Cancer Cell Membrane-Coated NPs as a Biomimetic Strategy for Precision Tumor Therapy
Abstract
1. Introduction
2. Biological Functions and Mechanisms of CCM-Coated NPs
2.1. Enhancing Drug Delivery by Targeting Tumor, Avoiding Phagocytosis and Penetrating Tumor Microenvironment (TME)
2.2. Serving as Biomimetic Nanovaccines and Cancer Immunotherapy Platforms
| CCM Source | NPs Coated | Processing Technology | Application/Outcome | Year | Ref. |
|---|---|---|---|---|---|
| B16-OVA (B16 melanoma, mouse) | AECM@PC7A (Antigen-enriched B16 cancer cell membrane coated on STING-activating polymer) | Ultrasonic method | Robust CD8+ T cell response, strong anti-tumor immunity, neoantigen specificity, memory generation, and metastasis suppression | 2025 | [36] |
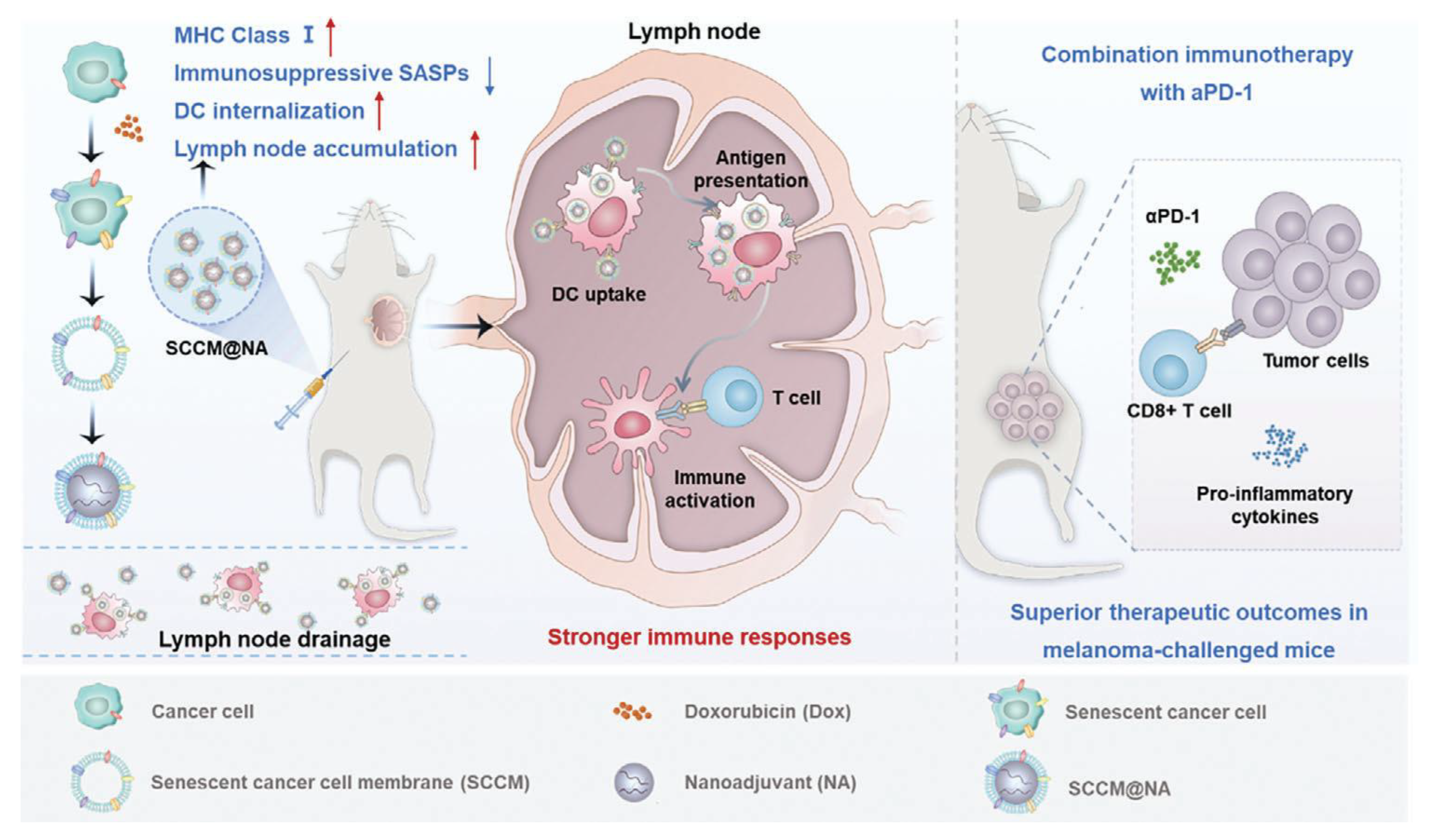
| B16-F10 (melanoma, mouse) | SCCM@NA (Senescent cancer cell membrane-coated on CpG-loaded Mesoporous Silica Nanoadjuvant) | Sonication & Extrusion | Enhanced DC internalization, improved lymph node targeting, robust CD8+ T cell activation, strong antitumor immunity, synergy with αPD-1, suppression of metastasis | 2024 | [37] |
| CT26 (colon cancer, mouse) | LDH-based nanovaccine coated with CT26 CCM (LGCMB) | Extrusion method | Activated dendritic cells, enhanced CD8+ T cell response, strong CRC tumor suppression | 2023 | [35] |
| CT26 (colon cancer, mouse) | PLGA/gambogic acid(GA) NPs coated with CT26 CCM (CCM-PLGA/GA) | Extrusion method | Dual mechanism: direct GA-mediated killing & immune modulation by CCM antigens | 2023 | [39] |
| RM-1 (prostate cancer, mouse) | PMBEOx-COOH NPs loaded with R837 and coated with RM-1 CCM (SCNPs/R837) | Extrusion method | Triggered strong lymph node DC activation; synergized with anti-PD1 to suppress prostate tumors | 2023 | [40] |
| 4T1 (breast cancer, mouse) | Aluminum phosphate NPs loaded with CpG and coated with B16-F10 CCM (APMC) | Extrusion method | Significantly reduced tumor size (avg. ≤ 646 mm) vs. control; prolonged survival with combo therapy | 2022 | [41] |
| ID8 (ovarian cancer, mouse) | CaCO3 NPs loaded with Dox in the core and Ce6 in the ID8 CCM shell(MC/Dox/Ce6) | Extrusion method | Strongest CD3+/CD8+ fluorescence, smallest tumors via ROS-PDT and ICD induction | 2022 | [42] |
| C1498 (AML, mouse) | PLGA NPs loaded with CpG-ODN 1826 and coated with C1498 CCM (AMC NPs) | Ultrasonic method | Increased survival to 4.4 weeks vs. 2.7 weeks in WCL group; 85% survival at week 21 | 2022 | [33] |
| 4T1 (breast cancer, mouse) | PLGA NPs loaded with R837 and coated with 4T1 CCM (CCMsP@R837) | Extrusion method | 75% mice survived >50 days; increased CD8+ T and memory T cells; decreased Tregs | 2021 | [43] |
| B16-OVA (melanoma, mouse) | B16 Cell membrane vesicles (CMVs) with CpG and dendritic cell (DC)-specific intercellular adhesion molecule (ICAM)-3 grabbing nonintegrin (DC-SIGN)-targeting aptamer | Extrusion method | Robust antitumor response via CpG/TLR9 and DC-SIGN-mediated DC targeting | 2021 | [44] |
| 4T1 (breast cancer, mouse) | Calcium oxide NPs loaded with DOX and Ce6, coated with 4T1 CCM | Extrusion method | Minimal drug release at pH 7.4; dual tumor inhibition (primary: ≤126 mm; distant: ≤89 mm) | 2021 | [45] |
| B16-F10 (melanoma, mouse) | Aluminum phosphate NPs loaded with CpG, coated with B16-F10 CCM | Extrusion method | Extended median survival to 29 days; strongest tumor suppression among groups | 2020 | [34] |
3. Fabrication and Characterization of CCM-Coated NPs
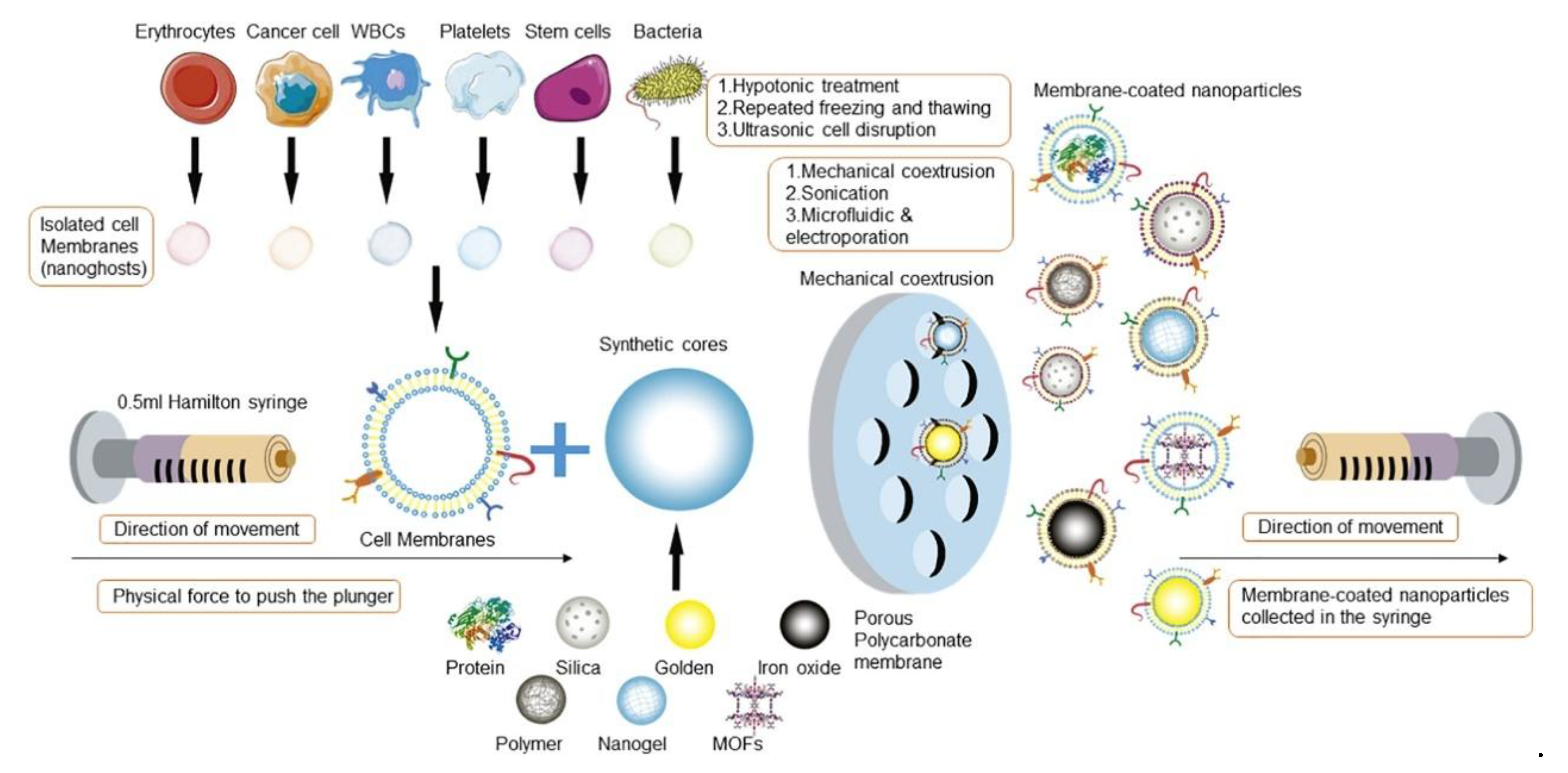
3.1. Cell Membrane Extraction
3.2. Membrane-NP Fusion and Coating
3.3. Membrane-Coated NP Characterization
4. Applications of CCM-NPs in Various Cancer Therapies
4.1. Glioblastomas (GBM)
4.2. Breast Cancer
| CCMs Source | NPs Coated | Processing Technology | Application/Outcome | Year | Ref. |
|---|---|---|---|---|---|
| 4T1 (breast cancer, Mouse) | CAMD@CM(coating COF nanospheres with 4T1 cell membrane and loading with Dox) | Ultrasonic method | ICD induction, antigen delivery, DC activation, suppressed tumor growth and metastasis | 2025 | [83] |
| 4T1 (breast cancer, Mouse) | MSF@CCM (mesoporous silica-loaded FeOOH core coated with 4T1 cell membrane) | Extrusion method | Enhanced tumor accumulation, immune evasion, and ultrasound-mediated deep tumor penetration; triggered ferroptosis and achieved 96.5% tumor growth inhibition. | 2025 | [84] |
| 4T1 (breast cancer, Mouse) | DTX@CHMSN (docetaxel-loaded HMSN coated with 4T1 cell membrane) | Extrusion method | Improved water solubility of docetaxel, homologous targeting and immune evasion, enhanced accumulation at tumor site, reduced systemic toxicity, and improved therapeutic efficacy. | 2024 | [85] |
| 4T1(breast cancer, Mouse) | R&F@Au/MnO2-CM (siRNA & Au/MnO2 nanosensitizer with 4T1 membrane) | Extrusion method | Enhanced radiotherapy and immune activation, prolonging survival. | 2024 | [80] |
| 4T1(breast cancer, Mouse) | TNBC membrane-coated NIR-II/chemo/PD-L1 inhibitor nanoplatform | Extrusion method | Suppressed lung metastasis by 51.2% and extended tumor remission. | 2024 | [79] |
| 4T1(breast cancer, Mouse) | Fe3O4 NPs with ICG and R837, coated with hybrid TRM membrane | Ultrasonic method | Amplified photothermal/Fenton effect and activated CD8+ T cell immunity. | 2023 | [86] |
| 4T1(breast cancer, Mouse) | IR-1048 liposomes coated with 4T1 membrane | Extrusion method | Achieved 96.16% tumor cell killing and significantly inhibited tumor growth. | 2022 | [87] |
| 4T1(breast cancer, Mouse) | PB & DTX/R837-loaded PLGA nanospheres with 4T1 membrane | Ultrasonic method | Boosted tumor cell uptake and increased CTL infiltration from 17.3% to 35.5%. | 2021 | [28] |
| 4T1(breast cancer, Mouse) | Hybrid membrane-coated Dox-loaded poly(lactic-co-glycolic acid) (PLGA) NPs (DPLGA@[RAW-4T1] NPs) | Ultrasonic method | Achieved 88.9% anti-metastasis efficacy in a lung metastasis model. | 2020 | [82] |
| 4T1(breast cancer, Mouse) | CPPNs (PTX-loaded PLA NPs with 4T1 membrane) | Extrusion method | Enhanced anti-tumor efficacy and reduced PTX toxicity in vitro and in vivo. | 2020 | [81] |
4.3. Other Tumors
5. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef]
- Chen, S.; Cao, Z.; Prettner, K.; Kuhn, M.; Yang, J.; Jiao, L.; Wang, Z.; Li, W.; Geldsetzer, P.; Bärnighausen, T.; et al. Estimates and Projections of the Global Economic Cost of 29 Cancers in 204 Countries and Territories From 2020 to 2050. JAMA Oncol. 2023, 9, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Guida, F.; Kidman, R.; Ferlay, J.; Schüz, J.; Soerjomataram, I.; Kithaka, B.; Ginsburg, O.; Mailhot Vega, R.B.; Galukande, M.; Parham, G.; et al. Global and regional estimates of orphans attributed to maternal cancer mortality in 2020. Nat. Med. 2022, 28, 2563–2572. [Google Scholar] [CrossRef]
- Baulu, E.; Gardet, C.; Chuvin, N.; Depil, S. TCR-engineered T cell therapy in solid tumors: State of the art and perspectives. Sci. Adv. 2023, 9, eadf3700. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Zhang, Y.; Huang, L. mRNA vaccine for cancer immunotherapy. Mol. Cancer 2021, 20, 41. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.; Soper, B.; Shopland, L. Cytokine release syndrome and cancer immunotherapies—Historical challenges and promising futures. Front. Immunol. 2023, 14, 1190379. [Google Scholar] [CrossRef]
- Siegler, E.L.; Kenderian, S.S. Neurotoxicity and Cytokine Release Syndrome After Chimeric Antigen Receptor T Cell Therapy: Insights Into Mechanisms and Novel Therapies. Front. Immunol. 2020, 11, 1973. [Google Scholar] [CrossRef]
- Gao, Y.; Joshi, M.; Zhao, Z.; Mitragotri, S. PEGylated therapeutics in the clinic. Bioeng. Transl. Med. 2024, 9, e10600. [Google Scholar] [CrossRef]
- Muro, S. Challenges in design and characterization of ligand-targeted drug delivery systems. J. Control. Release 2012, 164, 125–137. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, S.; Zhang, S.; Wang, L.; Yuan, H.; Hu, F. Cell membrane coated-nanoparticles for cancer immunotherapy. Acta Pharm. Sin. B 2022, 12, 3233–3254. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Gao, H.; Zhang, X.; Sun, L.; Huang, Y.; Zhang, J.; Ding, B. Cell Membrane-Coated Biomimetic Nanoparticles in Cancer Treatment. Pharmaceutics 2024, 16, 531. [Google Scholar] [CrossRef]
- Sultana, P.; Kim, Y.K.; Cho, S.J.; Asadujjaman, M.; Jee, J.P. Advances in cell membrane-coated nanoparticles: Multifunctional platforms for targeted drug delivery, precision phototherapy, and enhanced immunotherapy. Biomater. Sci. 2025, 13, 5232–5259. [Google Scholar] [CrossRef]
- Han, H.; Bártolo, R.; Li, J.; Shahbazi, M.A.; Santos, H.A. Biomimetic platelet membrane-coated nanoparticles for targeted therapy. Eur. J. Pharm. Biopharm. 2022, 172, 1–15. [Google Scholar] [CrossRef]
- Wu, Y.; Wan, S.; Yang, S.; Hu, H.; Zhang, C.; Lai, J.; Zhou, J.; Chen, W.; Tang, X.; Luo, J.; et al. Macrophage cell membrane-based nanoparticles: A new promising biomimetic platform for targeted delivery and treatment. J. Nanobiotechnol. 2022, 20, 542. [Google Scholar] [CrossRef]
- Song, Y.; Shou, X.; Sheng, B.; Mei, J.; Shi, K.; Shang, L.; Zhu, X. Cell Membranes from Tumor-Tropic MSCs Screened by a Microfluidic Chip for Drug Nanoparticles Encapsulation and Cancer Targeted Therapy. Adv. Healthc. Mater. 2023, 12, e2202904. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, X. Stem cell membrane-camouflaged targeted delivery system in tumor. Mater. Today Bio 2022, 16, 100377. [Google Scholar] [CrossRef]
- Zeng, S.; Tang, Q.; Xiao, M.; Tong, X.; Yang, T.; Yin, D.; Lei, L.; Li, S. Cell membrane-coated nanomaterials for cancer therapy. Mater. Today Bio 2023, 20, 100633. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, S.; Nie, G.; Li, N. Unlocking the power of nanomedicine: Cell membrane-derived biomimetic cancer nanovaccines for cancer treatment. Med 2024, 5, 660–688. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Thangaraj, P.; Wang, L.; Cao, Q.; Kim, J.H. Nanovaccines: An effective therapeutic approach for cancer therapy. Biomed. Pharmacother. 2024, 170, 115992. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, S.; Xue, N. Research progress of cancer cell membrane coated nanoparticles for the diagnosis and therapy of breast cancer. Front. Oncol. 2023, 13, 1270407. [Google Scholar] [CrossRef] [PubMed]
- Scully, M.A.; Wilkins, D.E.; Dang, M.N.; Hoover, E.C.; Aboeleneen, S.B.; Day, E.S. Cancer Cell Membrane Wrapped Nanoparticles for the Delivery of a Bcl-2 Inhibitor to Triple-Negative Breast Cancer. Mol. Pharm. 2023, 20, 3895–3913. [Google Scholar] [CrossRef]
- Harris, J.C.; Sterin, E.H.; Day, E.S. Membrane-Wrapped Nanoparticles for Enhanced Chemotherapy of Acute Myeloid Leukemia. ACS Biomater. Sci. Eng. 2022, 8, 4439–4448. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, S.; Xu, R.; Tang, Y.; Xia, X. Cancer cell membrane-coated nanoparticles: A promising anti-tumor bionic platform. RSC Adv. 2024, 14, 10608–10637. [Google Scholar] [CrossRef]
- Yang, Q.; Lai, S.K. Anti-PEG immunity: Emergence, characteristics, and unaddressed questions. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 655–677. [Google Scholar] [CrossRef]
- Estapé Senti, M.; de Jongh, C.A.; Dijkxhoorn, K.; Verhoef, J.J.F.; Szebeni, J.; Storm, G.; Hack, C.E.; Schiffelers, R.M.; Fens, M.H.; Boross, P. Anti-PEG antibodies compromise the integrity of PEGylated lipid-based nanoparticles via complement. J. Control. Release 2022, 341, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.H.; Hu, C.M.; Luk, B.T.; Gao, W.; Copp, J.A.; Tai, Y.; O’Connor, D.E.; Zhang, L. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett. 2014, 14, 2181–2188. [Google Scholar] [CrossRef] [PubMed]
- Nie, D.; Dai, Z.; Li, J.; Yang, Y.; Xi, Z.; Wang, J.; Zhang, W.; Qian, K.; Guo, S.; Zhu, C.; et al. Cancer-Cell-Membrane-Coated Nanoparticles with a Yolk-Shell Structure Augment Cancer Chemotherapy. Nano Lett. 2020, 20, 936–946. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, L.; Li, L.; Tan, M.; Liu, W.; Liu, S.; Xie, Z.; Zhang, W.; Wang, Z.; Cao, Y.; et al. Cancer cell membrane-coated nanoparticles for bimodal imaging-guided photothermal therapy and docetaxel-enhanced immunotherapy against cancer. J. Nanobiotechnol. 2021, 19, 449. [Google Scholar] [CrossRef] [PubMed]
- Nagel, G.; Sousa-Herves, A.; Wedepohl, S.; Calderón, M. Matrix Metalloproteinase-sensitive Multistage Nanogels Promote Drug Transport in 3D Tumor Model. Theranostics 2020, 10, 91–108. [Google Scholar] [CrossRef]
- Iaccarino, G.; Profeta, M.; Vecchione, R.; Netti, P.A. Matrix metalloproteinase-cleavable nanocapsules for tumor-activated drug release. Acta Biomater. 2019, 89, 265–278. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, Y.; Xin, Y.; Jiang, C.; Yan, B.; Zhai, S. Interactions Between Nanoparticles and Dendritic Cells: From the Perspective of Cancer Immunotherapy. Front. Oncol. 2018, 8, 404. [Google Scholar] [CrossRef]
- Tai, Y.; Chen, M.; Wang, F.; Fan, Y.; Zhang, J.; Cai, B.; Yan, L.; Luo, Y.; Li, Y. The role of dendritic cells in cancer immunity and therapeutic strategies. Int. Immunopharmacol. 2024, 128, 111548. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.T.; Zhou, J.; Kroll, A.V.; Fang, R.H.; Yan, M.; Xiao, C.; Chen, X.; Kline, J.; Zhang, L.; Zhang, D.E. Acute myeloid leukemia cell membrane-coated nanoparticles for cancer vaccination immunotherapy. Leukemia 2022, 36, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Xu, Y.; Ji, W.; Li, L.; Qiu, L.; Zhou, S.; Qian, Z.; Zhang, H. Hybrid Membrane Nanovaccines Combined with Immune Checkpoint Blockade to Enhance Cancer Immunotherapy. Int. J. Nanomed. 2022, 17, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, B.; Sun, L.; Niu, X.; Li, L.; Xu, Z.P. “Trojan horse” nanoparticle-delivered cancer cell membrane vaccines to enhance cancer immunotherapy by overcoming immune-escape. Biomater. Sci. 2023, 11, 2020–2032. [Google Scholar] [CrossRef]
- Li, Y.; Fang, M.; Yu, H.; Wang, X.; Xue, S.; Jiang, Z.; Huang, Z.; Rong, S.; Wei, X.; Lu, Z.; et al. Neoantigen enriched biomimetic nanovaccine for personalized cancer immunotherapy. Nat. Commun. 2025, 16, 4783. [Google Scholar] [CrossRef]
- Yang, C.; Chen, Y.; Liu, J.; Zhang, W.; He, Y.; Chen, F.; Xie, X.; Tang, J.; Guan, S.; Shao, D.; et al. Leveraging Senescent Cancer Cell Membrane to Potentiate Cancer Immunotherapy Through Biomimetic Nanovaccine. Adv. Sci. 2024, 11, e2400630. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, H.; Li, N.; Wei, Y.; Lin, Y.; Deng, R.; Ding, H.; Lv, Y.; Ma, T.; Li, R.; et al. Neoadjuvant chemotherapy by liposomal doxorubicin boosts immune protection of tumor membrane antigens-based nanovaccine. Cell Rep. Med. 2025, 6, 101877. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, Q.; Xiao, J.; Zhang, X.; Han, X.; Shi, X.; Hu, J.; Li, L.; Qian, X. Cancer Cell Membrane-Coated Gambogic Acid Nanoparticles for Effective Anticancer Vaccination by Activating Dendritic Cells. Int. J. Nanomed. 2023, 18, 2261–2273. [Google Scholar] [CrossRef]
- Li, S.; Dong, S.; Wu, J.; Lv, X.; Yang, N.; Wei, Q.; Wang, C.; Chen, J. Surgically Derived Cancer Cell Membrane-Coated R837-Loaded Poly(2-Oxazoline) Nanoparticles for Prostate Cancer Immunotherapy. ACS Appl. Mater. Interfaces 2023, 15, 7878–7886. [Google Scholar] [CrossRef]
- Gan, J.; Du, G.; He, C.; Jiang, M.; Mou, X.; Xue, J.; Sun, X. Tumor cell membrane enveloped aluminum phosphate nanoparticles for enhanced cancer vaccination. J. Control. Release 2020, 326, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Song, J.; Wang, B.; Hua, H.; Zhu, H.; Guo, X.; Xiong, S.; Zhao, Y. Dendritic cell vaccine for the effective immunotherapy of breast cancer. Biomed. Pharmacother. 2020, 126, 110046. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Huang, Y.; Yang, Y.; Miao, Z.; Zhu, J.; Zhong, M.; Feng, C.; Tang, W.; Zhou, J.; Wang, L.; et al. Biomimetic cytomembrane nanovaccines prevent breast cancer development in the long term. Nanoscale 2021, 13, 3594–3601. [Google Scholar] [CrossRef]
- Liu, B.; Yang, Y.; Chao, Y.; Xiao, Z.; Xu, J.; Wang, C.; Dong, Z.; Hou, L.; Li, Q.; Liu, Z. Equipping Cancer Cell Membrane Vesicles with Functional DNA as a Targeted Vaccine for Cancer Immunotherapy. Nano Lett. 2021, 21, 9410–9418. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, W.; Huang, J.; Li, F.; Sheng, J.; Song, H.; Chen, Y. Development of a Dendritic Cell/Tumor Cell Fusion Cell Membrane Nano-Vaccine for the Treatment of Ovarian Cancer. Front. Immunol. 2022, 13, 828263. [Google Scholar] [CrossRef]
- Vijayan, V.; Uthaman, S.; Park, I.K. Cell Membrane-Camouflaged Nanoparticles: A Promising Biomimetic Strategy for Cancer Theragnostics. Polymers 2018, 10, 983. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Luo, J.; Chen, X.; Liu, W.; Chen, T. Cell Membrane Coating Technology: A Promising Strategy for Biomedical Applications. Nano-Micro Lett. 2019, 11, 100. [Google Scholar] [CrossRef]
- Fang, R.H.; Jiang, Y.; Fang, J.C.; Zhang, L. Cell membrane-derived nanomaterials for biomedical applications. Biomaterials 2017, 128, 69–83. [Google Scholar] [CrossRef]
- Zhai, Y.; Su, J.; Ran, W.; Zhang, P.; Yin, Q.; Zhang, Z.; Yu, H.; Li, Y. Preparation and Application of Cell Membrane-Camouflaged Nanoparticles for Cancer Therapy. Theranostics 2017, 7, 2575–2592. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Ye, R.; Jin, Q.; Yin, F.; Liu, N.; Wang, Y.; Zhang, Q.; Gao, T.; Zhao, Y. Cancer Cell-Mimicking Prussian Blue Nanoplatform for Synergistic Mild Photothermal/Chemotherapy via Heat Shock Protein Inhibition. ACS Appl. Mater. Interfaces 2024, 16, 20908–20919. [Google Scholar] [CrossRef]
- Xia, Q.; Zhang, Y.; Li, Z.; Hou, X.; Feng, N. Red blood cell membrane-camouflaged nanoparticles: A novel drug delivery system for antitumor application. Acta Pharm. Sin. B 2019, 9, 675–689. [Google Scholar] [CrossRef]
- Hu, C.M.; Fang, R.H.; Wang, K.C.; Luk, B.T.; Thamphiwatana, S.; Dehaini, D.; Nguyen, P.; Angsantikul, P.; Wen, C.H.; Kroll, A.V.; et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature 2015, 526, 118–121. [Google Scholar] [CrossRef]
- Rao, L.; Cai, B.; Bu, L.L.; Liao, Q.Q.; Guo, S.S.; Zhao, X.Z.; Dong, W.F.; Liu, W. Microfluidic Electroporation-Facilitated Synthesis of Erythrocyte Membrane-Coated Magnetic Nanoparticles for Enhanced Imaging-Guided Cancer Therapy. ACS Nano 2017, 11, 3496–3505. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.H.; Kroll, A.V.; Gao, W.; Zhang, L. Cell Membrane Coating Nanotechnology. Adv. Mater. 2018, 30, e1706759. [Google Scholar] [CrossRef]
- Thanuja, M.Y.; Anupama, C.; Ranganath, S.H. Bioengineered cellular and cell membrane-derived vehicles for actively targeted drug delivery: So near and yet so far. Adv. Drug Deliv. Rev. 2018, 132, 57–80. [Google Scholar] [CrossRef]
- Tsong, T.Y. Electroporation of cell membranes. Biophys. J. 1991, 60, 297–306. [Google Scholar] [CrossRef]
- Fan, Z.; Zhou, H.; Li, P.Y.; Speer, J.E.; Cheng, H. Structural elucidation of cell membrane-derived nanoparticles using molecular probes. J. Mater. Chem. B 2014, 2, 8231–8238. [Google Scholar] [CrossRef]
- Jin, J.; Bhujwalla, Z.M. Biomimetic Nanoparticles Camouflaged in Cancer Cell Membranes and Their Applications in Cancer Theranostics. Front. Oncol. 2019, 9, 1560. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Ren, Y.; Li, H.; Tang, Y.; Yan, J.; Shen, Z.; Zhang, H.; Chen, F. Cancer Cell-Membrane Biomimetic Boron Nitride Nanospheres for Targeted Cancer Therapy. Int. J. Nanomed. 2021, 16, 2123–2136. [Google Scholar] [CrossRef]
- Copp, J.A.; Fang, R.H.; Luk, B.T.; Hu, C.M.; Gao, W.; Zhang, K.; Zhang, L. Clearance of pathological antibodies using biomimetic nanoparticles. Proc. Natl. Acad. Sci. USA 2014, 111, 13481–13486. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, W.; Li, Y.; Chang, J.; Tian, F.; Zhao, F.; Ma, Y.; Sun, J. Microfluidic Sonication To Assemble Exosome Membrane-Coated Nanoparticles for Immune Evasion-Mediated Targeting. Nano Lett. 2019, 19, 7836–7844. [Google Scholar] [CrossRef]
- Hu, C.M.; Zhang, L.; Aryal, S.; Cheung, C.; Fang, R.H.; Zhang, L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl. Acad. Sci. USA 2011, 108, 10980–10985. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yu, W.; Seitsonen, J.; Xu, W.; Lehto, V.P. Correct Identification of the Core-Shell Structure of Cell Membrane-Coated Polymeric Nanoparticles. Chem.–A Eur. J. 2022, 28, e202200947. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Huang, B.Q. Immunogold labeling for electron microscopy: Strategy and problem solving. In Plant Microtechniques and Protocols; Springer: Berlin/Heidelberg, Germany, 2015; pp. 225–249. [Google Scholar]
- Alleva, M.; Baranyai, Z.; Esteban-Pérez, N.; Martínez-Vicente, P.; Martín-Rapún, R.; Moros, M.; Martínez de la Fuente, J. Förster Resonance Energy Transfer (FRET) Demonstrates In Vitro Chitosan-Coated Nanocapsules Suitability for Intranasal Brain Delivery. ACS Appl. Mater. Interfaces 2025, 17, 26348–26360. [Google Scholar] [CrossRef] [PubMed]
- Mülhopt, S.; Diabaté, S.; Dilger, M.; Adelhelm, C.; Anderlohr, C.; Bergfeldt, T.; Gómez de la Torre, J.; Jiang, Y.; Valsami-Jones, E.; Langevin, D.; et al. Characterization of Nanoparticle Batch-To-Batch Variability. Nanomaterials 2018, 8, 311. [Google Scholar] [CrossRef]
- Ou, A.; Wang, Y.; Zhang, J.; Huang, Y. Living Cells and Cell-Derived Vesicles: A Trojan Horse Technique for Brain Delivery. Pharmaceutics 2023, 15, 1257. [Google Scholar] [CrossRef]
- Fan, Q.; Kuang, L.; Wang, B.; Yin, Y.; Dong, Z.; Tian, N.; Wang, J.; Yin, T.; Wang, Y. Multiple Synergistic Effects of the Microglia Membrane-Bionic Nanoplatform on Mediate Tumor Microenvironment Remodeling to Amplify Glioblastoma Immunotherapy. ACS Nano 2024, 18, 14469–14486. [Google Scholar] [CrossRef]
- Ren, Y.; Miao, C.; Tang, L.; Liu, Y.; Ni, P.; Gong, Y.; Li, H.; Chen, F.; Feng, S. Homotypic Cancer Cell Membranes Camouflaged Nanoparticles for Targeting Drug Delivery and Enhanced Chemo-Photothermal Therapy of Glioma. Pharmaceuticals 2022, 15, 157. [Google Scholar] [CrossRef]
- Jiménez-Boland, D.; Robles-Fernández, A.; Martín-Rodríguez, A.; Cuadros, M.; Traverso, J.; Sánchez-Moreno, P.; Bramini, M. Breaking Barriers in Glioblastoma Targeting through Advanced Nanoparticle Cell Membrane Coating. ACS Appl. Mater. Interfaces 2025, 147, 35288–35303. [Google Scholar] [CrossRef]
- Zou, Y.; Sun, Y.; Wang, Y.; Zhang, D.; Yang, H.; Wang, X.; Zheng, M.; Shi, B. Cancer cell-mitochondria hybrid membrane coated Gboxin loaded nanomedicines for glioblastoma treatment. Nat. Commun. 2023, 14, 4557. [Google Scholar] [CrossRef]
- Lu, G.; Wang, X.; Li, F.; Wang, S.; Zhao, J.; Wang, J.; Liu, J.; Lyu, C.; Ye, P.; Tan, H.; et al. Engineered biomimetic nanoparticles achieve targeted delivery and efficient metabolism-based synergistic therapy against glioblastoma. Nat. Commun. 2022, 13, 4214. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Cui, Y.; Fan, Y.; Chen, M.; Yang, G.; Wang, Y.; Yang, M.; Li, Z.; Gong, W.; Yang, Y.; et al. Hybrid membrane-coated nanosuspensions for multi-modal anti-glioma therapy via drug and antigen delivery. J. Nanobiotechnol. 2021, 19, 378. [Google Scholar] [CrossRef]
- Fan, Y.; Hao, W.; Cui, Y.; Chen, M.; Chu, X.; Yang, Y.; Wang, Y.; Gao, C. Cancer Cell Membrane-Coated Nanosuspensions for Enhanced Chemotherapeutic Treatment of Glioma. Molecules 2021, 26, 5103. [Google Scholar] [CrossRef]
- Han, S.; Lee, Y.; Lee, M. Biomimetic cell membrane-coated DNA nanoparticles for gene delivery to glioblastoma. J. Control. Release 2021, 338, 22–32. [Google Scholar] [CrossRef]
- Katsura, C.; Ogunmwonyi, I.; Kankam, H.K.; Saha, S. Breast cancer: Presentation, investigation and management. Br. J. Hosp. Med. 2022, 83, 1–7. [Google Scholar] [CrossRef]
- Gupta, P.; Neupane, Y.R.; Parvez, S.; Kohli, K. Recent advances in targeted nanotherapeutic approaches for breast cancer management. Nanomedicine 2021, 16, 2605–2631. [Google Scholar] [CrossRef]
- Xiong, W.; Cheng, Z.; Chen, H.; Liang, H.; Wang, M.; Chen, Y.; Ying, J.; Cai, Y.; Chai, J.; Dou, K.J.A.F.M. Biomimetic Tumor Cell Membrane-Encapsulated Nanoparticles Combine NIR-II Photothermal Therapy and Chemotherapy for Enhanced Immunotherapy in Triple-Negative Breast Cancer. Adv. Funct. Mater. 2024, 34, 2410841. [Google Scholar] [CrossRef]
- Wang, D.; Lin, S.; Li, T.; Yang, X.; Zhong, X.; Chen, Q.; Jiang, G.; Li, C. Cancer cell membrane-coated siRNA-Decorated Au/MnO2 nanosensitizers for synergistically enhanced radio-immunotherapy of breast cancer. Mater. Today Bio 2024, 29, 101275. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Xu, Y.; Guo, X.; Yuan, C.; Mu, D.; Xiao, Y. Cancer cell membrane-coated biomimetic platform for targeted therapy of breast cancer in an orthotopic mouse model. J. Biomater. Sci. Polym. Ed. 2020, 31, 1538–1551. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Yu, X.; You, B.; Wu, Y.; Wang, R.; Han, L.; Wang, Y.; Gao, S.; Yuan, Y. Macrophage-cancer hybrid membrane-coated nanoparticles for targeting lung metastasis in breast cancer therapy. J. Nanobiotechnol. 2020, 18, 92. [Google Scholar] [CrossRef]
- Lin, D.; Lv, W.; Qian, M.; Jiang, G.; Lin, X.; Gantulga, D.; Wang, Y. Engineering cell membrane-camouflaged COF-based nanosatellite for enhanced tumor-targeted photothermal chemoimmunotherapy. Biomaterials 2025, 314, 122869. [Google Scholar] [CrossRef]
- Guo, J.; Pan, X.; Wu, Q.; Li, P.; Wang, C.; Liu, S.; Zhang, H.; Huang, Z.; Mou, X.; Liu, H.; et al. Bio-barrier-adaptable biomimetic nanomedicines combined with ultrasound for enhanced cancer therapy. Signal Transduct. Target. Ther. 2025, 10, 137. [Google Scholar] [CrossRef]
- Liu, M.; Sun, Y.; Wei, Q.; Zhang, A.; Wang, S.; Wang, D.; Dong, Z.; Ma, X.; Yan, R.; Wang, Y. 4T1 Cell Membrane Biomimetic Nanovehicle for Enhanced Breast Cancer Treatment. ACS Med. Chem. Lett. 2025, 16, 51–58. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, W.; Zhou, Y.; Zheng, X.; Fu, Y.; Liu, H.; Wan, Z.; Zhao, Y. Intelligent Nanoplatform Integrating Macrophage and Cancer Cell Membrane for Synergistic Chemodynamic/Immunotherapy/Photothermal Therapy of Breast Cancer. ACS Appl. Mater. Interfaces 2023, 15, 59117–59133. [Google Scholar] [CrossRef]
- Xu, M.; Yang, Y.; Yuan, Z. Breast Cancer Cell Membrane Camouflaged Lipid Nanoparticles for Tumor-Targeted NIR-II Phototheranostics. Pharmaceutics 2022, 14, 1367. [Google Scholar] [CrossRef]
- Wang, D.; Liu, C.; You, S.; Zhang, K.; Li, M.; Cao, Y.; Wang, C.; Dong, H.; Zhang, X. Bacterial Vesicle-Cancer Cell Hybrid Membrane-Coated Nanoparticles for Tumor Specific Immune Activation and Photothermal Therapy. ACS Appl. Mater. Interfaces 2020, 12, 41138–41147. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, X.; Liu, C.; Wu, Y.; Wen, Y.; Zheng, R.; Xu, C.; Tian, J.; Peng, Q.; Zheng, X.; et al. Engineering a nano-drug delivery system to regulate m6A modification and enhance immunotherapy in gastric cancer. Acta Biomater. 2025, 191, 412–427. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Han, R.; Sun, Y.; Chen, W.; Zhao, L.; Guan, X.; Zhang, W. A minimalist cancer cell membrane-shielded biomimetic nanoparticle for nasopharyngeal carcinoma active-targeting therapy. Colloids Surf. B Biointerfaces 2024, 238, 113909. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, X.; Lu, Y.; Zhu, X.; Zheng, W.; Chen, K.; Liu, S.; Wu, J.; Guan, W. Improved Photodynamic Therapy Based on Glutaminase Blockage via Tumor Membrane Coated CB-839/IR-780 Nanoparticles. Small 2024, 20, e2305174. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, Y.; Song, J.; Liu, X.; Liu, S.; Yang, N.; Wang, L.; Liu, Y.; Zhao, Y.; Zhou, W.; et al. Tumor Cell-Targeting and Tumor Microenvironment-Responsive Nanoplatforms for the Multimodal Imaging-Guided Photodynamic/Photothermal/Chemodynamic Treatment of Cervical Cancer. Int. J. Nanomed. 2024, 19, 5837–5858. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Zhang, L.; Liu, Q.; Jiang, F.; Hou, W.; Wang, Y.; Fang, H.; Zhang, Y. Cancer Cell Membrane-Enveloped Dexamethasone Normalizes the Tumor Microenvironment and Enhances Gynecologic Cancer Chemotherapy. ACS Nano 2023, 17, 16703–16714. [Google Scholar] [CrossRef]
- Fu, L.; Zhang, W.; Zhou, X.; Fu, J.; He, C. Tumor cell membrane-camouflaged responsive nanoparticles enable MRI-guided immuno-chemodynamic therapy of orthotopic osteosarcoma. Bioact. Mater. 2022, 17, 221–233. [Google Scholar] [CrossRef]
- Xiong, J.; Wu, M.; Chen, J.; Liu, Y.; Chen, Y.; Fan, G.; Liu, Y.; Cheng, J.; Wang, Z.; Wang, S.; et al. Cancer-Erythrocyte Hybrid Membrane-Camouflaged Magnetic Nanoparticles with Enhanced Photothermal-Immunotherapy for Ovarian Cancer. ACS Nano 2021, 15, 19756–19770. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, Y.; Xu, X.; Wang, F.; Shen, W.; Leng, X.; Zhao, J.; Liu, B.; Wang, Y.; Liu, P. Surface PEGylated Cancer Cell Membrane-Coated Nanoparticles for Codelivery of Curcumin and Doxorubicin for the Treatment of Multidrug Resistant Esophageal Carcinoma. Front. Cell Dev. Biol. 2021, 9, 688070. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Wu, J.; Jin, L.; Hong, L.; Wang, F.; Mao, Z.; Wu, M. Cancer cell membrane-coated gold nanorods for photothermal therapy and radiotherapy on oral squamous cancer. J. Mater. Chem. B 2020, 8, 7253–7263. [Google Scholar] [CrossRef]
- Yang, M.; Zhou, J.; Lu, L.; Deng, D.; Huang, J.; Tang, Z.; Shi, X.; Lo, P.C.; Lovell, J.F.; Zheng, Y.; et al. Tumor cell membrane-based vaccines: A potential boost for cancer immunotherapy. Exploration 2024, 4, 20230171. [Google Scholar] [CrossRef]
- Li, A.; Zhao, J.; Fu, J.; Cai, J.; Zhang, P. Recent advances of biomimetic nano-systems in the diagnosis and treatment of tumor. Asian J. Pharm. Sci. 2021, 16, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.H.; Ye, P.J.; Zhou, Y.C.; He, D.X.; Wei, H.; Yu, C.Y. Cell membrane-camouflaged nanoparticles as drug carriers for cancer therapy. Acta Biomater. 2020, 105, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Zhao, J.; Pan, J.; Liu, C.; Guo, X.; Zhou, S. Personalized Nanovaccine Coated with Calcinetin-Expressed Cancer Cell Membrane Antigen for Cancer Immunotherapy. Nano Lett. 2021, 21, 8418–8425. [Google Scholar] [CrossRef]
- Yang, R.; Xu, J.; Xu, L.; Sun, X.; Chen, Q.; Zhao, Y.; Peng, R.; Liu, Z. Cancer Cell Membrane-Coated Adjuvant Nanoparticles with Mannose Modification for Effective Anticancer Vaccination. ACS Nano 2018, 12, 5121–5129. [Google Scholar] [CrossRef] [PubMed]
| Membrane Source | Main Advantages | Main Limitations |
|---|---|---|
| Cancer cell membrane (CCM) | Enables homotypic targeting through tumor antigen recognition; carries immune evasion proteins (e.g., CD47); improves tumor accumulation and selective uptake | Applicable mainly to cancer settings; potential safety concerns from oncogenic proteins; variability in membrane composition and scalability issues |
| Red blood cell (RBC) membrane | Readily available; well-established isolation methods; strong immune evasion and prolonged circulation time | Does not provide tumor-specific targeting; limited ability to direct nanoparticles to tumor tissues |
| Platelet membrane | Natural adhesion to damaged vasculature and circulating tumor cells; contributes to immune evasion; useful in metastasis prevention | Limited availability; possible pro-thrombotic activity; less tumor selectivity compared with CCM |
| Immune cell membrane (e.g., macrophage, T cell, NK cell) | Intrinsic affinity for inflammatory and tumor microenvironments; potential to modulate immune response; can facilitate tissue penetration | Limited cell sources; risk of immunogenicity; functional properties depend on immune cell type |
| Stem cell membrane | Tropism toward tumor and injured tissues; possesses immune evasion properties; potential for regenerative applications | Safety concerns related to stemness-associated factors; technical challenges in large-scale preparation |
| CCMs Source | NPs Coated | Processing Technology | Application/Outcome | Year | Ref. |
|---|---|---|---|---|---|
| U87-MG (GBM cell, human) | Carboxylate-modified micro-sphere FluoSpheres® (PS-NPs) were coated with the isolated U87-MG Cell membrane | Extrusion method | Enhance tumor targeting and BBB penetration for improved glioblastoma therapy. | 2025 | [71] |
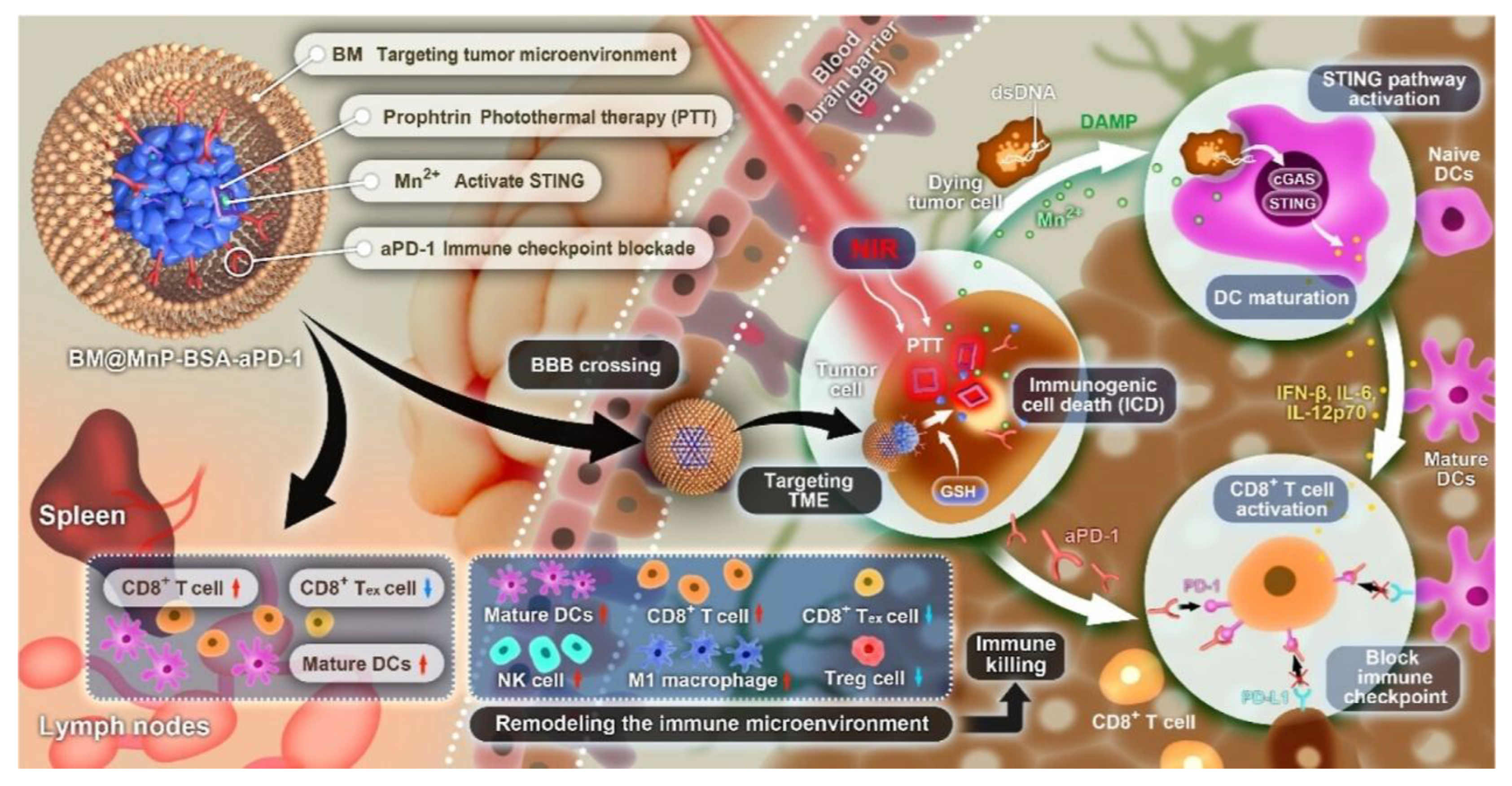
| U87-MG (GBM cell, human) | Amphiphilic CB [7]-PEG-Ce6 micelles loaded with MTIC | Extrusion method | Crosses BBB, targets TME, enhances metal immunotherapy & PTT, blocks immune checkpoints | 2024 | [69] |
| U87-MG (GBMU87 MG cell, human) | HM-NPs@G: Gboxin-loaded NPs coated with hybrid cancer cell-mitochondria membrane | Extrusion method | Prolongs Gboxin circulation, improves BBB permeability and tumor accumulation | 2023 | [72] |
| U251 (Glioma cells, human) | M@HLPC NPs: Self-assembled Hb, LOX, CPO-Ce6 particles coated with U251 membrane | Extrusion method | Strong anti-tumor efficacy via LA metabolic therapy & chemiexcited PDT in CDX & PDX models | 2022 | [73] |
| BV2 (microglia cell, mouse) | GQDs/DOX@CCMs: Graphene QDs & DOX coated with BV2 CCM | Extrusion method | Stable for 7 days at ~34 °C; 2x fluorescence in BV2 vs. MCF-7; effective for chemophotothermal therapy | 2022 | [70] |
| C6 (microglia cell, mouse) | DNS-[C6&DC]m: DTX nanosuspension coated with hybrid C6 & dendritic cell membrane | Ultrasonic method | Significantly prolonged survival (65 d) vs. DTX (37 d) and DNS (42 d) | 2021 | [74] |
| C6 (microglia cell, mouse) | HCPT-NS/CCM:10-hydroxycamptothecin nanosuspension camouflaged with C6 CCM | Ultrasonic method | 2x DiR fluorescence in tumors; significantly extended survival vs. saline/HCPT/HCPT-NS | 2021 | [75] |
| C6 (Glioblastoma cells, rat) | PEI25k/pDNA complexes coated with C6 CCM | Ultrasonic method | Reduced toxicity, higher HSVtk gene expression | 2021 | [76] |
| CCM Source | NPs Coated | Coating Method | Application/Outcome | Year | Ref |
|---|---|---|---|---|---|
| SGC-7901 (gastric cancer, human) or MFC (gastric cancer, mouse) | PLGA-STM-TAT and PLGA-STM TAT@CCM-YSA | Extrusion method | Tumor targeting, m6A modulation, enhanced anti-gastric cancer immunity | 2025 | [89] |
| CNE-2 (Nasopharyngeal carcinoma (NPC), human) | PAMAM dendrimer loaded with DOX, coated with CNE-2 CCM | Ultrasonic method | Prolonged circulation, tumor targeting, systemic anti-NPC efficacy | 2024 | [90] |
| AGS (gastric cancer, human) | IRCB@M: hybrid NP inhibiting glutamine metabolism and enhancing ROS-mediated PDT | Extrusion method | Dual-pathway PDT booster via glutamine metabolism inhibition | 2024 | [91] |
| HeLa (cervical cancer, human) | HMnO2 NPs loaded with ICG, coated with HeLa CCM | Extrusion method | Strong NIR-triggered antitumor effect with high biocompatibility | 2024 | [92] |
| U14 (cervical cancer, mouse) | (dexamethasone) Dex@PLGA-CM | Ultrasonic &Extrusion method | TME modulation, homologous tumor targeting, enhanced Doxil penetration, improved anti-gynecologic cancer efficacy | 2023 | [93] |
| K7M2 (osteosarcoma, mouse) | MnO2 NPs, functionalized with K7M2 CCM | Extrusion method | Reduced tumor volume; prolonged survival > 55 days | 2022 | [94] |
| ID8 & RBC (hybrid, ovarian, mouse) | Fe3O4 magnetic NPs loaded with ICG, coated with hybrid ID8 ovarian and RBC membranes | Ultrasonic method | ICG accumulation 2.9x higher in hybrid IRM vs. single-membrane NPs | 2021 | [95] |
| TE10 (esophageal cancer, human) | PLGA NPs co-loaded with DOX and curcumin, coated with PEGylated TE10 CCM | Extrusion method | Prolonged tumor suppression, reduced cardiotoxicity | 2021 | [96] |
| KB (oral cancer, human) | Gold nanorods coated with KB oral cancer cell membranes (GNR@Mem) | Extrusion method | 4.7x higher uptake; 95.6% inhibition with PTT & RT | 2020 | [97] |
| B16-F10 & OMVs (melanoma, mouse) | Hollow polydopamine NPs coated with hybrid OMVs and B16-F10 melanoma membranes | Ultrasonic method | 87x targeting specificity; 45-day survival post NIR-PTT | 2020 | [88] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.; Li, W.; Aboushanab, A.R.; Sun, J. Cancer Cell Membrane-Coated NPs as a Biomimetic Strategy for Precision Tumor Therapy. Pharmaceutics 2025, 17, 1322. https://doi.org/10.3390/pharmaceutics17101322
Lin J, Li W, Aboushanab AR, Sun J. Cancer Cell Membrane-Coated NPs as a Biomimetic Strategy for Precision Tumor Therapy. Pharmaceutics. 2025; 17(10):1322. https://doi.org/10.3390/pharmaceutics17101322
Chicago/Turabian StyleLin, Junyi, Wei Li, Alaa R. Aboushanab, and Jingjing Sun. 2025. "Cancer Cell Membrane-Coated NPs as a Biomimetic Strategy for Precision Tumor Therapy" Pharmaceutics 17, no. 10: 1322. https://doi.org/10.3390/pharmaceutics17101322
APA StyleLin, J., Li, W., Aboushanab, A. R., & Sun, J. (2025). Cancer Cell Membrane-Coated NPs as a Biomimetic Strategy for Precision Tumor Therapy. Pharmaceutics, 17(10), 1322. https://doi.org/10.3390/pharmaceutics17101322






