Virtual Bioequivalence Assessment and Dissolution Safe Space Exploration for Fixed-Dose Metformin–Glyburide Tablet Using Physiologically Based Biopharmaceutics Modeling
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Software
2.3. In Vitro Dissolution Tests
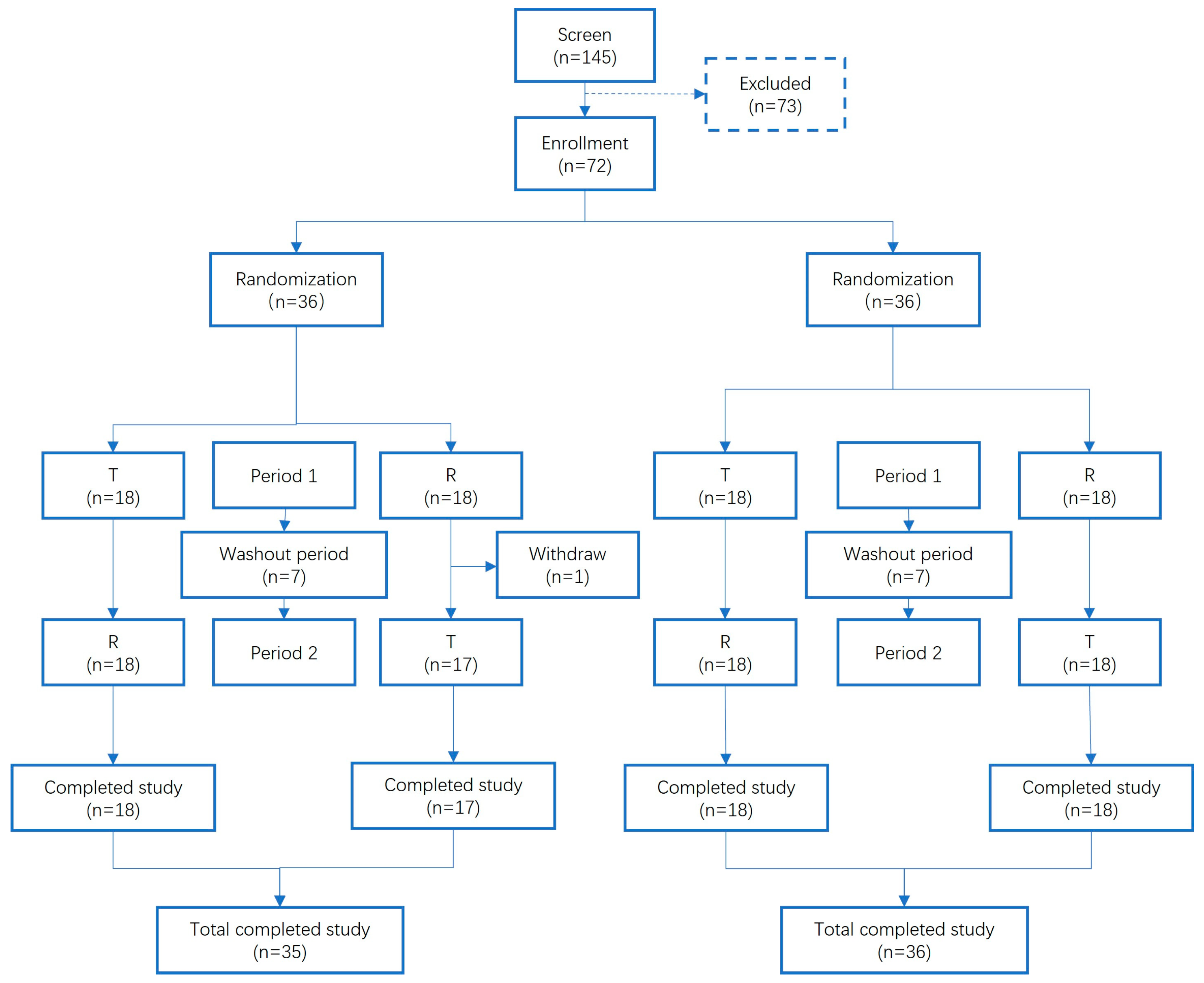
2.4. In Vivo Pharmacokinetic Studies
2.5. In Silico Model Development
2.5.1. Physiology and Pharmacokinetic Parameters
2.5.2. Model Development
2.5.3. Model Validation
2.6. Virtual Bioequivalence Study Design
2.6.1. Assessment of Parameter Sensitivity
2.6.2. Virtual Population Construction and BE Analysis
2.7. Development of Safe Space
3. Results
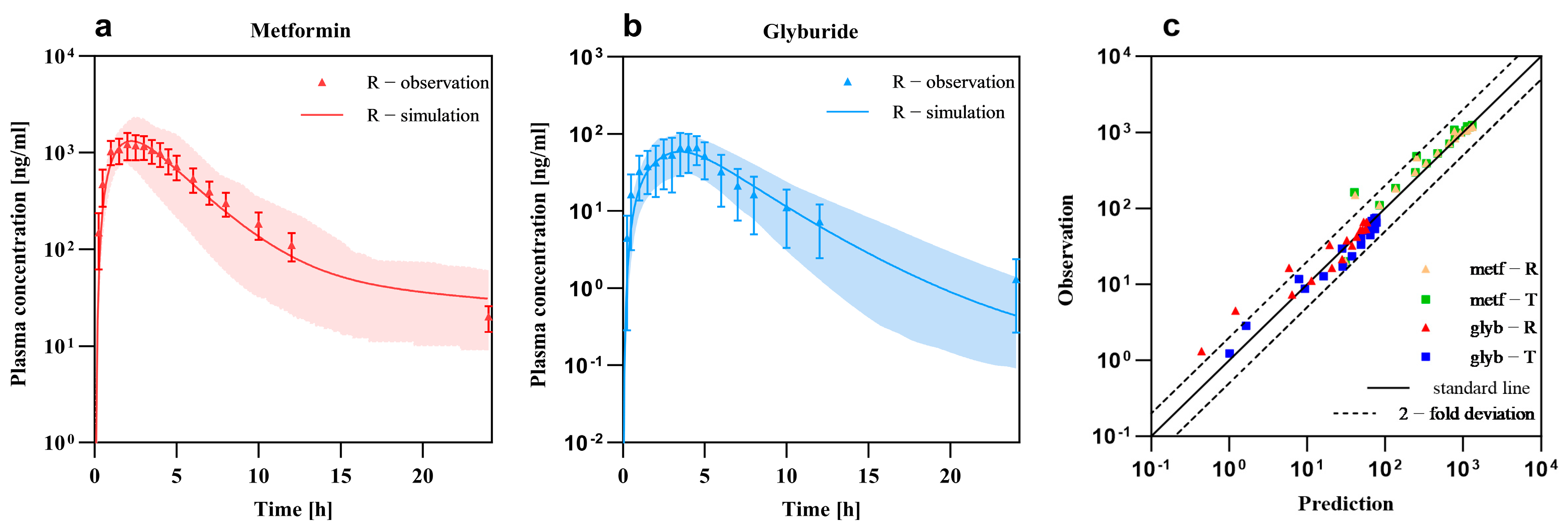
3.1. PBPK Models and Validation
3.2. Virtual Bioequivalence Study
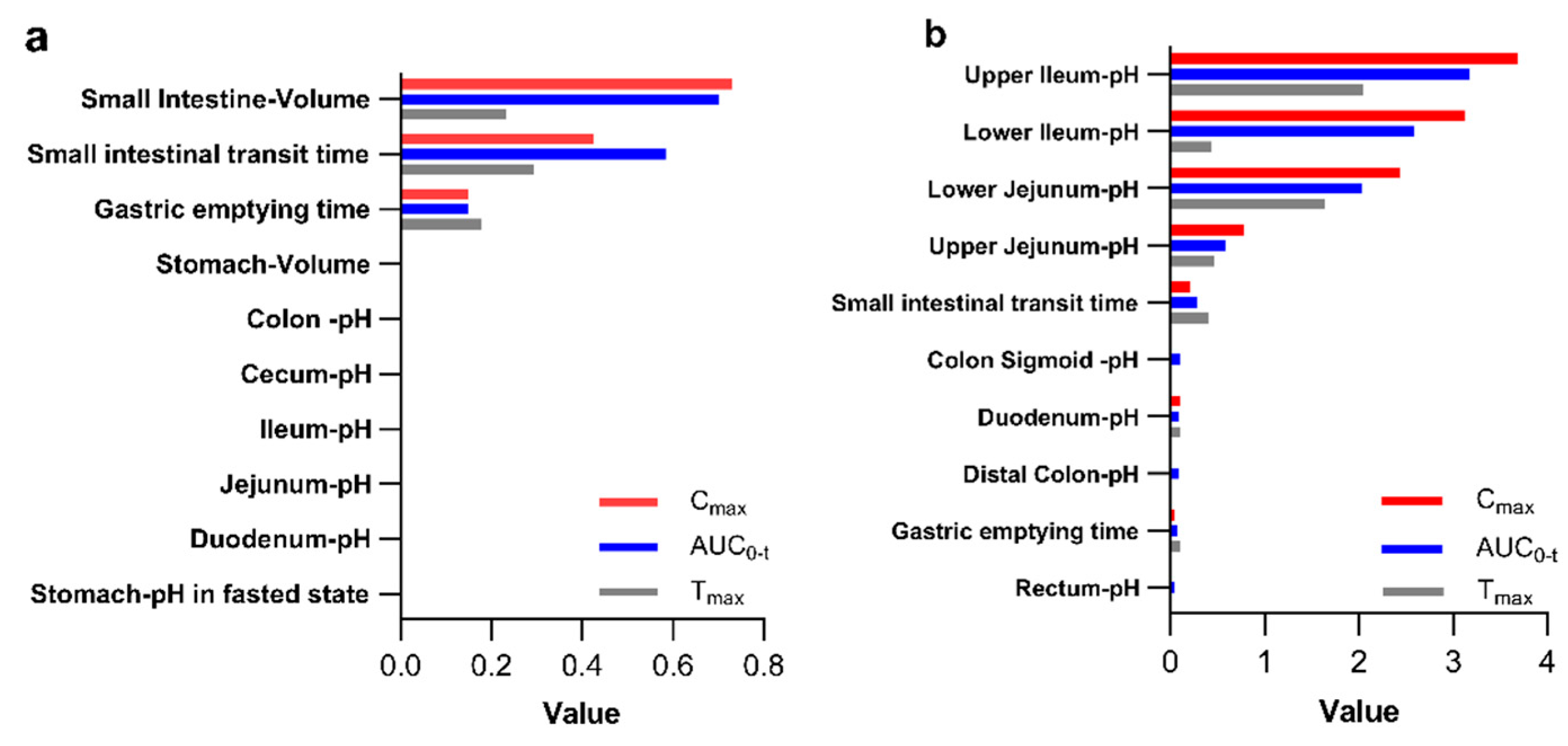
3.2.1. Sensitivity Analysis
3.2.2. Virtual Population
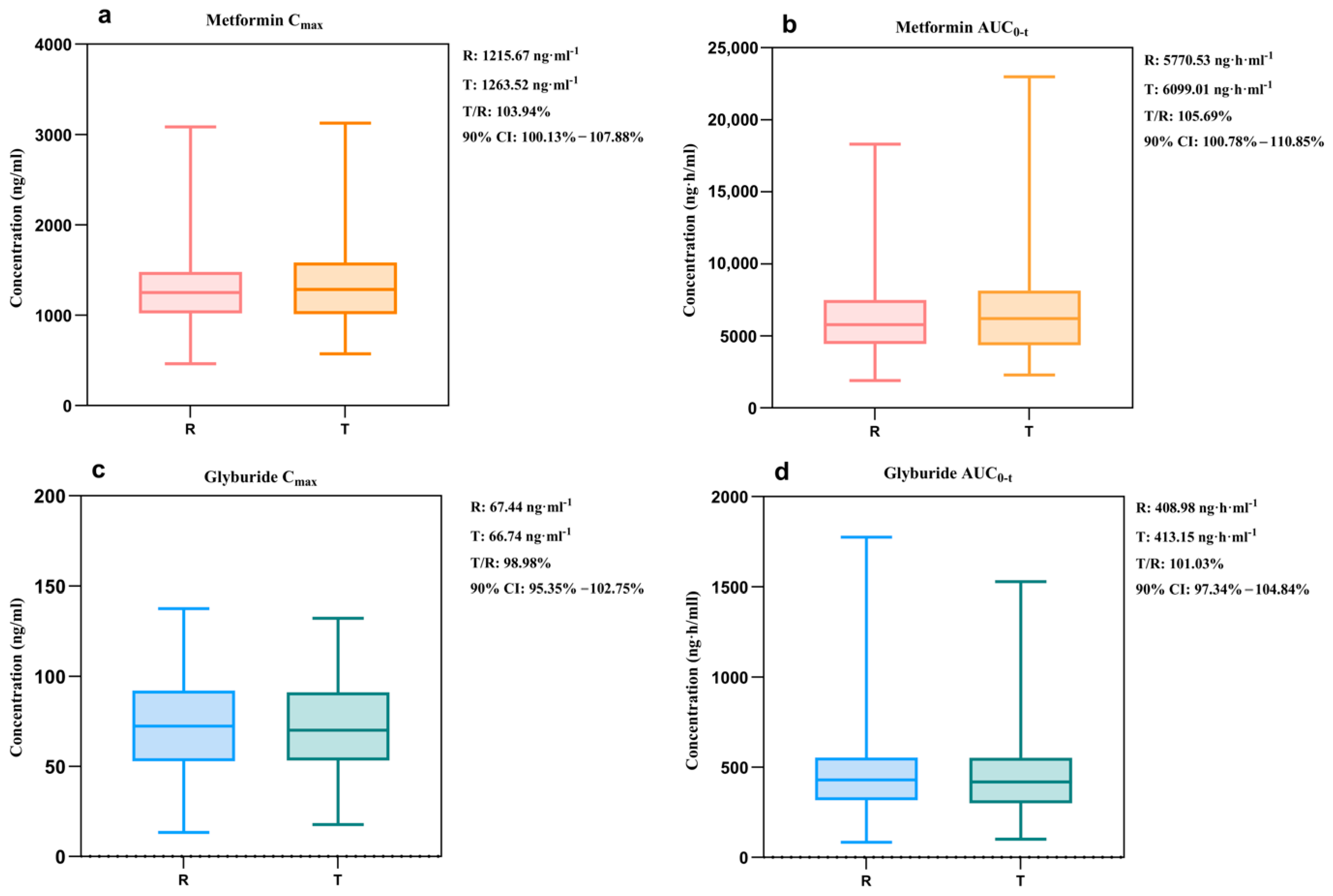
3.2.3. Virtual Bioequivalence Analysis
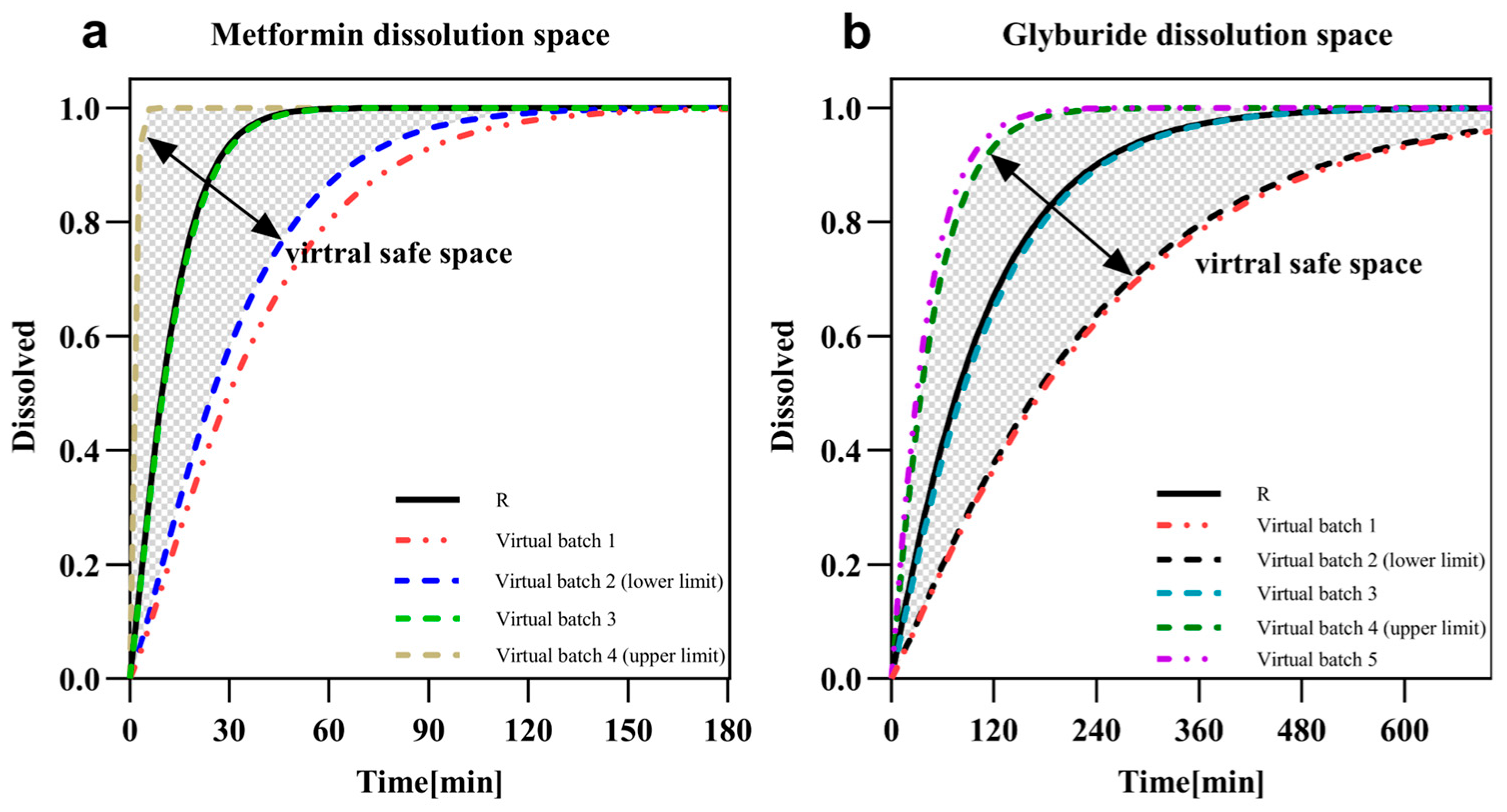
3.3. Dissolution Safe Spaces
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FDC | fixed-dose combination |
| BE | bioequivalence |
| API | active pharmaceutical ingredient |
| PBBM | physiologically based biopharmaceutics modeling |
| PBPK | physiologically based pharmacokinetic |
| PK | pharmacokinetics |
| VBE | virtual bioequivalence |
| GI | gastrointestinal |
| MIDD | model-informed drug development |
| BCS | biopharmaceutics classification system |
| PMAT | plasma membrane monoamine transporter |
| FE | fold errors |
| PE | predict errors |
| IIV | inter-individual variability |
| BSV | between-subject variability |
| WSV | within-subject variability |
References
- U.S. Food and Drug Administration (FDA). Hypertension: Developing Fixed-Combination Drug Products for Treatment; Guidance for Industry; Center for Drug Evaluation and Research (CDER): Silver Spring, MD, USA, 2018. Available online: https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm (accessed on 28 September 2025).
- Fillinger, L.; Walter, S.; Ley, M.; Kęska-Izworska, K.; Dehkordi, L.G.; Kratochwill, K.; Perco, P. Computational Modeling Approaches and Regulatory Pathways for Drug Combinations. Drug Discov. Today 2025, 30, 104345. [Google Scholar] [CrossRef]
- Sen, S.; Ganta, B.; Rachel, V.N.; Gogikar, S.K.; Singh, V.; Sonti, R.; Dikundwar, A.G. Mapping Advantages and Challenges in Analytical Development for Fixed Dose Combination Products, a Review. J. Pharm. Sci. 2024, 113, 2028–2043. [Google Scholar] [CrossRef]
- IDF Diabetes Atlas. 2025. Available online: https://diabetesatlas.org/resources/idf-diabetes-atlas-2025/ (accessed on 28 September 2025).
- The American Diabetes Association Releases the Standards of Care in Diabetes—2024. Available online: https://diabetes.org/newsroom/press-releases/american-diabetes-association-releases-standards-care-diabetes-2024 (accessed on 28 September 2025).
- NICE. Type 2 Diabetes in Adults: Management. Available online: https://www.nice.org.uk/guidance/ng28 (accessed on 28 September 2025).
- Shuster, D.L.; Shireman, L.M.; Ma, X.; Shen, D.D.; Flood Nichols, S.K.; Ahmed, M.S.; Clark, S.; Caritis, S.; Venkataramanan, R.; Haas, D.M.; et al. Pharmacodynamics of Glyburide, Metformin, and Glyburide/Metformin Combination Therapy in the Treatment of Gestational Diabetes Mellitus. Clin. Pharmacol. Ther. 2020, 107, 1362–1372. [Google Scholar] [CrossRef]
- Huang, X.; Huang, J.; Li, X.; Chen, L. Pharmacokinetic and Bioequivalence Studies of 2 Metformin Glibenclamide Tablets in Healthy Chinese Subjects Under Fasting and Fed Conditions. Clin. Pharmacol. Drug Dev. 2023, 12, 509–517. [Google Scholar] [CrossRef]
- International Council for Harmonisation. ICH M13A: Bioequivalence for Immediate-Release Solid Oral Dosage Forms; ICH: Geneva, Switzerland, 2023. [Google Scholar]
- European Medicines Agency. Guideline on Clinical Development of Fixed Combination Medicinal Products; European Medicines Agency (EMA): Amsterdam, The Netherlands, 2017. [Google Scholar]
- Center for Drug Evaluation. Technical Guideline for Bioavailability and Bioequivalence Studies of Chemical Drug Products; National Medical Products Administration (NMPA): Beijing, China, 2005. [Google Scholar]
- Bhattiprolu, A.K.; Kollipara, S.; Boddu, R.; Arumugam, A.; Khan, S.M.; Ahmed, T. A Semi-Mechanistic Physiologically Based Biopharmaceutics Model to Describe Complex and Saturable Absorption of Metformin: Justification of Dissolution Specifications for Extended Release Formulation. Aaps Pharmscitech 2024, 25, 193. [Google Scholar] [CrossRef]
- Hanke, N.; Türk, D.; Selzer, D.; Ishiguro, N.; Ebner, T.; Wiebe, S.; Müller, F.; Stopfer, P.; Nock, V.; Lehr, T. A Comprehensive Whole-Body Physiologically Based Pharmacokinetic Drug–Drug–Gene Interaction Model of Metformin and Cimetidine in Healthy Adults and Renally Impaired Individuals. Clin. Pharmacokinet. 2020, 59, 1419–1431. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Z.; Li, P.; Kong, W.; Liu, X.; Liu, L. A Whole-Body Physiologically Based Pharmacokinetic Model Characterizing Interplay of OCTs and MATEs in Intestine, Liver and Kidney to Predict Drug-Drug Interactions of Metformin with Perpetrators. Pharmaceutics 2021, 13, 698. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guo, H.; Liu, C.; Zhong, Z.; Liu, L.; Liu, X. Prediction of Drug Disposition in Diabetic Patients by Means of a Physiologically Based Pharmacokinetic Model. Clin. Pharmacokinet. 2015, 54, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Klumpp, L.; Dressman, J. Physiologically Based Pharmacokinetic Model Outputs Depend on Dissolution Data and Their Input: Case Examples Glibenclamide and Dipyridamole. Eur. J. Pharm. Sci. 2020, 151, 105380. [Google Scholar] [CrossRef]
- Food and Drug Administration. The Use of Physiologically Based Pharmacokinetic Analyses—Biopharmaceutics Applications for Oral Drug Product Development, Manufacturing Changes, and Controls: Guidance for Industry (Draft); U.S. Food and Drug Administration (U.S. FDA): Silver Spring, MD, USA, 2020. [Google Scholar]
- Derbalah, A.; Abdulla, T.; De Sousa Mendes, M.; Wu, Q.; Stader, F.; Jamei, M.; Gardner, I.; Sepp, A. Accelerating Biologics PBPK Modelling with Automated Model Building: A Tutorial. Pharmaceutics 2025, 17, 604. [Google Scholar] [CrossRef]
- Mackie, C.; Arora, S.; Seo, P.; Moody, R.; Rege, B.; Pepin, X.; Heimbach, T.; Tannergren, C.; Mitra, A.; Suarez-Sharp, S.; et al. Physiologically Based Biopharmaceutics Modeling (PBBM): Best Practices for Drug Product Quality, Regulatory and Industry Perspectives: 2023 Workshop Summary Report. Mol. Pharm. 2024, 21, 2065–2080. [Google Scholar] [CrossRef]
- Arora, S.; Pepin, X.; Jamei, M.; Sharma, P.; Heimbach, T.; Wagner, C.; Bransford, P.; Kollipara, S.; Ahmed, T.; Hingle, M.; et al. Development of a Physiologically Based Biopharmaceutics Model Report Template: Considerations for Improved Quality in View of Regulatory Submissions. Mol. Pharm. 2025, 22, 2735–2746. [Google Scholar] [CrossRef]
- Kuepfer, L.; Niederalt, C.; Wendl, T.; Schlender, J.-F.; Willmann, S.; Lippert, J.; Block, M.; Eissing, T.; Teutonico, D. Applied Concepts in PBPK Modeling: How to Build a PBPK/PD Model. CPT Pharmacomet. Syst. Pharmacol. 2016, 5, 516–531. [Google Scholar] [CrossRef] [PubMed]
- Graham, G.G.; Punt, J.; Arora, M.; Day, R.O.; Doogue, M.P.; Duong, J.K.; Furlong, T.J.; Greenfield, J.R.; Greenup, L.C.; Kirkpatrick, C.M.; et al. Clinical Pharmacokinetics of Metformin. Clin. Pharmacokinet. 2011, 50, 81–98. [Google Scholar] [CrossRef]
- Desai, D.; Wong, B.; Huang, Y.; Ye, Q.; Tang, D.; Guo, H.; Huang, M.; Timmins, P. Surfactant-Mediated Dissolution of Metformin Hydrochloride Tablets: Wetting Effects versus Ion Pairs Diffusivity. J. Pharm. Sci. 2014, 103, 920–926. [Google Scholar] [CrossRef]
- Zhou, M.; Xia, L.; Wang, J. Metformin Transport by a Newly Cloned Proton-Stimulated Organic Cation Transporter (Plasma Membrane Monoamine Transporter) Expressed in Human Intestine. Drug Metab. Dispos. 2007, 35, 1956–1962. [Google Scholar] [CrossRef]
- Greupink, R.; Schreurs, M.; Benne, M.S.; Huisman, M.T.; Russel, F.G.M. Semi-Mechanistic Physiologically-Based Pharmacokinetic Modeling of Clinical Glibenclamide Pharmacokinetics and Drug–Drug-Interactions. Eur. J. Pharm. Sci. 2013, 49, 819–828. [Google Scholar] [CrossRef]
- Shirasaka, Y.; Seki, M.; Hatakeyama, M.; Kurokawa, Y.; Uchiyama, H.; Takemura, M.; Yasugi, Y.; Kishimoto, H.; Tamai, I.; Wang, J.; et al. Multiple Transport Mechanisms Involved in the Intestinal Absorption of Metformin: Impact on the Nonlinear Absorption Kinetics. J. Pharm. Sci. 2022, 111, 1531–1541. [Google Scholar] [CrossRef] [PubMed]
- Bristol-Myers Squibb Company. GLUCOVANCE® (Glyburide and Metformin Hydrochloride) Tablets: Highlights of Prescribing Information; Initial U.S. Approval; Bristol-Myers Squibb Company: New York, NY, USA, 2000. [Google Scholar]
- Proks, P.; Kramer, H.; Haythorne, E.; Ashcroft, F.M. Binding of Sulphonylureas to Plasma Proteins—A KATP Channel Perspective. PLoS ONE 2018, 13, e0197634. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An Add-In Program for Modeling and Comparison of Drug Dissolution Profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef]
- Najjar, A.; Hamadeh, A.; Krause, S.; Schepky, A.; Edginton, A. Global Sensitivity Analysis of Open Systems Pharmacology Suite Physiologically Based Pharmacokinetic Models. CPT Pharmacomet. Syst. Pharmacol. 2024, 13, 2052–2067. [Google Scholar] [CrossRef]
- European Medicines Agency. Guideline on the Reporting of Physiologically Based Pharmacokinetic (PBPK) Modelling and Simulation. EMA/CHMP/458101/2016. 2018. Available online: https://www.ema.europa.eu/en/Documents/Scientific-Guideline/Guideline-Reporting-Physiologically-Based-Pharmacokinetic-Pbpk-Modelling-Simulation_en.pdf (accessed on 28 January 2021).
- Niemi, M.; Backman, J.T.; Neuvonen, M.; Neuvonen, P.J.; Kivistö, K.T. Effects of Rifampin on the Pharmacokinetics and Pharmacodynamics of Glyburide and Glipizide. Clin. Pharmacol. Ther. 2001, 69, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Lilja, J.J.; Niemi, M.; Fredrikson, H.; Neuvonen, P.J. Effects of Clarithromycin and Grapefruit Juice on the Pharmacokinetics of Glibenclamide. Br. J. Clin. Pharmacol. 2007, 63, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Varma, M.V.S.; Scialis, R.J.; Lin, J.; Bi, Y.-A.; Rotter, C.J.; Goosen, T.C.; Yang, X. Mechanism-Based Pharmacokinetic Modeling to Evaluate Transporter-Enzyme Interplay in Drug Interactions and Pharmacogenetics of Glyburide. AAPS J. 2014, 16, 736–748. [Google Scholar] [CrossRef] [PubMed]
- Donahue, S.R.; Turner, K.C.; Patel, S. Pharmacokinetics and Pharmacodynamics of Glyburide/Metformin Tablets (GlucovanceTM) Versus Equivalent Doses of Glyburide and Metformin in Patients with Type 2 Diabetes. Clin. Pharmacokinet. 2002, 41, 1301–1309. [Google Scholar] [CrossRef]
- Arnqvist, H.J.; Karlberg, B.E.; Melander, A. Pharmacokinetics and Effects of Glibenclamide in Two Formulations, HB 419 and HB 420, in Type 2 Diabetes. Ann. Clin. Res. 1983, 15 (Suppl. S37), 21–25. [Google Scholar]
- Desai, D.; Wang, J.; Wen, H.; Li, X.; Timmins, P. Formulation Design, Challenges, and Development Considerations for Fixed Dose Combination (FDC) of Oral Solid Dosage Forms. Pharm. Dev. Technol. 2013, 18, 1265–1276. [Google Scholar] [CrossRef]
- Belayneh, A.; Molla, F.; Kahsay, G. Formulation and Optimization of Monolithic Fixed-Dose Combination of Metformin HCl and Glibenclamide Orodispersible Tablets. Adv. Pharm. Pharm. Sci. 2020, 2020, 3546597. [Google Scholar] [CrossRef]
- Bhattiprolu, A.K.; Kollipara, S.; Boddu, R.; Ahmed, T. Justification of Widened Dissolution Specifications of an Extended-Release Product Using Physiologically Based Biopharmaceutics Modeling. Xenobiotica 2024, 54, 781–795. [Google Scholar] [CrossRef]
- Tannergren, C.; Arora, S.; Babiskin, A.; Borges, L.; Chatterjee, P.; Cheng, Y.-H.; Dallmann, A.; Govada, A.; Heimbach, T.; Hingle, M.; et al. Current State and New Horizons in Applications of Physiologically Based Biopharmaceutics Modeling (PBBM): A Workshop Report. Mol. Pharm. 2025, 22, 5–27. [Google Scholar] [CrossRef]
- Loisios-Konstantinidis, I.; Cristofoletti, R.; Fotaki, N.; Turner, D.B.; Dressman, J. Establishing Virtual Bioequivalence and Clinically Relevant Specifications Using in Vitro Biorelevant Dissolution Testing and Physiologically-Based Population Pharmacokinetic Modeling. Case Example. Eur. J. Pharm. Sci. 2020, 143, 105170. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, N.; Charbe, N.; Plano, D.; Shoyaib, A.A.; Pal, A.; Boyce, H.; Zhao, L.; Wu, F.; Polli, J.; Dressman, J.; et al. A Physiologically Based Biopharmaceutics Modeling (PBBM) Framework for Characterizing Formulation-Dependent Food Effects: Paving the Road towards Fed State Virtual BE Studies for Itraconazole Amorphous Solid Dispersions. Eur. J. Pharm. Sci. 2025, 209, 107047. [Google Scholar] [CrossRef]
- Gonzalez-Alvarez, I.; Ruiz-Picazo, A.; Selles-Talavera, R.; Figueroa-Campos, A.; Merino, V.; Bermejo, M.; Gonzalez-Alvarez, M. In Vivo Predictive Dissolution and Biopharmaceutic-Based In Silico Model to Explain Bioequivalence Results of Valsartan, a Biopharmaceutics Classification System Class IV Drug. Pharmaceutics 2024, 16, 390. [Google Scholar] [CrossRef]
- Guideline for the Testing and Comparison of Dissolution Curves of Ordinary Oral Solid Preparations. 2016. Available online: https://www.nmpa.gov.cn/directory/web/nmpa/zhuanti/ypqxgg/ggzhcfg/20160318210001633.html (accessed on 28 September 2025).
- Therapeutic Goods Administration. ICH M9 Guideline on Biopharmaceutics Classification System-Based Biowaivers. 2024. Available online: https://www.ema.europa.eu/en/ich-m9-biopharmaceutics-classification-system-based-biowaivers-scientific-guideline (accessed on 28 September 2025).
- Abuhelwa, A.Y.; Williams, D.B.; Upton, R.N.; Foster, D.J.R. Food, gastrointestinal pH, and models of oral drug absorption. Eur. J. Pharm. Biopharm. 2017, 112, 234–248. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Unit | Value | Source |
|---|---|---|---|
| Metformin | |||
| Physicochemical properties | |||
| LogP | Log unit | −1.43 | [22] |
| MW | g/mol | 129.16 | Pubchem |
| pKa1 | \ | 2.8 | [23] |
| pKa2 | \ | 11.5 | [23] |
| Solubility (Ref-pH 6.8) | g/L | 350.9 | [23] |
| Absorption | |||
| Intestinal permeability (transcellular) | cm/min | 5.80 × 10−8 | optimized |
| PMAT Hill coefficient | \ | 2.64 | [24] |
| PMAT Km | μmol/L | 1320 | optimized |
| PMAT Vmax | 1/min | 53.36 | optimized |
| Distribution | |||
| Fraction unbound | % | 100 | Drugbank |
| Elimination | |||
| TSmax_spec | μmol/L/min | 85.42 | optimized |
| Km(kidney) | μmol/L | 65.6 | optimized |
| Formulation | |||
| 50% dissolved time | min | 9.7 | optimized |
| 10 | experiment | ||
| Dissolution shape | \ | 1.37 | optimized |
| 1.22 | optimized | ||
| Glyburide | |||
| Physicochemical properties | |||
| LogP | Log unit | 3.75 | Pubchem |
| MW | g/mol | 494 | Pubchem |
| pKa | \ | 5.3 | [25] |
| Solubility at Reference pH | g/L | 2.06 × 10−3 | Pubchem |
| Reference pH | \ | 8.31 | optimized |
| Absorption | |||
| Intestinal permeability (transcellular) | cm/min | 6.27 × 10−5 | optimized |
| Distribution | |||
| Fraction unbound | % | 1 | Pubchem |
| Elimination | |||
| Total hepatic clearance | 1/min | 4.15 | optimized |
| Formulation | |||
| 50% dissolved time | min | 77.22 | optimized |
| 82.78 | optimized | ||
| Dissolution shape | \ | 1.06 | optimized |
| 1.10 | optimized | ||
| Compounds | PK Parameters | Formulations | FE | PE |
|---|---|---|---|---|
| Metformin | AUC0-t | T | 0.95 | 0.05 |
| R | 0.97 | 0.03 | ||
| Cmax | T | 1.001 | 0.001 | |
| R | 1.07 | 0.07 | ||
| Glyburide | AUC0-t | T | 1.07 | 0.07 |
| R | 1.13 | 0.13 | ||
| Cmax | T | 0.80 | 0.20 | |
| R | 0.80 | 0.20 |
| Virtual Batch | Dissolution Time (50% Dissolved) | Parameters | T | R | T/R % | 90% CI |
|---|---|---|---|---|---|---|
| 1 | 30 min | Cmax (ng·mL−1) | 1418.05 | 1215.67 | 116.65% | 112.75–120.68% |
| AUC0-t (ng·h·mL−1) | 7030.46 | 5770.53 | 121.83% | 116.62–127.28% | ||
| 2 | 25 min | Cmax (ng·mL−1) | 1387.88 | 1215.67 | 114.17% | 110.31–118.15% |
| AUC0-t (ng·h·mL−1) | 6801.63 | 5770.53 | 117.87% | 112.75–123.21% | ||
| 3 | 10 min | Cmax (ng·mL−1) | 1263.52 | 1215.67 | 103.94% | 100.13–107.88% |
| AUC0-t (ng·h·mL−1) | 6099.01 | 5770.53 | 105.69% | 100.78–110.85% | ||
| 4 | 1 min | Cmax (ng·mL−1) | 1197.00 | 1215.67 | 98.46% | 94.64–102.45% |
| AUC0-t (ng·h·mL−1) | 5798.47 | 5769.15 | 100.51% | 95.63–105.58% |
| Virtual Batch | Dissolution Time (50% Dissolved) | Parameters | T | R | T/R % | 90% CI |
|---|---|---|---|---|---|---|
| 1 | 175 min | Cmax (ng·mL−1) | 55.32 | 67.44 | 82.03% | 79.16–84.98% |
| AUC0-t (ng·h·mL−1) | 412.71 | 408.98 | 100.91% | 97.04–104.94% | ||
| 2 | 170 min | Cmax (ng·mL−1) | 56.01 | 67.44 | 83.05% | 80.16–86.02% |
| AUC0-t (ng·h·mL−1) | 414.75 | 408.98 | 101.41% | 97.54–105.43% | ||
| 3 | 83 min | Cmax (ng·mL−1) | 66.74 | 67.44 | 98.98% | 95.35–102.75% |
| AUC0-t (ng·h·mL−1) | 413.15 | 408.98 | 101.03% | 97.34–104.84% | ||
| 4 | 35 min | Cmax (ng·mL−1) | 65.33 | 67.44 | 96.87% | 92.75–101.17% |
| AUC0-t (ng·h·mL−1) | 343.75 | 408.98 | 84.05% | 80.60–87.65% | ||
| 5 | 30 min | Cmax (ng·mL−1) | 64.19 | 67.44 | 95.18% | 91.04–99.49% |
| AUC0-t (ng·h·mL−1) | 331.65 | 408.98 | 81.09% | 77.67–84.66% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Shen, C.; Xiao, Y.; Wang, L. Virtual Bioequivalence Assessment and Dissolution Safe Space Exploration for Fixed-Dose Metformin–Glyburide Tablet Using Physiologically Based Biopharmaceutics Modeling. Pharmaceutics 2025, 17, 1352. https://doi.org/10.3390/pharmaceutics17101352
Zhao C, Shen C, Xiao Y, Wang L. Virtual Bioequivalence Assessment and Dissolution Safe Space Exploration for Fixed-Dose Metformin–Glyburide Tablet Using Physiologically Based Biopharmaceutics Modeling. Pharmaceutics. 2025; 17(10):1352. https://doi.org/10.3390/pharmaceutics17101352
Chicago/Turabian StyleZhao, Chenshuang, Chaozhuang Shen, Yumeng Xiao, and Ling Wang. 2025. "Virtual Bioequivalence Assessment and Dissolution Safe Space Exploration for Fixed-Dose Metformin–Glyburide Tablet Using Physiologically Based Biopharmaceutics Modeling" Pharmaceutics 17, no. 10: 1352. https://doi.org/10.3390/pharmaceutics17101352
APA StyleZhao, C., Shen, C., Xiao, Y., & Wang, L. (2025). Virtual Bioequivalence Assessment and Dissolution Safe Space Exploration for Fixed-Dose Metformin–Glyburide Tablet Using Physiologically Based Biopharmaceutics Modeling. Pharmaceutics, 17(10), 1352. https://doi.org/10.3390/pharmaceutics17101352







