Pioglitazone-Based Combination Approaches for Non-Small-Cell Lung Cancer
Abstract
1. Introduction
2. Mechanisms of Action of PPARγ Agonists
3. Implications of Pioglitazone and Pioglitazone-Based Combination Approaches for NSCLC
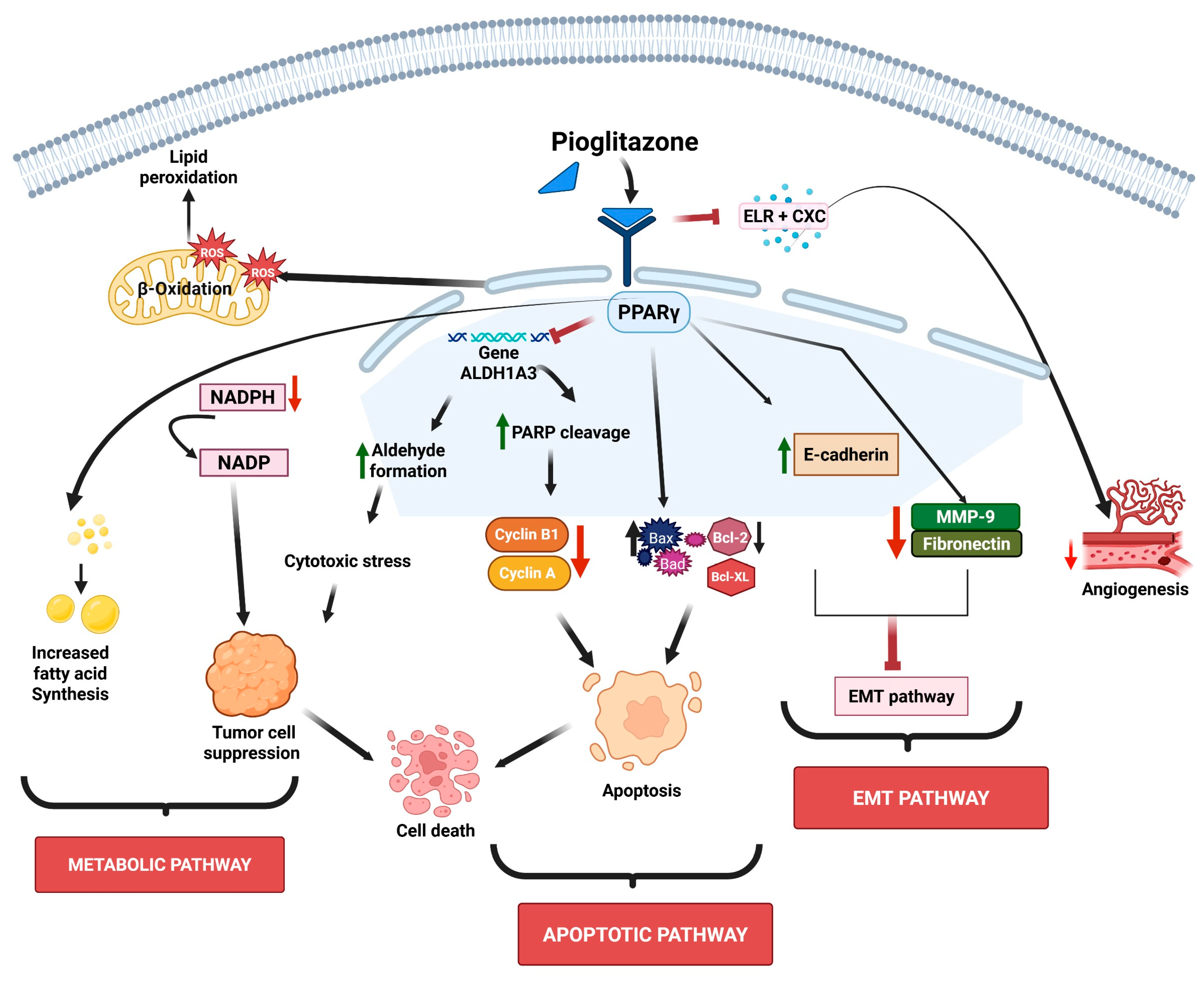
3.1. Evidence from In Vitro and In Vivo Studies
3.1.1. Pioglitazone’s Effects on Metabolic Pathways
3.1.2. Pioglitazone Effects on Chemical Carcinogens-Induced Tumorigenesis
3.1.3. Pioglitazone’s Effects on the Targeting of Cell Signaling Pathways
3.1.4. Pioglitazone’s Effects in Combination Therapy Efficacy
| Drug | Cell Lines/ Mouse Models | Targets | Findings | Refs. |
|---|---|---|---|---|
| Pioglitazone | H1770, H3255, A549, H2347, Calu6, H1993, H2073, HEK 293 and HBEC Athymic mice | PPARγ | Pioglitazone-mediated sumolyation of PPARγ induced tumor suppression via disrupting redox balance | [35] |
| Pioglitazone | HBEC, H1993, H1299 | PPARγ | Pioglitazone inhibited ALDH1A3 and production of aldehydes and induced ROS generation, which resulted in the restriction of cell growth | [66] |
| Pioglitazone | FVB/N | PPARγ | Pioglitazone caused a reduction in high-grade dysplasia, reversal of EMT-associated gene changes, and complete elimination of basal cells in bronchial epithelium | [67] |
| Pioglitazone | A549, H1299, p53 wild-type (p53wt/wt) and mutant (p53wt/Ala135Val) mice. Female NIH Swiss mice. | Caspase-3, Ki-67 | Pioglitazone suppressed lung cancer progression and caused induction of apoptosis | [68] |
| Pioglitazone | A/J mice | PPARγ | Pioglitazone inhibited the formation of lung tumors | [69] |
| Pioglitazone | A549 Cells SCID mice | PPARγ | Pioglitazone resulted in the suppression of tumor proliferation and pro-angiogenic chemokines (ELR + CXC) | [75] |
| Pioglitazone and Rosiglitazone | A427 and A549 | PGE2 | PPARγ agonists reduced PGE2 levels to suppress cancer growth | [76] |
| Pioglitazone | NCI-H358 cells and Nude mice | E-cadherin | E-cadherin loss is associated with brain metastasis. Pioglitazone enhances E-cadherin expression and suppresses EMT. | [78] |
| Pioglitazone | H1299, H460, A549, H1975, HCC827, and Beas2B | MAPK, NF-κB, EGFR/AKT, TNFα, TGF β/SMAD, Myc, R-Ras, and Transketolase | Pioglitazone inhibited tumor cell proliferation, reduced invasiveness, induced apoptosis, and modulated cancer cell metabolism | [17] |
| Pioglitazone and metformin | B(a)P mouse | PPARγ | Combination therapy resulted in significant reduction in adenomas | [79] |
| Pioglitazone and aerosolized budesonide | A549, H1299 B(a)P mouse, | Combination therapy inhibited lung adenomas and reduced tumor burden | [80] | |
| Pioglitazone and celecoxib | Balb/c mice | NFκB and COX-2 | Combination therapy caused reduced lung tissue weight and improved histopathological features, indicating effective tumor suppression | [21] |
| Pioglitazone and gefitinib | HCC827, H16650, BEAS-2B, | PTEN, Akt, EGFR TKI | Combination therapy induced apoptosis and autophagy to inhibit cell growth | [83] |
3.2. Evidence from Clinical Studies
| Drug | Study Type/ Population | Assessments | Dosing | Findings | Refs. |
|---|---|---|---|---|---|
| Pioglitazone | Randomized phase II trial with the goal of improving precancerous lung lesions in current and former smokers (92 high-risk smokers) | Histology, Ki-67, Inflammation | 30 mg | No significant histological improvement, trend toward benefit in former smokers | [87] |
| Pioglitazone | Pilot window trial in 6 patients with stage IA–IIIA NSCLC (smokers/ex-smokers) | Ki-67 expression; gene expression in airway tissue | 45 mg | 20% median reduction in Ki-67; all patients showed decreased proliferation; immune gene modulation observed (inflammatory/B cell survival, and increased complement/chemokine signaling | [88] |
4. Conclusions and Future Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| NSCLC | Non-small-cell lung cancer |
| PPARγ | Peroxisome proliferator-activated receptor-gamma |
| MAPK | Mitogen-activated protein kinase |
| EMT | Epithelial-to-mesenchymal transition |
| NR1C3 | Nuclear receptor superfamily 1 group C member 3 |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| ALDHs | Aldehyde dehydrogenase |
| ROS | Reactive oxygen species |
| POX | Proline oxidase |
| PTEN | Phosphatase and tensin homolog |
| ERK | Extracellular signal-regulated kinase |
| GADD153 | Growth Arrest and DNA Damage |
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer Statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Riely, G.J.; Wood, D.E.; Ettinger, D.S.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; et al. Non–Small Cell Lung Cancer, Version 4.2024, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc Netw. 2024, 22, 249–274. [Google Scholar] [CrossRef]
- Siegel Mph, R.L.; Giaquinto, A.N.; Ahmedin, J.; Dvm, J.; Siegel, R.L. Cancer Statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Khodabakhshi, Z.; Mostafaei, S.; Arabi, H.; Oveisi, M.; Shiri, I.; Zaidi, H. Non-Small Cell Lung Carcinoma Histopathological Subtype Phenotyping Using High-Dimensional Multinomial Multiclass CT Radiomics Signature. Comput. Biol. Med. 2021, 136, 104752. [Google Scholar] [CrossRef] [PubMed]
- Hendrixson, M.; Gladkiy, Y.; Thyagarajan, A.; Sahu, R.P. Efficacy of Sorafenib-Based Therapies for Non-Small Cell Lung Cancer. Med. Sci. 2024, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Sekido, Y.; Fong, K.M.; Minna, J.D. Progress in Understanding the Molecular Pathogenesis of Human Lung Cancer. Biochim. Biophys. Acta 1998, 1378, F21–F59. [Google Scholar] [CrossRef] [PubMed]
- Rodak, O.; Peris-Díaz, M.D.; Olbromski, M.; Podhorska-Okołów, M.; Dzięgiel, P. Current Landscape of Non-Small Cell Lung Cancer: Epidemiology, Histological Classification, Targeted Therapies, and Immunotherapy. Cancers 2021, 13, 4705. [Google Scholar] [CrossRef]
- Tan, N.; Li, Y.; Ying, J.; Chen, W. Histological Transformation in Lung Adenocarcinoma: Insights of Mechanisms and Therapeutic Windows. J. Transl. Int. Med. 2024, 12, 452. [Google Scholar] [CrossRef]
- Lu, T.; Yang, X.; Huang, Y.; Zhao, M.; Li, M.; Ma, K.; Yin, J.; Zhan, C.; Wang, Q. Trends in the Incidence, Treatment, and Survival of Patients with Lung Cancer in the Last Four Decades. Cancer Manag. Res. 2019, 11, 943. [Google Scholar] [CrossRef]
- Leiter, A.; Veluswamy, R.R.; Wisnivesky, J.P. The Global Burden of Lung Cancer: Current Status and Future Trends. Nat. Rev. Clin. Oncol. 2023, 20, 624–639. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, M.; Shang, J. PPARγ Modulators in Lung Cancer: Molecular Mechanisms, Clinical Prospects, and Challenges. Biomolecules 2024, 14, 190. [Google Scholar] [CrossRef]
- Zou, K.; Sun, P.; Huang, H.; Zhuo, H.; Qie, R.; Xie, Y.; Luo, J.; Li, N.; Li, J.; He, J.; et al. Etiology of Lung Cancer: Evidence from Epidemiologic Studies. J. Natl. Cancer Cent. 2022, 2, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Alduais, Y.; Zhang, H.; Fan, F.; Chen, J.; Chen, B. Non-Small Cell Lung Cancer (NSCLC): A Review of Risk Factors, Diagnosis, and Treatment. Medicine 2023, 102, e32899. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.; Kim, S.Y.; Cheng, H. Update 2020: Management of Non-Small Cell Lung Cancer. Lung 2020, 198, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Araghi, M.; Mannani, R.; Heidarnejad maleki, A.; Hamidi, A.; Rostami, S.; Safa, S.H.; Faramarzi, F.; Khorasani, S.; Alimohammadi, M.; Tahmasebi, S.; et al. Recent Advances in Non-Small Cell Lung Cancer Targeted Therapy; An Update Review. Cancer Cell Int. 2023, 23, 162. [Google Scholar] [CrossRef]
- Guo, Q.; Liu, L.; Chen, Z.; Fan, Y.; Zhou, Y.; Yuan, Z.; Zhang, W. Current Treatments for Non-Small Cell Lung Cancer. Front. Oncol. 2022, 12, 945102. [Google Scholar] [CrossRef]
- Ciaramella, V.; Sasso, F.C.; Di Liello, R.; Corte, C.M.D.; Barra, G.; Viscardi, G.; Esposito, G.; Sparano, F.; Troiani, T.; Martinelli, E.; et al. Activity and Molecular Targets of Pioglitazone via Blockade of Proliferation, Invasiveness and Bioenergetics in Human NSCLC. J. Exp. Clin. Cancer Res. 2019, 38, 178. [Google Scholar] [CrossRef]
- Wan, Y.; Evans, R.M. Rosiglitazone Activation of PPARγ Suppresses Fractalkine Signaling. J. Mol. Endocrinol. 2009, 44, 135. [Google Scholar] [CrossRef]
- Sharma, V.; Patial, V. Peroxisome Proliferator-Activated Receptor Gamma and Its Natural Agonists in the Treatment of Kidney Diseases. Front. Pharmacol. 2022, 13, 991059. [Google Scholar] [CrossRef]
- Ahmadian, M.; Suh, J.M.; Hah, N.; Liddle, C.; Atkins, A.R.; Downes, M.; Evans, R.M. PPARγ Signaling and Metabolism: The Good, the Bad and the Future. Nat. Med. 2013, 19, 557–566. [Google Scholar] [CrossRef]
- Kiran, A.V.V.V.R.; Kumari, G.K.; Krishnamurthy, P.T. Preliminary Evaluation of Anticancer Efficacy of Pioglitazone Combined with Celecoxib for the Treatment of Non-Small Cell Lung Cancer. Investig. New Drugs 2022, 40, 1–9. [Google Scholar] [CrossRef]
- Orasanu, G.; Ziouzenkova, O.; Devchand, P.R.; Nehra, V.; Hamdy, O.; Horton, E.S.; Plutzky, J. The PPARγ Agonist Pioglitazone Represses Inflammation In A PPARα-Dependent Manner In Vitro and In Vivo In Mice. J. Am. Coll. Cardiol. 2008, 52, 869. [Google Scholar] [CrossRef] [PubMed]
- Deeks, E.D.; Keam, S.J. Rosiglitazone. Drugs 2007, 67, 2747–2779. [Google Scholar] [CrossRef] [PubMed]
- See, L.C.; Wu, C.Y.; Tsai, C.Y.; Lee, C.C.; Chen, J.J.; Jenq, C.C.; Chen, C.Y.; Chen, Y.C.; Yen, C.L.; Yang, H.Y. PPAR-γ Agonist Pioglitazone and the Risks of Malignancy among Type2 Diabetes Mellitus Patients. Acta Diabetol. 2025, 62, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Minge, C.E.; Ryan, N.K.; Van Der Hoek, K.H.; Robker, R.L.; Norman, R.J. Troglitazone Regulates Peroxisome Proliferator-Activated Receptors and Inducible Nitric Oxide Synthase in Murine Ovarian Macrophages. Biol. Reprod. 2006, 74, 153–160. [Google Scholar] [CrossRef]
- Reddy, A.T.; Lakshmi, S.P.; Reddy, R.C. PPARγ as a Novel Therapeutic Target in Lung Cancer. PPAR Res. 2016, 2016, 8972570. [Google Scholar] [CrossRef]
- Chi, T.; Wang, M.; Wang, X.; Yang, K.; Xie, F.; Liao, Z.; Wei, P. PPAR-γ Modulators as Current and Potential Cancer Treatments. Front. Oncol. 2021, 11, 737776. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Phan, L.M.; Yeung, S.C.J.; Lee, M.H. Cancer Metabolic Reprogramming: Importance, Main Features, and Potentials for Precise Targeted Anti-Cancer Therapies. Cancer Biol. Med. 2014, 11, 1. [Google Scholar] [CrossRef]
- Andela, V.B.; Altuwaijri, S.; Wood, J.; Rosier, R.N. Inhibition of β-Oxidative Respiration Is a Therapeutic Window Associated with the Cancer Chemo-Preventive Activity of PPARγ Agonists. FEBS Lett. 2005, 579, 1765–1769. [Google Scholar] [CrossRef]
- Singh, S.; Brocker, C.; Koppaka, V.; Chen, Y.; Jackson, B.C.; Matsumoto, A.; Thompson, D.C.; Vasiliou, V. Aldehyde Dehydrogenases in Cellular Responses to Oxidative/Electrophilic Stress. Free Radic. Biol. Med. 2012, 56, 89. [Google Scholar] [CrossRef]
- Dinavahi, S.S.; Bazewicz, C.G.; Gowda, R.; Robertson, G.P. Aldehyde Dehydrogenase Inhibitors for Cancer Therapeutics. Trends Pharmacol. Sci. 2019, 40, 774–789. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Lu, L.; Zander, D.S.; Sreerama, L.; Coco, D.; Moreb, J.S. ALDH1A1 and ALDH3A1 Expression in Lung Cancers: Correlation with Histologic Type and Potential Precursors. Lung Cancer 2008, 59, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Zavala, J.S.; Calleja, L.F.; Moreno-Sánchez, R.; Yoval-Sánchez, B. Role of Aldehyde Dehydrogenases in Physiopathological Processes. Chem. Res. Toxicol. 2019, 32, 405–420. [Google Scholar] [CrossRef]
- Phan, A.N.H.; Vo, V.T.A.; Hua, T.N.M.; Kim, M.K.; Jo, S.Y.; Choi, J.W.; Kim, H.W.; Son, J.; Suh, Y.A.; Jeong, Y. PPARγ Sumoylation-Mediated Lipid Accumulation in Lung Cancer. Oncotarget 2017, 8, 82491. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495. [Google Scholar] [CrossRef]
- Sharma, A.; Boise, L.H.; Shanmugam, M. Cancer Metabolism and the Evasion of Apoptotic Cell Death. Cancers 2019, 11, 1144. [Google Scholar] [CrossRef]
- Budihardjo, I.; Oliver, H.; Lutter, M.; Luo, X.; Wang, X. Biochemical Pathways of Caspase Activation during Apoptosis. Annu. Rev. Cell Dev. Biol. 1999, 15, 269–290. [Google Scholar] [CrossRef]
- Riedl, S.J.; Shi, Y. Molecular Mechanisms of Caspase Regulation during Apoptosis. Nat. Rev. Mol. Cell Biol. 2004, 5, 897–907. [Google Scholar] [CrossRef]
- Ki, Y.K.; Jin, H.A.; Hyae, G.C. Apoptotic Action of Peroxisome Proliferator-Activated Receptor-Gamma Activation in Human Non Small-Cell Lung Cancer Is Mediated via Proline Oxidase-Induced Reactive Oxygen Species Formation. Mol. Pharmacol. 2007, 72, 674–685. [Google Scholar] [CrossRef]
- Ni, J.; Zhou, L.-l.; Ding, L.; Zhao, X.; Cao, H.; Fan, F.; Li, H.; Lou, R.; Du, Y.; Dong, S.; et al. PPARγ Agonist Efatutazone and Gefitinib Synergistically Inhibit the Proliferation of EGFR-TKI-Resistant Lung Adenocarcinoma Cells via the PPARγ/PTEN/Akt Pathway. Exp. Cell Res. 2017, 361, 246–256. [Google Scholar] [CrossRef]
- Li, M.; Lee, T.W.; Yim, A.P.C.; Mok, T.S.K.; Chen, G.G. Apoptosis Induced by Troglitazone Is Both Peroxisome Proliferator-Activated Receptor-Gamma- and ERK-Dependent in Human Non-Small Lung Cancer Cells. J. Cell Physiol. 2006, 209, 428–438. [Google Scholar] [CrossRef]
- Satoh, T.; Toyoda, M.; Hoshino, H.; Monden, T.; Yamada, M.; Shimizu, H.; Miyamoto, K.; Mori, M. Activation of Peroxisome Proliferator-Activated Receptor-Gamma Stimulates the Growth Arrest and DNA-Damage Inducible 153 Gene in Non-Small Cell Lung Carcinoma Cells. Oncogene 2002, 21, 2171–2180. [Google Scholar] [CrossRef] [PubMed]
- Nazim, U.M.; Moon, J.H.; Lee, Y.J.; Seol, J.W.; Park, S.Y. PPARγ Activation by Troglitazone Enhances Human Lung Cancer Cells to TRAIL-Induced Apoptosis via Autophagy Flux. Oncotarget 2017, 8, 26819–26831. [Google Scholar] [CrossRef] [PubMed]
- Keshamouni, V.G.; Reddy, R.C.; Arenberg, D.A.; Joel, B.; Thannickal, V.J.; Kalemkerian, G.P.; Standiford, T.J. Peroxisome Proliferator-Activated Receptor-γ Activation Inhibits Tumor Progression in Non-Small-Cell Lung Cancer. Oncogene 2004, 23, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Matthews, H.K.; Bertoli, C.; de Bruin, R.A.M. Cell Cycle Control in Cancer. Nat. Rev. Mol. Cell Biol. 2022, 23, 74–88. [Google Scholar] [CrossRef]
- Han, S.; Sidell, N.; Fisher, P.B.; Roman, J. Up-Regulation of P21 Gene Expression by Peroxisome Proliferator-Activated Receptor Gamma in Human Lung Carcinoma Cells. Clin. Cancer Res. 2004, 10, 1911–1919. [Google Scholar] [CrossRef]
- Gandalovičová, A.; Vomastek, T.; Rosel, D.; Brábek, J. Cell Polarity Signaling in the Plasticity of Cancer Cell Invasiveness. Oncotarget 2016, 7, 25022. [Google Scholar] [CrossRef]
- Shabani, H.K.; Kitange, G.; Tsunoda, K.; Anda, T.; Tokunaga, Y.; Shibata, S.; Kaminogo, M.; Hayashi, T.; Ayabe, H.; Iseki, M. Immunohistochemical Expression of E-Cadherin in Metastatic Brain Tumors. Brain Tumor Pathol. 2003, 20, 7–12. [Google Scholar] [CrossRef]
- Leber, M.F.; Efferth, T. Molecular Principles of Cancer Invasion and Metastasis (Review). Int. J. Oncol. 2009, 34, 881–895. [Google Scholar] [CrossRef]
- Castaneda, M.; den Hollander, P.; Kuburich, N.A.; Rosen, J.M.; Mani, S.A. Mechanisms of Cancer Metastasis. Semin. Cancer Biol. 2022, 87, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Reka, A.K.; Kurapati, H.; Narala, V.R.; Bommer, G.; Chen, J.; Standiford, T.J.; Keshamouni, V.G. Peroxisome Proliferator-Activated Receptor-g Activation Inhibits Tumor Metastasis by Antagonizing Smad3-Mediated Epithelial-Mesenchymal Transition. Mol. Cancer Ther. 2010, 9, 3221–3232. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, R.; Li, H.; Winn, R.A.; Sorenson, A.L.; Weiser-Evans, M.C.M.; Nemenoff, R.A. Peroxisome Proliferator-Activated Receptor-γ Inhibits Transformed Growth of Non-Small Cell Lung Cancer Cells through Selective Suppression of Snail. Neoplasia 2010, 12, 224. [Google Scholar] [CrossRef] [PubMed]
- Han, S.W.; Ritzenthaler, J.D.; Rivera, H.N.; Roman, J. Peroxisome Proliferator-Activated Receptor-γ Ligands Suppress Fibronectin Gene Expression in Human Lung Carcinoma Cells: Involvement of Both CRE and Sp1. Am. J. Physiol. Lung Cell Mol. Physiol. 2005, 289, 419–428. [Google Scholar] [CrossRef]
- Wu, S.; Zhu, C.; Tang, D.; Dou, Q.P.; Shen, J.; Chen, X. The Role of Ferroptosis in Lung Cancer. Biomark. Res. 2021, 9, 82. [Google Scholar] [CrossRef]
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and Function. Cell Death Differ. 2016, 23, 369–379. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Shabanian, N.; Shourmij, M.; Nashtahosseini, Z.; Ali, S.A.-J.; Alameedee, G.A.A.; Amiri, F.T.; Zefrei, F.J.; Farzipour, S. The Role of Pioglitazone as a Ferroptosis Inhibitor in Mitigating Radiation-Induced Damage in Testicular Tissue of Mice. Curr. Pharm. Biotechnol. 2025, 26, e13892010395182. [Google Scholar] [CrossRef]
- Tomanek, R.J.; Schatteman, G.C. Angiogenesis: New Insights and Therapeutic Potential. Anat. Rec. 2000, 261, 126–135. [Google Scholar] [CrossRef]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in Cancer. Vasc. Health Risk Manag. 2006, 2, 213. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor Angiogenesis: Causes, Consequences, Challenges and Opportunities. Cell Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Saman, H.; Raza, S.S.; Uddin, S.; Rasul, K. Inducing Angiogenesis, a Key Step in Cancer Vascularization, and Treatment Approaches. Cancers 2020, 12, 1172. [Google Scholar] [CrossRef]
- Jin, M.Z.; Jin, W.L. The Updated Landscape of Tumor Microenvironment and Drug Repurposing. Signal Transduct. Targeted Ther. 2020, 5, 166. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sorenson, A.L.; Poczobutt, J.; Amin, J.; Joyal, T.; Sullivan, T.; Crossno, J.T.; Weiser-Evans, M.C.M.; Nemenoff, R.A. Activation of PPARγ in Myeloid Cells Promotes Lung Cancer Progression and Metastasis. PLoS ONE 2011, 6, e28133. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.S.; Yip, Y.S.; Lim, E.K.Y.; Wahli, W.; Tan, N.S. PPARs and Tumor Microenvironment: The Emerging Roles of the Metabolic Master Regulators in Tumor Stromal-Epithelial Crosstalk and Carcinogenesis. Cancers 2021, 13, 2153. [Google Scholar] [CrossRef] [PubMed]
- Hua, T.N.M.; Namkung, J.; Phan, A.N.H.; Vo, V.T.A.; Kim, M.K.; Jeong, Y.; Choi, J.W. PPARgamma-Mediated ALDH1A3 Suppression Exerts Anti-Proliferative Effects in Lung Cancer by Inducing Lipid Peroxidation. J. Recept. Signal Transduct. Res. 2018, 38, 191–197. [Google Scholar] [CrossRef]
- Dwyer-Nield, L.D.; McArthur, D.G.; Hudish, T.M.; Hudish, L.I.; Mirita, C.; Sompel, K.; Smith, A.J.; Alavi, K.; Ghosh, M.; Merrick, D.T.; et al. PPARgamma Agonism Inhibits Progression of Premalignant Lesions in a Murine Lung Squamous Cell Carcinoma Model. Int. J. Cancer 2022, 151, 2195–2205. [Google Scholar] [CrossRef]
- Wang, Y.; James, M.; Wen, W.; Lu, Y.; Szabo, E.; Lubet, R.A.; You, M. Chemopreventive Effects of Pioglitazone on Chemically Induced Lung Carcinogenesis in Mice. Mol. Cancer Ther. 2010, 9, 3074–3082. [Google Scholar] [CrossRef]
- Li, M.Y.; Yuan, H.; Ma, L.T.; Kong, A.W.Y.; Hsin, M.K.Y.; Yip, J.H.Y.; Underwood, M.J.; Chen, G.G. Roles of Peroxisome Proliferator–Activated Receptor–α and –γ in the Development of Non–Small Cell Lung Cancer. Am. J. Respir. Cell Mol. Biol. 2012, 43, 674–683. [Google Scholar] [CrossRef]
- Hecht, S.S.; Isaacs, S.; Trushin, N. Lung Tumor Induction in A/J Mice by the Tobacco Smoke Carcinogens 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanone and Benzo[a]Pyrene: A Potentially Useful Model for Evaluation of Chemopreventive Agents. Carcinogenesis 1994, 15, 2721–2725. [Google Scholar] [CrossRef]
- Tuveson, D.A.; Jacks, T. Modeling Human Lung Cancer in Mice: Similarities and Shortcomings. Oncogene 1999, 18, 5318–5324. [Google Scholar] [CrossRef] [PubMed]
- Titis, A.P.; Forkert, P.G. Strain-Related Differences in Bioactivation of Vinyl Carbamate and Formation of DNA Adducts in Lungs of A/J, CD-1, and C57BL/6 Mice. Toxicol. Sci. 2001, 59, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Riolobos, L.; Gad, E.A.; Treuting, P.M.; Timms, A.E.; Hershberg, E.A.; Corulli, L.R.; Rodmaker, E.; Disis, M.L. The Effect of Mouse Strain, Sex and Carcinogen Dose on Toxicity and the Development of Lung Dysplasia and Squamous Cell Carcinomas in Mice. Cancer Prev. Res. 2019, 12, 507. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Nishioka, Y.; Izumi, K.; Tsuruo, T.; Tanaka, T.; Miyasaka, M.; Sone, S. Novel Metastasis Model of Human Lung Cancer in SCID Mice Depleted of NK Cells. Int. J. Cancer 1996, 67, 211–217. [Google Scholar] [CrossRef]
- Keshamouni, V.G.; Arenberg, D.A.; Reddy, R.C.; Newstead, M.J.; Anthwal, S.; Standiford, T.J. PPAR-γ Activation Inhibits Angiogenesis by Blocking ELR+CXC Chemokine Production in Non-Small Cell Lung Cancer. Neoplasia 2005, 7, 294. [Google Scholar] [CrossRef]
- Hazra, S.; Batra, R.K.; Tai, H.H.; Sharma, S.; Cui, X.; Dubinett, S.M. Pioglitazone and Rosiglitazone Decrease Prostaglandin E2 in Non–Small-Cell Lung Cancer Cells by Up-Regulating 15-Hydroxyprostaglandin Dehydrogenase. Mol. Pharmacol. 2007, 71, 1715–1720. [Google Scholar] [CrossRef]
- Petersen, I.; Hidalgo, A.; Petersen, S.; Schlüns, K.; Schewe, C.; Pacyna-Gengelbach, M.; Goeze, A.; Krebber, B.; Knösel, T.; Kaufmann, O.; et al. Chromosomal Imbalances in Brain Metastases of Solid Tumors. Brain Pathol. 2006, 10, 395. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Yang, S.H.; Lee, J.E.; Cho, D.G.; Kim, H.K.; Kim, S.H.; Kim, I.S.; Hong, J.T.; Sung, J.H.; Son, B.C.; et al. E-Cadherin as a Predictive Marker of Brain Metastasis in Nonsmallcell Lung Cancer, and Its Regulation by Pioglitazone in a Preclinical Model. J. Neurooncol. 2012, 109, 219–227. [Google Scholar] [CrossRef]
- Seabloom, D.E.; Galbraith, A.R.; Haynes, A.M.; Antonides, J.D.; RWuertz, B.; Miller, W.A.; Miller, K.A.; Steele, V.E.; Miller, M.S.; Clapper, M.L.; et al. Fixed-Dose Combinations of Pioglitazone & Metformin for Lung Cancer Prevention. Cancer Prev. Res. 2017, 10, 116–123. [Google Scholar] [CrossRef]
- Fu, H.; Zhang, J.; Pan, J.; Zhang, Q.; Lu, Y.; Wen, W.; Lubet, R.A.; Szabo, E.; Chen, R.; Wang, Y.; et al. Chemoprevention of Lung Carcinogenesis by the Combination of Aerosolized Budesonide and Oral Pioglitazone in A/J Mice. Mol. Carcinog. 2011, 50, 913–921. [Google Scholar] [CrossRef]
- Zhou, H.Q.; Zhang, Y.X.; Chen, G.; Yu, Q.T.; Zhang, H.; Wu, G.W.; Wu, D.; Lin, Y.C.; Zhu, J.F.; Chen, J.H.; et al. Gefitinib (an EGFR Tyrosine Kinase Inhibitor) plus Anlotinib (an Multikinase Inhibitor) for Untreated, EGFR-Mutated, Advanced Non-Small Cell Lung Cancer (FL-ALTER): A Multicenter Phase III Trial. Signal Transduct. Target. Ther. 2024, 9, 215. [Google Scholar] [CrossRef]
- Sos, M.L.; Koker, M.; Weir, B.A.; Heynck, S.; Rabinovsky, R.; Zander, T.; Seeger, J.M.; Weiss, J.; Fischer, F.; Frommolt, P.; et al. PTEN Loss Contributes to Erlotinib Resistance in EGFR-Mutant Lung Cancer by Activation of Akt and EGFR. Cancer Res. 2009, 69, 3256. [Google Scholar] [CrossRef]
- To, K.K.W.; Wu, W.K.K.; Loong, H.H.F. PPARgamma Agonists Sensitize PTEN-Deficient Resistant Lung Cancer Cells to EGFR Tyrosine Kinase Inhibitors by Inducing Autophagy. Eur. J. Pharmacol. 2018, 823, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Keith, R.L.; Blatchford, P.J.; Merrick, D.T.; Bunn, P.A.; Bagwell, B.; Dwyer-Nield, L.D.; Jackson, M.K.; Geraci, M.W.; Miller, Y.E. A Randomized Phase II Trial of Pioglitazone for Lung Cancer Chemoprevention in High-Risk Current and Former Smokers. Cancer Prev. Res. 2019, 12, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Hickey, A.J.; Maloney, S.E.; Kuehl, P.J.; Phillips, J.E.; Wolff, R.K. Practical Considerations in Dose Extrapolation from Animals to Humans. J. Aerosol Med. Pulm. Drug Deliv. 2024, 37, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Selman, C.; Swindell, W.R. Putting a Strain on Diversity. EMBO J. 2018, 37, e100862. [Google Scholar] [CrossRef]
- Nair, A.; Morsy, M.A.; Jacob, S. Dose Translation between Laboratory Animals and Human in Preclinical and Clinical Phases of Drug Development. Drug Dev. Res. 2018, 79, 373–382. [Google Scholar] [CrossRef]
- Wigle, D.A.; Mandrekar, S.J.; Allen-Ziegler, K.; Gesthalter, Y.; Holland, P.; Aubry, M.-C.; Limburg, P.J.; Avi, S.; Szabo, E. Pioglitazone as a Candidate Chemoprevention Agent for Lung Cancer: A Pilot Window Trial in Early Stage NSCLC. J. Clin. Oncol. 2014, 32, 1581. [Google Scholar] [CrossRef]
- Pioglitazone to Treat Adults Undergoing Surgery for Non-Small Cell Lung Cancer. Available online: https://clinicaltrials.gov/study/NCT00923949 (accessed on 18 September 2025).
- Harrer, D.C.; Lüke, F.; Pukrop, T.; Ghibelli, L.; Gerner, C.; Reichle, A.; Heudobler, D. Peroxisome Proliferator-Activated Receptorα/γ Agonist Pioglitazone for Rescuing Relapsed or Refractory Neoplasias by Unlocking Phenotypic Plasticity. Front. Oncol. 2023, 13, 1289222. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, L.H.; Huang, B.; Wang, R.Y.; Yuan, S.X.; Tao, Q.F.; Xu, Y.; Sun, H.Y.; Lin, C.; Zhou, W.P. Pioglitazone, a PPARγ Agonist, Inhibits Growth and Invasion of Human Hepatocellular Carcinoma via Blockade of the Rage Signaling. Mol. Carcinog. 2015, 54, 1584–1595. [Google Scholar] [CrossRef]
- Lv, S.; Wang, W.; Wang, H.; Zhu, Y.; Lei, C. PPARγ Activation Serves as Therapeutic Strategy against Bladder Cancer via Inhibiting PI3K-Akt Signaling Pathway. BMC Cancer 2019, 19, 204. [Google Scholar] [CrossRef]
- Tokhanbigli, S.; Alavifard, H.; Aghdaei, H.A.; Zali, M.R.; Baghaei, K. Combination of Pioglitazone and Dendritic Cell to Optimize Efficacy of Immune Cell Therapy in CT26 Tumor Models. Bioimpacts 2023, 13, 333–346. [Google Scholar] [CrossRef]
- Alamdar, N.; Farivar, S.; Baghaei, K.; Hamidieh, A.A.; Soltaninejad, H.; Aghdaei, H.A.; Zali, M.R.; Saltanatpour, Z. Effect of Pioglitazone and Cetuximab on Colon Cancer Stem-like Cell (CCSLCs) Properties. Curr. Stem Cell Res. Ther. 2025, 20, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.; Wu, Y.-L.; Ahn, M.-J.; Garassino, M.C.; Kim, H.R.; Ramalingam, S.S.; Shepherd, F.A.; He, Y.; Akamatsu, H.; Theelen, W.S.M.E.; et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N. Engl. J. Med. 2017, 376, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Planchard, D.; Jänne, P.A.; Cheng, Y.; Yang, J.C.-H.; Yanagitani, N.; Kim, S.-W.; Sugawara, S.; Yu, Y.; Fan, Y.; Geater, S.L.; et al. Osimertinib with or without Chemotherapy in EGFR-Mutated Advanced NSCLC. N. Engl. J. Med. 2023, 389, 1935–1948. [Google Scholar] [CrossRef] [PubMed]
- Marrocco, I.; Giri, S.; Simoni-Nieves, A.; Gupta, N.; Rudnitsky, A.; Haga, Y.; Romaniello, D.; Sekar, A.; Zerbib, M.; Oren, R.; et al. L858R Emerges as a Potential Biomarker Predicting Response of Lung Cancer Models to Anti-EGFR Antibodies: Comparison of Osimertinib vs. Cetuximab. Cell Rep. Med. 2023, 4, 101142. [Google Scholar] [CrossRef]
- Kim, T.W.; Hong, D.W.; Hong, S.H. CB13, a Novel PPARγ Ligand, Overcomes Radio-Resistance via ROS Generation and ER Stress in Human Non-Small Cell Lung Cancer. Cell Death Dis. 2020, 11, 848. [Google Scholar] [CrossRef]
- Govindarajan, R.; Ratnasinghe, L.; Simmons, D.L.; Siegel, E.R.; Midathada, M.V.; Kim, L.; Kim, P.J.; Owens, R.J.; Lang, N.P. Thiazolidinediones and the Risk of Lung, Prostate, and Colon Cancer in Patients with Diabetes. J. Clin. Oncol. 2007, 25, 1476–1481. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, H.B.; Shi, X.F.; Song, Y. Conventional Hypoglycaemic Agents and the Risk of Lung Cancer in Patients with Diabetes: A Meta-Analysis. PLoS ONE 2014, 9, e99577. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aluru, S.; Thyagarajan, A.; Sahu, R.P. Pioglitazone-Based Combination Approaches for Non-Small-Cell Lung Cancer. Pharmaceutics 2025, 17, 1416. https://doi.org/10.3390/pharmaceutics17111416
Aluru S, Thyagarajan A, Sahu RP. Pioglitazone-Based Combination Approaches for Non-Small-Cell Lung Cancer. Pharmaceutics. 2025; 17(11):1416. https://doi.org/10.3390/pharmaceutics17111416
Chicago/Turabian StyleAluru, Sravya, Anita Thyagarajan, and Ravi P. Sahu. 2025. "Pioglitazone-Based Combination Approaches for Non-Small-Cell Lung Cancer" Pharmaceutics 17, no. 11: 1416. https://doi.org/10.3390/pharmaceutics17111416
APA StyleAluru, S., Thyagarajan, A., & Sahu, R. P. (2025). Pioglitazone-Based Combination Approaches for Non-Small-Cell Lung Cancer. Pharmaceutics, 17(11), 1416. https://doi.org/10.3390/pharmaceutics17111416







