Modern Approaches and Emerging Biological Therapies to Treat Fracture Nonunion
Abstract
1. Introduction
2. Clinical Context
2.1. Clinical Significance
2.2. Risk Factors
3. Pathophysiology
3.1. Bone Healing
3.2. Impaired Healing
4. Current Solutions to Address Nonunion
4.1. Nonsurgical
4.2. Surgical Solutions
4.2.1. Traditional Surgical Approaches
4.2.2. Surgical Solutions with Biologic Therapy
5. Emerging Strategies for Nonunion Repair
5.1. Exogenous Growth Factors
5.1.1. BMP
5.1.2. Alternative Molecular Mediators of Bone Healing
5.2. Immunomodulation
5.3. Scaffolds
5.3.1. Bioceramics
5.3.2. Natural Polymers
5.3.3. Hydrogels
5.4. Stem Cell-Based Therapy
5.5. Integration of Gene Therapy
6. Future Directions
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Huang, J.; Hu, J.; Ma, X.; Huang, Z.; Zhu, J.; Zhu, K.; Zhang, C. Global Burden, Trends and Forecast Analysis of Extremity Fractures Based on GBD 2021. Int. J. Surg. 2025, 111, 4884–4897. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhang, C.; Li, B.; Zhan, S.; Wang, S.; Song, C. Global Burden of Hip Fracture: The Global Burden of Disease Study. Osteoporos. Int. 2024, 35, 41–52. [Google Scholar] [CrossRef]
- Mills, L.A.; Aitken, S.A.; Simpson, A.H.R.W. The Risk of Non-Union per Fracture: Current Myths and Revised Figures from a Population of over 4 Million Adults. Acta Orthop. 2017, 88, 434–439. [Google Scholar] [CrossRef]
- Zura, R.; Xiong, Z.; Einhorn, T.; Watson, J.T.; Ostrum, R.F.; Prayson, M.J.; Della Rocca, G.J.; Mehta, S.; McKinley, T.; Wang, Z.; et al. Epidemiology of Fracture Nonunion in 18 Human Bones. JAMA Surg. 2016, 151, e162775. [Google Scholar] [CrossRef]
- Calori, G.M.; Mazza, E.; Colombo, M.; Ripamonti, C.; Tagliabue, L. Treatment of Long Bone Non-Unions with Polytherapy: Indications and Clinical Results. Injury 2011, 42, 587–590. [Google Scholar] [CrossRef]
- Roberts, L.; Ahmed, M.R.; Agrawal, D.K. Current Strategies in the Prevention and Management of Infection in Open Fractures. J. Orthop. Sports Med. 2025, 7, 218–229. [Google Scholar] [CrossRef]
- Kawamura, K.; Chung, K.C. Treatment of Scaphoid Fractures and Nonunions. J. Hand Surg. Am. 2008, 33, 988–997. [Google Scholar] [CrossRef]
- Jones, G.L.; McCluskey, G.M.; Curd, D.T. Nonunion of the Fractured Clavicle: Evaluation, Etiology, and Treatment. J. South. Orthop. Assoc. 2000, 9, 43–54. [Google Scholar]
- Mundi, R.; Pincus, D.; Schemitsch, E.; Ekhtiari, S.; Paterson, J.M.; Chaudhry, H.; Leis, J.A.; Redelmeier, D.A.; Ravi, B. Association Between Periprosthetic Joint Infection and Mortality Following Primary Total Hip Arthroplasty. J. Bone Jt. Surg. 2024, 106, 1546–1552. [Google Scholar] [CrossRef]
- Tian, R.; Zheng, F.; Zhao, W.; Zhang, Y.; Yuan, J.; Zhang, B.; Li, L. Prevalence and Influencing Factors of Nonunion in Patients with Tibial Fracture: Systematic Review and Meta-Analysis. J. Orthop. Surg. Res. 2020, 15, 377. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.S.; Jensen, N.M.; Gundtoft, P.H.; Kold, S.; Zura, R.; Viberg, B. Risk Factors for Nonunion Following Surgically Managed, Traumatic, Diaphyseal Fractures: A Systematic Review and Meta-Analysis. EFORT Open. Rev. 2022, 7, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Dickson, K.M.D.; Katzman, S.M.D.; Delgado, E.M.D. Delayed Unions and Nonunions of Open Tibial Fractures: Correlation with Arteriography Results. Clin. Orthop. Relat. Res. 1994, 302, 189–193. [Google Scholar] [CrossRef]
- Schnetz, M.; Wengert, A.; Ruckes, C.; Jakobi, T.; Klug, A.; Gramlich, Y. Open Fractures of the Lower Leg: Outcome and Risk-Factor Analysis for Fracture-Related Infection and Nonunion in a Single Center Analysis of 187 Fractures. Injury 2025, 56, 112303. [Google Scholar] [CrossRef]
- Westgeest, J.; Weber, D.; Dulai, S.K.; Bergman, J.W.; Buckley, R.; Beaupre, L.A. Factors Associated with Development of Nonunion or Delayed Healing After an Open Long Bone Fracture: A Prospective Cohort Study of 736 Subjects. J. Orthop. Trauma. 2016, 30, 149. [Google Scholar] [CrossRef]
- Castillo, I.A.; Heiner, J.A.; Meremikwu, R.I.; Kellam, J.; Warner, S.J. Where Are We in 2022? A Summary of 11,000 Open Tibia Fractures Over 4 Decades. J. Orthop. Trauma 2023, 37, e326–e334. [Google Scholar] [CrossRef]
- Thomas, J.D.; Kehoe, J.L. Bone Nonunion. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Ghanem, W.; Ezzeddine, H.; Saad, R.; Kiwan, E.; Dahdouh, R.; Fakih, O.; Sakhat, G.; Alam, E.; Najjar, J.; Assaf, F.; et al. State of the Nonunion: A Review of the Latest Literature. Orthop. Rev. 2025, 17, 129085. [Google Scholar] [CrossRef]
- Leow, J.M.; Clement, N.D.; Tawonsawatruk, T.; Simpson, C.J.; Simpson, A.H.R.W. The Radiographic Union Scale in Tibial (RUST) Fractures. Bone Jt. Res. 2016, 5, 116–121. [Google Scholar] [CrossRef]
- Mouliou, D.S. C-Reactive Protein: Pathophysiology, Diagnosis, False Test Results and a Novel Diagnostic Algorithm for Clinicians. Diseases 2023, 11, 132. [Google Scholar] [CrossRef]
- Solomin, L.N.; Semenistyy, A.A.; Komarov, A.V.; Khominets, V.V.; Sheridan, G.A.; Rozbruch, S.R. Universal Long Bone Nonunion Classification. Strateg. Trauma. Limb Reconstr. 2023, 18, 169–173. [Google Scholar] [CrossRef]
- Gómez-Barrena, E.; Rosset, P.; Lozano, D.; Stanovici, J.; Ermthaller, C.; Gerbhard, F. Bone Fracture Healing: Cell Therapy in Delayed Unions and Nonunions. Bone 2015, 70, 93–101. [Google Scholar] [CrossRef]
- Nicholson, J.; Makaram, N.; Simpson, A.; Keating, J. Fracture Nonunion in Long Bones: A Literature Review of Risk Factors and Surgical Management. Injury 2021, 52, S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Vincken, L.; Van Der Broeck, L.; Geurts, J.; Qiu Shao, S.S.; Poeze, M.; Blokhuis, T.J. The Effect of Post-Traumatic Long Bone Non-Unions on Health-Related Quality of Life. Injury 2023, 54, 110929. [Google Scholar] [CrossRef] [PubMed]
- Schottel, P.C.; O’Connor, D.P.; Brinker, M.R. Time Trade-Off as a Measure of Health-Related Quality of Life: Long Bone Nonunions Have a Devastating Impact. J. Bone Jt. Surg. Am. 2015, 97, 1406–1410. [Google Scholar] [CrossRef]
- Wichlas, F.; Tsitsilonis, S.; Disch, A.C.; Haas, N.P.; Hartmann, C.; Graef, F.; Schwabe, P. Long-Term Functional Outcome and Quality of Life after Successful Surgical Treatment of Tibial Nonunions. Int. Orthop. 2015, 39, 521–525. [Google Scholar] [CrossRef]
- Maisenbacher, T.C.; Rollmann, M.F.; Menger, M.M.; Braun, N.R.; Braun, B.J.; Herath, S.C.; Stuby, F.; Nuessler, A.K.; Histing, T.; Reumann, M.K. Direct and Indirect Costs of Long Bone Fracture Nonunions of the Lower Limb. Bone Jt. Res. 2025, 14, 341–350. [Google Scholar] [CrossRef]
- Hak, D.J.; Fitzpatrick, D.; Bishop, J.A.; Marsh, J.L.; Tilp, S.; Schnettler, R.; Simpson, H.; Alt, V. Delayed Union and Nonunions: Epidemiology, Clinical Issues, and Financial Aspects. Injury 2014, 45, S3–S7. [Google Scholar] [CrossRef]
- Noferesti, A.; Movahedi Aliabadi, M.; Bagheri, F. Anxiety in Trauma Patients with Nonunion Diaphyseal Bone Fractures. Int. Orthop. SICOT 2025, 49, 1821–1827. [Google Scholar] [CrossRef]
- Johnson, L.; Igoe, E.; Kleftouris, G.; Papachristos, I.V.; Papakostidis, C.; Giannoudis, P.V. Physical Health and Psychological Outcomes in Adult Patients with Long-Bone Fracture Non-Unions: Evidence Today. J. Clin. Med. 2019, 8, 1998. [Google Scholar] [CrossRef]
- Brinker, M.R.; Hanus, B.D.; Sen, M.; O’Connor, D.P. The Devastating Effects of Tibial Nonunion on Health-Related Quality of Life. J. Bone Jt. Surg. Am. 2013, 95, 2170–2176. [Google Scholar] [CrossRef]
- Alt, V.; Donell, S.T.; Chhabra, A.; Bentley, A.; Eicher, A.; Schnettler, R. A Health Economic Analysis of the Use of rhBMP-2 in Gustilo–Anderson Grade III Open Tibial Fractures for the UK, Germany, and France. Injury 2009, 40, 1269–1275. [Google Scholar] [CrossRef]
- Flores, M.J.; Brown, K.E.; O’Marr, J.M.; Adejuyigbe, B.; Rodarte, P.; Gomez-Alvarado, F.; Nwachuku, K.; Urva, M.; Shearer, D. The Economic Impact of Infection and/or Nonunion on Long-Bone Shaft Fractures: A Systematic Review. OTA Int. 2024, 7, e337. [Google Scholar] [CrossRef]
- Antonova, E.; Le, T.K.; Burge, R.; Mershon, J. Tibia Shaft Fractures: Costly Burden of Nonunions. BMC Musculoskelet. Disord. 2013, 14, 42. [Google Scholar] [CrossRef]
- Iliaens, J.; Onsea, J.; Hoekstra, H.; Nijs, S.; Peetermans, W.E.; Metsemakers, W.-J. Fracture-Related Infection in Long Bone Fractures: A Comprehensive Analysis of the Economic Impact and Influence on Quality of Life. Injury 2021, 52, 3344–3349. [Google Scholar] [CrossRef]
- Sprague, S.; Bhandari, M. An Economic Evaluation of Early versus Delayed Operative Treatment in Patients with Closed Tibial Shaft Fractures. Arch. Orthop. Trauma. Surg. 2002, 122, 315–323. [Google Scholar] [CrossRef]
- Busse, J.W.; Bhandari, M.; Sprague, S.; Johnson-Masotti, A.P.; Gafni, A. An Economic Analysis of Management Strategies for Closed and Open Grade I Tibial Shaft Fractures. Acta Orthop. 2005, 76, 705–712. [Google Scholar] [CrossRef]
- Chang, C.-J.; Jou, I.-M.; Wu, T.-T.; Su, F.-C.; Tai, T.-W. Cigarette Smoke Inhalation Impairs Angiogenesis in Early Bone Healing Processes and Delays Fracture Union. Bone Jt. Res. 2020, 9, 99–107. [Google Scholar] [CrossRef]
- Roper, P.M.; Abbasnia, P.; Vuchkovska, A.; Natoli, R.M.; Callaci, J.J. Alcohol-Related Deficient Fracture Healing Is Associated With Activation of FoxO Transcription Factors in Mice. J. Orthop. Res. 2016, 34, 2106–2115. [Google Scholar] [CrossRef]
- Claes, L.; Recknagel, S.; Ignatius, A. Fracture Healing under Healthy and Inflammatory Conditions. Nat. Rev. Rheumatol. 2012, 8, 133–144. [Google Scholar] [CrossRef]
- Eby, J.M.; Sharieh, F.; Callaci, J.J. Impact of Alcohol on Bone Health, Homeostasis and Fracture Repair. Curr. Pathobiol. Rep. 2020, 8, 75–86. [Google Scholar] [CrossRef]
- Huston, P. Why Osteoarthritis of the Knee Is Called “a Wound That Does Not Heal” and Why Tai Chi Is an Effective Treatment. Front. Med. 2023, 10, 1208326. [Google Scholar] [CrossRef]
- Jiao, H.; Xiao, E.; Graves, D.T. Diabetes and Its Effect on Bone and Fracture Healing. Curr. Osteoporos. Rep. 2015, 13, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, U.; Cariati, I.; Greggi, C.; Gasbarra, E.; Belluati, A.; Ciolli, L.; Maccauro, G.; Momoli, A.; Ripanti, S.; Falez, F.; et al. Skeletal System Biology and Smoke Damage: From Basic Science to Medical Clinic. Int. J. Mol. Sci. 2021, 22, 6629. [Google Scholar] [CrossRef] [PubMed]
- Zeckey, C.; Mommsen, P.; Andruszkow, H.; Macke, C.; Frink, M.; Stübig, T.; Hüfner, T.; Krettek, C.; Hildebrand, F. The Aseptic Femoral and Tibial Shaft Non-Union in Healthy Patients–An Analysis of the Health-Related Quality of Life and the Socioeconomic Outcome. Open Orthop. J. 2011, 5, 193–197. [Google Scholar] [CrossRef]
- Knäuper, V.; López-Otin, C.; Smith, B.; Knight, G.; Murphy, G. Biochemical Characterization of Human Collagenase-3. J. Biol. Chem. 1996, 271, 1544–1550. [Google Scholar] [CrossRef] [PubMed]
- Kosaki, N.; Takaishi, H.; Kamekura, S.; Kimura, T.; Okada, Y.; Minqi, L.; Amizuka, N.; Chung, U.-I.; Nakamura, K.; Kawaguchi, H.; et al. Impaired Bone Fracture Healing in Matrix Metalloproteinase-13 Deficient Mice. Biochem. Biophys. Res. Commun. 2007, 354, 846–851. [Google Scholar] [CrossRef]
- Xiong, D.-H.; Liu, X.-G.; Guo, Y.-F.; Tan, L.-J.; Wang, L.; Sha, B.-Y.; Tang, Z.-H.; Pan, F.; Yang, T.-L.; Chen, X.-D.; et al. Genome-Wide Association and Follow-Up Replication Studies Identified ADAMTS18 and TGFBR3 as Bone Mass Candidate Genes in Different Ethnic Groups. Am. J. Hum. Genet. 2009, 84, 388–398. [Google Scholar] [CrossRef]
- Bolamperti, S.; Villa, I.; Rubinacci, A. Bone Remodeling: An Operational Process Ensuring Survival and Bone Mechanical Competence. Bone Res. 2022, 10, 48. [Google Scholar] [CrossRef]
- Sheen, J.R.; Mabrouk, A.; Garla, V.V. Fracture Healing Overview. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Epari, D.R.; Taylor, W.R.; Heller, M.O.; Duda, G.N. Mechanical Conditions in the Initial Phase of Bone Healing. Clin. Biomech. 2006, 21, 646–655. [Google Scholar] [CrossRef]
- Wazen, R.M.; Currey, J.A.; Guo, H.; Brunski, J.B.; Helms, J.A.; Nanci, A. Micromotion-Induced Strain Fields Influence Early Stages of Repair at Bone-Implant Interfaces. Acta Biomater. 2013, 9, 6663–6674. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Q.; Zhang, J.; Qi, S.; Duan, Y.; Li, C. The Mechanotransduction Signaling Pathways in the Regulation of Osteogenesis. Int. J. Mol. Sci. 2023, 24, 14326. [Google Scholar] [CrossRef]
- Yavropoulou, M.P.; Yovos, J.G. The Molecular Basis of Bone Mechanotransduction. J. Musculoskelet. Neuronal Interact. 2016, 16, 221–236. [Google Scholar]
- Li, R.; Liang, L.; Dou, Y.; Huang, Z.; Mo, H.; Wang, Y.; Yu, B. Mechanical Strain Regulates Osteogenic and Adipogenic Differentiation of Bone Marrow Mesenchymal Stem Cells. Biomed. Res. Int. 2015, 2015, 873251. [Google Scholar] [CrossRef]
- Lafuente-Gracia, L.; Borgiani, E.; Nasello, G.; Geris, L. Towards in Silico Models of the Inflammatory Response in Bone Fracture Healing. Front. Bioeng. Biotechnol. 2021, 9, 703725. [Google Scholar] [CrossRef]
- Maruyama, M.; Rhee, C.; Utsunomiya, T.; Zhang, N.; Ueno, M.; Yao, Z.; Goodman, S.B. Modulation of the Inflammatory Response and Bone Healing. Front. Endocrinol. 2020, 11, 386. [Google Scholar] [CrossRef]
- Hu, K.; Shang, Z.; Yang, X.; Zhang, Y.; Cao, L. Macrophage Polarization and the Regulation of Bone Immunity in Bone Homeostasis. J. Inflamm. Res. 2023, 16, 3563–3580. [Google Scholar] [CrossRef]
- Chen, K.; Jiao, Y.; Liu, L.; Huang, M.; He, C.; He, W.; Hou, J.; Yang, M.; Luo, X.; Li, C. Communications Between Bone Marrow Macrophages and Bone Cells in Bone Remodeling. Front. Cell Dev. Biol. 2020, 8, 598263. [Google Scholar] [CrossRef] [PubMed]
- ElHawary, H.; Baradaran, A.; Abi-Rafeh, J.; Vorstenbosch, J.; Xu, L.; Efanov, J.I. Bone Healing and Inflammation: Principles of Fracture and Repair. Semin. Plast. Surg. 2021, 35, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Loi, F.; Córdova, L.A.; Pajarinen, J.; Lin, T.; Yao, Z.; Goodman, S.B. Inflammation, Fracture and Bone Repair. Bone 2016, 86, 119–130. [Google Scholar] [CrossRef]
- Maruyama, M.; Moeinzadeh, S.; Guzman, R.A.; Zhang, N.; Storaci, H.W.; Utsunomiya, T.; Lui, E.; Huang, E.E.; Rhee, C.; Gao, Q.; et al. The Efficacy of Lapine Preconditioned or Genetically Modified IL4 Over-Expressing Bone Marrow-Derived Mesenchymal Stromal Cells in Corticosteroid-Associated Osteonecrosis of the Femoral Head in Rabbits. Biomaterials 2021, 275, 120972. [Google Scholar] [CrossRef] [PubMed]
- Burgan, J.; Rahmati, M.; Lee, M.; Saiz, A.M. Innate Immune Response to Bone Fracture Healing. Bone 2025, 190, 117327. [Google Scholar] [CrossRef]
- Houschyar, K.S.; Tapking, C.; Borrelli, M.R.; Popp, D.; Duscher, D.; Maan, Z.N.; Chelliah, M.P.; Li, J.; Harati, K.; Wallner, C.; et al. Wnt Pathway in Bone Repair and Regeneration–What Do We Know So Far. Front. Cell Dev. Biol. 2018, 6, 170. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wu, S.; Chen, W.; Li, Y.-P. The Roles and Regulatory Mechanisms of TGF-β and BMP Signaling in Bone and Cartilage Development, Homeostasis and Disease. Cell Res. 2024, 34, 101–123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, J.; Zhou, Y.; Wu, G. The Roles of Bone Morphogenetic Proteins and Their Signaling in the Osteogenesis of Adipose-Derived Stem Cells. Tissue Eng. Part B Rev. 2014, 20, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Duffhues, G.; Williams, E.; Goumans, M.-J.; Heldin, C.-H.; ten Dijke, P. Bone Morphogenetic Protein Receptors: Structure, Function and Targeting by Selective Small Molecule Kinase Inhibitors. Bone 2020, 138, 115472. [Google Scholar] [CrossRef]
- Huang, W.; Yang, S.; Shao, J.; Li, Y.-P. Signaling and Transcriptional Regulation in Osteoblast Commitment and Differentiation. Front. Biosci. 2007, 12, 3068–3092. [Google Scholar] [CrossRef]
- Baniwal, S.K.; Shah, P.K.; Shi, Y.; Haduong, J.H.; DeClerck, Y.A.; Gabet, Y.; Frenkel, B. Runx2 Promotes Both Osteoblastogenesis and Novel Osteoclastogenic Signals in ST2 Mesenchymal Progenitor Cells. Osteoporos. Int. 2012, 23, 1399–1413. [Google Scholar] [CrossRef]
- Kushchayeva, Y.; Pestun, I.; Kushchayev, S.; Radzikhovska, N.; Lewiecki, E.M. Advancement in the Treatment of Osteoporosis and the Effects on Bone Healing. J. Clin. Med. 2022, 11, 7477. [Google Scholar] [CrossRef]
- Avin, K.G.; Dominguez, J.M.; Chen, N.X.; Hato, T.; Myslinski, J.J.; Gao, H.; Liu, Y.; McKinley, T.O.; Brown, K.M.; Moe, S.M.; et al. Single-Cell RNAseq Provides Insight into Altered Immune Cell Populations in Human Fracture Nonunions. J. Orthop. Res. 2023, 41, 1060–1069. [Google Scholar] [CrossRef]
- De Seny, D.; Cobraiville, G.; Leprince, P.; Fillet, M.; Collin, C.; Mathieu, M.; Hauzeur, J.-P.; Gangji, V.; Malaise, M.G. Biomarkers of Inflammation and Innate Immunity in Atrophic Nonunion Fracture. J. Transl. Med. 2016, 14, 258. [Google Scholar] [CrossRef]
- Vi, L.; Baht, G.S.; Whetstone, H.; Ng, A.; Wei, Q.; Poon, R.; Mylvaganam, S.; Grynpas, M.; Alman, B.A. Macrophages Promote Osteoblastic Differentiation In-Vivo: Implications in Fracture Repair and Bone Homeostasis. J. Bone Min. Res. 2015, 30, 1090–1102. [Google Scholar] [CrossRef]
- Schlundt, C.; El Khassawna, T.; Serra, A.; Dienelt, A.; Wendler, S.; Schell, H.; van Rooijen, N.; Radbruch, A.; Lucius, R.; Hartmann, S.; et al. Macrophages in Bone Fracture Healing: Their Essential Role in Endochondral Ossification. Bone 2018, 106, 78–89. [Google Scholar] [CrossRef]
- Spiller, K.L.; Nassiri, S.; Witherel, C.E.; Anfang, R.R.; Ng, J.; Nakazawa, K.R.; Yu, T.; Vunjak-Novakovic, G. Sequential Delivery of Immunomodulatory Cytokines to Facilitate the M1-to-M2 Transition of Macrophages and Enhance Vascularization of Bone Scaffolds. Biomaterials 2015, 37, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Flierl, M.A.; Perl, M.; Rittirsch, D.; Bartl, C.; Schreiber, H.; Fleig, V.; Schlaf, G.; Liener, U.; Brueckner, U.B.; Gebhard, F.; et al. The Role of C5a in the Innate Immune Response after Experimental Blunt Chest Trauma. Shock 2008, 29, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Recknagel, S.; Bindl, R.; Kurz, J.; Wehner, T.; Ehrnthaller, C.; Knöferl, M.W.; Gebhard, F.; Huber-Lang, M.; Claes, L.; Ignatius, A. Experimental Blunt Chest Trauma Impairs Fracture Healing in Rats. J. Orthop. Res. 2011, 29, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Recknagel, S.; Bindl, R.; Brochhausen, C.; Göckelmann, M.; Wehner, T.; Schoengraf, P.; Huber-Lang, M.; Claes, L.; Ignatius, A. Systemic Inflammation Induced by a Thoracic Trauma Alters the Cellular Composition of the Early Fracture Callus. J. Trauma. Acute Care Surg. 2013, 74, 531–537. [Google Scholar] [CrossRef]
- Schell, H.; Thompson, M.S.; Bail, H.J.; Hoffmann, J.-E.; Schill, A.; Duda, G.N.; Lienau, J. Mechanical Induction of Critically Delayed Bone Healing in Sheep: Radiological and Biomechanical Results. J. Biomech. 2008, 41, 3066–3072. [Google Scholar] [CrossRef]
- Schmidt-Bleek, K.; Schell, H.; Schulz, N.; Hoff, P.; Perka, C.; Buttgereit, F.; Volk, H.-D.; Lienau, J.; Duda, G.N. Inflammatory Phase of Bone Healing Initiates the Regenerative Healing Cascade. Cell Tissue Res. 2012, 347, 567–573. [Google Scholar] [CrossRef]
- Reed, A.A.C.; Joyner, C.J.; Brownlow, H.C.; Simpson, A.H.R.W. Human Atrophic Fracture Non-unions Are Not Avascular. J. Orthop. Res. 2002, 20, 593–599. [Google Scholar] [CrossRef]
- Fajardo, M.; Liu, C.-J.; Egol, K. Levels of Expression for BMP-7 and Several BMP Antagonists May Play an Integral Role in a Fracture Nonunion: A Pilot Study. Clin. Orthop. Relat. Res. 2009, 467, 3071–3078. [Google Scholar] [CrossRef]
- Kloen, P.; Loots, G.G.; Hamdy, R.C.; Smit, T.H. Bridging the Gap: Compressing Non-Unions for Proper Cellular Signaling. Med. Hypotheses 2022, 160, 110794. [Google Scholar] [CrossRef]
- Kwong, F.N.K.; Hoyland, J.A.; Freemont, A.J.; Evans, C.H. Altered Relative Expression of BMPs and BMP Inhibitors in Cartilaginous Areas of Human Fractures Progressing towards Nonunion. J. Orthop. Res. 2009, 27, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, M.; Liu, C.-J.; Ilalov, K.; Egol, K.A. Matrix Metalloproteinases That Associate with and Cleave Bone Morphogenetic Protein-2 In Vitro Are Elevated in Hypertrophic Fracture Nonunion Tissue. J. Orthop. Trauma 2010, 24, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.H. Advances in Regenerative Orthopedics. Mayo Clin. Proc. 2013, 88, 1323–1339. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.S.; Rayan, F.; Dhinsa, B.S.; Marsh, D. An Osteoconductive, Osteoinductive, and Osteogenic Tissue-Engineered Product for Trauma and Orthopaedic Surgery: How Far Are We? Stem Cells Int. 2012, 2012, 236231. [Google Scholar] [CrossRef]
- Mayfield, C.K.; Ayad, M.; Lechtholz-Zey, E.; Chen, Y.; Lieberman, J.R. 3D-Printing for Critical Sized Bone Defects: Current Concepts and Future Directions. Bioengineering 2022, 9, 680. [Google Scholar] [CrossRef]
- Patterson, J.; Lieberman, J. Segmental defect repair and nonunion. In Orthobiologics: Scientific and Clinical Solutions for Orthopaedic Surgeons; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2024. [Google Scholar]
- Mazzotta, A.; Stagni, C.; Rocchi, M.; Rani, N.; Del Piccolo, N.; Filardo, G.; Dallari, D. Bone Marrow Aspirate Concentrate/Platelet-Rich Fibrin Augmentation Accelerates Healing of Aseptic Upper Limb Nonunions. J. Orthop. Traumatol. 2021, 22, 21. [Google Scholar] [CrossRef]
- Laubach, M.; Suresh, S.; Herath, B.; Wille, M.-L.; Delbrück, H.; Alabdulrahman, H.; Hutmacher, D.W.; Hildebrand, F. Clinical Translation of a Patient-Specific Scaffold-Guided Bone Regeneration Concept in Four Cases with Large Long Bone Defects. J. Orthop. Transl. 2022, 34, 73–84. [Google Scholar] [CrossRef]
- Xie, C.; Wang, C.; Huang, Y.; Li, Q.; Tian, X.; Huang, W.; Yin, D. Therapeutic Effect of Autologous Bone Grafting with Adjuvant Bone Morphogenetic Protein on Long Bone Nonunion: A Systematic Review and Meta-Analysis. J. Orthop. Surg. Res. 2022, 17, 298. [Google Scholar] [CrossRef]
- Choi, W.; Kim, B.-S.; Cho, W.-T.; Lim, E.J.; Choi, J.S.; Ryu, Y.K.; Cho, J.-W.; Sakong, S.; Oh, J.-K. Efficacy and Safety of Recombinant Human Bone Morphogenetic Protein-2 (rhBMP-2) Combined with Autologous Bone for the Treatment of Long Bone Nonunion: A Report of a Prospective Case Series. Injury 2024, 55, 111711. [Google Scholar] [CrossRef]
- Moyal, A.J.; Li, A.W.; Adelstein, J.M.; Moon, T.J.; Napora, J.K. Bone Marrow Aspirate and Bone Marrow Aspirate Concentrate: Does the Literature Support Use in Long-Bone Nonunion and Provide New Insights into Mechanism of Action? Eur. J. Orthop. Surg. Traumatol. 2024, 34, 2871–2880. [Google Scholar] [CrossRef]
- Zhu, J.; Han, D.; Sun, Y.; Zhao, C. Platelet-Rich Plasma in the Treatment of Delayed Union and Nonunion Fractures: An Umbrella Meta-Analysis. J. Orthop. Sci. 2024, 30, 894–904. [Google Scholar] [CrossRef]
- Tanavalee, C.; Ngarmukos, S.; Amarase, C.; Tantavisut, S.; Jaruthien, N.; Tanavalee, A. A Randomized Controlled Trial of Teriparatide for Accelerating Bone Union and Improving Clinical Outcomes in Patients with Pertrochanteric Fracture Fixation. Sci. Rep. 2025, 15, 19465. [Google Scholar] [CrossRef] [PubMed]
- Pelled, G.; Sheyn, D.; Tawackoli, W.; Jun, D.S.; Koh, Y.; Su, S.; Cohn Yakubovich, D.; Kallai, I.; Antebi, B.; Da, X.; et al. BMP6-Engineered MSCs Induce Vertebral Bone Repair in a Pig Model: A Pilot Study. Stem Cells Int. 2016, 2016, 6530624. [Google Scholar] [CrossRef] [PubMed]
- Brunello, G.; Panda, S.; Schiavon, L.; Sivolella, S.; Biasetto, L.; Del Fabbro, M. The Impact of Bioceramic Scaffolds on Bone Regeneration in Preclinical In Vivo Studies: A Systematic Review. Materials 2020, 13, 1500. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Fang, W.; Wu, G.; Li, Y.; Pathak, J.L.; Liu, Y. Low Dose BMP2-Doped Calcium Phosphate Graft Promotes Bone Defect Healing in a Large Animal Model. Front. Cell Dev. Biol. 2020, 8, 613891. [Google Scholar] [CrossRef]
- DeBaun, M.R.; Salazar, B.P.; Bai, Y.; Gardner, M.J.; Yang, Y.P.; Pan, C.C.; Stahl, A.M.; Moeinzadeh, S.; Kim, S.; Lui, E.; et al. A Bioactive Synthetic Membrane Improves Bone Healing in a Preclinical Nonunion Model. Injury 2022, 53, 1368–1374. [Google Scholar] [CrossRef]
- De La Vega, R.E.; van Griensven, M.; Zhang, W.; Coenen, M.J.; Nagelli, C.V.; Panos, J.A.; Peniche Silva, C.J.; Geiger, J.; Plank, C.; Evans, C.H.; et al. Efficient Healing of Large Osseous Segmental Defects Using Optimized Chemically Modified Messenger RNA Encoding BMP-2. Sci. Adv. 2022, 8, eabl6242. [Google Scholar] [CrossRef]
- da Rocha, L.R.; Dias, R.B.; Fernandes, M.B.C.; Prinz, R.; Eirado, T.P.; de Souza Costa, I.; Monteiro, M.J.; da Silva, C.E.R.; Dos Santos, C.T.; Fogagnolo, F. A New Option for Bone Regeneration: A Rapid Methodology for Cellularization of Allograft with Human Bone Marrow Stromal Cells with in Vivo Bone-Forming Potential. Injury 2023, 54 (Suppl. 6), 110777. [Google Scholar] [CrossRef]
- Garot, C.; Schoffit, S.; Monfoulet, C.; Machillot, P.; Deroy, C.; Roques, S.; Vial, J.; Vollaire, J.; Renard, M.; Ghanem, H.; et al. 3D-Printed Osteoinductive Polymeric Scaffolds with Optimized Architecture to Repair a Sheep Metatarsal Critical-Size Bone Defect. Adv. Healthc. Mater. 2023, 12, e2301692. [Google Scholar] [CrossRef]
- Sun, L.; Niu, H.; Wu, Y.; Dong, S.; Li, X.; Kim, B.Y.S.; Liu, C.; Ma, Y.; Jiang, W.; Yuan, Y. Bio-Integrated Scaffold Facilitates Large Bone Regeneration Dominated by Endochondral Ossification. Bioact. Mater. 2024, 35, 208–227. [Google Scholar] [CrossRef]
- Li, X.; Asuncion, F.; Ominsky, M.; Niu, Q.-T.; Akesson, K.E.; Wang, J.; Lieberman, J.; Ke, H.Z. High-Dose Romosozumab Promoted Bone Regeneration of Critical-Size Ulnar Defect Filled with Demineralized Bone Matrix in Nonhuman Primates. J. Orthop. Transl. 2025, 54, 1–7. [Google Scholar] [CrossRef]
- Aleem, I.S.; Aleem, I.; Evaniew, N.; Busse, J.W.; Yaszemski, M.; Agarwal, A.; Einhorn, T.; Bhandari, M. Efficacy of Electrical Stimulators for Bone Healing: A Meta-Analysis of Randomized Sham-Controlled Trials. Sci. Rep. 2016, 6, 31724. [Google Scholar] [CrossRef] [PubMed]
- Griffin, X.L.; Costa, M.L.; Parsons, N.; Smith, N. Electromagnetic Field Stimulation for Treating Delayed Union or Non-Union of Long Bone Fractures in Adults. Cochrane Database Syst. Rev. 2011, 4, CD008471. [Google Scholar] [CrossRef] [PubMed]
- Leighton, R.; Watson, J.T.; Giannoudis, P.; Papakostidis, C.; Harrison, A.; Steen, R.G. Healing of Fracture Nonunions Treated with Low-Intensity Pulsed Ultrasound (LIPUS): A Systematic Review and Meta-Analysis. Injury 2017, 48, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Leighton, R.; Phillips, M.; Bhandari, M.; Zura, R. Low Intensity Pulsed Ultrasound (LIPUS) Use for the Management of Instrumented, Infected, and Fragility Non-Unions: A Systematic Review and Meta-Analysis of Healing Proportions. BMC Musculoskelet Disord 2021, 22, 532. [Google Scholar] [CrossRef]
- TRUST Investigators Writing Group; Busse, J.W.; Bhandari, M.; Einhorn, T.A.; Schemitsch, E.; Heckman, J.D.; Tornetta, P.; Leung, K.-S.; Heels-Ansdell, D.; Makosso-Kallyth, S.; et al. Re-Evaluation of Low Intensity Pulsed Ultrasound in Treatment of Tibial Fractures (TRUST): Randomized Clinical Trial. BMJ 2016, 355, i5351. [Google Scholar] [CrossRef][Green Version]
- White, N.J.; Patterson, E.D.; Dhaliwal, G.S.; Hildebrand, K.A. WECAN Low-Intensity Pulsed Ultrasound Versus Sham in the Treatment of Operatively Managed Scaphoid Nonunions: The SNAPU Randomized Controlled Trial. J. Bone Jt. Surg. 2024, 106, 1573–1582. [Google Scholar] [CrossRef]
- Mittal, K.K.; Gupta, H.; Kaushik, N. Reunion of Post Nail Aseptic Non-Union of Diaphyseal Femoral Fractures by Augmentation Plating, Decortication and Bone Grafting–Replacement for Exchange Nailing. Injury 2021, 52, 1529–1533. [Google Scholar] [CrossRef]
- Bégué, T.; Mouchantaf, M.; Aurégan, J.-C. Aseptic Humeral Shaft Nonunion. Orthop. Traumatol. Surg. Res. 2023, 109, 103462. [Google Scholar] [CrossRef]
- Ayalon, O.; Rettig, S.A.; Tedesco, L.J. Bone Graft and Fixation Options in the Surgical Management of Scaphoid Nonunion. J. Am. Acad. Orthop. Surg. 2024, 33, e989–e999. [Google Scholar] [CrossRef]
- Alford, A.I.; Nicolaou, D.; Hake, M.; McBride-Gagyi, S. Masquelet’s Induced Membrane Technique: Review of Current Concepts and Future Directions. J. Orthop. Res. 2021, 39, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Hoit, G.; Kain, M.S.; Sparkman, J.W.; Norris, B.L.; Conway, J.D.; Watson, J.T.; Tornetta, P.; Nauth, A. The Induced Membrane Technique for Bone Defects: Basic Science, Clinical Evidence, and Technical Tips. OTA Int. 2021, 4, e106(1–5). [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-H.; Bao, Q.-W.; Wang, S.-K.; Zhou, P.-Y.; Xu, S.-G. Mechanisms of the Masquelet Technique to Promote Bone Defect Repair and Its Influencing Factors. Chin. J. Traumatol. 2025, 28, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, J.; Masquelet, A.-C.; Boutroux, P.; Cambon-Binder, A. Induced-Membrane Treatment of Refractory Humeral Non-Union with or without Bone Defect. Orthop. Traumatol. Surg. Res. 2020, 106, 803–811. [Google Scholar] [CrossRef]
- Chloros, G.D.; Kanakaris, N.K.; Harwood, P.J.; Giannoudis, P.V. Induced Membrane Technique for Acute Bone Loss and Nonunion Management of the Tibia. OTA Int. 2022, 5, e170. [Google Scholar] [CrossRef]
- Griffin, J.T.; Landy, D.C.; Sneed, C.R.; Liu, B.; Kavolus, M.; Pectol, R.W.; Gitajn, I.L.; Oh, J.-K.; Aneja, A. Masquelet Technique for the Tibia: A Systematic Review and Meta-Analysis of Contemporary Outcomes. J. Orthop. Trauma 2023, 37, e36–e44. [Google Scholar] [CrossRef]
- Ma, X.-Y.; Liu, B.; Yu, H.-L.; Zhang, X.; Xiang, L.-B.; Zhou, D.-P. Induced Membrane Technique for the Treatment of Infected Forearm Nonunion: A Retrospective Study. J. Hand Surg. 2022, 47, 583.e1–583.e9. [Google Scholar] [CrossRef]
- Moreillon, P.; Que, Y.A.; Bayer, A.S. Pathogenesis of Streptococcal and Staphylococcal Endocarditis. Infect. Dis. Clin. North. Am. 2002, 16, 297–318. [Google Scholar] [CrossRef]
- Singh, S.; Toci, G.R.; Kapadia, K.; Colon, A.; Greenberg, P.; Iyer, H.; Katt, B.; Shah, A. Vascularized Bone Grafting Versus the 2-Stage Masquelet Technique for Upper-Extremity Bone Reconstruction: A Meta-Analysis. J. Hand Surg. Am. 2023, 48, 984–992. [Google Scholar] [CrossRef]
- Rouse, B.J.; Sheridan, G.A.; Page, B.J.; Fragomen, A.T.; Rozbruch, S.R. Hypertrophic Nonunion Management with Distraction Osteogenesis: A Scoping Review of the Literature. OTA Int. 2024, 7, e342. [Google Scholar] [CrossRef]
- Fragomen, A.T.; Wellman, D.; Rozbruch, S.R. The PRECICE Magnetic IM Compression Nail for Long Bone Nonunions: A Preliminary Report. Arch. Orthop. Trauma. Surg. 2019, 139, 1551–1560. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J. Orthop. Trauma. 2019, 33, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Barrena, E.; Ehrnthaller, C. Long Bone Uninfected Non-Union: Grafting Techniques. EFORT Open Rev. 2024, 9, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, F.; La Padula, G.; Torsiello, E.; Spiezia, F.; Oliva, F.; Maffulli, N. Strategies for Large Bone Defect Reconstruction after Trauma, Infections or Tumour Excision: A Comprehensive Review of the Literature. Eur. J. Med. Res. 2021, 26, 118. [Google Scholar] [CrossRef]
- Dimitriou, R.; Mataliotakis, G.I.; Angoules, A.G.; Kanakaris, N.K.; Giannoudis, P.V. Complications Following Autologous Bone Graft Harvesting from the Iliac Crest and Using the RIA: A Systematic Review. Injury 2011, 42, S3–S15. [Google Scholar] [CrossRef]
- Bray, C.C.; Walker, C.M.; Spence, D.D. Orthobiologics in Pediatric Sports Medicine. Orthop. Clin. North. Am. 2017, 48, 333–342. [Google Scholar] [CrossRef]
- Chahla, J.; Mannava, S.; Cinque, M.E.; Geeslin, A.G.; Codina, D.; LaPrade, R.F. Bone Marrow Aspirate Concentrate Harvesting and Processing Technique. Arthrosc. Tech. 2017, 6, e441–e445. [Google Scholar] [CrossRef]
- Holton, J.; Imam, M.; Ward, J.; Snow, M. The Basic Science of Bone Marrow Aspirate Concentrate in Chondral Injuries. Orthop. Rev. 2016, 8, 6659. [Google Scholar] [CrossRef]
- Kim, G.B.; Seo, M.-S.; Park, W.T.; Lee, G.W. Bone Marrow Aspirate Concentrate: Its Uses in Osteoarthritis. Int. J. Mol. Sci. 2020, 21, 3224. [Google Scholar] [CrossRef]
- Lin, K.; VandenBerg, J.; Putnam, S.M.; Parks, C.D.; Spraggs-Hughes, A.; McAndrew, C.M.; Ricci, W.M.; Gardner, M.J. Bone Marrow Aspirate Concentrate with Cancellous Allograft versus Iliac Crest Bone Graft in the Treatment of Long Bone Nonunions. OTA Int. 2019, 2, e012. [Google Scholar] [CrossRef]
- Van Vugt, T.A.G.; Geurts, J.A.P.; Blokhuis, T.J. Treatment of Infected Tibial Non-Unions Using a BMAC and S53P4 BAG Combination for Reconstruction of Segmental Bone Defects: A Clinical Case Series. Injury 2021, 52, S67–S71. [Google Scholar] [CrossRef] [PubMed]
- Brozovich, A.; Sinicrope, B.J.; Bauza, G.; Niclot, F.B.; Lintner, D.; Taraballi, F.; McCulloch, P.C. High Variability of Mesenchymal Stem Cells Obtained via Bone Marrow Aspirate Concentrate Compared with Traditional Bone Marrow Aspiration Technique. Orthop. J. Sports Med. 2021, 9, 23259671211058459. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, C.; Boffa, A.; de Girolamo, L.; Merli, G.; Kon, E.; Cattini, L.; Santo, E.; Grigolo, B.; Filardo, G. Bone Marrow Aspirate Concentrate Quality Is Affected by Age and Harvest Site. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 2140–2151. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, I.A.; Growney Kalaf, E.A.; Bowlin, G.L.; Sell, S.A. Platelet-Rich Plasma in Bone Regeneration: Engineering the Delivery for Improved Clinical Efficacy. Biomed. Res. Int. 2014, 2014, 392398. [Google Scholar] [CrossRef]
- Wang, S.; Chen, J.; Wu, J.; Peng, Z.; Wang, K.; Zhang, X.; Wu, F.; Jie, K. Based on the Diamond Concept, Application of Platelet-Rich Plasma in the Treatment of Aseptic Femoral Shaft Nonunion: A Retrospective Controlled Study on 66 Patients. Injury 2025, 56, 112325. [Google Scholar] [CrossRef]
- Oh, J.H.; Kim, W.; Park, K.U.; Roh, Y.H. Comparison of the Cellular Composition and Cytokine-Release Kinetics of Various Platelet-Rich Plasma Preparations. Am. J. Sports Med. 2015, 43, 3062–3070. [Google Scholar] [CrossRef]
- Sugaya, H.; Yoshioka, T.; Kato, T.; Taniguchi, Y.; Kumagai, H.; Hyodo, K.; Ohneda, O.; Yamazaki, M.; Mishima, H. Comparative Analysis of Cellular and Growth Factor Composition in Bone Marrow Aspirate Concentrate and Platelet-Rich Plasma. Bone Marrow Res. 2018, 2018, 1549826. [Google Scholar] [CrossRef]
- Sundman, E.A.; Cole, B.J.; Fortier, L.A. Growth Factor and Catabolic Cytokine Concentrations Are Influenced by the Cellular Composition of Platelet-Rich Plasma. Am. J. Sports Med. 2011, 39, 2135–2140. [Google Scholar] [CrossRef]
- Jing, N.; Hou, Y.-C.; Zhang, J.-C.; Xu, G.; Lei, M.; Tang, X.; Chen, W.; Ni, H.; Zhang, F. Cracking the Code: Understanding ESWT’s Role in Bone Fracture Healing. J. Orthop. Transl. 2025, 50, 403–412. [Google Scholar] [CrossRef]
- Phillips, M.R.; Harrison, A.; Mehta, S.; Nolte, P.A.; Bhandari, M.; Zura, R. A Scoping Review of Operative and Non-Invasive Management in the Treatment of Non-Unions. Injury 2022, 53, 3872–3878. [Google Scholar] [CrossRef]
- Gillman, C.E.; Jayasuriya, A.C. FDA-Approved Bone Grafts and Bone Graft Substitute Devices in Bone Regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 130, 112466. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Barrena, E.; Rosset, P.; Gebhard, F.; Hernigou, P.; Baldini, N.; Rouard, H.; Sensebé, L.; Gonzalo-Daganzo, R.M.; Giordano, R.; Padilla-Eguiluz, N.; et al. Feasibility and Safety of Treating Non-Unions in Tibia, Femur and Humerus with Autologous, Expanded, Bone Marrow-Derived Mesenchymal Stromal Cells Associated with Biphasic Calcium Phosphate Biomaterials in a Multicentric, Non-Comparative Trial. Biomaterials 2019, 196, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Nashi, N.; Kagda, F.H. Current Concepts of Bone Grafting in Trauma Surgery. J. Clin. Orthop. Trauma. 2023, 43, 102231. [Google Scholar] [CrossRef] [PubMed]
- Dave, U.; Rubin, J.; Shah, H.; Gerhold, C.; McCormick, J.R.; Bi, A.S.; Yuh, C.; Rossi, L.A.; Chahla, J. Bone Marrow Aspirate Concentrate (BMAC) Harvested in the Axial and Appendicular Skeleton Does Not Differ in Progenitor Cell Count: A Systematic Review and Meta-Analysis. J. Orthop. 2025, 63, 216–223. [Google Scholar] [CrossRef]
- Impieri, L.; Pezzi, A.; Hadad, H.; Peretti, G.M.; Mangiavini, L.; Rossi, N. Orthobiologics in Delayed Union and Non-Union of Adult Long Bones Fractures: A Systematic Review. Bone Rep. 2024, 21, 101760. [Google Scholar] [CrossRef]
- Lieberman, J.R.; Daluiski, A.; Stevenson, S.; Jolla, L.; Wu, L.; McALLISTER, P.; Lee, Y.P.; Kabo, J.M.; Finerman, G.A.M.; Berk, A.J.; et al. The Effect of Regional Gene Therapy with Bone Morphogenetic Protein-2-Producing Bone-Marrow Cells on the Repair of Segmental Femoral Defects in Rats. JBJS 1999, 81, 905. [Google Scholar] [CrossRef]
- Paulini, M.; Camal Ruggieri, I.N.; Ramallo, M.; Alonso, M.; Rodriguez-Cabello, J.C.; Esbrit, P.; Mardegan Issa, J.P.; Feldman, S. Recombinant Proteins-Based Strategies in Bone Tissue Engineering. Biomolecules 2021, 12, 3. [Google Scholar] [CrossRef]
- Miri, Z.; Haugen, H.J.; Loca, D.; Rossi, F.; Perale, G.; Moghanian, A.; Ma, Q. Review on the Strategies to Improve the Mechanical Strength of Highly Porous Bone Bioceramic Scaffolds. J. Eur. Ceram. Soc. 2024, 44, 23–42. [Google Scholar] [CrossRef]
- Percival, K.M.; Paul, V.; Husseini, G.A. Recent Advancements in Bone Tissue Engineering: Integrating Smart Scaffold Technologies and Bio-Responsive Systems for Enhanced Regeneration. Int. J. Mol. Sci. 2024, 25, 6012. [Google Scholar] [CrossRef]
- Rodriguez-Merchan, C.; Gomez-Castresana, F. Internal Fixation of Nonunions. Clin. Orthop. Relat. Res. 2004, 419, 13–20. [Google Scholar] [CrossRef]
- Schulze, F.; Lang, A.; Schoon, J.; Wassilew, G.I.; Reichert, J. Scaffold Guided Bone Regeneration for the Treatment of Large Segmental Defects in Long Bones. Biomedicines 2023, 11, 325. [Google Scholar] [CrossRef] [PubMed]
- Farjaminejad, S.; Farjaminejad, R.; Hasani, M.; Garcia-Godoy, F.; Abdouss, M.; Marya, A.; Harsoputranto, A.; Jamilian, A. Advances and Challenges in Polymer-Based Scaffolds for Bone Tissue Engineering: A Path Towards Personalized Regenerative Medicine. Polymers 2024, 16, 3303. [Google Scholar] [CrossRef] [PubMed]
- Saurav, S.; Sharma, P.; Kumar, A.; Tabassum, Z.; Girdhar, M.; Mamidi, N.; Mohan, A. Harnessing Natural Polymers for Nano-Scaffolds in Bone Tissue Engineering: A Comprehensive Overview of Bone Disease Treatment. CIMB 2024, 46, 585–611. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zeng, J.; Gan, D.; Ling, K.; He, M.; Li, J.; Lu, Y. Recent Strategies and Advances in Hydrogel-Based Delivery Platforms for Bone Regeneration. Nano-Micro Lett. 2024, 17, 73. [Google Scholar] [CrossRef]
- Cui, C.; Lin, F.; Xia, L.; Zhang, X. Mesenchymal Stem Cells Therapy for the Treatment of Non-Union Fractures: A Systematic Review and Meta-Analysis. BMC Musculoskelet Disord 2025, 26, 245. [Google Scholar] [CrossRef]
- Lalu, M.M.; McIntyre, L.; Pugliese, C.; Fergusson, D.; Winston, B.W.; Marshall, J.C.; Granton, J.; Stewart, D.J. Canadian Critical Care Trials Group Safety of Cell Therapy with Mesenchymal Stromal Cells (SafeCell): A Systematic Review and Meta-Analysis of Clinical Trials. PLoS ONE 2012, 7, e47559. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Hong, I.-S. Double-Edged Sword of Mesenchymal Stem Cells: Cancer-Promoting versus Therapeutic Potential. Cancer Sci. 2017, 108, 1939–1946. [Google Scholar] [CrossRef]
- Thurairajah, K.; Briggs, G.D.; Balogh, Z.J. Stem Cell Therapy for Fracture Non-Union: The Current Evidence from Human Studies. J. Orthop. Surg. 2021, 29, 23094990211036545. [Google Scholar] [CrossRef]
- Arjomandnejad, M.; Dasgupta, I.; Flotte, T.R.; Keeler, A.M. Immunogenicity of Recombinant Adeno-Associated Virus (AAV) Vectors for Gene Transfer. BioDrugs 2023, 37, 311–329. [Google Scholar] [CrossRef]
- Chu, X.; Xiong, Y.; Lu, L.; Wang, Y.; Wang, J.; Zeng, R.; Hu, L.; Yan, C.; Zhao, Z.; Lin, S.; et al. Research Progress of Gene Therapy Combined with Tissue Engineering to Promote Bone Regeneration. APL Bioeng. 2024, 8, 031502. [Google Scholar] [CrossRef]
- Cornetta, K.; Lin, T.-Y.; Pellin, D.; Kohn, D.B. Meeting FDA Guidance Recommendations for Replication-Competent Virus and Insertional Oncogenesis Testing. Mol. Ther. Methods Clin. Dev. 2023, 28, 28–39. [Google Scholar] [CrossRef]
- Gallo, M.C.; Elias, A.; Reynolds, J.; Ball, J.R.; Lieberman, J.R. Regional Gene Therapy for Bone Tissue Engineering: A Current Concepts Review. Bioengineering 2025, 12, 120. [Google Scholar] [CrossRef]
- De Pace, R.; Molinari, S.; Mazzoni, E.; Perale, G. Bone Regeneration: A Review of Current Treatment Strategies. JCM 2025, 14, 1838. [Google Scholar] [CrossRef]
- Kirankumar, S.; Gurusamy, N.; Rajasingh, S.; Sigamani, V.; Vasanthan, J.; Perales, S.G.; Rajasingh, J. Modern Approaches on Stem Cells and Scaffolding Technology for Osteogenic Differentiation and Regeneration. Exp. Biol. Med. 2022, 247, 433–445. [Google Scholar] [CrossRef]
- Wang, Z.; Ren, L.; Li, Z.; Qiu, Q.; Wang, H.; Huang, X.; Ma, D. Impact of Different Cell Types on the Osteogenic Differentiation Process of Mesenchymal Stem Cells. Stem Cells Int. 2025, 2025, 5551222. [Google Scholar] [CrossRef]
- Zhao, T.; Chu, Z.; Ma, J.; Ouyang, L. Immunomodulation Effect of Biomaterials on Bone Formation. J. Funct. Biomater. 2022, 13, 103. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Berry, D.; Moran, A.; He, F.; Tam, T.; Chen, L.; Chen, S. Controlled Growth Factor Release in 3D-Printed Hydrogels. Adv. Heal Mater. 2020, 9, e1900977. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis. Genes. Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Andrae, J.; Gallini, R.; Betsholtz, C. Role of Platelet-Derived Growth Factors in Physiology and Medicine. Genes. Dev. 2008, 22, 1276–1312. [Google Scholar] [CrossRef] [PubMed]
- Cohn-Schwartz, D.; Schary, Y.; Yalon, E.; Krut, Z.; Da, X.; Schwarz, E.M.; Gazit, D.; Pelled, G.; Gazit, Z. PTH-Induced Bone Regeneration and Vascular Modulation Are Both Dependent on Endothelial Signaling. Cells 2022, 11, 897. [Google Scholar] [CrossRef]
- Menger, M.M.; Tobias, A.L.; Bauer, D.; Bleimehl, M.; Scheuer, C.; Menger, M.D.; Histing, T.; Laschke, M.W. Parathyroid Hormone Stimulates Bone Regeneration in an Atrophic Non-Union Model in Aged Mice. J. Transl. Med. 2023, 21, 844. [Google Scholar] [CrossRef]
- Shin, J.-O.; Lee, J.-B.; Lee, S.; Kim, J.-W. Enhancing Bone Regeneration and Osseointegration Using rhPTH(1-34) and Dimeric R25CPTH(1-34) in an Osteoporotic Beagle Model. Elife 2024, 13, RP93830. [Google Scholar] [CrossRef]
- Zhang, S.; Ding, L.; Chen, G.; Zhang, J.; Ge, W.; Qu, Y. Enhanced Bone Regeneration via Local Low-Dose Delivery of PTH1-34 in a Composite Hydrogel. Front. Bioeng. Biotechnol. 2023, 11, 1209752. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Wang, L.; Zhou, Z.; Sun, Q.; Liu, D.; Chen, Y.; Hu, H.; Cai, Y.; Lin, S.; Yu, Z.; et al. Simultaneous Incorporation of PTH(1-34) and Nano-Hydroxyapatite into Chitosan/Alginate Hydrogels for Efficient Bone Regeneration. Bioact. Mater. 2021, 6, 1839–1851. [Google Scholar] [CrossRef] [PubMed]
- Ishack, S.; Mediero, A.; Wilder, T.; Ricci, J.L.; Cronstein, B.N. Bone Regeneration in Critical Bone Defects Using Three-Dimensionally Printed β-Tricalcium Phosphate/Hydroxyapatite Scaffolds Is Enhanced by Coating Scaffolds with Either Dipyridamole or BMP-2. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Mediero, A.; Wilder, T.; Perez-Aso, M.; Cronstein, B.N. Direct or Indirect Stimulation of Adenosine A2A Receptors Enhances Bone Regeneration as Well as Bone Morphogenetic Protein-2. FASEB J. 2015, 29, 1577–1590. [Google Scholar] [CrossRef]
- Witek, L.; Alifarag, A.M.; Tovar, N.; Lopez, C.D.; Cronstein, B.N.; Rodriguez, E.D.; Coelho, P.G. Repair of Critical-Sized Long Bone Defects Using Dipyridamole-Augmented 3D-Printed Bioactive Ceramic Scaffolds. J. Orthop. Res. 2019, 37, 2499–2507. [Google Scholar] [CrossRef]
- Marie, P.J.; Miraoui, H.; Sévère, N. FGF/FGFR Signaling in Bone Formation: Progress and Perspectives. Growth Factors 2012, 30, 117–123. [Google Scholar] [CrossRef]
- Karnes, J.M.; Daffner, S.D.; Watkins, C.M. Multiple Roles of Tumor Necrosis Factor-Alpha in Fracture Healing. Bone 2015, 78, 87–93. [Google Scholar] [CrossRef]
- Bolander, J.; Ji, W.; Leijten, J.; Teixeira, L.M.; Bloemen, V.; Lambrechts, D.; Chaklader, M.; Luyten, F.P. Healing of a Large Long-Bone Defect through Serum-Free In Vitro Priming of Human Periosteum-Derived Cells. Stem Cell Rep. 2017, 8, 758–772. [Google Scholar] [CrossRef]
- Hettiaratchi, M.H.; Krishnan, L.; Rouse, T.; Chou, C.; McDevitt, T.C.; Guldberg, R.E. Heparin-Mediated Delivery of Bone Morphogenetic Protein-2 Improves Spatial Localization of Bone Regeneration. Sci. Adv. 2020, 6, eaay1240. [Google Scholar] [CrossRef]
- Krishnan, L.; Priddy, L.B.; Esancy, C.; Li, M.-T.A.; Stevens, H.Y.; Jiang, X.; Tran, L.; Rowe, D.W.; Guldberg, R.E. Hydrogel-Based Delivery of rhBMP-2 Improves Healing of Large Bone Defects Compared with Autograft. Clin. Orthop. Relat. Res. 2015, 473, 2885–2897. [Google Scholar] [CrossRef] [PubMed]
- Minier, K.; Touré, A.; Fusellier, M.; Fellah, B.; Bouvy, B.; Weiss, P.; Gauthier, O. BMP-2 Delivered from a Self-Crosslinkable CaP/Hydrogel Construct Promotes Bone Regeneration in a Critical-Size Segmental Defect Model of Non-Union in Dogs. Vet. Comp. Orthop. Traumatol. 2014, 27, 411–421. [Google Scholar] [CrossRef]
- Yasuda, H.; Yano, K.; Wakitani, S.; Matsumoto, T.; Nakamura, H.; Takaoka, K. Repair of Critical Long Bone Defects Using Frozen Bone Allografts Coated with an rhBMP-2-Retaining Paste. J. Orthop. Sci. 2012, 17, 299–307. [Google Scholar] [CrossRef] [PubMed]
- El Bialy, I.; Jiskoot, W.; Reza Nejadnik, M. Formulation, Delivery and Stability of Bone Morphogenetic Proteins for Effective Bone Regeneration. Pharm. Res. 2017, 34, 1152–1170. [Google Scholar] [CrossRef] [PubMed]
- McKay, W.F.; Peckham, S.M.; Badura, J.M. A Comprehensive Clinical Review of Recombinant Human Bone Morphogenetic Protein-2 (INFUSE Bone Graft). Int. Orthop. 2007, 31, 729–734. [Google Scholar] [CrossRef]
- Zara, J.N.; Siu, R.K.; Zhang, X.; Shen, J.; Ngo, R.; Lee, M.; Li, W.; Chiang, M.; Chung, J.; Kwak, J.; et al. High Doses of Bone Morphogenetic Protein 2 Induce Structurally Abnormal Bone and Inflammation in Vivo. Tissue Eng. Part A 2011, 17, 1389–1399. [Google Scholar] [CrossRef]
- Cahill, K.S.; Chi, J.H.; Day, A.; Claus, E.B. Prevalence, Complications, and Hospital Charges Associated with Use of Bone-Morphogenetic Proteins in Spinal Fusion Procedures. JAMA 2009, 302, 58–66. [Google Scholar] [CrossRef]
- Smucker, J.D.; Rhee, J.M.; Singh, K.; Yoon, S.T.; Heller, J.G. Increased Swelling Complications Associated with Off-Label Usage of rhBMP-2 in the Anterior Cervical Spine. Spine 2006, 31, 2813–2819. [Google Scholar] [CrossRef]
- Carragee, E.J.; Chu, G.; Rohatgi, R.; Hurwitz, E.L.; Weiner, B.K.; Yoon, S.T.; Comer, G.; Kopjar, B. Cancer Risk after Use of Recombinant Bone Morphogenetic Protein-2 for Spinal Arthrodesis. J. Bone Jt. Surg. Am. 2013, 95, 1537–1545. [Google Scholar] [CrossRef]
- Kim, S.E.; Song, S.-H.; Yun, Y.P.; Choi, B.-J.; Kwon, I.K.; Bae, M.S.; Moon, H.-J.; Kwon, Y.-D. The Effect of Immobilization of Heparin and Bone Morphogenic Protein-2 (BMP-2) to Titanium Surfaces on Inflammation and Osteoblast Function. Biomaterials 2011, 32, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Quang Le, B.; Chun Tan, T.; Lee, S.-B.; Woong Jang, J.; Sik Kim, Y.; Soo Lee, J.; Won Choi, J.; Sathiyanathan, P.; Nurcombe, V.; Cool, S.M. A Biomimetic Collagen-Bone Granule-Heparan Sulfate Combination Scaffold for BMP2 Delivery. Gene 2021, 769, 145217. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-H.; Yun, Y.-P.; Park, Y.-E.; Lee, S.-H.; Yong, W.; Kundu, J.; Jung, J.W.; Shim, J.-H.; Cho, D.-W.; Kim, S.E.; et al. In Vitro and in Vivo Evaluation of Bone Formation Using Solid Freeform Fabrication-Based Bone Morphogenic Protein-2 Releasing PCL/PLGA Scaffolds. Biomed. Mater. 2014, 9, 025008. [Google Scholar] [CrossRef] [PubMed]
- Briquez, P.S.; Tsai, H.-M.; Watkins, E.A.; Hubbell, J.A. Engineered Bridge Protein with Dual Affinity for Bone Morphogenetic Protein-2 and Collagen Enhances Bone Regeneration for Spinal Fusion. Sci. Adv. 2021, 7, eabh4302. [Google Scholar] [CrossRef]
- Bosemark, P.; Isaksson, H.; McDonald, M.M.; Little, D.G.; Tägil, M. Augmentation of Autologous Bone Graft by a Combination of Bone Morphogenic Protein and Bisphosphonate Increased Both Callus Volume and Strength. Acta Orthop. 2013, 84, 106–111. [Google Scholar] [CrossRef]
- Bosemark, P.; Isaksson, H.; Tägil, M. Influence of Systemic Bisphosphonate Treatment on Mechanical Properties of BMP-Induced Calluses in a Rat Fracture Model: Comparison of Three-Point Bending and Twisting Test. J. Orthop. Res. 2014, 32, 721–726. [Google Scholar] [CrossRef]
- Kaipel, M.; Schützenberger, S.; Schultz, A.; Ferguson, J.; Slezak, P.; Morton, T.J.; Van Griensven, M.; Redl, H. BMP-2 but Not VEGF or PDGF in Fibrin Matrix Supports Bone Healing in a Delayed-union Rat Model. J. Orthop. Res. 2012, 30, 1563–1569. [Google Scholar] [CrossRef]
- Ihn, H.; Kang, H.; Iglesias, B. Regional Gene Therapy with Transduced Human Cells: The Influence of “Cell Dose” on Bone Repair. Tissue Eng. Part A 2021, 27, 1422–1433. [Google Scholar] [CrossRef]
- Panos, J.A.; Coenen, M.J.; Nagelli, C.V.; McGlinch, E.B.; Atasoy-Zeybek, A.; De Padilla, C.L.; Coghlan, R.F.; Johnstone, B.; Ferreira, E.; Porter, R.M.; et al. IL-1Ra Gene Transfer Potentiates BMP2-Mediated Bone Healing by Redirecting Osteogenesis toward Endochondral Ossification. Mol. Ther. 2023, 31, 420–434. [Google Scholar] [CrossRef]
- Hu, K.; Olsen, B.R. The Roles of Vascular Endothelial Growth Factor in Bone Repair and Regeneration. Bone 2016, 91, 30–38. [Google Scholar] [CrossRef]
- He, S.; Fang, J.; Zhong, C.; Ren, F.; Wang, M. Controlled pVEGF Delivery via a Gene-Activated Matrix Comprised of a Peptide-Modified Non-Viral Vector and a Nanofibrous Scaffold for Skin Wound Healing. Acta Biomater. 2022, 140, 149–162. [Google Scholar] [CrossRef]
- Orth, M.; Fritz, T.; Stutz, J.; Scheuer, C.; Ganse, B.; Bullinger, Y.; Lee, J.S.; Murphy, W.L.; Laschke, M.W.; Menger, M.D.; et al. Local Application of Mineral-Coated Microparticles Loaded with VEGF and BMP-2 Induces the Healing of Murine Atrophic Non-Unions. Front. Bioeng. Biotechnol. 2022, 9, 809397. [Google Scholar] [CrossRef]
- Novak, S.; Madunic, J.; Shum, L.; Vucetic, M.; Wang, X.; Tanigawa, H.; Ghosh, M.; Sanjay, A.; Kalajzic, I. PDGF Inhibits BMP2-Induced Bone Healing. NPJ Regen. Med. 2023, 8, 3. [Google Scholar] [CrossRef]
- Daniels, T.R.; Anderson, J.; Swords, M.P.; Maislin, G.; Donahue, R.; Pinsker, E.; Quiton, J.D. Recombinant Human Platelet-Derived Growth Factor BB in Combination With a Beta-Tricalcium Phosphate (rhPDGF-BB/β-TCP)-Collagen Matrix as an Alternative to Autograft. Foot Ankle Int. 2019, 40, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- DiGiovanni, C.W.; Lin, S.S.; Baumhauer, J.F.; Daniels, T.; Younger, A.; Glazebrook, M.; Anderson, J.; Anderson, R.; Evangelista, P.; Lynch, S.E.; et al. Recombinant Human Platelet-Derived Growth Factor-BB and Beta-Tricalcium Phosphate (rhPDGF-BB/β-TCP): An Alternative to Autogenous Bone Graft. J. Bone Jt. Surg. Am. 2013, 95, 1184–1192. [Google Scholar] [CrossRef] [PubMed]
- Hollinger, J.O.; Onikepe, A.O.; MacKrell, J.; Einhorn, T.; Bradica, G.; Lynch, S.; Hart, C.E. Accelerated Fracture Healing in the Geriatric, Osteoporotic Rat with Recombinant Human Platelet-derived Growth Factor-bb and an Injectable Beta-tricalcium Phosphate/Collagen Matrix. J. Orthop. Res. 2008, 26, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Nayak, V.V.; Costello, J.P.; Ehlen, Q.T.; Slavin, B.V.; Mirsky, N.A.; Kelly, S.; Suarez, C.; Daunert, S.; Witek, L.; Coelho, P.G. A rhPDGF-BB/Bovine Type I Collagen/β-TCP Mixture for the Treatment of Critically Sized Non-union Tibial Defects: An in Vivo Study in Rabbits. J. Orthop. Res. 2024, 42, 1998–2006. [Google Scholar] [CrossRef]
- Kitcharanant, N.; Chattipakorn, N.; Chattipakorn, S.C. The Effect of Intermittent Parathyroid Hormone on Bone Lengthening: Current Evidence to Inform Future Effective Interventions. Osteoporos. Int. 2023, 34, 1657–1675. [Google Scholar] [CrossRef]
- Andreassen, T.T.; Ejersted, C.; Oxlund, H. Intermittent Parathyroid Hormone (1-34) Treatment Increases Callus Formation and Mechanical Strength of Healing Rat Fractures. J. Bone Min. Res. 1999, 14, 960–968. [Google Scholar] [CrossRef]
- Cipriano, C.A.; Issack, P.S.; Shindle, L.; Werner, C.M.L.; Helfet, D.L.; Lane, J.M. Recent Advances Toward the Clinical Application of PTH (1-34) in Fracture Healing. HSS J. 2009, 5, 149–153. [Google Scholar] [CrossRef]
- Holzer, G.; Majeska, R.J.; Lundy, M.W.; Hartke, J.R.; Einhorn, T.A. Parathyroid Hormone Enhances Fracture Healing. A Preliminary Report. Clin. Orthop. Relat. Res. 1999, 366, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Kumabe, Y.; Lee, S.Y.; Waki, T.; Iwakura, T.; Takahara, S.; Arakura, M.; Kuroiwa, Y.; Fukui, T.; Matsumoto, T.; Matsushita, T.; et al. Triweekly Administration of Parathyroid Hormone (1–34) Accelerates Bone Healing in a Rat Refractory Fracture Model. BMC Musculoskelet. Disord. 2017, 18, 545. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Song, T.; Liu, Y.; Li, J.; Jiang, Q.; Song, Q.; Deng, Z. The Effectiveness and Safety of Parathyroid Hormone in Fracture Healing: A Meta-Analysis. Clinics 2019, 74, e800. [Google Scholar] [CrossRef] [PubMed]
- Eastman, K.; Gerlach, M.; Piec, I.; Greeves, J.; Fraser, W. Effectiveness of Parathyroid Hormone (PTH) Analogues on Fracture Healing: A Meta-Analysis. Osteoporos. Int. 2021, 32, 1531–1546. [Google Scholar] [CrossRef]
- Cameron, K.O.; Lefker, B.A.; Ke, H.Z.; Li, M.; Zawistoski, M.P.; Tjoa, C.M.; Wright, A.S.; DeNinno, S.L.; Paralkar, V.M.; Owen, T.A.; et al. Discovery of CP-533536: An EP2 Receptor Selective Prostaglandin E2 (PGE2) Agonist That Induces Local Bone Formation. Bioorganic Med. Chem. Lett. 2009, 19, 2075–2078. [Google Scholar] [CrossRef]
- Graham, S.; Gamie, Z.; Polyzois, I.; Narvani, A.A.; Tzafetta, K.; Tsiridis, E.; Helioti, M.; Mantalaris, A.; Tsiridis, E. Prostaglandin EP2 and EP4 Receptor Agonists in Bone Formation and Bone Healing: In Vivo and in Vitro Evidence. Expert. Opin. Investig. Drugs 2009, 18, 746–766. [Google Scholar] [CrossRef]
- Li, M.; Thompson, D.D.; Paralkar, V.M. Prostaglandin E2 Receptors in Bone Formation. Int. Orthop. 2007, 31, 767–772. [Google Scholar] [CrossRef]
- Kamolratanakul, P.; Hayata, T.; Ezura, Y.; Kawamata, A.; Hayashi, C.; Yamamoto, Y.; Hemmi, H.; Nagao, M.; Hanyu, R.; Notomi, T.; et al. Nanogel-Based Scaffold Delivery of Prostaglandin E(2) Receptor-Specific Agonist in Combination with a Low Dose of Growth Factor Heals Critical-Size Bone Defects in Mice. Arthritis Rheum. 2011, 63, 1021–1033. [Google Scholar] [CrossRef]
- Rundle, C.H.; Strong, D.D.; Chen, S.-T.; Linkhart, T.A.; Sheng, M.H.-C.; Wergedal, J.E.; Lau, K.-H.W.; Baylink, D.J. Retroviral-Based Gene Therapy with Cyclooxygenase-2 Promotes the Union of Bony Callus Tissues and Accelerates Fracture Healing in the Rat. J. Gene Med. 2008, 10, 229–241. [Google Scholar] [CrossRef]
- Abedi, N.; Sadeghian, A.; Kouhi, M.; Haugen, H.J.; Savabi, O.; Nejatidanesh, F. Immunomodulation in Bone Tissue Engineering: Recent Advancements in Scaffold Design and Biological Modifications for Enhanced Regeneration. ACS Biomater. Sci. Eng. 2025, 11, 1269–1290. [Google Scholar] [CrossRef]
- Xuan, Y.; Li, L.; Zhang, C.; Zhang, M.; Cao, J.; Zhang, Z. The 3D-Printed Ordered Bredigite Scaffold Promotes Pro-Healing of Critical-Sized Bone Defects by Regulating Macrophage Polarization. Int. J. Nanomed. 2023, 18, 917–932. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 Polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef] [PubMed]
- Mahon, O.R.; Browe, D.C.; Gonzalez-Fernandez, T.; Pitacco, P.; Whelan, I.T.; Von Euw, S.; Hobbs, C.; Nicolosi, V.; Cunningham, K.T.; Mills, K.H.G.; et al. Nano-Particle Mediated M2 Macrophage Polarization Enhances Bone Formation and MSC Osteogenesis in an IL-10 Dependent Manner. Biomaterials 2020, 239, 119833. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Zhang, Y.; Zeng, J.; Zhou, J.; Liu, S.; Wei, P.; Liu, H.; Yi, F.; Wan, Z.; Xiong, L.; et al. DLP Fabrication of HA Scaffold with Customized Porous Structures to Regulate Immune Microenvironment and Macrophage Polarization for Enhancing Bone Regeneration. Mater. Today Bio 2024, 24, 100929. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, J.; Guo, J.; Zhang, H.; Zhang, X.; Yin, C.; Wang, L.; Zhu, Y.; Yao, Q. 3D Molecularly Functionalized Cell-Free Biomimetic Scaffolds for Osteochondral Regeneration. Adv. Funct. Mater. 2019, 29, 1807356. [Google Scholar] [CrossRef]
- Bougioukli, S.; Jain, A.; Sugiyama, O.; Tinsley, B.A.; Tang, A.H.; Tan, M.H.; Adams, D.J.; Kostenuik, P.J.; Lieberman, J.R. Combination Therapy with BMP-2 and a Systemic RANKL Inhibitor Enhances Bone Healing in a Mouse Critical-Sized Femoral Defect. Bone 2016, 84, 93–103. [Google Scholar] [CrossRef]
- Ratanavaraporn, J.; Furuya, H.; Tabata, Y. Local Suppression of Pro-Inflammatory Cytokines and the Effects in BMP-2-Induced Bone Regeneration. Biomaterials 2012, 33, 304–316. [Google Scholar] [CrossRef]
- Hannink, G.; Arts, J.J.C. Bioresorbability, Porosity and Mechanical Strength of Bone Substitutes: What Is Optimal for Bone Regeneration? Injury 2011, 42 (Suppl. 2), S22–S25. [Google Scholar] [CrossRef]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent Advances in Bone Tissue Engineering Scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef]
- Eliaz, N.; Metoki, N. Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef]
- Chinnasami, H.; Dey, M.K.; Devireddy, R. Three-Dimensional Scaffolds for Bone Tissue Engineering. Bioengineering 2023, 10, 759. [Google Scholar] [CrossRef]
- Farazin, A.; Darghiasi, S.F. Advanced Polymeric Scaffolds for Bone Tissue Regeneration. Explor. BioMat-X 2025, 2, 101340. [Google Scholar] [CrossRef]
- Filippi, M.; Born, G.; Chaaban, M.; Scherberich, A. Natural Polymeric Scaffolds in Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 474. [Google Scholar] [CrossRef]
- Da Cunha, M.R.; Maia, F.L.M.; Iatecola, A.; Massimino, L.C.; Plepis, A.M.d.G.; Martins, V.d.C.A.; Da Rocha, D.N.; Mariano, E.D.; Hirata, M.C.; Ferreira, J.R.M.; et al. In Vivo Evaluation of Collagen and Chitosan Scaffold, Associated or Not with Stem Cells, in Bone Repair. J. Funct. Biomater. 2023, 14, 357. [Google Scholar] [CrossRef]
- Fu, F.; Zhu, X.; Qin, Z.; Wang, J.-J.; Xu, C.; Wang, L.-N.; Tu, Y.; Zhang, S.; Li, R.-X.; Li, X.-H.; et al. Differential Degradation Rate and Underlying Mechanism of a Collagen/Chitosan Complex in Subcutis, Spinal Cord and Brain Tissues of Rat. J. Mater. Sci: Mater. Med. 2018, 29, 35. [Google Scholar] [CrossRef] [PubMed]
- Azaman, F.A.; Brennan Fournet, M.E.; Sheikh Ab Hamid, S.; Zawawi, M.S.F.; Da Silva Junior, V.A.; Devine, D.M. Enhancement of Scaffold In Vivo Biodegradability for Bone Regeneration Using P28 Peptide Formulations. Pharmaceuticals 2023, 16, 876. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Huang, H.; Ayers, D.C.; Song, J. Modulating Viscoelasticity, Stiffness, and Degradation of Synthetic Cellular Niches via Stoichiometric Tuning of Covalent versus Dynamic Noncovalent Cross-Linking. ACS Cent. Sci. 2018, 4, 971–981. [Google Scholar] [CrossRef]
- Foudazi, R.; Zowada, R.; Manas-Zloczower, I.; Feke, D.L. Porous Hydrogels: Present Challenges and Future Opportunities. Langmuir 2023, 39, 2092–2111. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.; Douglas, S.; Michelle Willerth, S. Mechanically Stable Fibrin Scaffolds Promote Viability and Induce Neurite Outgrowth in Neural Aggregates Derived from Human Induced Pluripotent Stem Cells. Sci. Rep. 2017, 7, 6250. [Google Scholar] [CrossRef]
- Sun, S.; Cui, Y.; Yuan, B.; Dou, M.; Wang, G.; Xu, H.; Wang, J.; Yin, W.; Wu, D.; Peng, C. Drug Delivery Systems Based on Polyethylene Glycol Hydrogels for Enhanced Bone Regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1117647. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Ma, Z.; He, D.; Li, H. Modulating Degradation of Sodium Alginate/Bioglass Hydrogel for Improving Tissue Infiltration and Promoting Wound Healing. Bioact. Mater. 2021, 6, 3692–3704. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.R.; Adams, D.J.; Laurencin, C.T.; Nukavarapu, S.P. Optimally Porous and Biomechanically Compatible Scaffolds for Large-Area Bone Regeneration. Tissue Eng. Part A 2012, 18, 1376–1388. [Google Scholar] [CrossRef] [PubMed]
- Guda, T.; Walker, J.A.; Singleton, B.M.; Hernandez, J.W.; Son, J.-S.; Kim, S.-G.; Oh, D.S.; Appleford, M.R.; Ong, J.L.; Wenke, J.C. Guided Bone Regeneration in Long-Bone Defects with a Structural Hydroxyapatite Graft and Collagen Membrane. Tissue Eng. Part A 2013, 19, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Valtanen, R.S.; Yang, Y.P.; Gurtner, G.C.; Maloney, W.J.; Lowenberg, D.W. Synthetic and Bone Tissue Engineering Graft Substitutes: What Is the Future? Injury 2021, 52, S72–S77. [Google Scholar] [CrossRef]
- Cabrera Pereira, A.; Tovar, N.; Nayak, V.V.; Mijares, D.Q.; Smay, J.E.; Torroni, A.; Flores, R.L.; Witek, L. Direct Inkjet Writing Type 1 Bovine Collagen/Β-tricalcium Phosphate Scaffolds for Bone Regeneration. J. Biomed. Mater. Res. 2024, 112, e35347. [Google Scholar] [CrossRef]
- Tovar, N.; Witek, L.; Atria, P.; Sobieraj, M.; Bowers, M.; Lopez, C.D.; Cronstein, B.N.; Coelho, P.G. Form and Functional Repair of Long Bone Using 3D-printed Bioactive Scaffolds. J. Tissue Eng. Regen. Med. 2018, 12, 1986–1999. [Google Scholar] [CrossRef]
- Zhang, T.; Huang, L.; Zhang, X.; Guo, J.; Shen, Y.; Luo, Y. 3D Printed Bioceramic Scaffolds with Fully Interconnected Channel Networks for Enhanced Vascularized Bone Regeneration. Biomater. Sci. 2025, 13, 4830–4845. [Google Scholar] [CrossRef]
- Wang, X.; Nie, Z.; Chang, J.; Lu, M.L.; Kang, Y. Multiple Channels with Interconnected Pores in a Bioceramic Scaffold Promote Bone Tissue Formation. Sci. Rep. 2021, 11, 20447. [Google Scholar] [CrossRef]
- Lu, Q.; Diao, J.; Wang, Y.; Feng, J.; Zeng, F.; Yang, Y.; Kuang, Y.; Zhao, N.; Wang, Y. 3D Printed Pore Morphology Mediates Bone Marrow Stem Cell Behaviors via RhoA/ROCK2 Signaling Pathway for Accelerating Bone Regeneration. Bioact. Mater. 2023, 26, 413–424. [Google Scholar] [CrossRef]
- Joo, S.; Gwon, Y.; Kim, S.; Park, S.; Kim, J.; Hong, S. Piezoelectrically and Topographically Engineered Scaffolds for Accelerating Bone Regeneration. ACS Appl. Mater. Interfaces 2024, 16, 1999–2011. [Google Scholar] [CrossRef]
- Dai, X.; Yao, X.; Zhang, W.; Cui, H.; Ren, Y.; Deng, J.; Zhang, X. The Osteogenic Role of Barium Titanate/Polylactic Acid Piezoelectric Composite Membranes as Guiding Membranes for Bone Tissue Regeneration. Int. J. Nanomed. 2022, 17, 4339–4353. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Yong, S.; Jiao, Z.; Zhang, W.; Xi, Y.; Huang, D.; Bie, X.; Li, C.; Shi, G.; Zhao, Y.; et al. Piezoelectric Composites with BaTiO3 NPs as Guiding Membranes: Reconstructing the Bioelectric Microenvironment for Enhanced Bone Regeneration. J. Mater. Chem. C 2025, 13, 11850–11868. [Google Scholar] [CrossRef]
- Fernandez De Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.-M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone Substitutes: A Review of Their Characteristics, Clinical Use, and Perspectives for Large Bone Defects Management. J. Tissue Eng. 2018, 9, 2041731418776819. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Kim, G. Three-Dimensional Electrospun Polycaprolactone (PCL)/Alginate Hybrid Composite Scaffolds. Carbohydr. Polym. 2014, 114, 213–221. [Google Scholar] [CrossRef]
- Leng, Y.; Yang, F.; Wang, Q.; Li, Z.; Yuan, B.; Peng, C.; Ren, G.; Wang, Z.; Cui, Y.; Wang, Y.; et al. Material-Based Therapy for Bone Nonunion. Mater. Des. 2019, 183, 108161. [Google Scholar] [CrossRef]
- Sapudom, J.; Kongsema, M.; Methachittipan, A.; Damrongsakkul, S.; Kanokpanont, S.; Teo, J.C.M.; Khongkow, M.; Tonsomboon, K.; Thongnuek, P. Degradation Products of Crosslinked Silk Fibroin Scaffolds Modulate the Immune Response but Not Cell Toxicity. J. Mater. Chem. B 2023, 11, 3607–3616. [Google Scholar] [CrossRef]
- Godbout, C.; Ryan, G.; Ramnaraign, D.J.; Hegner, C.; Desjardins, S.; Gagnon, S.; Bates, B.D.; Whatley, I.; Schemitsch, E.H.; Nauth, A. Optimal Delivery of Endothelial Progenitor Cells in a Rat Model of Critical-size Bone Defects. J. Orthop. Res. 2024, 42, 193–201. [Google Scholar] [CrossRef]
- Liao, H.; Zhong, Z.; Liu, Z.; Li, L.; Ling, Z.; Zou, X. Bone Mesenchymal Stem Cells Co-Expressing VEGF and BMP-6 Genes to Combat Avascular Necrosis of the Femoral Head. Exp. Ther. Med. 2018, 15, 954–962. [Google Scholar] [CrossRef]
- Pek, Y.S.; Gao, S.; Arshad, M.S.M.; Leck, K.-J.; Ying, J.Y. Porous Collagen-Apatite Nanocomposite Foams as Bone Regeneration Scaffolds. Biomaterials 2008, 29, 4300–4305. [Google Scholar] [CrossRef]
- Schwartz, L.; Ganta, A.; Konda, S.; Leucht, P.; Rivero, S.; Egol, K.A. Tibial Plateau Fracture Surgical Care Using Standardized Protocols Over Time: A Single Center’s Longitudinal View. J. Orthop. Trauma. 2023, 37, 627–632. [Google Scholar] [CrossRef]
- Bouhadir, K.H.; Lee, K.Y.; Alsberg, E.; Damm, K.L.; Anderson, K.W.; Mooney, D.J. Degradation of Partially Oxidized Alginate and Its Potential Application for Tissue Engineering. Biotechnol. Prog. 2001, 17, 945–950. [Google Scholar] [CrossRef]
- Ivanovska, J.; Zehnder, T.; Lennert, P.; Sarker, B.; Boccaccini, A.R.; Hartmann, A.; Schneider-Stock, R.; Detsch, R. Biofabrication of 3D Alginate-Based Hydrogel for Cancer Research: Comparison of Cell Spreading, Viability, and Adhesion Characteristics of Colorectal HCT116 Tumor Cells. Tissue Eng. Part C Methods 2016, 22, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Boerckel, J.D.; Kolambkar, Y.M.; Dupont, K.M.; Uhrig, B.A.; Phelps, E.A.; Stevens, H.Y.; García, A.J.; Guldberg, R.E. Effects of Protein Dose and Delivery System on BMP-Mediated Bone Regeneration. Biomaterials 2011, 32, 5241–5251. [Google Scholar] [CrossRef] [PubMed]
- Priddy, L.B.; Chaudhuri, O.; Stevens, H.Y.; Krishnan, L.; Uhrig, B.A.; Willett, N.J.; Guldberg, R.E. Oxidized Alginate Hydrogels for Bone Morphogenetic Protein-2 Delivery in Long Bone Defects. Acta Biomater. 2014, 10, 4390–4399. [Google Scholar] [CrossRef] [PubMed]
- Meinel, L.; Kaplan, D.L. Silk Constructs for Delivery of Musculoskeletal Therapeutics. Adv. Drug Deliv. Rev. 2012, 64, 1111–1122. [Google Scholar] [CrossRef]
- Stuckensen, K.; Schwab, A.; Knauer, M.; Muiños-López, E.; Ehlicke, F.; Reboredo, J.; Granero-Moltó, F.; Gbureck, U.; Prósper, F.; Walles, H.; et al. Tissue Mimicry in Morphology and Composition Promotes Hierarchical Matrix Remodeling of Invading Stem Cells in Osteochondral and Meniscus Scaffolds. Adv. Mater. 2018, 30, 1706754. [Google Scholar] [CrossRef]
- Deininger, C.; Wagner, A.; Heimel, P.; Salzer, E.; Vila, X.M.; Weißenbacher, N.; Grillari, J.; Redl, H.; Wichlas, F.; Freude, T.; et al. Enhanced BMP-2-Mediated Bone Repair Using an Anisotropic Silk Fibroin Scaffold Coated with Bone-like Apatite. Int. J. Mol. Sci. 2021, 23, 283. [Google Scholar] [CrossRef]
- Feng, Y.; Guo, W.; Hu, L.; Yi, X.; Tang, F. Application of Hydrogels as Sustained-Release Drug Carriers in Bone Defect Repair. Polymers 2022, 14, 4906. [Google Scholar] [CrossRef]
- Huang, H.; Qi, X.; Chen, Y.; Wu, Z. Thermo-Sensitive Hydrogels for Delivering Biotherapeutic Molecules: A Review. Saudi Pharm. J. 2019, 27, 990–999. [Google Scholar] [CrossRef]
- Li, R.; Liang, J.; He, Y.; Qin, J.; He, H.; Lee, S.; Pang, Z.; Wang, J. Sustained Release of Immunosuppressant by Nanoparticle-Anchoring Hydrogel Scaffold Improved the Survival of Transplanted Stem Cells and Tissue Regeneration. Theranostics 2018, 8, 878–893. [Google Scholar] [CrossRef]
- Fan, Z.; Cheng, P.; Wang, D.; Zhao, Y.; Wang, Z.; Han, J. Design and Investigation of Salecan/Chitosan Hydrogel Formulations with Improved Antibacterial Performance and 3D Cell Culture Function. J. Biomater. Sci. Polym. Ed. 2020, 31, 2268–2284. [Google Scholar] [CrossRef]
- Whitehead, J.; Griffin, K.H.; Gionet-Gonzales, M.; Vorwald, C.E.; Cinque, S.E.; Leach, J.K. Hydrogel Mechanics Are a Key Driver of Bone Formation by Mesenchymal Stromal Cell Spheroids. Biomaterials 2021, 269, 120607. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.T.; Jeffreys, N.; Diba, M.; Mooney, D.J. Viscoelastic Biomaterials for Tissue Regeneration. Tissue Eng. Part C Methods 2022, 28, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, W.; Li, M.; Song, P.; Lei, H.; Gui, X.; Zhou, C.; Liu, L. Biomimetic Methacrylated Gelatin Hydrogel Loaded With Bone Marrow Mesenchymal Stem Cells for Bone Tissue Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 770049. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.M.; Conze, N.; Lewik, G.; Wallner, C.; Brune, J.C.; Dittfeld, S.; Jaurich, H.; Becerikli, M.; Dadras, M.; Harati, K.; et al. Bone Allografts Combined with Adipose-Derived Stem Cells in an Optimized Cell/Volume Ratio Showed Enhanced Osteogenesis and Angiogenesis in a Murine Femur Defect Model. J. Mol. Med. 2019, 97, 1439–1450. [Google Scholar] [CrossRef]
- Chatterjee, K.; Lin-Gibson, S.; Wallace, W.E.; Parekh, S.H.; Lee, Y.J.; Cicerone, M.T.; Young, M.F.; Simon, C.G. The Effect of 3D Hydrogel Scaffold Modulus on Osteoblast Differentiation and Mineralization Revealed by Combinatorial Screening. Biomaterials 2010, 31, 5051–5062. [Google Scholar] [CrossRef]
- McBeth, C.; Lauer, J.; Ottersbach, M.; Campbell, J.; Sharon, A.; Sauer-Budge, A.F. 3D Bioprinting of GelMA Scaffolds Triggers Mineral Deposition by Primary Human Osteoblasts. Biofabrication 2017, 9, 015009. [Google Scholar] [CrossRef]
- Yu, F.T.H.; Armstrong, J.K.; Tripette, J.; Meiselman, H.J.; Cloutier, G. A Local Increase in Red Blood Cell Aggregation Can Trigger Deep Vein Thrombosis: Evidence Based on Quantitative Cellular Ultrasound Imaging. J. Thromb. Haemost. 2011, 9, 481–488. [Google Scholar] [CrossRef]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, Properties, and Biomedical Applications of Gelatin Methacryloyl (GelMA) Hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, Y.; Li, S.; Xing, L.; Du, S.; Yuan, G.; Li, J.; Zhou, T.; Xiong, D.; Tan, H.; et al. Doubly Crosslinked Biodegradable Hydrogels Based on Gellan Gum and Chitosan for Drug Delivery and Wound Dressing. Int. J. Biol. Macromol. 2020, 164, 2204–2214. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Zhang, T.; Zan, Y.; Ni, T.; Cao, Y.; Wang, J.; Liu, M.; Pei, R. Injectable Hydrogels from Enzyme-Catalyzed Crosslinking as BMSCs-Laden Scaffold for Bone Repair and Regeneration. Mater. Sci. Eng. C 2019, 96, 841–849. [Google Scholar] [CrossRef]
- Liu, X.; Sun, S.; Wang, N.; Kang, R.; Xie, L.; Liu, X. Therapeutic Application of Hydrogels for Bone-Related Diseases. Front. Bioeng. Biotechnol. 2022, 10, 998988. [Google Scholar] [CrossRef]
- Shi, W.; Jiang, Y.; Wu, T.; Zhang, Y.; Li, T. Advancements in Drug-Loaded Hydrogel Systems for Bone Defect Repair. Regen. Ther. 2023, 25, 174–185. [Google Scholar] [CrossRef]
- Zheng, S.; Zhong, H.; Cheng, H.; Li, X.; Zeng, G.; Chen, T.; Zou, Y.; Liu, W.; Sun, C. Engineering Multifunctional Hydrogel With Osteogenic Capacity for Critical-Size Segmental Bone Defect Repair. Front. Bioeng. Biotechnol. 2022, 10, 899457. [Google Scholar] [CrossRef]
- Rust, P.A.; Kalsi, P.; Briggs, T.W.R.; Cannon, S.R.; Blunn, G.W. Will Mesenchymal Stem Cells Differentiate into Osteoblasts on Allograft? Clin. Orthop. Relat. Res. 2007, 457, 220–226. [Google Scholar] [CrossRef]
- Rasch, A.; Naujokat, H.; Wang, F.; Seekamp, A.; Fuchs, S.; Klüter, T. Evaluation of Bone Allograft Processing Methods: Impact on Decellularization Efficacy, Biocompatibility and Mesenchymal Stem Cell Functionality. PLoS ONE 2019, 14, e0218404. [Google Scholar] [CrossRef] [PubMed]
- Vallés, G.; Bensiamar, F.; Maestro-Paramio, L.; García-Rey, E.; Vilaboa, N.; Saldaña, L. Influence of Inflammatory Conditions Provided by Macrophages on Osteogenic Ability of Mesenchymal Stem Cells. Stem Cell Res. Ther. 2020, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Poliwoda, S.; Noor, N.; Downs, E.; Schaaf, A.; Cantwell, A.; Ganti, L.; Kaye, A.D.; Mosel, L.I.; Carroll, C.B.; Viswanath, O.; et al. Stem Cells: A Comprehensive Review of Origins and Emerging Clinical Roles in Medical Practice. Orthop. Rev. 2022, 14, 37498. [Google Scholar] [CrossRef] [PubMed]
- de Girolamo, L.; Bertolini, G.; Cervellin, M.; Sozzi, G.; Volpi, P. Treatment of Chondral Defects of the Knee with One Step Matrix-Assisted Technique Enhanced by Autologous Concentrated Bone Marrow: In Vitro Characterisation of Mesenchymal Stem Cells from Iliac Crest and Subchondral Bone. Injury 2010, 41, 1172–1177. [Google Scholar] [CrossRef]
- Horwitz, E.M.; Le Blanc, K.; Dominici, M.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Deans, R.J.; Krause, D.S.; Keating, A. Clarification of the Nomenclature for MSC: The International Society for Cellular Therapy Position Statement. Cytotherapy 2005, 7, 393–395. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, S.; Liu, Q. Identification of Differentially Expressed Genes by Single-Cell Transcriptional Profiling of Umbilical Cord and Synovial Fluid Mesenchymal Stem Cells. J. Cell Mol. Med. 2020, 24, 1945–1957. [Google Scholar] [CrossRef] [PubMed]
- Stanko, P.; Kaiserova, K.; Altanerova, V.; Altaner, C. Comparison of Human Mesenchymal Stem Cells Derived from Dental Pulp, Bone Marrow, Adipose Tissue, and Umbilical Cord Tissue by Gene Expression. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 2014, 158, 373–377. [Google Scholar] [CrossRef]
- Benavides-Castellanos, M.P.; Garzón-Orjuela, N.; Linero, I. Effectiveness of Mesenchymal Stem Cell-Conditioned Medium in Bone Regeneration in Animal and Human Models: A Systematic Review and Meta-Analysis. Cell Regen. 2020, 9, 5. [Google Scholar] [CrossRef]
- Ismail, H.D.; Phedy, P.; Kholinne, E.; Djaja, Y.P.; Kusnadi, Y.; Merlina, M.; Yulisa, N.D. Mesenchymal Stem Cell Implantation in Atrophic Nonunion of the Long Bones: A Translational Study. Bone Jt. Res. 2016, 5, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-S.; Chung, H.-J.; Kim, K.-I. Optimal Concentration of Mesenchymal Stem Cells for Fracture Healing in a Rat Model with Long Bone Fracture. World J. Stem Cells 2022, 14, 839–850. [Google Scholar] [CrossRef]
- Peters, A.; Toben, D.; Lienau, J.; Schell, H.; Bail, H.J.; Matziolis, G.; Duda, G.N.; Kaspar, K. Locally Applied Osteogenic Predifferentiated Progenitor Cells Are More Effective Than Undifferentiated Mesenchymal Stem Cells in the Treatment of Delayed Bone Healing. Tissue Eng. Part A 2009, 15, 2947–2954. [Google Scholar] [CrossRef]
- Buchert, J.M.; Lotz, B.; Diederichs, S.; Richter, W. Adipose-Derived Stromal Cells: Isolation, Expansion, and Differentiation. Methods Mol. Biol. 2023, 2598, 75–85. [Google Scholar] [CrossRef]
- Schubert, T.; Lafont, S.; Beaurin, G.; Grisay, G.; Behets, C.; Gianello, P.; Dufrane, D. Critical Size Bone Defect Reconstruction by an Autologous 3D Osteogenic-like Tissue Derived from Differentiated Adipose MSCs. Biomaterials 2013, 34, 4428–4438. [Google Scholar] [CrossRef]
- Granero-Moltó, F.; Weis, J.A.; Miga, M.I.; Landis, B.; Myers, T.J.; O’Rear, L.; Longobardi, L.; Jansen, E.D.; Mortlock, D.P.; Spagnoli, A. Regenerative Effects of Transplanted Mesenchymal Stem Cells in Fracture Healing. Stem Cells 2009, 27, 1887–1898. [Google Scholar] [CrossRef]
- Ji, X.; Yuan, X.; Ma, L.; Bi, B.; Zhu, H.; Lei, Z.; Liu, W.; Pu, H.; Jiang, J.; Jiang, X.; et al. Mesenchymal Stem Cell-Loaded Thermosensitive Hydroxypropyl Chitin Hydrogel Combined with a Three-Dimensional-Printed Poly(ε-Caprolactone)/Nano-Hydroxyapatite Scaffold to Repair Bone Defects via Osteogenesis, Angiogenesis and Immunomodulation. Theranostics 2020, 10, 725–740. [Google Scholar] [CrossRef]
- Lee, D.J.; Miguez, P.; Kwon, J.; Daniel, R.; Padilla, R.; Min, S.; Zalal, R.; Ko, C.-C.; Shin, H.W. Decellularized Pulp Matrix as Scaffold for Mesenchymal Stem Cell Mediated Bone Regeneration. J. Tissue Eng. 2020, 11, 2041731420981672. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Tofighi, A.; Wang, X.; Strunk, M.; Ricketts, T.; Chang, J.; Kaplan, D.L. Calcium Phosphate Combination Biomaterials as Human Mesenchymal Stem Cell Delivery Vehicles for Bone Repair. J. Biomed. Mater. Res. 2011, 97B, 235–244. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Šponer, P.; Filip, S.; Kučera, T.; Brtková, J.; Urban, K.; Palička, V.; Kočí, Z.; Syka, M.; Bezrouk, A.; Syková, E. Utilizing Autologous Multipotent Mesenchymal Stromal Cells Andβ-Tricalcium Phosphate Scaffold in Human Bone Defects: A Prospective, Controlled Feasibility Trial. BioMed Res. Int. 2016, 2016, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Maiti, S.K.; Shivakumar, M.U.; Mohan, D.; Kumar, N.; Singh, K.P. Mesenchymal Stem Cells of Different Origin-Seeded Bioceramic Construct in Regeneration of Bone Defect in Rabbit. Tissue Eng. Regen. Med. 2018, 15, 477–492. [Google Scholar] [CrossRef]
- Ninu, A.R.; Maiti, S.K.; Kumar, S.; Kumar, S.; Sangeetha, P.; Kritaniya, D.; Gupta, S.; Saxena, A.; Kumar, N. Isolation, Proliferation, Characterization and in Vivo Osteogenic Potential of Bone-Marrow Derived Mesenchymal Stem Cells (rBMSC) in Rabbit Model. Indian. J. Exp. Biol. 2017, 55, 79–87. [Google Scholar]
- Li, X.; Zhang, R.; Tan, X.; Li, B.; Liu, Y.; Wang, X. Synthesis and Evaluation of BMMSC-Seeded BMP-6/nHAG/GMS Scaffolds for Bone Regeneration. Int. J. Med. Sci. 2019, 16, 1007–1017. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, S.H.; Kang, B.; Kim, W.H.; Yun, H.; Kweon, O. Comparison of Osteogenesis between Adipose-Derived Mesenchymal Stem Cells and Their Sheets on Poly-ε-Caprolactone/β-Tricalcium Phosphate Composite Scaffolds in Canine Bone Defects. Stem Cells Int. 2016, 2016, 8414715. [Google Scholar] [CrossRef]
- Hao, Z.; Lu, J.; Wang, S.; Wu, H.; Zhang, Y.; Xu, S. Stem Cell-derived Exosomes: A Promising Strategy for Fracture Healing. Cell Prolif. 2017, 50, e12359. [Google Scholar] [CrossRef]
- Hu, J.-C.; Zheng, C.-X.; Sui, B.-D.; Liu, W.-J.; Jin, Y. Mesenchymal Stem Cell-Derived Exosomes: A Novel and Potential Remedy for Cutaneous Wound Healing and Regeneration. World J. Stem Cells 2022, 14, 318–329. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, L.; Gao, Z.; Chen, G.; Zhang, C. Bone Marrow Stromal/Stem Cell-Derived Extracellular Vesicles Regulate Osteoblast Activity and Differentiation In Vitro and Promote Bone Regeneration In Vivo. Sci. Rep. 2016, 6, 21961. [Google Scholar] [CrossRef]
- Narayanan, R.; Huang, C.-C.; Ravindran, S. Hijacking the Cellular Mail: Exosome Mediated Differentiation of Mesenchymal Stem Cells. Stem Cells Int. 2016, 2016, 3808674. [Google Scholar] [CrossRef]
- Ankrum, J.A.; Ong, J.F.; Karp, J.M. Mesenchymal stem cells: Immune evasive, not immune privileged. Nat. Biotechnol. 2014, 32, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Sanabria-de la Torre, R.; Quiñones-Vico, M.I.; Fernández-González, A.; Sánchez-Díaz, M.; Montero-Vílchez, T.; Sierra-Sánchez, Á.; Arias-Santiago, S. Alloreactive Immune Response Associated to Human Mesenchymal Stromal Cells Treatment: A Systematic Review. J. Clin. Med. 2021, 10, 2991. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, K.E.; Trzaska, K.A.; Fernandes, H.; Bryan, M.; Taborga, M.; Srinivas, V.; Packman, K.; Patel, P.S.; Rameshwar, P. Mesenchymal Stem Cells in Early Entry of Breast Cancer into Bone Marrow. PLoS ONE 2008, 3, e2563. [Google Scholar] [CrossRef]
- Ridge, S.M.; Sullivan, F.J.; Glynn, S.A. Mesenchymal Stem Cells: Key Players in Cancer Progression. Mol. Cancer 2017, 16, 31. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, G.; Lieber, R.; Gazit, D.; Pelled, G. Recent Advances and Future of Gene Therapy for Bone Regeneration. Curr. Osteoporos. Rep. 2018, 16, 504–511. [Google Scholar] [CrossRef]
- De la Vega, R.E.; Atasoy-Zeybek, A.; Panos, J.A.; VAN Griensven, M.; Evans, C.H.; Balmayor, E.R. Gene Therapy for Bone Healing: Lessons Learned and New Approaches. Transl. Res. 2021, 236, 1–16. [Google Scholar] [CrossRef]
- Watson-Levings, R.S.; Palmer, G.D.; Levings, P.P.; Dacanay, E.A.; Evans, C.H.; Ghivizzani, S.C. Gene Therapy in Orthopaedics: Progress and Challenges in Pre-Clinical Development and Translation. Front. Bioeng. Biotechnol. 2022, 10, 901317. [Google Scholar] [CrossRef]
- Al-Dosari, M.S.; Gao, X. Nonviral Gene Delivery: Principle, Limitations, and Recent Progress. AAPS J. 2009, 11, 671–681. [Google Scholar] [CrossRef]
- Bez, M.; Sheyn, D.; Tawackoli, W.; Avalos, P.; Shapiro, G.; Giaconi, J.C.; Da, X.; David, S.B.; Gavrity, J.; Awad, H.A.; et al. In Situ Bone Tissue Engineering via Ultrasound-Mediated Gene Delivery to Endogenous Progenitor Cells in Mini-Pigs. Sci. Transl. Med. 2017, 9, eaal3128. [Google Scholar] [CrossRef]
- Bez, M.; Foiret, J.; Shapiro, G.; Pelled, G.; Ferrara, K.W.; Gazit, D. Nonviral Ultrasound-Mediated Gene Delivery in Small and Large Animal Models. Nat. Protoc. 2019, 14, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Feichtinger, G.A.; Hofmann, A.T.; Slezak, P.; Schuetzenberger, S.; Kaipel, M.; Schwartz, E.; Neef, A.; Nomikou, N.; Nau, T.; Van Griensven, M.; et al. Sonoporation Increases Therapeutic Efficacy of Inducible and Constitutive BMP2/7 In Vivo Gene Delivery. Hum. Gene Ther. Methods 2014, 25, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Wan, C.; Ramaswamy, G.; Clemens, T.L.; Ponnazhagan, S. Mesenchymal Stem Cells Expressing Osteogenic and Angiogenic Factors Synergistically Enhance Bone Formation in a Mouse Model of Segmental Bone Defect. Mol. Ther. 2010, 18, 1026–1034. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Lin, K.-J.; Kao, C.-Y.; Chen, M.-C.; Lo, W.-H.; Yen, T.-C.; Chang, Y.-H.; Hu, Y.-C. The Role of Adipose-Derived Stem Cells Engineered with the Persistently Expressing Hybrid Baculovirus in the Healing of Massive Bone Defects. Biomaterials 2011, 32, 6505–6514. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Gallo, M.C.; Bell, J.A.; Mayfield, C.K.; Ball, J.R.; Ayad, M.; Lechtholz-Zey, E.; Chang, S.W.; Sugiyama, O.; Evseenko, D.; et al. Ex Vivo Regional Gene Therapy Compared to Recombinant BMP-2 for the Treatment of Critical-Size Bone Defects: An In Vivo Single-Cell RNA-Sequencing Study. Bioengineering 2025, 12, 29. [Google Scholar] [CrossRef]
- Razi Soofiyani, S.; Baradaran, B.; Lotfipour, F.; Kazemi, T.; Mohammadnejad, L. Gene Therapy, Early Promises, Subsequent Problems, and Recent Breakthroughs. Adv. Pharm. Bull. 2013, 3, 249–255. [Google Scholar] [CrossRef]
- Baltzer, A.W.A.; Lattermann, C.; Whalen, J.D.; Wooley, P.; Weiss, K.; Grimm, M.; Ghivizzani, S.C.; Robbins, P.D.; Evans, C.H. Genetic Enhancement of Fracture Repair: Healing of an Experimental Segmental Defect by Adenoviral Transfer of the BMP-2 Gene. Gene Ther. 2000, 7, 734–739. [Google Scholar] [CrossRef]
- Evans, C.H.; Liu, F.J.; Glatt, V. Use of Genetically Modified Muscle and Fat Grafts to Repair Defects in Bone and Cartilage. Eur. Cell Mater. 2009, 18, 96–111. [Google Scholar] [CrossRef]
- Virk, M.S.; Sugiyama, O.; Park, S.H. Same Day” Ex-Vivo Regional Gene Therapy: A Novel Strategy to Enhance Bone Repair. Mol. Ther. 2011, 19, 960–968. [Google Scholar] [CrossRef]
- Bougioukli, S.; Alluri, R.; Pannell, W.; Sugiyama, O.; Vega, A.; Tang, A.; Skorka, T.; Park, S.H.; Oakes, D.; Lieberman, J.R. Ex Vivo Gene Therapy Using Human Bone Marrow Cells Overexpressing BMP-2: “Next-Day” Gene Therapy versus Standard “Two Step” Approach. Bone 2019, 128, 115032. [Google Scholar] [CrossRef]
- Chang, P.-C.; Seol, Y.-J.; Cirelli, J.A.; Pellegrini, G.; Jin, Q.; Franco, L.M.; Goldstein, S.A.; Chandler, L.A.; Sosnowski, B.; Giannobile, W.V. PDGF-B Gene Therapy Accelerates Bone Engineering and Oral Implant Osseointegration. Gene Ther. 2010, 17, 95–104. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ito, H.; Koefoed, M.; Tiyapatanaputi, P.; Gromov, K.; Goater, J.J.; Carmouche, J.; Zhang, X.; Rubery, P.T.; Rabinowitz, J.; Samulski, R.J.; et al. Remodeling of Cortical Bone Allografts Mediated by Adherent rAAV-RANKL and VEGF Gene Therapy. Nat. Med. 2005, 11, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Wright, V.; Usas, A.; Gearhart, B.; Shen, H.-C.; Cummins, J.; Huard, J. Synergistic Enhancement of Bone Formation and Healing by Stem Cell-Expressed VEGF and Bone Morphogenetic Protein-4. J. Clin. Investig. 2002, 110, 751–759. [Google Scholar] [CrossRef]
- Alden, T.D.; Pittman, D.D.; Beres, E.J.; Hankins, G.R.; Kallmes, D.F.; Wisotsky, B.M.; Kerns, K.M.; Helm, G.A. Percutaneous Spinal Fusion Using Bone Morphogenetic Protein-2 Gene Therapy. J. Neurosurg. Spine 1999, 90, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Kaihara, S.; Bessho, K.; Okubo, Y.; Sonobe, J.; Kawai, M.; Iizuka, T. Simple and Effective Osteoinductive Gene Therapy by Local Injection of a Bone Morphogenetic Protein-2-Expressing Recombinant Adenoviral Vector and FK506 Mixture in Rats. Gene Ther. 2004, 11, 439–447. [Google Scholar] [CrossRef]
- Liu, F.; Ferreira, E.; Porter, R.M. Rapid and Reliable Healing of Critical Size Bone Defects with Genetically Modified Sheep Muscle. Eur. Cell Mater. 2015, 30, 118–131. [Google Scholar] [CrossRef]
- Shirley, J.L.; de Jong, Y.P.; Terhorst, C.; Herzog, R.W. Immune Responses to Viral Gene Therapy Vectors. Mol. Ther. 2020, 28, 709–722. [Google Scholar] [CrossRef]
- Alden, T.D.; Pittman, D.D.; Hankins, G.R.; Beres, E.J.; Engh, J.A.; Das, S.; Hudson, S.B.; Kerns, K.M.; Kallmes, D.F.; Helm, G.A. In Vivo Endochondral Bone Formation Using a Bone Morphogenetic Protein 2 Adenoviral Vector. Hum. Gene Ther. 1999, 10, 2245–2253. [Google Scholar] [CrossRef]
- RE, V.; MJ, C.; SA, M. Effects of FK506 on the Healing of Diaphyseal, Critical Size Defects in the Rat Femur. Eur. Cell Mater. 2020, 40, 160–171. [Google Scholar] [CrossRef]
- Bell, J.A.; Collon, K.; Mayfield, C.; Gallo, M.C.; Chang, S.W.; Sugiyama, O.; Tang, A.H.; Hollis, R.; Chopra, S.; Kohn, D.B.; et al. Biodistribution of Lentiviral Transduced Adipose-Derived Stem Cells for “Ex-Vivo” Regional Gene Therapy for Bone Repair. Gene Ther. 2023, 30, 826–834. [Google Scholar] [CrossRef]
- Vakhshori, V.; Bougioukli, S.; Sugiyama, O. Ex Vivo Regional Gene Therapy with Human Adipose-Derived Stem Cells for Bone Repair. Bone 2020, 138, 115524. [Google Scholar] [CrossRef]
- Bougioukli, S.; Saitta, B.; Sugiyama, O.; Tang, A.H.; Elphingstone, J.; Evseenko, D.; Lieberman, J.R. Lentiviral Gene Therapy for Bone Repair Using Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells. Hum. Gene Ther. 2019, 30, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Feeley, B.T.; Conduah, A.H.; Sugiyama, O.; Krenek, L.; Chen, I.S.Y.; Lieberman, J.R. In Vivo Molecular Imaging of Adenoviral versus Lentiviral Gene Therapy in Two Bone Formation Models. J. Orthop. Res. 2006, 24, 1709–1721. [Google Scholar] [CrossRef] [PubMed]
- Sonobe, J.; Okubo, Y.; Kaihara, S.; Miyatake, S.-I.; Bessho, K. Osteoinduction by Bone Morphogenetic Protein 2-Expressing Adenoviral Vector: Application of Biomaterial to Mask the Host Immune Response. Hum. Gene Ther. 2004, 15, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Bauso, L.V.; La Fauci, V.; Longo, C.; Calabrese, G. Bone Tissue Engineering and Nanotechnology: A Promising Combination for Bone Regeneration. Biology 2024, 13, 237. [Google Scholar] [CrossRef]
- Siqueira, F.S.; Gomes, A.D.J.; Lunardi, C.N. Integrating Nanotechnology With 3D Printing for Drug Delivery: Bone Regeneration and Beyond. Nano Select 2025, 6, e70030. [Google Scholar] [CrossRef]
- Cui, Y.; Li, N.; Wu, J.; Wang, X.; Zhou, P.; Bai, J.; Liu, Y. Navigating the Landscape of Materials-Based RNA Delivery Systems in Bone Healing. Adv. Funct. Mater. 2025, e09383. [Google Scholar] [CrossRef]
- Iyer, S.S. Epigenetic Regulation of Bone Healing: Implications for Fracture Repair and Clinical Treatment Strategies. Yale J. Biol. Med. 2025, 98, 159–170. [Google Scholar] [CrossRef]
- Park, S.-Y.; Lee, J.-K.; Lee, S.-H.; Kim, D.-S.; Jung, J.-W.; Kim, J.H.; Baek, S.-W.; You, S.; Hwang, D.-Y.; Han, D.K. Multifunctional Vitamin D-Incorporated PLGA Scaffold with BMP/VEGF-Overexpressed Tonsil-Derived MSC via CRISPR/Cas9 for Bone Tissue Regeneration. Mater. Today Bio 2024, 28, 101254. [Google Scholar] [CrossRef]
- Da Silva, V.A.; Sharma, R.; Shteinberg, E.; Patel, V.; Bhardwaj, L.; Garay, T.; Yu, B.; Willerth, S.M. Machine Learning Approaches to 3D Models for Drug Screening. Biomed. Mater. Devices 2024, 2, 695–720. [Google Scholar] [CrossRef]
- Degenhart, C.; Engelhardt, L.; Niemeyer, F.; Erne, F.; Braun, B.; Gebhard, F.; Schütze, K. Computer-Based Mechanobiological Fracture Healing Model Predicts Non-Union of Surgically Treated Diaphyseal Femur Fractures. J. Clin. Med. 2023, 12, 3461. [Google Scholar] [CrossRef] [PubMed]
- Donie, C.; Reumann, M.K.; Hartung, T.; Braun, B.J.; Histing, T.; Endo, S.; Hirche, S. Predictive Model Development to Identify Failed Healing in Patients after Non-Union Fracture Surgery. In Proceedings of the 2024 46th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 15–19 July 2024; IEEE: Piscataway, NJ, USA, 2024; pp. 1–4. [Google Scholar] [CrossRef]
- Yu, H.; Mu, Q.; Wang, Z.; Guo, Y.; Zhao, J.; Wang, G.; Wang, Q.; Meng, X.; Dong, X.; Wang, S.; et al. A Study on Early Diagnosis for Fracture Non-Union Prediction Using Deep Learning and Bone Morphometric Parameters. Front. Med. 2025, 12, 1547588. [Google Scholar] [CrossRef] [PubMed]
- Breulmann, F.L.; Hatt, L.P.; Schmitz, B.; Wehrle, E.; Richards, R.G.; Della Bella, E.; Stoddart, M.J. Prognostic and Therapeutic Potential of microRNAs for Fracture Healing Processes and Non-Union Fractures: A Systematic Review. Clin. Transl. Med. 2023, 13, e1161. [Google Scholar] [CrossRef] [PubMed]
- Chitwood, J.R.; Chakraborty, N.; Hammamieh, R.; Moe, S.M.; Chen, N.X.; Kacena, M.A.; Natoli, R.M. Predicting Fracture Healing with Blood Biomarkers: The Potential to Assess Patient Risk of Fracture Nonunion. Biomarkers 2021, 26, 703–717. [Google Scholar] [CrossRef]
- Ribitsch, I.; Baptista, P.M.; Lange-Consiglio, A.; Melotti, L.; Patruno, M.; Jenner, F.; Schnabl-Feichter, E.; Dutton, L.C.; Connolly, D.J.; van Steenbeek, F.G.; et al. Large Animal Models in Regenerative Medicine and Tissue Engineering: To Do or Not to Do. Front. Bioeng. Biotechnol. 2020, 8, 972. [Google Scholar] [CrossRef]
- Dai, K.; Xu, X.; Tang, T.; Zhu, Z.; Yu, C.; Xu, M.; Zhu, L.; Hao, Y.; Lou, J. BMP-2 gene modified tissue-engineered bone repairing segmental tibial bone defects in goats. Zhonghua Yi Xue Za Zhi 2003, 83, 1345–1349. [Google Scholar]
- Arinzeh, T.L.; Peter, S.J.; Archambault, M.P.; van den Bos, C.; Gordon, S.; Kraus, K.; Smith, A.; Kadiyala, S. Allogeneic Mesenchymal Stem Cells Regenerate Bone in a Critical-Sized Canine Segmental Defect. J. Bone Jt. Surg. Am. 2003, 85, 1927–1935. [Google Scholar] [CrossRef]
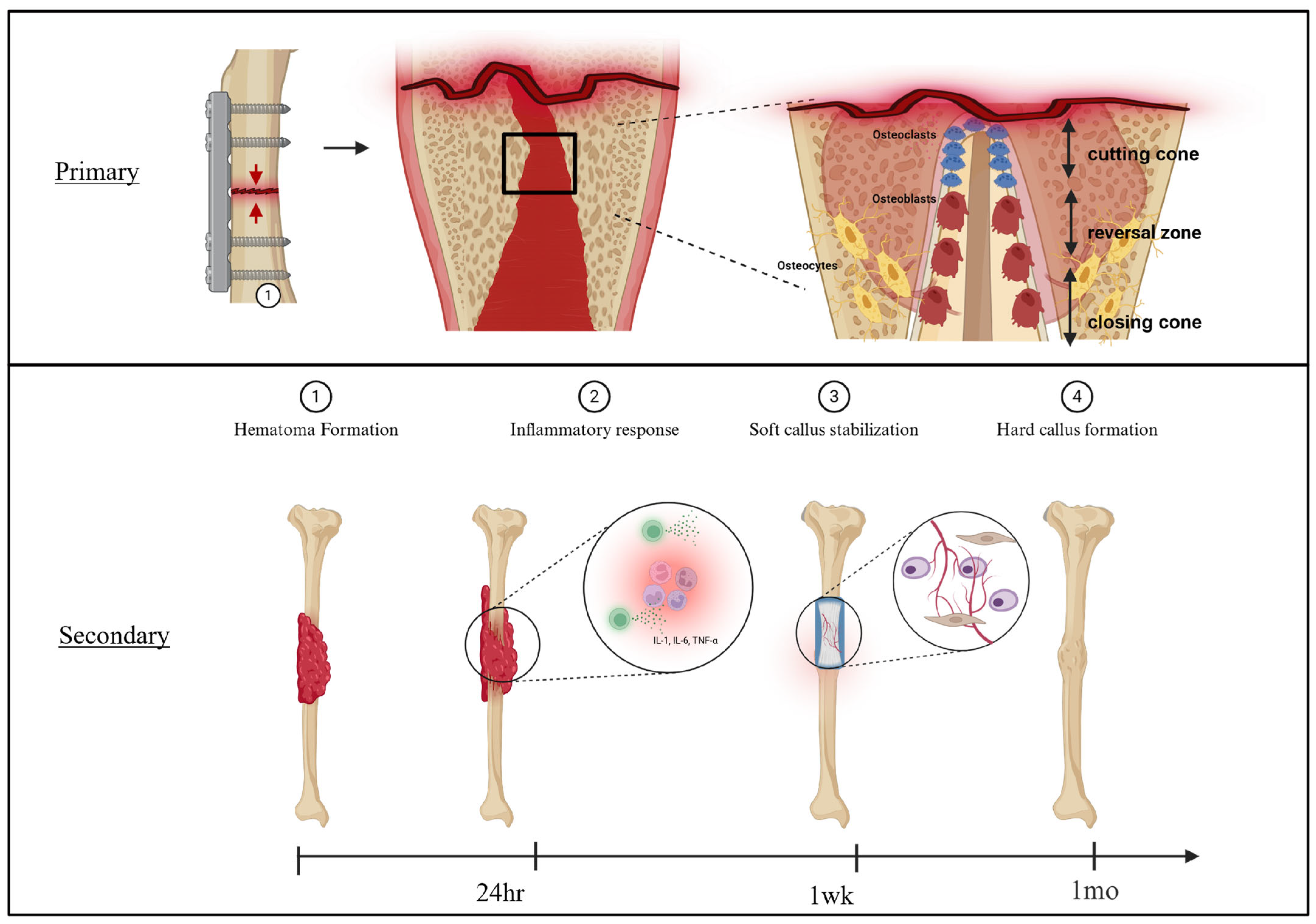
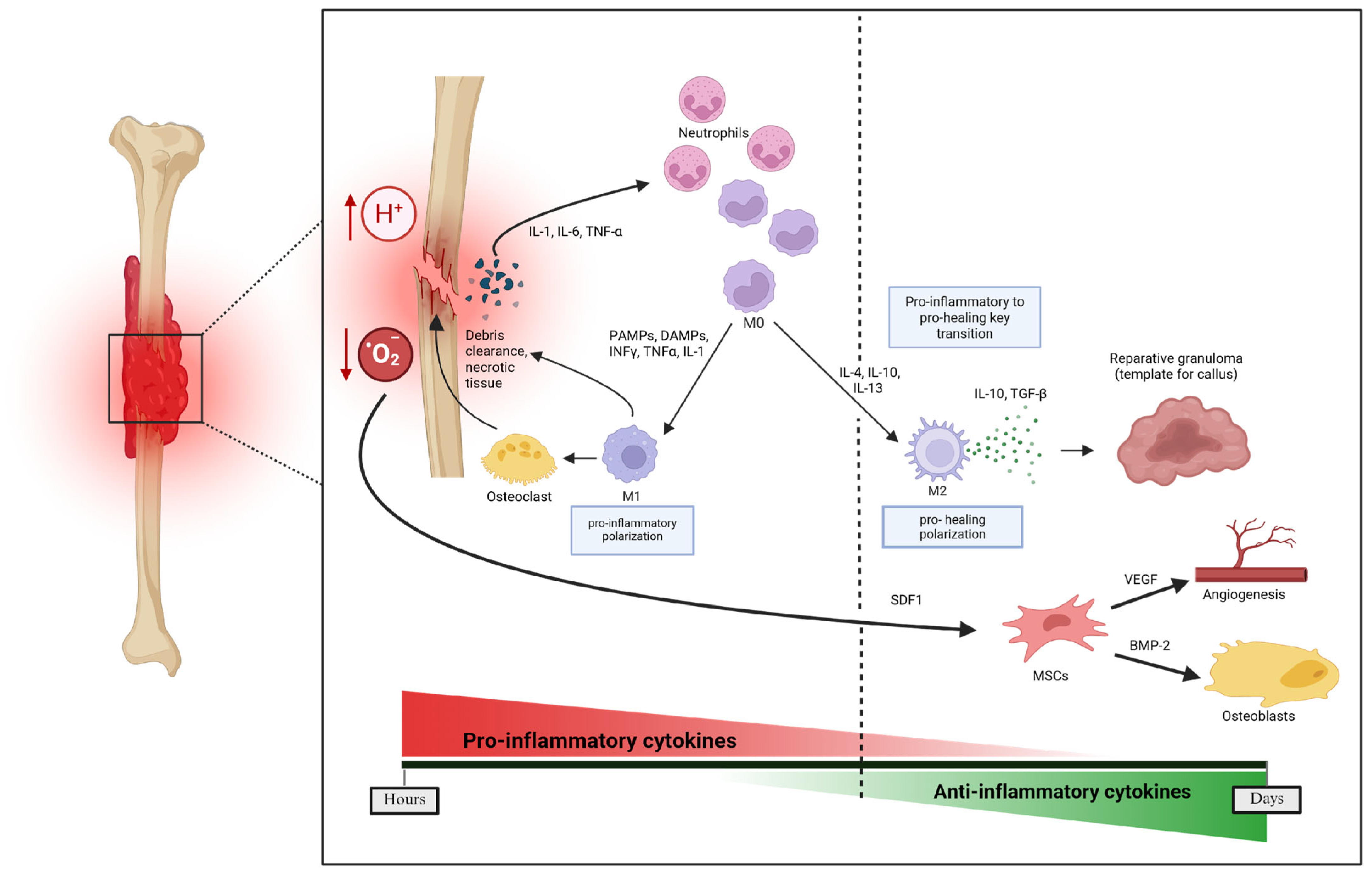
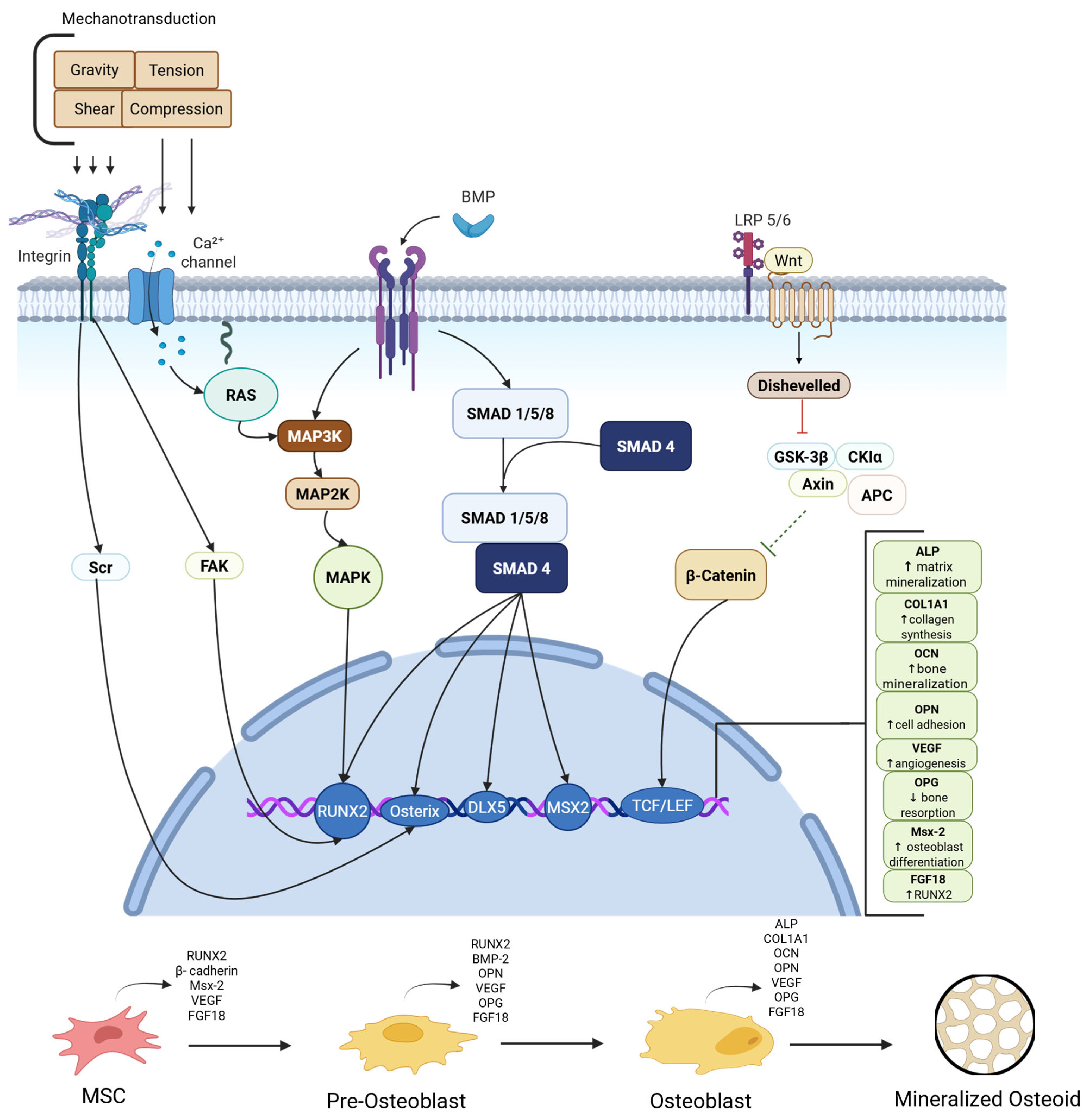
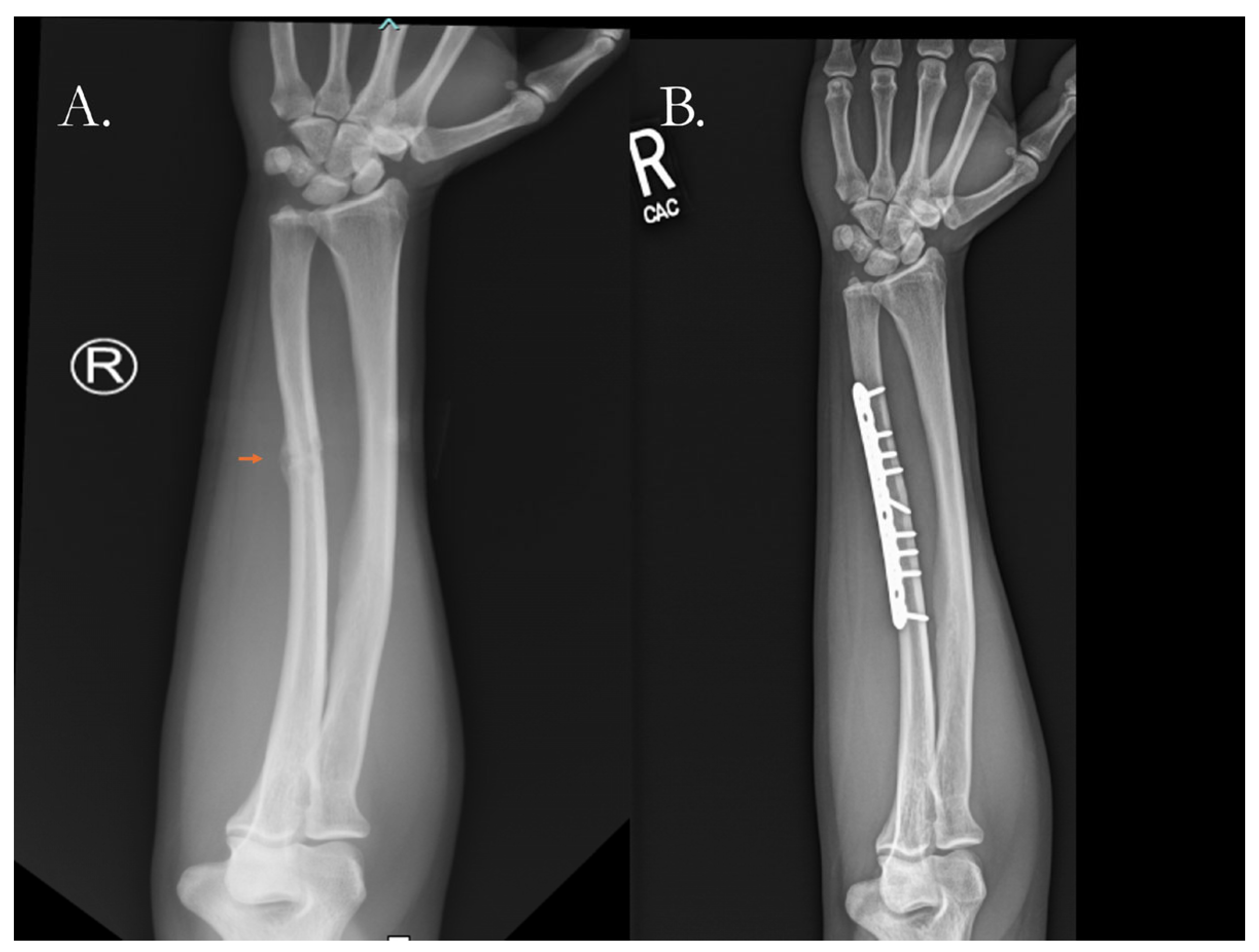
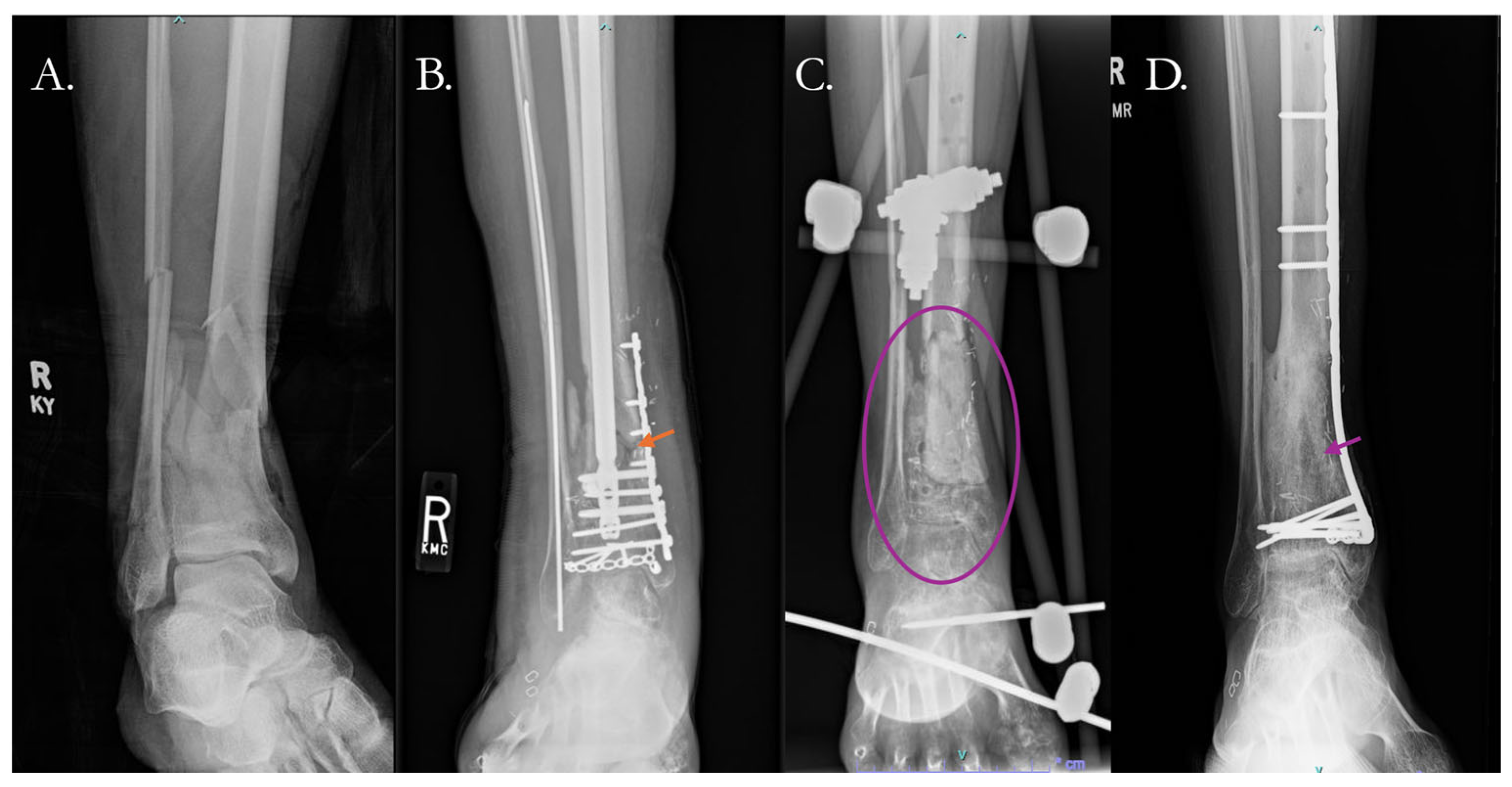
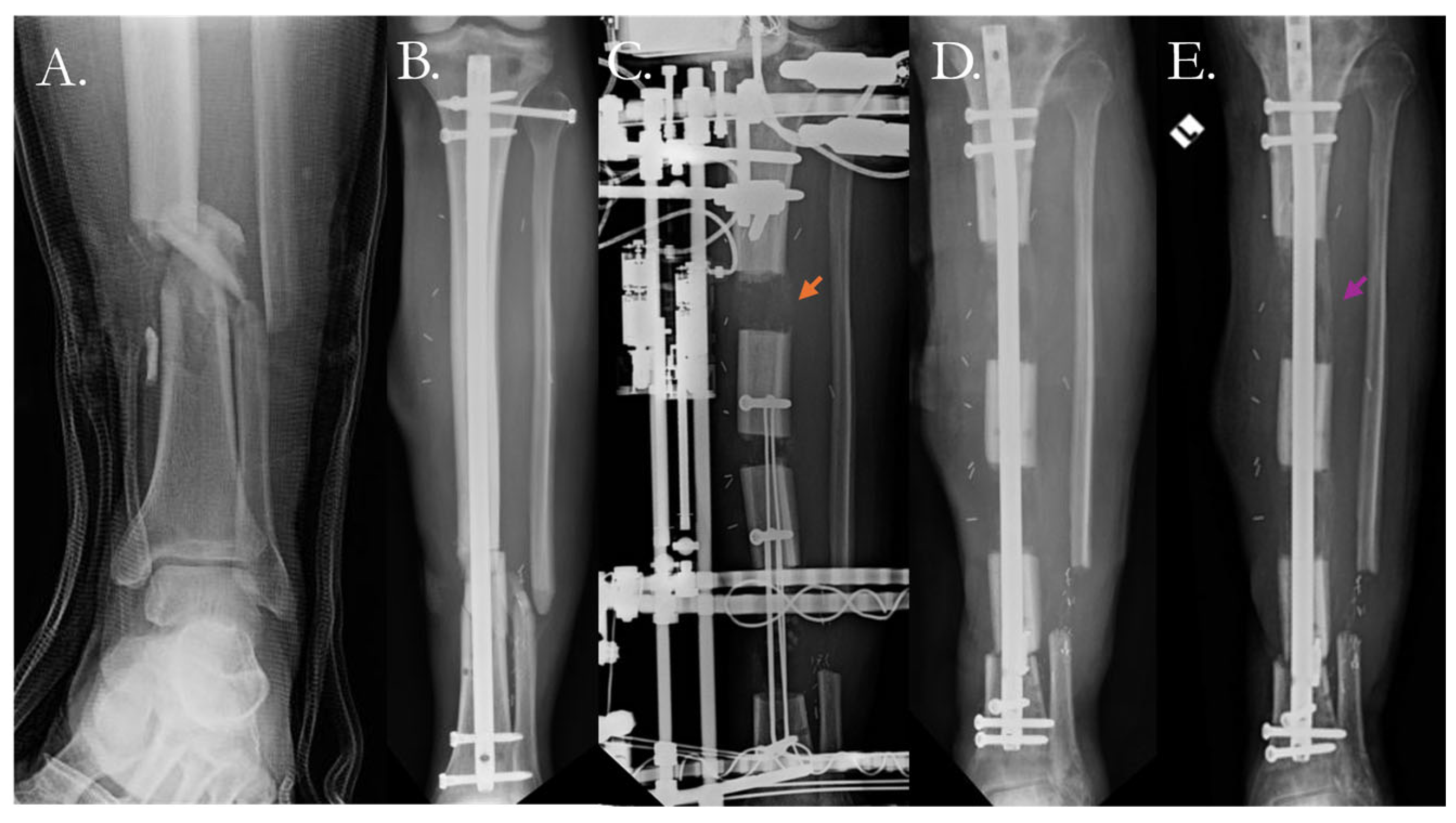
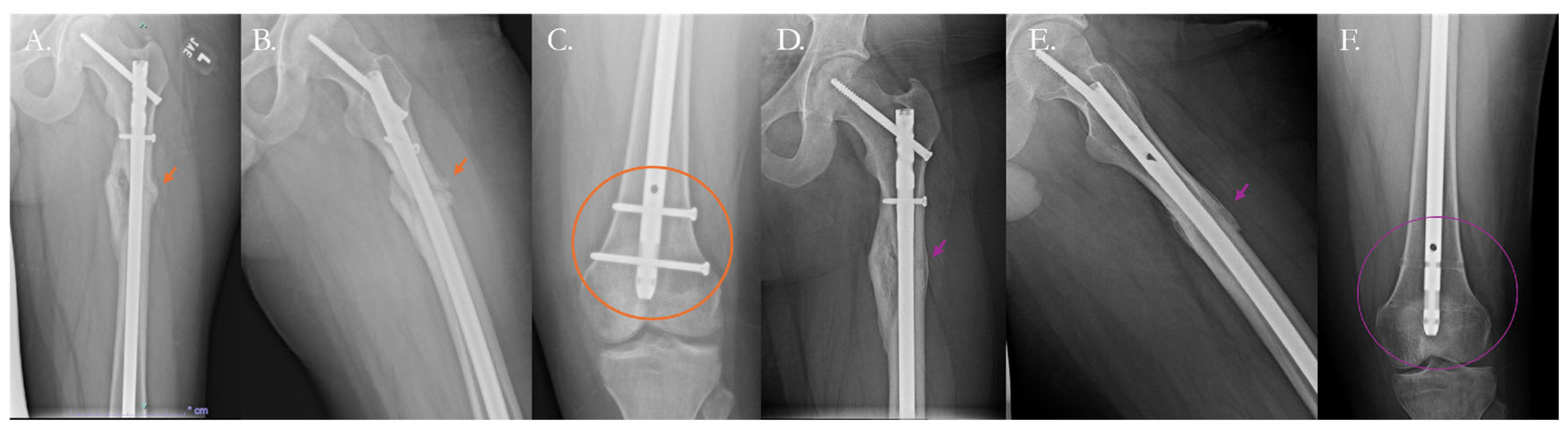
| Study (Year) | Population/Model | Intervention | Comparator | N | Outcomes | Safety Notes | Reference |
|---|---|---|---|---|---|---|---|
| Clinical Studies | |||||||
| Mazzotta et al. (2021) | Retrospective cohort of upper-limb aseptic nonunions | BMAC and PRF applied on lyophilized bone chip or bone graft following fixation | Fixation without addition of PRP/BMAC | 45 | Accelerated healing processes of lesions up to 6 cm in the upper limb | No adverse reaction reported | [89] |
| Laubach (2022) | Prospective pilot of large lower extremity bone defects | Patient specific 3D printed mPCL-TCP scaffolds with autologous bone graft | None | 4 | Three of the cases showed evidence of bone formation/bony fusion and were weight bearing within 9 months of scaffold placement. One case, which also included the use of BMP2, achieved bony fusion and underwent hardware removal | No adverse reaction reported | [90] |
| Xie (2022) | Meta-analysis of long bone nonunions | Autologous bone graft with rhBMP | Autologous bone graft alone | 394 | While the combination of ABG with rhBMP demonstrated better postoperative limb function compared to ABG alone, it did not result in improved healing times or postoperative pain. | No adverse reaction reported | [91] |
| Choi et al. (2024) | Prospective case series of long bone nonunion or bone defect | rhBMP-2 combined with autologous bone and hydroxyapatite carrier granules | None | 24 | All patients achieved union at 12 months | Reported that no adverse effects or development of BMP2 antibodies were observed. | [92] |
| Moyal (2024) | Systematic review of long bone nonunion or delayed union | Bone marrow aspirate (BM) and bone marrow aspirate concentrate (BMAC) | Scaffolds (porous collagen + bovine fibrillar collagen, demineralized bone matrix (DBM) or DBM composite, allogenic graft, bioactive glass, iliac bone autograft) | 25 studies (encompassing approximately 580 patients) | BM and BMAC can lead to satisfactory union rats but the data is heterogeneous and higher quality studies are needed to understand BM/BMAC efficacy in relation to nonunion | Studies included 2 deep wound infection and 1 heterotopic ossification | [93] |
| Zhu (2024) | Umbrella meta-analysis of nonunion and delayed union | PRP | Any control group | 5 meta-analyses (13 studies, 1362 patients) | As a whole, PRP used in the treatment of nonunion led to superior healing rates and improved healing time but when looking at individual studies PRP did not lead to improved healing rates but still led to improved healing time. | Adverse events found to be nonsignificant; Reported postoperative infections as adverse event following PRP | [94] |
| Tanavalee (2025) | Randomized controlled trial of patients >50 years old with pertrochanteric fractures undergoing surgical fixation | Teriparatide | Placebo | 50 | Teriparatide improved healing times of pertrochanteric fractures but did not lead to superior functional quality metrics (Harris hip score, time up and go test) or a significant difference in bone mineral density loss compared to the placebo. | No drug-related adverse events; Bruise at injection site at wk2/placebo group had skin itching around the injection area | [95] |
| Selected Preclinical Studies | |||||||
| Pelled (2015) | Minipigs with lumbar vertebrae critical-size cylindrical bone defect, 15 mm in depth and 4 mm in diameter | Allogeneic BMP6 producing mesenchymal stem cells in fibrin gel | Fibrin only | 6 | Higher bone regeneration with increased connectivity density and bone volume on microCT analysis. Significance was not reached, likely due to N = 3 per group. | No adverse reactions reported | [96] |
| Brunello (2020) | Systematic review | Bioceramics | Empty defect | 13 studies (6 in rats; 7 in rabbits) | Due to heterogeneity in protocols no meta-analysis could be performed. Higher healing proportion in treated defects | No adverse reactions reported | [97] |
| Liu (2021) | 6 sheep with femoral or humeral 8 or 13 mm defects | Interior or surface BMP2 coated Biomimetic Calcium Phosphate (BioCaP) Granules | Empty defect; autologous bone graft; Demineralized bone graft; BioCaP alone | 72 defects | Significantly greater bone volume and density in BMP2 BioCaP treated defects than empty defects, demineralized bone graft, and BioCaP alone; No differences between autologous or interior/surface BMP2 BioCP | No adverse reactions reported | [98] |
| DeBaun (2022) | 8mm critical sized rat femoral defect | PCL/TCP scaffold enveloped by BMP2 or PDGF or BMP2+PDGF microspheres | Empty defect or PMMA spacer followed by PCL/TCP scaffold | 40 | Significantly higher radiographic healing scores in BMP2 treated subjects; No differences between BMP2 versus BMP2+PDGF | 1/40 had a deep wound infection; No intervention-based safety concerns | [99] |
| De la Vega (2022) | 5mm critical sized rat femoral defect | Chemically modified mRNA encoding for BMP2 implanted on a collagen sponge | Collagen sponge containing rhBMP2 | 190 | cmRNA was faster as restoring mechanical strength and initiating bony bridging likely through endochondral ossification | No adverse reactions reported | [100] |
| da Rocha et al. (2023) | Paravertebral implantation in immunodeficient mice | Bone allograft with BMSC | β-TCP scaffold with and without BMSC; Bone allograft without BMSCs | 12 | Bone allograft with BMSC had significantly higher ALP activity and bone formation assessed via Alizarin red staining | No adverse reactions reported | [101] |
| Garot (2023) | 25mm sheep metatarsal defect | 3D printed polylactic acid scaffold with polyelectrolyte film coating delivering BMP2 | Scaffold without BMP2 | 24 | Cubic scaffolds with BMP2 (5/7 with full bridging) showed significantly improved bone formation than controls (0/4 with any bridging) and gyroid scaffold (3/7 with full bridging) on microCT with trends towards improved bone formation | No adverse reactions reported | [102] |
| Sun (2024) | 5mm critical sized rat femoral defect and 2cm critical sized beagle radial defect | Mesoporous bioactive glass (MBG) with deforoxime-induced hypoxia-mimicking scaffold with BMP2 embedded PEGylated poly | BMP2 loaded MBG | 12 rats; 12 beagles | Significantly higher vessel volume, bone volume percent, and bone mineral density with higher type I collagen versus type II collagen in the experimental rat group; Significantly higher bone volume percent (85% vs. 64%) and bone mineral density (1.3 vs. 0.9 g/cm3) in beagle group; Observed that BMP2 volume could be reduced to 1/10 using this system | High heterotopic ossification rates in the high (10 microgram) BMP2 dose groups versus low dose (1 microgram) (55% vs. 10%) | [103] |
| Li (2025) | 5 mm cynomolgus monkey ulnar defect | Romosozumab, a sclerostin antibody | Control vehicle | 22 | New bone volume and new bone area within the defect region were 118% and 105% greater, respectively | No adverse reactions reported | [104] |
| Intervention | Advantages | Limitations | References | |
|---|---|---|---|---|
| Clinical | ||||
| External Bone Growth Stimulation | - Noninvasive | - No proven clinical benefit in healing nonunion | [107,108,142,143] | |
| Bone grafting | - Can contain osteogenic and osteoinductive cells - Provides structural support and a scaffold for bone conduction | - Donor site morbidity - Limited availability | [144,145,146] | |
| Autologous Orthobiologics | - Contains important growth factors for osteoinduction - Relative ease of acquisition with low donor site morbidity | - Cost and availability - Does not provide structural support - Inconsistent growth factor and cell counts across harvests | [93,147,148] | |
| Recombinant Growth Factors | - Can be osteoinductive | - Difficulty in achieving local, therapeutic levels | [149,150] | |
| Preclinical | ||||
| Scaffolds | Bioceramics | - Mimics the mechanotransductive properties of the ECM - Provides a foundation for bone conduction - Its micropores facilitate angiogenesis, cell adhesion, and bone deposition | - Brittle and fragile on their own - No inherent osteoinduction - Nonideal resorption kinetics | [151,152,153,154] |
| Natural Polymers | - Resembles ECM - Low relative cost - Generally high biocompatibility and biodegradable | - Variability in release profiles of bioactive molecules, composition, and strength - Technological limitations in fabrication | [152,153,154,155,156] | |
| Hydrogels | - Can form to shape of defect site - Environment responsive - Elution dose and rates can be modulated to achieve therapeutic targets | - Do not provide structural support - Degradation products may not be biocompatible and induce a local tissue response | [152,153,154,157] | |
| Stem cells | - Differentiate into osteogenic cells directly to promote union - Overall favorable safety profile with limited adverse events | - Cost of manufacturing and testing - Potential for immunogenicity with allogeneic cell therapy - Risk of oncogenesis - May require additional factors to promote union | [158,159,160,161] | |
| Gene therapy | - Allows for sustained, local delivery of key osteoinductive proteins - Limited off target effects - Potential for synergistic multi-gene delivery to optimize healing | - Risk of immunogenicity and resulting impairment of efficacy - Risk of mutagenesis - High regulatory burden for clinical translation | [162,163,164,165] | |
| Combined therapy | - Combine the advantages of each therapy modality and reduce the limitations | - Cost - Optimal dosing and delivery mechanisms remain poorly defined | [166,167] | |
| Growth Factor | Mechanistic Target | Biologic Effects | References |
|---|---|---|---|
| VEGF | VEGF tyrosine kinase receptor; MAPK pathway; Endothelial cells | Angiogenesis; enhanced vascularization; improved graft survival | [171] |
| PDGF | PDGF tyrosine kinase receptor; MAPK pathway; Fibroblasts, smooth muscle cells, and glia cells | Fibroblast proliferation; wound healing; extracellular matrix production | [172] |
| PTH | PTH1 receptor (Gs/cAMP/PKA and PLC pathways); Osteoblasts and osteocytes | Intermittent dosing stimulates bone formation; improved callus strength; enhanced osteoblast differentiation and survival | [173,174,175,176,177] |
| DIPY | Adenosine receptor (A2A, A2B [ADORA2A/ADORA2B]) signaling; inhibits adenosine reuptake leading to osteoblast activation | Promotes osteogenesis; enhanced bone regeneration around scaffolds and implants; reduces osteoclast activity | [148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180] |
| FGF | (FGFR1–4); RAS/MAPK and STAT signaling pathways; Osteoblasts, chondrocytes, endothelial cells | Promotes angiogenesis and vascularization, osteogenesis through osteoblast differentiation; enhances chondrogenesis and endochondral ossification | [181] |
| TNF-alpha | TNFR1/TNFR2 (TNFRSF1A/TNFRSF1B) activates NF-kB and MAPK pathway; Immune regulation and MSC recruitment | Promotes early regulation of the initial inflammatory phase of fracture healing and MSC migration; Enhances osteoblast differentiation | [182] |
| Material | Pore Size (μm) | Strength (MPa) | Degradation Kinetics | Notes | Reference |
|---|---|---|---|---|---|
| Bone (cortical) | minimum >100 | 100–200 | Not applicable | Ideal material properties of therapeutic scaffolds may differ from these ranges depending on mechanism of effect | [231] |
| Calcium-Phosphate ceramics | 250–350 | 100–350 | >95% at 90 days | Variability in measures dependent on composite scaffold design | [232,233] |
| Synthetic Polymers | Engineering allows for modifiable pore sizes | 35–2300 | variable, ranging from 1 to >24 months | PLA/PCL fibers much stronger with longer degradation times versus PLGA/PGA | [234,235] |
| Natural Polymers | 50–400 | 0.05–60 | variable, average range from 1 to 3 months | Silk fibroin and chitosan are stronger with longer degradation times versus hyaluronic acid, gelatin, aliginate, and collagen-based polymers | [236,237,238] |
| Hydrogels | 0.01–100 | 10–90 | days to ~8 weeks | Generally low structural support and longevity unless modified with embedded synthetic polymers | [236,237,239,240,241,242,243,244] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wier, J.; Shelby, H.; Bergren, S.; Patterson, J.T.; Lieberman, J.R. Modern Approaches and Emerging Biological Therapies to Treat Fracture Nonunion. Pharmaceutics 2025, 17, 1457. https://doi.org/10.3390/pharmaceutics17111457
Wier J, Shelby H, Bergren S, Patterson JT, Lieberman JR. Modern Approaches and Emerging Biological Therapies to Treat Fracture Nonunion. Pharmaceutics. 2025; 17(11):1457. https://doi.org/10.3390/pharmaceutics17111457
Chicago/Turabian StyleWier, Julian, Hannah Shelby, Sarah Bergren, Joseph T. Patterson, and Jay R. Lieberman. 2025. "Modern Approaches and Emerging Biological Therapies to Treat Fracture Nonunion" Pharmaceutics 17, no. 11: 1457. https://doi.org/10.3390/pharmaceutics17111457
APA StyleWier, J., Shelby, H., Bergren, S., Patterson, J. T., & Lieberman, J. R. (2025). Modern Approaches and Emerging Biological Therapies to Treat Fracture Nonunion. Pharmaceutics, 17(11), 1457. https://doi.org/10.3390/pharmaceutics17111457








