Advances in PCL, PLA, and PLGA-Based Technologies for Anticancer Drug Delivery
Abstract
1. Introduction
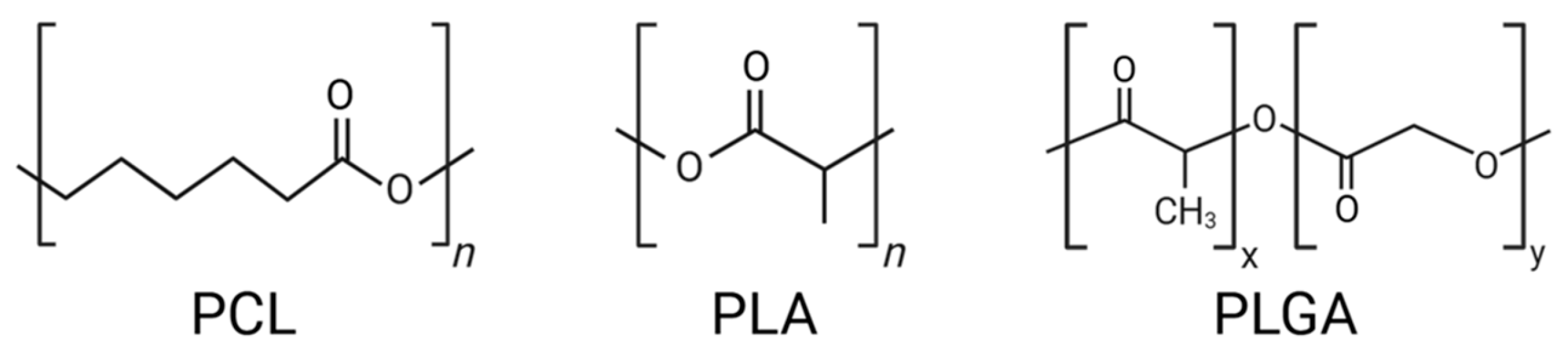
2. Structural and Physicochemical Properties of PCL, PLA, and PLGA
2.1. Chemical Composition and Biodegradability
2.2. Thermal and Mechanical Properties
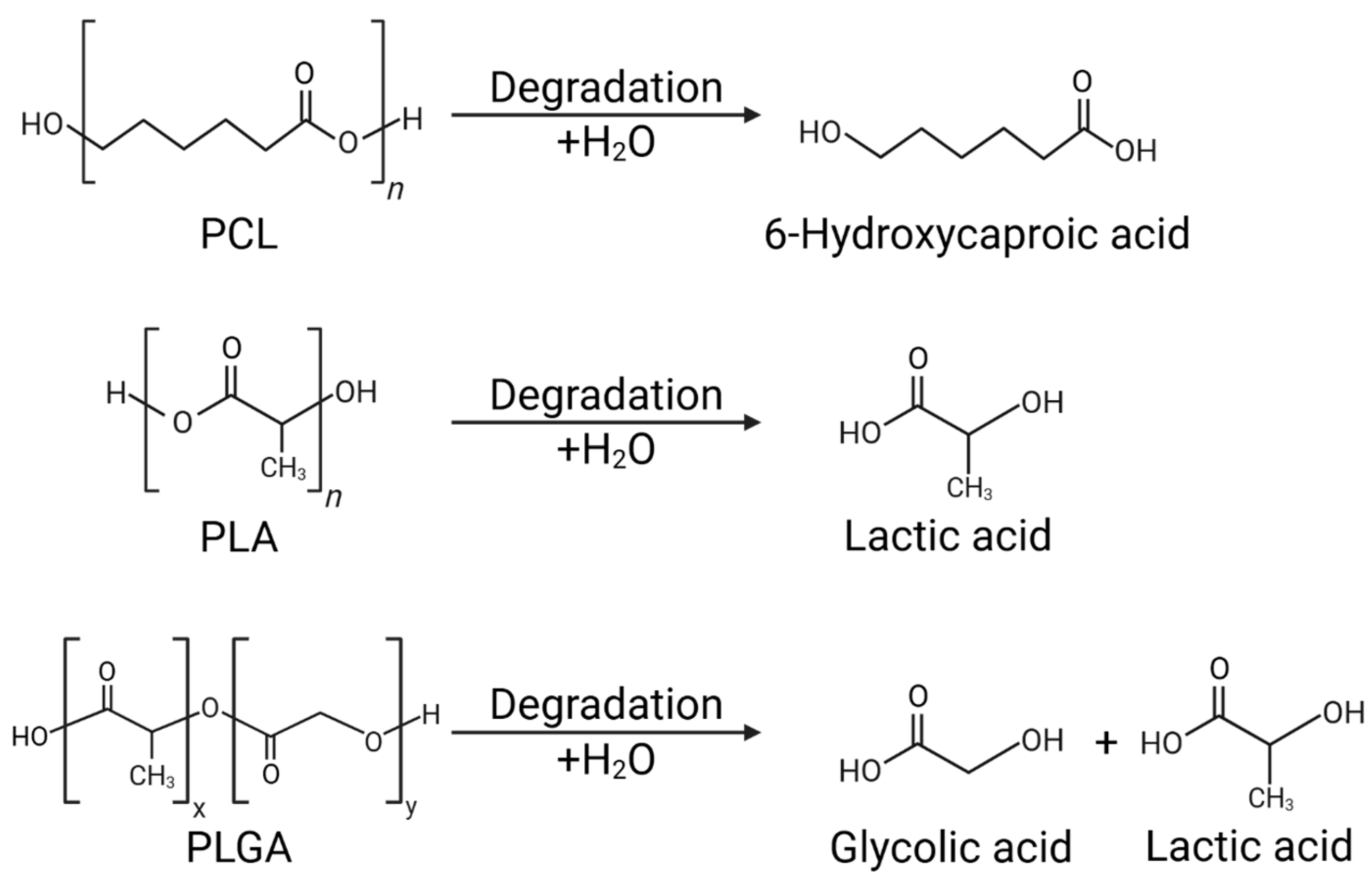
2.3. Degradation Mechanisms and Their Impact on Drug Release Kinetics
| Polymer | PCL | PLA | PLGA |
|---|---|---|---|
| Chemical Composition | Semi-crystalline aliphatic polyester; synthesized by ROP of ε-caprolactone. | Aliphatic polyester; derived from L-lactide and D-lactide (chiral isomers). | Copolymer of lactic acid (LA) and glycolic acid (GA); tunable LA:GA ratio. |
| Crystallinity | 20–33% (high crystallinity). | Varies by D/L isomer ratio; low D = crystalline, high D = amorphous. | Amorphous to semi-crystalline depending on LA:GA ratio. |
| Melting Point | 58–61 °C | 150–160 °C | Not well-defined; varies by LA:GA ratio, typically amorphous (no sharp Tm). |
| Glass Transition | ≈−60 °C | ≈60 °C | 40–60 °C (higher LA → higher Tg). |
| Mechanical Properties | Flexible; low tensile strength; strength increases with crystallinity. | Tensile strength: 50–70 MPa; Elastic modulus: 3–4 GPa; brittle if low crystallinity. | Properties depend on LA:GA; higher LA = more rigid, higher GA = more hydrophilic and weaker. |
| Biodegradation Behavior | Very slow hydrolytic degradation; complete biodegradation may take years. | Hydrolytic degradation into lactic acid → metabolized to CO2 + H2O. | Hydrolytic degradation; fastest at 50:50 LA:GA ratio; metabolites excreted safely. |
| Degradation Rate | Slowest (months–years). | Intermediate (weeks–months depending on crystallinity/MW). | Tunable (days–months depending on LA:GA ratio, 50:50 degrades fastest). |
| Drug Release Characteristics | Minimal burst release; stable zero-order release; good for long-term implants. | Three-stage release profile; tunable from days to months. | Highly tunable; hydrophilic drugs accelerate degradation, hydrophobic drugs prolong release. |
3. Synthesis and Functionalization of PCL, PLA, and PLGA for Drug Delivery
3.1. Ring-Opening Polymerization and Copolymerization Strategies
3.2. Surface Modifications and Functional Group Incorporation
3.3. Smart Polymer Design for Controlled Drug Release
4. Nano- and Micro-Scale Drug Delivery Systems Based on PCL, PLA, and PLGA
4.1. Polymeric Micelles: Self-Assembly and Drug Encapsulation Efficiency
4.2. Nanoparticles and Nanocapsules: Passive vs. Active Targeting Approaches
4.3. Hydrogels and Scaffolds: Applications in Localized Drug Delivery
4.4. Microspheres and Implants: Sustained Drug Release for Long-Term Therapy
5. Mechanisms of Drug Release and Controlled Delivery
5.1. Diffusion, Degradation, and Erosion-Controlled Release
5.2. Stimuli-Responsive Systems (pH-Sensitive, Temperature-Sensitive, Enzyme-Triggered)
5.3. Dual and Multi-Modal Drug Delivery Strategies
6. Active and Passive Targeting Strategies for Enhanced Efficacy
6.1. Enhanced Permeability and Retention (EPR) Effect for Passive Targeting
6.2. Ligand-Functionalized Nanoparticles for Receptor-Mediated Targeting
6.3. Multi-Functionalized Polymeric Carriers for Precision Medicine
7. Clinical and Preclinical Applications in Anticancer Therapy
7.1. Current Status of FDA-Approved Formulations Based on PCL, PLA, and PLGA
7.2. Recent Advancements in Preclinical Research and Animal Studies
7.3. Challenges in Clinical Translation and Regulatory Considerations
8. Conclusions
8.1. Summary of Key Findings
8.2. Implications for Future Research and Clinical Applications
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DDS | Drug Delivery System |
| PCL | Polycaprolactone |
| PLA | Polylactic Acid |
| PLGA | Poly(lactic-co-glycolic acid) |
| LA | Lactic Acid |
| GA | Glycolic Acid |
| T_g | Glass Transition Temperature |
| MPa | Megapascal |
| GPa | Gigapascal |
| ROP | Ring-Opening Polymerization |
| PEG | Polyethylene Glycol |
| EDC | 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide |
| NHS | N-hydroxysuccinimide |
| EPR | Enhanced Permeability and Retention (tumor targeting effect) |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| EGFR | Epidermal Growth Factor Receptor |
| GSH | Glutathione |
| PAA | Poly(acrylic acid) |
| PMAA | Poly(methacrylic acid) |
| PDMAEMA | Poly(N,N-dimethylaminoethyl methacrylate) |
| TME | Tumor Microenvironment |
| PTT | Photothermal Therapy |
| PDT | Photodynamic Therapy |
| PDA | Polydopamine |
| BP | Black Phosphorus |
| ICG | Indocyanine Green |
| GNR | Gold Nanorods |
| PSiNPs | Porous Silicon Nanoparticles |
| TPZ | Tirapazamine |
| DNA | Deoxyribonucleic Acid |
| cRGD | Cyclic Arginine–Glycine–Aspartic Acid peptide |
| FDA | Food and Drug Administration |
| GNO | Glial Neuro-Oncology |
References
- Kumari, P.; Ghosh, B.; Biswas, S. Nanocarriers for cancer-targeted drug delivery. J. Drug Target. 2016, 24, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.K.; Kim, S.W. Recent advances in polymeric drug delivery systems. Biomater. Res. 2020, 24, 12. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, A.; Giuliano, E.; Venkateswararao, E.; Fresta, M.; Bulotta, S.; Awasthi, V.; Cosco, D. Biodegradable Polymeric Nanoparticles for Drug Delivery to Solid Tumors. Front. Pharmacol. 2021, 12, 601626. [Google Scholar] [CrossRef] [PubMed]
- Masood, F. Polymeric nanoparticles for targeted drug delivery system for cancer therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 60, 569–578. [Google Scholar] [CrossRef]
- Archer, E.; Torretti, M.; Madbouly, S. Biodegradable polycaprolactone (PCL) based polymer and composites. Phys. Sci. Rev. 2023, 8, 4391–4414. [Google Scholar] [CrossRef]
- Karlsson, J.; Vaughan, H.J.; Green, J.J. Biodegradable Polymeric Nanoparticles for Therapeutic Cancer Treatments. Annu. Rev. Chem. Biomol. Eng. 2018, 9, 105–127. [Google Scholar] [CrossRef]
- Mahar, R.; Chakraborty, A.; Nainwal, N.; Bahuguna, R.; Sajwan, M.; Jakhmola, V. Application of PLGA as a Biodegradable and Biocompatible Polymer for Pulmonary Delivery of Drugs. AAPS PharmSciTech 2023, 24, 39. [Google Scholar] [CrossRef]
- Jana, P.; Shyam, M.; Singh, S.; Jayaprakash, V.; Dev, A.; Misra, S. Biodegradable polymers in drug delivery and oral vaccination. Eur. Polym. J. 2021, 142, 110155. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef]
- Avramović, N.; Mandić, B.; Savić-Radojević, A.; Simić, T. Polymeric Nanocarriers of Drug Delivery Systems in Cancer Therapy. Pharmaceutics 2020, 12, 298. [Google Scholar] [CrossRef]
- Vlachopoulos, A.; Karlioti, G.; Balla, E.; Daniilidis, V.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Christodoulou, E.; Koumentakou, I.; Karavas, E.; et al. Poly(Lactic Acid)-Based Microparticles for Drug Delivery Applications: An Overview of Recent Advances. Pharmaceutics 2022, 14, 359. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhang, B.; Sun, R.; Liu, W.; Zhu, Q.; Zhang, X.; Wang, R.; Chen, C. PLGA-based biodegradable microspheres in drug delivery: Recent advances in research and application. Drug Deliv. 2021, 28, 1397–1418. [Google Scholar] [CrossRef] [PubMed]
- Geszke-Moritz, M.; Moritz, M. Biodegradable Polymeric Nanoparticle-Based Drug Delivery Systems: Comprehensive Overview, Perspectives and Challenges. Polymers 2024, 16, 2536. [Google Scholar] [CrossRef] [PubMed]
- Malikmammadov, E.; Endogan Tanir, T.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef]
- Batakliev, T.; Georgiev, V.; Kalupgian, C.; Muñoz, P.A.R.; Ribeiro, H.; Fechine, G.J.M.; Andrade, R.J.E.; Ivanov, E.; Kotsilkova, R. Physico-chemical Characterization of PLA-based Composites Holding Carbon Nanofillers. Appl. Compos. Mater. 2021, 28, 1175–1192. [Google Scholar] [CrossRef]
- De, R.; Mahata, M.K.; Kim, K.-T. Structure-Based Varieties of Polymeric Nanocarriers and Influences of Their Physicochemical Properties on Drug Delivery Profiles. Adv. Sci. 2022, 9, e2105373. [Google Scholar] [CrossRef]
- Fernández-Tena, A.; Pérez-Camargo, R.A.; Dinçkal, S.; Yildiz, U.H. Effect of Molecular Weight on the Crystallization and Melt Memory of Poly(ε-caprolactone) (PCL). Macromolecules 2023, 56, 4602–4620. [Google Scholar] [CrossRef]
- Ruz-Cruz, M.A.; Herrera-Franco, P.J.; Flores-Johnson, E.A.; Moreno-Chulim, M.V.; Galera-Manzano, L.M.; Valadez-González, A. Thermal and mechanical properties of PLA-based multiscale cellulosic biocomposites. J. Mater. Res. Technol. 2022, 18, 485–495. [Google Scholar] [CrossRef]
- Cespi, M.; Bonacucina, G.; Tiboni, M.; Casettari, L.; Cambriani, A.; Fini, F.; Perinelli, D.R.; Palmieri, G.F. Insights in the rheological properties of PLGA-PEG-PLGA aqueous dispersions: Structural properties and temperature-dependent behaviour. Polymer 2021, 213, 123216. [Google Scholar] [CrossRef]
- Dias, J.R.; Sousa, A.; Augusto, A.; Bártolo, P.J.; Granja, P.L. Electrospun Polycaprolactone (PCL) Degradation: An In Vitro and In Vivo Study. Polymers 2022, 14, 3397. [Google Scholar] [CrossRef]
- Abu Bakar, A.A.; Zainuddin, M.Z.; Adam, A.N.; Noor, I.S.; Tamchek, N.B.; Alauddin, M.S.; Ghazali, M.I. The study of mechanical properties of poly(lactic) acid PLA-based 3D printed filament under temperature and environmental conditions. Mater. Today Proc. 2022, 67, 652–658. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Zeng, Q.; Guan, J.; Gao, H.; Zhang, L.; Zhong, J.; Lai, W.-Y.; Wang, Q. Designing Polymer Electrolytes via Ring-Opening Polymerization for Advanced Lithium Batteries. Adv. Energy Mater. 2023, 14, 2302876. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, K.; Wang, J.; Chen, M.; Dai, W.; Liu, R. Recent Advances and Future Developments in the Preparation of Polypeptides via N-Carboxyanhydride (NCA) Ring-Opening Polymerization. J. Am. Chem. Soc. 2024, 146, 24189–24208. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Storti, G.; Morbidelli, M. Kinetics of Ring-Opening Polymerization of L,L-Lactide. Ind. Eng. Chem. Res. 2011, 50, 7927–7940. [Google Scholar] [CrossRef]
- Nieboer, V.; Fanjul-Mosteirín, N.; Olsén, P.; Odelius, K. Mastering Macromolecular Architecture by Controlling Backbiting Kinetics during Anionic Ring-Opening Polymerization. Macromolecules 2024, 57, 3397–3406. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Shlyakhtin, A.V.; Tavtorkin, A.N.; Kosarev, M.A.; Gavrilov, D.E.; Komarov, P.D.; Ilyin, S.O.; Karchevsky, S.G.; Ivchenko, P.V. Mechanistic study of transesterification in TBD-catalyzed ring-opening polymerization of methyl ethylene phosphate. Eur. Polym. J. 2019, 118, 393–403. [Google Scholar] [CrossRef]
- Nieboer, V.; Fanjul-Mosteirín, N.; Olsén, P.; Odelius, K. Lewis-Pair Derived Activated Lactone Initiator (ALI) Complex for Rapid, Controlled, Bench Stable and Selective Ring-Opening Polymerization of (Macro)lactones. Eur. Polym. J. 2023, 201, 112594. [Google Scholar] [CrossRef]
- Fowler, H.R.; O’Shea, R.; Sefton, J.; Howard, S.C.; Muir, B.W.; Stockman, R.A.; Taresco, V.; Irvine, D.J. Rapid, Highly Sustainable Ring-Opening Polymerization via Resonant Acoustic Mixing. ACS Sustain. Chem. Eng. 2025, 13, 1916–1926. [Google Scholar] [CrossRef]
- Conen, N.; Fuchs, M.; Hoffmann, A.; Herres-Pawlis, S.; Jupke, A. Taking the Next Step—Model-Based Analysis of Robust and Non-Toxic Zn Catalysts for the Ring Opening Polymerization of Lactide in the Polymer Melt. Adv. Sustain. Syst. 2022, 7, 2200359. [Google Scholar] [CrossRef]
- Sharma, S.; Dang, S. Nanocarrier-Based Drug Delivery to Brain: Interventions of Surface Modification. Curr. Neuropharmacol. 2023, 21, 517–535. [Google Scholar] [CrossRef]
- Mannu, R.; Karthikeyan, V.; Velu, N.; Arumugam, C.; Roy, V.A.L.; Gopalan, A.-I.; Saianand, G.; Sonar, P.; Lee, K.-P.; Kim, W.-J.; et al. Polyethylene Glycol Coated Magnetic Nanoparticles: Hybrid Nanofluid Formulation, Properties and Drug Delivery Prospects. Nanomaterials 2021, 11, 440. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.M.; El-Shatoury, E.H.; El-Araby, M.M.I. Antibacterial and anticancer activities of three novel lectin-conjugated chitosan nanoparticles. Appl. Microbiol. Biotechnol. 2024, 108, 524. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, X.; Wang, Y.; Xun, Z.; Li, S. Application of PLGA in Tumor Immunotherapy. Polymers 2024, 16, 1253. [Google Scholar] [CrossRef] [PubMed]
- El-Hammadi, M.M.; Arias, J.L. Recent Advances in the Surface Functionalization of PLGA-Based Nanomedicines. Nanomaterials 2022, 12, 354. [Google Scholar] [CrossRef]
- Ahmad, F.; Salem-Bekhit, M.M.; Khan, F.; Alshehri, S.; Khan, A.; Ghoneim, M.M.; Wu, H.-F.; Taha, E.I.; Elbagory, I. Unique Properties of Surface-Functionalized Nanoparticles for Bio-Application: Functionalization Mechanisms and Importance in Application. Nanomaterials 2022, 12, 1333. [Google Scholar] [CrossRef]
- Liang, H.-Y.; Lee, W.-K.; Hsu, J.-T.; Shih, J.-Y.; Ma, T.-L.; Vo, T.T.T.; Lee, C.-W.; Cheng, M.-T.; Lee, I.-T. Polycaprolactone in Bone Tissue Engineering: A Comprehensive Review of Innovations in Scaffold Fabrication and Surface Modifications. J. Funct. Biomater. 2024, 15, 243. [Google Scholar] [CrossRef]
- Młotek, M.; Gadomska-Gajadhur, A.; Sobczak, A.; Kruk, A.; Perron, M.; Krawczyk, K. Modification of PLA Scaffold Surface for Medical Applications. Appl. Sci. 2021, 11, 1815. [Google Scholar] [CrossRef]
- Balcerak-Woźniak, A.; Dzwonkowska-Zarzycka, M.; Kabatc-Borcz, J. A Comprehensive Review of Stimuli-Responsive Smart Polymer Materials-Recent Advances and Future Perspectives. Materials 2024, 17, 4255. [Google Scholar] [CrossRef]
- Fattah-Alhosseini, A.; Chaharmahali, R.; Alizad, S.; Kaseem, M.; Dikici, B. A review of smart polymeric materials: Recent developments and prospects for medicine applications. Hybrid Adv. 2024, 5, 100178. [Google Scholar] [CrossRef]
- Yu, Z.; Shen, X.; Yu, H.; Tu, H.; Chittasupho, C.; Zhao, Y. Smart Polymeric Nanoparticles in Cancer Immunotherapy. Pharmaceutics 2023, 15, 775. [Google Scholar] [CrossRef]
- Liang, J.; Yang, B.; Zhou, X.; Han, Q.; Zou, J.; Cheng, L. Stimuli-responsive drug delivery systems for head and neck cancer therapy. Drug Deliv. 2021, 28, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Farjadian, F.; Ghasemi, S.; Akbarian, M.; Hoseini-Ghahfarokhi, M.; Moghoofei, M.; Doroudian, M. Physically stimulus-responsive nanoparticles for therapy and diagnosis. Front. Chem. 2022, 10, 952675. [Google Scholar] [CrossRef] [PubMed]
- Dallaev, R. Smart and Biodegradable Polymers in Tissue Engineering and Interventional Devices: A Brief Review. Polymers 2025, 17, 1976. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zeng, Z.; Zhu, R.; Liu, A.; Huo, M. Polymerization-Induced Self-Assembly Toward Micelle-Crosslinked Tough and Ultrastretchable Hydrogels. Chem. Mater. 2022, 34, 6408–6419. [Google Scholar] [CrossRef]
- Hasannia, M.; Aliabadi, A.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Synthesis of block copolymers used in polymersome fabrication: Application in drug delivery. J. Control. Release 2022, 341, 95–117. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Z. Improved encapsulation capacity of casein micelles with modified structure. J. Food Eng. 2022, 333, 111138. [Google Scholar] [CrossRef]
- Kocabay, Ö.G.; İsmail, O. Preparation and optimization of biodegradable self-assembled PCL-PEG-PCL nano-sized micelles for drug delivery systems. Int. J. Polym. Mater. Polym. Biomater. 2020, 70, 328–337. [Google Scholar] [CrossRef]
- Kuru, M.M.; Dalgakıran, E.A.; Kaçar, G. Investigation of morphology, micelle properties, drug encapsulation and release behavior of self-assembled PEG-PLA-PEG block copolymers: A coarse-grained molecular simulations study. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127445. [Google Scholar] [CrossRef]
- Wang, M.; Lin, Y.; Gao, J.; Liu, D. DPD simulations on morphologies and structures of blank PLGA-b-PEG-b-PLGA polymeric micelles and docetaxel-loaded PLGA-b-PEG-b-PLGA polymeric micelles. RSC Adv. 2022, 12, 12078–12088. [Google Scholar] [CrossRef]
- Gao, X.; Wang, W.; Rao, A.; Zhao, F.; Men, K.; Yang, B.; Liu, X.; Huang, M.; Gou, M.; Qian, Z.; et al. Preparation, characterization and application of star-shaped PCL/PEG micelles for the delivery of doxorubicin in the treatment of colon cancer. Int. J. Nanomed. 2013, 8, 971–982. [Google Scholar] [CrossRef]
- Phan, Q.T.; Le, M.H.; Le, T.T.H.; Tran, T.H.H.; Xuan, P.N.; Ha, P.T. Characteristics and cytotoxicity of folate-modified curcumin-loaded PLA-PEG micellar nano-systems with various PLA:PEG ratios. Int. J. Pharm. 2016, 507, 32–40. [Google Scholar] [CrossRef]
- Song, Z.; Feng, R.; Sun, M.; Guo, C.; Gao, Y.; Li, L.; Zhai, G. Curcumin-loaded PLGA-PEG-PLGA triblock copolymeric micelles: Preparation, pharmacokinetics and distribution in vivo. J. Colloid Interface Sci. 2011, 354, 116–123. [Google Scholar] [CrossRef]
- Argenziano, M.; Arpicco, S.; Brusa, P.; Cavalli, R.; Chirio, D.; Dosio, F.; Gallarate, M.; Peira, E.; Stella, B.; Ugazio, E. Developing Actively Targeted Nanoparticles to Fight Cancer: Focus on Italian Research. Pharmaceutics 2021, 13, 1538. [Google Scholar] [CrossRef] [PubMed]
- Keerikkadu, M.; Bangera, P.D.; Tippavajhala, V.K.; Rathnanand, M. An Overview on Lipid Nanocapsules: Exploring the Role in Precision Cancer Treatment and Lymphatic Drug Distribution. Adv. Pharm. Bull. 2025, 15, 248. [Google Scholar] [CrossRef] [PubMed]
- Agwa, M.M.; Marzouk, R.E.; Sabra, S.A. Advances in active targeting of ligand-directed polymeric nanomicelles via exploiting overexpressed cellular receptors for precise nanomedicine. RSC Adv. 2024, 14, 23520–23542. [Google Scholar] [CrossRef] [PubMed]
- Poudel, S.; Napit, P.R.; Briski, K.P.; Mattheolabakis, G. Oral Delivery of Nucleic Acids with Passive and Active Targeting to the Intestinal Tissue Using Polymer-Based Nanocarriers. Pharmaceutics 2021, 13, 1075. [Google Scholar] [CrossRef]
- Taiariol, L.; Chaix, C.; Farre, C.; Moreau, E. Click and Bioorthogonal Chemistry: The Future of Active Targeting of Nanoparticles for Nanomedicines? Chem. Rev. 2022, 122, 340–384. [Google Scholar] [CrossRef]
- Naik, H.; Sonju, J.J.; Singh, S.; Chatzistamou, I.; Shrestha, L.; Gauthier, T.; Jois, S. Lipidated Peptidomimetic Ligand-Functionalized HER2 Targeted Liposome as Nano-Carrier Designed for Doxorubicin Delivery in Cancer Therapy. Pharmaceuticals 2021, 14, 221. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, L.; Wang, J.; Zhang, H.; Zhang, Z.; Xing, G.; Wang, X.; Liu, M. Drug-loaded PEG-PLGA nanoparticles for cancer treatment. Front. Pharmacol. 2022, 13, 990505. [Google Scholar] [CrossRef]
- Chiu, H.I.; Abdul Samad, N.; Fang, L.; Lim, V. Cytotoxicity of targeted PLGA nanoparticles: A systematic review. RSC Adv. 2021, 11, 9433–9449. [Google Scholar] [CrossRef]
- Feng, R.; Song, Z.; Zhai, G. Preparation and in vivo pharmacokinetics of curcumin-loaded PCL-PEG-PCL triblock copolymeric nanoparticles. Int. J. Nanomed. 2012, 7, 4089–4098. [Google Scholar] [CrossRef]
- Lu, J.; Chuan, X.; Zhang, H.; Dai, W.; Wang, X.; Wang, X.; Zhang, Q. Free paclitaxel loaded PEGylated-paclitaxel nanoparticles: Preparation and comparison with other paclitaxel systems in vitro and in vivo. Int. J. Pharm. 2014, 471, 525–535. [Google Scholar] [CrossRef]
- Fonseca, C.; Simões, S.; Gaspar, R. Paclitaxel-loaded PLGA nanoparticles: Preparation, physicochemical characterization and in vitro anti-tumoral activity. J. Control. Release 2002, 83, 273–286. [Google Scholar] [CrossRef]
- Baretta, R.; Raucci, A.; Cinti, S.; Frasconi, M. Porous hydrogel scaffolds integrating Prussian Blue nanoparticles: A versatile strategy for electrochemical (bio)sensing. Sens. Actuators B Chem. 2023, 376, 132985. [Google Scholar] [CrossRef]
- Hsu, X.-L.; Wu, L.-C.; Hsieh, J.-Y.; Huang, Y.-Y. Nanoparticle-Hydrogel Composite Drug Delivery System for Potential Ocular Applications. Polymers 2021, 13, 642. [Google Scholar] [CrossRef] [PubMed]
- Arun, A.; Malrautu, P.; Laha, A.; Luo, H.; Ramakrishna, S. Collagen Nanoparticles in Drug Delivery Systems and Tissue Engineering. Appl. Sci. 2021, 11, 11369. [Google Scholar] [CrossRef]
- Koch, F.; Thaden, O.; Conrad, S.; Tröndle, K.; Finkenzeller, G.; Zengerle, R.; Kartmann, S.; Zimmermann, S.; Koltay, P. Mechanical properties of polycaprolactone (PCL) scaffolds for hybrid 3D-bioprinting with alginate-gelatin hydrogel. J. Mech. Behav. Biomed. Mater. 2022, 130, 105219. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Z.; Wei, X.; Chen, B.; Luo, Y. 3D printed hydrogel/PCL core/shell fiber scaffolds with NIR-triggered drug release for cancer therapy and wound healing. Acta Biomater. 2021, 131, 314–325. [Google Scholar] [CrossRef]
- Das, M.; Sharabani-Yosef, O.; Eliaz, N.; Mandler, D. Hydrogel-integrated 3D-printed poly(lactic acid) scaffolds for bone tissue engineering. J. Mater. Res. 2021, 36, 3833–3842. [Google Scholar] [CrossRef]
- Nguyen, H.T.-T.; Nguyen, L.T.-T.; Ha, A.C.; Huynh, P.D. Evaluation of Ibuprofen Prolonged Release of Biomedical PLA-PEG-PLA Hydrogel via Degradation Mechanism. Int. J. Biomater. 2023, 2023, 5005316. [Google Scholar] [CrossRef]
- Visan, A.I.; Negut, I. Development and Applications of PLGA Hydrogels for Sustained Delivery of Therapeutic Agents. Gels 2024, 10, 497. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Zhang, Y.; Wang, Q.; Ren, G.; Wang, Y.; Zhou, S.; Wang, Q.; Peng, C.; Cheng, X. Thermosensitive vancomycin@PLGA-PEG-PLGA/HA hydrogel as an all-in-one treatment for osteomyelitis. Int. J. Pharm. 2022, 627, 122225. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Chen, H.; Qin, J.; Wei, C.; Liang, J.; Liu, T.; Kong, D.; Lv, F. Thermosensitive porphyrin-incorporated hydrogel with four-arm PEG-PCL copolymer (II): Doxorubicin loaded hydrogel as a dual fluorescent drug delivery system for simultaneous imaging tracking in vivo. Drug Deliv. 2017, 24, 641–650. [Google Scholar] [CrossRef]
- De la Riva, B.; Nowak, C.; Sánchez, E.; Hernández, A.; Schulz-Siegmund, M.; Pec, M.K.; Delgado, A.; Evora, C. VEGF-controlled release within a bone defect from alginate/chitosan/PLA-H scaffolds. Eur. J. Pharm. Biopharm. 2009, 73, 50–58. [Google Scholar] [CrossRef]
- Wang, M.; Chen, J.; Li, W.; Zang, F.; Liu, X.; Qin, S. Paclitaxel-nanoparticles-loaded double network hydrogel for local treatment of breast cancer after surgical resection. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 114, 111046. [Google Scholar] [CrossRef]
- Zhu, B.; Chen, Y.; Lu, W.; Zhang, Q.; Gao, S.; Sun, L.; Chen, S.; Hu, R. Development of a Long-Term Drug Delivery System with Biodegradable Polymers. J. Drug Deliv. Sci. Technol. 2022, 68, 102955. [Google Scholar] [CrossRef]
- Cai, H.; Li, A.; Qi, F.; Liu, R.; Tang, X.; Li, D.; Gu, Y.; Liu, J. Application of biodegradable microsphere injections: An anticancer perspective. Mater. Adv. 2024, 5, 3094–3112. [Google Scholar] [CrossRef]
- Nwazojie, C.C.; Obayemi, J.D.; Salifu, A.A.; Borbor-Sawyer, S.M.; Uzonwanne, V.O.; Onyekanne, C.E.; Akpan, U.M.; Onwudiwe, K.C.; Oparah, J.C.; Odusanya, O.S.; et al. Targeted drug-loaded PLGA-PCL microspheres for specific and localized treatment of triple negative breast cancer. J. Mater. Sci. Mater. Med. 2023, 34, 41. [Google Scholar] [CrossRef]
- Soares, I.; Faria, J.; Marques, A.; Ribeiro, I.A.C.; Baleizão, C.; Bettencourt, A.; Ferreira, I.M.M.; Baptista, A.C. Drug Delivery from PCL/Chitosan Multilayer Coatings for Metallic Implants. ACS Omega 2022, 7, 23096–23106. [Google Scholar] [CrossRef]
- Butreddy, A.; Gaddam, R.P.; Kommineni, N.; Dudhipala, N.; Voshavar, C. PLGA/PLA-Based Long-Acting Injectable Depot Microspheres in Clinical Use: Production and Characterization Overview for Protein/Peptide Delivery. Int. J. Mol. Sci. 2021, 22, 8884. [Google Scholar] [CrossRef]
- Wang, P.; Wang, M.; Wei, X.; Xie, L.; Tian, L.; Yang, Z.; Zhou, Z.; Chen, H. In vitro and in vivo degradation profile, biocompatibility of poly-L-lactic acid porous microspheres. Int. J. Biol. Macromol. 2024, 272, 132876. [Google Scholar] [CrossRef]
- Istratov, V.; Gomzyak, V.; Baranov, O.; Markova, G.; Mezhuev, Y.; Vasnev, V. Preparation and Hydrolytic Degradation of Hydroxyapatite-Filled PLGA Composite Microspheres. J. Compos. Sci. 2023, 7, 346. [Google Scholar] [CrossRef]
- Bassand, C.; Freitag, J.; Benabed, L.; Verin, J.; Siepmann, F.; Siepmann, J. PLGA implants for controlled drug release: Impact of the diameter. Eur. J. Pharm. Biopharm. 2022, 177, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Aydın, Ö.; Aydın, B.; Tezcaner, A.; Keskin, D. Study on physiochemical structure and in vitro release behaviors of doxycycline-loaded PCL microspheres. J. Appl. Polym. Sci. 2015, 132, 41768. [Google Scholar] [CrossRef]
- Soo, P.L.; Cho, J.; Grant, J.; Ho, E.; Piquette-Miller, M.; Allen, C. Drug release mechanism of paclitaxel from a chitosan-lipid implant system: Effect of swelling, degradation and morphology. Eur. J. Pharm. Biopharm. 2008, 69, 149–157. [Google Scholar]
- Ruan, G.; Feng, S.-S. Preparation and characterization of poly(lactic acid)-poly(ethylene glycol)-poly(lactic acid) (PLA–PEG–PLA) microspheres for controlled release of paclitaxel. Biomaterials 2003, 24, 5037–5044. [Google Scholar] [CrossRef]
- Feng, T.; Tian, H.; Xu, C.; Lin, L.; Xie, Z.; Lam, M.H.-W.; Liang, H.; Chen, X. Synergistic co-delivery of doxorubicin and paclitaxel by porous PLGA microspheres for pulmonary inhalation treatment. Eur. J. Pharm. Biopharm. 2014, 88, 1086–1093. [Google Scholar] [CrossRef]
- Bayer, I.S. Controlled Drug Release from Nanoengineered Polysaccharides. Pharmaceutics 2023, 15, 1364. [Google Scholar] [CrossRef]
- Lakshani, N.; Wijerathne, H.S.; Sandaruwan, C.; Kottegoda, N.; Karunarathne, V. Release Kinetic Models and Release Mechanisms of Controlled-Release and Slow-Release Fertilizers. ACS Agric. Sci. Technol. 2023, 3, 939–956. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, H.; Liyanage, A.; Qiu, J.; Thushara, D.; Bao, B.; Zhao, S. Collective diffusion of charged nanoparticles in a microchannel under electric field. Chem. Eng. Sci. 2022, 248, 117264. [Google Scholar] [CrossRef]
- Engler, L.G.; Farias, N.C.; Crespo, J.S.; Gately, N.M.; Major, I.; Pezzoli, R.; Devine, D.M. Designing Sustainable Polymer Blends: Tailoring Mechanical Properties and Degradation Behaviour in PHB/PLA/PCL Blends in a Seawater Environment. Polymers 2023, 15, 2874. [Google Scholar] [CrossRef]
- Haroosh, H.J.; Dong, Y.; Jasim, S.; Ramakrishna, S. Morphological Structures and Drug Release Effect of Multiple Electrospun Nanofibre Membrane Systems Based on PLA, PCL and PCL/Magnetic Nanoparticle Composites. J. Nanomater. 2022, 2022, 5190163. [Google Scholar] [CrossRef]
- Li, T.; Sun, H.; Han, H.; Zhang, C.; Li, B.; Huang, J.; Sun, D. Ultrafast Bulk Degradation of Polylactic Acid by Artificially Cultured Diatom Frustules. Compos. Sci. Technol. 2022, 223, 109410. [Google Scholar] [CrossRef]
- Moya-López, C.; Juan, A.; Donizeti, M.; Valcárcel, J.; Vázquez, J.A.; Solano, E.; Chapron, D.; Bourson, P.; Bravo, I.; Alonso-Moreno, C.; et al. Multifunctional PLA/Gelatin Bionanocomposites for Tailored Drug Delivery Systems. Pharmaceutics 2022, 14, 1138. [Google Scholar] [CrossRef] [PubMed]
- Samanta, D.; Koithan, J.A.; Muliana, A.H.; Pharr, M. Effect of Mechanical Loading on PLGA Biodegradation. Polym. Degrad. Stab. 2025, 240, 111485. [Google Scholar] [CrossRef]
- Bassand, C.; Vérin, J.; Lamatsch, M.; Siepmann, F.; Siepmann, J. How agarose gels surrounding PLGA implants limit swelling and slow down drug release. J. Control. Release 2022, 343, 255–266. [Google Scholar] [CrossRef]
- Sun, R.; Chen, Y.; Pei, Y.; Wang, W.; Zhu, Z.; Zheng, Z.; Yang, L.; Sun, L. The drug release of PLGA-based nanoparticles and their application in treatment of gastrointestinal cancers. Heliyon 2024, 10, e38165. [Google Scholar] [CrossRef]
- Jiménez-Jiménez, S.; Cordero-Sánchez, S.; Mejía-Hernández, J.-G.; Villalobos-García, R.; Villegas-Cortez, J. Monte Carlo simulation methods-based models for analyzing the kinetics of drug delivery from controlled-release systems. Braz. J. Pharm. Sci. 2025, 61, e24249. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, Y.; Dheer, D.; Shankar, R. Stimuli responsiveness of recent biomacromolecular systems (concept to market): A review. Int. J. Biol. Macromol. 2024, 261, 129901. [Google Scholar] [CrossRef]
- Das, S.S.; Bharadwaj, P.; Bilal, M.; Barani, M.; Rahdar, A.; Taboada, P.; Bungau, S.; Kyzas, G.Z. Stimuli-Responsive Polymeric Nanocarriers for Drug Delivery, Imaging, and Theragnosis. Polymers 2020, 12, 1397. [Google Scholar] [CrossRef]
- Li, M.; Zhao, G.; Su, W.-K.; Shuai, Q. Enzyme-Responsive Nanoparticles for Anti-tumor Drug Delivery. Front. Chem. 2020, 8, 647. [Google Scholar] [CrossRef] [PubMed]
- Alsehli, M. Polymeric nanocarriers as stimuli-responsive systems for targeted tumor (cancer) therapy: Recent advances in drug delivery. Saudi Pharm. J. 2020, 28, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Dachani, S.R.; Vashi, A.; Mundada, A.B.; Mundada, P.A.; Suman, S.R. Innovative polymers in pharmaceutical chemistry: Revolutionizing drug delivery systems. Polym.-Plast. Technol. Mater. 2024, 63, 911–933. [Google Scholar] [CrossRef]
- Sethuraman, V.; Janakiraman, K.; Krishnaswami, V.; Kandasamy, R. Recent Progress in Stimuli-Responsive Intelligent Nano Scale Drug Delivery Systems: A Special Focus Towards pH-Sensitive Systems. Curr. Drug Targets 2021, 22, 947–966. [Google Scholar] [CrossRef]
- Ma, B.; Shi, J.; Zhang, Y.; Li, Z.; Yong, H.; Zhou, Y.-N.; Liu, S.; A, S.; Zhou, D. Enzymatically Activatable Polymers for Disease Diagnosis and Treatment. Adv. Mater. 2023, 36, e2306358. [Google Scholar] [CrossRef]
- Wei, D.; Sun, Y.; Zhu, H.; Fu, Q. Stimuli-Responsive Polymer-Based Nanosystems for Cancer Theranostics. ACS Nano 2023, 17, 23223–23261. [Google Scholar] [CrossRef]
- Sun, Y.; Davis, E. Nanoplatforms for Targeted Stimuli-Responsive Drug Delivery: A Review of Platform Materials and Stimuli-Responsive Release and Targeting Mechanisms. Nanomaterials 2021, 11, 746. [Google Scholar] [CrossRef]
- Yang, G.; Ding, J.; Chen, X. Bioactive poly(amino acid)s for multi-modal cancer therapy. WIREs Nanomed. Nanobiotechnol. 2024, 16, e1985. [Google Scholar] [CrossRef]
- Li, M.; Xuan, Y.; Zhang, W.; Zhang, S.; An, J. Polydopamine-containing nano-systems for cancer multi-mode diagnoses and therapies: A review. Int. J. Biol. Macromol. 2023, 247, 125826. [Google Scholar] [CrossRef]
- Wu, H.; Wei, M.; Xu, Y.; Li, Y.; Zhai, X.; Su, P.; Ma, Q.; Zhang, H. PDA-Based Drug Delivery Nanosystems: A Potential Approach for Glioma Treatment. Int. J. Nanomed. 2022, 17, 3751–3775. [Google Scholar] [CrossRef]
- Neerooa, B.N.H.M.; Ooi, L.-T.; Shameli, K.; Dahlan, N.A.; Islam, J.M.M.; Pushpamalar, J.; Teow, S.-Y. Development of polymer-assisted nanoparticles and nanogels for cancer therapy: An update. Gels 2021, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, P.; Dwivedi, D.; Tiwari, S.; Singh, V.K. Recent Applications of Natural Polymers in the Formulation of Nanotechnology-Based Drug Delivery Systems. Curr. Drug Targets 2022, 22, 947–966. [Google Scholar]
- Noori, F.; Osanloo, M.; Moradi, H.R.; Ghaderi Jafarbeigloo, H.; Jirehnezhadyan, M.; Kouhpayeh, S.A.; Tirgare, M.; Bozorgi, A.; Goodarzi, A. Fabrication, characterization, and in vivo implantation of eugenol-loaded nanogels and PCL/Cs electrospun nanofibers for wound healing applications. J. Bioact. Compat. Polym. 2023, 38, 480–492. [Google Scholar] [CrossRef]
- Fletcher, N.L.; Kempe, K.; Thurecht, K.J. Next-Generation Polymeric Nanomedicines for Oncology: Perspectives and Future Directions. Macromol. Rapid Commun. 2020, 41, e2000319. [Google Scholar] [CrossRef]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef]
- Henderson, A.R.; Ilan, I.S.; Lee, E. A bioengineered lymphatic vessel model for studying lymphatic endothelial cell–cell junction and barrier function. Microcirculation 2021, 28, e12730. [Google Scholar] [CrossRef]
- Visan, A.I.; Popescu-Pelin, G.; Gherasim, O.; Mihailescu, A.; Socol, M.; Zgura, I.; Chiritoiu, M.; Sima, L.E.; Antohe, F.; Ivan, L.; et al. Long-Term Evaluation of Dip-Coated PCL-Blend-PEG Coatings in Simulated Conditions. Polymers 2020, 12, 717. [Google Scholar] [CrossRef]
- Shinde, V.R.; Revi, N.; Murugappan, S.; Singh, S.P.; Rengan, A.K. Enhanced permeability and retention effect: A key facilitator for solid tumor targeting by nanoparticles. Photodiagn. Photodyn. Ther. 2022, 39, 102915. [Google Scholar] [CrossRef]
- Shipunova, V.O.; Sogomonyan, A.S.; Zelepukin, I.V.; Nikitin, M.P.; Deyev, S.M. PLGA Nanoparticles Decorated with Anti-HER2 Affibody for Targeted Delivery and Photoinduced Cell Death. Molecules 2021, 26, 3955. [Google Scholar] [CrossRef]
- Ejigah, V.; Owoseni, O.; Bataille-Backer, P.; Ogundipe, O.D.; Fisusi, F.A.; Adesina, S.K. Approaches to Improve Macromolecule and Nanoparticle Accumulation in the Tumor Microenvironment by the Enhanced Permeability and Retention Effect. Polymers 2022, 14, 2601. [Google Scholar] [CrossRef]
- Zhou, X.-Q.; Li, Y.-P.; Dang, S.-S. Precision targeting in hepatocellular carcinoma: Exploring ligand-receptor mediated nanotherapy. World J. Hepatol. 2024, 16, 164–176. [Google Scholar] [CrossRef]
- Kumar, V.; Tiwari, O.; Finkelstein-Zuta, G.; Rencus-Lazar, S.; Gazit, E. Design of Functional RGD Peptide-Based Biomaterials for Tissue Engineering. Pharmaceutics 2023, 15, 345. [Google Scholar] [CrossRef] [PubMed]
- Mennati, A.; Rostamizadeh, K.; Fathi, M. Dual silencing of integrin αvβ3 receptor and insulin-like growth factor 1 receptor using mPEG-PCL/DDAB hybrid nanoparticle loaded siRNA in breast cancer therapy: An in vitro study on MCF-7 cells. Int. J. Biol. Macromol. 2025, 294, 139334. [Google Scholar] [CrossRef] [PubMed]
- Ellebæk, S.; Brix, S.; Grandal, M.; Lantto, J.; Horak, I.D.; Kragh, M.; Poulsen, T.T. Pan-HER—An antibody mixture targeting EGFR, HER2 and HER3 abrogates preformed and ligand-induced EGFR homo- and heterodimers. Int. J. Cancer 2016, 139, 2095–2105. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.; Tchistiakova, L.; Scott, N. Implications of receptor-mediated endocytosis and intracellular trafficking dynamics in the development of antibody drug conjugates. MAbs 2013, 5, 13–21. [Google Scholar] [CrossRef]
- Yusuf, M.; Khan, M.; Alrobaian, M.M.; Alghamdi, S.A.; Warsi, M.H.; Sultana, S.; Khan, R.A. Brain-targeted polysorbate-80 coated PLGA thymoquinone nanoparticles for the treatment of Alzheimer’s disease, with biomechanistic insights. J. Drug Deliv. Sci. Technol. 2021, 61, 102214. [Google Scholar] [CrossRef]
- Sharma, S.; Dang, S. Polysorbate 80 surface modified PLGA nanoparticles: An in-vitro evaluation of cellular uptake and cytotoxicity on Neuro-2a cells. J. Microencapsul. 2023, 40, 534–548. [Google Scholar] [CrossRef]
- Matiyani, M.; Jali, H.; Ghodrat, S.; Rahimi, M.; Hosseini, S.M.; Yousefi, M. Development of multi-functionalized graphene oxide based novel drug nanocarrier for delivery of quercetin and curcumin. Int. J. Biol. Macromol. 2023, 254, 124389. [Google Scholar]
- Eom, J.; Kwak, Y.; Nam, C. Electrospinning fabrication of magnetic nanoparticles-embedded polycaprolactone sorbent with enhanced sorption capacity and recovery speed for spilled oil removal. Chemosphere 2022, 303, 135063. [Google Scholar] [CrossRef]
- Vieira, J.; Maurmann, N.; Venturini, J.; Pranke, P.; Fernandes, D.; Pérez Bergmann, C. PCL-coated magnetic Fe3O4 nanoparticles: Synthesis, characterization and application in stem cells. Mater. Today Commun. 2022, 31, 103416. [Google Scholar] [CrossRef]
- Machado, M.G.C.; de Oliveira, M.A.; Lanna, E.G.; Siqueira, R.P.; Pound-Lana, G.; Branquinho, R.T.; Mosqueira, V.C.F. Photodynamic therapy with the dual-mode association of IR780 to PEG-PLA nanocapsules and the effects on human breast cancer cells. Biomed. Pharmacother. 2022, 145, 112464. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Yang, C.; Shan, M.; Jia, H.; Zhang, S.; Chen, X.; Liu, W.; Liu, X.; Chen, J.; Wang, X. Synergistic poly(lactic acid) antibacterial surface combining superhydrophobicity for anti-adhesion and chlorophyll for photodynamic therapy. Langmuir 2022, 38, 8987–8998. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, X.; Wang, J.; Zhang, Y.; Guo, P.; Lv, Y.; Ma, G.; Wei, W.; Wang, S. Versatile PLGA-Based Drug Delivery Systems for Tumor Immunotherapy. Small Methods 2025, 9, 2401623. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C.V.; Gonçalves, V.; da Silva, M.C.; Bañobre-López, M.; Gallo, J. PLGA-Based Composites for Various Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 2034. [Google Scholar] [CrossRef]
- Chwalisz, K. Clinical development of the GnRH agonist leuprolide acetate depot. Fertil. Steril. Rep. 2022, 4, 33–39. [Google Scholar] [CrossRef]
- Liang, Y.; Yang, R.; Wei, Y.; Huang, B.; Chen, Y.; Zhang, X.; Yao, J.; Wang, G.; Mao, H.; Shi, H.; et al. Multicenter, prospective, single-arm clinical study to investigate the efficacy and safety of Zoladex (Goserelin acetate) 10.8 mg prior to surgery in Chinese premenopausal women with symptomatic uterine fibroids. Gynecol. Endocrinol. 2024, 40, 2427190. [Google Scholar] [CrossRef]
- Han, B.; Tang, H.; Liang, Q.; Zhu, M.; Xie, Y.; Chen, J.; Li, Q.; Jia, J.; Li, Y.; Ren, Z.; et al. Preparation of long-acting microspheres loaded with octreotide for the treatment of portal hypertensive. Drug Deliv. 2021, 28, 719–732. [Google Scholar] [CrossRef]
- Wang, S.-J.; Wang, P.-X.; Ding, H.-L.; Qi, Y.-Y.; Song, Y.-R.; Mu, X.-N. Cellular and molecular mechanisms of arenobufagin in cancer therapy: A systematic review. Discov. Oncol. 2025, 16, 1207. [Google Scholar] [CrossRef]
- Dwidar, Z.; Awadin, W.F.; El-Adl, M.; Abomosallam, M.; ELzeer, A.A.; Abdellatif, A.M. Artemisinin-loaded polylactic acid nanoparticles alleviate 1, 2 N,N-dimethylhydrazine-induced colorectal cancer in Albino rats. Cancer Nanotechnol. 2025, 16, 18. [Google Scholar] [CrossRef]
- Yadav, B.; Chauhan, M.; Shekhar, S.; Kumar, A.; Mehata, A.K.; Nayak, A.K.; Dutt, R.; Garg, V.; Kailashiya, V.; Muthu, M.S.; et al. RGD-decorated PLGA nanoparticles improved effectiveness and safety of cisplatin for lung cancer therapy. Int. J. Pharm. 2023, 633, 122587. [Google Scholar] [CrossRef]
- Remmers, R.C.P.A.; Neumann, K. Reaching new lights: A review on photo-controlled nanomedicines and their in vivo evaluation. Biomater. Sci. 2023, 11, 1607–1624. [Google Scholar] [CrossRef] [PubMed]
- Alvi, M.; Yaqoob, A.; Rehman, K.; Shoaib, S.M.; Akash, M.S.H. PLGA-based nanoparticles for the treatment of cancer: Current strategies and perspectives. AAPS Open 2022, 8, 12. [Google Scholar] [CrossRef]
- Lu, Y.; Cheng, D.; Niu, B.; Wang, X.; Wu, X.; Wang, A. Properties of Poly (Lactic-co-Glycolic Acid) and Progress of Poly (Lactic-co-Glycolic Acid)-Based Biodegradable Materials in Biomedical Research. Pharmaceuticals 2023, 16, 454. [Google Scholar] [CrossRef] [PubMed]
- Tracey, S.R.; Smyth, P.; Barelle, C.J.; Scott, C.J. Development of next generation nanomedicine-based approaches for the treatment of cancer: We’ve barely scratched the surface. Biochem. Soc. Trans. 2021, 49, 2253–2269. [Google Scholar] [CrossRef]
- Varenne, F.; Vauthier, C. Practical guidelines for the characterization and quality control of nanoparticles in the pharmaceutical industry. In Emerging Technologies for Nanoparticle Manufacturing; Springer: Cham, Switzerland, 2021; pp. 487–508. [Google Scholar] [CrossRef]
- Park, I.-H.; Sohn, J.-H.; Kim, S.-B.; Lee, K.-S.; Chung, J.-S.; Lee, S.-H.; Kim, T.-Y.; Jung, K.-H.; Cho, E.-K.; Kim, Y.-S.; et al. An open-label, randomized, parallel, phase III trial evaluating the efficacy and safety of polymeric micelle-formulated paclitaxel compared to conventional Cremophor EL-based paclitaxel for recurrent or metastatic HER2-negative breast cancer. Cancer Res. Treat. 2017, 49, 569–577. [Google Scholar] [CrossRef]
- Kim, G.-Y.; Kim, J.-H.; Lee, T.; Bae, B.-C.; Baik, H.; Kim, T.; Kim, J.; Kang, D.-W.; Kim, J.-H.; Kim, D.; et al. In vitro and in vivo evaluations of a 3-month sustained-release microsphere depot formulation of leuprolide acetate. J. Pharm. Investig. 2022, 52, 129–138. [Google Scholar] [CrossRef]
- Martins, C.; Pacheco, C.; Faria, P.; Sarmento, B. Nanomedicine approaches for treating glioblastoma. Nanomedicine 2023, 18, 1135–1138. [Google Scholar] [CrossRef]
- Creemers, J.H.A.; Pawlitzky, I.; Grosios, K.; Gileadi, U.; Middleton, M.R.; Gerritsen, W.R.; Mehra, N.; Rivoltini, L.; Walters, I.; Figdor, C.G.; et al. Assessing the safety, tolerability and efficacy of PLGA-based immunomodulatory nanoparticles in patients with advanced NY-ESO-1-positive cancers: A first-in-human phase I open-label dose-escalation study protocol. BMJ Open 2021, 11, e050725. [Google Scholar] [CrossRef]
- Siqueira, D.D.; Luna, C.B.B.; Araújo, E.M.; Barros, A.B.S.; Wellen, R.M.R. Approaches on PCL/macaíba biocomposites: Mechanical, thermal, morphological properties and crystallization kinetics. Polym. Adv. Technol. 2021, 32, 3572–3585. [Google Scholar] [CrossRef]
| Polymer | Formulation | Loaded Drug | Key Findings | Reference |
|---|---|---|---|---|
| Star-shaped PCL/PEG (SSMPEG-PCL) | Star-shaped micelle prepared by radical polymerization of acrylated MPEG–PCL | Doxorubicin (Dox) | Demonstrated high stability (low CMC), pH-responsive drug release, and superior anticancer efficacy in vitro and in vivo. | [50] |
| PLA–PEG/ PLA–PEG–Fol | Micellar nanoparticles with or without folate modification | Curcumin (CUR) | Improved solubility and cellular uptake; folate-modified micelles showed enhanced targeting and cytotoxicity toward HepG2 cells. | [51] |
| PLGA–PEG–PLGA | Triblock copolymeric micelles prepared by solvent–dialysis method | Curcumin (CUR) | Increased drug loading efficiency, extended plasma half-life, and altered biodistribution with reduced RES uptake. | [52] |
| Polymer | Formulation | Loaded Drug | Key Findings | Reference |
|---|---|---|---|---|
| PCL–PEG–PCL triblock copolymer | Self-assembled nanoparticles prepared by ultrasonic emulsion and solvent evaporation | Curcumin (CUR) | High encapsulation efficiency (≈95%), particle size ~60 nm, sustained release up to 96 h, and 4-fold higher AUC and longer circulation time vs. free CUR. | [61] |
| PEG-PLA (and PEG-PTX hybrid) | PEG-PTX/PTX nanoparticles vs. PEG-PLA/PTX micelles (thin-film hydration method) | Paclitaxel (PTX) | PEG-PTX/PTX showed better stability, higher tumor uptake, stronger antitumor efficacy, and lower toxicity than PEG-PLA/PTX or Taxol®. | [62] |
| PLGA | Paclitaxel-loaded PLGA nanoparticles (nanoprecipitation method) | Paclitaxel (PTX) | Spherical NPs < 200 nm with ~90% drug loading; biphasic release (initial burst + sustained), enhanced cytotoxicity vs. Taxol®, suitable for i.v. use. | [63] |
| Polymer | Formulation | Loaded Drug/ Application | Key Findings | Reference |
|---|---|---|---|---|
| PCL (Four-arm PEG–PCL hydrogel) | Thermosensitive porphyrin-incorporated hydrogel (POR–PEG–PCL) | Doxorubicin (Dox) | Dual fluorescent system enabled real-time imaging, sustained release, and effective tumor inhibition. | [73] |
| PLA (PLA-H coated chitosan scaffold) | Composite alginate/chitosan/PLA-H scaffold containing VEGF-loaded microspheres | VEGF | PLA-H coating allowed controlled VEGF release (~5 weeks), maintained >90% bioactivity, induced angiogenesis and bone regeneration at defect sites. | [74] |
| PLGA (Double-network hydrogel) | PLGA–PVA/collagen double-network hydrogel (PTX–NPs–DN hydrogel) | Paclitaxel (PTX) | Sustained local release (>10 days), reduced systemic toxicity, and strong antitumor efficacy post-surgery. | [75] |
| Polymer | Formulation | Loaded Drug/ Application | Key Findings | Reference |
|---|---|---|---|---|
| PCL | Doxycycline-loaded PCL microspheres (single emulsion–solvent evaporation) | Doxycycline | Sustained antibiotic release for 3 months; release kinetics mainly diffusion-controlled; tunable by PCL molecular weight (14 vs. 65 kDa). | [84] |
| PLA | Chitosan–lipid implant containing PLA–PEG/PLA nanoparticles | Paclitaxel (PTX) | Localized sustained PTX release up to 4 weeks in ascites fluid; strong correlation between in vitro and in vivo release; effective tumor suppression in ovarian cancer model. | [85] |
| PLA–PEG–PLA | PLA–PEG–PLA microspheres (O/W solvent evaporation) | Paclitaxel (PTX) | Hydrophilic PEG block improved porosity and drug release (~50% in 1 month); enhanced compatibility vs. PLGA microspheres. | [86] |
| PLGA | Porous PLGA microspheres (W/O/W double emulsion with ammonium bicarbonate) | Doxorubicin (DOX) + Paclitaxel (PTX) | Dual-drug encapsulation achieved synergistic cytotoxicity; optimal DOX/PTX ratio (2:1) for lung cancer inhalation; reduced systemic toxicity. | [87] |
| Polymer | Formulation Type | Brand Name (Active Ingredient) | FDA Application No. (NDA) | Approval Year | Indication(s) | Key Features | References |
|---|---|---|---|---|---|---|---|
| PLA/PLGA | Injectable microspheres | Lupron Depot® (Leuprolide) | NDA 019732/S012 | 1998 | Hormone- dependent prostate and breast cancer | Sustained release for 1–3 months, reduced dosing frequency, improved patient compliance | [135] |
| PLA | Biodegradable implant | Zoladex® (Goserelin) | NDA 019726/S24 & NDA 020578/S3 | 1998 | Hormone- dependent prostate cancer, some breast cancers | Biodegradable implant enabling long-term hormone suppression minimally invasive administration | [136] |
| PLGA | Long-acting injection (LAR) | Sandostatin® LAR (Octreotide) | NDA 021008 | 1998 | Neuroendocrine tumors, carcinoid syndrome | Monthly injection; stable long-term release | [137] |
| Polymer | Formulation/ Modification | Loaded Drug(s) | Animal Model | Key Findings | References |
|---|---|---|---|---|---|
| PEG-PLA micelles | PEG-PLA micelles | Arenobufagin | Mouse tumor model | Tumor inhibition rate 72.9%; 1.28× higher than free drug; reduced toxicity | [138] |
| PLA nanoparticles | PLA nanoparticles | Artemisinin | Rat colon cancer model | Significant reduction in tumor number and size compared to free drug | [139] |
| PLGA nanoparticles (RGD-modified with upconversion nanomaterials) | PLGA + RGD peptides, cisplatin + nanomaterials | Cisplatin + UCNPs | Animal lung cancer model | Antitumor efficacy 4.6× higher than cisplatin injection; sustained release ~72 h; reduced lung tissue damage | [140] |
| PCL/PLGA blended microspheres (EphA2 ligand conjugated) | PCL-PLGA microspheres + EphA2 targeting | Not specified (anticancer drug) | Triple-negative breast cancer (TNBC) model | Inhibited local tumor regrowth; sustained release >90 days; proposed as injectable/implantable depot | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Kwak, J.; Lim, M.; Lim, S.Y.; Chae, S.; Ha, S.-J.; Won, Y.-W.; Kim, H.-O.; Lim, K.S. Advances in PCL, PLA, and PLGA-Based Technologies for Anticancer Drug Delivery. Pharmaceutics 2025, 17, 1354. https://doi.org/10.3390/pharmaceutics17101354
Kim Y, Kwak J, Lim M, Lim SY, Chae S, Ha S-J, Won Y-W, Kim H-O, Lim KS. Advances in PCL, PLA, and PLGA-Based Technologies for Anticancer Drug Delivery. Pharmaceutics. 2025; 17(10):1354. https://doi.org/10.3390/pharmaceutics17101354
Chicago/Turabian StyleKim, Yeongbeom, Jaewoo Kwak, Minyeong Lim, Su Yeon Lim, Sehyun Chae, Suk-Jin Ha, Young-Wook Won, Hyun-Ouk Kim, and Kwang Suk Lim. 2025. "Advances in PCL, PLA, and PLGA-Based Technologies for Anticancer Drug Delivery" Pharmaceutics 17, no. 10: 1354. https://doi.org/10.3390/pharmaceutics17101354
APA StyleKim, Y., Kwak, J., Lim, M., Lim, S. Y., Chae, S., Ha, S.-J., Won, Y.-W., Kim, H.-O., & Lim, K. S. (2025). Advances in PCL, PLA, and PLGA-Based Technologies for Anticancer Drug Delivery. Pharmaceutics, 17(10), 1354. https://doi.org/10.3390/pharmaceutics17101354









