Photodynamic Therapy with 5-Aminolevulinic Acid Versus Topical Corticosteroids in the Treatment of Oral Lichen Planus: A Randomized Clinical Trial with Lesion Site-Specific Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Characteristics
2.2. Treatment Protocols
- Group 1 (ALA-PDT):
- Group 2 (CT):
2.3. Clinical Evaluation
2.4. Statistical Analysis
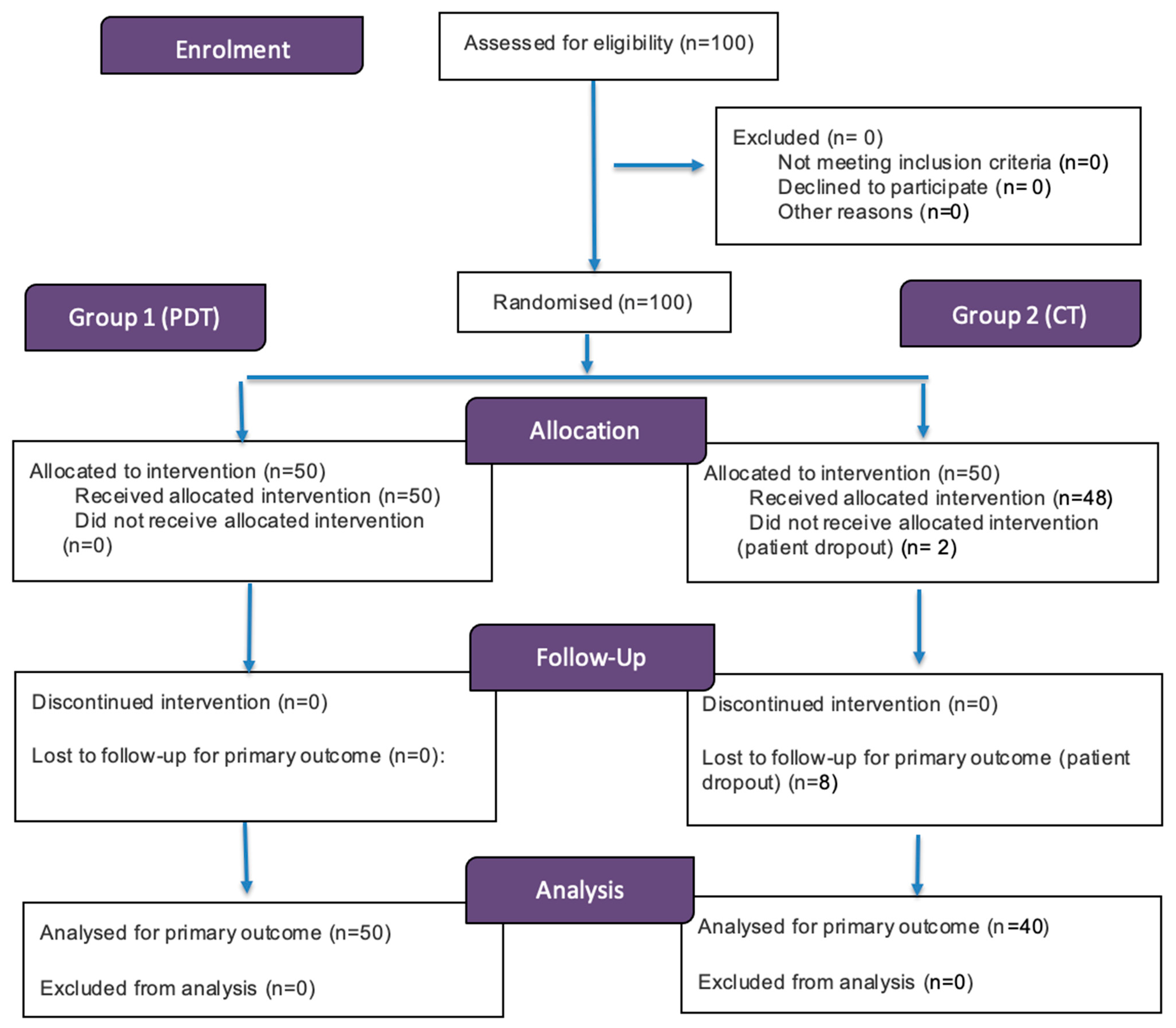
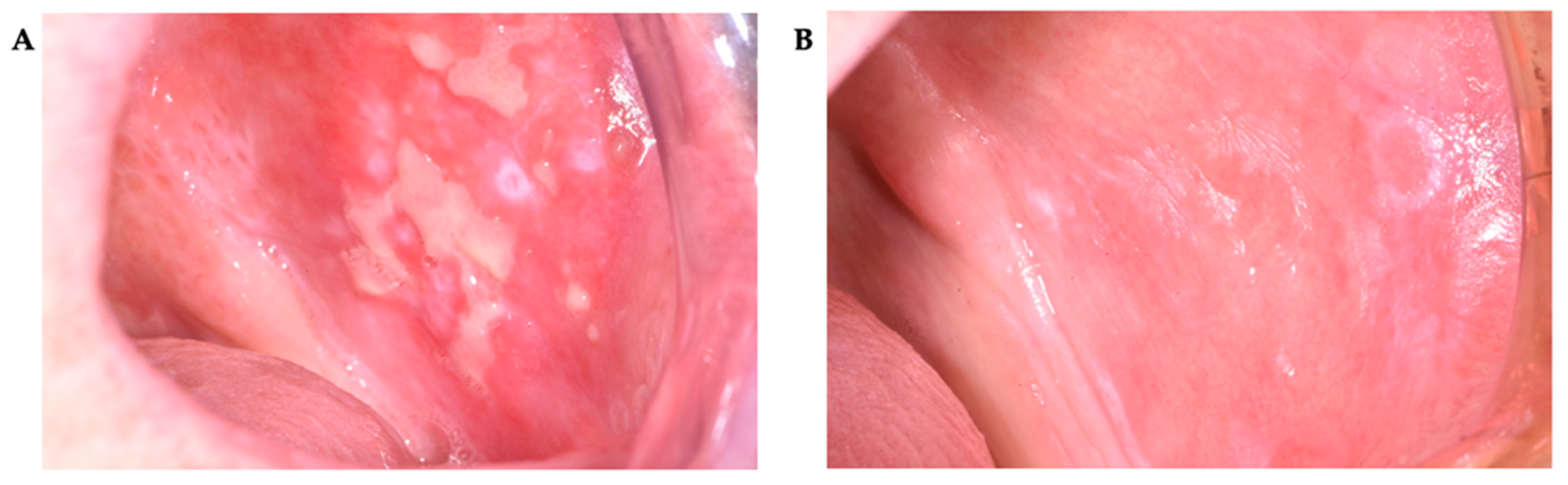
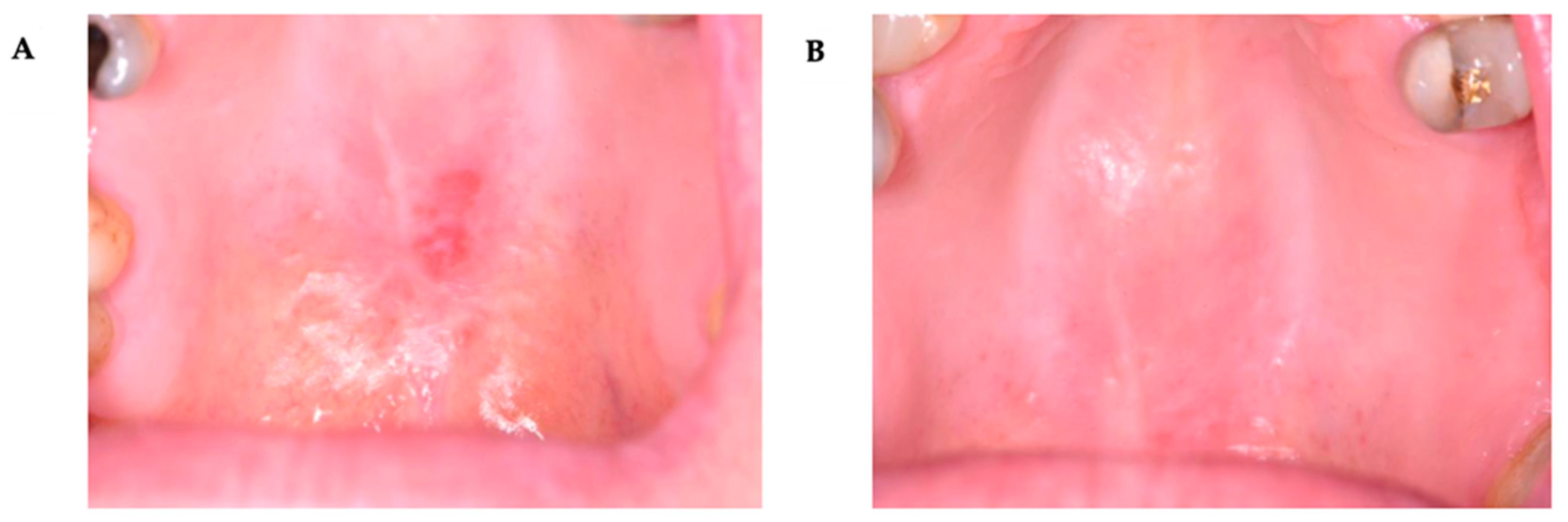
3. Results
3.1. Lesion Dimensions by Treatment and Mucosal Location
3.2. REU Index
3.3. Pain Intensity (VAS)
4. Discussion
Side Effects
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; van der Waal, I. Disease Scoring Systems for Oral Lichen Planus: A Critical Appraisal. Med. Oral Patol. Oral Cir. Bucal 2015, 20, e199–e204. [Google Scholar] [CrossRef]
- Barbosa, N.G.; Silveira, É.J.D.; Lima, E.N.A.; Oliveira, P.T.; Soares, M.S.M.; de Medeiros, A.M.C. Factors Associated with Clinical Characteristics and Symptoms in a Case Series of Oral Lichen Planus. Int. J. Dermatol. 2015, 54, e1–e6. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tang, X.; Zheng, X.; Ge, S.; Wen, H.; Lin, X.; Chen, Z.; Lu, L. Global Prevalence and Incidence Estimates of Oral Lichen Planus: A Systematic Review and Meta-Analysis. JAMA Dermatol. 2020, 156, 172–181. [Google Scholar] [CrossRef] [PubMed]
- González-Moles, M.Á.; Warnakulasuriya, S.; González-Ruiz, I.; González-Ruiz, L.; Ayén, Á.; Lenouvel, D.; Ruiz-Ávila, I.; Ramos-García, P. Worldwide Prevalence of Oral Lichen Planus: A Systematic Review and Meta-Analysis. Oral Dis. 2021, 27, 813–828. [Google Scholar] [CrossRef]
- Vičić, M.; Hlača, N.; Kaštelan, M.; Brajac, I.; Sotošek, V.; Prpić Massari, L. Comprehensive Insight into Lichen Planus Immunopathogenesis. Int. J. Mol. Sci. 2023, 24, 3038. [Google Scholar] [CrossRef]
- Liu, J.; Ye, Z.; Mayer, J.G.; Hoch, B.A.; Green, C.; Rolak, L.; Cold, C.; Khor, S.S.; Zheng, X.; Miyagawa, T.; et al. Phenome-Wide Association Study Maps New Diseases to the Human Major Histocompatibility Complex Region. J. Med. Genet. 2016, 53, 681–689. [Google Scholar] [CrossRef]
- Boch, K.; Langan, E.A.; Kridin, K.; Zillikens, D.; Ludwig, R.J.; Bieber, K. Lichen Planus. Front. Med. 2021, 8, 737813. [Google Scholar] [CrossRef]
- De Porras-Carrique, T.; Ramos-Garcia, P.; Aguilar-Diosdado, M.; Warnakulasuriya, S.; Gonzalez-Moles, M.A. Autoimmune Disorders in Oral Lichen Planus: A Systematic Review and Meta-Analysis. Oral Dis. 2022, 28, 1523–1535. [Google Scholar] [CrossRef]
- MohanKumar, K.P.; Nachiammai, N.; Madhushankari, G.S. Association of Oral Manifestations in Ulcerative Colitis: A Pilot Study. J. Oral Maxillofac. Pathol. 2018, 22, 199–203. [Google Scholar] [CrossRef]
- Garcia-Pola, M.J.; Llorente-Pendas, S.; Seoane-Romero, J.M.; Berasaluce, M.J.; Garcia-Martin, J.M. Thyroid Disease and Oral Lichen Planus as Comorbidity: A Prospective Case-Control Study. Dermatology 2016, 232, 214–219. [Google Scholar] [CrossRef]
- De Porras-Carrique, T.; Gonzalez-Moles, M.A.; Warnakulasuriya, S.; Ramos-Garcia, P. Depression, Anxiety, and Stress in Oral Lichen Planus: A Systematic Review and Meta-Analysis. Clin. Oral Investig. 2022, 26, 1391–1408. [Google Scholar] [CrossRef]
- Ma, S.H.; Tai, Y.H.; Dai, Y.X.; Chang, Y.T.; Chen, T.J.; Chen, M.H. Association between Hepatitis C Virus Infection and Subsequent Chronic Inflammatory Skin Disease. J. Dermatol. 2021, 48, 1884–1891. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Yang, L.; Wen, L.; Lu, H.; Chen, Q.; Wang, Z. Crosstalk between the Oral Microbiota, Mucosal Immunity, and the Epithelial Barrier Regulates Oral Mucosal Disease Pathogenesis. Mucosal Immunol. 2021, 14, 1247–1258. [Google Scholar] [CrossRef] [PubMed]
- Rasul, T.F.; Anderson, J.; Bergholz, D.R.; Faiz, A.; Prasad, R.R. Gold Dental Implant-Induced Oral Lichen Planus. Cureus 2022, 14, e21852. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Jauniaux, E. Oxidative Stress. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef]
- Sankari, S.L.; Babu, N.A.; Rajesh, E.; Kasthuri, M. Apoptosis in Immune-Mediated Diseases. J. Pharm. Bioallied Sci. 2015, 7, S200–S202. [Google Scholar] [CrossRef]
- Ergun, S.; Troşala, Ş.C.; Warnakulasuriya, S.; Özel, S.; Önal, A.E.; Ofluoğlu, D.; Güven, Y.; Tanyeri, H. Evaluation of Oxidative Stress and Antioxidant Profile in Patients with Oral Lichen Planus. J. Oral Pathol. Med. 2011, 40, 286–293. [Google Scholar] [CrossRef]
- Hassan, I.; Keen, A.; Majid, S.; Hassan, T. Evaluation of the Antioxidant Status in Patients of Lichen Planus in Kashmir Valley—A Hospital-Based Study. J. Saudi Soc. Dermatol. Dermatol. Surg. 2013, 17, 13–16. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, Y.; Tang, F.; Chen, Q. Immune Mechanisms Involved in the Coexistence of Oral Lichen Planus and Autoimmune Thyroid Diseases. Zhejiang Da Xue Xue Bao Yi Xue Ban 2021, 50, 222–228. [Google Scholar] [CrossRef]
- Łukaszewska-Kuska, M.; Ślebioda, Z.; Dorocka-Bobkowska, B. The Effectiveness of Topical Forms of Dexamethasone in the Treatment of Oral Lichen Planus—A Systematic Review. Oral Dis. 2022, 28, 2063–2071. [Google Scholar] [CrossRef]
- Aleinikov, A.; Jordan, R.C.; Main, J.H. Topical Steroid Therapy in Oral Lichen Planus: Review of a Novel Delivery Method in 24 Patients. J. Can. Dent. Assoc. 1996, 62, 324–327. [Google Scholar]
- Thongprasom, K.; Dhanuthai, K. Steroids in the Treatment of Lichen Planus: A Review. J. Oral Sci. 2008, 50, 377–385. [Google Scholar] [CrossRef]
- Didona, D.; Caposiena Caro, R.D.; Sequeira Santos, A.M.; Solimani, F.; Hertl, M. Therapeutic Strategies for Oral Lichen Planus: State of the Art and New Insights. Front. Med. 2022, 9, 997190. [Google Scholar] [CrossRef] [PubMed]
- Solimani, F.; Forchhammer, S.; Schloegl, A.; Ghoreschi, K.; Meier, K. Lichen Planus—A Clinical Guide. J. Dtsch. Dermatol. Ges. 2021, 19, 864–882. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.T.; Madsen, L.S.; Saunders, D.P.; Napenas, J.J.; McCreary, C.; Ni Riordain, R.; Pedersen, A.M.L.; Fedele, S.; Cook, R.J.; Abdelsayed, R.; et al. Efficacy and Safety of a Novel Mucoadhesive Clobetasol Patch for Treatment of Erosive Oral Lichen Planus: A Phase 2 Randomized Clinical Trial. J. Oral Pathol. Med. 2022, 51, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Li, C.; Hong, Y.; Yang, L.; Huang, Y.; Cheng, B. Short-Term Clinical Evaluation of Intralesional Triamcinolone Acetonide Injection for Ulcerative Oral Lichen Planus. J. Oral Pathol. Med. 2006, 35, 327–331. [Google Scholar] [CrossRef]
- Hunt, K.M.; Klager, S.; Kwak, Y.J.; Sami, N. Successful Systemic Treatment Outcomes of Lichen Planus: A Single-Center Retrospective Review. Dermatol. Ther. 2021, 34, e14903. [Google Scholar] [CrossRef]
- Ericson-Neilsen, W.; Kaye, A.D. Steroids: Pharmacology, Complications, and Practice Delivery Issues. Ochsner J. 2014, 14, 203–207. [Google Scholar]
- Malhotra, A.K.; Khaitan, B.K.; Sethuraman, G.; Sharma, V.K. Betamethasone Oral Mini-Pulse Therapy Compared with Topical Triamcinolone Acetonide (0.1%) Paste in Oral Lichen Planus: A Randomized Comparative Study. J. Am. Acad. Dermatol. 2008, 58, 596–602. [Google Scholar] [CrossRef]
- He, Y.; Deng, J.; Zhao, Y.; Tao, H.; Dan, H.; Xu, H.; Chen, Q. Efficacy Evaluation of Photodynamic Therapy for Oral Lichen Planus: A Systematic Review and Meta-Analysis. BMC Oral Health 2020, 20, 302. [Google Scholar] [CrossRef]
- Akram, Z.; Javed, F.; Hosein, M.; Al-Qahtani, M.A.; Alshehri, F.; Alzahrani, A.I.; Vohra, F. Photodynamic Therapy in the Treatment of Symptomatic Oral Lichen Planus: A Systematic Review. Photodermatol. Photoimmunol. Photomed. 2018, 34, 167–174. [Google Scholar] [CrossRef]
- Maloth, K.N.; Velpula, N.; Kodangal, S.; Sangmesh, M.; Vellamchetla, K.; Ugrappa, S.; Meka, N. Photodynamic Therapy—A Non-Invasive Treatment Modality for Precancerous Lesions. J. Lasers Med. Sci. 2016, 7, 30–36. [Google Scholar] [CrossRef]
- Sulewska, M.; Duraj, E.; Sobaniec, S.; Graczyk, A.; Milewski, R.; Wróblewska, M.; Pietruski, J.; Pietruska, M. A Clinical Evaluation of the Efficacy of Photodynamic Therapy in the Treatment of Erosive Oral Lichen Planus: A Case Series. Photodiagn. Photodyn. Ther. 2017, 18, 12–19. [Google Scholar] [CrossRef]
- Sulewska, M.; Duraj, E.; Sobaniec, S.; Graczyk, A.; Milewski, R.; Wróblewska, M.; Pietruski, J.; Pietruska, M. A Clinical Evaluation of Efficacy of Photodynamic Therapy in Treatment of Reticular Oral Lichen Planus: A Case Series. Photodiagn. Photodyn. Ther. 2019, 25, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Rakesh, N.; Clint, J.B.; Reddy, S.S.; Nagi, R.; Chauhan, P.; Sharma, S.; Sharma, P.; Kaur, A.; Shetty, B.; Ashwini, S.; et al. Clinical Evaluation of Photodynamic Therapy for the Treatment of Refractory Oral Lichen Planus—A Case Series. Photodiagn. Photodyn. Ther. 2018, 24, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Sulewska, M.E.; Tomaszuk, J.; Sajewicz, E.; Pietruski, J.; Starzyńska, A.; Pietruska, M. Treatment of Reticular Oral Lichen Planus with Photodynamic Therapy: A Case Series. J. Clin. Med. 2023, 12, 875. [Google Scholar] [CrossRef]
- Sulewska, M.; Pietruska, A.; Tomaszuk, J.; Szymańska, E.; Winnicka, K.; Narolewska, J.; Pietruska, M. Efficacy of Photodynamic Therapy with 5-Aminolevulinic Acid-Loaded Oromucosal Emulgel in Patients with Oral Lichen Planus: A Randomized Controlled Clinical Study. Int. Dent. J. 2025, 75, 103925. [Google Scholar] [CrossRef]
- Aghahosseini, F.; Arbabi-Kalati, F.; Fashtami, L.A.; Djavid, G.E.; Fateh, M.; Beitollahi, J.M. Methylene Blue-Mediated Photodynamic Therapy: A Possible Alternative Treatment for Oral Lichen Planus. Lasers Surg. Med. 2006, 38, 33–38. [Google Scholar] [CrossRef]
- Aghahosseini, F.; Arbabi-Kalati, F.; Fashtami, L.A.; Fatch, M.; Djavid, G.E. Treatment of Oral Lichen Planus with Photodynamic Therapy Mediated by Methylene Blue: A Case Report. Med. Oral Patol. Oral Cir. Bucal 2006, 11, e126–e129. [Google Scholar]
- Sadaksharam, J.; Nayaki, K.T.; Panneer Selvam, N. Treatment of Oral Lichen Planus with Methylene Blue Mediated Photodynamic Therapy—A Clinical Study. Photodermatol. Photoimmunol. Photomed. 2012, 28, 97–101. [Google Scholar] [CrossRef]
- Prasanna, S.W.; Ingle, E.; Aruna, P.R.; Pravada, C.; Koteeswaran, D.; Ganesan, S. Photodynamic Therapy of Oral Leukoplakia and Oral Lichen Planus Using Methylene Blue: A Pilot Study. J. Innov. Opt. Health Sci. 2015, 8, 1540005–1540011. [Google Scholar] [CrossRef]
- Bakhtiari, S.; Mojahedi, S.M.; Azari-Marhabi, S.; Namdari, M.; Rankohi, Z.E. Comparing Clinical Effects of Photodynamic Therapy as a Novel Method with Topical Corticosteroid for Treatment of Oral Lichen Planus. Photodiagn. Photodyn. Ther. 2017, 20, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, D.; Moussa, E.; Alnouaem, M. Evaluation of Photodynamic Therapy in Treatment of Oral Erosive Lichen Planus in Comparison with Topically Applied Corticosteroids. Photodiagn. Photodyn. Ther. 2017, 19, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Kvaal, S.I.; Angell-Petersen, E.; Warloe, T. Photodynamic Treatment of Oral Lichen Planus. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 115, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Sobaniec, S.; Bernaczyk, P.; Pietruski, J.; Cholewa, M.; Skurska, A.; Dolińska, E.; Duraj, E.; Tokajuk, G.; Paniczko, A.; Olszewska, E.; et al. Clinical Assessment of the Efficacy of Photodynamic Therapy in the Treatment of Oral Lichen Planus. Lasers Med. Sci. 2013, 28, 311–316. [Google Scholar] [CrossRef]
- Pavlic, V.; Vujic-Aleksic, V. Phototherapy Approaches in Treatment of Oral Lichen Planus. Photodermatol. Photoimmunol. Photomed. 2013, 29, 15–24. [Google Scholar] [CrossRef]
- Jajarm, H.H.; Falaki, F.; Sanatkhani, M.; Ahmadzadeh, M.; Ahrari, F.; Shafaee, H. A Comparative Study of Toluidine Blue-Mediated Photodynamic Therapy versus Topical Corticosteroids in the Treatment of Erosive-Atrophic Oral Lichen Planus: A Randomized Clinical Controlled Trial. Lasers Med. Sci. 2014, 29, 1329–1335. [Google Scholar] [CrossRef]
- Szymańska, E.; Potaś, J.; Baranowski, M.; Czarnomysy, R.; Sulewska, M.E.; Basa, A.; Pietruska, M.; Bielawski, K.; Winnicka, K. Evaluation of Oromucosal Natural Gum-Based Emulgels as Novel Strategy for Photodynamic Therapy of Oral Premalignant Lesions. Pharmaceutics 2023, 15, 2512. [Google Scholar] [CrossRef]
- Szymańska, E.; Potaś, J.; Maciejczyk, M.; Sulewska, M.E.; Pietruska, M.; Zalewska, A.; Pietruska, A.; Winnicka, K. Preliminary Assessment of Polysaccharide-Based Emulgels Containing Delta-Aminolevulinic Acid for Oral Lichen Planus Treatment. Pharmaceuticals 2023, 16, 1534. [Google Scholar] [CrossRef]
- Piboonniyom, S.O.; Treister, N.; Pitiphat, W.; Woo, S.B. Scoring System for Monitoring Oral Lichenoid Lesions: A Preliminary Study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2005, 99, 696–703. [Google Scholar] [CrossRef]
- Huskisson, E.C. Measurement of Pain. Lancet 1974, 2, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- Kurosaki, Y.; Takatori, T.; Nishimura, H.; Nakayama, T.; Kimura, T. Regional Variation in Oral Mucosal Drug Absorption: Permeability and Degree of Keratinization in Hamster Oral Cavity. Pharm. Res. 1991, 8, 1297–1301. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, S.; Gupta, D.; Pallagatti, S.; Singla, I.; Gupta, R.; Goel, V. Role of Topical Drugs in Treatment of Oral Mucosal Diseases: A Literature Review. N. Y. State Dent. J. 2013, 79, 58–64. [Google Scholar]
- Coderch, L.; Alonso, C.; Calpena, A.C.; Pérez-García, M.L.; Clares-Naveros, B.; Ramos, A.; Martí, M. Permeation Protection by Waterproofing Mucosal Membranes. Pharmaceutics 2023, 15, 2698. [Google Scholar] [CrossRef]
- Jurczyszyn, K.; Trzeciakowski, W.; Kozakiewicz, M.; Kida, D.; Malec, K.; Karolewicz, B.; Konopka, T.; Zborowski, J. Fractal Dimension and Texture Analysis of Lesion Autofluorescence in the Evaluation of Oral Lichen Planus Treatment Effectiveness. Materials 2021, 14, 5448. [Google Scholar] [CrossRef]
- Umashankar, M.S.; Madhav, N.V.S. A Smart Oro-Soft Palate Mucosal Drug Delivery: Credentials and Future Trends. Marmara Pharm. J. 2015, 19, 208–221. [Google Scholar] [CrossRef]
- Trakarnboonkij, J.; Tanya, S.; Sarideechaigul, W.; Subarnbhesaj, A.; Tabbon, P.; Sattayut, S. Recalcitrant Oral Lichen Planus Involving Bilateral Buccal Mucosae Treated with a Combination of Photodynamic and Photobiomodulation Therapies: Case Report. F1000Research 2024, 13, 152. [Google Scholar] [CrossRef]
- Zborowski, J.; Kida, D.; Szarwaryn, A.; Nartowski, K.; Rak, P.; Jurczyszyn, K.; Konopka, T. A Comparison of Clinical Efficiency of Photodynamic Therapy and Topical Corticosteroid in Treatment of Oral Lichen Planus: A Split-Mouth Randomised Controlled Study. J. Clin. Med. 2021, 10, 3673. [Google Scholar] [CrossRef]
- Lavaee, F.; Shadmanpour, M. Comparison of the Effect of Photodynamic Therapy and Topical Corticosteroid on Oral Lichen Planus Lesions. J. Biophotonics 2019, 25, 1954–1963. [Google Scholar] [CrossRef]
- Saleh, W.; Tageldin, S.; Khashaba, E.; Darwish, M.; Elnagdy, S.; Khashaba, O. Could Photodynamic Therapy Be Utilized as a Treatment Modality for Oral Lichen Planus? Photodiagn. Photodyn. Ther. 2020, 30, 101792. [Google Scholar] [CrossRef]
- Mirza, S.; Rehman, N.; Alrahla, A.; Alamri, W.A.R.; Vohra, F. Efficacy of Photodynamic Therapy and Low-Level Laser Therapy against Steroid Therapy in the Treatment of Erosive-Atrophic Oral Lichen Planus. Photodiagn. Photodyn. Ther. 2018, 21, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Waingade, M.; Medikeri, R.S.; Gaikwad, S. Effectiveness of Hyaluronic Acid in the Management of Oral Lichen Planus: A Systematic Review and Meta-Analysis. J. Dent. Anesth. Pain Med. 2022, 22, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Nagi, R.; Muthukrishnan, A.; Rakesh, N. Effectiveness of Photodynamic Therapy in the Management of Symptomatic Oral Lichen Planus—A Systematic Review. J. Oral Biol. Craniofac. Res. 2023, 13, 353–359. [Google Scholar] [CrossRef]
- Gulzar, M.A.; Gul, N.; Alvi, F.D.; Khattak, Y.R.; Hasan, U.S.; Haneef, M.B.; Ahmad, I. Comparison of Photodynamic Therapy and Corticosteroid Therapy in Management of Oral Lichen Planus: A Systematic Review of Randomized Controlled Trials. Photodiagn. Photodyn. Ther. 2023, 44, 103747. [Google Scholar] [CrossRef] [PubMed]
- Park, H.K.; Hurwitz, S.; Woo, S.B. Oral Lichen Planus: REU Scoring System Correlates with Pain. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 75–82. [Google Scholar] [CrossRef]
- Selvam, N.P.; Sadaksharam, J.; Singaravelu, G.; Ramu, R. Treatment of Oral Leukoplakia with Photodynamic Therapy: A Pilot Study. J. Cancer Res. Ther. 2015, 11, 464–467. [Google Scholar] [CrossRef]
- Yan, Y.; Li, Z.; Tian, X.; Zeng, X.; Chen, Q.; Wang, J. Laser-Assisted Photodynamic Therapy in Proliferative Verrucous Oral Leukoplakia. Photodiagn. Photodyn. Ther. 2022, 39, 103002. [Google Scholar] [CrossRef]
- Said, Z.; Murdoch, C.; Hansen, J.; Madsen, L.S.; Colley, H.E. Corticosteroid Delivery Using Oral Mucosa Equivalents for the Treatment of Inflammatory Mucosal Diseases. Eur. J. Oral Sci. 2021, 129, e12761. [Google Scholar] [CrossRef]
- Addy, M. The Oral Mucosal Absorption and Tissue Distribution of Triamcinolone Acetonide in the Dog Studied by Autoradiography. Arch. Oral Biol. 1980, 25, 809–817. [Google Scholar] [CrossRef]
- Wiśniewski, P.; Sulewska, M.; Tomaszuk, J.; Zalewska, A.; Zięba, S.; Pietruska, A.; Szymańska, E.; Winnicka, K.; Maciejczyk, M.; Żendzian-Piotrowska, M.; et al. Effects of Photodynamic Therapy and Glucocorticosteroids on Salivary Oxidative Stress in Oral Lichen Planus: A Randomized Clinical Trial. Antioxidants 2025, 14, 1017. [Google Scholar] [CrossRef]
- Leong, X.Y.; Gopinath, D.; Syeed, S.M.; Veettil, S.K.; Shetty, N.Y.; Menon, R.K. Comparative Efficacy and Safety of Interventions for the Treatment of Oral Lichen Planus: A Systematic Review and Network Meta-Analysis. J. Clin. Med. 2023, 12, 2763. [Google Scholar] [CrossRef]
- Andabak-Rogulj, A.; Vindiš, E.; Aleksijević, L.H.; Škrinjar, I.; Juras, D.V.; Aščić, A.; Brzak, B.L. Different Treatment Modalities of Oral Lichen Planus—A Narrative Review. Dent. J. 2023, 11, 26. [Google Scholar] [CrossRef]
- Chen, H.M.; Yu, C.H.; Tu, P.C.; Yeh, C.Y.; Tsai, T.; Chiang, C.P. Successful Treatment of Oral Verrucous Hyperplasia and Oral Leukoplakia with Topical 5-Aminolevulinic Acid-Mediated Photodynamic Therapy. Lasers Surg. Med. 2005, 37, 114–122. [Google Scholar] [CrossRef]
- Sandhu, S.; Klein, B.A.; Al-Hadlaq, M.; Chirravur, P.; Bajonaid, A.; Xu, Y.; Intini, R.; Hussein, M.; Vacharotayangul, P.; Sroussi, H.; et al. Oral Lichen Planus: Comparative Efficacy and Treatment Costs—A Systematic Review. BMC Oral Health 2022, 22, 161. [Google Scholar] [CrossRef]


| Variable | N (%) |
|---|---|
| Gender | |
| Male | 18 (20%) |
| Female | 72 (80%) |
| Age | |
| Average age (M ± SD) | 60 ± 11.7 years |
| Lesion location | |
| Non-keratinized mucosa | 132 (81.99%) |
| Keratinized mucosa | 29 (18.01%) |
| Group | Sex | Age < 40 | Age ≥ 40 ≤ 65 | Age > 65 | Total |
|---|---|---|---|---|---|
| PDT | M | 0 | 3 | 4 | 7 |
| F | 5 | 23 | 15 | 43 | |
| CT | M | 3 | 4 | 4 | 11 |
| F | 0 | 18 | 11 | 29 | |
| Total | 8 | 48 | 34 | 90 |
| Time Point | Type of Mucosa. | Mean | Standard Deviation | 95% CI (Min) | 95% CI (Max) | Median | Minimum | Maximum | p (Friedman Test) |
|---|---|---|---|---|---|---|---|---|---|
| T0 | NK | 2.54 | 1.86 | 2.22 | 2.86 | 2.25 | 0.15 | 12.00 | <0.0001 |
| T1 | 1.06 | 1.38 | 0.83 | 1.30 | 1.00 | 0.00 | 10.00 | ||
| T2 | 1.00 | 1.59 | 0.73 | 1.28 | 0.50 | 0.00 | 12.00 | ||
| T0 | K | 1.72 | 0.82 | 1.41 | 2.04 | 1.50 | 0.72 | 4.00 | <0.0001 |
| T1 | 1.12 | 1.03 | 0.73 | 1.51 | 1.00 | 0.00 | 4.00 | ||
| T2 | 1.27 | 1.36 | 0.76 | 1.79 | 0.75 | 0.00 | 4.00 |
| Location | Group | Time Point | Mean | SD | 95% CI (Min) | 95% CI (Max) | Median | Min | Max | p (Friedman Test) |
|---|---|---|---|---|---|---|---|---|---|---|
| Non-keratinized mucosa | 1 | T0 | 2.64 | 1.75 | 2.23 | 3.05 | 2.25 | 0.15 | 9.0 | <0.0001 |
| T1 | 1.05 | 0.99 | 0.81 | 1.28 | 1.0 | 0.0 | 4.0 | |||
| T2 | 0.56 | 0.92 | 0.34 | 0.78 | 0.0 | 0.0 | 3.0 | |||
| 2 | T0 | 2.43 | 2.0 | 1.91 | 2.94 | 2.25 | 0.35 | 12.0 | <0.0001 | |
| T1 | 1.08 | 1.74 | 0.63 | 1.53 | 0.55 | 0.0 | 10.0 | |||
| T2 | 1.54 | 2.02 | 1.02 | 2.06 | 1.0 | 0.0 | 12.0 | |||
| Keratinized mucosa | 1 | T0 | 1.83 | 0.84 | 1.45 | 2.21 | 1.5 | 0.75 | 4.0 | <0.0001 |
| T1 | 1.14 | 1.15 | 0.62 | 1.67 | 1.0 | 0.0 | 4.0 | |||
| T2 | 1.11 | 1.34 | 0.5 | 1.72 | 0.5 | 0.0 | 4.0 | |||
| 2 | T0 | 1.44 | 0.76 | 0.8 | 2.08 | 1.25 | 0.72 | 2.5 | 0.0035 | |
| T1 | 1.06 | 0.66 | 0.51 | 1.61 | 0.88 | 0.4 | 2.0 | |||
| T2 | 1.69 | 1.4 | 0.52 | 2.86 | 1.25 | 0.0 | 3.75 |
| Time Point | Type of Mucosa | Mean | Standard Deviation | 95% CI (Min) | 95% CI (Max) | Median | Minimum | Maximum | p (Friedman Test) |
|---|---|---|---|---|---|---|---|---|---|
| T0 | NK | 1.49 | 0.90 | 1.34 | 1.65 | 1.00 | 1.00 | 5.00 | <0.0001 |
| T1 | 0.84 | 0.58 | 0.74 | 0.94 | 1.00 | 0.00 | 2.00 | ||
| T2 | 0.69 | 0.67 | 0.57 | 0.80 | 1.00 | 0.00 | 3.00 | ||
| T0 | K | 1.83 | 0.71 | 1.56 | 2.10 | 2.00 | 1.00 | 3.00 | <0.0006 |
| T1 | 1.24 | 0.91 | 0.89 | 1.59 | 1.00 | 0.00 | 5.00 | ||
| T2 | 1.21 | 1.05 | 0.81 | 1.61 | 1.00 | 0.00 | 5.00 |
| Location | Group | Time Point | Mean | SD | 95% CI (Min) | 95% CI (Max) | Median | Min | Max | p (Friedman Test) |
|---|---|---|---|---|---|---|---|---|---|---|
| Non-keratinized mucosa | 1 | T0 | 1.65 | 1.08 | 1.4 | 1.91 | 1.0 | 1.0 | 5.0 | <0.0001 |
| T1 | 0.99 | 0.54 | 0.86 | 1.11 | 1.0 | 0.0 | 2.0 | |||
| T2 | 0.68 | 0.73 | 0.51 | 0.85 | 1.0 | 0.0 | 3.0 | |||
| 2 | T0 | 1.3 | 0.56 | 1.15 | 1.45 | 1.0 | 1.0 | 3.0 | <0.0001 | |
| T1 | 0.67 | 0.57 | 0.52 | 0.81 | 1.0 | 0.0 | 2.0 | |||
| T2 | 0.7 | 0.59 | 0.55 | 0.85 | 1.0 | 0.0 | 3.0 | |||
| Keratinized mucosa | 1 | T0 | 1.86 | 0.73 | 1.53 | 2.19 | 2.0 | 1.0 | 3.0 | 0.0014 |
| T1 | 1.33 | 0.97 | 0.89 | 1.77 | 1.0 | 0.0 | 5.0 | |||
| T2 | 1.1 | 1.14 | 0.58 | 1.61 | 1.0 | 0.0 | 5.0 | |||
| 2 | T0 | 1.75 | 0.71 | 1.16 | 2.34 | 2.0 | 1.0 | 3.0 | 0.1462 | |
| T1 | 1.0 | 0.76 | 0.37 | 1.63 | 1.0 | 0.0 | 2.0 | |||
| T2 | 1.5 | 0.76 | 0.87 | 2.13 | 1.0 | 1.0 | 3.0 |
| Time Point | Type of Mucosa | Median | Minimum | Maximum | Q1 | Q3 | p (Friedman Test) |
|---|---|---|---|---|---|---|---|
| T0 | NK | 3.00 | 2.00 | 10.00 | 3.00 | 4.00 | <0.0001 |
| T1 | 1.50 | 0.00 | 4.00 | 1.00 | 2.00 | ||
| T2 | 1.00 | 0.00 | 4.00 | 0.00 | 2.00 | ||
| T0 | K | 4.00 | 2.00 | 10.00 | 3.00 | 5.00 | <0.0001 |
| T1 | 2.00 | 0.00 | 5.00 | 1.00 | 3.00 | ||
| T2 | 2.00 | 0.00 | 6.00 | 1.00 | 2.00 |
| Location | Group | Time Point | Median | Min | Max | Q1 | Q3 | p (Friedman Test) |
|---|---|---|---|---|---|---|---|---|
| Non-keratinized mucosa | 1 | T0 | 3.0 | 2.0 | 10.0 | 3.0 | 4.5 | <0.0001 |
| T1 | 2.0 | 0.0 | 4.0 | 1.0 | 2.0 | |||
| T2 | 0.0 | 0.0 | 3.0 | 0.0 | 1.0 | |||
| 2 | T0 | 3.0 | 2.0 | 7.0 | 3.0 | 4.0 | <0.0001 | |
| T1 | 1.0 | 0.0 | 4.0 | 0.0 | 2.0 | |||
| T2 | 2.0 | 0.0 | 4.0 | 1.0 | 3.0 | |||
| Keratinized mucosa | 1 | T0 | 4.0 | 3.0 | 10.0 | 3.0 | 5.0 | <0.0001 |
| T1 | 2.0 | 0.0 | 3.0 | 1.0 | 3.0 | |||
| T2 | 1.0 | 0.0 | 3.0 | 0.0 | 2.0 | |||
| 2 | T0 | 4.0 | 2.0 | 6.0 | 3.0 | 5.5 | 0.0018 | |
| T1 | 2.0 | 0.0 | 5.0 | 1.0 | 3.0 | |||
| T2 | 3.0 | 0.0 | 6.0 | 1.5 | 4.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pietruska, A.; Sulewska, M.; Wiśniewski, P.; Tomaszuk, J.; Szymańska, E.; Winnicka, K.; Narolewska, J.; Pietruska, M. Photodynamic Therapy with 5-Aminolevulinic Acid Versus Topical Corticosteroids in the Treatment of Oral Lichen Planus: A Randomized Clinical Trial with Lesion Site-Specific Analysis. Pharmaceutics 2025, 17, 1381. https://doi.org/10.3390/pharmaceutics17111381
Pietruska A, Sulewska M, Wiśniewski P, Tomaszuk J, Szymańska E, Winnicka K, Narolewska J, Pietruska M. Photodynamic Therapy with 5-Aminolevulinic Acid Versus Topical Corticosteroids in the Treatment of Oral Lichen Planus: A Randomized Clinical Trial with Lesion Site-Specific Analysis. Pharmaceutics. 2025; 17(11):1381. https://doi.org/10.3390/pharmaceutics17111381
Chicago/Turabian StylePietruska, Aleksandra, Magdalena Sulewska, Patryk Wiśniewski, Jagoda Tomaszuk, Emilia Szymańska, Katarzyna Winnicka, Joanna Narolewska, and Małgorzata Pietruska. 2025. "Photodynamic Therapy with 5-Aminolevulinic Acid Versus Topical Corticosteroids in the Treatment of Oral Lichen Planus: A Randomized Clinical Trial with Lesion Site-Specific Analysis" Pharmaceutics 17, no. 11: 1381. https://doi.org/10.3390/pharmaceutics17111381
APA StylePietruska, A., Sulewska, M., Wiśniewski, P., Tomaszuk, J., Szymańska, E., Winnicka, K., Narolewska, J., & Pietruska, M. (2025). Photodynamic Therapy with 5-Aminolevulinic Acid Versus Topical Corticosteroids in the Treatment of Oral Lichen Planus: A Randomized Clinical Trial with Lesion Site-Specific Analysis. Pharmaceutics, 17(11), 1381. https://doi.org/10.3390/pharmaceutics17111381







