Angiotensin II/Angiotensin I Ratio as a New Pharmacodynamic Parameter for Population Modelling in Healthy Adults and Children with Heart Failure Treated with Enalapril
Abstract
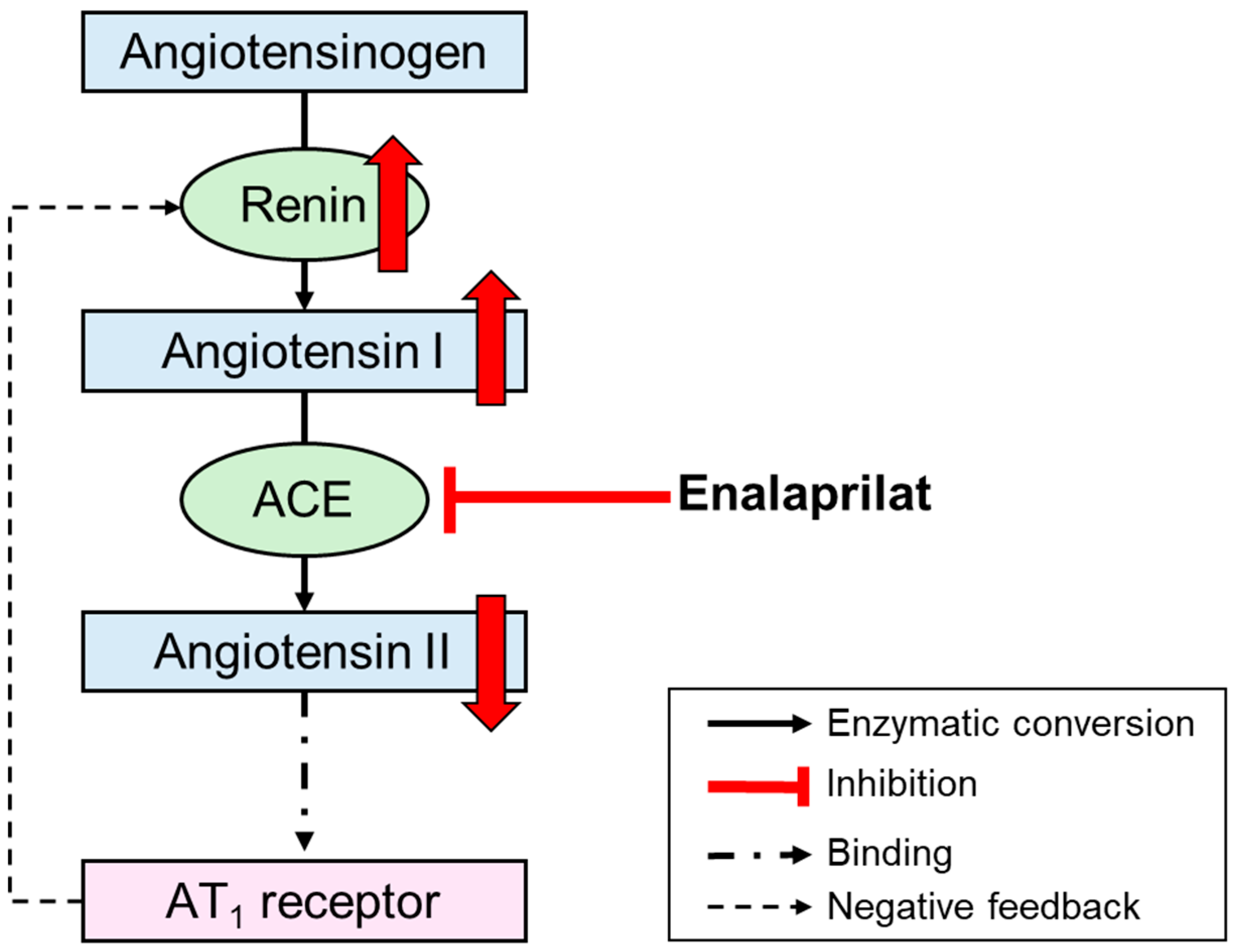
1. Introduction
2. Materials and Methods
2.1. Adult Data
2.1.1. Study Design and Investigated Population
2.1.2. Dosing
2.1.3. Sampling
2.1.4. Analytical Methods for Samples from Healthy Adults
2.2. Paediatric Data
2.2.1. Study Design and Investigated Population
2.2.2. Dosing
2.2.3. Sampling
2.2.4. Analytical Methods for Paediatric Samples
2.3. Software
2.4. Population Pharmacokinetic/Pharmacodynamic Modelling for Healthy Adults
2.4.1. Model Development
2.4.2. Model Evaluation
2.5. Comparison of the Time Course of the Effect in Healthy Adults and Children with Heart Failure
2.6. Change in the Angiotensin II/Angiotensin I Ratio After Initial Enalapril Dose in Children with Heart Failure
2.7. Population Pharmacodynamic Modelling for Children with Heart Failure
2.7.1. Model Development
2.7.2. Model Evaluation
3. Results
3.1. Adult Data
3.2. Paediatric Data
| Characteristic | Number of Observations (%) | Mean (SD) | Median (Range) |
|---|---|---|---|
| Healthy adults (n = 9) | |||
| Age (years) | 9 (100) | 22.7 (4.0) | 21 (19–30) |
| Weight (kg) | 9 (100) | 69.8 (13.6) | 74 (47–88) |
| Body mass index (kg/m2) | 9 (100) | 21.3 (2.4) | 22 (18–25) |
| Sex | |||
| Male | 6 (66.7) | - | - |
| Female | 3 (33.3) | - | - |
| Children with heart failure (n = 27) | |||
| Age (years) | 54 (100) | 0.49 (0.47) | 0.36 (0.07–2.24) |
| Weight (kg) | 54 (100) | 5.5 (2.2) | 4.8 (3.2–13.0) |
| Ross score | 54 (100) | 4.0 (2.6) | 4 (0–9) |
| Sex | |||
| Male | 12 (44.4) | - | - |
| Female | 15 (55.6) | - | - |
| Aetiology of heart failure | |||
| Dilated cardiomyopathy | 3 (11.1) | - | - |
| Congenital heart disease | 24 (88.9) | - | - |
3.3. Population Pharmacokinetic/Pharmacodynamic Modelling for Healthy Adults
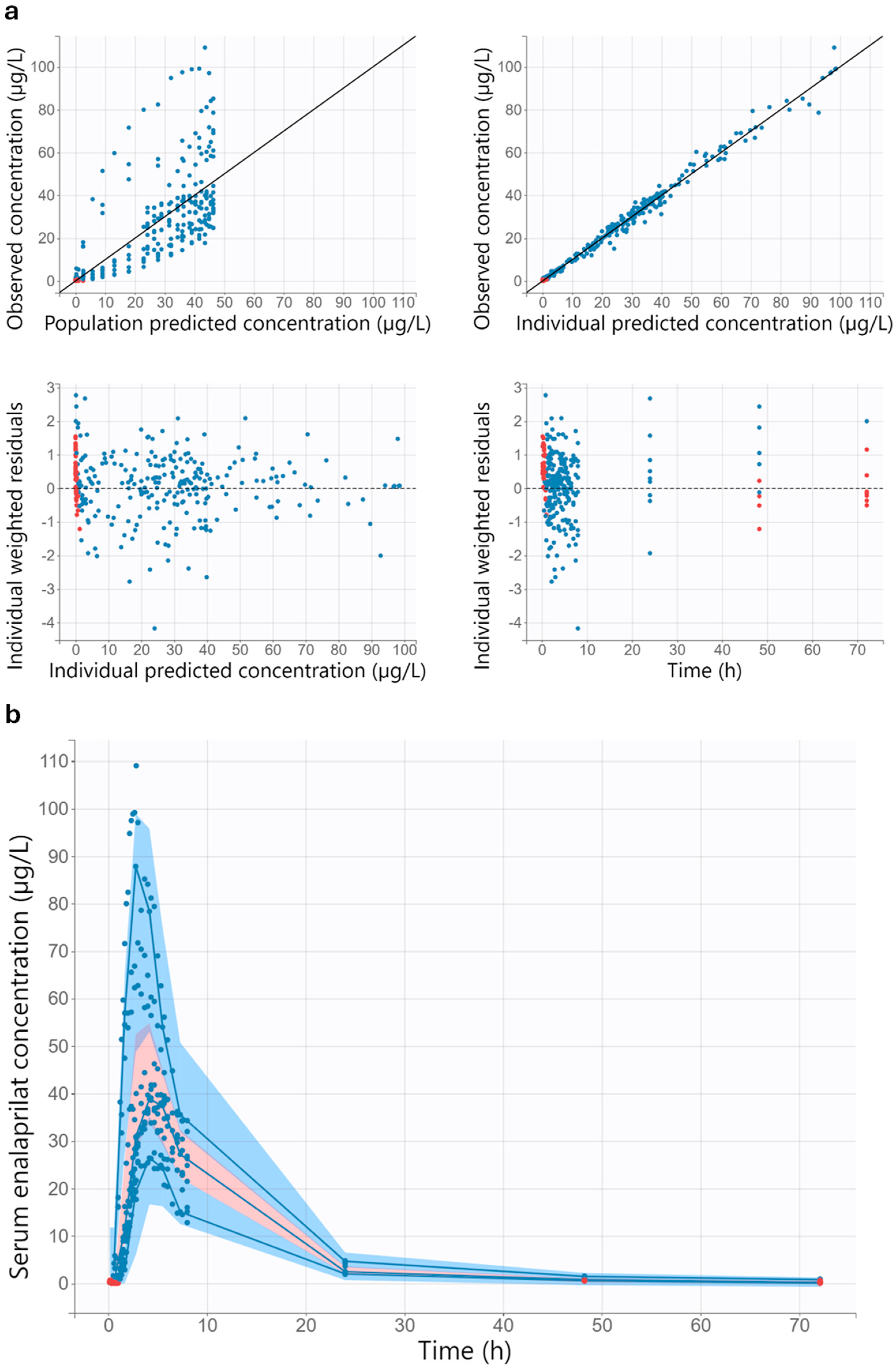
3.3.1. Population Pharmacokinetic/Pharmacodynamic Model
3.3.2. Model Evaluation
3.4. Comparison of the Time Course of the Effect in Healthy Adults and Children with Heart Failure
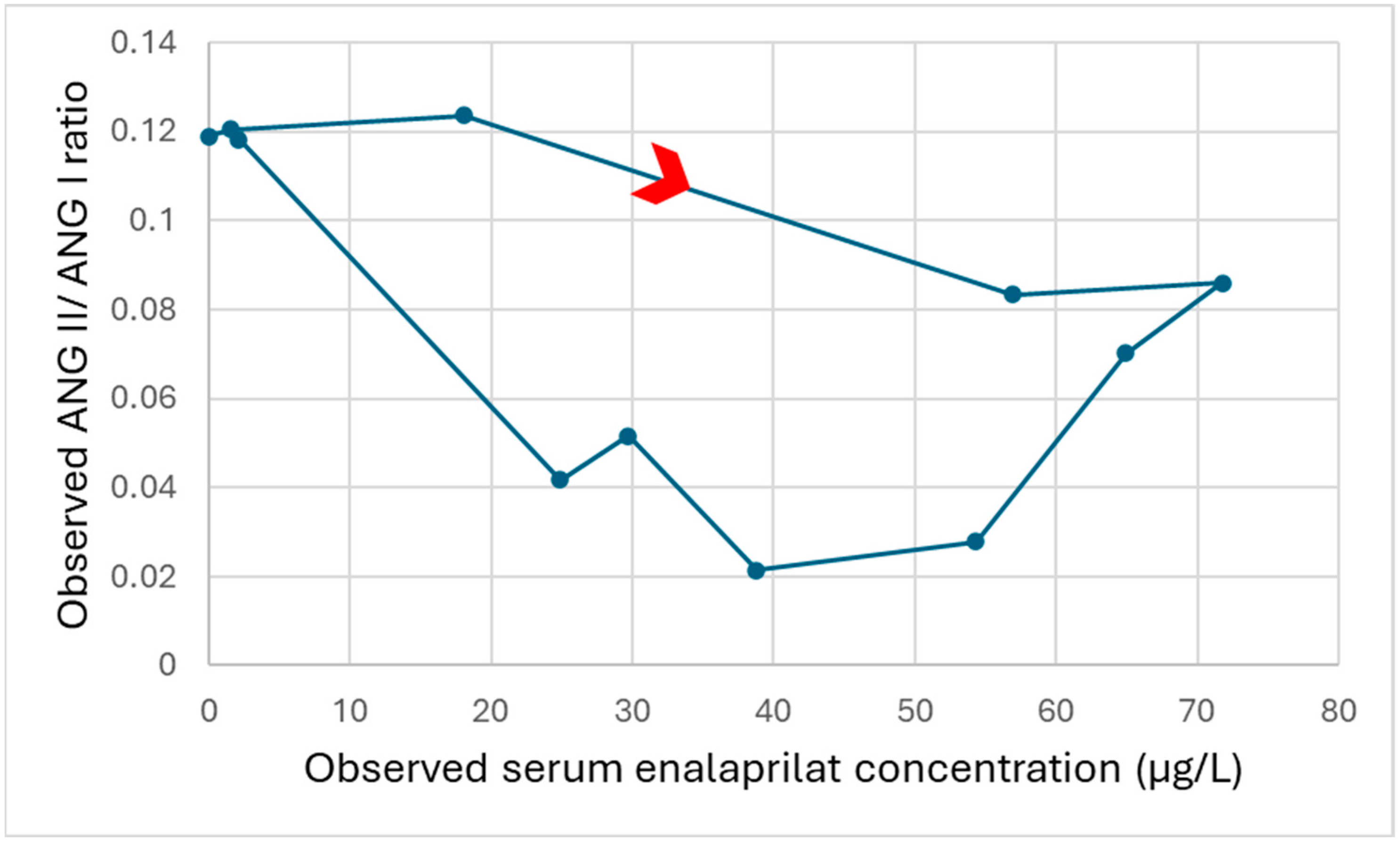
3.5. Change in the Angiotensin II/Angiotensin I Ratio After Initial Enalapril Dose in Children with Heart Failure
3.6. Population Pharmacodynamic Modelling for Children with Heart Failure
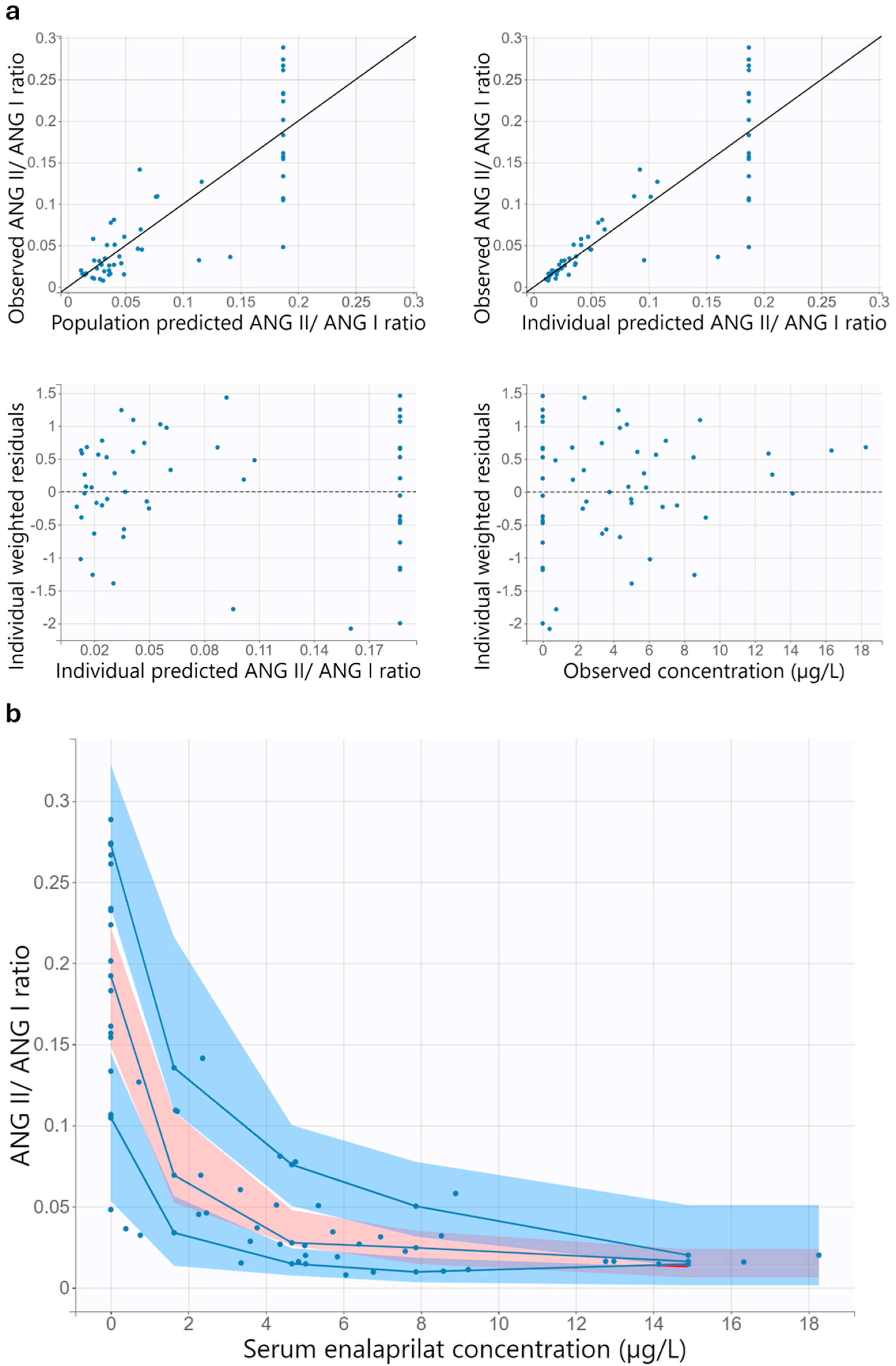
3.6.1. Population Pharmacodynamic Model
3.6.2. Model Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE | Angiotensin-converting enzyme |
| ANG I | Angiotensin I |
| ANG II | Angiotensin II |
| AT1 receptor | Angiotensin II type 1 receptor |
| CL/F | Apparent clearance of enalaprilat |
| COSSAC | Conditional sampling use for stepwise approach based on correlation tests |
| CV | Coefficient of variation |
| E0 | Baseline effect |
| EDTA | Ethylenediaminetetraacetic acid |
| Emax | Maximum effect |
| γ | Sigmoidicity factor |
| IC50 | Half-maximal inhibitory concentration |
| IIV | Interindividual variability |
| Imax | Maximum inhibition |
| ka | Absorption rate constant |
| ke0 | Effect compartment transfer rate constant |
| ktr | Transit rate constant |
| LENA | Labeling of Enalapril from Neonates up to Adolescents |
| LLOQ | Lower limit of quantification |
| Mtt | Mean transit time |
| NT-proBNP | N-terminal pro-B-type natriuretic peptide |
| Q/F | Apparent intercompartmental clearance |
| SD | Standard deviation |
| V1/F | Apparent central volume of distribution |
| V2/F | Apparent peripheral volume of distribution |
| Operational Definitions | |
| Phase II/III study | Seamless study design that combines the objectives of classical phase II and phase III studies to reduce the time required and the number of subjects. |
| Pharmacokinetic bridging study | Pharmacokinetic study with the objective of determining a dose that achieves similar exposure in children as in adults. Proof of efficacy is waived, as it is assumed that the exposure–effect relationship is similar in adults and children. |
References
- Bajcetic, M.; de Wildt, S.N.; Dalinghaus, M.; Breitkreutz, J.; Klingmann, I.; Lagler, F.B.; Keatley-Clarke, A.; Breur, J.M.; Male, C.; Jovanovic, I.; et al. Orodispersible minitablets of enalapril for use in children with heart failure (LENA): Rationale and protocol for a multicentre pharmacokinetic bridging study and follow-up safety study. Contemp. Clin. Trials Commun. 2019, 15, 100393. [Google Scholar] [CrossRef]
- Bijelic, M.; Djukic, M.; Vukomanovic, V.; Parezanovic, V.; Lazic, M.; Pavlovic, A.; Popovic, S.; Parezanovic, M.; Stefanovic, I.; Djordjevic, S.; et al. Clinical and Hemodynamic Outcomes with Enalapril Orodispersible Minitablets in Young Children with Heart Failure Due to Congenital Heart Disease. J. Clin. Med. 2024, 13, 4976. [Google Scholar] [CrossRef]
- Shaddy, R.; Canter, C.; Halnon, N.; Kochilas, L.; Rossano, J.; Bonnet, D.; Bush, C.; Zhao, Z.; Kantor, P.; Burch, M.; et al. Design for the sacubitril/valsartan (LCZ696) compared with enalapril study of pediatric patients with heart failure due to systemic left ventricle systolic dysfunction (PANORAMA-HF study). Am. Heart J. 2017, 193, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Shaddy, R.; Burch, M.; Kantor, P.F.; Solar-Yohay, S.; Garito, T.; Zhang, S.; Kocun, M.; Mao, C.; Cilliers, A.; Wang, X.; et al. Sacubitril/Valsartan in Pediatric Heart Failure (PANORAMA-HF): A Randomized, Multicenter, Double-Blind Trial. Circulation 2024, 150, 1756–1766. [Google Scholar] [CrossRef] [PubMed]
- EMA. Aqumeldi (Enalapril): European Public Assessment Report. Available online: https://www.ema.europa.eu/en/documents/overview/aqumeldi-epar-medicine-overview_en.pdf (accessed on 19 August 2025).
- EMA. Entresto (Sacubitril/Valsartan): European Public Assessment Report. Available online: https://www.ema.europa.eu/en/documents/overview/entresto-epar-summary-public_en.pdf (accessed on 19 August 2025).
- Smeets, N.J.L.; Schreuder, M.F.; Dalinghaus, M.; Male, C.; Lagler, F.B.; Walsh, J.; Laer, S.; de Wildt, S.N. Pharmacology of enalapril in children: A review. Drug Discov. Today 2020, 25, 1957–1970. [Google Scholar] [CrossRef] [PubMed]
- Steichert, M.; Cawello, W.; Laeer, S. Population Pharmacokinetic Analysis of Enalapril and Enalaprilat in Newly Treated Children with Heart Failure: Implications for Safe Dosing of Enalapril (LENA Studies). Clin. Pharmacokinet. 2025, 64, 1103–1118. [Google Scholar] [CrossRef]
- Steichert, M.; Cawello, W.; Bajcetic, M.; Breur, J.M.P.J.; Dalinghaus, M.; Male, C.; de Wildt, S.N.; Läer, S. Influence of Age, Heart Failure and ACE Inhibitor Treatment on Plasma Renin Activity in Children: Insights from a Systematic Review and the European LENA Project. Front. Biosci. 2023, 28, 335. [Google Scholar] [CrossRef]
- Suessenbach, F.K.; Burckhardt, B.B. Levels of angiotensin peptides in healthy and cardiovascular/renal-diseased paediatric population-an investigative review. Heart Fail. Rev. 2019, 24, 709–723. [Google Scholar] [CrossRef]
- Makowski, N. Development and Validation of a Low-Volume LC-HRMS Assay for the Analysis of Aldosterone, Its Precursor and Main Metabolite Tailored for Paediatric Research in a GCLP-Compliant Environment. Ph.D. Thesis, Heinrich Heine University Düsseldorf, Düsseldorf, Germany, 2019. [Google Scholar]
- Masarone, D.; Valente, F.; Rubino, M.; Vastarella, R.; Gravino, R.; Rea, A.; Russo, M.G.; Pacileo, G.; Limongelli, G. Pediatric Heart Failure: A Practical Guide to Diagnosis and Management. Pediatr. Neonatol. 2017, 58, 303–312. [Google Scholar] [CrossRef]
- Hockings, N.; Ajayi, A.A.; Reid, J.L. Age and the pharmacokinetics of angiotensin converting enzyme inhibitors enalapril and enalaprilat. Br. J. Clin. Pharmacol. 1986, 21, 341–348. [Google Scholar] [CrossRef]
- Donnelly, R.; Meredith, P.A.; Elliott, H.L.; Reid, J.L. Kinetic-dynamic relations and individual responses to enalapril. Hypertension 1990, 15, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Ríos, J.; Hermida, R.C.; Rodriguez-Fernandez, M. Dosing time optimization of antihypertensive medications by including the circadian rhythm in pharmacokinetic-pharmacodynamic models. PLoS Comput. Biol. 2022, 18, e1010711. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, A.A.; Campbell, B.C.; Kelman, A.W.; Howie, C.; Meredith, P.A.; Reid, J.L. Pharmacodynamics and population pharmacokinetics of enalapril and lisinopril. Int. J. Clin. Pharmacol. Res. 1985, 5, 419–427. [Google Scholar] [PubMed]
- Zapater, P.; Novalbos, J.; Gallego-Sandín, S.; Hernández, F.T.; Abad-Santos, F. Gender differences in angiotensin-converting enzyme (ACE) activity and inhibition by enalaprilat in healthy volunteers. J. Cardiovasc. Pharmacol. 2004, 43, 737–744. [Google Scholar] [CrossRef]
- Claassen, K.; Willmann, S.; Eissing, T.; Preusser, T.; Block, M. A detailed physiologically based model to simulate the pharmacokinetics and hormonal pharmacodynamics of enalapril on the circulating endocrine Renin-Angiotensin-aldosterone system. Front. Physiol. 2013, 4, 4. [Google Scholar] [CrossRef]
- Ramusovic, S.; Laeer, S. An integrated physiology-based model for the interaction of RAA system biomarkers with drugs. J. Cardiovasc. Pharmacol. 2012, 60, 417–428. [Google Scholar] [CrossRef]
- van Nguyen, A.; Zhang, L.; Kagan, L.; Rowland, M.; Mager, D.E. Target Reserve and Turnover Parameters Determine Rightward Shift of Enalaprilat Potency From its Binding Affinity to the Angiotensin Converting Enzyme. J. Pharm. Sci. 2024, 113, 167–175. [Google Scholar] [CrossRef]
- Kechagia, I.-A.; Kalantzi, L.; Dokoumetzidis, A. Extrapolation of enalapril efficacy from adults to children using pharmacokinetic/pharmacodynamic modelling. J. Pharm. Pharmacol. 2015, 67, 1537–1545. [Google Scholar] [CrossRef]
- De Cock, R.F.W.; Piana, C.; Krekels, E.H.J.; Danhof, M.; Allegaert, K.; Knibbe, C.A.J. The role of population PK-PD modelling in paediatric clinical research. Eur. J. Clin. Pharmacol. 2011, 67 (Suppl. 1), 5–16. [Google Scholar] [CrossRef]
- Nussberger, J.; Wuerzner, G.; Jensen, C.; Brunner, H.R. Angiotensin II suppression in humans by the orally active renin inhibitor Aliskiren (SPP100): Comparison with enalapril. Hypertension 2002, 39, E1–E8. [Google Scholar] [CrossRef]
- Juillerat, L.; Nussberger, J.; Ménard, J.; Mooser, V.; Christen, Y.; Waeber, B.; Graf, P.; Brunner, H.R. Determinants of angiotensin II generation during converting enzyme inhibition. Hypertension 1990, 16, 564–572. [Google Scholar] [CrossRef]
- Biollaz, J.; Schelling, J.L.; Des Jacot Combes, B.; Brunner, D.B.; Desponds, G.; Brunner, H.R.; Ulm, E.H.; Hichens, M.; Gomez, H.J. Enalapril maleate and a lysine analogue (MK-521) in normal volunteers; relationship between plasma drug levels and the renin angiotensin system. Br. J. Clin. Pharmacol. 1982, 14, 363–368. [Google Scholar] [CrossRef]
- Seguchi, M.; Nakazawa, M.; Momma, K. Effect of enalapril on infants and children with congestive heart failure. Cardiol. Young 1992, 2, 14–19. [Google Scholar] [CrossRef]
- Burckhardt, B.B.; Tins, J.; Ramusovic, S.; Läer, S. Tailored Assays for Pharmacokinetic and Pharmacodynamic Investigations of Aliskiren and Enalapril in Children: An Application in Serum, Urine, and Saliva. J. Pediatr. Pharmacol. Ther. 2015, 20, 431–452. [Google Scholar] [CrossRef] [PubMed]
- Laeer, S.; Cawello, W.; Burckhardt, B.B.; Ablonczy, L.; Bajcetic, M.; Breur, J.M.P.J.; Dalinghaus, M.; Male, C.; de Wildt, S.N.; Breitkreutz, J.; et al. Enalapril and Enalaprilat Pharmacokinetics in Children with Heart Failure Due to Dilated Cardiomyopathy and Congestive Heart Failure after Administration of an Orodispersible Enalapril Minitablet (LENA-Studies). Pharmaceutics 2022, 14, 1163. [Google Scholar] [CrossRef]
- Suessenbach, F.K. The Renin-Angiotensin-Aldosterone System in the Paediatric Population: Reliable Highly Sensitive Bioanalytical Investigations via Immunoassay and Innovative Multiplex High-resolution Mass Spectrometry. Ph.D. Thesis, Heinrich Heine University Düsseldorf, Düsseldorf, Germany, 2020. [Google Scholar]
- Ayral, G.; Si Abdallah, J.-F.; Magnard, C.; Chauvin, J. A novel method based on unbiased correlations tests for covariate selection in nonlinear mixed effects models: The COSSAC approach. CPT Pharmacometr. Syst. Pharmacol. 2021, 10, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.E.; Karlsson, M.O.; Dunne, A.; Ludden, T.M. Likelihood based approaches to handling data below the quantification limit using NONMEM VI. J. Pharmacokinet. Pharmacodyn. 2008, 35, 401–421. [Google Scholar] [CrossRef]
- Faisal, M.; Cawello, W.; Burckhardt, B.B.; de Hoon, J.; Laer, S. Simultaneous Semi-Mechanistic Population Pharmacokinetic Modeling Analysis of Enalapril and Enalaprilat Serum and Urine Concentrations from Child Appropriate Orodispersible Minitablets. Front. Pediatr. 2019, 7, 281. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, W.; Muscará, M.N.; Martins, A.R.; Moreno, H.; Mendes, G.B.; de Nucci, G. Bioequivalence study of two enalapril maleate tablet formulations in healthy male volunteers. Pharmacokinetic versus pharmacodynamic approach. Eur. J. Clin. Pharmacol. 1996, 50, 399–405. [Google Scholar] [CrossRef]
- Louizos, C.; Yáñez, J.A.; Forrest, M.L.; Davies, N.M. Understanding the hysteresis loop conundrum in pharmacokinetic/pharmacodynamic relationships. J. Pharm. Pharm. Sci. 2014, 17, 34–91. [Google Scholar] [CrossRef]
- Triebel, H.; Castrop, H. The renin angiotensin aldosterone system. Pflügers Arch. 2024, 476, 705–713. [Google Scholar] [CrossRef]
- Broughton Pipkin, F.; Smales, O.R.; O’Callaghan, M. Renin and angiotensin levels in children. Arch. Dis. Child. 1981, 56, 298–302. [Google Scholar] [CrossRef] [PubMed]
- van Acker, K.J.; Scharpé, S.L.; Lynen, P.J.; Amery, A.K. Comparative study of active and inactive plasma renin in healthy infants and adults. J. Clin. Chem. Clin. Biochem. 1983, 21, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Dutertre, J.P.; Billaud, E.M.; Autret, E.; Chantepie, A.; Oliver, I.; Laugier, J. Inhibition of angiotensin converting enzyme with enalapril maleate in infants with congestive heart failure. Br. J. Clin. Pharmacol. 1993, 35, 528–530. [Google Scholar] [CrossRef] [PubMed]
- Kearns, G.L.; Abdel-Rahman, S.M.; Alander, S.W.; Blowey, D.L.; Leeder, J.S.; Kauffman, R.E. Developmental pharmacology--drug disposition, action, and therapy in infants and children. N. Engl. J. Med. 2003, 349, 1157–1167. [Google Scholar] [CrossRef]


| Parameter | Unit | Estimate | Relative Standard Error (%) |
|---|---|---|---|
| ktr | h−1 | 5.31 | 25.7 |
| Mtt | h | 1.46 | 11.7 |
| ka | h−1 | 1.19 | 8.6 |
| CL/F | L/h | 36.39 | 10.2 |
| V1/F | L | 223.71 | 15.3 |
| Q/F | L/h | 6.38 | 13.7 |
| V2/F | L | 108.26 | 27.2 |
| ke0 | h−1 | 0.48 | 21.9 |
| γ | - | 2.02 | 14.9 |
| E0 | - | 0.043 | 39.1 |
| IC50 | µg/L | 30.01 | 27.8 |
| Interindividual variability | |||
| IIV ktr | CV% | 72.89 | 25.6 |
| IIV Mtt | CV% | 33.7 | 24.1 |
| IIV CL/F | CV% | 30.17 | 25.2 |
| IIV V1/F | CV% | 46.61 | 23.6 |
| IIV E0 | CV% | 141.9 | 24.0 |
| IIV IC50 | CV% | 79.35 | 30.4 |
| Correlations | |||
| Correlation V1/F, CL/F | - | 0.84 | 12.5 |
| Residual variability pharmacokinetic model | |||
| Proportional error | - | 0.072 | 12.2 |
| Additive error | µg/L | 0.42 | 8.7 |
| Residual variability pharmacodynamic model | |||
| Proportional error | - | 0.41 | 8.9 |
| Parameter | Unit | Estimate | Relative Standard Error (%) |
|---|---|---|---|
| E0 | - | 0.19 | 8.2 |
| IC50 | µg/L | 1.19 | 17.9 |
| Interindividual variability | |||
| IIV IC50 | CV% | 59.92 | 24.6 |
| Residual variability | |||
| Proportional error | - | 0.37 | 14.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steichert, M.; Cawello, W.; Burckhardt, B.B.; Suessenbach, F.K.; Laeer, S.; on behalf of the LENA Consortium. Angiotensin II/Angiotensin I Ratio as a New Pharmacodynamic Parameter for Population Modelling in Healthy Adults and Children with Heart Failure Treated with Enalapril. Pharmaceutics 2025, 17, 1345. https://doi.org/10.3390/pharmaceutics17101345
Steichert M, Cawello W, Burckhardt BB, Suessenbach FK, Laeer S, on behalf of the LENA Consortium. Angiotensin II/Angiotensin I Ratio as a New Pharmacodynamic Parameter for Population Modelling in Healthy Adults and Children with Heart Failure Treated with Enalapril. Pharmaceutics. 2025; 17(10):1345. https://doi.org/10.3390/pharmaceutics17101345
Chicago/Turabian StyleSteichert, Melina, Willi Cawello, Bjoern B. Burckhardt, Fabian K. Suessenbach, Stephanie Laeer, and on behalf of the LENA Consortium. 2025. "Angiotensin II/Angiotensin I Ratio as a New Pharmacodynamic Parameter for Population Modelling in Healthy Adults and Children with Heart Failure Treated with Enalapril" Pharmaceutics 17, no. 10: 1345. https://doi.org/10.3390/pharmaceutics17101345
APA StyleSteichert, M., Cawello, W., Burckhardt, B. B., Suessenbach, F. K., Laeer, S., & on behalf of the LENA Consortium. (2025). Angiotensin II/Angiotensin I Ratio as a New Pharmacodynamic Parameter for Population Modelling in Healthy Adults and Children with Heart Failure Treated with Enalapril. Pharmaceutics, 17(10), 1345. https://doi.org/10.3390/pharmaceutics17101345






