Niosomes as Vesicular Carriers: From Formulation Strategies to Stimuli-Responsive Innovative Modulations for Targeted Drug Delivery
Abstract
1. Introduction
2. Formulation Aspects of NIOs
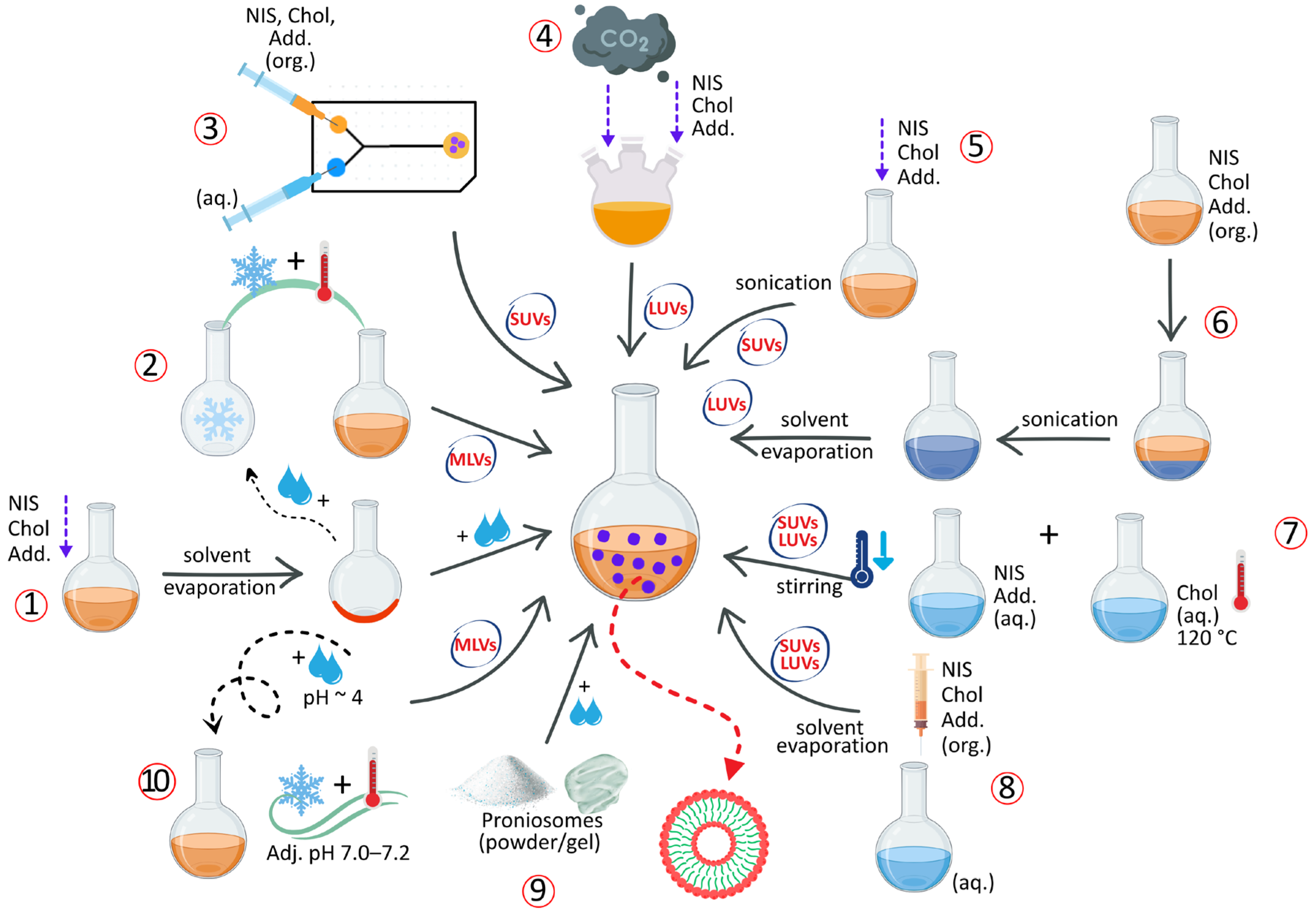
2.1. Methods of NIOs Preparation
2.2. Formulation Factors Affecting NIOs Characteristics
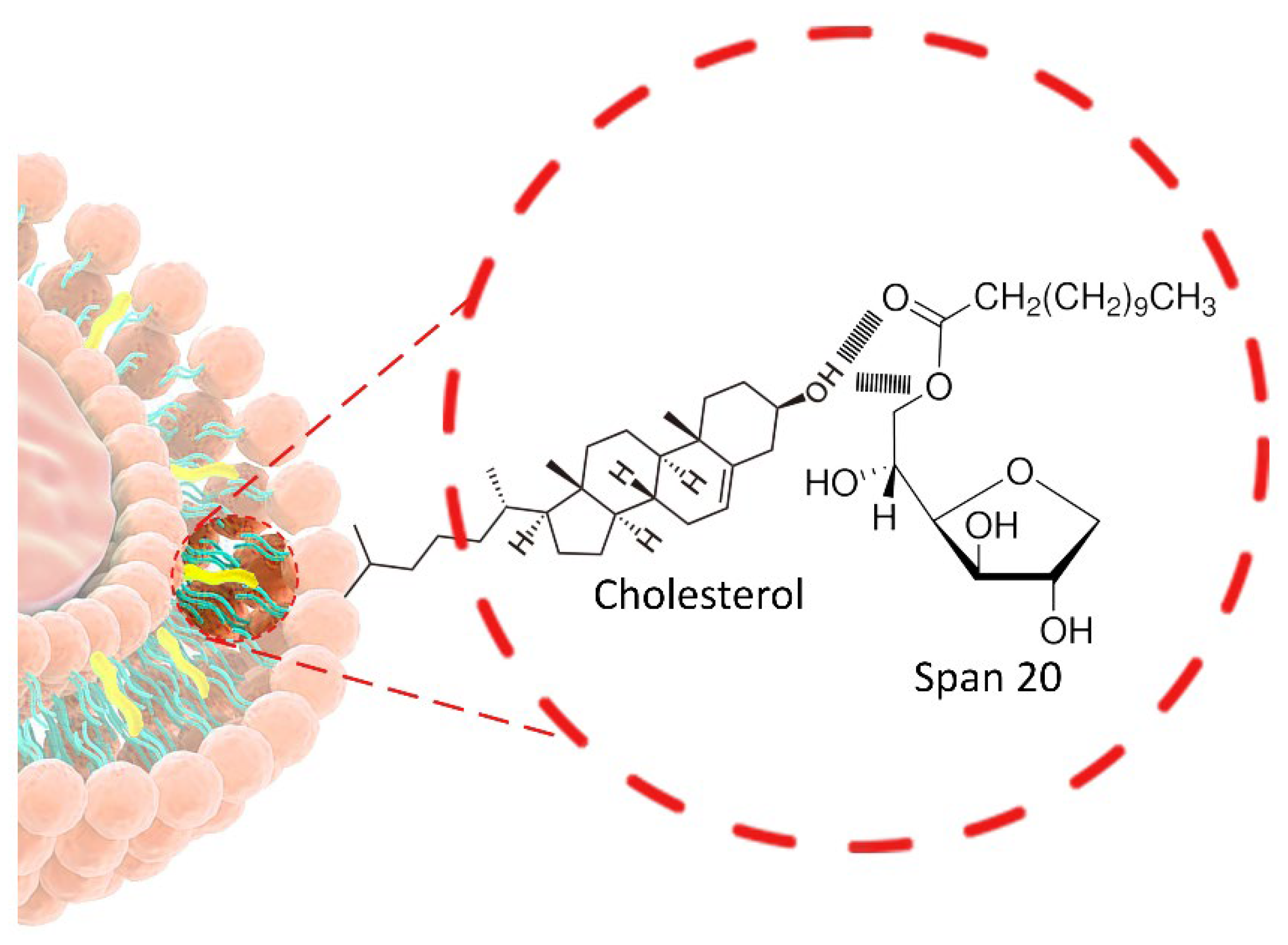
2.2.1. Non-Ionic Surfactant (NISs)
- The Hydrophilic–Lipophilic Balance
- Critical Packing Parameter
- Gel-Liquid Transition Temperature
2.2.2. Additive Agents
2.2.3. Preparation Conditions
2.2.4. Characteristics of the Encapsulated APIs
2.2.5. Resistance to Osmotic Stress
2.3. Preparation Methods for NIOs
2.3.1. Thin-Film Hydration Method
2.3.2. The Freeze–Thaw Method
2.3.3. Transmembrane pH Gradient Method
2.3.4. The “Bubble” Method
2.3.5. Solvent Injection Method
2.3.6. Sonication Method
2.3.7. The Reverse-Phase Evaporation Method
2.3.8. Microfluidization Method
2.3.9. The Heating Method
2.3.10. Proniosomes Technology
- Lipid Injection Method: a solvent-free modification of solvent injection technique, in which molten NISs, Chol, and additives are injected into a vigorously stirred and preheated aqueous phase, resulting in the formation of a niosomal suspension [9].
- Emulsion Method: involves the formation of an O/W emulsion by homogenizing an organic solution of NISs and Chol with an aqueous phase, followed by evaporation of the organic solvent to generate NIOs [45].
- Handjani-Vila Method: involves mixing NISs and Chol with an aqueous phase under controlled temperature and agitation (or by ultracentrifugation), leading to the formation of a lamellar liquid-crystalline phase composed of homogeneous vesicles [104].
- Supercritical Carbon Dioxide (scCO2) Method: developed by Manosroi et al. [142], this solvent-free, one-step technique produces LUVs by mixing formulation components with supercritical CO2 in a high-pressure glass cell under controlled temperature and pressure conditions (60 °C, 200 bar), eliminating the need for toxic organic solvents [115].
- Enzymatic Method: utilizes esterase-mediated cleavage of Chol esters or polyoxyethylene derivatives within mixed micellar systems, promoting the in situ formation of MLVs [33].
- The Ball Milling Method: a recent high-energy mechanical process in which APIs, NISs and Chol are co-processed in a rotating milling container containing grinding balls. The mechanical impact and shear forces induce particle fragmentation and compaction, leading to the formation of uniform NIOs [143,144,145].
2.4. Purification and Characterization of NIOs
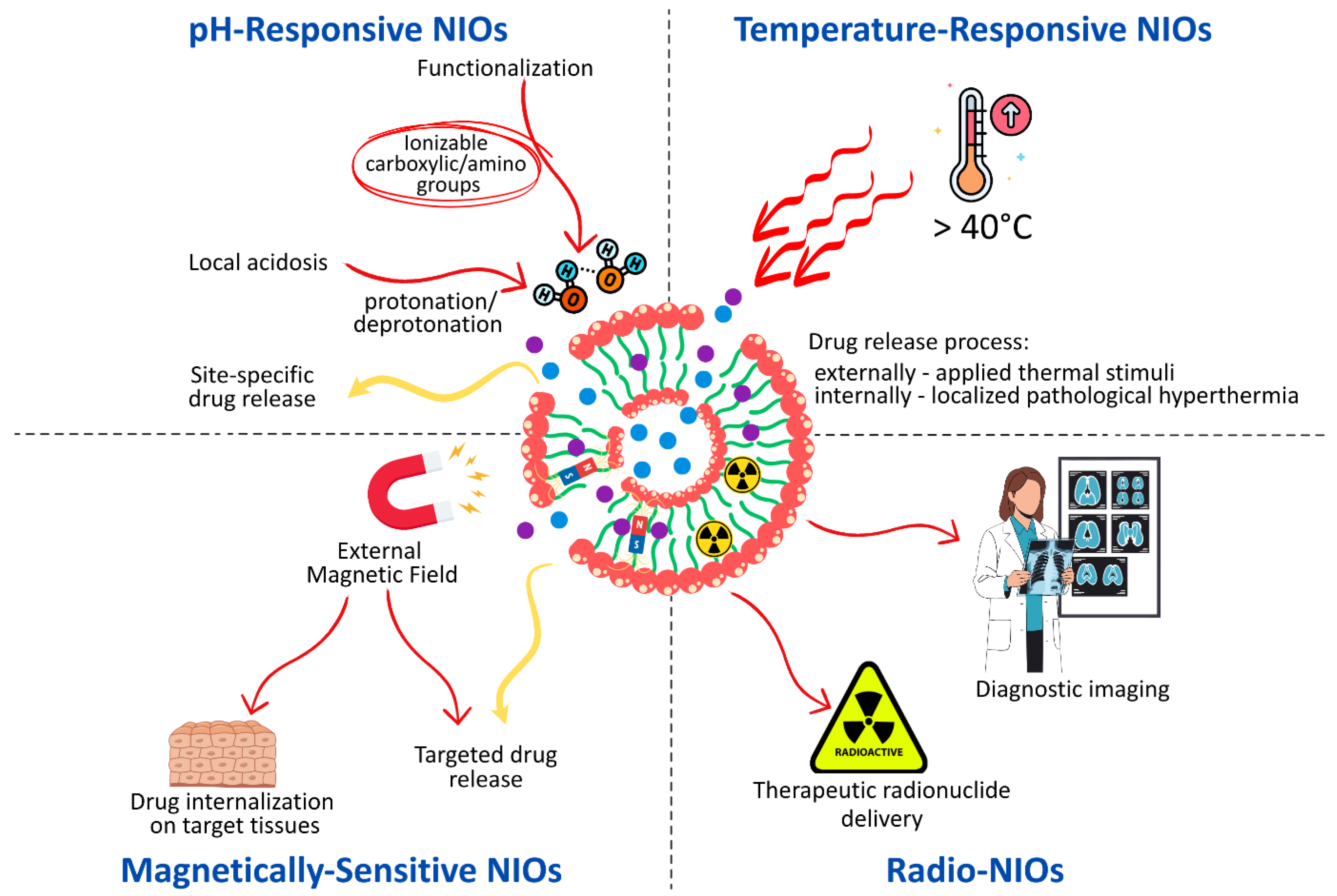
3. The Stimuli-Responsive NIOs—New Approaches for Targeting Drug Delivery
3.1. pH-Responsive NIOs
3.2. Temperature-Responsive NIOs
3.3. Magnetically Sensitive NIOs
3.4. Radio-NIOs
3.5. Multiple Stimuli-Responsive NIOs
3.6. Clinical Translation Status of Stimuli-Responsive NIOs and Conventional NIOs
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NIOs | niosomes |
| VDDSs | vesicular drug delivery systems |
| APIs | active pharmaceutical ingredients |
| NISs | non-ionic surfactants |
| Chol | cholesterol |
| CS | chitosan |
| EE | entrapment efficiency |
| Spans | sorbitan fatty acid esters |
| OEG | oligo(ethylene glycol) |
| POE | polyoxyethylene |
| EO | ethylene oxide |
| PO | propylene oxide |
| PPO | polypropylene oxide |
| PEO | polyethylene oxide |
| CMC | critical micelle concentration |
| HLB | hydrophilic–lipophilic balance |
| CPP | critical packing parameter |
| Tc | gel-liquid crystalline transition temperature |
| Lα | lamellar liquid crystalline |
| Lβ | gel phase |
| Lα | liquid-crystalline phase |
| DCP | diacetyl phosphate |
| STR | stearylamine |
| PEG | polyethylene glycol |
| MLVs | multilamellar vesicles |
| LUVs | large unilamellar vesicles |
| SUVs | small unilamellar vesicles |
| TFH | thin-film hydration |
| REV | reverse-phase evaporation |
| DLS | dynamic light scattering |
| SEM | scanning electron microscopy |
| STM | scanning tunneling microscopy |
| TEM | transmission electron microscopy |
| AFM | atomic force microscopy |
| FF-TEM | freeze fracture replication-electron microscopy |
| NS-TEM | negative-stained transmission electron microscopy |
| NMR | nuclear magnetic resonance |
| SAXS | small angle X-ray scattering |
| DPH | 1,6 diphenyl- 1,3,5-hexatriene |
| EDXD | energy-dispersive X-ray diffraction |
| CHEMS | cholesteryl hemisuccinate |
| ATRA | all-trans retinoic acid |
| HGSOC | high-grade serous ovarian cancer |
| CFZ | carfilzomib |
| MTX | methotrexate |
| PTX | paclitaxel |
| CCI | chronic constriction injury |
| DMG | N,N-dimethyl-glycine |
| DSPE | 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine |
| FA | folic acid |
| GLY | glycine |
| HA | hyaluronic acid |
| HaCaT | human adult low calcium high temperature keratinocytes |
| HCC | hepatocellular carcinoma |
| HD-PAA | hexadecyl-poly(acrylic acid) polymers |
| HFF | human foreskin fibroblasts |
| HUVEC | Human Umbilical Vein Endothelial Cell |
| MMG | N-methyl-glycine |
| PEG-PMMI-CholC6 | PEG-Poly(monomethyl itaconate)-CholC6 |
| pHLIP | pH-Low Insertion Peptide Platform |
| Pyr | N-(1-Pyrenyl) |
| R18 | octadecyl rhodamine B chloride |
| ROS | reactive oxygen species |
| LCST | lower critical solution temperature |
| CQDs | carbon quantum dots |
| NIR | near-infrared Radiation |
| PCM | phase change material |
| L64 | Pluronic® L64 |
| L64ox | oxidized derivative of Pluronic® L64 |
| MIC | Minimum Inhibitory Concentration |
| MRI | magnetic resonance imaging |
| AMF | alternating magnetic field |
| AuNPs | gold nanoparticles |
| siRNA | small interfering RNA |
| DSPE-PEG | 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine–polyethylene glycol |
| HBE | Human Bronchial Epithelial cells |
| LFG | lifeguard |
| SERS | Surface-Enhanced Raman Scattering |
| Tf | transferrin |
| SPECT | single-photon emission computed tomography |
| EPR | Enhanced Permeability and Retention Effect |
| TPGS-DTPA | d-α-tocopherol polyethylene glycol 1000 succinate-diethylenetriaminepentaacetic acid |
| HMPAO | hexamethylpropyleneamine oxime |
| PEG-DSPE | 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy (polyethylene glycol)-2000] |
| GSH | glutathione |
| SPECT/CT | Single Photon Emission Computed Tomography/Computed Tomography |
| ACM | acemetacin |
| CdSe/ZnS QDs | cadmium selenide zinc sulfide quantum dots |
| DSPE-CA-PEG2000 | 1,2-distearoyl-sn-glycero-3-phosphoethanolamine–citraconic amide–polyethylene glycol |
| DPPC | 1,2-dipalmitoyl-sn-glycero-3-phosphocholine phospholipid |
| HER2+ | human epidermal growth factor receptor 2-positive |
References
- Salwa; Chevala, N.T.; Jitta, S.R.; Marques, S.M.; Vaz, V.M.; Kumar, L. Polymeric Microneedles for Transdermal Delivery of Nanoparticles: Frontiers of Formulation, Sterility, and Stability Aspects. J. Drug Deliv. Sci. Technol. 2021, 65, 102711. [Google Scholar] [CrossRef]
- Farjadian, F.; Ghasemi, A.; Gohari, O.; Roointan, A.; Karimi, M.; Hamblin, M.R. Nanopharmaceuticals and Nanomedicines Currently on the Market: Challenges and Opportunities. Nanomedicine 2019, 14, 93–126. [Google Scholar] [CrossRef]
- Su, S.M.; Kang, P. Recent Advances in Nanocarrier-Assisted Therapeutics Delivery Systems. Pharmaceutics 2020, 12, 837. [Google Scholar] [CrossRef]
- Marianecci, C.; Di Marzio, L.; Rinaldi, F.; Celia, C.; Paolino, D.; Alhaique, F.; Esposito, S.; Carafa, M. Niosomes from 80s to present: The state of the art. Adv. Colloid Interface Sci. 2014, 205, 187–206. [Google Scholar] [CrossRef]
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective Use of Nanocarriers as Drug Delivery Systems for the Treatment of Selected Tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [PubMed]
- Mirtaleb, M.S.; Shahraky, M.K.; Ekrami, E.; Mirtaleb, A. Advances in Biological Nano-Phospholipid Vesicles for Transdermal Delivery: A Review on Applications. J. Drug Deliv. Sci. Technol. 2021, 61, 102331. [Google Scholar] [CrossRef]
- Kodel, H.d.A.C.; Alizadeh, P.; Ebrahimi, S.N.; Machado, T.O.X.; Oliveira, M.B.P.P.; Fathi, F.; Souto, E.B. Liposomes and Niosomes: New Trends and Applications in the Delivery of Bioactive Agents for Cancer Therapy. Int. J. Pharm. 2025, 668, 124994. [Google Scholar] [CrossRef] [PubMed]
- Asghari Moghaddam, N.; Mohammadgholi, A.; Mojtahedi, F.; Akhtari, N.; Kaveh Farsani, N.; Noorbazargan, H. Enhanced Anticancer Efficacy of Oxaliplatin-Loaded PEGylated Niosomes in Breast Cancer Treatment. Cancer Nanotechnol. 2025, 16, 16. [Google Scholar] [CrossRef]
- Chen, S.; Hanning, S.; Falconer, J.; Locke, M.; Wen, J. Recent Advances in Non-Ionic Surfactant Vesicles (Niosomes): Fabrication, Characterization, Pharmaceutical and Cosmetic Applications. Eur. J. Pharm. Biopharm. 2019, 144, 18–39. [Google Scholar] [CrossRef]
- Carballo-Pedrares, N.; Kattar, A.; Concheiro, A.; Alvarez-Lorenzo, C.; Rey-Rico, A. Niosomes-Based Gene Delivery Systems for Effective Transfection of Human Mesenchymal Stem Cells. Mater. Sci. Eng. C 2021, 128, 112307. [Google Scholar] [CrossRef]
- Aparajay, P.; Dev, A. Functionalized Niosomes as a Smart Delivery Device in Cancer and Fungal Infection. Eur. J. Pharm. Sci. 2022, 168, 106052. [Google Scholar] [CrossRef]
- Ammar, H.O.; Haider, M.; Ibrahim, M.; El Hoffy, N.M. In Vitro and In Vivo Investigation for Optimization of Niosomal Ability for Sustainment and Bioavailability Enhancement of Diltiazem after Nasal Administration. Drug Deliv. 2017, 24, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Witika, B.A.; Bassey, K.E.; Demana, P.H.; Siwe-Noundou, X.; Poka, M.S. Current Advances in Specialised Niosomal Drug Delivery: Manufacture, Characterization and Drug Delivery Applications. Int. J. Mol. Sci. 2022, 23, 9668. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Hao, Y.; Yuan, L.; Pradhan, S.; Shrestha, K.; Pradhan, O.; Liu, H.; Li, W. Nano-Formulations for Transdermal Drug Delivery: A Review. Chin. Chem. Lett. 2018, 29, 1713–1724. [Google Scholar] [CrossRef]
- García-Manrique, P.; Machado, N.D.; Fernández, M.A.; Blanco-López, M.C.; Matos, M.; Gutiérrez, G. Effect of Drug Molecular Weight on Niosomes Size and Encapsulation Efficiency. Colloids Surf. B 2020, 186, 110711. [Google Scholar] [CrossRef] [PubMed]
- Wichayapreechar, P.; Anuchapreeda, S.; Phongpradist, R.; Rungseevijitprapa, W.; Ampasavate, C. Dermal Targeting of Centella asiatica Extract Using Hyaluronic Acid Surface Modified Niosomes. J. Liposome Res. 2020, 30, 197–207. [Google Scholar] [CrossRef]
- Pardakhty, A.; Moazeni, E.; Varshosaz, J.; Hajhashemi, V.A.; Najafabadi, A.R. Pharmacokinetic Study of Niosome-Loaded Insulin in Diabetic Rats. DARU J. Pharm. Sci. 2011, 19, 404–411. [Google Scholar]
- Abdelkader, H.; Wu, Z.; Al-Kassas, R.; Alany, R.G. Niosomes and Discomes for Ocular Delivery of Naltrexone Hydrochloride: Morphological, Rheological, Spreading Properties and Photo-Protective Effects. Int. J. Pharm. 2012, 433, 142–148. [Google Scholar] [CrossRef]
- Moghassemi, S.; Hadjizadeh, A.; Omidfar, K. Formulation and Characterization of Bovine Serum Albumin-Loaded Niosome. AAPS PharmSciTech 2017, 18, 27–33. [Google Scholar] [CrossRef]
- Moulahoum, H.; Sanli, S.; Timur, S.; Zihnioglu, F. Potential Effect of Carnosine Encapsulated Niosomes in Bovine Serum Albumin Modifications. Int. J. Biol. Macromol. 2019, 137, 583–591. [Google Scholar] [CrossRef]
- Manosroi, A.; Khanrin, P.; Werner, R.G.; Götz, F.; Manosroi, W.; Manosroi, J. Entrapment Enhancement of Peptide Drugs in Niosomes. J. Microencapsul. 2010, 27, 272–280. [Google Scholar] [CrossRef]
- Dabbagh Moghaddam, F.; Akbarzadeh, I.; Marzbankia, E.; Farid, M.; Khaledi, L.; Reihani, A.; Javidfar, M.; Mortazavi, P. Delivery of Melittin-Loaded Niosomes for Breast Cancer Treatment: An In Vitro and In Vivo Evaluation of Anti-Cancer Effect. Cancer Nanotechnol. 2021, 12, 14. [Google Scholar] [CrossRef]
- Obeid, M.A.; Teeravatcharoenchai, T.; Connell, D.; Niwasabutra, K.; Hussain, M.; Carter, K.; Ferro, V.A. Examination of the Effect of Niosome Preparation Methods in Encapsulating Model Antigens on the Vesicle Characteristics and Their Ability to Induce Immune Responses. J. Liposome Res. 2021, 31, 195–202. [Google Scholar] [CrossRef]
- Gogoi, H.; Mani, R.; Bhatnagar, R. A Niosome Formulation Modulates the Th1/Th2 Bias Immune Response in Mice and Also Provides Protection against Anthrax Spore Challenge. Int. J. Nanomed. 2018, 13, 7427–7440. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Gao, S.; Song, P.; Dagnaes-Hansen, F.; Jakobsen, M.; Kjems, J. Theranostic Niosomes for Efficient siRNA/microRNA Delivery and Activatable Near-Infrared Fluorescent Tracking of Stem Cells. ACS Appl. Mater. Interfaces 2018, 10, 19494–19503. [Google Scholar] [CrossRef] [PubMed]
- Al Qtaish, N.; Gallego, I.; Villate-Beitia, I.; Sainz-Ramos, M.; López-Méndez, T.B.; Grijalvo, S.; Eritja, R.; Soto-Sánchez, C.; Martínez-Navarrete, G.; Fernández, E.; et al. Niosome-Based Approach for In Situ Gene Delivery to Retina and Brain Cortex as Immune-Privileged Tissues. Pharmaceutics 2020, 12, 198. [Google Scholar] [CrossRef] [PubMed]
- López-Seijas, J.; Iglesias-Fente, A.; Miranda-Balbuena, D.; Rey-Rico, A. Exploiting Niosomes as Efficient Non-Viral Vectors for Enhanced Gene Transfer to Human Mesenchymal Stem Cells. J. Drug Deliv. Sci. Technol. 2025, 107, 106766. [Google Scholar] [CrossRef]
- Liu, F.R.; Jin, H.; Wang, Y.; Chen, C.; Li, M.; Mao, S.J.; Wang, Q.; Li, H. Anti-CD123 Antibody-Modified Niosomes for Targeted Delivery of Daunorubicin Against Acute Myeloid Leukemia. Drug Deliv. 2017, 24, 882–890. [Google Scholar] [CrossRef]
- Tajbakhsh, M.; Saeedi, M.; Morteza-Semnani, K.; Akbari, J.; Nokhodchi, A. Innovation of Testosome as a Green Formulation for the Transdermal Delivery of Testosterone Enanthate. J. Drug Deliv. Sci. Technol. 2020, 57, 101685. [Google Scholar] [CrossRef]
- Sharma, P.K.; Kushwaha, A.; Repka, M.A.; Murthy, S.N. Formulation Development and Pharmacokinetic Investigation of Self-Assembled Hybrid Niosomes for Oral Delivery of 17-Hydroxyprogesterone Caproate. J. Drug Deliv. Sci. Technol. 2021, 61, 102215. [Google Scholar] [CrossRef]
- Pardakhty, A.; Varshosaz, J.; Rouholamini, A. In Vitro Study of Polyoxyethylene Alkyl Ether Niosomes for Delivery of Insulin. Int. J. Pharm. 2007, 328, 130–141. [Google Scholar] [CrossRef]
- Moghassemi, S.; Parnian, E.; Hakamivala, A.; Darzianiazizi, M.; Vardanjani, M.M.; Kashanian, S.; Larijani, B.; Omidfar, K. Uptake and Transport of Insulin Across Intestinal Membrane Model Using Trimethyl Chitosan Coated Insulin Niosomes. Mater. Sci. Eng. C 2015, 46, 333–340. [Google Scholar] [CrossRef]
- Gharbavi, M.; Amani, J.; Kheiri-Manjili, H.; Danafar, H.; Sharafi, A. Niosome: A Promising Nanocarrier for Natural Drug Delivery through Blood-Brain Barrier. Adv. Pharmacol. Sci. 2018, 2018, 6847971. [Google Scholar] [CrossRef]
- Khoee, S.; Yaghoobian, M. Niosomes: A Novel Approach in Modern Drug Delivery Systems. In Nanostructures for Drug Delivery; Andronescu, E., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 207–237. [Google Scholar] [CrossRef]
- Verma, P.; Pathak, K. Therapeutic and Cosmeceutical Potential of Ethosomes: An Overview. J. Adv. Pharm. Technol. Res. 2010, 1, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Paolino, D.; Cosco, D.; Muzzalupo, R.; Trapasso, E.; Picci, N.; Fresta, M. Innovative Bola-Surfactant Niosomes as Topical Delivery Systems of 5-Fluorouracil for the Treatment of Skin Cancer. Int. J. Pharm. 2008, 353, 233–242. [Google Scholar] [CrossRef]
- Riccardi, D.; Baldino, L.; Reverchon, E. Liposomes, Transfersomes and Niosomes: Production Methods and Their Applications in the Vaccinal Field. J. Transl. Med. 2024, 22, 339. [Google Scholar] [CrossRef]
- Iacob, A.T.; Ababei-Bobu, A.; Chirliu, O.M.; Lupascu, F.G.; Vasincu, I.M.; Apotrosoaei, M.; Profire, B.S.; Tauser, G.R.; Lupascu, D.; Profire, L. A State-of-the-Art Review on Recent Biomedical Application of Polysaccharide-Based Niosomes as Drug Delivery Systems. Polymers 2025, 17, 1566. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, Z.; Beheshtizadeh, N.; Jaymand, M.; Jahanban-Esfahlan, A.; Akbari, M.; Jahanban Esfahlan, R. Engineered Niosomes for Cancer Therapy: Classification, Synthesis, and Clinical Applications. BioNanoScience 2025, 15, 34. [Google Scholar] [CrossRef]
- Aboul-Einien, M.H.; Kandil, S.M.; Abdou, E.M.; Diab, H.M.; Zaki, M.S.E. Ascorbic Acid Derivative-Loaded Modified Aspasomes: Formulation, In Vitro, Ex Vivo and Clinical Evaluation for Melasma Treatment. J. Liposome Res. 2020, 30, 54–67. [Google Scholar] [CrossRef]
- Salamah, M.; Volk, B.; Lekli, I.; Bak, I.; Gyöngyösi, A.; Kozma, G.; Kónya, Z.; Szalenkó-Tőkés, Á.; Kiricsi, Á.; Rovó, L.; et al. Preparation, and Ex Vivo and In Vivo Characterization of Favipiravir-Loaded Aspasomes and Niosomes for Nose-to-Brain Administration. Int. J. Nanomed. 2025, 20, 6489–6514. [Google Scholar] [CrossRef] [PubMed]
- Gaafar, P.M.; Abdallah, O.Y.; Farid, R.M.; Abdelkader, H. Preparation, Characterization and Evaluation of Novel Elastic Nano-Sized Niosomes (Ethoniosomes) for Ocular Delivery of Prednisolone. J. Liposome Res. 2014, 24, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Manosroi, A.; Chankhampan, C.; Ofoghi, H.; Manosroi, W.; Manosroi, J. Low Cytotoxic Elastic Niosomes Loaded with Salmon Calcitonin on Human Skin Fibroblasts. Hum. Exp. Toxicol. 2013, 32, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, H.; Alani, A.W.; Alany, R.G. Recent Advances in Non-Ionic Surfactant Vesicles (Niosomes): Self-Assembly, Fabrication, Characterization, Drug Delivery Applications and Limitations. Drug Deliv. 2014, 21, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Kazi, K.M.; Mandal, A.S.; Biswas, N.; Guha, A.; Chatterjee, S.; Behera, M.; Kuotsu, K. Niosome: A Future of Targeted Drug Delivery Systems. J. Adv. Pharm. Technol. Res. 2010, 1, 374–380. [Google Scholar] [CrossRef]
- Hamishehkar, H.; Rahimpour, Y.; Kouhsoltani, M. Niosomes as a Propitious Carrier for Topical Drug Delivery. Expert Opin. Drug Deliv. 2013, 10, 261–272. [Google Scholar] [CrossRef]
- Khan, R.; Irchhaiya, R. Niosomes: A Potential Tool for Novel Drug Delivery. J. Pharm. Investig. 2016, 46, 195–204. [Google Scholar] [CrossRef]
- Azeem, A.; Anwer, M.K.; Talegaonkar, S. Niosomes in Sustained and Targeted Drug Delivery: Some Recent Advances. J. Drug. Target. 2009, 17, 671–689. [Google Scholar] [CrossRef]
- Ge, X.; Wei, M.; He, S.; Yuan, W.E. Advances of Non-Ionic Surfactant Vesicles (Niosomes) and Their Application in Drug Delivery. Pharmaceutics 2019, 11, 55. [Google Scholar] [CrossRef]
- Bagheri, A.; Chu, B.S.; Yaakob, H. Niosomal Drug Delivery Systems: Formulation, Preparation and Applications. World Appl. Sci. J. 2014, 32, 1671–1685. [Google Scholar] [CrossRef]
- Amoabediny, G.; Haghiralsadat, F.; Naderinezhad, S.; Helder, M.N.; Akhoundi Kharanaghi, E.; Mohammadnejad Arough, J.; Zandieh-Doulabi, B. Overview of Preparation Methods of Polymeric and Lipid-Based (Niosome, Solid Lipid, Liposome) Nanoparticles: A Comprehensive Review. Int. J. Polym. Mater. 2018, 67, 383–400. [Google Scholar] [CrossRef]
- Masjedi, M.; Montahaei, T. An Illustrated Review on Nonionic Surfactant Vesicles (Niosomes) as an Approach in Modern Drug Delivery: Fabrication, Characterization, Pharmaceutical, and Cosmetic Applications. J. Drug. Deliv. Sci. Technol. 2021, 61, 102234. [Google Scholar] [CrossRef]
- Moghassemi, S.; Hadjizadeh, A. Nano-Niosomes as Nanoscale Drug Delivery Systems: An Illustrated Review. J. Contr. Release 2014, 185, 22–36. [Google Scholar] [CrossRef]
- Kumar, G.P.; Rajeshwarrao, P. Nonionic Surfactant Vesicular Systems for Effective Drug Delivery—An Overview. Acta Pharm. Sin. B 2011, 1, 208–219. [Google Scholar] [CrossRef]
- Durga Bhavani, G.; Veera Lakshmi, P. Recent Advances of Non-Ionic Surfactant-Based Nano-Vesicles (Niosomes and Proniosomes): A Brief Review of These in Enhancing Transdermal Delivery of Drug. Future J. Pharm. Sci. 2020, 6, 100. [Google Scholar] [CrossRef]
- Azmin, M.N.; Florence, A.T.; Handjani-Vila, R.M.; Stuart, J.F.; Vanlerberghe, G.; Whittaker, J.S. The Effect of Non-Ionic Surfactant Vesicle (Niosome) Entrapment on the Absorption and Distribution of Methotrexate in Mice. J. Pharm. Pharmacol. 1985, 37, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Baillie, A.J.; Florence, A.T.; Hume, L.R.; Muirhead, G.T.; Rogerson, A. The Preparation and Properties of Niosomes-Non-Ionic Surfactant Vesicles. J. Pharm. Pharmacol. 1985, 37, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Guo, A.; Zhu, X. Tween Surfactants: Adsorption, Self-Organization, and Protein Resistance. Surf. Sci. 2011, 605, 494–499. [Google Scholar] [CrossRef]
- Guinedi, A.S.; Mortada, N.D.; Mansour, S.; Hathout, R.M. Preparation and Evaluation of Reverse-Phase Evaporation and Multilamellar Niosomes as Ophthalmic Carriers of Acetazolamide. Int. J. Pharm. 2005, 306, 71–82. [Google Scholar] [CrossRef]
- Nasr, M.; Mansour, S.; Mortada, N.D.; Elshamy, A.A. Vesicular Aceclofenac Systems: A Comparative Study Between Liposomes and Niosomes. J. Microencapsul. 2008, 25, 499–512. [Google Scholar] [CrossRef]
- Gupta, M.; Vaidya, B.; Mishra, N.; Vyas, S.P. Effect of Surfactants on the Characteristics of Fluconazole Niosomes for Enhanced Cutaneous Delivery. Artif. Cells Blood Substit. Immobil. Biotechnol. 2011, 39, 376–384. [Google Scholar] [CrossRef]
- Ruckmani, K.; Sankar, V. Formulation and Optimization of Zidovudine Niosomes. AAPS PharmSciTech 2010, 11, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Primavera, R.; Palumbo, P.; Celia, C.; Cinque, B.; Carata, E.; Carafa, M.; Paolino, D.; Cifone, M.G.; Di Marzio, L. An Insight of In Vitro Transport of PEGylated Non-Ionic Surfactant Vesicles (NSVs) Across the Intestinal Polarized Enterocyte Monolayers. Eur. J. Pharm. Biopharm. 2018, 127, 432–442. [Google Scholar] [CrossRef]
- Krupka, T.M.; Exner, A.A. Structural Parameters Governing Activity of Pluronic Triblock Copolymers in Hyperthermia Cancer Therapy. Int. J. Hyperth. 2011, 27, 663–671. [Google Scholar] [CrossRef]
- Bodratti, A.M.; Alexandridis, P. Formulation of Poloxamers for Drug Delivery. J. Funct. Biomater. 2018, 9, 11. [Google Scholar] [CrossRef]
- Haroun, M.; Elsewedy, H.S.; Shehata, T.M.; Tratrat, C.; Al Dhubiab, B.E.; Venugopala, K.N.; Almostafa, M.M.; Kochkar, H.; Elnahas, H.M. Significant of Injectable Brucine PEGylated Niosomes in Treatment of MDA Cancer Cells. J. Drug Deliv. Sci. Technol. 2022, 71, 103322. [Google Scholar] [CrossRef]
- Kerdmanee, K.; Phaechamud, T.; Limsitthichaikoon, S. Thermoresponsive Azithromycin-Loaded Niosome Gel Based on Poloxamer 407 and Hyaluronic Interactions for Periodontitis Treatment. Pharmaceutics 2022, 14, 2032. [Google Scholar] [CrossRef]
- Khan, M.I.; Madni, A.; Peltonen, L. Development and In-Vitro Characterization of Sorbitan Monolaurate and Poloxamer 184 Based Niosomes for Oral Delivery of Diacerein. Eur. J. Pharm. Sci. 2016, 95, 88–95. [Google Scholar] [CrossRef]
- Kiwada, H.; Niimura, H.; Fujisaki, Y.; Yamada, S.; Kato, Y. Application of Synthetic Alkyl Glycoside Vesicles as Drug Carriers. I. Preparation and Physical Properties. Chem. Pharm. Bull. 1985, 33, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Manconi, M.; Sinico, C.; Valenti, D.; Lai, F.; Fadda, A.M. Niosomes as Carriers for Tretinoin. III. A Study into the In Vitro Cutaneous Delivery of Vesicle-Incorporated Tretinoin. Int. J. Pharm. 2006, 311, 11–19. [Google Scholar] [CrossRef]
- Patel, A.R.; Kulkarni, S.; Nandekar, T.D.; Vavia, P.R. Evaluation of Alkyl Polyglucoside as an Alternative Surfactant in the Preparation of Peptide-Loaded Nanoparticles. J. Microencapsul. 2008, 25, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Pei, X.; Cui, Z.; Song, B.; Jiang, J.; Binks, B.P. Recyclable Nonionic-Anionic Bola Surfactant as a Stabilizer of Size-Controllable and pH-Responsive Pickering Emulsions. Langmuir 2023, 39, 841–850. [Google Scholar] [CrossRef]
- Kamal, M.S. A review of gemini surfactants: Potential application in enhanced oil recovery. J. Surfact. Deterg. 2016, 19, 223–236. [Google Scholar] [CrossRef]
- Visscher, I.; Engberts, J.B.F.N.; Visscher, N.V. Vesicles of Mixtures of the Bolaform Amphiphile Sodium Di-n-decyl α,ω-Eicosanyl Bisphosphate and Sodium Di-n-decyl Phosphate. Langmuir 2000, 16, 52–58. [Google Scholar] [CrossRef]
- Zanela, T.M.P.; Latczuk, I.F.; Muniz, E.C.; Almeida, C.A.P. Synthesis of Bolaform Surfactants from Recycled Poly(ethylene Terephthalate) Waste. J. Clean. Prod. 2021, 320, 128762. [Google Scholar] [CrossRef]
- Pal, N.; Samanta, K.; Mandal, A. A Novel Family of Non-Ionic Gemini Surfactants Derived from Funflower Oil: Synthesis, Characterization and Physicochemical Evaluation. J. Mol. Liq. 2019, 275, 638–653. [Google Scholar] [CrossRef]
- Guerrero-Hernández, L.; Meléndez-Ortiz, H.I.; Cortez-Mazatan, G.Y.; Vaillant-Sánchez, S.; Peralta-Rodríguez, R.D. Gemini and Bicephalous Surfactants: A Review on Their Synthesis, Micelle Formation, and Uses. Int. J. Mol. Sci. 2022, 23, 1798. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.A.; Szostak, J.W. A Kinetic Study of the Growth of Fatty Acid Vesicles. Biophys. J. 2004, 87, 988–998. [Google Scholar] [CrossRef]
- Bandyopadhyay, P.; Johnson, M. Fatty Alcohols or Fatty Acids as Niosomal Hybrid Carrier: Effect on Vesicle Size, Encapsulation Efficiency and In Vitro Dye Release. Colloids Surf. B Biointerfaces 2007, 58, 68–71. [Google Scholar] [CrossRef]
- Morigaki, K.; Walde, P. Fatty Acid Vesicles. Curr. Opin. Colloid Interface Sci. 2007, 12, 75–80. [Google Scholar] [CrossRef]
- Zhang, Y.; Jing, Q.; Hu, H.; He, Z.; Wu, T.; Guo, T.; Feng, N. Sodium Dodecyl Sulfate Improved Stability and Transdermal Delivery of Salidroside-Encapsulated Niosomes via Effects on Zeta Potential. Int. J. Pharm. 2020, 580, 119183. [Google Scholar] [CrossRef]
- Khazaeli, P.; Pardakhty, A. Caffeine-Loaded Niosomes: Characterization and In Vitro Release Studies. Drug Deliv. 2007, 14, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Yasamineh, S.; Yasamineh, P.; Kalajahi, H.G.; Gholizadeh, O.; Yekanipour, Z.; Afkhami, H.; Eslami, M.; Kheirkhah, A.H.; Taghizadeh, M.; Yazdani, Y.; et al. A State-of-the-Art Review on the Recent Advances of Niosomes as a Targeted Drug Delivery System. Int. J. Pharm. 2022, 624, 121878. [Google Scholar] [CrossRef] [PubMed]
- Yeo, P.L.; Lim, C.L.; Chye, S.M.; Ling, A.P.K.; Koh, R.Y. Niosomes: A Review of Their Structure, Properties, Methods of Preparation, and Medical Applications. Asian Biomed. 2017, 11, 301–313. [Google Scholar] [CrossRef]
- Ag Seleci, D.; Seleci, M.; Walter, J.G.; Stahl, F.; Scheper, T. Niosomes as Nanoparticular Drug Carriers: Fundamentals and Recent Applications. J. Nanomater. 2016, 2016, 7372306. [Google Scholar] [CrossRef]
- Shafiepour, M.; Razavi, S.H.; Khanniri, E.; Jahan, F.M.; Nouri, M.; Afraei, M. Nanocarriers for Foods: A Review of Niosomes and Proniosomes in Bioactive Compounds. Food Humanit. 2025, 4, 100623. [Google Scholar] [CrossRef]
- Needham, D. Reverse Engineering of the Low Temperature-Sensitive Liposome (LTSL) for Treating Cancer. In Biomaterials for Cancer Therapeutics: Diagnosis, Prevention and Therapy; Park, K., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 270–348. [Google Scholar] [CrossRef]
- Kobierski, J.; Wnętrzak, A.; Chachaj-Brekiesz, A.; Dynarowicz-Latka, P. Predicting the packing parameter for lipids in monolayers with the use of molecular dynamics. Colloids Surf. B Biointerfaces 2022, 211, 112298. [Google Scholar] [CrossRef]
- Barba-Bon, A.; Nilam, M.; Hennig, A. Supramolecular Chemistry in the Biomembrane. ChemBioChem 2020, 21, 886–910. [Google Scholar] [CrossRef]
- Gonçalves, R.A.; Naidjonoka, P.; Nylander, T.; Miguel, M.G.; Lindman, B.; Lam, Y.M. Facile Control of Surfactant Lamellar Phase Transition and Adsorption Behavior. RSC Adv. 2020, 10, 18025–18034. [Google Scholar] [CrossRef]
- Gugliotti, M.; Politi, M.J. The Role of the Gel ⇔ Liquid-Crystalline Phase Transition in the Lung Surfactant Cycle. Biophys. Chem. 2001, 89, 243–251. [Google Scholar] [CrossRef]
- Jaschonek, S.; Cascella, M.; Gauss, J.; Diezemann, G.; Milano, G. Intramolecular Structural Parameters Are Key Modulators of the Gel-Liquid Transition in Coarse Grained Simulations of DPPC and DOPC Lipid Bilayers. Biochem. Biophys. Res. Commun. 2018, 498, 327–333. [Google Scholar] [CrossRef]
- Somjid, S.; Shinsuphan, N.; Temprom, L.; Krongsuk, S. Effects of Cholesterol and Temperature on Structural Properties and Dynamic Behavior of Niosome Bilayers With Melatonin Inclusion: A Coarse-Grained Simulation Study. J. Mol. Liq. 2022, 368, 120686. [Google Scholar] [CrossRef]
- Basiri, L.; Rajabzadeh, G.; Bostan, A. α-Tocopherol-Loaded Niosome Prepared by Heating Method and Its Release Behavior. Food Chem. 2017, 221, 620–628. [Google Scholar] [CrossRef]
- Nazari, M.; Kurdi, M.; Heerklotz, H. Classifying Surfactants with Respect to Their Effect on Lipid Membrane Order. Biophys. J. 2012, 102, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Duangjit, S.; Pamornpathomkul, B.; Opanasopit, P.; Rojanarata, T.; Obata, Y.; Takayama, K.; Ngawhirunpat, T. Role of the Charge, Carbon Chain Length, and Content of Surfactant on the Skin Penetration of Meloxicam-Loaded Liposomes. Int. J. Nanomed. 2014, 9, 2005–2017. [Google Scholar] [CrossRef]
- Varshosaz, J.; Pardakhty, A.; Hajhashemi, V.I.; Najafabadi, A.R. Development and Physical Characterization of Sorbitan Monoester Niosomes for Insulin Oral Delivery. Drug Deliv. 2003, 10, 251–262. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Tripathi, P.; Gupta, R.; Pandey, S. Niosomes: A Review on Niosomal Research in the Last Decade. J. Drug Deliv. Sci. Technol. 2020, 56, 101581. [Google Scholar] [CrossRef]
- Manosroi, A.; Wongtrakul, P.; Manosroi, J.; Sakai, H.; Sugawara, F.; Yuasa, M.; Abe, M. Characterization of Vesicles Prepared with Various Non-Ionic Surfactants Mixed with Cholesterol. Colloids Surf. B Biointerfaces 2003, 30, 129–138. [Google Scholar] [CrossRef]
- Abdelbary, G.; El-Gendy, N. Niosome-Encapsulated Gentamicin for Ophthalmic Controlled Delivery. AAPS PharmSciTech 2008, 9, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Xu, T.; Wang, C.; Gan, C. The Stabilization and Antioxidant Performances of Coenzyme Q10-Loaded Niosomes Coated by PEG and Chitosan. J. Mol. Liq. 2021, 325, 115194. [Google Scholar] [CrossRef]
- Miatmoko, A.; Safitri, S.A.; Aquila, F.; Cahyani, D.M.; Hariawan, B.S.; Hendrianto, E.; Hendradi, E.; Sari, R. Characterization and Distribution of Niosomes Containing Ursolic Acid Coated with Chitosan Layer. Res. Pharm. Sci. 2021, 16, 660–673. [Google Scholar] [CrossRef]
- Hasan, M.; Messaoud, G.B.; Michaux, F.; Tamayol, A.; Kahn, C.J.; Belhaj, N.; Linder, M.; Arab-Tehrany, E. Chitosan-Coated Liposomes Encapsulating Curcumin: Study of Lipid–Polysaccharide Interactions and Nanovesicle Behavior. RSC Adv. 2016, 6, 45290–45304. [Google Scholar] [CrossRef]
- Mahale, N.B.; Thakkar, P.D.; Mali, R.G.; Walunj, D.R.; Chaudhari, S.R. Niosomes: Novel Sustained Release Nonionic Stable Vesicular Systems—An Overview. Adv. Colloid Interface Sci. 2012, 183–184, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Basiri, L.; Rajabzadeh, G.; Bostan, A. Physicochemical Properties and Release Behavior of Span 60/Tween 60 Niosomes as Vehicle for α-Tocopherol Delivery. LWT 2017, 84, 471–478. [Google Scholar] [CrossRef]
- Arunothayanun, P.; Bernard, M.S.; Craig, D.Q.; Uchegbu, I.F.; Florence, A.T. The Effect of Processing Variables on the Physical Characteristics of Non-Ionic Surfactant Vesicles (Niosomes) Formed from a Hexadecyl Diglycerol Ether. Int. J. Pharm. 2000, 201, 7–14. [Google Scholar] [CrossRef]
- Varshosaz, J.; Taymouri, S.; Pardakhty, A.; Asadi-Shekaari, M.; Babaee, A. Niosomes of Ascorbic Acid and α-Tocopherol in the Cerebral Ischemia-Reperfusion Model in Male Rats. Biomed. Res. Int. 2014, 2014, 816103. [Google Scholar] [CrossRef]
- Rajnish, A.; Ajay, S. Release Studies of Ketoconazole Niosome Formulation. J. Glob. Pharma Technol. 2010, 2, 125–127. [Google Scholar]
- Mokhtar, M.; Sammour, O.A.; Hammad, M.A.; Megrab, N.A. Effect of Some Formulation Parameters on Flurbiprofen Encapsulation and Release Rates of Niosomes Prepared from Proniosomes. Int. J. Pharm. 2008, 361, 104–111. [Google Scholar] [CrossRef]
- Yeo, L.K.; Chaw, C.S.; Elkordy, A.A. The Effects of Hydration Parameters and Co-Surfactants on Methylene Blue-Loaded Niosomes Prepared by the Thin Film Hydration Method. Pharmaceuticals 2019, 12, 46. [Google Scholar] [CrossRef]
- Shirsand, S.; Para, M.; Nagendrakumar, D.; Kanani, K.; Keerthy, D. Formulation and Evaluation of Ketoconazole Niosomal Gel Drug Delivery System. Int. J. Pharm. Investig. 2012, 2, 201–207. [Google Scholar] [CrossRef]
- Joshi, S.; White, R.; Sahu, R.; Dennis, V.A.; Singh, S.R. Comprehensive Screening of Drug Encapsulation and Co-Encapsulation into Niosomes Produced Using a Microfluidic Device. Processes 2020, 8, 535. [Google Scholar] [CrossRef]
- Finan, J.D.; Guilak, F. The Effects of Osmotic Stress on the Structure and Function of the Cell Nucleus. J. Cell. Biochem. 2010, 109, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of Univalent Ions Across the Lamellae of Swollen Phospholipids. J. Mol. Biol. 1965, 13, 238–252. [Google Scholar] [CrossRef]
- Durak, S.; Rad, M.E.; Yetisgin, A.A.; Sutova, H.E.; Kutlu, O.; Cetinel, S.; Zarrabi, A. Niosomal Drug Delivery Systems for Ocular Disease—Recent Advances and Future Prospects. Nanomaterials 2020, 10, 1191. [Google Scholar] [CrossRef]
- Thabet, Y.; Elsabahy, M.; Eissa, N.G. Methods for Preparation of Niosomes: A Focus on Thin-Film Hydration Method. Methods 2022, 199, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, P.; Tripathi, P.; Pandey, S.; Gupta, R.; Khar, R.K.; Patil, P.R. Improved Dermal Delivery of Pentoxifylline Niosomes for the Management of Psoriasis: Development, Optimization and In-Vivo Studies in Imiquimod Induced Psoriatic Plaque Model. J. Drug Deliv. Sci. Technol. 2022, 75, 103643. [Google Scholar] [CrossRef]
- Faheela, M.K.; Malathi, S.; Monica Susai Mary, S.; Narayana Kalkura, S. In-Vitro Characterization of Pluronic P 123 Based Niosome for Targeted Delivery of Doxorubicin. Mater. Today Proc. 2022, 58, 795–801. [Google Scholar] [CrossRef]
- Esmaeili Rad, M.; Egil, A.C.; Ozaydin Ince, G.; Yuce, M.; Zarrabi, A. Optimization of Curcumin Loaded Niosomes for Drug Delivery Applications. Colloids Surf. A Physicochem. Eng. Asp. 2022, 654, 129921. [Google Scholar] [CrossRef]
- Abdelkader, H.; Ismail, S.; Kamal, A.; Alany, R.G. Design and Evaluation of Controlled-Release Niosomes and Discomes for Naltrexone Hydrochloride Ocular Delivery. J. Pharm. Sci. 2011, 100, 1833–1846. [Google Scholar] [CrossRef]
- Manosroi, A.; Khanrin, P.; Lohcharoenkal, W.; Werner, R.G.; Götz, F.; Manosroi, W.; Manosroi, J. Transdermal Absorption Enhancement Through Rat Skin of Gallidermin Loaded in Niosomes. Int. J. Pharm. 2010, 392, 304–310. [Google Scholar] [CrossRef]
- Rajera, R.; Nagpal, K.; Singh, S.K.; Mishra, D.N. Niosomes: A Controlled and Novel Drug Delivery System. Biol. Pharm. Bull. 2011, 34, 945–953. [Google Scholar] [CrossRef]
- Kulkarni, P.; Rawtani, D.; Rajpurohit, S.; Vasvani, S.; Barot, T. Self-Assembly Based Aerosolized Hyaluronic Acid (HA) Loaded Niosomes for Lung Delivery: An In-Vitro and In-Vivo Evaluation. J. Drug Deliv. Sci. Technol. 2022, 75, 103627. [Google Scholar] [CrossRef]
- Alyami, H.; Abdelaziz, K.; Dahmash, E.Z.; Iyire, A. Nonionic Surfactant Vesicles (Niosomes) for Ocular Drug Delivery: Development, Evaluation and Toxicological Profiling. J. Drug Deliv. Sci. Technol. 2020, 60, 102069. [Google Scholar] [CrossRef]
- Palanisamy, P.; Crossia, W.F.; Prakash, D.; Antonyraj, A.P. Nanoformulation of Stavudine-Loaded Niosomes: Enhancing Drug Delivery, Entrapment Efficiency, and Controlled Release for Improved Antiretroviral Therapy. J. Orthop. Rep. 2025, 100677, in press. [Google Scholar] [CrossRef]
- Bansal, S.; Aggarwal, G.; Chandel, P.; Harikumar, S.L. Design and Development of Cefdinir Niosomes for Oral Delivery. J. Pharm. Bioallied. Sci. 2013, 5, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Zubair, S.; Farazuddin, M.; Ahmad, E.; Khan, A.; Zia, Q.; Malik, A.; Mohammad, O. Development, Characterization and Efficacy of Niosomal Diallyl Disulfide in Treatment of Disseminated Murine Candidiasis. Nanomedicine 2013, 9, 247–256. [Google Scholar] [CrossRef]
- Aher, P.; Dandgavhal, K.; Bhandari, D.; Saindane, H.; Deore, N.; Amrutkar, S. Niosomes as a Potential Drug Delivery System. Int. J. Pharm. Sci. Rev. Res. 2021, 68, 21–27. [Google Scholar] [CrossRef]
- Sharma, R.; Dua, J.S.; Prasad, D.N.; Kaushal, S.; Puri, A. Formulation and Evaluation of Clindamycin phosphate Niosomes by using Reverse Phase Evaporation Method. J. Drug Deliv. Ther. 2019, 9, 515–523. [Google Scholar] [CrossRef]
- Junyaprasert, V.B.; Teeranachaideekul, V.; Supaperm, T. Effect of Charged and Non-Ionic Membrane Additives on Physicochemical Properties and Stability of Niosomes. AAPS PharmSciTech 2008, 9, 851–859. [Google Scholar] [CrossRef]
- Weng, H.; Liu, X.; Ren, Y.; Li, Y.; Li, X. Fingolimod Loaded Niosomes Attenuates Sevoflurane Induced Cognitive Impairments. Biomed. Microdevices 2022, 24, 5. [Google Scholar] [CrossRef]
- Naderi, M.; Saidi, S.; Barani, M. Advancements in Microfluidic-Based Synthesis of Niosomes for Cancer and Infection Therapy: A Comprehensive Review. J. Mol. Liq. 2025, 434, 128048. [Google Scholar] [CrossRef]
- Machado, N.D.; García-Manrique, P.; Fernández, M.A.; Blanco-López, M.C.; Matos, M.; Gutiérrez, G. Cholesterol Free Niosome Production by Microfluidics: Comparative with Other Conventional Methods. Chem. Eng. Res. Des. 2020, 162, 162–171. [Google Scholar] [CrossRef]
- Obeid, M.A.; Gany, S.A.S.; Gray, A.I.; Young, L.; Igoli, J.O.; Ferro, V.A. Niosome-Encapsulated Balanocarpol: Compound Isolation, Characterisation, and Cytotoxicity Evaluation Against Human Breast and Ovarian Cancer Cell Lines. Nanotechnology 2020, 31, 195101. [Google Scholar] [CrossRef]
- Jaber, S.A.; Saadh, M.; Abuhassan, Q.; Obeid, M.A. Curcumin-Loaded Niosomes Prepared by Microfluidic Mixing; Characterization and Cytotoxicity Evaluation. J. Pharm. Innov. 2025, 20, 125. [Google Scholar] [CrossRef]
- Radha, G.; Rani, T.S.; Sarvani, B. A Review on Proniosomal Drug Delivery System for Targeted Drug Action. J. Basic Clin. Pharm. 2013, 4, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; Chaudhary, A.; Chaudhary, A.; Kumar, A. Proniosomes: The Effective and Efficient Drug-Carrier System. Ther. Deliv. 2020, 11, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, Z.; Zeng, W.; Ge, S.; Lu, H.; Wu, C.; Ge, L.; Liang, D.; Xu, Y. Proniosome-Derived Niosomes for Tacrolimus Topical Ocular Delivery: In Vitro Cornea Permeation, Ocular Irritation, and In Vivo Anti-Allograft Rejection. Eur. J. Pharm. Sci. 2014, 62, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Prasad, V.; Chaurasia, S. Performance Evaluation of Non-Ionic Surfactant Based Tazarotene Encapsulated Proniosomal Gel for the Treatment of Psoriasis. Mater. Sci. Eng. C 2017, 79, 168–176. [Google Scholar] [CrossRef]
- Csongradi, C.; du Plessis, J.; Aucamp, M.E.; Gerber, M. Topical Delivery of Roxithromycin Solid-State Forms Entrapped in Vesicles. Eur. J. Pharm. Biopharm. 2017, 114, 96–107. [Google Scholar] [CrossRef]
- Kirby, C.; Gregoriadis, G. Dehydration-Rehydration Vesicles: A Simple Method for High Yield Drug Entrapment in Liposomes. Nat. Biotechnol. 1984, 2, 979–984. [Google Scholar] [CrossRef]
- Manosroi, A.; Chutoprapat, R.; Abe, M.; Manosroi, J. Characteristics of Niosomes Prepared by Supercritical Carbon Dioxide (scCO2) Fluid. Int. J. Pharm. 2008, 352, 248–255. [Google Scholar] [CrossRef]
- Temprom, L.; Krongsuk, S.; Thapphasaraphong, S.; Priperm, A.; Namuangruk, S. A Novel Preparation and Characterization of Melatonin Loaded Niosomes Based on Using a Ball Milling Method. Mater. Today Commun. 2022, 31, 103340. [Google Scholar] [CrossRef]
- Mawazi, S.M.; Ann, T.J.; Widodo, R.T. Exploring the Evolution of Niosomes: From Past Techniques to Future Advances in Preparation Methods—A Comprehensive Review. BioNanoScience 2024, 14, 1854–1875. [Google Scholar] [CrossRef]
- Mawazi, S.M.; Ge, Y.; Widodo, R.T. Niosome Preparation Techniques and Structure-An Illustrated Review. Pharmaceutics 2025, 17, 67. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Rejinold, N.S.; Jeong, S.D.; Kim, Y.C. Stimuli-Responsive Polypeptides for Biomedical Applications. Polymers 2018, 10, 830. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hu, W.; Cai, C.; Wu, Y.; Li, J.; Dong, S. Advanced Application of Stimuli-Responsive Drug Delivery System for Inflammatory Arthritis Treatment. Mater. Today Bio 2022, 14, 100223. [Google Scholar] [CrossRef]
- Liga, S.; Paul, C.; Moacă, E.A.; Péter, F. Niosomes: Composition, Formulation Techniques, and Recent Progress as Delivery Systems in Cancer Therapy. Pharmaceutics 2024, 16, 223. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, C.; Wang, Y.; Chen, H.; Zhang, X.; Luo, C.; Zhou, W.; Li, L.; Teng, L.; Yu, H.; et al. Smart Drug Delivery Systems for Precise Cancer Therapy. Acta Pharm. Sin. B 2022, 12, 4098–4121. [Google Scholar] [CrossRef]
- Wang, S.; Hu, X.; Wei, W.; Ma, G. Transformable Vesicles for Cancer Immunotherapy. Adv. Drug Deliv. Rev. 2021, 179, 113905. [Google Scholar] [CrossRef]
- Torchilin, V.P. Fundamentals of Stimuli-Responsive Drug and Gene Delivery Systems. In Stimuli-Responsive Drug Delivery Systems; Singh, A., Amiji, M.M., Eds.; Royal Society of Chemistry: Cambridge, UK, 2018; pp. 3–36. [Google Scholar] [CrossRef]
- Karimi, M.; Sahandi Zangabad, P.; Ghasemi, A.; Amiri, M.; Bahrami, M.; Malekzad, H.; Ghahramanzadeh Asl, H.; Mahdieh, Z.; Bozorgomid, M.; Ghasemi, A.; et al. Temperature-Responsive Smart Nanocarriers for Delivery of Therapeutic Agents: Applications and Recent Advances. ACS Appl. Mater. Interfaces 2016, 8, 21107–21133. [Google Scholar] [CrossRef]
- Abtahi, N.; Naghib, S.; Haghiralsadat, F.; Reza, J.; Hakimian, F.; Yazdian, F.; Tofighi, D. Smart Stimuli-Responsive Biofunctionalized Niosomal Nanocarriers for Programmed Release of Bioactive Compounds into Cancer Cells In Vitro and In Vivo. Nanotechnol. Rev. 2021, 10, 1895–1911. [Google Scholar] [CrossRef]
- Das, S.S.; Bharadwaj, P.; Bilal, M.; Barani, M.; Rahdar, A.; Taboada, P.; Bungau, S.; Kyzas, G.Z. Stimuli-Responsive Polymeric Nanocarriers for Drug Delivery, Imaging, and Theragnosis. Polymers 2020, 12, 1397. [Google Scholar] [CrossRef]
- Marzoli, F.; Marianecci, C.; Rinaldi, F.; Passeri, D.; Rossi, M.; Minosi, P.; Carafa, M.; Pieretti, S. Long-Lasting, Antinociceptive Effects of pH-Sensitive Niosomes Loaded with Ibuprofen in Acute and Chronic Models of Pain. Pharmaceutics 2019, 11, 62. [Google Scholar] [CrossRef]
- Barani, M.; Hajinezhad, M.R.; Sargazi, S.; Rahdar, A.; Shahraki, S.; Lohrasbi-Nejad, A.; Baino, F. In Vitro and In Vivo Anticancer Effect of pH-Responsive Paclitaxel-Loaded Niosomes. J. Mater. Sci. Mater. Med. 2021, 32, 147. [Google Scholar] [CrossRef]
- Momekova, D.B.; Gugleva, V.E.; Petrov, P.D. Nanoarchitectonics of Multifunctional Niosomes for Advanced Drug Delivery. ACS Omega 2021, 6, 33265–33273. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.; Kumar, L.; Mehta, S.; Mehta, A. Stimuli-Sensitive Systems–An Emerging Delivery System for Drugs. Artif. Cells Nanomed. Biotechnol. 2015, 43, 299–310. [Google Scholar] [PubMed]
- Meoli, C.M.; Riccardi, D.; Baldino, L. Optimization of Chitosan-Coated Niosomes Encapsulating Doxycycline Hyclate for pH-Responsive Drug Release. Ind. Eng. Chem. Res. 2025, 64, 6292–6301. [Google Scholar] [CrossRef]
- Giuli, M.V.; Hanieh, P.N.; Forte, J.; Fabiano, M.G.; Mancusi, A.; Natiello, B.; Rinaldi, F.; Del Favero, E.; Ammendolia, M.G.; Marianecci, C.; et al. pH-Sensitive Niosomes for ATRA Delivery: A Promising Approach to Inhibit Pin1 in High-Grade Serous Ovarian Cancer. Int. J. Pharm. 2024, 649, 123672. [Google Scholar] [CrossRef]
- Gugleva, V.; Mihaylova, R.; Momekov, G.; Kamenova, K.; Forys, A.; Trzebicka, B.; Petrova, M.; Ugrinova, I.; Momekova, D.; Petrov, P.D. pH-Responsive Niosome-Based Nanocarriers of Antineoplastic Agents. RSC Adv. 2024, 14, 11124–11140. [Google Scholar] [CrossRef]
- Khodabakhsh, F.; Bourbour, M.; Yaraki, M.T.; Bazzazan, S.; Bakhshandeh, H.; Ahangari Cohan, R.; Tan, Y.N. pH-Responsive PEGylated Niosomal Nanoparticles as an Active-Targeting Cyclophosphamide Delivery System for Gastric Cancer Therapy. Molecules 2022, 27, 5418. [Google Scholar] [CrossRef]
- Rezaei, T.; Rezaei, M.; Karimifard, S.; Mahmoudi Beram, F.; Dakkali, M.S.; Heydari, M.; Afshari-Behbahanizadeh, S.; Mostafavi, E.; Bokov, D.O.; Ansari, M.J.; et al. Folic Acid-Decorated pH-Responsive Nanoniosomes with Enhanced Endocytosis for Breast Cancer Therapy: In Vitro Studies. Front. Pharmacol. 2022, 13, 851242. [Google Scholar] [CrossRef]
- Hajinezhad, M.R.; Fathi-Karkan, S.; Roostaee, M.; Sargazi, S.; Mirinejad, S.; Sheervalilou, R.; Sargazi, S.; Barani, M. Engineering pH-Responsive Niosomes with Ergosterol and CHEMS for Controlled Carfilzomib Release: Insights from In Vitro and In Vivo Studies. Drug Dev. Ind. Pharm. 2025, 51, 1122–1137. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, J.; Deng, L.; Hu, H.; Hu, J.; Zheng, G. Galactose-Modified PH-Sensitive Niosomes for Controlled Release and Hepatocellular Carcinoma Target Delivery of Tanshinone IIA. AAPS PharmSciTech 2021, 22, 96. [Google Scholar] [CrossRef]
- Barani, M.; Reza Hajinezhad, M.; Sargazi, S.; Zeeshan, M.; Rahdar, A.; Pandey, S.; Khatami, M.; Zargari, F. Simulation, In Vitro, and In Vivo Cytotoxicity Assessments of Methotrexate-Loaded pH-Responsive Nanocarriers. Polymers 2021, 13, 3153. [Google Scholar] [CrossRef]
- Sargazi, S.; Hosseinikhah, S.; Zargari, F.; Chauhana, N.; Hassanisaadi, M.; Amani, S. pH-Responsive Cisplatin-Loaded Niosomes: Synthesis, Characterization, Cytotoxicity Study and Interaction Analyses by Simulation Methodology. Nanofabrication 2021, 6, 1–15. [Google Scholar] [CrossRef]
- Tila, D.; Yazdani-Arazi, S.N.; Ghanbarzadeh, S.; Arami, S.; Pourmoazzen, Z. pH-Sensitive, Polymer Modified, Plasma Stable Niosomes: Promising Carriers for Anti-Cancer Drugs. EXCLI J. 2015, 14, 21–32. [Google Scholar] [CrossRef]
- Akbarzadeh, I.; Shayan, M.; Bourbour, M.; Moghtaderi, M.; Noorbazargan, H.; Eshrati Yeganeh, F.; Saffar, S.; Tahriri, M. Preparation, Optimization and In-Vitro Evaluation of Curcumin-Loaded Niosome@calcium Alginate Nanocarrier as a New Approach for Breast Cancer Treatment. Biology 2021, 10, 173. [Google Scholar] [CrossRef]
- Pereira, M.C.; Pianella, M.; Wei, D.; Moshnikova, A.; Marianecci, C.; Carafa, M.; Andreev, O.A.; Reshetnyak, Y.K. pH-Sensitive pHLIP® Coated Niosomes. Mol. Membr. Biol. 2016, 33, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, F.; Del Favero, E.; Rondelli, V.; Pieretti, S.; Bogni, A.; Ponti, J.; Rossi, F.; Di Marzio, L.; Paolino, D.; Marianecci, C.; et al. pH-Sensitive Niosomes: Effects on Cytotoxicity and on Inflammation and Pain in Murine Models. J. Enzyme Inhib. Med. Chem. 2017, 32, 538–546. [Google Scholar] [CrossRef]
- Masotti, A.; Vicennati, P.; Alisi, A.; Marianecci, C.; Rinaldi, F.; Carafa, M.; Ortaggi, G. Novel Tween® 20 Derivatives Enable the Formation of Efficient pH-Sensitive Drug Delivery Vehicles for Human Hepatoblastoma. Bioorg. Med. Chem. Lett. 2010, 20, 3021–3025. [Google Scholar] [CrossRef] [PubMed]
- Mazzotta, E.; Tavano, L.; Muzzalupo, R. Thermo-Sensitive Vesicles in Controlled Drug Delivery for Chemotherapy. Pharmaceutics 2018, 10, 150. [Google Scholar] [CrossRef]
- Tavano, L.; Oliviero Rossi, C.; Picci, N.; Muzzalupo, R. Spontaneous Temperature-Sensitive Pluronic(®) Based Niosomes: Triggered Drug Release Using Mild Hyperthermia. Int. J. Pharm. 2016, 511, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Arshad, N.; Shaheen, F.; Khan, I.N.; Naeem, S.; Riaz, M.; Siddique, M.I.; Ayesha, M.; Waqar, M.A. A Comprehensive Review on Niosomes: Novel Manufacturing Techniques, Factors Influencing Formation, Applications and Recent Advances. Int. J. Polym. Mater. Polym. Biomater. 2025, 74, 1331–1348. [Google Scholar] [CrossRef]
- Mazzotta, E.; Romeo, M.; Hafidi, Z.; Perez, L.; Perrotta, I.D.; Muzzalupo, R. Design of Thermosensitive Niosomes by Eutectic Mixture of Natural Fatty Acids. Pharmaceutics 2024, 16, 909. [Google Scholar] [CrossRef] [PubMed]
- Khakbaz, F.; Mirzaei, M.; Mahani, M. Lecithin Sensitized Thermo-Sensitive Niosome Using NIR-Carbon Dots for Breast Cancer Combined Chemo-Photothermal Therapy. J. Photochem. Photobiol. A Chem. 2023, 434, 114236. [Google Scholar] [CrossRef]
- Damera, D.P.; Nag, A. Tuning the Phase Transition Temperature of Hybrid Span60-L64 Thermoresponsive Niosomes: Insights from Fluorescence and Raman Spectroscopy. J. Mol. Liq. 2021, 340, 117110. [Google Scholar] [CrossRef]
- Canaparo, R.; Foglietta, F.; Giuntini, F.; Della Pepa, C.; Dosio, F.; Serpe, L. Recent Developments in Antibacterial Therapy: Focus on Stimuli-Responsive Drug-Delivery Systems and Therapeutic Nanoparticles. Molecules 2019, 24, 1991. [Google Scholar] [CrossRef]
- Tavano, L.; Vivacqua, M.; Carito, V.; Muzzalupo, R.; Caroleo, M.C.; Nicoletta, F. Doxorubicin Loaded Magneto-Niosomes for Targeted Drug Delivery. Colloids Surf. B Biointerfaces 2013, 102, 803–807. [Google Scholar] [CrossRef]
- Drake, P.; Amalina, I.; Sari, R.; Ruiz, A.; Ramazan, S.; Hope, G.; Pancholi, D.; Miatmoko, A. Magnetically Induced Drug Release from Niosome-Based Nanocarriers Loaded with Doxorubicin. Soft Matter 2025, 21, 6197–6206. [Google Scholar] [CrossRef]
- Barani, M.; Nematollahi, M.H.; Zaboli, M.; Mirzaei, M.; Torkzadeh-Mahani, M.; Pardakhty, A.; Karam, G.A. In Silico and In Vitro Study of Magnetic Niosomes for Gene Delivery: The Effect of Ergosterol and Cholesterol. Mater. Sci. Eng. C 2019, 94, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, R.; Mirjafary, Z.; Zarghami, N.; Saeidian, H. Efficient PEGylated Magnetic Nanoniosomes for Co-Delivery of Artemisinin and Metformin: A New Frontier in Chemotherapeutic Efficacy and Cancer Therapy. Sci. Rep. 2024, 14, 27380. [Google Scholar] [CrossRef]
- Maurer, V.; Altin, S.; Ag Seleci, D.; Zarinwall, A.; Temel, B.; Vogt, P.M.; Strauß, S.; Stahl, F.; Scheper, T.; Bucan, V.; et al. In-Vitro Application of Magnetic Hybrid Niosomes: Targeted siRNA-Delivery for Enhanced Breast Cancer Therapy. Pharmaceutics 2021, 13, 394. [Google Scholar] [CrossRef]
- Maurer, V.; Zarinwall, A.; Wang, Z.; Wundrack, S.; Wundrack, N.; Ag Seleci, D.; Helm, V.; Otenko, D.; Frank, C.; Schaper, F.; et al. All-in-One Superparamagnetic and SERS-Active Niosomes for Dual-Targeted In Vitro Detection of Breast Cancer Cells. Sens. Diagn. 2022, 1, 469–484. [Google Scholar] [CrossRef]
- Davarpanah, F.; Khalili Yazdi, A.; Barani, M.; Mirzaei, M.; Torkzadeh-Mahani, M. Magnetic Delivery of Antitumor Carboplatin by Using PEGylated-Niosomes. DARU J. Pharm. Sci. 2018, 26, 57–64. [Google Scholar] [CrossRef]
- De Silva, L.; Fu, J.Y.; Htar, T.T.; Muniyandy, S.; Kasbollah, A.; Wan Kamal, W.H.B.; Chuah, L.H. Characterization, Optimization, and In Vitro Evaluation of Technetium-99m-Labeled Niosomes. Int. J. Nanomed. 2019, 14, 1101–1117. [Google Scholar] [CrossRef] [PubMed]
- Ekinci, M.; Çalışkan, E.E.; Çakar, B.; İlem-Özdemir, D.; Uyanıkgil, Y.; Çetin Uyanıkgil, E.Ö. [99mTc]Technetium-Labeled Niosomes: Radiolabeling, Quality Control, and In Vitro Evaluation. ACS Omega 2023, 8, 6279–6288. [Google Scholar] [CrossRef]
- Almasi, A.; Shahhosseini, S.; Haeri, A.; Daha, F.J.; Geramifar, P.; Dadashzadeh, S. Radiolabeling of Preformed Niosomes with [99mTc]: In Vitro Stability, Biodistribution, and In Vivo Performance. AAPS PharmSciTech 2018, 19, 3859–3870. [Google Scholar] [CrossRef] [PubMed]
- Shewaiter, M.A.; Selim, A.A.; Moustafa, Y.M.; Gad, S.; Rashed, H.M. Radioiodinated Acemetacin Loaded Niosomes as a Dual Anticancer Therapy. Int. J. Pharm. 2022, 628, 122345. [Google Scholar] [CrossRef] [PubMed]
- De Silva, L.; Fu, J.Y.; Htar, T.T.; Wan Kamal, W.H.B.; Kasbollah, A.; Muniyandy, S.; Chuah, L.H. Biodistribution Study of Niosomes in Tumor-Implanted BALB/C Mice Using Scintigraphic Imaging. Front. Pharmacol. 2022, 12, 778396. [Google Scholar] [CrossRef]
- Munekane, M.; Kosugi, A.; Yamasaki, M.; Watanabe, Y.; Kannaka, K.; Sano, K.; Yamasaki, T.; Ogawara, K.I.; Mukai, T. Biodistribution Study of Indium-111-Labeled PEGylated Niosomes as Novel Drug Carriers for Tumor-Targeting. J. Drug Deliv. Sci. Technol. 2022, 75, 103648. [Google Scholar] [CrossRef]
- Raza, A.; Rasheed, T.; Nabeel, F.; Hayat, U.; Bilal, M.; Iqbal, H.M.N. Endogenous and Exogenous Stimuli-Responsive Drug Delivery Systems for Programmed Site-Specific Release. Molecules 2019, 24, 1117. [Google Scholar] [CrossRef]
- Akhlaghi, M.; Taebpour, M.; Lotfabadi, N.; Naghib, S.; Jalili, N.; Farahmand, L.; Haghiralsadat, B.; Rahmanian, M.; Tofighi, D. Synthesis and Characterization of Smart Stimuli-Responsive Herbal Drug-Encapsulated Nanoniosome Particles for Efficient Treatment of Breast Cancer. Nanotechnol. Rev. 2022, 11, 1364–1385. [Google Scholar] [CrossRef]
- Saharkhiz, S.; Nasri, N.; Naderi, N.; Saharkhiz, S.; Zarepour, A.; Ghalehshahi, S.S.; Goodarzi, R.; Khosravi, A.; Zarrabi, A. Dual-Ligand Functionalized pH- and Thermo-Sensitive Niosomes for Precise Targeted Therapy and Imaging of HER-2-Positive Breast Cancer. Mater. Today Chem. 2025, 47, 102829. [Google Scholar] [CrossRef]
- Jamshidifar, E.; Eshrati Yeganeh, F.; Shayan, M.; Tavakkoli Yaraki, M.; Bourbour, M.; Moammeri, A.; Akbarzadeh, I.; Noorbazargan, H.; Hossein-Khannazer, N. Super Magnetic Niosomal Nanocarrier as a New Approach for Treatment of Breast Cancer: A Case Study on SK-BR-3 and MDA-MB-231 Cell Lines. Int. J. Mol. Sci. 2021, 22, 7948. [Google Scholar] [CrossRef]
- Bahrami-Banan, F.; Sheikhha, M.H.; Ghasemi, N.; Rahmani, M.; Hemati, M.; Safdari, M.; Haghiralsadat, F. Synthesis of a Stimuli-Sensitive PEGylated Nanoniosomal Doxorubicin for the Treatment of Acute Myeloid Leukemia: An In Vitro Study. Nanomed. J. 2023, 10, 77–84. [Google Scholar] [CrossRef]
- Sankar, V.; Babu, E.; Siram, K.; Penmetsa, D.S.; Kabila, B.; Srinavas, R.C.; Rai, R. Formulation and Clinical Evaluation of Triamcinolone Acetonide Niosomes: Effect of Iontophoresis on the Permeation Across Skin. Pharm. Nanotechnol. 2013, 1, 282–289. [Google Scholar] [CrossRef]
- Arafa, M.G.; Ghalwash, D.; El-Kersh, D.M.; Elmazar, M.M. Propolis-based Niosomes as Oromuco-Adhesive Films: A Randomized Clinical Trial of a Therapeutic Drug Delivery Platform for the Treatment of Oral Recurrent Aphthous Ulcers. Sci. Rep. 2018, 8, 18056. [Google Scholar] [CrossRef] [PubMed]
- Farajzadeh, S.; Ahmadi, R.; Mohammadi, S.; Pardakhty, A.; Khalili, M.; Aflatoonian, M. Evaluation of the Efficacy of Intralesional Glucantime Plus Niosomal Zinc Sulphate in Comparison with Intralesional Glucantime Plus Cryotherapy in the Treatment of Acute Cutaneous Leishmaniasis, a Randomized Clinical Trial. J. Parasit. Dis. 2018, 42, 616–620. [Google Scholar] [CrossRef]
- Khalili, M.; Mehrabadi, R.; Mohammadi, S.; Pardakhty, A.; Amiri, R.; Aflatoonian, M. The Efficacy of Immunocryosurgery with Combined 2% Niosomal Zinc Sulfate Suspension and Cryotherapy in the Treatment of Verruca vulgaris. J. Kerman Univ. Med. Sci. 2022, 29, 139–145. [Google Scholar] [CrossRef]
- Damrongrungruang, T.; Paphangkorakit, J.; Limsitthichaikoon, S.; Khampaenjiraroch, B.; Davies, M.J.; Sungthong, B.; Priprem, A. Anthocyanin Complex Niosome Gel Accelerates Oral Wound Healing: In Vitro and Clinical Studies. Nanomedicine 2021, 37, 102423. [Google Scholar] [CrossRef]
- Rectal Dexmedetomidine Niosomes for Postoperative Analgesia in Pediatric Cancer Patients (DEX-NANO). Available online: https://clinicaltrials.gov/study/NCT05340725 (accessed on 31 October 2025).
- Tiwari, P.; Sinha, V.R.; Kaur, R. Chapter 4—Clinical Considerations on Micro- and Nanodrug Delivery Systems. In Drug Delivery Trends; Shegokar, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 77–101. ISBN 978-0-12-817870-6. [Google Scholar]
- Mali, N.; Darandale, S.; Vavia, P. Niosomes as a Vesicular Carrier for Topical Administration of Minoxidil: Formulation and In Vitro Assessment. Drug Deliv. Transl. Res. 2013, 3, 587–592. [Google Scholar] [CrossRef]
- Moammeri, A.; Chegeni, M.M.; Sahrayi, H.; Ghafelehbashi, R.; Memarzadeh, F.; Mansouri, A.; Akbarzadeh, I.; Abtahi, M.S.; Hejabi, F.; Ren, Q. Current Advances in Niosomes Applications for Drug Delivery and Cancer Treatment. Mater. Today Bio 2023, 23, 100837. [Google Scholar] [CrossRef] [PubMed]
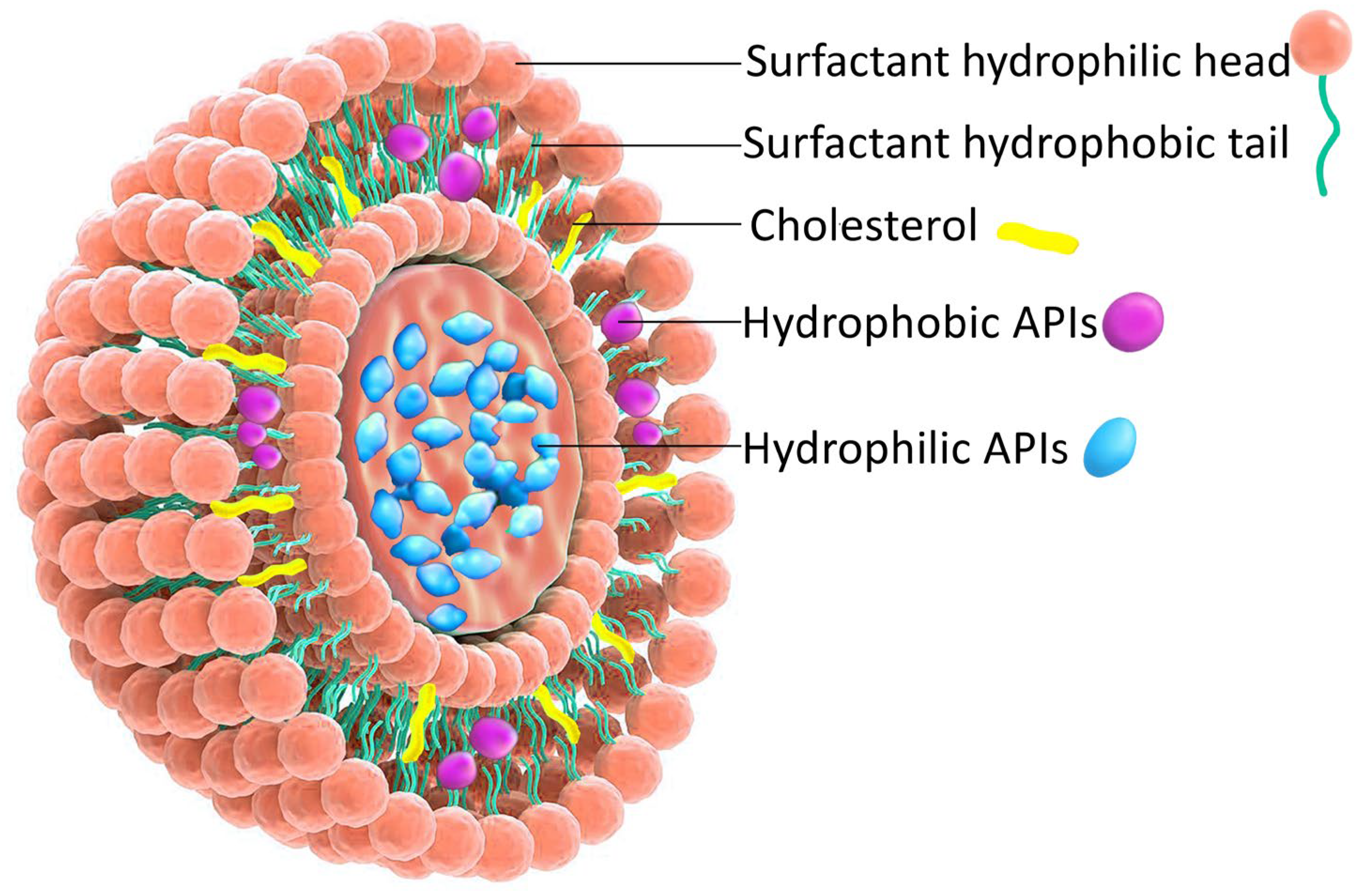
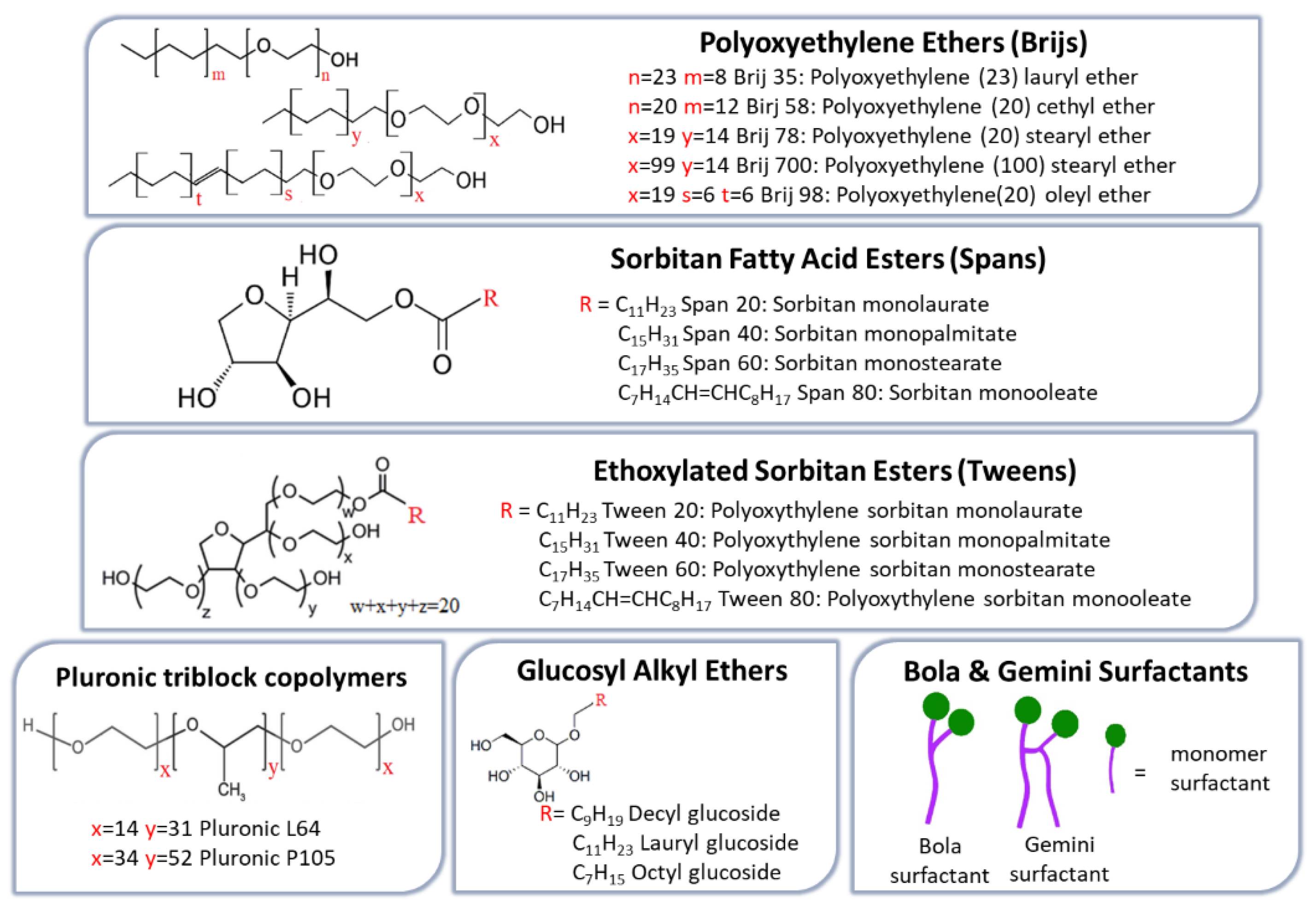
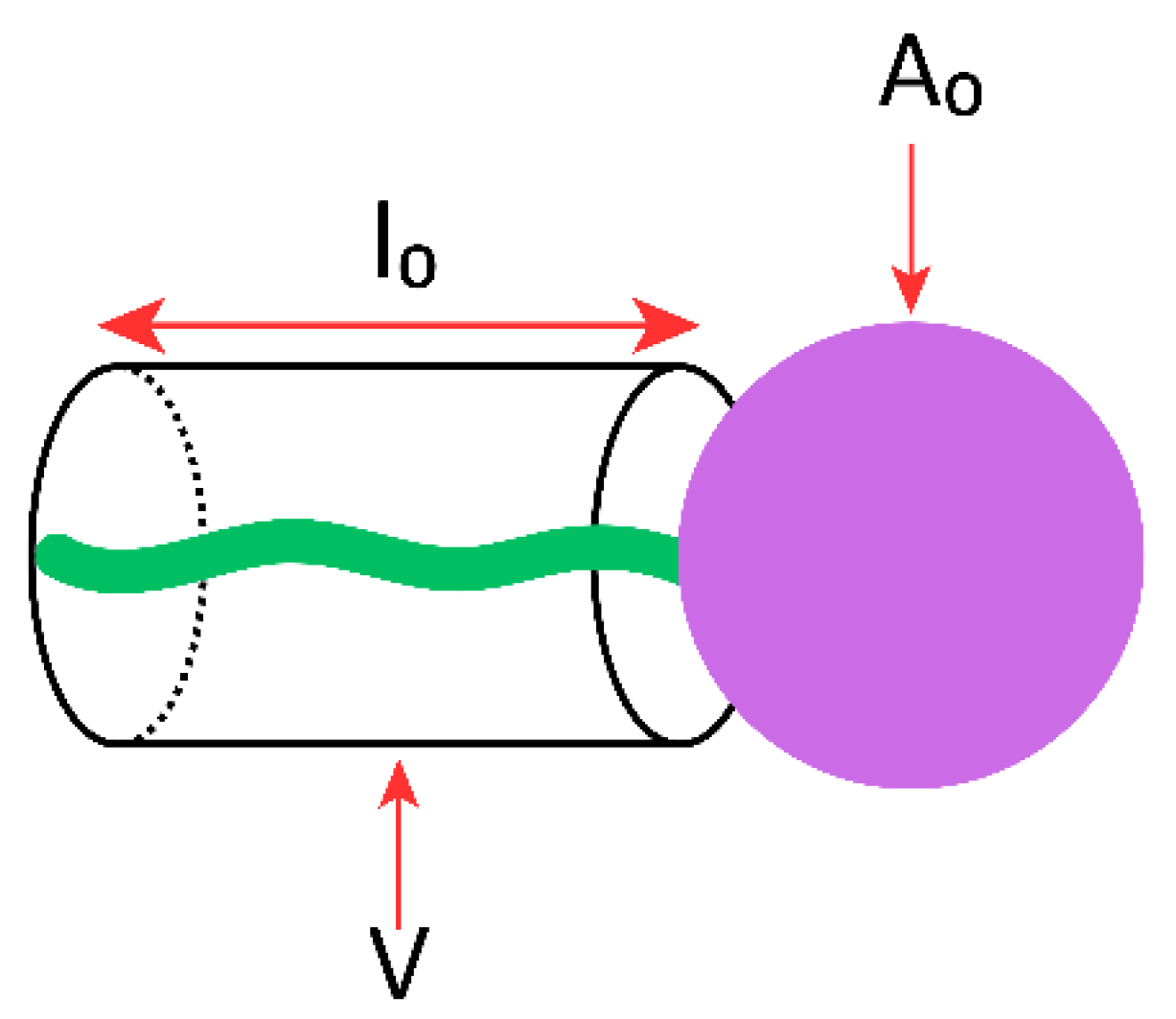
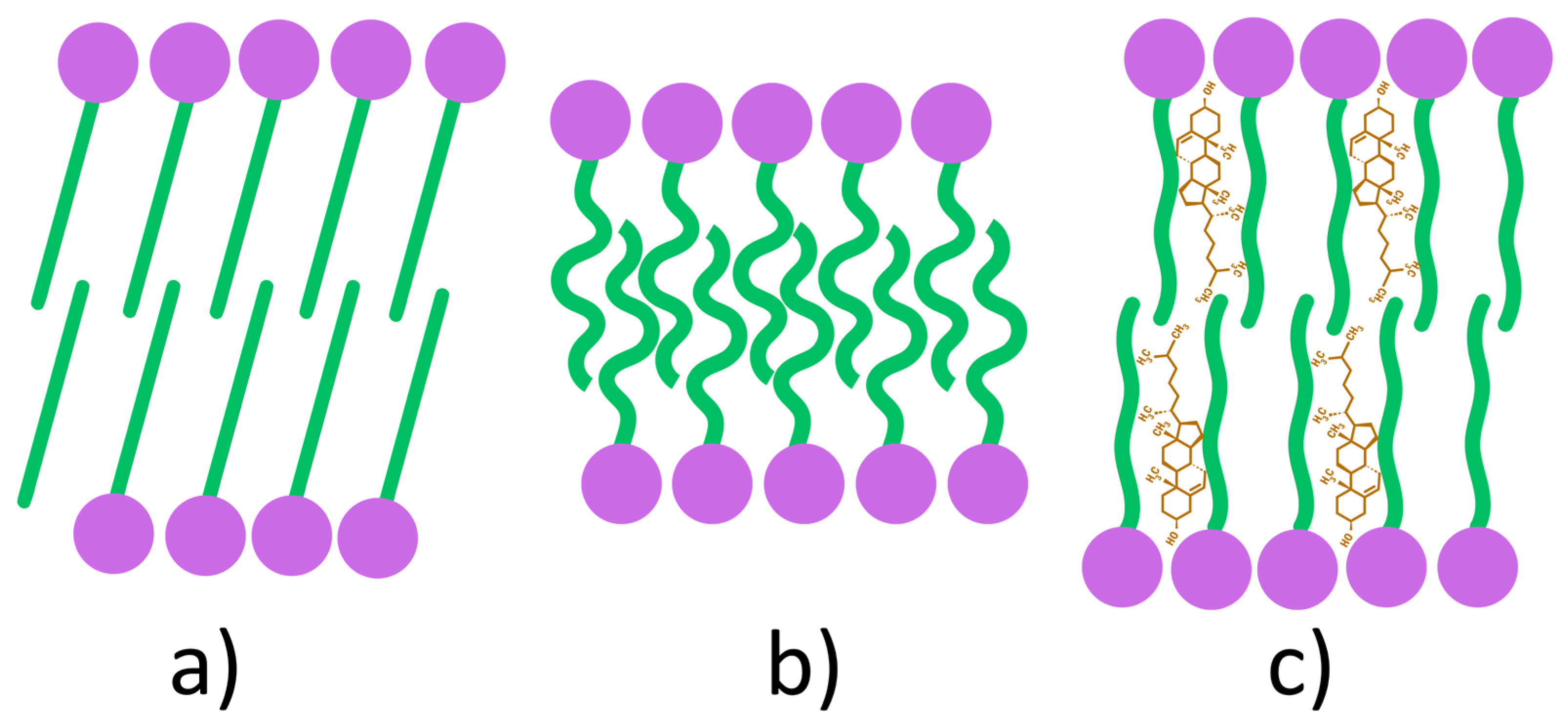
| Classification Criteria | Category | Representative Examples | Main Characteristics/Applications |
|---|---|---|---|
| Functionalized derivatives of NIOs | Ethosomes | Ethanol or isopropyl alcohol | Enhanced transdermal penetration |
| Bola-surfactant NIOs | Bola surfactants (α, ω-hexadecyl-bis-(1-aza-18-crown-6)) | Improved transdermal permeability | |
| Transfersomes | Edge activators | Ultra-flexible vesicles for membrane penetration | |
| Discomes | Cholesteryl poly-24-oxyethylene ether (Solulan C24) | Large disc-shaped vesicles for ocular drug delivery | |
| Aspasomes | Ascorbyl palmitate | Intrinsec antioxidant biological activity | |
| Elastic NIOs/Ethoniosomes | Ethanol or edge activators | Enhanced flexibility and deformability | |
| Polyhedral niosomes | Hexadecyl diglycerol ether (C16G2) or cholesteryl polyoxyethylene ether | Non-uniform spherical vesicles with a polygonal or faceted shape (typically 4–12 equal sides) | |
| Functionalization purpose | Conventional NIOs | No special modifications | Conventional drug delivery |
| Stealth NIOs (PEGylated) | PEG modification | Prolonged circulation, stealth behavior | |
| Targeted NIOs | Ligand-conjugated (antibody, peptide) | Targeted delivery to tumor/tissue | |
| Stimuli-responsive NIOs | Respond to pH, temperature, enzyme, or magnetic triggers | Controlled release and smart response | |
| Lamellar structure | Unilamellar NIOs | ~10–100 nm ~100–1000 nm | Small Unilamellar Vesicles (SUVs) Large Unilamellar Vesicles (LUVs) |
| Multilamellar NIOs (MLVs) | ~500–5000 nm | - | |
| Encapsulated molecule type | Hydrophilic NIOs | APIs in aqueous core | Monotherapy |
| Hydrophobic NIOs | APIs in bilayer | Monotherapy | |
| Co-loaded NIOs | Both hydrophilic and hydrophobic APIs | Combined therapies | |
| Encapsulated agent | Theranostic NIOs | Drug & imaging agent | Dual therapy and real-time tracking |
| Phytoniosomes | Plant-derived actives | ||
| Protein/Peptide-loaded NIOs | Proteins, enzymes or peptides | Targeted macromolecule delivery | |
| Gene-loaded NIOs | DNA, RNA, siRNA, miRNA | Gene therapy applications | |
| Hormone-loaded NIOs | Hormones (e.g., insulin, estradiol) | Controlled endocrine delivery | |
| Immunoniosomes | Antibody- or antigen-conjugated | Vaccine or immunotherapy systems | |
| Preparation method | Conventional hydration | Thin-film hydration of surfactants + Chol | Common lab-scale preparation |
| Proniosomes | Dried precursors in powder or gel | Enhanced stability, reconstitution in situ |
| Advantages | Disadvantages |
|---|---|
| High EE for both hydrophilic and hydrophobic drugs | Possible leakage of the entrapped drug |
| Biocompatible & biodegradable, being composed of non-toxic substances | Potential formation of niosomal aggregates during different preparation stages |
| Enhanced oral bioavailability and improved skin permeation | Risk of accumulation, fusion, or leakage of encapsulated drug in niosomal dispersions |
| Safe and non-toxic for administration via multiple delivery routes (oral, ocular, transdermal, parenteral etc.) | Variable encapsulation efficiency, especially for large hydrophilic molecules |
| Provide protection of encapsulated drugs against enzymatic degradation, oxidation, and other destabilizing processes | Potential local irritation depending on surfactants or excipients used |
| Effective carriers for targeted, controlled, and sustained drug delivery | Requirement of specialized equipment for certain preparation methods |
| Challenges in scaling up from laboratory to industrial production |
| CPP Value | Aggregation Shape | Surfactant Structural Characteristics | Schematic Representation |
|---|---|---|---|
| <1/2 | Spherical or cylindrical micelles | Very bulky head (e.g., ethoxylate group) and fairly small tail length (V < l0 × A0 values) |  |
| 1/2–1 | Geometrical packing (vesicles or flexible bilayers) | If V~l0 × A0 (CPP~1) the fairly symmetrical surfactant tends to be packed into cubic or simple lamellar liquid crystalline (Lα) phases, which when dispersed into water form vesicles |  |
| >1 | Inverted micelles | Cone-shaped surfactant with a very bulky tail and a small head and/or short tail (V > l0 × A0 values) |  |
| Formulation Factor | Key Material | Observed Effect on NIOs | Ref. |
|---|---|---|---|
| NISs | |||
| Alkyl ether surfactants | Alkyl glyceryl ethers; Brij series; | Stable, low-allergenic vesicles; suitable for macromolecule delivery. | [31,55,56,57] |
| Alkyl ester derivatives | Span series | Non-toxic, non-irritant; EE ↑ with chain length and saturation (Span 60 > Span 40 > Span 20 > Span 80); longer saturated chains ↑ bilayer rigidity and stability. | [58,59,60,61] |
| Alkyl ester derivatives | Tween series | Suitable for hydrophilic drug encapsulation; EE ↓ with chain length (Tween 20 > Tween 60 > Tween 40 > Tween 80); Tween 80 → gene delivery; Tween 20 → epithelial permeability | [58,62,63] |
| Pluronic triblock copolymers | EO–PO–EO type (poloxamers) | Improve EE and stability; suitable for injectable and thermo-responsive NIOs; Poloxamer 184 ↓ Chol need in mixed systems. | [64,65,66,67,68] |
| Glucosyl alkyl ethers (glucosides & alkyl polyglucosides) | Myristyl-, cetyl-, stearyl-glucosides; Octyl-/decyl-polyglucosides | Biodegradable, non-toxic; longer chains → stable vesicles; enhance transdermal and cutaneous drug delivery (e.g., tretinoin). | [69,70,71] |
| Bola & Gemini surfactants | Bola (α,ω-type) & Gemini dimers | Bola surfactants: ↑ water solubility, ↑ CMC, ↓ aggregation; Gemini surfactants: ↓ CMC, ↑ micelle stability and solubilization; both → non-toxic and non-hemolytic. | [72,73,74,75,76,77] |
| Fatty alcohols & fatty acids | Stearyl-, cetyl-, & myristyl-alcohol; Stearic-, palmitic-, myristic-, oleic-, linoleic-, octanoic-, & decanoic-acid | Form bilayer vesicles affecting size, stability, and EE; fatty alcohols → SUVs with controlled release; fatty acids → larger vesicles, stability pH-dependent (near pKa); mixtures improve bilayer stability. | [78,79,80] |
| Key NIS characteristics | |||
| Hydrophilic–Lipophilic Balance | Span, Tween, Brij series | HLB 4–8 → stable vesicles, ↑ EE (Span 60, Brij 72); high HLB → larger vesicles, ↓ EE (Tween 80, Brij 76); Chol needed for bilayer stabilization. | [59,60,61,81,84,85] |
| Critical Packing Parameter | - | Influences aggregation and bilayer assembly: ~ 0.5–1 → vesicles; <0.5 → micelles; >1 → inverted micelles. | [86,87,88] |
| Gel–Liquid Transition Temperature | - | Higher Tc → rigid, stable vesicles, ↑ EE; Lower Tc → flexible, permeable bilayers; Chol stabilizes bilayer via liquid-ordered phase. | [89,94,95,96,97] |
| Additive Agents | |||
| Membrane Additives | Chol | Intercalated in bilayer → ↑ rigidity, Tc, and EE, ↓ permeability; high-HLB surfactants need 30–50 mol % Chol; optimal surfactant/lipid ratio (10–30 mM) improves stability. | [33,34,52,54,98,99] |
| Surface additives (charge-inducing agents) | (–): DCP, phosphatidic acid; (+): stearylamine, stearyl pyridinium chloride | Provide electrostatic stabilization; ↑ EE, ↓ aggregation; optimal 2.5–5 mol%. | [4,33,45,85] |
| Steric Additives | PEG, CS | Improve colloidal stability via steric (PEG) or combined steric–electrostatic (CS) mechanisms; ↓ aggregation, ↑ rigidity, ↑ mucoadhesion, and prolong drug release. | [101,102,103] |
| Preparation Conditions | |||
| Hydration Temperature | - | Above Tc → stable bilayer formation; below Tc → defective vesicles; can effects vesicle morphology (polyhedral ↔ spherical); Chol improves thermal stability. | [105,106] |
| Hydration Medium pH | Phosphate buffer with various pH values | Acidic pH (5–5.5) enhances EE by favoring unionized APIs forms and better bilayer incorporation. | [54,107,108,109] |
| Hydration medium volume & time | - | Optimal hydration parameters → ↑ EE, stable vesicles; Short time or excess volume → large vesicles, drug leakage. | [62,98,110] |
| Other Formulation-Dependent Factors | |||
| Characteristics of the Encapsulated APIs | Hydrophilic vs. lipophilic drugs | Lipophilic APIs → ↑ EE (bilayer partitioning); Hydrophilic → ↓ EE (aqueous core); PEGylation limits size increase via steric hindrance. | [45,111,112] |
| Resistance to osmotic stress | Hypo-/hypertonic media | Hypertonic → vesicle shrinkage; hypotonic → swelling and accelerated drug release due to bilayer destabilization. | [45,98,113] |
| Specific Properties | Methodology | Reference |
|---|---|---|
| Purification | ||
| Dialysis of the aqueous niosomal dispersion against phosphate buffer, normal saline, or glucose solution | [4,9,33,83,86] | |
| Gel filtration chromatography (Sephadex) with elution in phosphate buffer or normal saline | ||
| Centrifugation/ultracentrifugation of the niosomal suspension, followed by removal of the supernatant and resuspension of the pellet | ||
| Characterization | ||
| Vesicle size and surface morphology | Dynamic light scattering (DLS); scanning electron microscopy (SEM); scanning tunneling microscopy (STM); transmission electron microscopy (TEM); cryo-TEM; atomic force microscopy (AFM); freeze fracture replication-electron microscopy (FF-TEM); negative-stained transmission electron microscopy (NS-TEM) | [11,13,53,85,112] |
| Size distribution and polydispersity index | Dynamic light scattering (DLS) | [13,34,53,86] |
| Charge of vesicle and zeta potential | Dynamic light scattering (DLS); microelectrophoresis; pH-sensitive fluorophores; high-performance capillary electrophoresis | [53,55,85,86,115,122] |
| Bilayer characterization | Lamellarity: AFM, nuclear magnetic resonance (NMR); small angle X-ray scattering (SAXS) Membrane rigidity: fluorescence polarization with DPH (1,6 diphenyl- 1,3,5-hexatriene) Bilayer thickness: fluorescence polarization combined with the in situ energy-dispersive X-ray diffraction (EDXD) | [11,13,44,85,86,115] |
| Vesicle stability | Evaluation of mean vesicle size, size distribution, and EE% during storage at various temperatures, combined with photodegradation studies (UV irradiation and fluorescent light exposure) | [33,34,54,86] |
| Entrapment efficiency | EE is determined by separating the unencapsulated (free) APIs from the vesicular fraction, followed by quantification of the encapsulated APIs content using appropriate spectrophotometric or chromatographic methods | [11,55,85,86,115,122] |
| In vitro drug release | The drug release profile is evaluated using the dialysis method, in which niosomal dispersions are placed within semipermeable membranes and subjected to controlled experimental conditions. Samples are periodically withdrawn from the external dialysate at predefined intervals, and the drug content is quantified using validated spectrophotometric or chromatographic analytical methods | [11,13,85,86,115] |
| In vivo studies | Drug biodistribution, tissue accumulation (in organs such as the liver, lungs, spleen, and bone marrow), and residence time are assessed in animal models following administration, depending on the route of delivery and the administered drug concentration | [53,55,98] |
| Specific Modulations | Biological Model and Therapeutic Agent | NIOs Composition and Preparation | Remarks and Applications | Ref. |
|---|---|---|---|---|
| CS-based pH-sensitive NIOs | In vitro model using doxycycline | Span 80/Tween 80; supercritical carbon dioxide-assisted process | CS coating decreases gastric release at pH 1.2 and enabled controlled intestinal/colonic delivery; diffusion mechanism remained Fickian, with coating layer acting as protective layer without altering release kinetics | [159] |
| Tween-based pH-sensitive NIOs | Human ovarian cancer cell lines (OVCAR-3, OVSAHO, Kuramochi) treated with all-trans retinoic acid (ATRA) | Tween (20, 21)/Chol; TFH method | NIOs loaded with ATRA showed stable encapsulation and serum compatibility; demonstrated pH-dependent release (slow at 7.4, fast at 5.5) and improved efficacy in HGSOC models | [160] |
| HD-PAA12/17-based pH-sensitive NIOs | Human tumor cell lines, urinary bladder carcinoma (T-24), cutaneous T-cell lymphoma (HUT-78, MJ), and lung carcinoma (H1299) and L929 murine fibroblasts (control) treated with curcumin and calcein (model marker) | Span 60/Tween 60/Chol/HD-PAA copolymers; TFH method | HD-PAA modification enabled pH-responsive drug release (in acidic media), enhanced internalization, and strong pro-apoptotic effect; most pronounced in T-24 bladder carcinoma cells | [161] |
| PEGylated surface-modified NIOs | AGS human gastric adenocarcinoma cell line (C131) treated with cyclophosphamide; HFF fibroblasts (C163) used as control | Span 20/Span 60/Chol/PEG 2000; TFH method | pH-responsive release under tumor-like conditions; enhanced cytotoxicity and apoptosis compared to free cyclophosphamide and non-PEG NIOs; selective G2/M cell cycle arrest, halting cell division at the G2 (Gap 2) to M (mitosis) transition, a key mechanism commonly triggered by cytotoxic agents | [162] |
| HA/FA/PEG-based surface-functionalized NIOs | MCF-7 (human breast cancer), 4T1 (mouse breast cancer) treated with 5-fluorouracil; MCF-10A (non-tumorigenic epithelial cells) used as control | Span 60/Chol; TFH method | Surface-functionalized NIOs enhanced tumor cell uptake and apoptosis; FA-decorated NIOs showed highest cytotoxicity, reduced migration, and strongest cellular uptake in acidic pH (5.4); sustained release at pH 7.4; ROS elevation and necrosis/apoptosis confirmed via flow cytometry | [163] |
| CHEMS-based pH-sensitive NIOs | Breast cancer cells treated with carfilzomib (CFZ) | Span 60/Tween 60/ergosterol/ CHEMS; TFH method | pH-dependent release (74.39% at pH 5.4 vs. 54.55% at pH 7.4); enhanced cytotoxicity in breast cancer cells (IC50 = 0.0415 µM vs. 0.0714 µM for free CFZ); synergistic effect with doxorubicin (combination index <1); safe ≤ 2 mg/kg, toxicity at 4 mg/kg | [164] |
| HCC: HepG2, Huh7 tumor cells (in vitro); AKT/c-Met–induced HCC mouse model (in vivo); treated with Tanshinone IIA | Span 80/Chol/CHEMS; ethanol injection method | Functionalization with galactose ligands enhanced selective uptake in hepatic tumors; pH-sensitive release increased antitumor efficacy of Tanshinone IIA; significant apoptosis induction (Annexin V/PI staining); G0/G1 cell cycle arrest; improved pharmacokinetics and biodistribution (higher plasma and liver retention); superior tumor suppression in AKT/c-Met–induced HCC mice | [165] | |
| CHEMS-based pH-sensitive NIOs | MCF7 breast cancer cells treated with methotrexate (MTX); HUVECs (control); Wistar rats (in vivo toxicity) | Tween 60/Span 60/ergosterol/ CHEMS; TFH method | pH-dependent release (initial burst release, 42.1% in 4 h, followed by a slower release phase up to 24 h, reaching 79.4% at pH 5.4, vs. a sustained release of 51.2% after 24 h at pH 7.4); lower IC50 in MCF7 cells for pH-MTX/NIOs vs. free MTX (9.46 vs. 84.03 μg/mL); reduced toxicity in normal cells and in vivo (2 mg/kg safe; 4 mg/kg induced liver/kidney damage) | [166] |
| MCF7 human breast cancer cells treated with cisplatin | Span 60/Tween 60/ergosterol/ CHEMS; TFH method | Sustained and pH-responsive release (faster at pH 5.4 vs. delayed at pH 7.4); enhanced cytotoxicity vs. free cisplatin; improved cell-killing effect; high EE% (89%) | [167] | |
| MCF-7 (breast cancer), OVCAR-3 (ovarian cancer) treated with mitoxantrone; HUVECs used as control | CHEMS/ PEG-PMMI-CholC6 copolymer; modified ethanol injection method | PEG-PMMI-CholC6 copolymer conferred pH-sensitivity and plasma stability; enhanced mitoxantrone release in acidic conditions (pH 6.5); higher cytotoxicity in tumor cells vs. conventional NIOs; lower toxicity in HUVECs | [168] | |
| MCF7 (human breast cancer), HeLa (human cervical cancer) treated with paclitaxel; HUVECs used as control | Span 60/Tween 60/ergosterol/ CHEMS; TFH method | pH-responsive release (faster at pH 5.2 vs. 7.4); EE% of 77%; improved antitumor efficacy (breast, cervical); lower IC50 vs. free PTX; reduced toxicity at 2.5 mg/kg dose; safer profile in normal cells | [156] | |
| Calcium alginate-coated pH-sensitive NIOs | MDA-MB-231 and SKBR3 (breast cancer cells) treated with curcumin; MCF-10A (normal breast epithelial cells) used as control | Span 80/Chol/calcium alginate coating; TFH method | pH-dependent release: sustained at pH 7.4, accelerated at pH 3; enhanced apoptosis and improved selectivity toward cancer cells; biocompatibility in normal cells | [169] |
| pH-sensitive Tween 20 derivatives with modified polar head groups | CD-1 mice with chemically induced inflammatory and neuropathic pain, treated with ibuprofen | Tween 20 derivatives (GLY)/Chol; TFH method | Bilayer destabilization at low pH enabling site- specific ibuprofen release; antinociceptive effects in multiple mouse pain models: writhing, capsaicin, zymosan-induced hyperalgesia, and CCI-induced allodynia; superior performance vs. free ibuprofen | [155] |
| pH LIP-based surface-functionalized NIOs | A549 lung carcinoma, 4T1 mammary carcinoma cells; tumor tissue (mouse model); R18 (fluorescent membrane tracer) | Span 20/Chol - pHLIP conjugates (DSPE-pHLIP or Pyr-pHLIP); TFH method | pH-sensitive targeting enabled selective accumulation in tumors with 2–3 fold increased uptake and prolonged circulation vs. PEG-NIOs; confirmed pH-dependent uptake and minimal toxicity; effective tumor imaging and biodistribution | [170] |
| pH-sensitive Tween 20 derivatives with modified polar head groups | Inflamed tissues in CD-1 mice treated with ibuprofen or lidocaine; in vitro cytotoxicity assessed on HaCaT (human keratinocytes) and Balb/3T3 (mouse fibroblasts) | Tween 20 or Tween 20 (GLY)/Chol; TFH method | Improved pH-responsive release via bilayer destabilization by protonation of glycine residues; non-cytotoxic; modulated in vivo antinociceptive and anti-inflammatory activity (formalin and zymosan paw edema models); higher bilayer fluidity vs. non-pH-sensitive NIOs | [171] |
| Hepatoblastoma cells; calcein (model marker) | Tween 20 derivatives (GLY, MMG, DMG)/Chol; TFH method | Proton sponge effect of Tween-20 derivatives enabled pH-sensitive intracellular delivery and enhanced calcein release; efficient uptake in 15 min; low toxicity (>93% viability) | [172] |
| Specific Modulations | Biological Model and Therapeutic Agent | NIOs Composition and Preparation | Remarks and Applications | Ref. |
|---|---|---|---|---|
| Fatty acid-based thermo-responsive NIOs | Gram-positive (Bacillus subtilis, Staphylococcus epidermidis, Staphylococcus aureus, Listeria monocytogenes, Enterococcus faecalis) and Gram-negative (Escherichia coli, Acinetobacter baumannii, Klebsiella pneumoniae) bacteria exposed to tetracycline | Span 60/Chol/PCM (lauric & stearic acids eutectic mixture); TFH method | Temperature-triggered release system with low leakage at 37 °C and enhanced release at 42 °C; improved antibacterial efficacy (MIC for S. epidermidis reduced from 112.81 to 14.10 μM at 42 °C); promising candidate for heat-augmented infection therapy | [176] |
| FA-functionalized thermo-sensitive NIOs | Human breast cancer cells (MCF-7, MDA-MB-231, SKBR-3) and normal breast epithelial cells (MCF-10A) treated with doxorubicin and/or CQDs (photothermal & bioimaging agents) | Tween 60/Span 40/Chol/FA/ lecithin; TFH method | Thermo-sensitive folate-targeted NIOs with CQDs: lecithin enhanced membrane fluidity and enabled thermally triggered doxorubicin release >41 °C; effective chemo-photothermal cytotoxicity in cancer cells; selective uptake via folate receptors | [177] |
| Pluronic® L64-based thermo-responsive NIOs | In vitro release study: calcein (model marker), 5-Fluorouracil (API) | Pluronic® L64 (or L64ox)/Chol; TFH method | Thermo-sensitive release enhanced at 42 °C vs. 25–27 °C; temperature-dependent release attributed to surfactant structure, not drug type; promising stealth and targeted delivery | [174] |
| Physicochemical model (1-Naphthol, fluorescent model) | Span 60/Pluronic® L64/Chol; TFH method | Tunable phase transition (Tc ~ 40 °C); fluorescence & Raman analysis confirmed bilayer transition; suitable for thermo-responsive drug delivery under mild hyperthermia | [178] |
| Specific Modulations | Biological Model and Therapeutic Agent | NIOs Composition and Preparation | Remarks and Applications | Ref. |
|---|---|---|---|---|
| Fe3O4-based magnetically responsive NIOs | In vitro release study; doxorubicin as model drug | Span 60/Chol/Fe3O4; TFH method | AMF-triggered burst release (86% in 3 h vs. 3% in 30 days); 1st order kinetics; magnetic loading conferred precise AMF-responsive control and enhanced efficiency | [181] |
| Human embryonic kidney cell line (HEK-293T) exposed to plasmid DNA/protamine complex | Span 60/Tween 60/Chol or ergosterol/Fe3O4; TFH method | Enhanced uptake, magnetic responsiveness, and gene expression in HEK-293T cells; ergosterol-based vesicles showed smaller size, better stability, slower plasmid release, and higher transfection efficiency vs. Chol-based ones; magnetic nanoparticles facilitated guided delivery and enhanced performance under magnetic stimulus | [182] | |
| Human chronic myelogenous leukemia cell (K562) treated with doxorubicin | Tween 60 or Pluronic L64/Fe3O4; TFH method | Magnetically triggered release of doxorubicin via co-encapsulation of Fe3O4 nanoparticles; sustained drug release (50% in 5 h vs. 100% in 3 h for free drug); low inherent cytotoxicity of carriers supports tumor-targeted applications | [180] | |
| PEGylated magnetically responsive NIOs | A549 lung cancer cells treated with metformin (MET) and artemisinin (ART); HBE normal epithelial cells used as control | Span 60/Chol/Fe3O4/ PEG 4000; TFH method | PEGylated Fe3O4-loaded NIOs co-delivering ART and MET: synergistic cytotoxicity, enhanced uptake and apoptosis in A549 cells; magnetic field (1.3 T) improved drug targeting and reduced viability (from 60% to 45%); no significant cytotoxicity in HBE cells | [183] |
| Breast cancer cells (BT-474) treated with LFG-specific siRNA and chemotherapeutics (erlotinib/trastuzumab) | Span 60/Chol/Fe3O4/DSPE-PEG; TFH method | PEG-maleimide-functionalized Fe3O4-NIOs enhanced siRNA protection, intracellular uptake, and gene silencing; induced apoptosis and amplified drug efficacy (erlotinib/trastuzumab); magnetic field further boosted uptake | [184] | |
| Breast cancer cells (MCF-7) treated with AuNPs (SERS probe) | Span 60/Chol/Fe3O4/ DSPE-PEG; TFH method | Tf-functionalized Fe3O4/AuNP-loaded NIOs enabled dual targeting (magnetic and Tf-mediated) and SERS-based sensing; high intracellular accumulation in MCF-7 cells; PEGylated niosomal shell provided enhanced structural stability and contamination-free performance, supporting accurate tumor monitoring | [185] | |
| Breast cancer cells (MCF-7) treated with carboplatin | Tween 60/Span 60/ Chol/Fe3O4@SiO2/PEG 6000; TFH method | PEGylated Fe3O4@SiO2 NIOs enabled magnetic targeting and sustained carboplatin release; enhanced cytotoxicity observed in MCF-7 cells with external magnetic field (38% vs. 57%); PEGylation reduced premature release and improved bioavailability | [186] |
| Specific Modulations | Biological Model and Therapeutic/Diagnostic Payload | NIOs Composition and Preparation | Radiolabeling Strategy | Remarks and Applications | Ref. |
|---|---|---|---|---|---|
| Directly radiolabeled NIOs | Human colorectal adenocarcinoma (HT-29) cells exposed to [99mTc]-radiolabeled NIOs | Span 60/Tween 60/Chol; TFH method | Direct [99mTc] labeling via SnCl2 reduction; reduced Tc coordinates with functional groups of the NIO bilayer | High radiolabeling efficiency (>95%), stability up to 6 h in biological media, and enhanced uptake in HT-29 cells versus free [99mTc]; suitable for passive targeting via EPR effect; outperforming chelator-based methods | [188] |
| Ehrlich ascites carcinoma cells (solid tumors induced in mice) exposed to intravenously administered [131I]-ACM-loaded NIOs | Span 60/Chol; ether injection method | Direct [131I] radiolabeling of ACM via electrophilic substitution using chloramine-T | Enables dual chemo-radiotherapeutic functionality: ACM mediates PGE2-dependent chemotherapy, while [131I] delivers tumor-targeted radiotherapy with enhanced uptake, tumor regression, metastasis suppression, and tissue-specific accumulation; supports passive targeting via the EPR effect | [190] | |
| Chelator-mediated radiolabeled PEGylated NIOs | Mouse breast tumor cells (4T1), exposed to [99mTc]-radiolabeled PEGylated NIOs for passive tumor targeting | Span 60/Chol/TPGS-DTPA; Sonication method | Surface chelation of [99mTc] using TPGS-DTPA in the presence of SnCl2 as a reducing agent | Stable [99mTc]-NIOs with high radiolabeling efficiency (98%) and in vitro/in vivo stability; tumor accumulation confirmed via scintigraphic imaging and biodistribution; high tumor-to-muscle uptake ratio via EPR; validated prototype for passive tumor targeting | [187,191] |
| Colon-26 tumor-bearing BALB/c mice, exposed to [111In]-DTPA-radiolabeled PEGylated NIOs for biodistribution analysis | Span (20/40/60/80)/ Chol/DCP/ PEG-DSPE; TFH method | Remote chelation of [111In] into DTPA-containing PEGylated NIOs using oxine as a carrier | Remote [111In] labeling of PEGylated NIOs enabled high labeling efficiency and serum stability; Span 20-based NIOs showed enhanced tumor accumulation, while Span 80-based NIOs exhibited reduced circulation stability and increased spleen uptake | [192] | |
| Chelator-mediated radiolabeling of GSH-loaded NIOs | 4T1 tumor-bearing BALB/c mice, exposed to [99mTc]-HMPAO-radio-complexincorporated via GSH-mediated passive diffusion into preformed NIOs | Tween 60/Brij 35/Chol; TFH method | Chelator-mediated encapsulation using [99mTc]-HMPAO; passive bilayer diffusion followed by GSH-driven trapping in the aqueous core | [99mTc]-HMPAO-labeled GSH-NIOs exhibited higher tumor accumulation and prolonged blood circulation vs. free tracer; radiolabeling stability ensured by GSH entrapment; enhanced tumor uptake via EPR effect; successful in vivo tracking by SPECT/CT | [189] |
| Specific Modulations | Biological Model and Therapeutic Agent | NIOs Composition and Preparation | Remarks and Applications | Ref. |
|---|---|---|---|---|
| Dual pH/thermo-sensitive PEGylated NIOs based on DPPC composition | Breast cancer cells (MCF-7 and BT-474) exposed to Hedera helix extract (HHE) or Glycyrrhiza glabra extract (GGE) encapsulated in responsive NIOs | Tween 60 or Span 60/Chol/DSPE-PEG/DPPC; TFH method | Dual-stimuli responsive release: enhanced extract release at 42 °C and acidic pH (4.5–5.2); triphasic release behavior (intrinsic pH-sensitivity via pH-cleavable DSPE-PEG and thermo-sensitivity via DPPC phase transition); superior cytotoxicity of NIOs-HHE vs. NIOs-GGE; antibacterial activity confirmed; enhanced cellular uptake with reduced IC50 vs. free extracts; upregulation of p53 by 60% and down-regulation of MCL-1 genes by 33%, confirming improved anticancer mechanism | [194] |
| Dual pH/thermo-sensitive NIOs co-functionalized with FA and trastuzumab | SKBR-3 (HER2+ breast cancer cells), treated with gemcitabine and CdSe/ZnS QDots-loaded NIOs | Span 60/Tween 60/Chol/DPPC/DSPE-CA-PEG2000/DSPE–PEG/DSPE-PEG-Maleimide; TFH method | FA (via DSPE–PEG) and trastuzumab (via PEG–Maleimide–thiol linkage) as dual-targeting ligands; DPPC as thermo-sensitive lipid (Tc > 41 °C); DSPE–CA–PEG2000 as pH-sensitive polymer (cleavable in acidic tumor microenvironment); high entrapment efficiency (~96.9%) and stability up to 6 months at 4 °C (EE 94.8%); triggered release up to 92% at 42 °C/pH 6.5; 4.5-fold higher cytotoxicity and >2600-fold cellular uptake in SKBR-3 vs. controls; induced 42% of total apoptosis to the cells via p53 activation and MCL-1 suppression; theranostic potential via CdSe/ZnS QDots | [195] |
| Dual pH/magnetic-sensitive NIOs incorporating NiCoFe2O4@Silica nanoparticles | MDA-MB-231 (triple-negative breast cancer), SK-BR-3 (HER2+ breast cancer) and MCF-10A (non-tumorigenic) cells treated with NiCoFe2O4@Silica@NIO co-loaded with curcumin and letrozole | Magnetic NiCoFe2O4 core coated with a porous silica shell (reservoir for letrozole), further encapsulated in a Span 80/Chol/DCP niosomal bilayer containing curcumin; TFH method | Magnetic NiCoFe2O4@Silica@NIOs enabled dual-drug co-delivery: magnetic NiCoFe2O4 core for guidance and MRI tracking and letrozole–curcumin as synergistic therapeutic payloads (theranostic function); pH-dependent release profile; enhanced cytotoxicity in MDA-MB-231 and SK-BR-3 cells vs. free drugs; reduced migration and apoptosis induction confirmed by gene and flow-cytometry analyses; selective biocompatibility in MCF-10A cells confirmed via low uptake; one of the lowest IC50 values reported for this drug combo; apoptosis induction, increased cellular uptake, and significant cell-cycle arrest; migration inhibition (~59% vs. 100% controls); selective biocompatibility in MCF-10A cells; magnetically guided, pH-responsive co-delivery with synergistic anticancer selectivity | [196] |
| Type of Niosomal DDS/Formulation Considerations | Medical Applications | Preclinical/Clinical Tests and Findings | Clinical Trial Number | Ref. |
|---|---|---|---|---|
| Ab-modified NIOs carrying daunorubicine/Anti-CD123 antibody thiolated with Traut’s reagent, Mal-PEG2000-DSPE obtained by post-insertion method | Acute myeloid leukemia | Ex vivo studies on NB4 & THP-1 cells; THP-1 NOD/SCID xenograft; flow cytometry analysis confirmed precise targeting of daunorubicin to NB4 and THP-1 cell lines, proportional to the density of the antibody on the niosomal surface. | - | [28] |
| Thermo-responsive PEGylated NIOs carrying doxorubicin/Span 60, Chol, SPC80, DSPE-mPEG, obtained by TFH method | Acute myeloid leukemia | Ex vivo studies on KG-1 cells; thermo-responsive NIOs significantly enhanced the cytotoxic effect of doxorubicin on the leukemia cell line KG-1; through PEG coating and temperature-sensitive properties, the obtained NIOs demonstrated prolonged circulation, superior cellular penetration, and controlled drug release. | - | [197] |
| pH-responsive NIOs carrying antineoplastic agents/Span 60 : Tween60 : Chol : HD-PAA17 and Cur obtained by TFH method | Lymphoma | Ex vivo studies—a panel of tumor cell lines of different origin namely urinary bladder carcinoma (T-24), and cutaneous T-cell lymphoma (HUT-78 and MJ); pH-sensitive Cur-loaded NIOs demonstrated greater colony formation inhibition and enhanced antineoplastic and anti-inflammatory activity compared to free Cur. | - | [161] |
| NIOs carrying Triamcinolone Acetonide/Brij 52, Span, Cetrimide, Chol, Oleic Acid, Drug obtained by TFH method | Keloids, joint damage in rheumatoid arthritis and psoriatic arthritis/Transdermal/with iontophoresis | Clinical study by histamine wheal suppression test, clinical trial-initial phase, small number of human subjects; compared to conventional preparations even without the application of non-invasive methods such as iontophoresis | Unknow status | [198] |
| NIOs propolis oromucodhesive film/Span 60 and Chol, polymer combination (EU-L100, HPMC and PVA) prepared by solvent casting technique | Aphthous ulcers/topical application | Completed clinical trial; NIOs improving local drug penetration and retention, through the NIS, and favored accelerated wound healing. | NCT03615820 | [199] |
| NIOs zinc sulfate/Span 60, Chol TFH method | Leishmaniasis/ topical application | Randomized clinical trial; an increased absorption of the niosomal formulation in the target organ with a therapy efficacy similar to the standard protocol (cryotherapy plus intralesional Glucantime). It is recommended as a second-line treatment. | - | [200] |
| NIOs/Brij 52, Chol, zinc sulfate prepared by TFH method | Verruca vulgaris/topical application | A triple-blind randomized clinical trial; a significant remission of wart Verruca vulgaris lesions following the administration of a 2% niosomal zinc oxide suspension and cryotherapy compared with 2% conventional zinc sulfate suspension combined with cryotherapy. | - | [201] |
| NIOs gel carrying anthocyanin complex (AC)/prepared by TFH method | Oral wounds/topical application | A randomized block placebo-controlled double-blind clinical trial; the in vitro results indicated that the administration of NIOs increased cell viability, proliferation, migration and expression of proteins with a role in promoting wound healing; the clinical study results showed that the AC niosome gel significantly accelerated wound healing compared to triamcinolone acetonide gel. | - | [202] |
| NIOs carrying Dexmedetomidine/Chol, Span 60, DCP, dexmedetomidine obtained by thin TFH method | Postoperative analgesia in pediatric cancer patients/rectal administration | Phase 2/3 clinical trial; improved pain management, a viable alternative to intravenous methods, concerns about absorption variability and patient comfort need addressing. | NCT05340725 | [203] |
| NIOs carrying salbutamol sulfate/Span 60, Chol obtained by reverse-phase evaporation method | Pulmonary disease/Inhalation route | Clinical trial phase I; lack of results obtained | NCT03059017 | [204] |
| NIOs gel carrying melatonin/gel (HPMC, CS, and P407), NIOs (Span 60, Chol, melatonin) obtained by TFH method | Sleep induction/transmucosal administration | Clinical trial phase I/II, status Unknown; transmucosal option for melatonin administration with substantial prolonged systemic delivery. | NCT02845778 | [204] |
| NIOs carrying minoxidil/(Span 60, Chol, Tween 20) obtained by ethanol injection method. | Alopecia areata/topical administration | Without FDA-defined phases; in vitro studies showed that can increase transfollicular, transepidermal absorption, controlled release, improved efficacy and superior safety profile compared to conventional topical minoxidil | NCT05587257 | [205] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ababei-Bobu, A.; Profire, B.-Ș.; Iacob, A.-T.; Chirliu, O.-M.; Lupașcu, F.G.; Profire, L. Niosomes as Vesicular Carriers: From Formulation Strategies to Stimuli-Responsive Innovative Modulations for Targeted Drug Delivery. Pharmaceutics 2025, 17, 1473. https://doi.org/10.3390/pharmaceutics17111473
Ababei-Bobu A, Profire B-Ș, Iacob A-T, Chirliu O-M, Lupașcu FG, Profire L. Niosomes as Vesicular Carriers: From Formulation Strategies to Stimuli-Responsive Innovative Modulations for Targeted Drug Delivery. Pharmaceutics. 2025; 17(11):1473. https://doi.org/10.3390/pharmaceutics17111473
Chicago/Turabian StyleAbabei-Bobu, Andra, Bianca-Ștefania Profire, Andreea-Teodora Iacob, Oana-Maria Chirliu, Florentina Geanina Lupașcu, and Lenuța Profire. 2025. "Niosomes as Vesicular Carriers: From Formulation Strategies to Stimuli-Responsive Innovative Modulations for Targeted Drug Delivery" Pharmaceutics 17, no. 11: 1473. https://doi.org/10.3390/pharmaceutics17111473
APA StyleAbabei-Bobu, A., Profire, B.-Ș., Iacob, A.-T., Chirliu, O.-M., Lupașcu, F. G., & Profire, L. (2025). Niosomes as Vesicular Carriers: From Formulation Strategies to Stimuli-Responsive Innovative Modulations for Targeted Drug Delivery. Pharmaceutics, 17(11), 1473. https://doi.org/10.3390/pharmaceutics17111473











