Cardiopulmonary and Immune Alterations in the Ts65Dn Mouse Model of Down Syndrome and Modulation by Epigallocatechin-3-Gallate-Enriched Green Tea Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Animal Model
2.3. GTE-EGCG Treatment
2.4. Experimental Design
2.5. Longitudinal Lung Imaging Using µCT
2.6. Contrast-Enhanced Cardiac µCT
2.7. Echocardiographic Analysis
2.8. Pulmonary Lung Function Measurements
2.9. Immunological Analysis
2.10. Histopathological and Histochemical Analysis
2.11. Phase-Contrast µCT
2.12. Statistical Analyses
3. Results
3.1. Tracing Body Weight Development Showed a Genotype Effect upon GTE-EGCG Administration
3.2. Characterization of the Structural and Functional Pulmonary Phenotype of Ts65Dn Mice Throughout Development and Modulatory Effects of GTE-EGCG
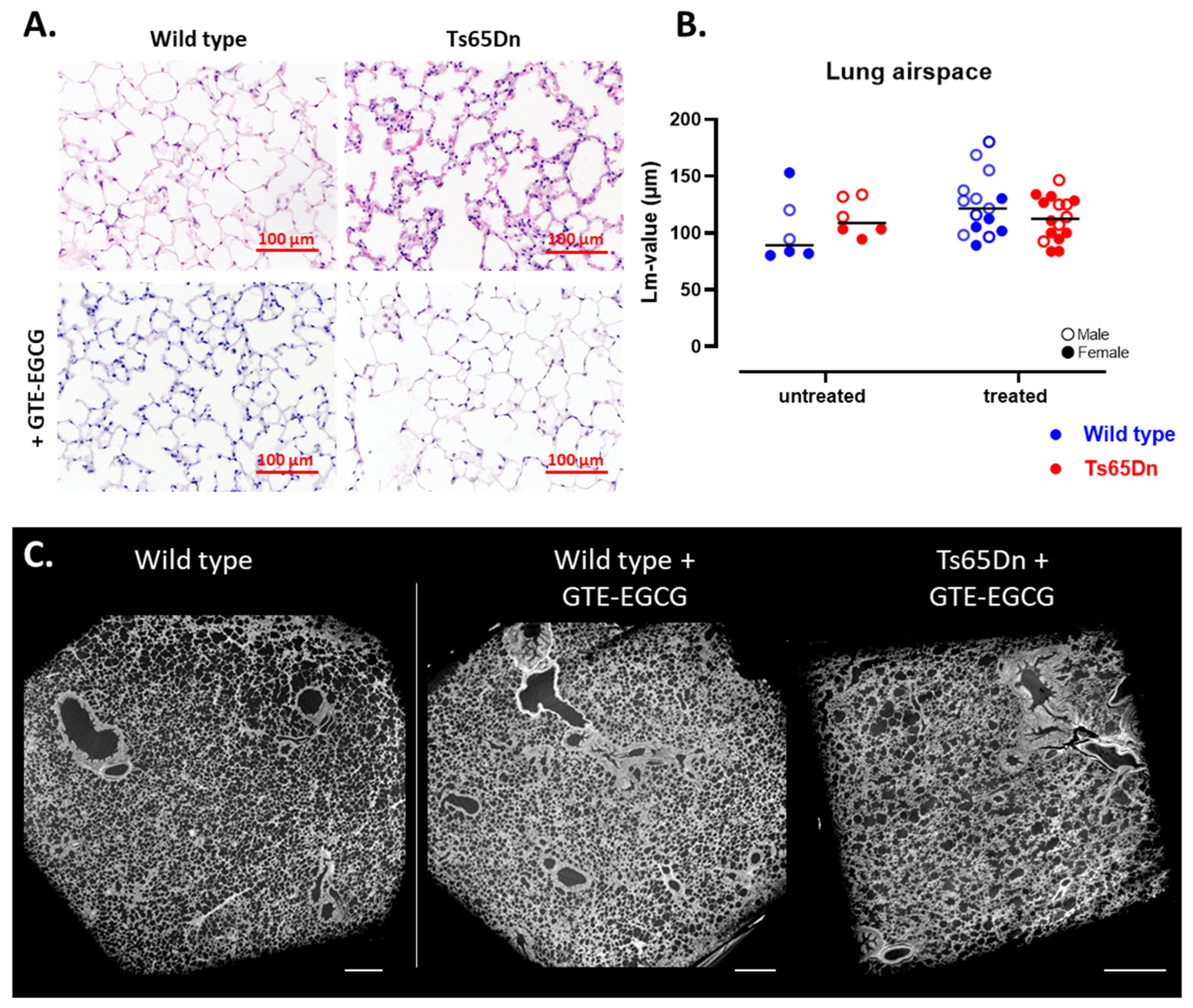
3.2.1. Structural Lung Development Is Similar in WT and Ts65Dn Mice
3.2.2. GTE-EGCG Administration Alters the Lung Maturation Trajectory
3.2.3. WT and Ts65Dn Littermates Present with Equal Pulmonary Lung Function and Airway Reactivity
3.2.4. GTE-EGCG Reduces Inspiratory Capacity and Sensitizes for Airway Hyperreactivity
3.3. Characterization of the Structural and Functional Cardiovascular Phenotype of Ts65Dn Mice and Impact of GTE-EGCG Modulation
3.3.1. Ts65Dn Mice Present with a High Variation in Arterial Vessel Thickness
3.3.2. GTE-EGCG Administration Decreases Arterial Wall Thickness in Ts65Dn Mice
3.3.3. Microstructural Analysis Reveals RV Hypertrophy in Ts65Dn Mice
3.3.4. GTE-EGCG Administration Affects Both LV and RV Cardiac Function and Structure
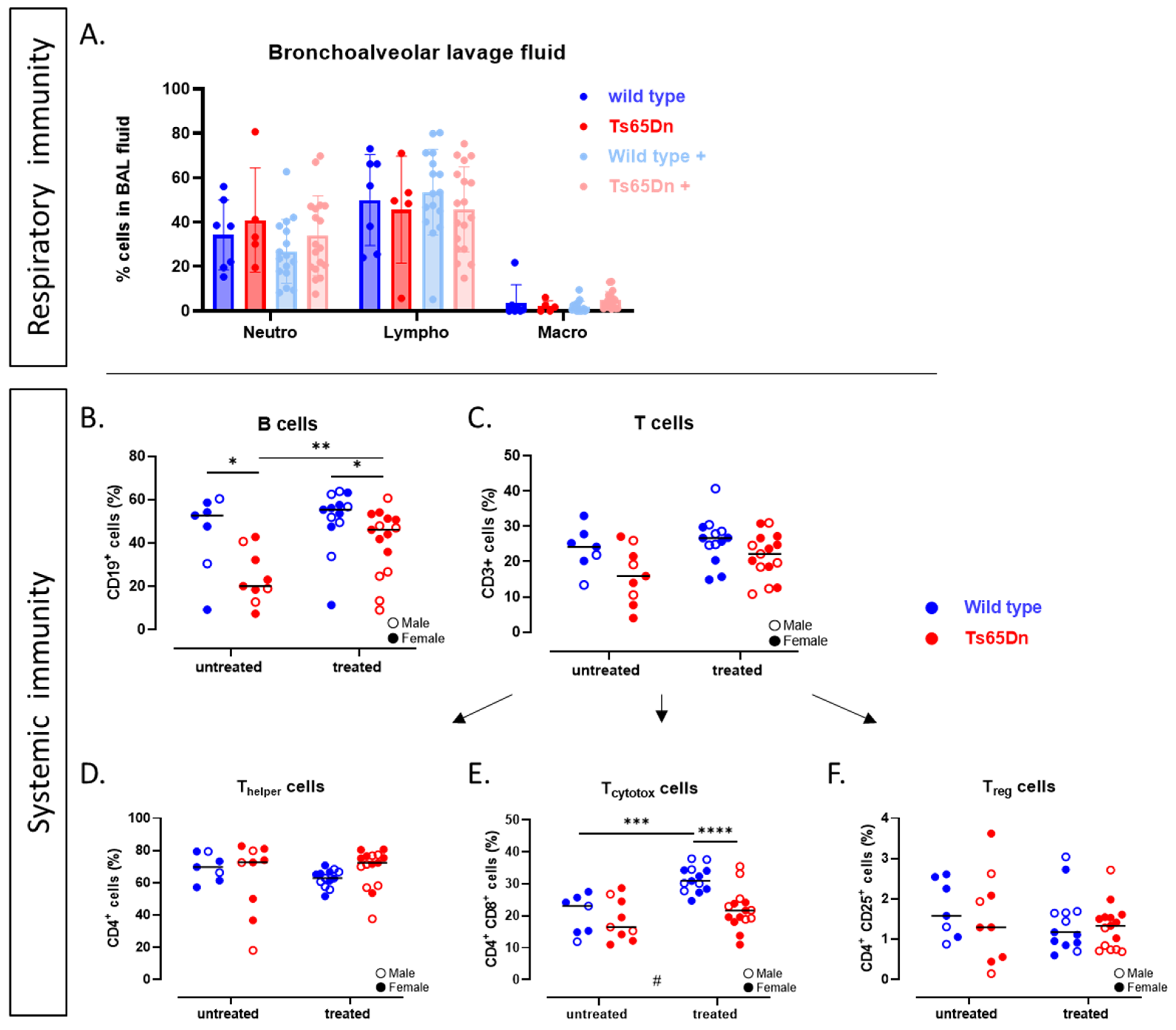
3.4. The Systemic and Pulmonary Immunological Status of Ts65Dn Mice and Effect of GTE-EGCG
3.4.1. Ts65Dn Mice Have Less B-Cells
3.4.2. GTE-EGCG Administration Alleviates B-Cell Numbers in Ts65Dn Mice
4. Discussion
4.1. Genotype, but No Treatment Effects in Body Mass Development
4.2. Insight into the Cardiopulmonary and Immunological Phenotype of Ts65Dn Mice
4.3. Is There an Effect of Prenatal GTE-EGCG Administration on Airway Development and Cardiac Function?
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Potier, M.-C.; Reeves, R.H. Editorial: Intellectual Disabilities in Down Syndrome from Birth and Throughout Life: Assessment and Treatment. Front. Behav. Neurosci. 2016, 10, 120. [Google Scholar] [CrossRef]
- Frid, C.; Drott, P.; Lundell, B.; Rasmussen, F.; Annerén, G. Mortality in Down’s Syndrome in Relation to Congenital Malformations. J. Intellect. Disabil. Res. 1999, 43, 234–241. [Google Scholar] [CrossRef]
- Bush, D.; Abman, S.H.; Galambos, C. Prominent Intrapulmonary Bronchopulmonary Anastomoses and Abnormal Lung Development in Infants and Children with Down Syndrome. J. Pediatr. 2017, 180, 156–162.e1. [Google Scholar] [CrossRef] [PubMed]
- Bush, D.; Galambos, C.; Ivy, D.D.; Abman, S.H.; Wolter-Warmerdam, K.; Hickey, F. Clinical Characteristics and Risk Factors for Developing Pulmonary Hypertension in Children with Down Syndrome. J. Pediatr. 2018, 202, 212–219.e2. [Google Scholar] [CrossRef] [PubMed]
- Maris, M.; Verhulst, S.; Wojciechowski, M.; Van de Heyning, P.; Boudewyns, A. Prevalence of Obstructive Sleep Apnea in Children with Down Syndrome. Sleep 2016, 39, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Benhaourech, S.; Drighil, A.; Hammiri, A. Congenital Heart Disease and Down Syndrome: Various Aspects of a Confirmed Association. Cardiovasc. J. Afr. 2016, 27, 287–290. [Google Scholar] [CrossRef]
- Bruijn, M.; van der Aa, L.B.; van Rijn, R.R.; Bos, A.P.; van Woensel, J.B.M. High Incidence of Acute Lung Injury in Children with Down Syndrome. Intensive Care Med. 2007, 33, 2179–2182. [Google Scholar] [CrossRef]
- Zhang, Y.; Che, M.; Yuan, J.; Yu, Y.; Cao, C.; Qin, X.-Y.; Cheng, Y. Aberrations in Circulating Inflammatory Cytokine Levels in Patients with Down Syndrome: A Meta-Analysis. Oncotarget 2017, 8, 84489–84496. [Google Scholar] [CrossRef]
- Khan, I.; Malinge, S.; Crispino, J. Myeloid Leukemia in Down Syndrome. Crit. Rev. Oncog. 2011, 16, 25–36. [Google Scholar] [CrossRef]
- Colvin, K.L.; Yeager, M.E. What People with Down Syndrome Can Teach Us about Cardiopulmonary Disease. Eur. Respir. Rev. 2017, 26, 16. [Google Scholar] [CrossRef]
- Sullivan, K.D.; Lewis, H.C.; Hill, A.A.; Pandey, A.; Jackson, L.P.; Cabral, J.M.; Smith, K.P.; Liggett, L.A.; Gomez, E.B.; Galbraith, M.D.; et al. Trisomy 21 Consistently Activates the Interferon Response. eLife 2016, 5, e16220. [Google Scholar] [CrossRef]
- Waugh, K.A.; Minter, R.; Baxter, J.; Chi, C.; Tuttle, K.D.; Eduthan, N.P.; Galbraith, M.D.; Kinning, K.T.; Andrysik, Z.; Araya, P.; et al. Triplication of the interferon receptor locus contributes to hallmarks of Down syndrome in a mouse model. Nat. Genet. 2023, 55, 1034–1047. [Google Scholar] [CrossRef]
- Gillenwater, L.A.; Galbraith, M.D.; Rachubinski, A.L.; Eduthan, N.P.; Sullivan, K.D.; Espinosa, J.M.; Costello, J.C. Integrated Analysis of Immunometabolic Interactions in Down Syndrome. Sci. Adv. 2024, 10, eadq3073. [Google Scholar] [CrossRef]
- Craven, V.E.; Daw, W.J.; Wan, J.W.Y.; Elphick, H.E. Respiratory and Airway Disorders in Children with Down Syndrome: A Review of the Clinical Challenges and Management. Front. Pediatr. 2025, 13, 1553984. [Google Scholar] [CrossRef]
- Crété, N.; Gosset, P.; Théophile, D.; Duterque-Coquillaud, M.; Blouin, J.L.; Fayssettes, C.; Sinet, P.M.; Créau-Goldberg, N. Mapping the Down Syndrome Chromosome Region. Eur. J. Hum. Genet. 1993, 1, 51–63. [Google Scholar] [CrossRef]
- Korenberg, J.R.; Aaltonen, J.; Brahe, C.; Cabin, D.; Creau, N.; Delabar, J.M.; Doering, J.; Gardiner, K.; Hubert, R.S.; Ives, J.; et al. Report of the Sixth International Workshop on Human Chromosome 21 Mapping 1996. Cytogenet. Cell Genet. 1997, 79, 21–52. [Google Scholar] [CrossRef]
- Belichenko, N.P.; Belichenko, P.V.; Kleschevnikov, A.M.; Salehi, A.; Reeves, R.H.; Mobley, W.C. The “Down Syndrome Critical Region” Is Sufficient in the Mouse Model to Confer Behavioral, Neurophysiological, and Synaptic Phenotypes Characteristic of Down Syndrome. J. Neurosci. 2009, 29, 5938–5948. [Google Scholar] [CrossRef] [PubMed]
- Herault, Y.; Delabar, J.M.; Fisher, E.M.C.; Tybulewicz, V.L.J.; Yu, E.; Brault, V. Rodent Models in Down Syndrome Research: Impact and Future Opportunities. Dis. Models Mech. 2017, 10, 1165–1186. [Google Scholar] [CrossRef] [PubMed]
- Richtsmeier, J.T.; Baxter, L.L.; Reeves, R.H. Parallels of Craniofacial Maldevelopment in down Syndrome and Ts65Dn Mice. Dev. Dyn. 2000, 217, 137–145. [Google Scholar] [CrossRef]
- Moore, C.S. Postnatal Lethality and Cardiac Anomalies in the Ts65Dn Down Syndrome Mouse Model. Mamm. Genome 2006, 17, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Duchon, A.; Raveau, M.; Chevalier, C.; Nalesso, V.; Sharp, A.J.; Herault, Y. Identification of the Translocation Breakpoints in the Ts65Dn and Ts1Cje Mouse Lines: Relevance for Modeling down Syndrome. Mamm. Genome 2011, 22, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Reinholdt, L.G.; Ding, Y.; Gilbert, G.T.; Czechanski, A.; Solzak, J.P.; Roper, R.J.; Johnson, M.T.; Donahue, L.R.; Lutz, C.; Davisson, M.T. Molecular Characterization of the Translocation Breakpoints in the Down Syndrome Mouse Model Ts65Dn. Mamm. Genome 2011, 22, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yu, T.; Morishima, M.; Pao, A.; LaDuca, J.; Conroy, J.; Nowak, N.; Matsui, S.-I.; Shiraishi, I.; Yu, Y.E. Duplication of the Entire 22.9 Mb Human Chromosome 21 Syntenic Region on Mouse Chromosome 16 Causes Cardiovascular and Gastrointestinal Abnormalities. Hum. Mol. Genet. 2007, 16, 1359–1366. [Google Scholar] [CrossRef]
- Lana-Elola, E.; Watson-Scales, S.; Slender, A.; Gibbins, D.; Martineau, A.; Douglas, C.; Mohun, T.; Fisher, E.M.; Tybulewicz, V.L. Genetic Dissection of Down Syndrome-Associated Congenital Heart Defects Using a New Mouse Mapping Panel. eLife 2016, 5, e11614. [Google Scholar] [CrossRef]
- Duchon, A.; Del Mar Muñiz Moreno, M.; Chevalier, C.; Nalesso, V.; Andre, P.; Fructuoso-Castellar, M.; Mondino, M.; Po, C.; Noblet, V.; Birling, M.-C.; et al. Ts66Yah, a Mouse Model of Down Syndrome with Improved Construct and Face Validity. Dis. Models Mech. 2022, 15, dmm049721. [Google Scholar] [CrossRef]
- Starbuck, J.M.; Llambrich, S.; Gonzàlez, R.; Albaigès, J.; Sarlé, A.; Wouters, J.; González, A.; Sevillano, X.; Sharpe, J.; De La Torre, R.; et al. Green Tea Extracts Containing Epigallocatechin-3-Gallate Modulate Facial Development in Down Syndrome. Sci. Rep. 2021, 11, 4715. [Google Scholar] [CrossRef] [PubMed]
- Catuara-Solarz, S.; Espinosa-Carrasco, J.; Erb, I.; Langohr, K.; Notredame, C.; Gonzalez, J.R.; Dierssen, M. Principal Component Analysis of the Effects of Environmental Enrichment and (-)-Epigallocatechin-3-Gallate on Age-Associated Learning Deficits in a Mouse Model of Down Syndrome. Front. Behav. Neurosci. 2015, 9, 330. [Google Scholar] [CrossRef]
- Stagni, F.; Giacomini, A.; Guidi, S.; Ciani, E.; Bartesaghi, R. Timing of Therapies for Down Syndrome: The Sooner, the Better. Front. Behav. Neurosci. 2015, 9, 265. [Google Scholar] [CrossRef]
- De la Torre, R.; De Sola, S.; Pons, M.; Duchon, A.; de Lagran, M.M.; Farré, M.; Fitó, M.; Benejam, B.; Langohr, K.; Rodriguez, J.; et al. Epigallocatechin-3-Gallate, a DYRK1A Inhibitor, Rescues Cognitive Deficits in Down Syndrome Mouse Models and in Humans. Mol. Nutr. Food Res. 2014, 58, 278–288. [Google Scholar] [CrossRef]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Scientific Opinion on the Safety of Green Tea Catechins. EFSA J. 2018, 16, e05239. [Google Scholar] [CrossRef]
- De la Torre, R.; de Sola, S.; Hernandez, G.; Farré, M.; Pujol, J.; Rodriguez, J.; Espadaler, J.M.; Langohr, K.; Cuenca-Royo, A.; Principe, A.; et al. Safety and Efficacy of Cognitive Training plus Epigallocatechin-3-Gallate in Young Adults with Down’s Syndrome (TESDAD): A Double-Blind, Randomised, Placebo-Controlled, Phase 2 Trial. Lancet Neurol. 2016, 15, 801–810. [Google Scholar] [CrossRef]
- Cieuta-Walti, C.; Cuenca-Royo, A.; Langohr, K.; Rakic, C.; López-Vílchez, M.Á.; Lirio, J.; González-Lamuño Leguina, D.; González, T.B.; García, J.G.; Roure, M.R.; et al. Safety and Preliminary Efficacy on Cognitive Performance and Adaptive Functionality of Epigallocatechin Gallate (EGCG) in Children with Down Syndrome. A Randomized Phase Ib Clinical Trial (PERSEUS Study). Genet. Med. 2022, 24, 2004–2013. [Google Scholar] [CrossRef]
- Abeysekera, I.; Thomas, J.; Georgiadis, T.M.; Berman, A.G.; Hammond, M.A.; Dria, K.J.; Wallace, J.M.; Roper, R.J. Differential Effects of Epigallocatechin-3-Gallate Containing Supplements on Correcting Skeletal Defects in a Down Syndrome Mouse Model. Mol. Nutr. Food Res. 2016, 60, 717–726. [Google Scholar] [CrossRef]
- Stringer, M.; Abeysekera, I.; Thomas, J.; LaCombe, J.; Stancombe, K.; Stewart, R.J.; Dria, K.J.; Wallace, J.M.; Goodlett, C.R.; Roper, R.J. Epigallocatechin-3-Gallate (EGCG) Consumption in the Ts65Dn Model of Down Syndrome Fails to Improve Behavioral Deficits and Is Detrimental to Skeletal Phenotypes. Physiol. Behav. 2017, 177, 230–241. [Google Scholar] [CrossRef]
- Goodlett, C.R.; Stringer, M.; LaCombe, J.; Patel, R.; Wallace, J.M.; Roper, R.J. Evaluation of the Therapeutic Potential of Epigallocatechin-3-Gallate (EGCG) via Oral Gavage in Young Adult Down Syndrome Mice. Sci. Rep. 2020, 10, 10426. [Google Scholar] [CrossRef] [PubMed]
- Jamal, R.; LaCombe, J.; Patel, R.; Blackwell, M.; Thomas, J.R.; Sloan, K.; Wallace, J.M.; Roper, R.J. Increased Dosage and Treatment Time of Epigallocatechin-3-Gallate (EGCG) Negatively Affects Skeletal Parameters in Normal Mice and Down Syndrome Mouse Models. PLoS ONE 2022, 17, e0264254. [Google Scholar] [CrossRef] [PubMed]
- Cerbin-Koczorowska, M.; Waszyk-Nowaczyk, M.; Bakun, P.; Goslinski, T.; Koczorowski, T. Current View on Green Tea Catechins Formulations, Their Interactions with Selected Drugs, and Prospective Applications for Various Health Conditions. Appl. Sci. 2021, 11, 4905. [Google Scholar] [CrossRef]
- Ludwig, A.; Lorenz, M.; Grimbo, N.; Steinle, F.; Meiners, S.; Bartsch, C.; Stangl, K.; Baumann, G.; Stangl, V. The Tea Flavonoid Epigallocatechin-3-Gallate Reduces Cytokine-Induced VCAM-1 Expression and Monocyte Adhesion to Endothelial Cells. Biochem. Biophys. Res. Commun. 2004, 316, 659–665. [Google Scholar] [CrossRef]
- Hayek, T.; Fuhrman, B.; Vaya, J.; Rosenblat, M.; Belinky, P.; Coleman, R.; Elis, A.; Aviram, M. Reduced Progression of Atherosclerosis in Apolipoprotein E–Deficient Mice Following Consumption of Red Wine, or Its Polyphenols Quercetin or Catechin, Is Associated with Reduced Susceptibility of LDL to Oxidation and Aggregation. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2744–2752. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y.; Chiba, T.; Miura, S.; Tomita, I.; Umegaki, K.; Ikeda, M.; Tomita, T. Green Tea Polyphenols (Flavan 3-Ols) Prevent Oxidative Modification of Low Density Lipoproteins: An Ex Vivo Study in Humans. J. Nutr. Biochem. 2000, 11, 216–222. [Google Scholar] [CrossRef]
- Yang, T.T.C.; Koo, M.W.L. Inhibitory Effect of Chinese Green Tea on Endothelial Cell-Induced LDL Oxidation. Atherosclerosis 2000, 148, 67–73. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, Y.; Liu, G. Epigallocatechin Gallate (EGCG) Attenuates Myocardial Hypertrophy and Fibrosis Induced by Transverse Aortic Constriction via Inhibiting the Akt/mTOR Pathway. Pharm. Biol. 2021, 59, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Romero, P.; Borralleras, C.; Bosch-Morató, M.; Guivernau, B.; Albericio, G.; Muñoz, F.J.; Pérez-Jurado, L.A.; Campuzano, V. Epigallocatechin-3-Gallate Improves Cardiac Hypertrophy and Short-Term Memory Deficits in a Williams-Beuren Syndrome Mouse Model. PLoS ONE 2018, 13, e0194476. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, C.; Frank, D.; Will, R.; Jaschinski, C.; Frauen, R.; Katus, H.A.; Frey, N. DYRK1A Is a Novel Negative Regulator of Cardiomyocyte Hypertrophy. J. Biol. Chem. 2009, 284, 17320–17327. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.-Y.; Zeng, Y.-F.; Guo, Q.-H.; Liu, J.-J.; Yin, N.; Liu, Y.; Zeng, W.-J. Cardioprotective Effect of Epigallocatechin Gallate in Myocardial Ischemia/Reperfusion Injury and Myocardial Infarction: A Meta-Analysis in Preclinical Animal Studies. Sci. Rep. 2023, 13, 14050. [Google Scholar] [CrossRef]
- Wilasrusmee, K.T.; Sitticharoon, C.; Keadkraichaiwat, I.; Maikaew, P.; Pongwattanapakin, K.; Chatree, S.; Sririwichitchai, R.; Churintaraphan, M. Epigallocatechin Gallate Enhances Sympathetic Heart Rate Variability and Decreases Blood Pressure in Obese Subjects: A Randomized Control Trial. Sci. Rep. 2024, 14, 21628. [Google Scholar] [CrossRef]
- Shaw, P.R.; Klein, J.A.; Aziz, N.M.; Haydar, T.F. Longitudinal Neuroanatomical and Behavioral Analyses Show Phenotypic Drift and Variability in the Ts65Dn Mouse Model of Down Syndrome. Dis. Models Mech. 2020, 13, dmm046243. [Google Scholar] [CrossRef]
- Llambrich, S.; González-Colom, R.; Wouters, J.; Roldán, J.; Salassa, S.; Wouters, K.; Van Bulck, V.; Sharpe, J.; Callaerts-Vegh, Z.; Vande Velde, G.; et al. Green Tea Catechins Modulate Skeletal Development with Effects Dependent on Dose, Time, and Structure in a down Syndrome Mouse Model. Nutrients 2022, 14, 4167. [Google Scholar] [CrossRef]
- Vande Velde, G.; Poelmans, J.; De Langhe, E.; Hillen, A.; Vanoirbeek, J.; Himmelreich, U.; Lories, R.J. Longitudinal Micro-CT Provides Biomarkers of Lung Disease That Can Be Used to Assess the Effect of Therapy in Preclinical Mouse Models, and Reveal Compensatory Changes in Lung Volume. Dis. Model. Mech. 2016, 9, 91–98. [Google Scholar] [CrossRef]
- Vande Velde, G.; De Langhe, E.; Poelmans, J.; Dresselaers, T.; Lories, R.J.; Himmelreich, U. Magnetic Resonance Imaging for Noninvasive Assessment of Lung Fibrosis Onset and Progression: Cross-Validation and Comparison of Different Magnetic Resonance Imaging Protocols with Micro–Computed Tomography and Histology in the Bleomycin-Induced Mouse Model. Investig. Radiol. 2014, 49, 691–698. [Google Scholar] [CrossRef]
- Seldeslachts, L.; Cawthorne, C.; Kaptein, S.F.; Boudewijns, R.; Thibaut, H.J.; Sanchez Felipe, L.; Sharma, S.; Schramm, G.; Weynand, B.; Dallmeier, K.; et al. Use of Micro-Computed Tomography to Visualize and Quantify COVID-19 Efficiency in Free-Breathing Hamsters. In Vaccine Design; Thomas, S., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2022; Volume 2410, pp. 177–192. ISBN 978-1-07-161883-7. [Google Scholar]
- Stypmann, J.; Engelen, M.A.; Troatz, C.; Rothenburger, M.; Eckardt, L.; Tiemann, K. Echocardiographic Assessment of Global Left Ventricular Function in Mice. Lab. Anim. 2009, 43, 127–137. [Google Scholar] [CrossRef]
- Devos, F.C.; Maaske, A.; Robichaud, A.; Pollaris, L.; Seys, S.; Lopez, C.A.; Verbeken, E.; Tenbusch, M.; Lories, R.; Nemery, B.; et al. Forced Expiration Measurements in Mouse Models of Obstructive and Restrictive Lung Diseases. Respir. Res. 2017, 18, 123–137. [Google Scholar] [CrossRef]
- Verbeken, E.K.; Cauberghs, M.; Van De Woestijne, K.P. Membranous Bronchioles and Connective Tissue Network of Normal and Emphysematous Lungs. J. Appl. Physiol. 1996, 81, 2468–2480. [Google Scholar] [CrossRef] [PubMed]
- Groshong, S.D.; Tomashefski, J.F.; Cool, C.D. Pulmonary Vascular Disease. In Dail and Hammar’s Pulmonary Pathology; Springer: New York, NY, USA, 2008. [Google Scholar]
- Dunnill, M.S. Quantitative Methods in the Study of Pulmonary Pathology. Thorax 1962, 17, 320–328. [Google Scholar] [CrossRef]
- Geens, J.H.; Jacobs, S.; Claus, P.; Trenson, S.; Leunens, V.; Vantichelen, I.; Rega, F.R.; Verbeken, E.K.; Burkhoff, D.; Meyns, B. Partial Mechanical Circulatory Support in an Ovine Model of Post-Infarction Remodeling. J. Heart Lung Transplant. 2013, 32, 815–822. [Google Scholar] [CrossRef]
- Gustschin, A.; Riedel, M.; Taphorn, K.; Petrich, C.; Gottwald, W.; Noichl, W.; Busse, M.; Francis, S.E.; Beckmann, F.; Hammel, J.U.; et al. High-Resolution and Sensitivity Bi-Directional x-Ray Phase Contrast Imaging Using 2D Talbot Array Illuminators. Optica 2021, 8, 1588. [Google Scholar] [CrossRef] [PubMed]
- Zdora, M.-C.; Thibault, P.; Zhou, T.; Koch, F.J.; Romell, J.; Sala, S.; Last, A.; Rau, C.; Zanette, I. X-Ray Phase-Contrast Imaging and Metrology through Unified Modulated Pattern Analysis. Phys. Rev. Lett. 2017, 118, 203903. [Google Scholar] [CrossRef]
- Llambrich, S.; Tielemans, B.; Saliën, E.; Atzori, M.; Wouters, K.; Van Bulck, V.; Platt, M.; Vanherp, L.; Gallego Fernandez, N.; Grau De La Fuente, L.; et al. Pleiotropic Effects of Trisomy and Pharmacologic Modulation on Structural, Functional, Molecular, and Genetic Systems in a Down Syndrome Mouse Model. eLife 2023, 12, RP89763. [Google Scholar] [CrossRef]
- Costa, A.C.S.; Stasko, M.R.; Schmidt, C.; Davisson, M.T. Behavioral Validation of the Ts65Dn Mouse Model for Down Syndrome of a Genetic Background Free of the Retinal Degeneration Mutation Pde6brd1. Behav. Brain Res. 2010, 206, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Heinen, M.; Hettich, M.M.; Ryan, D.P.; Schnell, S.; Paesler, K.; Ehninger, D. Adult-Onset Fluoxetine Treatment Does Not Improve Behavioral Impairments and May Have Adverse Effects on the Ts65Dn Mouse Model of Down Syndrome. Neural Plast. 2012, 2012, 467251. [Google Scholar] [CrossRef]
- Tallino, S.; Winslow, W.; Bartholomew, S.K.; Velazquez, R. Temporal and Brain Region-specific Elevations of Soluble Amyloid-β40–42 in the Ts65Dn Mouse Model of Down Syndrome and Alzheimer’s Disease. Aging Cell 2022, 21, e13590. [Google Scholar] [CrossRef]
- Blazek, J.D.; Gaddy, A.; Meyer, R.; Roper, R.J.; Li, J. Disruption of Bone Development and Homeostasis by Trisomy in Ts65Dn Down Syndrome Mice. Bone 2011, 48, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Blazek, J.D.; Malik, A.M.; Tischbein, M.; Arbones, M.L.; Moore, C.S.; Roper, R.J. Abnormal Mineralization of the Ts65Dn Down Syndrome Mouse Appendicular Skeleton Begins during Embryonic Development in a Dyrk1a-Independent Manner. Mech. Dev. 2015, 136, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.R.; Roper, R.J. Current Analysis of Skeletal Phenotypes in Down Syndrome. Curr. Osteoporos. Rep. 2021, 19, 338–346. [Google Scholar] [CrossRef]
- Scott-McKean, J.J.; Jones, R.; Johnson, M.W.; Mier, J.; Basten, I.A.; Stasko, M.R.; Costa, A.C.S. Emergence of Treadmill Running Ability and Quantitative Assessment of Gait Dynamics in Young Ts65Dn Mice: A Mouse Model for Down Syndrome. Brain Sci. 2023, 13, 743. [Google Scholar] [CrossRef]
- Myrelid, A.; Gustafsson, J.; Ollars, B.; Annerén, G. Growth Charts for Down’s Syndrome from Birth to 18 Years of Age. Arch. Dis. Child. 2002, 87, 97–103. [Google Scholar] [CrossRef] [PubMed]
- De Moraes, M.E.L.; Tanaka, J.L.O.; De Moraes, L.C.; Filho, E.M.; De Melo Castilho, J.C. Skeletal Age of Individuals with Down Syndrome. Spec. Care Dent. 2008, 28, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Zemel, B.S.; Pipan, M.; Stallings, V.A.; Hall, W.; Schadt, K.; Freedman, D.S.; Thorpe, P. Growth Charts for Children with Down Syndrome in the United States. Pediatrics 2015, 136, e1204–e1211. [Google Scholar] [CrossRef]
- Schloo, B.L.; Vawter, G.F.; Reid, L.M. Down Syndrome: Patterns of Disturbed Lung Growth. Hum. Pathol. 1991, 22, 919–923. [Google Scholar] [CrossRef]
- Lorenzo, L.P.E.; Shatynski, K.E.; Clark, S.; Yarowsky, P.J.; Williams, M.S. Defective Thymic Progenitor Development and Mature T-Cell Responses in a Mouse Model for Down Syndrome. Immunology 2013, 139, 447–458. [Google Scholar] [CrossRef]
- Illouz, T.; Biragyn, A.; Iulita, M.F.; Flores-Aguilar, L.; Dierssen, M.; De Toma, I.; Antonarakis, S.E.; Yu, E.; Herault, Y.; Potier, M.-C.; et al. Immune Dysregulation and the Increased Risk of Complications and Mortality Following Respiratory Tract Infections in Adults with Down Syndrome. Front. Immunol. 2021, 12, 621440. [Google Scholar] [CrossRef]
- Ram, G.; Chinen, J. Infections and Immunodeficiency in Down Syndrome: Immunodeficiency in Down Syndrome. Clin. Exp. Immunol. 2011, 164, 9–16. [Google Scholar] [CrossRef]
- Verstegen, R.H.J.; Chang, K.J.J.; Kusters, M.A.A. Clinical Implications of Immune-mediated Diseases in Children with Down Syndrome. Pediatr. Allergy Immunol. 2020, 31, 117–123. [Google Scholar] [CrossRef]
- Tielemans, B.; De Herdt, L.; Pollenus, E.; Vanhulle, E.; Seldeslachts, L.; Marain, F.; Belmans, F.; Ahookhosh, K.; Vanoirbeek, J.; Vermeire, K.; et al. A Multimodal Imaging-Supported Down Syndrome Mouse Model of RSV Infection. Viruses 2023, 15, 993. [Google Scholar] [CrossRef] [PubMed]
- Bush, D.S.; Ivy, D.D. Pulmonary Hypertension in the Population with Down Syndrome. Cardiol. Ther. 2022, 11, 33–47. [Google Scholar] [CrossRef]
- Galambos, C.; Minic, A.D.; Bush, D.; Nguyen, D.; Dodson, B.; Seedorf, G.; Abman, S.H. Increased Lung Expression of Anti-Angiogenic Factors in Down Syndrome: Potential Role in Abnormal Lung Vascular Growth and the Risk for Pulmonary Hypertension. PLoS ONE 2016, 11, e0159005. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Guignabert, C.; Bonnet, S.; Dorfmüller, P.; Klinger, J.R.; Nicolls, M.R.; Olschewski, A.J.; Pullamsetti, S.S.; Schermuly, R.T.; Stenmark, K.R.; et al. Pathology and Pathobiology of Pulmonary Hypertension: State of the Art and Research Perspectives. Eur. Respir. J. 2019, 53, 1–14. [Google Scholar] [CrossRef]
- Roper, R.J.; John, H.K.; Philip, J.; Lawler, A.; Reeves, R.H. Perinatal Loss of Ts65Dn Down Syndrome Mice. Genetics 2006, 172, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Villar, A.J.; Belichenko, P.V.; Gillespie, A.M.; Kozy, H.M.; Mobley, W.C.; Epstein, C.J. Identification and Characterization of a New Down Syndrome Model, Ts[Rb(12.1716)]2Cje, Resulting from a Spontaneous Robertsonian Fusion between T(1716)65Dn and mouseChromosome 12. Mamm. Genome 2005, 16, 79–90. [Google Scholar] [CrossRef]
- Colvin, K.L.; Nguyen, K.; Boncella, K.L.; Goodman, D.M.; Elliott, R.J.; Harral, J.W.; Bilodeaux, J.; Smith, B.J.; Yeager, M.E. Lung and Heart Biology of the Dp16 Mouse Model of down Syndrome: Implications for Studying Cardiopulmonary Disease. Genes 2023, 14, 1819. [Google Scholar] [CrossRef]
- Lana-Elola, E.; Aoidi, R.; Llorian, M.; Gibbins, D.; Buechsenschuetz, C.; Bussi, C.; Flynn, H.; Gilmore, T.; Watson-Scales, S.; Haugsten Hansen, M.; et al. Increased Dosage of DYRK1A Leads to Congenital Heart Defects in a Mouse Model of Down Syndrome. Sci. Transl. Med. 2024, 16, eadd6883. [Google Scholar] [CrossRef]
- Dunlevy, L.; Bennett, M.; Slender, A.; Lana-Elola, E.; Tybulewicz, V.L.; Fisher, E.M.C.; Mohun, T. Down’s Syndrome-like Cardiac Developmental Defects in Embryos of the Transchromosomic Tc1 Mouse. Cardiovasc. Res. 2010, 88, 287–295. [Google Scholar] [CrossRef]
- Ferrés, M.A.; Bianchi, D.W.; Siegel, A.E.; Bronson, R.T.; Huggins, G.S.; Guedj, F. Perinatal Natural History of the Ts1Cje Mouse Model of Down Syndrome: Growth Restriction, Early Mortality, Heart Defects, and Delayed Development. PLoS ONE 2016, 11, e0168009. [Google Scholar] [CrossRef]
- Wei, B.; Liu, M.; Zhong, X.; Yao, W.; Wei, M. Increased BBB Permeability Contributes to EGCG-Caused Cognitive Function Improvement in Natural Aging Rats: Pharmacokinetic and Distribution Analyses. Acta Pharmacol. Sin. 2019, 40, 1490–1500. [Google Scholar] [CrossRef] [PubMed]
- Forcano, L.; Fauria, K.; Soldevila-Domenech, N.; Minguillón, C.; Lorenzo, T.; Cuenca-Royo, A.; Menezes-Cabral, S.; Pizarro, N.; Boronat, A.; Molinuevo, J.L.; et al. Prevention of Cognitive Decline in Subjective Cognitive Decline APOE Ε4 Carriers after EGCG and a Multimodal Intervention (PENSA): Study Design. Alzheimer’s Dement. 2021, 7, e12155. [Google Scholar] [CrossRef] [PubMed]
- Almatroodi, S.A.; Almatroudi, A.; Khan, A.A.; Alhumaydhi, F.A.; Alsahli, M.A.; Rahmani, A.H. Potential Therapeutic Targets of Epigallocatechin Gallate (EGCG), the Most Abundant Catechin in Green Tea, and Its Role in the Therapy of Various Types of Cancer. Molecules 2020, 25, 3146. [Google Scholar] [CrossRef]
- Chen, X.; Man, G.C.W.; Hung, S.W.; Zhang, T.; Fung, L.W.Y.; Cheung, C.W.; Chung, J.P.W.; Li, T.C.; Wang, C.C. Therapeutic Effects of Green Tea on Endometriosis. Crit. Rev. Food Sci. Nutr. 2021, 63, 3222–3235. [Google Scholar] [CrossRef]
- Dhatwalia, S.K.; Kumar, M.; Dhawan, D.K. Role of EGCG in Containing the Progression of Lung Tumorigenesis—A Multistage Targeting Approach. Nutr. Cancer 2018, 70, 334–349. [Google Scholar] [CrossRef]
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green Tea Catechin, Epigallocatechin-3-Gallate (EGCG): Mechanisms, Perspectives and Clinical Applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef]
- Zhou, J.-R.; Yu, L.; Mai, Z.; Blackburn, G.L. Combined Inhibition of Estrogen-Dependent Human Breast Carcinoma by Soy and Tea Bioactive Components in Mice. Int. J. Cancer 2004, 108, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Zanoli, L.; Briet, M.; Empana, J.P.; Cunha, P.G.; Mäki-Petäjä, K.M.; Protogerou, A.D.; Tedgui, A.; Touyz, R.M.; Schiffrin, E.L.; Spronck, B.; et al. Vascular Consequences of Inflammation: A Position Statement from the ESH Working Group on Vascular Structure and Function and the ARTERY Society. J. Hypertens. 2020, 38, 1682–1698. [Google Scholar] [CrossRef]
- Huang, A.-C.; Cheng, H.-Y.; Lin, T.-S.; Chen, W.-H.; Lin, J.-H.; Lin, J.-J.; Lu, C.-C.; Chiang, J.-H.; Hsu, S.-C.; Wu, P.-P.; et al. Epigallocatechin Gallate (EGCG), Influences a Murine WEHI-3 Leukemia Model In Vivo Through Enhancing Phagocytosis of Macrophages and Populations of T- and B-Cells. In Vivo 2013, 27, 627–634. [Google Scholar] [PubMed]
- Chu, K.O.; Wang, C.C.; Chu, C.Y.; Choy, K.W.; Pang, C.P.; Rogers, M.S. Uptake and Distribution of Catechins in Fetal Organs Following in Utero Exposure in Rats. Hum. Reprod. 2007, 22, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Souchet, B.; Duchon, A.; Gu, Y.; Dairou, J.; Chevalier, C.; Daubigney, F.; Nalesso, V.; Créau, N.; Yu, Y.; Janel, N.; et al. Prenatal Treatment with EGCG Enriched Green Tea Extract Rescues GAD67 Related Developmental and Cognitive Defects in Down Syndrome Mouse Models. Sci. Rep. 2019, 9, 3914. [Google Scholar] [CrossRef] [PubMed]
- Warburton, D.; El-Hashash, A.; Carraro, G.; Tiozzo, C.; Sala, F.; Rogers, O.; Langhe, S.D.; Kemp, P.J.; Riccardi, D.; Torday, J.; et al. Lung Organogenesis. Curr. Top. Dev. Biol. 2010, 90, 73–158. [Google Scholar] [CrossRef]
- Bates, M.L.; Vasileva, A.; Flores, L.D.M.; Pryakhina, Y.; Buckman, M.; Tomasson, M.H.; DeRuisseau, L.R. Sex Differences in Cardiovascular Disease and Dysregulation in Down Syndrome. Am. J. Physiol. Heart Circ. Physiol. 2023, 324, H542–H552. [Google Scholar] [CrossRef]
- Gupta, M.; Dhanasekaran, A.R.; Gardiner, K.J. Mouse Models of Down Syndrome: Gene Content and Consequences. Mamm. Genome 2016, 27, 538–555. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tielemans, B.; Llambrich, S.; Seldeslachts, L.; Cremer, J.; Tsui, H.C.; Jonckheere, A.-C.; Marain, N.F.; Riedel, M.; Wouters, J.; Herzen, J.; et al. Cardiopulmonary and Immune Alterations in the Ts65Dn Mouse Model of Down Syndrome and Modulation by Epigallocatechin-3-Gallate-Enriched Green Tea Extract. Pharmaceutics 2025, 17, 1366. https://doi.org/10.3390/pharmaceutics17111366
Tielemans B, Llambrich S, Seldeslachts L, Cremer J, Tsui HC, Jonckheere A-C, Marain NF, Riedel M, Wouters J, Herzen J, et al. Cardiopulmonary and Immune Alterations in the Ts65Dn Mouse Model of Down Syndrome and Modulation by Epigallocatechin-3-Gallate-Enriched Green Tea Extract. Pharmaceutics. 2025; 17(11):1366. https://doi.org/10.3390/pharmaceutics17111366
Chicago/Turabian StyleTielemans, Birger, Sergi Llambrich, Laura Seldeslachts, Jonathan Cremer, Hung Chang Tsui, Anne-Charlotte Jonckheere, Nora Fopke Marain, Mirko Riedel, Jens Wouters, Julia Herzen, and et al. 2025. "Cardiopulmonary and Immune Alterations in the Ts65Dn Mouse Model of Down Syndrome and Modulation by Epigallocatechin-3-Gallate-Enriched Green Tea Extract" Pharmaceutics 17, no. 11: 1366. https://doi.org/10.3390/pharmaceutics17111366
APA StyleTielemans, B., Llambrich, S., Seldeslachts, L., Cremer, J., Tsui, H. C., Jonckheere, A.-C., Marain, N. F., Riedel, M., Wouters, J., Herzen, J., Leszczyński, B., Verbeken, E., Vanoirbeek, J., & Vande Velde, G. (2025). Cardiopulmonary and Immune Alterations in the Ts65Dn Mouse Model of Down Syndrome and Modulation by Epigallocatechin-3-Gallate-Enriched Green Tea Extract. Pharmaceutics, 17(11), 1366. https://doi.org/10.3390/pharmaceutics17111366








