Comparative In Vitro Evaluation of Buccal Films, Microcapsules, and Liposomal Systems for Naringin and Citrus × paradisi L. Peel Extract: Effects of Encapsulation Strategy and Compound Origin on Release Profiles
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Extract
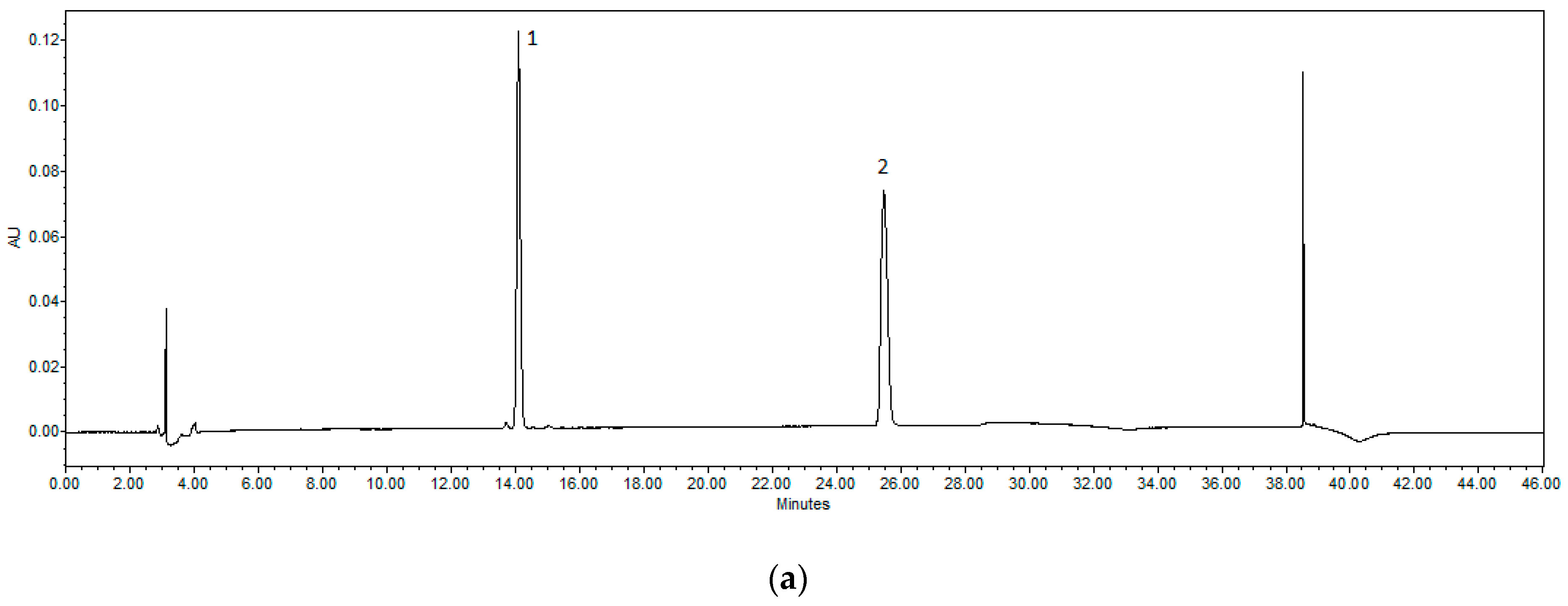
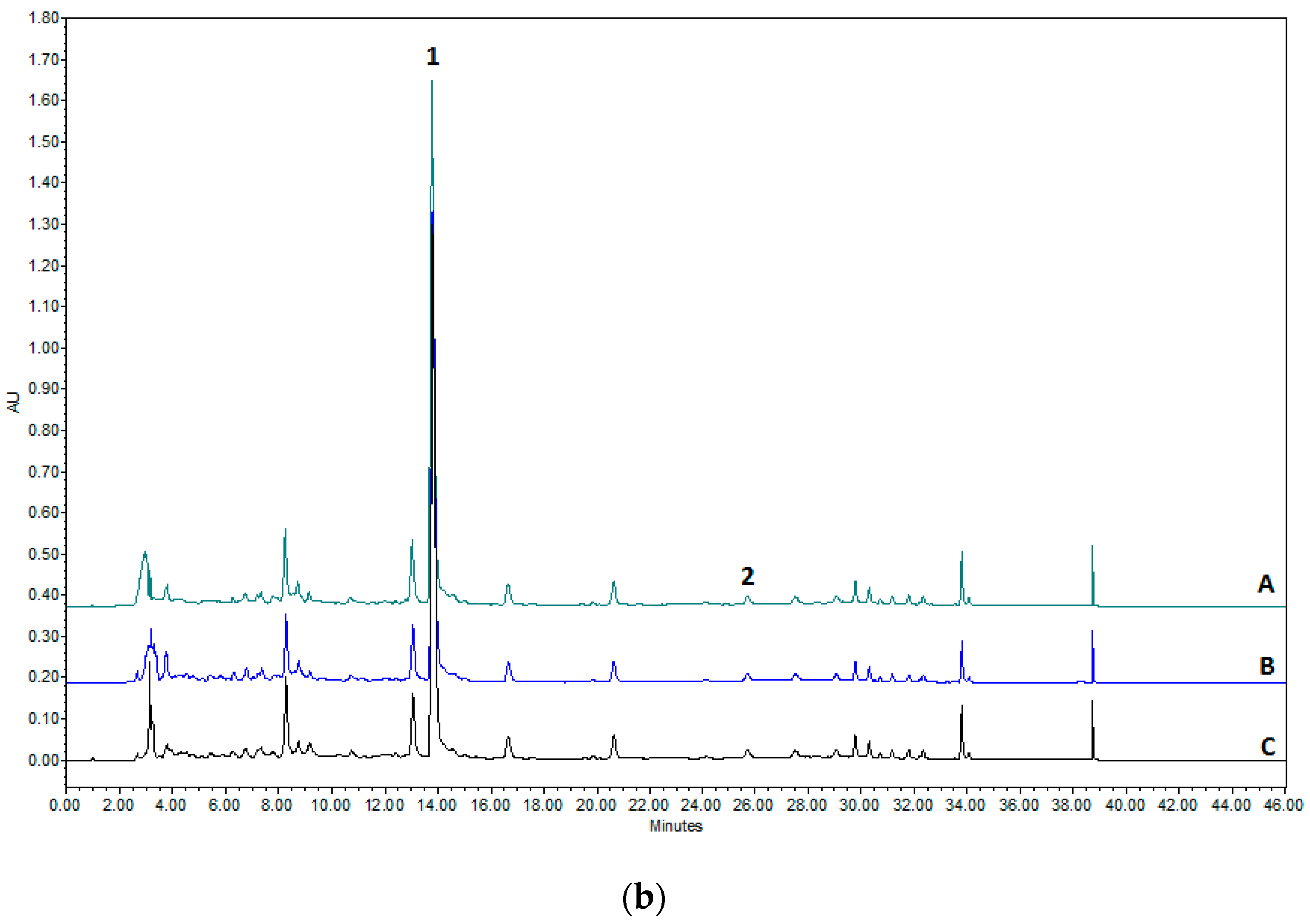
2.2. HPLC Methodology for the Quantification of Naringin and Naringenin
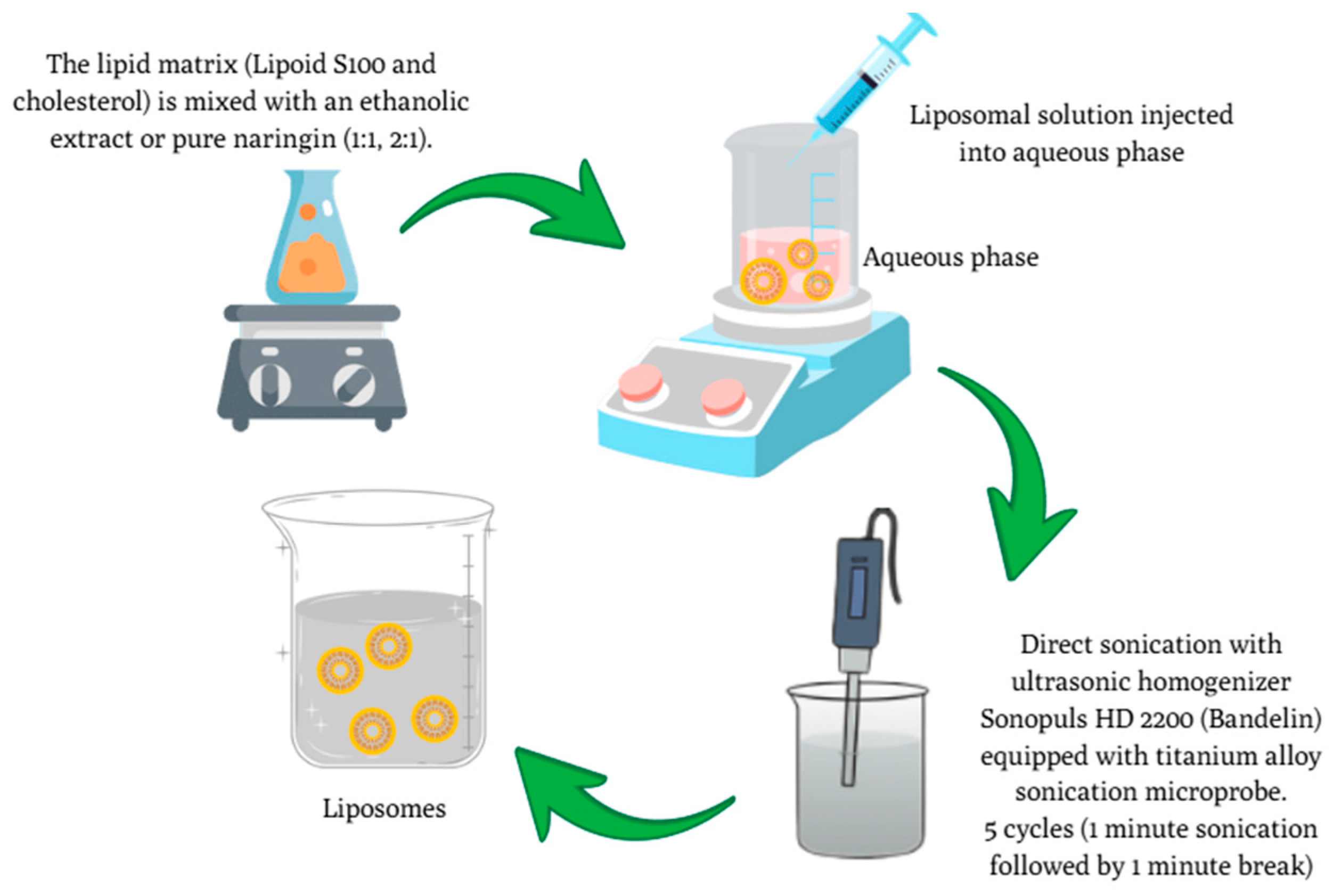
2.3. Preparation of C. paradisi Peel Extracts and Naringin-Loaded Liposomes
2.4. Characterisation of Particle Size Distribution and Zeta Potential
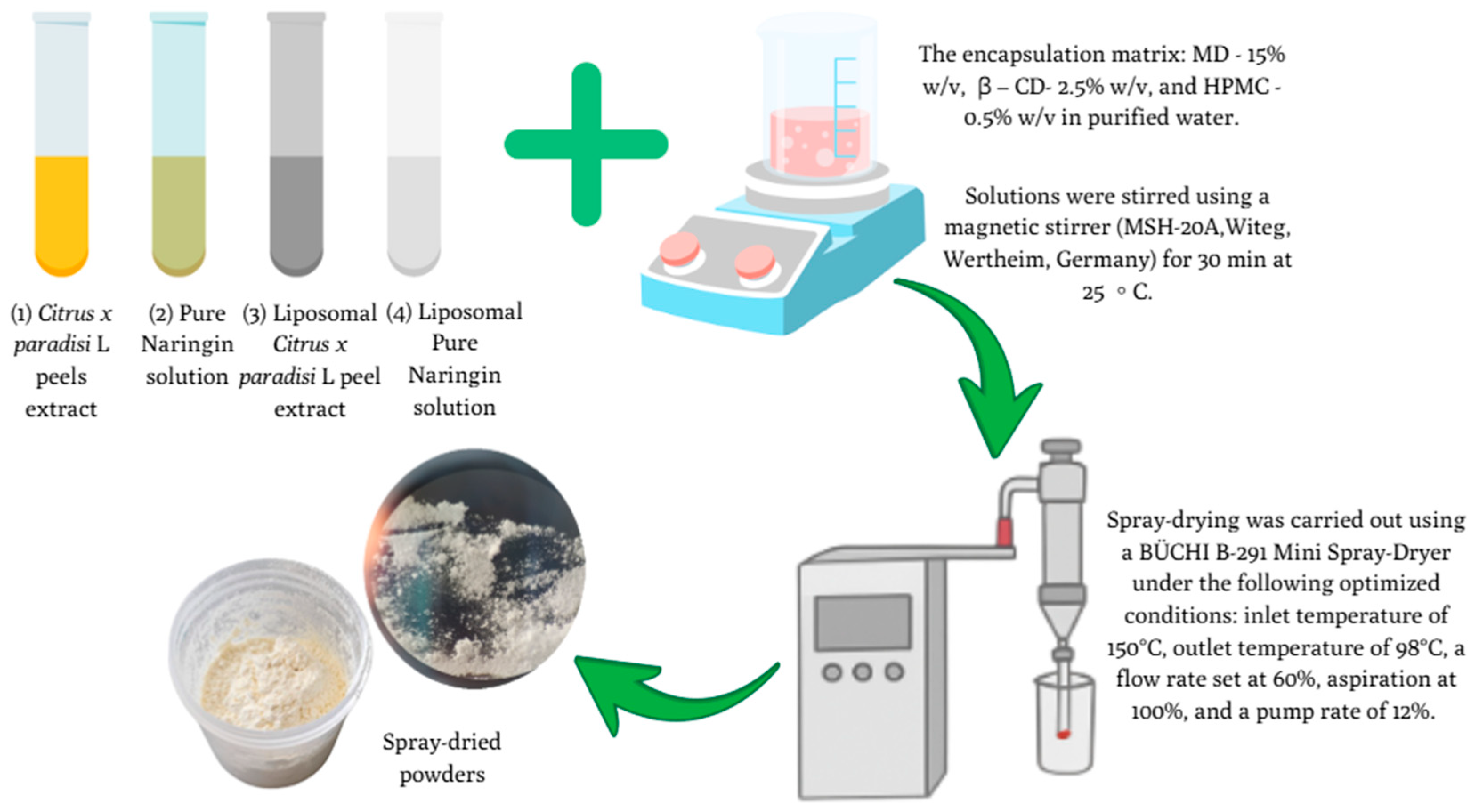
2.5. Spray-Drying Microencapsulation of C. paradisi Peel Extracts and NR Samples
2.6. SEM Analysis of Microcapsules: Morphological Evaluation
2.7. Encapsulation Efficiency (EE)
2.8. Characterisation of Spray-Dried Powders
2.8.1. Yield Calculation
2.8.2. Evaluation of Moisture Content in Spray-Dried Powders
2.8.3. Aqueous Solubility Determination of the Spray-Dried Powders
2.8.4. In Vitro Release Study of NR from Microcapsules and Liposomal Powders
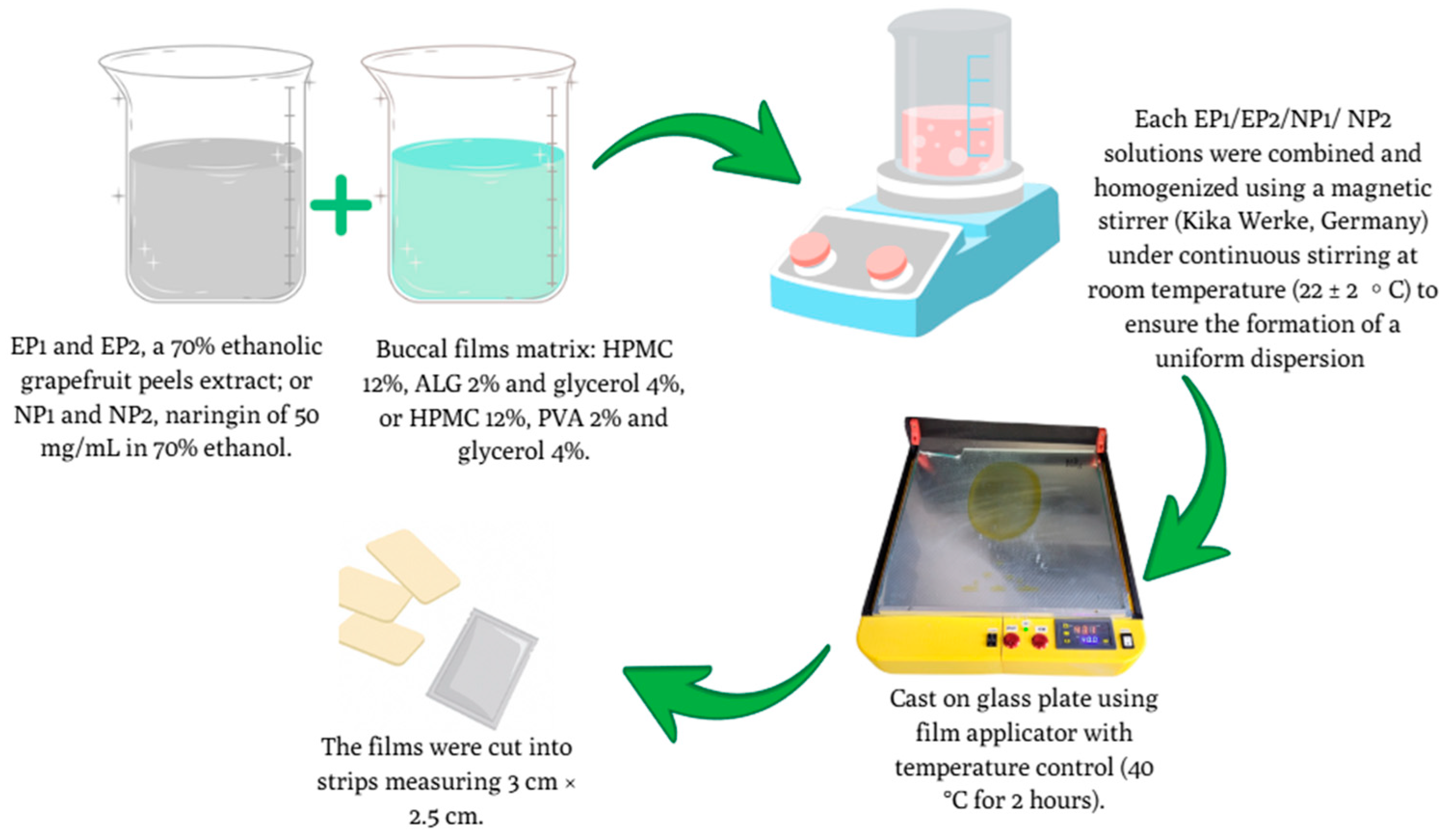
2.9. Preparation of Buccal Films
2.9.1. Texture Analysis of Buccal Films
2.9.2. Buccal Film Moisture Content Determination
2.9.3. In Vitro Release Profile of NR from Buccal Films
2.10. Statistical Analysis
3. Results and Discussion
3.1. Evaluation of Flavonoid Content in Hydroalcoholic Peel Extracts of C. paradisi
3.2. Characterisation of Liposomal Formulations: Particle Size, PDI, and Zeta Potential
3.3. Characterisation of Spray-Dried Microcapsules and Spray-Dried Liposomes
3.3.1. Powder Yield, Moisture Content, and Encapsulation Efficiency
3.3.2. Encapsulation Efficiency
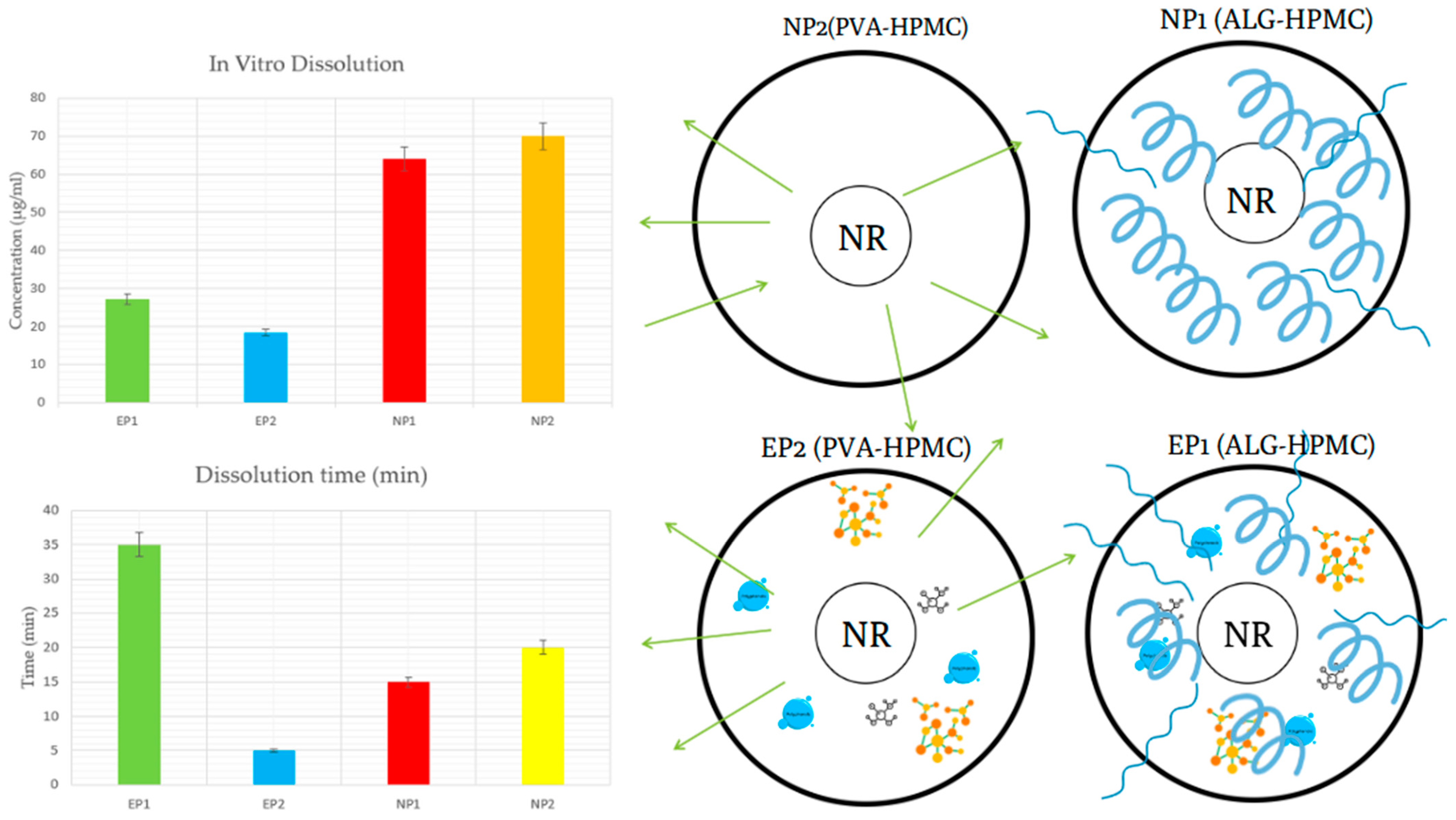
3.3.3. Solubility and Dissolution Efficiency
3.3.4. Theoretical and Dissolved Amounts of NR
3.3.5. Scanning Electron Microscopy of Spray-Dried Powder
3.4. In Vitro Release Profile in Simulated Gastrointestinal Fluids
3.4.1. NR Release in Gastric Phase (30–90 min)
3.4.2. NR Release in Intestinal Phase (120–180 min)
4. Physical Characterisation of Buccal Films
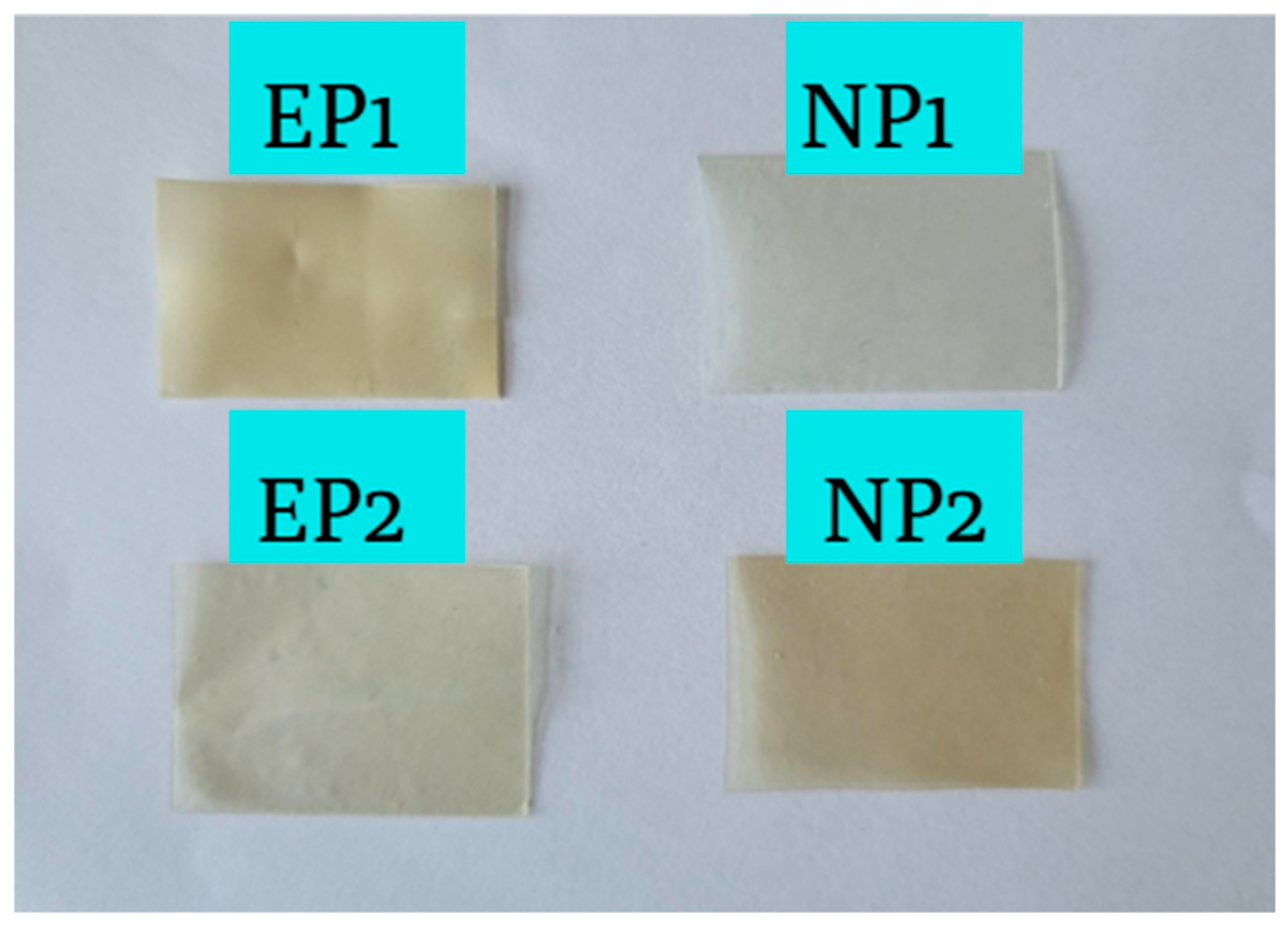
4.1. Visual Appearance of Buccal Films
4.2. Solubility, Moisture Content, and Mucoadhesive Properties of Buccal Films
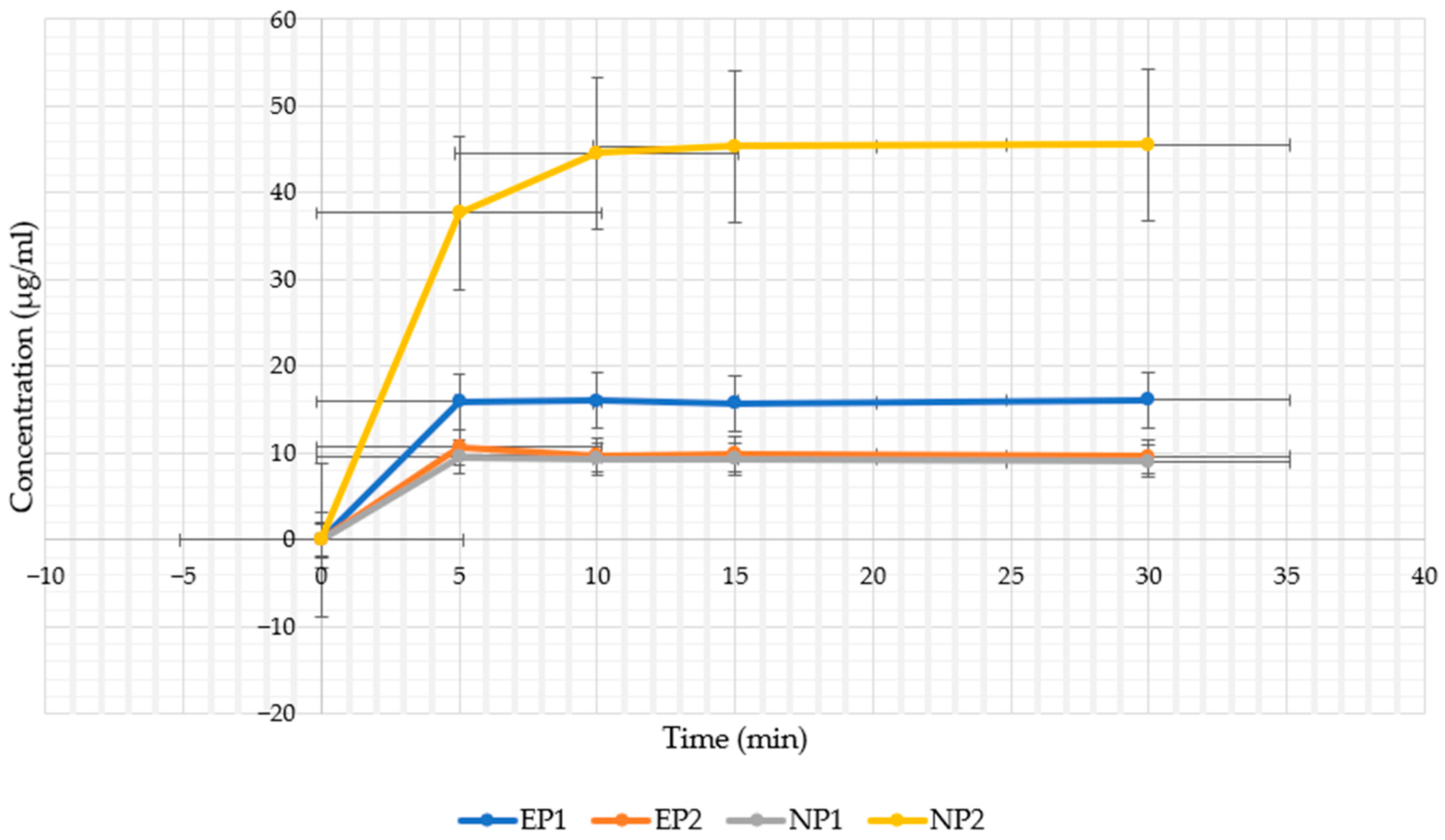
4.3. In Vitro Release Profile in Artificial Saliva
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Russo, C.; Maugeri, A.; Lombardo, G.E.; Musumeci, L.; Barreca, D.; Rapisarda, A.; Cirmi, S.; Navarra, M. The Second Life of Citrus Fruit Waste: A Valuable Source of Bioactive Compounds. Molecules 2021, 26, 5991. [Google Scholar] [CrossRef]
- Eom, S.; Lee, B.-B.; Lee, S.; Park, Y.; Yeom, H.D.; Kim, T.-H.; Nam, S.-H.; Lee, J.H. Antioxidative and Analgesic Effects of Naringin through Selective Inhibition of Transient Receptor Potential Vanilloid Member 1. Antioxidants 2021, 11, 64. [Google Scholar] [CrossRef]
- Ginwala, R.; Bhavsar, R.; Chigbu, D.G.I.; Jain, P.; Khan, Z.K. Potential Role of Flavonoids in Treating Chronic Inflammatory Diseases with a Special Focus on the Anti-Inflammatory Activity of Apigenin. Antioxidants 2019, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Stabrauskiene, J.; Kopustinskiene, D.M.; Lazauskas, R.; Bernatoniene, J. Naringin and Naringenin: Their Mechanisms of Action and the Potential Anticancer Activities. Biomedicines 2022, 10, 1686. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, R.; Gao, Y.; Zheng, Y.; Liao, L.; Cao, Y.; Li, J.; Zhou, W. Encapsulation of Hydrophobic and Low-Soluble Polyphenols into Nanoliposomes by pH-Driven Method: Naringenin and Naringin as Model Compounds. Foods 2021, 10, 963. [Google Scholar] [CrossRef]
- Bai, Y.; Peng, W.; Yang, C.; Zou, W.; Liu, M.; Wu, H.; Fan, L.; Li, P.; Zeng, X.; Su, W. Pharmacokinetics and Metabolism of Naringin and Active Metabolite Naringenin in Rats, Dogs, Humans, and the Differences Between Species. Front. Pharmacol. 2020, 11, 364. [Google Scholar] [CrossRef]
- Ravetti, S.; Garro, A.G.; Gaitán, A.; Murature, M.; Galiano, M.; Brignone, S.G.; Palma, S.D. Naringin: Nanotechnological Strategies for Potential Pharmaceutical Applications. Pharmaceutics 2023, 15, 863. [Google Scholar] [CrossRef]
- Budel, R.G.; da Silva, D.A.; Moreira, M.P.; Dalcin, A.J.F.; da Silva, A.F.; Nazario, L.R.; Majolo, J.H.; Lopes, L.Q.S.; Santos, R.C.V.; Antunes Soares, F.A.; et al. Toxicological Evaluation of Naringin-Loaded Nanocapsules In Vitro and In Vivo. Colloids Surf. B Biointerfaces 2020, 188, 110754. [Google Scholar] [CrossRef] [PubMed]
- Stabrauskiene, J.; Pudziuvelyte, L.; Bernatoniene, J. Optimizing Encapsulation: Comparative Analysis of Spray-Drying and Freeze-Drying for Sustainable Recovery of Bioactive Compounds from Citrus × Paradisi L. Peels. Pharmaceuticals 2024, 17, 596. [Google Scholar] [CrossRef]
- Elkhoury, K.; Sanchez-Gonzalez, L.; Lavrador, P.; Almeida, R.; Gaspar, V.; Kahn, C.; Cleymand, F.; Arab-Tehrany, E.; Mano, J.F. Gelatin Methacryloyl (GelMA) Nanocomposite Hydrogels Embedding Bioactive Naringin Liposomes. Polymers 2020, 12, 2944. [Google Scholar] [CrossRef]
- Caddeo, C.; Pucci, L.; Gabriele, M.; Carbone, C.; Fernàndez-Busquets, X.; Valenti, D.; Pons, R.; Vassallo, A.; Fadda, A.M.; Manconi, M. Stability, Biocompatibility and Antioxidant Activity of PEG-Modified Liposomes Containing Resveratrol. Int. J. Pharm. 2018, 538, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Pearce, K.; Cairncross, S.I.; Benjeddou, M. Liposomal-Naringenin Radiosensitizes Triple-Negative Breast Cancer MDA-MB-231 Cells In Vitro. IET Nanobiotechnol. 2024, 2024, 3786627. [Google Scholar] [CrossRef]
- Ruwizhi, N.; Aderibigbe, B.A. The Efficacy of Cholesterol-Based Carriers in Drug Delivery. Molecules 2020, 25, 4330. [Google Scholar] [CrossRef]
- Krithika, R.; Ravilla, L.; Perumal, S.; Padmini, R. Formulation and Characterization of Naringin—Niosomes for Sustained Drug Delivery and Wound Healing Properties. J. Adv. Zool. 2023, 44, 1579–1598. [Google Scholar] [CrossRef]
- Moammeri, A.; Chegeni, M.M.; Sahrayi, H.; Ghafelehbashi, R.; Memarzadeh, F.; Mansouri, A.; Akbarzadeh, I.; Abtahi, M.S.; Hejabi, F.; Ren, Q. Current Advances in Niosomes Applications for Drug Delivery and Cancer Treatment. Mater. Today Bio. 2023, 23, 100837. [Google Scholar] [CrossRef]
- Imam, S.; Gilani, S.; Zafar, A.; Jumah, M.; Ali, R.; Ahmed, M.; Alshehri, S. Preparation and Optimization of Naringin Oral Nanocarrier: In Vitro Characterization and Antibacterial Activity. Coatings 2022, 12, 1230. [Google Scholar] [CrossRef]
- Kaur, N.; Sharma, P.; Li, X.; Jasti, B. Sublingual Permeability of Model Drugs in New Zealand White Rabbits: In Vitro-In Vivo Correlation. Int. J. Pharm. 2025, 668, 124998. [Google Scholar] [CrossRef]
- Mohamed, E.; Abu Hashim, I.; Yusif, R.; Shaaban, A.; El-Sheakh, A.; Hamed, M.; Badria, F. Polymeric Micelles for Potentiated Antiulcer and Anticancer Activities of Naringin. Int. J. Nanomed. 2018, 13, 1009–1027. [Google Scholar] [CrossRef]
- Stabrauskiene, J.; Marksa, M.; Ivanauskas, L.; Bernatoniene, J. Optimization of Naringin and Naringenin Extraction from Citrus × Paradisi L. Using Hydrolysis and Excipients as Adsorbent. Pharmaceutics 2022, 14, 890. [Google Scholar] [CrossRef] [PubMed]
- Stabrauskiene, J.; Marksa, M.; Ivanauskas, L.; Viskelis, P.; Viskelis, J.; Bernatoniene, J. Citrus × Paradisi L. Fruit Waste: The Impact of Eco-Friendly Extraction Techniques on the Phytochemical and Antioxidant Potential. Nutrients 2023, 15, 1276. [Google Scholar] [CrossRef] [PubMed]
- Raudone, L.; Zymone, K.; Marksa, M.; Cesoniene, L. Phenolic Diversity in Viburnum L. Inflorescences: A Comparative Study of V. Opulus, V. Trilobum, and V. Sargentii. Sci. Hortic. 2025, 350, 114327. [Google Scholar] [CrossRef]
- Ang, S.-S.; Thoo, Y.Y.; Siow, L.F. Encapsulation of Hydrophobic Apigenin into Small Unilamellar Liposomes Coated with Chitosan Through Ethanol Injection and Spray Drying. Food Bioprocess Technol. 2024, 17, 424–439. [Google Scholar] [CrossRef]
- Németh, Z.; Csóka, I.; Semnani Jazani, R.; Sipos, B.; Haspel, H.; Kozma, G.; Kónya, Z.; Dobó, D.G. Quality by Design-Driven Zeta Potential Optimisation Study of Liposomes with Charge Imparting Membrane Additives. Pharmaceutics 2022, 14, 1798. [Google Scholar] [CrossRef] [PubMed]
- Pudziuvelyte, L.; Siauruseviciute, A.; Morkuniene, R.; Lazauskas, R.; Bernatoniene, J. Influence of Technological Factors on the Quality of Chitosan Microcapsules with Boswellia serata L. Essential Oil. Pharmaceutics 2022, 14, 1259. [Google Scholar] [CrossRef]
- Ghaferi, M.; Asadollahzadeh, M.J.; Akbarzadeh, A.; Ebrahimi Shahmabadi, H.; Alavi, S.E. Enhanced Efficacy of PEGylated Liposomal Cisplatin: In Vitro and In Vivo Evaluation. Int. J. Mol. Sci. 2020, 21, 559. [Google Scholar] [CrossRef]
- Kubiliene, A.; Munius, E.; Songailaite, G.; Kokyte, I.; Baranauskaite, J.; Liekis, A.; Sadauskiene, I. A Comparative Evaluation of Antioxidant Activity of Extract and Essential Oil of Origanum onites L. In Vivo. Molecules 2023, 28, 5302. [Google Scholar] [CrossRef]
- Ferreira, L.M.D.M.C.; Pereira, R.R.; Carvalho-Guimarães, F.B.D.; Remígio, M.S.D.N.; Barbosa, W.L.R.; Ribeiro-Costa, R.M.; Silva-Júnior, J.O.C. Microencapsulation by Spray Drying and Antioxidant Activity of Phenolic Compounds from Tucuma Coproduct (Astrocaryum Vulgare Mart.) Almonds. Polymers 2022, 14, 2905. [Google Scholar] [CrossRef] [PubMed]
- Thuong Nhan, N.P.; Tan Thanh, V.; Huynh Cang, M.; Lam, T.D.; Cam Huong, N.; Hong Nhan, L.T.; Thanh Truc, T.; Tran, Q.T.; Bach, L.G. Microencapsulation of Lemongrass (Cymbopogon Citratus) Essential Oil Via Spray Drying: Effects of Feed Emulsion Parameters. Processes 2020, 8, 40. [Google Scholar] [CrossRef]
- Kazlauskaite, J.A.; Matulyte, I.; Marksa, M.; Bernatoniene, J. Nutmeg Essential Oil, Red Clover, and Liquorice Extracts Microencapsulation Method Selection for the Release of Active Compounds from Gel Tablets of Different Bases. Pharmaceutics 2023, 15, 949. [Google Scholar] [CrossRef] [PubMed]
- Pudžiuvelytė, L.; Drulytė, E.; Bernatonienė, J. Nitrocellulose Based Film-Forming Gels with Cinnamon Essential Oil for Covering Surface Wounds. Polymers 2023, 15, 1057. [Google Scholar] [CrossRef]
- Bolko Seljak, K.; Grilc, B.; Gašperlin, M.; Gosenca Matjaž, M. Ibuprofen-Loaded, Nanocellulose-Based Buccal Films: The Development and Evaluation of Promising Drug Delivery Systems for Special Populations. Gels 2025, 11, 163. [Google Scholar] [CrossRef]
- Khan, M.S.; Ahmad, R.N.K.; Badruddoja, A.; Akhtar, M.M.J.S.; Rafeeque, S.B.M.; Ahmed, A.A.M.Z.; Shafiullah, F.S.M.; Kha-war, M.M.A.A.; Ahmed, W.A.N.; Iftekhar, A.A. Development and Evaluation of Mucoadhesive Buccal Films for the Sustained Release of Diclofenac Sodium: An Innovative Approach for Pain Management. World J. Adv. Eng. Technol. Sci. 2024, 13, 814–830. [Google Scholar] [CrossRef]
- Hassan, A.A.A.; Kristó, K.; Ibrahim, Y.H.-E.Y.; Regdon, G.; Sovány, T. Quality by Design-Guided Systematic Development and Optimization of Mucoadhesive Buccal Films. Pharmaceutics 2023, 15, 2375. [Google Scholar] [CrossRef] [PubMed]
- Maslii, Y.; Herbina, N.; Dene, L.; Ivanauskas, L.; Matulis, G.; Bernatoniene, J. Mucoadhesive Polymeric Film with Plant-Based Compounds for Dental Applications: Formulation, Characterization and Evaluation. Pharm. Dev. Technol. 2025, 30, 505–520. [Google Scholar] [CrossRef]
- Matulyte, I.; Marksa, M.; Bernatoniene, J. Development of Innovative Chewable Gel Tablets Containing Nutmeg Essential Oil Microcapsules and Their Physical Properties Evaluation. Pharmaceutics 2021, 13, 873. [Google Scholar] [CrossRef]
- Chen, J.; Li, S.; Xu, J.; Ding, F.; Wang, Z.; Cheng, Y.; Deng, X. Concentration and Distribution of Main Bitter Compounds in Fruit Tissues of ‘Oroblanco’ (Citrus grandis L.× Citrus paradisi Macf.). Sci. Hortic. 2015, 193, 84–89. [Google Scholar] [CrossRef]
- Lindsay, S.; Tumolva, O.; Khamiakova, T.; Coppenolle, H.; Kovarik, M.; Shah, S.; Holm, R.; Perrie, Y. Can We Simplify Liposome Manufacturing Using a Complex DoE Approach? Pharmaceutics 2024, 16, 1159. [Google Scholar] [CrossRef]
- Opatha, S.A.T.; Titapiwatanakun, V.; Chutoprapat, R. Transfersomes: A Promising Nanoencapsulation Technique for Transdermal Drug Delivery. Pharmaceutics 2020, 12, 855. [Google Scholar] [CrossRef]
- Soema, P.C.; Willems, G.-J.; Jiskoot, W.; Amorij, J.-P.; Kersten, G.F. Predicting the Influence of Liposomal Lipid Composition on Liposome Size, Zeta Potential and Liposome-Induced Dendritic Cell Maturation Using a Design of Experiments Approach. Eur. J. Pharm. Biopharm. 2015, 94, 427–435. [Google Scholar] [CrossRef]
- Koehler, J.K.; Schnur, J.; Heerklotz, H.; Massing, U. Screening for Optimal Liposome Preparation Conditions by Using Dual Centrifugation and Time-Resolved Fluorescence Measurements. Pharmaceutics 2021, 13, 2046. [Google Scholar] [CrossRef]
- Chen, C.; Chen, C.; Li, Y.; Gu, R.; Yan, X. Characterization of Lipid-Based Nanomedicines at the Single-Particle Level. Fundam. Res. 2023, 3, 488–504. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, K.; Kaplan, M.; Çalış, S. Effects of Nanoparticle Size, Shape, and Zeta Potential on Drug Delivery. Int. J. Pharm. 2024, 666, 124799. [Google Scholar] [CrossRef]
- Calandra, P.; Abe, A.A.; Scavo, A.; Bruno, L.; Rossi, C.O.; Caputo, P. Novel Microscopic Approach to Particle Size Evaluation in Colloidal Systems. Appl. Sci. 2024, 14, 3567. [Google Scholar] [CrossRef]
- Mardani, M.; Siahtiri, S.; Besati, M.; Baghani, M.; Baniassadi, M.; Nejad, A.M. Microencapsulation of Natural Products Using Spray Drying; an Overview. J. Microencapsul. 2024, 41, 649–678. [Google Scholar] [CrossRef]
- Kyriakoudi, A.; Spanidi, E.; Mourtzinos, I.; Gardikis, K. Innovative Delivery Systems Loaded with Plant Bioactive Ingredients: Formulation Approaches and Applications. Plants 2021, 10, 1238. [Google Scholar] [CrossRef]
- Safaeian Laein, S.; Samborska, K.; Can Karaca, A.; Mostashari, P.; Akbarbaglu, Z.; Sarabandi, K.; Jafari, S.M. Strategies for Further Stabilization of Lipid-Based Delivery Systems with a Focus on Solidification by Spray-Drying. Trends Food Sci. Technol. 2024, 146, 104412. [Google Scholar] [CrossRef]
- Buljeta, I.; Pichler, A.; Šimunović, J.; Kopjar, M. Polysaccharides as Carriers of Polyphenols: Comparison of Freeze-Drying and Spray-Drying as Encapsulation Techniques. Molecules 2022, 27, 5069. [Google Scholar] [CrossRef]
- Cegledi, E.; Garofulić, I.E.; Zorić, Z.; Roje, M.; Dragović-Uzelac, V. Effect of Spray Drying Encapsulation on Nettle Leaf Extract Powder Properties, Polyphenols and Their Bioavailability. Foods 2022, 11, 2852. [Google Scholar] [CrossRef]
- Papoutsis, K.; Golding, J.B.; Vuong, Q.; Pristijono, P.; Stathopoulos, C.E.; Scarlett, C.J.; Bowyer, M. Encapsulation of Citrus By-Product Extracts by Spray-Drying and Freeze-Drying Using Combinations of Maltodextrin with Soybean Protein and ι-Carrageenan. Foods 2018, 7, 115. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, M.; Lin, X.; Zheng, X.; Qi, H.; Chen, J.; Zeng, X.; Bai, W.; Xiao, G. Biological Activities and Solubilization Methodologies of Naringin. Foods 2023, 12, 2327. [Google Scholar] [CrossRef] [PubMed]
- Katukam, L.P.; Padakanti, A.P.; Chella, N. Upscaling Co-Amorphous Formulation of Naringin-Quinacrine Dihydrochloride Translating from Laboratory to Pilot Scale Using Spray Drying for Improved Physicochemical and Mechanical Properties. Powder Technol. 2025, 451, 120458. [Google Scholar] [CrossRef]
- Da Costa, R.S.; Teixeira, C.B.; Gabbay Alves, T.V.; Ribeiro-Costa, R.M.; Casazza, A.A.; Aliakbarian, B.; Converti, A.; Silva Júnior, J.O.C.; Perego, P. Optimization of Spray Drying Conditions to Microencapsulate Cupuassu (Theobroma grandiflorum) Seed by-Product Extract. Nat. Prod. Res. 2019, 33, 2600–2608. [Google Scholar] [CrossRef] [PubMed]
- Mohammadalinejhad, S.; Kurek, M.A. Microencapsulation of Anthocyanins—Critical Review of Techniques and Wall Materials. Appl. Sci. 2021, 11, 3936. [Google Scholar] [CrossRef]
- Gaćina, N.; Elez Garofulić, I.; Zorić, Z.; Pedisić, S.; Dragović-Uzelac, V. Influence of Encapsulation Parameters on the Retention of Polyphenols in Blackthorn Flower Extract. Processes 2022, 10, 2517. [Google Scholar] [CrossRef]
- Dejeu, I.L.; Vicaș, L.G.; Marian, E.; Ganea, M.; Frenț, O.D.; Maghiar, P.B.; Bodea, F.I.; Dejeu, G.E. Innovative Approaches to Enhancing the Biomedical Properties of Liposomes. Pharmaceutics 2024, 16, 1525. [Google Scholar] [CrossRef]
- Ashfaq, R.; Rasul, A.; Asghar, S.; Kovács, A.; Berkó, S.; Budai-Szűcs, M. Lipid Nanoparticles: An Effective Tool to Improve the Bioavailability of Nutraceuticals. Int. J. Mol. Sci. 2023, 24, 15764. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, W.; Zhang, J.; Juan, B.; Zhu, Y.; Zhu, L.; Zhao, Y.; Daglia, M.; Xiao, X.; He, Y. Advances in Intestinal-Targeted Release of Phenolic Compounds. Nutrients 2025, 17, 2598. [Google Scholar] [CrossRef]
- Desai, S.D.; Kundu, I.; Swamy, N.P.; Crull, G.B.; Pan, D.; Zhao, J.; Shah, R.P.; Venkatesh, C.; Vig, B.; Varia, S.A.; et al. Cross-Linking of Poly (Vinyl Alcohol) Films under Acidic and Thermal Stress. Eur. J. Pharm. Sci. 2020, 152, 105429. [Google Scholar] [CrossRef]
- Bayer, I.S. Recent Advances in Mucoadhesive Interface Materials, Mucoadhesion Characterization, and Technologies. Adv. Mater. Inter. 2022, 9, 2200211. [Google Scholar] [CrossRef]
- Al-Sahaf, Z.; Raimi-Abraham, B.; Licciardi, M.; De Mohac, L.M. Influence of Polyvinyl Alcohol (PVA) on PVA-Poly-N-Hydroxyethyl-Aspartamide (PVA-PHEA) Microcrystalline Solid Dispersion Films. AAPS PharmSciTech 2020, 21, 267. [Google Scholar] [CrossRef]
- Hazt, B.; Read, D.J.; Harlen, O.G.; Poon, W.C.K.; O’Connell, A.; Sarkar, A. Mucoadhesion across Scales: Towards the Design of Protein-Based Adhesives. Adv. Colloid Interface Sci. 2024, 334, 103322. [Google Scholar] [CrossRef]
- Lai, J.; Azad, A.K.; Sulaiman, W.M.A.W.; Kumarasamy, V.; Subramaniyan, V.; Alshehade, S.A. Alginate-Based Encapsulation Fabrication Technique for Drug Delivery: An Updated Review of Particle Type, Formulation Technique, Pharmaceutical Ingredient, and Targeted Delivery System. Pharmaceutics 2024, 16, 370. [Google Scholar] [CrossRef]
- Shipp, L.; Liu, F.; Kerai-Varsani, L.; Okwuosa, T.C. Buccal Films: A Review of Therapeutic Opportunities, Formulations & Relevant Evaluation Approaches. J. Control. Release 2022, 352, 1071–1092. [Google Scholar] [CrossRef]
- Pérez Zamora, C.M.; Michaluk, A.G.; Chiappetta, D.A.; Nuñez, M.B. Herbal Buccal Films with In Vitro Antibacterial and Anti-Inflammatory Effects. J. Herb. Med. 2022, 31, 100527. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Boddu, S.H.S.; Gorain, B.; Sreeharsha, N.; Shah, J. An Updated Overview of the Emerging Role of Patch and Film-Based Buccal Delivery Systems. Pharmaceutics 2021, 13, 1206. [Google Scholar] [CrossRef] [PubMed]
- El Sharawy, A.M.; Shukr, M.H.; Elshafeey, A.H. Formulation and Optimization of Duloxetine Hydrochloride Buccal Films: In Vitro and In Vivo Evaluation. Drug Deliv. 2017, 24, 1762–1769. [Google Scholar] [CrossRef]
- Elgharbawy, A.S.; El Demerdash, A.-G.M.; Sadik, W.A.; Kasaby, M.A.; Lotfy, A.H.; Osman, A.I. Enhancing the Biodegradability, Water Solubility, and Thermal Properties of Polyvinyl Alcohol through Natural Polymer Blending: An Approach toward Sustainable Polymer Applications. Polymers 2024, 16, 2141. [Google Scholar] [CrossRef]
- Dołowacka-Jóźwiak, A.; Nawrot-Hadzik, I.; Matkowski, A.; Ciecieląg, T.; Gawin-Mikołajewicz, A.; Dudek-Wicher, R.; Prochoń, M.; Markowska, D.; Adamski, R.; Wiater, A.; et al. Mucoadhesive PVA Film for Sustained Resveratrol Delivery: Formulation, Characterization, and Release Profile. Molecules 2025, 30, 2642. [Google Scholar] [CrossRef] [PubMed]
- Pamlényi, K.; Kristó, K.; Jójárt-Laczkovich, O.; Regdon, G. Formulation and Optimization of Sodium Alginate Polymer Film as a Buccal Mucoadhesive Drug Delivery System Containing Cetirizine Dihydrochloride. Pharmaceutics 2021, 13, 619. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, P.; Liu, T.; Zhou, L.; Zhang, L.; Lin, R.; Yang, G.; Wang, W.; Li, Y. Solubility of Naringin in Ethanol and Water Mixtures from 283.15 to 318.15K. J. Mol. Liq. 2015, 203, 98–103. [Google Scholar] [CrossRef]




| Composition | |||
|---|---|---|---|
| Formulation ID * | Lipoid S100 | Cholesterol | Total Lipid Phase: Extract or Naringin Ratio |
| EL1 | 100 mg | 10 mg | 1:1 |
| EL2 | 200 mg | 20 mg | 2:1 |
| NL1 | 100 mg | 10 mg | 1:1 |
| NL2 | 200 mg | 20 mg | 2:1 |
| Component | EP1 | EP2 | NP1 | NP2 |
|---|---|---|---|---|
| (HPMC) | 12% | 12% | 12% | 12% |
| (ALG) | 2% | – | 2% | – |
| (PVA) | – | 2% | – | 2% |
| Glycerol | 4% | 4% | 4% | 4% |
| 70% Ethanolic Peel Extract/NR Solution | 70% | 70% | 70% | 70% |
| Purified Water | 30% | 30% | 30% | 30% |
| Formulation | Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|
| EL1 | 101.5 ± 5.08 a | 0.362 ± 0.018 c | −17.5 ± 0.88 b |
| EL2 | 93.93 ± 4.70 b | 0.144 ± 0.007 a | −20.3 ± 1.02 bc |
| NL1 | 98.57 ± 4.93 ab | 0.225 ± 0.011 b | −10.4 ± 0.52 a |
| NL2 | 96.96 ± 4.85 ab | 0.151 ± 0.017 c | −25.8 ± 1.29 c |
| Sample ID | Powder Yield (%) | Moisture Content (%) | Encapsulation Efficiency (EE) (%) | Solubility NR (µg/mL) |
|---|---|---|---|---|
| ES | 43.00 ± 2.15 | 4.12 ± 0.206 | 90.91 ± 4.54 | 138.80 ± 4.25 * |
| ELS | 41.05 ± 1.60 | 3.81 ± 0.19 | 99.36 ± 4.96 * | 17.36 ± 1.01 |
| NS | 36.70 ± 1.83 | 4.21 ± 0.21 | 81.08 ± 4.05 | 306.42 ± 10.34 * |
| NLS | 38.15 ± 1.91 | 5.58 ± 0.279 * | 94.60 ± 4.73 * | 93.32 ± 6.01 |
| Sample ID | Theoretical Amount of NR (mg) | Dissolved Amount of NR (mg) | Dissolution Efficiency DE (%) |
|---|---|---|---|
| NS | 163.82 ± 2.10 | 9.19 ± 0.31 * | 5.6 ± 0.28 |
| NLS | 6.55 ± 0.22 | 2.80 ± 0.18 * | 42.7 ± 2.13 |
| ES | 4.53 ± 0.15 | 4.16 ± 0.12 | 91.8 ± 4.59 |
| ELS | 0.94 ± 0.05 | 0.52 ± 0.03 | 55.3 ± 2.76 |
| Sample ID * | In Vitro Dissolution Test (µg/mL) | Dissolution Efficiency (%) | Moisture Content (%) | Peak Force (Adhesiveness N) | Work of Adhesion (N·s) |
|---|---|---|---|---|---|
| EP1 | 27.08 ± 1.42 | 40.2 | 13.48 ± 0.45 | 0.02 ± 0.005 | −0.53 ± 0.12 |
| EP2 | 18.45 ± 1.05 | 26.6 | 15.25 ± 0.52 | 0.07 ± 0.01 | −0.25 ± 0.008 |
| NP1 | 63.99 ± 2.64 | 37.5 | 11.46 ± 0.40 | 0.08 ± 0.01 | 0.46 ± 0.10 |
| NP2 | 69.97 ± 3.01 | 40.9 | 11.49 ± 0.42 | 0.09 ± 0.01 | 0.47 ± 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stabrauskiene, J.; Marksa, M.; Bernatoniene, J. Comparative In Vitro Evaluation of Buccal Films, Microcapsules, and Liposomal Systems for Naringin and Citrus × paradisi L. Peel Extract: Effects of Encapsulation Strategy and Compound Origin on Release Profiles. Pharmaceutics 2025, 17, 1311. https://doi.org/10.3390/pharmaceutics17101311
Stabrauskiene J, Marksa M, Bernatoniene J. Comparative In Vitro Evaluation of Buccal Films, Microcapsules, and Liposomal Systems for Naringin and Citrus × paradisi L. Peel Extract: Effects of Encapsulation Strategy and Compound Origin on Release Profiles. Pharmaceutics. 2025; 17(10):1311. https://doi.org/10.3390/pharmaceutics17101311
Chicago/Turabian StyleStabrauskiene, Jolita, Mindaugas Marksa, and Jurga Bernatoniene. 2025. "Comparative In Vitro Evaluation of Buccal Films, Microcapsules, and Liposomal Systems for Naringin and Citrus × paradisi L. Peel Extract: Effects of Encapsulation Strategy and Compound Origin on Release Profiles" Pharmaceutics 17, no. 10: 1311. https://doi.org/10.3390/pharmaceutics17101311
APA StyleStabrauskiene, J., Marksa, M., & Bernatoniene, J. (2025). Comparative In Vitro Evaluation of Buccal Films, Microcapsules, and Liposomal Systems for Naringin and Citrus × paradisi L. Peel Extract: Effects of Encapsulation Strategy and Compound Origin on Release Profiles. Pharmaceutics, 17(10), 1311. https://doi.org/10.3390/pharmaceutics17101311







