Escitalopram Dose Optimization During Pregnancy: A PBPK Modeling Approach
Abstract
1. Introduction
2. Materials and Methods
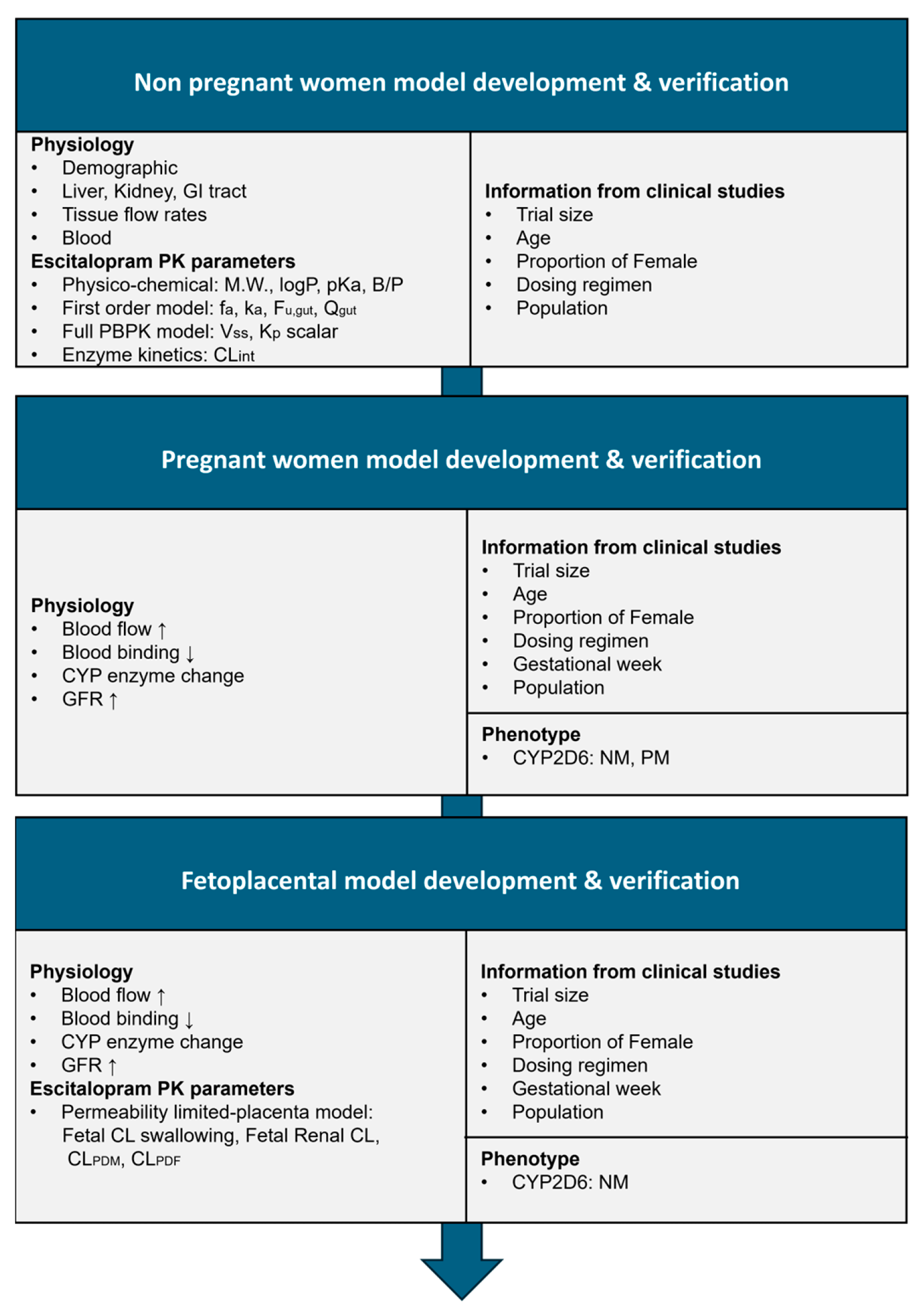
2.1. Software and Workflow
2.2. Development of the Nonpregnant Women Model
2.3. Development of the Pregnant Women Model
2.4. Development of the Fetoplacental Model
2.5. Development of Pregnant Women and Fetoplacental Model Based on CYP2C19 Phenotypes
2.6. Evaluation of the Physiologically Based Pharmacokinetic Model
2.7. Sensitivity Analysis of the Physiologically Based Pharmacokinetic Model
2.8. Dose Optimization Strategy
3. Results
3.1. Development and Verification of the Escitalopram Prediction Model in Nonpregnant Women
3.2. Development and Verification of the Escitalopram Prediction Model in Pregnant Women
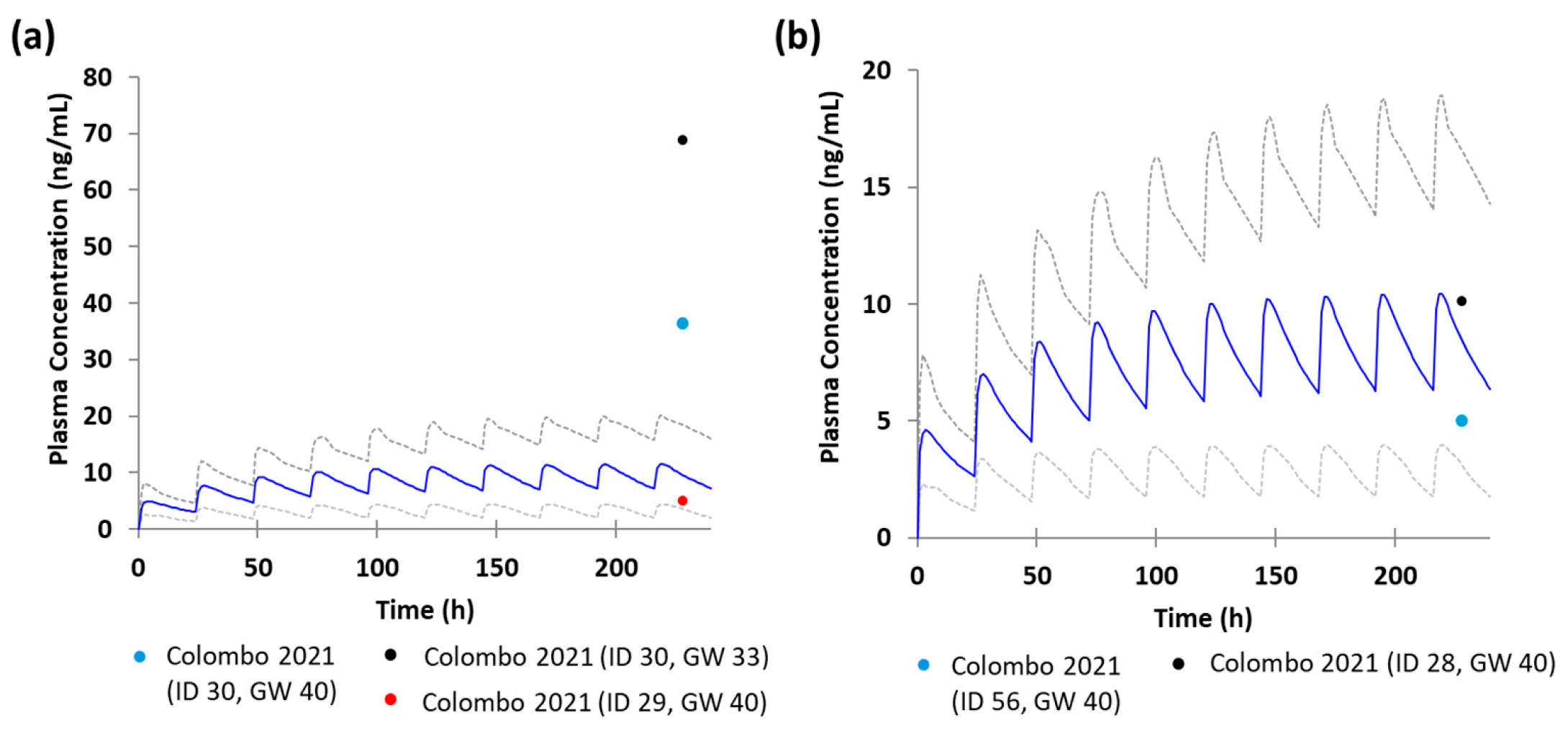
3.3. Development and Verification of the Escitalopram Prediction Model in the Fetoplacental Unit
3.4. Development and Verification of the Escitalopram Prediction Model in Pregnant Women and the Fetoplacental Unit Based on CYP2C19 Phenotypes
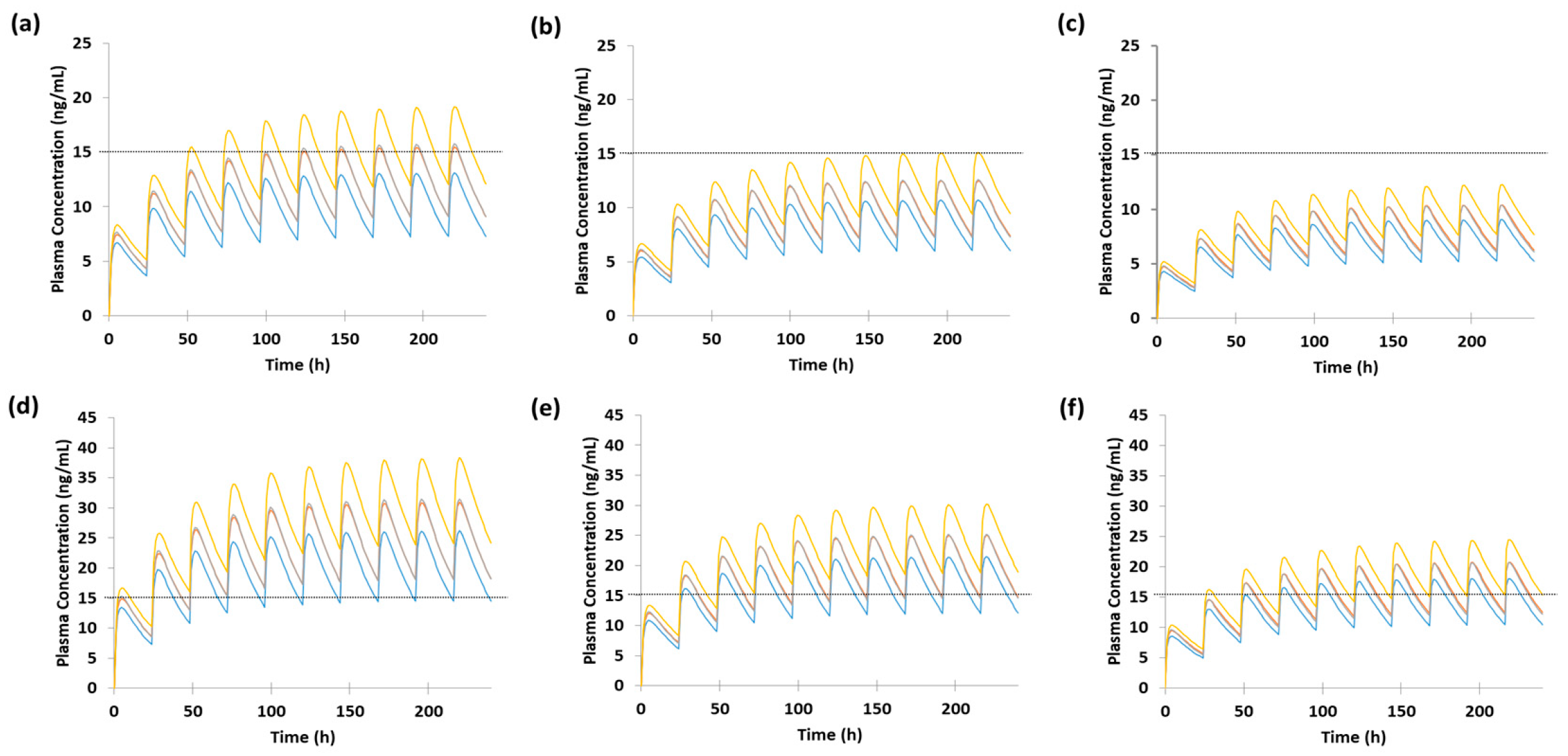
3.5. Dose Optimization of Escitalopram in Pregnant Women Based on Model Predictions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PBPK | Physiologically based pharmacokinetic |
| Cmax | Maximum plasma concentration |
| AUC | Area under the curve |
| B/P | Blood to plasma ratio |
| fa | Fraction absorbed |
| fu,gut | Fraction unbound in gut enterocytes |
| CLR | Renal clearance |
| ka | Absorption rate constant |
| Qgut | Hybrid parameter representing drug absorption rate from the gut lumen, removal of drug from the enterocyte by enterocytic blood supply, and enterocyte volume |
| Kp scalar | Tissue to plasma partition coefficient |
| Vss | Steady state volume of distribution |
| GW | Gestational week |
| CLPDM | Maternal-placental barrier |
| CLPDF | Placental-fetal barrier |
| IM | Intermediate metabolizer |
| NM | Normal metabolizer |
| UM | Ultrarapid metabolizer |
| PM | Poor metabolizer |
References
- Tauqeer, F.; Ceulemans, M.; Gerbier, E.; Passier, A.; Oliver, A.; Foulon, V.; Panchaud, A.; Lupattelli, A.; Nordeng, H. Mental Health of Pregnant and Postpartum Women During the Third Wave of the Covid-19 Pandemic: A European Cross-Sectional Study. BMJ Open 2023, 13, e063391. [Google Scholar] [CrossRef]
- Field, T. Prenatal Depression Risk Factors, Developmental Effects and Interventions: A Review. J. Preg. Child Health 2017, 4, 301. [Google Scholar] [CrossRef]
- Leung, B.M.; Kaplan, B.J. Perinatal Depression: Prevalence, Risks, and the Nutrition Link—A Review of the Literature. J. Am. Diet. Assoc. 2009, 109, 1566–1575. [Google Scholar] [CrossRef]
- Talge, N.M.; Neal, C.; Glover, V. Early Stress, Translational Research and Prevention Science Network: Fetal and Neonatal Experience on Child and Adolescent Mental Health. Antenatal Maternal Stress and Long-Term Effects on Child Neurodevelopment: How and Why? J. Child Psychol. Psychiatry 2007, 48, 245–261. [Google Scholar] [CrossRef] [PubMed]
- Vigod, S.N.; Wilson, C.A.; Howard, L.M. Depression in Pregnancy. BMJ 2016, 352, i1547. [Google Scholar] [CrossRef]
- Jain, A.; Shagana, J.; Dhanraj, M.; Nirosa, T. Physiological Changes in Pregnancy. Drug Interv. Today 2018, 10, 1594–1597. [Google Scholar]
- Abduljalil, K.; Furness, P.; Johnson, T.N.; Rostami-Hodjegan, A.; Soltani, H. Anatomical, Physiological and Metabolic Changes with Gestational Age During Normal Pregnancy: A Database for Parameters Required in Physiologically Based Pharmacokinetic Modelling. Clin. Pharmacokinet 2012, 51, 365–396. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.M.; Williams, O.A.; Magides, A.D.; Reilly, C.S. Gastric emptying is delayed at 8–12 weeks’ gestation. Br. J. Anaesth. 1994, 73, 237–238. [Google Scholar] [CrossRef]
- Mattison, D.R.; Blann, E.; Malek, A. Physiological Alterations During Pregnancy: Impact on Toxicokinetics. Fundam. Appl. Toxicol. 1991, 16, 215–218. [Google Scholar] [CrossRef]
- McGready, R.; Stepniewska, K.; Seaton, E.; Cho, T.; Cho, D.; Ginsberg, A.; Edstein, M.D.; Ashley, E.; Looareesuwan, S.; White, N.J.; et al. Pregnancy and use of oral contraceptives reduces the biotransformation of proguanil to cycloguanil. Eur. J. Clin. Pharmacol. 2003, 59, 553–557. [Google Scholar] [CrossRef]
- Gallitelli, V.; Franco, R.; Guidi, S.; Puri, L.; Parasiliti, M.; Vidiri, A.; Eleftheriou, G.; Perelli, F.; Cavaliere, A.F. Depression Treatment in Pregnancy: Is It Safe, or Is It Not? Int. J. Environ. Res. Public Health 2024, 21, 404. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration Label for Lexapro® (Escitalopram) Tablets, for Oral Use. Available online: https://www.Accessdata.Fda.Gov/Drugsatfda_Docs/Label/2024/021323s058lbl.Pdf (accessed on 18 November 2024).
- Jukić, M.M.; Haslemo, T.; Molden, E.; Ingelman-Sundberg, M. Impact of CYP2C19 Genotype on Escitalopram Exposure and Therapeutic Failure: A Retrospective Study Based on 2,087 Patients. Am. J. Psychiatry 2018, 175, 463–470. [Google Scholar] [CrossRef]
- Stika, C.S.; Avram, M.J.; George Jr, A.L.; Yang, A.; Ciolino, J.D.; Jeong, H.; Venkataramanan, R.; Caritis, S.N.; Costantine, M.M.; Wisner, K.L. Changes in S-Citalopram Plasma Concentrations across Pregnancy and Postpartum. Clin. Pharmacol. 2025, 118, 106–117. [Google Scholar] [CrossRef]
- Bérard, A.; Sheehy, O.; Zhao, J.; Vinet, É.; Bernatsky, S.; Abrahamowicz, M. SSRI and SNRI use during pregnancy and the risk of persistent pulmonary hypertension of the newborn. Br. J. Clin. Pharmacol. 2017, 83, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Abbiati, R.A.; Manca, D. A modeling tool for the personalization of pharmacokinetic predictions. Comput. Chem. Eng. 2016, 91, 28–37. [Google Scholar] [CrossRef]
- Jogiraju, V.K.; Avvari, S.; Gollen, R.; Taft, D.R. Application of physiologically based pharmacokinetic modeling to predict drug disposition in pregnant populations. Biopharm. Drug Dispos. 2017, 38, 426–438. [Google Scholar] [CrossRef]
- Jang, Y.J.; Kim, D.K.; Lim, S.; Hong, E. Impact of CYP2C19 Phenotype on Escitalopram Response in Geriatrics: Based on Physiologically-Based Pharmacokinetic Modeling and Clinical Observation. Clin. Pharmacol. Ther. 2024, 117, 826–835. [Google Scholar] [CrossRef]
- Anderson, K.N.; Lind, J.N.; Simeone, R.M.; Bobo, W.V.; Mitchell, A.A.; Riehle-Colarusso, T.; Polen, K.N.; Reefhuis, J. Maternal Use of Specific Antidepressant Medications During Early Pregnancy and the Risk of Selected Birth Defects. JAMA Psychiatry 2020, 77, 1246–1255. [Google Scholar] [CrossRef]
- Rampono, J.; Simmer, K.; Ilett, K.F.; Hackett, L.P.; Doherty, D.A.; Elliot, R.; Kok, C.H.; Coenen, A.; Forman, T. Placental Transfer of SSRI and SNRI Antidepressants and Effects on the Neonate. Pharmacopsychiatry 2009, 42, 95–100. [Google Scholar] [CrossRef]
- Préta, L.H.; Bouazza, N.; Foissac, F.; Froelicher, L.; Urien, S.; Dauvilliers, A.; Dinnall, A.; Buth, V.; Benaboud, S.; Tréluyer, J.M.; et al. Comparison of human transplacental transfer of escitalopram, sertraline and paroxetine: An ex vivo cotyledon perfusion study. Placenta 2025, 168, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.; Giordano, F.; Giorgetti, F.; Di Bernardo, I.; Bosi, M.F.; Varinelli, A.; Cafaro, R.; Pileri, P.; Cetin, I.; Clementi, E.; et al. Correlation between pharmacokinetics and pharmacogenetics of Selective Serotonin Reuptake Inhibitors and Selective Serotonin and Noradrenaline Reuptake Inhibitors and maternal and neonatal outcomes: Results from a naturalistic study in patients with affective disorders. Hum. Psychopharmacol. Clin. Exp. 2020, 36, e2772. [Google Scholar] [CrossRef]
- Rao, N. The Clinical Pharmacokinetics of Escitalopram. Clin. Pharmacokinet. 2007, 46, 281–290. [Google Scholar] [CrossRef]
- Sit, D.; Perel, J.M.; Wisniewski, S.R.; Helsel, J.C.; Luther, J.F.; Wisner, K.L. Mother-Infant Antidepressant Concentrations, Maternal Depression, and Perinatal Events. J. Clin. Psychiatry 2011, 72, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Sit, D.K.; Perel, J.M.; Helsel, J.C.; Wisner, K.L. Changes in Antidepressant Metabolism and Dosing Across Pregnancy and Early Postpartum. J. Clin. Psychiatry 2008, 69, 652–658. [Google Scholar] [CrossRef]
- Søgaard, B.; Mengel, H.; Rao, N.; Larsen, F. The Pharmacokinetics of Escitalopram After Oral and Intravenous Administration of Single and Multiple Doses to Healthy Subjects. J. Clin. Pharmacol. 2005, 45, 1400–1406. [Google Scholar] [CrossRef]
- Overø, K.F. Kinetics of citalopram in man; plasma levels in patients. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 1982, 6, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Steere, B.; Baker, J.A.R.; Hall, S.D.; Guo, Y. Prediction of In Vivo Clearance and Associated Variability of CYP2C19 Substrates by Genotypes in Populations Utilizing a Pharmacogenetics-Based Mechanistic Model. Drug Metab. Dispos. 2015, 43, 870–883. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Sharma, P.; Yeo, K.R.; Higashimori, M.; Xu, H.; Al-Huniti, N.; Zhou, D. Assessing pharmacokinetic differences in Caucasian and East Asian (Japanese, Chinese and Korean) populations driven by CYP2C19 polymorphism using physiologically-based pharmacokinetic modelling. Eur. J. Pharm. Sci. 2019, 139, 105061. [Google Scholar] [CrossRef]
- Hoogdalem, M.W.; Tanaka, R.; Abduljalil, K.; Johnson, T.N.; Wexelblatt, S.L.; Akinbi, H.T.; Vinks, A.A.; Mizuno, T. Forecasting Fetal Buprenorphine Exposure through Maternal–Fetal Physiologically Based Pharmacokinetic Modeling. Pharmaceutics 2024, 16, 375. [Google Scholar] [CrossRef]
- Romão, M.A.W.; Pinto, L.; Cavalli, R.C.; Duarte, G.; de Moraes, N.V.; Abduljalil, K.; Moreira, F.d.L. Mechanistic Framework to Predict Maternal-Placental-Fetal Pharmacokinetics of Nifedipine Employing Physiologically Based Pharmacokinetic Modeling Approach. J. Clin. Pharmacol. 2024, 64, 568–577. [Google Scholar] [CrossRef]
- Blackburn, S.T. Maternal, Fetal, & Neonatal Physiology: A Clinical Perspective; Elsevier Health Sciences: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Johnsen, S.L.; Rasmussen, S.; Wilsgaard, T.; Sollien, R.; Kiserud, T. Longitudinal reference ranges for estimated fetal weight. Acta Obstet. Et Gynecol. Scand. 2006, 85, 286–297. [Google Scholar] [CrossRef]
- Ezuruike, U.; Blenkinsop, A.; Pansari, A.; Abduljalil, K. Quantification of Fetal Renal Function Using Fetal Urine Production Rate and Its Reflection on the Amniotic and Fetal Creatinine Levels During Pregnancy. Front. Pediatr. 2022, 10, 841495. [Google Scholar] [CrossRef]
- Rhodin, M.M.; Anderson, B.J.; Peters, A.M.; Coulthard, M.G.; Wilkins, B.; Cole, M.; Chatelut, E.; Grubb, A.; Veal, G.J.; Keir, M.J.; et al. Human renal function maturation: A quantitative description using weight and postmenstrual age. Pediatr. Nephrol. 2009, 24, 67–76. [Google Scholar] [CrossRef]
- Jeong, H.-C.; Kim, M.-G.; Wei, Z.; Lee, K.-R.; Lee, J.; Song, I.-S.; Shin, K.-H. Integration of a Physiologically Based Pharmacokinetic and Pharmacodynamic Model for Tegoprazan and Its Metabolite: Application for Predicting Food Effect and Intragastric pH Alterations. Pharmaceutics 2022, 14, 1298. [Google Scholar] [CrossRef]
- Thompson, E.J.; Jeong, A.; Helfer, V.E.; Shakhnovich, V.; Edginton, A.; Balevic, S.J.; James, L.P.; Collier, D.N.; Anand, R.; Gonzalez, D.; et al. Physiologically-based pharmacokinetic modeling of pantoprazole to evaluate the role of CYP2C19 genetic variation and obesity in the pediatric population. CPT: Pharmacometrics Syst. Pharmacol. 2024, 13, 1394–1408. [Google Scholar] [CrossRef]
- Liu, D.; Li, L.; Rostami-Hodjegan, A.; Bois, F.Y.; Jamei, M. Considerations and Caveats when Applying Global Sensitivity Analysis Methods to Physiologically Based Pharmacokinetic Models. AAPS J. 2020, 22, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hiemke, C.; Bergemann, N.; Clement, H.; Conca, A.; Deckert, J.; Domschke, K.; Eckermann, G.; Egberts, K.; Gerlach, M.; Greiner, C.; et al. Consensus Guidelines for Therapeutic Drug Monitoring in Neuropsychopharmacology: Update 2017. Pharmacopsychiatry 2018, 51, 9–62. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, R.; Tateno, A.; Kim, W.; Sakayori, T.; Ogawa, K.; Okubo, Y. Time-course of serotonin transporter occupancy by single dose of three SSRIs in human brain: A positron emission tomography study with [11C]DASB. Psychiatry Res. Neuroimaging 2016, 251, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Howes, O.D.; Kim, B.H.; Chon, M.W.; Seo, S.; Turkheimer, F.E.; Lee, J.S.; Lee, Y.S.; Kwon, J.S. Regional Differences in Serotonin Transporter Occupancy by Escitalopram: An [11C]DASB PK-PD Study. Clin Pharmacokinet. 2017, 56, 371–381. [Google Scholar] [CrossRef]
- Lanzenberger, R.; Kranz, G.S.; Haeusler, D.; Akimova, E.; Savli, M.; Hahn, A.; Mitterhauser, M.; Spindelegger, C.; Philippe, C.; Fink, M.; et al. Prediction of SSRI treatment response in major depression based on serotonin transporter interplay between median raphe nucleus and projection areas. NeuroImage 2012, 63, 874–881. [Google Scholar] [CrossRef]
- Heikkinen, T.; Ekblad, U.; Kero, P.; Ekblad, S.; Laine, K. Citalopram in Pregnancy and Lactation. Clin. Pharmacol. Ther. 2002, 72, 184–191. [Google Scholar] [CrossRef]
- Kumar, B.J.; Mahalingam, V.T.; M, G.K. The impact of CYP2C19 genotypes on steady-state plasma concentration of escitalopram in South Indian population with Major Depressive Disorder. Pharmacia 2024, 71, 1–6. [Google Scholar] [CrossRef]
- Tsuchimine, S.; Ochi, S.; Tajiri, M.; Suzuki, Y.; Sugawara, N.; Inoue, Y.B.; Yasui-Furukori, N. Effects of Cytochrome P450 (CYP) 2C19 Genotypes on Steady-State Plasma Concentrations of Escitalopram and its Desmethyl Metabolite in Japanese Patients With Depression. Ther. Drug Monit. 2018, 40, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, T. PM Frequencies of Major CYPs in Asians and Caucasians. Drug Metab. Rev. 2003, 35, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Ewing, G.; Tatarchuk, Y.; Appleby, D.; Schwartz, N.; Kim, D. Placental Transfer of Antidepressant Medications: Implications for Postnatal Adaptation Syndrome. Clin. Pharmacokinet. 2015, 54, 359–370. [Google Scholar] [CrossRef]
- Loebstein, R.; Lalkin, A.; Koren, G. Pharmacokinetic Changes During Pregnancy and Their Clinical Relevance. Clin. Pharmacokinet. 1997, 33, 328–343. [Google Scholar] [CrossRef]

| Parameters | Values | References |
|---|---|---|
| Mol. weight (g/mol) | 324.39 | - |
| LogPo:w | 1.34 | Drugbank online Escitalopram |
| Compound type | Monoprotic Base | - |
| pKa | 9.5 | Drugbank online |
| B/P | 2.0 | [27] |
| fup | 0.44 | [29] |
| Absorption model | First-order model | - |
| fa | 1.0 | [29] |
| ka, (1/h) | 0.19 | Parameter estimation |
| fugut | 1.0 | [28] |
| Qgut | 5.69 | Parameter estimation |
| Distribution model | Full PBPK model | - |
| VSS (L/kg) | 13.513 | Predicted by the Simcyp® simulator |
| Kp Scalar | 0.92 | Parameter estimation |
| Enzyme kinetics parameters | CLint (μL/min/pmol of isoform): CYP2C19: 0.774 CYP2D6: 0.505 CYP3A4: 0.0155 | [29] |
| CLR(L/h) | 4.0 | [29] |
| Transport (Permeability Ltd. organ) | Permeability limited- placenta model | - |
| Fetal CL swallowing (L/h/kg fetal weight) | 0.00844 | Calculated by Equation (2) (see methods in Section 2.4) |
| Fetal CLR (L/h/kg fetal weight) | 0.044 | Calculated by Equation (3) (see methods in Section 2.4) |
| CLPDM and CLPDF | 0.80902 | Predicted by the Simcyp® simulator |
| Dose (mg) | Gestational Week | Predicted Escitalopram Parameters | ||
|---|---|---|---|---|
| Cmax (ng/mL) | Tmax (h) | AUC (ng·h/mL) | ||
| 10 | 0 | 18.38 (-) | 4.00 | 364.05 (-) |
| 20 | 13.70 (↓25.46%) | 3.96 | 268.98 (↓26.11%) | |
| 35 | 10.46 (↓43.09%) | 3.27 | 204.14 (↓43.92%) | |
| 20 | 0 | 36.76 (-) | 4.01 | 728.11 (-) |
| 20 | 27.39 (↓25.49%) | 3.97 | 537.97 (↓26.11%) | |
| 35 | 20.93 (↓43.06%) | 3.27 | 408.28 (↓43.93%) | |
| CYP2C19 Phenotype | Gestational Week | Predicted Escitalopram Parameters | ||
|---|---|---|---|---|
| Cmax (ng/mL) | Tmax (h) | AUC (ng·h/mL) | ||
| IM | 35 | 11.57 | 3.31 | 227.88 |
| NM | 40 | 10.52 | 3.06 | 202.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.-Y.; Yang, E.; Shin, K.-H. Escitalopram Dose Optimization During Pregnancy: A PBPK Modeling Approach. Pharmaceutics 2025, 17, 1341. https://doi.org/10.3390/pharmaceutics17101341
Choi S-Y, Yang E, Shin K-H. Escitalopram Dose Optimization During Pregnancy: A PBPK Modeling Approach. Pharmaceutics. 2025; 17(10):1341. https://doi.org/10.3390/pharmaceutics17101341
Chicago/Turabian StyleChoi, Seo-Yeon, Eunsol Yang, and Kwang-Hee Shin. 2025. "Escitalopram Dose Optimization During Pregnancy: A PBPK Modeling Approach" Pharmaceutics 17, no. 10: 1341. https://doi.org/10.3390/pharmaceutics17101341
APA StyleChoi, S.-Y., Yang, E., & Shin, K.-H. (2025). Escitalopram Dose Optimization During Pregnancy: A PBPK Modeling Approach. Pharmaceutics, 17(10), 1341. https://doi.org/10.3390/pharmaceutics17101341







