Dual-Cross-Linked Alginate Hydrogels as a Strategy to Improve the Antifungal Properties of Posaconazole
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture Materials
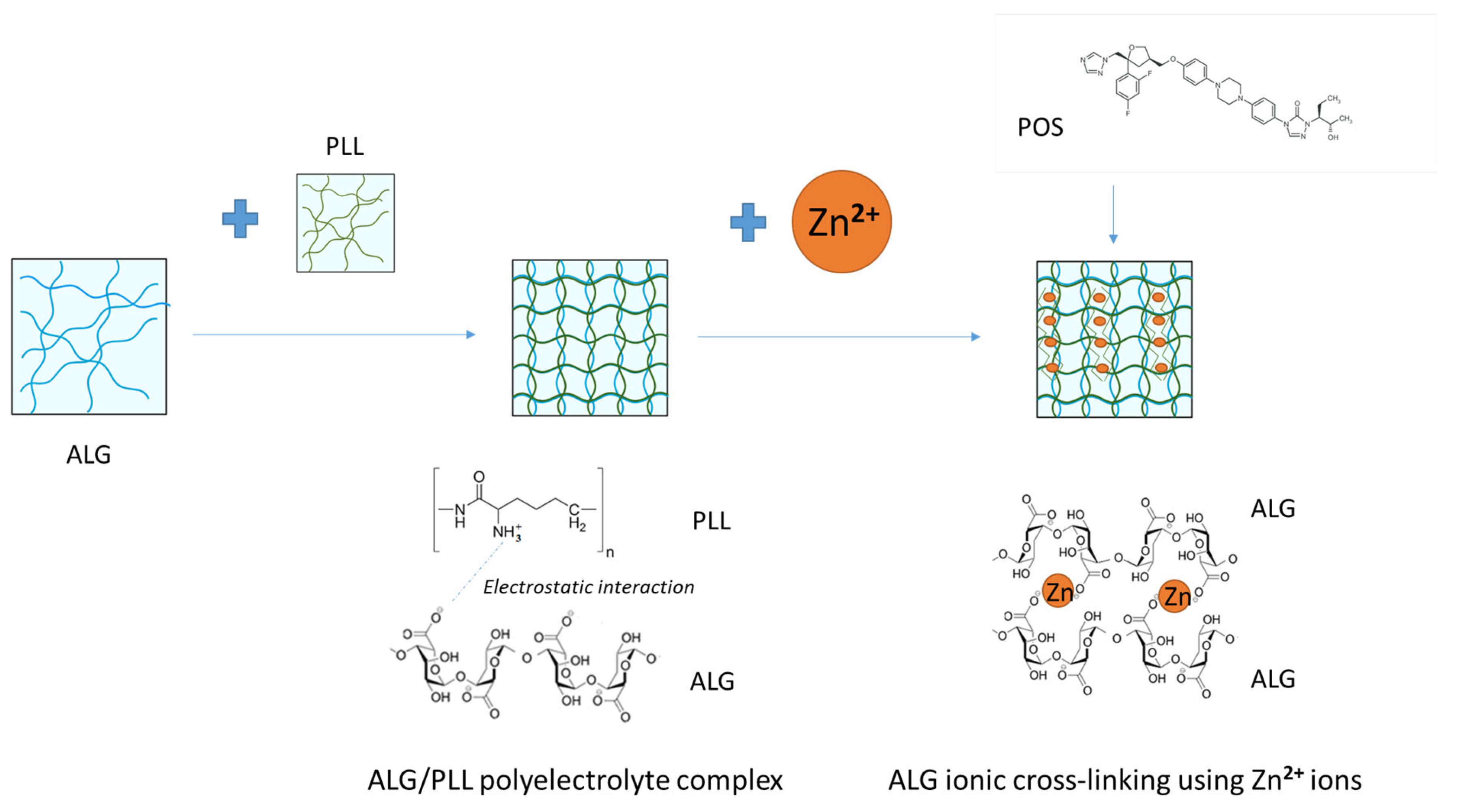
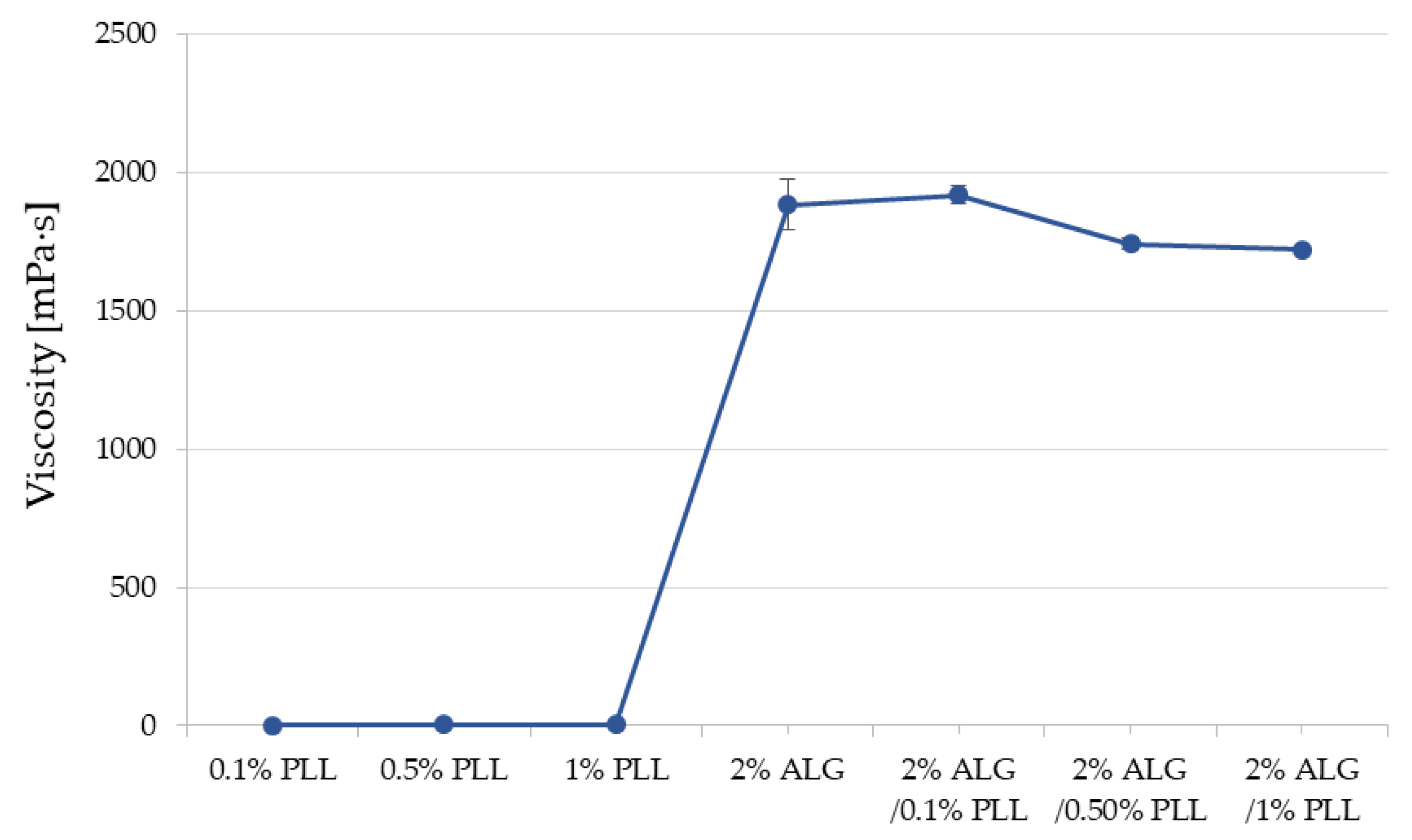
2.2. ALG/PLL Polyelectrolyte Complex Development and Evaluation
2.3. Dual-Cross-Linked Hydrogels Formation
2.4. Dual-Cross-Linked Hydrogel Assessment
2.4.1. Solid-State Characterization
2.4.2. POS Content and Particle Analysis
High-Performance Liquid Chromatography (HPLC) Method
2.4.3. Viscosity and Rheological Properties Analysis
2.4.4. Texture Analysis and Bioadhesiveness
2.4.5. In Vitro POS Release
2.4.6. Thermal Analysis Performance and Attenuated Total Reflectance–Fourier Transform Infrared Spectroscopy (ATR–FTIR)
2.4.7. Antifungal Activity
Agar Diffusion Method
Activity of Tested Formulations Against Planktonic and Biofilm Forms of Candida Fungi
2.4.8. Assessment of Biocompatibility of Tested Formulations Against Human Skin Fibroblasts
2.5. Statistical Analysis
3. Results
3.1. Turbidity and Viscosity Characteristics
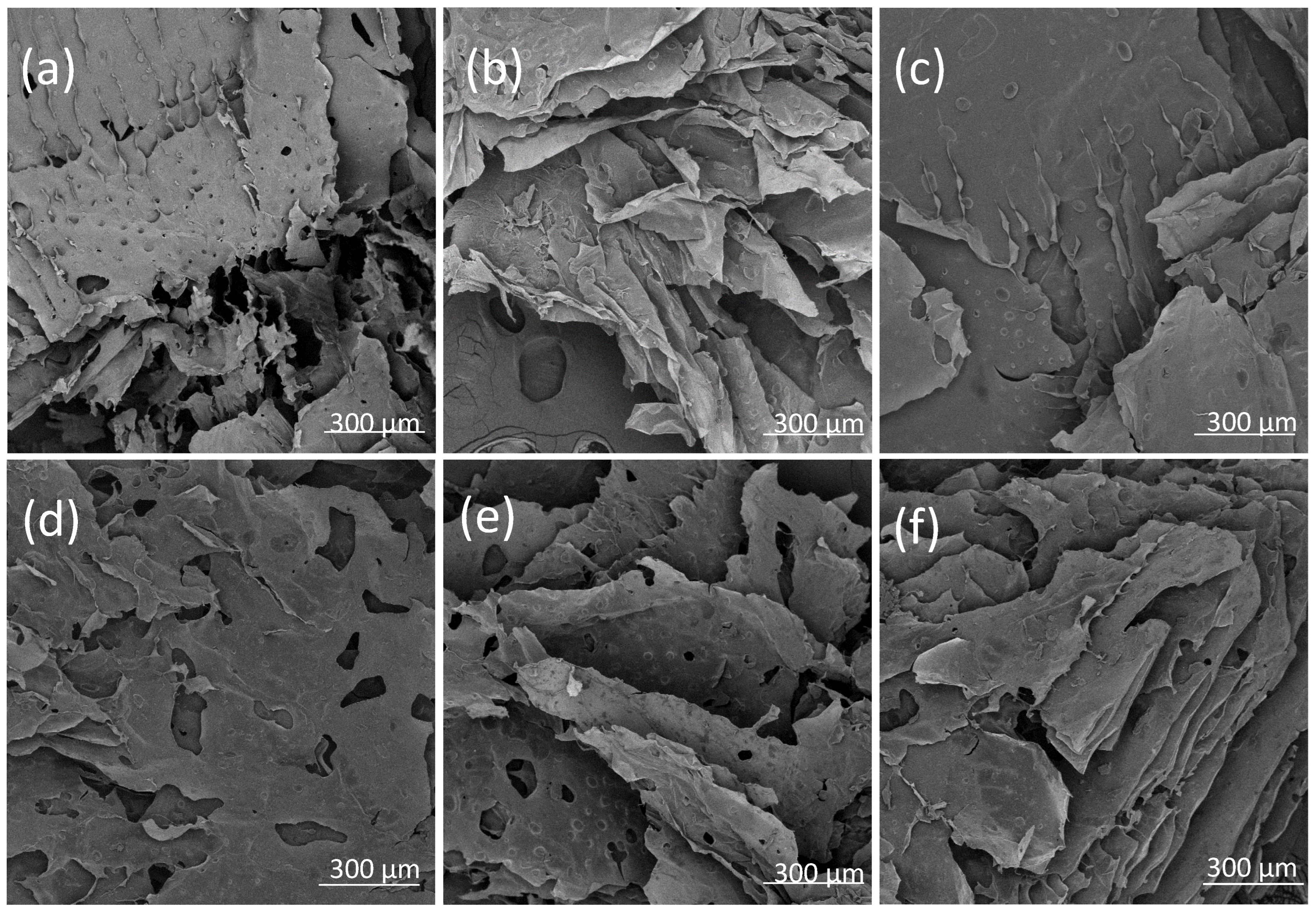
3.2. Assessment of ALG Dual-Cross-Linked Hydrogel Properties
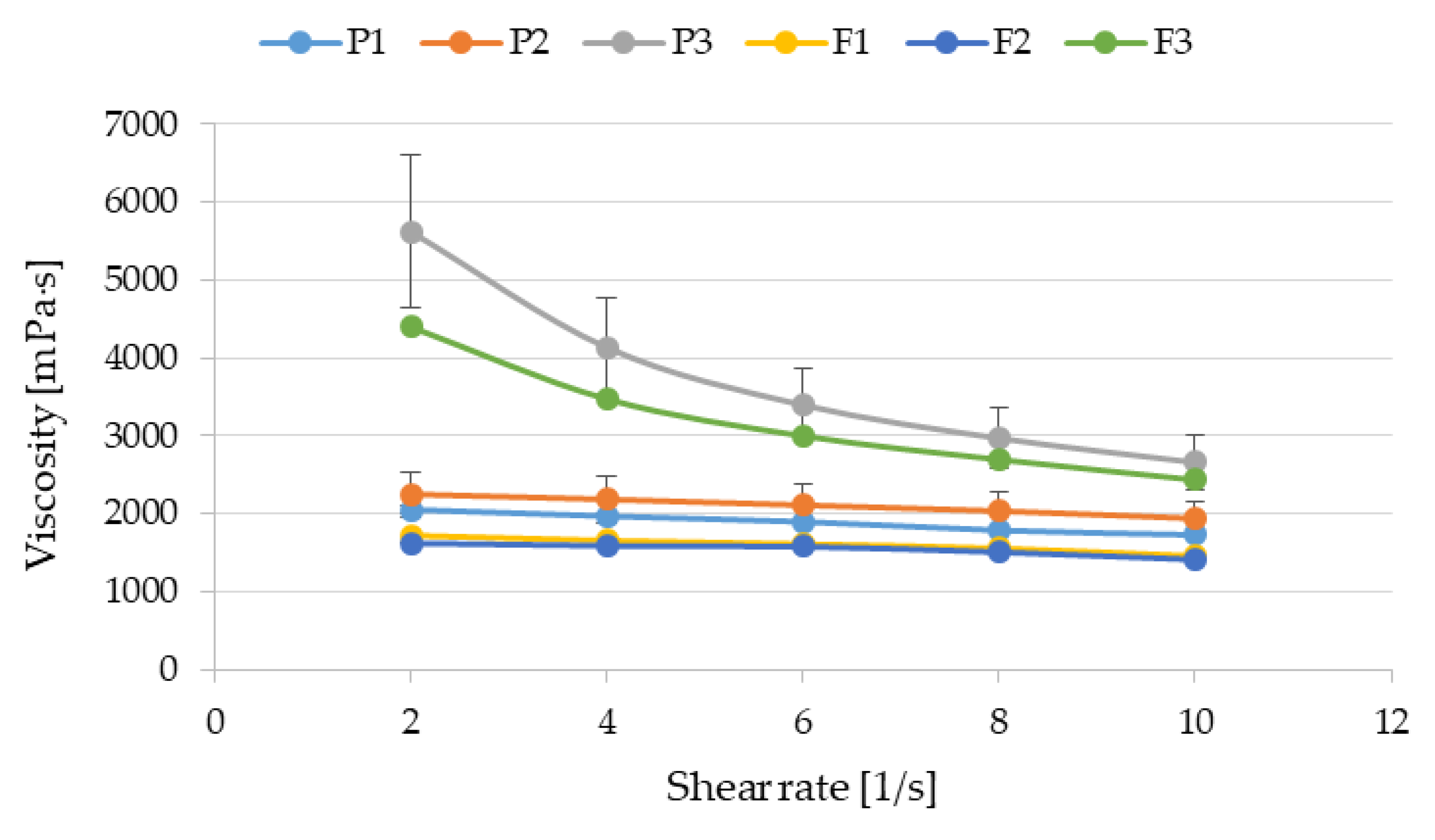
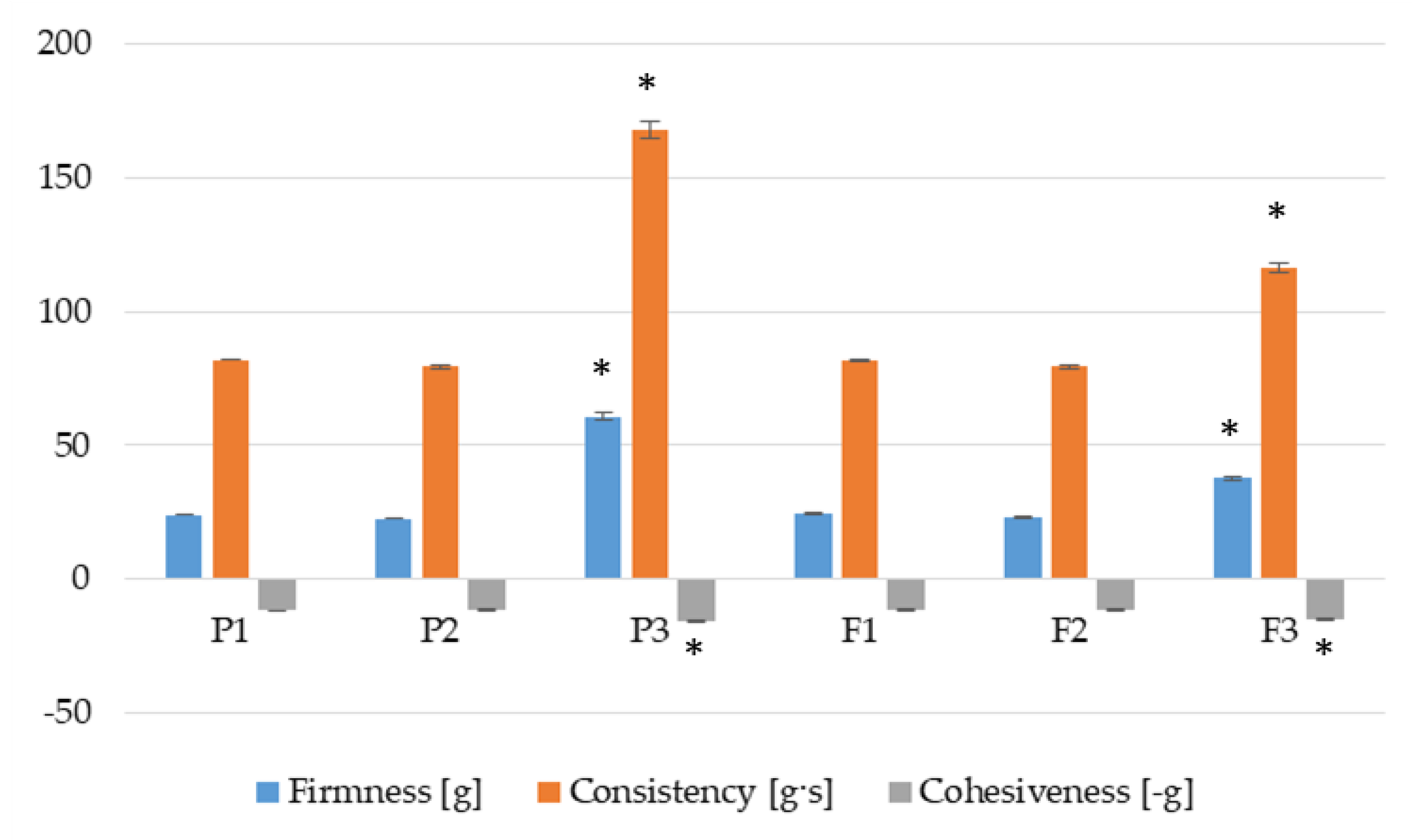
3.3. Flow Curves and Textural Properties
3.4. Bioadhesive Properties
3.5. POS Release
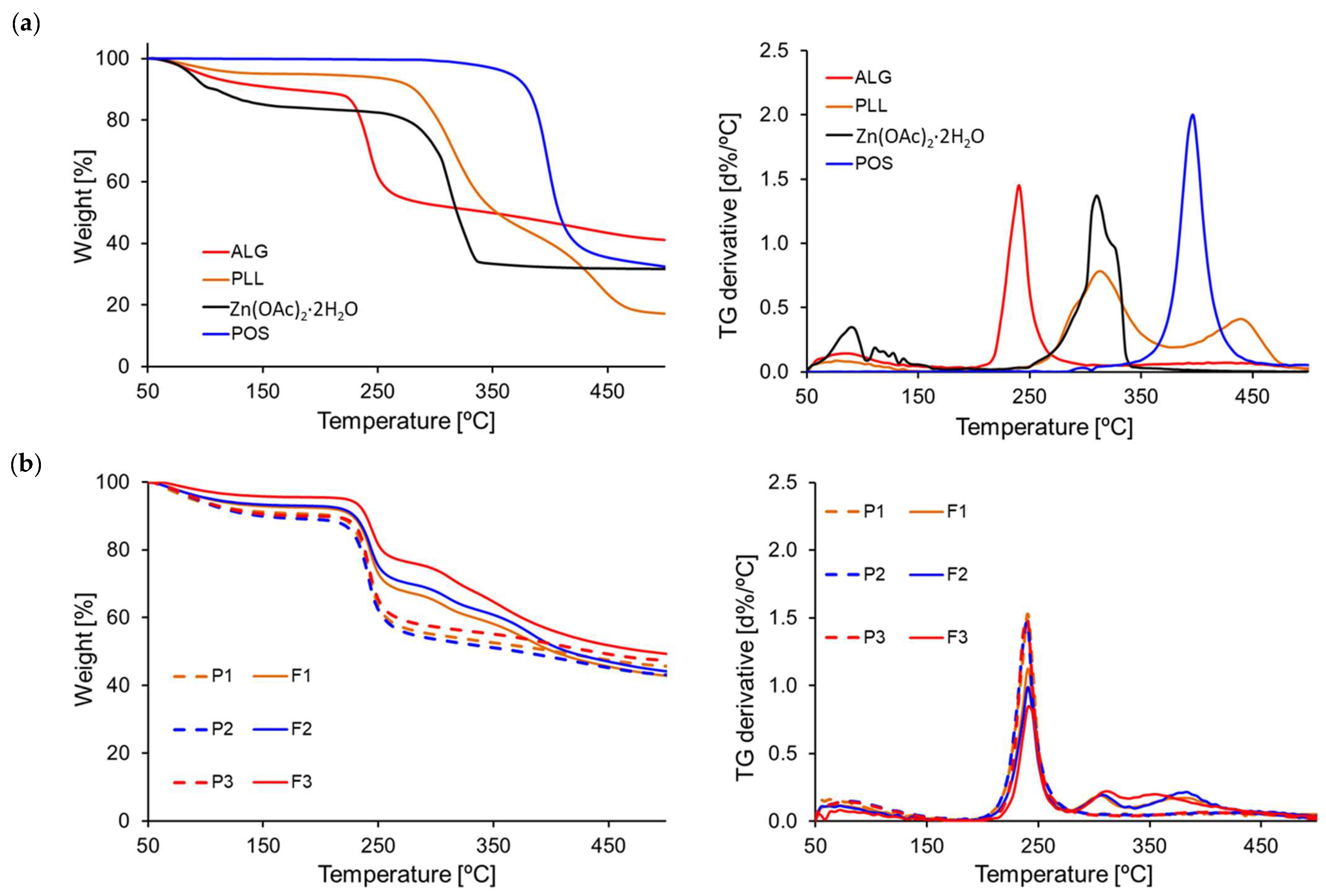
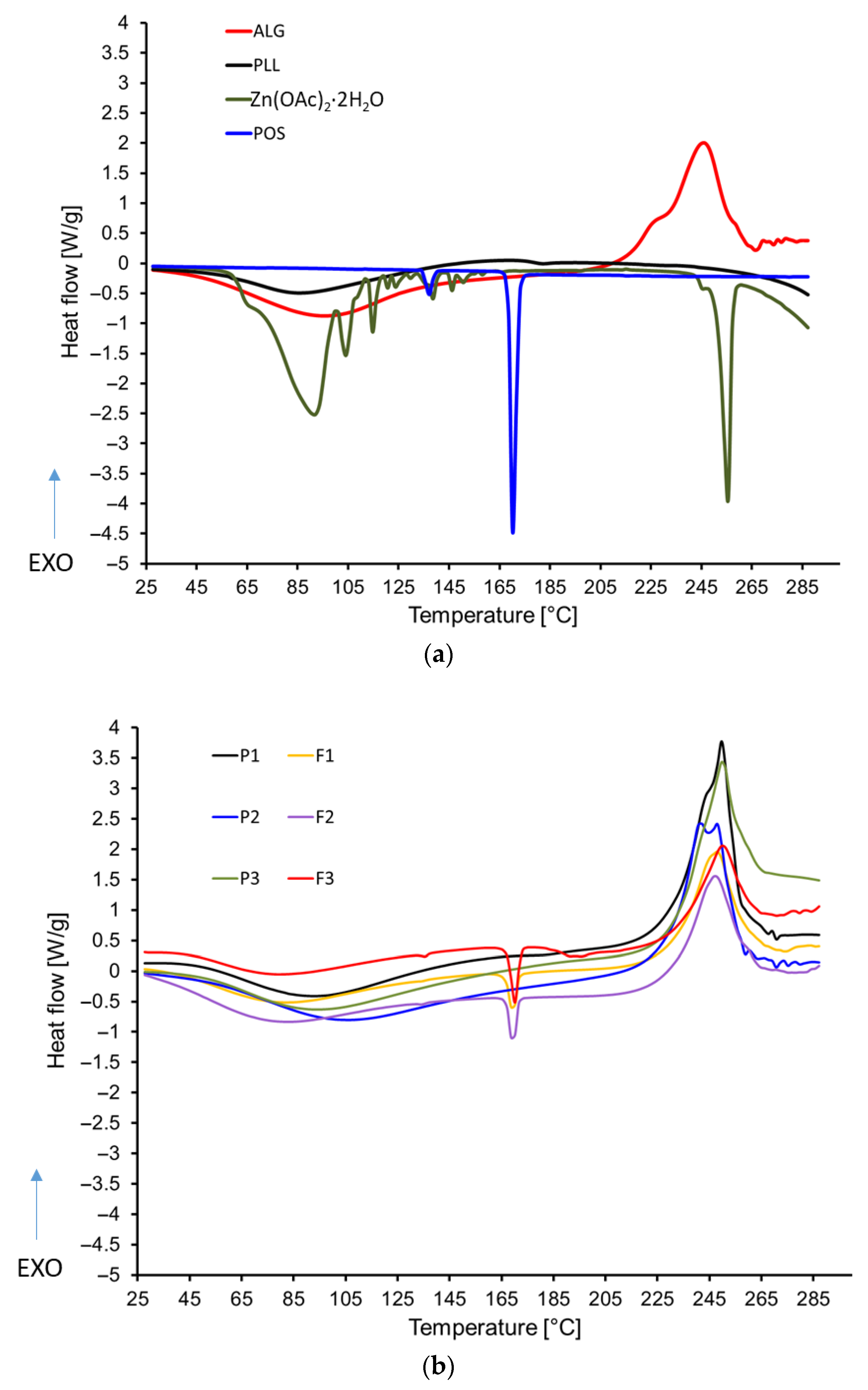
3.6. Thermal Analysis Results
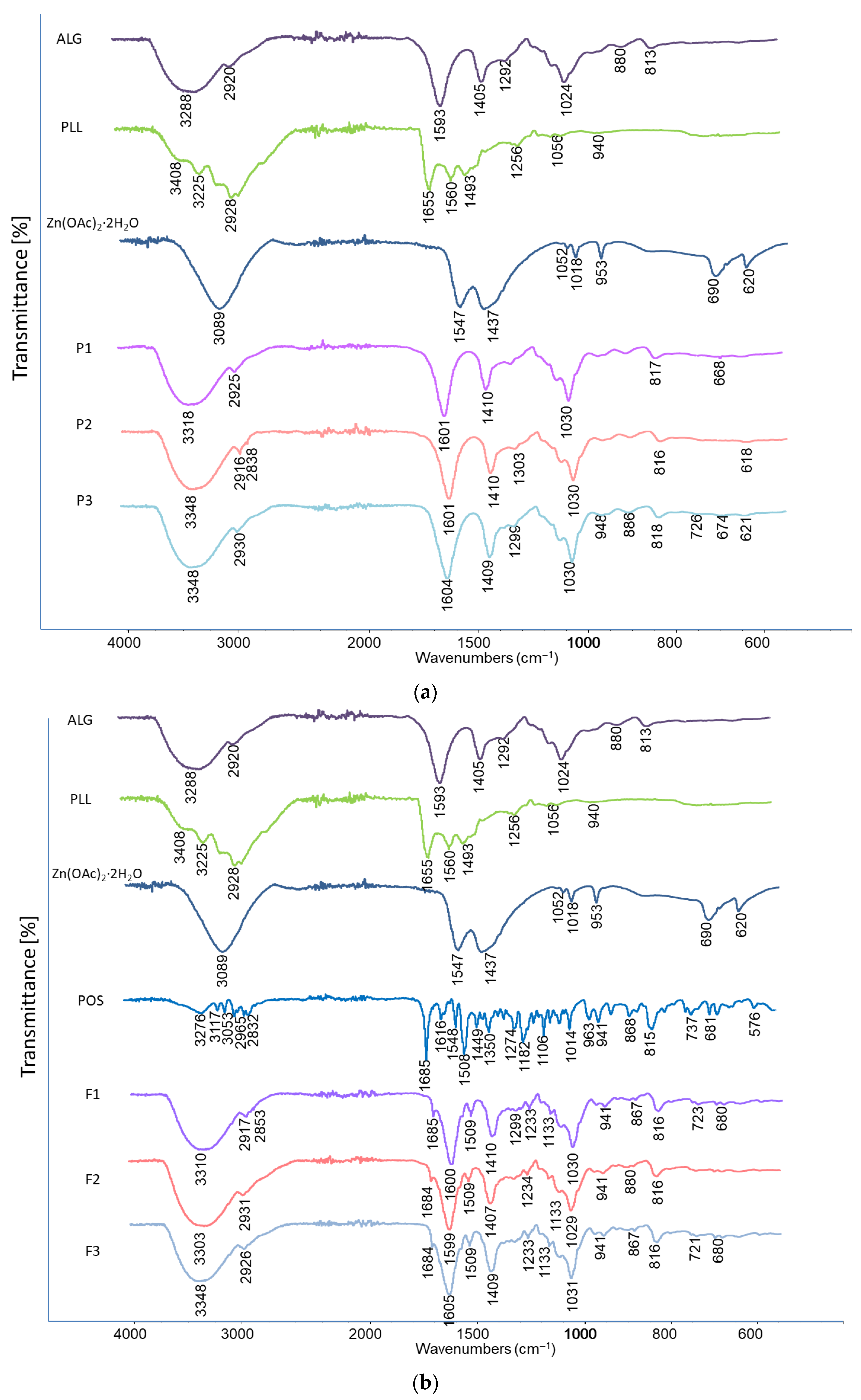
3.7. Thermal Analysis and Attenuated Total Reflectance–Fourier Transform Infrared Spectroscopy Evaluation
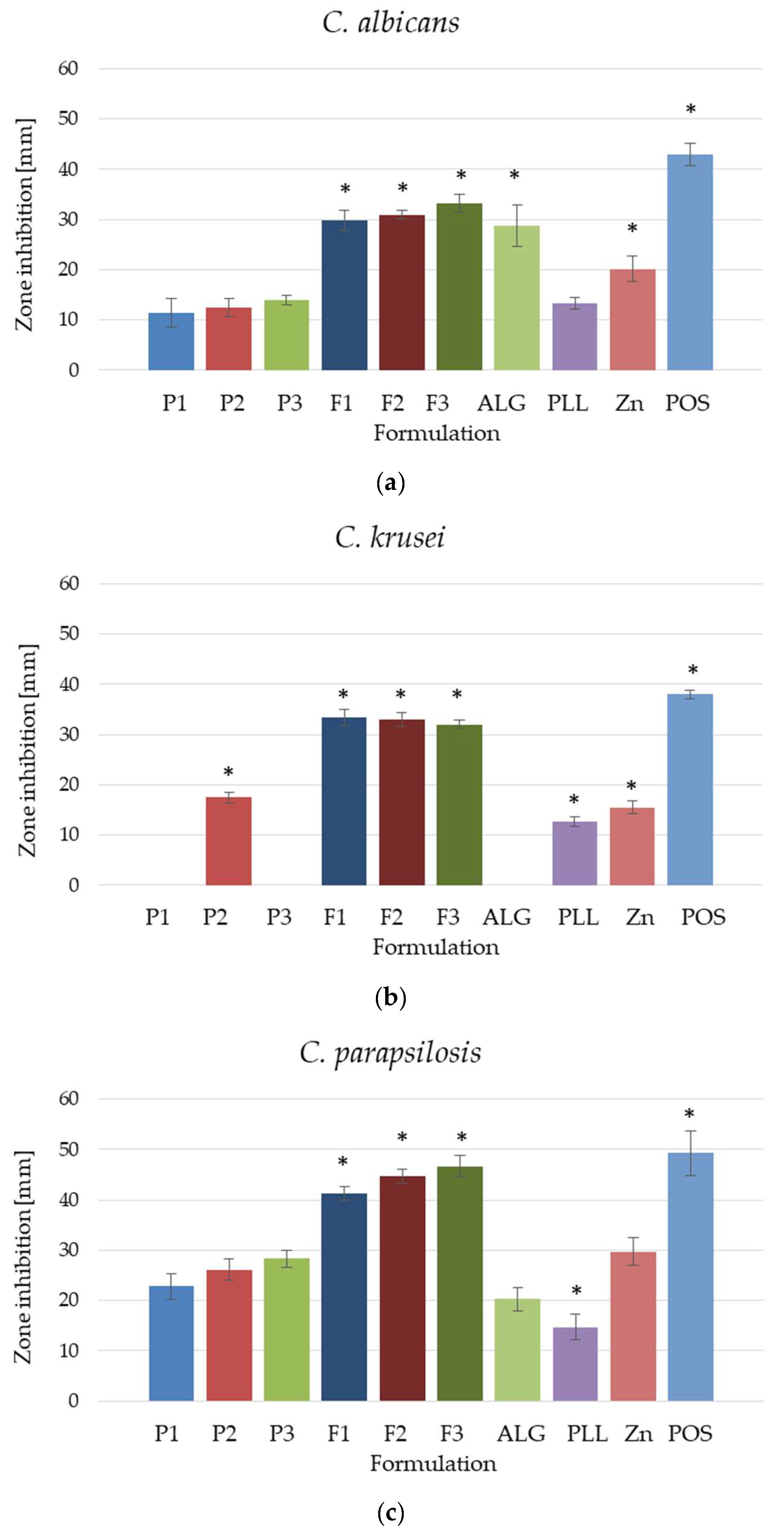
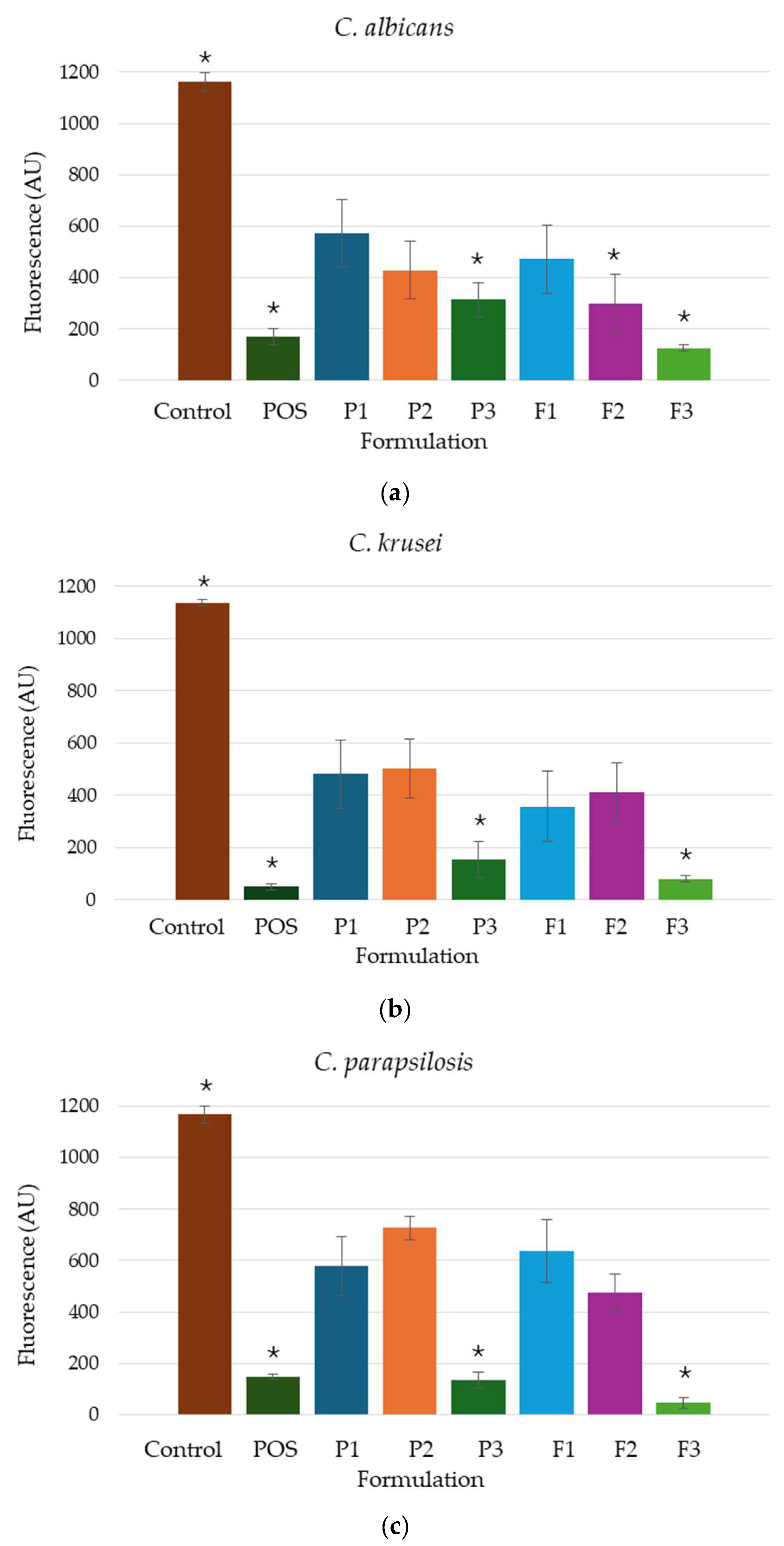
3.8. Fungicidal Assessment
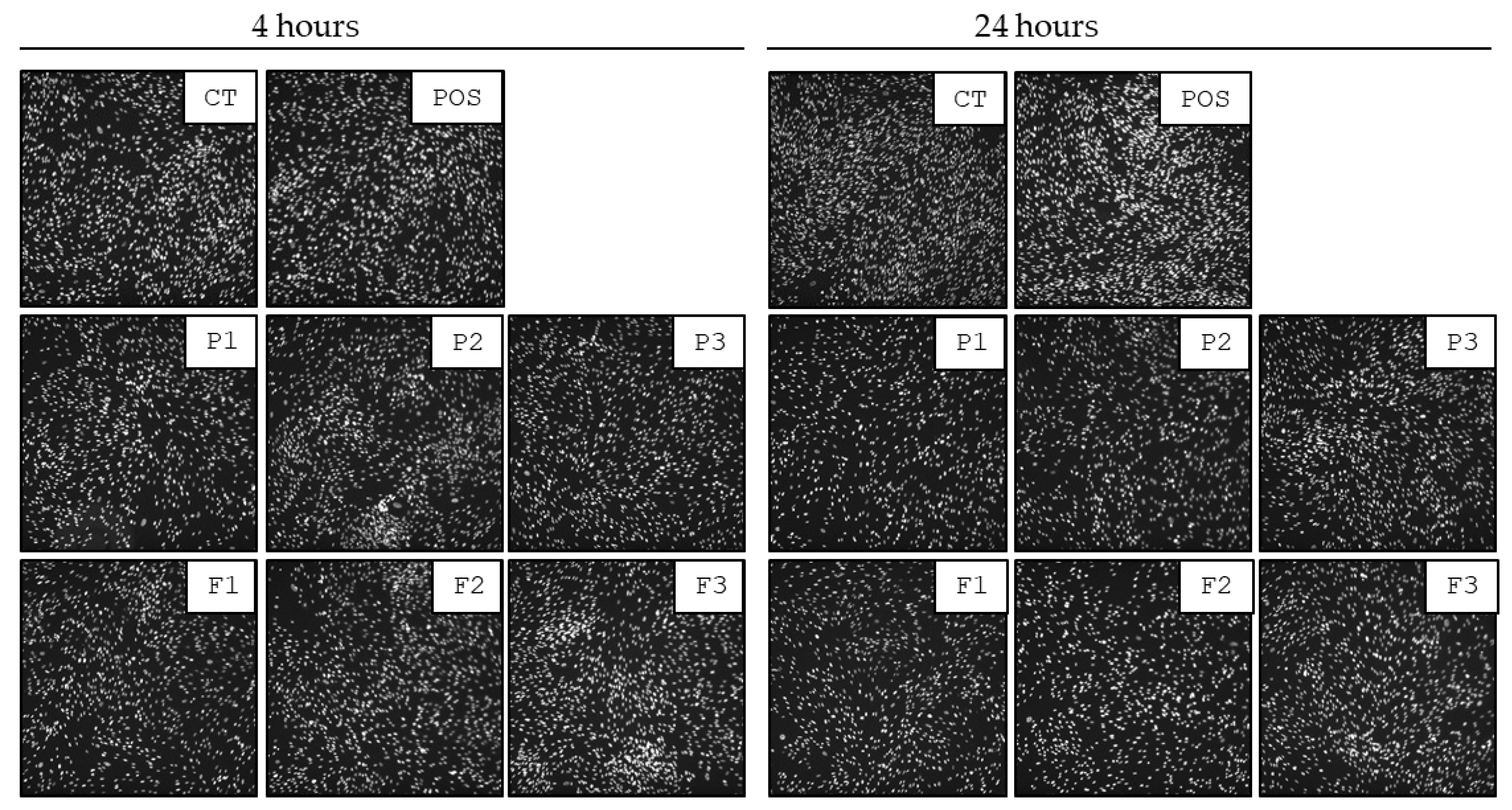
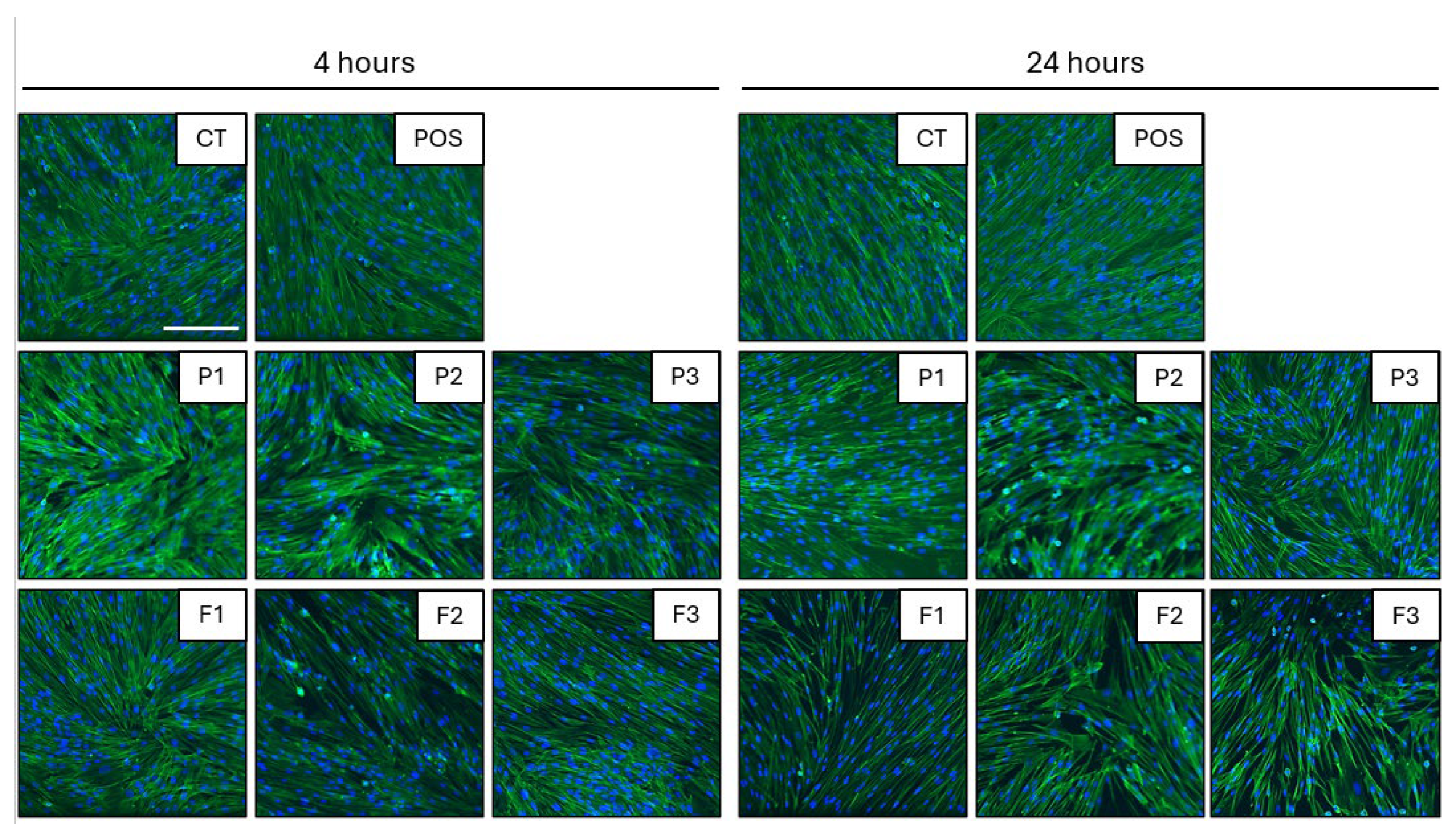
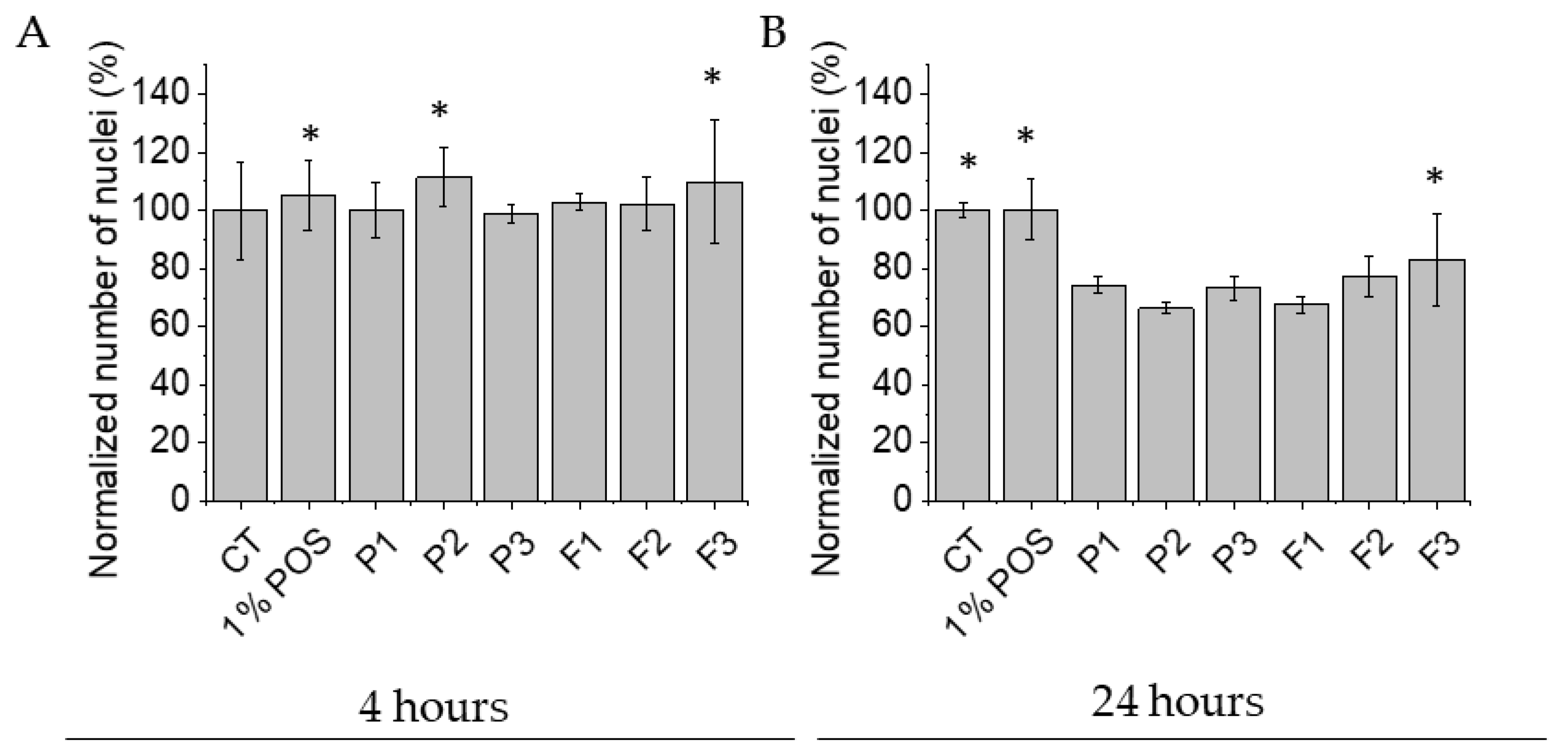
3.9. Biocompatibility Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALG | Sodium alginate |
| ATR-FTIR | Attenuated Total Reflectance–Fourier Transform Infrared Spectroscopy |
| CFU | Colony-forming unit |
| CLSI | Clinical and Laboratory Standards Institute |
| CT | Untreated cells |
| DSC | Differential scanning calorimetry analysis |
| DMSO | Dimethyl sulfoxide |
| EMEM | Eagle’s Minimum Essential Medium |
| FBS | Fetal bovine serum |
| FDA | Food and Drug Administration |
| FTIR | Fourier Transform Infrared Spectroscopy |
| GRAS | Generally Regarded as Safe |
| HPLC | High-Performance Liquid Chromatography |
| ISO | International Organization for Standardization |
| NTU | Nephelometric turbidity unit |
| PEC | Polyelectrolyte complex |
| PLL | ε-poly-L-lysine |
| POS | Posaconazole |
| ROS | Reactive oxygen species |
| SEM | Scanning Electron Microscope |
| TGA | Thermogravimetric analysis |
| Zn2+ | Zinc acetate dihydrate |
References
- Almoshari, Y. Novel hydrogels for topical applications: An updated comprehensive review based on source. Gels 2022, 8, 174. [Google Scholar] [CrossRef]
- Herbig, M.E.; Evers, D.-H.; Gorissen, S.; Köllmer, M. Rational design of topical semi-solid dosage forms-how far are we? Pharmaceutics 2023, 15, 1822. [Google Scholar] [CrossRef] [PubMed]
- Mollah, M.Z.I.; Zahid, H.M.; Mahal, Z.; Faruque, M.R.I.; Khandaker, M.U. The usages and potential uses of alginate for healthcare applications. Front. Mol. Biosci. 2021, 8, 719972. [Google Scholar] [CrossRef] [PubMed]
- Szekalska, M.; Puciłowska, A.; Szymańska, E.; Ciosek, P.; Winnicka, K. Alginate: Current use and future perspectives in pharmaceutical and biomedical applications. Int. J. Polym. Sci. 2016, 2016, 7697031. [Google Scholar] [CrossRef]
- Chan, L.W.; Jin, Y.; Heng, P.W.S. Cross-linking mechanisms of calcium and zinc in production of alginate microspheres. Int. J. Pharm. 2002, 242, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhu, L.; Zhu, T.; Jian, Y.; Ding, Y.; Zhou, M.; Feng, X. Zinc supplementation reduces Candida infections in pediatric intensive care unit: A randomized placebo-controlled clinical trial. J. Clin. Biochem. Nutr. 2019, 64, 170–173. [Google Scholar] [CrossRef]
- Potaś, J.; Winnicka, K. The potential of polyelectrolyte multilayer films as drug delivery materials. Int. J. Mol. Sci. 2022, 23, 3496. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Vad, B.S.; Stenvang, M.; Otzen, D.E.; Meyer, R.L. The antimicrobial mechanism of action of epsilon-poly-l-lysine. Appl. Environ. Microbiol. 2014, 80, 7758–7770. [Google Scholar] [CrossRef]
- Thakur, M.; Dan, A. Poly-l-lysine-functionalized green-light-emitting carbon dots as a fluorescence turn-on sensor for ultrasensitive detection of endotoxin. ACS Appl. Bio Mater. 2021, 4, 3410–3422. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Re: GRAS Notice No. GRN 000661. Available online: https://www.fda.gov/downloads/food/ingredientspackaginglabeling/GRAS/noticeinventory/ucm549588.pdf (accessed on 4 June 2025).
- Chen, S.; Huang, S.; Li, Y.; Zhou, C. Recent advances in epsilon-poly-L-lysine and L-lysine-based dendrimer synthesis, modification, and biomedical applications. Front. Chem. 2021, 9, 659304. [Google Scholar] [CrossRef]
- Jin, F.; Liao, S.; Li, W.; Jiang, C.; Wei, Q.; Xia, X.; Wang, Q. Amphiphilic sodium alginate-polylysine hydrogel with high antibacterial efficiency in a wide pH range. Carbohydr. Polym. 2023, 299, 120195. [Google Scholar] [CrossRef]
- Yu, J.; Tavsanli, B.; Tamminga, M.J.; Gillies, E.R. Compact polyelectrolyte complexes of poly(l-lysine) and anionic polysaccharides. Biomacromolecules 2024, 25, 5160–5168. [Google Scholar] [CrossRef]
- Spadari, C.D.C.; Lopes, L.B.; Ishida, K. Potential use of alginate-based carriers as antifungal delivery system. Front. Microbiol. 2017, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Szekalska, M.; Sosnowska, K.; Wróblewska, M.; Basa, A.; Winnicka, K. Does the freeze–thaw technique affect the properties of the alginate/chitosan glutamate gels with posaconazole as a model antifungal drug? Int. J. Mol. Sci. 2022, 23, 6775. [Google Scholar] [CrossRef] [PubMed]
- Szekalska, M.; Wróblewska, M.; Trofimiuk, M.; Basa, A.; Winnicka, K. Alginate oligosaccharides affect mechanical properties and antifungal activity of alginate buccal films with posaconazole. Mar. Drugs 2019, 17, 692. [Google Scholar] [CrossRef]
- Ianas, V.; Matthias, K.R.; Klotz, S.A. Role of posaconazole in the treatment of oropharyngeal candidiasis. Infect. Drug Resist. 2010, 3, 45–51. [Google Scholar] [CrossRef]
- Leung, S.; Poulakos, M.; Machin, J. Posaconazole: An update of its clinical use. Pharmacy 2015, 3, 210–268. [Google Scholar] [CrossRef]
- Dolton, M.J.; Ray, J.E.; Chen, S.C.-A.; Ng, K.; Pont, L.; McLachlan, A.J. Multicenter study of posaconazole therapeutic drug monitoring: Exposure-response relationship and factors affecting concentration. Antimicrob. Agents Chemother. 2012, 56, 5503–5510. [Google Scholar] [CrossRef]
- DrugBank Online. Database for Drug and Target Info. Available online: https://go.drugbank.com/drugs/dB01263 (accessed on 1 August 2025).
- Begum, B.; Koduru, T.S.; Madni, S.N.; Fathima Anjum, N.; Seetharaman, S.; Veeranna, B.; Gupta, V.K. Dual-self-crosslinking effect of alginate-di-aldehyde with natural and synthetic co-polymers as injectable in situ-forming biodegradable hydrogel. Gels 2024, 10, 649. [Google Scholar] [CrossRef]
- Jia, L.; Jiang, J.; Ren, A.; Wei, Z.; Xiang, T.; Zhou, S. Ultra-fast cryogenic self-healing ionic hydrogel for flexible wearable bioelectronics. Chem. Eng. J. 2024, 495, 153734. [Google Scholar] [CrossRef]
- Li, T.; Wei, H.; Zhang, Y.; Wan, T.; Cui, D.; Zhao, S.; Zhang, T.; Ji, Y.; Algadi, H.; Guo, Z.; et al. Sodium alginate reinforced polyacrylamide/xanthan gum double network ionic hydrogels for stress sensing and self-powered wearable device applications. Carbohydr. Polym. 2023, 309, 120678. [Google Scholar] [CrossRef]
- Ebel, F.; Ramírez-Reveco, A.; Strobel, P.; Wagenknecht, L.; Rodríguez, N.; Bosch, P.; Rivarola, C. Optimized protocol for high-vacuum scanning electron microscopy analysis of polyacrylamide hydrogel-attached sperm cells in a binary system. Microsc. Res. Tech. 2024, 87, 1122–1127. [Google Scholar] [CrossRef]
- Wróblewska, M.; Szymańska, E.; Szekalska, M.; Winnicka, K. Different types of gel carriers as metronidazole delivery systems to the oral mucosa. Polymers 2020, 12, 680. [Google Scholar] [CrossRef]
- Tang, P.H. Determination of posaconazole in plasma/serum by high-performance liquid chromatography with fluorescence detection. Separations 2017, 4, 16. [Google Scholar] [CrossRef]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Rex, J.H. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 3rd ed.; CLSI DocumentM27-A3; Clinical & Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Habibi, A.; Kasapis, S.; Truong, T. Effect of hydrogel particle size embedded into oleogels on the physico-functional properties of hydrogel-in-oleogel (bigels). LWT 2022, 163, 113501. [Google Scholar] [CrossRef]
- The United States Pharmacopeia. The United States Pharmacopeial Convention; The United States Pharmacopeia: Rockville, MD, USA, 2016; USP 41–NF 36. [Google Scholar]
- Brinke, A.S.T.; Mehlich, A.; Doberenz, C.; Janssens-Böcker, C. Acidification of the skin and maintenance of the physiological skin pH value by buffered skin care products formulated around pH 4. J. Cosmet. Dermatol. Sci. Appl. 2021, 11, 44–57. [Google Scholar] [CrossRef]
- Siboro, S.A.P.; Anugrah, D.S.B.; Ramesh, K.; Park, S.-H.; Kim, H.-R.; Lim, K.T. Tunable porosity of covalently crosslinked alginate-based hydrogels and its significance in drug release behavior. Carbohydr. Polym. 2021, 260, 117779. [Google Scholar] [CrossRef]
- Stojkov, G.; Niyazov, Z.; Picchioni, F.; Bose, R.K. Relationship between structure and rheology of hydrogels for various applications. Gels 2021, 7, 255. [Google Scholar] [CrossRef]
- Hashemnejad, S.M.; Kundu, S. Rheological properties and failure of alginate hydrogels with ionic and covalent crosslinks. Soft Matter 2019, 15, 7852–7862. [Google Scholar] [CrossRef]
- Jalalvandi, E.; Shavandi, A. Shear thinning/self-healing hydrogel based on natural polymers with secondary photocrosslinking for biomedical applications. J. Mech. Behav. Biomed. Mater. 2019, 90, 191–201. [Google Scholar] [CrossRef]
- Soltani, S.; Emadi, R.; Javanmard, S.H.; Kharaziha, M.; Rahmati, A. Shear-thinning and self-healing nanohybrid alginate-graphene oxide hydrogel based on guest-host assembly. Int. J. Biol. Macromol. 2021, 180, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, P.; Tang, C.; Liao, H.; Zhang, W.; Yang, R.; Shi, T.; Tan, X.; Chi, B. Injectable shear-thinning sodium alginate hydrogels with sustained submucosal lift for endoscopic submucosal dissection. Int. J. Biol. Macromol. 2022, 223, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Hurler, J.; Engesland, A.; Poorahmary Kermany, B.; Škalko-Basnet, N. Improved texture analysis for hydrogel characterization: Gel cohesiveness, adhesiveness, and hardness. J. Appl. Polym. Sci. 2012, 125, 180–188. [Google Scholar] [CrossRef]
- Dejeu, I.L.; Vicaș, L.G.; Vlaia, L.L.; Jurca, T.; Mureșan, M.E.; Pallag, A.; Coneac, G.H.; Olariu, I.V.; Muț, A.M.; Bodea, A.S.; et al. Study for evaluation of hydrogels after the incorporation of liposomes embedded with caffeic acid. Pharmaceuticals 2022, 15, 175. [Google Scholar] [CrossRef]
- Desai, S.R.; Bhagwat, D.A.; Singh, A.K.; Kumbhar, R.B.; Khan, J. Bioadhesive polymers in transdermal formulations for skin disorders. Curr. Opin. Pharmacol. 2025, 83, 102544. [Google Scholar] [CrossRef]
- Ye, R.; Xu, H.; Wan, C.; Peng, S.; Wang, L.; Xu, H.; Aguilar, Z.P.; Xiong, Y.; Zeng, Z.; Wei, H. Antibacterial activity and mechanism of action of ε-poly-l-lysine. Biochem. Biophys. Res. Commun. 2013, 439, 148–153. [Google Scholar] [CrossRef]
- Boddupalli, B.M.; Mohammed, Z.N.K.; Nath, R.A.; Banji, D. Mucoadhesive drug delivery system: An overview. J. Adv. Pharm. Technol. Res. 2010, 1, 381. [Google Scholar] [CrossRef]
- Teng, L.; Shao, Z.-W.; He, Y.-S.; Lu, J.-Y.; Zou, D.-R.; Feng, C.-L.; Dong, C.-M. A glycosylated and catechol-crosslinked ε-polylysine hydrogel: Simple preparation and excellent wound hemostasis and healing properties. Chin. J. Polym. Sci. 2022, 40, 1110–1119. [Google Scholar] [CrossRef]
- Mazia, D.; Schatten, G.; Sale, W. Adhesion of cells to surfaces coated with polylysine. Applications to electron microscopy. J. Cell Biol. 1975, 66, 198–200. [Google Scholar] [CrossRef]
- Dong, H.; Liu, C.; Zhang, X.; Si, D.; Luo, Y.; Jia, J.; Han, M.; Su, Y.; Zhou, H.; Zeng, W. Gelatin and zinc ion-cooperated triple crosslinked hydrogels with high mechanical properties and ultrasensitivity for multimodal sensing and handwriting recognition. Int. J. Biol. Macromol. 2025, 304, 140869. [Google Scholar] [CrossRef]
- Tundisi, L.L.; Yang, R.; Borelli, L.P.P.; Alves, T.; Mehta, M.; Chaud, M.V.; Mazzola, P.G.; Kohane, D.S. Enhancement of the mechanical and drug-releasing properties of Poloxamer 407 hydrogels with casein. Pharm. Res. 2021, 38, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as drug delivery systems: A review of current characterization and evaluation techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Metters, A.T. Hydrogels in controlled release formulations: Network design and mathematical modeling. Adv. Drug Deliv. Rev. 2006, 58, 1379–1408. [Google Scholar] [CrossRef] [PubMed]
- Hoppel, M.; Tabosa, M.A.M.; Bunge, A.L.; Delgado-Charro, M.B.; Guy, R.H. Assessment of drug delivery kinetics to epidermal targets in vivo. AAPS J. 2021, 23, 49. [Google Scholar] [CrossRef]
- Iliopoulos, F.; Sil, B.C.; Evans, C.L. The role of excipients in promoting topical and transdermal delivery: Current limitations and future perspectives. Front. Drug Deliv. 2022, 2, 1049848. [Google Scholar] [CrossRef]
- Binder, L.; Mazál, J.; Petz, R.; Klang, V.; Valenta, C. The role of viscosity on skin penetration from cellulose ether-based hydrogels. Skin Res. Technol. 2019, 25, 725–734. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar]
- Varaprasad, K.; Nùñez, D.; Ide, W.; Jayaramudu, T.; Sadiku, E.R. Development of high alginate comprised hydrogels for removal of Pb(II) Ions. J. Mol. Liq. 2020, 298, 112087. [Google Scholar] [CrossRef]
- Felix, C.B.; Chen, W.-H.; Ubando, A.T.; Park, Y.-K.; Lin, K.-Y.A.; Pugazhendhi, A.; Nguyen, T.-B.; Dong, C.-D. A comprehensive review of thermogravimetric analysis in lignocellulosic and algal biomass gasification. Chem. Eng. J. 2022, 445, 136730. [Google Scholar] [CrossRef]
- Flores-Hernández, C.G.; Cornejo-Villegas, M.D.L.A.; Moreno-Martell, A.; Del Real, A. Synthesis of a biodegradable polymer of poly (sodium alginate/ethyl acrylate). Polymers 2021, 13, 504. [Google Scholar] [CrossRef]
- Dudek, G.; Turczyn, R. New type of alginate/chitosan microparticle membranes for highly efficient pervaporative dehydration of ethanol. RSC Adv. 2018, 8, 39567–39578. [Google Scholar] [CrossRef] [PubMed]
- Arshad, R.; Tabish, T.A.; Naseem, A.A.; Hassan, M.R.U.; Hussain, I.; Hussain, S.S.; Shahnaz, G. Development of poly-L-lysine multi-functionalized muco-penetrating self-emulsifying drug delivery system (SEDDS) for improved solubilization and targeted delivery of ciprofloxacin against intracellular Salmonella typhi. J. Mol. Liq. 2021, 333, 115972. [Google Scholar] [CrossRef]
- David, S.E.; Timmins, P.; Conway, B.R. Impact of the counterion on the solubility and physicochemical properties of salts of carboxylic acid drugs. Drug Dev. Ind. Pharm. 2012, 38, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Rozenberg, M.; Shoham, G. FTIR spectra of solid poly-l-lysine in the stretching NH mode range. Biophys. Chem. 2007, 125, 166–171. [Google Scholar] [CrossRef]
- Tam, S.K.; Dusseault, J.; Polizu, S.; Ménard, M.; Hallé, J.-P.; Yahia, L. Physicochemical model of alginate–poly-l-lysine microcapsules defined at the micrometric/nanometric scale using ATR-FTIR, XPS, and ToF-SIMS. Biomaterials 2005, 26, 6950–6961. [Google Scholar] [CrossRef]
- Deepathomas, D.; Latha, M.S.; Kurienthomas, K. Zinc alginate beads for the controlled release of rifampicin. Orient. J. Chem. 2018, 34, 428–433. [Google Scholar] [CrossRef]
- Cavalheiro, M.; Teixeira, M.C. Candida biofilms: Threats, challenges, and promising strategies. Front. Med. 2018, 5, 28. [Google Scholar] [CrossRef]
- Blankenship, J.R.; Mitchell, A.P. How to build a biofilm: A fungal perspective. Curr. Opin. Microbiol. 2006, 9, 588–594. [Google Scholar] [CrossRef]
- Ajetunmobi, O.H.; Badali, H.; Romo, J.A.; Ramage, G.; Lopez-Ribot, J.L. Antifungal therapy of Candida biofilms: Past, present and future. Biofilm 2023, 5, 100126. [Google Scholar] [CrossRef]
- Fozouni, L.; Tahaei, M. Anticandidal effects of zinc oxide nanoparticles on fluconazole-resistant Candida isolates causing diarrhea in calves, in vitro. Arch. Razi Inst. 2022, 78, 499. [Google Scholar] [CrossRef]
- Das, B.; Khan, M.I.; Jayabalan, R.; Behera, S.K.; Yun, S.-I.; Tripathy, S.K.; Mishra, A. Understanding the antifungal mechanism of Ag@ZnO core-shell nanocomposites against Candida krusei. Sci. Rep. 2016, 6, 36403. [Google Scholar] [CrossRef] [PubMed]
- Roselletti, E.; Pericolini, E.; Nore, A.; Takacs, P.; Kozma, B.; Sala, A.; De Seta, F.; Comar, M.; Usher, J.; Brown, G.D.; et al. Zinc prevents vaginal candidiasis by inhibiting expression of an inflammatory fungal protein. Sci. Transl. Med. 2023, 15, eadi3363. [Google Scholar] [CrossRef] [PubMed]
- Hariyadi, D.M.; Islam, N. Current status of alginate in drug delivery. Adv. Pharmacol. Pharm. Sci. 2020, 2020, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Padilla-Garfias, F.; Ríos-Cifuentes, L.; Sánchez, N.S.; Calahorra, M.; Peña, A. Study of the mechanism of ε-poly-l-lysine as an antifungal on Candida albicans and Saccharomyces cerevisiae. Biochim. Biophys. Acta BBA Gen. Subj. 2022, 1866, 130197. [Google Scholar] [CrossRef]
- Greco, I.; Machrafi, H.; Minetti, C.; Risaliti, C.; Bandini, A.; Cialdai, F.; Monici, M.; Iorio, C.S. Hydrogel formulation for biomimetic fibroblast cell culture: Exploring effects of external stresses and cellular responses. Int. J. Mol. Sci. 2024, 25, 5600. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Standards Organization: Geneva, Switzerland, 2009. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:10993:-5:ed-3:v1:en (accessed on 4 June 2025).






| Formulation | ALG (g) | PLL (g) | Zn2+ (g) | POS (g) | Water (Up To) |
|---|---|---|---|---|---|
| P1 | 2.0 | – | – | – | 100 |
| P2 | 2.0 | 0.025 | – | – | 100 |
| P3 | 2.0 | 0.025 | 0.2 | – | 100 |
| F1 | 2.0 | – | – | 1.0 | 100 |
| F2 | 2.0 | 0.025 | – | 1.0 | 100 |
| F3 | 2.0 | 0.025 | 0.2 | 1.0 | 100 |
| Formulation | pH | Mean POS Particle Size (μm) | Drug Content (%) | Viscosity * (mPa∙s) |
|---|---|---|---|---|
| P1 | 5.04 ± 0.04 | – | – | 2050.55 ± 57.28 |
| P2 | 5.37 ± 0.01 * | – | – | 2248.99 ± 286.42 |
| P3 | 6.09 ± 0.02 * | – | – | 5622.47 ± 973.84 * |
| F1 | 5.15 ± 0.01 * | 22.87 ± 8.13 | 108.00 ± 3.25 | 1719.81 ± 57.28 |
| F2 | 5.48 ± 0.02 * | 25.44 ± 9.46 | 106.83 ± 3.76 | 1620.59 ± 57.28 |
| F3 | 6.20 ± 0.01 * | 23.52 ± 8.12 | 109.62 ± 1.79 | 4398.75 ± 206.54 * |
| Formulation | Zero-Order Kinetics | First-Order Kinetics | Highuchi Model | Korsmeyer–Peppas Model | |||||
|---|---|---|---|---|---|---|---|---|---|
| R2 | K | R2 | K | R2 | K | R2 | K | n | |
| F1 | 0.955 | 4830.700 | 0.90 | 0.41 | 0.97 | 13,362.00 | 0.971 | 0.77 | 0.94 |
| F2 | 0.77 | 963.68 | 0.76 | 0.13 | 0.93 | 2726.70 | 0.88 | 0.25 | 0.70 |
| F3 | 0.85 | 926.61 | 0.86 | 0.15 | 0.79 | 2436.00 | 0.74 | 0.24 | 0.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sosnowska, K.; Szekalska, M.; Piktel, E.; Bucki, R.; Wolska, E.; Misztalewska-Turkowicz, I.; Markiewicz, K.H.; Wilczewska, A.Z.; Winnicka, K. Dual-Cross-Linked Alginate Hydrogels as a Strategy to Improve the Antifungal Properties of Posaconazole. Pharmaceutics 2025, 17, 1055. https://doi.org/10.3390/pharmaceutics17081055
Sosnowska K, Szekalska M, Piktel E, Bucki R, Wolska E, Misztalewska-Turkowicz I, Markiewicz KH, Wilczewska AZ, Winnicka K. Dual-Cross-Linked Alginate Hydrogels as a Strategy to Improve the Antifungal Properties of Posaconazole. Pharmaceutics. 2025; 17(8):1055. https://doi.org/10.3390/pharmaceutics17081055
Chicago/Turabian StyleSosnowska, Katarzyna, Marta Szekalska, Ewelina Piktel, Robert Bucki, Eliza Wolska, Iwona Misztalewska-Turkowicz, Karolina Halina Markiewicz, Agnieszka Zofia Wilczewska, and Katarzyna Winnicka. 2025. "Dual-Cross-Linked Alginate Hydrogels as a Strategy to Improve the Antifungal Properties of Posaconazole" Pharmaceutics 17, no. 8: 1055. https://doi.org/10.3390/pharmaceutics17081055
APA StyleSosnowska, K., Szekalska, M., Piktel, E., Bucki, R., Wolska, E., Misztalewska-Turkowicz, I., Markiewicz, K. H., Wilczewska, A. Z., & Winnicka, K. (2025). Dual-Cross-Linked Alginate Hydrogels as a Strategy to Improve the Antifungal Properties of Posaconazole. Pharmaceutics, 17(8), 1055. https://doi.org/10.3390/pharmaceutics17081055










