Comparative Analysis of Classic and Novel Antitussives on Cough Suppression in Guinea Pigs
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cough Induction Protocol and Recording Analysis
2.3. Cough Quantification and Acoustic Data Analysis
2.4. Statistical Analysis
3. Results
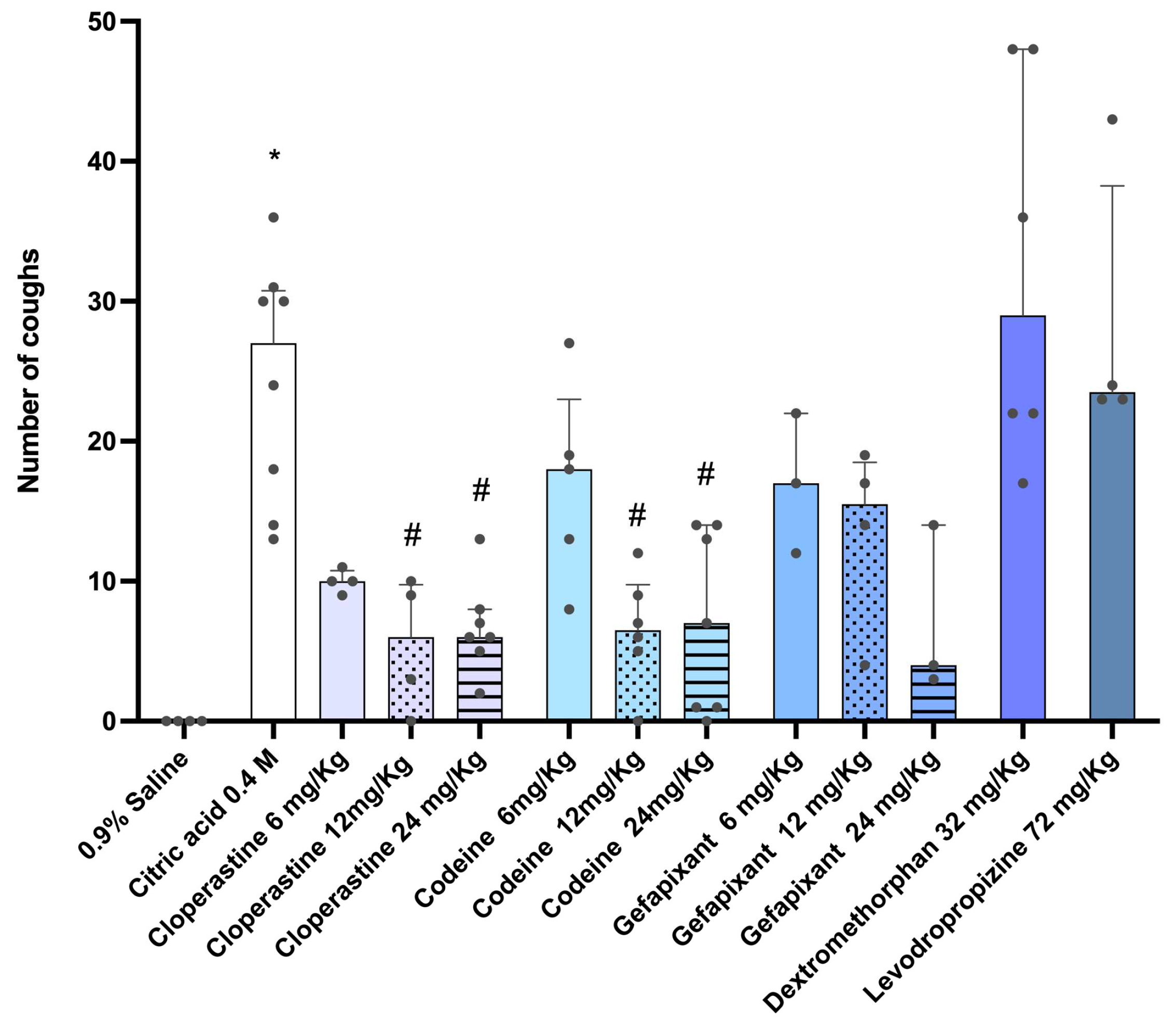
3.1. Evaluation of Antitussive Effects
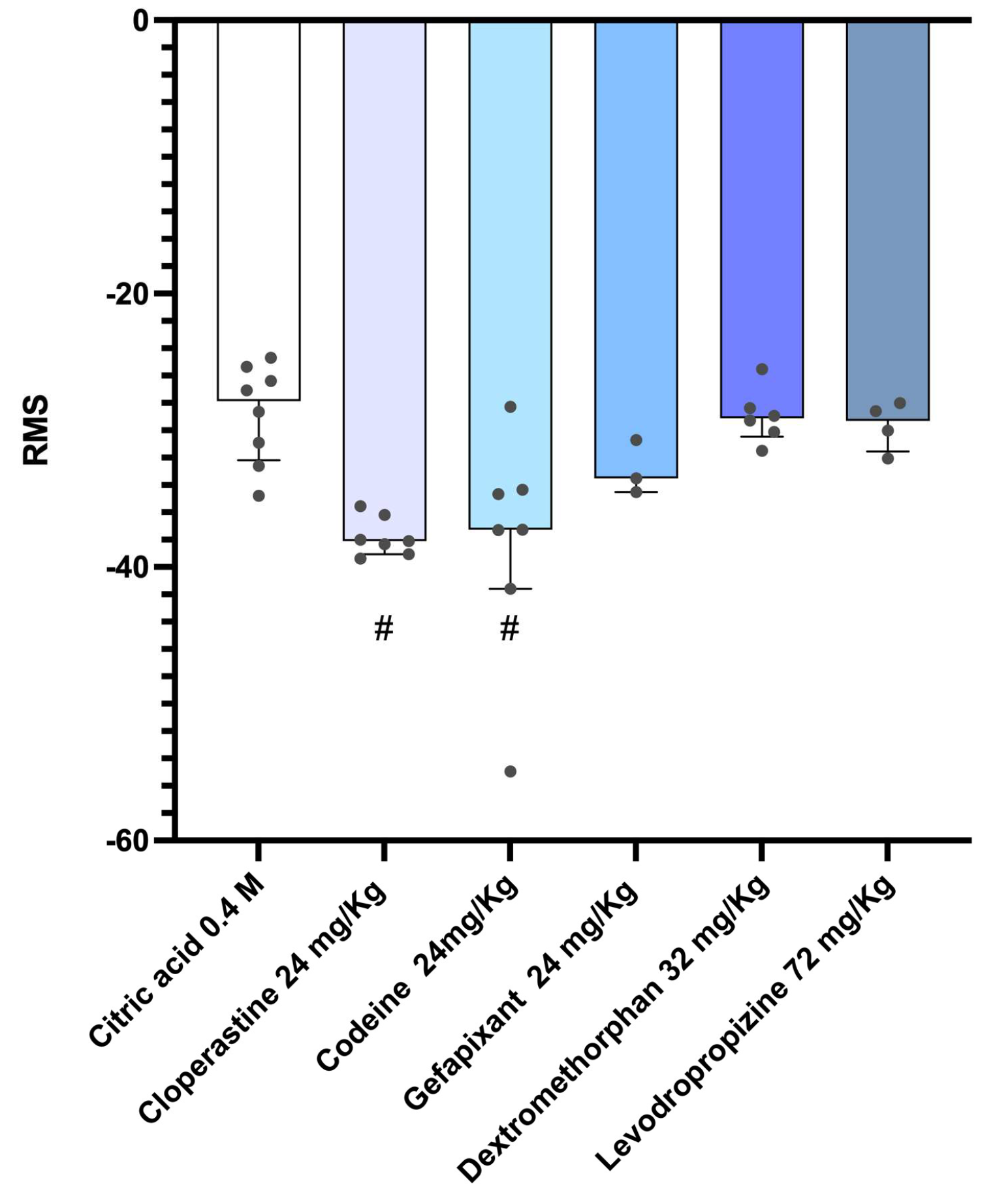
3.2. Analysis of Cough Intensity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murgia, V.; Manti, S.; Licari, A.; De Filippo, M.; Ciprandi, G.; Marseglia, G.L. Upper Respiratory Tract Infection-Associated Acute Cough and the Urge to Cough: New Insights for Clinical Practice. Pediatr. Allergy Immunol. Pulmonol. 2020, 33, 3–11. [Google Scholar] [CrossRef]
- Galway, N.C.; Shields, M.D. The child with an incessant dry cough. Paediatr. Respir. Rev. 2019, 30, 58–64. [Google Scholar] [CrossRef]
- Gilchrist, F.J. An approach to the child with a wet cough. Paediatr. Respir. Rev. 2019, 31, 75–81. [Google Scholar] [CrossRef]
- Mahashur, A. Chronic dry cough: Diagnostic and management approaches. Lung India 2015, 32, 44–49. [Google Scholar] [CrossRef]
- Dicpinigaitis, P.V.; Morice, A.H.; Birring, S.S.; McGarvey, L.; Smith, J.A.; Canning, B.J.; Page, C. Antitussive Drugs—Past, Present, and Future. Pharmacol. Rev. 2014, 66, 468–512. [Google Scholar] [CrossRef]
- Lai, K.; Pan, J.; Chen, R.; Liu, B.; Luo, W.; Zhong, N. Epidemiology of cough in relation to China. Cough 2013, 9, 18. [Google Scholar] [CrossRef]
- Song, W.-J.; Chang, Y.-S.; Faruqi, S.; Kim, J.-Y.; Kang, M.-G.; Kim, S.; Jo, E.-J.; Kim, M.-H.; Plevkova, J.; Park, H.-W.; et al. The global epidemiology of chronic cough in adults: A systematic review and meta-analysis. Eur. Respir. J. 2015, 45, 1479–1481. [Google Scholar] [CrossRef]
- Roventa, D.L.C.; Pieper-Fürst, U.; Acikel, C.; Santos, D.; Sent, U.; Mösges, R. Effectiveness and Tolerability of Ectoin® Mouth and Throat Spray Althaea Honey (ERS09) for Sore Throat due to Acute Pharyngitis and Dry Cough: A Multicentre, Actively Controlled, Open Label Study in Germany. J. Clin. Med. 2023, 12, 5813. [Google Scholar] [CrossRef]
- Romanova, J.; Rydlovskaya, A.; Mochalov, S.; Proskurina, O.; Gorokh, Y.; Nebolsin, V. The Effect of Anti-Chemokine Oral Drug XC8 on Cough Triggered by The Agonists of TRPA1 But Not TRPV1 Channels in Guinea Pigs. Pulm. Ther. 2022, 8, 105–122. [Google Scholar] [CrossRef]
- Morice, A.H.; Kantar, A.; Dicpinigaitis, P.V.; Birring, S.S.; McGarvey, L.P.; Chung, K.F. Treating acute cough: Wet versus dry–have we got the paradigm wrong? ERJ Open Res. 2015, 1, 00055–2015. [Google Scholar] [CrossRef]
- Mazzone, S.B.; McGarvey, L. Mechanisms and Rationale for Targeted Therapies in Refractory and Unexplained Chronic Cough. Clin. Pharmacol. Ther. 2021, 109, 619–636. [Google Scholar] [CrossRef]
- AEMPS. Boletín Mensual de la AEMPS Sobre Medicamentos de Uso Humano del Mes de Julio de 2019. Agencia Espa-ñola de Medicamentos y Productos Sanitarios 2019. Available online: https://www.aemps.gob.es/informa/boletines-aemps/boletin-mensual-de-la-aemps-sobre-medicamentos-de-uso-humano-del-mes-de-julio-de-2019/ (accessed on 29 July 2025).
- Birring, S.S. Controversies in the evaluation and management of chronic cough. Am. J. Respir. Crit. Care Med. 2011, 183, 708–715. [Google Scholar] [CrossRef]
- Catania, M.A.; Cuzzocrea, S. Pharmacological and clinical overview of cloperastine in treatment of cough. Ther. Clin. Risk Manag. 2011, 7, 83–92. [Google Scholar] [CrossRef]
- Lee, S.; Nam, K.-Y.; Oh, J.; Lee, S.; Cho, S.-M.; Choi, Y.-W.; Cho, J.-Y.; Lee, B.-J.; Hong, J.H. Evaluation of the effects of food on levodropropizine controlled-release tablet and its pharmacokinetic profile in comparison to that of immediate-release tablet. Drug Des. Dev. Ther. 2018, 12, 1413–1420. [Google Scholar] [CrossRef]
- Dicpinigaitis, P.V.; McGarvey, L.P.; Canning, B.J. P2 × 3-Receptor Antagonists as Potential Antitussives: Summary of Current Clinical Trials in Chronic Cough. Lung 2020, 198, 609–616. [Google Scholar] [CrossRef]
- Takahama, K.; Wakuda, I.; Fukushima, H.; Isohama, Y.; Kai, H.; Miyata, T. Differential effect of codeine on coughs caused by mechanical stimulation of two different sites in the airway of guinea pigs. Eur. J. Pharmacol. 1997, 329, 93–97. [Google Scholar] [CrossRef]
- Takahama, K.; Shirasaki, T. Central and peripheral mechanisms of narcotic antitussives: Codeine-sensitive and -resistant coughs. Cough 2007, 3, 8. [Google Scholar] [CrossRef]
- Luo, Y.; Li, P.; Zhang, C.; Zheng, Y.; Wang, S.; Nie, Y.; Zhang, K.-J.; Su, W.-W. Effects of four antitussives on airway neurogenic inflammation in a guinea pig model of chronic cough induced by cigarette smoke exposure. Inflamm. Res. 2013, 62, 1053–1061. [Google Scholar] [CrossRef]
- Folkerts, G.; van der Linde, H.J.; Omini, C.; Nijkamp, F.P. Virus-induced airway inflammation and hyperresponsiveness in the guinea-pig is inhibited by levodropropizine. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1993, 348, 213–219. [Google Scholar] [CrossRef]
- Takahara, A.; Fujiwara, K.; Ohtsuki, A.; Oka, T.; Namekata, I.; Tanaka, H. Effects of the antitussive drug cloperastine on ventricular repolarization in halothane-anesthetized guinea pigs. J. Pharmacol. Sci. 2012, 120, 165–175. [Google Scholar] [CrossRef]
- Luo, H.-Y.; Long, H.-Z.; Zhou, Z.-W.; Xu, S.-G.; Li, F.-J.; Cheng, Y.; Wen, D.-D.; Deng, P.; Gao, L.-C. Pharmacokinetics, Bioequivalence and Safety of Cloperastine in Chinese Healthy Subjects Under Fasting and Postprandial Conditions. Drugs R D 2022, 22, 311–320. [Google Scholar] [CrossRef]
- Guo, C.-R.; Zhang, Z.-Z.; Zhou, X.; Sun, M.-Y.; Li, T.-T.; Lei, Y.-T.; Gao, Y.-H.; Li, Q.-Q.; Yue, C.-X.; Gao, Y.; et al. Chronic cough relief by allosteric modulation of P2X3 without taste disturbance. Nat. Commun. 2023, 14, 5844. [Google Scholar] [CrossRef]
- Brown, C.; Fezoui, M.; Selig, W.M.; Schwartz, C.E.; Ellis, J.L. Antitussive activity of sigma-1 receptor agonists in the guinea-pig. Br. J. Pharmacol. 2004, 141, 233–240. [Google Scholar] [CrossRef]
- Xiang, A.; Uchida, Y.; Nomura, A.; Iijima, H.; Dong, F.; Zhang, M.J.; Hasegawa, S. Effects of airway inflammation on cough response in the guinea pig. J. Appl. Physiol. (1985) 1998, 85, 1847–1854. [Google Scholar] [CrossRef]
- Brozmanova, M.; Javorkova, N.; Hajtmanova, E.; Zamecnik, L.; Javorka, M.; Hanacek, J.; Tatar, M. Influence of chest gamma-irradiation on cough response in awake guinea pigs. J. Physiol. Pharmacol. 2007, 58 (Suppl. S5), 67–74. [Google Scholar]
- Ferrari, S.; Silva, M.; Guarino, M.; Aerts, J.M.; Berckmans, D. Cough sound analysis to identify respiratory infection in pigs. Comput. Electron. Agric. 2008, 64, 318–325. [Google Scholar] [CrossRef]
- Bergmann, M.; Haasenritter, J.; Beidatsch, D.; Schwarm, S.; Hörner, K.; Bösner, S.; Grevenrath, P.; Schmidt, L.; Viniol, A.; Donner-Banzhoff, N.; et al. Prevalence, aetiologies and prognosis of the symptom cough in primary care: A systematic review and meta-analysis. BMC Fam. Pract. 2021, 22, 151. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kang, S.-Y.; Yoo, Y.; An, J.; Park, S.-Y.; Lee, J.-H.; Lee, S.-E.; Kim, M.-H.; Kanemitsu, Y.; Chang, Y.-S.; et al. Epidemiology of adult chronic cough: Disease burden, regional issues, and recent findings. Asia Pac. Allergy 2021, 11, e38. [Google Scholar] [CrossRef]
- Lee, K.K.; Davenport, P.W.; Smith, J.A.; Irwin, R.S.; McGarvey, L.; Mazzone, S.B.; Birring, S.S.; Abu Dabrh, A.; Altman, K.W.; Barker, A.F.; et al. Global Physiology and Pathophysiology of Cough: Part 1: Cough Phenomenology—CHEST Guideline and Expert Panel Report. Chest 2021, 159, 282–293. [Google Scholar] [CrossRef]
- Lee, K.K.; Matos, S.; Ward, K.; Rafferty, G.F.; Moxham, J.; Evans, D.H.; Birring, S.S. Sound: A non-invasive measure of cough intensity. BMJ Open Respir. Res. 2017, 4, e000178. [Google Scholar] [CrossRef]
- Zhuang, J.; Zhao, L.; Gao, X.; Xu, F. An advanced recording and analysis system for the differentiation of guinea pig cough responses to citric acid and prostaglandin E2 in real time. PLoS ONE 2019, 14, e0217366. [Google Scholar] [CrossRef]
- Lee, S.P.; Lee, S.M.; Lee, B.-J.; Kang, S.-Y. Effectiveness and Safety of Codeine and Levodropropizine in Patients With Chronic Cough. J. Korean Med. Sci. 2022, 37, e275. [Google Scholar] [CrossRef]
- Johnson, C.M.; Cui, N.; Xing, H.; Wu, Y.; Jiang, C. The antitussive cloperastine improves breathing abnormalities in a Rett Syndrome mouse model by blocking presynaptic GIRK channels and enhancing GABA release. Neuropharmacology 2020, 176, 108214. [Google Scholar] [CrossRef]
- Gregori-Puigjané, E.; Setola, V.; Hert, J.; Crews, B.A.; Irwin, J.J.; Lounkine, E.; Marnett, L.; Roth, B.L.; Shoichet, B.K. Identifying mechanism-of-action targets for drugs and probes. Proc. Natl. Acad. Sci. USA 2012, 109, 11178–11183. [Google Scholar] [CrossRef]
- Li, H.; Zhang, C.; Wang, J.; Jiang, Y.; Fawcett, J.P.; Gu, J. Simultaneous quantitation of paracetamol, caffeine, pseudoephedrine, chlorpheniramine and cloperastine in human plasma by liquid chromatography–tandem mass spectrometry. J. Pharm. Biomed. Anal. 2010, 51, 716–722. [Google Scholar] [CrossRef]
- Manti, S.; Tosca, M.A.; Licari, A.; Brambilla, I.; Foiadelli, T.; Ciprandi, G.; Marseglia, G.L. Cough Remedies for Children and Adolescents: Current and Future Perspectives. Pediatr. Drugs 2020, 22, 617–634. [Google Scholar] [CrossRef]
- Kum, E.; Patel, M.; Diab, N.; Wahab, M.; Zeraatkar, D.; Chu, D.K.; O’bYrne, P.M.; Guyatt, G.H.; Satia, I. Efficacy and Tolerability of Gefapixant for Treatment of Refractory or Unexplained Chronic Cough: A Systematic Review and Dose-Response Meta-Analysis. JAMA 2023, 330, 1359–1369. [Google Scholar] [CrossRef]
- McGarvey, L.P.; Birring, S.S.; Morice, A.H.; Dicpinigaitis, P.V.; Pavord, I.D.; Schelfhout, J.; Nguyen, A.M.; Li, Q.; Tzontcheva, A.; Iskold, B.; et al. Efficacy and safety of gefapixant, a P2X3 receptor antagonist, in refractory chronic cough and unexplained chronic cough (COUGH-1 and COUGH-2): Results from two double-blind, randomised, parallel-group, placebo-controlled, phase 3 trials. Lancet 2022, 399, 909–923. [Google Scholar] [CrossRef]
- McGarvey, L.; Sher, M.; Shvarts, Y.G.; Lu, S.; Wu, W.-C.; Xu, P.; Schelfhout, J.; La Rosa, C.; Nguyen, A.M.; Reyfman, P.A.; et al. The Efficacy and Safety of Gefapixant in a Phase 3b Trial of Patients with Recent-Onset Chronic Cough. Lung 2023, 201, 111–118. [Google Scholar] [CrossRef]
- Markham, A. Gefapixant: First Approval. Drugs 2022, 82, 691–695. [Google Scholar] [CrossRef]
- Catena, E.; Daffonchio, L. Efficacy and tolerability of levodropropizine in adult patients with non-productive cough. Comparison with dextromethorphan. Pulm. Pharmacol. Ther. 1997, 10, 89–96. [Google Scholar] [CrossRef]
- Meeves, S.G.; Cruz-Rivera, M.; Leyva, R.A.; Wilson, B.L.; Moreira, S.A.; Gelotte, C.K.; Jayawardena, S. Objective and self-reported evidence of dextromethorphan antitussive efficacy in children, aged 6–11 years, with acute cough due to the common cold. Pediatr. Pulmonol. 2023, 58, 2229–2239. [Google Scholar] [CrossRef]
- Martinak, B.; Bolis, R.A.; Black, J.R.; Fargason, R.E.; Birur, B. Dextromethorphan in Cough Syrup: The Poor Man’s Psychosis. Psychopharmacol. Bull. 2017, 47, 59–63. [Google Scholar]
- Zanasi, A.; Lanata, L.; Fontana, G.; Saibene, F.; Dicpinigaitis, P.; De Blasio, F. Levodropropizine for treating cough in adult and children: A meta-analysis of published studies. Multidiscip. Respir. Med. 2015, 10, 19. [Google Scholar] [CrossRef]
- Song, J.-W.; Jang, Y.-S.; Jung, M.-C.; Kim, J.-H.; Choi, J.-H.; Park, S.; Hwang, Y.I.; Jang, S.H.; Jung, K.-S. Levodropropizine-Induced Anaphylaxis: Case Series and Literature Review. Allergy Asthma Immunol. Res. 2017, 9, 278–280. [Google Scholar] [CrossRef]
- Daffonchio, L.; Hernandez, A.; Melillo, G.; Clavenna, G.; Omini, C. Effectiveness of levodropropizine against cigarette smoke-induced airway hyperreactivity: Possible mechanism. Eur. J. Pharmacol. Environ. Toxicol. Pharmacol. 1993, 228, 257–261. [Google Scholar] [CrossRef]
- Yamawaki, I.; Geppetti, P.; Bertrand, C.; Huber, O.; Daffonchio, L.; Omini, C.; Nadel, J.A. Levodropropizine reduces capsaicin- and substance P-induced plasma extravasation in the rat trachea. Eur. J. Pharmacol. 1993, 243, 1–6. [Google Scholar] [CrossRef]
- Laude, E.A.; Higgins, K.S.; Morice, A.H. A comparative study of the effects of citric acid, capsaicin and resiniferatoxin on the cough challenge in guinea-pig and man. Pulm. Pharmacol. 1993, 6, 171–175. [Google Scholar] [CrossRef]
- Corboz, M.R.; Rivelli, M.A.; Egan, R.W.; Tulshian, D.; Matasi, J.; Fawzi, A.B.; Benbow, L.; Smith-Torhan, A.; Zhang, H.; Hey, J.A. Nociceptin inhibits capsaicin-induced bronchoconstriction in isolated guinea pig lung. Eur. J. Pharmacol. 2000, 402, 171–179. [Google Scholar] [CrossRef]
- Belvisi, M.G.; Hele, D.J. Cough: Citric acid and nerves. Drug Discov. Today Dis. Models 2006, 3, 237–241. [Google Scholar] [CrossRef]
- Plevkova, J.; Jakusova, J.; Brozmanova, M.; Biringerova, Z.; Buday, T. Advancing cough research: Methodological insights into cough challenge in guinea pig models using double chamber vs whole-body plethysmography. Respir. Physiol. Neurobiol. 2024, 327, 104302. [Google Scholar] [CrossRef]
- Plevkova, J.; Brozmanova, M.; Matloobi, A.; Poliacek, I.; Honetschlager, J.; Buday, T. Animal models of cough. Respir. Physiol. Neurobiol. 2021, 290, 103656. [Google Scholar] [CrossRef]
- Venkatasamy, R.; McKenzie, A.; Page, C.P.; Walker, M.J.; Spina, D. Use of within-group designs to test anti-tussive drugs in conscious guinea-pigs. J. Pharmacol. Toxicol. Methods 2010, 61, 157–162. [Google Scholar] [CrossRef]
- Sterusky, M.; Plevkova, J.; Grendar, M.; Buday, T. Female Guinea Pig Model for Cough Studies and Its Response to Most Common Tussive Substances. Physiol. Res. 2020, 69, S171–S179. [Google Scholar] [CrossRef]
- Plevkova, J.; Buday, T.; Kavalcikova Bogdanova, N.; Kovacikova, L.; Ruzinak, R. Role of gender in basic cough research. Respir. Physiol. Neurobiol. 2017, 245, 53–56. [Google Scholar] [CrossRef]
- Drake, M.G.; McGarvey, L.P.; Morice, A.H. From bench to bedside: The role of cough hypersensitivity in chronic cough. Clin. Transl. Med. 2023, 13, e1343. [Google Scholar] [CrossRef]
- Belvisi, M.G.; Birrell, M.A.; Khalid, S.; Wortley, M.A.; Dockry, R.; Coote, J.; Holt, K.; Dubuis, E.; Kelsall, A.; Maher, S.A.; et al. Neurophenotypes in Airway Diseases. Insights from Translational Cough Studies. Am. J. Respir. Crit. Care Med. 2016, 193, 1364–1372. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montero, P.; Roger, I.; Esposito, E.; Milara, J.; Cortijo, J. Comparative Analysis of Classic and Novel Antitussives on Cough Suppression in Guinea Pigs. Pharmaceutics 2025, 17, 1423. https://doi.org/10.3390/pharmaceutics17111423
Montero P, Roger I, Esposito E, Milara J, Cortijo J. Comparative Analysis of Classic and Novel Antitussives on Cough Suppression in Guinea Pigs. Pharmaceutics. 2025; 17(11):1423. https://doi.org/10.3390/pharmaceutics17111423
Chicago/Turabian StyleMontero, Paula, Inés Roger, Erika Esposito, Javier Milara, and Julio Cortijo. 2025. "Comparative Analysis of Classic and Novel Antitussives on Cough Suppression in Guinea Pigs" Pharmaceutics 17, no. 11: 1423. https://doi.org/10.3390/pharmaceutics17111423
APA StyleMontero, P., Roger, I., Esposito, E., Milara, J., & Cortijo, J. (2025). Comparative Analysis of Classic and Novel Antitussives on Cough Suppression in Guinea Pigs. Pharmaceutics, 17(11), 1423. https://doi.org/10.3390/pharmaceutics17111423




