Microfluidic Design of Ultradeformable Liposomes for Advanced Skin Delivery of Stellaria media Phytocomplex
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Stellaria Extract
2.3. Characterization of Stellaria Extract
2.4. Cell Viability Assay
2.5. Scalable Ultradeformable Liposomes Preparation
2.6. Physicochemical Characterization
2.7. Transmission Electron Microscopy
2.8. Entrapment Efficiency and Kinetic Release Profile
2.9. Long-Term Stability Studies
2.10. Deformability Assay
2.11. In Vitro Skin Permeation Studies
2.12. In Vivo Biosafety on Healthy Human Volunteers
2.13. Statistical Analysis
3. Results and Discussion
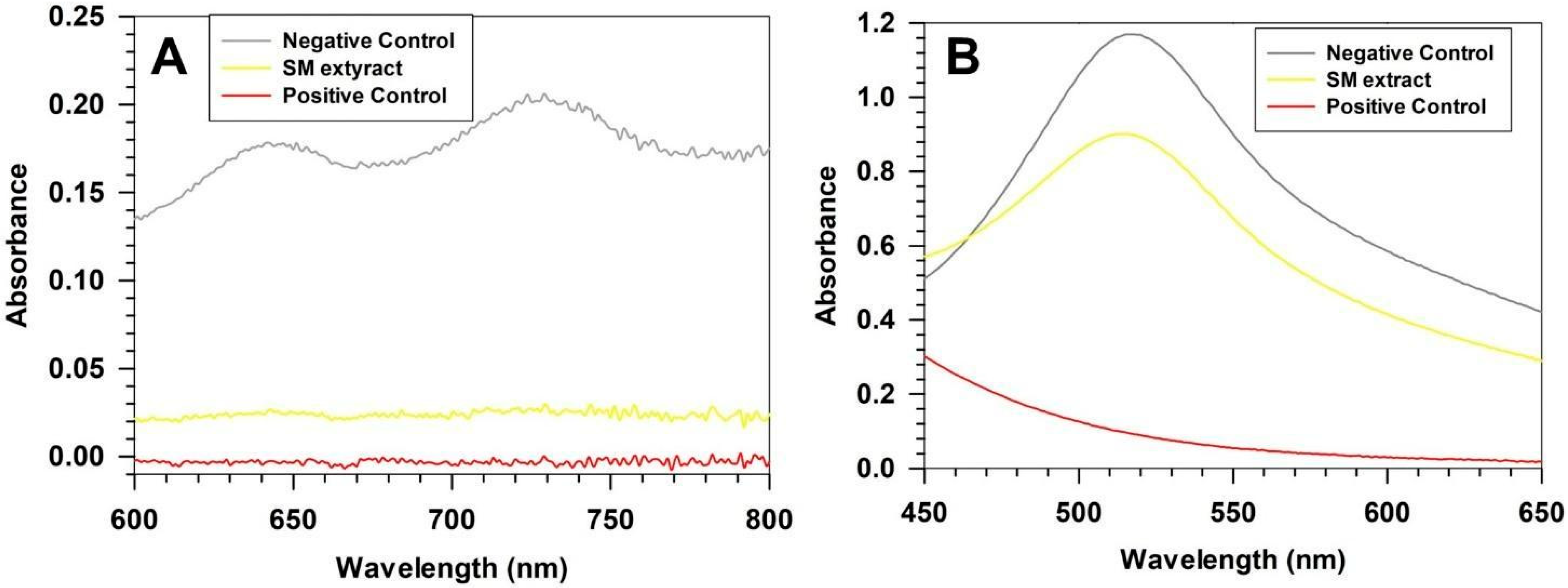
3.1. Chemical Characterization of SM Extract
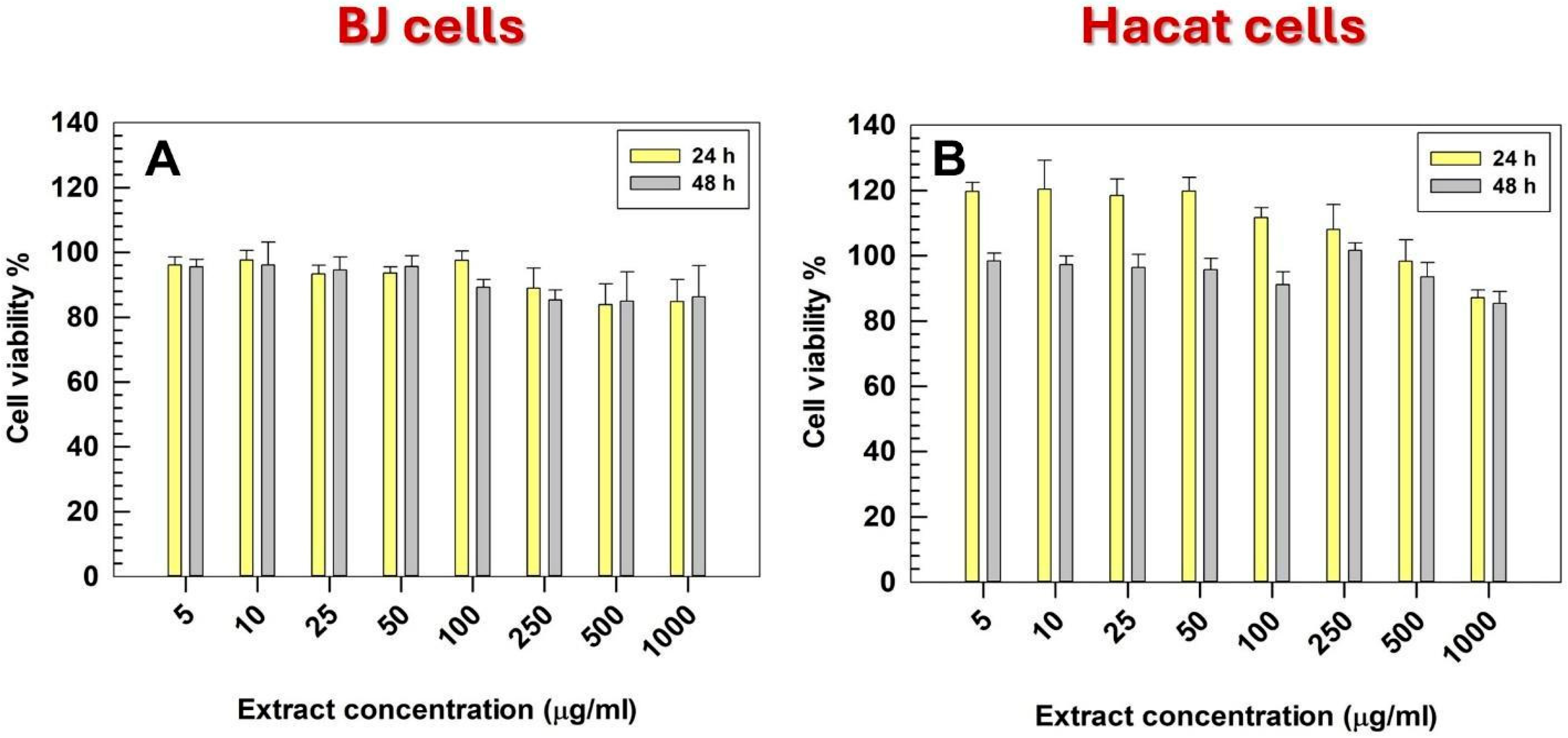
3.2. In Vitro Cell Viability
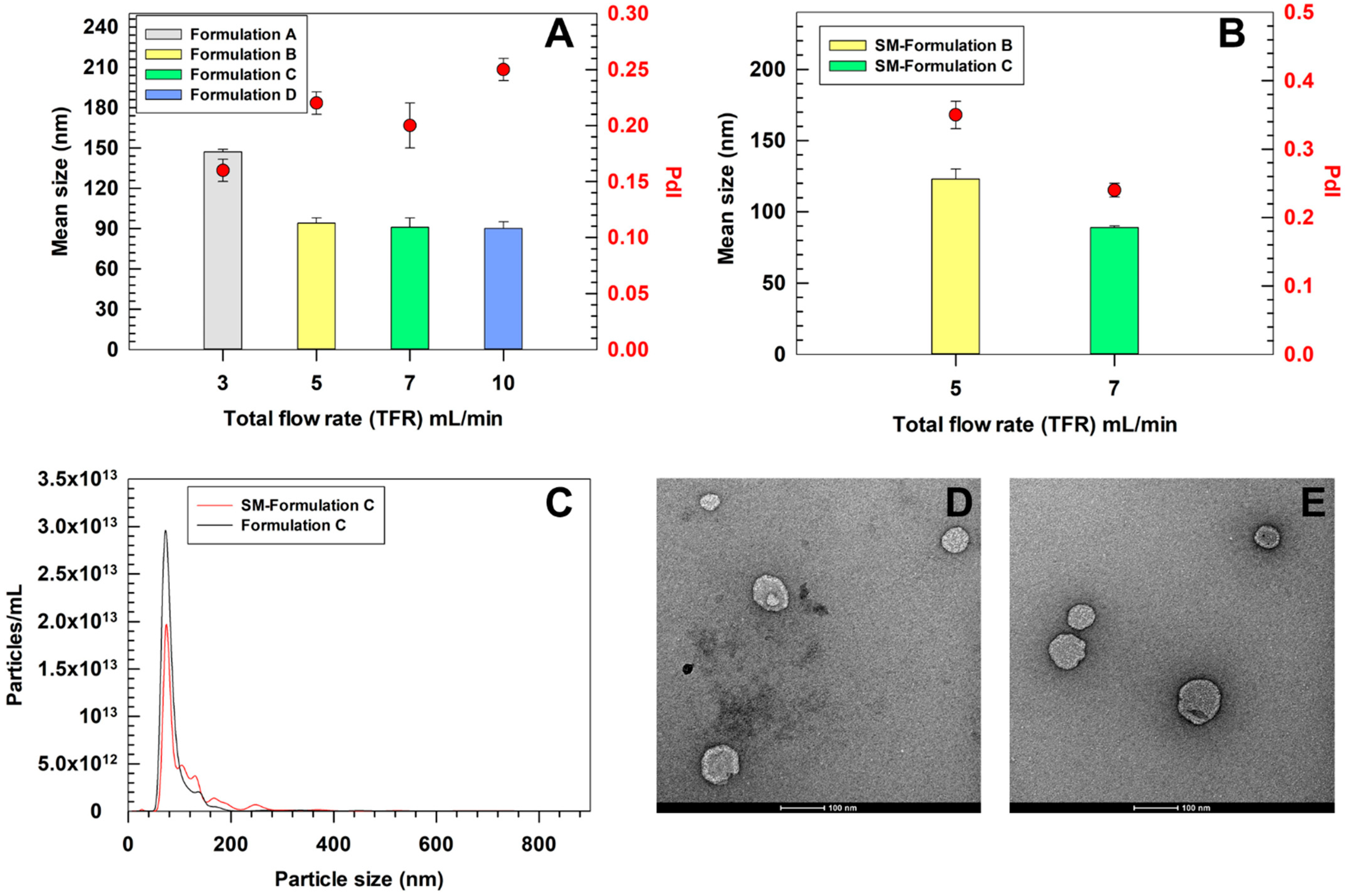
3.3. Scalable Preparation of SM-Ultradeformable Liposomes and Physicochemical Characterization
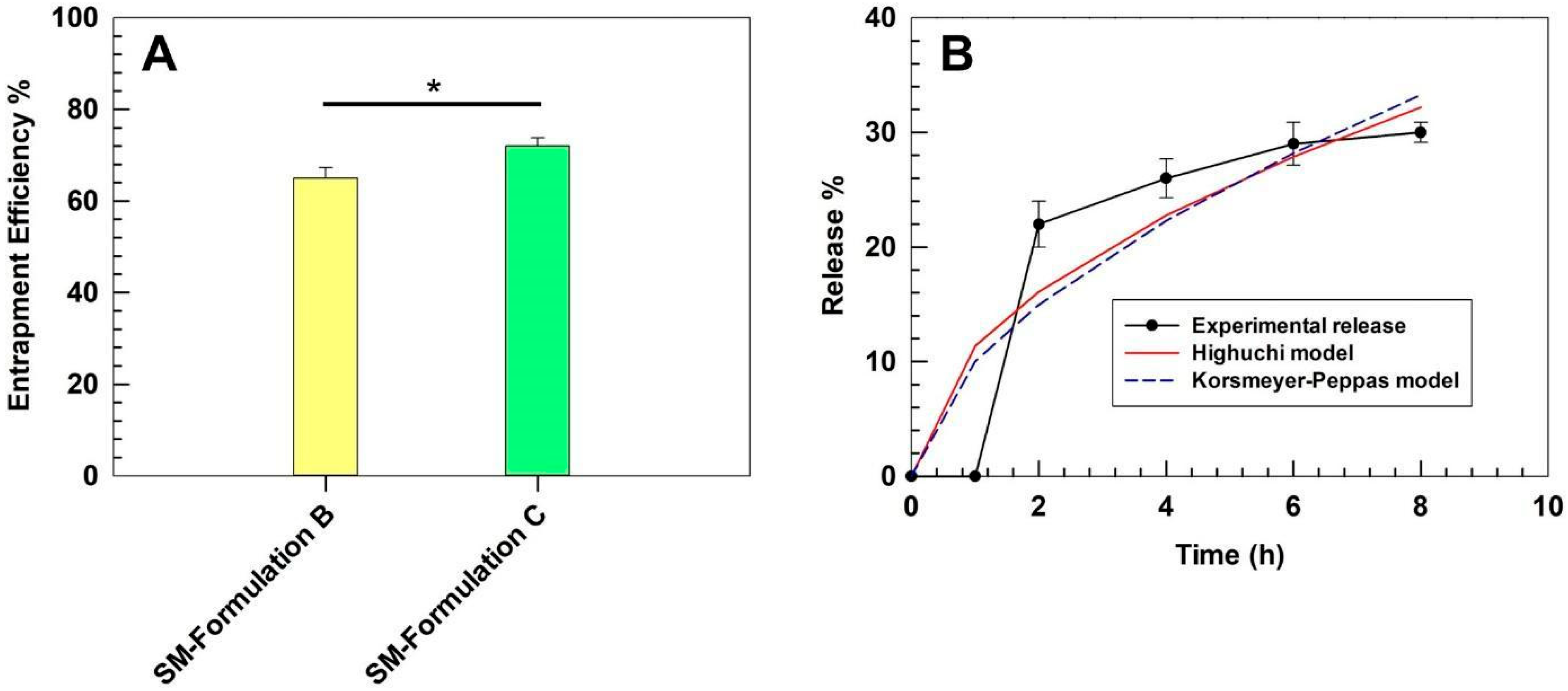
3.4. Entrapment Efficiency and Kinetics of Release of SM-Ultradeformable Liposomes
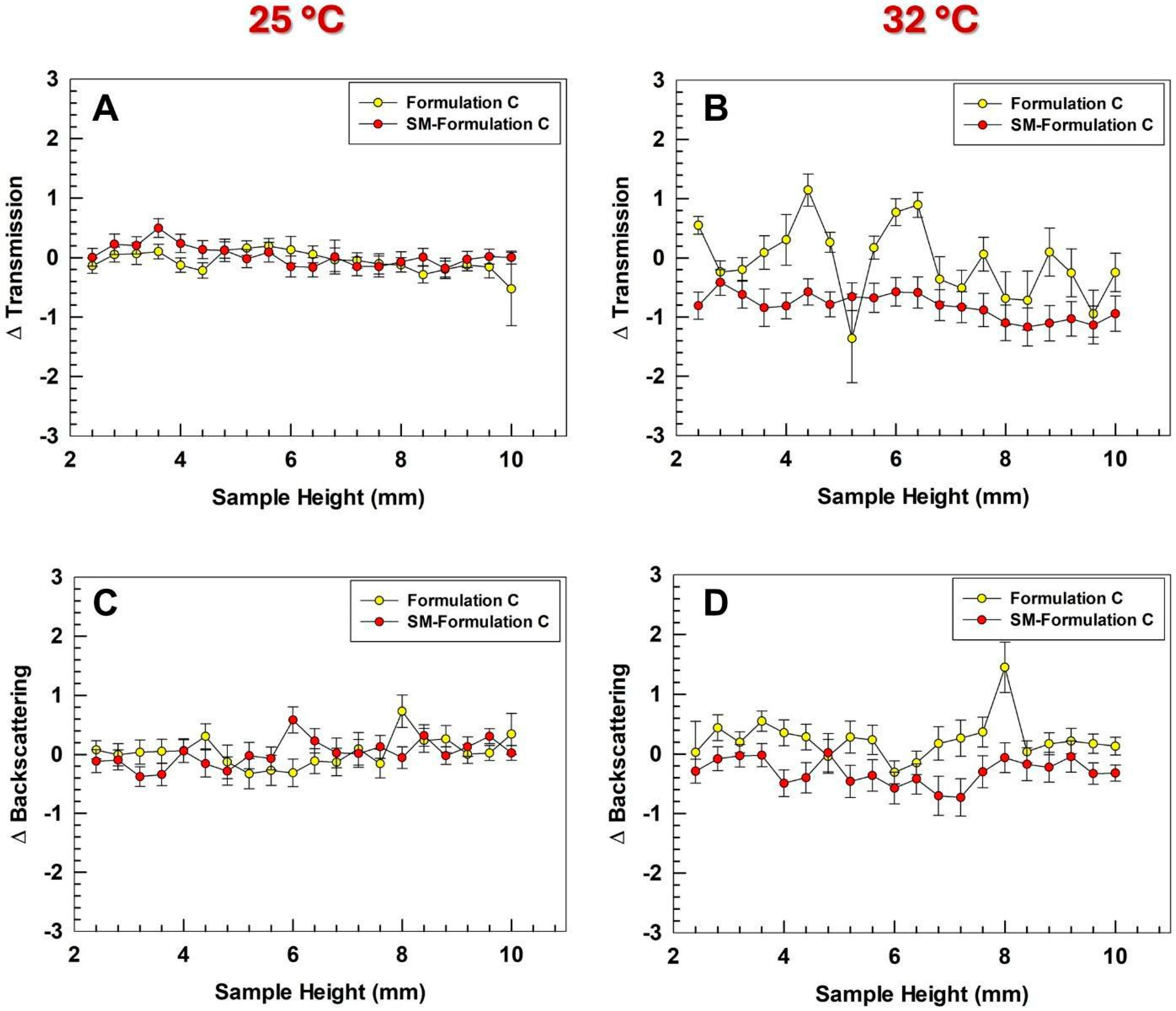
3.5. Long-Term Stability Studies
3.6. Deformability Index of Ultradeformable Liposomes
3.7. Percutaneous Permeation Studies
3.8. Skin Tolerability Studies on Healthy Human Volunteers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wagner, A.; Vorauer-Uhl, K. Liposome technology for industrial purposes. J. Drug Deliv. 2011, 2011, 591325. [Google Scholar] [CrossRef]
- Matharoo, N.; Mohd, H.; Michniak-Kohn, B. Transferosomes as a transdermal drug delivery system: Dermal kinetics and recent developments. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2024, 16, e1918. [Google Scholar] [CrossRef]
- Eugster, R.; Luciani, P. Liposomes: Bridging the gap from lab to pharmaceuticals. Curr. Opin. Colloid Interface Sci. 2025, 75, 101875. [Google Scholar] [CrossRef]
- Crommelin, D.J.A.; van Hoogevest, P.; Storm, G. The role of liposomes in clinical nanomedicine development. What Now? Now What? J. Control. Release 2020, 318, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Boakye, C.H.A.; Patel, K.; Doddapaneni, R.; Bagde, A.; Behl, G.; Chowdhury, N.; Safe, S.; Singh, M. Ultra-flexible nanocarriers for enhanced topical delivery of a highly lipophilic antioxidative molecule for skin cancer chemoprevention. Colloids Surf. B Biointerfaces 2016, 143, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Cristiano, M.C.; d’Avanzo, N.; Mancuso, A.; Tarsitano, M.; Barone, A.; Torella, D.; Paolino, D.; Fresta, M. Ammonium Glycyrrhizinate and Bergamot Essential Oil Co-Loaded Ultradeformable Nanocarriers: An Effective Natural Nanomedicine for In Vivo Anti-Inflammatory Topical Therapies. Biomedicines 2022, 10, 1039. [Google Scholar] [CrossRef]
- Fernández-García, R.; Lalatsa, A.; Statts, L.; Bolás-Fernández, F.; Ballesteros, M.P.; Serrano, D.R. Transferosomes as nanocarriers for drugs across the skin: Quality by design from lab to industrial scale. Int. J. Pharm. 2020, 573, 118817. [Google Scholar] [CrossRef]
- Singh, A.; Fatima, Z.; Srivastava, D. A Comprehensive Review on Polyphenols based Nanovesicular System for Topical Delivery. Curr. Drug Deliv. 2025, 22, 123–139. [Google Scholar] [CrossRef]
- Wu, P.S.; Li, Y.S.; Kuo, Y.C.; Tsai, S.J.; Lin, C.C. Preparation and Evaluation of Novel Transfersomes Combined with the Natural Antioxidant Resveratrol. Molecules 2019, 24, 600. [Google Scholar] [CrossRef]
- Ashtiani, H.A.; Bishe, P.; Lashgari, N.-A.; Nilforoushzadeh, M.A.; Zare, S. Liposomes in cosmetics. J. Ski. Stem Cell 2016, 3, e65815. [Google Scholar] [CrossRef]
- Fytianos, G.; Rahdar, A.; Kyzas, G.Z. Nanomaterials in Cosmetics: Recent Updates. Nanomaterials 2020, 10, 979. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Iqbal, H.M. New insights on unique features and role of nanostructured materials in cosmetics. Cosmetics 2020, 7, 24. [Google Scholar] [CrossRef]
- Figueroa-Robles, A.; Antunes-Ricardo, M.; Guajardo-Flores, D. Encapsulation of phenolic compounds with liposomal improvement in the cosmetic industry. Int. J. Pharm. 2021, 593, 120125. [Google Scholar] [CrossRef]
- Halliwell, B. Understanding mechanisms of antioxidant action in health and disease. Nat. Rev. Mol. Cell Biol. 2024, 25, 13–33. [Google Scholar] [CrossRef]
- Anik, M.I.; Mahmud, N.; Masud, A.A.; Khan, M.I.; Islam, M.N.; Uddin, S.; Hossain, M.K. Role of Reactive Oxygen Species in Aging and Age-Related Diseases: A Review. ACS Appl. Bio Mater. 2022, 5, 4028–4054. [Google Scholar] [CrossRef]
- Liu, T.; Zhu, S.; Yang, Y.; Qin, W.; Wang, Z.; Zhao, Z.; Liu, T.; Wang, X.; Duan, T.; Liu, Y.; et al. Oroxylin A ameliorates ultraviolet radiation-induced premature skin aging by regulating oxidative stress via the Sirt1 pathway. Biomed. Pharmacother. 2024, 171, 116110. [Google Scholar] [CrossRef]
- Tang, Z.; Liu, Z.; Zhang, Y.; Luo, S.; Xu, Y.; Ren, L. Functional hyaluronic acid microneedles for skin photoaging based on collagen induction and oxidative stress regulation strategies. Int. J. Biol. Macromol. 2024, 277, 134080. [Google Scholar] [CrossRef]
- Ferreira, L.; Torres, B.; Hameed, H.; Vieira, A.C.; Singh, S.K.; Dua, K.; Veiga, F.; Pires, P.C.; Mazzola, P.G.; Paiva-Santos, A.C. Updated insights of active cosmetic ingredients against blue light: In vivo and in vitro evidence. J. Drug Deliv. Sci. Technol. 2024, 101, 106306. [Google Scholar] [CrossRef]
- Rusu, M.E.; Fizeșan, I.; Vlase, L.; Popa, D.S. Antioxidants in Age-Related Diseases and Anti-Aging Strategies. Antioxidants 2022, 11, 1868. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M. Plant-Derived Antioxidants: Significance in Skin Health and the Ageing Process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef]
- Choi, H.Y.; Lee, Y.J.; Kim, C.M.; Lee, Y.-M. Revolutionizing cosmetic ingredients: Harnessing the power of antioxidants, probiotics, plant extracts, and peptides in personal and skin care products. Cosmetics 2024, 11, 157. [Google Scholar] [CrossRef]
- Hoang, H.T.; Moon, J.-Y.; Lee, Y.-C. Natural antioxidants from plant extracts in skincare cosmetics: Recent applications, challenges and perspectives. Cosmetics 2021, 8, 106. [Google Scholar] [CrossRef]
- Miere, F.; Teușdea, A.C.; Laslo, V.; Cavalu, S.; Fritea, L.; Dobjanschi, L.; Zdrinca, M.; Zdrinca, M.; Ganea, M.; Pașc, P.; et al. Evaluation of in vitro wound-healing potential, antioxidant capacity, and antimicrobial activity of Stellaria media (L.) Vill. Appl. Sci. 2021, 11, 11526. [Google Scholar] [CrossRef]
- Miere, F.G.; Ganea, M.; Teodorescu, A.G.; Horvath, T.; Hanga-Farcas, A.; Csaba, N.; Zdinca, M.; Zdinca, M.; Dobjanschi, L. Characterization in terms of phytochemical content and medicinal potential of the Stellaria media plant extract. Pharmacophore 2023, 14, 45–55. [Google Scholar] [CrossRef]
- Oladeji, O.S.; Oyebamiji, A.K. Stellaria media (L.) Vill.—A plant with immense therapeutic potentials: Phytochemistry and pharmacology. Heliyon 2020, 6, e04150. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Li, T.S.; Su, H.L.; Lee, P.C.; Wang, H.D. Transdermal Delivery Systems of Natural Products Applied to Skin Therapy and Care. Molecules 2020, 25, 5051. [Google Scholar] [CrossRef]
- Ricci, A.; Stefanuto, L.; Gasperi, T.; Bruni, F.; Tofani, D. Lipid Nanovesicles for Antioxidant Delivery in Skin: Liposomes, Ufasomes, Ethosomes, and Niosomes. Antioxidants 2024, 13, 1516. [Google Scholar] [CrossRef]
- Olaru, O.T.; Nitulescu, G.M.; Codreanu, A.M.; Calmuc, V.A.; Venables, L.; van de Venter, M.; Gird, C.E.; Duta-Bratu, C.G.; Nitulescu, G. Inhibitory Effects on Staphylococcus aureus Sortase A by Aesculus sp. Extracts and Their Toxicity Evaluation. Plants 2024, 13, 1405. [Google Scholar] [CrossRef]
- Mare, R.; Pujia, R.; Maurotti, S.; Greco, S.; Cardamone, A.; Coppoletta, A.R.; Bonacci, S.; Procopio, A.; Pujia, A. Assessment of Mediterranean Citrus Peel Flavonoids and Their Antioxidant Capacity Using an Innovative UV-Vis Spectrophotometric Approach. Plants 2023, 12, 4046. [Google Scholar] [CrossRef]
- Settino, M.; Maurotti, S.; Tirinato, L.; Greco, S.; Coppoletta, A.R.; Cardamone, A.; Musolino, V.; Montalcini, T.; Pujia, A.; Mare, R. Zibibbo Grape Seeds’ Polyphenolic Profile: Effects on Bone Turnover and Metabolism. Pharmaceuticals 2024, 17, 1418. [Google Scholar] [CrossRef] [PubMed]
- Celia, C.; Cilurzo, F.; Trapasso, E.; Cosco, D.; Fresta, M.; Paolino, D. Ethosomes® and transfersomes® containing linoleic acid: Physicochemical and technological features of topical drug delivery carriers for the potential treatment of melasma disorders. Biomed. Microdevices 2012, 14, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Adamo, G.; Fierli, D.; Romancino, D.P.; Picciotto, S.; Barone, M.E.; Aranyos, A.; Božič, D.; Morsbach, S.; Raccosta, S.; Stanly, C.; et al. Nanoalgosomes: Introducing extracellular vesicles produced by microalgae. J. Extracell. Vesicles 2021, 10, e12081. [Google Scholar] [CrossRef]
- Palmosi, T.; Tolomeo, A.M.; Cirillo, C.; Sandrin, D.; Sciro, M.; Negrisolo, S.; Todesco, M.; Caicci, F.; Santoro, M.; Dal Lago, E.; et al. Small intestinal submucosa-derived extracellular matrix as a heterotopic scaffold for cardiovascular applications. Front. Bioeng. Biotechnol. 2022, 10, 1042434. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, A.; Cristiano, M.C.; d’Avanzo, N.; Panza, S.; Tarsitano, M.; Celia, C.; Paolino, D.; Fresta, M. Assessing effectiveness of multistage nanomedicines for multidrug therapy of vitiligo. J. Drug Deliv. Sci. Technol. 2025, 105, 106615. [Google Scholar] [CrossRef]
- van den Bergh, B.A.; Bouwstra, J.A.; Junginger, H.E.; Wertz, P.W. Elasticity of vesicles affects hairless mouse skin structure and permeability. J. Control. Release 1999, 62, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.C.; Gagliardi, A.; Mancuso, A.; Barone, A.; Tarsitano, M.; Cosco, D.; Cristiano, M.C.; Fresta, M.; Paolino, D. Oleic acid-based vesicular nanocarriers for topical delivery of the natural drug thymoquinone: Improvement of anti-inflammatory activity. J. Control. Release 2022, 352, 74–86. [Google Scholar] [CrossRef]
- Gelker, M.; Müller-Goymann, C.C.; Viöl, W. Permeabilization of human stratum corneum and full-thickness skin samples by a direct dielectric barrier discharge. Clin. Plasma Med. 2018, 9, 34–40. [Google Scholar] [CrossRef]
- Ziemlewska, A.; Zagórska-Dziok, M.; Nizioł-Łukaszewska, Z. Assessment of cytotoxicity and antioxidant properties of berry leaves as by-products with potential application in cosmetic and pharmaceutical products. Sci. Rep. 2021, 11, 3240. [Google Scholar] [CrossRef]
- Souto, E.B.; Macedo, A.S.; Dias-Ferreira, J.; Cano, A.; Zielińska, A.; Matos, C.M. Elastic and Ultradeformable Liposomes for Transdermal Delivery of Active Pharmaceutical Ingredients (APIs). Int. J. Mol. Sci. 2021, 22, 9743. [Google Scholar] [CrossRef]
- Paolino, D.; Cosco, D.; Cilurzo, F.; Trapasso, E.; Morittu, V.M.; Celia, C.; Fresta, M. Improved in vitro and in vivo collagen biosynthesis by asiaticoside-loaded ultradeformable vesicles. J. Control. Release 2012, 162, 143–151. [Google Scholar] [CrossRef]
- Avadhani, K.S.; Manikkath, J.; Tiwari, M.; Chandrasekhar, M.; Godavarthi, A.; Vidya, S.M.; Hariharapura, R.C.; Kalthur, G.; Udupa, N.; Mutalik, S. Skin delivery of epigallocatechin-3-gallate (EGCG) and hyaluronic acid loaded nano-transfersomes for antioxidant and anti-aging effects in UV radiation induced skin damage. Drug Deliv. 2017, 24, 61–74. [Google Scholar] [CrossRef]
- Forbes, N.; Hussain, M.T.; Briuglia, M.L.; Edwards, D.P.; Horst, J.H.T.; Szita, N.; Perrie, Y. Rapid and scale-independent microfluidic manufacture of liposomes entrapping protein incorporating in-line purification and at-line size monitoring. Int. J. Pharm. 2019, 556, 68–81. [Google Scholar] [CrossRef]
- Levy, E.S.; Yu, J.; Estevez, A.; Mao, J.; Liu, L.; Torres, E.; Leung, D.; Yen, C.W. A Systematic Approach for Liposome and Lipodisk Preclinical Formulation Development by Microfluidic Technology. AAPS J. 2021, 23, 111. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, G.; Hui, Y.; Ranaweera, S.; Zhao, C.X. Microfluidic Nanoparticles for Drug Delivery. Small 2022, 18, e2106580. [Google Scholar] [CrossRef]
- Zook, J.M.; Vreeland, W.N. Effects of temperature, acyl chain length, and flow-rate ratio on liposome formation and size in a microfluidic hydrodynamic focusing device. Soft Matter 2010, 6, 1352–1360. [Google Scholar] [CrossRef]
- Balbino, T.A.; Aoki, N.T.; Gasperini, A.A.; Oliveira, C.L.; Azzoni, A.R.; Cavalcanti, L.P.; de la Torre, L.G. Continuous flow production of cationic liposomes at high lipid concentration in microfluidic devices for gene delivery applications. Chem. Eng. J. 2013, 226, 423–433. [Google Scholar] [CrossRef]
- Simon, C., Jr.; Borgos, S.E.; Calzolai, L.; Nelson, B.; Parot, J.; Petersen, E.J.; Roesslein, M.; Xu, X.; Caputo, F. Orthogonal and complementary measurements of properties of drug products containing nanomaterials. J. Control. Release 2023, 354, 120–127. [Google Scholar] [CrossRef]
- Joyce, P.; Allen, C.J.; Alonso, M.J.; Ashford, M.; Bradbury, M.S.; Germain, M.; Kavallaris, M.; Langer, R.; Lammers, T.; Peracchia, M.T.; et al. A translational framework to DELIVER nanomedicines to the clinic. Nat. Nanotechnol. 2024, 19, 1597–1611. [Google Scholar] [CrossRef] [PubMed]
- Wadher, K.; Trivedi, S.; Rarokar, N.; Umekar, M. Development and assessment of rutin loaded transfersomes to improve ex vivo membrane permeability and in vitro efficacy. Hybrid. Adv. 2024, 5, 100144. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Jakubek, Z.J.; Chen, S.; Zaifman, J.; Tam, Y.Y.C.; Zou, S. Lipid Nanoparticle and Liposome Reference Materials: Assessment of Size Homogeneity and Long-Term −70 °C and 4 °C Storage Stability. Langmuir 2023, 39, 2509–2519. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Y.; Xu, X.; Deng, L.; Feng, L.; Han, J.; Liu, W. Effect of oligosaccharides as lyoprotectants on the stability of curcumin-loaded nanoliposomes during lyophilization. Food Chem. 2023, 410, 135436. [Google Scholar] [CrossRef] [PubMed]
- Popova, A.V.; Hincha, D.K. Effects of flavonol glycosides on liposome stability during freezing and drying. Biochim. Biophys. Acta (BBA) Biomembr. 2016, 1858, 3050–3060. [Google Scholar] [CrossRef]
- Filipe, V.; Hawe, A.; Jiskoot, W. Critical evaluation of Nanoparticle Tracking Analysis (NTA) by NanoSight for the measurement of nanoparticles and protein aggregates. Pharm. Res. 2010, 27, 796–810. [Google Scholar] [CrossRef]
- Chan, M.Y.; Dowling, Q.M.; Sivananthan, S.J.; Kramer, R.M. Particle Sizing of Nanoparticle Adjuvant Formulations by Dynamic Light Scattering (DLS) and Nanoparticle Tracking Analysis (NTA). In Vaccine Adjuvants; Humana Press: New York, NY, USA, 2017; Volume 1494, pp. 239–252. [Google Scholar] [CrossRef]
- Dayan, N.; Touitou, E. Carriers for skin delivery of trihexyphenidyl HCl: Ethosomes vs. liposomes. Biomaterials 2000, 21, 1879–1885. [Google Scholar] [CrossRef]
- Zhang, Y.; Pu, C.; Tang, W.; Wang, S.; Sun, Q. Effects of four polyphenols loading on the attributes of lipid bilayers. J. Food Eng. 2020, 282, 110008. [Google Scholar] [CrossRef]
- Neupane, R.; Boddu, S.H.; Renukuntla, J.; Babu, R.J.; Tiwari, A.K. Alternatives to biological skin in permeation studies: Current trends and possibilities. Pharmaceutics 2020, 12, 152. [Google Scholar] [CrossRef]
- Schoenfelder, H.; Liu, Y.; Lunter, D.J. Systematic investigation of factors, such as the impact of emulsifiers, which influence the measurement of skin barrier integrity by in-vitro trans-epidermal water loss (TEWL). Int. J. Pharm. 2023, 638, 122930. [Google Scholar] [CrossRef] [PubMed]
- Guillot, A.J.; Martínez-Navarrete, M.; Garrigues, T.M.; Melero, A. Skin drug delivery using lipid vesicles: A starting guideline for their development. J. Control. Release 2023, 355, 624–654. [Google Scholar] [CrossRef]
- Vasudevan, S.; Vogt, W.C.; Weininger, S.; Pfefer, T.J. Melanometry for objective evaluation of skin pigmentation in pulse oximetry studies. Commun. Med. 2024, 4, 138. [Google Scholar] [CrossRef] [PubMed]



| Formulation | FRR | TFR (μL/min) | Water FR (μL/min) | Organic FR (μL/min) |
|---|---|---|---|---|
| A | 3:1 | 3000 | 2250 | 750 |
| B | 3:1 | 5000 | 3750 | 1250 |
| C | 3:1 | 7000 | 5250 | 1750 |
| D | 3:1 | 10,000 | 7500 | 2500 |
| POLYPHENOLS | |||
|---|---|---|---|
| Mean ABS 760 nm | Mean GAE (mg/mL) ± SD | Mean GAE (mg/g) ± SD | |
| 0.634 ± 0.053 | 0.131 ± 0.011 | 13.1 ± 1.1 | |
| ANTIOXIDANT ACTIVITY | |||
| ABTS ASSAY | Mean ABS 730 nm | Mean I (%) ± SD | AAE (µg) ±SD |
| CTRL (−) | 0.1995 ± 0.0064 | --- | --- |
| CTRL (+) | 0.0035 ± 0.0021 | ~98 | --- |
| Stellaria Extract | 0.030 ± 0.001 (***) | 84.96 ± 0.71 (***) | 23.09 ± 0.19 |
| DPPH ASSAY | Mean ABS 517 nm | Mean I (%) ± SD | AAE (µg) ± SD |
| CTRL (−) | 1.1696 ± 0.0003 | --- | --- |
| CTRL (+) | 0.0924 ± 0.0016 | ~92 | --- |
| Stellaria Extract | 0.8977 ± 0.0017 (***) | 23.25 ± 0.14 (***) | 12.58 ± 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciriolo, L.; d’Avanzo, N.; Mancuso, A.; Cristiano, M.C.; Barone, A.; Mare, R.; Tolomeo, A.M.; Comaniciu, A.I.; Nitulescu, G.; Olaru, O.T.; et al. Microfluidic Design of Ultradeformable Liposomes for Advanced Skin Delivery of Stellaria media Phytocomplex. Pharmaceutics 2025, 17, 1390. https://doi.org/10.3390/pharmaceutics17111390
Ciriolo L, d’Avanzo N, Mancuso A, Cristiano MC, Barone A, Mare R, Tolomeo AM, Comaniciu AI, Nitulescu G, Olaru OT, et al. Microfluidic Design of Ultradeformable Liposomes for Advanced Skin Delivery of Stellaria media Phytocomplex. Pharmaceutics. 2025; 17(11):1390. https://doi.org/10.3390/pharmaceutics17111390
Chicago/Turabian StyleCiriolo, Luigi, Nicola d’Avanzo, Antonia Mancuso, Maria Chiara Cristiano, Antonella Barone, Rosario Mare, Anna Maria Tolomeo, Alexandra I. Comaniciu, Georgiana Nitulescu, Octavian Tudorel Olaru, and et al. 2025. "Microfluidic Design of Ultradeformable Liposomes for Advanced Skin Delivery of Stellaria media Phytocomplex" Pharmaceutics 17, no. 11: 1390. https://doi.org/10.3390/pharmaceutics17111390
APA StyleCiriolo, L., d’Avanzo, N., Mancuso, A., Cristiano, M. C., Barone, A., Mare, R., Tolomeo, A. M., Comaniciu, A. I., Nitulescu, G., Olaru, O. T., Cilurzo, F., Paolino, D., & Fresta, M. (2025). Microfluidic Design of Ultradeformable Liposomes for Advanced Skin Delivery of Stellaria media Phytocomplex. Pharmaceutics, 17(11), 1390. https://doi.org/10.3390/pharmaceutics17111390













