Ginseng-Derived Carbon Quantum Dots Enhance Systemic Exposure of Bioactive Ginsenosides and Amplify Energy Metabolism in Mice
Abstract
1. Introduction
2. Methods
2.1. Reagents and Materials
2.2. Preparation of G-CQDs
2.3. Preparation of G-AE
2.4. Structural and Surface Characterization of G-CQDs
2.5. In Vivo Experimental Design
2.6. Energy Metabolism Detection
2.7. Compositional Analysis In Vitro and Vivo
2.7.1. Preparation of Samples for In Vitro Analysis
2.7.2. Preparation of Drug-Containing Serum
2.7.3. UHPLC-Q/Orbitrap/LTQ MS Analysis Conditions
2.7.4. Data Processing Methods
2.8. Correlation Analysis
2.9. Statistical Analysis
3. Result
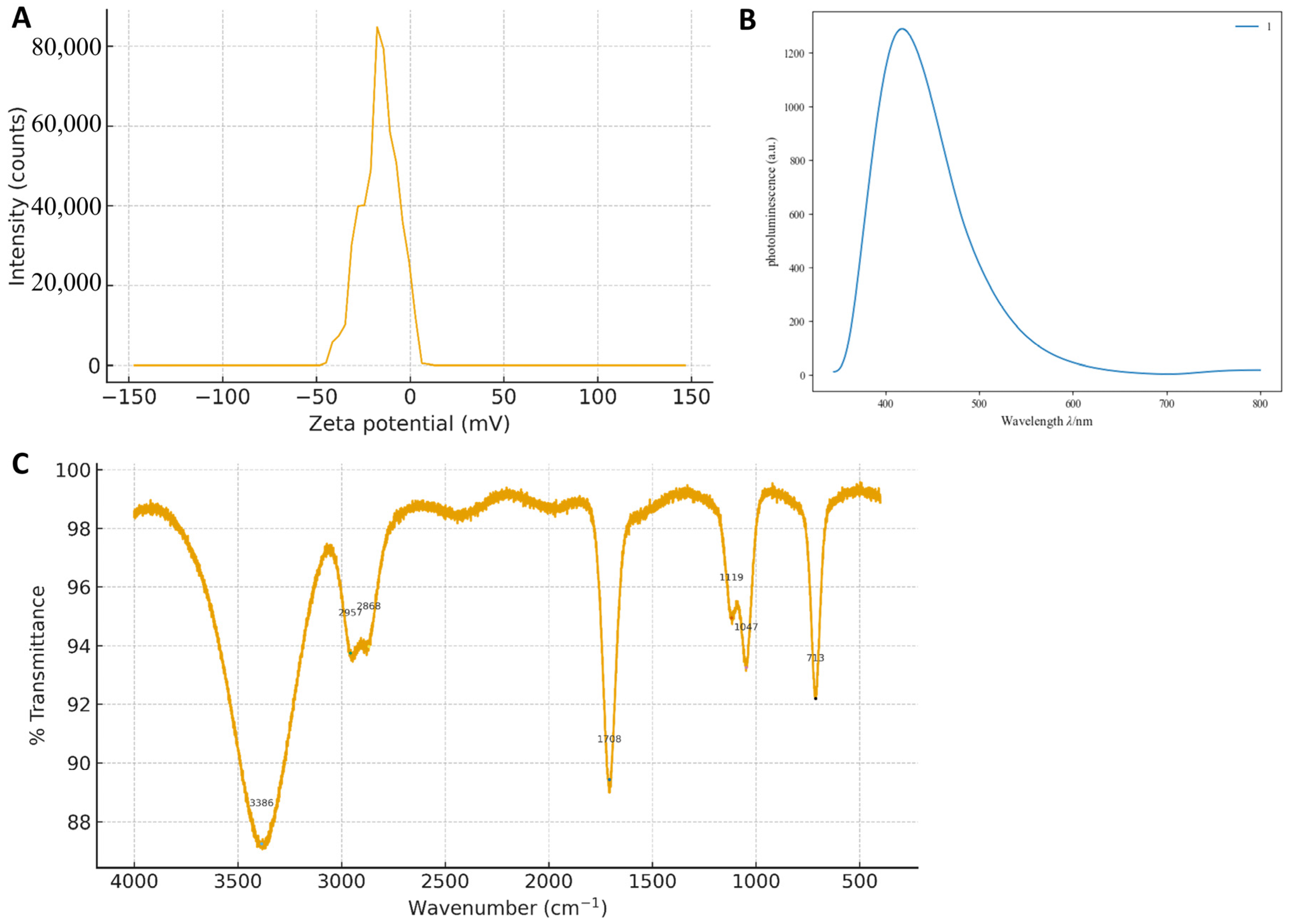
3.1. Structural and Surface Characterization of G-CQDs
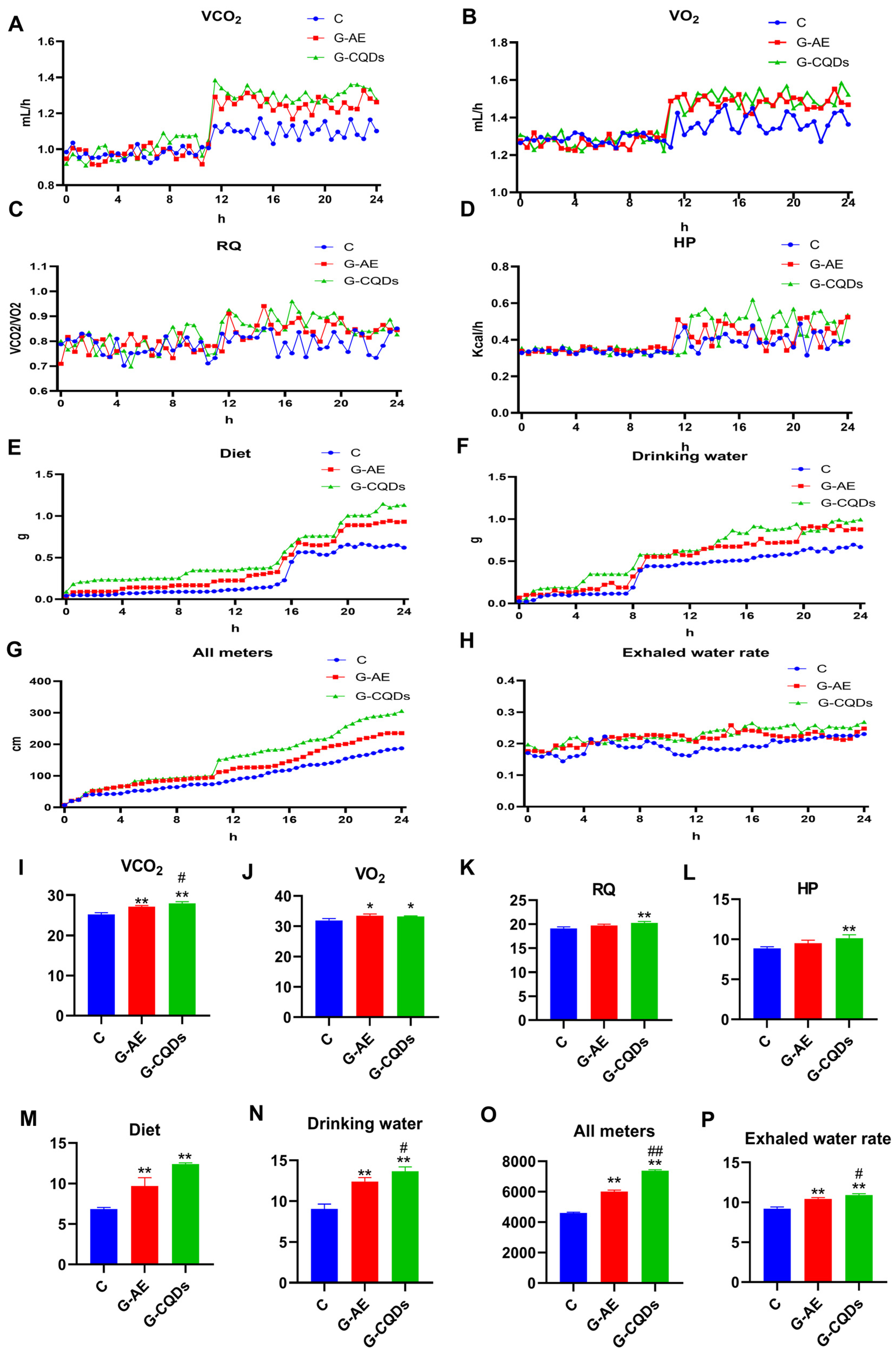
3.2. G-CQDs Elicit Superior Systemic Energy Metabolism Enhancement over G-AE
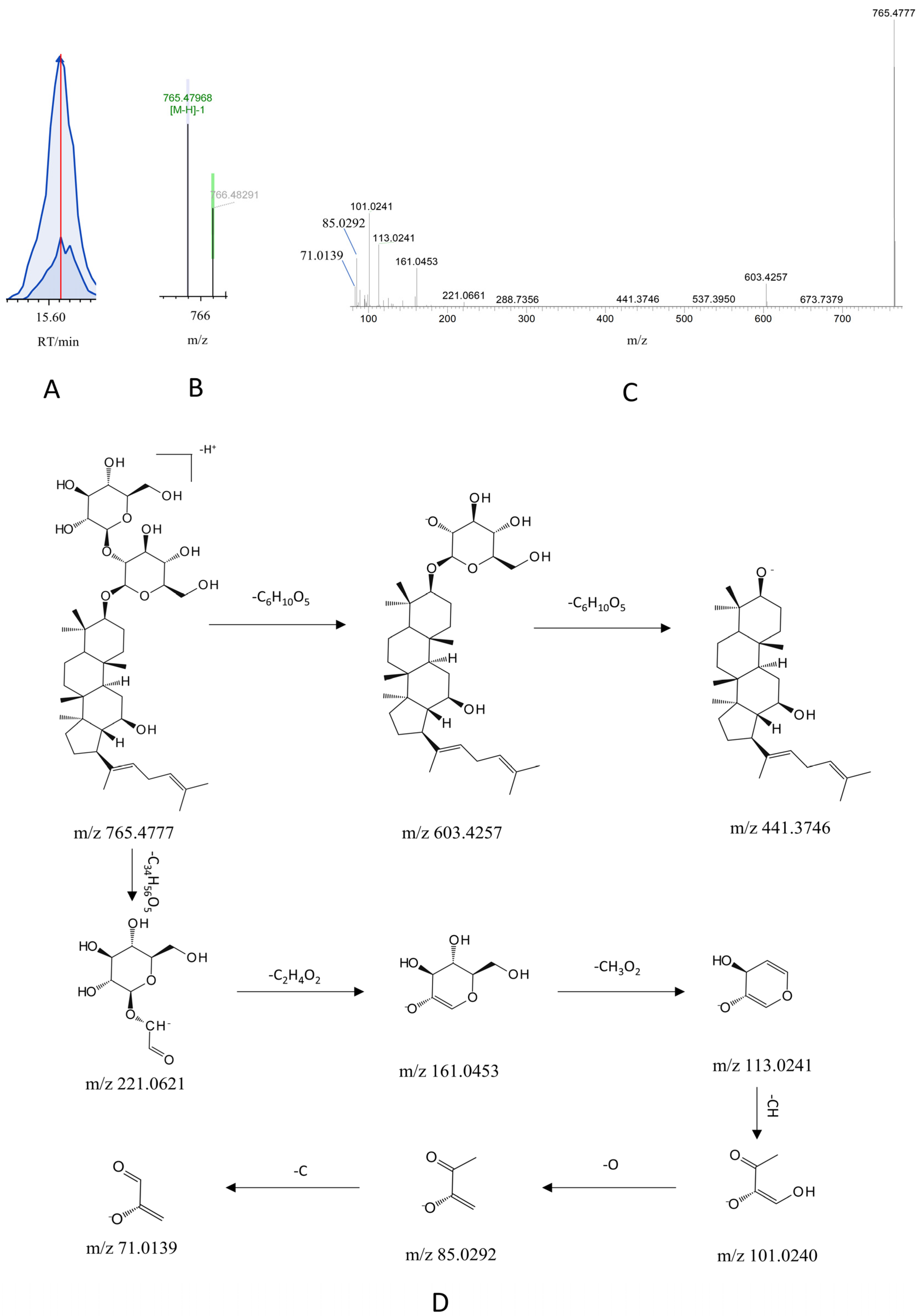
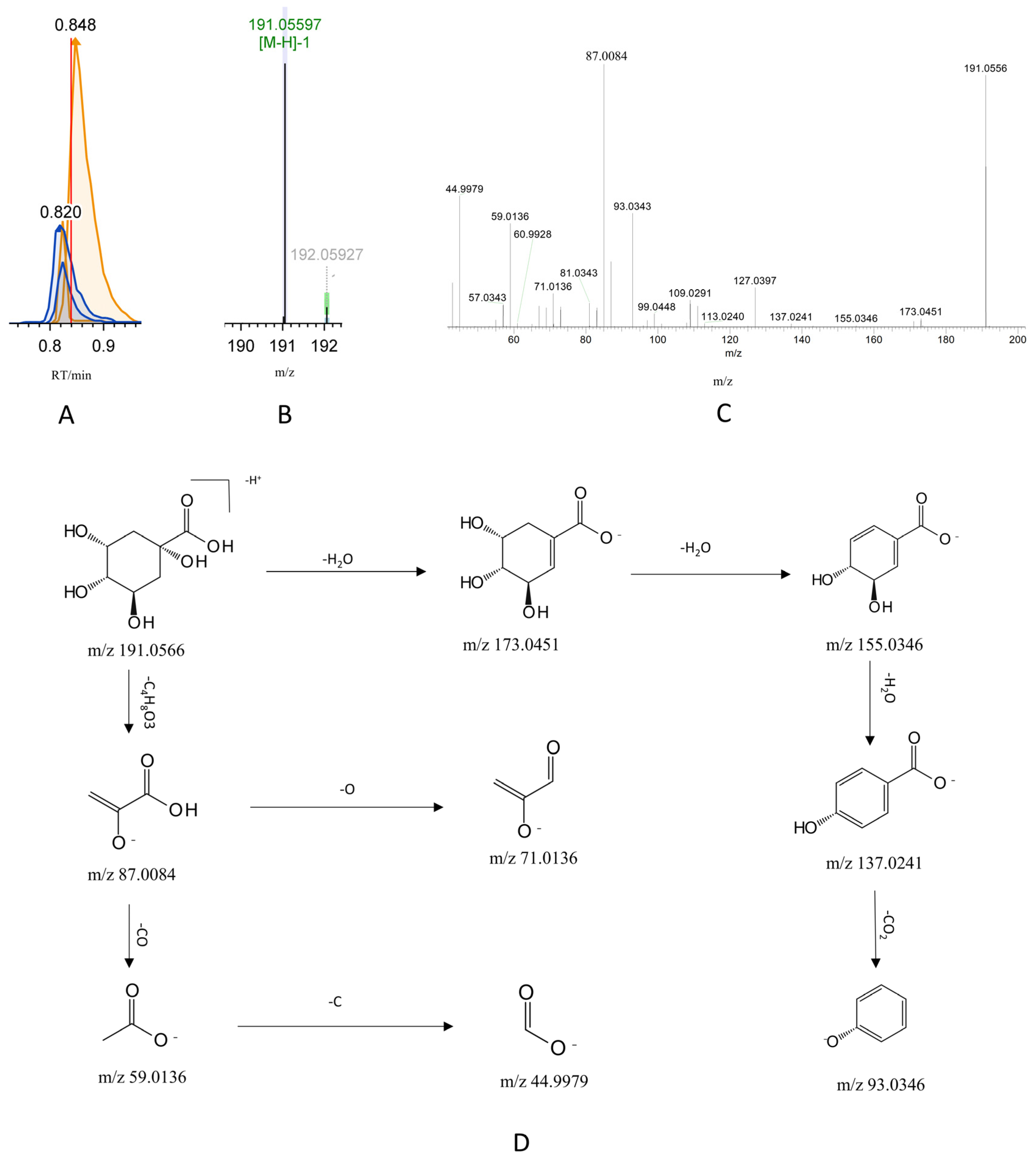
3.3. Comprehensive Mass Spectrometry Profiling Identifies 110 Chemical Constituents in G-CQDs’ Nanocomplex
3.4. In Vivo Tracing Reveals 35 Prototype Components of G-CQDs
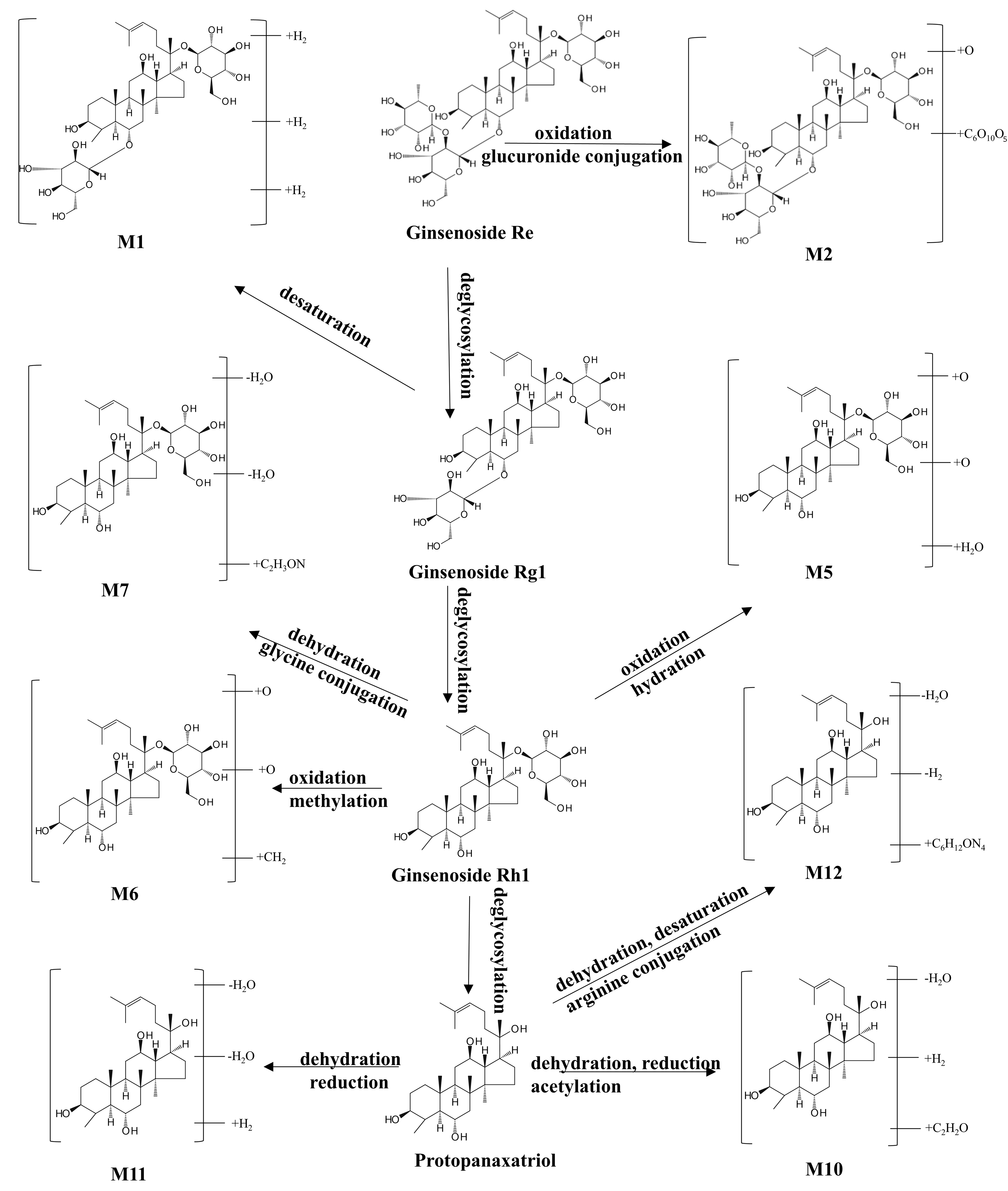
3.5. Metabolic Mapping Identifies 29 Phase I and II Metabolites of G-CQDs
3.6. G-CQDs Markedly Enhance Systemic Exposure of Bioactive Components In Vivo
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUC | area under the curve |
| CD | Compound Discoverer |
| CQDs | carbon quantum dots |
| DDA | data-dependent acquisition |
| FTIR | Fourier-transform infrared |
| G-AE | ginseng aqueous extract |
| G-CQDs | ginseng-derived carbon quantum dots |
| HP | heat production |
| HRTEM | high-resolution transmission electron microscopy |
| OXPHOS | oxidative phosphorylation |
| RQ | respiratory quotient |
| RT | retention time |
| SPF | specific pathogen-free |
| TEM | transmission electron microscopy |
| UV–Vis | ultraviolet–visible |
| VO2- | oxygen consumption |
| VCO2- | carbon dioxide production |
References
- Brazil, R. Speeding up ginseng growth to aid drug discovery. Nature 2025, 642, 264. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Ye, J.; Fan, P.; Zheng, R.; Wang, S.; Peng, Y.; Ruan, Y.; Yan, X.; Zhang, Z.; Chu, S.; et al. Targeting and reprogramming microglial phagocytosis of neutrophils by ginsenoside Rg1 nanovesicles promotes stroke recovery. Bioact. Mater. 2025, 47, 181–197. [Google Scholar] [CrossRef]
- Tang, X.; Yang, X.; Yu, Y.; Wu, M.; Li, Y.; Zhang, Z.; Jia, G.; Wang, Q.; Tu, W.; Wang, Y.; et al. Carbon quantum dots of ginsenoside Rb1 for application in a mouse model of intracerebral Hemorrhage. J. Nanobiotechnol. 2024, 22, 125. [Google Scholar] [CrossRef]
- Ma, C.; Lin, Q.; Xue, Y.; Ju, Z.; Deng, G.; Liu, W.; Sun, Y.; Guan, H.; Cheng, X.; Wang, C. Pharmacokinetic studies of ginsenosides Rk1 and Rg5 in rats by UFLC-MS/MS. Biomed. Chromatogr. 2021, 35, e5108. [Google Scholar] [CrossRef]
- Yadav, P.; Ambudkar, S.V.; Rajendra Prasad, N. Emerging nanotechnology-based therapeutics to combat multidrug-resistant cancer. J. Nanobiotechnol. 2022, 20, 423. [Google Scholar] [CrossRef] [PubMed]
- Ratan, Z.A.; Haidere, M.F.; Hong, Y.H.; Park, S.H.; Lee, J.-O.; Lee, J.; Cho, J.Y. Pharmacological potential of ginseng and its major component ginsenosides. J. Ginseng Res. 2020, 45, 199–210. [Google Scholar] [CrossRef]
- Dahan, A.; Yarmolinsky, L.; Nakonechny, F.; Semenova, O.; Khalfin, B.; Ben-Shabat, S. Etrog Citron (Citrus medica) as a Novel Source of Antimicrobial Agents: Overview of Its Bioactive Phytochemicals and Delivery Approaches. Pharmaceutics 2025, 17, 761. [Google Scholar] [CrossRef]
- Saravanan, A.; Maruthapandi, M.; Das, P.; Ganguly, S.; Margel, S.; Luong, J.H.T.; Gedanken, A. Applications of N-Doped Carbon Dots as Antimicrobial Agents, Antibiotic Carriers, and Selective Fluorescent Probes for Nitro Explosives. ACS Appl. Bio Mater. 2020, 3, 8023–8031. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Q.; Zhou, S. Carbon-based hybrid nanogels: A synergistic nanoplatform for combined biosensing, bioimaging, and responsive drug delivery. Chem. Soc. Rev. 2018, 47, 4198–4232. [Google Scholar] [CrossRef]
- Zhao, S.; Yue, G.; Liu, X.; Qin, S.; Wang, B.; Zhao, P.; Ragauskas, A.J.; Wu, M.; Song, X. Lignin-based carbon quantum dots with high fluorescence performance prepared by supercritical catalysis and solvothermal treatment for tumor-targeted labeling. Adv. Compos. Hybrid Mater. 2023, 6, 73. [Google Scholar] [CrossRef]
- Chen, Z.; Yuan, Z.; Yang, S.; Zhu, Y.; Xue, M.; Zhang, J.; Leng, L. Brain Energy Metabolism: Astrocytes in Neurodegenerative Diseases. CNS Neurosci. Ther. 2023, 29, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Yang, Y.; Metwaly, A.M.; Xue, Y.; Shi, Y.; Dou, D. The Chinese herbal formulae (Yitangkang) exerts an antidiabetic effect through the regulation of substance metabolism and energy metabolism in type 2 diabetic rats. J. Ethnopharmacol. 2019, 239, 111942. [Google Scholar] [CrossRef] [PubMed]
- McHill, A.W.; Thosar, S.S.; Bowles, N.P.; Butler, M.P.; Ordaz-Johnson, O.; Emens, J.S.; Purnell, J.Q.; Gillingham, M.; Shea, S.A. Obesity alters the circadian profiles of energy metabolism and glucose regulation in humans. Obesity 2024, 32, 315–323. [Google Scholar] [CrossRef]
- Balusamy, S.R.; Perumalsamy, H.; Huq, M.A.; Yoon, T.H.; Mijakovic, I.; Thangavelu, L.; Yang, D.C.; Rahimi, S. A comprehensive and systemic review of ginseng-based nanomaterials: Synthesis, targeted delivery, and biomedical applications. Med. Res. Rev. 2023, 43, 1374–1410. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S.; Wang, J.; Guo, X.; Song, Y.; Fu, K.; Gao, Z.; Liu, D.; He, W.; Yang, L.-L. Energy metabolism in health and diseases. Signal Transduct. Target. Ther. 2025, 10, 69. [Google Scholar] [CrossRef]
- Cheng, K.T.; Wang, Y.S.; Chou, H.C.; Chang, C.C.; Lee, C.K.; Juan, S.H. Kinsenoside-mediated lipolysis through an AMPK-dependent pathway in C3H10T1/2 adipocytes: Roles of AMPK and PPARα in the lipolytic effect of kinsenoside. Phytomedicine 2015, 22, 641–647. [Google Scholar] [CrossRef]
- Li, F.; Li, Y.; Yang, X.; Han, X.; Jiao, Y.; Wei, T.; Yang, D.; Xu, H.; Nie, G. Highly Fluorescent Chiral N-S-Doped Carbon Dots from Cysteine: Affecting Cellular Energy Metabolism. Angew. Chem. Int. Ed. Engl. 2018, 57, 2377–2382. [Google Scholar] [CrossRef]
- González-González, R.B.; González, L.T.; Madou, M.; Leyva-Porras, C.; Martinez-Chapa, S.O.; Mendoza, A. Synthesis, Purification, and Characterization of Carbon Dots from Non-Activated and Activated Pyrolytic Carbon Black. Nanomaterials 2022, 12, 298. [Google Scholar] [CrossRef] [PubMed]
- Tejwan, N.; Sharma, A.; Thakur, S.; Das, J. Green synthesis of a novel carbon dots from red Korean ginseng and its application for Fe2+ sensing and preparation of nanocatalyst. Inorg. Chem. Commun. 2021, 134, 108985. [Google Scholar] [CrossRef]
- Qiu, C.; Jiang, L.; Gao, Y.; Sheng, L. Effects of oxygen-containing functional groups on carbon materials in supercapacitors: A review. Mater. Des. 2023, 230, 111952. [Google Scholar] [CrossRef]
- Russo, C.; Stanzione, F.; Tregrossi, A.; Ciajolo, A. Infrared spectroscopy of some carbon-based materials relevant in combustion: Qualitative and quantitative analysis of hydrogen. Carbon 2014, 74, 127–138. [Google Scholar] [CrossRef]
- Basak, S.; Das, T.K. Enzyme-Immobilized Oxoammonium Nanogels: A Biocompatible and Injectable Platform for Enhanced Enzyme Stability and Reusability. Biomacromolecules 2025, 26, 8289–8302. [Google Scholar] [CrossRef]
- Amendola, V.; Meneghetti, M. Size Evaluation of Gold Nanoparticles by UV−vis Spectroscopy. J. Phys. Chem. C 2009, 113, 4277–4285. [Google Scholar] [CrossRef]
- Mohan, H.; Fagan, A.; Giordani, S. Carbon Nanomaterials (CNMs) in Cancer Therapy: A Database of CNM-Based Nanocarrier Systems. Pharmaceutics 2023, 15, 1545. [Google Scholar] [CrossRef]
- Chan, M.-H.; Chen, B.-G.; Ngo, L.T.; Huang, W.-T.; Li, C.-H.; Liu, R.-S.; Hsiao, M. Natural Carbon Nanodots: Toxicity Assessment and Theranostic Biological Application. Pharmaceutics 2021, 13, 1874. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Kong, H.; Zhang, M.; Cheng, J.; Luo, J.; Zhao, Y.; Qu, H. Haemostatic Nanoparticles-Derived Bioactivity of from Selaginella tamariscina Carbonisata. Molecules 2020, 25, 446. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, M.; Cheng, J.; Zhang, Y.; Luo, J.; Liu, Y.; Kong, H.; Qu, H.; Zhao, Y. Effect of Lonicerae japonicae Flos Carbonisata-Derived Carbon Dots on Rat Models of Fever and Hypothermia Induced by Lipopolysaccharide. Int. J. Nanomed. 2020, 15, 4139–4149. [Google Scholar] [CrossRef]
- Wang, J.; Tian, N.; Tian, T.; Xiao, L.; Zhou, X.; Liu, G.; Zhang, Z.; Zhao, Y.; Guo, J.; Lin, Q.; et al. Low toxicity ginsenoside Rg1-carbon nanodots as a potential therapeutic agent for human non-small cell lung cancer. Colloids Surf. B Biointerfaces 2025, 246, 114392. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, A.; Gopinath, P. Green synthesis of multifunctional carbon dots from coriander leaves and their potential application as antioxidants, sensors and bioimaging agents. Analyst 2015, 140, 4260–4269. [Google Scholar] [CrossRef]
- Wang, Z.; Han, J.; Guo, Z.; Wu, H.; Liu, Y.; Wang, W.; Zhang, C.; Liu, J. Ginseng-based carbon dots inhibit the growth of squamous cancer cells by increasing ferroptosis. Front. Oncol. 2023, 13, 1097692. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xiao, L.; Wang, J.; Tian, T.; Liu, G.; Zhao, Y.; Guo, J.; Zhang, W.; Wang, J.; Chen, C.; et al. Carbon nanodots constructed by ginsenosides and their high inhibitory effect on neuroblastoma. J. Nanobiotechnol. 2023, 21, 244. [Google Scholar] [CrossRef]
- Yao, H.; Li, J.; Song, Y.; Zhao, H.; Wei, Z.; Li, X.; Jin, Y.; Yang, B.; Jiang, J. Synthesis of ginsenoside Re-based carbon dots applied for bioimaging and effective inhibition of cancer cells. Int. J. Nanomed. 2018, 13, 6249–6264. [Google Scholar] [CrossRef]
- Mandal, S.; Adhikari, S.; Murmu, M.; Kim, B.; Kim, D. Graphene and Carbon Quantum Dots: Competing Carbons in Harmonized Photoelectrochemical Platforms. Small 2025, 21, e05846. [Google Scholar] [CrossRef]
- Tu, L.; Li, Q.; Qiu, S.; Li, M.; Shin, J.; Wu, P.; Singh, N.; Li, J.; Ding, Q.; Hu, C.; et al. Recent developments in carbon dots: A biomedical application perspective. J. Mater. Chem. B 2023, 11, 3038–3053. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, T.; Shin, G.H.; Kim, J.T. Carbon Dots: Opportunities and Challenges in Cancer Therapy. Pharmaceutics 2023, 15, 1019. [Google Scholar] [CrossRef]
- Panico, S.; Capolla, S.; Bozzer, S.; Toffoli, G.; Bo, M.D.; Macor, P. Biological Features of Nanoparticles: Protein Corona Formation and Interaction with the Immune System. Pharmaceutics 2022, 14, 2605. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Yamada, K.; Miyake, M.; Onoue, S. Recent Advancements in the Development of Nanocarriers for Mucosal Drug Delivery Systems to Control Oral Absorption. Pharmaceutics 2023, 15, 2708. [Google Scholar] [CrossRef] [PubMed]
- Crunkhorn, S. Cancer: Disrupting energy metabolism. Nat. Rev. Drug. Discov. 2018, 17, 708. [Google Scholar]
- Xia, W.; Wang, Y.; Yue, J.; Fu, X. Insights into Q-markers of honey-fried licorice in treating spleen deficiency based on substance and energy metabolism regulation. Phytomedicine 2024, 127, 155498. [Google Scholar] [CrossRef]
- Wang, W.; Zhan, W.; Liang, M.; Huang, Y.; Liu, Y.; Wang, L.; Bei, W.; Guo, J. Ginsenoside Rb1 ameliorates the abnormal hepatic glucose metabolism by activating STAT3 in T2DM mice. J. Funct. Foods 2023, 104, 105534. [Google Scholar] [CrossRef]
- Wen, S.; Zou, Z.-R.; Cheng, S.; Guo, H.; Hu, H.-S.; Zeng, F.-Z.; Mei, X.-F. Ginsenoside Rb1 improves energy metabolism after spinal cord injury. Neural Regen. Res. 2023, 18, 1332–1338. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, M.-H.; Yang, T.; Qin, T.-Y.; Qin, L.-L.; Hu, Y.-M.; Zhang, C.-F.; Sun, B.-J.; Ding, L.; Wu, L.-L.; et al. Mechanisms for Improving Hepatic Glucolipid Metabolism by Cinnamic Acid and Cinnamic Aldehyde: An Insight Provided by Multi-Omics. Front. Nutr. 2021, 8, 794841. [Google Scholar] [CrossRef]
- Mercola, J. Linoleic acid, mitochondria, gut microbiome, and metabolic health: A mechanistic review. Adv. Redox Res. 2025, 15, 100128. [Google Scholar] [CrossRef]
- Yin, F.; Li, P.; Liu, C.; Zheng, Y.; Yan, G.; Wang, M.; Wang, Y.; Chen, X.; Yan, X.; Han, J.; et al. Spatially resolved multi-omics reveals the renal cortex-metabolic reprogramming of Shenhua Tablet for intervention on IgA nephropathy. Phytomedicine 2025, 141, 156742. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Li, P.; Sun, H.; Zheng, Y.; Liu, C.; Chen, X.; Guan, S.; Yin, F.; Wang, X. Integrated metabolomics and mass spectrometry imaging analysis reveal the efficacy and mechanism of Huangkui capsule on type 2 diabetic nephropathy. Phytomedicine 2025, 138, 156397. [Google Scholar] [CrossRef]
- Wang, H.; Tang, C.; Xiang, Y.; Zou, C.; Hu, J.; Yang, G.; Zhou, W. Tea polyphenol-derived nanomedicine for targeted photothermal thrombolysis and inflammation suppression. J. Nanobiotechnol. 2024, 22, 146. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Yu, B.; Lan, X.; Cui, X.; Zhao, D.; Qiu, L.; Wang, H.; Wang, W.; Chen, L.; Jin, L.; et al. Synthesis, bioactivity evaluation and theoretical study of nicotinamide derivatives containing diphenyl ether fragments as potential succinate dehydrogenase inhibitors. J. Mol. Struct. 2024, 1308, 138331. [Google Scholar] [CrossRef]
- Fu, B.; Liu, W.; Wang, Y.; Li, G.; Wang, Y.; Huang, X.; Shi, H.; Qin, C. Design and Synthesis of Thiourea-Conjugating Organic Arsenic D-Glucose with Anticancer Activities. Molecules 2024, 29, 2850. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Sun, X.; Yang, B.; Lin, C.; Zhang, X.; Sun, H.; Meng, X.; Bai, Y.; Zhang, T.; Yan, G.; et al. Ginseng-Derived Carbon Quantum Dots Enhance Systemic Exposure of Bioactive Ginsenosides and Amplify Energy Metabolism in Mice. Pharmaceutics 2025, 17, 1485. https://doi.org/10.3390/pharmaceutics17111485
Liu H, Sun X, Yang B, Lin C, Zhang X, Sun H, Meng X, Bai Y, Zhang T, Yan G, et al. Ginseng-Derived Carbon Quantum Dots Enhance Systemic Exposure of Bioactive Ginsenosides and Amplify Energy Metabolism in Mice. Pharmaceutics. 2025; 17(11):1485. https://doi.org/10.3390/pharmaceutics17111485
Chicago/Turabian StyleLiu, Huiqiang, Xin Sun, Bo Yang, Chuan Lin, Xiwu Zhang, Hui Sun, Xiangcai Meng, Yufeng Bai, Tao Zhang, Guangli Yan, and et al. 2025. "Ginseng-Derived Carbon Quantum Dots Enhance Systemic Exposure of Bioactive Ginsenosides and Amplify Energy Metabolism in Mice" Pharmaceutics 17, no. 11: 1485. https://doi.org/10.3390/pharmaceutics17111485
APA StyleLiu, H., Sun, X., Yang, B., Lin, C., Zhang, X., Sun, H., Meng, X., Bai, Y., Zhang, T., Yan, G., Han, Y., & Wang, X. (2025). Ginseng-Derived Carbon Quantum Dots Enhance Systemic Exposure of Bioactive Ginsenosides and Amplify Energy Metabolism in Mice. Pharmaceutics, 17(11), 1485. https://doi.org/10.3390/pharmaceutics17111485





