Generation and Purification of RANKL-Derived Small-Fragment Variants for Osteoclast Inhibition
Abstract
1. Introduction
2. Materials and Methods
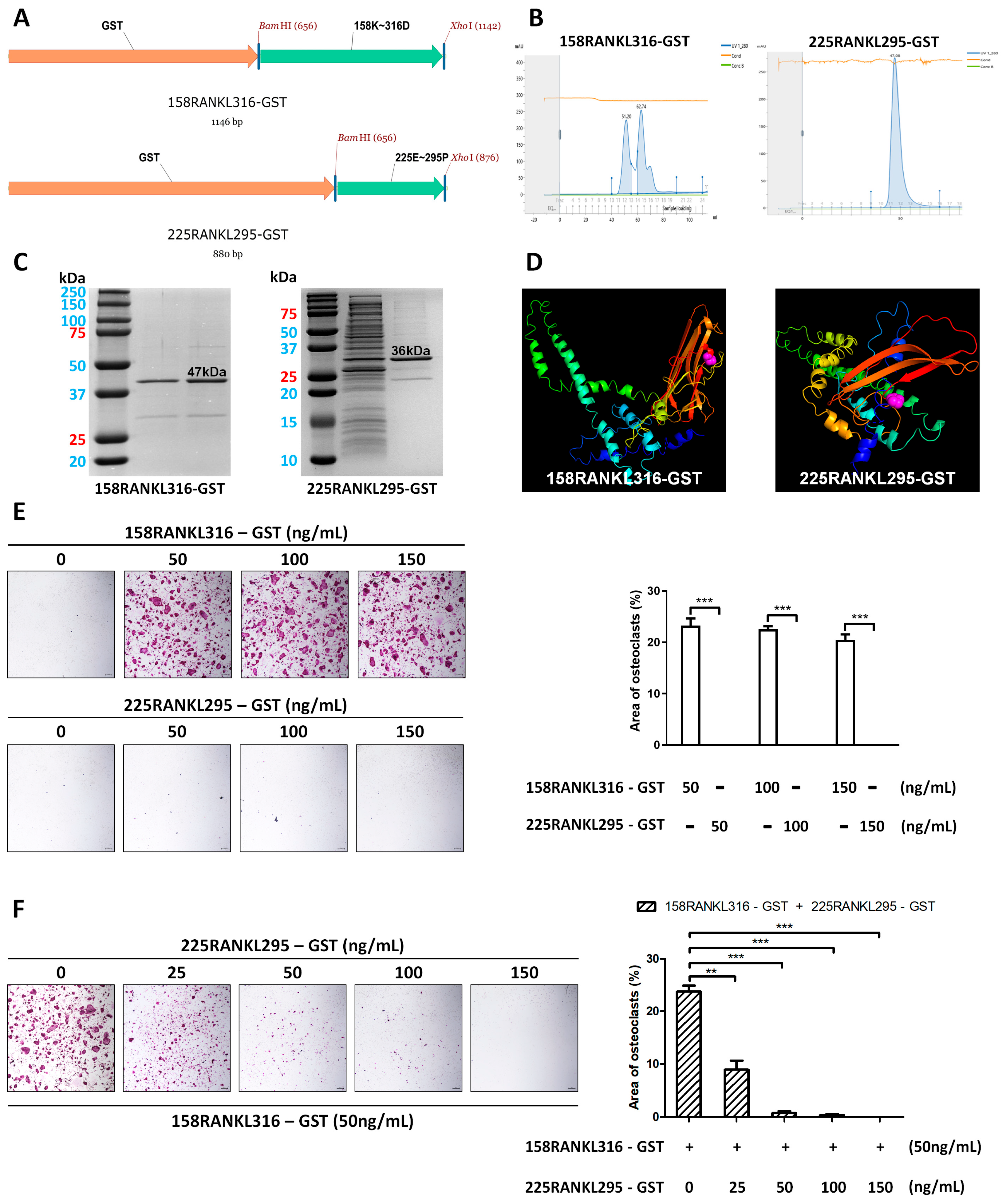
2.1. Construction of a GST-Tagged RANKL Variant Expression Vector
2.2. Initial Chromatography for the Purification of GST-Tagged RANKL Variants
2.3. Three-Dimensional Structure Simulation of a Small RANKL-Derived Protein
2.4. Biological Activity Test of Purified RANKL Variants
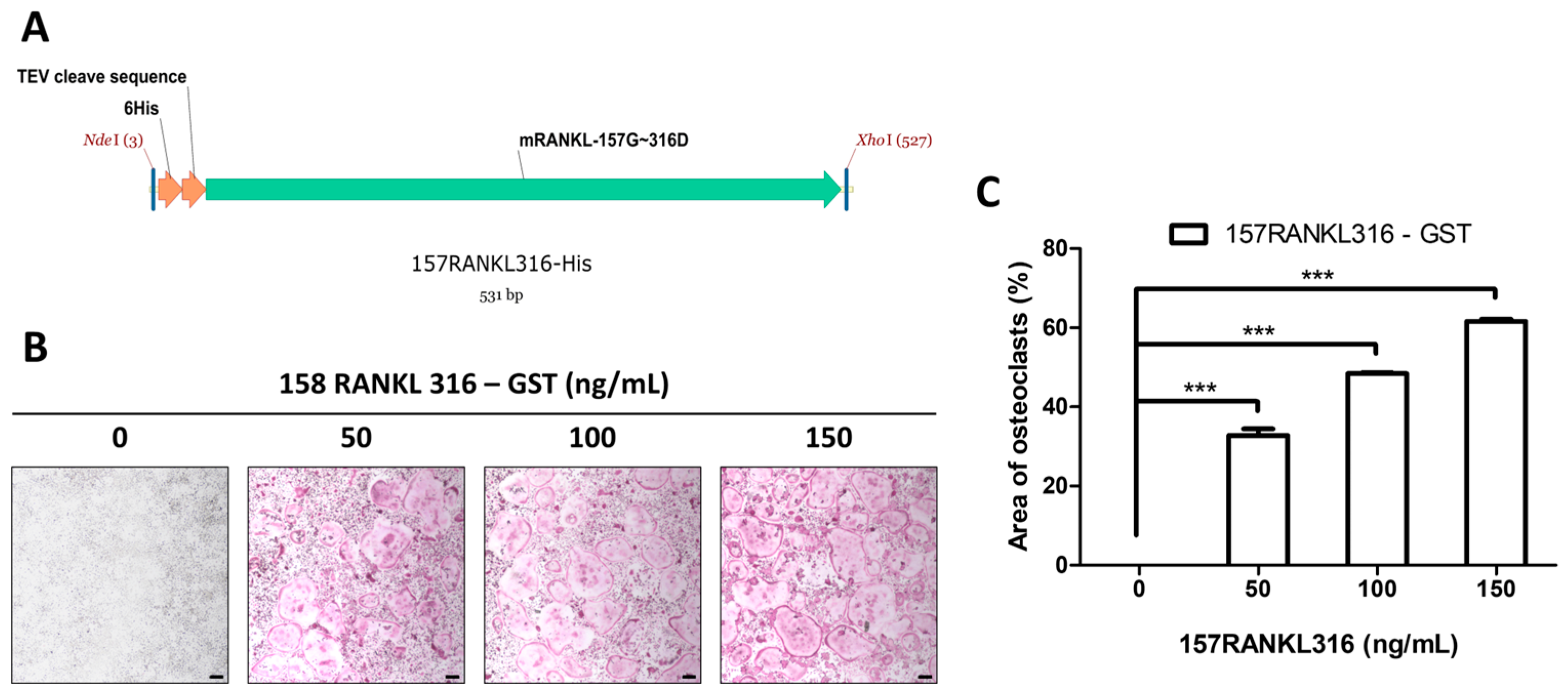
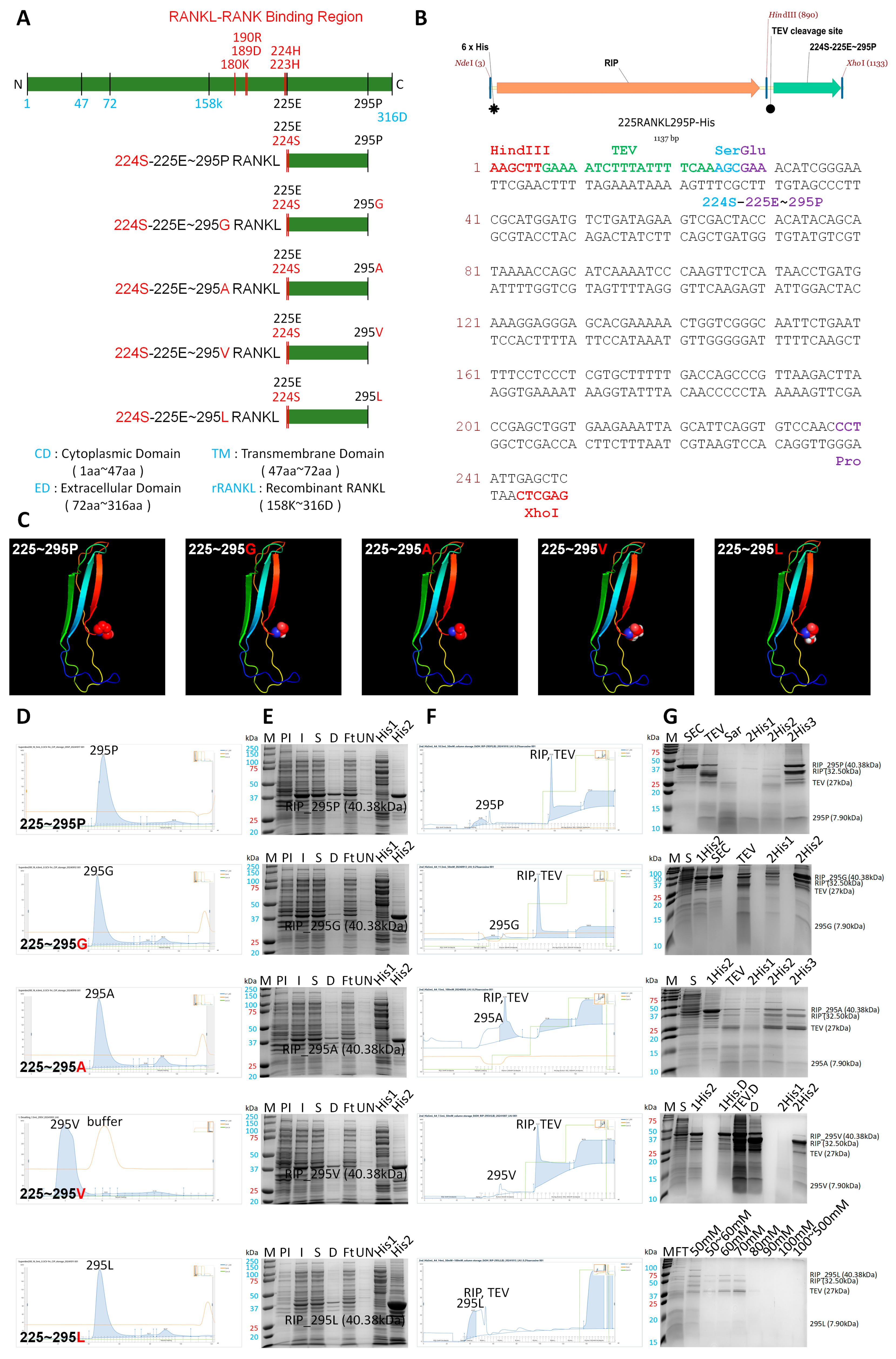
2.5. Construction of His-Tagged RANKL Variant Expression Vector
2.6. Initial Chromatography for the Purification of the RANKL Variant Using His Trap Affinity Chromatography
2.7. Secondary Chromatography for the Purification of RANKL Variants Using Size-Exclusion Chromatography
2.8. Cleavage of His-Tag by TEV Enzyme and Tertiary Chromatography for the Purification of the Unbound Fraction in RANKL Variants
2.9. SDS-PAGE
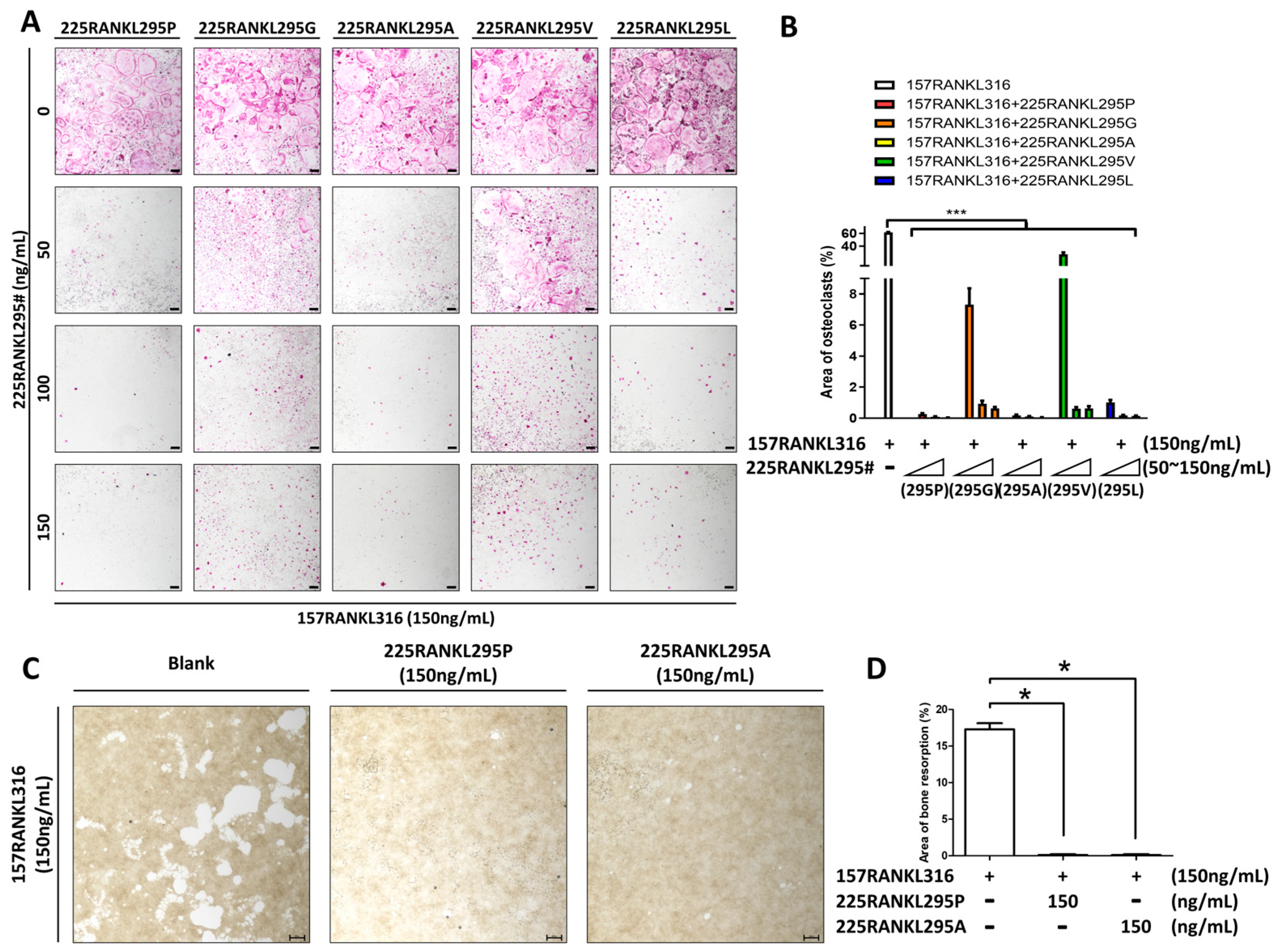
2.10. Bone Resorption Pit Formation Assay
2.11. Statistical Analysis
3. Results
3.1. GST-Tagged 158RANKL316 and 225RANKL295 Proteins
3.2. Generation of 157RAKL316 and Effects on Osteoclasts
3.3. Generation and Purification of 225RANKL295P and Modified Proteins
3.4. TRAP Analysis and Bone Resorption by Modified Proteins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RANKL | Receptor activator of nuclear factor kappa B ligand |
| RANK | Receptor activator of nuclear factor kappa B |
| LGR4 | Leucine-rich repeat-containing G protein-coupled receptor 4 |
| GST | Glutathione S-transferase |
| His | 6× histidine |
| Trap | Tartrate-resistant acid phosphatase |
| TNF | Tumor necrosis factor |
| GSK-3beta | Glycogen synthase kinase-3 beta |
| TEV | Tobacco etch virus |
| PCR | Polymerase chain reaction |
| LB | Luria–Bertani |
| IPTG | Isopropyl β-D-1-thiogalactopyranoside |
| OD | Optical density |
| PBS | Phosphate-buffered saline |
| FPLC | Fast protein liquid chromatography |
| BMMs | Bone marrow macrophages |
| APC | Ammonium persulfate |
| TEMED | Tetramethylethylenediamine |
| M-CSF | Macrophage colony stimulation factor |
References
- Ono, T.; Hayashi, M.; Sasaki, F.; Nakashima, T. RANKL biology: Bone metabolism, the immune system, and beyond. Inflamm. Regen. 2020, 40, 2. [Google Scholar] [CrossRef] [PubMed]
- Kearns, A.E.; Khosla, S.; Kostenuik, P.J. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr. Rev. 2008, 29, 155–192. [Google Scholar] [CrossRef] [PubMed]
- Ta, H.M.; Nguyen, G.T.; Jin, H.M.; Choi, J.; Park, H.; Kim, N.; Hwang, H.Y.; Kim, K.K. Structure-based development of a receptor activator of nuclear factor-kappaB ligand (RANKL) inhibitor peptide and molecular basis for osteopetrosis. Proc. Natl. Acad. Sci. USA 2010, 107, 20281–20286. [Google Scholar] [CrossRef]
- Huang, D.; Zhao, C.; Li, R.; Chen, B.; Zhang, Y.; Sun, Z.; Wei, J.; Zhou, H.; Gu, Q.; Xu, J. Identification of a binding site on soluble RANKL that can be targeted to inhibit soluble RANK-RANKL interactions and treat osteoporosis. Nat. Commun. 2022, 13, 5338. [Google Scholar] [CrossRef]
- Sobacchi, C.; Menale, C.; Crisafulli, L.; Ficara, F. Role of RANKL Signaling in Bone Homeostasis. Physiology 2025, 40, 46–66. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, N.K.; Lee, S.Y. Current Understanding of RANK Signaling in Osteoclast Differentiation and Maturation. Mol. Cells 2017, 40, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Yang, Z.; Ma, Y.; Yue, Z.; Lin, H.; Qu, G.; Huang, J.; Dai, W.; Li, C.; Zheng, C.; et al. LGR4 is a receptor for RANKL and negatively regulates osteoclast differentiation and bone resorption. Nat. Med. 2016, 22, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Filipowska, J.; Kondegowda, N.G.; Leon-Rivera, N.; Dhawan, S.; Vasavada, R.C. LGR4, a G Protein-Coupled Receptor with a Systemic Role: From Development to Metabolic Regulation. Front. Endocrinol. 2022, 13, 867001. [Google Scholar] [CrossRef]
- Jang, Y.; Lee, H.; Cho, Y.; Choi, E.; Jo, S.; Sohn, H.M.; Kim, B.C.; Ko, Y.J.; Lim, W. An LGR4 agonist activates the GSK-3beta pathway to inhibit RANK-RANKL signaling during osteoclastogenesis in bone marrow-derived macrophages. Int. J. Mol. Med. 2024, 53, 10. [Google Scholar] [CrossRef]
- Kim, B.C.; Cho, Y.J.; Jang, Y.; Ko, K.Y.; Lee, C.M.; Lim, W. Role of endosomal RANKL-LGR4 signaling during osteoclast differentiation. J. Mol. Med. 2025, 103, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.J.; Sohn, H.M.; Jang, Y.; Park, M.; Kim, B.; Kim, B.; Park, J.I.; Hyun, H.; Jeong, B.; Hong, C.; et al. A novel modified RANKL variant can prevent osteoporosis by acting as a vaccine and an inhibitor. Clin. Transl. Med. 2021, 11, e368. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Cho, Y.; Ko, Y.; Moon, Y.; Lee, C.M.; Lim, W. Advanced mutant receptor activator of nuclear factor kappa-Beta ligand development with low affinity for osteoprotegerin. Clin. Transl. Med. 2025, 15, e70195. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, T.; Shimamura, M.; Etani, Y.; Noguchi, T.; Fukuda, Y.; Ochiai, N.; Goshima, A.; Miura, T.; Hirao, M.; Sugimoto, A.; et al. RANKL-derived peptide MHP1-AcN attenuates ovariectomy-induced osteoporosis by targeting RANK and TNFR1 in mice. Bone 2025, 194, 117440. [Google Scholar] [CrossRef]
- Ju, N.; Shimamura, M.; Hayashi, H.; Ikeda, Y.; Yoshida, S.; Nakamura, A.; Morishita, R.; Rakugi, H.; Nakagami, H. Preventative effects of the partial RANKL peptide MHP1-AcN in a mouse model of imiquimod-induced psoriasis. Sci. Rep. 2019, 9, 15434. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Cho, Y.J.; Ko, Y.; Lee, H.; Kim, B.; Lee, C.M.; Lim, W. LGR4 binding small RANKL fragment inhibits RANKL-induced bone resorption. Eur. Cells Mater. 2025, 53, 15–27. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef]
- Lu, R.M.; Hwang, Y.C.; Liu, I.J.; Lee, C.C.; Tsai, H.Z.; Li, H.J.; Wu, H.C. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 2020, 27, 1. [Google Scholar] [CrossRef]
- Todaro, B.; Ottalagana, E.; Luin, S.; Santi, M. Targeting Peptides: The New Generation of Targeted Drug Delivery Systems. Pharmaceutics 2023, 15, 1648. [Google Scholar] [CrossRef]
- Xiao, W.; Jiang, W.; Chen, Z.; Huang, Y.; Mao, J.; Zheng, W.; Hu, Y.; Shi, J. Advance in peptide-based drug development: Delivery platforms, therapeutics and vaccines. Signal Transduct. Target. Ther. 2025, 10, 74. [Google Scholar] [CrossRef] [PubMed]
- Trabbic-Carlson, K.; Liu, L.; Kim, B.; Chilkoti, A. Expression and purification of recombinant proteins from Escherichia coli: Comparison of an elastin-like polypeptide fusion with an oligohistidine fusion. Protein Sci. 2004, 13, 3274–3284. [Google Scholar] [CrossRef] [PubMed]
- Raran-Kurussi, S.; Waugh, D.S. Expression and Purification of Recombinant Proteins in Escherichia coli with a His(6) or Dual His(6)-MBP Tag. In Protein Crystallography; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2017; Volume 1607, pp. 1–15. [Google Scholar] [CrossRef]
- Raran-Kurussi, S.; Cherry, S.; Zhang, D.; Waugh, D.S. Removal of Affinity Tags with TEV Protease. In Heterologous Gene Expression in E. coli; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2017; Volume 1586, pp. 221–230. [Google Scholar] [CrossRef]
- Young, C.L.; Britton, Z.T.; Robinson, A.S. Recombinant protein expression and purification: A comprehensive review of affinity tags and microbial applications. Biotechnol. J. 2012, 7, 620–634. [Google Scholar] [CrossRef]
- Francis, D.M.; Page, R. Strategies to optimize protein expression in E. coli. Curr. Protoc. Protein Sci. 2010, 61, 5.24.1–5.24.29. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, A.F.; Turchetto, J.; Saez, N.J.; Peysson, F.; Ramond, L.; Duhoo, Y.; Blemont, M.; Fernandes, V.O.; Gama, L.T.; Ferreira, L.M.; et al. Gene design, fusion technology and TEV cleavage conditions influence the purification of oxidized disulphide-rich venom peptides in Escherichia coli. Microb. Cell Fact. 2017, 16, 4. [Google Scholar] [CrossRef]
- Kapust, R.B.; Tozser, J.; Copeland, T.D.; Waugh, D.S. The P1’ specificity of tobacco etch virus protease. Biochem. Biophys. Res. Commun. 2002, 294, 949–955. [Google Scholar] [CrossRef]
- Dempster, D.W.; Lambing, C.L.; Kostenuik, P.J.; Grauer, A. Role of RANK ligand and denosumab, a targeted RANK ligand inhibitor, in bone health and osteoporosis: A review of preclinical and clinical data. Clin. Ther. 2012, 34, 521–536. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, D.; Haeussler, D.J.; Matsui, R.; Burgoyne, J.R.; Cohen, R.A.; Bachschmid, M.M. Regulation of cell physiology and pathology by protein S-glutathionylation: Lessons learned from the cardiovascular system. Antioxid. Redox Signal. 2012, 16, 524–542. [Google Scholar] [CrossRef]
- Lee, G.; Ko, Y.; Park, M.; Kim, B.; Hyun, H.; Lim, W. Recombinant DNA cloning of the active region of the receptor activator of NF-κB ligand (RANKL) gene and its role in osteoclastogenesis. Biotechnol. Bioprocess Eng. 2017, 22, 686–692. [Google Scholar] [CrossRef]
- Lee, B.G.; Kim, M.K.; Kim, B.W.; Suh, S.W.; Song, H.K. Structures of the ribosome-inactivating protein from barley seeds reveal a unique activation mechanism. Acta Crystallogr. Sect. D Biol. Crystallogr. 2012, 68, 1488–1500. [Google Scholar] [CrossRef] [PubMed]
- Stirpe, F.; Gilabert-Oriol, R. Ribosome-Inactivating Proteins: An Overview. In Plant Toxins; Gopalakrishnakone, P., Carlini, C.R., Ligabue-Braun, R., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 1–29. [Google Scholar]
- Yang, Y.X.; Wang, X.Y.; Lin, T.; Sun, Y.; Yu, Y.C.; Zhu, Z.H. Opportunities and challenges for ribosome-inactivating proteins in traditional Chinese medicine plants. Toxicon 2023, 234, 107278. [Google Scholar] [CrossRef] [PubMed]
- Mark, J.K.K.; Lim, C.S.Y.; Nordin, F.; Tye, G.J. Expression of mammalian proteins for diagnostics and therapeutics: A review. Mol. Biol. Rep. 2022, 49, 10593–10608. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Upadhyay, V.; Upadhyay, A.K.; Singh, S.M.; Panda, A.K. Protein recovery from inclusion bodies of Escherichia coli using mild solubilization process. Microb. Cell Fact. 2015, 14, 41. [Google Scholar] [CrossRef] [PubMed]
| Oligo | Sequence (5′ --> 3′) | Note |
|---|---|---|
| 224S-5′ | AAGCTTGAAAATCTTTATTTTCAAAGCGAAACATCGGGAAGCGTACCTACAG | S-225E |
| P295P-3′ | CTCGAGTTACGGGTTGGACACCTGAATGCTAATTTCT | Non-polar |
| P295G-3′ | CTCGAGTTAGCCGTTGGACACCTGAATGCTAATTTCT | Non-polar |
| P295A-3′ | CTCGAGTTACGCGTTGGACACCTGAATGCTAATTTCT | Non-polar |
| P295V-3′ | CTCGAGTTACACGTTGGACACCTGAATGCTAATTTCT | Non-polar |
| P295L-3′ | CTCGAGTTACAGGTTGGACACCTGAATGCTAATTTCT | Non-polar |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Park, H.; Kim, K.; Ko, Y.; Lee, C.-M.; Lim, W. Generation and Purification of RANKL-Derived Small-Fragment Variants for Osteoclast Inhibition. Pharmaceutics 2025, 17, 1385. https://doi.org/10.3390/pharmaceutics17111385
Lee H, Park H, Kim K, Ko Y, Lee C-M, Lim W. Generation and Purification of RANKL-Derived Small-Fragment Variants for Osteoclast Inhibition. Pharmaceutics. 2025; 17(11):1385. https://doi.org/10.3390/pharmaceutics17111385
Chicago/Turabian StyleLee, Hyungjun, Hyungseok Park, Kabsun Kim, Youngjong Ko, Chang-Moon Lee, and Wonbong Lim. 2025. "Generation and Purification of RANKL-Derived Small-Fragment Variants for Osteoclast Inhibition" Pharmaceutics 17, no. 11: 1385. https://doi.org/10.3390/pharmaceutics17111385
APA StyleLee, H., Park, H., Kim, K., Ko, Y., Lee, C.-M., & Lim, W. (2025). Generation and Purification of RANKL-Derived Small-Fragment Variants for Osteoclast Inhibition. Pharmaceutics, 17(11), 1385. https://doi.org/10.3390/pharmaceutics17111385






