Aptamer-Modified Magnetic Nanoparticles as Targeted Drug Delivery Systems for Hepatocellular Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Chemicals and Reagents
2.1.2. Cell Cultures
2.2. Methods
2.2.1. Aptamer Solution Preparation
2.2.2. Magnetic Nanoparticle Synthesis
2.2.3. Aptamer Functionalization
2.2.4. SOR Loading and Release
2.2.5. Zeta Potential and Hydrodynamic Size
2.2.6. UV-Vis Spectrophotometry
2.2.7. Transmission Electron Microscopy
2.2.8. X-Ray Diffraction
2.2.9. Fourier-Transform Infrared Spectroscopy
2.2.10. X-Ray Photoelectron Spectroscopy
2.2.11. Electrochemical Impedance Spectroscopy
2.2.12. Cytotoxicity and Cell Internalization Studies
2.2.13. Evaluation of Cellular Uptake
2.2.14. Statistical Analysis
3. Results
3.1. MNP Synthesis
3.2. Optimization of Aptamer Functionalization and SOR Loading
3.3. Zeta Potential and Hydrodynamic Size
3.4. SOR Loading and Release
3.5. TEM
3.6. XRD
3.7. FTIR Spectroscopy
3.8. XPS
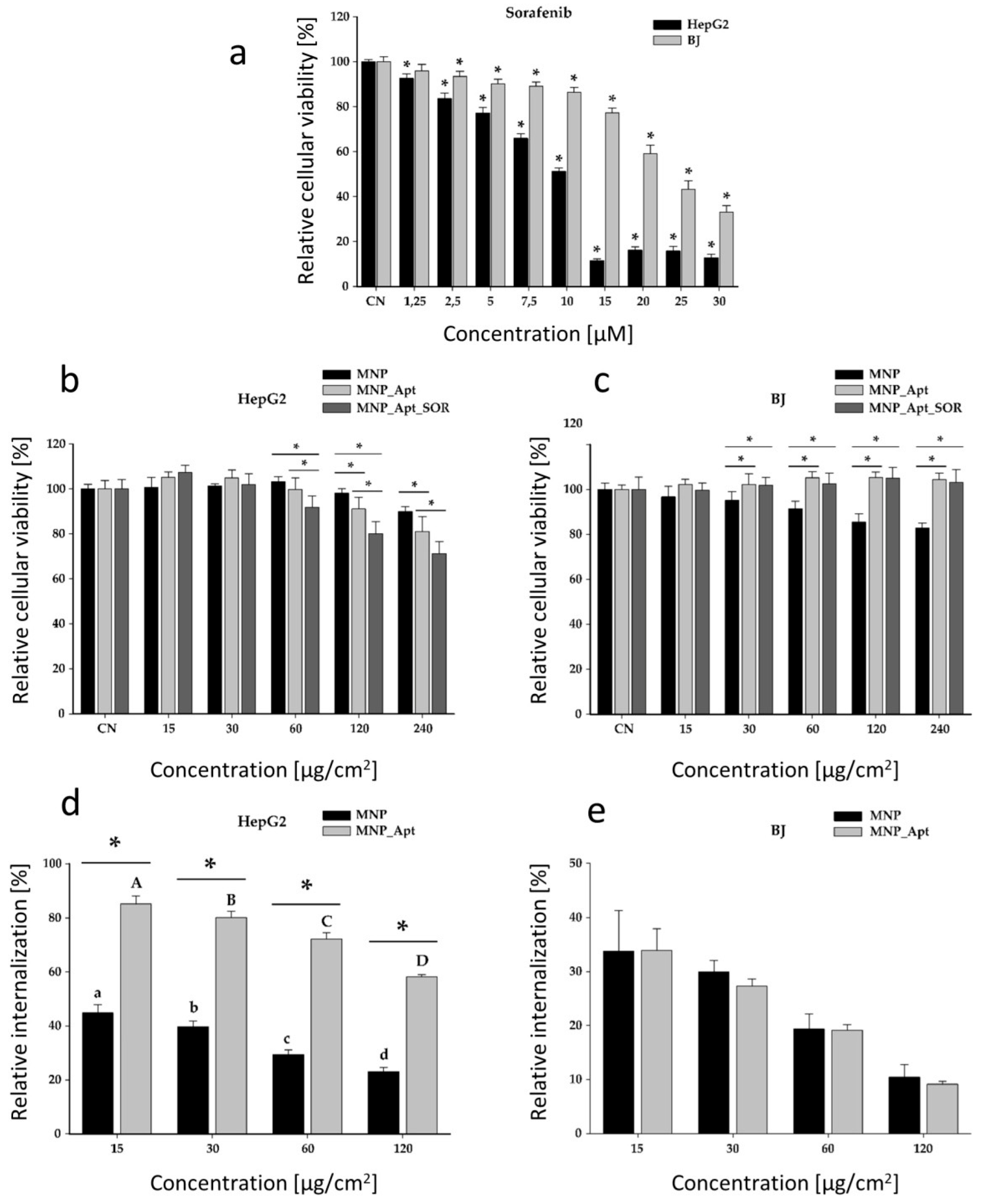
3.9. In Vitro Cytotoxicity and Cellular Uptake Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BJ | Human normal foreskin fibroblasts |
| DMEM | Dulbecco’s Modified Eagle Medium |
| EDC | N-ethyl-N-(3-(dimethylamino)propyl)carbodiimide |
| EE | Encapsulation Efficiency |
| EIS | Electrochemical Impedance Spectroscopy |
| EMEM | Eagle’s Minimum Essential Medium |
| FBS | Fetal Bovine Serum |
| FTIR | Fourier-Transform Infrared Spectroscopy |
| HCC | Hepatocellular Carcinoma |
| LC | Loading Capacity |
| MNP | Magnetic Nanoparticles |
| MNP@AZA | Magnetic Nanoparticles modified with Azelaic Acid |
| MNP_Apt | Magnetic Nanoparticles modified with Azelaic Acid and Aptamer TLS11a |
| MNP_Apt_SOR | Magnetic Nanoparticles modified with Azelaic Acid, Aptamer TLS11a and Sorafenib |
| NHS | N-hydroxysuccinimide |
| PBS | Phosphate-Buffered Solution |
| PDI | Polydispersity Index |
| Rct | Resistance to Charge Transfer |
| SAED | Selected Area Electron Diffraction |
| SD | Standard Deviation |
| SOR | Sorafenib |
| SPIONs | Superparamegnetic iron oxide nanoparticles |
| TEM | Transmission Electron Microscopy |
| XPS | X-ray Photoelectron Spectroscopy |
| XRD | X-ray Diffraction |
References
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 2021, 7, 6. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Llovet, J.M.; De Baere, T.; Kulik, L.; Haber, P.K.; Greten, T.F.; Meyer, T.; Lencioni, R. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 293–313. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.; Park, S.Y.; Lee, S.-W.; Kang, Z.; Jin, Y.S.; Kim, I.W. Dissolution enhancement of sorafenib tosylate by co-milling with tetradecanol post-extracted using supercritical carbon dioxide. Pharmazie 2020, 75, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Chen, Z.; Zhang, W.; Cheng, Y.; Zhang, B.; Wu, F.; Wang, Q.; Wang, S.; Rong, D.; Reiter, F.P.; et al. The mechanisms of sorafenib resistance in hepatocellular carcinoma: Theoretical basis and therapeutic aspects. Signal Transduct. Target. Ther. 2020, 5, 87. [Google Scholar] [CrossRef]
- Abdellatif, A.A.H.; Ali, A.T.; Bouazzaoui, A.; Alsharidah, M.; Al Rugaie, O.; Tolba, N.S. Formulation of polymeric nanoparticles loaded sorafenib; Evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines. Nanotechnol. Rev. 2022, 11, 987–1004. [Google Scholar] [CrossRef]
- Li, Z.; Ye, L.; Liu, J.; Lian, D.; Li, X. Sorafenib-loaded nanoparticles based on biodegradable dendritic polymers for enhanced therapy of hepatocellular carcinoma. Int. J. Nanomedicine 2020, 15, 1469–1480. [Google Scholar] [CrossRef]
- Bartos, A.; Iancu, I.; Ciobanu, L.; Onaciu, A.; Moldovan, C.; Moldovan, A.; Moldovan, R.C.; Tigu, A.B.; Stiufiuc, G.F.; Toma, V.; et al. Hybrid Lipid Nanoformulations for Hepatoma Therapy: Sorafenib Loaded Nanoliposomes-A Preliminary Study. Nanomaterials 2022, 12, 2833. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.H.; Ye, Q.F.; Miao, X.Y.; Liu, X.; Huang, S.Q.; Xiong, L.; Wen, Y.; Zhang, Z.J. Current status of sorafenib nanoparticle delivery systems in the treatment of hepatocellular carcinoma. Theranostics 2021, 11, 5464–5490. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, R.; Chai, W.; Du, X. Low-density lipoprotein decorated silica nanoparticles co-delivering sorafenib and doxorubicin for effective treatment of hepatocellular carcinoma. Drug Deliv. 2018, 25, 2007–2014. [Google Scholar] [CrossRef]
- Zheng, G.; Zhao, R.; Xu, A.; Shen, Z.; Chen, X.; Shao, J. Co-delivery of sorafenib and siVEGF based on mesoporous silica nanoparticles for ASGPR mediated targeted HCC therapy. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2018, 111, 492–502. [Google Scholar] [CrossRef]
- Elsayed, M.M.; Mostafa, M.E.; Alaaeldin, E.; Sarhan, H.A.; Shaykoon, M.S.; Allam, S.; Ahmed, A.R.; Elsadek, B.E. Design And Characterisation Of Novel Sorafenib-Loaded Carbon Nanotubes With Distinct Tumour-Suppressive Activity In Hepatocellular Carcinoma. Int. J. Nanomed. 2019, 14, 8445–8467. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Tang, X.; Wu, X.; Feng, X. Biosynthesis of sorafenib coated graphene nanosheets for the treatment of gastric cancer in patients in nursing care. J. Photochem. Photobiol. B. 2019, 191, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Iacobazzi, R.M.; Vischio, F.; Arduino, I.; Canepa, F.; Laquintana, V.; Notarnicola, M.; Scavo, M.P.; Bianco, G.; Fanizza, E.; Lopedota, A.A.; et al. Magnetic implants in vivo guiding sorafenib liver delivery by superparamagnetic solid lipid nanoparticles. J. Colloid Interface Sci. 2022, 608, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Alpdemir, Ş.; Vural, T.; Kara, G.; Bayram, C.; Haberal, E.; Denkbaş, E.B. Magnetically responsive, sorafenib loaded alginate microspheres for hepatocellular carcinoma treatment. IET Nanobiotechnol. 2020, 14, 623–627. [Google Scholar] [CrossRef]
- Dahiya, M.; Awasthi, R.; Yadav, J.P.; Sharma, S.; Dua, K.; Dureja, H. Chitosan based sorafenib tosylate loaded magnetic nanoparticles: Formulation and in-vitro characterization. Int. J. Biol. Macromol. 2023, 242, 124919. [Google Scholar] [CrossRef]
- Depalo, N.; Iacobazzi, R.M.; Valente, G.; Arduino, I.; Villa, S.; Canepa, F.; Laquintana, V.; Fanizza, E.; Striccoli, M.; Cutrignelli, A.; et al. Sorafenib delivery nanoplatform based on superparamagnetic iron oxide nanoparticles magnetically targets hepatocellular carcinoma. Nano Res. 2017, 10, 2431–2448. [Google Scholar] [CrossRef]
- Ebadi, M.; Buskaran, K.; Bullo, S.; Hussein, M.Z.; Fakurazi, S.; Pastorin, G. Synthesis and cytotoxicity study of magnetite nanoparticles coated with polyethylene glycol and sorafenib–zinc/aluminium layered double hydroxide. Polymers 2020, 12, 2716. [Google Scholar] [CrossRef]
- Ebadi, M.; Bullo, S.; Buskara, K.; Hussein, M.Z.; Fakurazi, S.; Pastorin, G. Release of a liver anticancer drug, sorafenib from its PVA/LDH- and PEG/LDH-coated iron oxide nanoparticles for drug delivery applications. Sci. Rep. 2020, 10, 21521. [Google Scholar] [CrossRef]
- Ebadi, M.; Rifqi Md Zain, A.; Tengku Abdul Aziz, T.H.; Mohammadi, H.; Tee, C.A.T.; Rahimi Yusop, M. Formulation and Characterization of Fe3O4@PEG Nanoparticles Loaded Sorafenib; Molecular Studies and Evaluation of Cytotoxicity in Liver Cancer Cell Lines. Polymers 2023, 15, 971. [Google Scholar] [CrossRef]
- Pusta, A.; Tertis, M.; Crăciunescu, I.; Turcu, R.; Mirel, S.; Cristea, C. Recent Advances in the Development of Drug Delivery Applications of Magnetic Nanomaterials. Pharmaceutics 2023, 15, 1872. [Google Scholar] [CrossRef]
- Attia, N.F.; El-Monaem, E.M.A.; El-Aqapa, H.G.; Elashery, S.E.A.; Eltaweil, A.S.; El Kady, M.; Khalifa, S.A.M.; Hawash, H.B.; El-Seedi, H.R. Iron oxide nanoparticles and their pharmaceutical applications. Appl. Surf. Sci. Adv. 2022, 11, 100284. [Google Scholar] [CrossRef]
- Ștefan, G.; Hosu, O.; De Wael, K.; Lobo-Castañón, M.J.; Cristea, C. Aptamers in biomedicine: Selection strategies and recent advances. Electrochim. Acta 2021, 376, 137994. [Google Scholar] [CrossRef]
- DeRosa, M.C.; Lin, A.; Mallikaratchy, P.; McConnell, E.M.; McKeague, M.; Patel, R.; Shigdar, S. In vitro selection of aptamers and their applications. Nat. Rev. Methods Prim. 2023, 3, 54. [Google Scholar] [CrossRef]
- He, J.; Duan, Q.; Ran, C.; Fu, T.; Liu, Y.; Tan, W. Recent progress of aptamer–drug conjugates in cancer therapy. Acta Pharm. Sin. B 2023, 13, 1358–1370. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.Y.; Tariq, I.; Farhan Sohail, M.; Amin, M.U.; Ali, S.; Pinnapireddy, S.R.; Ali, A.; Schäfer, J.; Bakowsky, U. Selective anti-ErbB3 aptamer modified sorafenib microparticles: In vitro and in vivo toxicity assessment. Eur. J. Pharm. Biopharm. 2019, 145, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, C.; Liu, J.; Hu, S.; Yu, J.; Yin, Q.; Tian, H.; Ding, Z.; Qi, G.; Wang, L.; et al. Aptamer-mediated hollow MnO(2) for targeting the delivery of sorafenib. Drug Deliv. 2023, 30, 28–39. [Google Scholar] [CrossRef]
- Zhang, B.-C.; Luo, B.-Y.; Zou, J.-J.; Wu, P.-Y.; Jiang, J.-L.; Le, J.-Q.; Zhao, R.-R.; Chen, L.; Shao, J.-W. Co-delivery of Sorafenib and CRISPR/Cas9 Based on Targeted Core–Shell Hollow Mesoporous Organosilica Nanoparticles for Synergistic HCC Therapy. ACS Appl. Mater. Interfaces 2020, 12, 57362–57372. [Google Scholar] [CrossRef]
- Shangguan, D.; Meng, L.; Cao, Z.C.; Xiao, Z.; Fang, X.; Li, Y.; Cardona, D.; Witek, R.P.; Liu, C.; Tan, W. Identification of liver cancer-specific aptamers using whole live cells. Anal. Chem. 2008, 80, 721–728. [Google Scholar] [CrossRef]
- Crǎciunescu, I.; Palade, P.; Iacob, N.; Ispas, G.M.; Stanciu, A.E.; Kuncser, V.; Turcu, R.P. High-Performance Functionalized Magnetic Nanoparticles with Tailored Sizes and Shapes for Localized Hyperthermia Applications. J. Phys. Chem. C 2021, 125, 11132–11146. [Google Scholar] [CrossRef]
- Fizesan, I.; Iacovita, C.; Pop, A.; Kiss, B.; Dudric, R.; Stiufiuc, R.; Lucaciu, C.M.; Loghin, F. The Effect of Zn-Substitution on the Morphological, Magnetic, Cytotoxic, and In Vitro Hyperthermia Properties of Polyhedral Ferrite Magnetic Nanoparticles. Pharmaceutics 2021, 13, 2148. [Google Scholar] [CrossRef] [PubMed]
- Tertis, M.; Leva, P.I.; Bogdan, D.; Suciu, M.; Graur, F.; Cristea, C. Impedimetric aptasensor for the label-free and selective detection of Interleukin-6 for colorectal cancer screening. Biosens. Bioelectron. 2019, 137, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Németh, Z.; Csóka, I.; Semnani Jazani, R.; Sipos, B.; Haspel, H.; Kozma, G.; Kónya, Z.; Dobó, D.G. Quality by Design-Driven Zeta Potential Optimisation Study of Liposomes with Charge Imparting Membrane Additives. Pharmaceutics 2022, 14, 1798. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems—A review (Part 1). Trop. J. Pharm. Res. 2013, 12, 255–264. [Google Scholar] [CrossRef]
- Dahiya, M.; Awasthi, R.; Dua, K.; Dureja, H. Sorafenib tosylate loaded superparamagnetic nanoparticles: Development, optimization and cytotoxicity analysis on HepG2 human hepatocellular carcinoma cell line. J. Drug Deliv. Sci. Technol. 2023, 79, 104044. [Google Scholar] [CrossRef]
- Ebadi, M.; Buskaran, K.; Bullo, S.; Hussein, M.Z.; Fakurazi, S.; Pastorin, G. Drug delivery system based on magnetic iron oxide nanoparticles coated with (polyvinyl alcohol-zinc/aluminium-layered double hydroxide-sorafenib). Alexandria Eng. J. 2021, 60, 733–747. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, Z.; Dong, Y.; Yu, D.; Jiang, F.; Han, Y.; Chen, Y. Magnetic Fe3O4@MOF@aptamer-mediated entropy-driven fluorescence biosensor for multiplexed and DNA extraction- and amplification-free detection of pathogenic bacteria. Chem. Eng. J. 2024, 499, 155978. [Google Scholar] [CrossRef]
- Jiang, L.-F.; Chen, B.-C.; Chen, B.; Li, X.-J.; Liao, H.-L.; Zhang, W.-Y.; Wu, L. Aptamer-functionalized Fe3O4 magnetic nanoparticles as a solid-phase extraction adsorbent for the selective extraction of berberine from Cortex phellodendri. J. Sep. Sci. 2017, 40, 2933–2940. [Google Scholar] [CrossRef]
- Ishii, M.; Nakahira, M.; Yamanaka, T. Infrared absorption spectra and cation distributions in (Mn, Fe)3O4. Solid State Commun. 1972, 11, 209–212. [Google Scholar] [CrossRef]
- Namduri, H.; Nasrazadani, S. Quantitative analysis of iron oxides using Fourier transform infrared spectrophotometry. Corros. Sci. 2008, 50, 2493–2497. [Google Scholar] [CrossRef]
- Nalbandian, L.; Patrikiadou, E.; Zaspalis, V.; Patrikidou, A.; Hatzidaki, E.; Papandreou, C.N. Magnetic Nanoparticles in Medical Diagnostic Applications: Synthesis, Characterization and Proteins Conjugation. Curr. Nanosci. 2016, 12, 455–468. [Google Scholar] [CrossRef]
- Stan, G.E.; Marcov, D.A.; Popa, A.C.; Husanu, M.A. Polymer-like and diamond-like carbon coatings prepared by RF-PECVD for biomedical applications. Dig. J. Nanomater. Biostructures 2010, 5, 705–718. [Google Scholar]
- Mohrig, J.R.M.; Hammond, C.N.; Schatz, P.F. Infrared spectroscopy. In Techniques in Organic Chemistry; Freeman: New York, NY, USA, 2006. [Google Scholar]
- Han, Y.; Han, L.; Yao, Y.; Li, Y.; Liu, X. Key factors in FTIR spectroscopic analysis of DNA: The sampling technique, pretreatment temperature and sample concentration. Anal. Methods 2018, 10, 2436–2443. [Google Scholar] [CrossRef]
- Babić, S.D.; Serec, K. Sodium and manganese salt DNA thin films: An infrared spectroscopy study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 241, 118646. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database. J. Chem. Educ. 1993, 70, A25. [Google Scholar] [CrossRef]
- Malvindi, M.A.; De Matteis, V.; Galeone, A.; Brunetti, V.; Anyfantis, G.C.; Athanassiou, A.; Cingolani, R.; Pompa, P.P. Toxicity Assessment of Silica Coated Iron Oxide Nanoparticles and Biocompatibility Improvement by Surface Engineering. PLoS ONE 2014, 9, e85835. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Buton, N.; Megier, C.; Motelica-Heino, M. Biocompatible Layers Obtained from Functionalized Iron Oxide Nanoparticles in Suspension. Coatings 2019, 9, 773. [Google Scholar] [CrossRef]
- Hanot, C.C.; Choi, Y.S.; Anani, T.B.; Soundarrajan, D.; David, A.E. Effects of Iron-Oxide Nanoparticle Surface Chemistry on Uptake Kinetics and Cytotoxicity in CHO-K1 Cells. Int. J. Mol. Sci. 2016, 17, 54. [Google Scholar] [CrossRef]
- Meng, Y.Q.; Shi, Y.N.; Zhu, Y.P.; Liu, Y.Q.; Gu, L.W.; Liu, D.D.; Ma, A.; Xia, F.; Guo, Q.Y.; Xu, C.C.; et al. Recent trends in preparation and biomedical applications of iron oxide nanoparticles. J. Nanobiotechnol. 2024, 22, 24. [Google Scholar] [CrossRef]
- Chen, Y.; Hou, S. Recent progress in the effect of magnetic iron oxide nanoparticles on cells and extracellular vesicles. Cell Death Discov. 2023, 9, 195. [Google Scholar] [CrossRef] [PubMed]
- Safi, M.; Sarrouj, H.; Sandre, O.; Mignet, N.; Berret, J.-F. Interactions between sub-10-nm iron and cerium oxide nanoparticles and 3T3 fibroblasts: The role of the coating and aggregation state. Nanotechnology 2010, 21, 145103. [Google Scholar] [CrossRef] [PubMed]
- Iacoviță, C.; Fizeșan, I.; Nitica, S.; Florea, A.; Barbu-Tudoran, L.; Dudric, R.; Pop, A.; Vedeanu, N.; Crisan, O.; Tetean, R.; et al. Silica Coating of Ferromagnetic Iron Oxide Magnetic Nanoparticles Significantly Enhances Their Hyperthermia Performances for Efficiently Inducing Cancer Cells Death In Vitro. Pharmaceutics 2021, 13, 2026. [Google Scholar] [CrossRef] [PubMed]
- Oh, N.; Park, J.-H. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int. J. Nanomedicine 2014, 9, 51–63. [Google Scholar] [CrossRef] [PubMed]








| Aptamer functionalization | ||
| Optimized Parameter | Conditions | Decrease in A (%) |
| Incubation time (h) | ½ | 5.1 |
| 1 | 11.1 | |
| 2 | 11.8 | |
| 24 | 20.0 | |
| 48 | 24.2 | |
| Incubation temperature (°C) | 4 | 0.83 |
| 25 | 11.1 | |
| 37 | 13.4 | |
| SOR incubation | ||
| Optimized parameter | Conditions | EE (%) |
| SOR concentration (mg/mL) | 1 | 14.8 |
| 5 | 44.3 | |
| Incubation time (h) | 24 | 44.3 |
| 48 | 41.7 | |
| Conditions | Rct (Ω) |
|---|---|
| Bare electrode | 300 |
| MNP@AZA | 472 |
| MNP@AZA-activated | 259 |
| MNP@AZA_Apt ½ h | 399 |
| MNP@AZA_Apt 1 h | 584 |
| MNP@AZA_Apt 2 h | 602 |
| MNP@AZA_Apt 24 h | 721 |
| Media | λmax | Calibration Curve Equation | R2 |
|---|---|---|---|
| Ethanol 96% | 266 | A = 0.0707[SOR] + 0.2313 | 0.9834 |
| PBS pH 5.5 | 262 | A = 0.0212[SOR] + 0.0141 | 0.9894 |
| PBS pH 7.4:ethanol (1:3) | 269 | A = 75.62[SOR] − 0.0019 | 0.9974 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pusta, A.; Tertis, M.; Ciocan, B.; Turcu, R.; Crăciunescu, I.; Diculescu, V.C.; Stan, G.E.; Bulat, S.; Porfire, A.; Petru, A.-E.; et al. Aptamer-Modified Magnetic Nanoparticles as Targeted Drug Delivery Systems for Hepatocellular Carcinoma. Pharmaceutics 2025, 17, 1292. https://doi.org/10.3390/pharmaceutics17101292
Pusta A, Tertis M, Ciocan B, Turcu R, Crăciunescu I, Diculescu VC, Stan GE, Bulat S, Porfire A, Petru A-E, et al. Aptamer-Modified Magnetic Nanoparticles as Targeted Drug Delivery Systems for Hepatocellular Carcinoma. Pharmaceutics. 2025; 17(10):1292. https://doi.org/10.3390/pharmaceutics17101292
Chicago/Turabian StylePusta, Alexandra, Mihaela Tertis, Bianca Ciocan, Rodica Turcu, Izabell Crăciunescu, Victor C. Diculescu, George E. Stan, Stefan Bulat, Alina Porfire, Andreea-Elena Petru, and et al. 2025. "Aptamer-Modified Magnetic Nanoparticles as Targeted Drug Delivery Systems for Hepatocellular Carcinoma" Pharmaceutics 17, no. 10: 1292. https://doi.org/10.3390/pharmaceutics17101292
APA StylePusta, A., Tertis, M., Ciocan, B., Turcu, R., Crăciunescu, I., Diculescu, V. C., Stan, G. E., Bulat, S., Porfire, A., Petru, A.-E., Fizeșan, I., Mirel, S., & Cristea, C. (2025). Aptamer-Modified Magnetic Nanoparticles as Targeted Drug Delivery Systems for Hepatocellular Carcinoma. Pharmaceutics, 17(10), 1292. https://doi.org/10.3390/pharmaceutics17101292














