaceutics 2025, 17, 167. https://doi.org/10.3390/pharmaceutics17020167
Budama-Kilinc Y, Kurtur OB, Gok B, Kecel-Gunduz S, Alpay-Karaoglu S, Yılmaz Atalı P, Kartal M. Production of Prophylactic Nanoformulation for Dental Caries and Investigation of Its Effectiveness by In Vitro and In Silico Methods. Pharmaceutics. 2025; 17(2):167. https://doi.org/10.3390/pharmaceutics17020167
Chicago/Turabian StyleBudama-Kilinc, Yasemin, Ozan Baris Kurtur, Bahar Gok, Serda Kecel-Gunduz, Sengul Alpay-Karaoglu, Pınar Yılmaz Atalı, and Murat Kartal. 2025. "Production of Prophylactic Nanoformulation for Dental Caries and Investigation of Its Effectiveness by In Vitro and In Silico Methods" Pharmaceutics 17, no. 2: 167. https://doi.org/10.3390/pharmaceutics17020167
APA StyleBudama-Kilinc, Y., Kurtur, O. B., Gok, B., Kecel-Gunduz, S., Alpay-Karaoglu, S., Yılmaz Atalı, P., & Kartal, M. (2025). Production of Prophylactic Nanoformulation for Dental Caries and Investigation of Its Effectiveness by In Vitro and In Silico Methods. Pharmaceutics, 17(2), 167. https://doi.org/10.3390/pharmaceutics17020167








