Albumin Nanoparticles in Cancer Therapeutics: Clinical Status, Challenges, and Future Directions
Abstract
1. Introduction
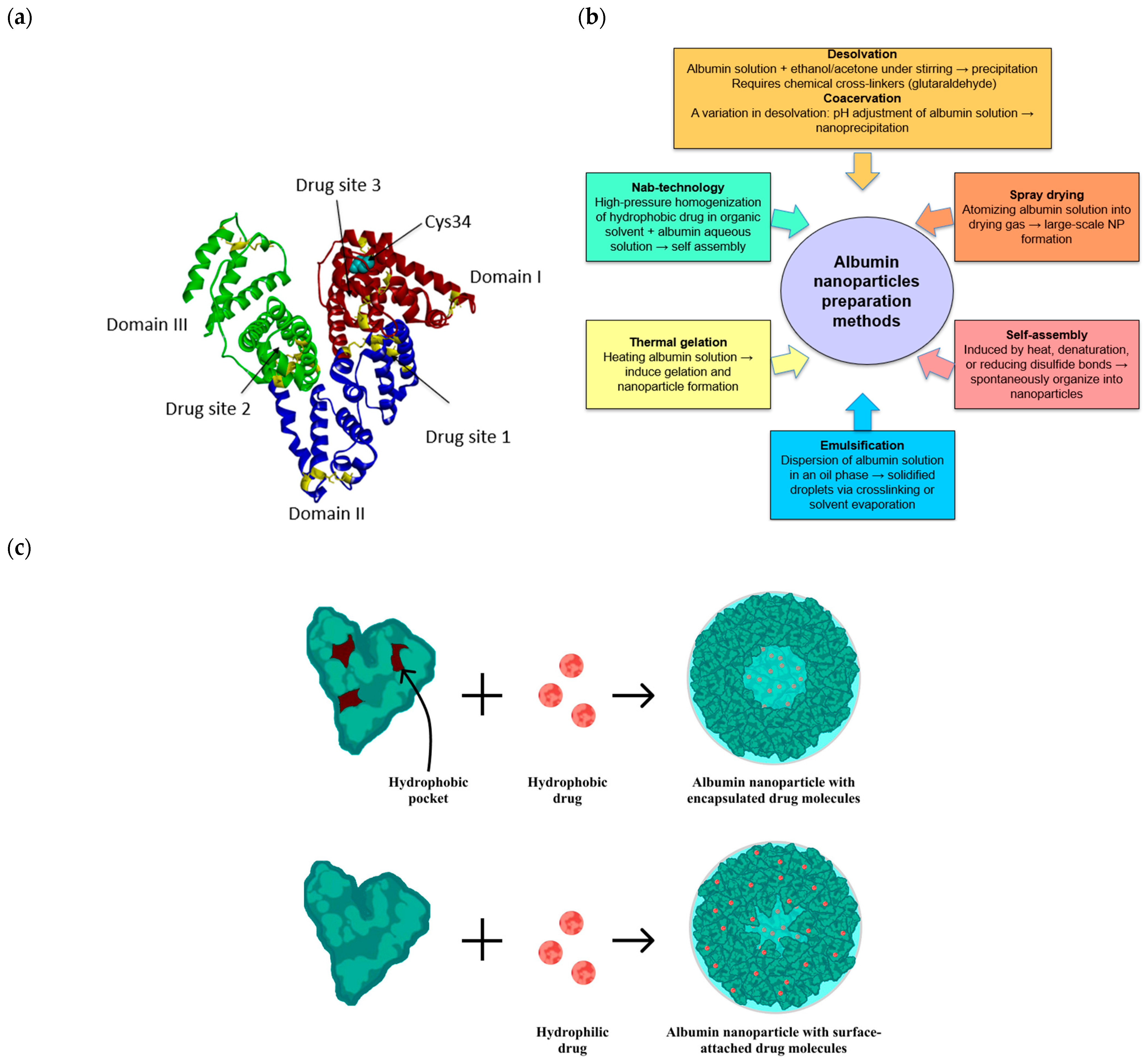
2. Albumin Nanoparticles: Properties and Methods of Preparation
3. Clinical Applications in Cancer Treatment
4. Challenges
5. Future Directions for Albumin Nanoparticles in Cancer Treatment
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krieghoff-Henning, E.; Folkerts, J.; Penzkofer, A.; Weg-Remers, S. Cancer—An overview. Med. Monatsschr Pharm. 2017, 40, 48–54. [Google Scholar]
- World Health Organization. Cancer [Internet]; WHO: Geneva, Switzerland, 2025; Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 23 August 2025).
- Pich, O.; Bailey, C.; Watkins, T.B.; Zaccaria, S.; Jamal-Hanjani, M.; Swanton, C. The translational challenges of precision oncology. Cancer Cell. 2022, 40, 458–478. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. All Cancers Fact Sheet [Internet]. GLOBOCAN 2022 Version 1.1; Updated 8 February 2024. Available online: https://gco.iarc.who.int/media/globocan/factsheets/cancers/39-all-cancers-fact-sheet.pdf (accessed on 20 August 2025).
- International Agency for Research on Cancer. Cancer Tomorrow: Isotype Visualization, Year 2050 [Internet]; GLOBOCAN 2022 v1.1; Updated Feb 2024; IARC: Lyon, France; Available online: https://gco.iarc.fr/tomorrow/en/dataviz/isotype?years=2050 (accessed on 20 August 2025).
- Zheng, H.C. The molecular mechanisms of chemoresistance in cancers. Oncotarget 2017, 8, 59950–59964. [Google Scholar] [CrossRef] [PubMed]
- Gyanani, V.; Haley, J.C.; Goswami, R. Challenges of Current Anticancer Treatment Approaches with Focus on Liposomal Drug Delivery Systems. Pharmaceuticals 2021, 14, 835. [Google Scholar] [CrossRef] [PubMed]
- Bentzen, S.M. Preventing or reducing late side effects of radiation therapy: Radiobiology meets molecular pathology. Nat. Rev. Cancer 2006, 6, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Panichpisal, K.; Kurtzman, N.; Nugent, K. Cisplatin nephrotoxicity: A review. Am. J. Med. Sci. 2007, 334, 115–124. [Google Scholar] [CrossRef]
- Young, A.C.; Mercer, B.; Perren, T.J.; Dodwell, D. Anthracycline-induced cardiomyopathy in siblings with early breast cancer. Ann. Oncol. 2011, 22, 1692. [Google Scholar] [CrossRef]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of ATPdependent transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef]
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef]
- Martinho, N.; Damgé, C.; Reis, C.P. Recent Advances in Drug Delivery Systems. J. Biomater. Nanobiotechnol. 2011, 2, 510–526. [Google Scholar] [CrossRef]
- Afzal, O.; Altamimi, A.S.A.; Nadeem, M.S.; Alzarea, S.I.; Almalki, W.H.; Tariq, A.; Mubeen, B.; Murtaza, B.N.; Iftikhar, S.; Riaz, N.; et al. Nanoparticles in Drug Delivery: From History to Therapeutic Applications. Nanomaterials 2022, 12, 4494. [Google Scholar] [CrossRef]
- Lohcharoenkal, W.; Wang, L.; Chen, Y.C.; Rojanasakul, Y. Protein Nanoparticles as Drug Delivery Carriers for Cancer Therapy. BioMed Res. Int. 2014, 2014, e180549. [Google Scholar] [CrossRef]
- Kratz, F. Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. J. Control. Release 2008, 132, 171–183. [Google Scholar] [CrossRef]
- Karimi, M.; Bahrami, S.; Ravari, S.B.; Zangabad, P.S.; Mirshekari, H.; Bozorgomid, M.; Shahreza, S.; Sori, M.; Hamblin, M.R. Albumin nanostructures as advanced drug delivery systems. Expert. Opin. Drug Deliv. 2016, 13, 1609–1623. [Google Scholar] [CrossRef]
- Bertucci, C.; Domenici, E. Reversible and Covalent Binding of Drugs to Human Serum Albumin: Methodological Approaches and Physiological Relevance. Curr. Med. Chem. 2002, 9, 1463–1481. [Google Scholar] [CrossRef] [PubMed]
- Ghuman, J.; Zunszain, P.A.; Petitpas, I.; Bhattacharya, A.A.; Otagiri, M.; Curry, S. Structural basis of the drug-binding specificity of human serum albumin. J. Mol. Biol. 2005, 353, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Heath, R.J. Structural and biochemical features of human serum albumin essential for eukaryotic cell culture. Int. J. Mol. Sci. 2021, 22, 8411. [Google Scholar] [CrossRef]
- Bujacz, A. Structures of bovine, equine and leporine serum albumin. Biol. Crystallogr. 2012, 68, 1278–1289. [Google Scholar] [CrossRef]
- Shahapurmath, S.; Sharannavar, B.R.; Koli, R. Serum albumin nanoparticles: Ligand functionalization for enhanced targeted therapeutics in precision medicine. Med. Drug Discov. 2025, 27, 100218. [Google Scholar] [CrossRef]
- Al-Harthi, S.; Alshehri, A.; Alhudhali, L.; Alghrably, M.; Bennici, G.; Almohaywi, M.; Jaremko, Ł.; Jaremko, M. Albumin as a Drug Delivery System: Mechanisms, Applications, and Innovations. Nanoparticle Drug Delivery—A Comprehensive Overview; IntechOpen: London, UK, 2025. [Google Scholar] [CrossRef]
- An, F.-F.; Zhang, X.-H. Strategies for Preparing Albumin-based Nanoparticles for Multifunctional Bioimaging and Drug Delivery. Theranostics 2017, 7, 3667–3689. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Albumin-based nanoparticles as potential controlled release drug delivery systems. J. Control. Release 2012, 157, 168–182. [Google Scholar] [CrossRef]
- Adick, A.; Hoheisel, W.; Schneid, S.; Mulac, D.; Azhdari, S.; Langer, K. Challenges of nanoparticle albumin bound (nabTM) technology: Comparative study of Abraxane® with a newly developed albumin-stabilized itraconazole nanosuspension. Eur. J. Pharm. Biopharm. 2023, 193, 129–143. [Google Scholar] [CrossRef]
- Bessone, F.; Dianzani, C.; Argenziano, M.; Cangemi, L.; Spagnolo, R.; Maione, F.; Giraudo, E.; Cavalli, R. Albumin nanoformulations as an innovative solution to overcome doxorubicin chemoresistance. Cancer Drug Resist. 2021, 4, 192–207. [Google Scholar] [CrossRef] [PubMed]
- Gradishar, W.J. Albumin-bound paclitaxel: A next-generation taxane. Expert. Opin. Pharmacother. 2006, 7, 1041–1053. [Google Scholar] [CrossRef]
- Cho, H.; Jeon, S.I.; Ahn, C.-H.; Shim, M.K.; Kim, K. Emerging Albumin-Binding Anticancer Drugs for Tumor-Targeted Drug Delivery: Current Understandings and Clinical Translation. Pharmaceutics 2022, 14, 728. [Google Scholar] [CrossRef]
- Wagner, A.J.; Ravi, V.; Riedel, R.F.; Ganjoo, K.; Van Tine, B.A.; Chugh, R.; Cranmer, L.; Gordon, E.M.; Hornick, J.L.; Du, H.; et al. Phase II Trial of nab- Sirolimus in Patients With Advanced Malignant Perivascular Epithelioid Cell Tumors (AMPECT): Long-Term Efficacy and Safety Update. J. Clin. Oncol. 2024, 42, 1472–1476. [Google Scholar] [CrossRef] [PubMed]
- Hornok, V. Serum Albumin Nanoparticles: Problems and Prospects. Polymers 2021, 13, 3759. [Google Scholar] [CrossRef]
- Raj, S.; Khurana, S.; Choudhari, R.; Kesari, K.K.; Kamal, M.A.; Garg, N.; Ruokolainen, J.; Das, B.C.; Kumar, D. Specific targeting cancer cells with nanoparticles and drug delivery in cancer therapy. Semin. Cancer Biol. 2021, 69, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Hermeling, S.; Crommelin, D.J.A.; Schellekens, H.; Jiskoot, W. Structure-immunogenicity relationships of therapeutic proteins. Pharm. Res. 2004, 21, 897–903. [Google Scholar] [CrossRef]
- Merodio, M.; Arnedo, A.; Renedo, M.; Irache, J.M. Ganciclovir-loaded albumin nanoparticles: Characterization and in vitro release properties. Eur. J. Pharm. Sci. 2001, 12, 251–259. [Google Scholar] [CrossRef]
- Truong-Le, V.L.; Walsh, S.M.; Schweibert, E.; Mao, H.-Q.; Guggino, W.B.; August, J.; Leong, K.W. Gene transfer by DNA-gelatin nanospheres. Arch. Biochem. Biophys. 1999, 361, 47–56. [Google Scholar] [CrossRef]
- Amighi, F.; Emam-Djomeh, Z.; Labbafi-Mazraeh-Shahi, M. Effect of different cross-linking agents on the preparation of bovine serum albumin nanoparticles. J. Iran. Chem. Soc. 2020, 17, 1223–1235. [Google Scholar] [CrossRef]
- Niknejad, H.; Mahmoudzadeh, R. Comparison of different crosslinking methods for preparation of docetaxel-loaded albumin nanoparticles. Iran. J. Pharm. Res. IJPR 2015, 14, 385. [Google Scholar] [PubMed Central]
- Luo, R.; Lin, M.; Zhang, C.; Shi, J.; Zhang, S.; Chen, Q.; Hu, Y.; Zhang, M.; Zhang, J.; Gao, F. Genipin-crosslinked human serum albumin coating using a tannic acid layer for enhanced oral administration of curcumin in the treatment of ulcerative colitis. Food Chem. 2020, 330, 127241. [Google Scholar] [CrossRef] [PubMed]
- Barbinta-Patrascu, M.-E.; Iftimie, S.; Cazacu, N.; Stan, D.L.; Costas, A.; Balan, A.E.; Chilom, C.G. Bio-Entities Based on Albumin Nanoparticles and Biomimetic Cell Membranes: Design, Characterization and Biophysical Evaluation. Coatings 2023, 13, 671. [Google Scholar] [CrossRef]
- Park, C.; Baek, N.; Loebenberg, R.; Lee, B.-J. Importance of the fatty acid chain length on in vitro and in vivo anticancer activity of fattigation-platform albumin nanoparticles in human colorectal cancer xenograft mice model. J. Control. Release 2020, 324, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Kuche, K.; Yadav, V.; Dharshini, M.; Ghadi, R.; Chaudhari, D.; Date, T.; Jain, S. Synergistic anticancer therapy via ferroptosis using modified bovine serum albumin nanoparticles loaded with sorafenib and simvastatin. Int. J. Biol. Macromol. 2023, 253, 127254. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Y.; Yang, N.; Yin, W.; Yang, H.; Li, C.; Zhuang, Y.; Song, Z.; Cheng, X.; Shi, S.; et al. Self-assembly of maltose-albumin nanoparticles for efficient targeting delivery and therapy in liver cancer. Int. J. Biol. Macromol. 2024, 258, 128691. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Yao, F.; An, Y.; Li, X.; Yang, X.-D. Novel Nanotherapeutics for Cancer Immunotherapy by PD-L1-Aptamer-Functionalized and Fexofenadine-Loaded Albumin Nanoparticles. Molecules 2023, 28, 2556. [Google Scholar] [CrossRef]
- Jiang, Q.; Yao, F.; An, Y.; Lai, X.; Li, X.; Yu, Z.; Yang, X.-D. Novel nanotherapeutics for cancer immunotherapy by albumin nanoparticles functionalized with PD-1 and PD-L1 aptamers. Cancer Nanotechnol. 2024, 15, 3. [Google Scholar] [CrossRef]
- Ibrahim, A.I.; Sahyon, H.A.; Attia, A.M.; El-Shehawy, A.A. Novel protocatechuic acid encapsulated bovine serum albumin functionalized folic acid nanoparticles for targeted therapy in urethane-induced lung cancer model. Sci. Rep. 2025, 15, 25793. [Google Scholar] [CrossRef] [PubMed]
- Xiong, B.; Liu, H.; Yi, M.; Li, Y.; Huang, Y.; Guo, W.; Lin, Y.; Liu, P.; Lu, B. A pH/GSH dual-responsive, folic acid-targeted drug delivery system based on bovine serum albumin nanoparticles for cancer therapy. Part. Part. Syst. Charact. 2024, 41, 2400184. [Google Scholar] [CrossRef]
- Li, Q.; Meng, F.; Xie, M.; Nishikawa, M.; Kusamori, K.; Giri, A.K.; Yang, L.; Li, Q.; Gu, H.; Chen, Z.; et al. Folate-targeted pH-sensitive albumin nanoparticles loaded with Baicalin enhance tamoxifen efficacy in estrogen receptor α-positive breast cancer. Int. J. Biol. Macromol. 2025, 318, 145155. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yao, S.-Y.; Luo, J.; Ding, C.; Huang, Q.; Yang, Y.; Shi, Z.; Lin, J.; Pan, Y.-C.; Zeng, X.; et al. Engineered hypoxia-responsive albumin nanoparticles mediating mitophagy regulation for cancer therapy. Nat. Commun. 2025, 16, 596. [Google Scholar] [CrossRef] [PubMed]

| Treatment | Type | Clinical Stage | Clinical Application | Refs. |
|---|---|---|---|---|
| Abraxane® Nabpaclitaxel (ABI-007) | HSA-bound paclitaxel NP | Approved by FDA | Metastatic breast cancer, non-small cell lung cancer, pancreatic cancer | [28,32] |
| Fyarro® Nab-rapamycin (ABI-009) | Albumin-bound rapamycin NP | Approved by FDA | Advanced malignant PEComa in adults | [30] |
| Nab-docetaxel (ABI-008) | Albumin-bound docetaxel NP | Phase I/II * | Prostate and breast cancers | [31] |
| Nab-thiocolchicine dimer (ABI-011) | Albumin-bound thiocolchicine dimer | Phase I * | Advanced solid tumor malignancies and lymphomas | [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadri, H.; Alshatfa, M.; Alsalloum, F.Z.; Elhissi, A.; Daou, A.; Khoder, M. Albumin Nanoparticles in Cancer Therapeutics: Clinical Status, Challenges, and Future Directions. Pharmaceutics 2025, 17, 1290. https://doi.org/10.3390/pharmaceutics17101290
Kadri H, Alshatfa M, Alsalloum FZ, Elhissi A, Daou A, Khoder M. Albumin Nanoparticles in Cancer Therapeutics: Clinical Status, Challenges, and Future Directions. Pharmaceutics. 2025; 17(10):1290. https://doi.org/10.3390/pharmaceutics17101290
Chicago/Turabian StyleKadri, Hachemi, Mesk Alshatfa, Feras Z. Alsalloum, Abdelbary Elhissi, Anis Daou, and Mouhamad Khoder. 2025. "Albumin Nanoparticles in Cancer Therapeutics: Clinical Status, Challenges, and Future Directions" Pharmaceutics 17, no. 10: 1290. https://doi.org/10.3390/pharmaceutics17101290
APA StyleKadri, H., Alshatfa, M., Alsalloum, F. Z., Elhissi, A., Daou, A., & Khoder, M. (2025). Albumin Nanoparticles in Cancer Therapeutics: Clinical Status, Challenges, and Future Directions. Pharmaceutics, 17(10), 1290. https://doi.org/10.3390/pharmaceutics17101290








