Autophagy-Targeting Stapled Peptide Utilizes Macropinocytosis for Cell Entry to Potentiate Anti-Proliferative Autosis in Small-Cell Lung Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Cell Culture
2.2. Reagents and Antibodies
2.3. Cell Viability Assays
2.4. Immunoblot Analysis
2.5. Flow Cytometry
2.6. Seahorse Analysis
2.7. Intracellular Ca2+ Measurement
2.8. Animal Study
3. Results
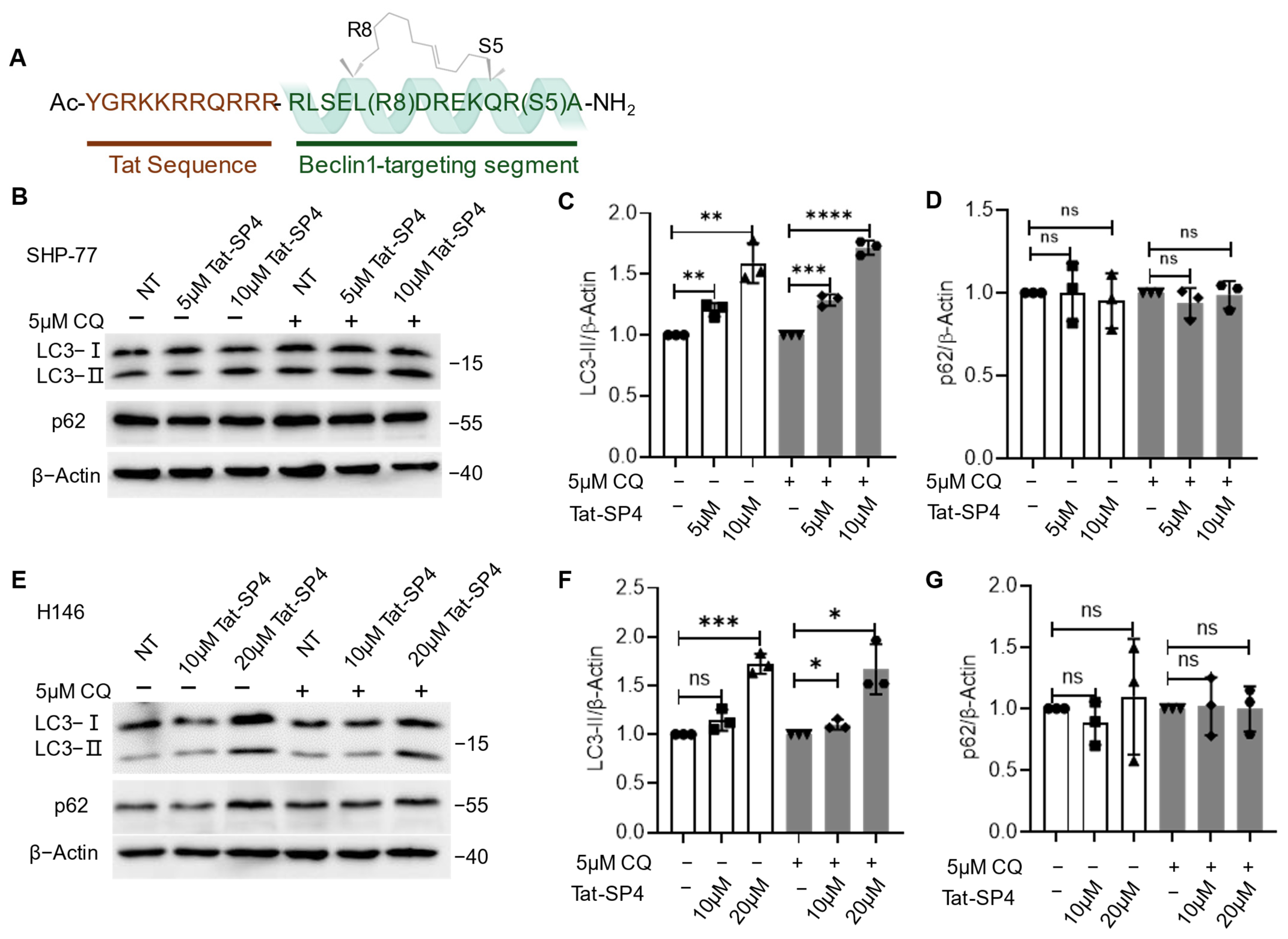
3.1. Beclin 1-Targeting Stapled Peptide Tat-SP4 Promotes Autophagy in SCLC Cells
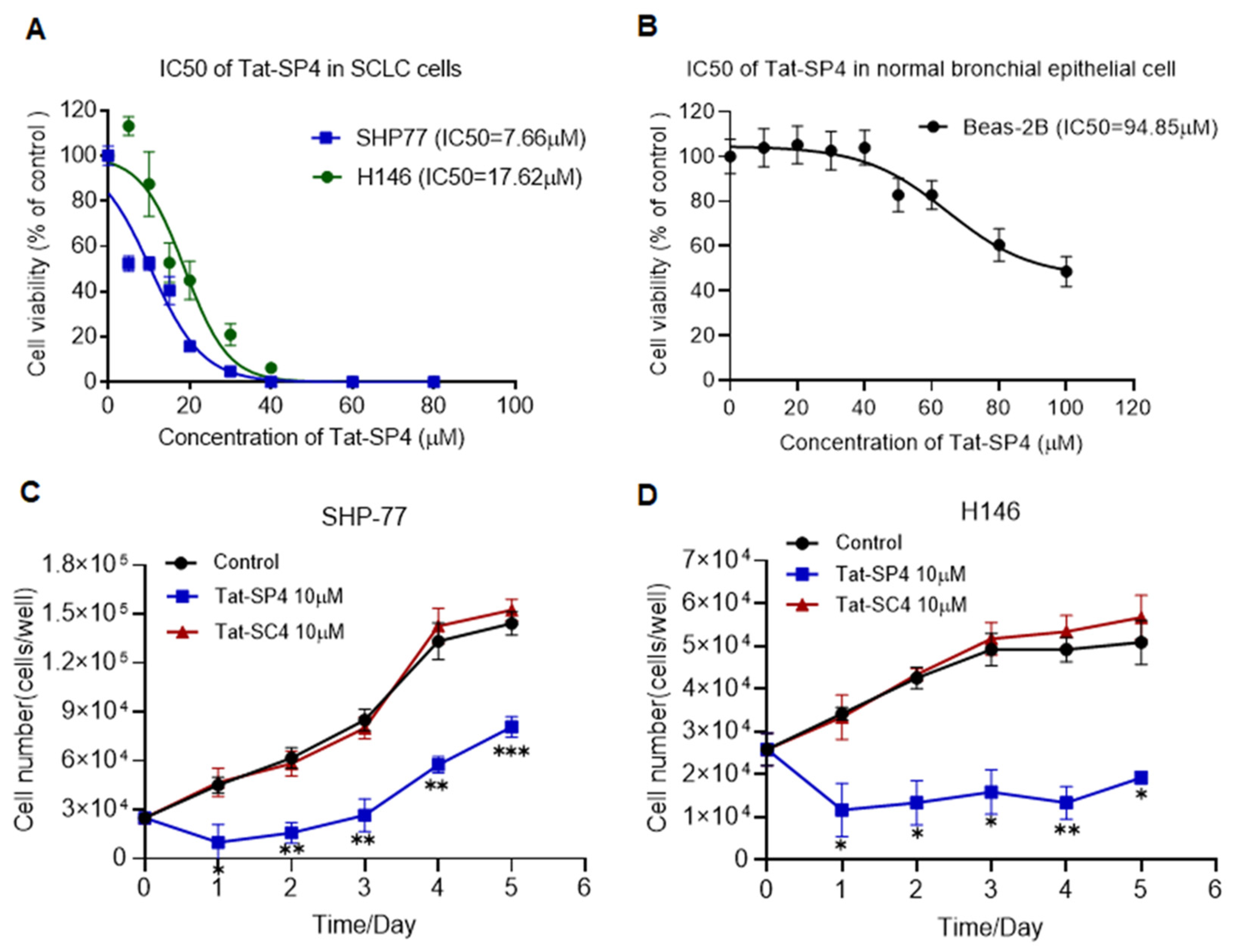
3.2. Tat-SP4 Inhibits Proliferation of SCLC Cells In Vitro
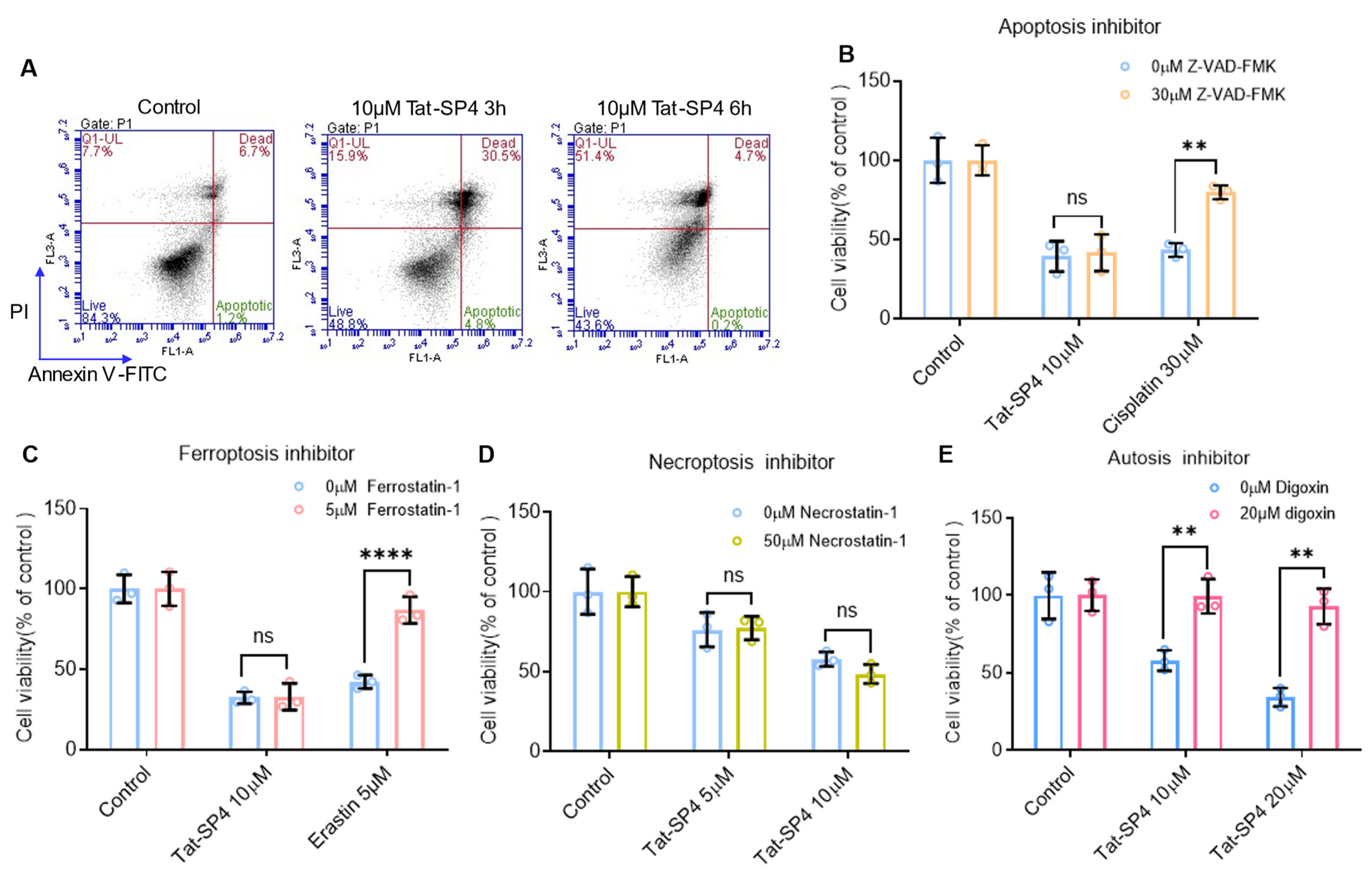
3.3. Tat-SP4 Induces Autotic Cell Death in SCLC Cells
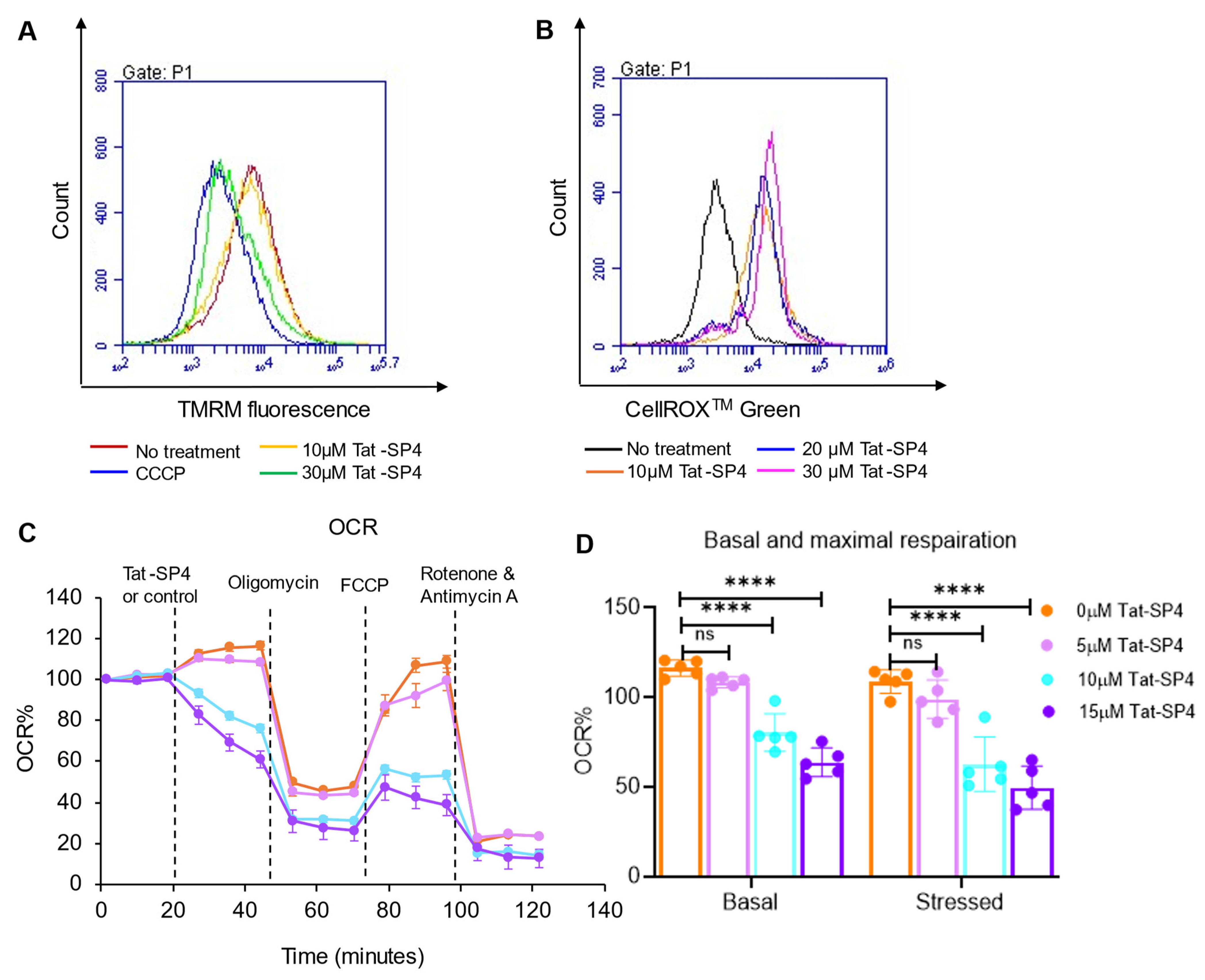
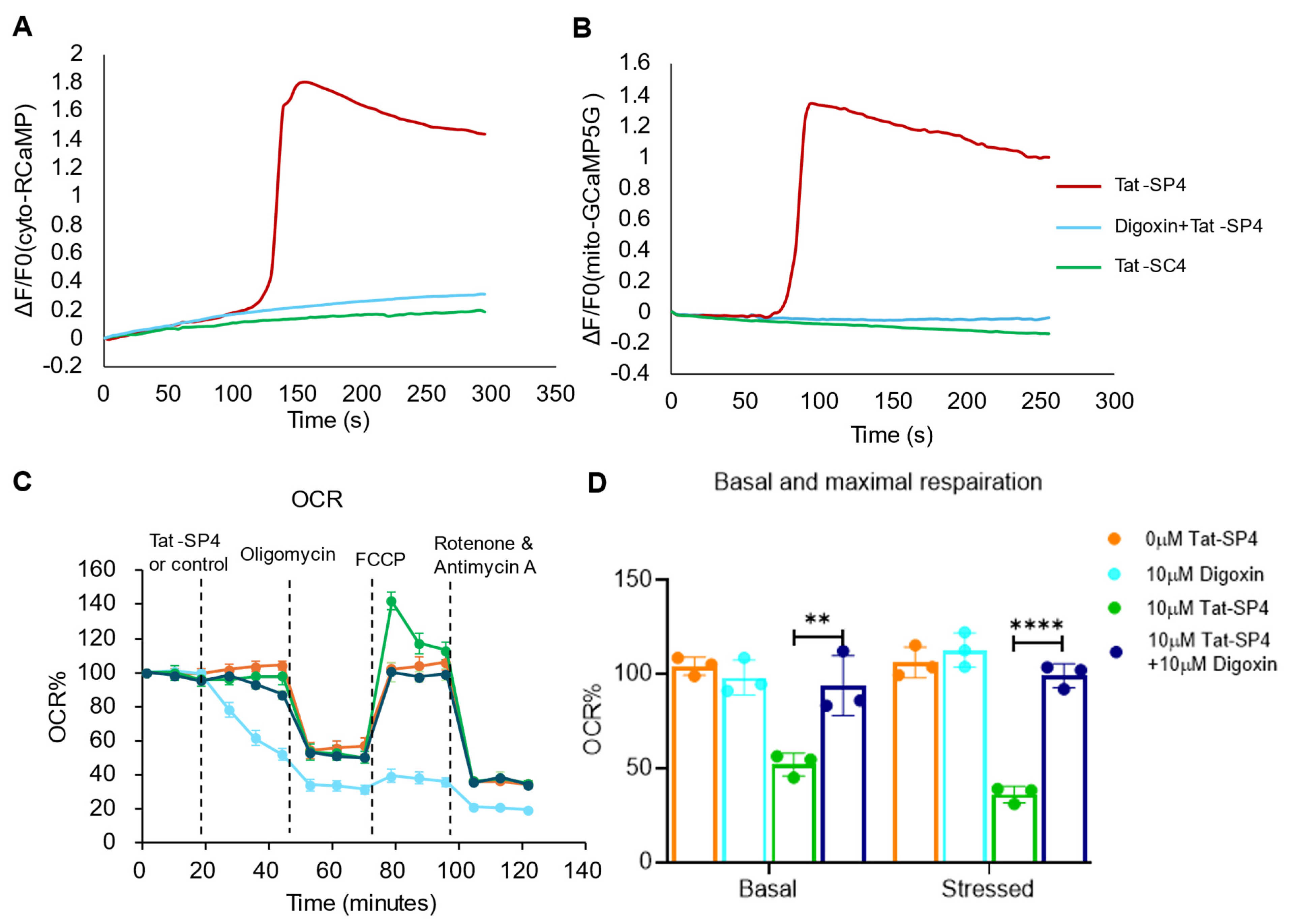
3.4. Tat-SP4 Causes Mitochondrial Dysfunction and Impairs OXPHOS Activity
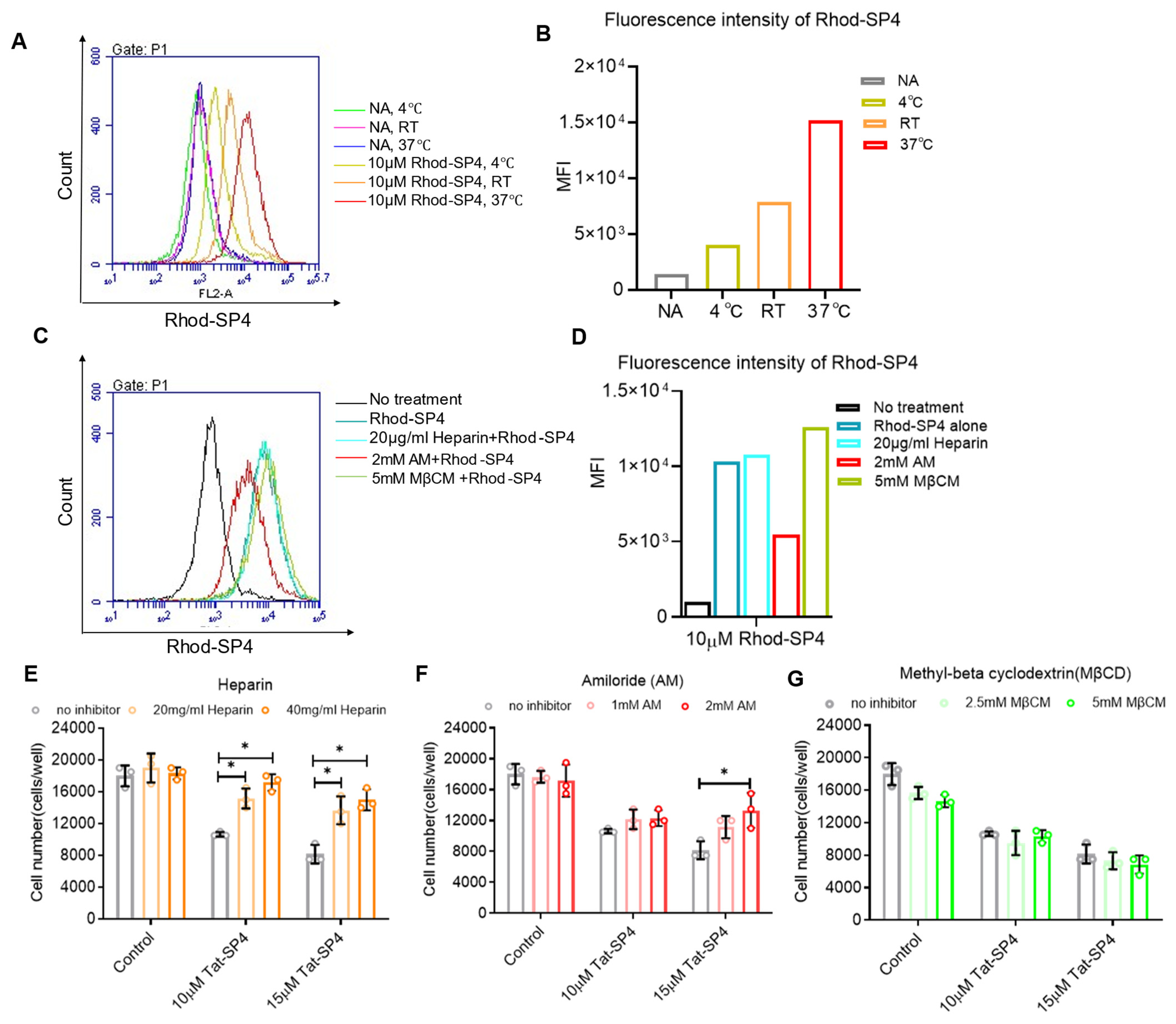
3.5. The Cell Entry of Tat-SP4 Is Mediated by Macropinocytosis
3.6. Ca2+ Influx Can Serve as a Surrogate Signal to Track Macropinocytosis-Mediated Cell Entry of Tat-SP4
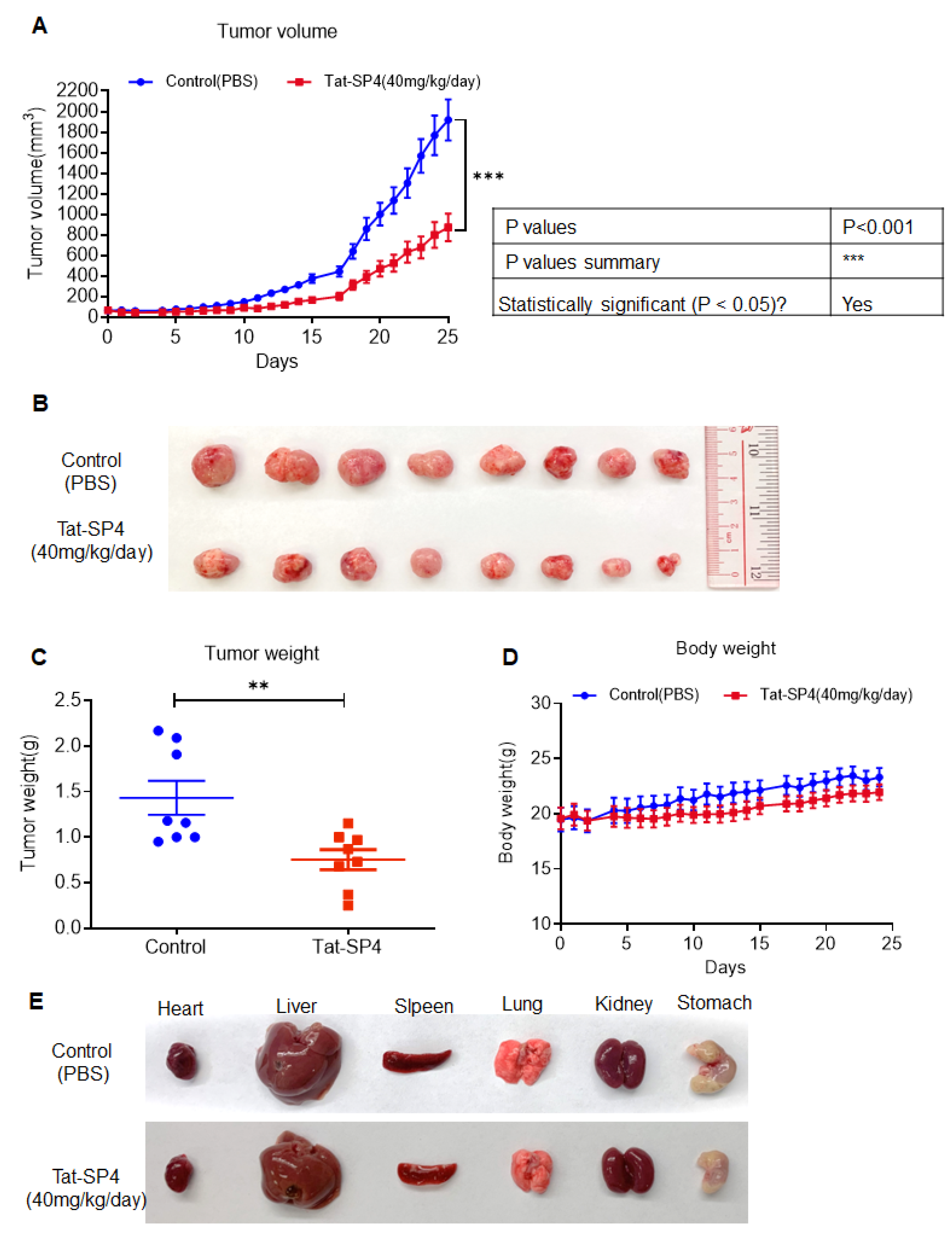
3.7. Tat-SP4 Exerts Potent Anti-Tumor Effect in SHP-77 Xenograft Model
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Wang, Q.; Gümüş, Z.H.; Colarossi, C.; Memeo, L.; Wang, X.; Kong, C.Y.; Boffetta, P. SCLC: Epidemiology, Risk Factors, Genetic Susceptibility, Molecular Pathology, Screening, and Early Detection. J. Thorac. Oncol. 2023, 18, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Simon, G.R. and H. Wagner, Small cell lung cancer. Chest 2003, 123 (Suppl. S1), 259s–271s. [Google Scholar] [CrossRef] [PubMed]
- Saltos, A.; Shafique, M.; Chiappori, A. Update on the Biology, Management, and Treatment of Small Cell Lung Cancer (SCLC). Front. Oncol. 2020, 10, 1074. [Google Scholar] [CrossRef]
- LaraLara, P.N.; Natale, R.; Crowley, J.; Lenz, H.J.; Redman, M.W.; Carleton, J.E.; Jett, J.; Langer, C.J.; Kuebler, J.P.; Dakhil, S.R.; et al. Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: Clinical and pharmacogenomic results from SWOG S0124. J. Clin. Oncol. 2009, 27, 2530–2535. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; et al. Durvalumab plus platinum–etoposide versus platinum–etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomised, controlled, open-label, phase 3 trial. Lancet 2019, 394, 1929–1939. [Google Scholar] [CrossRef]
- Melosky, B.; Cheema, P.K.; Brade, A.; McLeod, D.; Liu, G.; Price, P.W.; Jao, K.; Schellenberg, D.D.; Juergens, R.; Leighl, N.; et al. Prolonging Survival: The Role of Immune Checkpoint Inhibitors in the Treatment of Extensive-Stage Small Cell Lung Cancer. Oncologist 2020, 25, 981–992. [Google Scholar] [CrossRef]
- Knelson, E.H.; Patel, S.A.; Sands, J.M. PARP Inhibitors in Small-Cell Lung Cancer: Rational Combinations to Improve Responses. Cancers 2021, 13, 727. [Google Scholar] [CrossRef]
- Giffin, M.J.; Cooke, K.; Lobenhofer, E.K.; Estrada, J.C.; Zhan, J.; Deegen, P.; Thomas, M.; Murawsky, C.M.; Werner, J.; Liu, S.; et al. AMG 757, a Half-Life Extended, DLL3-Targeted Bispecific T-Cell Engager, Shows High Potency and Sensitivity in Preclinical Models of Small-Cell Lung Cancer. Clin. Cancer Res. 2021, 27, 1526–1537. [Google Scholar] [CrossRef]
- White, E. Deconvoluting the context-dependent role for autophagy in cancer. Nat. Rev. Cancer 2012, 12, 401–410. [Google Scholar] [CrossRef]
- Sutherland, K.D.; Proost, N.; Brouns, I.; Adriaensen, D.; Song, J.-Y.; Berns, A. Cell of origin of small cell lung cancer: Inactivation of Trp53 and Rb1 in distinct cell types of adult mouse lung. Cancer Cell 2011, 19, 754–764. [Google Scholar] [CrossRef]
- Hao, Y.; Li, M.; Liu, W.; Ma, Z.; Liu, Z. Autophagic flux modulates tumor heterogeneity and lineage plasticity in SCLC. Front. Oncol. 2024, 14, 1509183. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Tai, X.-J.; Cheng, W.; Wu, Y.; He, D.; Wang, L.-F.; Liu, H.; Zhang, S.-Y.; Sun, Y.-T.; Liu, H.-Z.; et al. Metformin inhibits the growth of SCLC cells by inducing autophagy and apoptosis via the suppression of EGFR and AKT signalling. Sci. Rep. 2025, 15, 6081. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Liu, R.; Dong, X.; Zhong, Q. Beclin orthologs: Integrative hubs of cell signaling, membrane trafficking, and physiology. Trends Cell Biol. 2015, 25, 533–544. [Google Scholar] [CrossRef]
- Wu, S.; He, Y.; Qiu, X.; Yang, W.; Liu, W.; Li, X.; Li, Y.; Shen, H.-M.; Wang, R.; Yue, Z.; et al. Targeting the potent Beclin 1-UVRAG coiled-coil interaction with designed peptides enhances autophagy and endolysosomal trafficking. Proc. Natl. Acad. Sci. USA 2018, 115, E5669–E5678. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shan, G.; Li, N.; Chen, J.; Ji, C.; Li, X.; Jiang, L.; Lee, T.K.W.; Keng, V.W.; Zhao, Y. An autophagy-inducing stapled peptide induces mitochondria dysfunction and triggers autotic cell death in triple-negative breast cancer. Cell Death Discov. 2023, 9, 303. [Google Scholar] [CrossRef]
- Li, N.; Zhang, X.; Chen, J.; Gao, S.; Wang, L.; Zhao, Y. Perturbation of Autophagy by a Beclin 1-Targeting Stapled Peptide Induces Mitochondria Stress and Inhibits Proliferation of Pancreatic Cancer Cells. Cancers 2023, 15, 953. [Google Scholar] [CrossRef]
- Gao, S.; Li, N.; Zhang, X.; Chen, J.; Ko, B.C.; Zhao, Y. An autophagy-inducing stapled peptide promotes c-MET degradation and overrides adaptive resistance to sorafenib in c-MET+ hepatocellular carcinoma. Biochem. Biophys. Rep. 2023, 33, 101412. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Gao, S.; Li, N.; Keng, V.; Zhao, Y. A Beclin 1-targeting stapled peptide synergizes with erlotinib to potently inhibit proliferation of non-small-cell lung cancer cells. Biochem. Biophys. Res. Commun. 2022, 636, 125–131. [Google Scholar] [CrossRef]
- Yang, Q.; Qiu, X.; Zhang, X.; Yu, Y.; Li, N.; Wei, X.; Feng, G.; Li, Y.; Zhao, Y.; Wang, R. Optimization of beclin 1-targeting stapled peptides by staple scanning leads to enhanced antiproliferative potency in cancer cells. J. Med. Chem. 2021, 64, 13475–13486. [Google Scholar] [CrossRef]
- Szczepny, A.; Rogers, S.; Jayasekara, W.S.N.; Park, K.; A McCloy, R.; Cochrane, C.R.; Ganju, V.; A Cooper, W.; Sage, J.; Peacock, C.D.; et al. The role of canonical and non-canonical Hedgehog signaling in tumor progression in a mouse model of small cell lung cancer. Oncogene 2017, 36, 5544–5550. [Google Scholar] [CrossRef]
- Lim, J.; Lachenmayer, M.L.; Wu, S.; Liu, W.; Kundu, M.; Wang, R.; Komatsu, M.; Oh, Y.J.; Zhao, Y.; Yue, Z. Proteotoxic stress induces phosphorylation of p62/SQSTM1 by ULK1 to regulate selective autophagic clearance of protein aggregates. PLoS Genet. 2015, 11, e1004987. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Lim, J.; Wang, Q.; Purtell, K.; Wu, S.; Palomo, G.M.; Tan, H.; Manfredi, G.; Zhao, Y.; Peng, J.; et al. ALS-FTLD-linked mutations of SQSTM1/p62 disrupt selective autophagy and NFE2L2/NRF2 anti-oxidative stress pathway. Autophagy 2020, 16, 917–931. [Google Scholar] [CrossRef]
- Liu, Y.; Levine, B. Autosis and autophagic cell death: The dark side of autophagy. Cell Death Differ. 2015, 22, 367–376. [Google Scholar] [CrossRef]
- Liu, Y.; Shoji-Kawata, S.; Sumpter, R.M., Jr.; Wei, Y.; Ginet, V.; Zhang, L.; Posner, B.; Tran, K.A.; Green, D.R.; Xavier, R.J.; et al. Autosis is a Na+,K+-ATPase-regulated form of cell death triggered by autophagy-inducing peptides, starvation, and hypoxia-ischemia. Proc. Natl. Acad. Sci. USA 2013, 110, 20364–20371. [Google Scholar] [CrossRef]
- Chaban, Y.; Boekema, E.J.; Dudkina, N.V. Structures of mitochondrial oxidative phosphorylation supercomplexes and mechanisms for their stabilisation. Biochim. Biophys. Acta (BBA)—Bioenerg. 2014, 1837, 418–426. [Google Scholar] [CrossRef]
- Madani, F.; Lindberg, S.; Langel, Ü.; Futaki, S.; Gräslund, A. Mechanisms of cellular uptake of cell-penetrating peptides. J. Biophys. 2011, 2011, 414729. [Google Scholar] [CrossRef]
- Zhang, M.S.; Di Cui, J.; Lee, D.; Yuen, V.W.-H.; Chiu, D.K.-C.; Goh, C.C.; Cheu, J.W.-S.; Tse, A.P.-W.; Bao, M.H.-R.; Wong, B.P.Y.; et al. Hypoxia-induced macropinocytosis represents a metabolic route for liver cancer. Nat. Commun. 2022, 13, 954. [Google Scholar] [CrossRef]
- Mauthe, M.; Orhon, I.; Rocchi, C.; Zhou, X.; Luhr, M.; Hijlkema, K.-J.; Coppes, R.P.; Engedal, N.; Mari, M.; Reggiori, F. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 2018, 14, 1435–1455. [Google Scholar] [CrossRef]
- Sotelo, J.; Briceño, E.; López-González, M.A. Adding chloroquine to conventional treatment for glioblastoma multiforme: A randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 2006, 144, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Bock, J.E.; Gavenonis, J.; Kritzer, J.A. Getting in shape: Controlling peptide bioactivity and bioavailability using conformational constraints. ACS Chem. Biol. 2013, 8, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Finicle, B.T.; Jayashankar, V.; Edinger, A.L. Nutrient scavenging in cancer. Nat. Rev. Cancer 2018, 18, 619–633. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Gao, S.; Zhang, X.; Li, N.; Yu, Y.; Wang, L.; Feng, Y.; Lao, Y.; Zhao, Y. Autophagy-Targeting Stapled Peptide Utilizes Macropinocytosis for Cell Entry to Potentiate Anti-Proliferative Autosis in Small-Cell Lung Cancer. Pharmaceutics 2025, 17, 1521. https://doi.org/10.3390/pharmaceutics17121521
Chen J, Gao S, Zhang X, Li N, Yu Y, Wang L, Feng Y, Lao Y, Zhao Y. Autophagy-Targeting Stapled Peptide Utilizes Macropinocytosis for Cell Entry to Potentiate Anti-Proliferative Autosis in Small-Cell Lung Cancer. Pharmaceutics. 2025; 17(12):1521. https://doi.org/10.3390/pharmaceutics17121521
Chicago/Turabian StyleChen, Jingyi, Shan Gao, Xiaozhe Zhang, Na Li, Yingting Yu, Lei Wang, Yu Feng, Yuanzhi Lao, and Yanxiang Zhao. 2025. "Autophagy-Targeting Stapled Peptide Utilizes Macropinocytosis for Cell Entry to Potentiate Anti-Proliferative Autosis in Small-Cell Lung Cancer" Pharmaceutics 17, no. 12: 1521. https://doi.org/10.3390/pharmaceutics17121521
APA StyleChen, J., Gao, S., Zhang, X., Li, N., Yu, Y., Wang, L., Feng, Y., Lao, Y., & Zhao, Y. (2025). Autophagy-Targeting Stapled Peptide Utilizes Macropinocytosis for Cell Entry to Potentiate Anti-Proliferative Autosis in Small-Cell Lung Cancer. Pharmaceutics, 17(12), 1521. https://doi.org/10.3390/pharmaceutics17121521





