The Aptamer bi-(AID-1-T) Synergizes with Radiation to Inhibit Proliferation of Human Glioma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Treatment with Aptamers
2.3. Cell Proliferation Assessment by MTS Assay
2.4. Cell Migration Assessment by Transwell Inserts
2.5. Immunocytochemistry (ICC)
2.6. Radiation
2.7. Transcriptome Analysis
- STAR (version 2.7.11) was used to index the GRCh38.p14 reference genome assembly (NCBI RefSeq GCF_000001405.40) and align the RNA-seq data.
- Gene expression levels were assessed using HTSeq (v. 2.0.5).
- Differential expression analysis was performed using DESeq2 (version 1.44.0).
- Over-representation analysis (ORA) for gene ontology terms was performed using the ClusterProfiler package (version 4.12.0) in R.
2.8. Statistics
3. Results
3.1. The Effect of Combined Radiotherapy and bi-(AID-1-T) Aptamer Treatment on the Proliferative and Migration Potential of High-Grade Human Glioma Cell Cultures
3.2. Transcriptome Analysis of Human Glioblastoma G01 Cell Culture After Exposure to 20 Gy Radiation and bi-(AID-1-T) Aptamer
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weller, M.; Wick, W.; Aldape, K.; Brada, M.; Berger, M.; Pfister, S.M.; Nishikawa, R.; Rosenthal, M.; Wen, P.Y.; Stupp, R.; et al. Glioma. Nat. Rev. Dis. Primers 2015, 1, 15017. [Google Scholar] [CrossRef]
- Grochans, S.; Cybulska, A.M.; Simińska, D.; Korbecki, J.; Kojder, K.; Chlubek, D.; Baranowska-Bosiacka, I. Epidemiology of Glioblastoma Multiforme. Cancers 2022, 14, 2412. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015–2019. Neuro Oncol. 2022, 24, v1–v95. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-A.; Chang, C.-Y.; Hsueh, K.-W.; Su, H.-L.; Chiou, T.-W.; Lin, S.-Z.; Harn, H.-J. Migration/Invasion of Malignant Gliomas and Implications for Therapeutic Treatment. Int. J. Mol. Sci. 2018, 19, 1115. [Google Scholar] [CrossRef] [PubMed]
- Goenka, A.; Tiek, D.; Song, X.; Huang, T.; Hu, B.; Cheng, S.-Y. The Many Facets of Therapy Resistance and Tumor Recurrence in Glioblastoma. Cells 2021, 10, 484. [Google Scholar] [CrossRef]
- Cuddapah, V.A.; Robel, S.; Watkins, S.; Sontheimer, H. A Neurocentric Perspective on Glioma Invasion. Nat. Rev. Neurosci. 2014, 15, 455–465. [Google Scholar] [CrossRef]
- Qi, X.T.; Zhan, J.S.; Xiao, L.M.; Li, L.; Xu, H.X.; Fu, Z.B.; Zhang, Y.H.; Zhang, J.; Jia, X.H.; Ge, G.; et al. The Unwanted Cell Migration in the Brain: Glioma Metastasis. Neurochem. Res. 2017, 42, 1847–1863. [Google Scholar] [CrossRef]
- Tsuji, Y.; Nonoguchi, N.; Okuzaki, D.; Wada, Y.; Motooka, D.; Hirota, Y.; Toho, T.; Yoshikawa, N.; Furuse, M.; Kawabata, S.; et al. Chronic Pathophysiological Changes in the Normal Brain Parenchyma Caused by Radiotherapy Accelerate Glioma Progression. Sci. Rep. 2021, 11, 22110. [Google Scholar] [CrossRef]
- Merrick, M.; Mimlitz, M.J.; Weeder, C.; Akhter, H.; Bray, A.; Walther, A.; Nwakama, C.; Bamesberger, J.; Djam, H.; Abid, K.; et al. In Vitro Radiotherapy and Chemotherapy Alter Migration of Brain Cancer Cells before Cell Death. Biochem. Biophys. Rep. 2021, 27, 101071. [Google Scholar] [CrossRef]
- Brennan, C.W.; Verhaak, R.G.W.; McKenna, A.; Campos, B.; Noushmehr, H.; Salama, S.R.; Zheng, S.; Chakravarty, D.; Sanborn, J.Z.; Berman, S.H.; et al. The Somatic Genomic Landscape of Glioblastoma. Cell 2013, 155, 462–477. [Google Scholar] [CrossRef]
- Pouyan, A.; Ghorbanlo, M.; Eslami, M.; Jahanshahi, M.; Ziaei, E.; Salami, A.; Mokhtari, K.; Shahpasand, K.; Farahani, N.; Meybodi, T.E.; et al. Glioblastoma Multiforme: Insights into Pathogenesis, Key Signaling Pathways, and Therapeutic Strategies. Mol. Cancer 2025, 24, 58. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Dutra, A.; Pak, E.; Labrie, J.E.; Gerstein, R.M.; Pandolfi, P.P.; Recht, L.D.; Ross, A.H. EGFRvIII Expression and PTEN Loss Synergistically Induce Chromosomal Instability and Glial Tumors. Neuro Oncol. 2009, 11, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhou, H.; Fu, M.; Xu, H.; Huang, H.; Zhong, M.; Zhang, M.; Hua, W.; Lv, K.; Zhu, G. TMEM64 Aggravates the Malignant Phenotype of Glioma by Activating the Wnt/β-Catenin Signaling Pathway. Int. J. Biol. Macromol. 2024, 260, 129332. [Google Scholar] [CrossRef]
- Wu, X.; Fu, M.; Ge, C.; Zhou, H.; Huang, H.; Zhong, M.; Zhang, M.; Xu, H.; Zhu, G.; Hua, W.; et al. M6A-Mediated Upregulation of LncRNA CHASERR Promotes the Progression of Glioma by Modulating the MiR-6893-3p/TRIM14 Axis. Mol. Neurobiol. 2024, 61, 5418–5440. [Google Scholar] [CrossRef]
- Zeng, W.-J.; Zhang, L.; Cao, H.; Li, D.; Zhang, H.; Xia, Z.; Peng, R. A Novel Inflammation-Related LncRNAs Prognostic Signature Identifies LINC00346 in Promoting Proliferation, Migration, and Immune Infiltration of Glioma. Front. Immunol. 2022, 13, 810572. [Google Scholar] [CrossRef]
- Li, F.; Zhou, K.; Gao, L.; Zhang, B.; Li, W.; Yan, W.; Song, X.; Yu, H.; Wang, S.; Yu, N.; et al. Radiation Induces the Generation of Cancer Stem Cells: A Novel Mechanism for Cancer Radioresistance. Oncol. Lett. 2016, 12, 3059–3065. [Google Scholar] [CrossRef]
- Auffinger, B.; Spencer, D.; Pytel, P.; Ahmed, A.U.; Lesniak, M.S. The Role of Glioma Stem Cells in Chemotherapy Resistance and Glioblastoma Multiforme Recurrence. Expert Rev. Neurother. 2015, 15, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Ramón y Cajal, S.; Sesé, M.; Capdevila, C.; Aasen, T.; De Mattos-Arruda, L.; Diaz-Cano, S.J.; Hernández-Losa, J.; Castellví, J. Clinical Implications of Intratumor Heterogeneity: Challenges and Opportunities. J. Mol. Med. 2020, 98, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Qazi, M.A.; Vora, P.; Venugopal, C.; Sidhu, S.S.; Moffat, J.; Swanton, C.; Singh, S.K. Intratumoral Heterogeneity: Pathways to Treatment Resistance and Relapse in Human Glioblastoma. Ann. Oncol. 2017, 28, 1448–1456. [Google Scholar] [CrossRef]
- Shi, Z.; Hu, C.; Zheng, X.; Sun, C.; Li, Q. Feedback Loop between Hypoxia and Energy Metabolic Reprogramming Aggravates the Radioresistance of Cancer Cells. Exp. Hematol. Oncol. 2024, 13, 55. [Google Scholar] [CrossRef]
- Bouleftour, W.; Rowinski, E.; Louati, S.; Sotton, S.; Wozny, A.-S.; Moreno-Acosta, P.; Mery, B.; Rodriguez-Lafrasse, C.; Magne, N. A Review of the Role of Hypoxia in Radioresistance in Cancer Therapy. Med. Sci. Monit. 2021, 27, e934116. [Google Scholar] [CrossRef]
- Li, Z.; Bao, S.; Wu, Q.; Wang, H.; Eyler, C.; Sathornsumetee, S.; Shi, Q.; Cao, Y.; Lathia, J.; McLendon, R.E.; et al. Hypoxia-Inducible Factors Regulate Tumorigenic Capacity of Glioma Stem Cells. Cancer Cell 2009, 15, 501–513. [Google Scholar] [CrossRef]
- Menegakis, A.; Klompmaker, R.; Vennin, C.; Arbusà, A.; Damen, M.; van den Broek, B.; Zips, D.; van Rheenen, J.; Krenning, L.; Medema, R.H. Resistance of Hypoxic Cells to Ionizing Radiation Is Mediated in Part via Hypoxia-Induced Quiescence. Cells 2021, 10, 610. [Google Scholar] [CrossRef] [PubMed]
- Valable, S.; Gérault, A.N.; Lambert, G.; Leblond, M.M.; Anfray, C.; Toutain, J.; Bordji, K.; Petit, E.; Bernaudin, M.; Pérès, E.A. Impact of Hypoxia on Carbon Ion Therapy in Glioblastoma Cells: Modulation by LET and Hypoxia-Dependent Genes. Cancers 2020, 12, 2019. [Google Scholar] [CrossRef]
- Mahabir, R.; Tanino, M.; Elmansuri, A.; Wang, L.; Kimura, T.; Itoh, T.; Ohba, Y.; Nishihara, H.; Shirato, H.; Tsuda, M.; et al. Sustained Elevation of Snail Promotes Glial-Mesenchymal Transition after Irradiation in Malignant Glioma. Neuro Oncol. 2014, 16, 671–685. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Hong, Y.; Lee, S.; Liu, P.; Lim, J.; Lee, Y.; Lee, T.; Chang, K.; Hong, Y. Therapeutic Implications for Overcoming Radiation Resistance in Cancer Therapy. Int. J. Mol. Sci. 2015, 16, 26880–26913. [Google Scholar] [CrossRef]
- Walker, A.J.; Ruzevick, J.; Malayeri, A.A.; Rigamonti, D.; Lim, M.; Redmond, K.J.; Kleinberg, L. Postradiation Imaging Changes in the CNS: How Can We Differentiate Between Treatment Effect and Disease Progression? Future Oncol. 2014, 10, 1277–1297. [Google Scholar] [CrossRef]
- Carlos-Reyes, A.; Muñiz-Lino, M.A.; Romero-Garcia, S.; López-Camarillo, C.; Hernández-de la Cruz, O.N. Biological Adaptations of Tumor Cells to Radiation Therapy. Front. Oncol. 2021, 11, 718636. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Xiang, J. Aptamers, the Nucleic Acid Antibodies, in Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 2793. [Google Scholar] [CrossRef]
- Morita, Y.; Leslie, M.; Kameyama, H.; Volk, D.; Tanaka, T. Aptamer Therapeutics in Cancer: Current and Future. Cancers 2018, 10, 80. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, L.; Liang, Z.; Kou, Z.; Chen, Y.; Shi, G.; Li, X.; Liang, Y.; Wang, F.; Shi, Y. Effects of Aptamer to U87-EGFRvIII Cells on the Proliferation, Radiosensitivity, and Radiotherapy of Glioblastoma Cells. Mol. Ther. Nucleic Acids 2018, 10, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, P.; Ma, J.; Li, D.; Yang, H.; Chen, W.; Jiang, Y. Enhancement of Radiosensitization by Silver Nanoparticles Functionalized with Polyethylene Glycol and Aptamer As1411 for Glioma Irradiation Therapy. Int. J. Nanomed. 2019, 14, 9483–9496. [Google Scholar] [CrossRef]
- Cesarini, V.; Scopa, C.; Silvestris, D.A.; Scafidi, A.; Petrera, V.; Del Baldo, G.; Gallo, A. Aptamer-Based In Vivo Therapeutic Targeting of Glioblastoma. Molecules 2020, 25, 4267. [Google Scholar] [CrossRef]
- Pavlova, S.; Fab, L.; Dzarieva, F.; Ryabova, A.; Revishchin, A.; Panteleev, D.; Antipova, O.; Usachev, D.; Kopylov, A.; Pavlova, G. Unite and Conquer: Association of Two G-Quadruplex Aptamers Provides Antiproliferative and Antimigration Activity for Cells from High-Grade Glioma Patients. Pharmaceuticals 2024, 17, 1435. [Google Scholar] [CrossRef]
- Pavlova, S.; Fab, L.; Savchenko, E.; Ryabova, A.; Ryzhova, M.; Revishchin, A.; Pronin, I.; Usachev, D.; Kopylov, A.; Pavlova, G. The Bi-(AID-1-T) G-Quadruplex Has a Janus Effect on Primary and Recurrent Gliomas: Anti-Proliferation and Pro-Migration. Pharmaceuticals 2024, 17, 74. [Google Scholar] [CrossRef]
- Golovin, A.; Dzarieva, F.; Rubetskaya, K.; Shamadykova, D.; Usachev, D.; Pavlova, G.; Kopylov, A. In Silico Born Designed Anti-EGFR Aptamer Gol1 Has Anti-Proliferative Potential for Patient Glioblastoma Cells. Int. J. Mol. Sci. 2025, 26, 1072. [Google Scholar] [CrossRef]
- Roxo, C.; Kotkowiak, W.; Pasternak, A. G-Quadruplex-Forming Aptamers—Characteristics, Applications, and Perspectives. Molecules 2019, 24, 3781. [Google Scholar] [CrossRef]
- Carvalho, J.; Mergny, J.-L.; Salgado, G.F.; Queiroz, J.A.; Cruz, C. G-Quadruplex, Friend or Foe: The Role of the G-Quartet in Anticancer Strategies. Trends Mol. Med. 2020, 26, 848–861. [Google Scholar] [CrossRef]
- Murnyák, B.; Kouhsari, M.C.; Hershkovitch, R.; Kálmán, B.; Marko-Varga, G.; Klekner, Á.; Hortobágyi, T. PARP1 Expression and Its Correlation with Survival Is Tumour Molecular Subtype Dependent in Glioblastoma. Oncotarget 2017, 8, 46348–46362. [Google Scholar] [CrossRef] [PubMed]
- Schiewer, M.J.; Knudsen, K.E. Transcriptional Roles of PARP1 in Cancer. Mol. Cancer Res. 2014, 12, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Legatova, V.; Samoylenkova, N.; Arutyunyan, A.; Tashlitsky, V.; Zavyalova, E.; Usachev, D.; Pavlova, G.; Kopylov, A. Covalent Bi-Modular Parallel and Antiparallel G-Quadruplex DNA Nanocostructs Reduce Viability of Patient Glioma Primary Cell Cultures. Int. J. Mol. Sci. 2021, 22, 3372. [Google Scholar] [CrossRef]
- Antipova, O.; Samoylenkova, N.; Savchenko, E.; Zavyalova, E.; Revishchin, A.; Pavlova, G.; Kopylov, A. Bimodular Antiparallel G-Quadruplex Nanoconstruct with Antiproliferative Activity. Molecules 2019, 24, 3625. [Google Scholar] [CrossRef]
- Kopylov, A.M.; Samoylenkova, N.; Bizayeva, A.; Arutyunyan, A.; Tashlitsky, V.; Golbin, D.; Usachev, D.; Pavlova, G. P13.19 Bi-Modular G-Quadruplex DNA-Crypto-Aptamers Diminish Viability of Glioma Primary Cell Cultures of Patients. Neuro Oncol. 2021, 23, ii36–ii37. [Google Scholar] [CrossRef]
- Lan, X.-Y.; Kalkowski, L.; Chu, C.-Y.; Jablonska, A.; Li, S.; Kai, M.; Gao, Y.; Janowski, M.; Walczak, P. Unlocking the Potential of Ultra-High Dose Fractionated Radiation for Effective Treatment of Glioblastoma in Mice. J. Cancer 2024, 15, 4060–4071. [Google Scholar] [CrossRef]
- Waqar, M.; Trifiletti, D.M.; McBain, C.; O’Connor, J.; Coope, D.J.; Akkari, L.; Quinones-Hinojosa, A.; Borst, G.R. Early Therapeutic Interventions for Newly Diagnosed Glioblastoma: Rationale and Review of the Literature. Curr. Oncol. Rep. 2022, 24, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, D.; Subham, S.; Jeppson, J.D.; Aguilar, B.; Wong, R.A.; Hibbard, J.C.; Hui, S.; Wong, J.Y.C.; Forman, S.J.; Alizadeh, D.; et al. Evaluation of the Immunomodulatory Effects of Radiation for Chimeric Antigen Receptor T Cell Therapy in Glioblastoma Multiforme. Cells 2024, 13, 1075. [Google Scholar] [CrossRef]
- Liljedahl, E.; Konradsson, E.; Linderfalk, K.; Gustafsson, E.; Petersson, K.; Ceberg, C.; Redebrandt, H.N. Comparable Survival in Rats with Intracranial Glioblastoma Irradiated with Single-Fraction Conventional Radiotherapy or FLASH Radiotherapy. Front. Oncol. 2024, 13, 1309174. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hormuth, D.A.; Yang, J.; Yankeelov, T.E. A Multi-Compartment Model of Glioma Response to Fractionated Radiation Therapy Parameterized via Time-Resolved Microscopy Data. Front. Oncol. 2022, 12, 811415. [Google Scholar] [CrossRef]
- Oprita, A.; Baloi, S.-C.; Staicu, G.-A.; Alexandru, O.; Tache, D.E.; Danoiu, S.; Micu, E.S.; Sevastre, A.-S. Updated Insights on EGFR Signaling Pathways in Glioma. Int. J. Mol. Sci. 2021, 22, 587. [Google Scholar] [CrossRef]
- Saadeh, F.S.; Mahfouz, R.; Assi, H.I. EGFR as a Clinical Marker in Glioblastomas and Other Gliomas. Int. J. Biol. Markers 2018, 33, 22–32. [Google Scholar] [CrossRef]
- Huang, P.H.; Xu, A.M.; White, F.M. Oncogenic EGFR Signaling Networks in Glioma. Sci. Signal 2009, 2, re6. [Google Scholar] [CrossRef]
- Rodriguez, S.M.B.; Kamel, A.; Ciubotaru, G.V.; Onose, G.; Sevastre, A.-S.; Sfredel, V.; Danoiu, S.; Dricu, A.; Tataranu, L.G. An Overview of EGFR Mechanisms and Their Implications in Targeted Therapies for Glioblastoma. Int. J. Mol. Sci. 2023, 24, 11110. [Google Scholar] [CrossRef]
- Moses-Gardner, A.; Guo, P.; Moses, M.; Smith, E. ITGA-2 Is a Novel Therapeutic Target for Glioblastoma and High-Grade Gliomas. Cancer Res. 2018, 78, 2914. [Google Scholar] [CrossRef]
- Ren, Y.; Xu, J.; Zhang, Z.; Yu, R. ITGA1 Promotes Glioma Cell Proliferation and Affects Immune Cell Infiltration in Low-Grade Glioma. Mediat. Inflamm. 2024, 2024, 6147483. [Google Scholar] [CrossRef]
- Ren, D.; Zhao, J.; Sun, Y.; Li, D.; Meng, Z.; Wang, B.; Fan, P.; Liu, Z.; Jin, X.; Wu, H. Overexpressed ITGA2 Promotes Malignant Tumor Aggression by Up-Regulating PD-L1 Expression through the Activation of the STAT3 Signaling Pathway. J. Exp. Clin. Cancer Res. 2019, 38, 485. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Moses-Gardner, A.; Huang, J.; Smith, E.R.; Moses, M.A. ITGA2 as a Potential Nanotherapeutic Target for Glioblastoma. Sci. Rep. 2019, 9, 6195. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.M.; Shi, D.M.; Dai, Q.; Cheng, X.J.; Yao, W.Y.; Sun, P.H.; Ding, Y.F.; Qiao, M.M.; Wu, Y.L.; Jiang, S.H.; et al. Tumor Suppressor XAF1 Induces Apoptosis, Inhibits Angiogenesis and Inhibits Tumor Growth in Hepatocellular Carcinoma. Oncotarget 2014, 5, 5403–5415. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, Q.; Berglund, A.E.; Wang, D.; MacAulay, R.J.; Mulé, J.J.; Etame, A.B. Paradoxical Epigenetic Regulation of XAF1 Mediates Plasticity towards Adaptive Resistance Evolution in MGMT-Methylated Glioblastoma. Sci. Rep. 2019, 9, 14072. [Google Scholar] [CrossRef]
- Jeong, S.-I.; Kim, J.-W.; Ko, K.-P.; Ryu, B.-K.; Lee, M.-G.; Kim, H.-J.; Chi, S.-G. XAF1 Forms a Positive Feedback Loop with IRF-1 to Drive Apoptotic Stress Response and Suppress Tumorigenesis. Cell Death Dis. 2018, 9, 806. [Google Scholar] [CrossRef]
- Lee, M.; Ko, K.-P.; Chi, S.-G. XAF1 Enhances Temozolomide Induced Autophagic Cell Death through AMPK Signaling Pathway. Ann. Oncol. 2019, 30, v12. [Google Scholar] [CrossRef]
- Wang, X.; Liao, Z.; Bai, Z.; He, Y.; Duan, J.; Wei, L. MiR-93-5p Promotes Cell Proliferation through Down-Regulating PPARGC1A in Hepatocellular Carcinoma Cells by Bioinformatics Analysis and Experimental Verification. Genes 2018, 9, 51. [Google Scholar] [CrossRef]
- Egashira, S.; Jinnin, M.; Ajino, M.; Shimozono, N.; Okamoto, S.; Tasaki, Y.; Hirano, A.; Ide, M.; Kajihara, I.; Aoi, J.; et al. Chronic Sun Exposure-Related Fusion Oncogenes EGFR-PPARGC1A in Cutaneous Squamous Cell Carcinoma. Sci. Rep. 2017, 7, 12654. [Google Scholar] [CrossRef] [PubMed]
- Martins, E.P.; Gonçalves, C.S.; Pojo, M.; Carvalho, R.; Ribeiro, A.S.; Miranda-Gonçalves, V.; Taipa, R.; Pardal, F.; Pinto, A.A.; Custódia, C.; et al. Cadherin-3 Is a Novel Oncogenic Biomarker with Prognostic Value in Glioblastoma. Mol. Oncol. 2022, 16, 2611–2631. [Google Scholar] [CrossRef] [PubMed]
- Noronha, C.; Ribeiro, A.S.; Taipa, R.; Castro, D.S.; Reis, J.; Faria, C.; Paredes, J. Cadherin Expression and EMT: A Focus on Gliomas. Biomedicines 2021, 9, 1328. [Google Scholar] [CrossRef]
- Martins, E.P.; Pojo, M.; Gonçalves, C.S.; Carvalho, R.; Ribeiro, A.S.; Pardal, F.; Pinto, A.A.; Sousa, N.; Paredes, J.; Costa, B.M. PO-105 CDH3/P-Cadherin as a Novel Biomarker in Glioblastoma: Functional and Prognostic Insights. ESMO Open 2018, 3, A61–A62. [Google Scholar] [CrossRef]
- Paredes, J.; Albergaria, A.; Oliveira, J.T.; Jerónimo, C.; Milanezi, F.; Schmitt, F.C. P-Cadherin Overexpression Is an Indicator of Clinical Outcome in Invasive Breast Carcinomas and Is Associated with CDH3 Promoter Hypomethylation. Clin. Cancer Res. 2005, 11, 5869–5877. [Google Scholar] [CrossRef]
- Kannan, K.; Coarfa, C.; Chao, P.-W.; Luo, L.; Wang, Y.; Brinegar, A.E.; Hawkins, S.M.; Milosavljevic, A.; Matzuk, M.M.; Yen, L. Recurrent BCAM-AKT2 Fusion Gene Leads to a Constitutively Activated AKT2 Fusion Kinase in High-Grade Serous Ovarian Carcinoma. Proc. Natl. Acad. Sci. USA 2015, 112, E1272–E1277. [Google Scholar] [CrossRef]
- Akiyama, H.; Iwahana, Y.; Suda, M.; Yoshimura, A.; Kogai, H.; Nagashima, A.; Ohtsuka, H.; Komiya, Y.; Tashiro, F. The FBI1/Akirin2 Target Gene, BCAM, Acts as a Suppressive Oncogene. PLoS ONE 2013, 8, e78716. [Google Scholar] [CrossRef]
- Burela, S.; He, M.; Trontzas, I.P.; Gavrielatou, N.; Schalper, K.A.; Langermann, S.; Flies, D.B.; Rimm, D.L.; Aung, T.N. BCAM (Basal Cell Adhesion Molecule) Protein Expression in Different Tumor Populations. Discov. Oncol. 2024, 15, 381. [Google Scholar] [CrossRef] [PubMed]
- Bartolini, A.; Cardaci, S.; Lamba, S.; Oddo, D.; Marchiò, C.; Cassoni, P.; Amoreo, C.A.; Corti, G.; Testori, A.; Bussolino, F.; et al. BCAM and LAMA5 Mediate the Recognition between Tumor Cells and the Endothelium in the Metastatic Spreading of KRAS-Mutant Colorectal Cancer. Clin. Cancer Res. 2016, 22, 4923–4933. [Google Scholar] [CrossRef]
- Kannan, M.B.; Solovieva, V.; Blank, V. The Small MAF Transcription Factors MAFF, MAFG and MAFK: Current Knowledge and Perspectives. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2012, 1823, 1841–1846. [Google Scholar] [CrossRef]
- Moon, E.J.; Mello, S.S.; Li, C.G.; Chi, J.-T.; Thakkar, K.; Kirkland, J.G.; Lagory, E.L.; Lee, I.J.; Diep, A.N.; Miao, Y.; et al. The HIF Target MAFF Promotes Tumor Invasion and Metastasis through IL11 and STAT3 Signaling. Nat. Commun. 2021, 12, 4308. [Google Scholar] [CrossRef]
- Yu, Z.; Chen, Y.; Chen, S.; Ye, W.; Li, R.; Fu, Y.; Chen, Y.; Fu, W.; Wei, X.; Yu, Q.; et al. Oligoadenylate Synthetase-like Aggravated Newcastle Disease Virus–Induced Necroptosis in Glioma Cells. Front. Oncol. 2025, 15, 1574214. [Google Scholar] [CrossRef]
- Li, S.-B.; Yuan, L.; Zhu, Q.-Y.; Zhang, G.; Liu, Y.-T.; Gao, M.-H.; Han, F.; Lin, Z.-R.; Zhang, H.; Tang, L.-Q.; et al. OASL Enhances MRNA Translation and Reprograms Lipid Metabolism to Promote Cancer Progression. Cell Rep. 2025, 44, 115901. [Google Scholar] [CrossRef]
- Xing, X.; Li, X.-Q.; Yin, S.-Q.; Ma, H.-T.; Xiao, S.-Y.; Tulamaiti, A.; Yang, Y.; Jiang, S.-H.; Hu, L.-P.; Zhang, Z.-G.; et al. OASL Promotes Immune Evasion in Pancreatic Ductal Adenocarcinoma by Enhancing Autolysosome-Mediated Degradation of MHC-I. Theranostics 2025, 15, 2104–2120. [Google Scholar] [CrossRef]
- Choi, U.Y.; Kang, J.-S.; Hwang, Y.S.; Kim, Y.-J. Oligoadenylate Synthase-like (OASL) Proteins: Dual Functions and Associations with Diseases. Exp. Mol. Med. 2015, 47, e144. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Makale, M.T.; McDonald, C.R.; Hattangadi-Gluth, J.A.; Kesari, S. Mechanisms of Radiotherapy-Associated Cognitive Disability in Patients with Brain Tumours. Nat. Rev. Neurol. 2017, 13, 52–64. [Google Scholar] [CrossRef]
- Greene-Schloesser, D.; Robbins, M.E.; Peiffer, A.M.; Shaw, E.G.; Wheeler, K.T.; Chan, M.D. Radiation-Induced Brain Injury: A Review. Front. Oncol. 2012, 2, 73. [Google Scholar] [CrossRef]
- Brandsma, D.; Stalpers, L.; Taal, W.; Sminia, P.; van den Bent, M.J. Clinical Features, Mechanisms, and Management of Pseudoprogression in Malignant Gliomas. Lancet Oncol. 2008, 9, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, F.H.; Pruitt, A. Assumptions in the Radiotherapy of Glioblastoma. Neurology 1980, 30, 907. [Google Scholar] [CrossRef]
- Liang, B.C.; Thornton, A.F.; Sandler, H.M.; Greenberg, H.S. Malignant Astrocytomas: Focal Tumor Recurrence after Focal External Beam Radiation Therapy. J. Neurosurg. 1991, 75, 559–563. [Google Scholar] [CrossRef]
- Chen, L.; Chaichana, K.L.; Kleinberg, L.; Ye, X.; Quinones-Hinojosa, A.; Redmond, K. Glioblastoma Recurrence Patterns near Neural Stem Cell Regions. Radiother. Oncol. 2015, 116, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma Stem Cells Promote Radioresistance by Preferential Activation of the DNA Damage Response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef]
- Eckerdt, F.; Platanias, L.C. Emerging Role of Glioma Stem Cells in Mechanisms of Therapy Resistance. Cancers 2023, 15, 3458. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Y.; Yu, T.-S.; McKay, R.M.; Burns, D.K.; Kernie, S.G.; Parada, L.F. A Restricted Cell Population Propagates Glioblastoma Growth after Chemotherapy. Nature 2012, 488, 522–526. [Google Scholar] [CrossRef]
- Shankar, A.; Kumar, S.; Iskander, A.; Varma, N.R.; Janic, B.; deCarvalho, A.; Mikkelsen, T.; Frank, J.A.; Ali, M.M.; Knight, R.A.; et al. Subcurative Radiation Significantly Increases Cell Proliferation, Invasion, and Migration of Primary Glioblastoma Multiforme In Vivo. Chin. J. Cancer 2014, 33, 148–158. [Google Scholar] [CrossRef]
- Multhoff, G.; Rödel, F.; Pockley, A.G.; Gaipl, U.S. Frontiers Research Topic: Radiation-Induced Effects and the Immune System. Front. Oncol. 2013, 3, 55. [Google Scholar] [CrossRef]
- Franco, M.S.; Raulefs, S.; Schilling, D.; Combs, S.E.; Schmid, T.E. Impact of Radiation on Invasion and Migration of Glioma In Vitro and In Vivo. Cancers 2024, 16, 3900. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Yoo, K.-C.; Cui, Y.-H.; Uddin, N.; Lim, E.-J.; Kim, M.-J.; Nam, S.-Y.; Kim, I.-G.; Suh, Y.; Lee, S.-J. Radiation Promotes Malignant Progression of Glioma Cells through HIF-1alpha Stabilization. Cancer Lett. 2014, 354, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Faustino, A.C.; Viani, G.A.; Hamamura, A.C. Patterns of Recurrence and Outcomes of Glioblastoma Multiforme Treated with Chemoradiation and Adjuvant Temozolomide. Clinics 2020, 75, e1553. [Google Scholar] [CrossRef]
- Liu, G.; Yuan, X.; Zeng, Z.; Tunici, P.; Ng, H.; Abdulkadir, I.R.; Lu, L.; Irvin, D.; Black, K.L.; Yu, J.S. Analysis of Gene Expression and Chemoresistance of CD133+ Cancer Stem Cells in Glioblastoma. Mol. Cancer 2006, 5, 67. [Google Scholar] [CrossRef]
- Singh, S.K.; Hawkins, C.; Clarke, I.D.; Squire, J.A.; Bayani, J.; Hide, T.; Henkelman, R.M.; Cusimano, M.D.; Dirks, P.B. Identification of Human Brain Tumour Initiating Cells. Nature 2004, 432, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Samatov, T.R.; Wicklein, D.; Tonevitsky, A.G. L1CAM: Cell Adhesion and More. Prog. Histochem. Cytochem. 2016, 51, 25–32. [Google Scholar] [CrossRef]
- Kiefel, H.; Bondong, S.; Hazin, J.; Ridinger, J.; Schirmer, U.; Riedle, S.; Altevogt, P. L1CAM: A Major Driver for Tumor Cell Invasion and Motility. Cell Adh Migr. 2012, 6, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Altevogt, P.; Doberstein, K.; Fogel, M. L1CAM in Human Cancer. Int. J. Cancer 2016, 138, 1565–1576. [Google Scholar] [CrossRef]
- Schäfer, M.K.E.; Altevogt, P. L1CAM Malfunction in the Nervous System and Human Carcinomas. Cell. Mol. Life Sci. 2010, 67, 2425–2437. [Google Scholar] [CrossRef]
- Kiefel, H.; Bondong, S.; Pfeifer, M.; Schirmer, U.; Erbe-Hoffmann, N.; Schäfer, H.; Sebens, S.; Altevogt, P. EMT-Associated up-Regulation of L1CAM Provides Insights into L1CAM-Mediated Integrin Signalling and NF-ΚB Activation. Carcinogenesis 2012, 33, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Sarji, M.; Ankawa, R.; Yampolsky, M.; Fuchs, Y. A near Death Experience: The Secret Stem Cell Life of Caspase-3. Semin. Cell Dev. Biol. 2025, 171, 103617. [Google Scholar] [CrossRef]
- Feng, X.; Zhu, F.; Dai, L.; Liu, X.; Shao, L.; Hao, L.; Cang, S.; Cheng, J. Caspase-3 in Glioma Indicates an Unfavorable Prognosis by Involving Surrounding Angiogenesis and Tumor Cell Repopulation. J. Neurooncol 2023, 163, 313–325. [Google Scholar] [CrossRef]
- An, S.; Song, I.H.; Woo, C.G. Diagnostic Value of Nestin Expression in Adult Gliomas. Int. J. Surg. Pathol. 2023, 31, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Naito, Z. Neuroepithelial Stem Cell Marker Nestin Regulates the Migration, Invasion and Growth of Human Gliomas. Oncol. Rep. 2011, 26, 91–99. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, H.; Hu, J.; Fu, H.; Qu, Y.; Yang, Y.; Cai, K.Q.; Efimov, A.; Wu, M.; Yen, T.; et al. Nestin Is Required for Spindle Assembly and Cell-Cycle Progression in Glioblastoma Cells. Mol. Cancer Res. 2021, 19, 1651–1665. [Google Scholar] [CrossRef]
- Senbanjo, L.T.; Chellaiah, M.A. CD44: A Multifunctional Cell Surface Adhesion Receptor Is a Regulator of Progression and Metastasis of Cancer Cells. Front. Cell Dev. Biol. 2017, 5, 18. [Google Scholar] [CrossRef]
- Mooney, K.L.; Choy, W.; Sidhu, S.; Pelargos, P.; Bui, T.T.; Voth, B.; Barnette, N.; Yang, I. The Role of CD44 in Glioblastoma Multiforme. J. Clin. Neurosci. 2016, 34, 1–5. [Google Scholar] [CrossRef]
- Shaalan, F.; Ballout, N.; Chamoun, W.T. Insights Into the Role of Bmi-1 Deregulation in Promoting Stemness and Therapy Resistance in Glioblastoma: A Narrative Review. Cancer Med. 2025, 14, e70566. [Google Scholar] [CrossRef]
- Cenci, T.; Martini, M.; Montano, N.; D’Alessandris, Q.G.; Falchetti, M.L.; Annibali, D.; Savino, M.; Bianchi, F.; Pierconti, F.; Nasi, S.; et al. Prognostic Relevance of C-Myc and BMI1 Expression in Patients With Glioblastoma. Am. J. Clin. Pathol. 2012, 138, 390–396. [Google Scholar] [CrossRef]
- Jiang, L.; Song, L.; Wu, J.; Yang, Y.; Zhu, X.; Hu, B.; Cheng, S.-Y.; Li, M. Bmi-1 Promotes Glioma Angiogenesis by Activating NF-ΚB Signaling. PLoS ONE 2013, 8, e55527. [Google Scholar] [CrossRef]
- Conde, M.; Michen, S.; Wiedemuth, R.; Klink, B.; Schröck, E.; Schackert, G.; Temme, A. Chromosomal Instability Induced by Increased BIRC5/Survivin Levels Affects Tumorigenicity of Glioma Cells. BMC Cancer 2017, 17, 889. [Google Scholar] [CrossRef]
- Wang, F.; Bao, M.; Xu, J.; Shi, L.; Niu, R.; Wang, T.; Liu, J. Scutellarin Inhibits the Glioma Cell Proliferation by Downregulating BIRC5to Promote Cell Apoptosis. J. Cell Mol. Med. 2023, 27, 1975–1987. [Google Scholar] [CrossRef]
- Jiang, X.; Shi, Y.; Chen, X.; Xu, H.; Huang, X.; Li, L.; Pu, J. The N6-Methylandenosine-Related Gene BIRC5 as a Prognostic Biomarker Correlated with Cell Migration and Immune Cell Infiltrates in Low Grade Glioma. Front. Mol. Biosci. 2022, 9, 773662. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Wang, B.; Ding, M.; Huo, Y.; Hu, H.; Cai, R.; Zhou, T.; Gao, Z.; Wang, Z.; Chen, D. Elevated E2F7 Expression Predicts Poor Prognosis in Human Patients with Gliomas. J. Clin. Neurosci. 2016, 33, 187–193. [Google Scholar] [CrossRef]
- Chen, K.; Lei, H.; Liu, X.; Wang, S. The Roles of E2F7 in Cancer: Current Knowledge and Future Prospects. Heliyon 2024, 10, e34362. [Google Scholar] [CrossRef]
- Meng, J.; Qian, W.; Yang, Z.; Gong, L.; Xu, D.; Huang, H.; Jiang, X.; Pu, Z.; Yin, Y.; Zou, J. E2F7 as a Dual Regulator of Tumor Suppression and Chemoresistance in Glioblastoma Multiforme 2023. Res. Sq. 2024. [Google Scholar] [CrossRef]
- He, C.; Sheng, L.; Pan, D.; Jiang, S.; Ding, L.; Ma, X.; Liu, Y.; Jia, D. Single-Cell Transcriptomic Analysis Revealed a Critical Role of SPP1/CD44-Mediated Crosstalk Between Macrophages and Cancer Cells in Glioma. Front. Cell Dev. Biol. 2021, 9, 779319. [Google Scholar] [CrossRef]
- Wang, T.; Zhou, J.; Chen, G.; Xu, X.; Shen, H. SPP1 Is Associated with Glioma Malignancy and Immunosuppressive Regulation in 916 Samples. Neurol. Res. 2025, 47, 666–676. [Google Scholar] [CrossRef]
- Safaee, S.; Fardi, M.; Hemmat, N.; Khosravi, N.; Derakhshani, A.; Silvestris, N.; Baradaran, B. Silencing ZEB2 Induces Apoptosis and Reduces Viability in Glioblastoma Cell Lines. Molecules 2021, 26, 901. [Google Scholar] [CrossRef]
- Qi, S.; Song, Y.; Peng, Y.; Wang, H.; Long, H.; Yu, X.; Li, Z.; Fang, L.; Wu, A.; Luo, W.; et al. ZEB2 Mediates Multiple Pathways Regulating Cell Proliferation, Migration, Invasion, and Apoptosis in Glioma. PLoS ONE 2012, 7, e38842. [Google Scholar] [CrossRef] [PubMed]
- Myung, J.K.; Ah Choi, S.; Kim, S.-K.; Ik Kim, S.; Park, J.W.; Park, S.-H. The Role of ZEB2 Expression in Pediatric and Adult Glioblastomas. Anticancer. Res. 2021, 41, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Codó, P.; Weller, M.; Kaulich, K.; Schraivogel, D.; Silginer, M.; Reifenberger, G.; Meister, G.; Roth, P. Control of Glioma Cell Migration and Invasiveness by GDF-15. Oncotarget 2016, 7, 7732–7746. [Google Scholar] [CrossRef]
- Zhu, S.; Yang, N.; Guan, Y.; Wang, X.; Zang, G.; Lv, X.; Deng, S.; Wang, W.; Li, T.; Chen, J. GDF15 Promotes Glioma Stem Cell-like Phenotype via Regulation of ERK1/2–c-Fos–LIF Signaling. Cell Death Discov. 2021, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Chen, Y.; Hu, S.; Gao, L.; Tang, N.; Liu, R.; Qin, Y.; Ren, C.; Du, S. GDF15 Expression in Glioma Is Associated with Malignant Progression, Immune Microenvironment, and Serves as a Prognostic Factor. CNS Neurosci. Ther. 2022, 28, 158–171. [Google Scholar] [CrossRef]
- Guo, M.; Goudarzi, K.M.; Abedi, S.; Pieber, M.; Sjöberg, E.; Behnan, J.; Zhang, X.-M.; Harris, R.A.; Bartek, J.; Lindström, M.S.; et al. SFRP2 Induces a Mesenchymal Subtype Transition by Suppression of SOX2 in Glioblastoma. Oncogene 2021, 40, 5066–5080. [Google Scholar] [CrossRef]
- Wu, Q.; Yin, X.; Zhao, W.; Xu, W.; Chen, L. Downregulation of SFRP2 Facilitates Cancer Stemness and Radioresistance of Glioma Cells via Activating Wnt/β-Catenin Signaling. PLoS ONE 2021, 16, e0260864. [Google Scholar] [CrossRef]
- Suzuki, H.; Kaneko, M.K.; Kato, Y. Roles of Podoplanin in Malignant Progression of Tumor. Cells 2022, 11, 575. [Google Scholar] [CrossRef]
- Gharahkhani, R.; Pourhadi, M.; Mirdamadi, N.S.; Dana, N.; Rafiee, L.; Nedaeinia, R.; Javanmard, S.H. Effect of Anti-Podoplanin on Malignant Glioma Cell Viability, Invasion and Tumor Cell-Induced Platelet Aggregation. Arch. Med. Res. 2022, 53, 461–468. [Google Scholar] [CrossRef]
- Leng, T.; Li, M.; Shen, J.; Liu, M.; Li, X.; Sun, H.; Branigan, D.; Zeng, Z.; Si, H.; Li, J.; et al. Suppression of TRPM7 Inhibits Proliferation, Migration, and Invasion of Malignant Human Glioma Cells. CNS Neurosci. Ther. 2015, 21, 252–261. [Google Scholar] [CrossRef]
- Liu, M.; Inoue, K.; Leng, T.; Guo, S.; Xiong, Z. TRPM7 Channels Regulate Glioma Stem Cell through STAT3 and Notch Signaling Pathways. Cell Signal 2014, 26, 2773–2781. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Guo, A.A.; King, P.; Guo, S.; Saafir, T.; Jiang, Y.; Liu, M. TRPM7 Induces Tumorigenesis and Stemness Through Notch Activation in Glioma. Front. Pharmacol. 2020, 11, 590723. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.-N.; Lim, H.S.; Liu, L.; Chang, J.W.; Lim, Y.C.; Rha, K.S.; Koo, B.S. LAMB3 Mediates Metastatic Tumor Behavior in Papillary Thyroid Cancer by Regulating C-MET/Akt Signals. Sci. Rep. 2018, 8, 2718. [Google Scholar] [CrossRef]
- Wang, R.; Liao, Z.; Liu, C.; Yu, S.; Xiang, K.; Wu, T.; Feng, J.; Ding, S.; Yu, T.; Cheng, G.; et al. CRABP2 Promotes Cell Migration and Invasion by Activating PI3K/AKT and MAPK Signalling Pathways via Upregulating LAMB3 in Prostate Cancer. J. Biochem. 2024, 176, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Nasarre, C.; Koncina, E.; Labourdette, G.; Cremel, G.; Roussel, G.; Aunis, D.; Bagnard, D. Neuropilin-2 Acts as a Modulator of Sema3A-Dependent Glioma Cell Migration. Cell Adh Migr. 2009, 3, 383–389. [Google Scholar] [CrossRef]
- Lee, J.; Shin, Y.J.; Lee, K.; Cho, H.J.; Sa, J.K.; Lee, S.-Y.; Kim, S.-H.; Lee, J.; Yoon, Y.; Nam, D.-H. Anti-SEMA3A Antibody: A Novel Therapeutic Agent to Suppress Glioblastoma Tumor Growth. Cancer Res. Treat. 2018, 50, 1009–1022. [Google Scholar] [CrossRef]
- Liu, B.; Dong, H.; Lin, X.; Yang, X.; Yue, X.; Yang, J.; Li, Y.; Wu, L.; Zhu, X.; Zhang, S.; et al. RND3 Promotes Snail 1 Protein Degradation and Inhibits Glioblastoma Cell Migration and Invasion. Oncotarget 2016, 7, 82411–82423. [Google Scholar] [CrossRef]
- Almarán, B.; Ramis, G.; Fernández de Mattos, S.; Villalonga, P. Rnd3 Is a Crucial Mediator of the Invasive Phenotype of Glioblastoma Cells Downstream of Receptor Tyrosine Kinase Signalling. Cells 2022, 11, 3716. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, J.; Yang, L.; Sun, K.; Jiang, Q.; Dong, F.; Lu, W.; Chen, R.; Chen, Y. Elevated INHBA Promotes Tumor Progression of Cervical Cancer. Technol. Cancer Res. Treat. 2024, 23, 15330338241234798. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Liu, Y. INHBA Promotes the Proliferation, Migration and Invasion of Colon Cancer Cells through the Upregulation of VCAN. J. Int. Med. Res. 2021, 49, 03000605211014998. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Qin, L.; Peng, X.; Hu, Y.; Liu, B. INHBA Gene Silencing Inhibits Gastric Cancer Cell Migration and Invasion by Impeding Activation of the TGF-β Signaling Pathway. J. Cell Physiol. 2019, 234, 18065–18074. [Google Scholar] [CrossRef]
- Chien, M.-H.; Yang, Y.-C.; Ho, K.-H.; Ding, Y.-F.; Chen, L.-H.; Chiu, W.-K.; Chen, J.-Q.; Tung, M.-C.; Hsiao, M.; Lee, W.-J. Cyclic Increase in the ADAMTS1-L1CAM-EGFR Axis Promotes the EMT and Cervical Lymph Node Metastasis of Oral Squamous Cell Carcinoma. Cell Death Dis. 2024, 15, 82. [Google Scholar] [CrossRef]
- de Assis Lima, M.; da Silva, S.V.; Serrano-Garrido, O.; Hülsemann, M.; Santos-Neres, L.; Rodríguez-Manzaneque, J.C.; Hodgson, L.; Freitas, V.M. Metalloprotease ADAMTS-1 Decreases Cell Migration and Invasion Modulating the Spatiotemporal Dynamics of Cdc42 Activity. Cell Signal 2021, 77, 109827. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, J.; Yang, Z. The Roles of ADAMTS in Angiogenesis and Cancer. Tumor Biol. 2015, 36, 4039–4051. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, J.; Wang, K.; Zhao, Y.; Liu, D. ADAMTS1 as Potential Prognostic Biomarker Promotes Malignant Invasion of Glioma. Int. J. Clin. Oncol. 2023, 28, 52–68. [Google Scholar] [CrossRef]
- Meng, M.; Zhou, H.; He, Y.; Chen, L.; Wang, W.; Yang, L.; Wang, Z.; Zhang, L.; Wang, S. CDH6 as a Prognostic Indicator and Marker for Chemotherapy in Gliomas. Front. Genet. 2022, 13, 949552. [Google Scholar] [CrossRef] [PubMed]
- Gugnoni, M.; Sancisi, V.; Gandolfi, G.; Manzotti, G.; Ragazzi, M.; Giordano, D.; Tamagnini, I.; Tigano, M.; Frasoldati, A.; Piana, S.; et al. Cadherin-6 Promotes EMT and Cancer Metastasis by Restraining Autophagy. Oncogene 2017, 36, 667–677. [Google Scholar] [CrossRef] [PubMed]
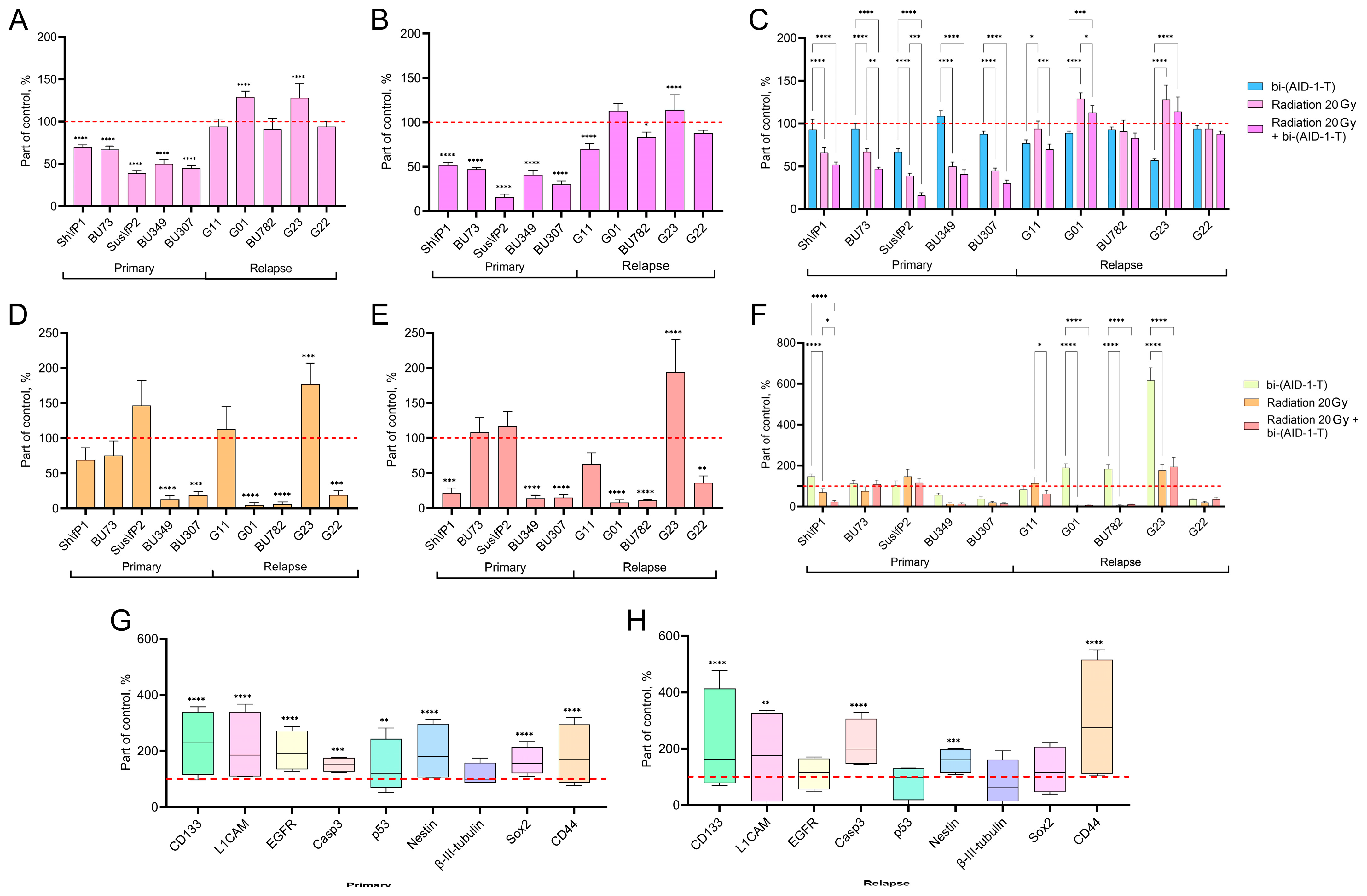
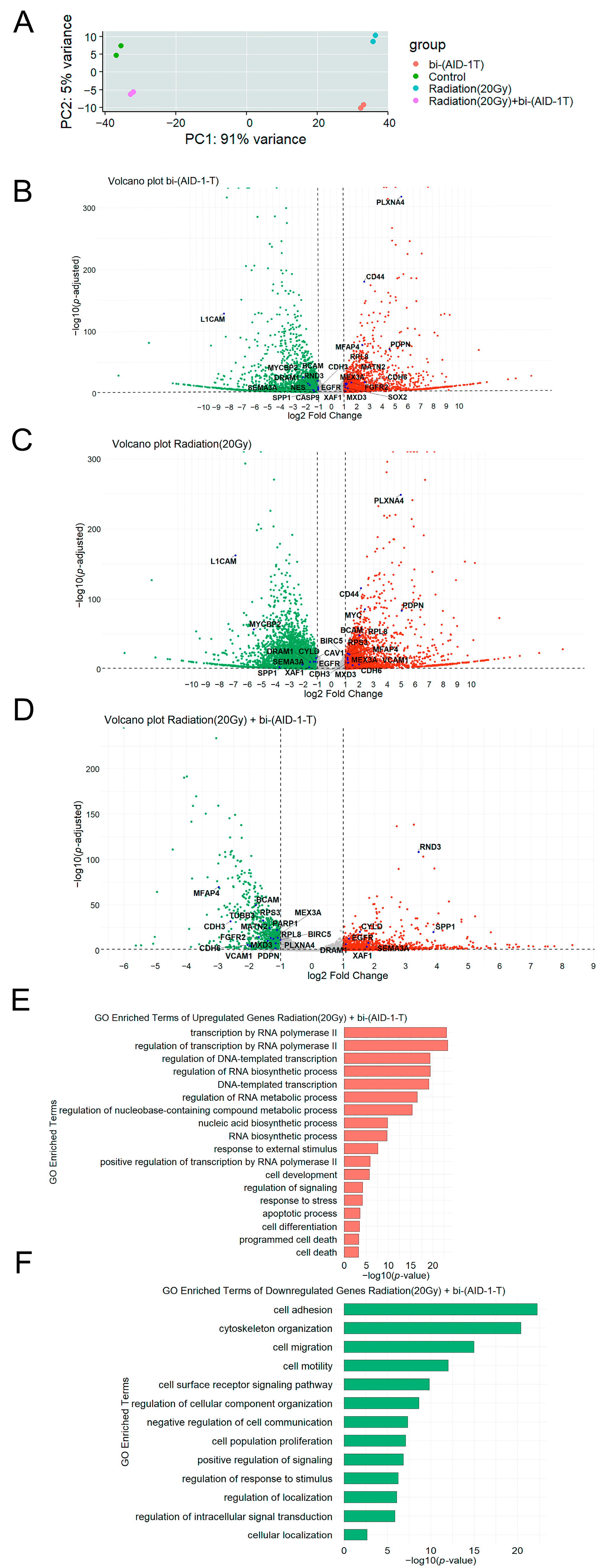
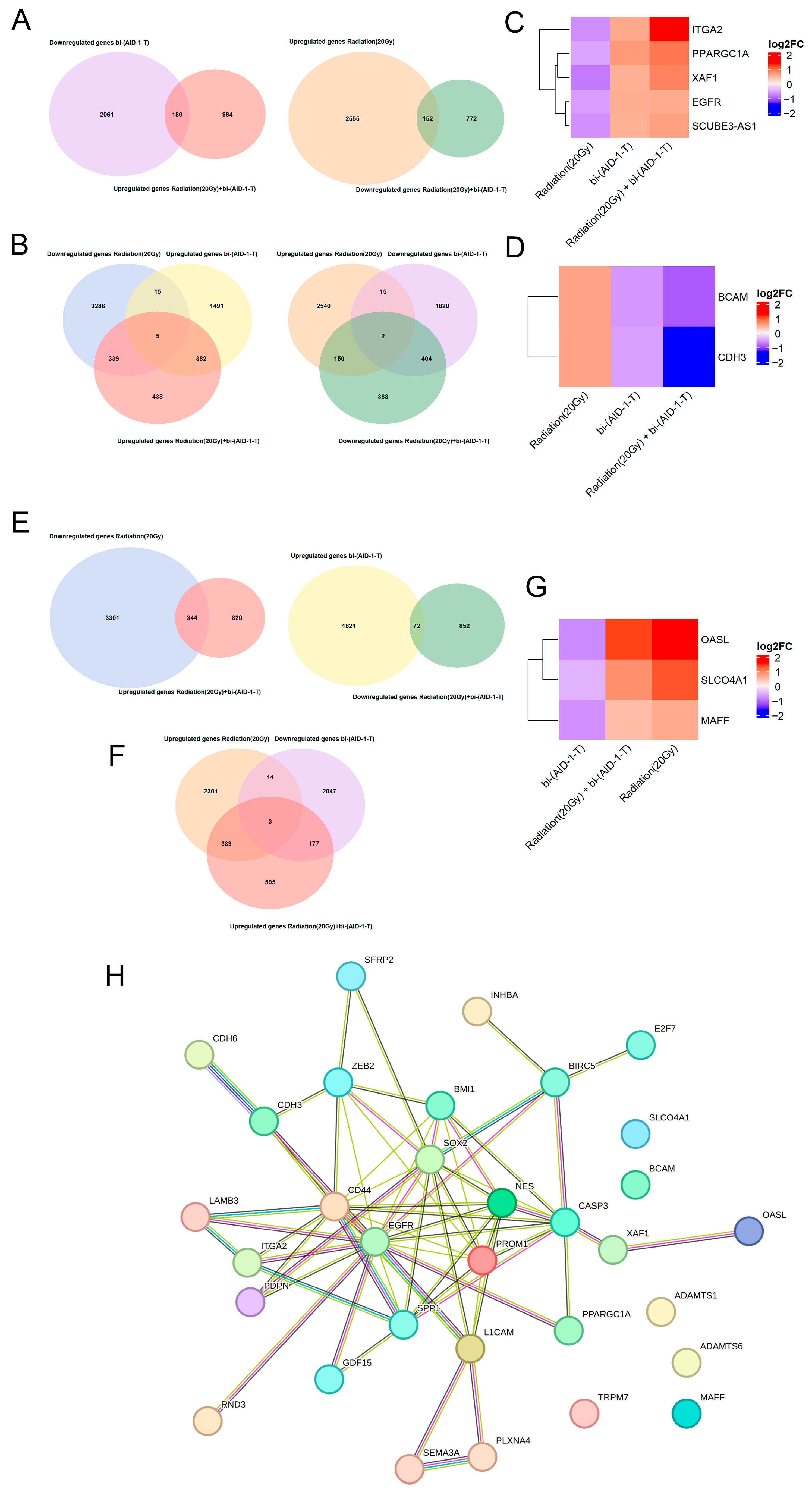
| Culture Name | Grade | Diagnosis | |
|---|---|---|---|
| Primary tumor | Sh\fP1 | 4 | Glioblastoma |
| BU73 | 3 | Anaplastic astrocytoma | |
| Sus\fP2 | 4 | Glioblastoma | |
| BU349 | 4 | Glioblastoma | |
| BU307 | 4 | Glioblastoma | |
| Relapse | G-11 | 3–4 | Anaplastic astrocytoma |
| G-01 | 4 | Glioblastoma | |
| BU782 | 4 | Glioblastoma | |
| G-23 | 4 | Glioblastoma | |
| G22 | 4 | Glioblastoma |
| bi-(AID-1-T) | Radiation | Radiation + bi-(AID-1-T) | |
|---|---|---|---|
| Migration | ↑↑ | ↓ | ↓↓ |
| Proliferation | ↓↓ | ↑↑ | ↓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlova, S.; Rubetskaya, K.; Fab, L.; Savchenko, E.; Samoylenkova, N.; Revishchin, A.; Ryabova, A.; Antipina, N.; Galkin, M.; Golanov, A.; et al. The Aptamer bi-(AID-1-T) Synergizes with Radiation to Inhibit Proliferation of Human Glioma Cells. Pharmaceutics 2025, 17, 1442. https://doi.org/10.3390/pharmaceutics17111442
Pavlova S, Rubetskaya K, Fab L, Savchenko E, Samoylenkova N, Revishchin A, Ryabova A, Antipina N, Galkin M, Golanov A, et al. The Aptamer bi-(AID-1-T) Synergizes with Radiation to Inhibit Proliferation of Human Glioma Cells. Pharmaceutics. 2025; 17(11):1442. https://doi.org/10.3390/pharmaceutics17111442
Chicago/Turabian StylePavlova, Svetlana, Ksenia Rubetskaya, Lika Fab, Ekaterina Savchenko, Nadezhda Samoylenkova, Alexander Revishchin, Anastasia Ryabova, Natalia Antipina, Mikhail Galkin, Andrey Golanov, and et al. 2025. "The Aptamer bi-(AID-1-T) Synergizes with Radiation to Inhibit Proliferation of Human Glioma Cells" Pharmaceutics 17, no. 11: 1442. https://doi.org/10.3390/pharmaceutics17111442
APA StylePavlova, S., Rubetskaya, K., Fab, L., Savchenko, E., Samoylenkova, N., Revishchin, A., Ryabova, A., Antipina, N., Galkin, M., Golanov, A., Usachev, D., Kopylov, A., & Pavlova, G. (2025). The Aptamer bi-(AID-1-T) Synergizes with Radiation to Inhibit Proliferation of Human Glioma Cells. Pharmaceutics, 17(11), 1442. https://doi.org/10.3390/pharmaceutics17111442








