Therapeutic and Formulation Innovations in the Management of Canine Otitis Externa
Abstract
1. Introduction
2. Basic Anatomy of the Canine External Ear
3. Canine Otitis Externa
3.1. Diagnosis of Canine Otitis Externa
3.2. Medical Management of Canine Otitis Externa
3.3. Alternative and Novel Therapies for the Treatment of Canine Otitis Externa
4. Considerations for Otic Formulation Development
4.1. Physicochemical Properties of Active Ingredients
4.2. Safety Profiles
4.3. Osmotic Pressure
4.4. Bioadhesion
4.5. Viscosity
4.6. Formulation pH
4.7. Application Devices
5. Future Trends of COE Treatment
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singh, A.T.S.; Das, G.; Gupta, D.K.; Mishra, A.; Dawar, P. Epidemiological studies on otitis externa in dogs. Int. J. Adv. Biochem. Res. 2024, 8, 48–52. [Google Scholar] [CrossRef]
- Kumar, S.; Hussain, K.; Sharma, R.; Chhibber, S.; Sharma, N. Prevalence of Canine Otitis Externa in Jammu. J. Anim. Res. 2014, 4, 121–129. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Centenaro, S.; Beribe, F.; Laus, F.; Cerquetella, M.; Spaterna, A.; Guidetti, G.; Canello, S.; Terrazzano, G. Clinical evaluation of an antiinflammatory and antioxidant diet effect in 30 dogs affected by chronic otitis externa: Preliminary results. Vet. Res. Commun. 2016, 40, 29–38. [Google Scholar] [CrossRef]
- Perry, L.R.; MacLennan, B.; Korven, R.; Rawlings, T.A. Epidemiological study of dogs with otitis externa in Cape Breton, Nova Scotia. Can. Vet. J. 2017, 58, 168–174. [Google Scholar]
- Mittal, A.; Kumar, S. Role of pH of External Auditory Canal in Acute Otitis Externa. Indian J. Otolaryngol. Head Neck Surg. 2014, 66, 86–91. [Google Scholar] [CrossRef]
- Bajwa, J. Canine otitis externa—Treatment and complications. Can. Vet. J. 2019, 60, 97–99. [Google Scholar]
- Forsythe, P.J. Acute otitis externa: The successful first-opinion ear consultation. In Pract. 2016, 38, 2–6. [Google Scholar] [CrossRef]
- Nuttall, T. Successful management of otitis externa. In Pract. 2016, 38, 17–21. [Google Scholar] [CrossRef]
- Strain, G.M.; Merchant, S.R.; Neer, T.M.; Tedford, B.L. Ototoxicity assessment of a gentamicin sulfate otic preparation in dogs. Am. J. Vet. Res. 1995, 56, 532–538. [Google Scholar] [CrossRef]
- Merchant, S.R.; Neer, T.M.; Tedford, B.; Twedt, A.C.; Cheramine, P.M. Ototoxicity assessment of a chlorhexidine otic preparation in dogs. Prog. Vet. Neurol. 2015, 4, 72–75. [Google Scholar]
- Rosychuk, R.A.W. Otitis externa: A quick guide to management. In Proceedings of the Central Veterinary Conference (CVC), Kansas City, MO, USA, 27–30 August 2005. [Google Scholar]
- Kim, S.-H.; Kim, S.; Jun, H.K.; Kim, D.-H. Efficacy of aromatherapy for the treatment of otitis externa in dogs. Korean J. Vet. Res. 2009, 49, 85–89. [Google Scholar]
- Hawkins, C.; Harper, D.; Burch, D.; Anggard, E.; Soothill, J. Topical treatment of Pseudomonas aeruginosa otitis of dogs with a bacteriophage mixture: A before/after clinical trial. Vet. Microbiol. 2010, 146, 309–313. [Google Scholar] [CrossRef]
- Shin, J.-C.; Kim, S.-H.; Park, H.-J.; Seo, K.-W.; Song, K.-H. Effect of Aromatherapy and Apipuncture on Malassezia-related Otitis Externa in Dogs. J. Vet. Clin. 2012, 29, 470–473. [Google Scholar]
- Nuttall, T.; Cole, L.K. Evidence-based veterinary dermatology: A systematic review of interventions for treatment of Pseudomonas otitis in dogs. Vet. Dermatol. 2007, 18, 69–77. [Google Scholar] [CrossRef]
- Liu, X.; Li, M.; Smyth, H.; Zhang, F. Otic drug delivery systems: Formulation principles and recent developments. Drug Dev. Ind. Pharm. 2018, 44, 1395–1408. [Google Scholar] [CrossRef]
- Seo, M.; Oh, T.; Bae, S. Antibiofilm activity of silver nanoparticles against biofilm forming Staphylococcus pseudintermedius isolated from dogs with otitis externa. Vet. Med. Sci. 2021, 7, 1551–1557. [Google Scholar] [CrossRef]
- Ghibaudo, G. Ear Anatomy. In Manual to Veterinary Video-Oto-Endoscopy: Use and Utility in Canine and Feline Ear Diseases; Ghibaudo, G., Ed.; Springer International Publishing: Cham, Germany, 2022; pp. 29–54. [Google Scholar]
- Cole, L. Anatomy and physiology of the canine ear. Vet. Dermatol. 2009, 20, 412–421, Corrected in Vet. Dermatol. 2010, 21, 221–231. [Google Scholar] [CrossRef]
- Harvey, R.G.; Paterson, S. Otitis Externa: An Essential Guide to Diagnosis and Treatment; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- O’Neill, D.G.; Volk, A.V.; Soares, T.; Church, D.B.; Brodbelt, D.C.; Pegram, C. Frequency and predisposing factors for canine otitis externa in the UK—A primary veterinary care epidemiological view. Canine Med. Genet. 2021, 8, 7. [Google Scholar] [CrossRef]
- Tesin, N.; Stojanovic, D.; Stancic, I.; Kladar, N.; Ruzic, Z.; Spasojevic, J.; Tomanic, D.; Kovacevic, Z. Prevalence of the microbiological causes of canine otitis externa and the antibiotic susceptibility of the isolated bacterial strains. Pol. J. Vet. Sci. 2023, 26, 449–459. [Google Scholar] [CrossRef]
- Song, Y.; Abdella, S.; Afinjuomo, F.; Weir, E.J.; Tan, J.Q.E.; Hill, P.; Page, S.W.; Garg, S. Physicochemical properties of otic products for Canine Otitis Externa: Comparative analysis of marketed products. BMC Vet. Res. 2023, 19, 39. [Google Scholar] [CrossRef]
- Paterson, S. Discovering the causes of otitis externa. In Pract. 2016, 38, 7–11. [Google Scholar] [CrossRef]
- Fernández, G.; Barboza, G.; Villalobos, A.; Parra, O.; Finol, G.; Ramirez, R.A. Isolation and identification of microorganisms present in 53 dogs suffering otitis externa. Rev. Cient. 2006, 16, 23–30. [Google Scholar]
- Kiss, G.; Radvanyi, S.Z.; Szigeti, G. New combination for the therapy of canine otitis externa. I. Microbiology of otitis externa. J. Small Anim. Pract. 1997, 38, 51–56. [Google Scholar] [CrossRef]
- Manju, R.; Roshan, K.; Suhsovan, R. Prevalence of Canine Otitis Externa, Etiology and Clinical Practice in and around Durg District of Chhattisgarh State, India. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 269–274. [Google Scholar] [CrossRef]
- De Martino, L.; Nocera, F.P.; Mallardo, K.; Nizza, S.; Masturzo, E.; Fiorito, F.; Iovane, G.; Catalanotti, P. An update on microbiological causes of canine otitis externa in Campania Region, Italy. Asia Pac. J. Trop. Biomed. 2016, 6, 384–389. [Google Scholar] [CrossRef]
- Nuttall, T. Managing recurrent otitis externa in dogs: What have we learned and what can we do better? J. Am. Vet. Med. Assoc. 2023, 261, S10–S22. [Google Scholar] [CrossRef]
- Jacobson, L.S. Diagnosis and medical treatment of otitis externa in the dog and cat. J. S. Afr. Vet. Assoc. 2002, 73, 162–170. [Google Scholar] [CrossRef]
- Bond, R. Self assessment test: Selecting ear drops for dogs with otitis externa. In Pract. 2012, 34, 392–399. [Google Scholar] [CrossRef]
- Cohen, M.; Bohling, M.W.; Wright, J.C.; Welles, E.A.; Spano, J.S. Evaluation of sensitivity and specificity of cytologic examination: 269 cases (1999-2000). J. Am. Vet. Med. Assoc. 2003, 222, 964–967. [Google Scholar] [CrossRef]
- Pinto, D.; Cruz, E.; Branco, D.; Linares, C.; Carvalho, C.; Silva, A.; Chorão, M.; Schmitt, F. Cytohistological Correlation in Pleural Effusions Based on the International System for Reporting Serous Fluid Cytopathology. Diagnostics 2021, 11, 1126. [Google Scholar] [CrossRef]
- Koch, S.N.; Torres, S.M.F.; Plumb, D.C. Topical Agents. In Canine and Feline Dermatology Drug Handbook; Wiley-Blackwell: Ames, IA, USA, 2012; pp. 219–393. [Google Scholar]
- Forsythe, P.; Paterson, S. Ciclosporin 10 years on: Indications and efficacy. Vet. Rec. 2014, 174, 13–21. [Google Scholar] [CrossRef]
- Cosgrove, S.B.; Cleaver, D.M.; King, V.L.; Gilmer, A.R.; Daniels, A.E.; Wren, J.A.; Stegemann, M.R. Long-term compassionate use of oclacitinib in dogs with atopic and allergic skin disease: Safety, efficacy and quality of life. Vet. Dermatol. 2015, 26, 171–179. [Google Scholar] [CrossRef]
- Nuttall, T.J.; Marsella, R.; Rosenbaum, M.R.; Gonzales, A.J.; Fadok, V.A. Update on pathogenesis, diagnosis, and treatment of atopic dermatitis in dogs. J. Am. Vet. Med. Assoc. 2019, 254, 1291–1300. [Google Scholar] [CrossRef]
- Marsella, R.; Doerr, K.; Gonzales, A.; Rosenkrantz, W.; Schissler, J.; White, A. Oclacitinib 10 years later: Lessons learned and directions for the future. J. Am. Vet. Med. Assoc. 2023, 261, S36–S47. [Google Scholar] [CrossRef] [PubMed]
- Drechsler, Y.; Dong, C.; Clark, D.E.; Kaur, G. Canine Atopic Dermatitis: Prevalence, Impact, and Management Strategies. Vet. Med. 2024, 15, 15–29. [Google Scholar] [CrossRef]
- Panel, A.I.D.A. Antibiotic Prescribing Detailed Guidelines, 2nd ed.; Australasian Infectious Diseases Advisory Panel: Sydney, Australia, 2022; pp. 103–108. [Google Scholar]
- Brissot, H.; Cervantes, S.; Guardabassi, L.; Hibbert, A.; Lefebvre, H.; Mateus, A.; Noli, C.; Nuttall, T.; Pomba, C.; Schulz, B. GRAM Experts Guidance for the Rational use of Antimicrobials—Recommendations for Dogs and Cats; Ceva Santé Animale: Libourne, France, 2016; pp. 146–151. [Google Scholar]
- Sim, J.X.F.; Khazandi, M.; Chan, W.Y.; Trott, D.J.; Deo, P. Antimicrobial activity of thyme oil, oregano oil, thymol and carvacrol against sensitive and resistant microbial isolates from dogs with otitis externa. Vet. Dermatol. 2019, 30, 524-e159. [Google Scholar] [CrossRef]
- Roh, D.-H.; Kwon, Y.-B.; Kim, H.-W.; Ham, T.-W.; Yoon, S.-Y.; Kang, S.-Y.; Han, H.-J.; Lee, H.-J.; Beitz, A.J.; Lee, J.-H. Acupoint stimulation with diluted bee venom (apipuncture) alleviates thermal hyperalgesia in a rodent neuropathic pain model: Involvement of spinal alpha2-adrenoceptors. J. Pain 2004, 5, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, H.; Coles, M.; Poole, D.; Lund, L.; Page, R. Update on antimicrobial susceptibilities of bacterial isolates from canine and feline otitis externa. Can. Vet. J. 2006, 47, 253–255. [Google Scholar]
- Zou, J.; Feng, H.; Mannerström, M.; Heinonen, T.; Pyykkö, I. Toxicity of silver nanoparticle in rat ear and BALB/c 3T3 cell line. J. Nanobiotechnol. 2014, 12, 52. [Google Scholar] [CrossRef] [PubMed]
- Zahra, G.; Esmaeil, K.; Mohammad, F.; Rashidy-Pour, A.; Mahdi, M.; Mahdi, A.; Ali, K. Combined effects of the exposure to silver nanoparticles and noise on hearing function and cochlea structure of the male rats. Life Sci. 2022, 304, 120724. [Google Scholar] [CrossRef]
- Barbara, M.; Margani, V.; Covelli, E.; Filippi, C.; Volpini, L.; El-Borady, O.M.; El-Kemary, M.; Elzayat, S.; Elfarargy, H.H. The use of nanoparticles in otoprotection. Front. Neurol. 2022, 13, 912647. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, R.M.; Schwartz, S.R.; Cannon, C.R.; Roland, P.S.; Simon, G.R.; Kumar, K.A.; Huang, W.W.; Haskell, H.W.; Robertson, P.J. Clinical Practice Guideline: Acute Otitis Externa. Otolaryngol. Head Neck Surg. 2014, 150, S1–S24. [Google Scholar] [CrossRef] [PubMed]
- Administration, F.a.D.; Medicine, C.f.V. Infectious Otitis Externa Drugs for Topical Use in Dogs Guidance for Industry; Center for Veterinary Medicine: Laurel, MD, USA, 2023. [Google Scholar]
- Iliopoulos, F.; Sil, B.C.; Evans, C.L. The role of excipients in promoting topical and transdermal delivery: Current limitations and future perspectives. Front. Drug Deliv. 2022, 2, 1049848. [Google Scholar] [CrossRef]
- Medicine, F.C.f.V. FDA Approves Treatment for Yeast Ear Infections in Dogs. Available online: https://www.fda.gov/animal-veterinary/cvm-updates/fda-approves-treatment-yeast-ear-infections-dogs (accessed on 11 June 2024).
- Authority, A.P.a.V.M. New Veterinary Chemical Product Containing a New Veterinary Active Constituent:Mometamax Ultra Ear Drops Suspension for Dogs Containing Posaconazole; Australian Pesticides and Veterinary Medicines Authority: Canberra, Australia, 2023; pp. 12–17. [Google Scholar]
- Roberts, M.S.; Cheruvu, H.S.; Mangion, S.E.; Alinaghi, A.; Benson, H.A.E.; Mohammed, Y.; Holmes, A.; van der Hoek, J.; Pastore, M.; Grice, J.E. Topical drug delivery: History, percutaneous absorption, and product development. Adv. Drug Deliv. Rev. 2021, 177, 113929. [Google Scholar] [CrossRef]
- Waters, L.J.; Bhuiyan, A.K.M.M.H. Ionisation effects on the permeation of pharmaceutical compounds through silicone membrane. Colloids Surf. B Biointerfaces 2016, 141, 553–557. [Google Scholar] [CrossRef]
- Baba, H.; Ueno, Y.; Hashida, M.; Yamashita, F. Quantitative prediction of ionization effect on human skin permeability. Int. J. Pharm. 2017, 522, 222–233. [Google Scholar] [CrossRef]
- National Library of Medicine-PubChem. 2024. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 9 September 2025).
- Redgrave, L.S.; Sutton, S.B.; Webber, M.A.; Piddock, L.J.V. Fluoroquinolone resistance: Mechanisms, impact on bacteria, and role in evolutionary success. Trends Microbiol. 2014, 22, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Ihrke, P.J.; Papich, M.G.; Demanuelle, T.C. The use of fluoroquinolones in veterinary dermatology. Vet. Dermatol. 1999, 10, 193–204. [Google Scholar] [CrossRef]
- Steyger, P.S.; Cunningham, L.L.; Esquivel, C.R.; Watts, K.L.; Zuo, J. Editorial: Cellular Mechanisms of Ototoxicity. Front. Cell. Neurosci. 2018, 12, 75. [Google Scholar] [CrossRef]
- Hao, J.; Li, S.K. Inner ear drug delivery: Recent advances, challenges, and perspective. Eur. J. Pharm. Sci. 2019, 126, 82–92. [Google Scholar] [CrossRef]
- Rapalli, V.K.; Singhvi, G. Dermato-pharmacokinetic: Assessment tools for topically applied dosage forms. Expert Opin. Drug Deliv. 2021, 18, 423–426. [Google Scholar] [CrossRef]
- Wani, T.U.; Mohi-ud-Din, R.; Majeed, A.; Kawoosa, S.; Pottoo, F.H. Skin Permeation of Nanoparticles: Mechanisms Involved and Critical Factors Governing Topical Drug Delivery. Curr. Pharm. Des. 2020, 26, 4601–4614. [Google Scholar] [CrossRef] [PubMed]
- Cevc, G.; Blume, G. Lipid Vesicles Penetrate into Intact Skin Owing to the Transdermal Osmotic Gradients and Hydration Force. Biochim. Biophys. Acta 1992, 1104, 226–232. [Google Scholar] [CrossRef]
- Björklund, S.; Engblom, J.; Thuresson, K.; Sparr, E. A water gradient can be used to regulate drug transport across skin. J. Control. Release 2010, 143, 191–200. [Google Scholar] [CrossRef]
- Brahmbhatt, D. Bioadhesive drug delivery systems: Overview and recent advances. Int. J. Chem. Life Sci. 2017, 6, 2016–2024. [Google Scholar] [CrossRef]
- Singh, J.; Nayak, P. pH-responsive polymers for drug delivery: Trends and opportunities. J. Polym. Sci. 2023, 61, 2828–2850. [Google Scholar] [CrossRef]
- Lim, C.; Lee, D.W.; Israelachvili, J.N.; Jho, Y.; Hwang, D.S. Contact time- and pH-dependent adhesion and cohesion of low molecular weight chitosan coated surfaces. Carbohydr. Polym. 2015, 117, 887–894. [Google Scholar] [CrossRef]
- Secker, B. Developing Bacteriophage and the Predatory Bacterium Bdellovibrio Bacteriovorus As an Alternative Therapy for Canine Otitis Externa. Ph.D. Thesis, University of Nottingham, Nottingham, UK, 2004. [Google Scholar]
- Houtsaeger, C.; Pasmans, F.; Claes, I.; Vandenabeele, S.; Haesebrouck, F.; Lebeer, S.; Boyen, F. The role of the microbiome in allergic dermatitis-related otitis externa: A multi-species comparative review. Front. Vet. Sci. 2024, 11, 1413684. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.C.; Calixto, G.; Hatakeyama, I.N.; Luz, G.M.; Gremião, M.P.D.; Chorilli, M. Rheological, mechanical, and bioadhesive behavior of hydrogels to optimize skin delivery systems. Drug Dev. Ind. Pharm. 2013, 39, 1750–1757. [Google Scholar] [CrossRef] [PubMed]
- Deuschle, V.C.K.N.; Deuschle, R.A.N.; Bortoluzzi, M.R.; Athayde, M.L. Physical chemistry evaluation of stability, spreadability, in vitro antioxidant, and photo-protective capacities of topical formulations containing Calendula officinalis L. leaf extract. Braz. J. Pharm. Sci. 2015, 51, 63–75. [Google Scholar] [CrossRef]
- Donthi, M.R.; Munnangi, S.R.; Krishna, K.V.; Saha, R.N.; Singhvi, G.; Dubey, S.K. Nanoemulgel: A Novel Nano Carrier as a Tool for Topical Drug Delivery. Pharmaceutics 2023, 15, 164. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, Y.H.; Namjoshi, S.N.; Telaprolu, K.C.; Jung, N.; Shewan, H.M.; Stokes, J.R.; Benson, H.A.E.; Grice, J.E.; Raney, S.G.; Rantou, E.; et al. Impact of Different Packaging Configurations on A Topical Cream Product. Pharm. Res. 2024, 41, 2043–2056. [Google Scholar] [CrossRef]
- Meyer, T. Pharmaceutical Compositions for the Treatment of Inner Ear Disorders. EP1928405B1, 12 April 2007. [Google Scholar]
- Dangre, P.V.; Kattekar, K.R.; Shirolkar, S.V. Development and evaluation of in-situ gelling otic formulations of chloramphenicol using poloxamer 407. Indo Am. J. Pharm. Res. 2013, 3, 8000–8007. [Google Scholar]
- Matousek, J.L.; Campbell, K.L.; Kakoma, I.; Solter, P.F.; Schaeffer, D.J. Evaluation of the effect of pH on in vitro growth of Malassezia pachydermatis. Can. J. Vet. Res. 2003, 67, 56–59. [Google Scholar]
- Ahmed, I.; Kasraian, K. Pharmaceutical challenges in veterinary product development. Adv. Drug Deliv. Rev. 2002, 54, 871–882. [Google Scholar] [CrossRef]
- Boda, C.; Liege, P.; Rème, C.A. Evaluation of Owner Compliance with Topical Treatment of Acute Otitis Externa in Dogs: A Comparative Study of Two Auricular Formulations. Int. J. Appl. Res. Vet. Med. 2011, 9, 157–165. [Google Scholar]
- De, M.; McDonald, P.; Vaughan-Jones, R. Variability Of Ear Drops In Normal Population: An Accurate Delivery Device Required. Internet J. Otorhinolaryngol. 2006, 6, 1–4. [Google Scholar]
- Caneschi, A.; Bardhi, A.; Barbarossa, A.; Zaghini, A. The Use of Antibiotics and Antimicrobial Resistance in Veterinary Medicine, a Complex Phenomenon: A Narrative Review. Antibiotics 2023, 12, 487. [Google Scholar] [CrossRef]
- Horsman, S.; Zaugg, J.; Meler, E.; Mikkelsen, D.; Soares Magalhaes, R.J.; Gibson, J.S. Molecular Epidemiological Characteristics of Staphylococcus pseudintermedius, Staphylococcus coagulans, and Coagulase-Negative Staphylococci Cultured from Clinical Canine Skin and Ear Samples in Queensland. Antibiotics 2025, 14, 80. [Google Scholar] [CrossRef] [PubMed]
- Gyetvai, B. Development of Advanced Antimicrobial Combinations for the Treatment of Canine Otitis Externa. Ph.D. Thesis, University of Veterinary Medicine, Budapest, Hungary, 2018. [Google Scholar]
- Saengchoowong, S.; Jitvaropas, R.; Poomipak, W.; Praianantathavorn, K.; Payungporn, S. Identification of bacteria associated with canine otitis externa based on 16S rDNA high-throughput sequencing. Braz. J. Microbiol. 2023, 54, 3283–3290. [Google Scholar] [CrossRef] [PubMed]
- Borriello, G.; Paradiso, R.; Catozzi, C.; Brunetti, R.; Roccabianca, P.; Riccardi, M.G.; Cecere, B.; Lecchi, C.; Fusco, G.; Ceciliani, F.; et al. Cerumen microbial community shifts between healthy and otitis affected dogs. PLoS ONE 2020, 15, e0241447. [Google Scholar] [CrossRef] [PubMed]
- Land, M.; Hauser, L.; Jun, S.R.; Nookaew, I.; Leuze, M.R.; Ahn, T.H.; Karpinets, T.; Lund, O.; Kora, G.; Wassenaar, T.; et al. Insights from 20 years of bacterial genome sequencing. Funct. Integr. Genom. 2015, 15, 141–161. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, D.H. Alternatives to conventional antimicrobial drugs: A review of future prospects. Vet. Dermatol. 2012, 23, 299–304. [Google Scholar] [CrossRef]
- Soto, S.M. Role of efflux pumps in the antibiotic resistance of bacteria embedded in a biofilm. Virulence 2013, 4, 223–229. [Google Scholar] [CrossRef]
- Panchal, V.; Brenk, R. Riboswitches as Drug Targets for Antibiotics. Antibiotics 2021, 10, 45. [Google Scholar] [CrossRef]
- Kasai, T.; Fukui, Y.; Aoki, K.; Ishii, Y.; Tateda, K. Changes in the ear canal microbiota of dogs with otitis externa. J. Appl. Microbiol. 2021, 130, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Weese, J.S. The canine and feline skin microbiome in health and disease. Vet. Dermatol. 2013, 24, 137–145. [Google Scholar] [CrossRef]
- Khoo, X.; Simons, E.J.; Chiang, H.H.; Hickey, J.M.; Sabharwal, V.; Pelton, S.I.; Rosowski, J.J.; Langer, R.; Kohane, D.S. Formulations for trans-tympanic antibiotic delivery. Biomaterials 2013, 34, 1281–1288. [Google Scholar] [CrossRef]
- Shau, P.A.; Dangre, P.V.; Potnis, V.V. Formulation of Thermosensitive in situ Otic Gel for Topical Management of Otitis Media. Indian J. Pharm. Sci. 2015, 77, 764–770. [Google Scholar] [CrossRef]
- Kim, D.-K. Nanomedicine for Inner Ear Diseases: A Review of Recent In Vivo Studies. BioMed Res. Int. 2017, 2017, 3098230. [Google Scholar] [CrossRef]
- Zou, J.; Feng, H.; Sood, R.; Kinnunen, P.K.; Pyykko, I. Biocompatibility of liposome nanocarriers in the rat inner ear after intratympanic administration. Nanoscale Res. Lett. 2017, 12, 372. [Google Scholar] [CrossRef] [PubMed]
- Bu, M.; Tang, J.; Wei, Y.; Sun, Y.; Wang, X.; Wu, L.; Liu, H. Enhanced bioavailability of nerve growth factor with phytantriol lipid-based crystalline nanoparticles in cochlea. Int. J. Nanomed. 2015, 10, 6879–6889. [Google Scholar] [CrossRef] [PubMed]

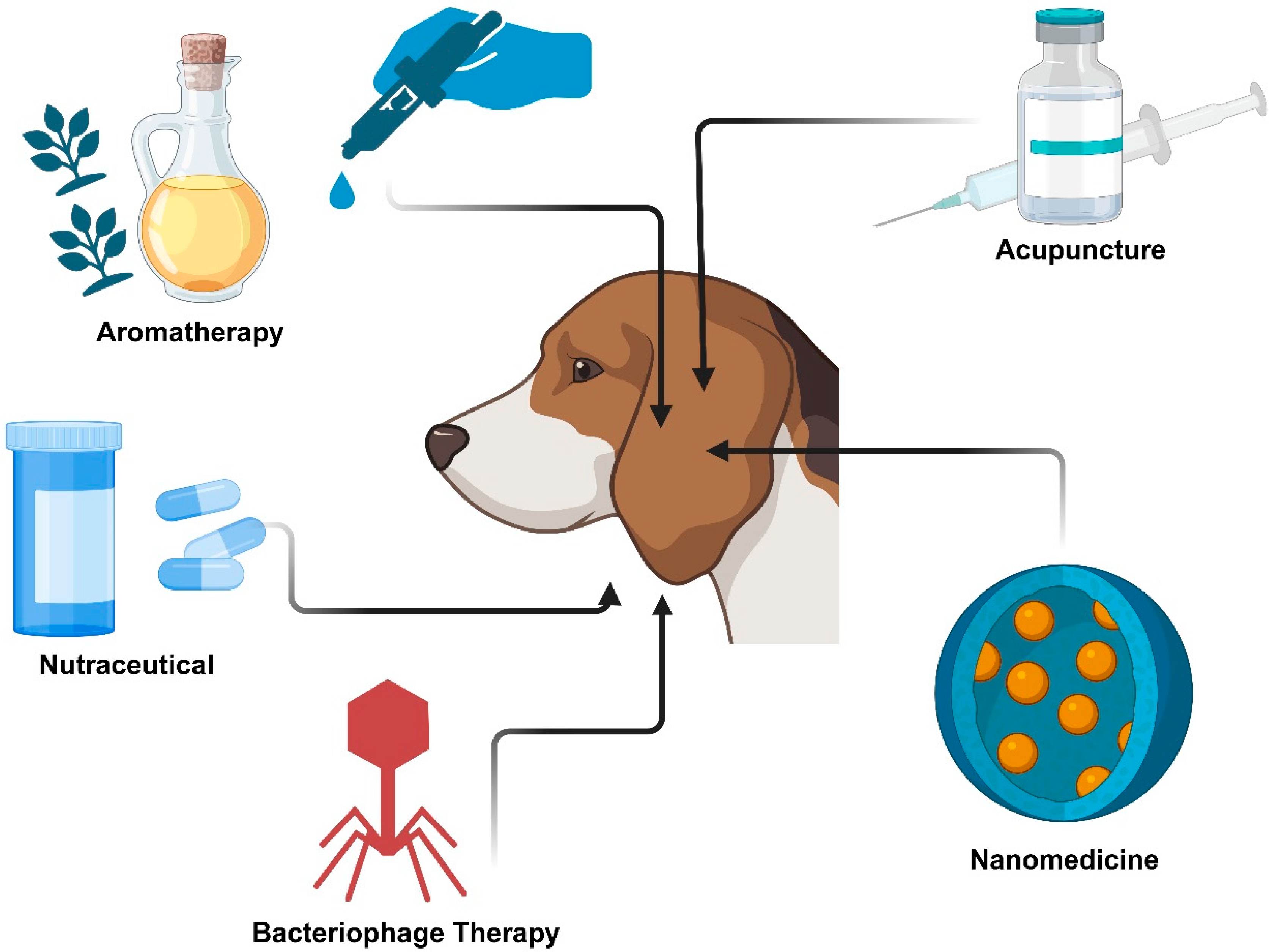
| Therapy | Examples | Study Design | Sample Size | Comparator | Otitis Type | Route of Administration | Duration of Treatment | Outcomes | Evidence Quality Rating | Limitations of the Study | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aromatherapy | Volatile essential oil (mixture of sweet almond oil, bergamot oil, lavender oil, tea tree oil, and roman chamomile oil) administered topically | Controlled experimental study; 2-week intervention comparing topical aroma-oil to standard antibiotic therapy | Control: 5 dogs Experimental: 6 dogs | Amoxicillinclavulanic acid; ciprofloxacin; and ketoconazole | Present dogs | Topically to ear canal | Twice daily for two weeks | The treatment group showed a significantly lower bacterial count (p < 0.05) than the control | Moderate | A small number of dogs were used. Further studies are required to evaluate the safety and effectiveness of aromatherapy. | [12] |
| Oregano oil, thyme oil, carvacrol and thymol | In vitro experimental study evaluating antimicrobial activity of essential oils and phenolic constituents against bacterial and fungal isolates from canine otitis externa | 100 isolates | Ampicillin, gentamicin, and amphotericin B | Present | in vitro | NA | MIC90: 0.015 to 0.03% for Gram-positive bacteria and P. mirabilis MIC90: 0.09 to 0.25% for P. aeruginosa and M. pachydermatis | Moderate | In vivo investigation required | [42] | |
| Acupuncture | Injection of apitoxin in combination with topical aroma oil | Prospective controlled experimental study; 2-week intervention comparing aromatherapy + apipuncture to ketoconazole in dogs with Malassezia-related otitis externa | Control: 5 dogs Experimental: 5 dogs | Ketoconazole | Malassezia- related otitis externa | Topical and injection | 2 weeks | No hepatotoxicity was observed as compared to the control group (Ketoconazole) and similar antifungal activity | Moderate | Limited samples used for Malaissezia-related otitis externa | [14] |
| Bacteriophages | Topical composition containing 1 × 105 plaque forming units of bacteriophage strains active against P. aeruginosa | Prospective uncontrolled clinical trial evaluating single-dose topical bacteriophage therapy for chronic Pseudomonas aeruginosa otitis in dog | Control: 0 Experimental:10 Dogs | None | Chronic Pseudomonas aeruginosa otitis | Topical | A single dose of topical preparation, observation after 48 h of administration | Drop in clinical scores after 48 h (signifying improving conditions) against Pseudomonas aeruginosa otitis | Low | Small study | [13] |
| Nutraceuticals | Microcapsules composed of 60–80% of hydrolyzed fish oils, 20–40% minerals and other therapeutic materials (Tea tree oil Melaleuca alternifolia 0.00343%, Linden oil Tilia platyphyllos scapoli et cordata, 0.0147% Garlic Allium sativum L., 0.0245%, Rosa canina L., 0.098%, and Zinc, 0.00479%) | Prospective randomized controlled trial; 90-day intervention comparing nutraceutical diet + topical treatment to standard diet + topical treatment in dogs with chronic bilateral otitis externa | Control: 15 dogs Experimental: 15 dogs | Placebo | Chronic bilateral otitis externa | Oral | Once a day for 90 days In addition, all dogs (treatment and control groups) were treated with OTOMAX- 8 drops a day for 7 days | Significantly decreased mean score intensity of chronic bilateral otitis externa over a period of 90 days intervention (p < 0.0001) | High | Further studies required with a larger sample and extended observation period. | [3] |
| Nanomedicine | Silver nanoparticles | In vitro experimental study evaluating dose-dependent antibiofilm activity of silver nanoparticles against S. pseudintermedius isolates from dogs with otitis externa | 10 isolates | Untreated bacterial isolates | Otitis externa present | in vitro | Microtiter plate and Congo red agar method | Notable antibiofilm activity depends on the dosage (20 and 10 µg/mL) against Staphylococcus pseudintermedius (isolated from dogs suffering from otitis externa). | Moderate | Ten strains were insufficient to represent the S. pseudintermedius bacterial species, and more work was required to match with in vivo conditions of otitis externa, the study confirmed the inhibition of early biofilm formation for 24 h but did not assess the complete suppression of mature biofilm. | [17] |
| Type | Drugs | Molecular Weight (g/mol) | XLogP3 | PubChem Compound ID |
|---|---|---|---|---|
| Antibacterial | Bacitracin zinc | 1486.1 | −4.1 * | 70687193 |
| Fusidic acid diethanolamine | 621.8 | 5.5 * | 46174083 | |
| Enrofloxacin | 359.4 | −0.2 | 71188 | |
| Florfenicol | 358.2 | 0.8 | 114811 | |
| Framycetin sulfate | 712.7 | −9.0 * | 197162 | |
| Gentamicin sulfate | 575.7 | −4.1 * | 6419933 | |
| Marbofloxacin | 362.4 | −0.5 | 60651 | |
| Orbifloxacin | 395.4 | 0.9 a | 60605 | |
| Polymyxin B sulfate | 1301.6 | Not applicable (large cyclic peptide, no reported LogP) | 56842110 | |
| Antifungal | Clotrimazole | 344.8 | 5 | 2812 |
| Ketoconazole | 531.4 | 4.3 | 47576 | |
| Miconazole nitrate | 479.1 | 5.3 * | 68553 | |
| Nystatin | 926.1 | −0.2 a | 6433272 | |
| Posaconazole | 700.8 | 4.6 | 468595 | |
| Terbinafine hydrochloride | 327.9 | 5.6 * | 5282481 | |
| Steroid | Betamethasone valerate | 476.6 | 3.6 | 16533 |
| Dexamethasone acetate | 434.5 | 2.8 | 236702 | |
| Hydrocortisone aceponate | 460.6 | 3.3 | 68921 | |
| Mometasone furoate | 521.4 | 3.9 | 441336 | |
| Prednisolone acetate | 402.5 | 2.4 | 5834 | |
| Triamcinolone acetonide | 434.5 | 2.5 | 6436 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Kim, S.; Mukhopadhyay, S.; Youssef, S.H.; Tan, J.Q.E.; Weir, E.J.; Page, S.W.; Garg, S. Therapeutic and Formulation Innovations in the Management of Canine Otitis Externa. Pharmaceutics 2025, 17, 1332. https://doi.org/10.3390/pharmaceutics17101332
Song Y, Kim S, Mukhopadhyay S, Youssef SH, Tan JQE, Weir EJ, Page SW, Garg S. Therapeutic and Formulation Innovations in the Management of Canine Otitis Externa. Pharmaceutics. 2025; 17(10):1332. https://doi.org/10.3390/pharmaceutics17101332
Chicago/Turabian StyleSong, Yunmei, Sangseo Kim, Songhita Mukhopadhyay, Souha H. Youssef, Jin Quan Eugene Tan, Emily Josephine Weir, Stephen W. Page, and Sanjay Garg. 2025. "Therapeutic and Formulation Innovations in the Management of Canine Otitis Externa" Pharmaceutics 17, no. 10: 1332. https://doi.org/10.3390/pharmaceutics17101332
APA StyleSong, Y., Kim, S., Mukhopadhyay, S., Youssef, S. H., Tan, J. Q. E., Weir, E. J., Page, S. W., & Garg, S. (2025). Therapeutic and Formulation Innovations in the Management of Canine Otitis Externa. Pharmaceutics, 17(10), 1332. https://doi.org/10.3390/pharmaceutics17101332








