RNA Therapeutics: Delivery Problems and Solutions—A Review
Abstract
1. Introduction
2. siRNA Design Principles and Algorithms
3. RNA Therapeutic Target Types and Approaches to Their Delivery
3.1. Molecular Targets of RNA Therapeutics
3.1.1. Historical Context and Early Approaches
3.1.2. RNA Interference (RNAi) and siRNA-Based Therapeutics
3.1.3. MicroRNA (miRNA) Therapeutics: Mimics and Inhibitors
3.1.4. mRNA Vaccines and Therapeutic Applications
3.1.5. Other RNA-Based Approaches
3.2. Non-Viral Systems for RNA Delivery
3.3. Viral Vectors for RNA Delivery
4. Discussion
Future Perspectives
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lo Re, V.; Newcomb, C.W.; Carbonari, D.M.; Mezochow, A.K.; Hennessy, S.; Rentsch, C.T.; Park, L.S.; Tate, J.P.; Bräu, N.; Bhattacharya, D.; et al. Hepatotoxicity Score: A New Method to Adjust for Use of Potentially Hepatotoxic Medications by Chronic Liver Disease Status. Pharmacoepidemiol. Drug 2024, 33, e70069. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.K. Genetic and Genomic Approaches to the Study of Drug-Induced Liver Injury. Liver Int. 2025, 45, e16191. [Google Scholar] [CrossRef] [PubMed]
- Wouters, O.J.; McKee, M.; Luyten, J. Estimated Research and Development Investment Needed to Bring a New Medicine to Market, 2009–2018. JAMA 2020, 323, 844. [Google Scholar] [CrossRef]
- El-Shabrawi, M.H.F.; Kamal, N.M. Medical Management of Chronic Liver Diseases in Children (Part I): Focus on Curable or Potentially Curable Diseases. Pediatr. Drugs 2011, 13, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Zhong, L.; Weng, Y.; Peng, L.; Huang, Y.; Zhao, Y.; Liang, X.-J. Therapeutic siRNA: State of the Art. Sig Transduct. Target. Ther. 2020, 5, 101. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-Y.; DeRuiter, S.L.; Turner, D.L. RNA Interference by Expression of Short-Interfering RNAs and Hairpin RNAs in Mammalian Cells. Proc. Natl. Acad. Sci. USA 2002, 99, 6047–6052. [Google Scholar] [CrossRef]
- Wang, X.-B.; Wu, Q.; Ito, T.; Cillo, F.; Li, W.-X.; Chen, X.; Yu, J.-L.; Ding, S.-W. RNAi-Mediated Viral Immunity Requires Amplification of Virus-Derived siRNAs in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2010, 107, 484–489. [Google Scholar] [CrossRef]
- Lu, A.; Zhang, H.; Zhang, X.; Wang, H.; Hu, Q.; Shen, L.; Schaffhausen, B.S.; Hou, W.; Li, L. Attenuation of SARS Coronavirus by a Short Hairpin RNA Expression Plasmid Targeting RNA-Dependent RNA Polymerase. Virology 2004, 324, 84–89. [Google Scholar] [CrossRef]
- Roberts, T.C.; Morris, K.V.; Weinberg, M.S. Perspectives on the Mechanism of Transcriptional Regulation by Long Non-Coding RNAs. Epigenetics 2014, 9, 13–20. [Google Scholar] [CrossRef]
- Elbashir, S.M.; Harborth, J.; Lendeckel, W.; Yalcin, A.; Weber, K.; Tuschl, T. Duplexes of 21-Nucleotide RNAs Mediate RNA Interference in Cultured Mammalian Cells. Nature 2001, 411, 494–498. [Google Scholar] [CrossRef]
- Peer, D. Harnessing RNAi Nanomedicine for Precision Therapy. Mol. Cell Ther. 2014, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Kara, G.; Calin, G.A.; Ozpolat, B. RNAi-Based Therapeutics and Tumor Targeted Delivery in Cancer. Adv. Drug Deliv. Rev. 2022, 182, 114113. [Google Scholar] [CrossRef]
- Pallathadka, H.; Jabir, M.; Rasool, K.H.; Hanumanthaiah, M.; Sharma, N.; Pramanik, A.; Rab, S.O.; Jawad, S.F.; Oghenemaro, E.F.; Mustafa, Y.F. siRNA-Based Therapy for Overcoming Drug Resistance in Human Solid Tumours: Molecular and Immunological Approaches. Human. Immunol. 2025, 86, 111221. [Google Scholar] [CrossRef]
- Soutschek, J.; Akinc, A.; Bramlage, B.; Charisse, K.; Constien, R.; Donoghue, M.; Elbashir, S.; Geick, A.; Hadwiger, P.; Harborth, J.; et al. Therapeutic Silencing of an Endogenous Gene by Systemic Administration of Modified siRNAs. Nature 2004, 432, 173–178. [Google Scholar] [CrossRef]
- Shin, H.; Park, S.; Yim, Y.; Kim, J.; Choi, C.; Won, C.; Min, D. Recent Advances in RNA Therapeutics and RNA Delivery Systems Based on Nanoparticles. Adv. Ther. 2018, 1, 1800065. [Google Scholar] [CrossRef]
- Micklefield, J. Backbone Modification of Nucleic Acids: Synthesis, Structure and Therapeutic Applications. Curr. Med. Chem. 2001, 8, 1157–1179. [Google Scholar] [CrossRef]
- Chernikov, I.V.; Vlassov, V.V.; Chernolovskaya, E.L. Current Development of siRNA Bioconjugates: From Research to the Clinic. Front. Pharmacol. 2019, 10, 444. [Google Scholar] [CrossRef]
- Seth, P.P.; Tanowitz, M.; Bennett, C.F. Selective Tissue Targeting of Synthetic Nucleic Acid Drugs. J. Clin. Investig. 2019, 129, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Obika, S.; Nanbu, D.; Hari, Y.; Morio, K.; In, Y.; Ishida, T.; Imanishi, T. Synthesis of 2′-O,4′-C-Methyleneuridine and -Cytidine. Novel Bicyclic Nucleosides Having a Fixed C3, -Endo Sugar Puckering. Tetrahedron Lett. 1997, 38, 8735–8738. [Google Scholar] [CrossRef]
- Morita, K.; Hasegawa, C.; Kaneko, M.; Tsutsumi, S.; Sone, J.; Ishikawa, T.; Imanishi, T.; Koizumi, M. 2′-O, 4′-C-Ethylene-Bridged Nucleic Acids (ENA) with Nuclease-Resistance and High Affnity for RNA. Nucleic Acids Symp. Ser. 2001, 1, 241–242. [Google Scholar] [CrossRef] [PubMed]
- Burel, S.A.; Hart, C.E.; Cauntay, P.; Hsiao, J.; Machemer, T.; Katz, M.; Watt, A.; Bui, H.; Younis, H.; Sabripour, M.; et al. Hepatotoxicity of High Affinity Gapmer Antisense Oligonucleotides Is Mediated by RNase H1 Dependent Promiscuous Reduction of Very Long Pre-mRNA Transcripts. Nucleic Acids Res. 2016, 44, 2093–2109. [Google Scholar] [CrossRef]
- Ray, K.K.; Wright, R.S.; Kallend, D.; Koenig, W.; Leiter, L.A.; Raal, F.J.; Bisch, J.A.; Richardson, T.; Jaros, M.; Wijngaard, P.L.J.; et al. Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol. N. Engl. J. Med. 2020, 382, 1507–1519. [Google Scholar] [CrossRef]
- Fatima, M.; Park, P.-G.; Hong, K.-J. Clinical Advancements in mRNA Vaccines against Viral Infections. Clin. Immunol. 2025, 271, 110424. [Google Scholar] [CrossRef]
- Angart, P.; Vocelle, D.; Chan, C.; Walton, S. Design of siRNA Therapeutics from the Molecular Scale. Pharmaceuticals 2013, 6, 440–468. [Google Scholar] [CrossRef]
- Fajardo, L.E.; Macalalad, M.A.; Odchimar, N.M.; De Guzman, J.C.; Orosco, F. Computational Design and Validation of siRNA Molecules to Silence Oncogenic CTNNB1 mRNA as a Potential Therapeutic Strategy against Hepatitis B/C Virus-Associated Hepatocellular Carcinoma. Pharmacia 2024, 71, 1–14. [Google Scholar] [CrossRef]
- Cipriano, C.; Noce, S.; Mereu, S.; Santini, M. Algorithms Going Wild—A Review of Machine Learning Techniques for Terrestrial Ecology. Ecol. Model. 2025, 506, 111164. [Google Scholar] [CrossRef]
- Auslander, N.; Gussow, A.B.; Koonin, E.V. Incorporating Machine Learning into Established Bioinformatics Frameworks. Int. J. Mol. Sci. 2021, 22, 2903. [Google Scholar] [CrossRef] [PubMed]
- Mandelli, C.; Crippa, G. Machine Learning Reveals Intrinsic Determinants of siRNA Efficacy. bioRxiv 2025. [Google Scholar] [CrossRef]
- Bofill-De Ros, X. Toward Learning the Rules That Predict siRNA Efficacy. Mol. Ther. Nucleic Acids 2023, 33, 543–544. [Google Scholar] [CrossRef]
- Richter, M.; Admasu, A. siRNA Features—Automated Machine Learning of 3D Molecular Fingerprints and Structures for Therapeutic Off-Target Data. Int. J. Mol. Sci. 2025, 26, 6795. [Google Scholar] [CrossRef] [PubMed]
- Muscat, G.E.O.; Rea, S.; Downes, M. Identification of a Regulatory Function for an Orphan Receptor in Muscle: COUP-TF II Affects the Expression of the myoD Gene Family during Myogenesis. Nucl. Acids Res. 1995, 23, 1311–1318. [Google Scholar] [CrossRef]
- Klessig, D.F.; Quinlan, M.P.; Grodzicker, T. Proteins Containing Only Half of the Coding Information of Early Region 1b of Adenovirus Are Functional in Human Cells Transformed with the Herpes Simplex Virus Type 1 Thymidine Kinase Gene and Adenovirus Type 2 DNA. J. Virol. 1982, 41, 423–434. [Google Scholar] [CrossRef]
- Izant, J.G.; Weintraub, H. Inhibition of Thymidine Kinase Gene Expression by Anti-Sense RNA: A Molecular Approach to Genetic Analysis. Cell 1984, 36, 1007–1015. [Google Scholar] [CrossRef]
- Melton, D.A. Injected Anti-Sense RNAs Specifically Block Messenger RNA Translation in Vivo. Proc. Natl. Acad. Sci. USA 1985, 82, 144–148. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.K.; Leonard, G.T.; Bandyopadhyay, T.; Stark, G.R.; Sen, G.C. Transcriptional Induction by Double-Stranded RNA Is Mediated by Interferon-Stimulated Response Elements without Activation of Interferon-Stimulated Gene Factor 3. J. Biol. Chem. 1995, 270, 19624–19629. [Google Scholar] [CrossRef]
- Hong, D.; Kurzrock, R.; Kim, Y.; Woessner, R.; Younes, A.; Nemunaitis, J.; Fowler, N.; Zhou, T.; Schmidt, J.; Jo, M.; et al. AZD9150, a next-Generation Antisense Oligonucleotide Inhibitor of STAT3 with Early Evidence of Clinical Activity in Lymphoma and Lung Cancer. Sci. Transl. Med. 2015, 7, 314ra185. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Hartley, G.P.; Couillault, C.; Yuan, Y.; Lin, H.; Nicholas, C.; Srinivasamani, A.; Dai, J.; Dumbrava, E.E.I.; Fu, S.; et al. Preclinical Study and Parallel Phase II Trial Evaluating Antisense STAT3 Oligonucleotide and Checkpoint Blockade for Advanced Pancreatic, Non-Small Cell Lung Cancer and Mismatch Repair-Deficient Colorectal Cancer. BMJ Oncol. 2024, 3, e000133. [Google Scholar] [CrossRef] [PubMed]
- Kamerkar, S.; Leng, C.; Burenkova, O.; Jang, S.C.; McCoy, C.; Zhang, K.; Dooley, K.; Kasera, S.; Zi, T.; Sisó, S.; et al. Exosome-Mediated Genetic Reprogramming of Tumor-Associated Macrophages by exoASO-STAT6 Leads to Potent Monotherapy Antitumor Activity. Sci. Adv. 2022, 8, eabj7002. [Google Scholar] [CrossRef]
- Sarker, D.; Plummer, R.; Meyer, T.; Sodergren, M.H.; Basu, B.; Chee, C.E.; Huang, K.-W.; Palmer, D.H.; Ma, Y.T.; Evans, T.R.J.; et al. MTL-CEBPA, a Small Activating RNA Therapeutic Upregulating C/EBP-α, in Patients with Advanced Liver Cancer: A First-in-Human, Multicenter, Open-Label, Phase I Trial. Clin. Cancer Res. 2020, 26, 3936–3946. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadou, E.; Seto, A.G.; Beatty, X.; Hermreck, M.; Gilles, M.-E.; Stroopinsky, D.; Pinter-Brown, L.C.; Pestano, L.; Marchese, C.; Avigan, D.; et al. Cobomarsen, an Oligonucleotide Inhibitor of miR-155, Slows DLBCL Tumor Cell Growth In Vitro and In Vivo. Clin. Cancer Res. 2021, 27, 1139–1149. [Google Scholar] [CrossRef]
- van der Ree, M.H.; van der Meer, A.J.; van Nuenen, A.C.; de Bruijne, J.; Ottosen, S.; Janssen, H.L.; Kootstra, N.A.; Reesink, H.W. Miravirsen Dosing in Chronic Hepatitis C Patients Results in Decreased microRNA-122 Levels without Affecting Other microRNAs in Plasma. Aliment. Pharmacol. Ther. 2016, 43, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Santolla, M.F.; Lappano, R.; Cirillo, F.; Rigiracciolo, D.C.; Sebastiani, A.; Abonante, S.; Tassone, P.; Tagliaferri, P.; Di Martino, M.T.; Maggiolini, M.; et al. miR-221 Stimulates Breast Cancer Cells and Cancer-Associated Fibroblasts (CAFs) through Selective Interference with the A20/c-Rel/CTGF Signaling. J. Exp. Clin. Cancer Res. 2018, 37, 94. [Google Scholar] [CrossRef]
- Gallant-Behm, C.L.; Piper, J.; Dickinson, B.A.; Dalby, C.M.; Pestano, L.A.; Jackson, A.L. A Synthetic microRNA-92a Inhibitor (MRG-110) Accelerates Angiogenesis and Wound Healing in Diabetic and Nondiabetic Wounds. Wound Repair. Regen. 2018, 26, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Gale, D.P.; Gross, O.; Wang, F.; Esteban de la Rosa, R.J.; Hall, M.; Sayer, J.A.; Appel, G.; Hariri, A.; Liu, S.; Maski, M.; et al. A Randomized Controlled Clinical Trial Testing Effects of Lademirsen on Kidney Function Decline in Adults with Alport Syndrome. Clin. J. Am. Soc. Nephrol. 2024, 19, 995–1004. [Google Scholar] [CrossRef]
- Meo, S.A.; Bukhari, I.A.; Akram, J.; Meo, A.S.; Klonoff, D.C. COVID-19 Vaccines: Comparison of Biological, Pharmacological Characteristics and Adverse Effects of Pfizer/BioNTech and Moderna Vaccines. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1663–1669. [Google Scholar] [CrossRef]
- Teo, S.P. Review of COVID-19 mRNA Vaccines: BNT162b2 and mRNA-1273. J. Pharm. Pract. 2022, 35, 947–951. [Google Scholar] [CrossRef] [PubMed]
- van Zandwijk, N.; Pavlakis, N.; Kao, S.; Clarke, S.; Lee, A.; Brahmbhatt, H.; Macdiarmid, J.; Pattison, S.; Leslie, F.; Huynh, Y.; et al. P1.02MesomiR 1: A Phase I Study of TargomiRs in Patients with Refractory Malignant Pleural Mesothelioma (MPM) and Lung Cancer (NSCLC). Ann. Oncol. 2015, 26, ii16. [Google Scholar] [CrossRef]
- Fuertes, T.; Ramiro, A.R.; de Yebenes, V.G. miRNA-Based Therapies in B Cell Non-Hodgkin Lymphoma. Trends Immunol. 2020, 41, 932–947. [Google Scholar] [CrossRef]
- Gallant-Behm, C.L.; Piper, J.; Lynch, J.M.; Seto, A.G.; Hong, S.J.; Mustoe, T.A.; Maari, C.; Pestano, L.A.; Dalby, C.M.; Jackson, A.L.; et al. A MicroRNA-29 Mimic (Remlarsen) Represses Extracellular Matrix Expression and Fibroplasia in the Skin. J. Investig. Dermatol. 2019, 139, 1073–1081. [Google Scholar] [CrossRef]
- Urits, I.; Swanson, D.; Swett, M.C.; Patel, A.; Berardino, K.; Amgalan, A.; Berger, A.A.; Kassem, H.; Kaye, A.D.; Viswanath, O. A Review of Patisiran (ONPATTRO®) for the Treatment of Polyneuropathy in People with Hereditary Transthyretin Amyloidosis. Neurol. Ther. 2020, 9, 301–315. [Google Scholar] [CrossRef]
- Nie, T.; Heo, Y.-A.; Shirley, M. Vutrisiran: A Review in Polyneuropathy of Hereditary Transthyretin-Mediated Amyloidosis. Drugs 2023, 83, 1425–1432. [Google Scholar] [CrossRef]
- Syed, Y.Y. Givosiran: A Review in Acute Hepatic Porphyria. Drugs 2021, 81, 841–848. [Google Scholar] [CrossRef]
- Kang, C. Lumasiran: A Review in Primary Hyperoxaluria Type 1. Drugs 2024, 84, 219–226. [Google Scholar] [CrossRef]
- Liu, A.; Zhao, J.; Shah, M.; Migliorati, J.M.; Tawfik, S.M.; Bahal, R.; Rasmussen, T.P.; Manautou, J.E.; Zhong, X. Nedosiran, a Candidate siRNA Drug for the Treatment of Primary Hyperoxaluria: Design, Development, and Clinical Studies. ACS Pharmacol. Transl. Sci. 2022, 5, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Dai, Q.; Zhou, Y.; Guan, J.; Wu, J.; Dong, Y.; Lv, J. Inclisiran in Cardiovascular Health: A Review of Mechanisms, Efficacy, and Future Prospects. Med. Sci. Monit. 2025, 31, e946439. [Google Scholar] [CrossRef]
- Young, G.; Lenting, P.J.; Croteau, S.E.; Nolan, B.; Srivastava, A. Antithrombin Lowering in Hemophilia: A Closer Look at Fitusiran. Res. Pract. Thromb. Haemost. 2023, 7, 100179. [Google Scholar] [CrossRef] [PubMed]
- Thielmann, M.; Corteville, D.; Szabo, G.; Swaminathan, M.; Lamy, A.; Lehner, L.J.; Brown, C.D.; Noiseux, N.; Atta, M.G.; Squiers, E.C.; et al. Teprasiran, a Small Interfering RNA, for the Prevention of Acute Kidney Injury in High-Risk Patients Undergoing Cardiac Surgery: A Randomized Clinical Study. Circulation 2021, 144, 1133–1144. [Google Scholar] [CrossRef]
- Levin, L.A.; Bhatti, M.T.; Klier, S.; Morgenstern, R.; Szanto, D.; Miller, N.R.; Kupersmith, M.J. Quark NAION Study Group A Randomized Sham-Controlled Phase 2/3 Trial of QPI-1007 for Acute Nonarteritic Anterior Ischemic Optic Neuropathy. Ophthalmology 2025. [Google Scholar] [CrossRef]
- Moreno-Montañés, J.; Bleau, A.-M.; Jimenez, A.I. Tivanisiran, a Novel siRNA for the Treatment of Dry Eye Disease. Expert. Opin. Investig. Drugs 2018, 27, 421–426. [Google Scholar] [CrossRef]
- Barratt, J.; Liew, A.; Yeo, S.C.; Fernström, A.; Barbour, S.J.; Sperati, C.J.; Villanueva, R.; Wu, M.-J.; Wang, D.; Borodovsky, A.; et al. Phase 2 Trial of Cemdisiran in Adult Patients with IgA Nephropathy: A Randomized Controlled Trial. Clin. J. Am. Soc. Nephrol. 2024, 19, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Gaya, A.; Munir, T.; Urbano-Ispizua, A.; Griffin, M.; Taubel, J.; Bush, J.; Bhan, I.; Borodovsky, A.; Wang, Y.; Badri, P.; et al. Results of a Phase 1/2 Study of Cemdisiran in Healthy Subjects and Patients with Paroxysmal Nocturnal Hemoglobinuria. EJHaem 2023, 4, 612–624. [Google Scholar] [CrossRef]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and Specific Genetic Interference by Double-Stranded RNA in Caenorhabditis Elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef]
- McManus, M.T.; Sharp, P.A. Gene Silencing in Mammals by Small Interfering RNAs. Nat. Rev. Genet. 2002, 3, 737–747. [Google Scholar] [CrossRef]
- Banerjee, D.; Slack, F. Control of Developmental Timing by Small Temporal RNAs: A Paradigm for RNA-mediated Regulation of Gene Expression. BioEssays 2002, 24, 119–129. [Google Scholar] [CrossRef]
- Betancur, J.G.; Tomari, Y. Dicer Is Dispensable for Asymmetric RISC Loading in Mammals. RNA 2012, 18, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Shin, C. Slicer-Independent Mechanism Drives Small-RNA Strand Separation during Human RISC Assembly. Nucleic Acids Res. 2015, 43, 9418–9433. [Google Scholar] [CrossRef]
- Hutvágner, G.; Zamore, P.D. A microRNA in a Multiple-Turnover RNAi Enzyme Complex. Science 2002, 297, 2056–2060. [Google Scholar] [CrossRef] [PubMed]
- Flores-Jasso, C.F.; Salomon, W.E.; Zamore, P.D. Rapid and Specific Purification of Argonaute-Small RNA Complexes from Crude Cell Lysates. RNA 2013, 19, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Seyhan, A.A.; Vlassov, A.V.; Ilves, H.; Egry, L.; Kaspar, R.L.; Kazakov, S.A.; Johnston, B.H. Complete, Gene-Specific siRNA Libraries: Production and Expression in Mammalian Cells. RNA 2005, 11, 837–846. [Google Scholar] [CrossRef]
- Meister, G.; Landthaler, M.; Patkaniowska, A.; Dorsett, Y.; Teng, G.; Tuschl, T. Human Argonaute2 Mediates RNA Cleavage Targeted by miRNAs and siRNAs. Mol. Cell 2004, 15, 185–197. [Google Scholar] [CrossRef]
- Mokhlis, H.A.; Bayraktar, R.; Kabil, N.N.; Caner, A.; Kahraman, N.; Rodriguez-Aguayo, C.; Zambalde, E.P.; Sheng, J.; Karagoz, K.; Kanlikilicer, P.; et al. The Modulatory Role of MicroRNA-873 in the Progression of KRAS-Driven Cancers. Mol. Ther. Nucleic Acids 2019, 14, 301–317. [Google Scholar] [CrossRef]
- Tahtasakal, R.; Hamurcu, Z.; Oz, A.B.; Balli, M.; Dana, H.; Gok, M.; Cinar, V.; Inanc, M.; Sener, E.F. miR-484 as an “OncomiR” in Breast Cancer Promotes Tumorigenesis by Suppressing Apoptosis Genes. Ann. Surg. Oncol. 2025, 32, 2994–3008. [Google Scholar] [CrossRef]
- Lin, D.; Shi, Y.; Hu, Y.; Du, X.; Tu, G. miR-329-3p Regulates Neural Stem Cell Proliferation by Targeting E2F1. Mol. Med. Rep. 2019, 19, 4137–4146. [Google Scholar] [CrossRef] [PubMed]
- Torrisi, R.; Vaira, V.; Giordano, L.; Destro, A.; Basilico, V.; Mazzara, S.; Salvini, P.; Gaudioso, G.; Fernandes, B.; Rudini, N.; et al. Predictors of Fulvestrant Long-Term Benefit in Hormone Receptor-Positive/HER2 Negative Advanced Breast Cancer. Sci. Rep. 2022, 12, 12789. [Google Scholar] [CrossRef]
- Chiorino, G.; Petracci, E.; Sehovic, E.; Gregnanin, I.; Camussi, E.; Mello-Grand, M.; Ostano, P.; Riggi, E.; Vergini, V.; Russo, A.; et al. Plasma microRNA Ratios Associated with Breast Cancer Detection in a Nested Case–Control Study from a Mammography Screening Cohort. Sci. Rep. 2023, 13, 12040. [Google Scholar] [CrossRef]
- Martínez, C.G.; Therapontos, S.; Lorente, J.A.; Lucena, M.A.; Ortega, F.G.; Serrano, M.J. Evaluating MicroRNAs as Diagnostic Tools for Lymph Node Metastasis in Breast Cancer: Findings from a Systematic Review and Meta-Analysis. Crit. Rev. Oncol./Hematol. 2025, 207, 104598. [Google Scholar] [CrossRef]
- Salum, G.M.; Elaraby, N.M.; Ahmed, H.A.; Abd El Meguid, M.; Fotouh, B.E.; Ashraf, M.; Elhusseny, Y.; Dawood, R.M. Evaluation of Tumorigenesis-Related miRNAs in Breast Cancer in Egyptian Women: A Retrospective, Exploratory Analysis. Sci. Rep. 2024, 14, 29757. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Pan, X. Exosome-Derived MicroRNA-221-3p Desensitizes Breast Cancer Cells to Adriamycin by Regulating PIK3r1-Mediated Glycose Metabolism. Cancer Biother. Radiopharm. 2024, 39, 463–475. [Google Scholar] [CrossRef]
- Ma, M.; He, M.; Jiang, Q.; Yan, Y.; Guan, S.; Zhang, J.; Yu, Z.; Chen, Q.; Sun, M.; Yao, W.; et al. MiR-487a Promotes TGF-Β1-Induced EMT, the Migration and Invasion of Breast Cancer Cells by Directly Targeting MAGI2. Int. J. Biol. Sci. 2021, 17, 4034–4035. [Google Scholar] [CrossRef] [PubMed]
- Holý, P.; Brynychová, V.; Šeborová, K.; Haničinec, V.; Koževnikovová, R.; Trnková, M.; Vrána, D.; Gatěk, J.; Kopečková, K.; Mrhalová, M.; et al. Integrative Analysis of mRNA and miRNA Expression Profiles and Somatic Variants in Oxysterol Signaling in Early-stage Luminal Breast Cancer. Mol. Oncol. 2023, 17, 2074–2089. [Google Scholar] [CrossRef]
- Parrella, P.; Barbano, R.; Jonas, K.; Fontana, A.; Barile, S.; Rendina, M.; Lo Mele, A.; Prencipe, G.; Ciuffreda, L.; Morritti, M.G.; et al. Tumor Suppressor miR-27a-5p and Its Significance for Breast Cancer. Biomedicines 2024, 12, 2625. [Google Scholar] [CrossRef]
- Duan, W.-L.; Wang, X.-J.; Guo, A.; Gu, L.-H.; Sheng, Z.-M.; Luo, H.; Yang, L.-X.; Wang, W.-H.; Zhang, B.-G. miR-141-3p Promotes Paclitaxel Resistance by Attenuating Ferroptosis via the Keap1-Nrf2 Signaling Pathway in Breast Cancer. J. Cancer 2024, 15, 5622–5635. [Google Scholar] [CrossRef]
- Tassone, P.; Di Martino, M.T.; Arbitrio, M.; Fiorillo, L.; Staropoli, N.; Ciliberto, D.; Cordua, A.; Scionti, F.; Bertucci, B.; Salvino, A.; et al. Safety and Activity of the First-in-Class Locked Nucleic Acid (LNA) miR-221 Selective Inhibitor in Refractory Advanced Cancer Patients: A First-in-Human, Phase 1, Open-Label, Dose-Escalation Study. J. Hematol. Oncol. 2023, 16, 68. [Google Scholar] [CrossRef]
- Skerritt, J.H. Considerations for mRNA Product Development, Regulation and Deployment Across the Lifecycle. Vaccines 2025, 13, 473. [Google Scholar] [CrossRef] [PubMed]
- Batisani, K. The Role of mRNA Vaccines in Infectious Diseases: A New Era of Immunization. Trop. Dis. Travel. Med. Vaccines 2025, 11, 12. [Google Scholar] [CrossRef]
- Haghmorad, D.; Eslami, M.; Orooji, N.; Halabitska, I.; Kamyshna, I.; Kamyshnyi, O.; Oksenych, V. mRNA Vaccine Platforms: Linking Infectious Disease Prevention and Cancer Immunotherapy. Front. Bioeng. Biotechnol. 2025, 13, 1547025. [Google Scholar] [CrossRef]
- Leong, K.Y.; Tham, S.K.; Poh, C.L. Revolutionizing Immunization: A Comprehensive Review of mRNA Vaccine Technology and Applications. Virol. J. 2025, 22, 71. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, H.G.; Gee, J.; Liu, R.; Marquez, P.L.; Zhang, B.; Strid, P.; Abara, W.E.; McNeil, M.M.; Myers, T.R.; Hause, A.M.; et al. Safety of mRNA Vaccines Administered during the Initial 6 Months of the US COVID-19 Vaccination Programme: An Observational Study of Reports to the Vaccine Adverse Event Reporting System and v-Safe. Lancet Infect. Dis. 2022, 22, 802–812. [Google Scholar] [CrossRef]
- Trougakos, I.P.; Terpos, E.; Alexopoulos, H.; Politou, M.; Paraskevis, D.; Scorilas, A.; Kastritis, E.; Andreakos, E.; Dimopoulos, M.A. Adverse Effects of COVID-19 mRNA Vaccines: The Spike Hypothesis. Trends Mol. Med. 2022, 28, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Barda, N.; Dagan, N.; Ben-Shlomo, Y.; Kepten, E.; Waxman, J.; Ohana, R.; Hernán, M.A.; Lipsitch, M.; Kohane, I.; Netzer, D.; et al. Safety of the BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Setting. N. Engl. J. Med. 2021, 385, 1078–1090. [Google Scholar] [CrossRef]
- Avci, E.; Abasiyanik, F. Autoimmune Hepatitis after SARS-CoV-2 Vaccine: New-Onset or Flare-Up? J. Autoimmun. 2021, 125, 102745. [Google Scholar] [CrossRef]
- Zin Tun, G.S.; Gleeson, D.; Al-Joudeh, A.; Dube, A. Immune-Mediated Hepatitis with the Moderna Vaccine, No Longer a Coincidence but Confirmed. J. Hepatol. 2022, 76, 747–749. [Google Scholar] [CrossRef]
- Patone, M.; Handunnetthi, L.; Saatci, D.; Pan, J.; Katikireddi, S.V.; Razvi, S.; Hunt, D.; Mei, X.W.; Dixon, S.; Zaccardi, F.; et al. Neurological Complications after First Dose of COVID-19 Vaccines and SARS-CoV-2 Infection. Nat. Med. 2021, 27, 2144–2153. [Google Scholar] [CrossRef]
- Ndeupen, S.; Qin, Z.; Jacobsen, S.; Bouteau, A.; Estanbouli, H.; Igyártó, B.Z. The mRNA-LNP Platform’s Lipid Nanoparticle Component Used in Preclinical Vaccine Studies Is Highly Inflammatory. iScience 2021, 24, 103479. [Google Scholar] [CrossRef]
- Karikó, K.; Ni, H.; Capodici, J.; Lamphier, M.; Weissman, D. mRNA Is an Endogenous Ligand for Toll-like Receptor 3. J. Biol. Chem. 2004, 279, 12542–12550. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cai, S.; Peng, J.; Friedberg, M.; Redington, A. Time Dependent Distribution of MicroRNA 144 after Intravenous Delivery. Microrna 2016, 5, 36–49. [Google Scholar] [CrossRef]
- Lockhart, J.H.; VanWye, J.; Banerjee, R.; Wickline, S.A.; Pan, H.; Totary-Jain, H. Self-Assembled miRNA-Switch Nanoparticles Target Denuded Regions and Prevent Restenosis. Mol. Ther. 2021, 29, 1744–1757. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Maeda, H. A New Concept for Macromolecular Therapeutics in Cancer Chemotherapy: Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs1. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- Jiang, A.Y.; Lathwal, S.; Meng, S.; Witten, J.; Beyer, E.; McMullen, P.; Hu, Y.; Manan, R.S.; Raji, I.; Langer, R.; et al. Zwitterionic Polymer-Functionalized Lipid Nanoparticles for the Nebulized Delivery of mRNA. J. Am. Chem. Soc. 2024, 146, 32567–32574. [Google Scholar] [CrossRef]
- Devoldere, J.; Wels, M.; Peynshaert, K.; Dewitte, H.; De Smedt, S.C.; Remaut, K. The Obstacle Course to the Inner Retina: Hyaluronic Acid-Coated Lipoplexes Cross the Vitreous but Fail to Overcome the Inner Limiting Membrane. Eur. J. Pharm. Biopharm. 2019, 141, 161–171. [Google Scholar] [CrossRef]
- Ortega, R.A.; Barham, W.J.; Kumar, B.; Tikhomirov, O.; McFadden, I.D.; Yull, F.E.; Giorgio, T.D. Biocompatible Mannosylated Endosomal-Escape Nanoparticles Enhance Selective Delivery of Short Nucleotide Sequences to Tumor Associated Macrophages. Nanoscale 2015, 7, 500–510. [Google Scholar] [CrossRef]
- Patni, H.; Chaudhary, R.; Kumar, A. Unleashing Nanotechnology to Redefine Tumor-Associated Macrophage Dynamics and Non-Coding RNA Crosstalk in Breast Cancer. Nanoscale 2024, 16, 18274–18294. [Google Scholar] [CrossRef]
- Chao, M.P.; Jaiswal, S.; Weissman-Tsukamoto, R.; Alizadeh, A.A.; Gentles, A.J.; Volkmer, J.; Weiskopf, K.; Willingham, S.B.; Raveh, T.; Park, C.Y.; et al. Calreticulin Is the Dominant Pro-Phagocytic Signal on Multiple Human Cancers and Is Counterbalanced by CD47. Sci. Transl. Med. 2010, 2, 63ra94. [Google Scholar] [CrossRef]
- Schürch, C.M.; Roelli, M.A.; Forster, S.; Wasmer, M.-H.; Brühl, F.; Maire, R.S.; Di Pancrazio, S.; Ruepp, M.-D.; Giger, R.; Perren, A.; et al. Targeting CD47 in Anaplastic Thyroid Carcinoma Enhances Tumor Phagocytosis by Macrophages and Is a Promising Therapeutic Strategy. Thyroid 2019, 29, 979–992. [Google Scholar] [CrossRef]
- Zhang, Y.; Rao, Y.; Lu, J.; Wang, J.; Ker, D.F.E.; Zhou, J.; Wang, D.M. The Influence of Biophysical Niche on Tumor-Associated Macrophages in Liver Cancer. Hepatol. Commun. 2024, 8, e0569. [Google Scholar] [CrossRef]
- Rannikko, J.H.; Hollmén, M. Clinical Landscape of Macrophage-Reprogramming Cancer Immunotherapies. Br. J. Cancer 2024, 131, 627–640. [Google Scholar] [CrossRef] [PubMed]
- Proia, T.A.; Singh, M.; Woessner, R.; Carnevalli, L.; Bommakanti, G.; Magiera, L.; Srinivasan, S.; Grosskurth, S.; Collins, M.; Womack, C.; et al. STAT3 Antisense Oligonucleotide Remodels the Suppressive Tumor Microenvironment to Enhance Immune Activation in Combination with Anti–PD-L1. Clin. Cancer Res. 2020, 26, 6335–6349. [Google Scholar] [CrossRef] [PubMed]
- Reilley, M.J.; McCoon, P.; Cook, C.; Lyne, P.; Kurzrock, R.; Kim, Y.; Woessner, R.; Younes, A.; Nemunaitis, J.; Fowler, N.; et al. STAT3 Antisense Oligonucleotide AZD9150 in a Subset of Patients with Heavily Pretreated Lymphoma: Results of a Phase 1b Trial. J. Immunother. Cancer 2018, 6, 119. [Google Scholar] [CrossRef]
- Sargent, B. Lonza’s Xcite® EV Technology: A Platform Approach for Producing Engineered Extracellular Vesicles. Available online: https://cellculturedish.com/lonzas-xcite-ev-technology-a-platform-approach-for-producing-engineered-extracellular-vesicles/ (accessed on 17 September 2025).
- Dooley, K.; McConnell, R.E.; Xu, K.; Lewis, N.D.; Haupt, S.; Youniss, M.R.; Martin, S.; Sia, C.L.; McCoy, C.; Moniz, R.J.; et al. A Versatile Platform for Generating Engineered Extracellular Vesicles with Defined Therapeutic Properties. Mol. Ther. 2021, 29, 1729–1743. [Google Scholar] [CrossRef]
- Voutila, J.; Reebye, V.; Roberts, T.C.; Protopapa, P.; Andrikakou, P.; Blakey, D.C.; Habib, R.; Huber, H.; Saetrom, P.; Rossi, J.J.; et al. Development and Mechanism of Small Activating RNA Targeting CEBPA, a Novel Therapeutic in Clinical Trials for Liver Cancer. Mol. Ther. 2017, 25, 2705–2714. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.P.; Sinigaglia, L.; Gomez, V.; Nicholls, J.; Habib, N.A. RNA Activation—A Novel Approach to Therapeutically Upregulate Gene Transcription. Molecules 2021, 26, 6530. [Google Scholar] [CrossRef]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Muchtaridi, M. Scale-up Polymeric-Based Nanoparticles Drug Delivery Systems: Development and Challenges. OpenNano 2022, 7, 100048. [Google Scholar] [CrossRef]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H. Nanoparticle Uptake: The Phagocyte Problem. Nano Today 2015, 10, 487–510. [Google Scholar] [CrossRef]
- Zelepukin, I.V.; Shevchenko, K.G.; Deyev, S.M. Rediscovery of Mononuclear Phagocyte System Blockade for Nanoparticle Drug Delivery. Nat. Commun. 2024, 15, 4366. [Google Scholar] [CrossRef]
- Islam, S.; Ahmed, M.M.S.; Islam, M.A.; Hossain, N.; Chowdhury, M.A. Advances in Nanoparticles in Targeted Drug Delivery–A Review. Results Surf. Interfaces 2025, 19, 100529. [Google Scholar] [CrossRef]
- Greish, K. Enhanced Permeability and Retention (EPR) Effect for Anticancer Nanomedicine Drug Targeting. In Cancer Nanotechnology; Grobmyer, S.R., Moudgil, B.M., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2010; Volume 624, pp. 25–37. ISBN 978-1-60761-608-5. [Google Scholar]
- Kuźnicki, J.; Janicka, N.; Białynicka-Birula, B.; Kuźnicki, W.; Chorążyczewska, H.; Deszcz, I.; Kulbacka, J. How to Use Macrophages Against Cancer. Cells 2024, 13, 1948. [Google Scholar] [CrossRef] [PubMed]
- Rupaimoole, R.; Slack, F.J. MicroRNA Therapeutics: Towards a New Era for the Management of Cancer and Other Diseases. Nat. Rev. Drug Discov. 2017, 16, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Yi, Y.; Kim, A.; Miyata, K. Small Delivery Vehicles of siRNA for Enhanced Cancer Targeting. Biomacromolecules 2018, 19, 2377–2390. [Google Scholar] [CrossRef] [PubMed]
- Shuhendler, A.J.; Prasad, P.; Leung, M.; Rauth, A.M.; DaCosta, R.S.; Wu, X.Y. A Novel Solid Lipid Nanoparticle Formulation for Active Targeting to Tumor αv β3 Integrin Receptors Reveals Cyclic RGD as A Double-Edged Sword. Adv. Healthc. Mater. 2012, 1, 600–608. [Google Scholar] [CrossRef]
- Kim, J.; Cho, H.; Lim, D.-K.; Joo, M.K.; Kim, K. Perspectives for Improving the Tumor Targeting of Nanomedicine via the EPR Effect in Clinical Tumors. Int. J. Mol. Sci. 2023, 24, 10082. [Google Scholar] [CrossRef] [PubMed]
- Gawali, P.; Saraswat, A.; Bhide, S.; Gupta, S.; Patel, K. Human Solid Tumors and Clinical Relevance of The Enhanced Permeation and Retention Effect: A ‘Golden Gate’ for Nanomedicine in Preclinical Studies? Nanomedicine 2023, 18, 169–190. [Google Scholar] [CrossRef]
- Lee, S.W.L.; Paoletti, C.; Campisi, M.; Osaki, T.; Adriani, G.; Kamm, R.D.; Mattu, C.; Chiono, V. MicroRNA Delivery through Nanoparticles. J. Control. Release 2019, 313, 80–95. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka-Puchta, L.; Sawicka, D.; Zapor, L.; Miranowicz-Dzierzawska, K. Assessing Cytotoxicity and Endoplasmic Reticulum Stress in Human Blood–Brain Barrier Cells Due to Silver and Copper Oxide Nanoparticles. J. Appl. Genet. 2025, 66, 87–103. [Google Scholar] [CrossRef]
- Cabral, H.; Makino, J.; Matsumoto, Y.; Mi, P.; Wu, H.; Nomoto, T.; Toh, K.; Yamada, N.; Higuchi, Y.; Konishi, S.; et al. Systemic Targeting of Lymph Node Metastasis through the Blood Vascular System by Using Size-Controlled Nanocarriers. ACS Nano 2015, 9, 4957–4967. [Google Scholar] [CrossRef] [PubMed]
- Rachamalla, H.K.; Mondal, S.K.; Deshpande, S.S.; Sridharan, K.; Javaji, K.; Jaggarapu, M.M.C.S.; Jinka, S.; Bollu, V.; Misra, S.; Banerjee, R. Efficient Anti-Tumor Nano-Lipoplexes with Unsaturated or Saturated Lipid Induce Differential Genotoxic Effects in Mice. Nanotoxicology 2019, 13, 1161–1175. [Google Scholar] [CrossRef]
- Li, B.; Zhao, Q.; Yang, H.; Wang, X.; Zhang, Z.; Gong, Y.; Wan, X. Long-Circulating and Targeted Liposomes Co-Loading Cisplatin and Mifamurtide: Formulation and Delivery in Osteosarcoma Cells. AAPS PharmSciTech 2024, 25, 272. [Google Scholar] [CrossRef]
- Gheybi, F.; Alavizadeh, S.H.; Rezayat, S.M.; Hatamipour, M.; Akhtari, J.; Faridi Majidi, R.; Badiee, A.; Jaafari, M.R. pH-Sensitive PEGylated Liposomal Silybin: Synthesis, In Vitro and In Vivo Anti-Tumor Evaluation. J. Pharm. Sci. 2021, 110, 3919–3928. [Google Scholar] [CrossRef]
- Griffiths, P.C.; Khayat, Z.; Tse, S.; Heenan, R.K.; King, S.M.; Duncan, R. Studies on the Mechanism of Interaction of a Bioresponsive Endosomolytic Polyamidoamine with Interfaces. 1. Micelles as Model Surfaces. Biomacromolecules 2007, 8, 1004–1012. [Google Scholar] [CrossRef]
- Du, L.; Gong, Y.; Zhang, X.; Sun, J.; Gao, F.; Shen, M.; Bai, H.; Yang, T.; Cheng, X.; Li, S.; et al. PD-L1 siRNA Hitched Polyethyleneimine-Elastase Constituting Nanovesicle Induces Tumor Immunogenicity and PD-L1 Silencing for Synergistic Antitumor Immunotherapy. J. Nanobiotechnol. 2024, 22, 442. [Google Scholar] [CrossRef]
- Al-Absi, M.Y.; Caprifico, A.E.; Calabrese, G. Chitosan and Its Structural Modifications for siRNA Delivery. Adv. Pharm. Bull. 2023, 13, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Zhang, Y.; Huang, M.; Luo, G.; Ma, Y.; Wang, X. Microdroplets Encapsulated with NFATc1-siRNA and Exosomes-Derived from MSCs Onto 3D Porous PLA Scaffold for Regulating Osteoclastogenesis and Promoting Osteogenesis. Int. J. Nanomed. 2024, 19, 3423–3440. [Google Scholar] [CrossRef]
- Wan, X.; Chen, C.; Zhan, J.; Ye, S.; Li, R.; Shen, M. Dendritic Polylysine Co-Delivery of Paclitaxel and siAXL Enhances the Sensitivity of Triple-Negative Breast Cancer Chemotherapy. Front. Bioeng. Biotechnol. 2024, 12, 1415191. [Google Scholar] [CrossRef]
- Padnya, P.; Shiabiev, I.; Pysin, D.; Gerasimova, T.; Ranishenka, B.; Stanavaya, A.; Abashkin, V.; Shcharbin, D.; Shi, X.; Shen, M.; et al. Non-Viral Systems Based on PAMAM-Calix-Dendrimers for Regulatory siRNA Delivery into Cancer Cells. Int. J. Mol. Sci. 2024, 25, 12614. [Google Scholar] [CrossRef]
- Akhmetova, D.R.; Rogova, A.; Tishchenko, Y.A.; Mitusova, K.A.; Postovalova, A.S.; Dovbysh, O.V.; Gavrilova, N.V.; Epifanovskaya, O.S.; Pyatiizbyantsev, T.A.; Shakirova, A.I.; et al. An Investigation of Nano- and Micron-Sized Carriers Based on Calcium Carbonate and Polylactic Acid for Oral Administration of siRNA. Expert. Opin. Drug Deliv. 2024, 21, 1279–1295. [Google Scholar] [CrossRef]
- Zhang, F.; Parayath, N.N.; Ene, C.I.; Stephan, S.B.; Koehne, A.L.; Coon, M.E.; Holland, E.C.; Stephan, M.T. Genetic Programming of Macrophages to Perform Anti-Tumor Functions Using Targeted mRNA Nanocarriers. Nat. Commun. 2019, 10, 3974. [Google Scholar] [CrossRef]
- Karlsson, J.; Rhodes, K.R.; Green, J.J.; Tzeng, S.Y. Poly(Beta-Amino Ester)s as Gene Delivery Vehicles: Challenges and Opportunities. Expert. Opin. Drug Deliv. 2020, 17, 1395–1410. [Google Scholar] [CrossRef]
- Wei, J.; Zhu, L.; Lu, Q.; Li, G.; Zhou, Y.; Yang, Y.; Zhang, L. Recent Progress and Applications of Poly(Beta Amino Esters)-Based Biomaterials. J. Control. Release 2023, 354, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Bao, Q.; Bao, M.; Xiao, H.; Ganbold, T.; Han, S.; Baigude, H. Tumor-Targeted Codelivery of CpG and siRNA by a Dual-Ligand-Functionalized Curdlan Nanoparticle. Biomacromolecules 2024, 25, 3360–3372. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Niu, H.; Zhao, X.; Huang, X.; Ding, Y.; Dang, K.; Yang, T.; Chen, Y.; Ma, J.; Liu, X.; et al. Targeted Anti-Cancer Therapy: Co-Delivery of VEGF siRNA and Phenethyl Isothiocyanate (PEITC) via cRGD-Modified Lipid Nanoparticles for Enhanced Anti-Angiogenic Efficacy. Asian J. Pharm. Sci. 2024, 19, 100891. [Google Scholar] [CrossRef]
- Tambe, P.; Salve, R.; Choudhary, P.; Kumar, P.; Jadhav, S.; Paknikar, K.M.; Gajbhiye, V. Targeted Silencing of the MCL-1 Gene Using Multi-Layered Dendrimer-Based Nanoconstructs Achieves Efficient Tumor Regression in Xenografted Mice Models. Int. J. Pharm. 2023, 634, 122659. [Google Scholar] [CrossRef]
- Samal, S.; Panda, G.P.; Shyamal, S.; Das, K.; Dash, M. Surface Engineered Osteoblast-Extracellular Vesicles Serve as an Efficient Carrier for Drug and Small RNA to Actively Target Osteosarcoma. ACS Biomater. Sci. Eng. 2024, 10, 7466–7481. [Google Scholar] [CrossRef]
- Hei, M.-W.; Zhan, Y.-R.; Chen, P.; Zhao, R.-M.; Tian, X.-L.; Yu, X.-Q.; Zhang, J. Lipoic Acid-Based Poly(Disulfide)s as Versatile Biomolecule Delivery Vectors and the Application in Tumor Immunotherapy. Mol. Pharm. 2023, 20, 3210–3222. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zhu, X.; Li, S.; Wang, P.; Fang, J. Liver-Targeted Delivery of Oligonucleotides with N-Acetylgalactosamine Conjugation. ACS Omega 2021, 6, 16259–16265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, H.; Zhen, W.; Jiang, T.; Cui, J. Advancement of Drugs Conjugated with GalNAc in the Targeted Delivery to Hepatocytes Based on Asialoglycoprotein Receptor. Carbohydr. Res. 2025, 552, 109426. [Google Scholar] [CrossRef]
- Springer, A.D.; Dowdy, S.F. GalNAc-siRNA Conjugates: Leading the Way for Delivery of RNAi Therapeutics. Nucleic Acid. Ther. 2018, 28, 109–118. [Google Scholar] [CrossRef]
- Bansal, R.; Prakash, J.; De Ruiter, M.; Poelstra, K. Targeted Recombinant Fusion Proteins of IFNγ and Mimetic IFNγ with PDGFβR Bicyclic Peptide Inhibits Liver Fibrogenesis In Vivo. PLoS ONE 2014, 9, e89878. [Google Scholar] [CrossRef]
- Shevelev, A.; Pozdniakova, N.; Generalov, E.; Tarasova, O. siRNA Therapeutics for the Treatment of Hereditary Diseases and Other Conditions: A Review. Int. J. Mol. Sci. 2025, 26, 8651. [Google Scholar] [CrossRef] [PubMed]
- Kalita, T.; Dezfouli, S.A.; Pandey, L.M.; Uludag, H. siRNA Functionalized Lipid Nanoparticles (LNPs) in Management of Diseases. Pharmaceutics 2022, 14, 2520. [Google Scholar] [CrossRef]
- Hosseini-Kharat, M.; Bremmell, K.E.; Prestidge, C.A. Why Do Lipid Nanoparticles Target the Liver? Understanding of Biodistribution and Liver-Specific Tropism. Mol. Ther. Methods Clin. Dev. 2025, 33, 101436. [Google Scholar] [CrossRef]
- Pattipeiluhu, R.; Arias-Alpizar, G.; Basha, G.; Chan, K.Y.T.; Bussmann, J.; Sharp, T.H.; Moradi, M.; Sommerdijk, N.; Harris, E.N.; Cullis, P.R.; et al. Anionic Lipid Nanoparticles Preferentially Deliver mRNA to the Hepatic Reticuloendothelial System. Adv. Mater. 2022, 34, 2201095. [Google Scholar] [CrossRef]
- Puhl, D.L.; D’Amato, A.R.; Gilbert, R.J. Challenges of Gene Delivery to the Central Nervous System and the Growing Use of Biomaterial Vectors. Brain Res. Bull. 2019, 150, 216–230. [Google Scholar] [CrossRef]
- Geng, G.; Xu, Y.; Hu, Z.; Wang, H.; Chen, X.; Yuan, W.; Shu, Y. Viral and Non-Viral Vectors in Gene Therapy: Current State and Clinical Perspectives. eBioMedicine 2025, 118, 105834. [Google Scholar] [CrossRef] [PubMed]
- Attia, M.S.; Kijanka, G.; Nguyen, N.-T.; Zhang, J.; An, H. Advances and Prospects of RNA Delivery Nanoplatforms for Cancer Therapy. Acta Pharm. Sin. B 2025, 15, 52–96. [Google Scholar] [CrossRef]
- Zare, M.; Pemmada, R.; Madhavan, M.; Shailaja, A.; Ramakrishna, S.; Kandiyil, S.P.; Donahue, J.M.; Thomas, V. Encapsulation of miRNA and siRNA into Nanomaterials for Cancer Therapeutics. Pharmaceutics 2022, 14, 1620. [Google Scholar] [CrossRef]
- Naeem, S.; Zhang, J.; Zhang, Y.; Wang, Y. Nucleic Acid Therapeutics: Past, Present, and Future. Mol. Ther. Nucleic Acids 2025, 36, 102440. [Google Scholar] [CrossRef]
- Kowalski, P.S.; Rudra, A.; Miao, L.; Anderson, D.G. Delivering the Messenger: Advances in Technologies for Therapeutic mRNA Delivery. Mol. Ther. 2019, 27, 710–728. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid Nanoparticles for mRNA Delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, Y.; He, G.; Guo, C.; Dong, J.; Wu, L. Development of mRNA Lipid Nanoparticles: Targeting and Therapeutic Aspects. Int. J. Mol. Sci. 2024, 25, 10166. [Google Scholar] [CrossRef]
- Ma, Y.; Li, S.; Lin, X.; Chen, Y. A Perspective of Lipid Nanoparticles for RNA Delivery. Exploration 2024, 4, 20230147. [Google Scholar] [CrossRef]
- Jung, H.N.; Lee, S.-Y.; Lee, S.; Youn, H.; Im, H.-J. Lipid Nanoparticles for Delivery of RNA Therapeutics: Current Status and the Role of in Vivo Imaging. Theranostics 2022, 12, 7509–7531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X.; Gao, Y.; Liu, S. Principles of Lipid Nanoparticle Design for mRNA Delivery. BMEMat 2025, 3, e12116. [Google Scholar] [CrossRef]
- Farjadian, F.; Mirkiani, S.; Ghasemiyeh, P.; Rahbar Kafshboran, H.; Mehdi-Alamdarlou, S.; Raeisi, A.; Esfandiarinejad, R.; Soleymani, S.; Goshtasbi, G.; Firouzabadi, N.; et al. Smart Nanogels as Promising Platform for Delivery of Drug, Gene, and Vaccine; Therapeutic Applications and Active Targeting Mechanism. Eur. Polym. J. 2024, 219, 113400. [Google Scholar] [CrossRef]
- Zhong, R.; Talebian, S.; Mendes, B.B.; Wallace, G.; Langer, R.; Conde, J.; Shi, J. Hydrogels for RNA Delivery. Nat. Mater. 2023, 22, 818–831. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.; Wu, Y.; Park, J.; Kim, J.S.; Li, Q.; Choi, J.; Shin, N.; Lan, M.; Cai, Y.; Lee, J.; et al. RNA Nanomedicine: Delivery Strategies and Applications. AAPS J. 2023, 25, 95. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Pujol, E.J.; Martínez, G.; Casado-Jurado, D.; Vázquez, J.; León-Barberena, J.; Rodríguez-Lucena, D.; Torres, Y.; Alcudia, A.; Begines, B. Hydrogels and Nanogels: Pioneering the Future of Advanced Drug Delivery Systems. Pharmaceutics 2025, 17, 215. [Google Scholar] [CrossRef]
- Espuche, B.; Moya, S.E.; Calderón, M. Nanogels: Smart Tools to Enlarge the Therapeutic Window of Gene Therapy. Int. J. Pharm. 2024, 653, 123864. [Google Scholar] [CrossRef]
- Zhang, L.; Haddouti, E.-M.; Welle, K.; Burger, C.; Kabir, K.; Schildberg, F.A. Local Cellular Responses to Metallic and Ceramic Nanoparticles from Orthopedic Joint Arthroplasty Implants. Int. J. Nanomed. 2020, 15, 6705–6720. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, J.; Wang, Z.; Liu, C.; Wang, T.; Kim, C.-J.; Durikova, H.; Fernandes, S.; Johnson, D.N.; De Rose, R.; et al. mRNA Delivery Enabled by Metal–Organic Nanoparticles. Nat. Commun. 2024, 15, 9664. [Google Scholar] [CrossRef]
- Vosoughi, P.; Naghib, S.M.; Kangarshahi, B.M.; Mozafari, M.R. A Review of RNA Nanoparticles for Drug/Gene/Protein Delivery in Advanced Therapies: Current State and Future Prospects. Int. J. Biol. Macromol. 2025, 295, 139532. [Google Scholar] [CrossRef]
- Sharma, A.R.; Lee, Y.-H.; Bat-Ulzii, A.; Bhattacharya, M.; Chakraborty, C.; Lee, S.-S. Recent Advances of Metal-Based Nanoparticles in Nucleic Acid Delivery for Therapeutic Applications. J. Nanobiotechnol. 2022, 20, 501. [Google Scholar] [CrossRef]
- Shahalaei, M.; Azad, A.K.; Sulaiman, W.M.A.W.; Derakhshani, A.; Mofakham, E.B.; Mallandrich, M.; Kumarasamy, V.; Subramaniyan, V. A Review of Metallic Nanoparticles: Present Issues and Prospects Focused on the Preparation Methods, Characterization Techniques, and Their Theranostic Applications. Front. Chem. 2024, 12, 1398979. [Google Scholar] [CrossRef] [PubMed]
- Eltaib, L. Polymeric Nanoparticles in Targeted Drug Delivery: Unveiling the Impact of Polymer Characterization and Fabrication. Polymers 2025, 17, 833. [Google Scholar] [CrossRef]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Mirón-Barroso, S.; Correia, J.; Frampton, A.; Lythgoe, M.; Clark, J.; Tookman, L.; Ottaviani, S.; Castellano, L.; Porter, A.; Georgiou, T.; et al. Polymeric Carriers for Delivery of RNA Cancer Therapeutics. ncRNA 2022, 8, 58. [Google Scholar] [CrossRef]
- Jiang, X.; Abedi, K.; Shi, J. Polymeric Nanoparticles for RNA Delivery. In Encyclopedia of Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2023; pp. 555–573. ISBN 978-0-12-822423-6. [Google Scholar]
- Paunovska, K.; Loughrey, D.; Dahlman, J.E. Drug Delivery Systems for RNA Therapeutics. Nat. Rev. Genet. 2022, 23, 265–280. [Google Scholar] [CrossRef]
- Rodrigues, A.F.; Rebelo, C.; Simões, S.; Paulo, C.; Pinho, S.; Francisco, V.; Ferreira, L. A Polymeric Nanoparticle Formulation for Targeted mRNA Delivery to Fibroblasts. Adv. Sci. 2023, 10, 2205475. [Google Scholar] [CrossRef]
- Mahor, A.; Singh, P.P.; Bharadwaj, P.; Sharma, N.; Yadav, S.; Rosenholm, J.M.; Bansal, K.K. Carbon-Based Nanomaterials for Delivery of Biologicals and Therapeutics: A Cutting-Edge Technology. C 2021, 7, 19. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Huang, X.-C.; Luo, Y.-L.; Chang, Y.-C.; Hsieh, Y.-Z.; Hsu, H.-Y. Non-Metallic Nanomaterials in Cancer Theranostics: A Review of Silica- and Carbon-Based Drug Delivery Systems. Sci. Technol. Adv. Mater. 2013, 14, 044407. [Google Scholar] [CrossRef] [PubMed]
- Zare-Zardini, H.; Hatamizade, N.; Haddadzadegan, N.; Soltaninejad, H.; Karimi-Zarchi, M. Advantages and Disadvantages of Using Carbon Nanostructures in Reproductive Medicine: Two Sides of the Same Coin. JBRA Assist. Reprod. 2022, 26, 142–144. [Google Scholar] [CrossRef] [PubMed]
- Makhado, B.P.; Oladipo, A.O.; Gumbi, N.N.; De Kock, L.A.; Andraos, C.; Gulumian, M.; Nxumalo, E.N. Unravelling the Toxicity of Carbon Nanomaterials—From Cellular Interactions to Mechanistic Understanding. Toxicol. Vitr. 2024, 100, 105898. [Google Scholar] [CrossRef]
- Palmero, P. Structural Ceramic Nanocomposites: A Review of Properties and Powders’ Synthesis Methods. Nanomaterials 2015, 5, 656–696. [Google Scholar] [CrossRef]
- Singh, D.; Singh, S.; Sahu, J.; Srivastava, S.; Singh, M.R. Ceramic Nanoparticles: Recompense, Cellular Uptake and Toxicity Concerns. Artif. Cells Nanomed. Biotechnol. 2016, 44, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Harshita, B.S.P.; Mishra, P.; Talegaonkar, S. Ceramic Nanoparticles: Fabrication Methods and Applications in Drug Delivery. Curr. Pharm. Des. 2015, 21, 6165–6188. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.A. Quantum Dots to Monitor RNAi Delivery and Improve Gene Silencing. Nucleic Acids Res. 2005, 33, e190. [Google Scholar] [CrossRef] [PubMed]
- Zahed, Z.; Hadi, R.; Imanzadeh, G.; Ahmadian, Z.; Shafiei, S.; Zadeh, A.Z.; Karimi, H.; Akbarzadeh, A.; Abbaszadeh, M.; Ghadimi, L.S.; et al. Recent Advances in Fluorescence Nanoparticles “Quantum Dots” as Gene Delivery System: A Review. Int. J. Biol. Macromol. 2024, 254, 127802. [Google Scholar] [CrossRef]
- Won, J.E.; Park, M.; Hong, S.-H.; Kim, Y.S.; Song, H. Quantum Dots as Biocompatible Small RNA Nanocarriers Modulating Macrophage Polarization to Treat Asherman’s Syndrome. npj Regen. Med. 2025, 10, 15. [Google Scholar] [CrossRef]
- Pareek, A.; Kumar, D.; Pareek, A.; Gupta, M.M. Advancing Cancer Therapy with Quantum Dots and Other Nanostructures: A Review of Drug Delivery Innovations, Applications, and Challenges. Cancers 2025, 17, 878. [Google Scholar] [CrossRef]
- He, Z.-Y.; Jin, Z.-H.; Zhan, M.; Qin, Z.; Li, Z.; Xu, T. Advances in Quantum Dot-Mediated siRNA Delivery. Chin. Chem. Lett. 2017, 28, 1851–1856. [Google Scholar] [CrossRef]
- Mondal, S.; Raut, J.; Sahoo, P. Gene Silencing and Gene Delivery in Therapeutics: Insights Using Quantum Dots. Front. Biosci. 2023, 28, 364. [Google Scholar] [CrossRef]
- Lee, T.; Yagati, A.K.; Pi, F.; Sharma, A.; Choi, J.-W.; Guo, P. Construction of RNA–Quantum Dot Chimera for Nanoscale Resistive Biomemory Application. ACS Nano 2015, 9, 6675–6682. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, D.-Y.; Huang, L. In Vivo Delivery of miRNAs for Cancer Therapy: Challenges and Strategies. Adv. Drug Deliv. Rev. 2015, 81, 128–141. [Google Scholar] [CrossRef]
- Dasgupta, I.; Chatterjee, A. Recent Advances in miRNA Delivery Systems. Methods Protoc. 2021, 4, 10. [Google Scholar] [CrossRef]
- McClorey, G.; Banerjee, S. Cell-Penetrating Peptides to Enhance Delivery of Oligonucleotide-Based Therapeutics. Biomedicines 2018, 6, 51. [Google Scholar] [CrossRef]
- Frank, A.M.; Weidner, T.; Brynza, J.; Uckert, W.; Buchholz, C.J.; Hartmann, J. CD8-Specific Designed Ankyrin Repeat Proteins Improve Selective Gene Delivery into Human and Primate T Lymphocytes. Human. Gene Ther. 2020, 31, 679–691. [Google Scholar] [CrossRef]
- Jayandharan, G.R. Gene and Cell Therapy; Springer Singapore Pte. Limited: Singapore, 2018; ISBN 978-981-13-0480-4. [Google Scholar]
- Gerrard, J.A.; Domigan, L.J. (Eds.) Protein Nanotechnology: Protocols, Instrumentation, and Applications, 3rd ed.; Methods in Molecular Biology; Humana: New York, NY, USA, 2020; ISBN 978-1-4939-9868-5. [Google Scholar]
- Zhang, J.-H.; Du, A.-L.; Wang, L.; Wang, X.-Y.; Gao, J.-H.; Wang, T.-Y. Episomal Lentiviral Vector-Mediated miR-145 Overexpression Inhibits Proliferation and Induces Apoptosis of Human Esophageal Carcinomas Cells. Recent Pat. Anti Cancer Drug Discov. 2016, 11, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Cavazzana-Calvo, M.; Payen, E.; Negre, O.; Wang, G.; Hehir, K.; Fusil, F.; Down, J.; Denaro, M.; Brady, T.; Westerman, K.; et al. Transfusion Independence and HMGA2 Activation after Gene Therapy of Human β-Thalassaemia. Nature 2010, 467, 318–322. [Google Scholar] [CrossRef]
- Aravalli, R. Development of MicroRNA Therapeutics for Hepatocellular Carcinoma. Diagnostics 2013, 3, 170–191. [Google Scholar] [CrossRef] [PubMed]
- Bofill-De Ros, X.; Gironella, M.; Fillat, C. MiR-148a- and miR-216a-Regulated Oncolytic Adenoviruses Targeting Pancreatic Tumors Attenuate Tissue Damage Without Perturbation of miRNA Activity. Mol. Ther. 2014, 22, 1665–1677. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, P.-H.; Kim, S.W.; Yun, C.-O. Enhancing the Therapeutic Efficacy of Adenovirus in Combination with Biomaterials. Biomaterials 2012, 33, 1838–1850. [Google Scholar] [CrossRef]
- Latronico, M.V.G.; Condorelli, G. Therapeutic Use of MicroRNAs in Myocardial Diseases. Curr. Heart Fail. Rep. 2011, 8, 193–197. [Google Scholar] [CrossRef]
- Aponte-Ubillus, J.J.; Barajas, D.; Peltier, J.; Bardliving, C.; Shamlou, P.; Gold, D. Molecular Design for Recombinant Adeno-Associated Virus (rAAV) Vector Production. Appl. Microbiol. Biotechnol. 2018, 102, 1045–1054. [Google Scholar] [CrossRef]
- Kuranda, K.; Mingozzi, F. AAV Vector-Based Gene Therapy, Progress and Current Challenges. In Safety and Efficacy of Gene-Based Therapeutics for Inherited Disorders; Brunetti-Pierri, N., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 77–112. ISBN 978-3-319-53455-8. [Google Scholar]
- Bhere, D.; Arghiani, N.; Lechtich, E.R.; Yao, Y.; Alsaab, S.; Bei, F.; Matin, M.M.; Shah, K. Simultaneous Downregulation of miR-21 and Upregulation of miR-7 Has Anti-Tumor Efficacy. Sci. Rep. 2020, 10, 1779. [Google Scholar] [CrossRef]
- Bhere, D.; Tamura, K.; Wakimoto, H.; Choi, S.H.; Purow, B.; Debatisse, J.; Shah, K. microRNA-7 Upregulates Death Receptor 5 and Primes Resistant Brain Tumors to Caspase-Mediated Apoptosis. Neuro Oncol. 2018, 20, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-S.; Lin, C.; Ma, H.; Xie, J.; Kaplan, F.S.; Gao, G.; Shim, J.-H. AAV-Mediated Targeting of the Activin A-ACVR1R206H Signaling in Fibrodysplasia Ossificans Progressiva. Biomolecules 2023, 13, 1364. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.A.; Zhang, C.; Huys, A.; Richardson, C.D. Human Ago2 Is Required for Efficient MicroRNA 122 Regulation of Hepatitis C Virus RNA Accumulation and Translation. J. Virol. 2011, 85, 2342–2350. [Google Scholar] [CrossRef] [PubMed]
- Pushko, P.; Pumpens, P.; Grens, E. Development of Virus-Like Particle Technology from Small Highly Symmetric to Large Complex Virus-Like Particle Structures. Intervirology 2013, 56, 141–165. [Google Scholar] [CrossRef]
- Yao, Y.; Jia, T.; Pan, Y.; Gou, H.; Li, Y.; Sun, Y.; Zhang, R.; Zhang, K.; Lin, G.; Xie, J.; et al. Using a Novel MicroRNA Delivery System to Inhibit Osteoclastogenesis. Int. J. Mol. Sci. 2015, 16, 8337–8350. [Google Scholar] [CrossRef]
- Callanan, J.; Stockdale, S.R.; Shkoporov, A.; Draper, L.A.; Ross, R.P.; Hill, C. RNA Phage Biology in a Metagenomic Era. Viruses 2018, 10, 386. [Google Scholar] [CrossRef]
- Pinto, A.M.; Silva, M.D.; Pastrana, L.M.; Bañobre-López, M.; Sillankorva, S. The Clinical Path to Deliver Encapsulated Phages and Lysins. FEMS Microbiol. Rev. 2021, 45, fuab019. [Google Scholar] [CrossRef]
- Naskalska, A.; Heddle, J.G. Virus-like Particles Derived from Bacteriophage MS2 as Antigen Scaffolds and RNA Protective Shells. Nanomedicine 2024, 19, 1103–1115. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, D.B.; Böker, K.O.; Schneider, S.; Eckermann-Felkl, E.; Schuder, A.; Komrakova, M.; Sehmisch, S.; Gruber, J. In Vivo siRNA Delivery Using JC Virus-like Particles Decreases the Expression of RANKL in Rats. Mol. Ther. Nucleic Acids 2016, 5, e298. [Google Scholar] [CrossRef]
- Chao, C.-N.; Yang, Y.-H.; Wu, M.-S.; Chou, M.-C.; Fang, C.-Y.; Lin, M.-C.; Tai, C.-K.; Shen, C.-H.; Chen, P.-L.; Chang, D.; et al. Gene Therapy for Human Glioblastoma Using Neurotropic JC Virus-like Particles as a Gene Delivery Vector. Sci. Rep. 2018, 8, 2213. [Google Scholar] [CrossRef]
- Armstrong, J.K.; Hempel, G.; Koling, S.; Chan, L.S.; Fisher, T.; Meiselman, H.J.; Garratty, G. Antibody against Poly(Ethylene Glycol) Adversely Affects PEG-asparaginase Therapy in Acute Lymphoblastic Leukemia Patients. Cancer 2007, 110, 103–111. [Google Scholar] [CrossRef]
- Galaway, F.A.; Stockley, P.G. MS2 Viruslike Particles: A Robust, Semisynthetic Targeted Drug Delivery Platform. Mol. Pharm. 2013, 10, 59–68. [Google Scholar] [CrossRef]
- Chen, W.; Kang, T.; Yuan, R.; Shao, C.; Jing, S. Immunogenicity and Protective Potency of Norovirus GII.17 Virus-like Particle-Based Vaccine. Biotechnol. Lett. 2020, 42, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Komiya, R. Biogenesis of Diverse Plant phasiRNAs Involves an miRNA-Trigger and Dicer-Processing. J. Plant Res. 2017, 130, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Verlaan, M.G.; Hutton, S.F.; Ibrahem, R.M.; Kormelink, R.; Visser, R.G.F.; Scott, J.W.; Edwards, J.D.; Bai, Y. The Tomato Yellow Leaf Curl Virus Resistance Genes Ty-1 and Ty-3 Are Allelic and Code for DFDGD-Class RNA–Dependent RNA Polymerases. PLoS Genet. 2013, 9, e1003399. [Google Scholar] [CrossRef]
- Modlich, U.; Navarro, S.; Zychlinski, D.; Maetzig, T.; Knoess, S.; Brugman, M.H.; Schambach, A.; Charrier, S.; Galy, A.; Thrasher, A.J.; et al. Insertional Transformation of Hematopoietic Cells by Self-Inactivating Lentiviral and Gammaretroviral Vectors. Mol. Ther. 2009, 17, 1919–1928. [Google Scholar] [CrossRef]
- Logan, A.C.; Haas, D.L.; Kafri, T.; Kohn, D.B. Integrated Self-Inactivating Lentiviral Vectors Produce Full-Length Genomic Transcripts Competent for Encapsidation and Integration. J. Virol. 2004, 78, 8421–8436. [Google Scholar] [CrossRef]

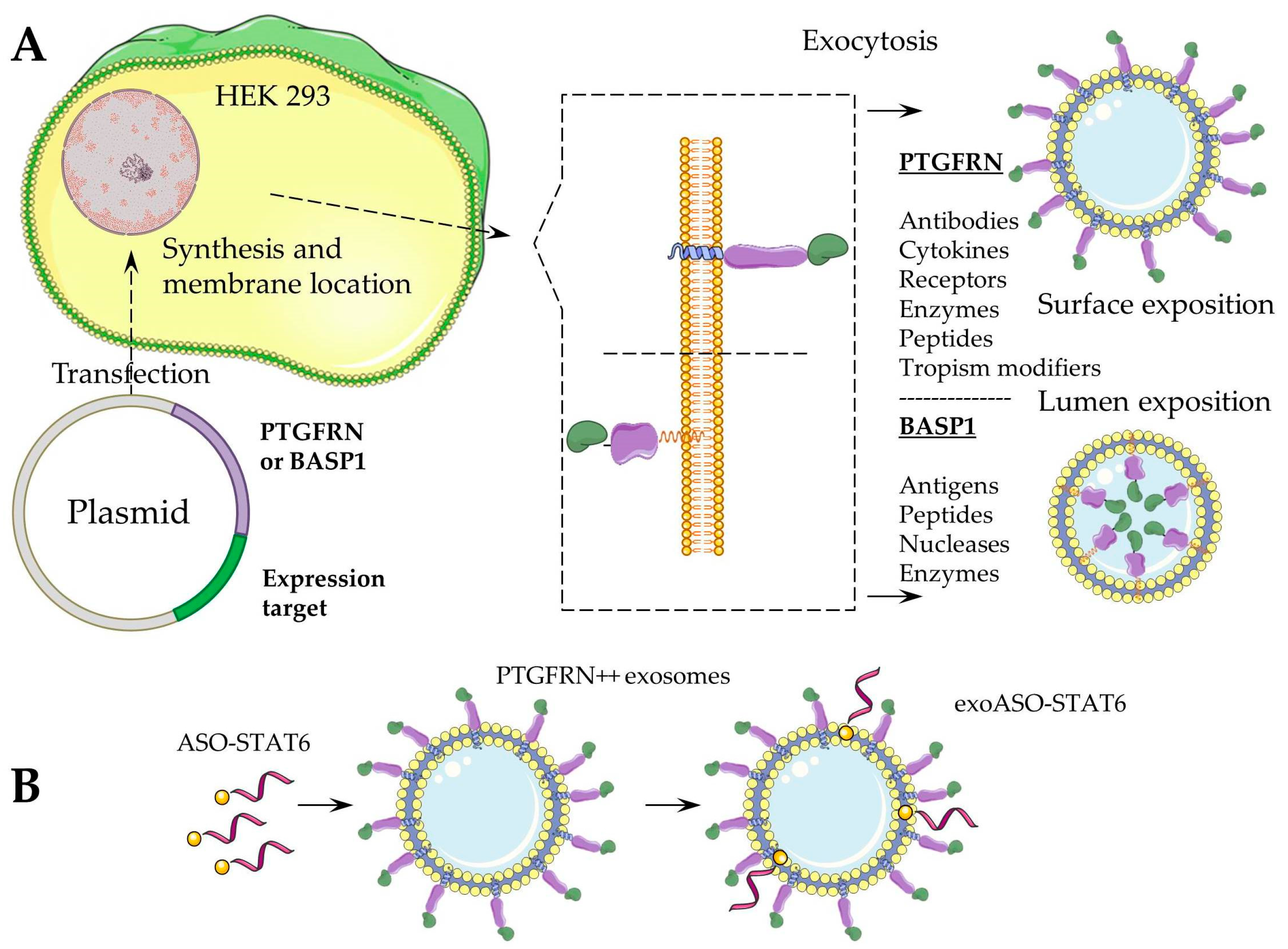
| RNA Type/Mechanism of Action | Drug Name | Molecular Target | Therapeutic Application | References |
|---|---|---|---|---|
| ss ASO (single-strand antisense oligonucleotide) induces gene silencing | Danvatirsen | STAT3 | Therapy of bladder, colorectal, pancreatic cancer, non-small cell lung cancer (NSCLC), head and neck tumours, malignant ascites cancer, acute myeloid leukaemia at the stage of non-Hodgkin’s lymphoma, myelodysplastic syndrome | [36,37] |
| exoASO-STAT6 | STAT6 | Therapy of advanced hepatocellular carcinoma (HCC), liver metastases from either primary gastric cancer or colorectal cancer (CRC) and oral squamous cell carcinoma | [38] | |
| saRNA (small activating RNA) target gene promoter regions and enhance the transcription of a desired gene | MTL-CEBPA | C/EBP-α | Combined therapy of hepatocellular carcinoma (HCC), cirrhosis of the liver, non-alcoholic steatohepatitis and liver metastases | [39] |
| miR inhibitor binds to and inhibits miRNAs involved in the pathogenesis process | Cobomarsen/MRG-106 | miR155 | Therapy of T-cell lymphoma (CTCL) [mycosis fungoides (MFs) subtype], chronic lymphocytic leukaemia (CLL), diffuse large B-cell lymphoma (DLBCL) | [40] |
| Miravirsen/SPC3649 | miR-122 (HCV 5′-UTR) | Chronic hepatitis C therapy | [41] | |
| LNA-i-miR-221 | miR-221 (CDKN1B/p27 and PTEN) | Refractory Multiple Myeloma, HCC | [42] | |
| MRG110 | miR-92 | Wound healing and preventing heart failure | [43] | |
| Lademirsen/RG012 | miR-21 | Treatment of Alport syndrome | [44] | |
| mRNA Antigen synthesis and presentation for immunisation | Spikevax | Spike protein | Prophylaxis of SARS-CoV-2 infection | [45] |
| BNT162b2/Comirnaty | Spike protein | Prophylaxis of SARS-CoV-2 infection | [46] | |
| ds miR-mimetic mimics lost oncosuppressive function miRs | TargomiR | miR-16 | Treatment of refractory malignant pleural mesothelioma (MPM) and lung cancer (NSCLC) | [47] |
| ds miR-mimetic | MRX34 | miR34 (MET, MYC, PDGFR-α, CDK4/6, BCL2, PD-L1, DGKζ) | Melanoma, lymphoma, renal cell carcinoma, multiple myeloma, NSCLC, primary liver cancer, SCLC | [48] |
| Remlarsen/MRG201 | miR-29 (collagens) | Prevention of fibrous and keloid scar formation | [49] | |
| siRNA suppresses the expression of key genes in disease pathogenesis through mRNA degradation | Patisiran/Onpattro/ALN-TTR02 | Transthyretin (TTR) | Treatment of hereditary transthyretin amyloidosis (familial amyloid polyneuropathy) (hATTR) | [50] |
| Vutrisiran/Amvuttra | Transthyretin (TTR) | Treatment of hATTR | [51] | |
| Givosiran/Givlaari | δ-aminolevulinate synthase 1 (ALAS1) | Treatment of the acute hepatic porphyria (AHP) | [52] | |
| Lumasiran/Oxlumo | Glyoxylate oxidase (GO) | Therapy of primary hyperoxaluria type 1 (PH1) | [53] | |
| Nedosiran/Rivfloza | Lactate dehydrogenase | Therapy of all types of primary hyperoxaluria | [54] | |
| Inclisiran/Leqvio | Proprotein convertase subtilisin/kexin type 9 (PCSK9) | Therapy for high low-density lipoprotein (LDL) cholesterol and an increased risk of premature atherosclerotic cardiovascular disease | [55] | |
| Fitusiran | Antithrombin | Therapy of haemophilia A or B | [56] | |
| Teprasiran | p53 antioncogene | Therapy of delayed graft function (DGF) | [57] | |
| Cosdosiran | Caspase 2 | Therapeutic for the nonarteritic anterior ischaemic optic neuropathy (NAION) and the primary angle closure glaucoma | [58] | |
| Tivanisiran | TRPV1 receptor | Treatment of dry eye disease | [59] | |
| Cemdisiran | Complement Component C5 | Therapy of immunoglobulin A nephropathy (IgAN), paroxysmal nocturnal haemoglobinuria, myasthenia gravis, atypical haemolytic uremic syndrome | [60,61] |
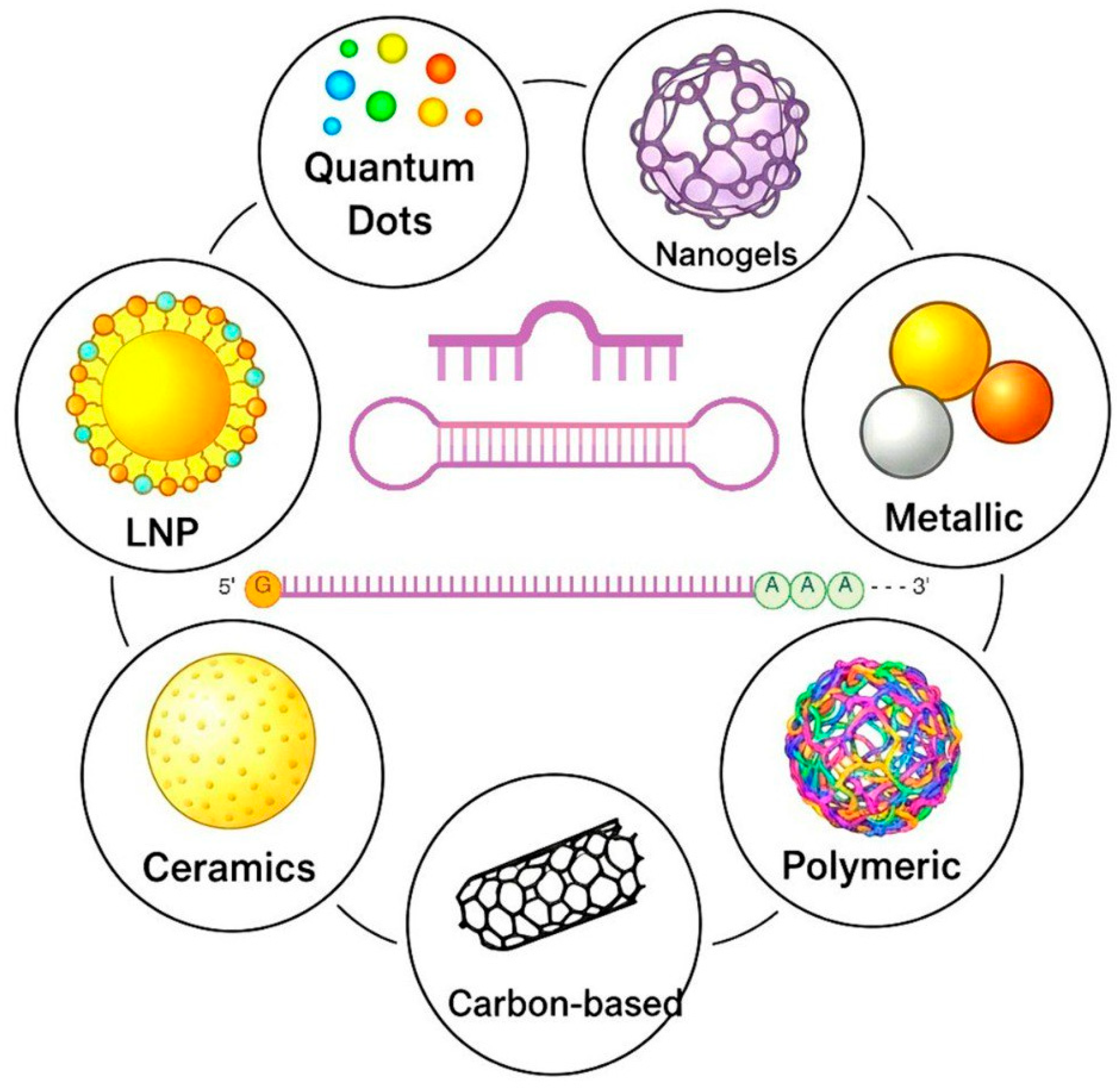
| Nanoparticles | Design Principal | Mechanism of Action | Advantages | Limitations | Reference |
|---|---|---|---|---|---|
| LNP | Chemical (the combination of the ionisable lipid, PEG-lipid, cholesterol, and a helper lipid) or biogenic particles (exosomes) from 50 to 200 nm | RNA electrostatic complexation and encapsulation. Clathrin-mediated endocytosis, receptor-mediated uptake, fusion with endosomal membrane | Efficacy and safety, RNA protection, effective endosomal escape, nontoxic, biocompatible, tunable formulation, longer circulation time, targeting capability, definite structure | Poor interaction with RNA for neutral lipids, toxicity and immunogenicity for cationic lipids, aggregation, high costs | [160,161,162,163,164] |
| Nanogels | Three-dimensional networks of cross-linked hydrophilic, natural, or synthetic polymers from 50 to 200 nm | Electrostatic interactions, physically trap RNA, covalent conjugation, and hybrids with other RNA carriers. Clathrin- or caveolae-mediated endocytosis, receptor-mediated uptake | High loading capacity, stability, stimuli-responsiveness, biocompatibility and biodegradability, protection of RNA, tunable properties, prolonged release, no need for multiple administrations | Complexity of synthesis, immunogenicity, non-specific uptake, aggregation | [165,166,167,168,169] |
| Metallic | Metall (Au, Al, Pt, Zn, Fe, etc.) particles from 1 to 100 nm with a high surface-area-to-volume ratio and quantum mechanical effects | Electrostatic interactions, physically trap RNA. Clathrin- or caveolae-mediated endocytosis, macropinocytosis, and targeted uptake, magnetic targeting | Versatility, high electrical and thermal conductivity, unique stimuli-responsive properties, biocompatibility, strong electrostatic interactions with RNA | Low thermal and corrosion resistance, disrupting multiple cellular functions, proinflammatory, activating/inhibiting different pathways, low biodegradability | [170,171,172,173,174] |
| Polymeric | Natural, synthetic, or semi-synthetic polymers from 1 to 1000 nm | Electrostatic interactions, physically trap RNA. Clathrin- or caveolae-mediated endocytosis, macropinocytosis, targeted uptake. Endosome escape. RNA can be linked via pH-cleavable linkers | Versatility, tunable properties, biocompatibility and biodegradability, low toxicity, efficient RNA complexation, endosomal escape, easy to functionalise | Polymers can be cytotoxic, immunogenic, off-target delivery, and endosomal escape | [175,176,177,178,179,180] |
| Carbon-based | Carbon particles from 1 to 100 nm (single-walled or multi-walled carbon nanotubes; 2D sheets of graphene or graphene oxide with large surface area; fullerenes; carbon dots; amorphous carbon nanoparticles; graphyne; nanodiamonds; carbyne) | Physically encapsulating through hydrophobic interactions and π–π stacking, cell membrane penetration and endocytosis release | Electrical and thermal conductivity, mechanical strength, high stability, high surface area, adjustable fluorescence | Aggregation, insolubility, non-biodegradability, agglomeration, immunogenicity, high cost, carcinogenicity, oxidative stress | [181,182,183,184] |
| Ceramics | Inorganic, nonmetallic particles (carbonates, phosphates, oxides, carbides, TiO2, Si3N4, etc.) up to 200 nm. Porous core, functionalised surface with charge-modifying groups or biocompatible polymers | Electrostatic interactions, physically trap RNA. Clathrin- or caveolae-mediated endocytosis, macropinocytosis, targeted uptake | Deeper penetration into capillaries, fenestrations, high heat and corrosion resistance, chemical stability, diverse electrical properties | Brittleness, disrupting multiple cellular functions, proinflammatory, activating/inhibiting different pathways, endosomal escape | [185,186,187] |
| Quantum dots | Semiconductor nanocrystal complexes from 10 to 100 nm synthesised with a core from 2 to 10 nm (CdSe, CdTe), a shell (ZnS) and a functionalised surface | Electrostatic interactions, RNA covalent conjugation or secondary carrier encapsulation within a. Clathrin- or caveolae-mediated endocytosis, macropinocytosis, targeted uptake, co-delivery | Bright, stable, and tunable fluorescence, cellular entry, dual-modality (carrying and tracing) | Cytotoxic, need of endosomolytic components for endosomal escape, immunogenicity | [188,189,190,191,192,193,194] |
| Delivery System | Phosphorothioate Backbone | Phosphodiester Backbone | ||||
|---|---|---|---|---|---|---|
| 2′-F and 2′-OMe Ribose | 2′-F, 2′-OMe Ribose, and dT | 2′-MOE, 2′-F, and 2′-OMe | LNA | 2′-F and 2′-OMe Ribose | No Modifications | |
| LNP | Patisiran MTL-CEBPA | |||||
| HEK-293-derived exosomes | exoASO-STAT6 | |||||
| E. coli-derived exosomes | TargomiR | |||||
| Triantennary GalNAc covalent modification | Vutrisiran Givosiran Lumasiran Nedosiran Fitusiran Cemdisiran | Inclisiran | ||||
| No | Miravirsen Remlarsen | Danvatirsen LNA-i-miR-221 | Teprasiran Cosdosiran | Tivanisiran | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pozdniakova, N.; Generalov, E.; Shevelev, A.; Tarasova, O. RNA Therapeutics: Delivery Problems and Solutions—A Review. Pharmaceutics 2025, 17, 1305. https://doi.org/10.3390/pharmaceutics17101305
Pozdniakova N, Generalov E, Shevelev A, Tarasova O. RNA Therapeutics: Delivery Problems and Solutions—A Review. Pharmaceutics. 2025; 17(10):1305. https://doi.org/10.3390/pharmaceutics17101305
Chicago/Turabian StylePozdniakova, Natalia, Evgenii Generalov, Alexei Shevelev, and Olga Tarasova. 2025. "RNA Therapeutics: Delivery Problems and Solutions—A Review" Pharmaceutics 17, no. 10: 1305. https://doi.org/10.3390/pharmaceutics17101305
APA StylePozdniakova, N., Generalov, E., Shevelev, A., & Tarasova, O. (2025). RNA Therapeutics: Delivery Problems and Solutions—A Review. Pharmaceutics, 17(10), 1305. https://doi.org/10.3390/pharmaceutics17101305









