Advances in Nasal Biopharmaceutics to Support Product Development and Therapeutic Needs
Abstract
1. Introduction to Nasal Drug Products
1.1. Compendial and Regulatory Requirements
1.2. Nasal Drug Product Bioequivalence
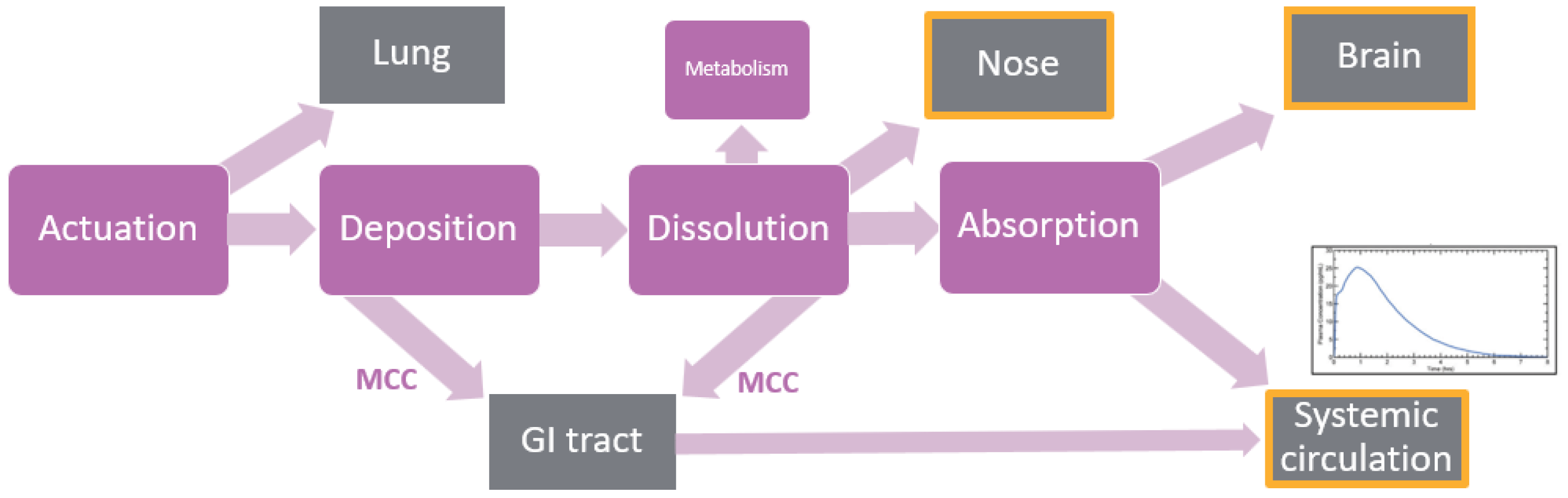
1.3. Nasal Biopharmaceutics
2. Physiologically Based Biopharmaceutics Modeling (PBBM)
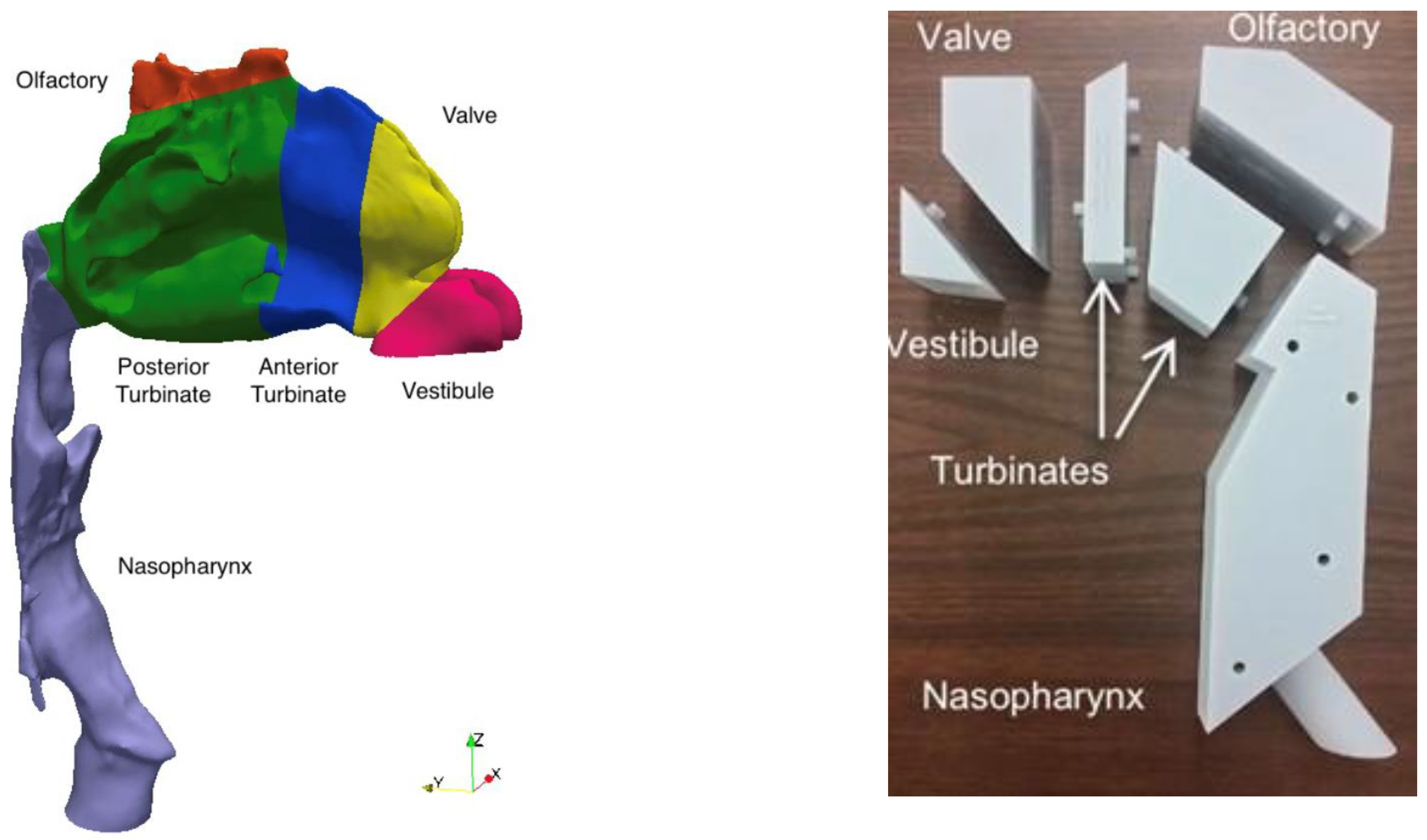
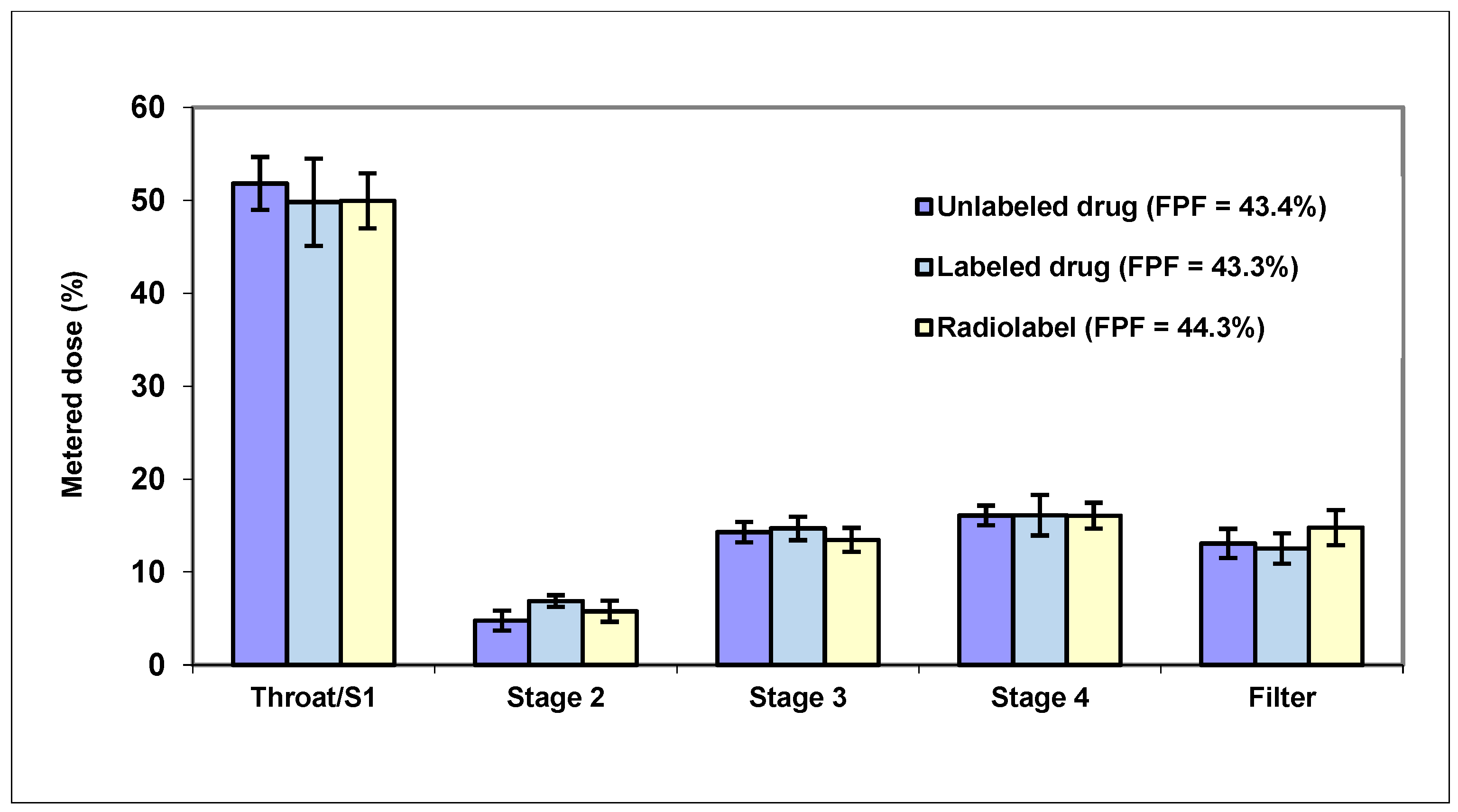
3. Modeling Deposition in the Nasal Cavity
4. In Vitro Biological Models for Nasal Biopharmaceutics
4.1. What Regions of the Nasal Cavity Do We Want to Model?
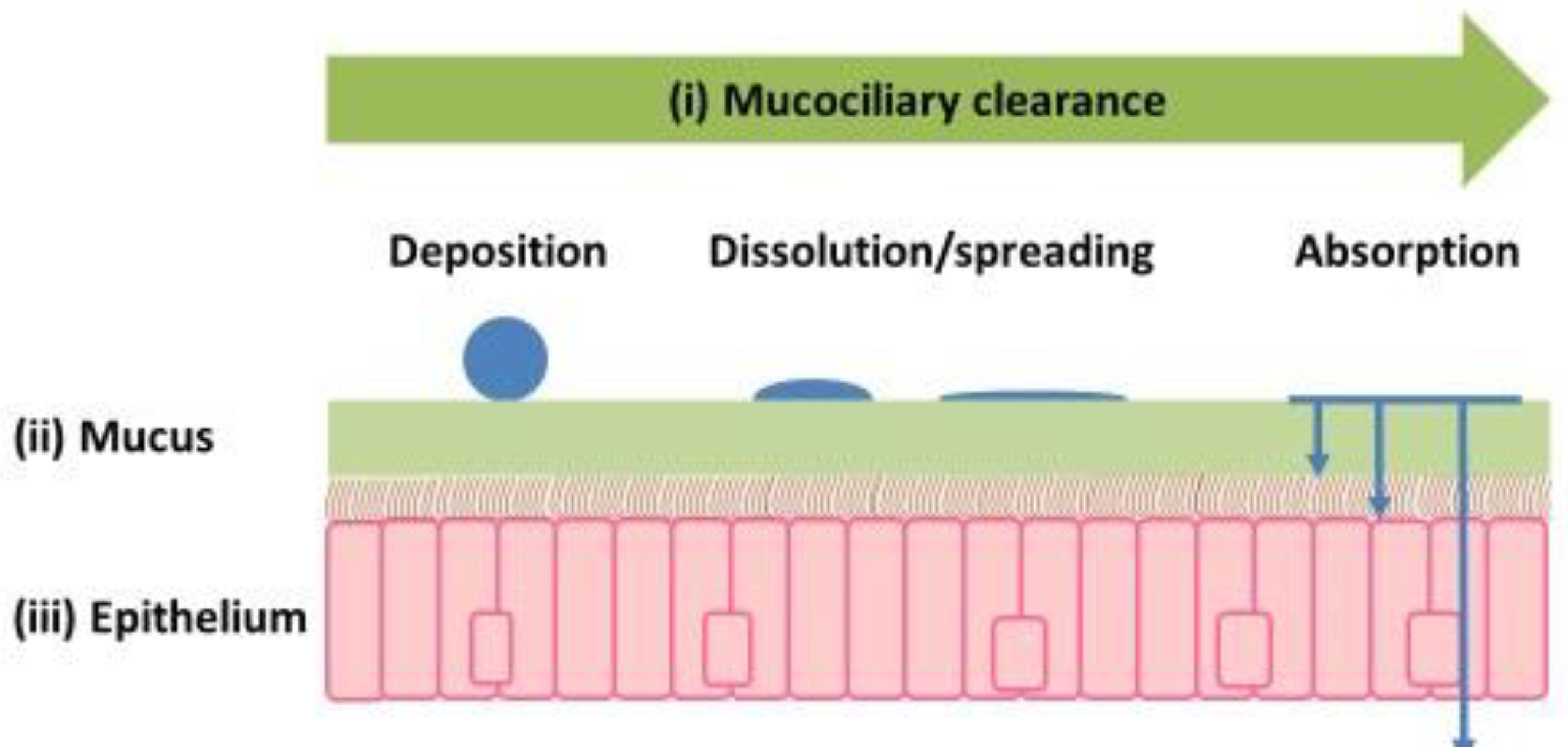
4.2. How Important Is Mucus in Nasal Drug Delivery?
4.3. How Best to Validate Existing and New In Vitro Cell Models
5. Non-Clinical In Vivo Models for Nasal Drug Product Development
6. Clinical Tools in Nasal Drug Product Development
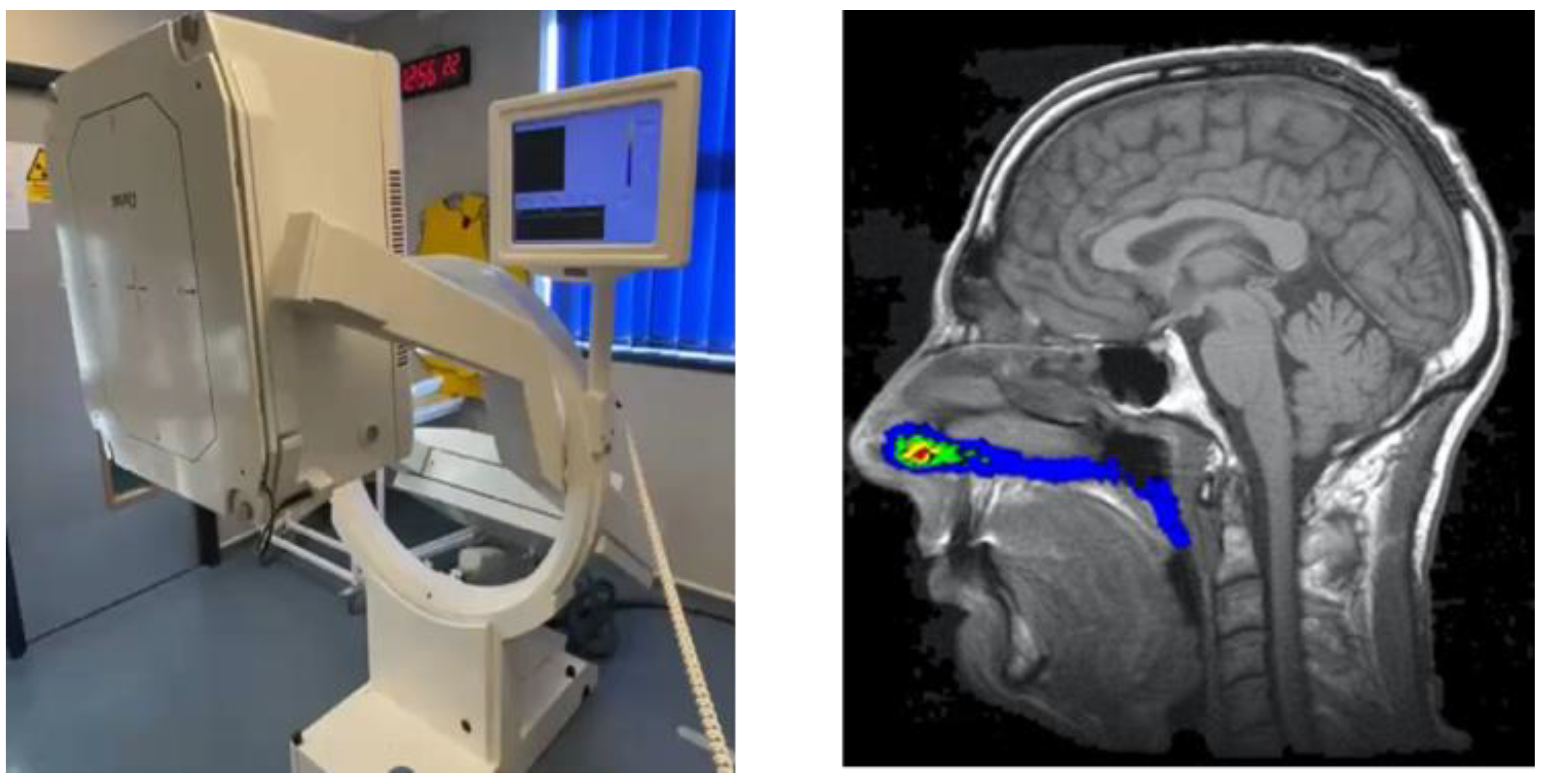
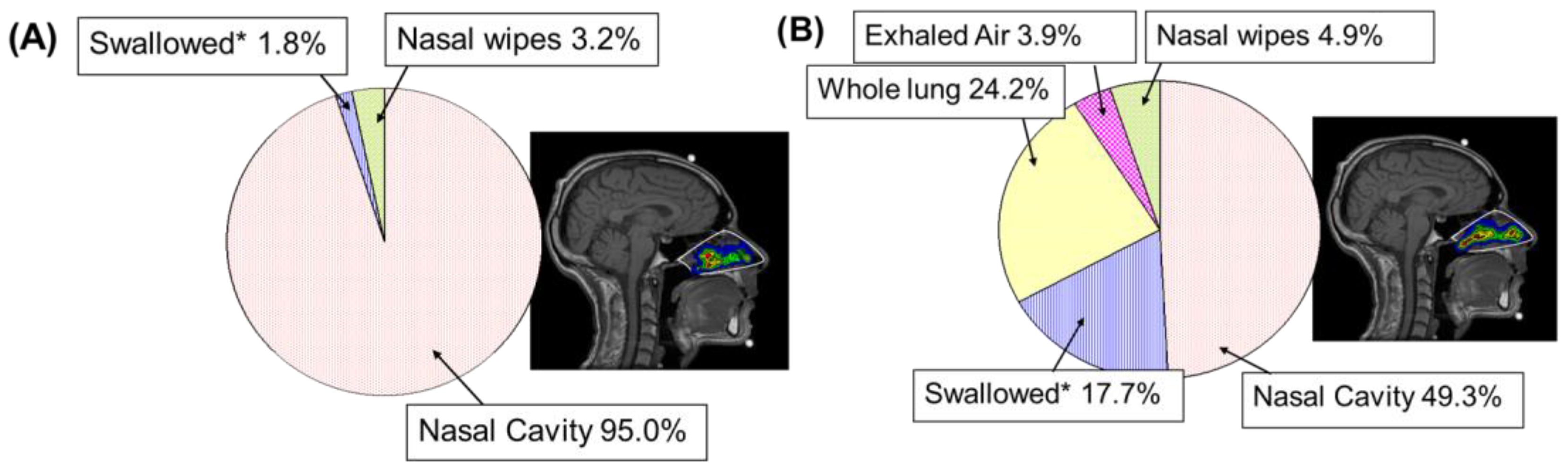
6.1. Gamma Scintigraphy
6.2. Nasal Wicks
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qayyum, S.N.; Ansari, R.S.; Ullah, I.; Siblini, D. The FDA approves the second OTC naloxone—A step toward opioid crisis mitigation. Int. J. Surg. 2023, 109, 4349–4350. [Google Scholar] [CrossRef] [PubMed]
- Borden, T.J.; Levien, T.L.; Baker, D.E. Nasal Glucagon. Hosp. Pharm. 2022, 57, 697–703. [Google Scholar] [CrossRef]
- Dworaczyk, D.A.; Hunt, A.L.; Di Spirito, M.; Lor, M.; Dretchen, K.L.; Lamson, M.J.; Pollock, J.; Ward, T. A 13.2 mg epinephrine intranasal spray demonstrates comparable pharmacokinetics, pharmacodynamics, and safety to a 0.3 mg epinephrine autoinjector. J. Allergy Clin. Immunol. Glob. 2024, 3, 100200. [Google Scholar] [CrossRef]
- Kehagia, E.; Papakyriakopoulou, P.; Valsami, G. Advances in intranasal vaccine delivery: A promising non-invasive route of immunization. Vaccine 2023, 41, 3589–3603. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, C.; Huang, Y.; Ma, Y.; Song, Q.; Chen, H.; Jiang, G.; Gao, X. Intranasal drug delivery: The interaction between nanoparticles and the nose-to-brain pathway. Adv. Drug Deliv. Rev. 2024, 207, 115196. [Google Scholar] [CrossRef]
- Research and Markets. Intranasal Drug and Vaccine Delivery Market Size, Share & Trends Analysis Report by Product, Dosage, Application, Distribution Channel, Region, and Segment Forecasts, 2025–2030. Research and Markets Reports October. Available online: https://www.researchandmarkets.com/reports/6024620/intranasal-drug-vaccine-delivery-market-size#src-pos-1 (accessed on 21 July 2025).
- Grand View Research. Nasal Drug Delivery Technology Market (2023–2030). Available online: https://www.grandviewresearch.com/industry-analysis/nasal-drug-delivery-technology-system-market#:~:text=The%20global%20nasal%20drug%20delivery,7.45%25%20from%202023%20to%202030 (accessed on 19 September 2025).
- Research and Markets. Intranasal Drug and Vaccine Delivery Market Size, Share & Trends Analysis Report by Product, Dosage, Application, Distribution Channel, Region, and Segment Forecasts, 2025–2030. Research and Markets Reports January. Available online: https://www.researchandmarkets.com/reports/516888/intranasal_drug_delivery_global_strategic?utm_source=BW&utm_medium=PressRelease&utm_code=j4zhdr&utm_campaign=1995813+-+Intranasal+Drug+Delivery+Research+Report+2024%3a+Nose-to-Brain+Drug+Delivery%3a+An+Evolving+Area+of+Interest&utm_exec=chdomspi (accessed on 22 September 2025).
- Scherließ, R. Nasal formulations for drug administration and characterization of nasal preparations in drug delivery. Therapeutic Delivery 2020, 11, 183–191. [Google Scholar] [CrossRef]
- FDA. Nasal Spray and Inhalation Solution, Suspension, and Spray Drug Products—Chemistry, Manufacturing, and Controls Documentation. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/nasal-spray-and-inhalation-solution-suspension-and-spray-drug-products-chemistry-manufacturing-and (accessed on 21 July 2025).
- EMA. Guideline on the Pharmaceutical Quality of Inhalation and Nasal Products. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-pharmaceutical-quality-inhalation-nasal-products_en.pdf (accessed on 21 July 2025).
- Bousquet, J.; Bachert, C.; Bernstein, J.; Canonica, G.W.; Carr, W.; Dahl, R.; Demoly, P.; Devillier, P.; Hellings, P.; Fokkens, W.; et al. Advances in pharmacotherapy for the treatment of allergic rhinitis; MP29-02 (a novel formulation of azelastine hydrochloride and fluticasone propionate in an advanced delivery system) fills the gaps. Expert. Opin. Pharmacother. 2015, 16, 913–928. [Google Scholar] [CrossRef] [PubMed]
- Schoenbrodt, T.; Egen, M.; Heyder, K.; Kohler, D.; Kranz, Y.; Mueller, C.; Schiewe, J. Method Development for Deposition Studies in a Nasal Cast. Respir. Drug Deliv. 2010, 2, 445–450. [Google Scholar]
- Kundoor, V.; Dalby, R.N. Assessment of nasal spray deposition pattern in a silicone human nose model using a color-based method. Pharm. Res. 2010, 27, 30–36. [Google Scholar] [CrossRef]
- Chen, J.Z.; Kiaee, M.; Martin, A.R.; Finlay, W.H. In vitro assessment of an idealized nose for nasal spray testing: Comparison with regional deposition in realistic nasal replicas. Int. J. Pharm. 2020, 582, 119341. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.; Suman, J.D. In Vitro Anatomical Models for Nasal Drug Delivery. Pharmaceutics 2022, 14, 1353. [Google Scholar] [CrossRef] [PubMed]
- Kiaee, M.; Wachtel, H.; Noga, M.L.; Martin, A.R.; Finlay, W.H. Regional deposition of nasal sprays in adults: A wide ranging computational study. Int. J. Numer. Methods Biomed. Eng. 2018, 34, e2968. [Google Scholar] [CrossRef]
- Calmet, H.; Inthavong, K.; Eguzkitza, B.; Lehmkuhl, O.; Houzeaux, G.; Vázquez, M. Nasal sprayed particle deposition in a human nasal cavity under different inhalation conditions. PLoS ONE 2019, 14, e0221330. [Google Scholar] [CrossRef] [PubMed]
- Doub, W.H.; Suman, J.M.; Copley, M.; Goodey, A.P.; Hosseini, S.; Mitchell, J.P. Laboratory Performance Testing of Aqueous Nasal Inhalation Products for Droplet/Particle Size Distribution: An Assessment from the International Pharmaceutical Aerosol Consortium on Regulation and Science (IPAC-RS). AAPS PharmSciTech 2023, 24, 208. [Google Scholar] [CrossRef]
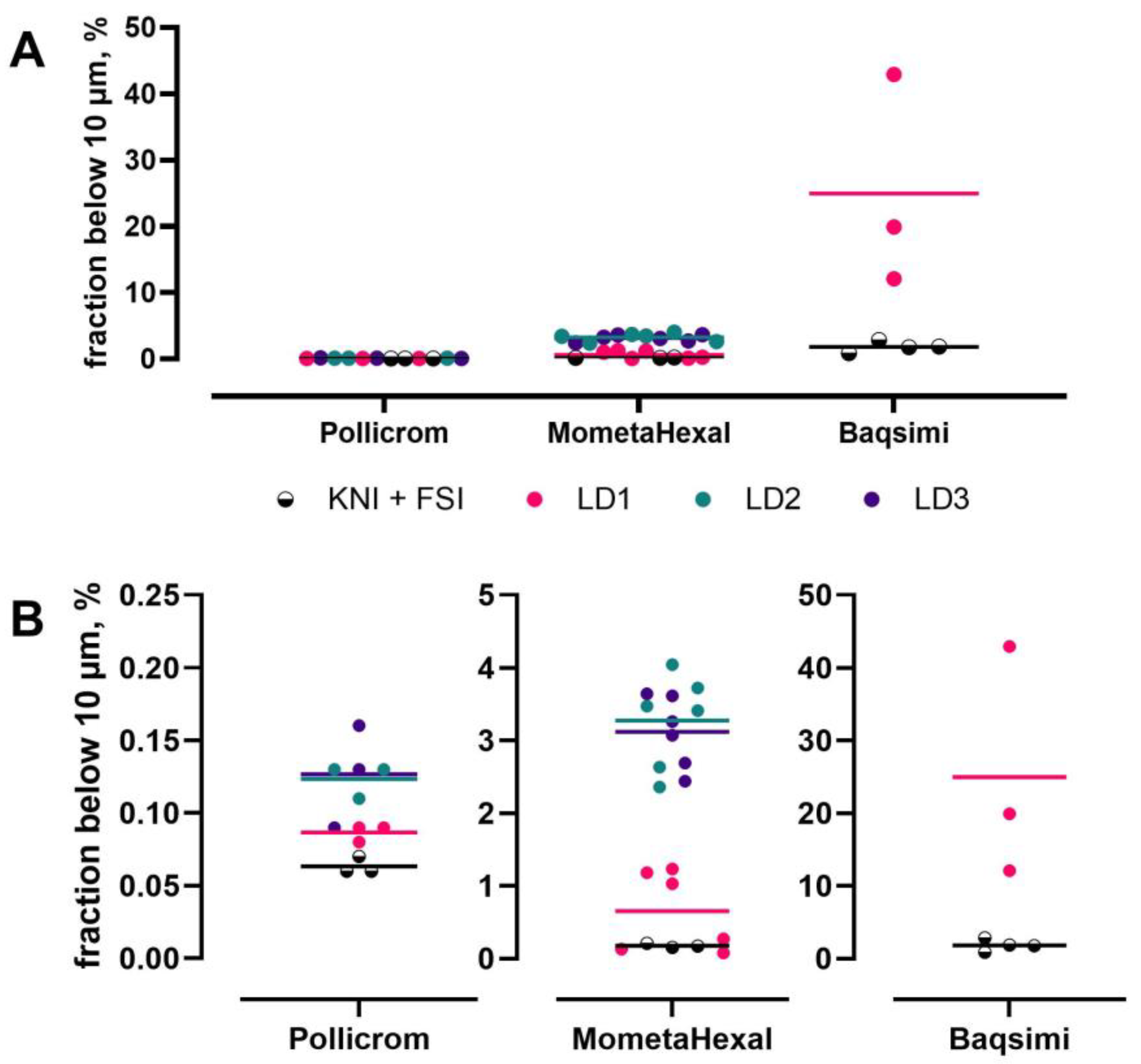
- Baltz, N.; Scherließ, R. Assessment of Mass Fraction Less Than 10 micron in Nasal Products–Method Considerations. In Proceedings of the Drug Delivery to the Lungs, Edinburgh, Scotland, 7–9 December 2022. [Google Scholar]
- Baltz, N.; Svensson, J.; Skogevall, M.; Ohlsson, A.; Svensson, M.; Scherließ, R. Advancing nasal formulation characterization: Considerations toward a robust and precise method to determine the mass fraction below 10 μm in nasal products. Aerosol Sci. Technol. 2024, 58, 1305–1317. [Google Scholar] [CrossRef]
- Williams, G.; Blatchford, C.; Mitchell, J.P. Evaluation of Nasal Inlet Ports Having Simplified Geometry for the Pharmacopeial Assessment of Mass Fraction of Dose Likely to Penetrate Beyond the Nasopharynx: A Preliminary Investigation. AAPS PharmSciTech 2018, 19, 3723–3733. [Google Scholar] [CrossRef]
- Baltz, N.; Scherließ, R. Assessment of Nasal Products–Proposing a New Inlet. In Proceedings of the Drug Delivery to the Lungs, Edinburgh, Scotland, 7–9 December 2022. [Google Scholar]
- Baltz, N.; Scherließ, R. Fraction below 10 µm in Nasal Products–A Comparative Study of Laser Diffraction and Aerodynamic Assessment. In Proceedings of the Drug Delivery to the Lungs, Edinburgh, UK, 11–13 December 2024. [Google Scholar]
- Anand, O.; Pepin, X.J.H.; Kolhatkar, V.; Seo, P. The Use of Physiologically Based Pharmacokinetic Analyses—In Biopharmaceutics Applications -Regulatory and Industry Perspectives. Pharm. Res. 2022, 39, 1681–1700. [Google Scholar] [CrossRef]
- Ding, X.; Kaminsky, L.S. Human extrahepatic cytochromes P450: Function in xenobiotic metabolism and tissue-selective chemical toxicity in the respiratory and gastrointestinal tracts. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 149–173. [Google Scholar] [CrossRef]
- Oliveira, P.; Fortuna, A.; Alves, G.; Falcao, A. Drug-metabolizing Enzymes and Efflux Transporters in Nasal Epithelium: Influence on the Bioavailability of Intranasally Administered Drugs. Curr. Drug Metab. 2016, 17, 628–647. [Google Scholar] [CrossRef] [PubMed]
- Ohkubo, K.; Baraniuk, J.N.; Hohman, R.; Merida, M.; Hersh, L.B.; Kaliner, M.A. Aminopeptidase activity in human nasal mucosa. J. Allergy Clin. Immunol. 1998, 102, 741–750. [Google Scholar] [CrossRef]
- Gonda, I. Mathematical modeling of deposition and disposition of drugs administered via the nose. Adv. Drug Deliv. Rev. 1998, 29, 179–184. [Google Scholar] [CrossRef]
- Dave, S.; Kleinstreuer, C.; Chari, S. An effective PBPK model predicting dissolved drug transfer from a representative nasal cavity to the blood stream. J. Aerosol Sci. 2022, 160, 105898. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration, Center for Drug Evaluation and Research. FY 2024 GDUFA Science and Research Report. 2024. Available online: https://www.fda.gov/media/186225/download (accessed on 22 September 2025).
- Schroeter, J.D.; Tewksbury, E.W.; Wong, B.A.; Kimbell, J.S. Experimental measurements and computational predictions of regional particle deposition in a sectional nasal model. J. Aerosol Med. Pulm. Drug Deliv. 2015, 28, 20–29. [Google Scholar] [CrossRef]
- Keeler, J.A.; Patki, A.; Woodard, C.R.; Frank-Ito, D.O. A Computational Study of Nasal Spray Deposition Pattern in Four Ethnic Groups. J. Aerosol Med. Pulm. Drug Deliv. 2016, 29, 153–166. [Google Scholar] [CrossRef]
- Cabrera, M.; Michelet, O.; Piazzoni, E.; Erra, B.; Santiago-Ribeiro, M.J.; Maia, S.; Williams, G.; Vecellio, L. Development of In Vitro Nasal Cast Imaging Techniques to Predict In Vivo Nasal Deposition. Respir. Drug Deliv. Eur. 2017, 2, 325–328. [Google Scholar]
- Manniello, M.D.; Hosseini, S.; Alfaifi, A.; Esmaeili, A.R.; Kolanjiyil, A.V.; Walenga, R.; Babiskin, A.; Sandell, D.; Mohammadi, R.; Schuman, T.; et al. In vitro evaluation of regional nasal drug delivery using multiple anatomical nasal replicas of adult human subjects and two nasal sprays. Int. J. Pharm. 2021, 593, 120103. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.; Alfaifi, A.; Esmaeili, A.R.; Edwards, D.; Schuman, T.; Longest, W.; Hindle, M.; Golshahi, L. Effects of nasal anatomical characteristics and administration parameters on delivery of locally-acting drugs with suspension nasal sprays in adults. J. Aerosol Sci. 2023, 167, 106101. [Google Scholar] [CrossRef]
- Kimbell, J.S.; Segal, R.A.; Asgharian, B.; Wong, B.A.; Schroeter, J.D.; Southall, J.P.; Dickens, C.J.; Brace, G.; Miller, F.J. Characterization of deposition from nasal spray devices using a computational fluid dynamics model of the human nasal passages. J. Aerosol Med. 2007, 20, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Inthavong, K.; Tian, Z.F.; Tu, J.Y.; Yang, W.; Xue, C. Optimising nasal spray parameters for efficient drug delivery using computational fluid dynamics. Comput. Biol. Med. 2008, 38, 713–726. [Google Scholar] [CrossRef]
- Rygg, A.; Hindle, M.; Longest, P.W. Linking Suspension Nasal Spray Drug Deposition Patterns to Pharmacokinetic Profiles: A Proof-of-Concept Study Using Computational Fluid Dynamics. J. Pharm. Sci. 2016, 105, 1995–2004. [Google Scholar] [CrossRef]
- Chen, J.; Finlay, W.H.; Vehring, R.; Martin, A.R. Characterizing regional drug delivery within the nasal airways. Expert. Opin. Drug Deliv. 2024, 21, 537–551. [Google Scholar] [CrossRef]
- Chen, J.; Martin, A.R.; Finlay, W.H. Recent In Vitro and In Silico Advances in the Understanding of Intranasal Drug Delivery. Curr. Pharm. Des. 2021, 27, 1482–1497. [Google Scholar] [CrossRef] [PubMed]
- Alfaifi, A.; Hosseini, S.; Esmaeili, A.R.; Walenga, R.; Babiskin, A.; Schuman, T.; Longest, W.; Hindle, M.; Golshahi, L. Anatomically realistic nasal replicas capturing the range of nasal spray drug delivery in adults. Int. J. Pharm. 2022, 622, 121858. [Google Scholar] [CrossRef]
- Kiaee, M.; Wachtel, H.; Noga, M.L.; Martin, A.R.; Finlay, W.H. An idealized geometry that mimics average nasal spray deposition in adults: A computational study. Comput. Biol. Med. 2019, 107, 206–217. [Google Scholar] [CrossRef]
- Chen, J.Z.; Finlay, W.H.; Martin, A. In Vitro Regional Deposition of Nasal Sprays in an Idealized Nasal Inlet: Comparison with In Vivo Gamma Scintigraphy. Pharm. Res. 2022, 39, 3021–3028. [Google Scholar] [CrossRef]
- Duong, K.; Aisenstat, M.; Chen, J.Z.; Murphy, B.; Tavernini, S.; Wang, H.; Reiz, B.; Zheng, J.; Whittal, R.; McClary, W.D.; et al. Characterization of Spray-Dried Powders Using a Coated Alberta Idealized Nasal Inlet. J. Aerosol Med. Pulm. Drug Deliv. 2025, 38, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Henriques, P.; Bicker, J.; Carona, A.; Miranda, M.; Vitorino, C.; Doktorovová, S.; Fortuna, A. Amorphous nasal powder advanced performance: In vitro/ex vivo studies and correlation with in vivo pharmacokinetics. J. Pharm. Investig. 2023, 53, 723–742. [Google Scholar] [CrossRef]
- Potts, J.C.; Penn, L.C.; Ahad, J.; Signorelli, V.; Jepras, T.J.; Mistry, S.K.; Mason, L.M. Investigations into the Relationship Between Spray Dried Powder Particle Size and Deposition in Nose and Lung Analogues when Actuated from a Nasal Device. In Proceedings of the Respiratory Drug Delivery, Antibes, France, 2–5 May 2023. [Google Scholar]
- Mygind, N.; Dahl, R. Anatomy, physiology and function of the nasal cavities in health and disease. Adv. Drug Deliv. Rev. 1998, 29, 3–12. [Google Scholar] [CrossRef]
- Amini, S.E.; Gouyer, V.; Portal, C.; Gottrand, F.; Desseyn, J.L. Muc5b is mainly expressed and sialylated in the nasal olfactory epithelium whereas Muc5ac is exclusively expressed and fucosylated in the nasal respiratory epithelium. Histochem. Cell Biol. 2019, 152, 167–174. [Google Scholar] [CrossRef]
- Kennel, C.; Gould, E.A.; Larson, E.D.; Salcedo, E.; Vickery, T.; Restrepo, D.; Ramakrishnan, V.R. Differential Expression of Mucins in Murine Olfactory Versus Respiratory Epithelium. Chem. Senses 2019, 44, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Batts, A.H.; Marriott, C.; Martin, G.P.; Bond, S.W.; Greaves, J.L.; Wilson, C.G. The use of a radiolabelled saccharin solution to monitor the effect of the preservatives thiomersal, benzalkonium chloride and EDTA on human nasal clearance. J. Pharm. Pharmacol. 1991, 43, 180–185. [Google Scholar] [CrossRef]
- Rogers, T.D.; Button, B.; Kelada, S.N.P.; Ostrowski, L.E.; Livraghi-Butrico, A.; Gutay, M.I.; Esther, C.R., Jr.; Grubb, B.R. Regional Differences in Mucociliary Clearance in the Upper and Lower Airways. Front. Physiol. 2022, 13, 842592. [Google Scholar] [CrossRef]
- McGhee, E.O.; Hart, S.M.; Urueña, J.M.; Sawyer, W.G. Hydration Control of Gel-Adhesion and Muco-Adhesion. Langmuir 2019, 35, 15769–15775. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, M.; Lethem, M.I.; Lansley, A.B. The effect of ingredients commonly used in nasal and inhaled solutions on the secretion of mucus in vitro. Int. J. Pharm. 2021, 608, 121054. [Google Scholar] [CrossRef]
- Lock, J.Y.; Carlson, T.L.; Carrier, R.L. Mucus models to evaluate the diffusion of drugs and particles. Adv. Drug Deliv. Rev. 2018, 124, 34–49. [Google Scholar] [CrossRef]
- Lechanteur, A.; das Neves, J.; Sarmento, B. The role of mucus in cell-based models used to screen mucosal drug delivery. Adv. Drug Deliv. Rev. 2018, 124, 50–63. [Google Scholar] [CrossRef]
- Liu, L.; Tian, C.; Dong, B.; Xia, M.; Cai, Y.; Hu, R.; Chu, X. Models to evaluate the barrier properties of mucus during drug diffusion. Int. J. Pharm. 2021, 599, 120415. [Google Scholar] [CrossRef]
- Mura, S.; Hillaireau, H.; Nicolas, J.; Kerdine-Römer, S.; Le Droumaguet, B.; Deloménie, C.; Nicolas, V.; Pallardy, M.; Tsapis, N.; Fattal, E. Biodegradable Nanoparticles Meet the Bronchial Airway Barrier: How Surface Properties Affect Their Interaction with Mucus and Epithelial Cells. Biomacromolecules 2011, 12, 4136–4143. [Google Scholar] [CrossRef]
- Meindl, C.; Stranzinger, S.; Dzidic, N.; Salar-Behzadi, S.; Mohr, S.; Zimmer, A.; Fröhlich, E. Permeation of Therapeutic Drugs in Different Formulations across the Airway Epithelium In Vitro. PLoS ONE 2015, 10, e0135690. [Google Scholar] [CrossRef]
- Cingolani, E.; Alqahtani, S.; Sadler, R.C.; Prime, D.; Stolnik, S.; Bosquillon, C. In vitro investigation on the impact of airway mucus on drug dissolution and absorption at the air-epithelium interface in the lungs. Eur. J. Pharm. Biopharm. 2019, 141, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.F.; Lethem, M.I.; Lansley, A.B. A comparison of three mucus-secreting airway cell lines (Calu-3, SPOC1 and UNCN3T) for use as biopharmaceutical models of the nose and lung. Eur. J. Pharm. Biopharm. 2021, 167, 159–174. [Google Scholar] [CrossRef]
- Sheikh, Z.; Bradbury, P.; Pozzoli, M.; Young, P.M.; Ong, H.X.; Traini, D. An in vitro model for assessing drug transport in cystic fibrosis treatment: Characterisation of the CuFi-1 cell line. Eur. J. Pharm. Biopharm. 2020, 156, 121–130. [Google Scholar] [CrossRef]
- Sibinovska, N.; Žakelj, S.; Kristan, K. Suitability of RPMI 2650 cell models for nasal drug permeability prediction. Eur. J. Pharm. Biopharm. 2019, 145, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Economou, E.C.; Marinelli, S.; Smith, M.C.; Routt, A.A.; Kravets, V.V.; Chu, H.W.; Spendier, K.; Celinski, Z.J. Magnetic Nanodrug Delivery Through the Mucus Layer of Air-Liquid Interface Cultured Primary Normal Human Tracheobronchial Epithelial Cells. Bionanoscience 2016, 6, 235–242. [Google Scholar] [CrossRef]
- Brinks, V.; Lipinska, K.; de Jager, M.; Beumer, W.; Button, B.; Livraghi-Butrico, A.; Henig, N.; Matthee, B. The Cystic Fibrosis-Like Airway Surface Layer Is not a Significant Barrier for Delivery of Eluforsen to Airway Epithelial Cells. J. Aerosol Med. Pulm. Drug Deliv. 2019, 32, 303–316. [Google Scholar] [CrossRef]
- Ladel, S.; Schlossbauer, P.; Flamm, J.; Luksch, H.; Mizaikoff, B.; Schindowski, K. Improved In Vitro Model for Intranasal Mucosal Drug Delivery: Primary Olfactory and Respiratory Epithelial Cells Compared with the Permanent Nasal Cell Line RPMI 2650. Pharmaceutics 2019, 11, 367. [Google Scholar] [CrossRef]
- Mahallawi, W.H.; Aljeraisi, T.M. In vitro cell culture model of human nasal-associated lymphoid tissue (NALT) to evaluate the humoral immune response to SARS-CoV-2 spike proteins. Saudi J. Biol. Sci. 2021, 28, 4516–4521. [Google Scholar] [CrossRef]
- Welch, J.; Wallace, J.; Lansley, A.B.; Roper, C. Evaluation of the toxicity of sodium dodecyl sulphate (SDS) in the MucilAir™ human airway model in vitro. Regul. Toxicol. Pharmacol. 2021, 125, 105022. [Google Scholar] [CrossRef] [PubMed]
- Mercier, C.; Jacqueroux, E.; He, Z.; Hodin, S.; Constant, S.; Perek, N.; Boudard, D.; Delavenne, X. Pharmacological characterization of the 3D MucilAir™ nasal model. Eur. J. Pharm. Biopharm. 2019, 139, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Gadhave, D.G.; Quadros, M.; Ugale, A.R.; Goyal, M.; Ayehunie, S.; Gupta, V. Mucoadhesive chitosan-poly (lactic-co-glycolic acid) nanoparticles for intranasal delivery of quetiapine—Development & characterization in physiologically relevant 3D tissue models. Int. J. Biol. Macromol. 2024, 267, 131491. [Google Scholar] [CrossRef]
- Furubayashi, T.; Inoue, D.; Nishiyama, N.; Tanaka, A.; Yutani, R.; Kimura, S.; Katsumi, H.; Yamamoto, A.; Sakane, T. Comparison of Various Cell Lines and Three-Dimensional Mucociliary Tissue Model Systems to Estimate Drug Permeability Using an In Vitro Transport Study to Predict Nasal Drug Absorption in Rats. Pharmaceutics 2020, 12, 79. [Google Scholar] [CrossRef]
- Wallace, J.; Jackson, G.R.; Kaluzhny, Y.; Ayehunie, S.; Lansley, A.B.; Roper, C.; Hayden, P.J. Evaluation of in vitro rat and human airway epithelial models for acute inhalation toxicity testing. Toxicol. Sci. 2023, 194, 178–190. [Google Scholar] [CrossRef]
- Balogh Sivars, K.; Sivars, U.; Hornberg, E.; Zhang, H.; Brändén, L.; Bonfante, R.; Huang, S.; Constant, S.; Robinson, I.; Betts, C.J.; et al. A 3D Human Airway Model Enables Prediction of Respiratory Toxicity of Inhaled Drugs In Vitro. Toxicol. Sci. 2018, 162, 301–308. [Google Scholar] [CrossRef]
- Gonçalves, V.S.S.; Matias, A.A.; Poejo, J.; Serra, A.T.; Duarte, C.M.M. Application of RPMI 2650 as a cell model to evaluate solid formulations for intranasal delivery of drugs. Int. J. Pharm. 2016, 515, 1–10. [Google Scholar] [CrossRef]
- Berger, J.T.; Voynow, J.A.; Peters, K.W.; Rose, M.C. Respiratory carcinoma cell lines. MUC genes and glycoconjugates. Am. J. Respir. Cell Mol. Biol. 1999, 20, 500–510. [Google Scholar] [CrossRef]
- Martin, J.; Rittersberger, R.; Treitler, S.; Kopp, P.; Ibraimi, A.; Koslowski, G.; Sickinger, M.; Dabbars, A.; Schindowski, K. Characterization of a primary cellular airway model for inhalative drug delivery in comparison with the established permanent cell lines CaLu3 and RPMI 2650. In Vitro Models 2024, 3, 183–203. [Google Scholar] [CrossRef] [PubMed]
- Wengst, A.; Reichl, S. RPMI 2650 epithelial model and three-dimensional reconstructed human nasal mucosa as in vitro models for nasal permeation studies. Eur. J. Pharm. Biopharm. 2010, 74, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.W.; Wu, T.Y.; Siah, Z.Y.; Liu, D.Z. Antioxidative NAC-Loaded Silk Nanoparticles with Opening Mucosal Tight Junctions for Nasal Drug Delivery: An In Vitro and In Vivo Study. Pharmaceutics 2022, 14, 1288. [Google Scholar] [CrossRef] [PubMed]
- Dolberg, A.; Reichl, S. Activity of Multidrug Resistance-associated Proteins 1–5 (MRP1–5) in the RPMI 2650 Cell Line and Explants of Human Nasal Turbinate. Mol. Pharm. 2017, 14, 1577–1590. [Google Scholar] [CrossRef]
- Dolberg, A.M.; Reichl, S. Expression of P-glycoprotein in excised human nasal mucosa and optimized models of RPMI 2650 cells. Int. J. Pharm. 2016, 508, 22–33. [Google Scholar] [CrossRef]
- Mercier, C.; Hodin, S.; He, Z.; Perek, N.; Delavenne, X. Pharmacological Characterization of the RPMI 2650 Model as a Relevant Tool for Assessing the Permeability of Intranasal Drugs. Mol. Pharm. 2018, 15, 2246–2256. [Google Scholar] [CrossRef]
- Dolberg, A.M.; Reichl, S. Expression analysis of human solute carrier (SLC) family transporters in nasal mucosa and RPMI 2650 cells. Eur. J. Pharm. Sci. 2018, 123, 277–294. [Google Scholar] [CrossRef]
- Stuetz, H.; Reihs, E.I.; Neuhaus, W.; Pflüger, M.; Hundsberger, H.; Ertl, P.; Resch, C.; Bauer, G.; Povoden, G.; Rothbauer, M. The Cultivation Modality and Barrier Maturity Modulate the Toxicity of Industrial Zinc Oxide and Titanium Dioxide Nanoparticles on Nasal, Buccal, Bronchial, and Alveolar Mucosa Cell-Derived Barrier Models. Int. J. Mol. Sci. 2023, 24, 5634. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, T.; Han, J.; Zhang, H.; Hu, S.; Wei, S.; Cao, M.; Song, Y.; Yin, D. Three-Dimensional Cultured Human Nasal Epithelial Cell Model for Testing Respiratory Toxicity and Neurotoxicity of Air Pollutants. Environ. Sci. Technol. 2025, 59, 6452–6463. [Google Scholar] [CrossRef] [PubMed]
- Clementino, A.R.; Marchi, C.; Pozzoli, M.; Bernini, F.; Zimetti, F.; Sonvico, F. Anti-Inflammatory Properties of Statin-Loaded Biodegradable Lecithin/Chitosan Nanoparticles: A Step Toward Nose-to-Brain Treatment of Neurodegenerative Diseases. Front. Pharmacol. 2021, 12, 716380. [Google Scholar] [CrossRef]
- Varga, P.; Németh, A.; Zeiringer, S.; Roblegg, E.; Budai-Szűcs, M.; Balla-Bartos, C.; Ambrus, R. Formulation and investigation of differently charged β-cyclodextrin-based meloxicam potassium containing nasal powders. Eur. J. Pharm. Sci. 2024, 202, 106879. [Google Scholar] [CrossRef]
- Wong, S.; Brown, A.D.; Abrahams, A.B.; Nurzak, A.N.; Eltaher, H.M.; Sykes, D.A.; Veprintsev, D.B.; Fone, K.C.F.; Dixon, J.E.; King, M.V. A Modified Cell-Penetrating Peptide Enhances Insulin and Oxytocin Delivery across an RPMI 2650 Nasal Epithelial Cell Barrier In Vitro. Pharmaceutics 2024, 16, 1267. [Google Scholar] [CrossRef]
- Fulcher, M.L.; Gabriel, S.E.; Olsen, J.C.; Tatreau, J.R.; Gentzsch, M.; Livanos, E.; Saavedra, M.T.; Salmon, P.; Randell, S.H. Novel human bronchial epithelial cell lines for cystic fibrosis research. Am. J. Physiol. Lung Cell Mol. Physiol. 2009, 296, L82–L91. [Google Scholar] [CrossRef]
- Randell, S.H.; Liu, J.Y.; Ferriola, P.C.; Kaartinen, L.; Doherty, M.M.; Davis, C.W.; Nettesheim, P. Mucin production by SPOC1 cells--an immortalized rat tracheal epithelial cell line. Am. J. Respir. Cell Mol. Biol. 1996, 14, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Grainger, C.I.; Greenwell, L.L.; Lockley, D.J.; Martin, G.P.; Forbes, B. Culture of Calu-3 cells at the air interface provides a representative model of the airway epithelial barrier. Pharm. Res. 2006, 23, 1482–1490. [Google Scholar] [CrossRef]
- Furuse, M.; Itoh, M.; Hirase, T.; Nagafuchi, A.; Yonemura, S.; Tsukita, S.; Tsukita, S. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J. Cell Biol. 1994, 127, 1617–1626. [Google Scholar] [CrossRef]
- Rotoli, B.M.; Barilli, A.; Visigalli, R.; Ferrari, F.; Frati, C.; Lagrasta, C.A.; Lascia, M.D.; Riccardi, B.; Puccini, P.; Dall’Asta, V. Characterization of ABC Transporters in EpiAirway™, a Cellular Model of Normal Human Bronchial Epithelium. Int. J. Mol. Sci. 2020, 21, 3190. [Google Scholar] [CrossRef]
- Foster, K.A.; Avery, M.L.; Yazdanian, M.; Audus, K.L. Characterization of the Calu-3 cell line as a tool to screen pulmonary drug delivery. Int. J. Pharm. 2000, 208, 1–11. [Google Scholar] [CrossRef]
- Loman, S.; Radl, J.; Jansen, H.M.; Out, T.A.; Lutter, R. Vectorial transcytosis of dimeric IgA by the Calu-3 human lung epithelial cell line: Upregulation by IFN-gamma. Am. J. Physiol. 1997, 272, L951–L958. [Google Scholar] [CrossRef] [PubMed]
- Søndergaard, H.B.; Brodin, B.; Nielsen, C.U. hPEPT1 is responsible for uptake and transport of Gly-Sar in the human bronchial airway epithelial cell-line Calu-3. Pflugers Arch. 2008, 456, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, K.O.; Topp, E.; Makagiansar, I.; Siahaan, T.; Yazdanian, M.; Audus, K.L. Multidrug resistance-associated protein-1 functional activity in Calu-3 cells. J. Pharmacol. Exp. Ther. 2001, 298, 1199–1205. [Google Scholar] [CrossRef]
- Sibinovska, N.; Žakelj, S.; Roškar, R.; Kristan, K. Suitability and functional characterization of two Calu-3 cell models for prediction of drug permeability across the airway epithelial barrier. Int. J. Pharm. 2020, 585, 119484. [Google Scholar] [CrossRef] [PubMed]
- Ingoglia, F.; Visigalli, R.; Rotoli, B.M.; Barilli, A.; Riccardi, B.; Puccini, P.; Dall’Asta, V. Functional characterization of the organic cation transporters (OCTs) in human airway pulmonary epithelial cells. Biochim. Biophys. Acta 2015, 1848, 1563–1572. [Google Scholar] [CrossRef]
- Mathia, N.R.; Timoszyk, J.; Stetsko, P.I.; Megill, J.R.; Smith, R.L.; Wall, D.A. Permeability characteristics of calu-3 human bronchial epithelial cells: In vitro-in vivo correlation to predict lung absorption in rats. J. Drug Target. 2002, 10, 31–40. [Google Scholar] [CrossRef]
- Jeong, M.H.; Han, Y.; Oh, I.S.; Kim, D.M.; Son, D.W.; Jung, M.S.; Yang, H.; Lee, K.; Shin, J.Y.; Kim, H.R.; et al. Pre-validation of a Calu-3 epithelium cytotoxicity assay for predicting acute inhalation toxicity of chemicals. Toxicol. In Vitro 2021, 75, 105136. [Google Scholar] [CrossRef]
- Jeong, M.H.; Kim, H.R.; Bang, I.J.; Yoo, S.H.; Lee, S.J.; Lee, K.H.; Chung, K.H. In vitro model for predicting acute inhalation toxicity by using a Calu-3 epithelium cytotoxicity assay. J. Pharmacol. Toxicol. Methods 2019, 98, 106576. [Google Scholar] [CrossRef]
- Florea, B.I.; Thanou, M.; Junginger, H.E.; Borchard, G. Enhancement of bronchial octreotide absorption by chitosan and N-trimethyl chitosan shows linear in vitro/in vivo correlation. J. Control Release 2006, 110, 353–361. [Google Scholar] [CrossRef]
- Ghadiri, M.; Canney, F.; Pacciana, C.; Colombo, G.; Young, P.M.; Traini, D. The use of fatty acids as absorption enhancer for pulmonary drug delivery. Int. J. Pharm. 2018, 541, 93–100. [Google Scholar] [CrossRef]
- Molina, S.A.; Stauffer, B.; Moriarty, H.K.; Kim, A.H.; McCarty, N.A.; Koval, M. Junctional abnormalities in human airway epithelial cells expressing F508del CFTR. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 309, L475–L487. [Google Scholar] [CrossRef]
- Feizi, S.; Awad, M.; Nepal, R.; Cooksley, C.M.; Psaltis, A.J.; Wormald, P.J.; Vreugde, S. Deferiprone-gallium-protoporphyrin (IX): A promising treatment modality against Mycobacterium abscessus. Tuberculosis 2023, 142, 102390. [Google Scholar] [CrossRef]
- Thomas, N.; Dong, D.; Richter, K.; Ramezanpour, M.; Vreugde, S.; Thierry, B.; Wormald, P.-J.; Prestidge, C.A. Quatsomes for the treatment of Staphylococcus aureus biofilm. J. Mater. Chem. B 2015, 3, 2770–2777. [Google Scholar] [CrossRef] [PubMed]
- Harkema, J.R.; Carey, S.A.; Wagner, J.G. The nose revisited: A brief review of the comparative structure, function, and toxicologic pathology of the nasal epithelium. Toxicol. Pathol. 2006, 34, 252–269. [Google Scholar] [CrossRef]
- Reznik, G.; Stinson, S.F. Experimental Nasal Carcinogenesis, 1st ed.; CRC Press: Boca Raton, FL, USA, 1983; Volume III. [Google Scholar]
- Newman, S.P.; Illum, L. Radionuclide imaging studies in the assessment of nasal drug delivery in humans. Am. J. Drug Deliv. 2004, 2, 101–112. [Google Scholar] [CrossRef]
- Greiff, L.; Wollmer, P.; Erjefält, I.; Pipkorn, U.; Persson, C.G. Clearance of 99mTc DTPA from guinea pig nasal, tracheobronchial, and bronchoalveolar airways. Thorax 1990, 45, 841–845. [Google Scholar] [CrossRef]
- Shah, S.A.; Berger, R.L.; McDermott, J.; Gupta, P.; Monteith, D.; Connor, A.; Lin, W. Regional deposition of mometasone furoate nasal spray suspension in humans. Allergy Asthma Proc. 2015, 36, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Ishii, K.; Newman, S. Optimization of Nasal Dosing Regimens for Olfactory Delivery. In Proceedings of the Controlled Release Society (CRS), Copenhagen, Denmark, 18–22 July 2009. [Google Scholar]
- Thwaites, R.S.; Ito, K.; Chingono, J.M.S.; Coates, M.; Jarvis, H.C.; Tunstall, T.; Anderson-Dring, L.; Cass, L.; Rapeport, G.; Openshaw, P.J.; et al. Nasosorption as a Minimally Invasive Sampling Procedure: Mucosal Viral Load and Inflammation in Primary RSV Bronchiolitis. J. Infect. Dis. 2017, 215, 1240–1244. [Google Scholar] [CrossRef] [PubMed]
- Bäckman, P.; Arora, S.; Couet, W.; Forbes, B.; de Kruijf, W.; Paudel, A. Advances in experimental and mechanistic computational models to understand pulmonary exposure to inhaled drugs. Eur. J. Pharm. Sci. 2018, 113, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Bäckman, P.; Cabal, A.; Clark, A.; Ehrhardt, C.; Forbes, B.; Hastedt, J.; Hickey, A.; Hochhaus, G.; Jiang, W.; Kassinos, S.; et al. iBCS: 2. Mechanistic Modeling of Pulmonary Availability of Inhaled Drugs versus Critical Product Attributes. Mol. Pharm. 2022, 19, 2040–2047. [Google Scholar] [CrossRef]
- Forbes, B.; Bäckman, P.; Cabal, A.; Clark, A.; Ehrhardt, C.; Hastedt, J.E.; Hickey, A.J.; Hochhaus, G.; Jiang, W.; Kassinos, S.; et al. iBCS: 4. Application of the Inhalation Biopharmaceutics Classification System to the Development of Orally Inhaled Drug Products. Mol. Pharm. 2025, 22, 1740–1751. [Google Scholar] [CrossRef] [PubMed]
| Model Input | Measurement Method | Typical Range/Units |
|---|---|---|
| Regional Deposition | In vitro nasal casts, gamma scintigraphy and computational fluid dynamic modeling | % of dose per region (e.g., anterior vs. posterior) |
| Dissolution/Spreading Under Volume-Limited Conditions | Simulated nasal fluid dissolution, e.g., Transwell systems | % dissolved with time (minutes to hours) |
| Mucus Thickness/Viscoelasticity | Imaging (confocal microscopy), histology, rheometry, microrheology | 5–20 µm, 1–1000 cP |
| Absorption/Permeation | Caco-2, PAMPA, mucus-secreting airway cell models (e.g., Calu-3, SPOC-1, UNCN3T, NuLi-1, RPMI 2560, MucilAir and EpiNasal) and ex vivo nasal tissue | 10−6 to 10−3 cm/s |
| Mucociliary Clearance Half-Life | Gamma scintigraphy and in vivo studies | 15–30 min (varies by region) |
| Enzymatic Degradation (e. g., Esterases) | Biochemical assays and LC-MS/MS profiling | Half-life: minutes to hours; enzyme activity varies by region |
| Epithelial Model | Source | Mucin Secretion | TEER Cells Cultured at ALI (Ω.cm2) | TJ Proteins | Transporter Functional Studies | Recommended Use Cases | ||
|---|---|---|---|---|---|---|---|---|
| Permeation | Toxicity | Enhancer Screening | ||||||
| Primary Cells | Various species including human, rat, sheep and pig nose/trachea/bronchus Produced in house or commercially supplied, e.g., MucilAir, human nasal, and EpiNasal, human nasal | MucilAir Yes [68] EpiNasal Yes (in house) | MucilAir 316 ± 31 [69] EpiNasal 110.7 ± 5.5 [70] | MucilAir Yes [69] EpiNasal Yes (in house) | MucilAir P-gp and BCRP [69] EpiNasal No studies | MucilAir Yes [71] EpiNasal Yes [70] | MucilAir Yes [72,73] EpiNasal Yes (in house) | MucilAir No studies EpiNasal Yes [70] |
| RPMI-2650 | Human nose, squamous cell carcinoma, cancer cell line | Yes [66,74] No [75] Low [76] | 75 [77] 66 ± 5 [66] 79.4 ± 5.2 [74] 55.1 ± 3.9 [78] 58 ± 5 [76] | Yes [78] | MRP1-5 Yes [79,80] P-gp and BCRP No [63] P-gp, MRP1, MRP2 and BCRP Yes [81] SLC transporters (PEPT2, OATP1A2, OATP4C1, OCT2, OCTN1 and OCTN2) [82] | Yes [63,74,77,81] | Yes [74,83,84] | Yes [85,86,87] |
| UNCN3T | Human bronchus, telomerase immortalized cell line | Yes [61,88] | 229 ± 20 [61] | Yes [61] | Yes [61] | Yes [61] | No studies | |
| SPOC-1 | Rat trachea, spontaneously immortalized cell line | Yes [61,89] | 217 ± 18 [61] | Yes [61] | Yes [61] | No studies | ||
| Calu-3 | Human lung adenocarcinoma, cancer cell line | Yes * [61,66,76] | 306 ± 53 [90] 368 ± 183 [61] 400 [91,92] 600 [93] 700–2500 [94] 624 ± 170 [76] | Yes [76,90] | PEPT1 [95] P-gp Yes [96,97] MRP1 Yes [92,96] MDR1 only in fully differentiated cells [92] BRCRP No [97] OCT1, OCT3 [98] | Yes [93,99] | Yes [83,100,101] | Yes [102,103] |
| NuLi-1 | Human bronchus, transformed cell line | Yes [62] Serous phenotype [104] | ~200 at 8 weeks ~450 at 6 weeks [104] | Yes [62,104] | No | Yes [62] | Yes [105,106] | No studies |
| Sprague-Dawley Rat | Guinea Pig | Beagle Dog | Rhesus Monkey | Human | |
|---|---|---|---|---|---|
| Body Weight | 250 g | 600 g | 10 kg | 7 kg | ~70 kg |
| Naris Cross-Section | 0.7 mm2 | 2.5 mm2 | 16.7 mm2 | 22.9 mm2 | 140 mm2 |
| Bend in Naris | 40° | 40° | 30° | 30° | |
| Length | 2.3 cm | 3.4 cm | 10 cm | 5.3 cm | 7–8 cm |
| Greatest Vertical Diameter | 9.6 mm | 12.8 mm | 23 mm | 27 mm | 40–45 mm |
| Surface Area (Both Sides of Nasal Cavity) | 10.4 cm2 | 27.4 cm2 | 220.7 cm2 | 61.6 cm2 | 181 cm2 |
| Volume (Both Sides) | 0.4 cm3 | 0.9 cm3 | 20 cm3 | 8 cm3 | 16–19 cm3 (does not include sinuses) |
| Bend in Nasopharynx | 15° | 30° | 30° | 80° | ~90° |
| Turbinate Complexity | Complex scroll | Complex scroll | Very complex membranous | Simple scroll | Simple scroll |
| Biopharmaceutics Facet | Unmet Needs |
|---|---|
| Characterization |
|
| Physiologically based biopharmaceutics modeling |
|
| Nasal deposition |
|
| Biological models |
|
| Non-clinical development |
|
| Clinical development |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forbes, B.; Goodacre, L.; Lansley, A.B.; Martin, A.R.; Palmer, H.; Patterson, C.; Roe, C.; Scherließ, R. Advances in Nasal Biopharmaceutics to Support Product Development and Therapeutic Needs. Pharmaceutics 2025, 17, 1321. https://doi.org/10.3390/pharmaceutics17101321
Forbes B, Goodacre L, Lansley AB, Martin AR, Palmer H, Patterson C, Roe C, Scherließ R. Advances in Nasal Biopharmaceutics to Support Product Development and Therapeutic Needs. Pharmaceutics. 2025; 17(10):1321. https://doi.org/10.3390/pharmaceutics17101321
Chicago/Turabian StyleForbes, Ben, Lucy Goodacre, Alison B. Lansley, Andrew R. Martin, Helen Palmer, Claire Patterson, Chris Roe, and Regina Scherließ. 2025. "Advances in Nasal Biopharmaceutics to Support Product Development and Therapeutic Needs" Pharmaceutics 17, no. 10: 1321. https://doi.org/10.3390/pharmaceutics17101321
APA StyleForbes, B., Goodacre, L., Lansley, A. B., Martin, A. R., Palmer, H., Patterson, C., Roe, C., & Scherließ, R. (2025). Advances in Nasal Biopharmaceutics to Support Product Development and Therapeutic Needs. Pharmaceutics, 17(10), 1321. https://doi.org/10.3390/pharmaceutics17101321







