Hacking Extracellular Vesicles: Using Vesicle-Related Tags to Engineer Mesenchymal Stromal Cell-Derived Extracellular Vesicles
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation, Culture, and Characterization of Canine MSC
2.2. RNA Isolation and cDNA Synthesis
2.3. Plasmids
2.4. MSC Transfection and Isolation of EVs
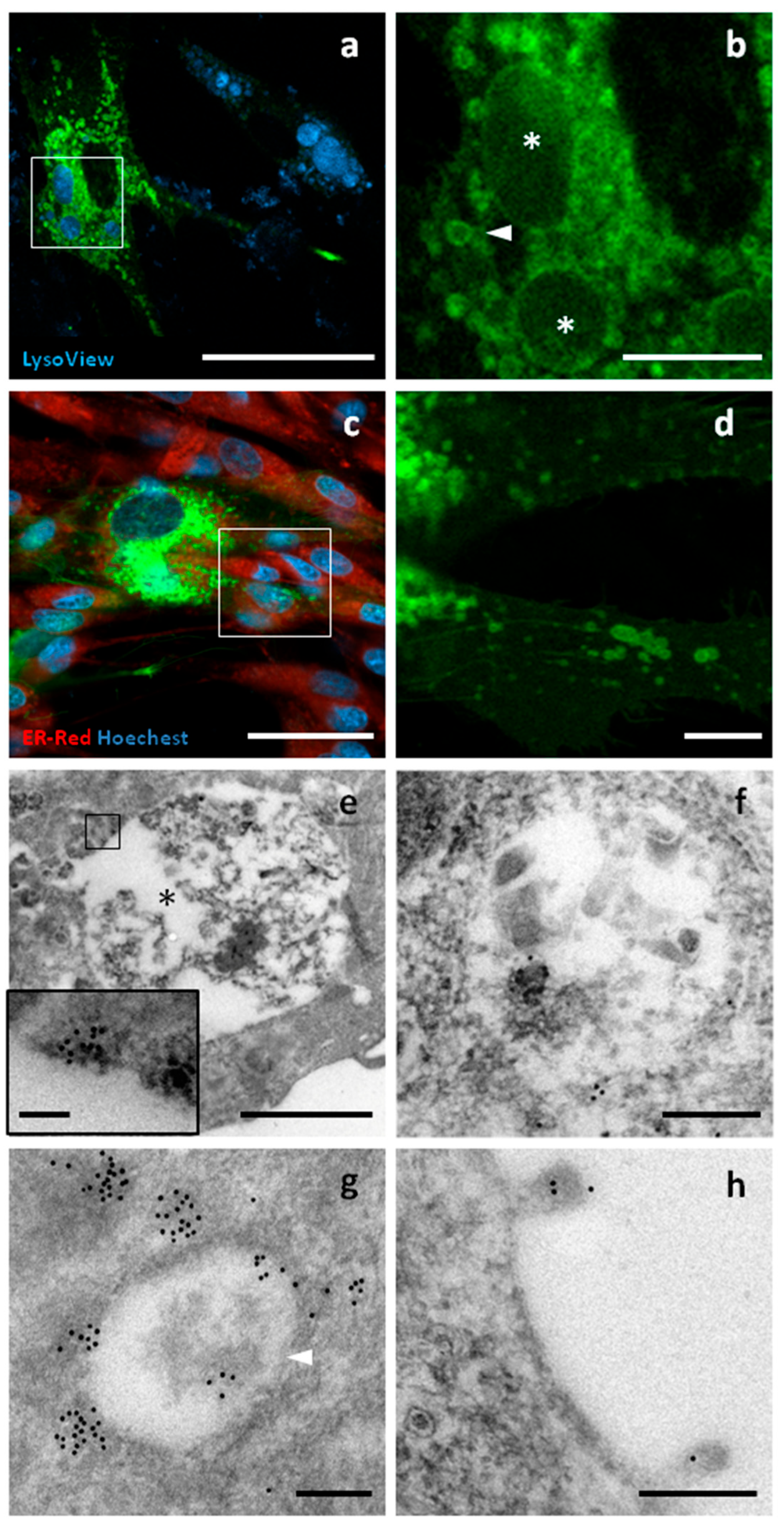
2.5. Confocal Microscopy of Living Cells
2.6. Immunoelectron Microscopy of Transfected c-Ad-MSCs
2.7. Western Blotting
3. Results
3.1. Canine Cells Meet the ISCT Inclusion Criteria for MSCs
3.2. DNAs Encoding EVs Markers Show Complete Identity Compared to the Annotated Sequence
3.3. Transfected Canine MSCs Express GFP
3.4. EVs from Transfected MSCs Contain GFP-Tagged Proteins
3.5. Palmitoylation Signal, CD63, and Syntenin-1 Confer Different Loading Efficiencies in EVs
3.6. tGFP Shows Different Intracellular Localizations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EVs | Extracellular vesicles |
| Ex | Exosomes |
| MVBs | Multivesicular bodies |
| MVs | Microvesicles |
| ABs | Apoptotic bodies |
| ESCRT | Endosomal sorting complex required for transport |
| MSCs | Mesenchymal Stromal Cells |
| c-Ad-MSCs | Canine adipose-derived MSCs |
| GFP | Green Fluorescent Protein |
| OVUD | Veterinary University Hospital |
| DMEM | Dulbecco’s modified Eagle Medium |
| SVF | Stromal Vascular Fraction |
| FBS | Fetal Bovine Serum |
| ISCT | International Society for Cell and Gene Therapy |
| DPBS | Dulbecco’s Phosphate-Buffered Saline |
References
- Meldolesi, J. Exosomes and Ectosomes in Intercellular Communication. Curr. Biol. 2018, 28, R435–R444. [Google Scholar] [CrossRef]
- Simeone, P.; Bologna, G.; Lanuti, P.; Pierdomenico, L.; Guagnano, M.T.; Pieragostino, D.; Del Boccio, P.; Vergara, D.; Marchisio, M.; Miscia, S.; et al. Extracellular vesicles as signaling mediators and disease biomarkers across biological barriers. Int. J. Mol. Sci. 2020, 21, 2514. [Google Scholar] [CrossRef]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Burrello, J.; Bianco, G.; Burrello, A.; Manno, C.; Maulucci, F.; Pileggi, M.; Nannoni, S.; Michel, P.; Bolis, S.; Melli, G.; et al. Extracellular Vesicle Surface Markers as a Diagnostic Tool in Transient Ischemic Attacks. Stroke 2021, 52, 3335–3347. [Google Scholar] [CrossRef]
- Tamura, T.; Yoshioka, Y.; Sakamoto, S.; Ichikawa, T.; Ochiya, T. Extracellular vesicles as a promising biomarker resource in liquid biopsy for cancer. Extracell. Vesicles Circ. Nucleic Acids 2021, 2, 148–174. [Google Scholar] [CrossRef]
- Simonazzi, A.; Cid, A.G.; Villegas, M.; Romero, A.I.; Palma, S.D.; Bermúdez, J.M. Nanotechnology applications in drug controlled release. In Drug Targeting and Stimuli Sensitive Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2018; pp. 81–116. [Google Scholar] [CrossRef]
- Villata, S.; Canta, M.; Cauda, V. Evs and bioengineering: From cellular products to engineered nanomachines. Int. J. Mol. Sci. 2020, 21, 6048. [Google Scholar] [CrossRef] [PubMed]
- Crivelli, B.; Chlapanidas, T.; Perteghella, S.; Lucarelli, E.; Pascucci, L.; Brini, A.T.; Ferrero, I.; Marazzi, M.; Pessina, A.; Torre, M.L.; et al. Mesenchymal stem/stromal cell extracellular vesicles: From active principle to next generation drug delivery system. J. Control. Release 2017, 262, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Corso, G.; Heusermann, W.; Trojer, D.; Görgens, A.; Steib, E.; Voshol, J.; Graff, A.; Genoud, C.; Lee, Y.; Hean, J.; et al. Systematic characterization of extracellular vesicles sorting domains and quantification at the single molecule–single vesicle level by fluorescence correlation spectroscopy and single particle imaging. J. Extracell. Vesicles 2019, 8, 1663043. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yu, J.; Kadungure, T.; Beyene, J.; Zhang, H.; Lu, Q. ARMMs as a versatile platform for intracellular delivery of macromolecules. Nat. Commun. 2018, 9, 960. [Google Scholar] [CrossRef]
- Teng, F.; Fussenegger, M. Shedding Light on Extracellular Vesicle Biogenesis and Bioengineering. Adv. Sci. 2020, 8, 2003505. [Google Scholar] [CrossRef]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brügger, B.; Simons, M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef]
- Van Niel, G.; Charrin, S.; Simoes, S.; Romao, M.; Rochin, L.; Saftig, P.; Marks, M.S.; Rubinstein, E.; Raposo, G. The Tetraspanin CD63 Regulates ESCRT-Independent and -Dependent Endosomal Sorting during Melanogenesis. Dev. Cell 2011, 21, 708–721. [Google Scholar] [CrossRef]
- Jurgielewicz, B.J.; Yao, Y.; Stice, S.L. Kinetics and Specificity of HEK293T Extracellular Vesicle Uptake using Imaging Flow Cytometry. Nanoscale Res. Lett. 2020, 15, 170. [Google Scholar] [CrossRef]
- Strohmeier, K.; Hofmann, M.; Hauser, F.; Sivun, D.; Puthukodan, S.; Karner, A.; Sandner, G.; Le Renard, P.-E.; Jacak, J.; Mairhofer, M. Crispr/cas9 genome editing vs. Over-expression for fluorescent extracellular vesicle-labeling: A quantitative analysis. Int. J. Mol. Sci. 2021, 23, 282. [Google Scholar] [CrossRef]
- Sung, B.H.; von Lersner, A.; Guerrero, J.; Krystofiak, E.S.; Inman, D.; Pelletier, R.; Zijlstra, A.; Ponik, S.M.; Weaver, A.M. A live cell reporter of exosome secretion and uptake reveals pathfinding behavior of migrating cells. Nat. Commun. 2020, 11, 2092. [Google Scholar] [CrossRef]
- Voga, M.; Adamic, N.; Vengust, M.; Majdic, G. Stem Cells in Veterinary Medicine—Current State and Treatment Options. Front. Vet.-Sci. 2020, 7, 278. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Mesenchymal stem cells: Time to change the name! Stem Cells Transl. Med. 2017, 6, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Keshtkar, S.; Azarpira, N.; Ghahremani, M.H. Mesenchymal stem cell-derived extracellular vesicles: Novel frontiers in regenerative medicine. Stem Cell Res. Ther. 2018, 9, 63. [Google Scholar] [CrossRef]
- Lopez-Verrilli, M.A.; Caviedes, A.; Cabrera, A.; Sandoval, S.; Wyneken, U.; Khoury, M. Mesenchymal stem cell-derived exosomes from different sources selectively promote neuritic outgrowth. Neuroscience 2016, 320, 129–139. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, B.; Shi, H.; Qian, H.; Xu, W. MSC-exosome: A novel cell-free therapy for cutaneous regeneration. Cytotherapy 2018, 20, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Huang, R.; Qiu, G.; Ge, M.; Wang, J.; Shu, Q.; Xu, J. Mesenchymal stromal cell-derived extracellular vesicles: Regenerative and immunomodulatory effects and potential applications in sepsis. Cell Tissue Res. 2018, 374, 1–15. [Google Scholar] [CrossRef]
- Vonk, L.A.; De Windt, T.S.; Slaper-Cortenbach, I.C.M.; Saris, D.B.F. Autologous, allogeneic, induced pluripotent stem cell or a combination stem cell therapy? Where are we headed in cartilage repair and why: A concise review. Stem Cell Res. Ther. 2015, 6, 94. [Google Scholar] [CrossRef]
- Neupane, M.; Chang, C.C.; Kiupel, M.; Yuzbasiyan-Gurkan, V. Isolation and characterization of canine adipose-derived mesenchymal stem cells. Tissue Eng. Part A 2008, 14, 1007–1015. [Google Scholar] [CrossRef]
- Ivanovska, A.; Grolli, S.; Borghetti, P.; Ravanetti, F.; Conti, V.; De Angelis, E.; Macchi, F.; Ramoni, R.; Martelli, P.; Gazza, F.; et al. Immunophenotypical characterization of canine mesenchymal stem cells from perivisceral and subcutaneous adipose tissue by a species-specific panel of antibodies. Res. Vet.-Sci. 2017, 114, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Hedayati, V.; Mousavi, A.; Razavi, K.; Cultrera, N.; Alagna, F.; Mariotti, R.; Hosseini-Mazinani, M.; Baldoni, L. Polymorphisms in the AOX2 gene are associated with the rooting ability of olive cuttings. Plant Cell Rep. 2015, 34, 1151–1164. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Scattini, G.; Pellegrini, M.; Severi, G.; Cagiola, M.; Pascucci, L. The Stromal Vascular Fraction from Canine Adipose Tissue Contains Mesenchymal Stromal Cell Subpopulations That Show Time-Dependent Adhesion to Cell Culture Plastic Vessels. Animals 2023, 13, 1175. [Google Scholar] [CrossRef]
- Cheng, Y.; Schorey, J.S. Targeting Soluble Proteins to Exosomes Using a Ubiquitin Tag. Biotechnol. Bioeng. 2015, 113, 1315–1324. [Google Scholar] [CrossRef]
- Geeurickx, E.; Tulkens, J.; Dhondt, B.; Van Deun, J.; Lippens, L.; Vergauwen, G.; Heyrman, E.; De Sutter, D.; Gevaert, K.; Impens, F.; et al. The generation and use of recombinant extracellular vesicles as biological reference material. Nat. Commun. 2019, 10, 3288. [Google Scholar] [CrossRef]
- Koumangoye, R.B.; Sakwe, A.M.; Goodwin, J.S.; Patel, T.; Ochieng, J. Detachment of breast tumor cells induces rapid secretion of exosomes which subsequently mediate cellular adhesion and spreading. PLoS ONE 2011, 6, e24234. [Google Scholar] [CrossRef]
- Lai, C.P.; Kim, E.Y.; Badr, C.E.; Weissleder, R.; Mempel, T.R.; Tannous, B.A.; Breakefield, X.O. Visualization and tracking of tumour extracellular vesicle delivery and RNA translation using multiplexed reporters. Nat. Commun. 2015, 6, 7029. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.; Losacco, J.; Stickney, Z.; Li, L.; Marriott, G.; Lu, B. Pseudotyping exosomes for enhanced protein delivery in mammalian cells. Int. J. Nanomed. 2017, 12, 3153–3170. [Google Scholar] [CrossRef] [PubMed]
- Sterzenbach, U.; Putz, U.; Low, L.H.; Silke, J.; Tan, S.S.; Howitt, J. Engineered Exosomes as Vehicles for Biologically Active Proteins. Mol. Ther. 2017, 25, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Niida, S.; Azuma, E.; Yanagibashi, T.; Muramatsu, M.; Huang, T.T.; Sagara, H.; Higaki, S.; Ikutani, M.; Nagai, Y.; et al. Inflammation-induced endothelial cell-derived extracellular vesicles modulate the cellular status of pericytes. Sci. Rep. 2015, 5, 8505. [Google Scholar] [CrossRef]
- Nabhan, J.F.; Hu, R.; Oh, R.S.; Cohen, S.N.; Lu, Q. Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proc. Natl. Acad. Sci. USA 2012, 109, 4146–4151. [Google Scholar] [CrossRef]
- Kim, B.Y.; Olzmann, J.A.; Barsh, G.S.; Chin, L.-S.; Li, L. Spongiform Neurodegeneration-associated E3 Ligase Mahogunin Ubiquitylates TSG101 and Regulates Endosomal Trafficking. Mol. Biol. Cell 2007, 18, 1129–1142. [Google Scholar] [CrossRef]
- Horgan, C.P.; Hanscom, S.R.; Kelly, E.E.; McCaffrey, M.W. Tumor susceptibility gene 101 (TSG101) is a novel binding-partner for the class II Rab11-FIPs. PLoS ONE 2012, 7, e32030. [Google Scholar] [CrossRef]
- Rauch, S.; Martin-Serrano, J. Multiple Interactions between the ESCRT Machinery and Arrestin-Related Proteins: Implications for PPXY-Dependent Budding. J. Virol. 2011, 85, 3546–3556. [Google Scholar] [CrossRef]
- Wu, A.Y.; Sung, Y.; Chen, Y.; Chou, S.T.; Guo, V.; Chien, J.C.; Ko, J.J.; Yang, A.L.; Huang, H.; Chuang, J.; et al. Multiresolution Imaging Using Bioluminescence Resonance Energy Transfer Identifies Distinct Biodistribution Profiles of Extracellular Vesicles and Exomeres with Redirected Tropism. Adv. Sci. 2020, 7, 2001467. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, X.; Chen, X.; Aramsangtienchai, P.; Tong, Z.; Lin, H. Protein Lipidation: Occurrence, Mechanisms, Biological Functions, and Enabling Technologies. Chem. Rev. 2018, 118, 919–988. [Google Scholar] [CrossRef]
- Shen, B.; Wu, N.; Yang, M.; Gould, S.J. Protein targeting to exosomes/microvesicles by plasma membrane anchors. J. Biol. Chem. 2011, 286, 14383–14395. [Google Scholar] [CrossRef]
- Baietti, M.F.; Zhang, Z.; Mortier, E.; Melchior, A.; DeGeest, G.; Geeraerts, A.; Ivarsson, Y.; Depoortere, F.; Coomans, C.; Vermeiren, E.; et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 2012, 14, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Hwangbo, C.; Tae, N.; Lee, S.; Kim, O.; Park, O.K.; Kim, J.; Kwon, S.-H.; Lee, J.-H. Syntenin regulates TGF-β1-induced Smad activation and the epithelial-to-mesenchymal transition by inhibiting caveolin-mediated TGF-β type i receptor internalization. Oncogene 2015, 35, 389–401. [Google Scholar] [CrossRef]
- Johnson, I.R.D.; Sorvina, A.; Logan, J.M.; Moore, C.R.; Heatlie, J.K.; Parkinson-Lawrence, E.J.; Selemidis, S.; O’leary, J.J.; Butler, L.M.; Brooks, D.A. A paradigm in immunochemistry, revealed by monoclonal antibodies to spatially distinct epitopes on syntenin-1. Int. J. Mol. Sci. 2019, 20, 6035. [Google Scholar] [CrossRef] [PubMed]
- Conceição, M.; Forcina, L.; Wiklander, O.P.; Gupta, D.; Nordin, J.Z.; Vrellaku, B.; McClorey, G.; Mäger, I.; Gӧrgens, A.; Lundin, P.; et al. Engineered extracellular vesicle decoy receptor-mediated modulation of the IL6 trans-signalling pathway in muscle. Biomaterials 2021, 266, 120435. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Pols, M.S.; Klumperman, J. Trafficking and function of the tetraspanin CD63. Exp. Cell Res. 2009, 315, 1584–1592. [Google Scholar] [CrossRef]
- Stickney, Z.; Losacco, J.; McDevitt, S.; Zhang, Z.; Lu, B. Development of exosome surface display technology in living human cells. Biochem. Biophys. Res. Commun. 2016, 472, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Kojima, R.; Bojar, D.; Rizzi, G.; Hamri, G.C.-E.; El-Baba, M.D.; Saxena, P.; Ausländer, S.; Tan, K.R.; Fussenegger, M. Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson’s disease treatment. Nat. Commun. 2018, 9, 1305. [Google Scholar] [CrossRef]
- Heusermann, W.; Hean, J.; Trojer, D.; Steib, E.; von Bueren, S.; Graff-Meyer, A.; Genoud, C.; Martin, K.; Pizzato, N.; Voshol, J.; et al. Exosomes surf on filopodia to enter cells at endocytic hot spots, traffic within endosomes, and are targeted to the ER. J. Cell Biol. 2016, 213, 173–184. [Google Scholar] [CrossRef]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Saba, E.; Sandhu, M.A.; Pelagalli, A. Canine Mesenchymal Stromal Cell Exosomes: State-of-the-Art Characterization, Functional Analysis and Applications in Various Diseases. Vet. Sci. 2024, 11, 187. [Google Scholar] [CrossRef] [PubMed]







| Protein Name | Gene ID | Protein Size | EVs Localization | Specific EVs Motif/Domain |
|---|---|---|---|---|
| Lck (Tyrosine-protein kinase Lck) | 478151 | 509 aa/58 kDa | membrane anchor | Palmitoylation signal in N-term (MGCSCSSNPE) |
| CD63 | 474391 | 238 aa/25.6 kDa | transmembrane | Unknown |
| SDCBP (Syntenin-1) | 482977 | 298 aa/32 kDa | luminal | (LYPXnL) |
| TSG101 (Tumour susceptibility gene 101) | 485406 | 391 aa/44 kDa | luminal | (PTAP) |
| Gene of Interest | Sequence Expected Size (bp) | Primer/Oligo Sequences | Thermal Conditions |
|---|---|---|---|
| CD63 | 740 | F: 5′-GGCAAGCTTCCATGGCGGTGGAAGG-3′ R: 5′-GAGAGTCGACCCCTACATGACTTCATAGCCAC-3′ for cloning in pBlueScriptII-SK plasmid | 98° × 10″ 65° × 15″ 72° × 1′ |
| TSG101 | 1255 | F: 5′-CCCTAAGCTTGCGGTGACTGGAGTGG-3′ R: 5′-GCTTTAAGTCGACCTCAATCTCCAGCTGAT-3′ for cloning in pBlueScriptII-SK plasmid | 98° × 10″ 57° × 20″ 72° × 1′10″ |
| SDCBP (Syntenin-1) | 1021 | F: 5′-AAAAGGTACCTCTGCAAAAATGTCTCTCTACCCA-3′ R: 5′-AAAAGTCGACTGGCTCCTGGAAAGCTTCA-3′ for cloning in pBlueScriptII-SK plasmid | 98° × 10″ 60° × 15″ 72° × 1′ |
| CD63 | 735 | F: 5′-GGCAAGCTTCCATGGCGGTGGAAGG-3′ R: 5′-AAAACGTCGACATGACTTCATAGCC-3′ for subcloning in pTagGFP2-N plasmid | 98° × 10″ 65° × 15″ 72° × 1′ |
| TSG101 | 1217 | F: 5′-CCCTAAGCTTGCGGTGACTGGAGTGG-3′ R: 5′-AAAACTCGAGTAGAGGTCACTGAGACC-3′ for subcloning in pTagGFP2-N plasmid | 98° × 10″ 57° × 20″ 72° × 1′10″ |
| SDCBP (Syntenin-1) | 921 | F: 5′-AAAAGTCGACTCTGCAAAAATGTCTCTCTACCC-3′ R: 5′-AAAACGTCGACACCTCAGGAATGGTGTG-3′ for subcloning in pTagGFP2-N plasmid | 98° × 10″ 60° × 15″ 72° × 1′ |
| Palm sequence | 45–37 | Sense: 5′-AGCTTGCCATGGGCTGTAGCTGCAGCTCAAACCCTGAAGCGGTAC-3′ Antisense: 5′-CGCTTCAGGGTTTGAGCTGCAGCTACAGCCCATGGCA-3′ | N.A. (denaturation at 80 °C and slow annealing in cooling water) |
| Tag and Relative Topology | Palmitoylated-GFP | CD63-GFP | Syntenin1-GFP | TSG101-GFP |
|---|---|---|---|---|
| GFP relative abundance in cell lysates (compared to untagged GFP) | 2 fold | 5 fold | 1,6 fold | Not detected |
| GFP relative abundance in Evs (compared to untagged GFP) | 4 fold | 6 fold | 7 fold | Not detected |
| Intracellular Localization | Cell membrane and Golgi apparatus  | Cell membrane, endosomes, endoplasmic reticulum | Cytoplasm | Focal spots, not defined |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scattini, G.; Pianigiani, G.; Capomaccio, S.; Ceccarini, M.R.; Mecocci, S.; Musa, L.; Avellini, L.; Barbato, O.; Bufalari, A.; Casagrande Proietti, P.; et al. Hacking Extracellular Vesicles: Using Vesicle-Related Tags to Engineer Mesenchymal Stromal Cell-Derived Extracellular Vesicles. Pharmaceutics 2025, 17, 1435. https://doi.org/10.3390/pharmaceutics17111435
Scattini G, Pianigiani G, Capomaccio S, Ceccarini MR, Mecocci S, Musa L, Avellini L, Barbato O, Bufalari A, Casagrande Proietti P, et al. Hacking Extracellular Vesicles: Using Vesicle-Related Tags to Engineer Mesenchymal Stromal Cell-Derived Extracellular Vesicles. Pharmaceutics. 2025; 17(11):1435. https://doi.org/10.3390/pharmaceutics17111435
Chicago/Turabian StyleScattini, Gabriele, Giulia Pianigiani, Stefano Capomaccio, Maria Rachele Ceccarini, Samanta Mecocci, Laura Musa, Luca Avellini, Olimpia Barbato, Antonello Bufalari, Patrizia Casagrande Proietti, and et al. 2025. "Hacking Extracellular Vesicles: Using Vesicle-Related Tags to Engineer Mesenchymal Stromal Cell-Derived Extracellular Vesicles" Pharmaceutics 17, no. 11: 1435. https://doi.org/10.3390/pharmaceutics17111435
APA StyleScattini, G., Pianigiani, G., Capomaccio, S., Ceccarini, M. R., Mecocci, S., Musa, L., Avellini, L., Barbato, O., Bufalari, A., Casagrande Proietti, P., Gialletti, R., Sulla, A., Beccari, T., & Pascucci, L. (2025). Hacking Extracellular Vesicles: Using Vesicle-Related Tags to Engineer Mesenchymal Stromal Cell-Derived Extracellular Vesicles. Pharmaceutics, 17(11), 1435. https://doi.org/10.3390/pharmaceutics17111435









